Introduction
The public health system in China is increasingly
burdened by a disproportionate increase in type 1 and type 2
diabetes (T2D) (1). While the
mortality rate of the diseases and their presentation differ
significantly, the overall morbidity and associated mortality rates
associated with both the diseases are increasing sharply (2); for example, the morbidity rate in the
elderly population with type 2 diabetes was recorded as 21.4 and
24.8% in 2001 and 2010, respectively, in China (2,3). A
multitude of factors, such as obesity, age, diet, physical
activity, and genetic and epigenetic modifications have been
identified to be responsible for the increasing disease burden in
the Chinese population (4).
Region-specific studies of diabetes have reported an
incidence rate of 1.01 per 100,000 person years across all age
groups in China (5), with an
increased incidence rate in males compared with females (6). On the other hand, the prevalence of
T2D has increased to 9% in the last three decades (7) and it has been demonstrated to be
associated with high rates of morbidity and mortality (8).
Diabetes-induced microvascular diseases, including
retinopathy, neuropathy and nephropathy, and macrovascular
diseases, such as cardiovascular disease, stroke and peripheral
disease, often arise due to the inadequate maintenance of glycemic
control (9). It has been
hypothesized that microvasculopathy may give rise to
macrovasculopathy through hypoxia and changes in the vasa vasorum
(10); the changes in the vasa
vasorum have been often associated with endothelial dysfunction
(11); however, the molecular
mechanisms driving endothelial cell plasticity under hyperglycemic
stress have received little attention.
Transcription box-3 (TBX-3) is a T-box transcription
factor with a known role in cell development, patterning, exit from
the cell cycle and apoptosis (12). Given the significance of this
transcription factor and its modifications over the expression
levels of its target genes in the normally quiescent endothelial
cells, the present study aimed to investigate whether TBX-3 had a
role in mediating endothelial dysfunction and its associated
vasculopathy. Additionally, TBX-3 has been previously reported to
have a role in promoting adipocyte self-renewal (13). Therefore, the present study also
aimed to determine whether the expression levels of TBX-3,
the gene coding for TBX-3, were upregulated in endothelial cells in
response to hyperglycemia, in addition to their contribution to the
neotransformation of cells.
To the best of our knowledge, the present study was
the first demonstration of TBX-3-mediated changes in in
vitro endothelial cells in a hyperglycemic environment. In
addition, the study further determined the molecular pathways of
TBX-3-mediated endothelial dysfunction.
Materials and methods
Clinical samples
The present study was conducted in accordance with
the Declaration of Helsinki (14)
and approved by the Medical Ethics Committee of Qiqihar Medical
University. Written informed consent was obtained from all
recruited subjects. A total of 15 patients with diabetes (age,
40–60 years; sex, 7 males and 8 females) who had a T2D diagnosis
for ≥10 years according to the WHO criteria (15) and 15 non-diabetic age-matched
participants (sex, 7 males and 8 females) were recruited to the
Department of Endocrinology, The Third Affiliated Hospital of
Qiqihar Medical University (Qiqihar, China) from January 2015 to
January 2016. The exclusion criteria were as follows: Missing
clinical data, the prescription of oral medication and a
pre-existing diagnosis of essential hypertension, other autoimmune
diseases, thyroid disease, renal disease, psychosis, acute
infectious disease, acute stage of myocardial infarction and
stroke, type 1 diabetes and other specific forms of diabetes, such
as chronic pancreatitis and steroid-induced diabetes. Blood glucose
levels of all patients were controlled using insulin through a
subcutaneous injection. Fresh blood samples (5 ml/sample) were
obtained after an overnight fast and centrifugation at 2,000 × g at
4°C for 15 min. The separated serum was stored in liquid nitrogen
immediately following centrifugation for use in subsequent
analysis.
Gene Expression Omnibus (GEO)
datasets
The GSE49524 dataset (16) was downloaded from the GEO database
(https://www.ncbi.nlm.nih.gov/geo). This
dataset comprised the gene expression data of three mothers with
gestational diabetes and three normal participants. The data
analysis was performed using GEO2R software (17).
Human umbilical vein endothelial cell
(HUVECs) culture and transfection
HUVECs were purchased from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences. HUVECs were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo
Fisher Scientific, Inc.), and maintained at 37°C in a humidified 5%
CO2 atmosphere. These cells were from the umbilical cord
vein (18). HUVECs were treated
with different concentrations of glucose (0, 10, 20, 30 or 40 mM;
Sigma-Aldrich; Merck KGaA) for 1 h at 37°C and then cultured for 24
h in the medium without glucose.
Small interfering (si)RNA targeting TBX-3
(5′-GAGGAUGUACAUUCACCCG-3′), sirtuin 1 (SIRT1;
5′-GATGAAGTTGACCTCCTCA-3′) and control siRNA
(5′-UUCUCCGAACGAGUCACG-3′), and TBX-3 overexpression plasmids, were
synthesized by Shanghai GenePharma Co., Ltd. The pcDNA3.1 plasmid
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for the
generation of the overexpression plasmids; an empty plasmid was
used as the control for the overexpression experiments. HUVECs were
transfected with 10 nM siRNA or 1 µg/100 µl overexpression plasmid
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Following transfection for 48 h at 37°C, the cells were used for
subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from cultured HUVECs and serum samples was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
cDNA was then reverse transcribed from 2 µg RNA using a commercial
PrimeScript™ RT Reagent kit (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocol. The following RT
temperature protocol was used: 37°C for 15 min and 85°C for 5 sec,
followed by maintenance at 4°C for qPCR. qPCR was subsequently
performed using a SYBR Green Real-Time PCR Master mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol, on a 7500 Fast Real Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR: Initial
denaturation for 2 min at 94°C; followed by 30 cycles of 94°C for
30 sec, annellation at 55°C for 30 sec, extension at 74°C for 1
min; and a final extension at 74°C for 5 min. The following primer
sequences were used for the qPCR: TBX-3 forward,
5′-TTCCACATTGTAAGAGCCAATG-3′ and reverse,
5′-CTTTGAGGTTCGTTGTCCCTAC-3′; SIRT1 forward,
5′-TGGCAAAGGAGCAGATTAGTAGG-3′ and reverse,
5′-CTGCCACAAGAACTAGAGGATAAGA-3′; and GAPDH forward,
5′-GGGCTGCTTTTAACTCTGGT-3′ and reverse, 5′-TGGCAGGTTTTTCTAGACGG-3′.
Expression levels were quantified using the 2−ΔΔCq
method (19) and analyzed using
7500 FAST software (version 1.4; Applied Biosystems; Thermo Fisher
Scientific, Inc.), with the expression levels of the mRNA
normalized to the endogenous control, GAPDH.
Western blotting
Total protein was extracted from HUVECs using
ice-cold RIPA lysis buffer [50 mM Tris pH 7.4, 150 mM NaCl, 1%
Triton X-100, 1% sodium deoxycholate, 0.1% SDS with
proteinase/phosphatase inhibitors (Thermo Fisher Scientific,
Inc.)]. Total protein was quantified using a bicinchoninic acid
assay (Beyotime Institute of Biotechnology) and 20 µg protein/lane
was separated via 10% SDS-PAGE. The separated proteins were
subsequently transferred onto a polyvinylidene difluoride membrane
(EMD Millipore) and blocked in 5% non-fat dried milk (Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. The membranes were
incubated with the following primary antibodies purchased from
Santa Cruz Biotechnology, Inc. overnight at 4°C: Anti-TBX-3 (1:500;
cat. no. sc-166623); anti-p21 (1:500; cat. no. sc-6246); anti-p27
(1:500; cat. no. sc-1641); anti-vascular endothelial growth factor
(VEGF; 1:500; cat. no. sc-7269); anti-SIRT1 (1:500; cat. no.
sc-74504); anti-phosphorylated (p)-AKT (1:200; cat. no. sc-293125);
anti-AKT (1:500; cat. no. sc-135829); and anti-GAPDH (1:1,000; cat.
no. sc-365062). Following the primary antibody incubation, the
membrane was washed 3 times with TBS-0.1% Tween and incubated with
horseradish peroxidase-conjugated anti-rabbit (1:10,000; cat. no.
sc-2357) or anti-goat (1:10,000; cat. no. sc-2354) secondary
antibodies (Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Protein bands were visualized using an enhanced
chemiluminescence kit (Thermo Fisher Scientific, Inc.) and the
protein expression levels were analyzed using ImageJ software
(version 1.45; National Institutes of Health).
MTS assay
HUVEC proliferation was analyzed using an MTS assay
kit (Promega Corporation), according to the manufacturer's
protocol. Briefly, 2×103 HUVECs/well were seeded into
96-well plates for 24 h at 37°C and the medium was subsequently
replaced with fresh DMEM, prior to the cells being cultured for
another 3 days at 37°C. Following culturing, 20 µl MTS reagent was
added to each well and the plates were incubated for 1 h at 37°C.
The absorbance was measured at 490 nm using a microplate reader
(Bio-Rad Laboratories, Inc.). Each experiment was performed for 6
replicates, independently three times.
Flow cytometric analysis of the cell
cycle
A total of 2×106 HUVECs/ml were collected
by centrifugation (1,000 × g; 5 min; 4°C) and washed twice with PBS
buffer, prior to being fixed with ice-cold 70% ethanol for 5 min at
4°C. The cells were incubated with 1 µg/ml RNase I (Sigma-Aldrich;
Merck KGaA) at 37°C for 1 h in the dark. Following the addition of
20 µg/ml propidium iodide at 37°C for 15 min, the samples were
analyzed using an LSR II flow cytometer (BD Biosciences). Cells
were analyzed using FlowJo version 10.0 software (FlowJo LLC).
Wound healing assay
HUVECs (1×106 cells/well) were seeded
into six-well plates and cultured 37°C in a 5% CO2
incubator until they reached 100% confluence in DMEM, supplemented
with FBS. Subsequently, an artificial homogenous wound was made in
the cell monolayer using a sterile 200-µl plastic micropipette tip.
The cells were cultured for a further 24 h in DMEM without FBS at
37°C. The wound closure area was visualized at 0 and 24 h
post-wound creation using a light microscope (magnification, ×50;
Nikon Corporation) and ImageJ software was used to measure the
distance of cell migration.
Transwell invasion assay
HUVECs (2×105 cells/well) were plated
into the upper chambers of Transwell plates (8-µm pore size) in
serum-free DMEM. Transwell membranes were precoated with Matrigel
(Corning Inc.) for 30 min at 37°C. DMEM supplemented with 10% FBS
was plated into the lower chambers. Following incubation for 24 h
at 37°C in a 5% CO2 incubator, the invasive cells in the
lower chamber were fixed with 100% methanol for 5 min at room
temperature and stained with 0.1% crystal violet solution for 1 min
at room temperature. Stained cells were counted in six randomly
selected visual fields under a light microscope (magnification,
×100; Nikon Corporation) and ImageJ software was used to analyze
the cell invasion.
Matrix metalloproteinases (MMPs)
activity detection
A Fluorokine MAP human MMP2 and MMP9 kit (R&D
Systems, Inc.) was used to detect MMP activity, according to the
manufacturer's protocol. Briefly, HUVECs (2×105
cells/well) were cultured in 24-well plates for 24 h at 37°C, and
the suspension was collected and centrifuged for 15 min at 10,000 ×
g at room temperature. Active forms of MMP2 and MMP9 levels were
measured at a wavelength of 340 nm/465 nm, respectively.
Tube formation
To analyze tube formation, 96-well plates were
precoated with Matrigel (250 µg/ml; Corning Inc.) at 37°C for 30
min to solidify. Subsequently, 1×104 HUVECs/well were
seeded into the plates and incubated at 37°C for 4 h. The
network-like structures ‘tubes’ were observed under a light
microscope (magnification, ×25; Nikon Corporation) and ImageJ
software was used to measure tube formation.
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 software (IBM Corp.) and data are expressed as the
mean ± SD. Statistical differences between groups were analyzed
using an unpaired Student's t-test or a one-way ANOVA followed by a
Bonferroni post hoc test for multiple comparisons. The correlation
between TBX-3 and SIRT1 expression levels was
determined using Pearson's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were repeated ≥3 times.
Results
Upregulation of TBX-3 in HUVECs and
serum of patients with diabetes
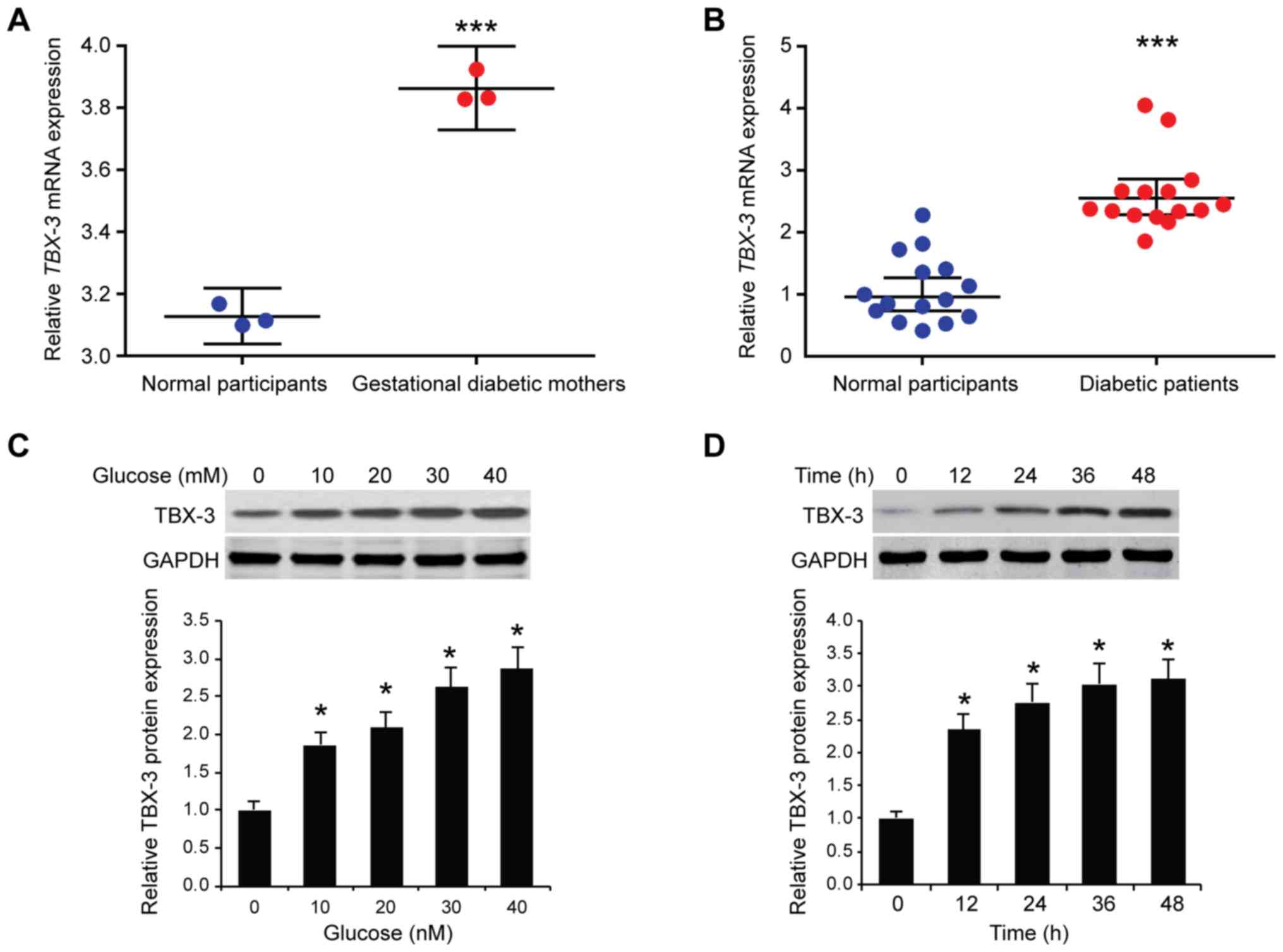
The differences in the mRNA expression levels of
TBX-3 in primary endothelial cells from the umbilical cord vein
between women with gestational diabetes and normal women was
investigated. TBX-3 mRNA expression levels were
significantly upregulated in gestational diabetic women obtained
from the Gene Expression Omnibus (GEO) dataset GSE49524 compared
with normal participants (P<0.001; Fig. 1A). Similarly, the expression levels
of TBX-3 were identified to be significantly upregulated in
the serum of 15 patients with diabetes compared with normal
participants (Fig. 1B).
Subsequently, HUVECs were treated with increasing concentrations of
glucose (0, 10, 20, 30 or 40 mM). Western blotting results revealed
that TBX-3 protein expression levels were significantly increased
by glucose in HUVECs in a dose-dependent manner compared with the
control group (P<0.05; Fig.
1C). Furthermore, HUVECs were treated with 30 mM glucose for
different time points (0, 12, 24, 36 or 48 h); the western blotting
results revealed that TBX-3 expression levels were increased by
glucose in a time-dependent manner (P<0.05; Fig. 1D).
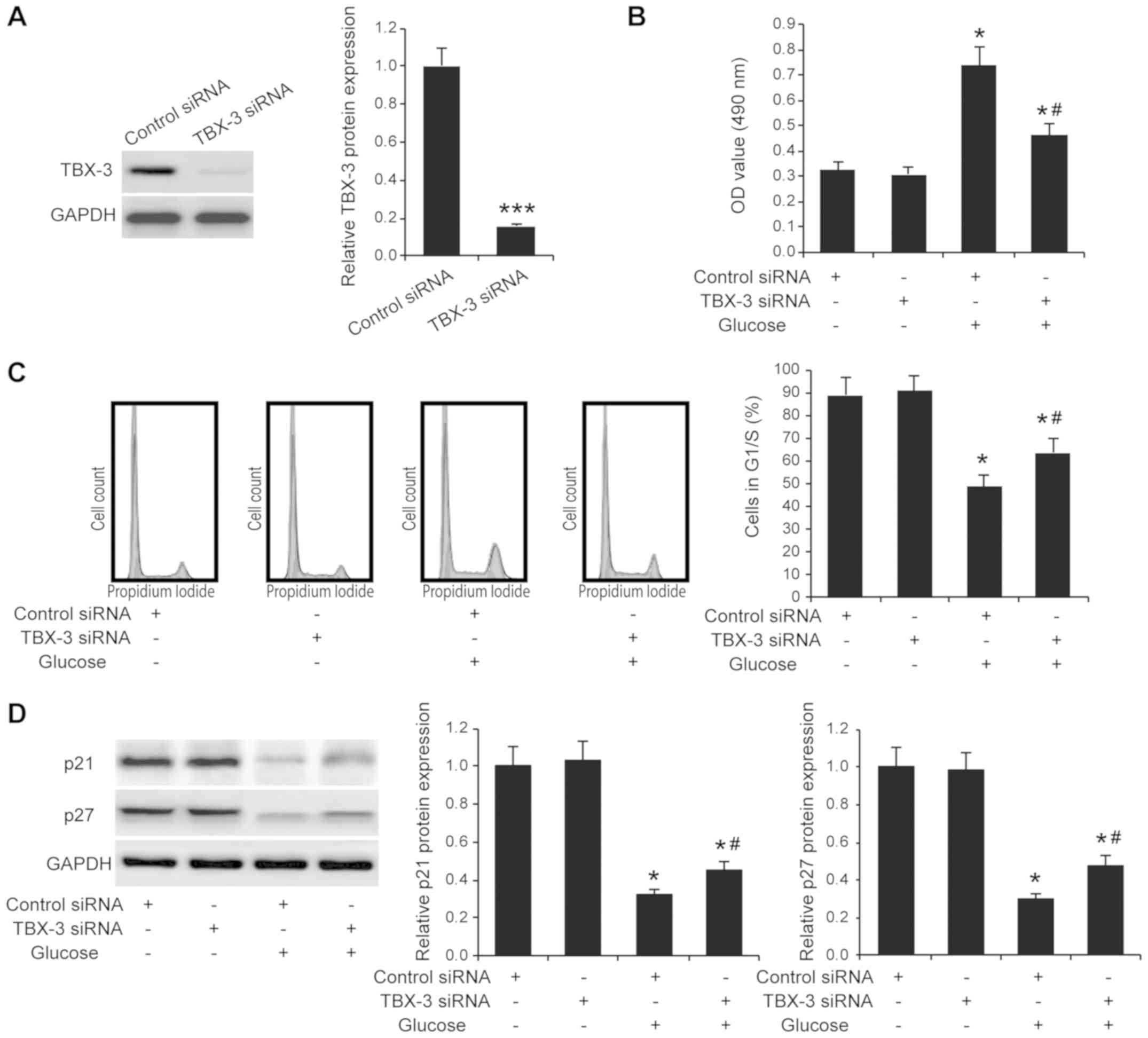
Effect of TBX-3 on cell proliferation
under high-glucose conditions
It has been reported that glucose alone exerts
effects on cell proliferation (20,21).
To investigate whether TBX-3 affected HUVEC function in a
high-glucose environment, TBX-3 expression was knocked down in
HUVECs using siRNA transfection. The transfection efficiency of the
siRNA was confirmed using western blotting (P<0.001; Fig. 2A). The TBX-3 knockdown HUVECs were
subjected to an MTS cell proliferation assay; it was identified
that TBX-3 knockdown did not affect HUVEC proliferation in a
non-glucose environment (P≥0.05; Fig.
2B); however, upon treatment with 30 mM glucose, the genetic
knockdown of TBX-3 significantly decreased the proliferation of
HUVECs compared with the HUVECs transfected with control siRNA and
treated with glucose (P<0.05; Fig.
2B). Cell cycle analysis further confirmed that TBX-3 knockdown
significantly inhibited cell proliferation in a high-glucose
environment (P<0.05; Fig. 2C).
In addition, the results of western blotting analysis revealed that
p21 and p27 expression levels were decreased in a high-glucose
environment; however, the genetic knockdown of TBX-3 significantly
increased the p21 and p27 expression levels compared with the
HUVECs transfected with the control siRNA and treated with glucose
(P<0.05; Fig. 2D).
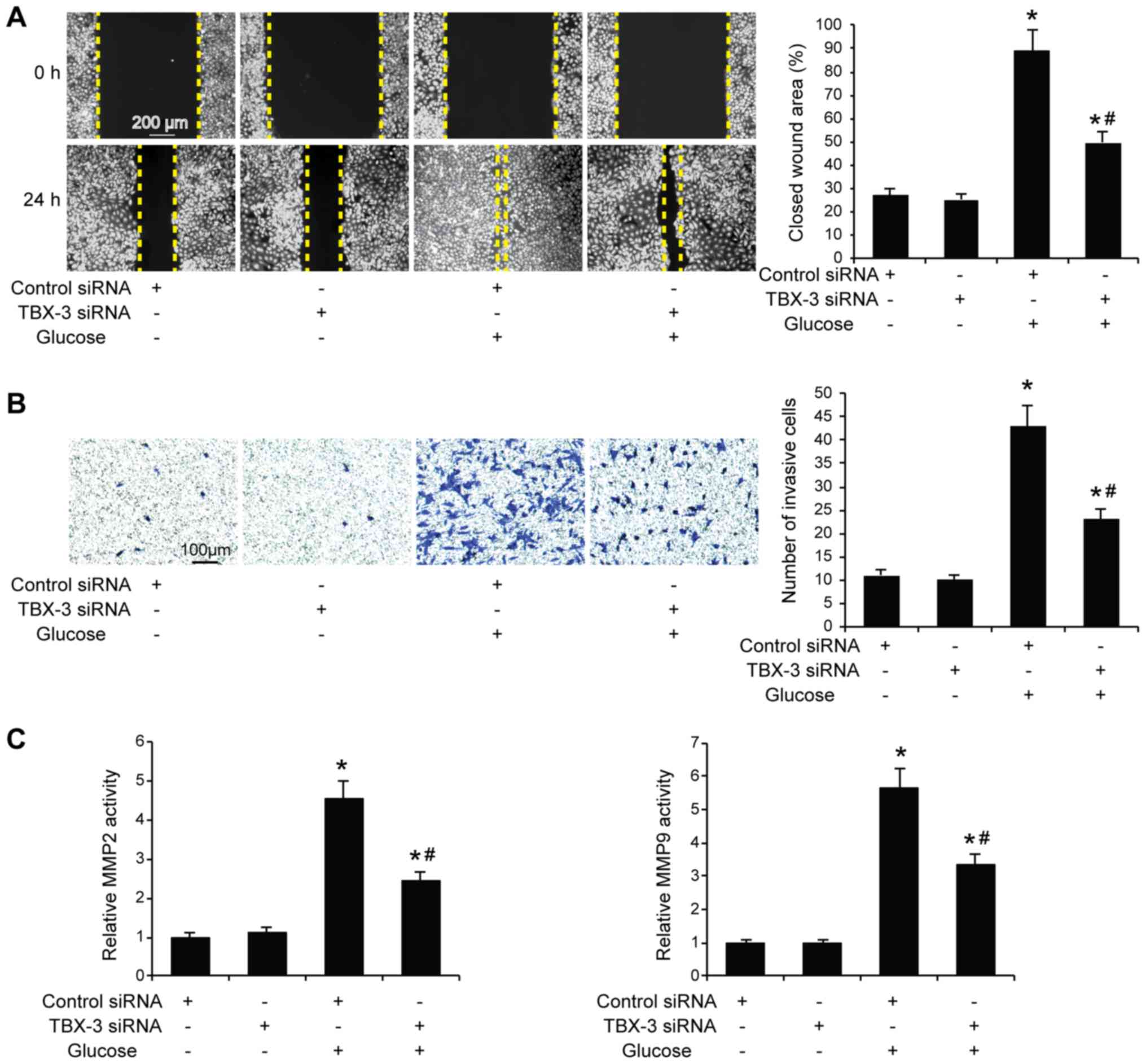
Effect of TBX-3 on migration, invasion
and angiogenesis induced by high glucose
Subsequently, whether TBX-3 knockdown affected HUVEC
migration, invasion and angiogenesis in a high-glucose environment
was investigated. The migratory and invasive abilities of HUVECs
were analyzed using wound healing and Transwell assays,
respectively, with and without high glucose. The results
demonstrated that the knockdown of TBX-3 in HUVECs significantly
decreased their migratory and invasive abilities in a high-glucose
environment compared with HUVECs transfected with control siRNA
with glucose treatment (P<0.05; Fig. 3A and B). As expected, the genetic
knockdown of TBX-3 significantly decreased the activity of MMP2 and
MMP9 compared with HUVECs transfected with control siRNA with
glucose treatment (P<0.05; Fig.
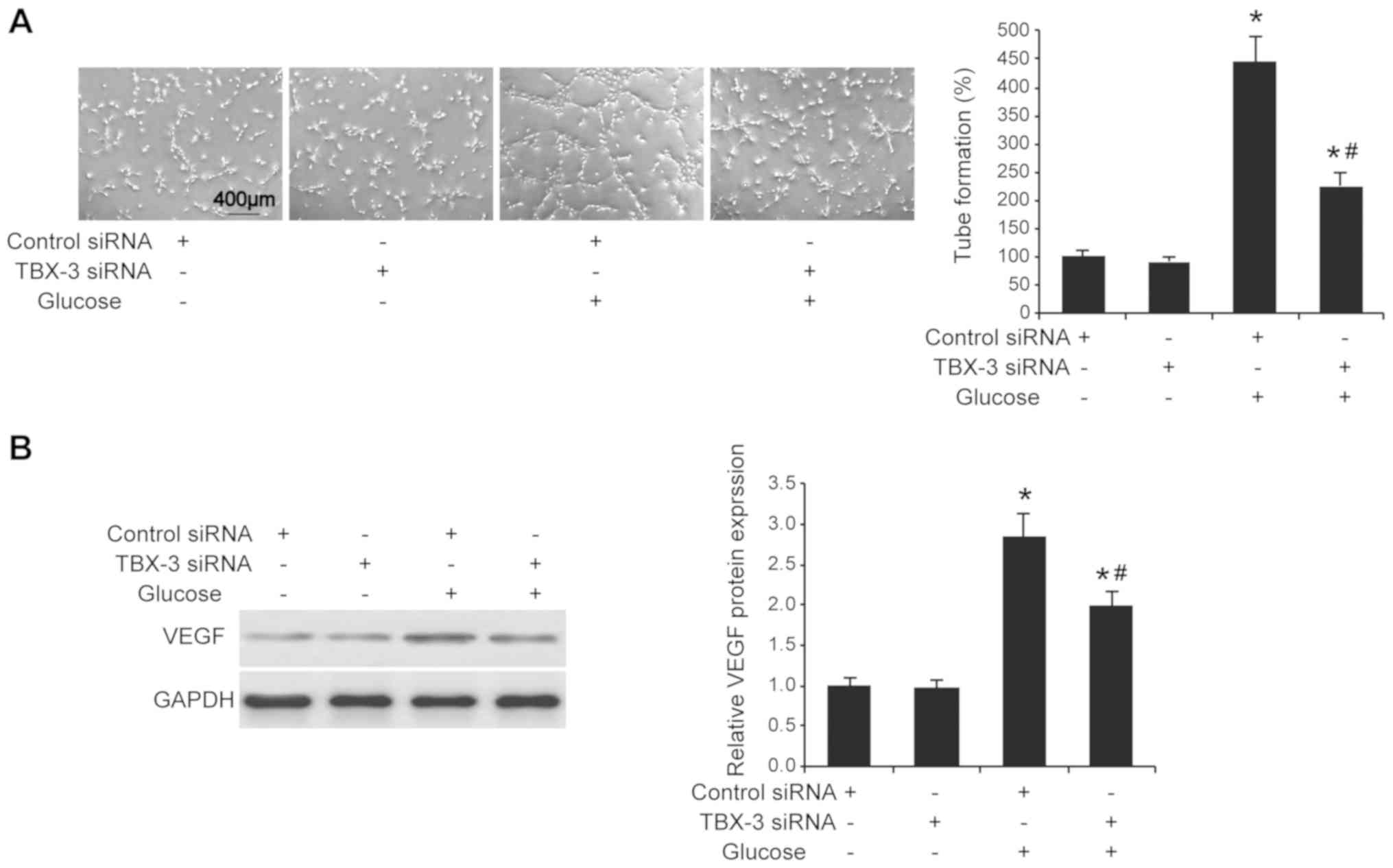
3C). The angiogenic potential of HUVECs was also investigated
using a tube formation assay; the knockdown of TBX-3 significantly
inhibited the number of tubes formed in a high-glucose environment
compared with HUVECs transfected with control siRNA with glucose
treatment (P<0.05; Fig. 4A).
Furthermore, the knockdown of TBX-3 also significantly decreased
the expression levels of VEGF in a high-glucose environment
compared with HUVECs transfected with control siRNA with glucose
treatment (P<0.05; Fig.
4B).
Correlation of the expression levels
between TBX-3 and SIRT1 in the serum of patients with diabetes
The molecular regulation of TBX-3 function in
glucose-affected HUVECs was further investigated. SIRT1 has been
identified to serve an important role in glucose metabolism
(22,23) and it was identified in one study to
affect the expression levels of TBX-3 during the differentiation of
stem cells (24). Thus, the
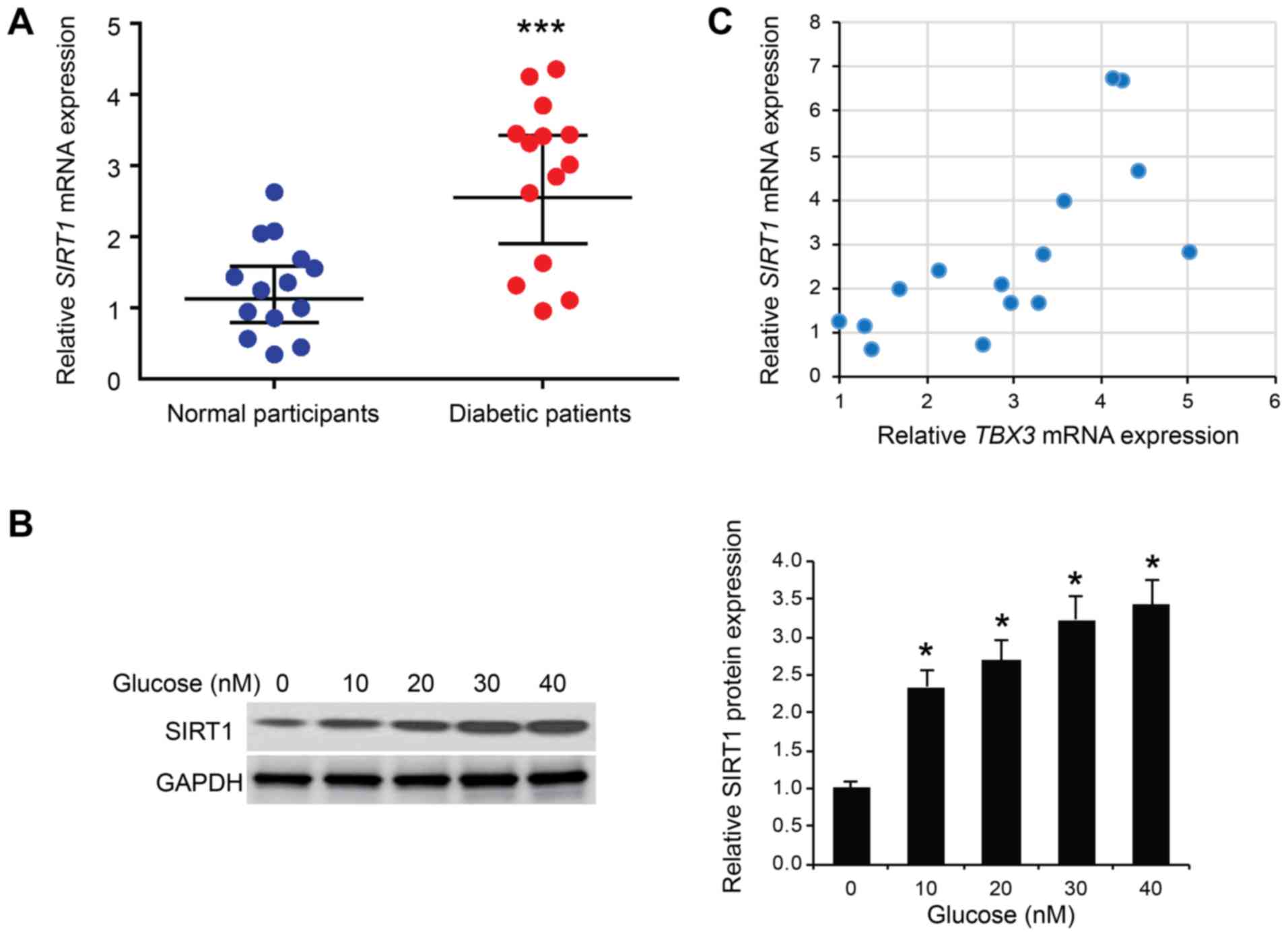
expression levels of SIRT1 mRNA were determined in the serum of 15
patients with type 2 diabetes. The expression levels of SIRT1 mRNA
were significantly increased in the serum of patients with diabetes
compared with the normal participants (P<0.001; Fig. 5A). Subsequently, HUVECs were
treated with different concentrations of glucose (0, 10, 20, 30 or
40 mM); the western blotting results revealed that SIRT1 protein
expression levels were significantly increased by glucose in HUVECs
in a dose-dependent manner (P<0.05; Fig. 5B). Subsequently, a positive
correlation was observed between SIRT1 and TBX-3 mRNA expression
levels in the serum of patients with diabetes (R=0.710; P<0.001;
Fig. 5C), suggesting that SIRT1
may positively regulate TBX-3 in endothelial cells.
TBX-3 affects HUVECs through
SIRT1-mediated AKT signaling
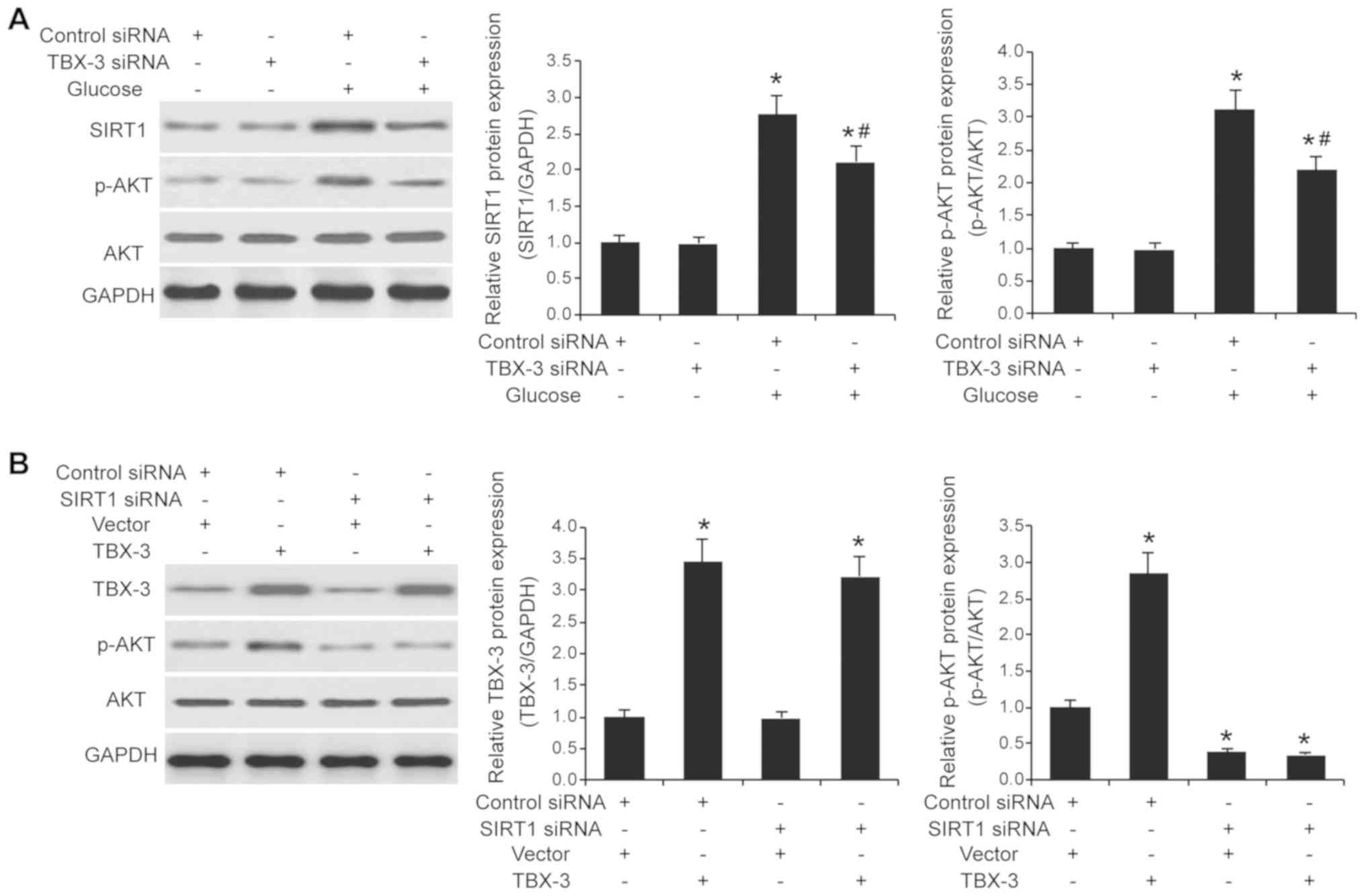
The expression levels of SIRT1 in TBX-3 knockdown
HUVECs treated with and without high glucose were further
investigated. The interference of TBX-3 expression affected SIRT1
expression levels; the genetic knockdown of TBX-3 significantly
increased SIRT1 expression levels in a high glucose environment
compared with the control siRNA group in a high glucose environment
(P<0.05; Fig. 6A). As AKT
signaling is downstream of SIRT1 (25), the expression levels of p-AKT and
AKT in TBX-3 knockdown HUVECs treated with and without high glucose
were subsequently investigated. The genetic knockdown of TBX-3
decreased the expression levels of p-AKT induced by high glucose
(P<0.05; Fig. 6A); however, it
had no effect on the total AKT expression levels (Fig. S1A). To investigate whether SIRT1
was required for TBX-3 function, the expression levels of
SIRT1 were knocked down using siRNA transfection
(P<0.001; Fig. S2). The
SIRT1-transfected cells were co-transfected to overexpress
TBX-3 concurrently (P<0.05; Fig. S3). The expression levels of p-AKT
and AKT were subsequently determined using western blotting in a
high-glucose environment (P<0.05; Fig. 6B and Fig. S1B). In HUVECs overexpressing
TBX-3, whilst having genetically decreased levels of
SIRT1, decreased expression levels of p-AKT were observed
compared with the control group; however, total AKT expression
levels in the HUVECs were not affected (Fig. S1B). These results indicated that
SIRT1 may regulate TBX-3-driven AKT signaling.
Discussion
Diabetes is a disease associated with high morbidity
rates around the world, with an estimated prevalence of 9.3%
(26), which commonly affects the
younger population and inflicts associated co-morbidities, that are
not only an economic burden but may also result in increased rates
of mortality (27). Diabetes and
its associated co-morbidities are often a burden on the overly
stretched public health systems of low- and middle-income nations,
such as China (27). The micro-
and macrovascular changes in diabetes, which are associated with
the endothelial dysfunction, have been identified to be regulators
of its co-morbidities (10). Thus,
the present study aimed to provide a molecular insight into the
breach of the endothelial cell barrier under hyperglycemic
conditions and the probable effects of this breach on endothelial
cell survival and function.
The present study demonstrated that TBX-3 expression
levels were increased in patients with diabetes compared with
normal recruited controls. These increases in TBX-3 expression
levels were associated with a concomitant increase in cellular
proliferation. These results associated with the role of TBX-3 in
promoting cellular proliferation are consistent with other studies;
for example, in chondrosarcomas, it was identified that TBX-3
mediated cellular proliferation through the repression of p21
(28). Furthermore, a previous
study in human embryonic stem cells demonstrated that the
overexpression of TBX-3 was associated with increased cellular
proliferation and an associated repression of NF-κB inhibitor β and
p14 (29). Meanwhile, another
study reported that the accelerated senescence of endothelial cells
was mediated through AKT activation, p21 expression and p53
accumulation (30). These data are
discordant with our present results, whereby the overexpression of
TBX-3 resulted in the activation of AKT signaling. However,
increased expression levels of p-AKT were not observed in SIRT1
knockdown cells following the introduction of the TBX-3
overexpression plasmid, which indicated that SIRT1 may be required
for TBX-3 function.
It has also been previously demonstrated that TBX-3
expression levels were increased in the G1-phase, rising to their
peak in the S-phase of the cell cycle; the presence of c-Myc in the
S-phase was revealed to be responsible for TBX-3 upregulation both
transcriptionally and translationally (31). In addition, c-Myc was identified to
have a binding site in the promoter region of TBX-3 and its
occupancy was revealed to be enhanced 600-fold during the S-phase;
however, a minimal occupancy of the promoter region by c-Myc in the
cells in the G1- and G2-phase was observed (31). These data supported our results
that there were significantly fewer cells in the G1-phase in the
presence of glucose alongside increased expression levels of TBX-3.
The partial rescue of the cellular phenotype observed in the
presence of TBX-3 siRNA and glucose may likely be due to the leaky
expression of TBX-3.
TBX-3 has also been identified to regulate VEGF
expression, which is known to be responsible for mediating
endothelial cell migration as a pre-requisite to angiogenesis
(32). VEGF mediates its action
through promoting the activation and consequently, the
differentiation of endothelial cells into stalk and phalanx cells,
thus forming the body of the sprout and a tip cell phenotype. Tip
cells are specialized filopodial extensions of endothelial cells
stimulated by VEGF (33). Previous
studies have also reported the involvement of transferrin, a
protein, in endothelial cell migration, angiogenesis and
neo-vascularization (34). In
addition, epigenetic modifications through long non-coding RNA were
found to be negatively correlated with the regulation of microRNA,
which subsequently influenced the proliferation, migration and
invasion of hemangioma-derived endothelial cells (35).
Furthermore, the present study revealed a positive
correlation between TBX-3 and SIRT1 expression levels in
endothelial cells. Contrary to the results of the present study, a
previous study in human embryonic stem cells demonstrated that the
increased expression levels of SIRT1 led to the downregulated
expression levels of TBX-3 and other genes involved in development
(24). Downregulated expression
levels of SIRT1 and TBX-3 have also been observed in lung specimens
derived from chronic obstructive pulmonary disease, which indicated
that SIRT1 and TBX-3 are not always negatively regulated (36). The SIRT1/TBX-3 axis is
microenvironment-dependent and undergoes function-driven regulation
(37). The results of a previous
in vivo study were consistent with the data from the present study,
observing the overexpression of SIRT1 in response to hyperglycemia;
the overexpression of SIRT1 significantly decreased the expression
levels of senescence-associated markers, such as p53, p21 and
plasminogen activator inhibitor-1 (38).
The failure to activate AKT in the absence of SIRT1
expression is also consistent with the hypothesis that SIRT1 binds
to the promoter region of the TBX-3 gene and regulates
transcription (37). AKT is under
transcriptional control of TBX-3, therefore the TBX-3-mediated
activation of AKT may be responsible for modulating the expression
of VEGF through the activation of specificity protein 1; however,
alternative mechanisms may also be plausible.
In conclusion, the present study hypothesized that
the tolerance to hyperglycemia, induction of cellular
proliferation, migration, invasion and angiogenesis in endothelial
cells may not be just a pathognomonic feature of diabetes but may
be a well-curated event with multiple molecular players. TBX-3 was
identified to be an important factor; TBX-3-mediated AKT activation
resulted in VEGF induction, which in turn was considered
responsible for the angiogenic phenotype of endothelial cells. It
was suggested that the TBX-3 expression levels in endothelial cells
may be regulated by SIRT1; as the genetic knockdown of these genes
resulted in partial or complete phenotype reversal, it could be
suggested that endothelium-targeted TBX-3 therapy may serve as a
therapeutic option for regulating diabetes-associated
co-morbidities arising from endothelial dysfunction. As the
delivery of gene therapy remains a challenge, TBX-3 may also be
used as an early biomarker for disease prognosis and
prevention.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by grants from the
Fundamental Research Business Expenses of Undergraduate
Universities in Heilongjiang Province (grant no. 2017-KYYWF-0740)
and the Scientific Research Project of Qiqihar (grant no.
SFGG-201961).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY, WZ, XZ performed the experiments, analyzed the
data and wrote the manuscript; DX and NW analyzed the data; ZY and
WZ wrote the manuscript; and WZ conceived and designed the study.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and approved by the Medical Ethics
Committee of Qiqihar Medical University (Heilongjiang, China).
Written informed consent was obtained from all recruited
subjects.
Patient consent for publication
Written informed consent was obtained from all
recruited subjects.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mao W, Yip CW and Chen W: Complications of
diabetes in China: Health system and economic implications. BMC
Public Health. 19:2692019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Wang L, Wang P, Liu R, Yang K, Qian
X, Fan J, Yu S, Li Y and Wang C: The dynamics of type 2 diabetes
mellitus prevalence and management rates among rural population in
Henan province, China. J Diabetes Res. 2017:90927592017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu M, Wang J, He Y, Jiang B, Wu L, Wang
Y, Di Z and Zeng J: Awareness, treatment and control of type 2
diabetes among Chinese elderly and its changing trend for past
decade. BMC Public Health. 16:2782016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu C and Jia W: Diabetes in China:
Epidemiology and genetic risk factors and their clinical utility in
personalized medication. Diabetes. 67:3–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weng J, Zhou Z, Guo L, Zhu D, Ji L, Luo X,
Mu Y and Jia W; T1D China Study Group, : Incidence of type 1
diabetes in China, 2010-13: Population based study. BMJ.
360:j52952018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Yu C, Wang Y, Bi Y, Liu Y and Zhang
ZJ: Trends in the incidence and mortality of diabetes in China from
1990 to 2017: A joinpoint and age-period-cohort analysis. Int J
Environ Res Public Health. 16:162019.
|
|
7
|
Yuan H, Li X, Wan G, Sun L, Zhu X, Che F
and Yang Z: Type 2 diabetes epidemic in East Asia: A 35-year
systematic trend analysis. Oncotarget. 9:6718–6727. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu
S, Li Y, Wang L, Liu Y, Yin P, et al: Cause-specific mortality for
240 causes in China during 1990–2013: A systematic subnational
analysis for the Global Burden of Disease Study 2013. Lancet.
387:251–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Evans JL and Goldfine ID: A new road for
treating the vascular complications of diabetes: So let's step on
the gas. Diabetes. 65:346–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chawla A, Chawla R and Jaggi S:
Microvasular and macrovascular complications in diabetes mellitus:
Distinct or continuum? Indian J Endocrinol Metab. 20:546–551. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kajino H, Goldbarg S, Roman C, Liu BM,
Mauray F, Chen YQ, Takahashi Y, Koch CJ and Clyman RI: Vasa vasorum
hypoperfusion is responsible for medial hypoxia and anatomic
remodeling in the newborn lamb ductus arteriosus. Pediatr Res.
51:228–235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Willmer T, Cooper A, Peres J, Omar R and
Prince S: The T-Box transcription factor 3 in development and
cancer. Biosci Trends. 11:254–266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papaioannou VE: The T-box gene family:
Emerging roles in development, stem cells and cancer. Development.
141:3819–3833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takashima A and Faller DV: Targeting the
RAS oncogene. Expert Opin Ther Targets. 17:507–531. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications. Part 1: Diagnosis and classification of diabetes
mellitus provisional report of a WHO consultation. Diabet Med.
15:539–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ambra R, Manca S, Palumbo MC, Leoni G,
Natarelli L, De Marco A, Consoli A, Pandolfi A and Virgili F:
Transcriptome analysis of human primary endothelial cells (HUVEC)
from umbilical cords of gestational diabetic mothers reveals
candidate sites for an epigenetic modulation of specific gene
expression. Genomics. 103:337–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clough E and Barrett T: The Gene
Expression Omnibus Database. Methods Mol Biol. 1418:93–110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HJ, Zhang Y, Georgescu SP, Johnson
KL, Kong D and Galper JB: Human umbilical vein endothelial cells
and human dermal microvascular endothelial cells offer new insights
into the relationship between lipid metabolism and angiogenesis.
Stem Cell Rev. 2:93–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Li Q, Yu W and Xiao Q: High glucose
and/or high insulin affects HIF-1 signaling by regulating AIP1 in
human umbilical vein endothelial cells. Diabetes Res Clin Pract.
109:48–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang SH, Zhang SG, Zhou P, Wei X, Mao XD,
Lin SG and Liu C: LncRNA MALAT1 affects high glucose-induced
endothelial cell proliferation, apoptosis, migration and
angiogenesis by regulating the PI3K/Akt signaling pathway. Eur Rev
Med Pharmacol Sci. 23:8551–8559. 2019.PubMed/NCBI
|
|
22
|
Ye X, Li M, Hou T, Gao T, Zhu WG and Yang
Y: Sirtuins in glucose and lipid metabolism. Oncotarget.
8:1845–1859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guclu A, Erdur FM and Turkmen K: The
emerging role of sirtuin 1 in cellular metabolism, diabetes
mellitus, diabetic kidney disease and hypertension, experimental
and clinical endocrinology and diabetes. Exp Clin Endocrinol
Diabetes. 124:131–139. 2016.PubMed/NCBI
|
|
24
|
Calvanese V, Lara E, Suárez-Alvarez B, Abu
Dawud R, Vázquez-Chantada M, Martínez-Chantar ML, Embade N,
López-Nieva P, Horrillo A, Hmadcha A, et al: Sirtuin 1 regulation
of developmental genes during differentiation of stem cells. Proc
Natl Acad Sci USA. 107:13736–13741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakanishi A, Wada Y, Kitagishi Y and
Matsuda S: Link between PI3K/AKT/PTEN pathway and NOX proteinin
diseases. Aging Dis. 5:203–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al IDF Diabetes Atlas Committee, : Global and
regional diabetes prevalence estimates for 2019 and projections for
2030 and 2045: Results from the International Diabetes Federation
Diabetes Atlas, 9th edition. Diabetes Res Clin Pract.
157:1078432019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harding JL, Pavkov ME, Magliano DJ, Shaw
JE and Gregg EW: Global trends in diabetes complications: A review
of current evidence. Diabetologia. 62:3–16. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Willmer T, Hare S, Peres J and Prince S:
The T-box transcription factor TBX3 drives proliferation by direct
repression of the p21(WAF1) cyclin-dependent kinase inhibitor. Cell
Div. 11:62016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Esmailpour T and Huang T: TBX3 promotes
human embryonic stem cell proliferation and neuroepithelial
differentiation in a differentiation stage-dependent manner. Stem
Cells. 30:2152–2163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rosso A, Balsamo A, Gambino R, Dentelli P,
Falcioni R, Cassader M, Pegoraro L, Pagano G and Brizzi MF: p53
Mediates the accelerated onset of senescence of endothelial
progenitor cells in diabetes. J Biol Chem. 281:4339–4347. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Willmer T, Peres J, Mowla S, Abrahams A
and Prince S: The T-Box factor TBX3 is important in S-phase and is
regulated by c-Myc and cyclin A-CDK2. Cell Cycle. 14:3173–3183.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lamalice L, Le Boeuf F and Huot J:
Endothelial cell migration during angiogenesis. Circ Res.
100:782–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daub JT and Merks RM: A cell-based model
of extracellular-matrix-guided endothelial cell migration during
angiogenesis. Bull Math Biol. 75:1377–1399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carlevaro MF, Albini A, Ribatti D, Gentili
C, Benelli R, Cermelli S, Cancedda R and Cancedda FD: Transferrin
promotes endothelial cell migration and invasion: Implication in
cartilage neovascularization. J Cell Biol. 136:1375–1384. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Chen B, Chi D, Zhang Y and Jiang W:
lncRNA CASC9 regulates cell migration and invasion in hemangioma
endothelial cells by targeting miR-125a-3p/Nrg1. OncoTargets Ther.
12:423–432. 2019. View Article : Google Scholar
|
|
36
|
Acquaah-Mensah GK, Malhotra D, Vulimiri M,
McDermott JE and Biswal S: Suppressed expression of T-box
transcription factors is involved in senescence in chronic
obstructive pulmonary disease. PLOS Comput Biol. 8:e10025972012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O'Callaghan C and Vassilopoulos A:
Sirtuins at the crossroads of stemness, aging, and cancer. Aging
Cell. 16:1208–1218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen H, Wan Y, Zhou S, Lu Y, Zhang Z,
Zhang R, Chen F, Hao D, Zhao X, Guo Z, et al: Endothelium-specific
SIRT1 overexpression inhibits hyperglycemia-induced upregulation of
vascular cell senescence. Sci China Life Sci. 55:467–473. 2012.
View Article : Google Scholar : PubMed/NCBI
|