Introduction
Cancer is the second leading cause of death
worldwide and nearly 1 in 6 deaths are due to it (1). According to the World Health
Organization, ~70.0% of deaths from cancer occur in low- and
middle-income countries (1). As a
serious malignant neoplasm, ovarian cancer is a relatively rare
type of cancer, but it is the most frequent cause of death from
gynecological cancer worldwide (2,3). For
instance, the number of new cases of ovarian cancer was
11.4/100,000 females per year and the number of deaths was
7.0/100,000 per year in the USA based on cases and deaths between
2012 and 2016 (4). Furthermore, it
has a poor prognosis, with the 5-year survival rate being only 40%
in the UK (5). Thus, an increasing
amount of anti-tumor drugs from plants have been studied due to
their affordability and relatively fewer side effects (6).
Radix ophiopogonis, recorded as a traditional
Chinese medicine in the Pharmacopoeia of the China (2015 edition)
(7), consists of the roots from
three botanical sources, namely Liriope spicata var.
prolifera, Ophiopogon japonicus and Liriope muscari,
which are widely distributed in East Asia, particularly in China
(mainly in the Provinces Hubei, Zhejiang, Sichuan and Fujian)
(8). Steroidal saponins are among
the major components of radix ophiopogonis and 72 steroidal
saponins have so far been isolated and identified (8–14).
Studies have indicated that the steroidal saponins of radix
ophiopogonis possess a marked anti-cancer activity. Among 15 types
of steroidal saponins isolated from the tuberous roots of
Ophiopogon japonicus, 5 different saponins were cytotoxic to
all tested cell lines (HepG2, HLE, BEL7402, BEL7403 and HeLa)
(9). Ophiopogonin B induces
autophagy in non-small cell lung cancer cells by inhibiting the
PI3K/Akt signaling pathway way (10) and autophagy and apoptosis of colon
cancer cells by activating the JNK/c-Jun signaling pathway
(11), sensitization to tumor
necrosis factor superfamily member 10-induced apoptosis (12) and suppression of the metastasis and
angiogenesis of A549 cells by inhibiting the EPH receptor A2/Akt
signaling pathway (13).
Ophiopogonin D inhibits the proliferation and leads to
chemosensitization of human lung cancer cells (12,14).
Ophiopogonin D induces apoptosis in androgen-independent human
prostate cancer cells (15). DT-13
inhibits breast cancer cell migration via modulation of
procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 in the adipocyte
microenvironment (16). DT-13
inhibits the proliferation of colorectal cancer cells via the
glycolytic metabolism and the adenosine monophosphate-activated
protein kinase/mTOR signaling pathway (17). DT-13 inhibits the proliferation and
metastasis of human prostate cancer cells by blocking the PI3K/Akt
pathway (18). DT-13 is also able
to induce autophagy and potentiate the anti-cancer effect of
nutrient deprivation (19) and
suppress cell adhesion and invasion by inhibiting matrix
metalloproteinase-2/9 (20) and
downregulating C-C motif chemokine receptor type 5 (CCR5) and
vascular endothelial growth factor in MDA-MB-435 cells (21). DT-13 inhibits gastric cancer cell
migration via downregulation of the CCR5-C-C motif chemokine ligand
5 axis (22) and attenuates human
lung cancer metastasis via regulating non-muscle myosin IIA (NMIIA)
activity under hypoxic conditions (23). The combination of DT-13 and
topotecan synergistically inhibits human gastric cancer (24) via MIIA-induced endocytosis of
epidermal growth factor receptor (EGFR) (25) and aerobic glycolysis via the
NMIIA/EGFR/hexokinase II axis (26), and potentiates the sensitivity of
gastric cancer cells to topotecan by inducing cell cycle arrest
(24). DT-13 synergistically
enhances vinorelbine-mediated mitotic arrest through inhibition of
the forkhead box M1-BICD cargo adaptor 2 axis in non-small cell
lung cancer cells (27) and
activation of the ERK signaling pathway in NCI-H1299 cells
(28).
However, the anti-cancer activities of steroidal
saponins on A2780 cells and their mechanisms of action remain to be
elucidated. A previous study by our group reported that
liriopesides B [chemical structure drawn with chemdraw 14.0
(CambridgeSoft) and presented in Fig.
1], one of the steroidal saponins from L. spicata var.
prolifera in Hubei, inhibits proliferation and induced cell
differentiation by decreasing cancer antigen 125 levels and
alkaline phosphatase (AKP) activity in A2780 cells (29). Therefore, the same concentration of
liriopesides B according to its IC50 value in A2780
cells for the same treatment time of 48 h (29) was selected to explore the effects
of liriopesides B on metastatic activity, cell cycle arrest and
apoptosis of A2780 cells in the present study.
Materials and methods
Materials
A2780 ovarian cancer cells were obtained from the
China Center for Type Culture Collection. Dulbecco's modified
Eagle's medium (DMEM) was provided by GE Healthcare. Fetal bovine
serum (FBS) was from Gibco (Thermo Fisher Scientific, Inc.).
Dimethyl sulfoxide (DMSO) was from Amresco, LLC. Crystal Violet,
Hoechst 33258 kits and Annexin V-FITC/PI Apoptosis Detection kit
were purchased from Wuhan Hualianke Biotechnology Co., Ltd.
Fibronectin was from Sigma-Aldrich (Merck KGaA). Matrigel was
obtained from BD Biosciences. Liriopesides B isolated from the
tuberous root of Liriope spicata var. prolifera was
identified by nuclear magnetic resonance spectroscopy with its
purity beyond 95% (Shanghai Yuanye BioTechnology Co., Ltd.) and
0.1% DMSO was used as a solvent (20). The other chemicals and solvents
used were all of the highest purity grade available.
Cell cultivation
A2780 cells were cultivated with DMEM containing 10%
FBS, 100 µg/ml streptomycin and 100 IU/ml penicillin at 37°C in a
humidified atmosphere with 5% CO2.
Cell invasion assay
The invasive abilities of A2780 cells were assessed
in Transwell chambers (8-µm pore size; Corning Inc.) using a
slightly modified method based on that reported by Nizamutdinova
et al (30). Cells were
treated with different concentrations of liriopesides B (0,
1×IC50, 5×IC50 and 10×IC50) in
serum-free culture medium for 24 h. Prior to seeding the cells, a
24-well plate and Transwell chambers were filled with 1X PBS to
moisten the chambers for 5 min. The upper side of the filter
membrane of the Transwell chamber was coated with 500 mg/l
fibronectin (10 µl), while the lower side of the filter membrane
was coated with 500 mg/l Matrigel (10 µl) and dried for 30 min at
37°C. Cells were suspended and seeded into the upper chamber in
serum-free media at a density of 1×105 cells in 0.5 ml
per chamber. The lower chamber of the 24-well plate was filled with
0.75 ml DMEM containing 10% FBS. After 48 h of incubation at 37°C,
cells that had transgressed through the lower side of the membrane
were fixed with 1 ml 4% formaldehyde for 10 min at room
temperature, the media was discarded and cells were washed with 1X
PBS once. Subsequently, 1 ml 0.5% crystal violet solution was added
to stain the cells for 30 min at room temperature, following which
the cells were washed with 1X PBS three times. Cells that had not
migrated through the membrane were removed with cotton swabs. The
number of cells in each visual field (five fields were examined)
were counted and a routine light microscope (IX53; Olympus
Corporation) was used to capture typical images (magnification,
×400). The rate of invasion was calculated as follows: Invasion
rate=(invaded cells in treatment group/invaded cells in control
group) ×100%.
Cell chemotaxis assay
The chemotactic movement experiment of A2780 cells
was performed in Transwell chambers using a slightly modified
method based on that reported by Nizamutdinova et al
(30). A2780 cells in the absence
or presence of liriopesides B (1×IC50, 5×IC50
and 10×IC50) were cultivated in serum-free culture
medium for 24 h. Prior to seeding the cells, a 24-well plate and
the Transwell chambers were filled with 1X PBS to moisten the
chambers for 5 min. Cells were suspended in DMEM with 1% FBS,
counted and seeded into the chambers at a density of
1×105 cells in 0.5 ml per chamber. The lower 24-well
plate was filled with 0.75 ml DMEM containing 10% FBS. After
incubation at 37°C for 48 h, 1 ml 4% formaldehyde per well was
added to fix the cells for 10 min at room temperature.
Subsequently, the media was discarded, the cells were washed with
1X PBS once and 1 ml 0.5% crystal violet solution was then added to
stain the cells for 30 min at room temperature, followed by washing
with 1X PBS three times and drying. Cells without chemotaxis on the
upper side of the filter were removed using cotton swabs. The
number of cells in each visual field (five fields were examined)
were counted and a routine light microscope (IX53; Olympus
Corporation) was used to capture typical images (magnification,
×400). The rate of chemotaxis was calculated as follows: Chemotaxis
rate=(chemotaxis cells in the treatment group/chemotaxis cells in
the control group) ×100%.
Cell cycle analysis
Cell cycle analysis was performed as reported
previously with certain modifications (31). Aftertreatment for 24, 48 and 72 h
with the indicated concentrations of liriopesides B (0,
1×IC50 and 10×IC50), A2780 cells were fixed
in ethyl alcohol and then kept overnight at −20°C. Cells were
washed twice with cold PBS. After incubation with 1 mg/ml RNase A
(100 µl; purchased from Thermo Fisher Scientific, Inc.) for 30 min
at 37°C, cells were stained with 400 µl 50 µg/ml propidium iodide
at 25°C in the dark for 20 min and analyzed with a flow cytometer
(FC500 MCL; Beckman Coulter, Inc.) and ModFit LT2.0 (Verity
Software House) was used to analysis the flow cytometry
results.
Hoechst 33258 staining
After incubation in the absence or presence of
indicated concentrations of liriopesides B (1×IC50 and
5×IC50) for 48 h, cells were washed with PBS three times
and fixed with 4% paraformaldehyde for 15 min at room temperature.
Cells were then washed with PBS twice and stained for 5 min using
200 µl Hoechst 33258 at room temperature. After washing five times
with PBS, cellular nuclear staining was examined at 460 nm under a
fluorescence microscope (TS100-F; Nikon Corporation; magnification,
×400). The evenly distributed cells were randomly chosen for image
capture.
Discrimination between early and late
apoptosis
Discrimination between early and late apoptosis was
performed as reported previously with certain modifications
(32). Cells were incubated for
24, 48 or 72 h with or without different concentrations of
liriopesides B (1×IC50 and 10×IC50). Adherent
and floating cells were harvested and washed twice with PBS, and
then suspended in 200 µl of 1X Binding Buffer, to which 10 µl
Annexin V-FITC and 10 µl propidium iodide were added. After the
cells were gently vortexed and incubated for 30 min in the dark,
300 µl of 1X Binding Buffer was added. The fluorescence of the
cells was detected with a flow cytometer (FC500 MCL; Beckman
Coulter, Inc.) and CXP Analysis 2.0 (Beckman Coulter, Inc.) was
used to analysis the flow cytometry results.
Reverse transcription-quantitative PCR
(RT-qPCR)
Gene expression levels were assessed as reported
previously with certain modifications (32). Relative quantification of gene
expression was performed by the 2−ΔΔCq method based on
Ct values for both target genes (E-CADHERIN, BCL-2, p21 and
p27) and reference gene (GAPDH) (33). Cells were incubated for 48 h in the
absence or presence of liriopesides B at different concentrations
(1×IC50, 5×IC50 or 10×IC50). Total
RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and were reverse transcribed into cDNA by using a
reverse transcription kit (Takara Biotechnology Co., Ltd). The 20
µl reaction system, including the total RNA, 5X reaction buffer,
Oligo DT18 primer, dNTP Mix, RiboLock RNase Inhibitor, RevertAid
M-MuLV RT and DNase-free ddH2O, was prepared and the
reverse transcription was performed as the follows: 42°C 60 min,
70°C 15 min then hold at 16°C. The SYBR Green Real Time PCR Master
Mix (Kapa Biosystems; Roche Diagnostics) was used to amplify the
resulting cDNA samples with the Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.). The amplification parameters were as
follows: 95°C for 3 min; 39 cycles of 95°C for 5 sec, 56°C for 10
sec and 72°C for 25 sec, and then 65°C for 5 sec and 95°C for 50
sec. The primer sequences used were designed with Primer Premier
v6.24 (Premier Biosoft International), except for BCL-2
(34), and they are listed in
Table I.
 | Table I.Sequences of primers for quantitative
PCR. |
Table I.
Sequences of primers for quantitative
PCR.
| Gene | Primer
sequence | Bp |
|---|
|
E-CADHERIN | F:
5′-CACCCTGGCTTTGACG-3′ | 119 |
|
| R:
3′-TAGGCTGTCCTTTGTCG-5′ |
|
| BCL-2 | F:
5′-GGGAGGATTGTGGCCTTCTT-3′ | 99 |
|
| R:
3′-TCATCCACAGGGCGATGTT-5′ |
|
| p21 | F:
5′-CTAATCCGCCCACAGG-3′ | 81 |
|
| R:
3′-TGAGACTAAGGCAGAAGATG-5′ |
|
| p27 | F:
5′-GCTTGCCCGAGTTCTA-3′ | 87 |
|
| R:
3′-TCCCGCTGACATCCTG-5′ |
|
| GAPDH | F:
5′-CCACTCCTCCACCTTTG-3′ | 106 |
|
| R:
3′-CACCACCCTGTTGCTGT-5′ |
|
Western blot analysis
Protein expression levels were assessed as
previously described by Sun et al (35) with certain modifications. A2780
cells treated with or without liriopesides B (1×IC50,
5×IC50 and 10×IC50) for 48 h were lysed in
lysis buffer (Cell lysate HR2005, Wuhan Hualianke Biotechnology
Co., Ltd.). The protein in the lysates was then quantified
according to a BCA protein concentration test kit (HR2006, Wuhan
Hualianke Biotechnology Co., Ltd.), and then separated by SDS-PAGE
on a 12% gel (10 µg protein per lane), transferred onto a
polyvinylidene difluoride membrane and blocked with 5% skimmed milk
powder overnight at 4°C. Membranes were then immunoblotted with
antibodies at 4°C overnight against E-cadherin (1:5,000; cat. no.
ab133597; Abcam), Bcl-2 (1:1,000; cat. no. ab32124; Abcam), p21
(1:1,000; cat. no. ab109199; Abcam), p27 (1:1,000; cat. no.
ab32034; Abcam) and GAPDH (1:1,000; cat. no. 2118; Cell Signaling
Technology, Inc.) was used as an endogenous reference. HRP labeled
Goat anti-rabbit IgG (1:10,000; cat. no. HR2031; Wuhan Hualianke
Biotechnology Co., Ltd. was added. and then incubated at 4°C
overnight. A fully automatic chemiluminescence analyzer
(Tanon-5200; Tanon Science and Technology Co., Ltd.) was used for
protein visualization and semi-quantitative protein analysis
(densitometry values are from chemiluminescence analyzer's
software).
Statistical analysis
In the experiment, all treatments and controls were
performed 3 times and values are expressed as the mean ± standard
deviation using SPSS 16.0 (SPSS, Inc.). The control and
experimental groups were compared using one-way analysis of
variance followed by the least-significant differences test for the
quantification of the cell cycle and cell apoptosis, and Dunnett's
post hoc test for all other data. P<0.05 was considered to
indicate a statistically significant difference.
Results
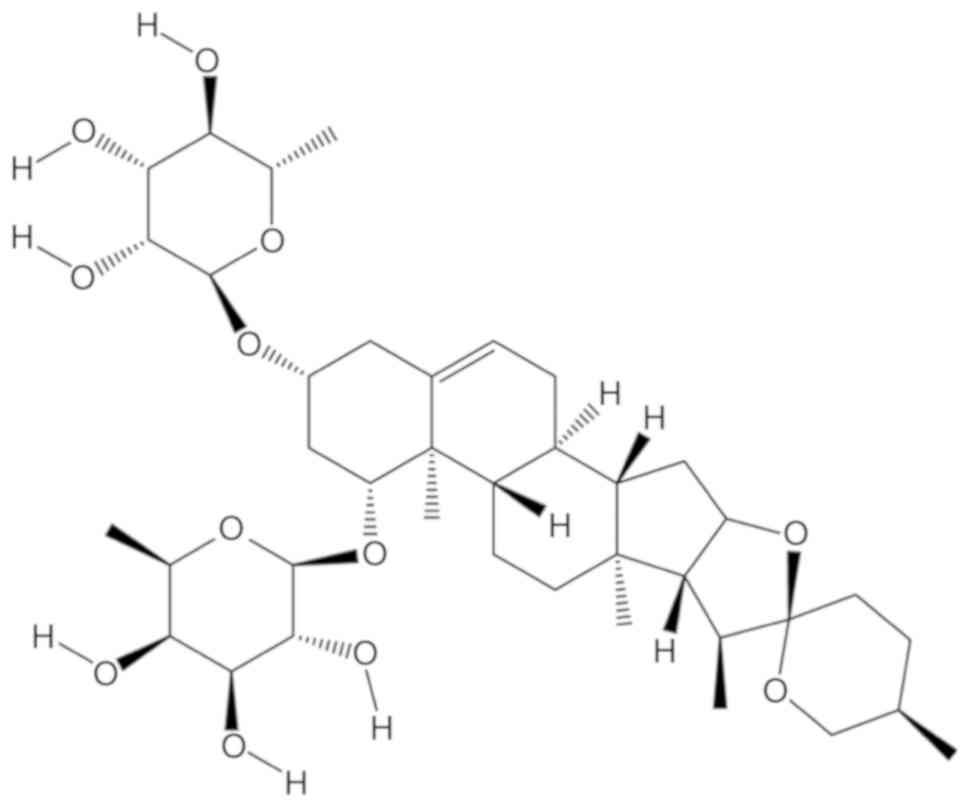
Effect of liriopesides B on invasive
ability of A2780 cells
The invasive ability of cells was examined using a
Transwell assay with Matrigel-coated filter membranes (Fig. 2). The results indicated that
liriopesides B significantly decreased the invasive rate in a
dose-dependent manner (P<0.001). The invasive rate was 45.316
when the concentration of liriopesides B was 1×IC50
value and 1.996 at 5×IC50 value, and when the
concentration of liriopesides B was 10×IC50 value, no
invasive cells were observed.
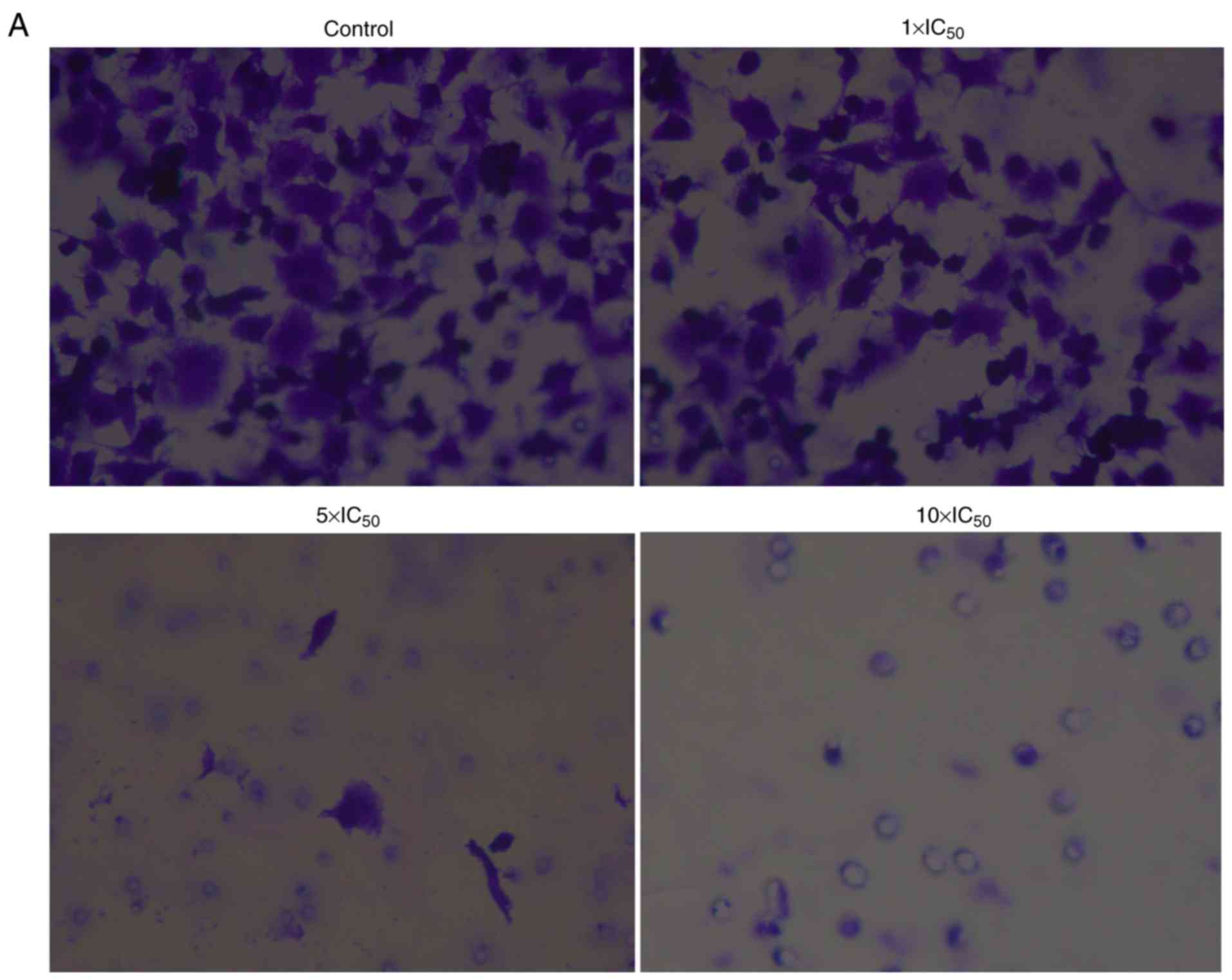
Chemotaxis assay
The chemotactic movement ability of A2780 cells was
also examined using Transwell chambers. As indicated in Fig. 3, liriopesides B significantly
decreased the chemotaxis rate in a dose-dependent manner
(P<0.001). The chemotaxis rate in the groups treated with
liriopesides B at 1×IC50, 5×IC50 and
10×IC50 value was 37.037, 0.777 and 0, respectively.
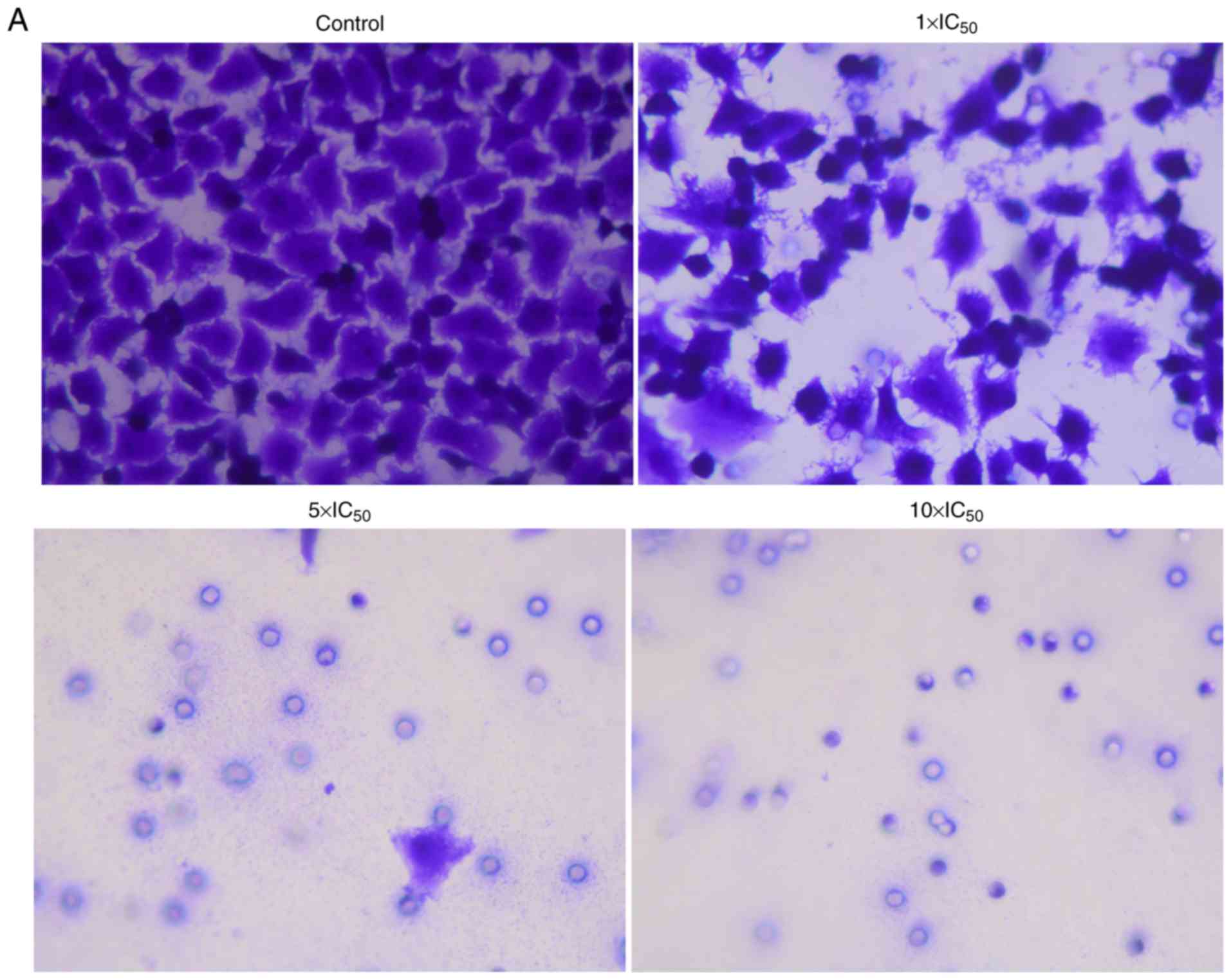
Cell cycle analysis
As presented in Fig.
4, different concentrations of liriopesides B significantly
affected the cell cycle distribution of A2780 cells following
incubation for 24, 48 and 72 h. Of note, the maximum G1-phase
percentage, 70.720%, occurred after treatment with liriopesides B
at 10×IC50 value for 24 h, followed by 69.157 and 56.527
at 48 and 72 h, respectively. After treatment with liriopesides B
at 10×IC50 value for 72 h, the cell cycle was blocked at
the G2/M phase. In short, liriopesides B caused cell cycle arrest
at the G1 phase after 24, 48 and 72 h, and at the G2/M phase with
liriopesides B at 10×IC50 value after 72 h.
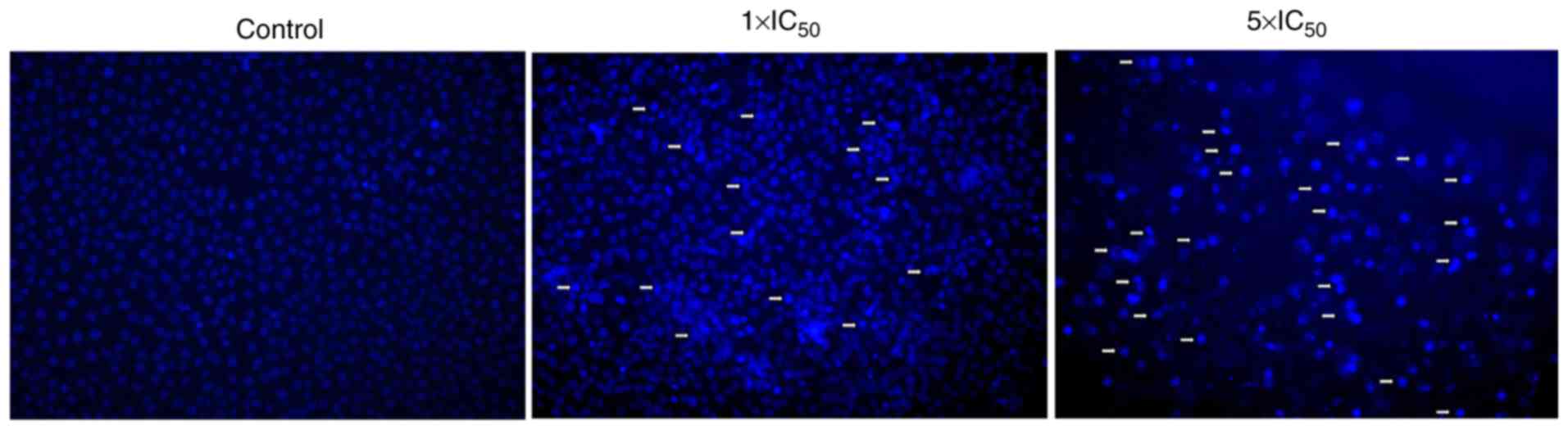
Hoechst 33258 assay
As illustrated in Fig.
5, Hoechst 33258 staining of A2780 cells revealed evident
apoptosis bodies (the cell membrane shrinks and invaginates,
divides and envelops the cytoplasm, and forms vesicular bodies
containing DNA and organelles termed apoptotic bodies),
condensation and margination of nuclear chromatin was present
following treatment with liriopesides B at 5×IC50 value
for 48 h, which indicated that liriopesides B induced apoptosis in
A2780 cells.
Effects of liriopesides B on early and
late apoptosis
The effect of liriopesides B on early and late
apoptosis of A2780 cells is illustrated in Fig. 6. Except for the proportions of
early apoptosis in A2780 cells at 72 h, a significant difference in
proportions was observed in cells induced by liriopesides B in
comparison with the control. The proportion of early apoptotic
cells was increased to the maximum (73.645%) following incubation
with liriopesides B at 1×IC50 for 48 h. As for the late
apoptotic cells, they increased in a dose-dependent manner and the
maximum percentage (61.730% at 1×IC50 liriopesides B and
92.975% at 10×IC50 liriopesides B) occurred in the
treatment group at 72 h. In conclusion, liriopesides B is able to
induce early and late apoptosis in A2780 cells.
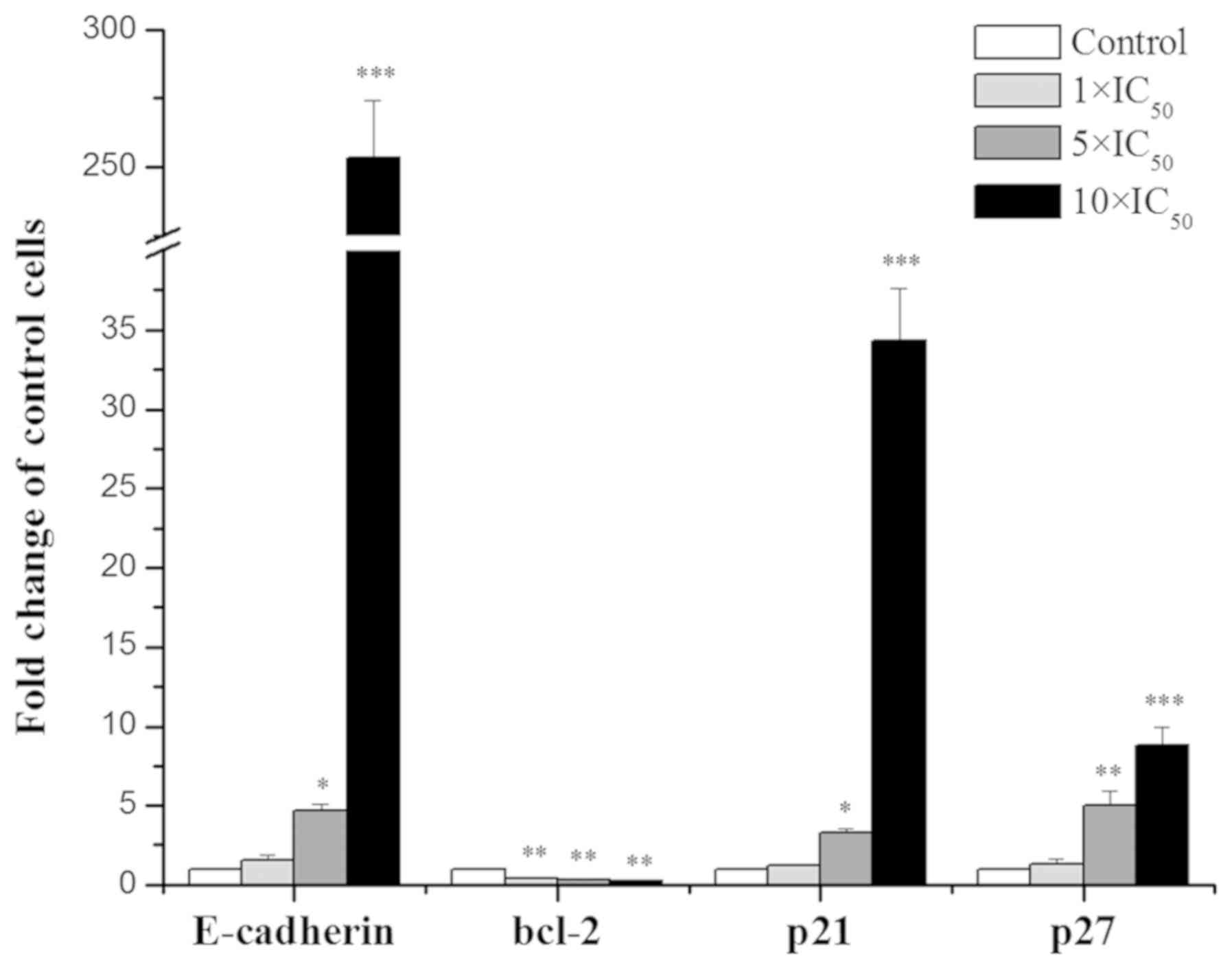
RT-qPCR
The effects of liriopesides Bon the mRNA levels of
four genes in A2780 cells are presented in Fig. 7. With the concentration of
liriopesides B increasing, the expression levels of the four genes
(E-CADHERIN, BCL-2, p21 and p27) were significantly
affected. Specifically, E-CADHERIN, p21 and p27 were
upregulated and BCL-2 was downregulated. The maximum fold
change occurred in A2780 cells treated with liriopesides B at
10×IC50 value, there was a 253.344-fold increase in
E-CADHERIN expression, 34.303 for p21 and 8.767 for p27, and
a minimum fold change of 0.240 for BCL-2 expression. In
brief, liriopesides B significantly increased the expression levels
of E-CADHERIN, p21 and p27, whereas it decreased the
expression of BCL-2.
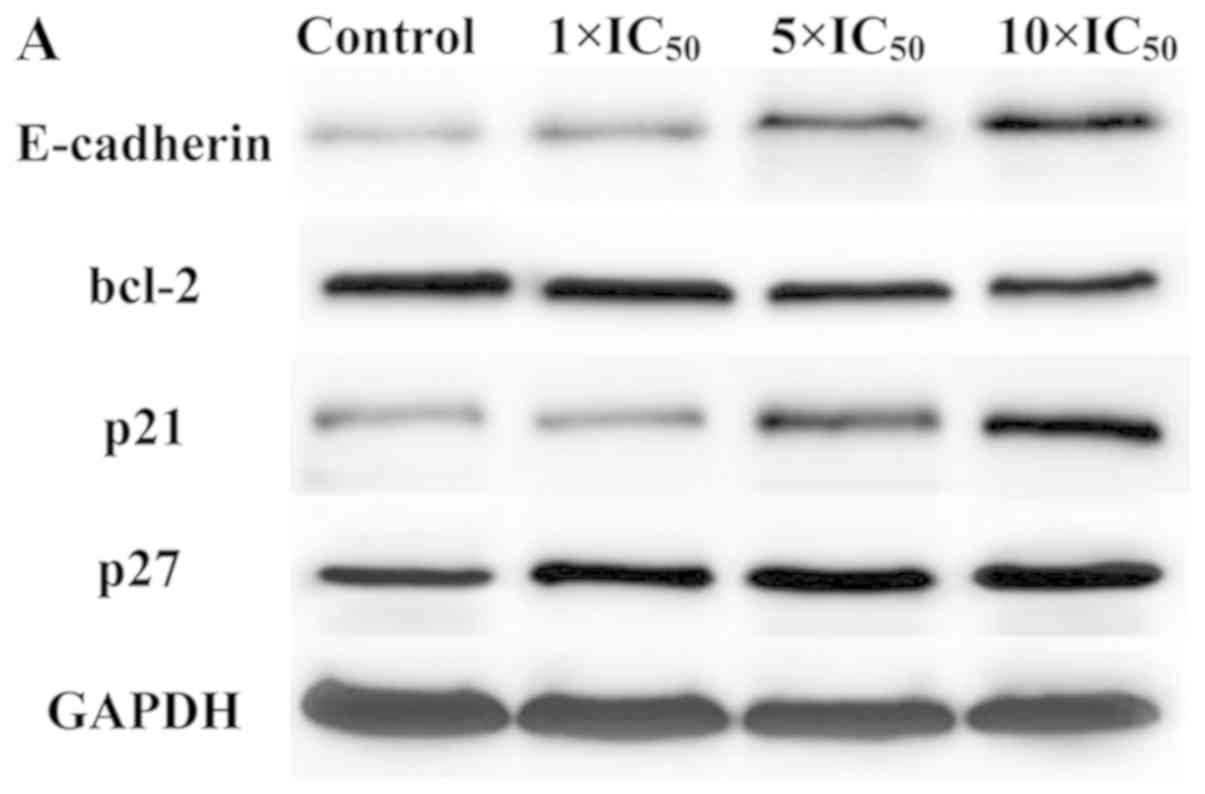
Western blot analysis
Western blotting was performed to detect whether
significant changes in the mRNA expression of the four genes
(E-CADHERIN, BCL-2, p21 and p27) detected by RT-qPCR
were consistent with the changes observed at the protein level. As
indicated in Fig. 8, liriopesides
B increased the levels of three proteins (E-cadherin, p21 and p27)
and decreased the protein levels of Bcl-2 in A2780 cells in a
dose-dependent manner. A significant difference was observed in the
levels of all four proteins in A2780 cells treated with higher
doses of liriopesides B (5×IC50 and 10×IC50),
and the expression of E-CADHERIN was increased after
treatment with all concentrations of liriopesides B in the present
study. As may be expected from the effects on each mRNA,
liriopesides B had the strongest effect on E-cadherin protein
levels in A2780 cells, with a maximum fold change of 1.717 at
10×IC50. Furthermore, liriopesides B also increased the
protein levels of p21 and p27, with maximum fold changes of 1.543
and 1.160, respectively, in A2780 cells treated with the highest
concentration of liriopesides B. Liriopesides B decreased protein
expression of BCL-2, with a fold change of 0.840. In brief,
western blot analysis confirmed that the changes in protein
expression levels were consistent with those in mRNA expression
levels.
Discussion
Traditional Chinese Medicine provides a rich
reservoir of small-molecule active components with the potential to
serve as anti-cancer drugs (36).
In the present study, the effects of liriopesides B on cell
metastatic activity, cell cycle arrest and apoptosis of cancer
cells were examined.
According to the World Health Organization, tumor
cell metastasis is a major cause of death from cancer (1) and has an important role in tumor
progression. The major steps of tumor development include cell
adhesion, migration and invasion. E-cadherin, one of the
calcium-dependent adhesion receptors, is an essential adhesive
system in the epithelium for stable cell-cell adhesion (37) and may be gradually lost in advanced
stages of cancer (3). Studies have
indicated that E-cadherin has an important role in the progression
of ovarian cancer (38–40). Insulin-like growth factor 1 is
reported to induce ovarian cancer cell proliferation via
downregulating E-cadherin (41).
Growth differentiation factor 8 is also able to induce the
migration of SKOV3 ovarian cancer cells by downregulating
E-cadherin (42). Downregulation
of E-cadherin expression by epidermal growth factor receptor ligand
amphiregulin (AREG) induces the invasion of SKOV3 and OVCAR5
ovarian cancer cells in a SNAIL-dependent manner (43) and small interfering RNA targeting
SNAIL is able to abolish AREG-induced cell invasion (44). Furthermore, transforming growth
factor-αis reported to induce human ovarian cancer cell invasion by
downregulating E-cadherin in a SNAIL-independent manner (45). MicroRNA-200a targeting E-cadherin
repressor zinc finger E-box binding homeobox 2 is able to inhibit
the migration and invasion of CD133/1+ ovarian cancer
stem cells (46). Taken together,
downregulation of E-cadherin may result in cancer cell
proliferation, migration and invasion, while upregulation of
E-cadherin may inhibit those processes associated with tumor cell
metastasis. In the present study, compared with untreated cells,
liriopesides B significantly suppressed A2780 cell invasion and
chemotaxis in a dose-dependent manner (P<0.001). The results are
consistent with those by Zhang et al (47). Furthermore, the gene expression
levels of E-cadherin were elevated by liriopesides B in a
dose-dependent manner and the maximum increase was 253.344-fold of
the control group at 10×IC50 liriopesides B. Western
blot analysis also indicated that the protein expression levels of
E-CADHERIN exhibited the most significant increases among
the four genes selected, which was consistent with the results of
the RT-qPCR analysis. In summary, liriopesides B was able to
inhibit migration and invasion of A2780 cells and induce cancer
cell apoptosis at least partially via upregulating
E-CADHERIN. Therefore, E-cadherin may prove to be an
important target in the preventative treatment of metastatic
ovarian cancer (48).
The cell cycle process may be initiated via a
variety of cyclin-dependent kinases (CDKs) in association with
their own cyclin partners. CDK inhibitors are able to restrain the
progression. CDK2, together with cyclin E, controls the G1/S-phase
transition. CDK1, in association with type-A and -B cyclins,
regulates the G2/M-phase transition. p21 and p27 are two important
inhibitory proteins of the cell cycle, which are able to block cell
cycle progression at the G1/S and G2/M checkpoints (49). To reveal the underlying molecular
mechanisms, two important cell cycle regulatory factors, p21 and
p27, were selected and changes in their expression levels were
measured by RT-qPCR (50). The
results indicated that liriopesides B treatment upregulated the
expression levels of the two genes in a dose-dependent manner, with
the maximum fold-change of control cells being 34.303 for
p21 and 8.767 for p27. Therefore, liriopesides B may
block the cell cycle at the G1 phase via upregulating the gene
expression levels of p21 and p27.
Apoptosis or programmed cell death, an orderly and
genetically controlled form of cell death, may be initiated by a
variety of extracellular stimuli, e.g., nutrition supply, DNA
damage and environmental pressure (51) and is also an important mechanism of
the cytotoxic effects induced by numerous anticancer drugs
(52), including trans
reveratorol, curcumin, isoflavones, matrine and oxymatrine.
Apoptosis is distinguished from necrosis in terms of cell shrinkage
and chromatin condensation, and fragmentation of nuclear components
in whole apoptotic cells was clearly observed in a morphological
sense without any damage to adjacent tissue (51). The efficacy of certain anticancer
drugs is determined by their ability to induce tumor cells to
undergo apoptosis (51). In the
present study, the Hoechst 33258 assay indicated that treatment
with liriopesides B for 48 h induced apoptosis with apparent
apoptosis bodies, chromatin condensation and margination in A2780
cells. The results were confirmed by flow cytometry, which
suggested that liriopesides B induced apoptosis in A2780 cells.
Liriopesides B at 1×IC50 mainly induced early apoptosis
at 24 h and the proportion of early apoptotic cells reached the
maximum (73.6%) at 48 h, and subsequently, late apoptosis became
prominent (61.7%) at 72 h. The ability to induce tumor cells to
undergo apoptosis depends on the expression of various oncogenes to
a large extent, including BCL-2, p53, c-MYC, BAX and
RAS (51). In the present
study, BCL-2 was selected to detect changes in gene
expression levels in the absence or presence of liriopesides B via
RT-qPCR analysis. The results indicated a significant difference in
the gene expression levels of BCL-2 (a multidomain
anti-apoptotic Bcl-2 family member). A previous study demonstrated
that liriopesides B may activate Bcl-2 via upregulating p53, which
initiates the intrinsic apoptotic pathway, releases cytochrome C
from the mitochondrial membrane, activates the caspase cascade and
finally leads to apoptosis in A2780 cells (52). Western blot analysis further
confirmed that the changes in the protein expression levels of
BCL-2 were consistent with the changes in its gene
expression levels.
In conclusion, the present study indicated that
liriopesides B may be able to suppress ovarian cancer metastasis,
cause cell cycle arrest at G1 phase and induce apoptosis in A2780
cells, which may be attributed to upregulation of the expression
levels of E-CADHERIN, p21 and p27, as well as
downregulation of the expression of BCL-2. Therefore,
liriopesides B may be considered as a candidate drug against human
ovarian cancer and the more detailed experiments, including
clarifying the main pathway and its side effects and effectiveness
in vivo, will be performed in the near future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW made substantial contributions to the conception
of the present study. HY and HW performed the experiments and wrote
the manuscript; YY contributed to the design of the present study
and interpreted the data. All authors read and approved the final
version of the manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO, . Cancer. September
12–2018
|
|
2
|
Yang ZJ, Zhao BB and Li L: The
significance of the change pattern of serum CA125 level for judging
prognosis and diagnosing recurrences of epithelial ovarian cancer.
J Ovarian Res. 9:572016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alshenawy HA: Immunohistochemical
expression of epidermal growth factor receptor, E-cadherin, and
matrix metalloproteinase-9 in ovarian epithelial cancer and
relation to patient deaths. Ann Diagn Pathol. 14:387–395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
SEER Cancer Stat Facts, . Ovarian Cancer.
National Cancer Institute. (Bethesda, MD). 2016.
|
|
5
|
Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj
A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E,
Cruickshank D, et al: Ovarian cancer screening and mortality in the
UK collaborative trial of ovarian cancer screening (UKCTOCS): A
randomised controlled trial. Lancet. 387:945–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engel N, Oppermann C, Falodun A and Kragl
U: Proliferative effects of five traditional Nigerian medicinal
plant extracts on human breast and bone cancer cell lines. J
Ethnopharmacol. 137:1003–1010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chinese Pharmacopoeia Commission, .
Pharmacopoeia of the People's Republic of China. China Medi Sci
Press. 26:2015.
|
|
8
|
Hao LZ: The research on the chemical
composition of steroidal saponins of Ophiopogonis Radix. Yanbian
University. 2007.
|
|
9
|
Li N, Zhang L, Zeng KW, Zhou Y, Zhang JY
and Che YY: Cytotoxic steroidal saponins from Ophiopogon
japonicus. Steroids. 78:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen M, Du Y, Qui M, Wang M, Chen K, Huang
Z, Jiang M, Xiong F, Chen J, Zhou J, et al: Ophiopogonin B-induced
autophagy in non-small cell lung cancer cells via inhibition of the
PI3K/Akt signaling pathway. Oncol Rep. 29:430–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao GY, Ma J, Lu P, Jiang X and Chang C:
Ophiopogonin B induces the autophagy and apoptosis of colon cancer
cells by activating JNK/c-Jun signaling pathway. Biomed
Pharmacother. 108:1208–1215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nazim UM, Jeong JK and Park SY:
Ophiopogonin B sensitizes TRAIL-induced apoptosis through
activation of autophagy flux and downregulates cellular FLICE-like
inhibitory protein. Oncotarget. 9:4161–4172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen M, Hu C, Guo Y, Jiang R, Jiang H,
Zhou Y, Fu H, Wu M and Zhang X: Ophiopogonin B suppresses the
metastasis and angiogenesis of A549 cells in vitro and in
vivo by inhibiting the EphA2/Akt signaling pathway. Oncol Rep.
40:1339–1347. 2018.PubMed/NCBI
|
|
14
|
Lee JH, Kim C, Lee SG, Sethi G and Ahn KS:
Ophiopogonin D, a steroidal glycoside abrogates STAT3 signaling
cascade and exhibits anti-cancer activity by causing GSH/GSSG
imbalance in lung carcinoma. Cancers (Basel). 10:4272018.
View Article : Google Scholar
|
|
15
|
Lu Z, Wang H, Zhu M, Song W, Wang J, Wu C,
Kong Y, Guo J, Li N, Liu J, et al: Ophiopogonin D′, a natural
product from radix ophiopogonis, induces in vitro and in vivo
RIPK1-dependent and caspase-independent apoptotic death in
androgen-independent human prostate cancer cells. Front Pharmacol.
9:4322018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He J, Wei X, Li S, Quan X, Li R, Du H,
Yuan S and Sun L: DT-13 suppresses breast cancer metastasis by
modulating PLOD2 in the adipocytes microenvironment. Phytomedicine.
59:1527782019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei X, Mao T, Li S, He J, Hou X, Li H,
Zhan M, Yang X, Li R, Xiao J, et al: DT-13 inhibited the
proliferation of colorectal cancer via glycolytic metabolism and
AMPK/mTOR signaling pathway. Phytomedicine. 54:120–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Wang Y, Zhu S, Liu Y, Peng X,
Zhang S, Zhang Z, Qiu Y, Jin M, Wang R, et al: DT-13 inhibits
proliferation and metastasis of human prostate cancer cells through
blocking PI3K/Akt pathway. Front Pharmacol. 9:14502018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Sun L, de Carvalho EL, Li X, Lv X,
Khan GJ, Semukunzi H, Yuan S and Lin S: DT-13, a saponin monomer of
dwarf lilyturf tuber, induces autophagy and potentiates anti-cancer
effect of nutrient deprivation. Eur J Pharmacol. 781:164–172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Liu J, Kou J, Yu J and Yu B:
DT-13 suppresses MDA-MB-435 cell adhesion and invasion by
inhibiting MMP-2/9 via the p38 MAPK pathway. Mol Med Rep.
6:1121–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren-Ping Z, Sen-Sen L, Yuan ST, Yu BY, Bai
XS, Sun L and Zhang LY: DT-13, a saponin of dwarf lilyturf tuber,
exhibits anti-cancer activity by down-regulating C-C chemokine
receptor type 5 and vascular endothelial growth factor in
MDA-MB-435 cells. Chin J Nat Med. 12:24–29. 2014.PubMed/NCBI
|
|
22
|
Lin SS, Fan W, Sun L, Li FF, Zhao RP,
Zhang LY, Yu BY and Yuan ST: The saponin DT-13 inhibits gastric
cancer cell migration through down-regulation of CCR5-CCL5 axis.
Chin J Nat Med. 12:833–840. 2014.PubMed/NCBI
|
|
23
|
Wei XH, Lin SS, Liu Y, Zhao RP, Khan GJ,
Du HZ, Mao TT, Yu BY, Li RM, Yuan ST and Sun L: DT-13 attenuates
human lung cancer metastasis via regulating NMIIA activity under
hypoxia condition. Oncol Rep. 36:991–999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du H, Liu Y, Chen X, Yu X, Hou X, Li H,
Zhan M, Lin S, Lu L, Yuan S and Sun L: DT-13 synergistically
potentiates the sensitivity of gastric cancer cells to topotecan
via cell cycle arrest in vitro and in vivo. Eur J Pharmacol.
818:124–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu XW, Lin S, Du HZ, Zhao RP, Feng SY, Yu
BY, Zhang LY, Li RM, Qian CM, Luo XJ, et al: Synergistic
combination of DT-13 and topotecan inhibits human gastric cancer
via myosin IIA-induced endocytosis of EGF receptor in vitro and in
vivo. Oncotarget. 7:32990–33003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu XW, Wei D, Gao YS, Du HZ, Yu BY, Li RM,
Qian CM, Luo XJ, Yuan ST, Wang JS and Sun L: Synergistic
combination of DT-13 and topotecan inhibits aerobic glycolysis in
human gastric carcinoma BGC-823 cells via NM IIA/EGFR/HK II axis. J
Cell Mol Med. 23:6622–6634. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Sun L, Li H, Lv X, Semukunzi H, Li
R, Yu J, Yuan S and Lin S: DT-13 synergistically enhanced
vinorelbine-mediated mitotic arrest through inhibition of
FOXM1-BICD2 axis in non-small-cell lung cancer cells. Cell Death
Dis. 8:e28102017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Sun L, Li H, Lv X, Semukunzi H, Li
R, Yu J, Yuan S and Lin S: DT-13, a saponin monomer 13 of the Dwarf
lilyturf tuber, synergized with vinorelbine to induce mitotic
arrest via activation of ERK signaling pathway in NCI-H1299 cells.
Biomed Pharmacother. 89:1277–1285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Yu H, Sun Y, Zhao H, Guo Z and Yu
B: Liriopesides B inhibited cell growth and decreased CA125 level
in human ovarian cancer A2780 cells. Nat Prod Res. 31:2198–2202.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nizamutdinova IT, Lee GW, Lee JS, Cho MK,
Son KH, Jeon SJ, Kang SS, Kim YS, Lee JH, Seo HG, et al: Tanshinone
I suppresses growth and invasion of human breast cancer cells,
MDA-MB-231, through regulation of adhesion molecules.
Carcinogenesis. 29:1885–1892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang CX, Liu SY, San JH and Yang M:
Matrine suppresses proliferation of Raji cells via inhibition of
notch signaling pathway. Chin J Biologicals. 30:1162–1167.
2017.
|
|
32
|
Wang H, Ao M, Wu J and Yu L: TNFα and
Fas/FasL pathways are involved in 9-methoxycamptothecin-induced
apoptosis in cancer cells with oxidative stress and G2/M cell cycle
arrest. Food Chem Toxicol. 55:396–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang N, Zhou LQ and Zhang XY:
Downregulation of the nucleosome-binding protein 1 (NSBP1) gene can
inhibit the in vitro and in vivo proliferation of prostate cancer
cells. Asian J Androl. 12:709–717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun P, Jin J, Wang Y, Ren X, Chen L, Lin Z
and Piao Y: Baicalein induces the apoptosis of gastric cancer
MGC-803 cells. Tumor. 37:1041–1046. 2017.
|
|
36
|
Tan W, Lu J, Huang M, Li Y, Chen M, Wu G,
Gong J, Zhong Z, Xu Z, Dang Y, et al: Anti-cancer natural products
isolated from chinese medicinal herbs. Chin Med. 6:272011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Braga V: Spatial integration of E-cadherin
adhesion, signalling and the epithelial cytoskeleton. Curr Opin
Cell Biol. 42:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Y, Liang J, Kang S, Dong Z, Wang N,
Xing H, Zhou R, Li X and Zhao X: E-cadherin gene polymorphisms and
haplotype associated with the occurrence of epithelial ovarian
cancer in Chinese. Gynecol Oncol. 108:409–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng JC, Chang HM, Xiong S, So WK and
Leung PC: Sprouty2 inhibits amphiregulin-induced down-regulation of
E-cadherin and cell invasion in human ovarian cancer cells.
Oncotarget. 7:81645–81660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Fan N and Yang J: Expression and
clinical significance of hypoxia-inducible factor 1α, Snail and
E-cadherin in human ovarian cancer cell lines. Mol Med Rep.
12:3393–3399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lau MT and Leung PC: The PI3K/Akt/mTOR
signaling pathway mediates insulin-like growth factor 1-induced
E-cadherin down-regulation and cell proliferation in ovarian cancer
cells. Cancer Lett. 326:191–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao J, Klausen C, Xiong S, Cheng JC,
Chang HM and Leung PC: Growth differentiation factor 8 induces
SKOV3 ovarian cancer cell migration and E-cadherin down-regulation.
Cell Signal. 28:1615–1622. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng JC, Qiu X, Chang HM and Leung PC:
HER2 mediates epidermal growth factor-induced down-regulation of
E-cadherin in human ovarian cancer cells. Biochem Biophys Res
Commun. 434:81–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
So WK, Fan Q, Lau MT, Qiu X, Cheng JC and
Leung PC: Amphiregulin induces human ovarian cancer cell invasion
by down-regulating E-cadherin expression. FEBS Lett. 588:3998–4007.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qiu X, Cheng JC, Klausen C, Fan Q, Chang
HM, So WK and Leung PC: Transforming growth factor-α induces human
ovarian cancer cell invasion by down-regulating E-cadherin in a
Snail-independent manner. Biochem Biophys Res Commun. 461:128–135.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu Q, Guo R, Lin M, Zhou B and Wang Y:
MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration
and invasion by targeting E-cadherin repressor ZEB2. Gynecol Oncol.
122:149–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Han Y, Zhai K, Sun M, Liu J, Yu B
and Kou J: Ophiopogonin-D suppresses MDA-MB-435 cell adhesion and
invasion by inhibiting matrix metalloproteinase-9. Mol Med Rep.
12:1493–1498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ansenberger K, Zhuge Y, Lagman JA,
Richards C, Barua A, Bahr JM and Hales DB: E-cadherin expression in
ovarian cancer in the laying hen, gallus domesticus, compared to
human ovarian cancer. Gynecol Oncol. 113:362–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Harada K and Ogden GR: An overview of the
cell cycle arrest protein, p21(WAF1). Oral Oncol. 36:3–7. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sørby LA, Andersen SN, Bukholm IR and
Jacobsen MB: Evaluation of suitable reference genes for
normalization of real-time reverse transcription PCR analysis in
colon cancer. J Exp Clin Cancer Res. 29:1442010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guchelaar HJ, Vermes A, Vermes I and
Haanen C: Apoptosis: Molecular mechanisms and implications for
cancer chemotherapy. Pharm World Sci. 19:119–125. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim R, Tanabe K, Uchida Y, Emi M, Inoue H
and Toge T: Current status of the molecular mechanisms of
anticancer drug-induced apoptosis. The contribution of
molecular-level analysis to cancer chemotherapy. Cancer Chemother
Pharmacol. 50:343–352. 2002. View Article : Google Scholar : PubMed/NCBI
|