Introduction
Liver transplantation is the most effective
therapeutic strategies for end-stage liver diseases; however, the
ever-increasing shortage of donor organs limits the development of
liver transplantation and leads to the use of livers from expanded
criteria donors (ECDs) (1). As a
result, marginal organs that are more susceptible to the harmful
effects of ischemia-reperfusion injury (IRI) are frequently used
for transplantation (2). IRI is a
complicated pathophysiological process that is difficult to avoid
during liver transplantation. Multiple mechanisms contribute to the
process of IRI, of which inflammation and oxidative stress display
major roles (3,4). After transplantation, patients who
receive marginal organs are more likely to develop early allograft
dysfunction, liver rejection and biliary complications, and thus
have a higher risk of unfavorable short- and long-term outcomes
(5). Therefore, the effective
maintenance and optimization of the quality of organs donated after
circulatory death (DCD) are urgent problems in the field of organ
transplantation.
In order to attenuate IRI, the organ preservation
method of HMP has received increasing interest worldwide (6,7). The
first clinical study on liver HMP confirmed its effectiveness for
DCD and ECD organs, with HMP displaying improved results compared
with cold storage (CS) (8).
Previous studies investigating the application of HMP for discarded
human livers verified the advantages of the technique (7,9).
Furthermore, the protective effects of HMP in DCD organs has been
reported. In a previous study, HMP was employed for 1–7 h and
displayed beneficial outcomes for all durations (10). However, the optimal duration of HMP
to reduce liver injury and maintain optimal liver viability, as
well as the exact molecular mechanisms underlying HMP-mediated
protection of DCD livers, have not been previously reported.
Lüer et al (11) reported that HMP can upregulate
kruppel-like factor 2 (KLF2) expression in livers. KLF2 is a
transcriptional regulator that has a zinc-finger structure and is
highly expressed in the lungs and vascular endothelium. KLF2
expression can be induced by laminar sheer stress, resulting in
atheroprotective, anticoagulant and anti-inflammatory effects
(12,13). Several studies have demonstrated
that KLF2 can inhibit the transcriptional activity of NF-κB, which
controls the transcription and expression of various cytokines and
adhesion molecules involved in inflammation and the immune
response. For example, NF-κB can reduce the expression of tumor
necrosis factor (TNF)-α and interleukin (IL)-1β to attenuate
inflammation (14–20). Wang et al (21) demonstrated that flow-stimulated
phosphorylation and nuclear export of histone deacetylase 5 (HDAC5)
results in the dissociation of HDAC5 and myocyte enhancer factor 2C
(MEF2C), which enhances the transcriptional activity of MEF2C and
induces the expression of KLF2 and endothelial nitric oxide
synthase (eNOS), increasing nitric oxide (NO) levels in human
umbilical vein endothelial cells (ECs) exposed to laminar flow.
eNOS is a Ca2+-, NADPH-, flavin- and biopterin-dependent
enzyme that can constitutively produce NO in various cells. The
activity of eNOS is tightly regulated by different mechanisms, of
which phosphorylation at specific amino acids can lead to
activation or inhibition of eNOS activity depending on the
localization in the protein sequence (22,23).
Among the numerous phosphorylation sites, Ser1177 is rapidly
phosphorylated after the application of fluid shear stress, which
results in eNOS activation to sustain moderate NO generation
(24). NO displays important
biological functions, including vasodilatation, scavenging of
superoxide, inhibition of platelet aggregation, and reduction of
proliferation and inflammation (22–25).
However, only a limited number of studies have investigated the
anti-inflammatory and antioxidant effects of the KLF2/NF-κB/eNOS/NO
signaling pathway during organ transplantation.
Although HMP has been widely researched, the optimal
duration of HMP to effectively attenuate IRI has not been
previously reported and the molecular mechanisms underlying the
protective effects of HMP are not completely understood. The
present study aimed to investigate the optimal duration of liver
HMP by comparing anti-inflammatory and antioxidant activities
during IRI. In addition, whether the protective effects of HMP were
exerted via the KLF2/NF-κB/eNOS/NO signaling was investigated.
Materials and methods
Animals
A total of 42 adult male Sprague Dawley (SD) rats
(age, 8–10 weeks; weight, 250–300 g) were purchased from the
Experimental Animal Culture Center of the Hubei Center for Disease
Control. All animals received standard care and were housed under
standard laboratory conditions (temperature, 25±2°C; relative
humidity, 55±5%; a 12 h light/dark cycle) in Zhongnan Hospital's
Animal Experiment Center of Wuhan University. Animals had free
access to food and water. The present study was approved by the
Ethical Committee of Wuhan University and carried out in accordance
with the Experimental Animal Management Ordinance (National Science
and Technology Committee of China) and the Guide for the Care and
Use of Laboratory Animals (National Institutes of Health) (26).
Establishment of the rat model of
DCD
Rats were anesthetized by the intraperitoneal
injection of pentobarbital sodium (50 mg/kg). An abdominal
longitudinal incision was made, and the hepatic artery, portal
vein, supra- and infrahepatic inferior vena cava, bile duct and
peripheral ligaments were freed to fully expose the liver. An
epidural guiding conduit was inserted (Jiangsu Changfeng Medical
Industry Co., Ltd.) into the bile duct. After ligation of the left
phrenic vein, the diaphragm was cut to induce bilateral
pneumothorax and cardiac arrest. The period of WI started from the
point of cardiac arrest (27).
After 30 min of WI, systemic heparinization was implemented by the
injection of 2 ml Ringer (Chimin Health Management Co., Ltd.;
www.chimin.cn) and 100 IU heparin (Hepatunn;
http://www.hepatunn.com/) via the right iliac
vein (28). Subsequently, the
hepatic artery was ligated and the liver was flushed in situ
with 20 ml 0–4°C Histidine Tryptophan Ketoglutarate (HTK) solution
(Dr Franz Koehler Chemie GmbH) via the portal vein intubation
[homemade pressure equalizer (PE) tubes; outer diameter, 2.1 mm;
inner diameter, 1.8 mm]. To collect the hepatic effluent, the
suprahepatic inferior vena cava was intubated using a PE catheter
(inner diameter, 3 mm) (29).
Preservation of DCD livers
SD rats were randomly divided into the HMP group and
the CS group. In the HMP group (n=18), the livers were obtained
after 30 min of WI and 0–4°C HTK solution (150 ml) was perfused via
the portal vein for 0, 1, 3, 5, 12 or 24 h at a rate of 0.5
ml/g/min (29). At each time
point, three livers were harvested. In the CS group (n=18), after
30 min of WI, the livers were maintained in CS (0-4°C) in HTK
solution (150 ml) for 0, 1, 3, 5, 12 or 24 h. At each time point,
three livers were harvested. The WI group consisted of livers
harvested at 0 h in both groups.
Evaluation of preservation
effects
SD rats were randomly divided into the HMP +
normothermic reperfusion (NR) group and the CS + NR group. In the
HMP + NR group (n=3), HMP was performed for 3 h followed by NR for
2 h using the isolated perfused rat liver (IPRL) system. In the CS
+ NR group (n=3), livers were maintained in CS for 3 h followed by
NR for 2 h using the IPRL system.
Liver perfusion system
A thermostat water bath (Jiaxingjunsi Electronics
Co., Ltd.) containing a liver perfusion box was used. The
temperature of the bath was controlled by heated water around the
box or by an ice-water mixture inside the box. The temperature of
the liver perfusion box was monitored using a temperature sensor
(Xinghe Electronics Co., Ltd.). A peristaltic pump (Longerpump
Technologies Inc.; http://www.longerpump.com/), a hollow-fiber membrane
oxygenator (Dongguan Kewei Medical Instrument Co., Ltd.) and a flow
meter (Hehua Mechanical and Electric Corporation; http://hqn640901.d17.cc/) were connected to complete
the perfusion system. The portal vein cannula was connected to the
baroreceptor of a BL-420F Biological Functional System (Chengdu
Taimeng Science and Technology Co., Ltd.; http://www.tme.com.cn/) to measure the portal
perfusion pressure.
IPRL system
After HMP or CS, livers were rewarmed for 20 min to
simulate the period of rewarming during transplantation.
Subsequently, the liver was connected to the IPRL system, which
consists of a liver perfusion system with an oxygen supply. The
temperature of the water bath was set to 40°C to maintain the
temperature of the liver container at 36.5±0.5°C. Krebs-Henseleit
buffer (Macgene; http://www.macgene.com/) with 4% dextran (Xian Wanlong
Pharmaceutical Co., Ltd.; http://www.xawanlong.com/) was used for reperfusion
(30). The perfusate was
oxygenated with 95% O2 + 5% CO2 gas to
maintain >500 mmHg oxygen pressure. During the 2 h perfusion,
the portal vein perfusion pressure was maintained at 10.3 mmHg
(10,31).
Biochemical examination
During the 2 h reperfusion, alanine transaminase
(ALT; cat. no. C009; Nanjing Jiancheng Bioengineering Institute),
aspartate transaminase (AST; cat. no. C010; Nanjing Jiancheng
Bioengineering Institute) and lactate dehydrogenase (LDH; cat. no.
A020; Nanjing Jiancheng Bioengineering Institute) levels were
measured in the hepatic effluent that was collected from the
suprahepatic vena cava every 60 min using commercial standard kits,
according to the manufacturer's protocol.
Oxygen consumption
To assess the metabolic activity of the livers,
hepatic oxygen consumption was analyzed every 60 min during the 2 h
reperfusion using an i-STAT pH-blood gas analyzer (Abbott Point of
Care, Inc.). Oxygen consumption (μmol/min/g liver) was calculated
according to the following formula: (Cin -
Cout) / portal flow (ml/min) / liver weight (g), where
Cin and Cout represent the oxygen
concentration of the liver inflow and outflow, respectively
(31).
Bile production
Bile was collected every 60 min via the epidural
guiding tube that was positioned in the bile duct. Bile flow is
expressed as µl/h/g liver, as the density of bile is almost equal
to that of water (10).
Liver histology
Liver samples were stored in 10% formaldehyde at
room temperature for 1–5 days, embedded in paraffin, and cut into
3-µm sections. Subsequently, sections were stained with hematoxylin
and eosin (HE) at room temperature (hematoxylin staining for 5–10
min and eosin staining for 1–3 min). The severity of hepatic injury
was assessed by pathologists who were blinded to the experiment
using a light microscope at ×200 magnification according to the
classification described by Suzuki et al (32). Breifly, sinusoidal congestion,
hepatocyte necrosis and ballooning degeneration were graded from 0
to 4, where 0 indicated no necrosis, congestion or ballooning, and
4 indicated severe congestion, ballooning degeneration or
hepatocyte necrosis (>60%).
Western blotting
Total protein was extracted from liver tissues using
RIPA lysis buffer (Beyotime Institue of Biotechnology; cat. no.
P0013B) containing protease inhibitor and quantified by the
bicinchoninic acid method. Protein (40 µg/lane) was separated via
10% SDS-PAGE and transferred onto PVDF membranes, which were
blocked with 5% skim milk (cat. no. G5002; Wuhan Servicebio
Technology Co., Ltd.) at room temperature for 2 h. Subsequently,
the membranes were incubated overnight at 4°C with the following
primary antibodies: anti-MEF2C (rabbit; 1:500; cat. no. 10056-1-AP;
ProteinTech Group, Inc.), anti-KLF2 (rabbit; 1:400; cat. no.
bs-2772R; Beijing Biosynthesis Biotechnology Co., Ltd.), anti-NF-κB
p65 (rabbit; 1:400; cat. no. bs-20355R; Beijing Biosynthesis
Biotechnology Co., Ltd.), anti-eNOS (rabbit; 1:1,000; cat. no.
20116-1-AP; ProteinTech Group, Inc.), anti-phosphorylated eNOS at
Ser1177 (rabbit; 1:1,000; cat. no. 9571, Cell Signaling Technology,
Inc.) and anti-β-actin (rabbit; 1:1500; cat. no. bs-0061R; Beijing
Biosynthesis Biotechnology Co., Ltd.). After washing with TBST
(Tris, 2.42 g; NaCl, 8 g; Tween, 1 ml; Distilled Water, 1,000 ml),
the membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit IgG (H+L) antibodies (1:3,000; cat. no. G1213;
Wuhan Servicebio Technology Co., Ltd.) at room temperature for 2 h.
Protein bands were visualized using chemiluminescence ECL reagent
(cat. no. AR1172; Boster Biological Technology). Protein expression
was quantified using ImageJ software (version 1.42q; National
Institutes of Health) with β-actin as the loading control.
ELISA
TNF-α (cat. no. ERC102a; QuantiCyto, http://www.neobioscience.net/pro_view-7421.html) and
IL-1β (cat. no. ERC007; QuantiCyto; http://www.neobioscience.net/ pro_view-7346.html)
expression levels in liver tissues and NO levels (cat. no. A012;
Nanjing Jiancheng Bioengineering Institute) in the perfusate were
measured using ELISA kits.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 17.0; SPSS, Inc.). Data are presented as the mean
± standard error. The normality of each results was analyzed by
homogeneity of variance analysis. Differences between two groups
were analyzed using the unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
HMP attenuates WI-induced pathological
liver injury
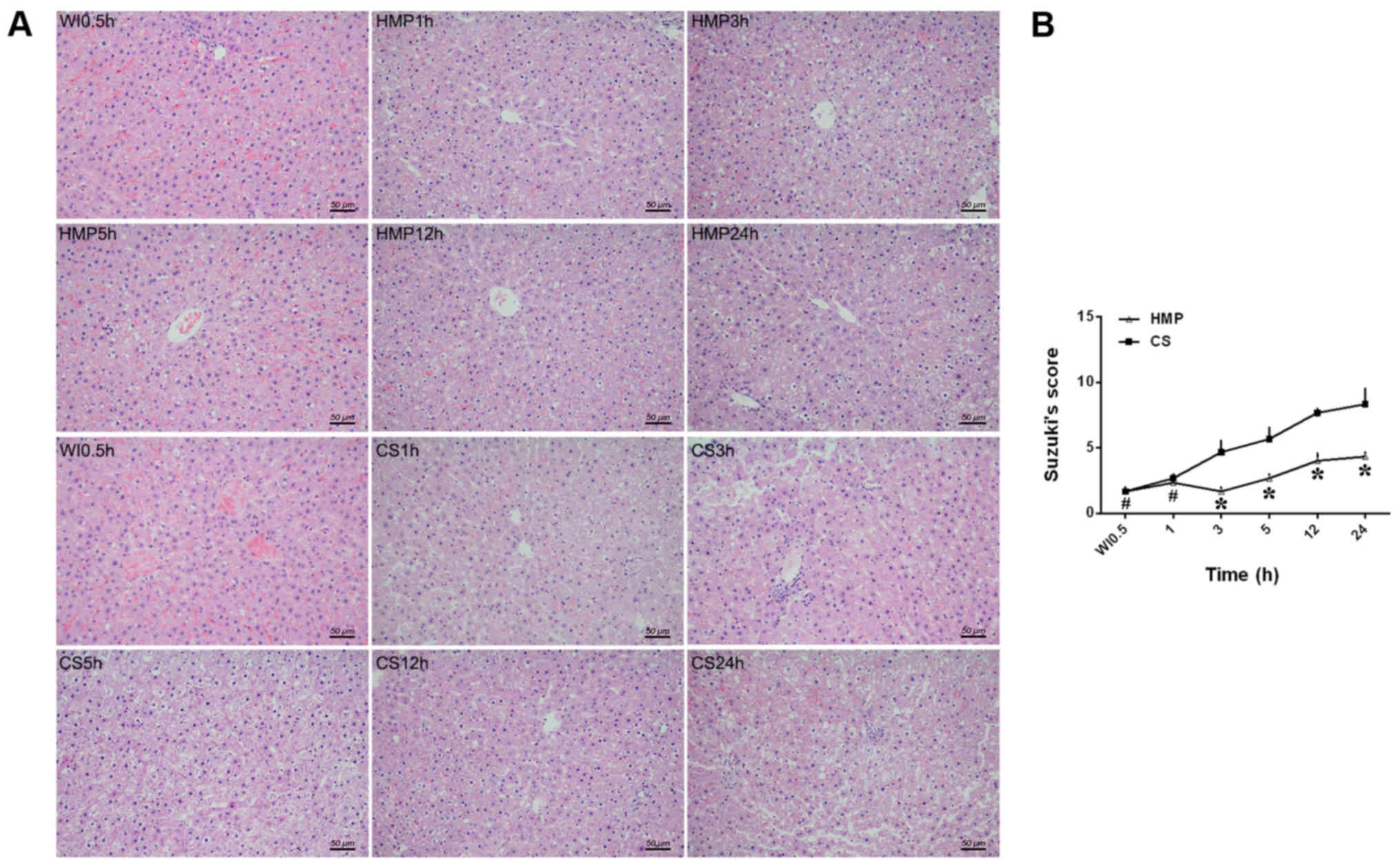
First, hepatic pathological injury was assessed
(Fig. 1). Injury was quantified
using a previously validated liver pathological damage scoring
system (32), where a higher score
indicated greater damage. After 30 min of WI, livers displayed
significant sinusoidal congestion, hepatocyte edema, anoxic
vacuoles, dot necrosis and high numbers of lymphocytes. In the
livers of the CS group, severe hepatocellular swelling/necrosis,
sinusoidal congestion and inflammatory cell infiltration was
observed. The livers of the HMP group displayed less damage
compared with the CS group (Fig.
1A). Moreover, the HMP group displayed a significantly lower
pathology score compared with the CS group at 3, 5, 12 and 24 h
(P<0.05), with the lowest score recorded at the 3 h timepoint.
At the 1 h timepoint, the difference in pathology score was not
significant between the HMP and CS groups (P>0.05; Fig. 1B). The results suggested that HMP
preserved DCD livers more effectively compared with CS.
HMP increases MEF2C and KLF2
expression and decreases NF-κB p65, TNF-α and IL-1β expression
during the ischemic phase
Subsequently, the protein expression levels of
MEF2C, KLF2 and NF-κB p65 were assessed by western blotting to
determine the effects of HMP on hepatic inflammation (Fig. 2). MEF2C and KLF2 expression levels
were significantly increased in the HMP group compared with the CS
group at 3, 5, 12 and 24 h (P<0.05; Fig. 2B and C). By contrast, the
expression levels of NF-κB p65 were significantly lower in the HMP
group compared with the CS group at 3, 5, 12 and 24 h (P<0.05;
Fig. 2D). TNF-α and IL-1β are
downstream genes of NF-κB p65 (33); therefore, the expression of the two
inflammatory cytokines was measured by ELISA to assess the degree
of NF-κB p65-induced inflammation. TNF-α and IL-1β levels were
significantly lower in the HMP group compared with the CS group at
3, 5, 12 and 24 h (P<0.05; Fig. 2E
and F). For all genes, the most notable difference between the
HMP group and the CS group was observed at the 3 h timepoint.
However, the expression levels of the examined genes were not
significantly different between the HMP and CS groups at the 1 h
timepoint (P>0.05).
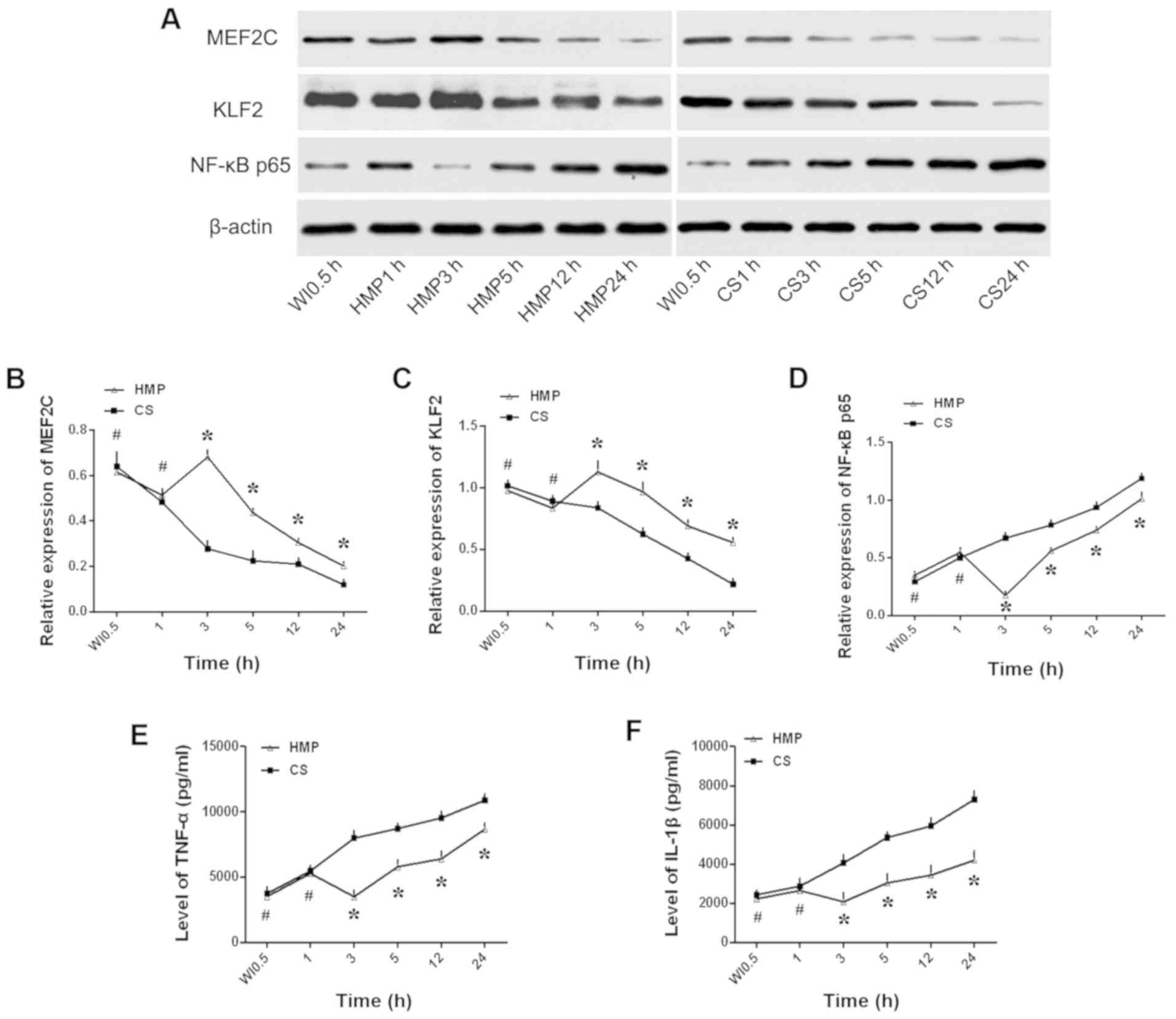 | Figure 2.Expression levels of MEF2C, KLF2,
NF-κB p65, TNF-α and IL-1β in liver tissues. Protein expression
levels were (A) determined by western blotting and semi-quantified
for (B) MEF2C, (C) KLF2 and (D) NF-κB p65. (E) TNF-α and (F) IL-1β
expression levels were assessed by ELISA. *P<0.05 vs. CS;
#P>0.05 vs. CS. MEF2C, myocyte enhancer factor 2;
KLF2, kruppel-like factor 2; TNF-α, tumor necrosis factor-α; IL-1β,
interleukin-1β; CS, cold storage; HMP, hypothermic machine
perfusion; WI, warm ischemia. |
HMP alleviates reperfusion-induced
hepatocellular injury
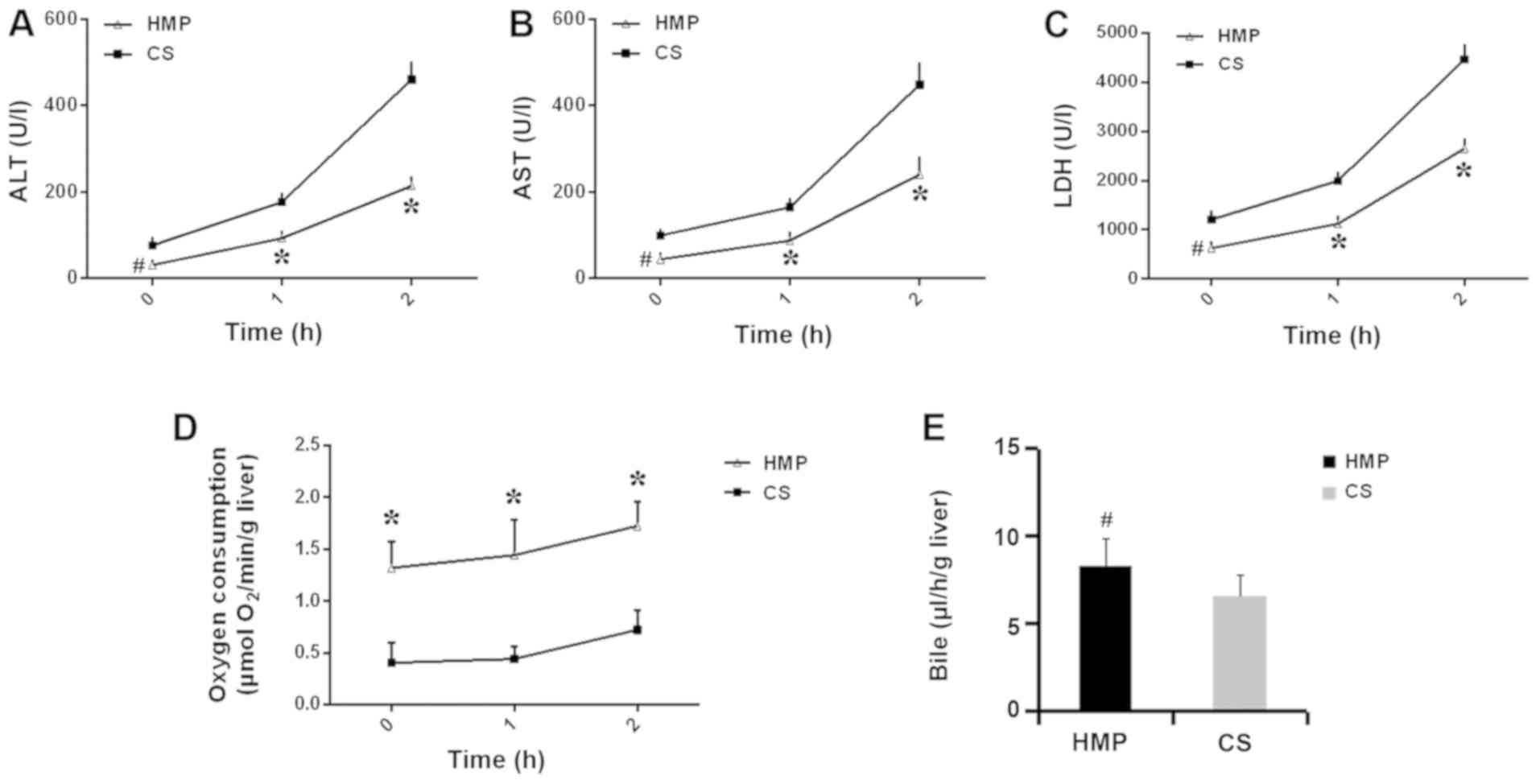
The protective effects of HMP were further assessed
using the IPRL system, which can imitate the physical environment.
The release of ALT, AST and LDH during reperfusion was measured.
ALT, AST and LDH concentrations sharply increased following
reperfusion in both the HMP and CS groups (Fig. 3). However, enzyme levels were
significantly lower in the HMP group compared with the CS group at
60 and 120 min (P<0.05; Fig.
3A-C). Livers in the HMP group displayed significantly higher
rates of oxygen consumption compared with the CS group at 0, 60 and
120 min (P<0.05; Fig. 3D). In
addition, bile production was slightly higher in the HMP group
compared with the CS group, but the difference was not
statistically significant at 120 min (P>0.05; Fig. 3E).
HMP increases MEF2C and KLF2
expression and decreases NF-κB p65, IL-1β and TNF-α expression
during the reperfusion phase
Based on the observation that HMP for 3 h increased
MEF2C and KLF2 expression and decreased NF-κB p65, TNF-α and IL-1β
expression (Fig. 2), the
protective mechanisms underlying HMP were further investigated by
evaluating protein expression after NR. MEF2C and KLF2 expression
levels were significantly increased in livers of the HMP + NR group
compared with the CS + NR group (P<0.05; Fig. 4A-C). By contrast, NF-κB p65, TNF-α
and IL-1β expression levels were significantly decreased in the HMP
+ NR group compared with the CS + NR group (P<0.05; Fig. 4D-F). The results indicated that HMP
may attenuate IRI-induced hepatic inflammation.
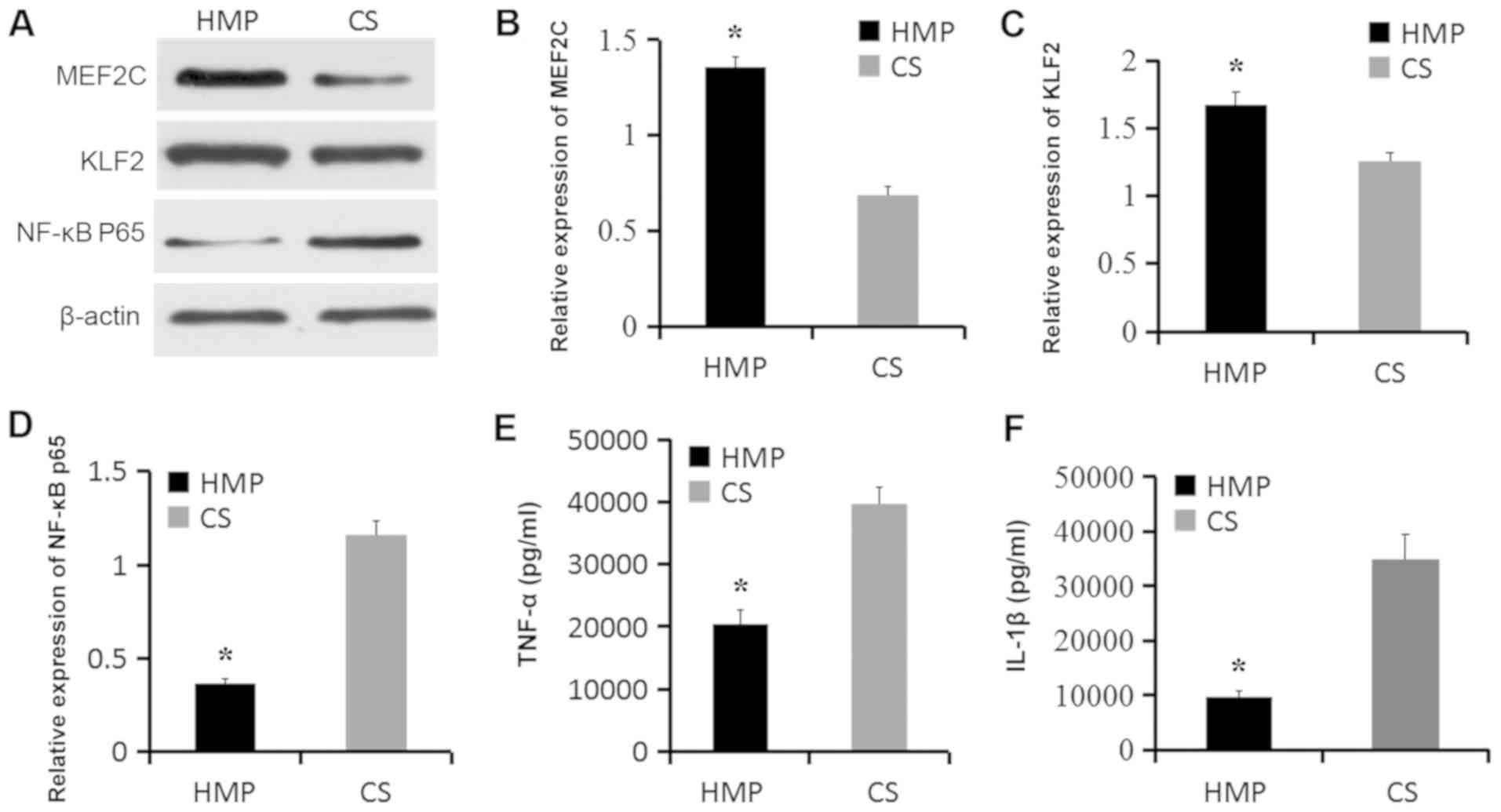 | Figure 4.Expression levels of MEF2C, KLF2,
NF-κB p65, TNF-α and IL-1β after NR. Protein expression levels were
(A) determined by western blotting and semi-quantified for (B)
MEF2C, (C) KLF2 and (D) NF-κB p65. Expression levels of the
inflammatory cytokines (E) TNF-α and (F) IL-1β were measured by
ELISA. *P<0.05 vs. CS. MEF2C, myocyte enhancer factor 2; KLF2,
kruppel-like factor 2; TNF-α, tumor necrosis factor-α; IL-1β,
interleukin-1β; NR, normothermic reperfusion; CS, cold storage;
HMP, hypothermic machine perfusion. |
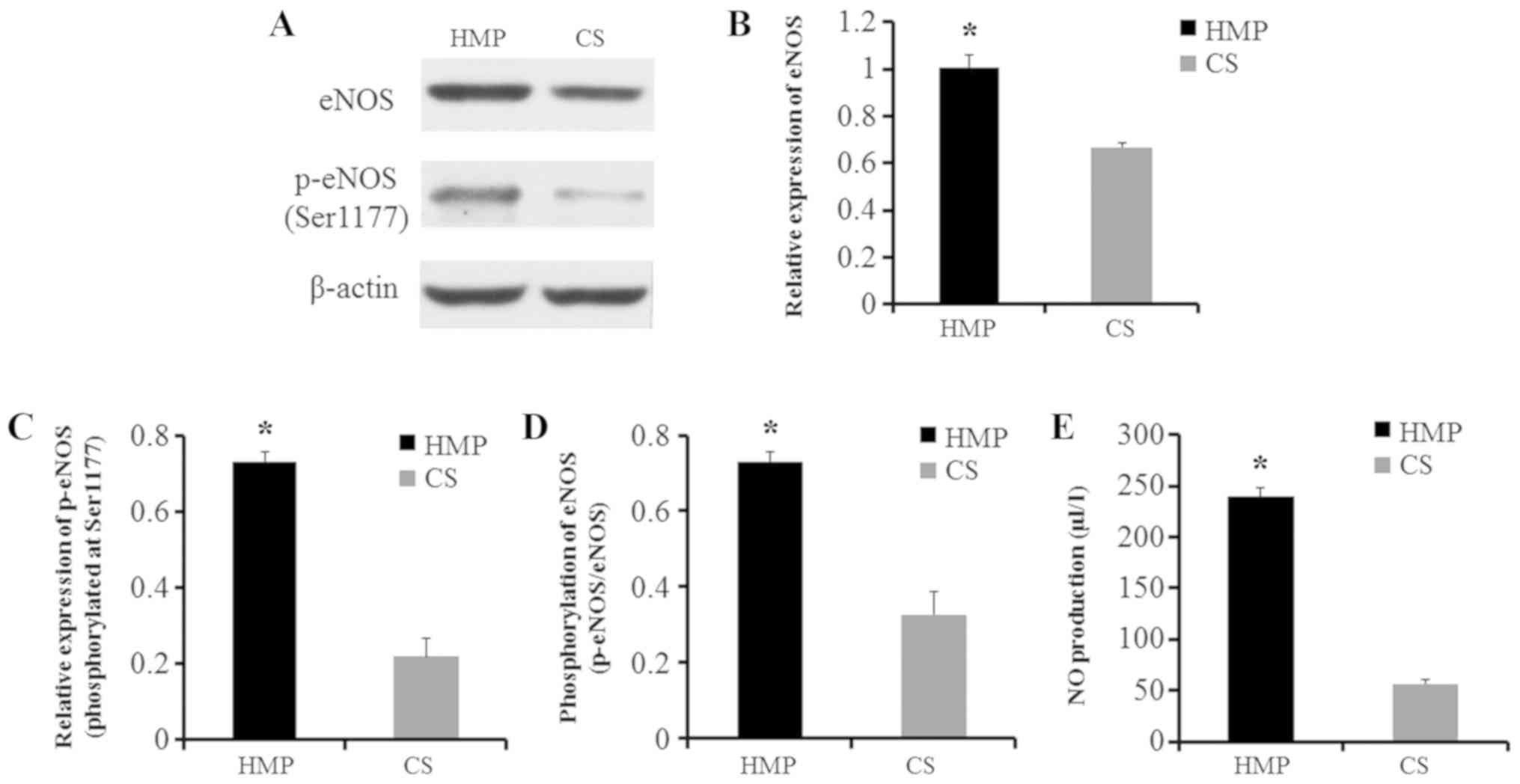
HMP increases eNOS and NO levels
during the reperfusion phase
Finally, whether HMP could reduce IRI-associated
oxidative stress was assessed by evaluating the expression of eNOS
and p-eNOS at Ser1177. In livers preserved by HMP, significantly
higher levels of eNOS and p-eNOS were observed compared with livers
preserved by CS, and the ratio of p-eNOS/eNOS was also
significantly increased(P<0.05; Fig. 5A-D). eNOS constitutively produces
NO; therefore, NO levels in the perfusate after 2 h of NR were also
measured by ELISA. NO levels were significantly higher in the HMP
group compared with the CS group (P<0.05; Fig. 5E). Therefore, the results indicated
that HMP reduced hepatic oxidative stress during IRI.
Discussion
The increased demand for organs has led to the use
of ECD and DCD organs, which often require longer periods of WI or
cold ischemia, resulting in a higher risk of serious injury and
unfavorable outcomes (2,6). Liver IRI is a complex
pathophysiological response that cannot be fully avoided during
liver transplantation. Oxidative stress and inflammatory responses
are the main mechanisms underlying liver damage during liver
transplantation (3,4). Donor organs with impaired function
severely threaten the short- and long-term prognosis of the patient
receiving the liver transplantation (1,2);
therefore, identifying strategies that focus on reducing liver IRI
and preserving organ quality are of high importance.
At present, HMP and CS are the two most commonly
used strategies for organ preservation (34). Numerous studies have demonstrated
that HMP preserves organs more effectively compared with CS
(7–10). HMP, which can cause vascular shear
stress by pumping perfusate, allows for organ optimization and
offers a platform for viability assessment, organ repair and
resuscitation (35,36). Despite the extensive studies on
HMP, the optimal perfusion time has not been reported and the
molecular mechanisms underlying HMP are not completely understood.
In the present study, a well-established animal model was used to
generate DCD livers, which were then preserved by HMP or CS. Liver
quality was assessed at six different time points (0, 1, 3, 5, 12
and 24 h). The results suggsted that HMP preserved DCD livers more
effectively compared with CS, which was consistent with our
previous studies (6,10,13).
In addition, morphological alterations to the liver tissues
indicated that the protective effect of HMP was most notable at the
3 h timepoint.
Vascular shear stress serves an important role in
the regulation of vascular function, while laminar shear stress
mechanically stimulates vascular endothelium and induces the
expression of flow-dependent vascular protective genes (12,21,37).
Moreover, laminar flow can activate a number of distinct signaling
pathways in ECs to modulate transcription factor activity (37), with the MEF2 transcription family
being an example. MEF2 proteins are members of the MCM1
agamous-deficiens-serum response factor family of transcription
factors, which bind to AT-rich sequences. There are four isoforms
of MEF2: MEF2A, MEF2B, MEF2C and MEF2D (38). MEF2C displays a key role during EC
angiogenesis (39), and Xu et
al (40) reported that MEF2C
suppresses EC inflammation by regulating NF-κB and KLF2 expression.
The most extensively studied in vitro targets of MEF2 in ECs
are KLF2 and KLF4, which regulate antithrombotic and
anti-inflammatory transcriptional signaling pathways (41). Parmar et al (42) demonstrated that overexpression of a
dominant-negative MEF2 variant prevents flow-mediated induction of
KLF2 expression in ECs. As an important flow-regulated molecule,
KLF2 has greatly advanced the understanding of the molecular
mechanisms underlying vascular homeostasis (12,15,37).
KLF2 interacts with NF-κB during the inflammatory immune response,
which activates NF-κB and leads to nuclear translocation. In the
nucleus, NF-κB regulates the expression of downstream inflammation
factors, such as TNF-α and IL-1β, via the p65 subunit, which serves
a critical role in kidney IRI (43,44).
In the present study, MEF2C and KLF2 expression levels were
significantly increased, whereas NF-κB p65, IL-1β and TNF-α
expression levels were significantly decreased in HMP livers
compared with CS livers. The results suggested that
KLF2/NF-κB-dependent inflammation was associated with the
protective effects of HMP in DCD livers.
However, the differences between HMP and CS at the 1
h timepoint were not statistically significant, which may be
explained by the fact that MEF2C and KLF2 are exclusively induced
by laminar flow and not by disturbed flow (37,42).
Based on the present findings it was speculated that during
perfusion, the perfusion flow is constant, but the vessels of the
liver are flexible. At the beginning of perfusion, intrahepatic
resistance may be relatively high due to thrombi, and the perfusion
flow may not effectively eliminate toxic metabolites and provide
adequate nutrients to the liver, thus inducing MEF2C and KLF2
expression. Therefore, sufficient perfusion times are required, and
this requires further investigation. The present study indicated
that the expression levels of MEF2C and KLF2 peaked after 3 h of
HMP and then gradually reduced with increased perfusion duration.
In addition, the levels of NF-κB p65, IL-1β, and TNF-α were lowest
at 3 h compared with the other timepoints, which was consistent
with the morphological alterations that were observed. A potential
explanation for the aforementioned results is that 3 h of HMP is
sufficient to achieve a steady laminar flow, allowing effective
liver perfusion and significant induction of MEF2C and KLF2
expression. Nevertheless, no matter how effective HMP may be, it
can only delay organ damage caused by cold ischemia (35). With prolonged perfusion times,
livers experienced edema, and because hepatic sinuses were not
elastic, the intrahepatic resistance increased and the perfusion
flow in liver sinuses decreased, which resulted in decreased MEF2C
and KLF2 expression. Therefore, the results indicated that the
optimal perfusion time for HMP to effectively inhibit inflammation
was 3 h.
Based on the aforementioned results, the IPRL system
was used to imitate transplantation and evaluate the protective
effects of HMP. A significant reduction in ALT, AST and LDH levels
was observed in HMP-perfused livers compared with CS-perfused
livers at different reperfusion time points. Moreover, liver oxygen
consumption was significantly higher in the HMP group compared with
the CS groups. Bile production was also higher in the HMP group
compared with the CS group, but the difference was not
statistically significant, possibly because the liver was only
perfused via the portal vein and not via the hepatic artery. The
hepatic artery is the only source of blood supply to the bile duct;
therefore, the biliary tract may not have received sufficient
oxygen supply in the present study (45). To elucidate the mechanisms
underlying the protective effects of HMP, the expression levels of
NF-κB and eNOS/NO signaling pathway-related proteins were measured.
The NF-κB signaling pathway serves an important role during the
inflammatory response (16,40),
and eNOS is the most important NOS isoform that constitutively
produces NO under physiological conditions (23). In ECs, the homeostasis of eNOS/NO
displays an important role in oxidative stress and the process IRI
(25,46). The results of the present study
indicated that HMP alleviated the inflammatory response and
oxidative stress injury during IRI by inducing KLF2 expression,
which inhibited NF-κB signaling and activated eNOS/NO
signaling.
The present study had a number of limitations. The
optimal perfusion time for DCD livers was determined by focusing
only on KLF2 levels rather than the total gene expression profile.
In addition, the ex vivo perfusion model used in the present
study only allowed for a short observation period after
reperfusion; therefore, long-term effects, including those of
transplantation, were not assessed and require further
investigation. Finally, the results were obtained using an ex
vivo model that was perfused via the portal vein, so the
effects of perfusion via the hepatic artery or both blood vessels
were not examined.
The present study further suggested that HMP was
more effective at preserving DCD livers compared with CS. The
results indicated that the optimal perfusion time for HMP was 3 h,
and that shorter or longer perfusion times did not achieve the
desired perfusion effect. In addition, the protective effects of
HMP were primarily exerted via attenuation of the
KLF2/NF-κB/eNOS-dependent inflammatory response and oxidative
stress.
Acknowledgements
The authors would like to thank the doctors and
students of Zhongnan Hospital of Wuhan University for their help in
this research, including Professor Guizhu Peng, Vice-Professor
Shaojun Ye, Vice-Professor Dawei Zhou, Dr Zhiping Xia, Mrs. Ling
Li, Mrs. Xin Zhou, Miss Ruizeng Luo, Miss Bingru Zhao, Mr. Juntao
Liang, Mr. Huikai Zhang and Mr. Wenhao Su.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81570079) and the
National Natural Science Foundation of China-Xinjiang joint fund
(grant no. U1403222).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH, YW and QY designed the study, performed the
experiments, analyzed the data and wrote the manuscript. WW and CZ
performed the experiments, contributed to the design of the study
and helped to write the manuscript. XH, CZ and ZZ contributed to
the establishment of the liver perfusion system. WH and ZL
established the rat model of DCD. WW and ZZ provided guidance and
revised the manuscript. YW and QY also provided overall guidance.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Wuhan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neuberger J: An update on liver
transplantation: A critical review. J Autoimmun. 66:51–59. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dar WA, Sullivan E, Bynon JS, Eltzschig H
and Ju C: Ischaemia reperfusion injury in liver transplantation:
Cellular and molecular mechanisms. Liver Int. 2019.39(5): 788–801.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion - from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhai Y, Petrowsky H, Hong JC, Busuttil RW
and Kupiec-Weglinski JW: Ischaemia-reperfusion injury in liver
transplantation - from bench to bedside. Nat Rev Gastroenterol
Hepatol. 10:79–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dengu F, Abbas SH, Ebeling G and Nasralla
D: Normothermic machine perfusion (NMP) of the liver as a platform
for therapeutic interventions during ex-vivo liver preservation: A
Review. J Clin Med. 9:E10462020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xue S, He W, Zeng X, Tang Z, Feng S, Zhong
Z, Xiong Y, Wang Y and Ye Q: Hypothermic machine perfusion
attenuates ischemia/reperfusion injury against rat livers donated
after cardiac death by activating the Keap1/Nrf2 ARE signaling
pathway. Mol Med Rep. 18:815–826. 2018.PubMed/NCBI
|
|
7
|
Guarrera JV, Henry SD, Samstein B, Reznik
E, Musat C, Lukose TI, Ratner LE, Brown RS Jr, Kato T and Emond JC:
Hypothermic machine preservation facilitates successful
transplantation of ‘orphan’ extended criteria donor livers. Am J
Transplant. 15:161–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guarrera JV, Henry SD, Samstein B,
Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, Ratner LE, Renz JF,
Lee HT, Brown RS Jr, et al: Hypothermic machine preservation in
human liver transplantation: The first clinical series. Am J
Transplant. 10:372–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monbaliu D, Liu Q, Libbrecht L, De Vos R,
Vekemans K, Debbaut C, Detry O, Roskams T, van Pelt J and Pirenne
J: Preserving the morphology and evaluating the quality of liver
grafts by hypothermic machine perfusion: A proof-of-concept study
using discarded human livers. Liver Transpl. 18:1495–1507. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng C, Hu X, He W, Wang Y, Li L, Xiong Y
and Ye Q: Hypothermic machine perfusion ameliorates inflammation
during ischemia reperfusion injury via sirtuin 1 mediated
deacetylation of nuclear factor κB p65 in rat livers donated after
circulatory death. Mol Med Rep. 16:8649–8656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lüer B, Fox M, Efferz P and Minor T:
Adding pulsatile vascular stimulation to venous systemic oxygen
persufflation of liver grafts. Artif Organs. 38:404–410. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doddaballapur A, Michalik KM, Manavski Y,
Lucas T, Houtkooper RH, You X, Chen W, Zeiher AM, Potente M,
Dimmeler S, et al: Laminar shear stress inhibits endothelial cell
metabolism via KLF2-mediated repression of PFKFB3. Arterioscler
Thromb Vasc Biol. 35:137–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Zhong Z, Lan J, Li M, Wang W, Yang
J, Tang C, Wang J, Ye S, Xiong Y, et al: Mechanisms of hypothermic
machine perfusion to decrease donation after cardiac death graft
inflammation: through the pathway of upregulating expression of
KLF2 and inhibiting TGF-β signaling. Artif Organs. 41:82–88. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hide D, Ortega-Ribera M, Garcia-Pagan JC,
Peralta C, Bosch J and Gracia-Sancho J: Effects of warm ischemia
and reperfusion on the liver microcirculatory phenotype of rats:
Underlying mechanisms and pharmacological therapy. Sci Rep.
6:221072016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nayak L, Lin Z and Jain MK: ‘Go with the
flow’: How Krüppel-like factor 2 regulates the vasoprotective
effects of shear stress. Antioxid Redox Signal. 15:1449–1461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marrone G, Maeso-Díaz R, García-Cardena G,
Abraldes JG, García-Pagán JC, Bosch J and Gracia-Sancho J: KLF2
exerts antifibrotic and vasoprotective effects in cirrhotic rat
livers: Behind the molecular mechanisms of statins. Gut.
64:1434–1443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fledderus JO, van Thienen JV, Boon RA,
Dekker RJ, Rohlena J, Volger OL, Bijnens AP, Daemen MJ, Kuiper J,
van Berkel TJ, et al: Prolonged shear stress and KLF2 suppress
constitutive proinflammatory transcription through inhibition of
ATF2. Blood. 109:4249–4257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boon RA, Fledderus JO, Volger OL, van
Wanrooij EJ, Pardali E, Weesie F, Kuiper J, Pannekoek H, ten Dijke
P and Horrevoets AJ: KLF2 suppresses TGF-beta signaling in
endothelium through induction of Smad7 and inhibition of AP-1.
Arterioscler Thromb Vasc Biol. 27:532–539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Das H, Kumar A, Lin Z, Patino WD, Hwang
PM, Feinberg MW, Majumder PK and Jain MK: Kruppel-like factor 2
(KLF2) regulates proinflammatory activation of monocytes. Proc Natl
Acad Sci USA. 103:6653–6658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Ha CH, Jhun BS, Wong C, Jain MK
and Jin ZG: Fluid shear stress stimulates phosphorylation-dependent
nuclear export of HDAC5 and mediates expression of KLF2 and eNOS.
Blood. 115:2971–2979. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chu H, Li H, Guan X, Yan H, Zhang X, Cui
X, Li X and Cheng M: Resveratrol protects late endothelial
progenitor cells from TNF-α-induced inflammatory damage by
upregulating Krüppel-like factor-2. Mol Med Rep. 17:5708–5715.
2018.PubMed/NCBI
|
|
23
|
Erkens R, Suvorava T, Kramer CM, Diederich
LD, Kelm M and Cortese-Krott MM: Modulation of Local and Systemic
Heterocellular Communication by Mechanical Forces: A Role of
Endothelial Nitric Oxide Synthase. Antioxid Redox Signal.
26:917–935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fleming I: Molecular mechanisms underlying
the activation of eNOS. Pflugers Arch. 459:793–806. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Z, Zhang X, Xiao Q, Ye S, Lai CH, Luo
J, Huang X, Wang W, Zeng C, Zhong Z, et al: Pretreatment Donors
after Circulatory Death with Simvastatin Alleviates Liver Ischemia
Reperfusion Injury through a KLF2-Dependent Mechanism in Rat. Oxid
Med Cell Longev. 2017:38619142017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. (8th).
National Academies Press (US). (Washington, DC). 2011.
|
|
27
|
Schlegel A, Kron P, Graf R, Dutkowski P
and Clavien PA: Warm vs. cold perfusion techniques to rescue rodent
liver grafts. J Hepatol. 61:1267–1275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kamada N and Calne RY: A surgical
experience with five hundred thirty liver transplants in the rat.
Surgery. 93:64–69. 1983.PubMed/NCBI
|
|
29
|
Minor T, Manekeller S, Sioutis M and
Dombrowski F: Endoplasmic and vascular surface activation during
organ preservation: Refining upon the benefits of machine
perfusion. Am J Transplant. 6:1355–1366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pizarro MD, Rodriguez JV, Mamprin ME,
Fuller BJ, Mann BE, Motterlini R and Guibert EE: Protective effects
of a carbon monoxide-releasing molecule (CORM-3) during hepatic
cold preservation. Cryobiology. 58:248–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Balaban CL, Rodriguez JV and Guibert EE:
Delivery of the bioactive gas hydrogen sulfide during cold
preservation of rat liver: Effects on hepatic function in an ex
vivo model. Artif Organs. 35:508–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suzuki S, Toledo-Pereyra LH, Rodriguez FJ
and Cejalvo D: Neutrophil infiltration as an important factor in
liver ischemia and reperfusion injury. Modulating effects of FK506
and cyclosporine. Transplantation. 55:1265–1272. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jha P and Das H: KLF2 in regulation of
NF-κB-mediated immune cell function and inflammation. Int J Mol
Sci. 18:E23832017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weissenbacher A, Vrakas G, Nasralla D and
Ceresa CDL: The future of organ perfusion and re-conditioning.
Transpl Int. 32:586–597. 2019.PubMed/NCBI
|
|
35
|
Schlegel A and Dutkowski P: Role of
hypothermic machine perfusion in liver transplantation. Transpl
Int. 28:677–689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Op den Dries S, Sutton ME, Karimian N, de
Boer MT, Wiersema-Buist J, Gouw AS, Leuvenink HG, Lisman T and
Porte RJ: Hypothermic oxygenated machine perfusion prevents
arteriolonecrosis of the peribiliary plexus in pig livers donated
after circulatory death. PLoS One. 9:e885212014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Niu N, Xu S, Xu Y, Little PJ and Jin ZG:
Targeting Mechanosensitive Transcription Factors in
Atherosclerosis. Trends Pharmacol Sci. 40:253–266. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McKinsey TA, Zhang CL and Olson EN: MEF2:
A calcium-dependent regulator of cell division, differentiation and
death. Trends Biochem Sci. 27:40–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Potthoff MJ and Olson EN: MEF2: A central
regulator of diverse developmental programs. Development.
134:4131–4140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu Z, Yoshida T, Wu L, Maiti D, Cebotaru L
and Duh EJ: Transcription factor MEF2C suppresses endothelial cell
inflammation via regulation of NF-κB and KLF2. J Cell Physiol.
230:1310–1320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu YW, Lowery AM, Sun LY, Singer HA, Dai
G, Adam AP, Vincent PA and Schwarz JJ: Endothelial Myocyte Enhancer
Factor 2c Inhibits Migration of Smooth Muscle Cells Through
Fenestrations in the Internal Elastic Lamina. Arterioscler Thromb
Vasc Biol. 37:1380–1390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Parmar KM, Nambudiri V, Dai G, Larman HB,
Gimbrone MA Jr and García-Cardeña G: Statins exert endothelial
atheroprotective effects via the KLF2 transcription factor. J Biol
Chem. 280:26714–26719. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Z, Nickkholgh A, Yi X, Bruns H, Gross
ML, Hoffmann K, Mohr E, Zorn M, Büchler MW and Schemmer P:
Melatonin protects kidney grafts from ischemia/reperfusion injury
through inhibition of NF-kB and apoptosis after experimental kidney
transplantation. J Pineal Res. 46:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang J, Xia J, Zhang Y, Xiao F, Wang J,
Gao H, Liu Y, Rong S, Yao Y, Xu G, et al: HMGB1-TLR4 signaling
participates in renal ischemia reperfusion injury and could be
attenuated by dexamethasone-mediated inhibition of the ERK/NF-κB
pathway. Am J Transl Res. 8:4054–4067. 2016.PubMed/NCBI
|
|
45
|
Carnevale ME, Balaban CL, Guibert EE,
Bottai H and Rodriguez JV: Hypothermic machine perfusion versus
cold storage in the rescuing of livers from non-heart-beating donor
rats. Artif Organs. 37:985–991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ajamieh H, Farrell GC, McCuskey RS, Yu J,
Chu E, Wong HJ, Lam W and Teoh NC: Acute atorvastatin is
hepatoprotective against ischaemia-reperfusion injury in mice by
modulating eNOS and microparticle formation. Liver Int.
35:2174–2186. 2015. View Article : Google Scholar : PubMed/NCBI
|