Introduction
Vascular cognitive impairment (VCI) comprises a
spectrum of clinical syndromes ranging from mild cognitive
impairment to dementia, and is caused by cerebrovascular risk
factors, such as hypertension, diabetes and smoking, and
cerebrovascular diseases (1–3). VCI
is likely to become the primary type of cognitive impairment in
developing countries, and thus is becoming a serious social health
problem (4). Although the
molecular pathogenesis of VCI is unclear, current studies suggest
that 3-N-butylphthalide (NBP), one of the chemical constituents in
celery oil, improves VCI, thus new therapeutic targets are likely
to be identified (5,6).
Silent information regulator 1 (SIRT1) is a member
of the sirtuin family that undergoes NAD+-dependent
acetylation, and acts as a regulatory protein associated with aging
and age-related degenerative diseases (7). SIRT1 plays an important role in a
number of physiological and pathological processes, including
senescence and metabolic regulation (8–10).
SIRT1 is also involved in the regulation of endothelial cell gene
expression after angiogenesis, suggesting that it may play an
important protective role in chronic cerebral ischemia (11).
Brain-derived neurotrophic factor (BDNF) is a major
neuroprotective factor with anti-apoptotic properties, which
protects and promotes nerve regeneration (12). BDNF exerts its biological function
by binding to its specific receptor, tropomyosin receptor kinase B
(TrkB) (12). High expression
levels of BDNF and TrkB are present in the hippocampus and cortex
of the brain (13,14). BDNF is the most effective
biologically active substance for protecting neurons and regulating
synaptic plasticity (15).
However, expression levels of BDNF in hippocampal neurons are
significantly decreased by hypoxia, suggesting that BDNF may play
an important role in the pathogenesis of VCI (16).
NBP is a novel drug for the treatment of ischemic
cerebral injury that has been independently developed and
researched in China (17). Several
studies have reported that NBP reduces focal cerebral infarction
volume in rats, improves brain energy metabolism disorders and
reduces apoptotic neuronal cell death (18–20).
NBP is a multi-target drug with several mechanisms, and thus has a
variety of neuroprotective effects in brain tissues (21–23).
The present study hypothesized that the regulation of the
SIRT1/BDNF signaling pathway may be involved in the underlying
mechanism of action of NBP. Therefore, the present study
investigated the neuroprotective effects of NBP on learning and
memory in a rat model of VCI induced by two-vessel occlusion, and
also examined the role of the SIRT1/BDNF signaling pathway.
Materials and methods
Animals and groups
In total, 60 specific pathogen-free (SPF)
Sprague-Dawley rats, (male; 2 months old; weight, 250–280 g) were
purchased from Liaoning Longevity Biotechnology Co., Ltd. (Liao
2010–0201). Rats were housed in a SPF animal experiment room at
24±2°C with 40–70% humidity under a 12 h light/dark cycle, and were
allowed free access to water and food. After 10 days of
acclimatization, the rats were divided randomly into six groups: i)
Sham operated control group (C group; n=10); ii) model group (M
group; n=10); iii) NBP-low-dose group (L-NBP group; 30 mg/kg/day;
n=10); iv) NBP-high-dose group (H-NBP group; 60 mg/kg/day; n=10);
v) SIRT1 inhibitor + NBP group (S+N group; NBP 60 mg/kg/day; n=10);
and vi) BDNF inhibitor + NBP group (B+N group; NBP 60 mg/kg/day;
n=10). Experiments were approved by The China Medical University
Animal Care and Use Committee, and adhered to The Chinese Academy
of Science Guidelines for Care and Use of Laboratory Animals.
Surgical procedure
The TrkB antagonist K-252α (EMD Millipore) and
sirtinol (Sigma-Aldrich; Merck KGaA) were used to inhibit BDNF and
SIRT1, respectively. Rats were anesthetized by intraperitoneal
injection of 10% chloral hydrate (300 mg/kg) and were then placed
in the prone position on a rat brain stereotaxic device. Successful
anesthesia was indicated when slow breathing, limb paralysis, dull
pain reflex and loss of righting reflex occurred; individual rats
showed mild abdominal distension but no signs of peritonitis after
anesthesia. The catheter-insertion point was marked using the
fontanelle as the zero point, Bregma after 1 mm and then right 1.5
mm. A lateral ventricle catheter was inserted vertically to a depth
of 3.5–3.8 mm 1 day before model induction surgery. Bilateral
common carotid artery ligation is the most common method of
inducing chronic cerebral ischemia in rats and has been widely used
in VCI research (24,25). The rats were weighed and injected
intraperitoneally with 10% chloral hydrate (300 mg/kg) for
anesthesia. A ~2 cm incision was made in the skin and subcutaneous
tissue around the midline of the neck, and the muscles were parted
to expose the common carotid arteries. The common carotid arteries
were peeled away, avoiding the vagus nerve and the bilateral common
carotid arteries were then ligated.
Experimental design
The detailed protocol is shown in Fig. 1. Rats in the S+N and B+N groups
underwent lateral ventricle catheterization and the experimental
reagents were injected slowly into the lateral ventricle, leaving
the needle in for 2–3 min. Rats in the B+N group were administered
1 µg/day K-252α and rats in the S+N group were administered 1 mg/kg
body weight/day sirtinol for 9 days. In addition to the C group,
all rats in each group underwent artery ligation. Rats in the C and
M groups were fed with vegetable oil (1 ml/100 g−1) as a
placebo. Rats in the H-NBP group and the two inhibitor groups were
given NBP 60 mg/kg/day, and rats in the L-NBP group received NBP 30
mg/kg/day. NBP (dissolved in vegetable oil) or vegetable oil alone
was administered via oral gavage once a day after bilateral common
carotid artery occlusion (BCCAO). The NBP/vegetable oil treatments
were administered for 28 consecutive days. During the experiment,
the rats were weighed every week. NBP soft capsules were purchased
from Shijiazhuang Pharmaceutical Co., Ltd. Then, 4 weeks after the
surgery there were 10 rats in the C group, 9 in the M group, 9 in
the H-NBP group, 9 in the L-NBP group, 8 in the S+N group and 8 in
the B+N group.
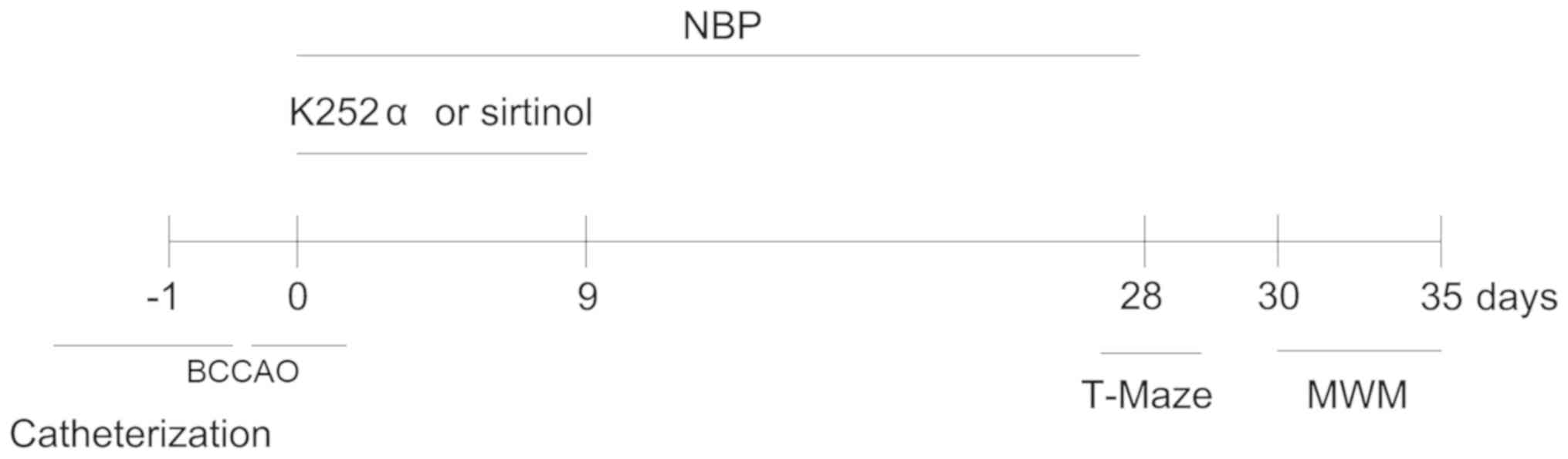 | Figure 1.Timeline for experiments. One day
prior to the operation, rats in the S+N and B+N groups underwent
lateral ventricle catheterization. With the exception of the C
group, all rats underwent vessel ligation, 24 h post-lateral
ventricle catheterization. Rats in the S+N and B+N groups were
administered K-252α and sirtinol by lateral ventricle for
consecutive 9 days, and were given NBP via oral gavage for 28
consecutive days, other groups were also fed with vegetable oil or
NBP via oral gavage for 28 consecutive days. T-maze tests were
performed on day 28 and MWM tests were conducted on day 30
post-operation. Nissl staining, western blot analysis,
immunofluorescence was performed on day 35. NBP,
3-N-Butylphthalide; BCCAO, bilateral common carotid artery
occlusion; MWM, Morris water maze; S+N, SIRT1 inhibitor + NBP (60
mg/kg) group; B+N, BDNF inhibitor + NBP (60 mg/kg); C, control; M,
model group. |
T-maze test
T-maze tests can be used to assess spatial working
memory (26). In the present
study, rats were subjected to T-maze tests after 4 weeks of NBP
treatment. Rats were maintained on a restricted feeding schedule at
85% of their free-feeding weight, and were habituated to the maze
and accustomed to food rewards (a small amount of sugar). Each
trial consisted of a sample run and a choice run. On the sample
run, rats had to enter either the left or right arm to get the
reward, while the other arm was blocked by a sliding door. On the
choice run, the blocked door was removed and the rats were allowed
to choose either arm freely. An interval of 10 sec was allowed
between the sample and choice runs. If the rats entered the
previously unvisited arm, they were rewarded. The delayed
alternation was then prolonged to 90 and 180 sec. Each daily
session consisted of five trials, and each rat ran one trial at a
time with an inter-trial interval of 10 min. The number of
corrections made by the rats when they entered the unvisited arm of
the T-maze was measured.
Morris water maze (MWM) test
The MWM is designed to test spatial learning in
rodents (27). Rats were trained
twice a day for 5 consecutive days with an interval of 3 h, and
each trial lasted for 90 sec. The rats started from a different
quadrant for each trial and the time to reach the platform (escape
latency) was recorded using a video camera. The digital images were
analyzed using water maze software (HVS Image 2020; HVS Image
Software Ltd.). Additional probe trials were conducted, with the
platform removed on the 6th day of the test. Swimming speed, times
of crossing the platform, time spent in the quadrant previously
containing the submerged platform and swimming distance were also
recorded and represented as an index of memory.
Nissl staining
Rats were deeply anesthetized by intraperitoneal
injection of sodium pentobarbital (200 mg/kg). When continuous
spontaneous breathing had arrested for 2–3 min and muscle
relaxation had occurred, the rats were perfused transcardially with
0.9% normal saline followed by 4% paraformaldehyde in 0.1 M sodium
phosphate buffer (pH 7.3). The brains were removed and immersed in
10% paraformaldehyde in sodium phosphate buffer for 24 h at 4°C for
post-fixation, and then embedded in a single paraffin block.
Sections were cut at 5 µm and stained with 1% toluidine blue at
60°C for 40 min, and Nissl-positive cells in the hippocampus were
examined using a light microscope at ×400 magnification (model
BX53; Olympus Corporation) by two independent investigators who
were blinded to the experimental conditions.
Immunofluorescence
Hippocampal tissues were removed and post-fixed in
4% paraformaldehyde (pH 7.4) at 4°C for 12 h, and then cut at a
thickness of 30 µm using a vibrating microtome (Leica VT1000 S;
Leica Biosystem GmbH). After blocking endogenous peroxidases and
nonspecific binding sites with 10% normal rabbit serum (cat. no.
ab166640; Abcam) for 1 h at room temperature, the sections were
incubated with primary rabbit anti-SIRT1 (1:800; cat. no. ab12193;
Abcam) and rabbit anti-BDNF (1:800; cat. no. ab108319; Abcam)
antibodies overnight at 4°C, followed by incubation with
FITC-conjugated goat anti rabbit IgG (1:1,000; cat. no. ab6717;
Abcam) and Cy3-conjugated goat anti-rabbit IgG (1:1,000; cat. no.
ab6939; Abcam) at room temperature for 1 h. After staining with
DAPI (Sigma-Aldrich; Merck KGaA) for 10 min at 37°C, the slides
were examined under a fluorescence microscope (Eclipse E800; Nikon
Corporation) at magnification ×200, with a SPOT advanced digital
camera (Diagnostic Instruments, Inc.). Fluorescent images were
merged using Andor IQ version 2.0 software (Andor Technology
Ltd.).
Western blotting
Rats were euthanized with an intraperitoneal
injection of sodium pentobarbital (200 mg/kg) and sacrificed on day
28 after the final administration of NBP. Hippocampal tissues were
dissected from rats and transferred into liquid nitrogen. For
protein extraction, isolated tissue was homogenized in RIPA lysis
buffer (cat. no. P0013B; Beyotime Institute of Biotechnology) on
ice for 1 h and centrifuged at 12,000 × g for 10 min at 4°C. The
supernatant was collected and the protein concentration was
quantified using a bicinchoninic acid protein assay kit
(Sigma-Aldrich; Merck KGaA). Proteins (30 µg/lane) were loaded on a
10% gel, resolved using SDS-PAGE and subsequently transferred onto
PVDF membranes (EMD Millipore). The membranes were probed with
primary antibodies against SIRT1 (1:1,000; cat. no. sc74465) and
BDNF (1:1,000; cat. no. sc65514) overnight at 4°C, followed by
incubation with a horseradish peroxidase-labeled secondary antibody
(1:1,500; cat. no. sc51625) at room temperature for 3 h (all
purchased from Santa Cruz Biotechnology, Inc.). The proteins were
then visualized by enhanced chemiluminescence (GE Healthcare)
according to the manufacturer's instructions. The densities of the
protein bands were analyzed quantitatively using Quantity-One
software version 4.6.3 (Bio-Rad Laboratories, Inc.). GAPDH
(1:5,000; cat. no. ab9485; Abcam) was used as a protein-loading
control.
Statistical analysis
Data are presented as the mean ± SD and were
analyzed using SPSS 16.0 statistical software (SPSS, Inc.).
Statistical significance was determined by one-way ANOVA followed
by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
NBP reverses spatial working memory
impairment in rats with VCI in the T-maze test
The present results suggested that rats with VCI (M
group) showed significantly reduced spontaneous alternation
behavior compared with the control group (P<0.05). In addition,
NBP (30 or 60 mg/kg) significantly attenuated the impairment of
spontaneous alternation behavior in rats with VCI (P<0.05),
including when the interval between the sample and choice run was
delayed by 90 or 180 sec (P<0.05). Therefore, the present
results suggested that NBP improved working memory deficits in VCI
rats (Fig. 2A).
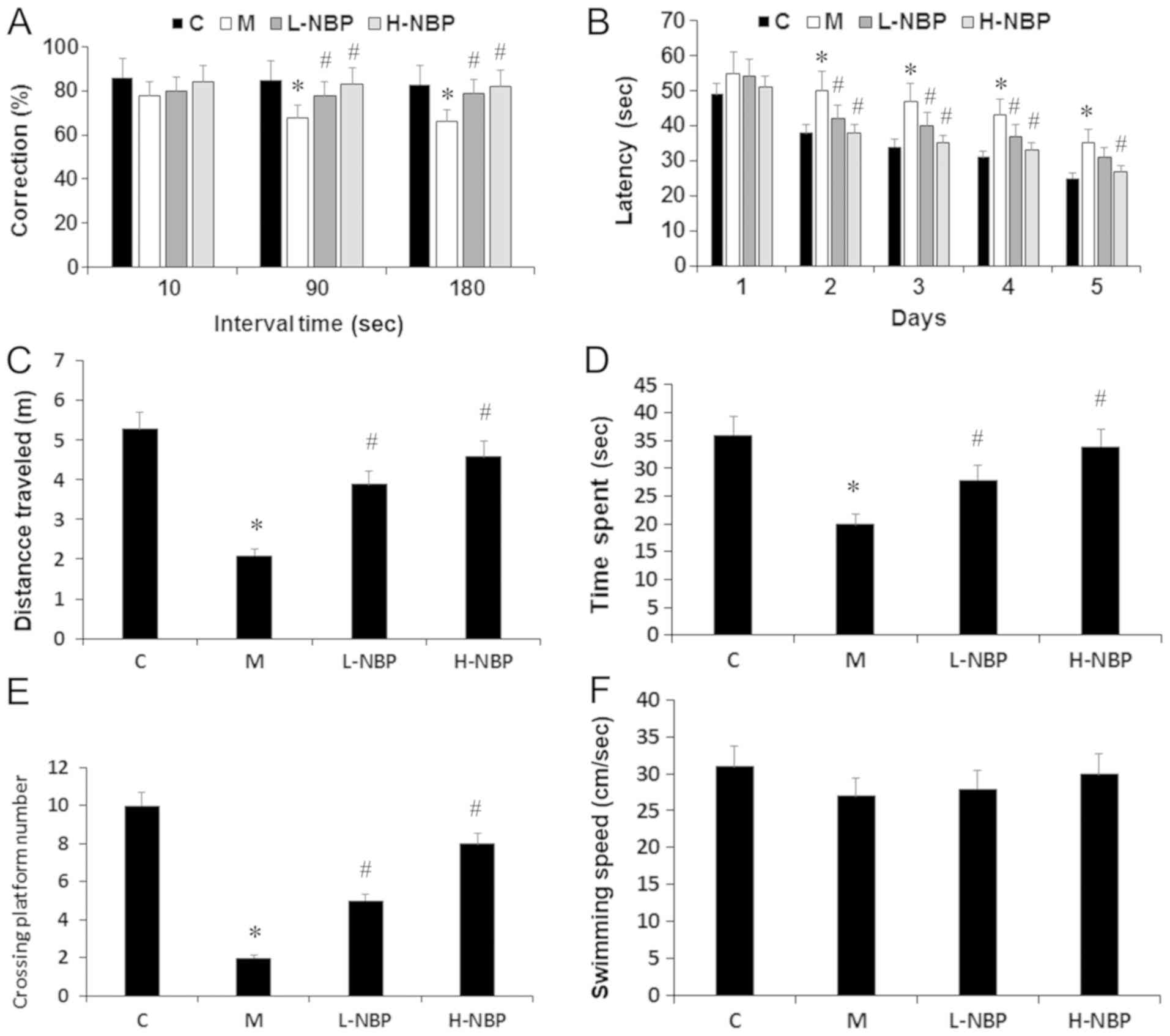 | Figure 2.Protective effects of NBP against
ischemic injury-induced spatial memory impairment in T-maze and MWM
test. (A) Correction alternation was examined using a T-maze and
tested using various intervals at 10, 90 and 180 sec delay. (B)
Latency to reach the platform during the training period in MWM
test. (C) Distance travelled, (D) time spent in the target quadrant
and (E) the crossing platform numbers during the probe trail in the
MWM test. (F) Swimming speed among the groups. Data are presented
as the mean ± SD. C, n=10; M, n=9; L-NBP, n=9; H-NBP, n=9.
*P<0.05 vs. C group. #P<0.05 vs. M group. NBP,
3-N-butylphthalide; C, control; M, model group; L-NBP, low dose NBP
group (30 mg/kg); H-NBP, high dose NBP group (60 mg/kg); MWM,
Morris water maze. |
NBP reverses learning and memory
deficits in rats with VCI in the MWM test
The escape latencies in the different groups are
shown in Fig. 2B. There was a
significant difference in training test performance among the four
different groups from day 2, VCI rats (M group) took longer to find
the platform compared with the controls (P<0.05), while
treatment with NBP (30 or 60 mg/kg) decreased the escape latency
compared with the VCI rats (P<0.05; Fig. 2B). In the probe test, the time
spent and the swimming distance in the target quadrant, as well as
the number of platform crossings were all significantly lower in
the M group compared with the control group (P<0.05). NBP (30 or
60 mg/kg) increased the time spent and the swimming distance in the
target quadrant, and improved the search performance as indicated
by a higher number of platform crossings compared with the M group
(P<0.05; Fig. 2C-E). However,
swimming speed was similar in all groups during the trials
(Fig. 2F). Collectively, the
present results suggested that NBP may improve learning and memory
impairments in VCI rats.
NBP reversal of cognitive deficits in
rats with VCI may be related to SIRT1 signaling
To examine the role of SIRT1 signaling in the
neuroprotective effect of NBP against VCI rats, the present study
investigated the cognitive performance of rats treated with NBP +
SIRT1 antagonist (S+N) using the MWM test. The present results
suggested that NBP reversed learning and memory deficits in rats
with VCI in the MWM test, as indicated by shorter escape latencies,
longer swimming distance and increased platform crossings (Fig. 2). However, the escape latencies of
the VCI rats significantly increased after treatment with NBP +
SIRT1 antagonist (S+N), the swimming distance was significantly
shorter and the number of platform crossings decreased compared
with the NBP group (P<0.05; Fig.
3). Therefore, the present results suggested that SIRT1
antagonists may partially inhibit the spatial memory and learning
improvements caused by NBP. However, this inhibition was not
significant in the NBP + BDNF antagonist treatment (B+N) group
(Fig. 3).
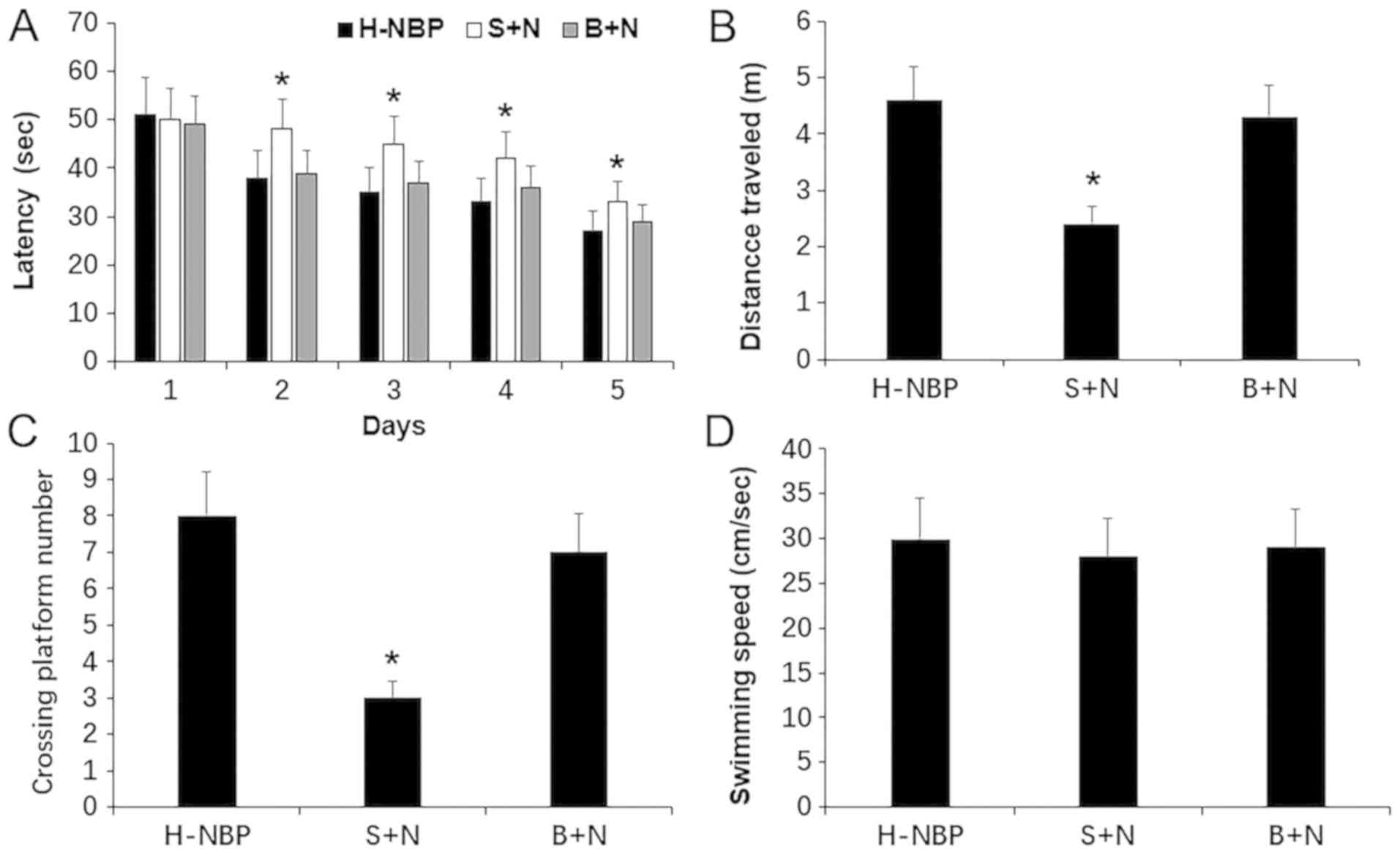 | Figure 3.Cognitive performance of NBP in
vascular cognitive impaired rats after the blockade of SIRT1
measured by MWM test. (A) Latency to reach the platform during the
training period in MWM test. (B) Representative swimming distance.
(C) Crossing platform numbers in the probe trail. (D) Swimming
speed among the groups. Data are presented as the mean ± SD. H-NBP,
n=9; S+N, n=8; B+N, n=8. *P<0.05 vs. H-NBP group. NBP,
3-N-butylphthalide; BDNF, brain-derived neurotrophic factor; SIRT1,
silent information regulator 1; H-NBP, high dose NBP group (60
mg/kg); MWM, Morris water maze; S+N, SIRT1 inhibitor + NBP (60
mg/kg) group; B+N, BDNF inhibitor + NBP (60 mg/kg). |
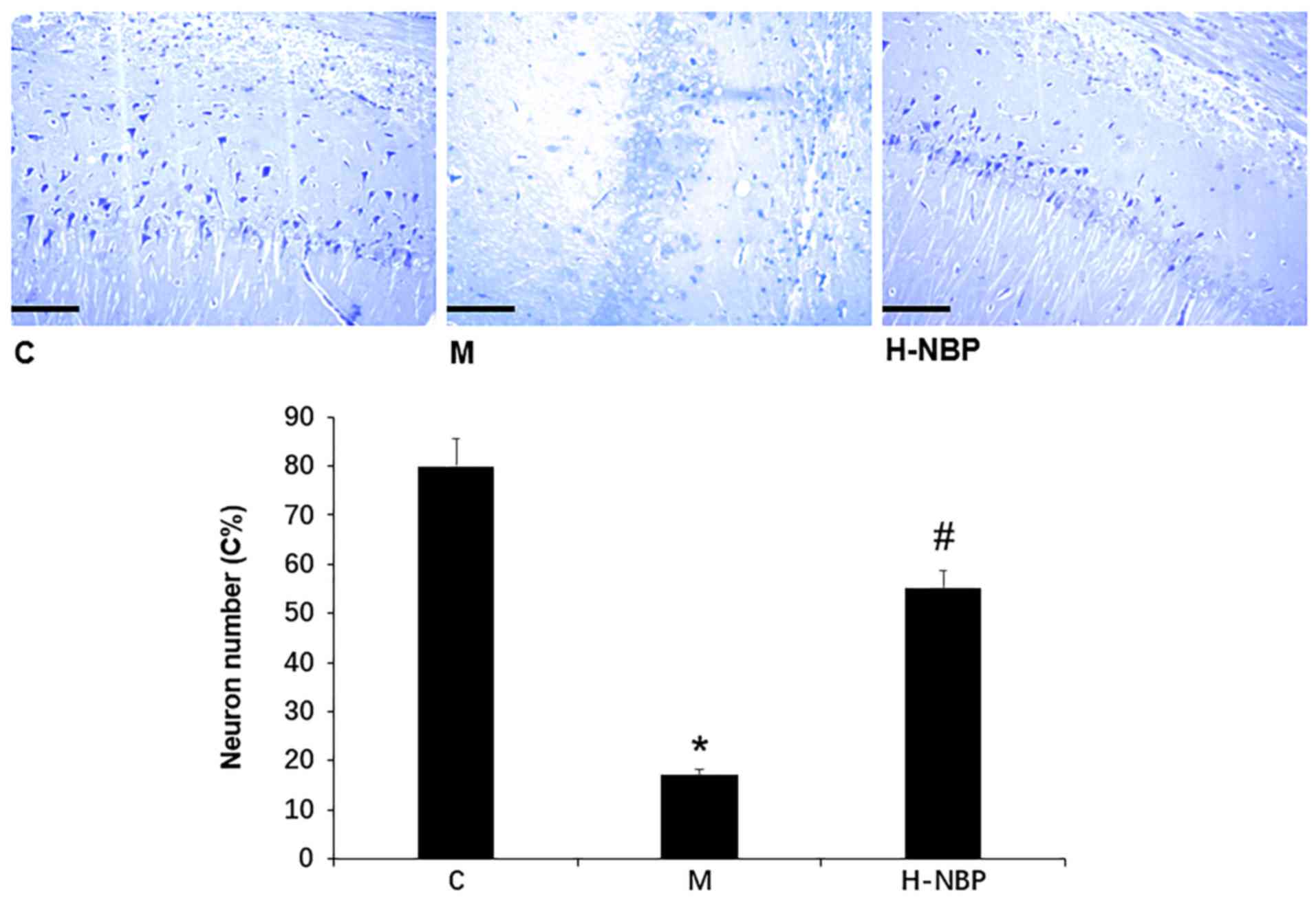
NBP suppresses neuronal loss in the
hippocampal cornu Ammonis 1 (CA1) region in VCI rats
The present results suggested that there were no
histopathologic abnormalities in the hippocampus in control rats
(Fig. 4). However, VCI rats showed
significant neuronal loss and neuronal structural degeneration in
the CA1 region, while these effects were attenuated by 60 mg/kg NBP
(H-NBP), as reflected by the density and morphology of neurons with
Nissl bodies (P<0.05). Collectively, the present results
suggested that NBP may inhibit hippocampal neuronal death in VCI
rats.
NBP upregulates SIRT1 and BDNF protein
expression levels in the hippocampus of VCI rats, which is reversed
by SIRT1 inhibition
The present study investigated the effects of NBP on
SIRT1 and BDNF expression levels using immunofluorescence.
SIRT1-positive and BDNF-positive cells were lacking in the VCI
group (M group) compared with the control group (C group). However,
the expression levels of these proteins was increased by 60 mg/kg
NBP treatment (H-NBP group) compared with the VCI group (P<0.05;
Fig. 5). The present results
suggested that BDNF-positive cells were significantly reduced by
SIRT1 inhibition (S+N group) compared with the H-NBP group
(P<0.05), but SIRT1-positive cells were not reduced by BDNF
inhibition.
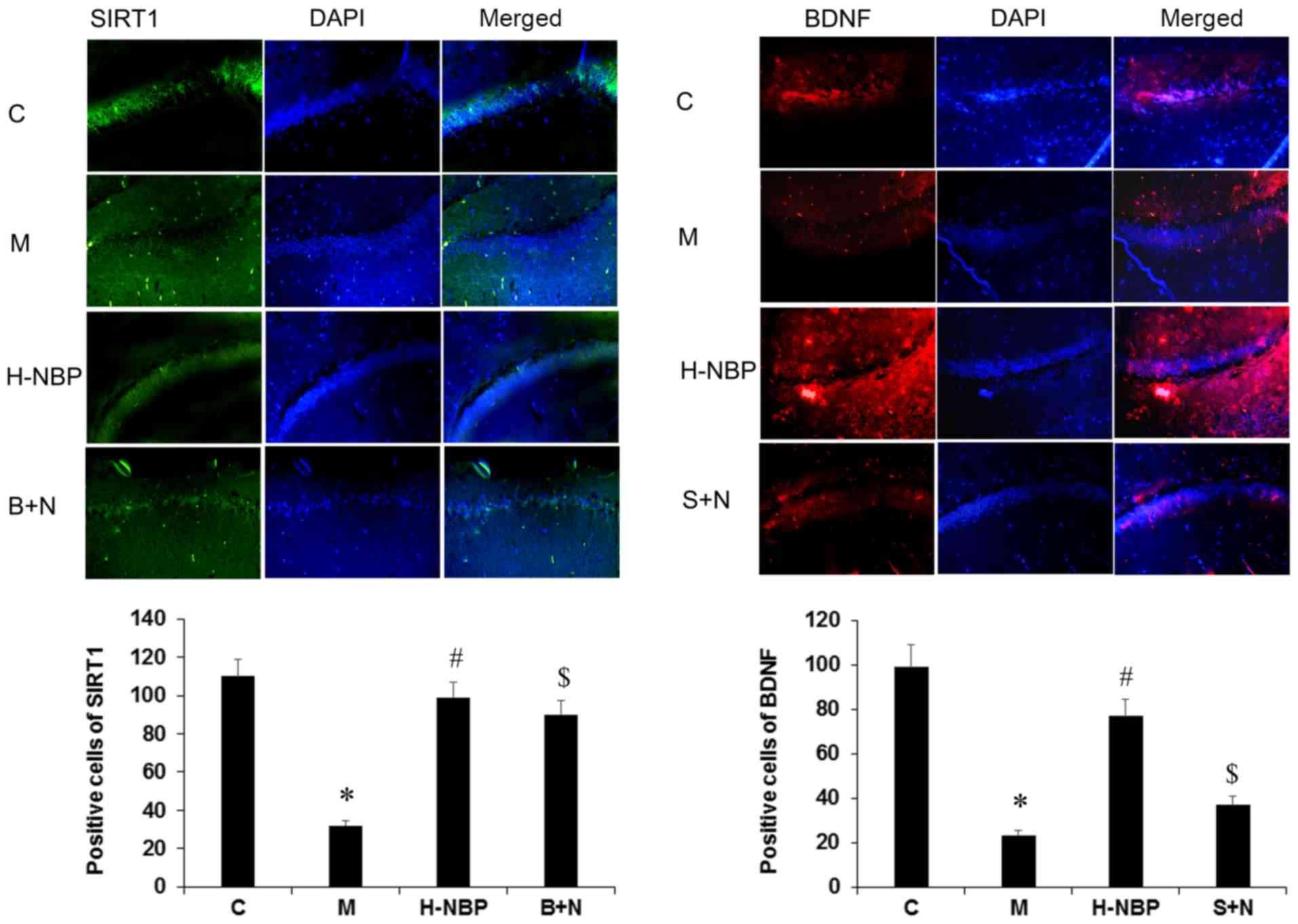 | Figure 5.Effects of NBP on the expression
levels of SIRT1 and BDNF in the hippocampus of VCI rats by
immunofluorescence analysis. Magnification, ×400. The intensity of
the red or green fluorescence signal represents the expression
level of the corresponding protein. The nucleus was counterstained
with DAPI, which is represented by blue fluorescence. NBP reversed
the decrease of SIRT1 and BDNF expression levels in VCI rats, as
reflected by the positive labeled cells. BDNF expression level was
reduced in S+N group, but SIRT1 expression level was not decreased
in B+N group, compared with H-NBP group. Data are presented as the
mean ± SD. *P<0.05 vs. C group. #P<0.05 vs. M
group. $P<0.05 vs. H-NBP group. NBP,
3-N-butylphthalide; BDNF, brain-derived neurotrophic factor; SIRT1,
silent information regulator 1; H-NBP, high dose NBP group (60
mg/kg); S+N, SIRT1 inhibitor + NBP (60 mg/kg) group; B+N, BDNF
inhibitor + NBP (60 mg/kg); C, control; M, model group; VCI,
vascular cognitive impairment. |
The present study also investigated the effect of
NBP on the expression levels of SIRT1 and BDNF by western blotting.
SIRT1 and BDNF protein expression levels were reduced in the VCI
rats (M group) compared with control (C group) rats, and this
effect was reversed by NBP treatment (P<0.05; Fig. 6). In addition, compared with the
H-NBP group, the use of a SIRT1 inhibitor significantly decreased
BDNF protein expression level in the hippocampus of VCI rats
(P<0.05), but use of a BDNF inhibitor had no effect on SIRT1
protein expression compared with the H-NBP group. Therefore, the
present results indicated that NBP increased SIRT1 and BDNF protein
expression levels in the hippocampus of VCI rats, and that SIRT1
may be the upstream signaling factor in the SIRT1/BDNF pathway.
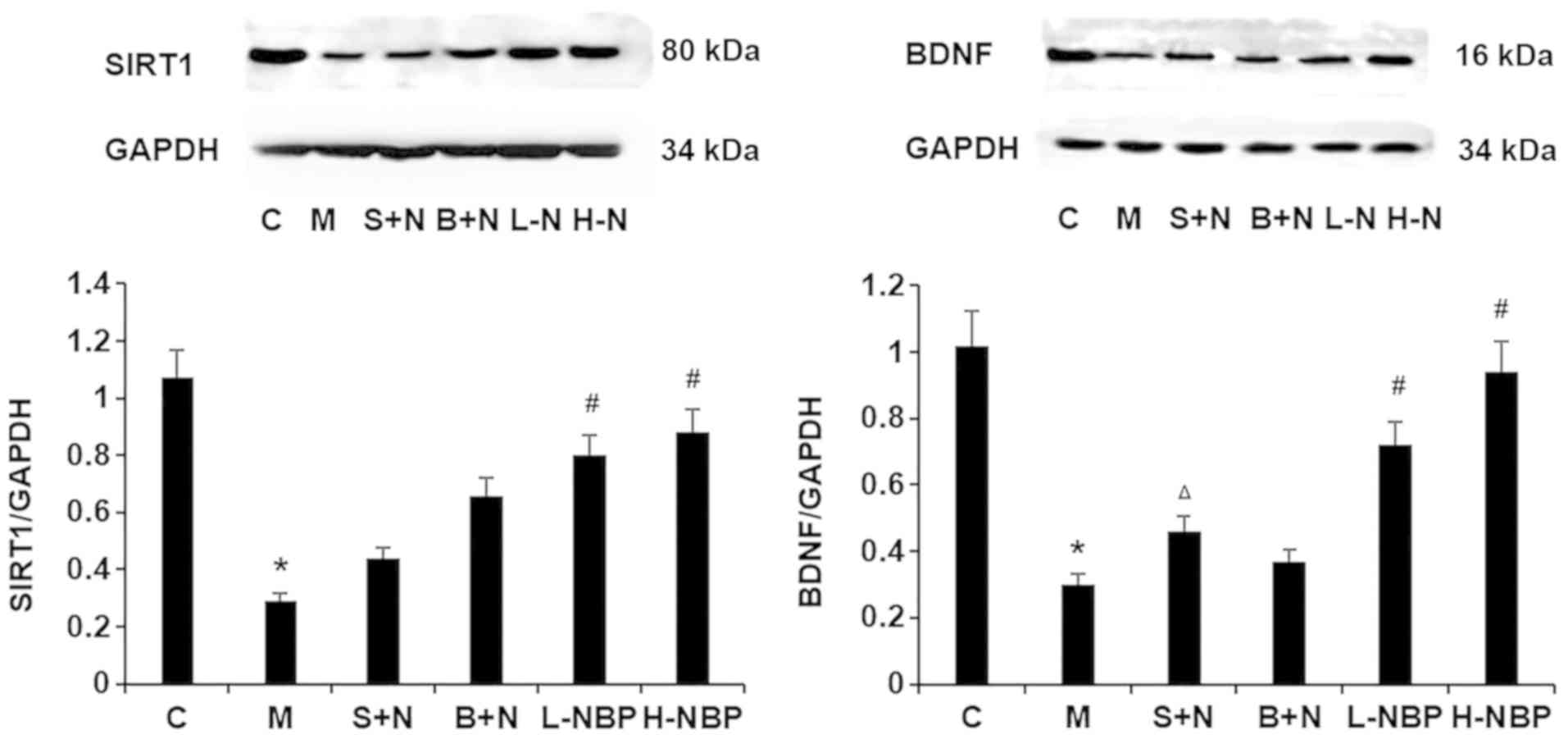 | Figure 6.Effects of NBP on the expression
levels of SIRT1 and BDNF in the hippocampus of vascular cognitive
impaired rats by western blotting. NBP increased SIRT1 and BDNF
protein expression levels. BDNF expression level was reduced in the
S+N group, but SIRT1 expression was not decreased in the B+N group,
compared with the H-NBP group. Data are presented as the mean ± SD.
*P<0.05 vs. C group. #P<0.05 vs. M group.
ΔP<0.05 vs. H-NBP group. NBP, 3-N-butylphthalide;
BDNF, brain-derived neurotrophic factor; SIRT1, silent information
regulator 1; H-NBP, high dose NBP group (60 mg/kg); L-NBP, low dose
NBP group (30 mg/kg); S+N, SIRT1 inhibitor + NBP (60 mg/kg) group;
B+N, BDNF inhibitor + NBP (60 mg/kg); C, control; M, model
group. |
Discussion
Vascular cognitive impairment (VCI) has been the
focus of noteworthy scientific research. VCI is a brain dysfunction
syndrome, mainly caused by cerebrovascular disease (28,29),
consisting of a progression from mild cognitive impairment to
dementia (30). However, VCI is
not a single disorder and there are currently no universally
acceptable criteria for its diagnosis (31,32).
Treatment of VCI is becoming increasingly important in countries
with aging populations, as early treatment could prevent or slow
the rate of progression from mild cognitive impairment to vascular
dementia. Therefore, there has been an increase in research aimed
at developing effective drugs for the treatment of VCI.
3-N-Butylphthalide (NBP) has been shown to be
effective not only in protecting against ischemic cerebral injury,
but also in ameliorating VCI in patients with dementia (33). The underlying mechanisms include
enhancement of antioxidation, improvement of mitochondrial
dysfunction, inhibition of neuronal apoptosis and autophagy,
reduction of endoplasmic reticulum stress and upregulation of the
Sonic hedgehog/Protein patched homolog 1 pathway (34–36).
However, further studies are needed to determine if the
neuroprotective effects of NBP in VCI are correlated with the
SIRT1/BDNF pathway. The present study investigated the therapeutic
efficacy of NBP against ischemic injury in VCI rats and examined
the modulatory effect of NBP on the SIRT1/BDNF signaling pathway.
BCCAO has been widely used to induce ischemic injury in VCI
research (37). The present
results suggested that NBP may protect against ischemic
injury-induced learning and memory impairment in both T-maze and
MWM tests in a BCCAO model of VCI in rats. The present results also
indicated that the neuroprotective effect of NBP may involve the
SIRT1-BDNF signaling pathway. Furthermore, NBP also increased
downstream BDNF expression levels by ameliorating upstream SIRT1
expression in the hippocampus of VCI rats.
Silent information regulator 1 (SIRT1) is closely
related to aging, and is expressed in the liver, skeletal muscle
and brain (38). SIRT1 brain
levels are notably higher compared with levels in other tissues in
mammals (39,40), especially in the hippocampus, which
is closely associated with learning and memory (41). SIRT1 has been shown to affect
complex biological processes, including cognitive decline (42,43).
The present results suggested that ischemic injury induced learning
and memory decline as well as decreasing SIRT1 expression levels in
rats, and this process could be reversed by NBP treatment.
Collectively, the present results suggested that the
neuroprotective effect of NBP may be partially via the upregulation
of SIRT1.
BDNF is a neuroprotective factor that is widely
distributed in the central nervous system and plays a significant
role in neurodegenerative diseases (44). BDNF binds to its receptor TrkB and
activates the downstream signaling pathways to regulate synaptic
function and maintain neuronal survival (45,46).
BDNF has been demonstrated to exert substantial neuroprotective
effects and to improve neurological deficits (47). The present results suggested that
cognitive impairment, as determined by T-maze and MWM tests, was
associated with decreased BDNF expression levels compared with
control rats, thus suggesting that BDNF was downregulated after
ischemic injury. However, this downregulation of BDNF was reversed
by NBP treatment.
Numerous studies have reported that NBP is a
multi-target drug and its multiple mechanisms of action is
effective in numerous conditions, such as Alzheimer's disease,
Parkinson's disease and demyelination diseases (48–50).
Previous studies revealed that NBP could support rebuilding of the
collateral circulation, and had antithrombotic, anti-apoptotic and
antioxidant effects, coupled with protection of mitochondrial
functions (5,51,52).
The present study investigated the SIRT1/BDNF signaling pathway in
rats with ischemic damage, and examined the relationship between
NBP treatment and this signaling pathway. To the best of our
knowledge, few studies have assessed the effect of NBP on VCI via
the SIRT1/BDNF signaling pathway. Zeng and Yang (53) reported that SIRT1 may protect
retinal neurons and visual function via the regulation of the
BDNF/TrkB signaling pathway. In addition, Jiang et al
(54) reported that SIRT1 had a
neuroprotective effect in patients with Huntington's disease, which
was associated with upregulation of the BDNF/TrkB signaling
pathway. The present results suggested that NBP could significantly
reduce the escape latency and swimming distance, increase the time
in the targeted quadrant and increase the expression levels of
SIRT1 and BDNF in VCI rats. In addition, the present results
suggested that NBP improved spatial learning in VCI rats via
activating the SIRT1/BDNF pathway. Injection of a SIRT1 inhibitor
prevented the NBP-induced improvement in cognition and the
elevation of BDNF expression levels, while injection of a BDNF
inhibitor could not reverse the process. Therefore, the present
results indicated that NBP increased SIRT1 expression level, which
activated the downstream signaling pathway molecule BDNF, and
resulted in partial recovery of some cognitive functions in VCI
rats.
In conclusion, NBP may improve the recovery of
cognitive function in rats after cerebral ischemic injury via
upregulation of the SIRT1/BDNF signaling pathway. The SIRT1 may be
the upstream regulatory element promoting the expression of BDNF
and thereby improving cognitive deficits in VCI rats.
Acknowledgements
Not applicable.
Funding
The current study was supported by National Key
R&D Program of China (grant no. 2018YFC1311600), Liaoning
Province Nature Science Foundation of China (grant no. 2015020471)
and Science and Technology Program of Shenyang (grant no.
17-230-930).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZ designed the study. WL established the rat
models. QZ and HL collected and analyzed all experimental data. AT
and WL drafted the manuscript and contributed substantially to its
revision. AT performed the statistical analysis. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Experiments were approved by The China Medical
University Animal Care and Use Committee, and adhered to The
Chinese Academy of Science Guidelines for Care and Use of
Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Du J, Ma M, Zhao Q, Fang L, Chang J, Wang
Y, Fei R and Song X: Mitochondrial bioenergetic deficits in the
hippocampi of rats with chronic ischemia-induced vascular dementia.
Neuroscience. 231:345–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akinyemi R, Mukaetova-Ladinska E, Attems
J, Ihara M and Kalaria RN: Vascular risk factors and
neurodegeneration in ageing related dementias: Alzheimer's disease
and vascular dementia. Curr Alzheimer Res. 10:642–653. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benisty S: Current concepts in vascular
dementia. Geriatr Psychol Neuropsychiatr Vieil. 11:171–180.
2013.(In French). PubMed/NCBI
|
|
4
|
Grinberg LT, Nitrini R, Suemoto CK, Lucena
Ferretti-Rebustini RE, Leite RE, Farfel JM, Santos E, Andrade MP,
Alho AT, Lima Mdo C, et al: Prevalence of dementia subtypes in a
developing country: A clinicopathological study. Clinics (Sao
Paulo). 68:1140–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi Q, Xu J, Lv P, Dong Y, Liu Z, Hu M,
Xiao Y, Jia Y, Jin W, Fan M, et al: DL-3-n-butylphthalide
alleviates vascular cognitive impairment induced by chronic
cerebral hypoperfusion by activating the Akt/Nrf2 signaling pathway
in the hippocampus of rats. Neurosci Lett. 672:59–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han QY, Zhang H, Zhang X, He DS, Wang SW,
Cao X, Dai YT, Xu Y and Han LJ: dl-3-n-butylphthalide preserves
white matter integrity and alleviates cognitive impairment in mice
with chronic cerebral hypoperfusion. CNS Neurosci Ther.
25:1042–1053. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
North BJ and Verdin E: Sirtuins:
Sir2-related NAD-dependent protein deacetylases. Genome Biol.
5:2242004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seo JS, Moon MH, Jeong JK, Seol JW, Lee
YJ, Park BH and Park SY: SIRT1, a histone deacetylase, regulates
prion protein-induced neuronal cell death. Neurobiol Aging.
33:1110–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zu G, Ji A, Zhou T and Che N:
Clinicopathological significance of SIRT1 expression in colorectal
cancer: A systematic review and meta analysis. Int J Surg.
26:32–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Liang Y and Vanhoutte PM: SIRT1
and AMPK in regulating mammalian senescence: A critical review and
a working model. FEBS Lett. 585:986–994. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Duan W, Li Y, Yan J, Yi W, Liang
Z, Wang N, Yi D and Jin Z: New role of silent information regulator
1 in cerebral ischemia. Neurobiol Aging. 34:2879–2888. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia Y, Wang CZ, Liu J, Anastasio NC and
Johnson KM: Brain-derived neurotrophic factor prevents
phencyclidine-induced apoptosis in developing brain by parallel
activation of both the ERK and PI-3K/Akt pathways.
Neuropharmacology. 58:330–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castillo DV and Escobar ML: A role for
MAPK and PI-3K signaling pathways in brain-derived neurotrophic
factor modification of conditioned taste aversion retention. Behav
Brain Res. 217:248–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pandey SC, Zhang H, Roy A and Misra K:
Central and medial amygdaloid brain-derived neurotrophic factor
signaling plays a critical role in alcohol-drinking and
anxiety-like behaviors. J Neurosci. 26:8320–8331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kramár EA, Chen LY, Lauterborn JC, Simmons
DA, Gall CM and Lynch G: BDNF upregulation rescues synaptic
plasticity in middle-aged ovariectomized rats. Neurobiol Aging.
33:708–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vetrovoĭ OV, Rybnikova TS and Samoĭlov MO:
Effect of hypoxic postconditioning on the expression of
antiapoptotic protein Bcl-2 and neurotrophin BDNF in CA1
hippocampal field of rats surviving severe hypoxia. Morfologiia.
145:16–20. 2014.(In Russian). PubMed/NCBI
|
|
17
|
Zhang T, Yan W, Li Q, Fu J, Liu K, Jia W,
Sun X and Liu X: 3-n-Butylphthalide (NBP) attenuated neuronal
autophagy and amyloid-β expression in diabetic mice subjected to
brain ischemia. Neurol Res. 33:396–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng Y, Xu SF, Wang L, Feng YP and Wang
XL: Effects of chiral NBP on cerebral infarct volume due to
transient focal cerebral ischemia. Zhongguo Xinyao Zazhi.
14:420–423. 2005.
|
|
19
|
Dong GX and Feng YP: Effects of
3-N-butylphthalide on cortical calcineurin and calpain activities
in focal cerebral ischemia rats. Yao Xue Xue Bao. 35:790–792.
2000.(In Chinese). PubMed/NCBI
|
|
20
|
Liu CL, Liao SJ, Zeng JS, Lin JW, Li CX,
Xie LC, Shi XG and Huang RX: Dl-3n-Butylphthalide prevents stroke
via improvement of cerebral microvessels in RHRSP. J Neurol Sci.
260:106–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng Y, Hu Y, Xu S, Feng N, Wang L and
Wang XL: L-3-n-butylphthalide regulates amyloid precursor protein
processing by PKC and MAPK pathways in SK-N-SH cells
over-expressing wild type human APP695. Neurosci Lett. 487:211–216.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng X, Peng Y, Liu M and Cui L:
DL-3-n-butylphthalide extends survival by attenuating glial
activation in a mouse model of amyotrophic lateral sclerosis.
Neuropharmacology. 62:1004–1010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng Y, Xu S, Chen G, Wang L, Feng Y and
Wang X: l-3-n-Butylphthalide improves cognitive impairment induced
by chronic cerebral hypoperfusion in rats. J Pharmacol Exp Ther.
321:902–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghanbarabadi M, Iranshahi M, Amoueian S,
Mehri S, Motamedshariaty VS and Mohajeri SA: Neuroprotective and
memory enhancing effects of auraptene in a rat model of vascular
dementia: Experimental study and histopathological evaluation.
Neurosci Lett. 623:13–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CH, Park JH, Ahn JH and Won MH:
Effects of melatonin on cognitive impairment and hippocampal
neuronal damage in a rat model of chronic cerebral hypoperfusion.
Exp Ther Med. 11:2240–2246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deacon RM and Rawlins JN: T-maze
alternation in the rodent. Nat Protoc. 1:7–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bowler JV: The concept of vascular
cognitive impairment. J Neurol Sci 203-204. 11–15. 2002. View Article : Google Scholar
|
|
29
|
Bowler JV: Modern concept of vascular
cognitive impairment. Br Med Bull. 83:291–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gorelick PB, Scuteri A, Black SE, Decarli
C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL,
Nyenhuis D, et al: Vascular contributions to cognitive impairment
and dementia: A statement for healthcare professionals from the
American heart association/American stroke association. Stroke.
42:2672–2713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Román GC, Sachdev P, Royall DR, Bullock
RA, Orgogozo JM, López-Pousa S, Arizaga R and Wallin A: Vascular
cognitive disorder: A new diagnostic category updating vascular
cognitive impairment and vascular dementia. Neurol Sci. 226:81–87.
2004. View Article : Google Scholar
|
|
32
|
O'Brien JT: Vascular cognitive impairment.
Am J Geriatr Psychiatry. 14:724–733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jia J, Wei C, Liang J, Zhou A, Zuo X, Song
H, Wu L, Chen X, Chen S, Zhang J, et al: The effects of
DL-3-n-butylphthalide in patients with vascular cognitive
impairment without dementia caused by subcortical ischemic small
vessel disease: A multicentre, randomized, double-blind,
placebo-controlled trial. Alzheimers Dement. 12:89–99. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen N, Zhou Z, Li J, Li BC, Feng JH, He
D, Luo Y, Zheng X, Luo J and Zhang J: 3-n-butylphthalide exerts
neuroprotective effects by enhancing anti-oxidation and attenuating
mitochondrial dysfunction in an in vitro model of ischemic stroke.
Drug Des Devel Ther. 12:4261–4271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu J, Huai Y, Meng N, Dong Y, Liu Z, Qi Q,
Hu M, Fan M, Jin W and Lv P: L-3-n-Butylphthalide activates
Akt/mTOR signaling, inhibits neuronal apoptosis and autophagy and
improves cognitive impairment in mice with repeated cerebral
ischemia-reperfusion injury. Neurochem Res. 42:2968–2981. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Niu XL, Jiang X, Xu GD, Zhang GM, Tang ZP,
Yin N, Li XQ, Yang YY and Lv PY: DL-3-n-butylphthalide alleviates
vascular cognitive impairment by regulating endoplasmic reticulum
stress and the Shh/Ptch1 signaling-pathway in rats. J Cell Physiol.
234:12604–12614. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xing M, Sun Q, Wang Y, Cheng Y and Zhang
N: Hydroxysafflor yellow A increases BDNF and NMDARs in the
hippocampus in a vascular dementia rat model. Brain Res.
1642:419–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ogawa T, Wakai C, Saito T, Murayama A,
Mimura Y, Youfu S, Nakamachi T, Kuwagata M, Satoh K and Shioda S:
Distribution of the longevity gene product, SIRT1, in developing
mouse organs. Congenit Anom (Kyoto). 51:70–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Michishita E, Park JY, Burneskis JM,
Barrett JC and Horikawa I: Evolutionarily conserved and
nonconserved cellular localizations and functions of human SIRT
proteins. Mol Biol Cell. 16:4623–4635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shan T, Wang Y, Wu T, Liu C, Guo J, Zhang
Y, Liu J and Xu Z: Porcine sirtuin 1 gene clone, expression
pattern, and regulation by resveratrol. J Anim Sci. 87:895–904.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Michán S, Li Y, Chou MH, Parrella E, Ge H,
Long JM, Allard JS, Lewis K, Miller M, Xu W, et al: SIRT1 is
essential for normal cognitive function and synaptic plasticity. J
Neurosci. 30:9695–9707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen YY, Zhang L, Shi DL, Song XH, Shen
YL, Zheng MZ and Wang LL: Resveratrol attenuates subacute systemic
inflammation-induced spatial memory impairment via inhibition of
astrocyte activation and enhancement of synaptophysin expression in
the hippocampus. Ann Clin Lab Sci. 47:17–24. 2017.PubMed/NCBI
|
|
43
|
Wang R, Zhang Y, Li J and Zhang C:
Resveratrol ameliorates spatial learning memory impairment induced
by Aβ1-42 in rats. Neuroscience. 344:39–47. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li XH, Chen C, Tu Y, Sun HT, Zhao ML,
Cheng SX, Qu Y and Zhang S: Sirt1 promotes axonogenesis by
deacetylation of Akt and inactivation of GSK3. Mol Neurobiol.
48:490–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li XH, Lv BL, Xie JZ, Liu J, Zhou XW and
Wang JZ: AGEs induce Alzheimer-like tau pathology and memory
deficit via RAGE-mediated GSK-3 activation. Neurobiol Aging.
33:1400–1410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Watzlawick R, Howells DW and Schwab JM:
Neuroprotection aftr traumatic brain injury. JAMA Neurol.
73:149–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kaplan GB, Vasterling JJ and Vedak PC:
Brain-derived neurotrophic factor in traumatic brain injury,
post-traumatic stress disorder, and their comorbid conditions: Role
in pathogenesis and treatment. Behav Pharmacol. 21:427–437. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xiang J, Pan J, Chen F, Zheng L, Chen Y,
Zhang S and Feng W: L-3-n-butylphthalide improves cognitive
impairment of APP/PS1 mice by BDNF/TrkB/PI3K/AKT pathway. Int J
Clin Exp Med. 7:1706–1713. 2014.PubMed/NCBI
|
|
49
|
Koppula S, Kumar H, More SV, Kim BW, Kim
IS and Choi DK: Recent advances on the neuroprotective potential of
antioxidants in experimental models of Parkinson's disease. Int J
Mol Sci. 13:10608–10629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu Y, Huang Q, Liu X and Wei X:
Dl-3-n-butylphthalide is effective for demyelination: A
case-combined study. Clin Neurol Neurosurg. 137:83–88. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang C, Zhao S, Zang Y, Gu F, Mao S, Feng
S, Hu L and Zhang C: The efficacy and safety of
Dl-3n-butylphthalide on progressive cerebral infarction: A
randomized controlled STROBE study. Medicine (Baltimore).
96:e72572017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang X, Wang L, Sheng X, Huang Z, Li T,
Zhang M, Xu J, Ji H, Yin J and Zhang Y: Design, synthesis and
biological evaluation of hydrogen sulfide releasing derivatives of
3-n-butylphthalide as potential antiplatelet and antithrombotic
agents. Org Biomol Chem. 12:5995–6004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zeng Y and Yang K: SIRT1uin 1 participates
in the process of age-related retinal degeneration. Biochem Biophys
Res Commun. 468:167–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang M, Wang J, Fu J, Du L, Jeong H, West
T, Xiang L, Peng Q, Hou Z, Cai H, et al: Neuroprotective role of
Sirt1 in mammalian models of Huntington's disease through
activation of multiple Sirt1 targets. Nat Med. 18:153–158. 2011.
View Article : Google Scholar : PubMed/NCBI
|