Introduction
Cervical cancer is the 4th most common malignant
tumor in women worldwide, with ~530,000 incident cases and 270,000
mortalities each year (1).
Infection with high risk human papillomaviruses (HPV) is a major
risk factor for cervical cancer, while environmental factors such
as smoking have also be shown to be correlated with cervical cancer
occurrence (2). HPV-positive
status can be detected in the majority of patients with cervical
cancer and cervical malignant lesions, however ~15% of patients are
HPV-negative and cannot be detected by the HPV test kit (3). As the initial stage of cervical
cancer is usually asymptomatic, many patients are diagnosed at an
advanced stage, and thus the effect of surgical resection is
limited (4). Therefore, it is
important to identify novel targets in order to develop new
therapeutic strategies for cervical cancer treatment.
Previous studies have indicated that microRNAs
(miRNAs/miRs) serve crucial roles in the cellular features of
tumors (5,6). miRNAs are potential regulators of
tumorigenesis and cancer development in various cancer types,
including cervical cancer (5,7–9).
miRNAs are expected to be used as a complementary treatment for
cancer types due to the ability of miRNAs to target various
physiological activities of cells, including proliferation,
apoptosis and survival (10,11).
Moreover, several miRNAs have been identified to act as epigenetic
drugs in glioblastoma, including miR-124, miR-101, miR-221 and
miR-222 (12). Additionally,
miRNAs not only act as cancer therapeutic targets, but also as
promising biomarkers for diagnosis and prognosis (13). It has been previously demonstrated
that miRNAs can be combined with chemotherapy agents for cancer
therapy (14). The combination of
miR-205 and gemcitabine can significantly inhibit the growth of
gemcitabine-resistant pancreatic cancer cells (15), and the combination of miR-34a and
paclitaxel improves anticancer activity in mice (16). An oncogenic role of miR-96 has been
revealed in various cancer types such as breast cancer,
hepatocellular carcinoma and prostate cancer (17–20).
He et al (21) reported
that miR-96 serves as a tumor suppressor in bladder cancer, while
Ma et al (22) showed that
miR-96 increases the proliferation and tumorigenicity of HeLa
cells. However, the specific role of miR-96 in the metastasis of
cervical cancer remains unknown.
Caveolin-1 (CAV-1) is an integral membrane protein
that serves as major structural component of caveolae. CAV-1 is
involved in cell adhesion and signal transduction, and has been
shown to be involved in tumorigenesis (23–25).
Zhou et al (26) reported
that miR-124 regulates the progression of bladder cancer by
targeting CAV-1. Furthermore, CAV-1 serves a role in clear cell
renal cell carcinoma as a target of miR-124-3p (27). However, the role of CAV-1 in the
progression of cervical cancer is not fully understood.
The aim of the present study was to investigate the
role of miR-96 in the progression of cervical cancer. It was
identified that overexpression of miR-96 promoted the
proliferation, migration and invasion of the cervical cancer SiHa
(HPV+) and C33A (HPV−) cell lines, and also
enhanced the Akt/mTOR signaling pathway. Moreover, the present
results suggested that miR-96 may bind to CAV-1 mRNA, which
indicates its possible oncogenic role in cervical cancer.
Materials and methods
Cell culture and transfection
The human cervical cancer SiHa (HPV+),
C33A (HPV−) and CaSki (HPV+) cell lines were
obtained from the Cell Bank of the Chinese Academy of Sciences.
Cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.), penicillin (100 U/ml; Sigma-Aldrich; Merck KGaA) and
streptomycin (100 mg/ml; Sigma-Aldrich; Merck KGaA) at 37°C in an
atmosphere of 5% CO2. The pCMV-MIR-miR-96
(5′-UUUGGCACUAGCACAUUUUUGCU-3′) vector was synthesized by Guangzhou
RiboBio Co., Ltd., and was transfected into SiHa and C33A cells
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.); the blank pCMV-MIR plasmid (Guangzhou RiboBio
Co., Ltd.) was used as the negative control (NC). Another group of
SiHa or C33A cells was co-transfected with pCMV-MIR-miR-96 and
pcDNA-3.1-CAV-1 (Guangzhou RiboBio Co., Ltd.) or pcDNA-3.1-CAV-1
alone using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). The miR-96 inhibitor
(5′-AGCAAAAAUGUGCUAGUGCCAAA-3′; Guangzhou RiboBio Co., Ltd.) was
transfected into CaSki cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.); miR control was used
as the negative control. Transfection complexes were added to the
medium at a final concentration of 50 nM. The cells were harvested
for further experiments 24 or 48 h later.
Reverse transcription-quantitative
PCR
After 24 h of transfection, cells were collected and
extracted using a miRNA Purification kit (CoWin Biosciences)
followed by RT with a miRNA cDNA Synthesis kit (cat. no. CW2141;
CoWin Biosciences). RT was conducted at 42°C for 50 min and 85°C
for 5 min. qPCR was then performed using a SYBR-Green miRNA qPCR
assay kit (cat. no. CW2142; CoWin Biosciences) according to the
manufacturer's instructions. qPCR thermocycling conditions were as
follows: Initial denaturation at 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 1 min, and final extension
at 72°C for 50 sec. Data were normalized using the endogenous U6.
The primers used in this study were synthesized from Guangzhou
RiboBio Co., Ltd. The U6 primer was 5′-CTCGCTTCGGCAGCACA-3′
(sense). The miR-96 primer was 5′-TTTGGCACTAGCACATTTTTGCT-3′
(sense). The anti-sense primers of miR-96 and U6 were obtained from
the universal primers included in the SYBR-Green miRNA qPCR assay
kit. The 2−ΔΔCq method was performed to analyze the
RT-qPCR data (28).
Cell Counting Kit-8 (CCK-8) assay
Cells transfected with plasmids for 24 h were
collected and seeded in a 96-well plate at a density of
1×103 cells per well at 37°C in a 5% CO2
atmosphere. At 0, 24, 48 and 72 h, 10 µl CCK-8 reagent (Beijing
Solarbio Science & Technology Co., Ltd.) was added to each well
before the experiment according to the manufacturer's instructions,
and incubated at 37°C for 1.5 h. Then, the optical density value
was measured using a microplate reader at a wavelength of 450 nm,
according to the manufacturer's protocol.
Colony formation assay
Cells were seeded into 60 mm dishes (500 cells/dish)
following 24 h of transfection, and cultured for 1–2 weeks until
visible colonies formed. The cells were fixed with 4%
paraformaldehyde at room temperature for 30 min then stained with
0.1% crystal violet at room temperature for 30 min, and the number
of colonies was counted. Images were obtained using a camera.
Transwell assay
Transwell chambers (8-µm pore; EMD Millipore) in
24-well plates were performed for the assessment of cell migration
and invasion abilities in vitro. Cells transfected with
plasmids for 24 h were suspended in serum-free medium, then
1×105 cells were placed into the upper chamber
pre-coated with or without Matrigel (BD Bioscience) for 1 h at
37°C. Then, 500 µl RPMI-1640 medium containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) was added to the lower chamber and
was cultured for 24 h. The remaining cells in the upper chamber
were removed with a cotton swab. Following washing with PBS, the
cells were fixed with 4% polyformaldehyde for 30 min at room
temperature and stained with 0.1% crystal violet for 20 min at room
temperature. The number of migrated or invaded cells was counted
and captured under a light microscope at magnification, ×100 (Nikon
Corporation).
Western blot analysis
After 48 h of transfection, RIPA lysis buffer (CoWin
Biosciences) was used to extract cell proteins. Proteins were
quantified using a bicinchoninic acid assay kit (Beijing Leagene
Biotech Co., Ltd.). Protein samples (20 µg) were resolved by 10%
SDS-PAGE gel and transferred onto a PVDF membrane (EMD Millipore).
Then, the membrane was blocked with 5% dried skimmed milk for 1 h
at room temperature and probed with primary antibodies (1:1,000) at
4°C overnight. Antibody binding was detected with horseradish
peroxidase-conjugated secondary antibodies (1:1,000; ProteinTech
Group, Inc.) for 1 h at room temperature and then chemiluminescence
(Enhanced Chemiluminescence kit; CoWin Biosciences) was performed.
GAPDH was used as the internal control, and the relative expression
of proteins was normalized to GAPDH The densitometry was quantified
using ImageJ software (v.1.48; National Institutes of Health). The
primary antibodies used were as follows: Akt (cat. no. 10176-2-AP;
ProteinTech Group, Inc.); phosphorylated(p)-Akt (Ser473; cat. no.
66444-1-Ig; ProteinTech Group, Inc.); mTOR (cat. no. 20657-1-AP;
ProteinTech Group, Inc.); p-mTOR (Ser2448; cat. no. 2971; Cell
Signaling Technology, Inc.); Cyclin D-1 (cat. no. 60186-1-lg;
ProteinTech Group, Inc.); P70S6K (cat. no. 14485-1-AP; ProteinTech
Group, Inc.); CAV-1 (cat. no. 16447-1-AP; ProteinTech Group, Inc.);
and GAPDH (cat. no. 10494-1-AP; ProteinTech Group, Inc.).
Dual-luciferase reporter assay
The potential target gene of miR-96 was predicted
using the online software StarBase (v2.0; http://starbase.sysu.edu.cn/starbase2/index.php)
(29). The wild-type (wt) or
mutated (mut) CAV-1 3′ untranslated regions (UTRs) were cloned into
the pmirGLO plasmids (Promega Corporation). Cells were
co-transfected with the pmirGLO-CAV-1-wt (2 µg) or
pmirGLO-CAV-1-mut (2 µg) plasmid and pCMV-MIR-miR-96 using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), and the blank plasmid was used as the negative
control. After transfection for 48 h, the luciferase activity was
examined using a Dual-Luciferase Reporter assay system (Promega
Corporation) according to the manufacturer's protocol. The firefly
luciferase expression was normalized to Renilla luciferase
activity.
Statistical analysis
Data are presented as the mean ± SD from three
independent experiments. GraphPad Prism 7.0 software (GraphPad
Software, Inc.) was used for statistical analyses. Group
comparisons were analyzed with a Student's t-test or one-way ANOVA
analysis followed by the Newman-Keuls method. P<0.05 was
considered to indicate a statistically significant difference.
Results
Overexpression of miR-96 enhances the
proliferation of cervical cancer cells
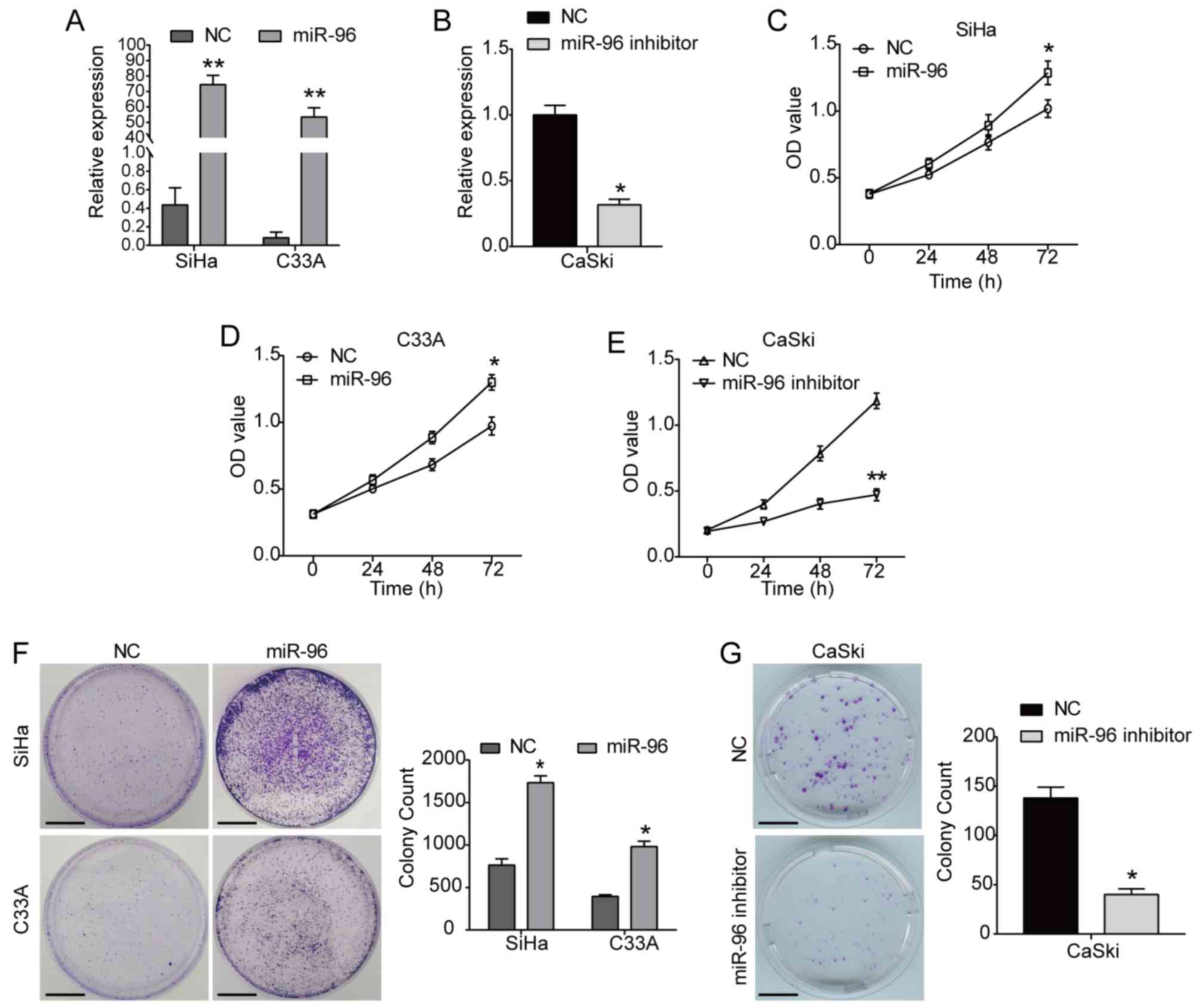
To investigate the functional role of miR-96 in the
progression of cervical cancer, the pCMV-MIR-miR-96 vector was
transfected into SiHa and C33A cells, which had an initial low
miR-96 expression level, to overexpress miR-96; the blank pCMV-MIR
vector was used as the NC (Figs.
1A and S1A). Additionally,
due to the innate high miR-96 expression level in CaSki cells,
CaSki cells were transfected with miR-96 inhibitor to downregulate
miR-96 (Figs. 1B and S1A). It was identified that the
overexpression of miR-96 significantly increased the viability of
SiHa cells compared with the NC group (Fig. 1C). Moreover, the viability of C33A
cells was also promoted by miR-96 overexpression (Fig. 1D), whereas the miR-96 inhibitor
significantly inhibited the viability of CaSki cells (Fig. 1E). In addition, the colony
formation assay results indicated that miR-96 overexpression
significantly increased the clonogenic capacities of both SiHa and
C33A cells, while the miR-96 inhibitor had the opposite effect on
CaSki cells (Fig. 1F and G).
miR-96 overexpression increases the
migratory and invasive abilities of cervical cancer cells
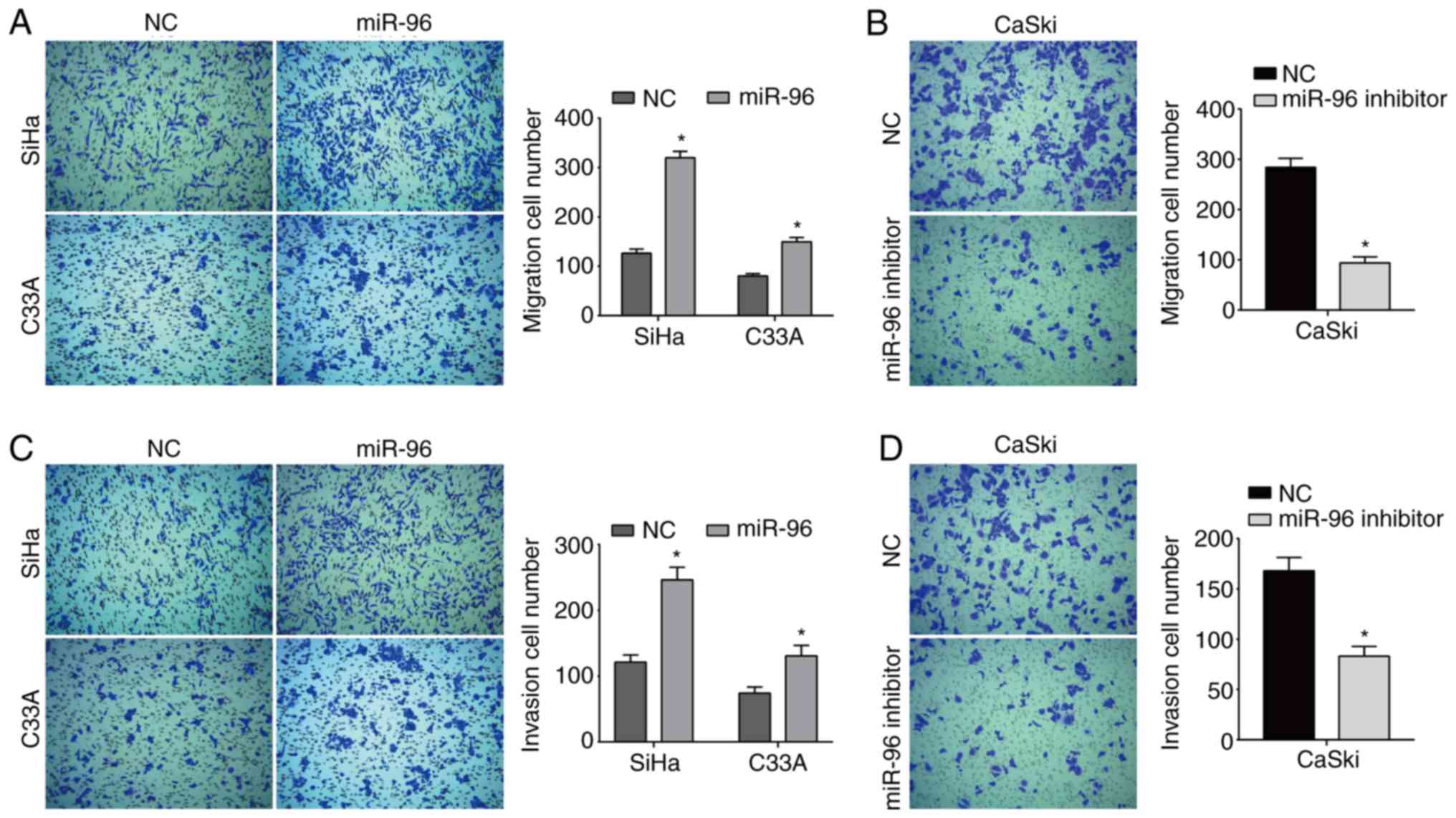
The present results suggested that overexpression of
miR-96 significantly increased cell migration in both SiHa and C33A
cells (Fig. 2A). Furthermore, a
significant decrease in cell migration was demonstrated in CaSki
cells transfected with the miR-96 inhibitor (Fig. 2B). Moreover, miR-96 overexpression
also increased the number of invaded cells compared with the NC
group (Fig. 2C), while the miR-96
inhibitor reduced the invasive ability of CaSki cells (Fig. 2D).
miR-96 increases activation of the
Akt/mTOR signaling pathway in cervical cancer cells
The Akt/mTOR signaling pathway serves an essential
role in cellular processes, including cell proliferation,
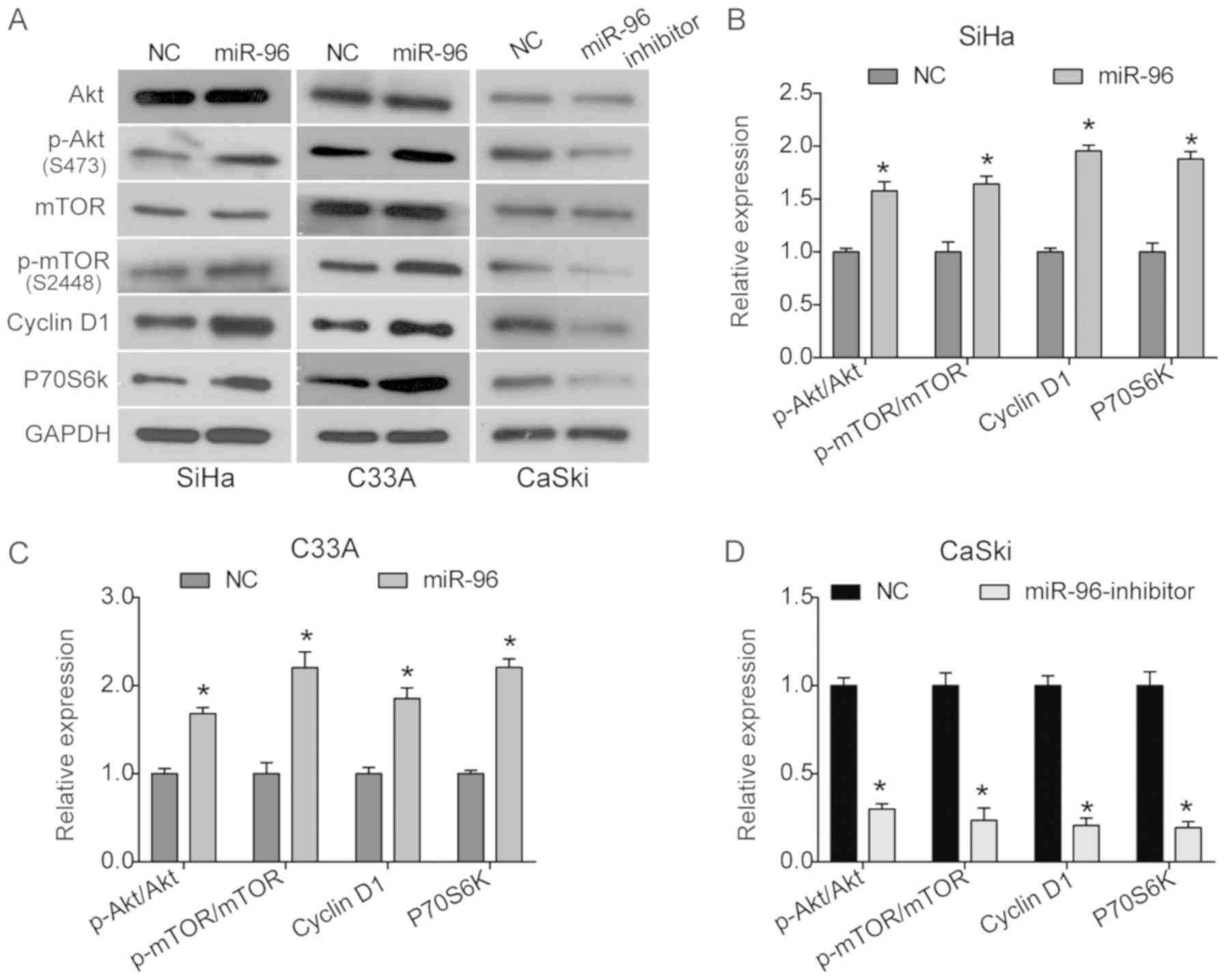
migration, survival and apoptosis. It was identified that there was
a significant increase in the levels of p-Akt and p-mTOR in SiHa
and C33A cells transfected with the pCMV-MIR-miR-96 vector, while
the total expression of Akt and mTOR was not affected (Fig. 3A-C). In addition, the expression
levels of the downstream proteins cyclin D1 and P70S6k were
significantly increased by miR-96 overexpression in both SiHa and
C33A cells (Fig. 3A-C). Moreover,
it was demonstrated that the miR-96 inhibitor significantly
inhibited the Akt signaling pathway by suppressing the expression
levels of p-Akt and p-mTOR in CaSki cells (Fig. 3A and D). Therefore, the present
results suggested that the Akt/mTOR signaling pathway may be
involved in the oncogenic role of miR-96 in cervical cancer.
miR-96 directly targets CAV-1 in
cervical cancer cells
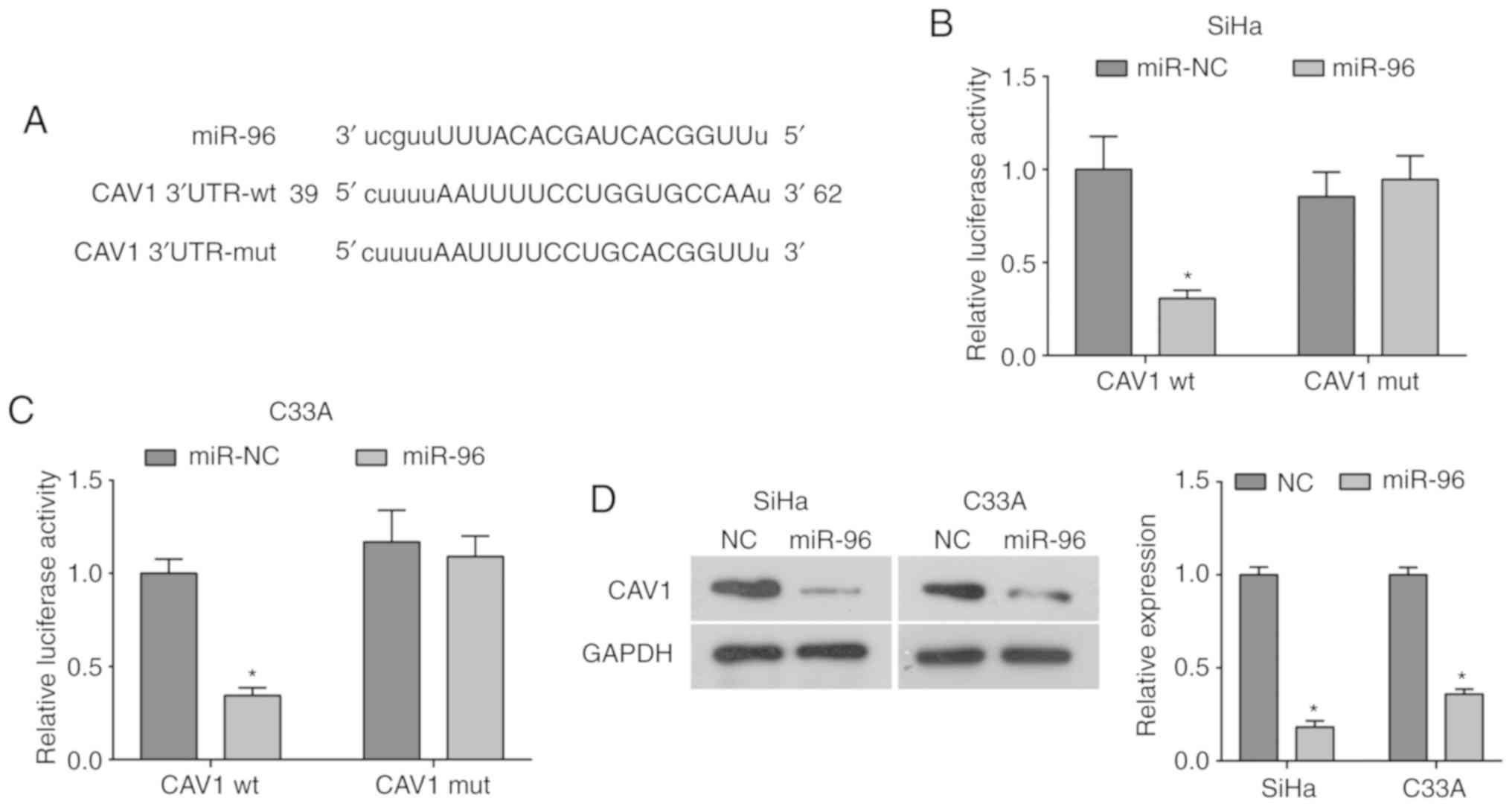
Using the bioinformatics database, the present study
identified that miR-96 has a conserved binding site in the 3′UTR of
CAV-1 mRNA (Fig. 4A). In order to
investigate this further, the pCMV-MIR-miR-96 vector or control
vector was co-infected with CAV-1 3′UTR-wt or CAV-1 3′UTR-mut into
SiHa and C33A cells. It was identified that miR-96 overexpression
could significantly decrease the luciferase activity of the CAV-1
3′UTR-wt, while the luciferase activity of the CAV-1 3′UTR-mut was
unaffected by miR-96 overexpression (Fig. 4B and C), suggesting that CAV-1 may
be a target of miR-96. Moreover, overexpression of miR-96
significantly decreased the protein expression level of CAV-1
compared with the NC group (Fig.
4D). Collectively, the present results indicated that miR-96
may directly target CAV-1 and inhibit its expression in cervical
cancer.
miR-96 increases cell proliferation,
migration and invasion by targeting CAV-1
To further investigate the molecular mechanism of
miR-96, SiHa and C33A cells were either transfected with
pcDNA-3.1-CAV-1 vector alone (Fig.
S1B and C) or co-transfected with pCMV-MIR-miR-96. The CCK-8
results suggested that the overexpression of CAV-1 significantly
inhibited the viabilities of SiHa and C33A cells compared with
control cells, suggesting an inhibitory effect of CAV-1 on cell
proliferation (Fig. 5A and B).
Moreover, co-transfection of CAV-1 and miR-96 decreased cell
viability compared with miR-96 alone (Fig. 5A and B). The Transwell assay
results suggested that the overexpression of CAV-1 inhibited the
migratory and invasive abilities of both SiHa and C33A cells.
Furthermore, the increased migratory and invasive abilities of SiHa
and C33A cells induced by miR-96 were also abolished by CAV-1
overexpression, as indicated by the co-transfection of miR-96 and
CAV-1 (Fig. 5C and D). The western
blot analysis results suggested that the overexpression of CAV-1
inhibited the activation of the Akt signaling pathway (Fig. 5E and F). Further, the upregulation
of p-Akt and p-mTOR induced by miR-96 was also demonstrated to be
rescued by CAV-1 co-transfection in SiHa cells (Fig. 5E and F). Collectively, the present
results suggested that miR-96 enhances cell proliferation,
migration and invasion by targeting CAV-1, which modulates the
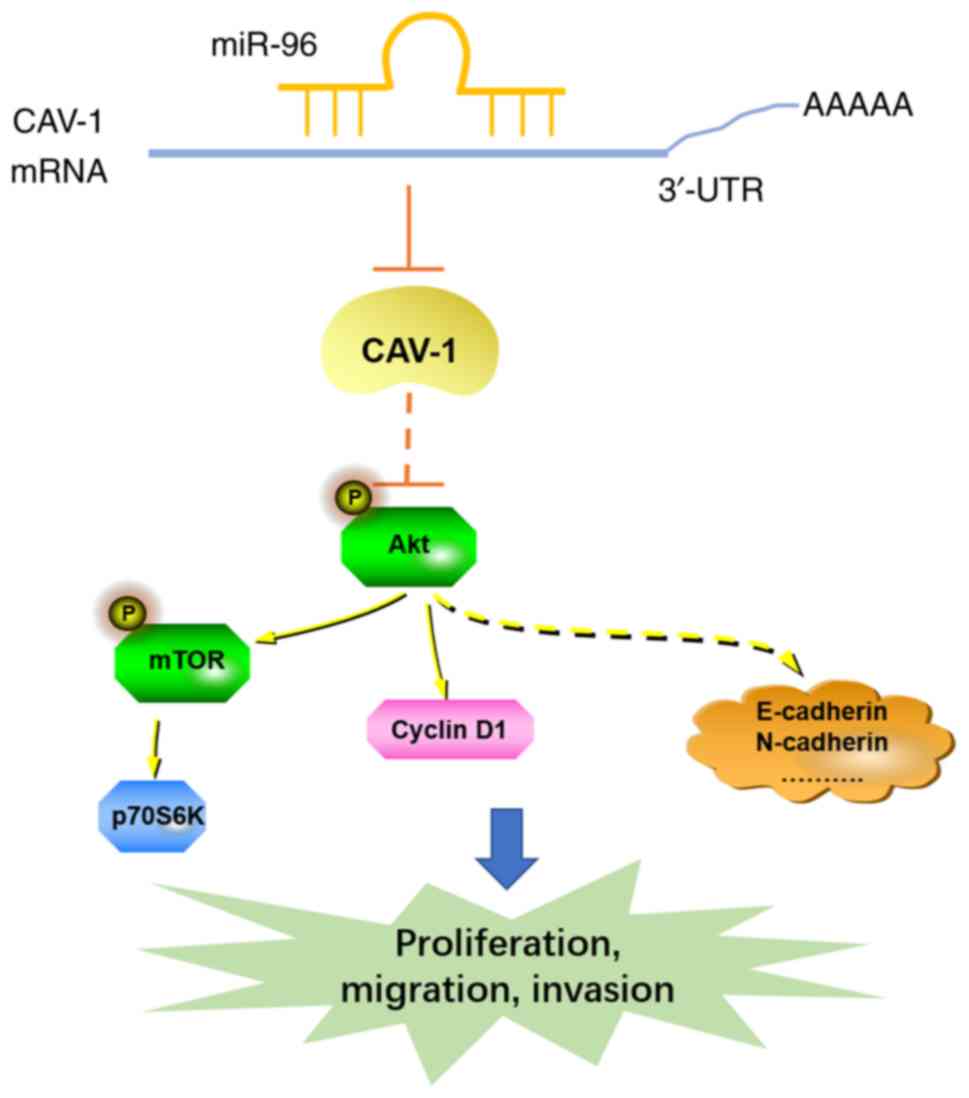
Akt/mTOR signaling pathway in cervical cancer (Fig. 6).
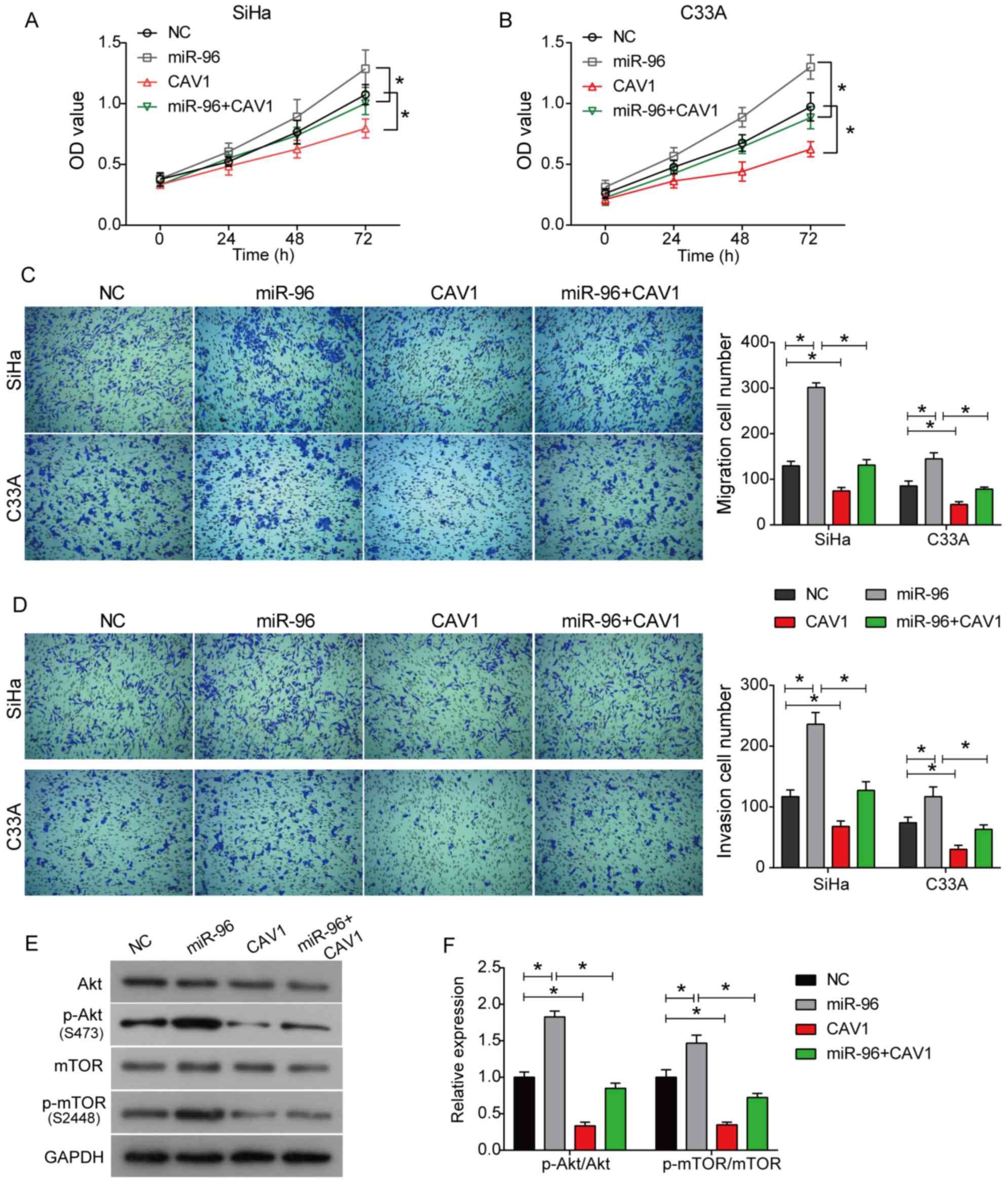 | Figure 5.miR-96 promotes cell proliferation,
migration and invasion in cervical cancer by targeting CAV-1. (A)
SiHa and (B) C33A cells were co-transfected with miR-96 and CAV-1,
or transfected with miR-96/CAV-1 alone, and then a Cell
Counting-Kit 8 assay was used to detect cell viability. Following
transfection for 24 h, a Transwell assay was performed to assess
(C) cell migration and (D) invasion. Original magnification, ×100.
(E) Protein expression levels of the Akt signaling
pathway-associated proteins in SiHa cells. (F) Quantitative
analysis of western blot analysis. *P<0.05. Data are presented
as the mean ± SD. miR-96, microRNA-96; NC, negative control; CAV-1,
caveolin 1; OD, optical density; p-, phosphorylated. |
Discussion
Previous studies have demonstrated that miR-96 is
associated with tumorigenesis by acting as an oncogene or a tumor
suppressor, depending on the tissue type (18,21).
The present results suggested that miR-96 was associated with the
growth and metastatic potential of HPV+ and
HPV− cervical cancer cells. Overexpression of miR-96 was
identified to facilitate the proliferative, migratory and invasive
abilities of SiHa and C33A cells. Moreover, Ma et al
(22) showed that miR-96 could
promote the proliferation of Hela cell by silencing protein
tyrosine phosphatase non-receptor type 9, which is in concordance
with the results of the present study. Therefore, miR-96 may serve
an oncogenic role in the progression of cervical cancer, and it may
be used as a potential target for cervical cancer therapy.
The role of CAV-1 in cancer biology is
controversial, with some previous studies suggesting that CAV-1
plays a negative regulatory role in tumor metastasis (24,25).
Overexpression of CAV-1 inhibits the migration of HeLa cells via
its caveolin scaffolding domain to regulate cell signaling
(30). However, a recent study
showed that silencing CAV-1 can inhibit the proliferation of lung
adenocarcinoma H522 cells, but overexpression of CAV-1 is
associated with a worse overall survival (25). Moreover, previous studies have
suggested that the expression level of CAV-1 is tissue-type
dependent, as CAV-1 is downregulated in ovarian cancer and colon
cancer, but upregulated in bladder cancer and breast cancer
(25,31). Furthermore, the function and
expression level of CAV-1 may be correlated with the grade and
stage of cancer (25,32,33).
CAV-1 often acts as a tumor suppressor with decreased expression
levels during the early stage of cancer progression, but enhances
tumor aggressive and metastatic functions in the advanced stage of
cancer (25,32,33).
Therefore, CAV-1 may serve a role as a tumor promotor and as a
tumor suppressor in cancer progression. The present results
suggested that CAV-1 acts as a tumor suppressor in cervical cancer,
and that CAV-1 overexpression may inhibit cell proliferation,
migration and invasion. Moreover, it was demonstrated that miR-96
can directly bind to CAV-1 mRNA and negatively regulate its
expression in cervical cancer cells. Furthermore, overexpression of
CAV-1 could reverse the increase in cell proliferation, migration
and invasion induced by miR-96. Collectively, the present results
indicated that miR-96 increased the growth and metastatic potential
of cervical cancer by targeting CAV-1.
In the present study, it was identified that the
overexpression of miR-96 upregulated the Akt/mTOR signaling pathway
in cervical cancer cells. The Akt/mTOR signaling pathway serves
essential roles in cancer cell activity, and is an important
therapeutic target in cancer treatment (34). Yang et al (35) reported that miR-96 mimics activate
the Akt/GSK-3β/β-catenin signaling pathway in hepatocellular
carcinoma, which is involved in the carcinogenic effect of miR-96.
Moreover, Song et al (36)
showed that miR-96 may inhibit the expression of Forkhead Box O1
(FOXO1) via the upregulation the Akt/FOXO1/Bim signaling pathway in
papillary thyroid carcinoma cells. Therefore, the present results
indicated that the Akt/mTOR signaling pathway may be involved in
the oncogenic role of miR-96 in cervical cancer. The effect of
miR-96 on the transport of CAV-1 and the association between the
miR-96/CAV-1/Akt signaling pathway should be further investigated
in future studies.
In summary, the present results suggested that
miR-96 exerted a carcinogenic effect by targeting CAV-1 in both
HPV+ and HPV− cervical cancer cells, and that
the Akt/mTOR signaling pathway may be involved in this process.
Therefore, miR-96 may function as a potential target in cervical
cancer therapy. However, the present study only investigated the
role of miR-96 in the biological function of HPV+ and
HPV− cervical cancer cells, thus further study is
required to investigate the role of miR-96 in the progression of
cervical cancer in vivo.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC, CL and BX conceived and designed the
experiments. All authors performed the experiments. SC, YZ and SZ
analyzed the data. YC wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CAV-1
|
caveolin 1
|
|
miRNA/miR
|
microRNA
|
References
|
1
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN and Gaffney DK: Cervical cancer: A global health
crisis. Cancer. 123:2404–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao B, Xie Z, Guo L, Wu J and Zhang H:
Stomatin-like protein 2 expression is associated with clinical
survival in patients with cervical cancer. Int J Clin Exp Pathol.
8:1804–1809. 2015.PubMed/NCBI
|
|
3
|
de Sanjose S, Quint WG, Alemany L, Geraets
DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin
HR, et al: Human papillomavirus genotype attribution in invasive
cervical cancer: A retrospective cross-sectional worldwide study.
Lancet Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lecuru F, Bats A, Mathevet P, Querleu D,
Leblanc E, Darai PM, Marret H, Collin C, Chatellier G and Gilaizeau
F: Impact of sentinel lymph node biopsy on staging of early
cervical cancer: Results of a prospective, multicenter study. J
Clin Oncol. 27:CRA55062009. View Article : Google Scholar
|
|
5
|
Zhang Z, Wang J, Li J, Wang X and Song W:
MicroRNA-150 promotes cell proliferation, migration, and invasion
of cervical cancer through targeting PDCD4. Biomed Pharmacother.
97:511–517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2011. View Article : Google Scholar
|
|
7
|
Esquela-Kerscher A and Slack F:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li GC, Cao XY, Li YN, Qiu YY, Li YN, Liu
XJ and Sun XX: MicroRNA-374b inhibits cervical cancer cell
proliferation and induces apoptosis through the p38/ERK signaling
pathway by binding to JAM-2. J Cell Physiol. 233:7379–7390. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang F, Shan S, Huo Y, Xie Z, Fang Y, Qi
Z, Chen F, Li Y and Sun B: MiR-155-5p inhibits PDK1 and promotes
autophagy via the mTOR pathway in cervical cancer. Int J Biochem
Cell Biol. 99:91–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kouji B, Iida M, Yanokura M, Kisu I, Iwata
T, Tominaga E, Tanaka K and Aoki D: MicroRNA in cervical cancer:
OncomiRs and tumor suppressor miRs in diagnosis and treatment.
ScientificWorldJournal. 2014:1780752014.PubMed/NCBI
|
|
11
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rasras S, Zibara K, Vosughi T and Zayeri
Z: Genetics and epigenetics of glioblastoma: Therapeutic
challenges. Clin Cancer Invest J. 7:43–49. 2018. View Article : Google Scholar
|
|
13
|
Takahashi RU, Prieto Vila M, Kohama I and
Ochiya T: Development of microRNA-based therapeutic approaches for
cancer patients. Cancer Sci. 110:1140–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chakraborty C, Sharma AR, Sharma G, Sarkar
BK and Lee SS: The novel strategies for next-generation cancer
treatment: miRNA combined with chemotherapeutic agents for the
treatment of cancer. Oncotarget. 9:10164–10174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mittal A, Chitkara D, Behrman SW and
Mahato RI: Efficacy of gemcitabine conjugated and miRNA-205
complexed micelles for treatment of advanced pancreatic cancer.
Biomaterials. 35:7077–7087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi S, Han L, Deng L, Zhang Y, Shen H,
Gong T, Zhang Z and Sun X: Dual drugs (microRNA-34a and
paclitaxel)-loaded functional solid lipid nanoparticles for
synergistic cancer cell suppression. J Control Release.
194:228–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Testoni D, Hayashi M, Cohen-Wolkowiez M,
Benjamin DK Jr, Lopes RD, Clark RH, Benjamin DK and Smith P:
Late-onset bloodstream infections in hospitalized term infants.
Pediatr Infect Dis J. 33:920–923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie W, Sun F, Chen L and Cao X: MiR-96
promotes breast cancer metastasis by suppressing MTSS1. Oncol Lett.
15:3464–3471. 2018.PubMed/NCBI
|
|
19
|
Yang S, Chen Z, Fan D, Zhang R, Zhang Y
and Wu S: MiR-182-5p and miR-96-5p increased hepatocellular
carcinoma cell mobility, proliferation and cisplatin resistance
partially by targeting RND3. RSC Advances. 8:34973–34983. 2018.
View Article : Google Scholar
|
|
20
|
Fendler A, Jung M, Stephan C, Erbersdobler
A, Jung K and Yousef GM: The antiapoptotic function of miR-96 in
prostate cancer by inhibition of FOXO1. PLoS One. 8:e808072013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He C, Zhang Q, Gu R, Lou Y and Liu W:
miR-96 regulates migration and invasion of bladder cancer through
epithelial-mesenchymal transition in response to transforming
growth factor-β1. J Cell Biochem. 119:7807–7817. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma X, Shi W, Peng L, Qin X and Hui Y:
MiR-96 enhances cellular proliferation and tumorigenicity of human
cervical carcinoma cells through PTPN9. Saudi J Biol Sci.
25:863–867. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sternberg PW and Schmid SL: Caveolin,
cholesterol and Ras signalling. Nat Cell Biol. 1:E35–E37. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeong J, Thike AA, Ikeda M, Lim JCT, Lee
B, Nakamura S, Iqbal J and Tan PH: Caveolin-1 expression as a
prognostic marker in triple negative breast cancers of Asian women.
J Clin Pathol. 71:161–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duregon E, Senetta R, Bertero L, Bussolati
B, Annaratone L, Pittaro A, Papotti M, Marchiò C and Cassoni P:
Caveolin 1 expression favors tumor growth and is associated with
poor survival in primary lung adenocarcinomas. Tumor Biol.
39:1010428317694312017. View Article : Google Scholar
|
|
26
|
Zhou W, He L, Dai Y, Zhang Y, Wang J and
Liu B: MicroRNA-124 inhibits cell proliferation, invasion and
migration by targeting CAV1 in bladder cancer. Exp Ther Med.
16:2811–2820. 2018.PubMed/NCBI
|
|
27
|
Butz H, Szabó PM, Khella HW, Nofech-Mozes
R, Patocs A and Yousef GM: miRNA-target network reveals miR-124 as
a key miRNA contributing to clear cell renal cell carcinoma
aggressive behaviour by targeting CAV1 and FLOT1. Oncotarget.
6:12543–12557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
Starbase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale clip-seq data. Nucleic Acids
Res. 42((Database issue)): D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okada S, Raja SA, Okerblom J, Boddu A,
Horikawa Y, Ray S, Okada H, Kawamura I, Murofushi Y, Murray F and
Patel HH: Deletion of caveolin scaffolding domain alters cancer
cell migration. Cell Cycle. 18:1268–1280. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Senetta R, Trevisan E, Rudà R, Maldi E,
Molinaro L, Lefranc F, Chiusa L, Lanotte M, Soffietti R and Cassoni
P: Caveolin 1 expression independently predicts shorter survival in
oligodendrogliomas. J Neuropathol Exp Neurol. 68:425–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shatz M and Liscovitch M: Caveolin-1: A
tumor-promoting role in human cancer. Int J Radiat Biol.
84:177–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goetz J, Lajoie P, M Wiseman S and Nabi I:
Caveolin-1 in tumor progression: The good, the bad and the ugly.
Cancer Metastasis Rev. 27:715–735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martini M, De Santis M, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang N, Zhou J, Li Q, Han F and Yu Z:
miR-96 exerts carcinogenic effect by activating
AKT/GSK-3β/β-catenin signaling pathway through targeting inhibition
of FOXO1 in hepatocellular carcinoma. Cancer Cell Int. 19:382019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song HM, Luo Y, Li DF, Wei CK, Hua KY,
Song JL, Xu H, Maskey N and Fang L: MicroRNA-96 plays an oncogenic
role by targeting FOXO1 and regulating AKT/FOXO1/Bim pathway in
papillary thyroid carcinoma cells. Int J Clin Exp Pathol.
8:9889–9900. 2015.PubMed/NCBI
|