Introduction
Posterior capsule opacification (PCO), which is the
most frequent long-term complication of modern cataract surgery, is
the result of proliferation and fibrogenesis of lens epithelial
cells (LECs) following surgical trauma, and may also result in
anterior capsular constriction and capsular bag fibrosis (1–4).
Inhibition of proliferation and fibrogenesis of LECs may
significantly improve the results of refraction cataract
surgery.
Transforming growth factor-β (TGF-β) is an
extensively well studied polypeptide growth factor, and consists of
three subtypes in humans, TGF-β1, TGF-β2 and TGF-β3. TGF-β1 and
TGF-β2 are both highly expressed in the human lens and ocular media
(5), and serve key roles in
regulating proliferation, migration and epithelial-mesenchymal
transition (EMT) of LECs in PCO (6–8).
TGF-β2 is most closely associated with trans-differentiation and
histopathological fibrosis of LECs, and is an important regulatory
factor in the pathogenesis and growth of LECs (9,10).
TGF-β2 expression is upregulated in aqueous humor and vitreous
bodies following surgical trauma (11). Binding of TGF-β2 to its receptor
activates the Smad protein family and results in the translocation
of membrane kinase receptors to the cell nucleus (12). Smad3 is a key protein involved in
EMT induced by TGF-β following trauma, and LEC-EMT is dependent on
the Smad3 pathway (13). Wormstone
et al (14) showed that the
increase in the expression of α-smooth muscle actin (SMA) induced
by TGF-β2 in LECs was significantly inhibited by a human monoclonal
anti-TGF-β2 antibody, CAT-152. Sun et al (15) also demonstrated that the
anti-TGF-β2 (anti-T) antibody significantly reduced migration and
EMT in LECs.
Research on peptides has increased significantly in
the past decade (16,17), and numerous therapeutic peptides
are in pre-clinical or clinical trials and ~60 peptide drugs have
already been approved (18,19).
These peptide-based therapeutics have certain advantages over drugs
based on small molecules or protein antibodies, including higher
biological activity, higher specificity and low levels of toxicity
(20). It has been demonstrated
that peptide drugs have potential applications in clinical fields,
including in metabolic diseases, oncology and cardiovascular
disease (19). Currently, a number
of peptides have been developed for treating ocular pathologies
(21,22). In previous studies, a small
molecule peptide H-RN has been demonstrated to exert
anti-inflammatory effects in an endotoxin-induced uveitis model,
and a decrease in the infiltration of inflammatory cells and
protein transudation, inhibition in the production of
pro-inflammatory mediators in aqueous humor and ocular tissues
(23–26). Furthermore, pathological changes in
the ocular tissues as a result of inflammation was ameliorated by
H-RN, and it exhibited no toxic effects on macrophages and human
umbilical vein endothelial cells (HUVECs), and significantly
suppressed lipopolysaccharide (LPS)-induced phosphorylation of
NF-κB-p65, possibly via the PI3K/Akt signaling pathway (23–26).
Furthermore, other similar functional small peptides have also been
identified by our laboratory (27–30).
In our previous studies, the peptide H-RN, derived
from hepatocyte growth factor (HGF) kringle 1 domain was designed
and demonstrated to exhibit anti-angiogenic activity in a mouse
model of vascular endothelial growth factor (VEGF)-induced corneal
neovascularization (23–26). In the present study, the effects of
H-RN on the development of EMT induced by TGF-β2 via the TGF-β/Smad
and Akt/mTOR signaling pathways in human LECs were
investigated.
Materials and methods
Cell culture and treatment
Human LEC line SRA01/04 was obtained from American
Type Culture Collection, and the cells were cultured in DMEM
(Thermo Fisher Scientific, Inc.) with 10% FBS (Thermo Fisher
Scientific, Inc.) in a humidified 37°C incubator with 5%
CO2. A total of 24 h prior to treatment, cells were
seeded in cell culture plates, and 30 min before treatment, 10
ng/ml recombinant human TGF-β2 (Cell Signaling Technology, Inc.)
was added to the cells with or without 50 µM H-RN (ChinaPeptides
Co., Ltd.) for 24 h (25), and
TGF-β2 inhibitor SB431542 (Sigma-Aldrich; Merck KGaA) was added
combined with TGF-β2 as a positive control or to untreated cells as
a negative control.
Reverse transcription-quantitative
(RT-q)PCR
Following the various aforementioned treatments,
cells with harvested and total RNA was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The mRNA levels of
different genes in the cells were measured using qPCR using an
EzQuick™ One-Step qPCR kit (Biomics Biotechnologies Co., Ltd.)
according to the manufacturer's protocol. Reverse transcription was
performed at 42°C for 30 min, and the following thermocycling
conditions: Initial denaturation at 95°C for 10 min, followed by 45
cycles of 95°C for 20 sec and final extension at 60°C for 1 min.
The housekeeping gene β-actin was used as an internal control. The
results of RT-qPCR were analyzed using the 2−ΔΔCq method
(31). The primer sequences used
are presented in Table I.
 | Table I.Sequences of reverse
transcription-quantitative PCR primers. |
Table I.
Sequences of reverse
transcription-quantitative PCR primers.
| Gene name | Sequences
(5′-3′) |
|---|
| α-SMA | F:
CTCCGGAGCGCAAATACTCT |
|
| R:
TGCTAGAGACAGAGAGGAGCA |
| FN | F:
ACAAGCATGTCTCTCTGCCA |
|
| R:
TCAGGAAACTCCCAGGGTGA |
| Cx43 | F:
AGCCACTAGCCATTGTGGAC |
|
| R:
CCACCTCCACCGGATCAAAA |
| E-cad | F:
TTGTCTGGCCACATCTTGACT |
|
| R:
CTGCAGCACTTTAGGCACTAT |
| β-actin | F:
TTGCCGACAGGATGCAGAAGGA |
|
| R:
AGGTGGACAGCGAGGCCAGGAT |
Western blotting
A total of 1×105 cells/well were plated
in a 6-well plate and grown for 24 h, cells were treated as
described above, and total proteins were extracted using RIPA lysis
buffer (Sigma-Aldrich; Merck KGaA) on ice. Denatured proteins were
quantified using the BCA method and then 50 µg protein per lane was
loaded on a 12% SDS-gel, resolved using SDS-PAGE and transferred to
a PVDF membrane (GE Healthcare). Subsequently, membranes were
blocked using 5% non-fat milk for 2 h at room temperature, then
incubated with rabbit anti-human α-SMA (1:2,000; ab5694),
fibronectin (FN) (1:1,000; ab2413), E cadherin (E-cad) (1:500;
ab15148), connexin 43 (Cx43) (1:1,000; ab11370), Akt (1:1,000,
ab179463), phosphorylated (p)-Akt (1:5,000; ab81283), mTOR
(1:1,000; ab134903), p-mTOR (1:1,000; ab109268), p70S6K1 (1:5,000;
ab32529), p-p70S6K1 (1:1,000; ab5231), Smad2 (1:2,000; ab40855),
p-Smad2 (1:300; ab53100), Smad3 (1:1,000; ab40854) or p-Smad3
(1:2,000; ab52903) antibody or a mouse anti-human β-actin antibody
(1:2,000; ab115777; all from Abcam) at 4°C overnight. After washing
with TBST (0.05% Tween-20), the membranes were incubated with a
horseradish peroxidase-conjugated IgG secondary antibody (1:2,000;
cat. no. ab205718; Abcam) for 2 h at room temperature. After
washing with TBST again, signals were visualized using ECL Western
Blotting Substrate (Promega Corporation). Densitometry analysis was
performed using ImageJ v1.51 (National Institutes of Health).
Immunofluorescence staining
Cells were seeded in 24-well plates that contained a
glass coverslip in each well and treated as described above. Once
cells had adhered, they were fixed with 4% paraformaldehyde at 4°C
for 30 min, washed with PBS, permeabilized with 0.5% Triton X-100
for 10 min at room temperature, washed with PBS and blocked with 1%
BSA in PBS for 30 min at room temperature. Subsequently, the cells
were incubated with α-SMA (1:50; cat. no. ab5694), FN (1:50; cat.
no. ab2413), Cx43 (1:1,000; cat. no. ab11370) or E-cad (1:50; cat.
no. ab15148) antibodies (all from Abcam) overnight at 4°C. The
following day, the cells were washed with PBS, and incubated with
an Alexa Fluor® 594 conjugated goat anti-rabbit IgG
(1:1,000; cat. no. ab150080; Abcam) at room temperature for 30 min.
The nucleus was stained using Hoechst 33258 (Thermo Fisher
Scientific, Inc.) for 10 min at room temperature. Fluorescent cells
were visualized using a fluorescence microscope (magnification,
×40).
Cell proliferation assay
Cell proliferation was assessed using the MTT
method. Briefly, 1×103 cells/well were plated in a
96-well plate and grown for 24 h. After cells were treated for 24,
48 and 72 h as described above, 10 µl of MTT (Promega Corporation)
was added to each well, and incubated at 37°C for 4 h away from
light. Subsequently, 150 µl DMSO was added to each well and
incubated at 37°C for 10 min. Fluorescence intensity of each well
was measured at 490 nm using a microplate reader (BioTek
Instruments, Inc.).
Wound-healing assay
Cell migration was assessed using a wound-healing
assay. Briefly, 1×105 cells/well were plated into a
96-well plate and grown for 24 h. A wound was created in the
confluent monolayer of cells using a 1-ml pipette tip and cell
culture medium was replaced with fresh DMEM containing 1% FBS
(32), then cells were cultured in
a humidified 37°C incubator with 5% CO2. After been
treated as described above for 4 h, cells were washed with PBS, and
images were taken using a light microscope after 0, 24 and 48 h
(magnification, ×4), and cell migration was calculated by relative
migration rate (%) = [1-(wound area at Tt/wound area at T0)] ×
100.
Hematoxylin and eosin (H&E)
staining
Cells were seeded into 24-well plates containing
glass coverslips, and treated as described above. Subsequently, the
glass coverslips were washed with PBS, fixed in 95% pre-chilled
ethanol for 10 min at room temperature and washed with PBS. This
was followed by staining with Harris hematoxylin solution for 10
min at room temperature, washing with PBS, and then staining with
eosin solution for 10 min, following which they were washed again
with PBS. Finally, cells were mounted using antifade mounting
medium, and the stained cells were observed under a light
microscope (magnification, ×20).
Statistical analysis
All experiments were performed three times
independently. Data are presented as the mean ± standard deviation.
Statistical analyses were performed using SPSS version 19.0 (IBM
Corp.). A comparison between two groups was performed using a
Student's t-test and the differences between multiple groups were
determined using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
H-RN suppresses the development of
TGF-β2-induced EMT
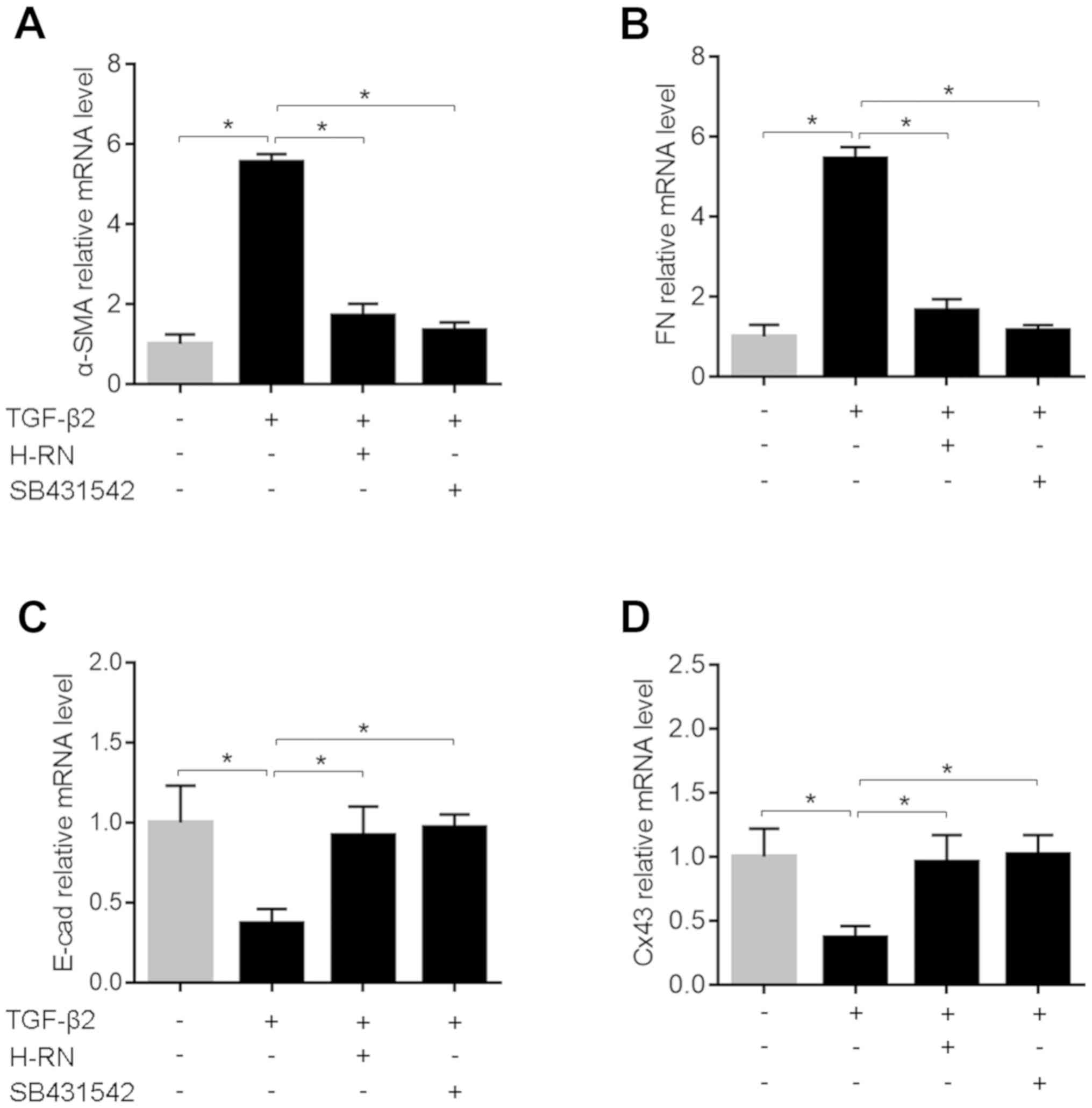
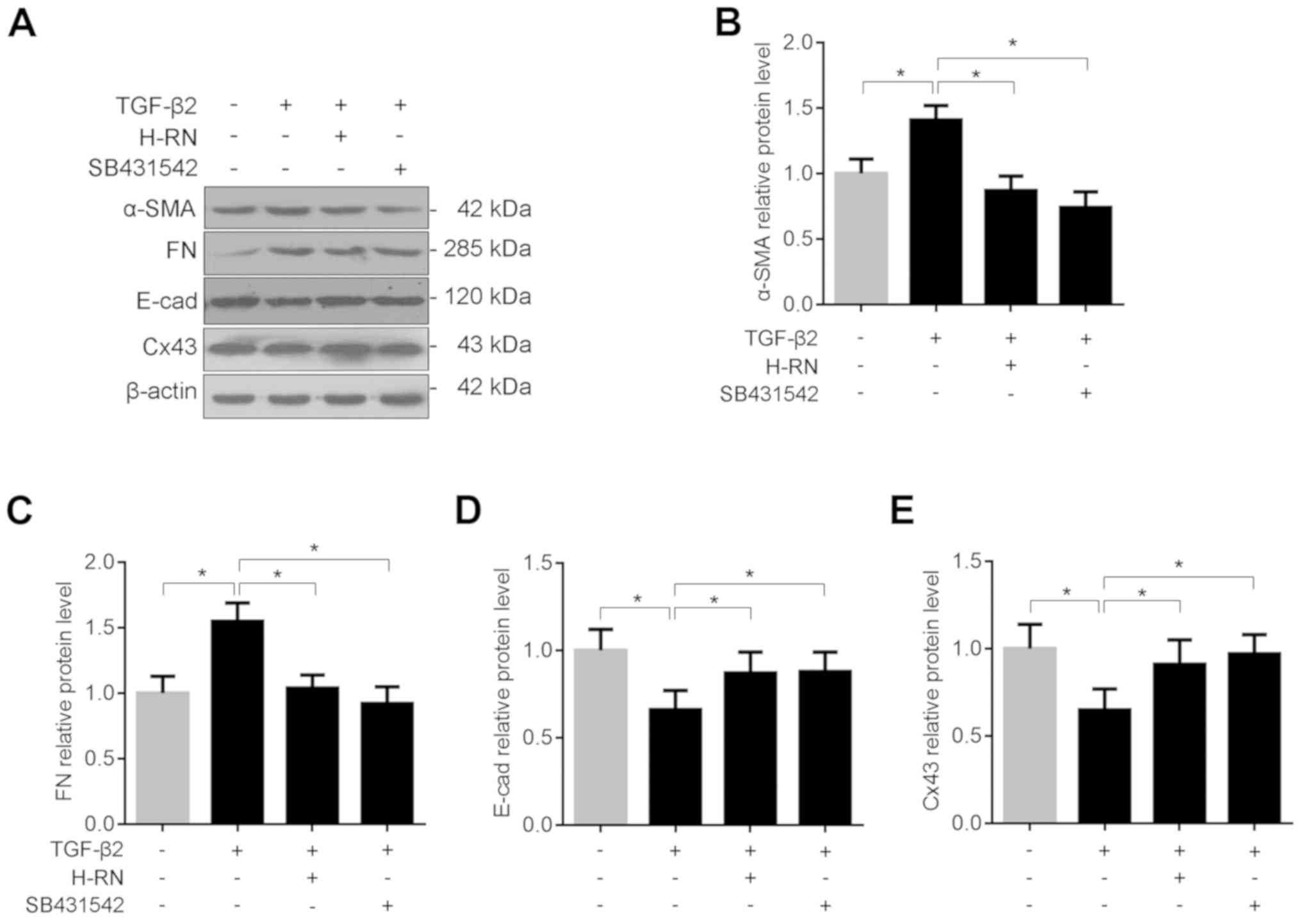
To investigate the effect of H-RN on TGF-β2-induced
EMT in human LECs, the mRNA and protein expression levels of EMT
markers: α-SMA, FN, E-cad and Cx43 were detected by RT-qPCR and
western blotting, respectively. Compared with the untreated cells,
the mRNA and protein expression levels of α-SMA and FN were
significantly increased, and the expression levels of E-cad and
Cx43 were significantly decreased in LECs induced by TGF-β2. The
expression of these EMT markers was increased by TGF-β2 and
decreased by H-RN significantly in LECs, and the same results were
observed when cells were treated with the TGF-β2 inhibitor SB431542
(Figs. 1 and 2).
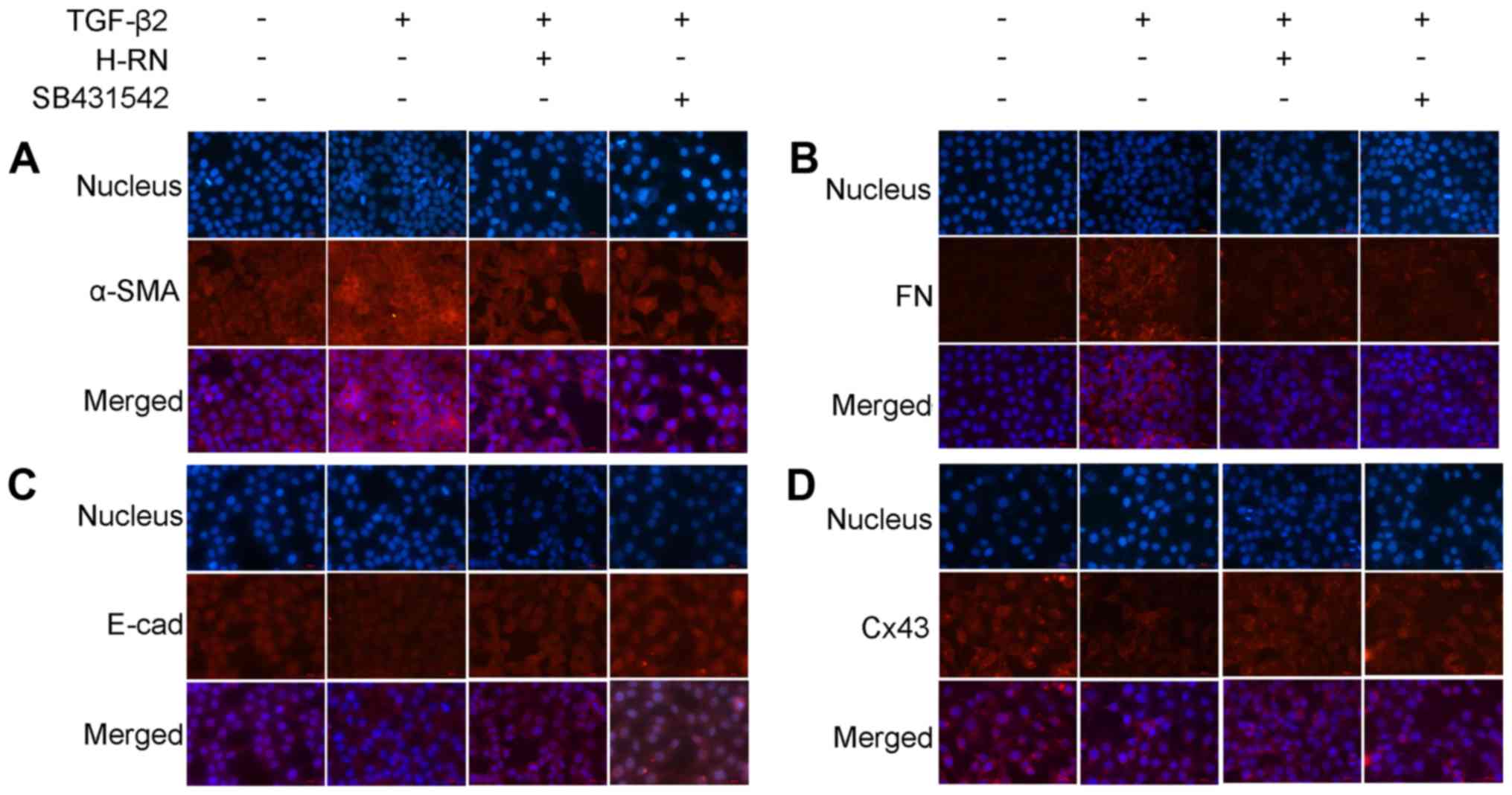
Expression of the EMT markers, α-SMA, FN, E-cad and
Cx43, in LECs were also determined by immunofluorescence, and the
results showed that the expression levels of these EMT markers were
consistent with the western blotting and RT-qPCR results (Fig. 3). Therefore, H-RN inhibited TGF-β2
induced EMT in LECs.
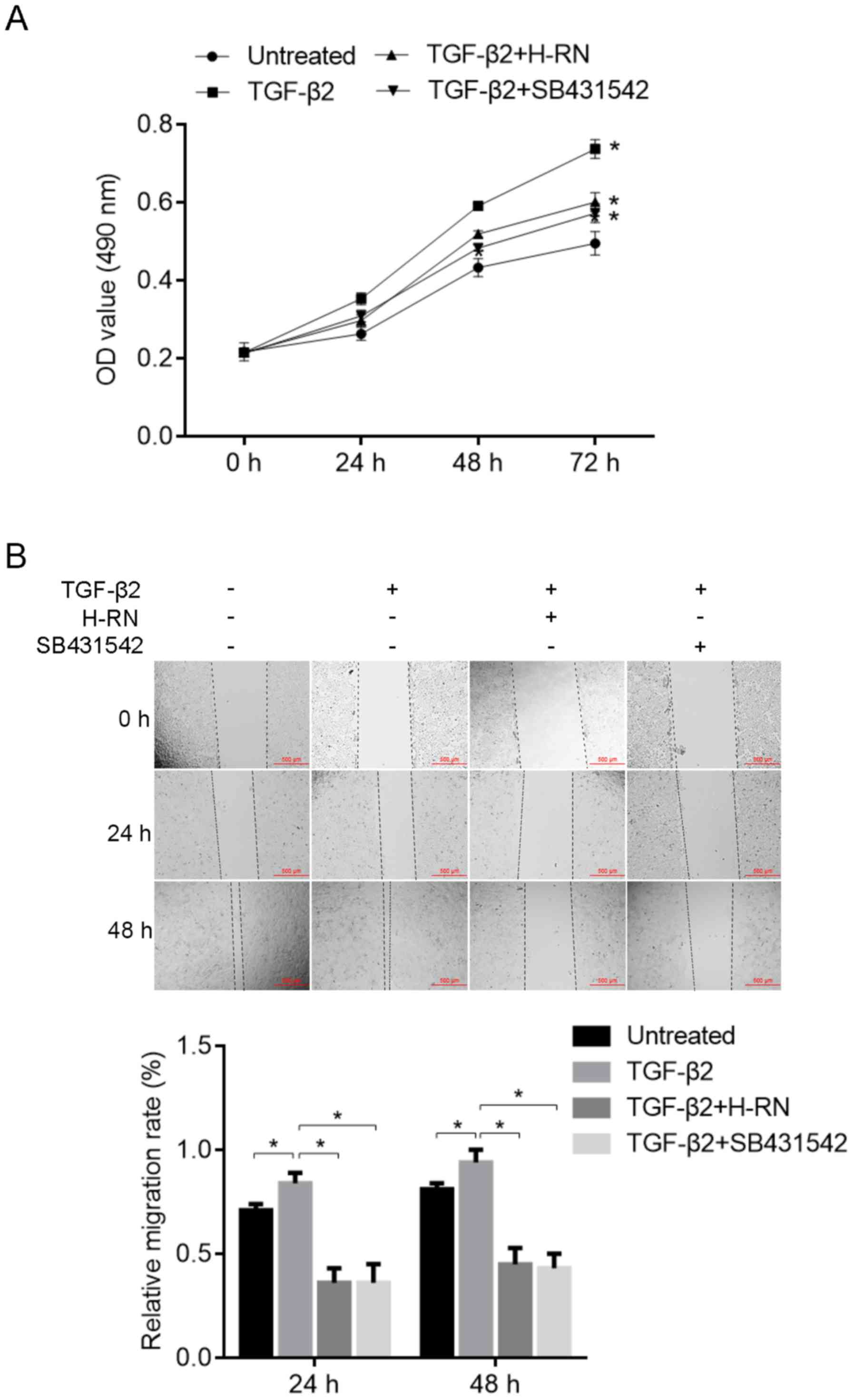
Proliferation and migration of LECs is
modulated by H-RN
MTT assays were used to assess the proliferation of
LECs treated with TGF-β2 and H-RN. The results showed that the
proliferation of LECs was increased by TGF-β2 significantly
(P<0.05), and the TGF-β2-induced increase in cell proliferation
was significantly inhibited by H-RN and SB431542 (P<0.05;
Fig. 4A), compared with the
untreated cells. The migration of LECs treated with TGF-β2 and H-RN
was assessed using a wound-healing assay. Compared with the
untreated cells, the migration of LECs was significantly increased
by TGF-β2 (P<0.05), and TGF-β2-induced cell migration was
significantly inhibited by H-RN or SB431542 (P<0.05; Fig. 4B).
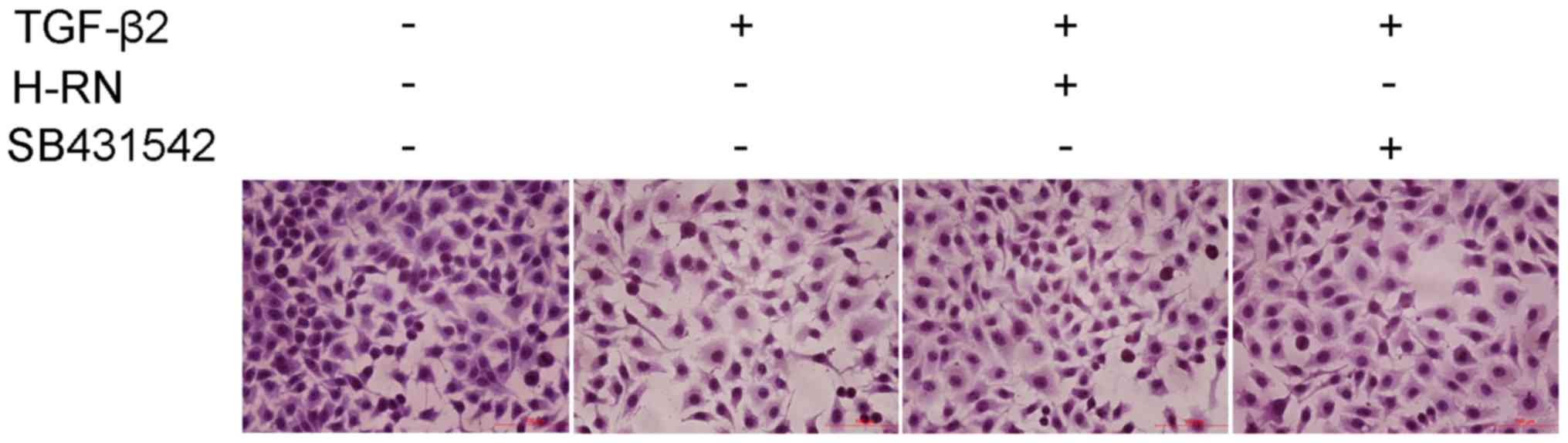
Effect of H-RN on the morphology of
LECs
H&E staining was used to observe the morphology
of LECs treated with TGF-β2 and H-RN. The results showed that the
morphology of the cells exhibited a more mesenchymal-like phenotype
when treated with TGF-β2. LECs exhibited altered morphologies,
appearing flattened and stretched instead of their typical
oval-like shape and additionally, adherence was reduced resulting
in an increase in the number of floating cells, and the gaps
between cells were increased as well. Treatment with H-RN or
SB431542 in conjunction with TGF-β notably reduced the
morphological changes (Fig.
5).
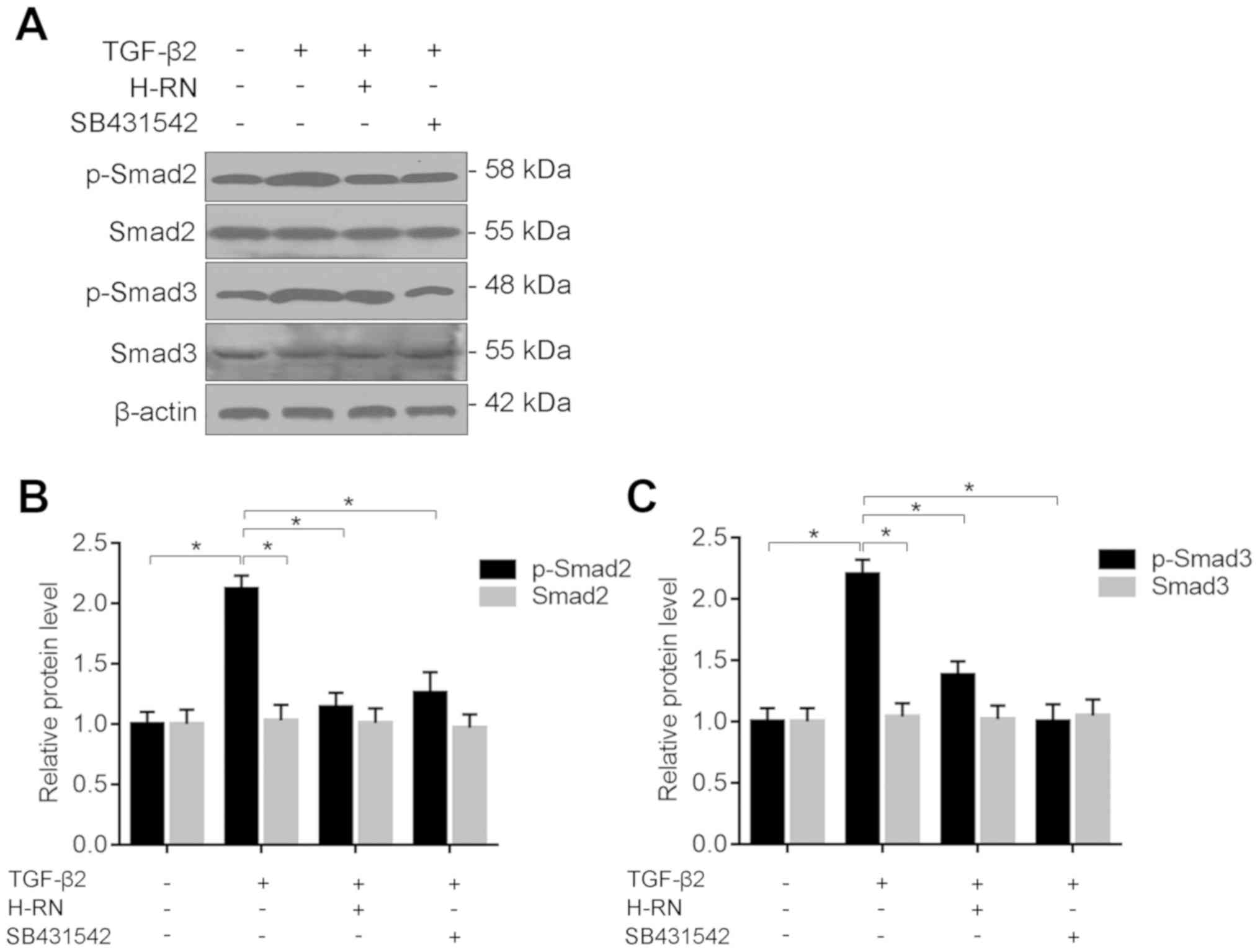
H-RN suppresses the development of EMT
via the TGF-β/Smad signaling pathway
To clarify the regulatory mechanism by which H-RN
regulated EMT in LECs, the phosphorylation of Smad2 and Smad3,
members of the TGF-β/Smad signaling pathway, were assessed using
western blotting. The results showed that Smad2 and Smad3
phosphorylation were increased by TGF-β2, and H-RN or SB431542
significantly inhibited this (Fig.
6). The results suggest that H-RN suppressed the development of
EMT, possibly via inhibition of the TGF-β/Smad signaling
pathway.
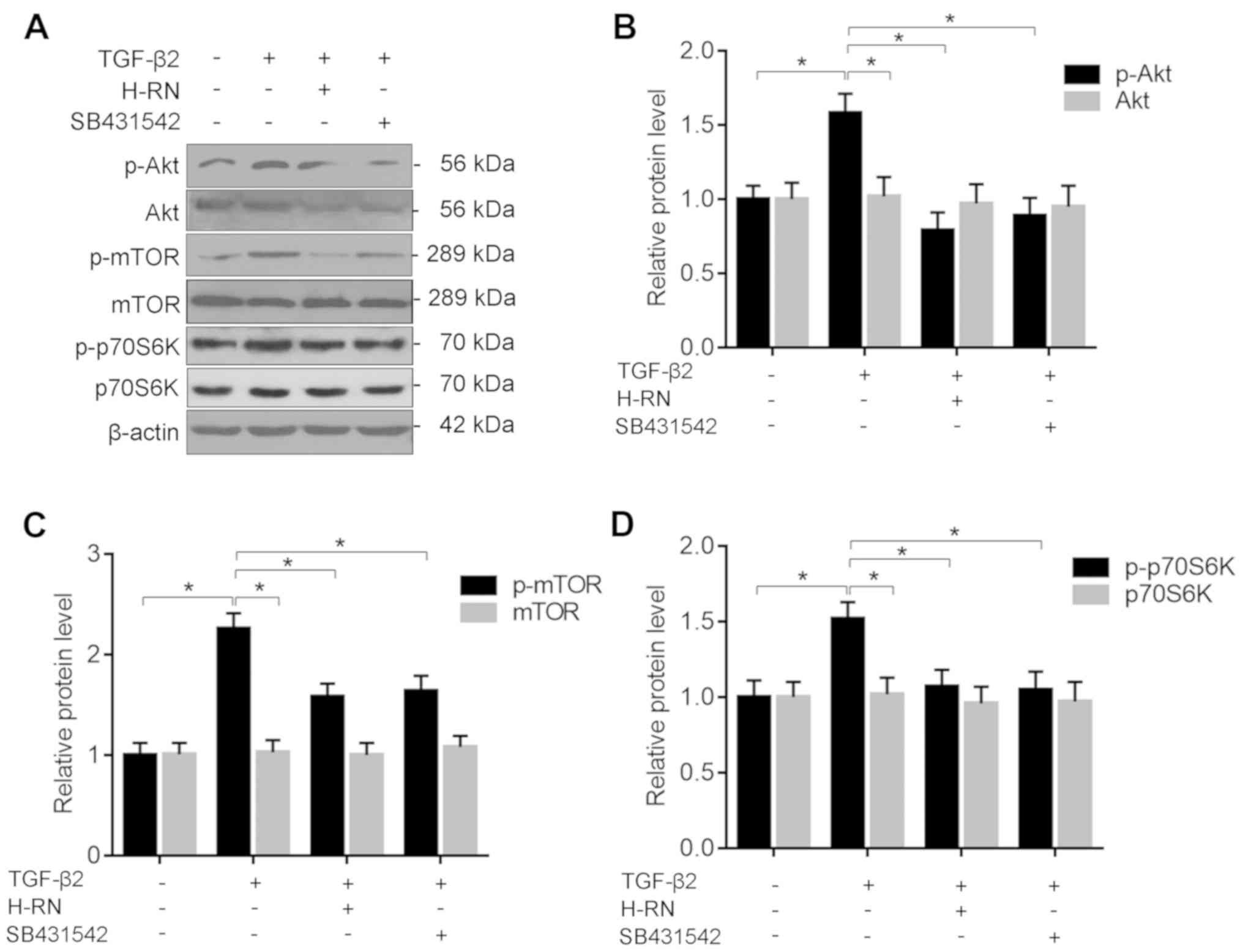
H-RN suppresses the development of EMT
via the Akt/mTOR signaling pathway
The effects of H-RN on the Akt/mTOR signaling
pathway were further investigated. The results showed that levels
of p-Akt, mTOR and P70S6K were increased significantly in TGF-β2
treated LECs, and H-RN or SB431542 significantly inhibited the
TGF-β-induced increase (Fig. 7).
The results further support the hypothesis that H-RN suppressed the
development of EMT, possibly via inhibition of the Akt/mTOR
signaling pathway.
Discussion
Lens epithelial cells (LECs) are essential for the
normal development and homeostatic maintenance of the lens
(33). Aberrant growth,
proliferation, migration, trans-differentiation and secretion of
components of the extracellular matrix of LECs following cataract
surgery may contribute to the development of various diseases of
the lens, such as Posterior capsule opacification (PCO) (1,2).
Numerous studies have shown that LECs can undergo
epithelial-mesenchymal transition (EMT), and these transformed
cells bear a morphological and molecular resemblance to PCO
(34–36). During EMT, there are a series of
changes, such as the formation of spindle-like cells accompanied by
wrinkling of the lens capsule, accumulation of extracellular matrix
and cell death as a result of apoptosis (37,38).
However, EMT is a complex mechanism, and involves different
signaling pathways from the microenvironment in vitro and
in vivo (39–41). The TGF-β subtypes, including TGF-β1
and TGF-β2 have been demonstrated to promote EMT in LECs (6–8). In
the present study, it was demonstrated that TGF-β2 treatment
significantly increased α-SMA and fibronectin expression levels,
decreased E-cadherin and Cx43 expression levels, and that EMT in
LECs was induced by TGF-β2. Also, the proliferation and migration
of LECs were increased by TGF-β2 significantly, although the cell
migration experiments may have been affected by the low confluence
of cells. Furthermore, the morphology of LECs was significantly
altered to a more mesenchymal-like phenotype when LECs were treated
with TGF-β2.
Hepatocyte growth factor (HGF) is a paracrine
cellular growth factor that regulates motility and morphogenic
development, and it has been shown to possess a major role in
embryonic organ development, specifically in myogenesis, in adult
organ regeneration and in wound healing (42,43).
HGF is also a potent stimulator of neo-angiogenesis and an
important angiogenic factor in vascular retinopathies where it acts
as a retinal angiogenesis regulator (44). HGF is comprised of an
amino-terminal domain (N), four kringle domains (K1-K4), and a
serine proteinase homology (SPH) domain (45). The kringle 1 domain, was reported
to exhibit antiangiogenic, antitumor and anti-inflammatory effects
(46–48). H-RN (amino acid sequence,
RNPRGEEGGPW; molecular weight: 1254.34 Da), is a novel peptide
derived from HGF kringle 1 domain, which was identified by Xu et
al (23). H-RN effectively
inhibited the proliferation, migration and tube formation of RF/6A
cells stimulated by VEGF, and was also shown to exhibit
antiangiogenic activity in vitro and in vivo
(23). Sun et al (24) also confirmed that H-RN exhibited
anti-angiogenic activity in HUVECs, and in a mouse model of
VEGF-induced corneal neovascularization the anti-angiogenic
activity of H-RN was associated with apoptosis and cell cycle
arrest, indicating a strategy for anti-angiogenic treatment in the
cornea. Wang et al (25)
further demonstrated that intravitreal treatment of H-RN suppressed
clinical manifestation, this included inhibiting ocular
inflammatory cytokine production and improving histopathological
scores in a concentration dependent manner, and H-RN was shown to
suppress tumor necrosis factor-α-induced adhesion molecule
expression and E-selectin. H-RN significantly suppressed
LPS-induced phosphorylation of NF-κB-p65 and inhibited PI3K-p85 and
Akt (Ser473) phosphorylation, which may result in the attenuation
of LPS-induced IκB kinase (IKK) complex activation and IκB
degradation, this study suggested that H-RN exhibits
anti-inflammatory effects possibly via the PI3K/Akt/IKK/NF-κB
signaling pathway (25). Several
signaling pathways mediate TGF-β induced EMT of LECs, and canonical
TGF-β/Smad signaling has been demonstrated to serve a crucial role
in the networks regulating EMT in LECs (13). In addition, noncanonical signaling
pathways including RhoA (5,41),
Akt (7,25) and ERK (7) are also involved. In the present
study, the effect of H-RN on the development of EMT induced by
TGF-β2 via Smad-dependent and Smad-independent pathways in human
LECs were investigated. Smad2 and Smad3 phosphorylation were
induced by TGF-β2, and phosphorylation of Akt, mTOR and P70S6K was
increased significantly, suggesting that H-RN suppressed the
development of EMT via the TGF-β/Smad and Akt/mTOR signaling
pathways.
In conclusion, to the best of our knowledge, the
present study is the first to report that H-RN exhibits anti-EMT
activity in human LECs induced by TGF-β2. EMT suppression by H-RN
may be mediated via the TGF-β/Smad and Akt/mTOR signaling pathways,
indicating a potential strategy for PCO treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81371069 and 81670898);
Scientific Research Foundation of Shanghai Municipal Commission of
Health and Family Planning (grant no. 201640266) and Scientific
Research Foundation of Nantong Municipal Health Commission Project
for Young People (grant no. WKZL2018015), and the 13th Five-Year
Science and Education Project, Nantong Key Medical Talents Fund for
Young People (grant no. 025).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XH and HZ conceived and designed the study. XH, YW
and PZ performed the experiments. XH and HZ analyzed and
interpreted the data. XH drafted the manuscript. YW, PZ and HZ
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ebihara Y, Kato S, Oshika T, Yoshizaki M
and Sugita G: Posterior capsule opacification after cataract
surgery in patients with diabetes mellitus. J Cataract Refract
Surg. 32:1184–1187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zukin LM, Pedler MG, Groman-Lupa S,
Pantcheva M, Ammar DA and Petrash JM: Aldose Reductase Inhibition
Prevents Development of Posterior Capsular Opacification in an In
Vivo Model of Cataract Surgery. Invest Ophthalmol Vis Sci.
59:3591–3598. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wormstone IM and Eldred JA: Experimental
models for posterior capsule opacification research. Exp Eye Res.
142:2–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valcourt U, Kowanetz M, Niimi H, Heldin CH
and Moustakas A: TGF-beta and the Smad signaling pathway support
transcriptomic reprogramming during epithelial-mesenchymal cell
transition. Mol Biol Cell. 16:1987–2002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao K, Ye PP, Tan J, Tang XJ and Shen Tu
XC: Involvement of PI3K/Akt pathway in TGF-beta2-mediated
epithelial mesenchymal transition in human lens epithelial cells.
Ophthalmic Res. 40:69–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nahomi RB, Pantcheva MB and Nagaraj RH:
αB-crystallin is essential for the TGF-β2-mediated epithelial to
mesenchymal transition of lens epithelial cells. Biochem J.
473:1455–1469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hou M, Bao X, Luo F, Chen X, Liu L and Wu
M: HMGA2 Modulates the TGFβ/Smad, TGFβ/ERK and Notch Signaling
Pathways in Human Lens Epithelial-Mesenchymal Transition. Curr Mol
Med. 18:71–82. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wormstone IM, Tamiya S, Anderson I and
Duncan G: TGF-beta2-induced matrix modification and cell
transdifferentiation in the human lens capsular bag. Invest
Ophthalmol Vis Sci. 43:2301–2308. 2002.PubMed/NCBI
|
|
9
|
Gotoh N, Perdue NR, Matsushima H, Sage EH,
Yan Q and Clark JI: An in vitro model of posterior capsular
opacity: SPARC and TGF-beta2 minimize epithelial-to-mesenchymal
transition in lens epithelium. Invest Ophthalmol Vis Sci.
48:4679–4687. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leight JL, Wozniak MA, Chen S, Lynch ML
and Chen CS: Matrix rigidity regulates a switch between
TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol
Biol Cell. 23:781–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu XJ, Chen MJ, Zhang KK, Yang J and Lu
Y: Elevated TGF-β2 level in aqueous humor of cataract patients with
high myopia: Potential risk factor for capsule contraction
syndrome. J Cataract Refract Surg. 42:232–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livitsanou M, Vasilaki E, Stournaras C and
Kardassis D: Modulation of TGFβ/Smad signaling by the small GTPase
RhoB. Cell Signal. 48:54–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng F, Li J, Yang X, Yuan X and Tang X:
Role of Smad3 signaling in the epithelial-mesenchymal transition of
the lens epithelium following injury. Int J Mol Med. 42:851–860.
2018.PubMed/NCBI
|
|
14
|
Wormstone IM, Anderson IK, Eldred JA,
Dawes LJ and Duncan G: Short-term exposure to transforming growth
factor beta induces long-term fibrotic responses. Exp Eye Res.
83:1238–1245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun CB, Teng WQ, Cui JT, Huang XJ and Yao
K: The effect of anti-TGF-β2 antibody functionalized intraocular
lens on lens epithelial cell migration and epithelial-mesenchymal
transition. Colloids Surf B Biointerfaces. 113:33–42. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Albericio F and Kruger HG: Therapeutic
peptides. Future Med Chem. 4:1527–1531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fosgerau K and Hoffmann T: Peptide
therapeutics: Current status and future directions. Drug Discov
Today. 20:122–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Craik DJ, Fairlie DP, Liras S and Price D:
The future of peptide-based drugs. Chem Biol Drug Des. 81:136–147.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lau JL and Dunn MK: Therapeutic peptides:
Historical perspectives, current development trends, and future
directions. Bioorg Med Chem. 26:2700–2707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato AK, Viswanathan M, Kent RB and Wood
CR: Therapeutic peptides: Technological advances driving peptides
into development. Curr Opin Biotechnol. 17:638–642. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohammed I, Said DG and Dua HS: Human
antimicrobial peptides in ocular surface defense. Prog Retin Eye
Res. 61:1–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brandt CR: Peptide therapeutics for
treating ocular surface infections. J Ocul Pharmacol Ther.
30:691–699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Zhao H, Zheng Y, Gu Q, Ma J and Xu
X: A novel antiangiogenic peptide derived from hepatocyte growth
factor inhibits neovascularization in vitro and in vivo. Mol Vis.
16:1982–1995. 2010.PubMed/NCBI
|
|
24
|
Sun Y, Su L, Wang Z, Xu Y and Xu X: H-RN,
a peptide derived from hepatocyte growth factor, inhibits corneal
neovascularization by inducing endothelial apoptosis and arresting
the cell cycle. BMC Cell Biol. 14:82013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Xu Y, Yu Q, Sun Q, Xu Y, Gu Q and
Xu X: H-RN, a novel antiangiogenic peptide derived from hepatocyte
growth factor inhibits inflammation in vitro and in vivo through
PI3K/AKT/IKK/NF-κB signal pathway. Biochem Pharmacol. 89:255–265.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu S, Xu X, Wang L, Su L, Gu Q, Wei F and
Liu K: Inhibitory effect of a novel peptide, H-RN, on keratitis
induced by LPS or poly(I:C) in vitro and in vivo via suppressing
NF-κB and MAPK activation. J Transl Med. 15:202017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin H, Yang X, Liu K, Gu Q and Xu X:
Effects of a novel peptide derived from human thrombomodulin on
endotoxin-induced uveitis in vitro and in vivo. FEBS Lett.
585:3457–3464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Y, Xu X, Jin H, Yang X, Gu Q and Liu K:
Effects of a thrombomodulin-derived peptide on monocyte adhesion
and intercellular adhesion molecule-1 expression in
lipopolysaccharide-induced endothelial cells. Mol Vis. 19:203–212.
2013.PubMed/NCBI
|
|
29
|
Zhu S, Xu X, Liu K, Gu Q and Yang X:
PAPep, a small peptide derived from human pancreatitis-associated
protein, attenuates corneal inflammation in vivo and in vitro
through the IKKα/β/IκBα/NF-κB signaling pathway. Pharmacol Res.
102:113–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang X, Jin H, Liu K, Gu Q and Xu X: A
novel peptide derived from human pancreatitis-associated protein
inhibits inflammation in vivo and in vitro and blocks NF-kappa B
signaling pathway. PLoS One. 6:e291552011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uboveja A, Satija YK, Siraj F, Sharma I
and Saluja D: p73 - NAV3 axis plays a critical role in suppression
of colon cancer metastasis. Oncogenesis. 9:122020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Slingsby C and Wistow GJ: Functions of
crystallins in and out of lens: Roles in elongated and post-mitotic
cells. Prog Biophys Mol Biol. 115:52–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu L and Xiao W: Notch1 signaling induces
epithelial-mesenchymal transition in lens epithelium cells during
hypoxia. BMC Ophthalmol. 17:1352017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu T, Zhang L, Wang Y, Zhang H, Li L and
Bao X: Dickkopf-1 inhibits Wnt3a-induced migration and
epithelial-mesenchymal transition of human lens epithelial cells.
Exp Eye Res. 161:43–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang G, Kang L, Chen J, Xue Y, Yang M,
Qin B, Yang L, Zhang J, Lu H and Guan H: CtBP2 Regulates
TGFβ2-Induced Epithelial-Mesenchymal Transition Through Notch
Signaling Pathway in Lens Epithelial Cells. Curr Eye Res.
41:1057–1063. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cerra A, Mansfield KJ and Chamberlain CG:
Exacerbation of TGF-beta-induced cataract by FGF-2 in cultured rat
lenses. Mol Vis. 9:689–700. 2003.PubMed/NCBI
|
|
38
|
Wei Z, Caty J, Whitson J, Zhang AD,
Srinivasagan R, Kavanagh TJ, Yan H and Fan X: Reduced Glutathione
Level Promotes Epithelial-Mesenchymal Transition in Lens Epithelial
Cells via a Wnt/β-Catenin-Mediated Pathway: Relevance for Cataract
Therapy. Am J Pathol. 187:2399–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sánchez-Duffhues G, García de Vinuesa A
and Ten Dijke P: Endothelial-to-mesenchymal transition in
cardiovascular diseases: Developmental signaling pathways gone
awry. Dev Dyn. 247:492–508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ha GH, Park JS and Breuer EK: TACC3
promotes epithelial-mesenchymal transition (EMT) through the
activation of PI3K/Akt and ERK signaling pathways. Cancer Lett.
332:63–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gulhati P, Bowen KA, Liu J, Stevens PD,
Rychahou PG, Chen M, Lee EY, Weiss HL, O'Connor KL, Gao T, et al:
mTORC1 and mTORC2 regulate EMT, motility, and metastasis of
colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res.
71:3246–3256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
De Silva DM, Roy A, Kato T, Cecchi F, Lee
YH, Matsumoto K and Bottaro DP: Targeting the hepatocyte growth
factor/Met pathway in cancer. Biochem Soc Trans. 45:855–870. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kataoka H, Kawaguchi M, Fukushima T and
Shimomura T: Hepatocyte growth factor activator inhibitors (HAI-1
and HAI-2): Emerging key players in epithelial integrity and
cancer. Pathol Int. 68:145–158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Colombo ES, Menicucci G, McGuire PG and
Das A: Hepatocyte growth factor/scatter factor promotes retinal
angiogenesis through increased urokinase expression. Invest
Ophthalmol Vis Sci. 48:1793–1800. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Madonna R, Cevik C, Nasser M and De
Caterina R: Hepatocyte growth factor: Molecular biomarker and
player in cardioprotection and cardiovascular regeneration. Thromb
Haemost. 107:656–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu Q, Zhang L, Shen X, Zhu Y, Zhang Q,
Zhou Q, Gan R, Zhang H, Zhong Y and Xie B: A novel and effective
human hepatocyte growth factor kringle 1 domain inhibits ocular
neovascularization. Exp Eye Res. 105:15–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chang PC, Wu HL, Lin HC, Wang KC and Shi
GY: Human plasminogen kringle 1–5 reduces atherosclerosis and
neointima formation in mice by suppressing the inflammatory
signaling pathway. J Thromb Haemost. 8:194–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jin J, Zhou KK, Park K, Hu Y, Xu X, Zheng
Z, Tyagi P, Kompella UB and Ma JX: Anti-inflammatory and
antiangiogenic effects of nanoparticle-mediated delivery of a
natural angiogenic inhibitor. Invest Ophthalmol Vis Sci.
52:6230–6237. 2011. View Article : Google Scholar : PubMed/NCBI
|