Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common malignancy and the fourth leading cause of cancer-related
death globally, and worldwide incidence increases by 3–4% per year
(1,2). Hepatitis B virus (HBV) or hepatitis C
virus (HCV) infection, alcohol consumption, diabetes, nonalcoholic
fatty liver disease and smoking are known to be major risk factors
for HCC (3,4). HCC is highly heterogeneous, and the
pathogenesis is extremely complex. The progression of HCC involves
multiple processes such as mutation and signaling pathway
maladjustment, reflecting the interaction among multiple genes
(5,6). Despite the development of various
drugs and breakthroughs in diagnosis, the prognosis of HCC remains
poor, with a 5-year survival rate of only 5% for patients with
advanced HCC (7). Timely and
effective assessment of prognosis is of great significance to guide
the treatment. At present, there are no biomarkers that effectively
predict the survival of patients with HCC, and thus, finding
effective prognostic biomarkers for patients with HCC is
crucial.
Long non-coding RNAs (lncRNAs), non-coding
transcripts >200 nucleotides, serve important cellular functions
such as in chromatin modification as well as transcriptional and
post-transcriptional regulation (8,9).
Increasing evidence demonstrates that aberrant expression of
lncRNAs is associated with the occurrence and development of
various human diseases, especially cancer (10–12).
For example, the overexpression of lncRNA HOX Transcript Antisense
Intergenic RNA (HOTAIR) was demonstrated to predict tumor
recurrence after liver transplantation (13). There was also a significant
association between HOTAIR expression and tumor progression in
patients with HCC (14–16). Increased biallelic expression of
H19 and IGF2 may participate in an epigenetic mechanism of HCC
development and progression (17).
The lncRNA GPC3-AS1 promotes HCC progression by epigenetic GPC3
activation (18). However, the
role of lncRNAs in alcohol-related HCC remains unclear.
Alcohol is a dose-related risk factor known to be
associated with more than 200 diseases, including HCC (19,20).
Heavy drinkers are at 3- to 10-fold higher risk of hepatocellular
carcinoma than non-drinkers (2).
In addition, the overall survival rate is lower for patients with
alcohol-related HCC than for those with non-alcohol-related HCC,
suggesting that there may be a link between alcohol and prognosis
(21). Thus, the present study
aimed to examine whether lncRNAs are differentially expressed in
the presence of alcohol consumption that may be used as prognostic
markers in HCC, and whether these differences might influence the
risk of HCC recurrence or death. Using data from The Cancer Genome
Atlas (TCGA), the present study developed a risk-scoring system
based on lncRNA levels that may be valuable for predicting the
prognosis of patients with alcohol-related HCC.
Materials and methods
Patient selection and data
collection
Profiles of lncRNA and mRNA expression in HCC
patients were downloaded from the University of California Santa
Cruz (UCSC) Xena server (https://xenabrowser.net/datapages/). Corresponding
clinical information was obtained from TCGA (version 09–14–2017 for
HCC). The patients in this dataset had histologically confirmed
HCC. Patient data included a complete lncRNA expression profile,
alcohol consumption status and survival data for determining OS and
RFS. A total of 113 patients with HCC and alcohol consumption, and
224 without alcohol consumption were selected (Table I). This study complied with TCGA
publication guidelines and policies (http://cancergenome.nih.gov/publications/publicationguidelines).
No ethics approval was required for this study since data were
obtained from TCGA.
 | Table I.Clinicopathological characteristics of
337 patients with alcohol- or non-alcohol-related hepatocellular
carcinoma. |
Table I.
Clinicopathological characteristics of
337 patients with alcohol- or non-alcohol-related hepatocellular
carcinoma.
|
| Patients (n=337) |
|---|
|
|
|
|---|
| Clinicopathological
characteristics | n | % |
|---|
| Age, years |
| ≤60 | 162 | 48.07 |
|
>60 | 175 | 51.93 |
| BMI |
|
<25 | 161 | 47.77 |
|
≥25 | 150 | 44.51 |
| Not
reported | 26 | 7.72 |
| Race |
|
Non-Asian | 186 | 55.19 |
|
Asian | 141 | 41.84 |
| Not
reported | 10 | 2.97 |
| Sex |
|
Female | 107 | 31.75 |
|
Male | 230 | 68.25 |
|
Hepatitisa |
| No | 189 | 56.08 |
|
Yes | 148 | 43.92 |
| Cirrhosis |
|
Non-cirrhosis | 124 | 36.80 |
|
Cirrhosis | 74 | 21.96 |
| Not
reported | 139 | 41.25 |
| Alcohol
consumption |
| No | 224 | 66.47 |
|
Yes | 113 | 33.53 |
| Histologic
grade |
|
G1-2 | 209 | 62.02 |
|
G3-4 | 123 | 36.50 |
| Not
reported | 5 | 1.418 |
| New tumor
event |
| No | 167 | 49.55 |
|
Yes | 154 | 45.70 |
| Not
reported | 16 | 4.75 |
| Pathologic
stage |
| Stage
I+II | 231 | 68.55 |
| Stage
III+IV | 82 | 24.33 |
| Not
reported | 24 | 7.12 |
| Cancer status |
| Tumor
free | 181 | 53.71 |
| With
tumor | 142 | 42.14 |
| Not
reported | 14 | 4.15 |
| Family cancer
history |
| No | 188 | 55.79 |
|
Yes | 108 | 32.05 |
| Not
reported | 41 | 12.17 |
| Residual tumor |
| R0 | 301 | 89.32 |
|
Non-R0 | 29 | 8.61 |
| Not
reported | 7 | 2.08 |
| Vascular
invasion |
|
Negative | 188 | 55.79 |
|
Positive | 95 | 28.19 |
| Not
reported | 54 | 16.02 |
Identification of lncRNAs
differentially expressed between alcohol-related or
non-alcohol-related HCC
After eliminating lncRNAs showing zero expression in
>50% of all patients, the edgeR package in R (https://www.r-project.org/) was used to identify
lncRNAs differentially expressed between patients with or without
alcohol consumption (22).
Differential expression was defined as log2fold change
(log2FC) >1 and false discovery rate (FDR) <0.05.
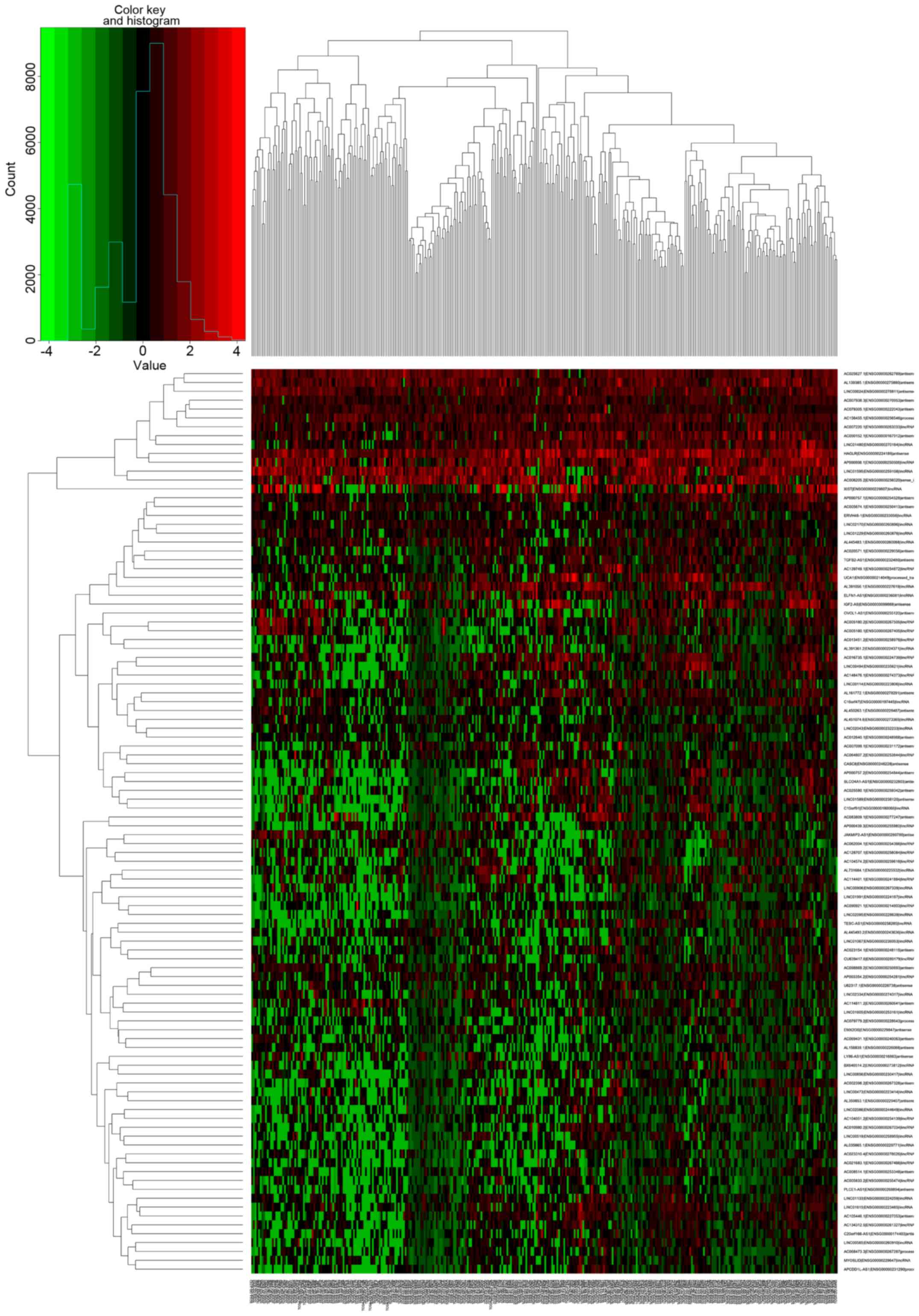
Differentially expressed lncRNAs were then presented in cluster
heat maps and volcano maps generated using the packages
gplots and heatmap in R.
Construction of lncRNA-based risk
scoring systems
Standardized expression of lncRNAs in multiple
tissues of the same patient were averaged. Univariate Cox analysis
was then performed to screen differentially expressed lncRNAs to
determine their significant relationship with OS or RFS, with the
threshold set at P=0.05. Selected lncRNAs were included in
subsequent multivariate Cox regression by the backward stepwise
method in order to identify the best model. The expression level of
each lncRNA was multiplied by the corresponding regression
coefficient β and linearly combined to generate a risk scoring
system:
Risk score=(β1 × expression level of lncRNA1) + (β2
× expression level of lncRNA2) + (β3 × expression level of lncRNA3)
+ (βn × expression level of lncRNAn).
This formula was used to calculate a risk score for
each patient. The prognosis prediction performance of this risk
score was assessed using time-dependent receiver operating
characteristic (ROC) curves within three years (23). Patients with HCC were divided into
a high- or a low-risk group according to the cut-off value of the
median risk score, as demonstrated in non-cluster heat maps.
Kaplan-Meier survival curves were generated and compared between
high- and low-risk groups. All these analyses were conducted using
R/Bioconductor (version 3.4.4, http://www.r-project.org/).
Prognostic performance of the risk
scoring systems
To validate the prognostic performance of the risk
scoring systems, univariate and multivariate Cox regression
analyses were performed to determine whether the risk score was an
independent factor for survival. This regression was performed in
SPSS 16.0 (SPSS, Inc.), and a significance threshold of P=0.05
(two-sided).
Co-expression and functional
enrichment analysis of related mRNAs
Pearson correlation was performed to screen for
relationships between lncRNAs in the risk scoring systems and mRNAs
based on data of 337 patients with HCC. Relationships were
considered significant if the mRNA expression co-varied with that
of lncRNAs with a two-sided absolute value of the Pearson
correlation coefficient >0.30 and a z-test P<0.01. To obtain
a deeper understanding of these mRNAs, enrichment analyses were
performed using the Genomes pathway in the Kyoto Encyclopedia of
Genes and Genomes by the package clusterProfiler in R
(24). P<0.05 was considered to
indicate a statistically significant difference.
Results
lncRNAs are differentially expressed
in alcohol-related HCC or non-alcohol-related HCC
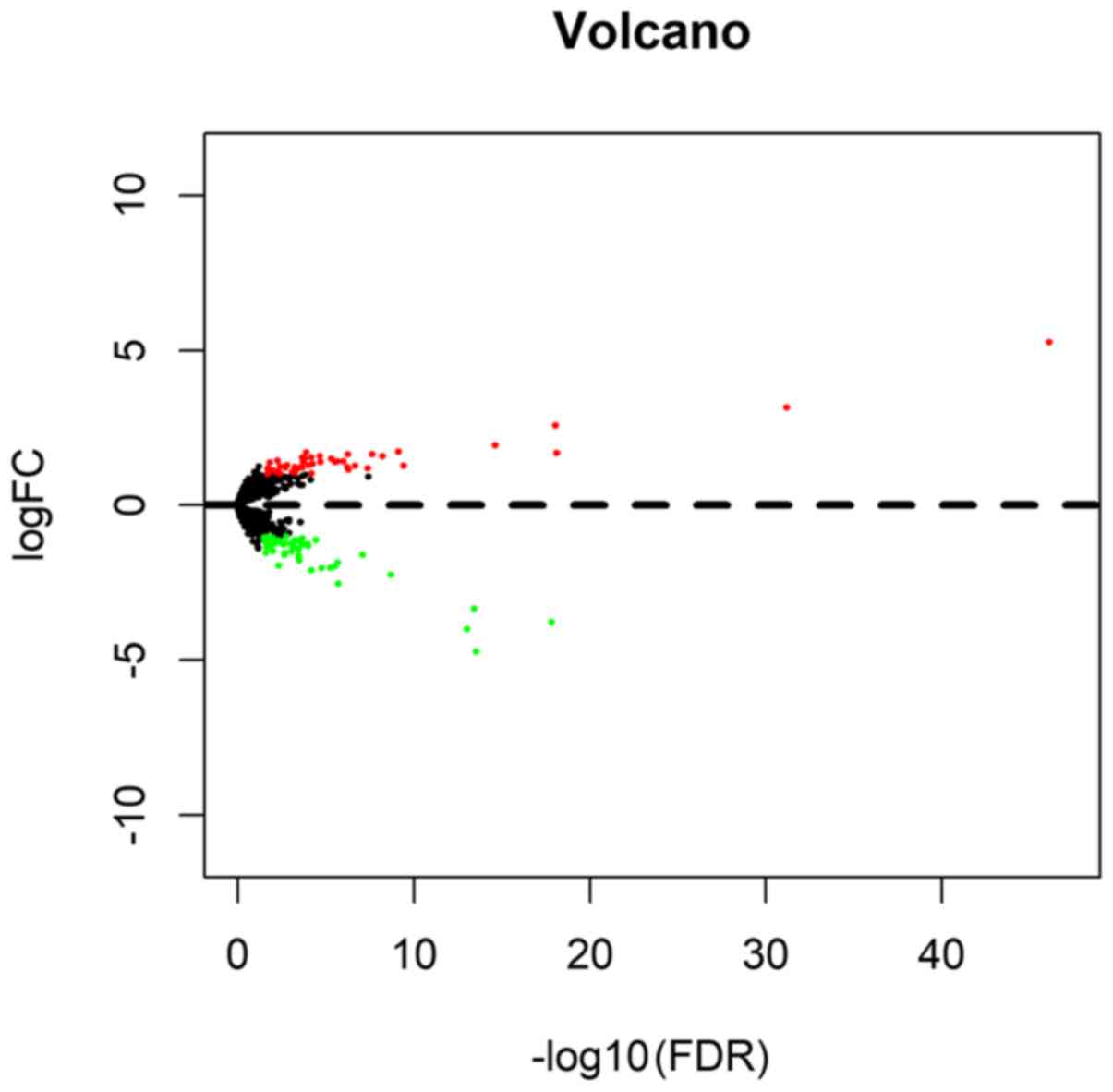
A total of 102 differentially expressed lncRNAs were
identified, 47 (46.08%) of which were upregulated and 55 (53.92%)
downregulated (Figs. 1 and
2); the first 20 up- and
downregulated lncRNAs, together with the corresponding values for
log2FC, P and FDR are demonstrated in Table II.
 | Table II.Differentially expressed lncRNAs in
patients with alcohol-related or non-alcohol-related hepatocellular
carcinoma. |
Table II.
Differentially expressed lncRNAs in
patients with alcohol-related or non-alcohol-related hepatocellular
carcinoma.
| Top 20 upregulated
lncRNAs | Top 20
downregulated lncRNAs |
|---|
|
|
|---|
| lncRNA | logFC | P-value | FDR | lncRNA | logFC | P-value | FDR |
|---|
| AC090921.1 | 5.26 |
1.35×10−50 |
7.79×10−47 | ERVH48-1 | −3.77 |
1.25×10−21 |
1.45×10−18 |
| AL451074.6 | 3.15 |
2.17×10−35 |
6.29×10−32 | AP000439.3 | −4.73 |
3.49×10−17 |
2.89×10−14 |
| AC007938.3 | 1.68 |
3.78×10−22 |
7.29×10−19 | AC139749.1 | −3.34 |
5.15×10−17 |
3.72×10−14 |
| AC105446.1 | 2.58 |
6.40×10−22 |
9.26×10−19 | LINC00473 | −4.00 |
1.51×10−16 |
9.73×10−14 |
| AC012640.1 | 1.94 |
2.45×10−18 |
2.36×10−15 | LINC01480 | −2.26 |
4.15×10−12 |
2.00×10−9 |
| AC025627.1 | 1.26 |
6.31×10−13 |
3.65×10−10 | AP000757.1 | −1.61 |
2.31×10−10 |
7.86×10−8 |
| AL445483.1 | 1.73 |
1.36×10−12 |
7.14×10−10 | UCA1 | −2.54 |
7.54×10−9 |
1.90×10−6 |
| MYOSLID | 1.59 |
1.35×10−11 |
6.03×10−9 | AC064807.2 | −1.85 |
9.35×10−9 |
2.16×10−6 |
| EMX2OS | 1.63 |
5.63×10−11 |
2.33×10−8 | AL359853.1 | −1.98 |
1.29×10−8 |
2.82×10−6 |
| AC156455.1 | 1.18 |
1.15×10−10 |
4.17×10−8 | PLCE1-AS1 | −2.03 |
2.86×10−8 |
5.71×10−6 |
| AP003354.2 | 1.27 |
6.76×10−10 |
2.17×10−7 | AC002398.2 | −2.03 |
9.19×10−8 |
1.77×10−5 |
| AC007220.1 | 1.14 |
1.70×10−9 |
5.18×10−7 | AC079305.1 | −1.12 |
2.12×10−7 |
3.71×10−5 |
| U62317.1 | 1.63 |
1.93×10−9 |
5.58×10−7 | AC007099.1 | −2.10 |
4.35×10−7 |
6.80×10−5 |
| LINC02043 | 1.22 |
2.25×10−9 |
6.19×10−7 | LINC01229 | −1.32 |
7.07×10−7 |
1.02×10−4 |
| AC068473.3 | 1.41 |
3.83×10−9 |
1.01×10−6 | AC098869.2 | −1.26 |
7.52×10−7 |
1.06×10−4 |
| AC069431.1 | 1.42 |
8.70×10−9 |
2×10−6 | LINC00624 | −1.28 |
1.16×10−6 |
1.51×10−4 |
| LINC01615 | 1.40 |
1.32×10−8 |
2.82×10−6 | AC005674.1 | −1.06 |
1.83×10−6 |
2.11×10−4 |
| AC079779.2 | 1.50 |
2.35×10−8 |
4.86×10−6 | AL161772.1 | −1.24 |
2.26×10−6 |
2.52×10−4 |
| AC062004.1 | 1.40 |
1.11×10−7 |
2.07×10−5 | BX640514.2 | −1.79 |
2.93×10−6 |
3.08×10−4 |
| AL391056.1 | 1.574 |
1.21×10−7 |
2.18×10−5 | AC023154.1 | −1.44 |
3.44×10−6 |
3.46×10−4 |
Risk-scoring system based on lncRNA
expression and OS
Univariate Cox analysis identified six lncRNAs that
were significantly associated with OS: AC012640.1, AC013451.2,
AC062004.1, LINC02334, AC090921.1 and LINC01605. The first four
were independent prognostic indicators of OS based on multivariate
Cox regression (Table III). The
resulting risk scoring system was: Risk score=(0.186 × AC012640.1)
+ (0.363 × AC013451.2) + (−0.243 × AC062004.1) + (−0.275 ×
LINC02334).
 | Table III.Four lncRNAs were correlated with
overall survival in the best statistical model. |
Table III.
Four lncRNAs were correlated with
overall survival in the best statistical model.
| lncRNA | β | HR | z | P-value |
|---|
| AC012640.1 | 0.186 | 1.205 | 1.71 | 0.088 |
| AC062004.1 | 0.243 | 0.784 | −1.98 | 0.047a |
| LINC02334 | −0.275 | 0.759 | −2.23 | 0.026a |
| AC013451.2 | 0.363 | 1.437 | 2.42 | 0.016a |
In this scoring system, increased expression of
AC012640.1 and AC013451.2 predicted worse OS (β>0), whereas
increased expression of AC062004.1 and LINC02334 predicted better
OS (β<0).
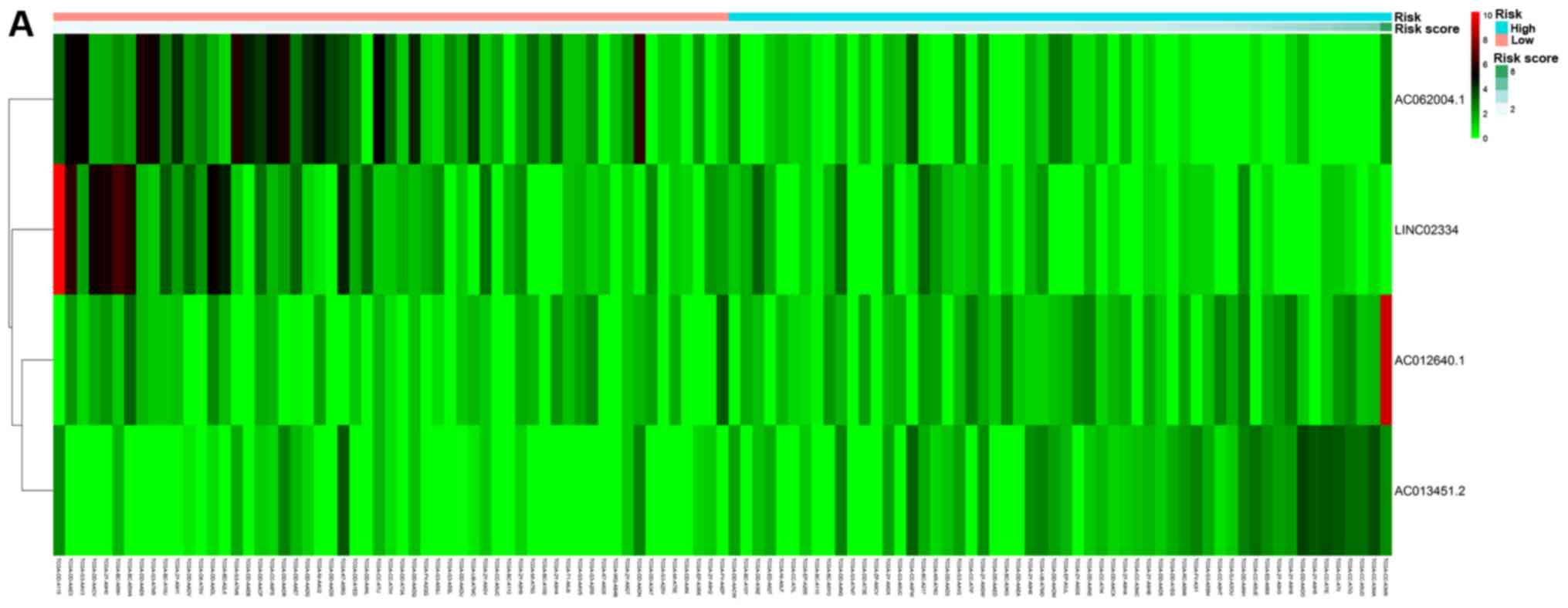
Based on their risk scores, patients were classified
as at low or high risk of poor OS using the median risk score as
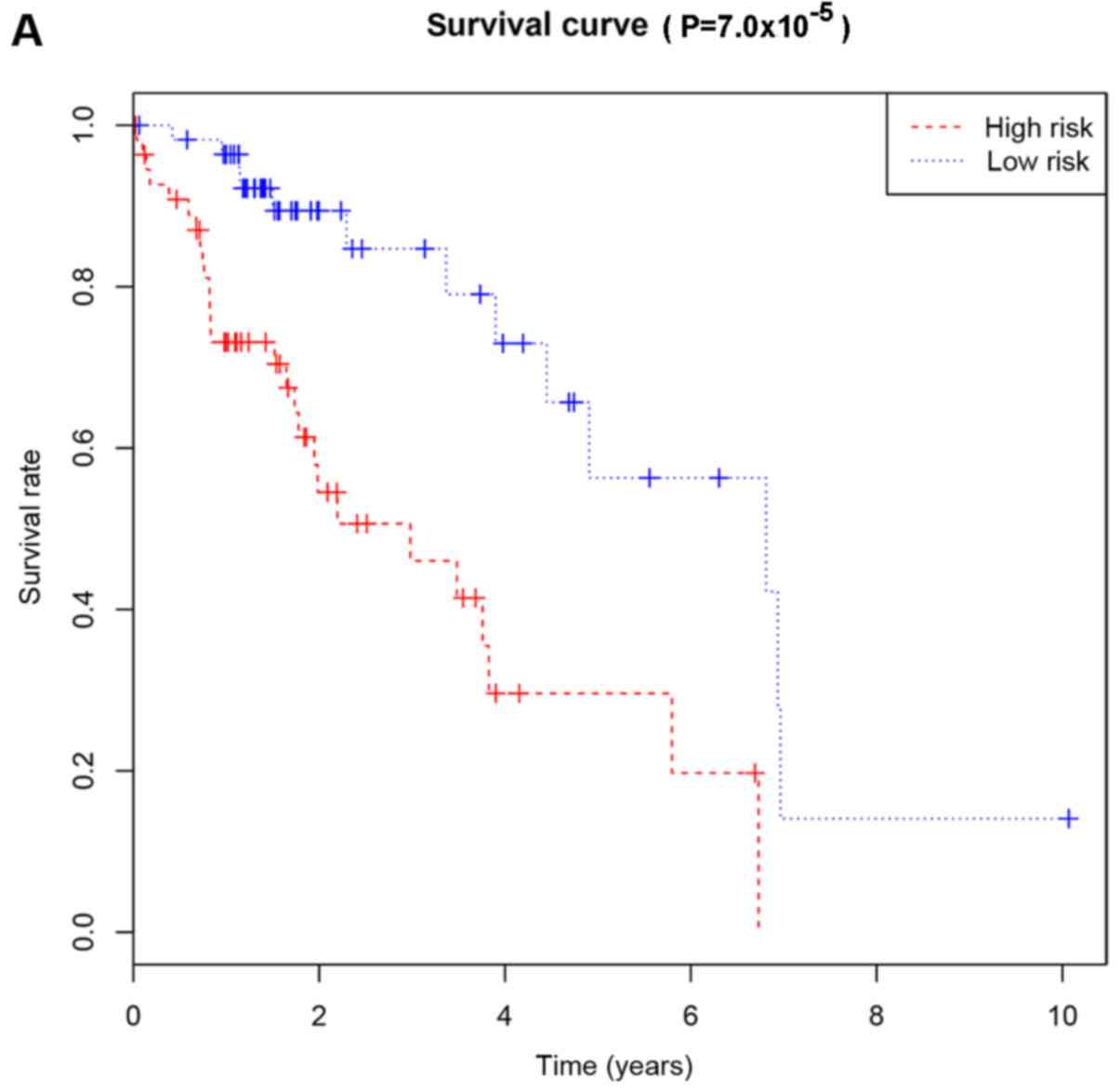
cutoff (Fig. 3A). Kaplan-Meier
curves demonstrated that patients with high risk had significantly
lower OS at 3 and 5 years compared with that of patients with low
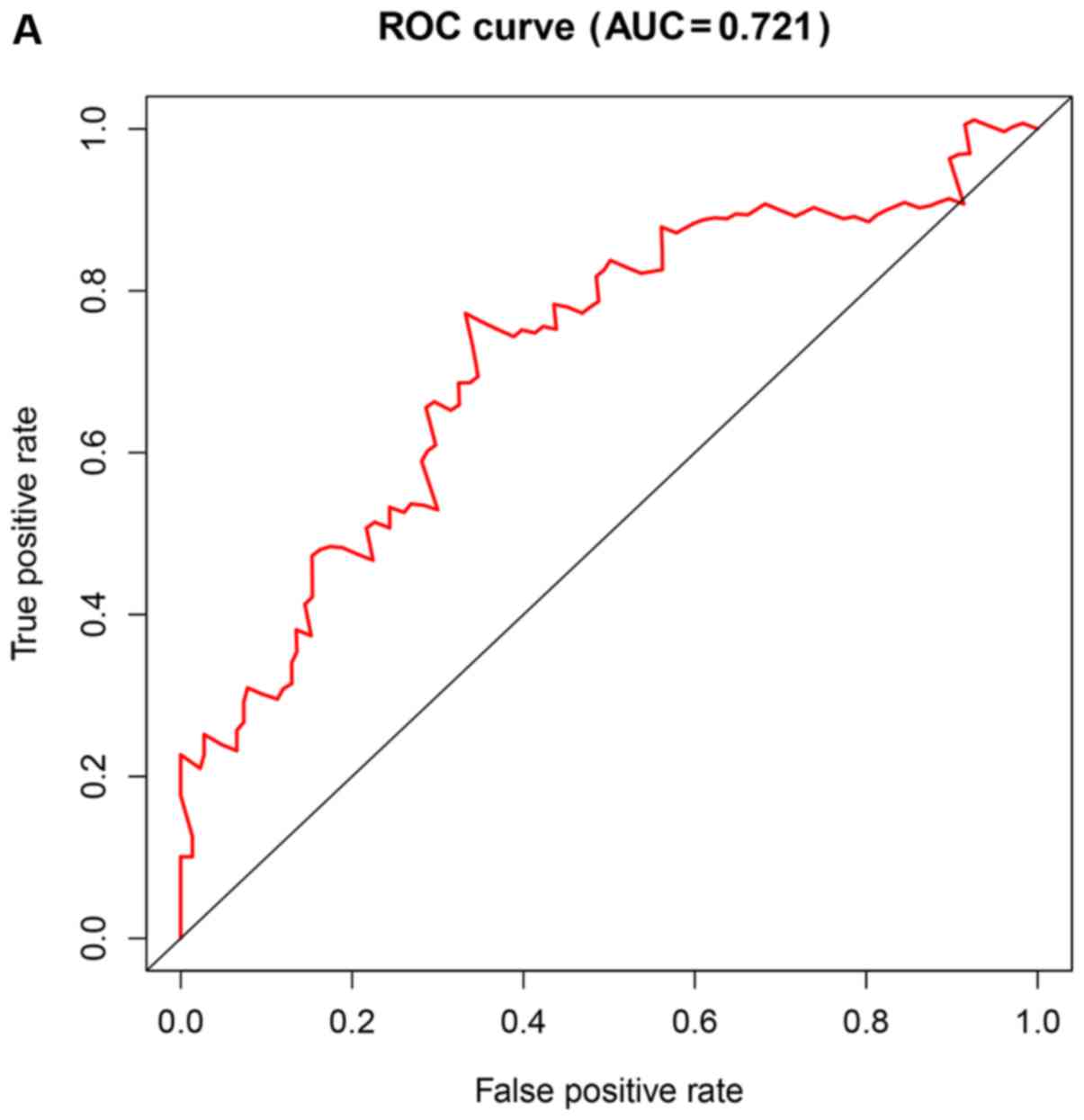
risk (Fig. 4A). The area under the
ROC curve (AUC) for the risk scoring system was 0.721 (Fig. 5A).
The risk scoring system was used to predict OS of
patients with different clinicodemographic characteristics. This is
an important test of the scoring system because of the
heterogeneity of HCC and the large number of factors that influence
prognosis.
Univariate analysis identified risk score, family
cancer history and vascular invasion as significantly associated
with OS, but not age, body mass index (BMI), ethnicity, sex,
hepatitis, cirrhosis, histological grade of cancer, new tumor
event, pathology stage, cancer status or residual tumor.
Multivariate Cox regression identified the following as independent
predictors of poor OS: Risk score [hazard ratio (HR) 3.393, 95%
confidence interval (CI) 1.597–7.210] and vascular invasion (HR
2.146, 95% CI 0.903–5.104, Table
V).
 | Table V.Univariate and multivariate Cox
regression analysis for overall survival. |
Table V.
Univariate and multivariate Cox
regression analysis for overall survival.
|
| Univariate Cox
regression | Multivariate Cox
regression |
|---|
|
|
|
|
|---|
| Variables | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Risk score
(high/low) | 0.003a | 4.949 | 1.735–14.117 | 0.001a | 3.393 | 1.597–7.210 |
| Age
(>60/≤60) | 0.202 | 1.816 | 0.726–4.543 |
|
|
|
| BMI | 0.060 |
|
|
|
|
|
|
<25 | Reference |
|
|
|
|
|
|
≥25 |
| 0.245 | 0.073–0.818 |
|
|
|
| Not
reported |
| 0.779 | 0.096–6.340 |
|
|
|
| Race | 0.423 |
|
|
|
|
|
|
Non-Asian | Reference |
|
|
|
|
|
|
Asian |
| 0.352 | 0.053–2.322 |
|
|
|
| Not
reported |
| 1.915 | 0.115–31.769 |
|
|
|
| Sex
(Male/Female) | 0.143 | 2.590 | 0.725–9.251 |
|
|
|
| Hepatitis B or
C | 0.542 |
|
|
|
|
|
| No | Reference |
|
|
|
|
|
|
Yes |
| 1.489 | 0.415–5.346 |
|
|
|
|
Cirrhosis | 0.216 |
|
|
|
|
|
|
Non-cirrhosis | Reference |
|
|
|
|
|
| Cirrhosis |
| 3.183 | 0.524–19.324 |
|
|
|
| Not
reported |
| 0.572 | 0.178–1.843 |
|
|
|
| Histologic
grade | 0.586 |
|
|
|
|
|
|
G1-2 | Reference |
|
|
|
|
|
|
G3-4 |
| 1.326 | 0.481–3.649 |
|
|
|
| New tumor
event | 0.406 |
|
|
|
|
|
| No | Reference |
|
|
|
|
|
|
Yes |
| 1.126 | 0.216–5.883 |
|
|
|
| Not
reported |
| 16.771 | 0.260–1080.40 |
|
|
|
| Pathologic
stage | 0.093 |
|
|
|
|
|
| Stage
I+II | Reference |
|
|
|
|
|
| Stage
III+IV |
| 2.857 | 0.935–8.730 |
|
|
|
| Not
reported |
| 3.173 | 0.740–13.617 |
|
|
|
| Cancer status | 0.998 |
|
|
|
|
|
| Tumor
free | Reference |
|
|
|
|
|
| With
tumor |
| 1.052 | 0.196–5.635 |
|
|
|
| Not
reported |
| 0.000 | 0.000 |
|
|
|
| Family cancer
history | 0.031 |
|
| 0.126 |
|
|
| No | Reference | Reference |
|
|
|
|
|
Yes |
| 0.667 | 0.234–1.899 |
| 0.868 | 0.419–1.799 |
| Not
reported |
| 0.076 | 0.011–0.528 |
| 0.206 | 0.045–0.953 |
| Residual tumor | 0.611 |
|
|
|
|
|
| R0 | Reference |
|
|
|
|
|
|
Non-R0 |
| 0.469 | 0.105–2.092 |
|
|
|
| Not
reported |
| 0.000 | 0.000 |
|
|
|
| Vascular
invasion |
| 0.006a |
| 0.005a |
|
|
|
Negative | Reference | Reference |
|
|
|
|
|
Positive |
| 3.019 | 0.798–11.416 |
| 2.146 | 0.903–5.104 |
| Not
reported |
| 8.383 | 2.264–31.038 |
| 3.577 | 1.652–7.748 |
Risk-scoring system based on lncRNA
expression and RFS
Univariate analysis identified 11 lncRNAs that were
significantly correlated with RFS: ERVH48-1, LINC02043, LINC01605,
AC062004.1, AL139385, AC007938.3, AC090921.1, AC025580.1,
AC012640.1, C10orf91, and LINC01589. Multivariate analysis
demonstrated the first five to be independent prognostic indicators
of RFS (Table IV). The resulting
risk scoring system was: Risk score=(0.3529 × ERVH48-1) + (0.3499 ×
LINC02043) + (0.1701 × LINC01605) + (−0.3531 × AC062004.1) +
(−0.1924 × AL139385).
 | Table IV.Five lncRNAs were correlated with
recurrence-free survival in the best statistical model. |
Table IV.
Five lncRNAs were correlated with
recurrence-free survival in the best statistical model.
| lncRNA | β | HR | z | P-value |
|---|
| ERVH48-1 | 0.3529 | 1.4232 | 2.59 | 0.0096a |
| LINC02043 | 0.3499 | 1.4189 | 3.13 | 0.0017a |
| AC062004.1 | −0.3531 | 0.7025 | −3.12 | 0.0018a |
| LINC01605 | 0.1701 | 1.1855 | 2.19 | 0.0285a |
| AL139385 | −0.1924 | 0.8249 | −2.23 | 0.0259a |
In this scoring system, increased expression of
ERVH48-1, LINC02043, and LINC01605 predicted worse RFS (β>0),
whereas increased expression of AC062004.1 and AL139385 predicted
better RFS (β<0).
Patients were classified as at low or high risk of
poor RFS (Fig. 3B). Kaplan-Meier
curves demonstrated that patients with high risk had significantly
lower RFS at 3 and 5 years compared with that of patients with low
risk (Fig. 4B). AUC for the risk
scoring system was 0.777 (Fig.
5B).
Univariate analysis identified that risk score and
vascular invasion were significantly correlated with RFS, but not
age, BMI, ethnicity, sex, hepatitis, cirrhosis, histology grade,
new tumor event, pathology stage, cancer status, family cancer
history or residual tumor. Multivariate analysis identified the
independent predictors to be risk score (HR 2.895, 95% CI
1.491–5.621) and vascular invasion (HR 2.398, 95% CI 1.104–5.210,
Table VI).
 | Table VI.Univariate and multivariate Cox
regression analysis for recurrence-free survival. |
Table VI.
Univariate and multivariate Cox
regression analysis for recurrence-free survival.
|
| Univariate Cox
regression | Multivariate Cox
regression |
|---|
|
|
|
|
|---|
| Variables | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Risk score
(high/low) | 0.009a | 4.883 | 1.485–16.051 | 0.002a | 2.895 | 1.491–5.621 |
| Age
(>60/≤60) | 0.064 | 2.343 | 0.952–5.767 |
|
|
|
| BMI | 0.255 |
|
|
|
|
|
|
<25 | Reference |
|
|
|
|
|
|
≥25 |
| 0.533 | 0.092–3.074 |
|
|
|
| Not
reported |
| 0.087 | 0.004–1.772 |
|
|
|
| Race | 0.403 |
|
|
|
|
|
|
Non-Asian | Reference |
|
|
|
|
|
|
Asian |
| 4.064 | 0.514–32.105 |
|
|
|
| Not
reported |
| 1.065 | 0.034–33.341 |
|
|
|
| Sex
(Male/Female) | 0.680 | 1.523 | 0.207–11.220 |
|
|
|
| Hepatitis B or
C | 0.210 |
|
|
|
|
|
| No | Reference |
|
|
|
|
|
|
Yes |
| 3.102 | 0.528–18.223 |
|
|
|
| Cirrhosis | 0.340 |
|
|
|
|
|
|
Non-cirrhosis | Reference |
|
|
|
|
|
|
Cirrhosis |
| 2.228 | 0.297–16.737 |
|
|
|
| Not
reported |
| 0.291 | 0.037–2.284 |
|
|
|
| Histologic
grade | 0.489 |
|
|
|
|
|
|
G1-2 | Reference |
|
|
|
|
|
|
G3-4 |
| 0.705 | 0.262–1.899 |
|
|
|
| New tumor
event | 0.860 |
|
|
|
|
|
| No | Reference |
|
|
|
|
|
|
Yes |
| 316078 |
0.000–3.495×1066 |
|
|
|
| Pathologic
stage | 0.464 |
|
|
|
|
|
| Stage
I+II | Reference |
|
|
|
|
|
| Stage
III+IV |
| 1.896 | 0.638–5.632 |
|
|
|
| Not
reported |
| 0.997 | 0.127–7.803 |
|
|
|
| Cancer status | 0.286 |
|
|
|
|
|
| Tumor
free | Reference |
|
|
|
|
|
| With
tumor |
| 2.632 | 0.323–21.431 |
|
|
|
| Not
reported |
| 11.747 | 0.399–345.797 |
|
|
|
| Family cancer
history | 0.242 |
|
|
|
|
|
| No | Reference |
|
|
|
|
|
|
Yes |
| 0.726 | 0.147–3.588 |
|
|
|
| Not
reported |
| 0.129 | 0.010–1.703 |
|
|
|
| Residual tumor | 0.749 |
|
|
|
|
|
| R0 |
| Reference |
|
|
|
|
|
Non-R0 |
| 0.930 | 0.090–9.614 |
|
|
|
| Not
reported |
| 0.321 | 0.015–6.981 |
|
|
|
| Vascular
invasion | 0.039a |
|
| 0.001a |
|
|
|
Negative | Reference | Reference |
|
|
|
|
|
Positive |
| 10.023 | 1.408–71.326 |
| 2.398 | 1.104–5.210 |
| Not
reported |
| 3.990 | 0.369–43.169 |
| 4.732 | 2.235–10.019 |
Functional analysis of co-expressed
lncRNA and mRNAs
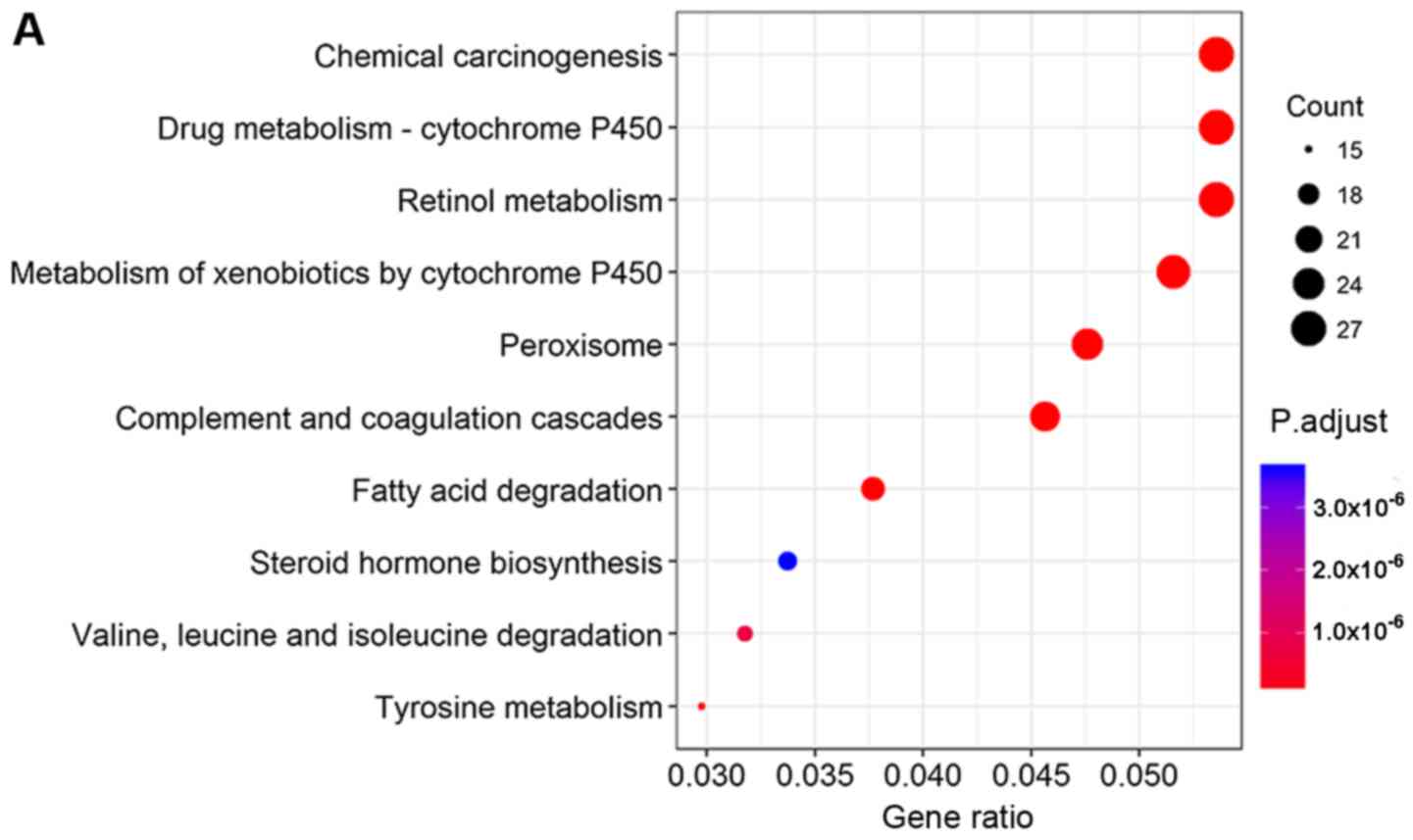
KEGG pathway analysis revealed that co-expressed
lncRNA and mRNAs that correlated with OS were involved mainly in
chemical carcinogenesis, cytochrome P450-mediated drug metabolism
and retinol metabolism (Fig. 6A).
Co-expressed lncRNAs and mRNAs that correlated with RFS were
involved mainly in cell cycle and carbon metabolism (Fig. 6B).
Discussion
HCC is a major health problem worldwide with poor
overall prognosis (25,26). Most patients with HCC are diagnosed
at advanced stages (III–IV) (27).
Earlier diagnosis and more reliable prognosis, based on suitable
biomarkers, are crucial for improving the management and therefore
outcomes of patients with HCC. Accumulating evidence has suggested
that the abnormal expression of lncRNAs is associated with the
recurrence, metastasis and prognosis of HCC (28–30).
Since the prognosis in HCC may differ depending on whether it is
alcohol-related or not, the present study developed a risk scoring
system based on lncRNA expression to evaluate the risk of poor OS
or RFS in alcohol-related patients with HCC. The results of the
present study may suggest good potential for lncRNAs to be
prognostic biomarkers in alcohol-related HCC.
The results of the present study demonstrated that
the risk-scoring system and vascular invasion were important
independent predictors of prognosis in the sample of patients with
HCC. The AUCs for OS and RFS risk scoring systems were high,
suggesting good predictive power. Thus, an lncRNA-based risk
scoring system may be used to estimate the risk scores of different
alcohol-related patients with HCC, predict survival and determine
treatment.
Previous studies have identified lncRNAs as
prognostic biomarkers for HCC using the TCGA database (31,32).
To the best of our knowledge, the present study is the first to
analyze alcohol-related HCC. The present study identified eight
lncRNAs as potential prognostic biomarkers for alcohol-related HCC.
Among them, LINC01605 has been demonstrated to be upregulated in
bladder cancer tissues and may be associated with poor prognosis
(33), whereas ERVH48-1 has been
identified as a prognostic biomarker for tongue squamous cell
carcinoma (34). The remaining
potential biomarkers from the present study (AC012640.1,
AC013451.2, AC062004.1, LINC02334, LINC02043, and AL139385) do not
appear to have been analyzed in detail. The eight lncRNAs in this
model appear to be involved in chemical carcinogenesis, metabolism
and the cell cycle. Investigating these lncRNA-mediated pathways
may provide new insights into the development of alcohol-related
HCC.
There are some limitations in this study. First, HCC
treatment types were not included in the multivariate Cox
regression due to lack of data. Second, Cox analyses may be less
accurate because some clinical data were missing for some patients.
Third, the sample was relatively small, and as a result the present
study could not divide the samples into training and test dataset
for determining and validating the model. Thus the findings of the
present study should be verified and extended in larger
studies.
Despite these limitations, the results of the
present study suggested that an lncRNA-based risk scoring system
may predict the risk of poor prognosis in patients with
alcohol-related HCC. Eight lncRNAs are independent
clinicopathological variables for alcohol-related HCC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Guangxi Key Research
and Development Program (grant. no. GuiKeAB16380215), Guangxi
First-class Discipline Project for Basic Medicine Sciences (grant.
no. GXFCDP-BMS-2018) and Guangxi Medical University Training
Program for Distinguished Young Scholars.
Availability of data and material
The datasets used during the present study are
included in this published article.
Authors' contributions
YUL. and JY designed and performed the research,
analyzed and interpreted the data, and drafted the manuscript. JW
collected and analyzed the data. YOL and JZ conceived the study,
designed the methodology and reviewed the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsushita H and Takaki A: Alcohol and
hepatocellular carcinoma. BMJ Open Gastroenterol. 6:e0002602019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrick JL, Kelly SP, Altekruse SF,
McGlynn KA and Rosenberg PS: Future of hepatocellular carcinoma
incidence in the United States forecast through 2030. J Clin Oncol.
34:1787–1794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zucman-Rossi J, Villanueva A, Nault JC and
Llovet JM: Genetic landscape and biomarkers of hepatocellular
carcinoma. Gastroenterology. 149:1226–1239 e1224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levrero M and Zucman-Rossi J: Mechanisms
of HBV-induced hepatocellular carcinoma. J Hepatol. 64 (1
Suppl):S84–S101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Louafi S, Boige V, Ducreux M, Bonyhay L,
Mansourbakht T, de Baere T, Asnacios A, Hannoun L, Poynard T and
Taïeb J: Gemcitabine plus oxaliplatin (GEMOX) in patients with
advanced hepatocellular carcinoma (HCC): Results of a phase II
study. Cancer. 109:1384–1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hung T and Chang HY: Long noncoding RNA in
genome regulation: Prospects and mechanisms. RNA Biol. 7:582–585.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: Novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheetham SW, Gruhl F, Mattick JS and
Dinger ME: Long noncoding RNAs and the genetics of cancer. Br J
Cancer. 108:2419–2425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hauptman N and Glavac D: Long non-coding
RNA in cancer. Int J Mol Sci. 14:4655–4669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Z, Zhou L, Wu LM, Lai MC, Xie HY,
Zhang F and Zheng SS: Overexpression of long non-coding RNA HOTAIR
predicts tumor recurrence in hepatocellular carcinoma patients
following liver transplantation. Ann Surg Oncol. 18:1243–1250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geng YJ, Xie SL, Li Q, Ma J and Wang GY:
Large intervening non-coding RNA HOTAIR is associated with
hepatocellular carcinoma progression. J Int Med Res. 39:2119–2128.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang L, Peng X, Li Y, Zhang X, Ma Y, Wu C,
Fan Q, Wei S, Li H and Liu J: Long non-coding RNA HOTAIR promotes
exosome secretion by regulating RAB35 and SNAP23 in hepatocellular
carcinoma. Mol Cancer. 18:782019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim KS and Lee YI: Biallelic expression of
the HI9 and ZGF2 genes in hepatocellular carcinoma. Cancer Lett.
119:143–148. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu XT, Yuan JH, Zhu TT, Li YY and Cheng
XY: Long noncoding RNA glypican 3 (GPC3) antisense transcript 1
promotes hepatocellular carcinoma progression via epigenetically
activating GPC3. FEBS J. 283:3739–3754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ganne-Carrie N and Nahon P: Hepatocellular
carcinoma in the setting of alcohol-related liver disease. J
Hepatol. 70:284–293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pimpin L, Cortez-Pinto H, Negro F,
Corbould E, Lazarus JV, Webber L and Sheron N; EASL HEPAHEALTH
Steering Committee, : Burden of liver disease in Europe:
Epidemiology and analysis of risk factors to identify prevention
policies. J Hepatol. 69:718–735. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bucci L, Garuti F, Camelli V, Lenzi B,
Farinati F, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di
Marco M, et al: Comparison between alcohol- and hepatitis C
virus-related hepatocellular carcinoma: Clinical presentation,
treatment and outcome. Aliment Pharmacol Ther. 43:385–399. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heagerty PJ, Lumley T and Pepe MS:
Time-dependent ROC curves for censored survival data and a
diagnostic marker. Biometrics. 56:337–344. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Budhu A, Forgues M, Ye QH, Jia HL, He P,
Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY and Wang XW:
Prediction of venous metastases, recurrence, and prognosis in
hepatocellular carcinoma based on a unique immune response
signature of the liver microenvironment. Cancer Cell. 10:99–111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin Y, Liang R, Qiu Y, Lv Y, Zhang J, Qin
G, Yuan C, Liu Z, Li Y, Zou D and Mao Y: Expression and gene
regulation network of RBM8A in hepatocellular carcinoma based on
data mining. Aging (Albany NY). 11:423–447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Y, Zheng S, Yao J, Li M, Yang G, Zhang
N, Zhang S and Zhong B: Decreased expression of protocadherin 20 is
associated with poor prognosis in hepatocellular carcinoma.
Oncotarget. 8:3018–3028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang X, Xie X, Xiao YF, Xie R, Hu CJ, Tang
B, Li BS and Yang SM: The emergence of long non-coding RNAs in the
tumorigenesis of hepatocellular carcinoma. Cancer Lett.
360:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiong H, Li B, He J, Zeng Y, Zhang Y and
He F: lncRNA HULC promotes the growth of hepatocellular carcinoma
cells via stabilizing COX-2 protein. Biochem Biophys Res Commun.
490:693–699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z, Li J, He T, Ouyang Y, Huang Y,
Liu Q, Wang P and Ding J: The competitive endogenous RNA regulatory
network reveals potential prognostic biomarkers for overall
survival in hepatocellular carcinoma. Cancer Sci. 110:2905–2923.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin P, Wen DY, Li Q, He Y, Yang H and Chen
G: Genome-wide analysis of prognostic lncRNAs, miRNAs, and mRNAs
forming a competing endogenous RNA network in hepatocellular
carcinoma. Cell Physiol Biochem. 48:1953–1967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qin Z, Wang Y, Tang J, Zhang L, Li R, Xue
J, Han P, Wang W, Qin C, Xing Q, et al: High LINC01605 expression
predicts poor prognosis and promotes tumor progression via
up-regulation of MMP9 in bladder cancer. Biosci Rep.
38:BSR201805622018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Cao R, Li Q, Yao M, Chen Y and
Zhou H: Comprehensive analysis of lncRNA-associated competing
endogenous RNA network in tongue squamous cell carcinoma. PeerJ.
7:e63972019. View Article : Google Scholar : PubMed/NCBI
|