Introduction
Non-healing bone defects due to trauma, infection
and tumor ablation restrict the development of oral implant
restoration (1). Therefore,
identifying ways to accelerate bone integration has become an
urgent requirement. Due to their strong osteogenesis, ease of
culture and availability, MC3T3-E1 and MDPC23 cells are considered
good candidates for alveolar bone regeneration. Osteogenesis is a
complex process that is strictly regulated by numerous pathways
(2). The molecular mechanism of
osteogenesis and mineralization remains unclear.
Circular RNAs (circRNAs) are a new class of
non-coding RNAs that serve important roles in multiple biological
processes (3,4). Due to advances in science and
technology, particularly in bioinformatics and RNA sequencing
technologies, it has been determined that numerous exon transcripts
can be used to form circRNAs by nonlinear reverse splicing or gene
rearrangement. circRNA molecules that exhibit a closed ring
structure are thought to serve an important role in bone metabolism
(5,6). For example, osteogenesis can be
enhanced by mm9_circ_009056, which upregulates bone morphogenetic
protein 7 by targeting microRNA (miR/miRNA)-22-3p (7). Additionally, the circRNA CDR1-AS can
activate Smad1/5/8 and p38 mitogen associated protein kinase
pathways to promote osteogenesis of periodontal ligament stem cells
by inhibiting miR-7 expression (8). Therefore, the present study attempted
to explain the molecular mechanism of osteogenesis and
mineralization via circRNAs.
The authors' previous study found that
mmu_circ_003795 was significantly upregulated in bone
marrow-derived stem cells (BMSCs) undergoing cell proliferation,
demonstrating that mmu_circ_003795 may serve an important role in
the proliferation of BMSCs (9).
The present study further investigated the function of
mmu_circ_003795 in the osteogenesis of MC3T3-E1 and MDPC23
cells.
Materials and methods
Cell culture
MC3T3-E1 (American Type Culture Collection) and
MDPC23 (Cellcook) cells were plated on 6-well plate culture dishes
at density of 1×105 cells/well. In addition, 2 ml
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) was added to each well. Cells were
maintained in a humidified atmosphere of 95% air and 5%
CO2 at 37°C.
Transfection of small interfering RNAs
(siRNAs), mimics and inhibitors
The siRNAs, mimics and inhibitors were designed and
synthesized by Guangzhou RiboBio Co., Ltd. (siRNA: Sense:
5′-GCUAGAACAGCAUGGUCCAdTdT-3′, antisense:
3′-dTdTCGAUCUUGUCGUACCAGGU-5′; inhibitors:
5′-UCCUCCCUCCCCUACCCGGUUCAAG-3′; mimic sense:
5′-AGGAGGGAGGGGAUGGGCCAAGUUC-3′, antisense:
5′-UCCUCCCUCCCCUACCCGGUUCAAG-3′) (Table I). MC3T3-E1 and MDPC23 cells were
seeded in 6-well plates at a density of 1×105/well. The
cells were transfected with 20 nM siRNA mimics or inhibitors using
GenMute™ siRNA Transfection kit and Transfection Buffer (SignaGen
Laboratories) according to the manufacturer's protocol. To ensure a
high silencing efficiency, the transfection medium with siRNAs was
changed every 72 h. Experiments were performed 24–72 h post
transfection.
 | Table I.The primers of the detected
genes. |
Table I.
The primers of the detected
genes.
| Name | Primer |
|---|
| COL15A1 | F:
5′-CTCGCGGGTTACATAAGGCT-3′ |
|
| R:
5′-GTAGAGGATAACCCGCTGGC-3′ |
| ALP | F:
5′-GAGGCATACGCCATCACATG-3′ |
|
| R:
5′-CCGATGGCACACCTGCTT-3′ |
| OCN | F:
5′-TCTGACAAAGCCTTCATGTCCA-3′ |
|
| R:
5′-AACGGTGGTGCCATAGAT-3′ |
| GAPDH | F:
5′-AAGAAGGTGGTGAAGCAGG-3′ |
|
| R:
5′-GAAGGTGGAAGAGTGGGAG-3′ |
| OSX | F:
5′-GCAAATGACTACCCACCCTT-3′ |
|
| R:
5′-ACGAGCCATAGGGATGAGTC-3′ |
| RUNX2 | F:
5′-ACTTGTGGCTGTTGTGATG-3′ |
|
| R:
5′-TTGCTGTTGCTGTTGTTG-3′ |
|
mmu_circRNA_003795 | F:
5′-CCTTAGCACCTGCCTTCTTG-3′ |
|
| R:
5′-AGTTCACCCACTGTGCCTCC-3′ |
|
mmu-miR-1249-5p |
5′-AGGAGGGAGGGGATGGGCCAAGTTC-3′ |
| U6 |
5′-GCGCGTCGTGAAGCGTTC-3′ |
|
Inhibitor-miR-1249-5p |
5′-UCCUCCCUCCCCUACCCGGUUCAAG-3′ |
|
Mimic-miR-1249-5p | F:
5′-AGGAGGGAGGGGAUGGGCCAAGUUC-3′ |
|
| R:
5′-UCCUCCCUCCCCUACCCGGUUCAAG-3′ |
Microarray hybridization
The MDPC23 cells were divided into two groups and
treated as before. In the subject group, 1×105 cells
were treated with the induction medium containing 20 nM siRNA of
mmu_circ_003795 for 7 days. In the control groups, the cells
(1×105) were treated with the induction medium
containing 20 nM negative control siRNA for 7 days. The sequences
of siRNA were as follows: (Sense 5′-GCUAGAACAGCAUGGUCCAdTdT-3′ and
antisense 3′-dTdTCGAUCUUGUCGUACCAGGU-5′). These two groups were
used to detect the expression of mRNAs and miRNAs following
silencing of mmu_circRNA_003795. Total RNA from each sample was
quantified using the NanoDrop ND-2000 (Thermo Fisher Scientific,
Inc.). The sample preparation and microarray hybridization were
performed based on the standard protocols of the BGI group
(10).
Detection of alkaline phosphatase
(ALP) activity
Cells were seeded into 48-well plates at a density
of 5×103 cells/well. The MDPC23 and MC3T3-E1 cells were
divided into two groups: The control group and the subject group.
In the subject group, the cells (5×103) were treated
with the induction medium containing 20 nm siRNA of mmu_circ_003795
for 14 days. In the control group, the cells (5×103)
were treated with the induction medium containing 20 nM negative
control siRNA for 14 days. The medium was changed every 3 days. An
Alkaline Phosphatase Staining Kit (BestBio, Inc.) was used to
detect the ALP activity, according to the manufacturer's
protocol.
Alizarin Red-S (ARS) staining
Mineralization in MC3T3-E1 and MDPC23 cells was
measured by staining with ARS. Cells were seeded in 48-well plates
at a density of 5×103 cells/well. As previously the
MDPC23 and MC3T3-E1 cells were divided into two groups. In the
subject group, the cells were treated with the induction medium
containing 20 nm siRNA of mmu_circ_003795. In the control group,
the cells were treated with the induction medium containing 20 nM
negative control siRNA. The medium was changed every 3 days.
Following culture for 21 days, cells were fixed with 4%
paraformaldehyde for 10 min after washing three times with PBS at
room temperature. Subsequently, the ARS solution (BestBio, Inc.)
was used to stain the cells at 37°C for 30 min. The stained cells
were observed by a stereo light microscope (Leica Microsystems
GmbH). For quantification analysis, 10% hexadecyl pyridinium
chloride monohydrate (CPC; Sigma-Aldrich; Merck KGaA) was used to
dissolve the mineralized nodules and then the absorbance was
measured at 562 nm using a Multiskan™ FC spectrophotometer (Thermo
Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and nucleic acid
electrophoresis
The total RNA of BMSCs was extracted using TRIzol
reagent (Thermo Fisher Scientific, Inc.). The quantity and quality
of RNA were determined using a NanoDrop 2000. Following RNA
extraction, SuperScript III reverse transcriptase (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to synthesize
complementary DNA (cDNA), according to the manufacturer's protocol.
In the reverse transcription reaction the temperature protocol was
as follows: 65°C for 5 min and 4°C for 1 min in the first step,
followed by 37°C for 1 min, 50°C for 60 min and 70°C for 15 min in
the second step. Subsequently, qPCR was performed using 12.5 µl
SYBR Premix EX Taq (Takara Bio, Inc.), 0.5 µl forward primer (10
pmol/l), 0.5 µl reverse primer (10 pmol/l;), 1 µl cDNA and
ddH2O to create a final volume of 25 µl. The primers
used are listed in Table I. The
reaction conditions were as follows: 95°C for 30 sec; and 95°C for
5 sec, 55°C for 30 sec and 72°C for 30 sec for 40 cycles. The
instrument automatically generated the cycle quantification (Cq)
value of mRNA and the internal reference gene GAPDH. Cq value
difference (ΔCq) indicated the relative expression of each mRNA
investigated (11).
Identification of differentially
expressed mRNAs
Quantile normalization of the original data and
subsequent data processing was performed using the R software
package (version 3.1.2) (12).
After quantile normalization of the original data was performed,
low-intensity filtering was carried out and the mRNAs with P
(present) or M (marginal) (‘all target values’) tagged in at least
one or two samples were retained for further analysis. When
comparing the two sets of contours, the ‘fold-change’ (namely, the
ratio of the group mean) between groups of mRNAs was calculated.
The statistical significance of the differences between groups was
determined using a t-test. mRNAs exhibiting a significant
difference in expression exhibited a fold-change >2 and
P<0.05. The sorting and filtering function of Microsoft Excel
2019 (Microsoft Corporation) was used to filter data, analyze
output and sort the mRNAs with differential expression according to
the fold-changes and P-values. In addition, gene ontology (GO)
analysis was performed to classify the related mRNAs (13). miRDB (http://www.mirdb.org/), TargetScan (http://www.targetscan.org/) and mirbases (http://www.mirbase.org/) were used to determine the
corresponding target genes. All differentially expressed miRNAs
were annotated in detail with information on the interaction
between mmu_circ_003795 and miRNAs. Cytoscape (version 3.6.0;
http://cytoscape.org) software was used to
generate a network map.
Western blotting
Radioimmunoprecipitation assay buffer [50 mM Tris
HCl (pH 7.4), 150 mM NaCl, 1% Nonidet P40 and 0.1% sodium dodecyl
sulfate] and phenylmethanesulfonyl fluoride were used at 4°C to
extract protein from the cells according to the standard procedure.
The concentration was determined using a bicinchoninic acid protein
assay kit (BestBio, Inc.). Total protein (10 µg/well) was separated
using 10% SDS-PAGE and then transferred to a polyvinylidene
difluoride membrane. Skimmed milk was used to block membranes for 1
h at room temperature, then the blots were incubated with primary
antibodies against OPN (1:1,000; catalog no. ab124830; Abcam),
COL15A1 (1:1,000; catalog no. PA5-69518; Thermo Fisher Scientific,
Inc.) and β-actin (1:5,000; catalog no. ab8226; Abcam) overnight at
4°C. Following washing with 0.1% Tween PBS-T, the blots were
incubated with goat anti-mouse horseradish peroxidase-conjugated
secondary antibody (1:5,000; catalog no. ab6799; Abcam) for 1 h at
room temperature. An enhanced chemiluminescent (ECL) kit (Beijing
Dingguo Changsheng Biotechnology Co., Ltd.) was used to detect the
proteins. ImageJ (version k1.45; National Institutes of Health) was
used to measure densitometry.
Statistical analysis
All data were analyzed using the SPSS 11.5 software
package (SPSS, Inc.). The data were expressed as the mean ±
standard deviation and the comparison of the data between groups
was performed using paired t-test or two-way analysis of variance
according to the nature of the data. Each experiment was repeated
three times. The data of three groups were evaluated using analysis
of variance and followed by Fisher's least significant difference
post hoc test when the variance was normal, otherwise Dunnett's T3
test was used. P<0.05 was considered to indicate a statistically
significant difference.
Results
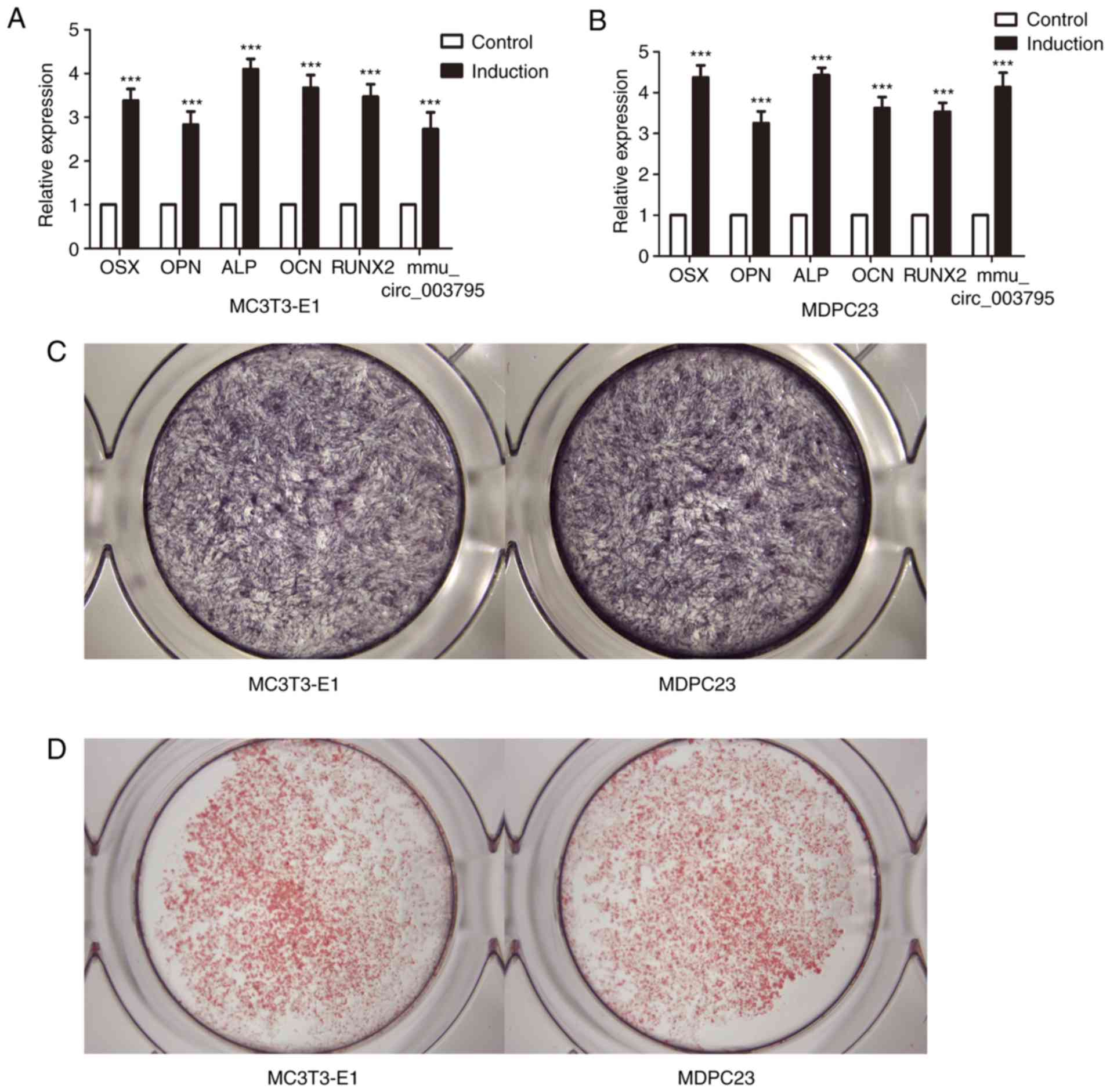
mmu_circ_003795 is upregulated during
osteoblast differentiation of MC3T3-E1 and MDPC23 cells
MC3T3-E1 and MDPC23 cells were cultured with
induction medium for 3 days. RT-qPCR was used to analyze the
expression levels of mmu_circRNA_003795 and the osteogenic marker
OPN, ALP, Sp7 transcription factor (OSX), osteocalcin (OCN) and
runt-related transcription factor 2 (RUNX2). The results
demonstrated that mmu_circRNA_003795, OPN, ALP, OSX, OCN and RUNX2
were increased (Fig. 1A). The ALP
and ARS staining were performed to verify the successful induction
(Fig. 1B and C). An siRNA sequence
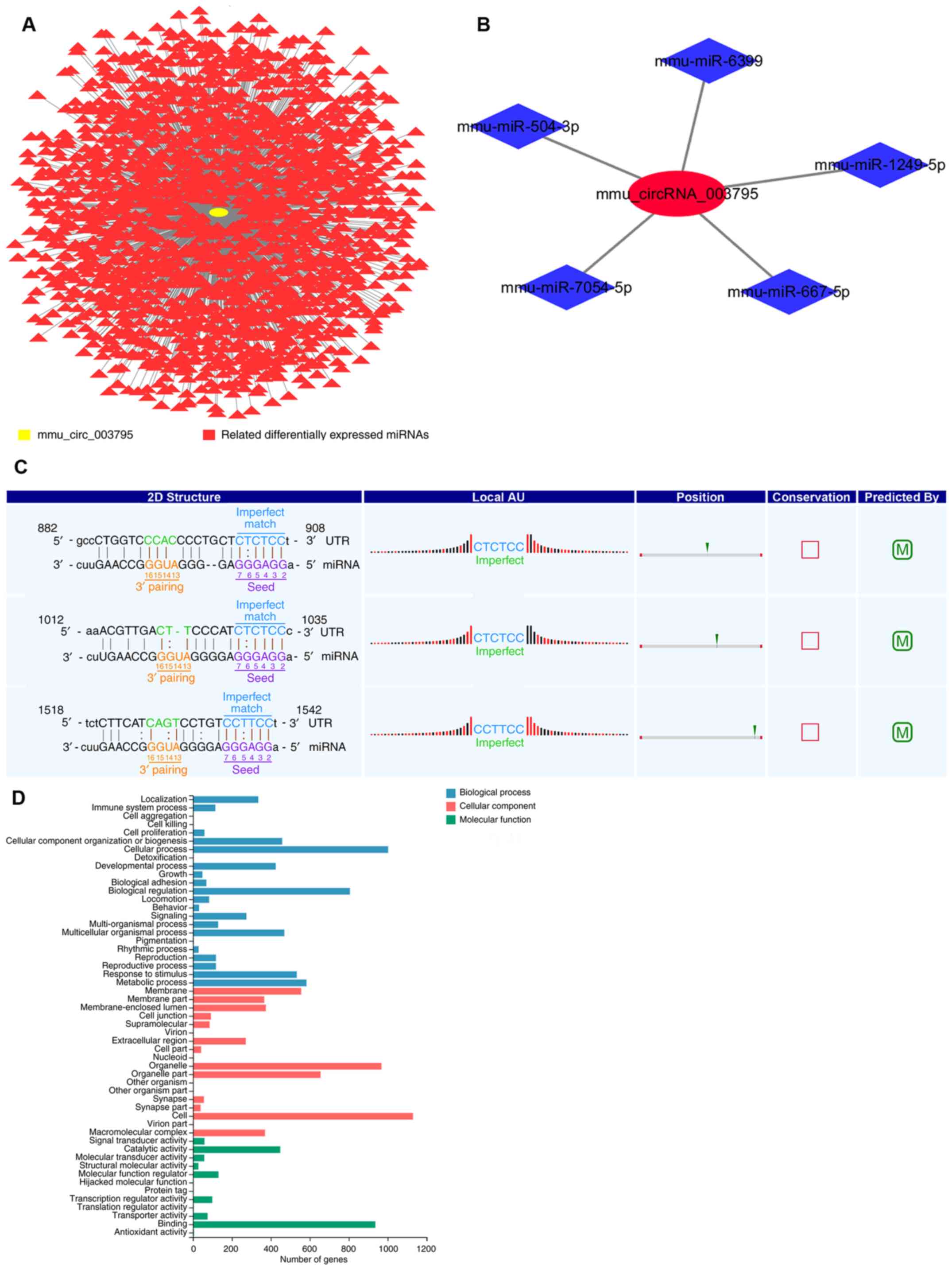
was designed to silence mmu_circRNA_003795. MDPC23 cells were
transfected with negative control RNAs or siRNAs for 7 days. In the
miRNA microarray, 1,729 miRNAs were different between the control
and subject groups (Fig. 2A). The
present study identified that mmu_circRNA_003795 is most likely
bound to mmu-miR-1249-5p, mmu-miR-504-3p, mmu-miR-6399,
mmu-miR-7054-5p and mmu-miR-667-5p (Fig. 2B). By comparing the aforementioned
data, the present study selected mmu-miR-1249-5p as the target
miRNA. The seed sequences of the circRNA were compared with miRNA
sequences to identify the miRNAs that potentially bind to circRNAs
(Fig. 2C).
Identification of differentially
expressed mRNAs
GO analysis was performed to classify the related
mRNAs. The analysis focused on ‘Biological Process’, ‘Cellular
Component’ and ‘Molecular Function’ terms that may be associated
with osteogenesis differentiation. The top GO terms were ranked by
number of genes (Fig. 2D). miRDB
(http://www.mirdb.org/), TargetScan (http://www.targetscan.org/) and mirbases (http://www.mirbase.org/) were used to determine the
corresponding target genes of miR1249-5p. The above data was
compared with the current mRNA microarray data. According to the
analysis, miR1249-5p and COL15A1 may have regulated osteoblast
differentiation and mineralization in MDPC23 cells.
Alterations in the expression of
miR1249-5p, COL15A1 and OPN following mmu_circRNA_003795
interference
siRNA was used to knockdown mmu_circRNA_003795 in
MC3T3-E1 and MDPC23 cells. Compared with the negative control
group, the expression of mmu_circRNA_003795 in the siRNA group was
decreased in both MC3T3-E1 and MDPC23 cells (Fig. 3A and B). Following successful
silencing of mmu_circRNA_003795, the expression of miR-1249-5p was
increased both in MC3T3-E1 and MDPC23 cells (Fig. 3C and D). Compared with the negative
control group, the expression of OPN was significantly decreased in
the mRNA microarray. It is known that OPN is one of the early
marker proteins involved in osteogenic differentiation (14). The present study speculates that
OPN may be involved in regulating osteoblast differentiation and
mineralization in MDPC23 cells. Therefore, the current study also
tried to verify the changes in OPN when interfering with circ003795
and miR-1249-5p. The expression levels of COL15A1 and OPN were
significantly decreased in both cell lines (Fig. 3E and F). Western blotting revealed
the same result; the western blot analysis demonstrated that
silencing of mmu_circRNA_003795 reduced the protein expression
levels of COL15A1 and OPN (Fig. 3G and
H).
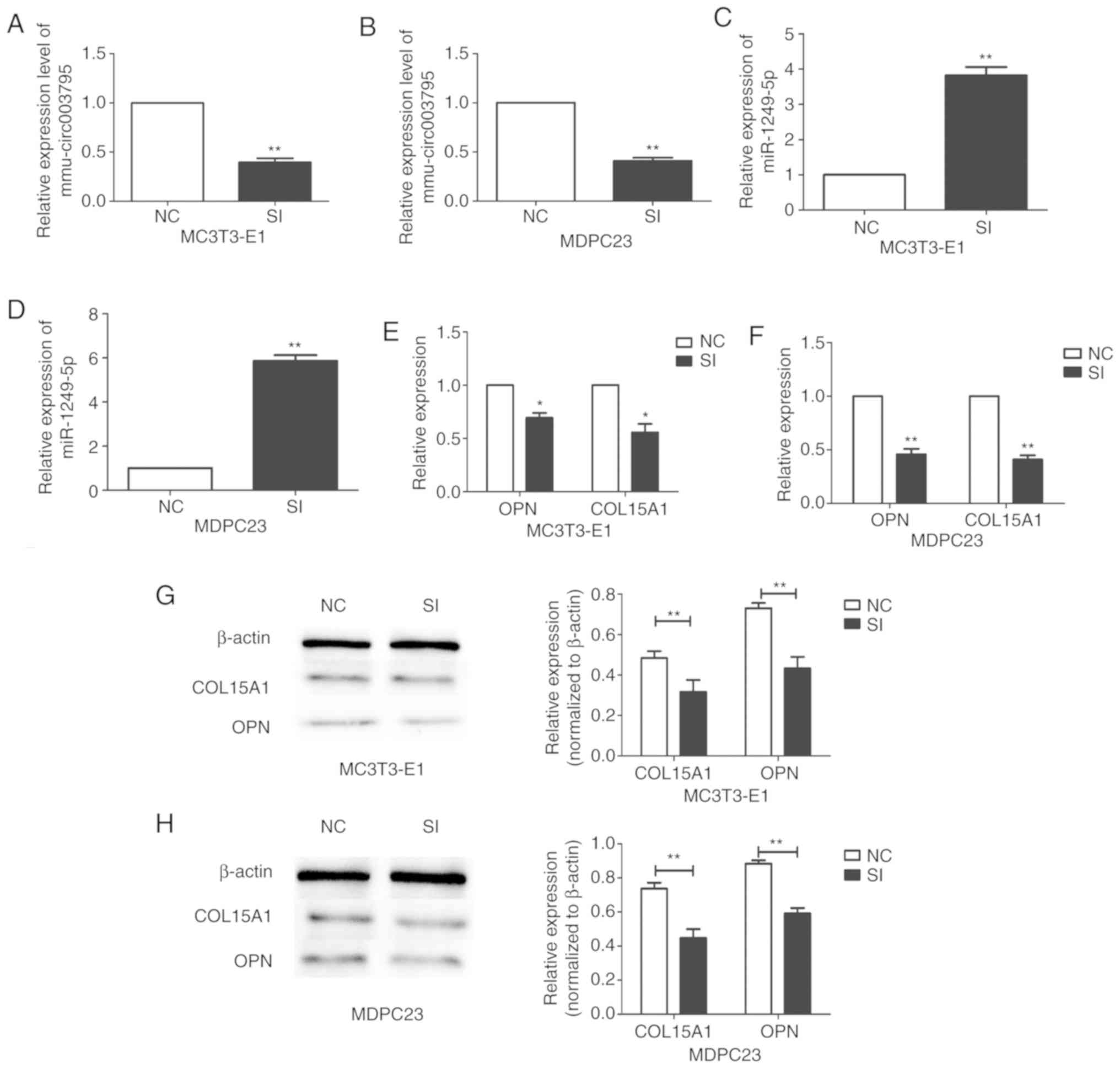 | Figure 3.Changes of miR-1249-5p, COL15A1 and
OPN expression following mmu_circRNA_003795 silencing. Expression
of mmu_circRNA_003795 in the interference and NC groups, as
analyzed by RT-qPCR in (A) MC3T3-E1 and (B) MDPC23 cells.
Successful transfection and knockdown, and increased expression of
miR-1249-5p in (C) MC3T3-E1 and (D) MDPC23 cells, and COL15A1 and
OPN also in (E) MC3T3-E1 and (F) MDPC23 cells following
mmu_circRNA_003795 silencing, as analyzed by RT-qPCR. Western
blotting analysis of COL15A1 and OPN following mmu_circRNA_003795
silencing in (G) MC3T3-E1 and (H) MDPC23 cells. *P<0.05 and
**P<0.01 vs. NC. circRNA, circular RNA; OPN, osteopontin;
RT-qPCR, reverse transcription-quantitative PCR; miR, microRNA;
COL15A1, collagen type XV α 1 chain; NC, negative control; SI,
small inhibitor. |
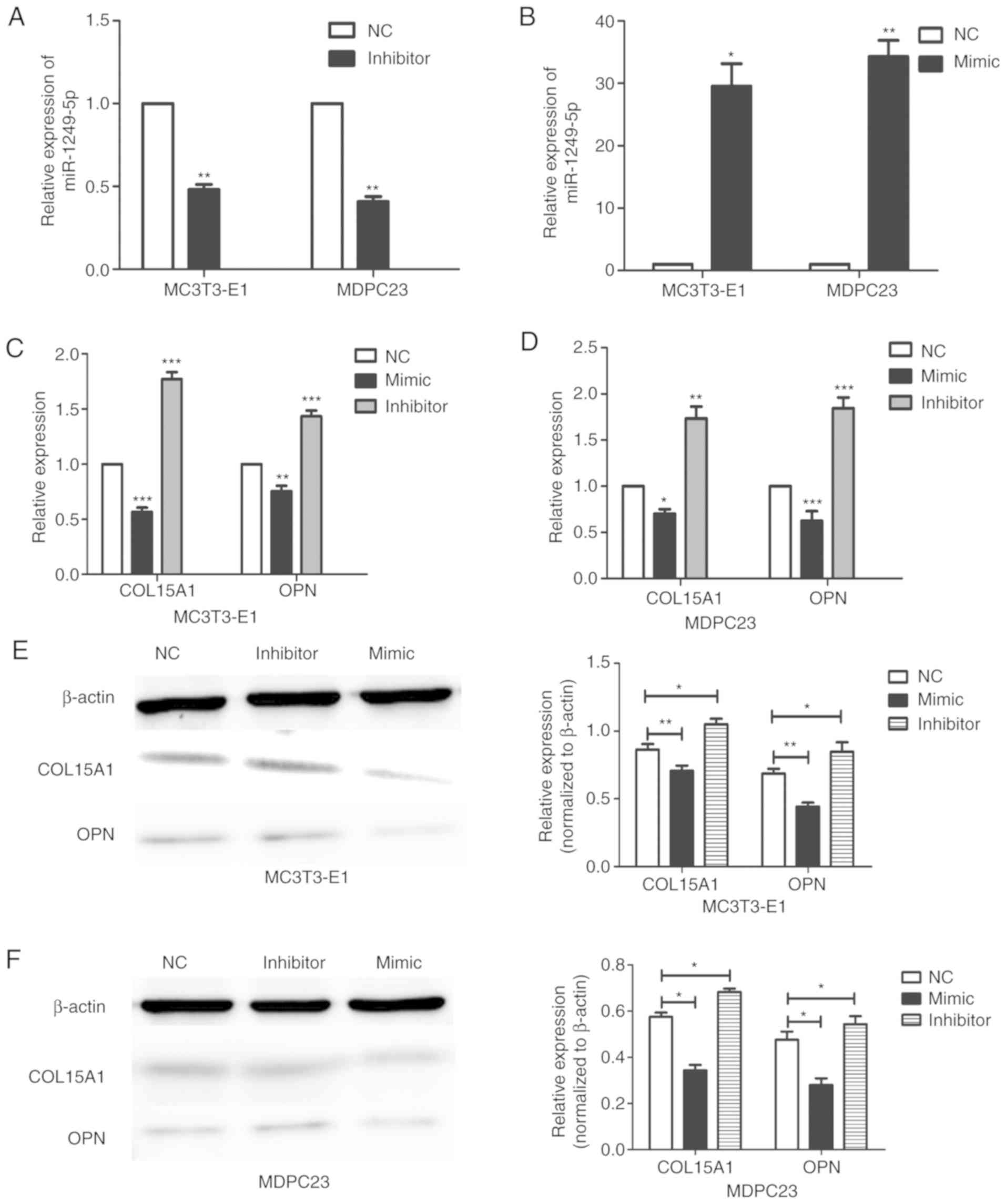
Alterations in the expression of
COL15A1 and OPN following miR1249-5p interference
An inhibitor and mimic were used to knockdown and
overexpress miR1249-5p, respectively, in MC3T3-E1 and MDPC23 cells.
Compared with the negative control group, the expression of
miR1249-5p was decreased in the inhibitor group and increased in
the mimic group in both MC3T3-E1 and MDPC23 cells (Fig. 4A and B). Following successful
knockdown of miR1249-5p, the expression levels of COL15A1 and OPN
were increased in MC3T3-E1 cells, whereas the expression levels of
COL15A1 and OPN were decreased when miR1249-5p was overexpressed
(Fig. 4C). This result was also
demonstrated in MDPC23 cells (Fig.
4D). Western blotting revealed the same result. When miR1249-5p
was upregulated or downregulated, the protein expression levels of
COL15A1 and OPN were downregulated and upregulated, respectively
(Fig. 4E and F). Compared with the
negative control group, the staining and activity of ALP in the
mmu_circRNA_003795 inhibition group was significantly reduced at 7
days (Fig. 5A). In addition, the
ARS staining at 21 days revealed the same result. Following
osteogenic induction for 21 days, the intensity of ARS staining in
the mmu_circRNA_003795 inhibition group was significantly reduced.
Compared with the negative control group, the matrix mineralization
of the mmu_circRNA_003795 inhibition group was decreased (Fig. 5B and C).
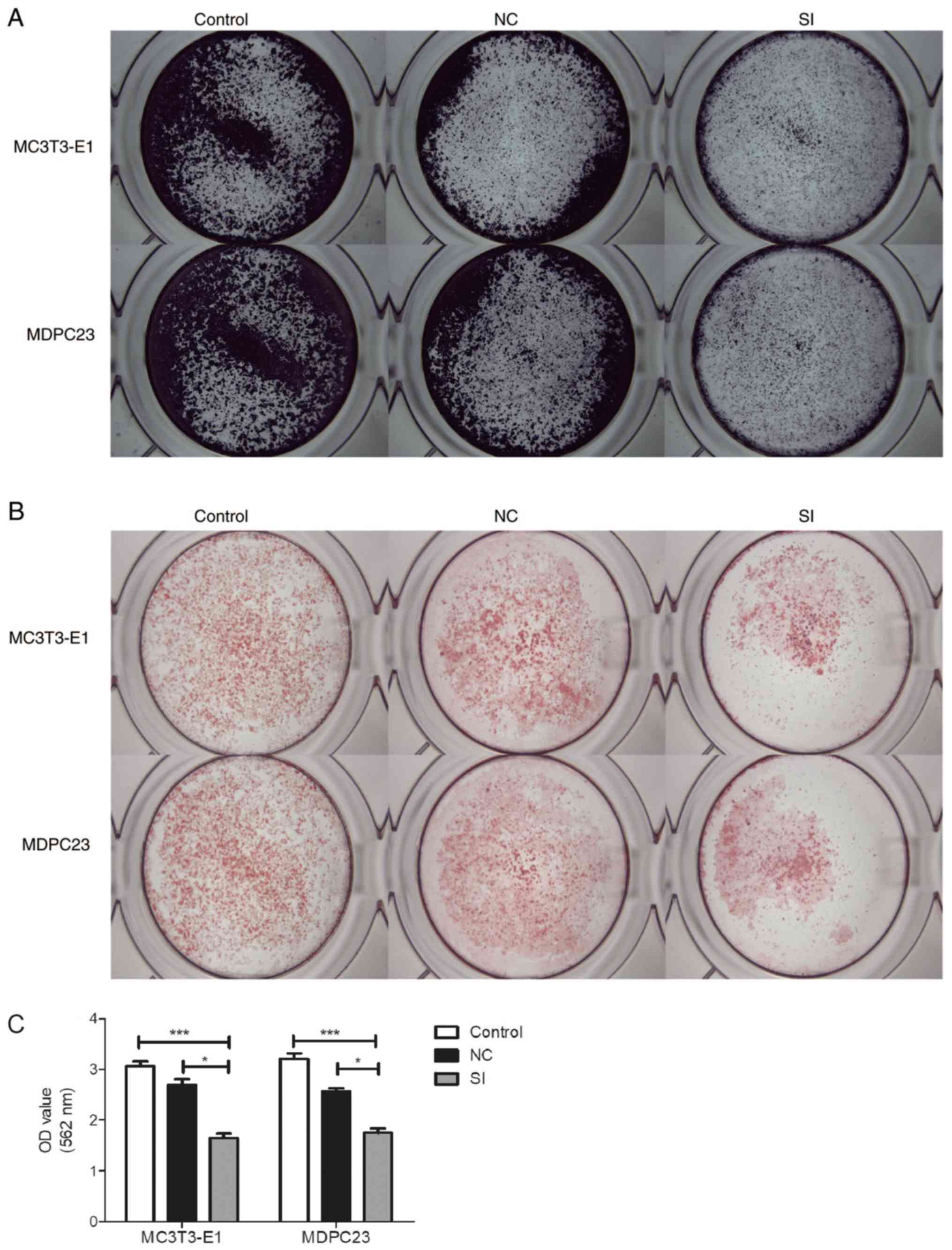 | Figure 5.Silencing of mmu_circ_003795 affects
osteogenesis of MC3T3-E1 and MDPC23 cells. (A) ALP assay of
MC3T3-E1 and MDPC23 cells 7 days after siRNA transfection. (B) ARS
assay of MC3T3-E1 and MDPC23 cells 21 days after siRNA
transfection. (C) Calcium nodules quantification assay. *P<0.05
and ***P<0.005. ARS, Alizarin Red-S; siRNA, small interfering
RNA; miR, microRNA;circRNA, circular RNA; ALP, alkaline
phosphatase, tissue nonspecific isozyme; OCN, osteocalcin; RUNX2,
runt-related transcription factor 2; OSX, Sp7 transcription factor;
COL15A1, collagen type XV α 1 chain; OPN, osteopontin; NC, negative
control. |
Discussion
circRNAs are circular RNA molecules that were first
identified in the 1970s (15).
Using electron microscopy, Hsu and Coca-Prados observed RNA could
be a cyclic form in the cytoplasm of eukaryotic cells (16). With the continuous improvement of
technology, circRNAs have become an increasingly important research
area with huge development potential. circRNAs are now understood
to exhibit structural stability, have conserved sequences and be
extensively expressed in mammals (17,18).
Additionally, circRNAs are tissue and time specific. MDPC23 cells
are mouse odontoblast cells derived from the oral dentin of mice,
while MC3T3-E1 cells are derived from the bone of mice. The tissue
specificity of the two may explain the differences in the
osteogenic mineralization ability when mmu_circRNA_003795 was
inhibited. However, this needs to be further investigated in future
experiments.
COL15A1, a novel marker in osteoblasts, is
associated with important biological functions in osteogenic
differentiation and mineralization. Lisignoli et al
(19) identified that COL15A1 is
differentially expressed between osteoblasts and MSCs that were
isolated from the same donors using high throughput technology.
Trošt et al (20) isolated
primary cultures of osteoblasts from osteoporotic and
non-osteoporotic human bone tissue samples. Using genome-wide gene
expression sequencing, this previous study found COL15A1 was
downregulated in osteoporotic bone tissue compared with
non-osteoporotic human bone tissue. However, Gabusi et al
reported that when chronically stimulated by Ca2+ at
certain concentrations, the osteogenic ability of human osteoblasts
was significantly enhanced, whereas the expression of COL15A1 was
reduced (21).
OPN is a protein widely distributed in various
tissues and cells, and it can participate in tissue repair,
metabolism and other functions. OPN is associated with a variety of
pathological processes, including cardiovascular disease, cancer,
diabetes and kidney stones. OPN is also associated with
physiological activities, such as cell viability, biomineralization
and wound healing (22–25). OPN can regulate osteoclast function
by influencing the expression levels of interleukin (IL)-10, IL-12
and IL-3 (26). Mineralized
tissues, such as tooth and bones, release OPN that is generated by
osteoclasts and osteoblasts. Additionally, OPN can enhance the
adhesion of osteoblasts, osteoclasts and bone cells (27). In the mineralized collagen matrix
during the formation of bone tissue, the adhesion of bone cells is
upregulated through concentrating OPN (26,28).
In the present study, MC3T3-E1 and MDPC23 cells were cultured in
osteogenic induction medium containing siRNA. When the
mineralization effect was tested by ALR staining after 21 days,
compared with the control group, it was identified that the
mineralized nodules in the 48-well plate were reduced, which may be
due to the siRNA inhibiting the expression of COL15A1 and OPN, and
ultimately affecting the cell adhesion and osteogenesis. Due to
their strong osteogenesis, ease of culture and availability,
MC3T3-E1 and MDPC23 cells are considered good candidates for
alveolar bone regeneration (29,30).
Therefore, it is important to understand the mechanism that
regulates the differentiation of MDPC23 and MC3T3-E1 cells.
circRNAs serve an important regulatory role in physiological
activities (31). As a result of
their abundant, stable and cell-specific expression, circRNAs are
ideal biomarkers for the diagnosis of cancer, Alzheimer's disease,
bone disease and other diseases (32–35).
However, only a few studies have investigated the role of circRNAs
during osteogenesis (36,37). Recently, the expression of circRNAs
in the MC3T3-E1 cell line during osteogenic differentiation was
studied (7). The present study
suggested that mmu_circ_003795 regulates the osteoblast
differentiation and mineralization in MC3T3-E1 and MDPC23 cells.
The current study identified the mRNAs that are associated with the
osteoblast differentiation and mineralization of MDPC23 cells.
The expression of corresponding parental genes can
be increased by circRNAs through polymerase II elongation mechanism
(17). Consequently, the present
study investigated the regulatory role of mmu_circ_003795 by
annotating the parental genes via GO analysis. The results revealed
a large number of GO terms in the cellular processes and biological
processes that were related to the osteogenic differentiation of
cells. Previous studies have often focused on signaling proteins
and osteogenic markers that play a key role in osteogenic
differentiation (38,39). For example, ALP, OCN and calcium
deposition have been largely studied (40). Whereas, only a few studies have
evaluated the expression profile of circRNAs in osteoblastic
differentiation (41,42). The present study suggested that
mmu_circ_003795 may play an important role in the differentiation
and mineralization of MC3T3-E1 and MDPC23 osteoblasts by targeting
COL15A1. The mRNA expression levels of OPN and COL15A1 were
decreased when siRNA was used to knockdown the expression of
mmu_circRNA_003795. Therefore, the silencing of mmu_circRNA_003795
expression confirmed the association between mmu_circRNA_003795,
mmu_miR_1249-5p, COL15A1 mRNA and OPN mRNA.
In conclusion, the preliminary observations of the
present study demonstrated that mmu_circRNA_003795 can regulate
osteoblast differentiation and mineralization in MC3T3-E1 and
MDPC23 cells by targeting COL15A1 and OPN.
Acknowledgements
The authors would like to thank Dr Yan Yongyong (Key
Laboratory of Oral Medicine, Guangzhou Institute of Oral Disease,
Stomatology Hospital of Guangzhou Medical University, Guangzhou,
Guangdong, P.R. China) for his great help in writing this
paper.
Funding
The present study was supported by the Science &
Technology Bureau of Guangdong Province (grant nos. 2017A050501041
and 2018B050502012) and the National Key R&D Program of China
(grant no. 2018YFB1106903).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, WR, ZZ, ZH, TL, FL, ZS, QJ, LG and XY performed
the experiments and analyzed the data. LG and ZZ designed the study
and wrote the article. All authors approved the final
submission.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bianco P, Robey PG and Simmons PJ:
Mesenchymal stem cells: Revisiting history, concepts, and assays.
Cell Stem Cell. 2:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Long T, Guo Z, Han L, Yuan X, Liu L, Jing
W, Tian W, Zheng XH, Tang W and Long J: Differential expression
profiles of circular RNAs during osteogenic differentiation of
mouse adipose-derived stromal cells. Calcif Tissue Int.
103:338–352. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng T, Yang L, Zheng Z, Li Y, Ren W, Wu C
and Guo L: Calcitonin gene-related peptide induces IL-6 expression
in RAW264.7 macrophages mediated by mmu_circRNA_007893. Mol Med
Rep. 16:9367–9374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang W, Dong R, Shu D, Du J, Fan Z and
Wang F: Differential long noncoding RNA/mRNA expression profiling
and functional network analysis during osteogenic differentiation
of human bone marrow mesenchymal stem cells. Stem Cell Res Ther.
8:302017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li P, Chen S, Chen H, Mo X, Li T, Shao Y,
Xiao B and Guo J: Using circular RNA as a novel type of biomarker
in the screening of gastric cancer. Clin Chim Acta. 444:132–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu C, Zheng Z, Ren W, Deng T, Li Y, Yang
L, Wu J, Huang Z, Li Z and Guo L: Mm9_circ_009056 enhances
osteogenesis by targeting BMP7 via CGRP-mediated miR-22-3p. Biochem
Biophys Res Commun. 501:199–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Zheng Y, Zheng Y, Huang Y, Zhang Y,
Jia L and Li W: Circular RNA CDR1as regulates osteoblastic
differentiation of periodontal ligament stem cells via the
miR-7/GDF5/SMAD and p38 MAPK signaling pathway. Stem Cell Res Ther.
9:2322018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren W, Yang L, Deng T, Wu C, Li Y, Wu J,
Huang Z, Du F and Guo L: Calcitonin gene related peptide regulates
FOSL2 expression and cell proliferation of BMSCs via
mmu_circRNA_003795. Mol Med Rep. 19:3732–3742. 2019.PubMed/NCBI
|
|
10
|
Chen K, Liu J, Liu S, Xia M, Zhang X, Han
D, Jiang Y, Wang C and Cao X: Methyltransferase SETD2-mediated
methylation of STAT1 is critical for interferon antiviral activity.
Cell. 170:492–506.e14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
R Core Team: R: A language and environment
for statistical computing. Version 3.1.2. R foundation for
statistical computing. (Vienna). 2014.PubMed/NCBI
|
|
13
|
Grossmann S, Bauer S, Robinson PN and
Vingron M: Improved detection of overrepresentation of
gene-ontology annotations with parent child analysis.
Bioinformatics. 23:3024–3031. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Standal T, Borset M and Sundan A: Role of
osteopontin in adhesion, migration, cell survival and bone
remodeling. Exp Oncol. 26:179–184. 2004.PubMed/NCBI
|
|
15
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu MT and Coca-Prados M: Electron
microscopic evidence for the circular form of RNA in the cytoplasm
of eukaryotic cells. Nature. 280:339–340. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lisignoli G, Codeluppi K, Todoerti K,
Manferdini C, Piacentini A, Zini N, Grassi F, Cattini L, Piva R,
Rizzoli V, et al: Gene array profile identifies collagen type XV as
a novel human osteoblast-secreted matrix protein. J Cell Physiol.
220:401–409. 2010. View Article : Google Scholar
|
|
20
|
Trošt Z, Trebše R, Preželj J, Komadina R,
Logar DB and Marc J: A microarray based identification of
osteoporosis-related genes in primary culture of human osteoblasts.
Bone. 46:72–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gabusi E, Manferdini C, Grassi F,
Piacentini A, Cattini L, Filardo G, Lambertini E, Piva R, Zini N,
Facchini A and Lisignoli G: Extracellular calcium chronically
induced human osteoblasts effects: Specific modulation of
osteocalcin and collagen type XV. J Cell Physiol. 227:3151–3161.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iaffaldano P, Ruggieri M, Viterbo RG,
Mastrapasqua M and Trojano M: The improvement of cognitive
functions is associated with a decrease of plasma Osteopontin
levels in Natalizumab treated relapsing multiple sclerosis. Brain
Behav Immun. 35:176–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clemente N, Raineri D, Cappellano G,
Boggio E, Favero F, Soluri MF, Dianzani C, Comi C, Dianzani U and
Chiocchetti A: Osteopontin bridging innate and adaptive immunity in
autoimmune diseases. J Immunol Res. 2016:76754372016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Standal T, Borset M and Sundan A: Role of
osteopontin in adhesion, migration, cell survival and bone
remodeling. Exp Oncol. 26:179–184. 2004.PubMed/NCBI
|
|
25
|
Coppola D, Szabo M, Boulware D, Muraca P,
Alsarraj M, Chambers AF and Yeatman TJ: Correlation of osteopontin
protein expression and pathological stage across a wide variety of
tumor histologies. Clin Cancer Res. 10:184–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nau GJ, Liaw L, Chupp GL, Berman JS, Hogan
BL and Young RA: Attenuated host resistance against Mycobacterium
bovis BCG infection in mice lacking osteopontin. Infect Immun.
67:4223–4230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reinholt FP, Hultenby K, Oldberg A and
Heinegård D: Osteopontin-a possible anchor of osteoclasts to bone.
Proc Natl Acad Sci USA. 87:4473–4475. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lenton S, Seydel T, Nylander T, Holt C,
Härtlein M, Teixeira S and Zaccai G: Dynamic footprint of
sequestration in the molecular fluctuations of osteopontin. J R Soc
Interf. 12:5062015. View Article : Google Scholar
|
|
29
|
Schwarz F, Golubovic V, Becker K and
Mihatovic I: Extracted tooth roots used for lateral alveolar ridge
augmentation: A proof-of-concept study. J Clin Periodontol.
43:345–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schwarz F, Schmucker A and Becker J:
Initial case report of an extracted tooth root used for lateral
alveolar ridge augmentation. J Clin Periodontol. 43:985–989. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Liu T, Wang X and He A: Circles
reshaping the RNA world: From waste to treasure. Mol Cancer.
16:582017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hansen TB, Kjems J and Damgaard CK:
Circular RNA and miR-7 in cancer. Cancer Res. 73:5609–5612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang F, Nazarali AJ and Ji S: Circular
RNAs as potential biomarkers for cancer diagnosis and therapy. Am J
Cancer Res. 6:1167–1176. 2016.PubMed/NCBI
|
|
34
|
Peng L, Yuan XQ and Li GC: The emerging
landscape of circular RNA ciRS-7 in cancer (Review). Oncol Rep.
33:2669–2674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Q, Zhang X, Hu X, Dai L, Fu X, Zhang J
and Ao Y: Circular RNA related to the chondrocyte ECM regulates
MMP13 expression by functioning as a MiR-136 ‘Sponge’ in human
cartilage degradation. Sci Rep. 6:225722016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng Y, Li X, Huang Y, Jia L and Li W:
The circular RNA landscape of periodontal ligament stem cells
during osteogenesis. J Periodontol. 88:906–914. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang X, Cen X, Zhang B, Liao Y, Zhu G,
Liu J and Zhao Z: Prospect of circular RNA in osteogenesis: A novel
orchestrator of signaling pathways. J Cell Physiol.
234:21450–21459. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu X and Li Z, Wan Q, Cheng X, Zhang J,
Pathak JL and Li Z: Inhibition of JAK2/STAT3 signaling suppresses
bone marrow stromal cells proliferation and osteogenic
differentiation, and impairs bone defect healing. Biol Chem.
399:1313–1323. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee SH, Park Y, Song M, Srikanth S, Kim S,
Kang MK, Gwack Y, Park NH, Kim RH and Shin KH: Orai1 mediates
osteogenic differentiation via BMP signaling pathway in bone marrow
mesenchymal stem cells. Biochem Biophys Res Commun. 473:1309–1314.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cotter EJ, Ip HS, Powderly WG and Doran
PP: Mechanism of HIV protein induced modulation of mesenchymal stem
cell osteogenic differentiation. BMC Musculoskelet Disord.
9:332008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Zheng Y, Zheng Y, Huang Y, Zhang Y,
Jia L and Li W: Circular RNA CDR1as regulates osteoblastic
differentiation of periodontal ligament stem cells via the
miR-7/GDF5/SMAD and p38 MAPK signaling pathway. Stem Cell Res Ther.
9:2322018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qian DY, Yan GB, Bai B, Chen Y, Zhang SJ,
Yao YC and Xia H: Differential circRNA expression profiles during
the BMP2-induced osteogenic differentiation of MC3T3-E1 cells.
Biomed Pharmacother. 90:492–499. 2017. View Article : Google Scholar : PubMed/NCBI
|