Introduction
In the last few decades, the incidence of
degenerative diseases, including neurodegenerative diseases, such
as Alzheimers disease, osteoporosis and osteoarthritis (OA) has
increased in Western countries (1,2)
These common pathologies are caused by intrinsic and extrinsic
factors, such as biological, environmental and lifestyle factors
(3). In addition, an increase in
life expectancy, as observed in France, United Kingdom, USA and
Netherlands (4), has led to a
higher incidence of degenerative diseases (5). Aging is often associated with
degenerative diseases caused by molecular and cellular damages
(6). Therefore, throughout ageing
impaired molecular signaling and disrupted cellular processes
induce loss of function in tissues with a consequent physical
decline (7). Nutrients that
increase longevity can counteract the detrimental consequences of
ageing (8) For example,
astaxanthin has been demonstrated to reduce oxidative stress and
apoptosis in model organisms (9,10),
and thus can be considered an anti-aging antidote. This review
highlights a possible role of astaxanthin in preventing or reducing
the negative effects of degenerative diseases and, in particular,
of pathologies related to the skeleton.
Skeletal degenerative diseases associated
with oxidative stress
Oxidative stress and inflammation serve an important
role in degenerative diseases. Reactive oxygen species (ROS)
overproduction, resulting from oxidative stress, damages various
biological molecules, including DNA, carbohydrates, proteins and
lipids, causing alterations during cellular growth and
differentiation processes, thus inducing apoptosis (7). Among degenerative diseases, bone
diseases are associated with oxidative stress (11). The bone remodeling process, where
old bone is replaced by newly formed bone, modulates redox state
changes and increases ROS production. Defective antioxidant systems
are also involved in the pathogenesis of bone loss (12). By inducing apoptosis in osteoblasts
(cells with bone forming activity), ROS impair osteogenesis and
mineralization. Increased osteoblastic apoptosis also promotes
osteoclast-induced bone resorption, with oxidative stress further
enhancing osteoclast differentiation (11,13,14).
In vitro experiments have demonstrated that
H2O2 increases the number and activation of
osteoclasts (15). In addition, as
ROS are involved in the regulation of inflammation, ROS
overproduction activates certain inflammatory factors such as tumor
necrosis factor-α (TNF-α) and lipopolysaccharide (LPS) (16). TNF-α is involved in bone
homeostasis regulation by activating different cellular pathways
such as the upregulation of DKK1 (17) or RANKL (18), the inhibition of matrix protein
genes expression as well as the stimulation of expression of genes
related in the osteoclastogenesis or by inducing the resistance to
1,25-dihydroxyvitamin D3 (1,25(OH)2D3) (19). Generally, TNF-α promotes bone
resorption by stimulating osteoclastogenesis and reduces bone
formation by inhibiting osteoblastogenesis (20).
Osteoclastogenesis and bone resorption are promoted
by ROS under physiological and pathological conditions. In
particular, ROS promote osteoclastogenesis by inducing receptor
activator of nuclear factor-κB (RANK) signaling (21,22).
Furthermore, oxidative stress affects fibronectin, an extracellular
bone matrix component, by inducing its partial degradation, thereby
impairing the proliferation and differentiation of osteoblasts
(23). Oxidative stress
involvement in the pathogenesis of osteoporosis has been
investigated in several studies. For example, oxidative stress was
demonstrated to increase bone resorption by inducing the expression
of osteoclast-stimulating cytokines such as interleukin (IL)-1,
TNF-α and IL-6 (23,24). In addition, postmenopausal females
were prone to oxidative stress damage due to reduced levels of
17β-estradiol (E2), a hormone with antioxidant activity (25).
TNF-α is associated with the pathogenesis of various
inflammatory diseases such as rheumatoid arthritis and ankylosing
spondylitis. Rheumatoid arthritis is characterized by inflammatory
processes that impair cartilage and bone, through TNF-α and IL-1
(26). TNF-α production in
rheumatoid arthritis promotes pro-inflammatory cytokines such as
IL-1 and IL-17, thereby increasing osteoclast activity and
promoting bone resorption (27).
TNF-α also increases the production of matrix metalloproteinases
(MMPs) and aggrecanases which degrade cartilage extracellular
matrix (20). Ankylosing
spondylitis is a chronic form of arthritis characterized by
inflammation and impairment of new bone formation (28). In particular, in ankylosing
spondylitis and spondyloarthritis disorders, bone resorption and
bone formation processes occur simultaneously in proximal
anatomical sites (29). Treatment
of patients with TNF inhibitors reduces disease symptoms, albeit
without recovering bone remodeling ability (30,31).
Astaxanthin: Structure and properties
Astaxanthin (3,3-dihydroxy-β,β-carotene-4,4-dione)
is a ketocarotenoid pigment belonging to the family of carotenoid
oxygenated derivatives, known as xanthophylls (32). Astaxanthin is a red pigment
commonly found in aquatic organisms such as shrimps, crabs and
Salmonidae (33) and is produced
primarily by microalgae and phytoplankton (34). Microalgae are at the base of the
food chain and are ingested by zooplankton and crustaceans, which
are the prey of certain fish (35). The pigment is absorbed by these
organisms and can accumulate in their tissue (36), giving them a distinctive
orange-pink color. Tetraterpene exerts a strong antioxidant
activity and is widely used in food, cosmetic and pharmaceutical
industries (32).
While most commercial astaxanthin, primarily
manufactured and distributed by Roche Diagnostics, is obtained from
chemical synthesis (37), the
greater demand for natural foods and the high cost of synthetic
production have stimulated research into the production of
astaxanthin from microorganisms. Consequently, this alternative
source is becoming more relevant in basic research and in
industrial applications, resulting in new companies and startups
focusing on natural astaxanthin production (38).
The microalgae Haematococcus pluvialis
(36) and the yeast
Xanthophyllomyces dendrorhous (39) are currently the most promising
sources of natural astaxanthin production, making them model
organisms for use in industry. Natural astaxanthin produced by
H. pluvialis exhibits greater antioxidant activity when
compared with synthetic preparation (40). However, the greater costs of algae
growth, production and active molecule extraction substantially
limits its distribution in the international market (41).
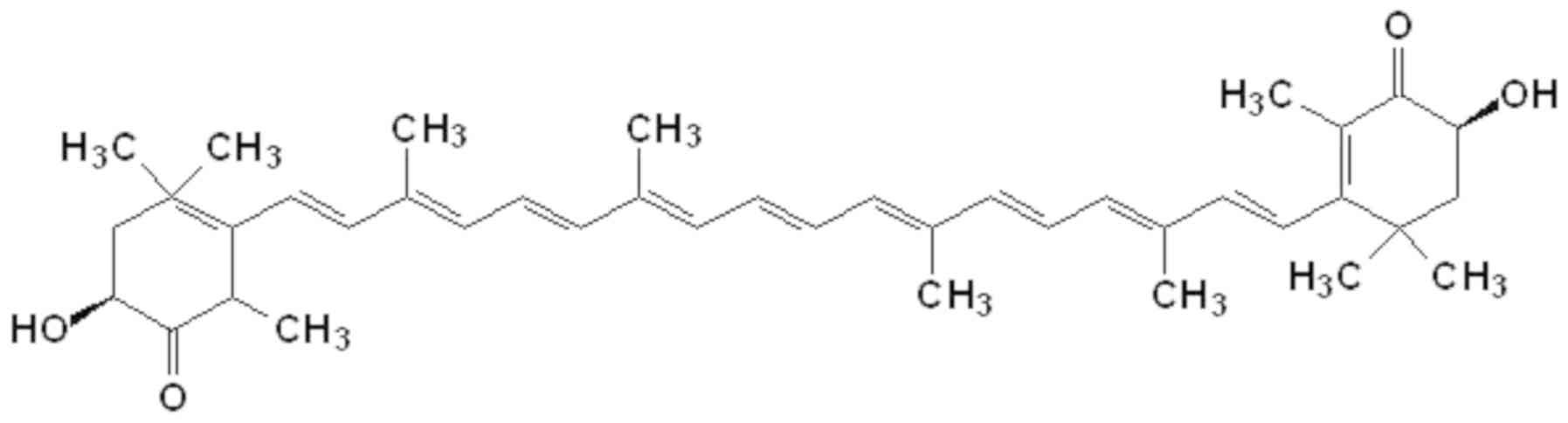
Astaxanthin is synthesized via the carotenoid
pathway, beginning with β-carotene (42), which is derived from geranylgeranyl
pyrophosphate synthesis. The central long chain formed by carbon
atoms linked by conjugated double bonds, alongside the two terminal
rings, are responsible for the chemical characteristics and light
absorption properties of astaxanthin (Fig. 1). Astaxanthin, together with other
carotenoids such as canthaxanthin, exhibits various antioxidant
activities, serving roles as free radical scavengers and potent
quenchers of ROS and reactive nitrogen species (RNS) (43). Astaxanthin possess antioxidant
activity that is 100 and ×10 greater than vitamin E and β-carotene,
respectively (44). It is
therefore known as a superantioxidant. Unlike other antioxidant
molecules, astaxanthin is free of pro-oxidative effects and
produces no toxicity at high dosages (45). In addition to its antioxidant
activity, astaxanthin exerts anti-inflammatory effects by
inhibiting the NF-κB signaling pathway (46).
Natural astaxanthin may be associated with other
compounds; for example, one or both hydroxyl groups may be
esterified with different fatty acids (oleic, palmitic and stearic
acid), forming a complex with proteins (carotenoproteins) or
lipoproteins (carotenolipoproteins) (47) Natural astaxanthin produced in algae
is always esterified, whereas its synthetic equivalent is not
(48,49).
Astaxanthin has a wide range of applications in
food, animal feeding supplementation (50) aquaculture (51) nutraceutical (52) cosmetic (53) and pharmaceutical industries
(54). Its antioxidant activity is
exploited in medicine for the prevention of or as an adjuvant in
various diseases such as cancer, dermatological disorders,
inflammation and cardiovascular damage, as well as for the general
enhancement of the immune response (38,45).
Delivery of astaxanthin
Although astaxanthin possesses a strong
anti-inflammatory activity (55),
its use in nutraceuticals and in the medical-pharmaceutical field
is limited. This is due to astaxanthin being highly unstable and
easily degradable when heated. The presence of oxygen and light are
therefore required for its production, extraction and
transformation, alongside additional technological processes such
as mechanical and chemical treatments using solvents (45,56).
The chemical structure of astaxanthin makes it insoluble in water,
which leads to difficulties in intestinal tract absorption if
administered orally. The insolubility of astaxanthin also makes its
parenteral administration difficult without solubilisers, such as
DMSO (57); however, such
solubilisers would change the chemical properties of the molecule
(58).
The production of astaxanthin derivatives as
prodrugs has been undertaken to overcome the aforementioned
difficulties (45). An alternative
approach is to encapsulate astaxanthin into particles with
biocompatible and biodegradable polymers, conferring water
solubility and a greater resistance to degradation; thus, specially
designed micro- or nano- delivery structures, such as micro- and
nano-dispersion systems, are under development (59,60).
Astaxanthin formulation into nanostructures was
initially employed to protect the active compound from thermal
degradation and other physicochemical parameters such as pH and
ionic strength (56,61). Furthermore, this innovative
approach has been used to improve yield during extraction from
microorganisms (62–64) or to achieve a more sustainable
production process (65). Another
process conferring stability to the active molecule is its
encapsulation into micro- and nano-alginate beads. This method is
mainly used to entrap astaxanthin oil derivatives and produce a
higher stability at room temperature over time, which is important
for the shelf-life of nutraceuticals and food products (66,67).
Since astaxanthin is mainly characterized by its low
water solubility, novel approaches have been developed to enhance
its bioavailability in cells using nanotechnology. The
nanoencapsulation system most commonly used to study astaxanthin in
an aqueous environment and for biomedical purposes is based on
liposomes (68–70). The liposomal system preserves
astaxanthin antioxidant activity as the nanoparticles used are
non-toxic and cellular uptake is more efficient when compared with
other delivery methods such as colloidal astaxanthin (71) or astaxanthin dissolved in dietary
oils (72). Furthermore,
astaxanthin can be coupled with other substances, such as vitamin E
derivatives, to study their synergistic effects (73,74).
Another polymer used to encapsulate astaxanthin is
chitosan, a D-glucosamine polysaccharide obtained by digestion of
the chitin shells of shrimps and other crustaceans with an alkaline
agent (75) Chitosan encapsulated
astaxanthin results in a non-toxic and biocompatible material with
antibacterial activity, which confers stability and solubility in
water, and increases bioavailability of the encapsulated active
compound (76). This biopolymer
can be used alone or in combination with other polymers, such as
proteins. For example, the chitosan and β-lactoglobulin
nanoparticle complex prolongs the release of astaxanthin and
improves its stability when subjected to the extreme pH conditions
of the gastrointestinal tract (77). Poly(lactic-co-glycolic acid) has
been approved by the Food and Drug Administration and the European
Medicines Agency together with chitosan for nanoparticle synthesis
(78,79). It is a biocompatible polymer that
degrades into lactic and glycolic acid monomers, which enter into
the Krebs cycle and are easily metabolized. The aforementioned
polymers have been used recently to prepare astaxanthin loaded
core-shell nanoparticles with good aggregation stability and
increased bioavailability of the active compound when administered
orally (60).
Role of astaxanthin in oxidative stress
Oxidative stress serves a central role in aging and
in the development of various degenerative, neurodegenerative and
inflammatory disorders, which are often the result of oxidative
stress that affects mitochondrial function, disrupting cellular
homeostasis (80).
Mitochondria regulate energy production at a
cellular level and generate high levels of ROS. ROS produced by
mitochondria are generally removed by superoxide dismutase,
catalase and glutathione peroxidase (81). However, pathological disorders can
induce an excessive production of mitochondrial ROS, the
accumulation of which impairs mitochondria and consequently leads
to cell damage (82). Damaged
mitochondria exhibit morphological fragmentation. As the
mitochondrial membrane potential is disrupted, their functionality
is reduced. In addition, oxidative stress due to the accumulation
of ROS, induces mitochondrial membrane permeabilization, leading to
the activation of the intrinsic apoptotic pathway (83).
Astaxanthin scavenges singlet status oxygen
molecules and free radicals, reducing lipid peroxidation (32). The ability to counteract excessive
oxidation, thus causing lipidic alteration, is important in
osteoporosis. It the authors previous study, increased levels of
modified lipoproteins derived from the oxidation of
arachidonate-containing phospholipids (Ox-PAPCs) were identified in
osteoporotic patient sera (84).
In addition, it was demonstrated that Ox-PAPCs impair the
osteogenic commitment of mesenchymal stem cells (MSCs) by
downregulating Runt-related transcription factor 2 and upregulating
adipogenic transcription factor peroxisome proliferator-activated
receptor gamma 2 (84).
Mutations and disrupted enzymatic activity occurring
in mitochondria are often associated with atherosclerosis (85). In particular, damaged endothelial
cells result from the overproduction of mitochondrial ROS in
atherosclerosis (86).
Antioxidants targeting mitochondria could prevent this dysfunction
to protect endothelial cells (87). Previous studies have suggested that
astaxanthin reduces oxidative stress affecting the mitochondria.
For example, the role of astaxanthin in maintaining mitochondrial
functions by modulating their redox state has been demonstrated
(88). It was also revealed that
astaxanthin maintained mitochondria in a reduced state in the
presence of H2O2 (88). Supplementation of astaxanthin to
increase ATP production and to potentiate the activity of the
mitochondrial respiratory chain has been proposed to counteract
age-associated diseases in dogs (89). Furthermore, astaxanthin can prevent
cytochrome c release due to mitochondria permeabilization,
consequently inhibiting apoptosis (81). As demonstrated in lung fibrosis,
astaxanthin can prevent H2O2-induced
apoptosis and bleomycin in alveolar epithelial cells (90). Pretreatment with astaxanthin in
cardiotoxicity models has been demonstrated to prevent
mitochondrial fragmentation, poly-ADP-ribose polymerase and caspase
activity, thus inhibiting apoptosis (91).
ROS can impair redox balance in muscle after
physical activity, inducing muscle fatigue and reducing exercise
performance (92). Upon
supplementation, astaxanthin accumulates in the liver, kidney and
muscle, where it reduces DNA and lipid peroxidation, thereby
preventing further muscle damage (93). It has been suggested that
astaxanthin, by reducing the accumulation of exercise-induced ROS,
can improve carnitine palmitoyl transferase 1 activity, increasing
fat oxidation (94,95). Astaxanthin has therefore been
proposed for use in physical activity to counteract the negative
effects of ROS production (96).
Effects of astaxanthin on bone and
cartilage
Search strategy. Studies aiming to evaluate of the
effects of astaxanthin on the skeletal system were collected from
public databases. In particular, 80 full articles were identified
by consulting the following databases: PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Science
(http://login.webofknowledge.com) and
Scopus (https://www.scopus.com) The search
strategy employed in all databases was as follows: Astaxanthin AND
bone/or mesenchymal cells/or osteoblasts/or skeletal/ or
chondrocytes/or cartilage. Duplicates were removed and an
independent screening of abstracts was performed for content
consistency. A total of 9 papers were included.
Astaxanthin and bone. In chronic metabolic diseases,
elevated levels of fatty acids impair the microenvironment of MSCs,
affecting their viability and the production of certain cytokines,
such IL-6, vascular endothelial growth factor and monocyte
chemoattractant protein-1 (97).
However, it has been demonstrated that astaxanthin supplementation
protects MSCs from fatty acid induced-cell death and inflammatory
effects (97). Antioxidant
molecules can induce osteogenic differentiation and improve bone
mineralization by counteracting oxidative stress (12,98).
Accordingly, it has been demonstrated that supplementation with
astaxanthin in a model of periodontitis reduced osteoclast activity
and increased osteoblast number in the mandible (99). It has been revealed that the
supplementation of astaxanthin improved bone quality in
ovariectomized mice, an in vivo model of osteoporosis
(100). In particular, the
authors observed reduced levels of bone resorption biomarkers, such
as serum calcium, inorganic phosphorus, alkaline phosphatase and
tartrate-resistant acid phosphatase (TRAP), and that bone mineral
density and microarchitecture were recovered after 6 weeks of
astaxanthin supplementation. In addition, by performing in
vitro experiments, the authors observed that astaxanthin
affected osteoclast activation by reducing the expression of
nuclear factor of activated T cells c1, dendritic cell-specific
transmembrane protein, TRAP and cathepsin K (101). Furthermore, a recent study
reported that in rats treated for 8 weeks with D-galactose (200
mg/kg), H. pluvialis biomass administration reduced bone
loss (101). In particular, the
authors observed that bone quality in treated rats improved via
regulation of the osteoprotegerin (OPG)/RANKL pathway. This pathway
regulates the crosstalk between osteoblasts and osteoclasts,
consequently affecting the bone remodeling process (102). The aforementioned studies suggest
the positive effects of astaxanthin in reducing bone loss. Its
supplementation may therefore represent a useful tool for
counteracting bone degenerative diseases.
Astaxanthin and cartilage. Previously published
works have suggested positive effects of astaxanthin in OA
experimental models. OA occurs due to chondrocyte dysfunction
following an inflammatory state, with an overproduction of MMPs
inducing the degeneration of articular cartilage (103). Two different studies have
reported reduced expressions of MMP-1, MMP-3 and MMP-13 in the
IL-1β stimulated chondrocytes of an in vitro experimental OA
model treated with astaxanthin (103,104). In particular, it has been
demonstrated that astaxanthin prevented MMP production by reducing
the phosphorylation of p38 and ERK1/2 activated by
mitogen-activated protein kinase (104), or by downregulating the
expression of NF-κB and activator protein-1, which are upstream
regulators of MMP production (103). The administration of astaxanthin
ameliorated cartilage status by reducing MMP production in an in
vivo OA model obtained by damaging the knee anterior cruciate
ligament in rabbits (105).
Therefore, the supplementation of astaxanthin can be considered a
beneficial treatment to counteract OA.
Conclusions
Degenerative diseases and in particular bone
diseases, cause physical disability and heavily impact public
healthcare (106). Oxidative
stress is considered an important cause of the pathogenesis of
chronic and degenerative diseases. Therefore, targeted actions
aimed at preventing or reducing the negative effects of oxidative
stress are required. The use of natural antioxidant molecules, such
as astaxanthin, may represent an effective strategy to counteract
bone degenerative diseases.
To the best of our knowledge, few studies aiming to
evaluate the use of astaxanthin in bone diseases have been
performed thus far. The results of the present review may stimulate
further investigations aimed at understanding and potentially
supporting the use of astaxanthin against bone diseases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors contributions
MTV, MP and MM conceptualized and designed the
study. MTV, MP, MGR, MM and LDC acquired and interpreted the data,
and wrote the manuscript. All the authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kopp W: How Western diet and lifestyle
drive the pandemic of obesity and civilization diseases. Diabetes
Metab Syndr Obes. 12:2221–2236. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Badley EM and Davis AM: Meeting the
challenge of the ageing of the population: Issues in access to
specialist care for arthritis. Best Pract Res Clin Rheumatol.
26:599–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naylor RM, Baker DJ and van Deursen JM:
Senescent cells: A novel therapeutic target for aging and
age-related diseases. Clin Pharmacol Ther. 93:105–116. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verstraeten SP, van Oers HA and Mackenbach
JP: Differences in life expectancy between four Western countries
and their Caribbean dependencies, 1980–2014. Eur J Public Health.
30:85–92. 2020.PubMed/NCBI
|
|
5
|
Montero JA, Lorda-Diez CI and Hurlé JM:
Regenerative medicine and connective tissues: Cartilage versus
tendon. J Tissue Eng Regen Med. 6:337–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Escobar KA, Cole NH, Mermier CM and
VanDusseldorp TA: Autophagy and aging: Maintaining the proteome
through exercise and caloric restriction. Aging Cell.
18:e128762019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohd Sahardi NF and Makpol S: Ginger
(Zingiber officinale Roscoe) in the prevention of ageing and
degenerative diseases: Review of current evidence. Evid Based
Complement Alternat Med. 2019:50543952019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sachdeva V, Roy A and Bharadvaja N:
Current prospective of nutraceuticals: A review. Curr Pharm
Biotechnol. Jan 29–2020.(Epub ahead of print). doi:
10.2174/1389201021666200130113441. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sj S, Veerabhadrappa B, Subramaniyan S and
Dyavaiah M: Astaxanthin enhances the longevity of Saccharomyces
cerevisiae by decreasing oxidative stress and apoptosis. FEMS
Yeast Res. Jan 1–2019.(Epub ahead of print). doi:
10.1093/femsyr/foy113. PubMed/NCBI
|
|
10
|
El-Baz FK, Hussein RA, Abdel Jaleel GA and
Saleh DO: Astaxanthin-rich Haematococcus pluvialis Algal
hepatic modulation in D-galactose-induced aging in rats: Role of
Nrf2. Adv Pharm Bull. 8:523–528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang X, Ma S, Li Y, Sun Y, Zhang K, Zhou Q
and Yu R: Evaluate the activity of sodium butyrate to prevent
osteoporosis in rats by promoting osteal GSK-3beta/Nrf2 signaling
and mitochondrial function. J Agric Food Chem. 68:6588–6603. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Domazetovic V, Marcucci G, Iantomasi T,
Brandi ML and Vincenzini MT: Oxidative stress in bone remodeling:
Role of antioxidants. Clin Cases Miner Bone Metab. 14:209–216.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Wang C, Qiu H, Yuan Y, Chen K, Cao
Z, Xiang Tan R, Tickner J, Xu J and Zou J: Asperpyrone A attenuates
RANKL-induced osteoclast formation through inhibiting NFATc1,
Ca2+ signalling and oxidative stress. J Cell Mol Med.
23:8269–8279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prideaux M, Kitase Y, Kimble M, OConnell
TM and Bonewald LF: Taurine, an osteocyte metabolite, protects
against oxidative stress-induced cell death and decreases
inhibitors of the Wnt/β-catenin signaling pathway. Bone.
137:1153742020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baek KH, Oh KW, Lee WY, Lee SS, Kim MK,
Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, et al: Association of
oxidative stress with postmenopausal osteoporosis and the effects
of hydrogen peroxide on osteoclast formation in human bone marrow
cell cultures. Calcif Tissue Int. 87:226–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lingappan K: NF-κB in oxidative stress.
Curr Opin Toxicol. 7:81–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li S, Yin Y, Yao L, Lin Z, Sun S, Zhang J
and Li X: TNF-α treatment increases DKK1 protein levels in primary
osteoblasts via upregulation of DKK1 mRNA levels and downregulation
of miR-335-5p. Mol Med Rep. May 18–2020.(Epub ahead of print). doi:
10.3892/mmr.2020.11152.
|
|
18
|
Marahleh A, Kitaura H, Ohori F, Kishikawa
A, Ogawa S, Shen WR, Qi J, Noguchi T, Nara Y and Mizoguchi I: TNF-α
directly enhances osteocyte RANKL expression and promotes
osteoclast formation. Front Immunol. 10:29252019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nanes MS: Tumor necrosis factor-alpha:
Molecular and cellular mechanisms in skeletal pathology. Gene.
321:1–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osta B, Benedetti G and Miossec P:
Classical and paradoxical effects of TNF-α on bone homeostasis.
Front Immunol. 5:482014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim
HH and Lee ZH: Reactive oxygen species mediate RANK signaling in
osteoclasts. Exp Cell Res. 301:119–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee NK, Choi YG, Baik JY, Han SY, Jeong
DW, Bae YS, Kim N and Lee SY: A crucial role for reactive oxygen
species in RANKL-induced osteoclast differentiation. Blood.
106:852–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Azizieh FY, Shehab D, Jarallah KA, Gupta R
and Raghupathy R: Circulatory levels of RANKL, OPG, and oxidative
stress markers in postmenopausal women with normal or low bone
mineral density. Biomark Insights. 14:11772719198438252019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Azizieh F, Raghupathy R, Shehab D,
Al-Jarallah K and Gupta R: Cytokine profiles in osteoporosis
suggest a proresorptive bias. Menopause. 24:1057–1064. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pansini F, Mollica G and Bergamini CM:
Management of the menopausal disturbances and oxidative stress.
Curr Pharm Des. 11:2063–2073. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gravallese EM, Harada Y, Wang JT, Gorn AH,
Thornhill TS and Goldring SR: Identification of cell types
responsible for bone resorption in rheumatoid arthritis and
juvenile rheumatoid arthritis. Am J Pathol. 152:943–951.
1998.PubMed/NCBI
|
|
27
|
Gravallese EM, Manning C, Tsay A, Naito A,
Pan C, Amento E and Goldring SR: Synovial tissue in rheumatoid
arthritis is a source of osteoclast differentiation factor.
Arthritis Rheum. 43:250–258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carter S, Braem K and Lories RJ: The role
of bone morphogenetic proteins in ankylosing spondylitis. Ther Adv
Musculoskelet Dis. 4:293–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hreggvidsdottir HS, Noordenbos T and
Baeten DL: Inflammatory pathways in spondyloarthritis. Mol Immunol.
57:28–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van der Heijde D, Salonen D, Weissman BN,
Landewé R, Maksymowych WP, Kupper H, Ballal S, Gibson E and Wong R;
Canadian (M03-606) study group; ATLAS study group, : Assessment of
radiographic progression in the spines of patients with ankylosing
spondylitis treated with adalimumab for up to 2 years. Arthritis
Res Ther. 11:R1272009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan JP, Peng J, Yin K and Wang JH:
Potential health-promoting effects of astaxanthin: A high-value
carotenoid mostly from microalgae. Mol Nutr Food Res. 55:150–165.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Milani A, Basirnejad M, Shahbazi S and
Bolhassani A: Carotenoids: Biochemistry, pharmacology and
treatment. Br J Pharmacol. 174:1290–1324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Novoveská L, Ross ME, Stanley MS,
Pradelles R, Wasiolek V and Sassi JF: Microalgal carotenoids: A
review of production, current markets, regulations, and future
direction. Mar Drugs. 17:6402019. View Article : Google Scholar
|
|
35
|
Davies BH: Carotenoid metabolism in
animals: A biochemists view. Pure Appl Chem. 57:679–684. 1985.
View Article : Google Scholar
|
|
36
|
Lorenz RT and Cysewski GR: Commercial
potential for Haematococcus microalgae as a natural source of
astaxanthin. Trends Biotechnol. 18:160–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roche F: Astaxanthin as a Pigmenter in
Salmon Feed, Color Additive Petition 7C02 1 1, United States Food
and Drug Administration. Astaxanthin: Human Food Safety Summary.
Hoffman-La Roche Ltd. (Basel). 431987.
|
|
38
|
Higuera-Ciapara I, Félix-Valenzuela L and
Goycoolea FM: Astaxanthin: A review of its chemistry and
applications. Crit Rev Food Sci Nutr. 46:185–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kothari D, Lee JH, Chon JW, Seo KH and Kim
SK: Improved astaxanthin production by Xanthophyllomyces
dendrorhous SK984 with oak leaf extract and inorganic phosphate
supplementation. Food Sci Biotechnol. 28:1171–1176. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Capelli B, Talbott S and Ding LX:
Astaxanthin sources: Suitability for human health and nutrition.
Funct Food Health Dis. 9:430–445. 2019. View Article : Google Scholar
|
|
41
|
McCoy M: Astaxanthin market a hard one to
crack. Chem Eng News. 77:15–17. 1999. View Article : Google Scholar
|
|
42
|
Fraser PD, Miura Y and Misawa N: In vitro
characterization of astaxanthin biosynthetic enzymes. J Biol Chem.
272:6128–6135. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tanaka T, Shnimizu M and Moriwaki H:
Cancer chemoprevention by carotenoids. Molecules. 17:3202–3242.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kavitha K, Kowshik J, Kishore TKK, Baba AB
and Nagini S: Astaxanthin inhibits NF-κB and Wnt/β-catenin
signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to
induce intrinsic apoptosis in a hamster model of oral cancer.
Biochim Biophys Acta. 1830:4433–4444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Satoh A, Tsuji S, Okada Y, Murakami N,
Urami M, Nakagawa K, Ishikura M, Katagiri M, Koga Y and Shirasawa
T: Preliminary clinical evaluation of toxicity and efficacy of a
new astaxanthin-rich Haematococcus pluvialis extract. J Clin
Biochem Nutr. 44:280–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bhuvaneswari S, Yogalakshmi B, Sreeja S
and Anuradha CV: Astaxanthin reduces hepatic endoplasmic reticulum
stress and nuclear factor-κB-mediated inflammation in high fructose
and high fat diet-fed mice. Cell Stress Chaperones. 19:183–191.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kidd P: Astaxanthin, cell membrane
nutrient with diverse clinical benefits and anti-aging potential.
Altern Med Rev. 16:355–364. 2011.PubMed/NCBI
|
|
48
|
Johnson EA and An GH: Astaxanthin from
microbial sources. Crit Rev Biotechnol. 11:297–326. 1991.
View Article : Google Scholar
|
|
49
|
Yuan JP, Gong XD and Chen F: Separation
and analysis of carotenoids and chlorophylls in Haematococcus
lacustris by high-performance liquid chromatography photodiode
array detection. J Agric Food Chem. 45:1952–1956. 1997. View Article : Google Scholar
|
|
50
|
Casella P, Iovine A, Mehariya S, Marino T,
Musmarra D and Molino A: Smart method for carotenoids
characterization in Haematococcus pluvialis red phase and
evaluation of astaxanthin thermal stability. Antioxidants.
9:4222020. View Article : Google Scholar
|
|
51
|
Shah MM, Liang Y, Cheng JJ and Daroch M
and Daroch M: Astaxanthin-producing green microalga
Haematococcus pluvialis: From single cell to high value
commercial products. Front Plant Sci. 7:5312016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
McCarty MF and Lerner A: Nutraceuticals
targeting generation and oxidant activity of peroxynitrite may aid
prevention and control of Parkinsons disease. Int J Mol Sci.
21:36242020. View Article : Google Scholar
|
|
53
|
Alves A, Sousa E, Kijjoa A and Pinto M:
Marine-derived compounds with potential use as cosmeceuticals and
nutricosmetics. Molecules. 25:25362020. View Article : Google Scholar
|
|
54
|
Menin B, Santabarbara S, Lami A, Musazzi
S, Villafiorita Monteleone F and Casazza AP: Non-endogenous
ketocarotenoid accumulation in engineered Synechocystis sp.
PCC 6803. Physiol Plant. 166:403–412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fakhri S, Abbaszadeh F, Dargahi L and
Jorjani M: Astaxanthin: A mechanistic review on its biological
activities and health benefits. Pharmacol Res. 136:1–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tachaprutinun A, Udomsup T, Luadthong C
and Wanichwecharungruang S: Preventing the thermal degradation of
astaxanthin through nanoencapsulation. Int J Pharm. 374:119–124.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Küçüködük A, Helvacioglu F, Haberal N,
Dagdeviren A, Bacanli D, Yilmaz G and Akkoyun I: Antiproliferative
and anti-apoptotic effect of astaxanthin in an oxygen-induced
retinopathy mouse model. Can J Ophthalmol. 54:65–74. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yuan JP and Chen F: Isomerization of
trans-astaxanthin to cis-isomers in organic solvents. J Agric Food
Chem. 47:3656–3660. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu Q, Hu S, Fleming E, Lee JY and Luo Y:
Chitosan-caseinate-dextran ternary complex nanoparticles for
potential oral delivery of astaxanthin with significantly improved
bioactivity. Int J Biol Macromol. 151:747–756. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu C, Zhang S, McClements DJ, Wang D and
Xu Y: Design of astaxanthin-loaded core-shell nanoparticles
consisting of chitosan oligosaccharides and poly
(lactic-co-glycolic acid): Enhancement of water solubility,
stability, and bioavailability. J Agric Food Chem. 67:5113–5121.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tamjidi F, Shahedi M, Varshosaz J and
Nasirpour A: Stability of astaxanthin-loaded nanostructured lipid
carriers as affected by pH, ionic strength, heat treatment,
simulated gastric juice and freeze-thawing. J Food Sci Technol.
54:3132–3141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Machado FR Jr, Trevisol TC, Boschetto DL,
Burkert JF, Ferreira SR, Oliveira JV and Burkert CA: Technological
process for cell disruption, extraction and encapsulation of
astaxanthin from Haematococcus pluvialis. J Biotechnol.
218:108–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bustamante A, Masson L, Velasco J, Del
Valle JM and Robert P: Microencapsulation of H. pluvialis
oleoresins with different fatty acid composition: Kinetic stability
of astaxanthin and alpha-tocopherol. Food Chem. 190:1013–1021.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen L, Wang JL, Ni H and Zhu MJ:
Disruption of Phaffia rhodozyma cells and preparation of
microencapsulated astaxanthin with high water solubility. Food Sci
Biotechnol. 28:111–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Salatti-Dorado JA, García-Gómez D,
Rodriguez-Ruiz V, Gueguen V, Pavon-Djavid G and Rubio S:
Multifunctional green supramolecular solvents for cost-effective
production of highly stable astaxanthin-rich formulations from
Haematococcus pluvialis. Food Chem. 279:294–302. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin SF, Chen YC, Chen RN, Chen LC, Ho HO,
Tsung YH, Sheu MT and Liu DZ: Improving the stability of
astaxanthin by microencapsulation in calcium alginate beads. PLoS
One. 11:e01536852016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Niizawa I, Espinaco BY, Zorrilla SE and
Sihufe GA: Natural astaxanthin encapsulation: Use of response
surface methodology for the design of alginate beads. Int J Biol
Macromol. 121:601–608. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hama S, Uenishi S, Yamada A, Ohgita T,
Tsuchiya H, Yamashita E and Kogure K: Scavenging of hydroxyl
radicals in aqueous solution by astaxanthin encapsulated in
liposomes. Biol Pharm Bull. 35:2238–2242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chiu CH, Chang CC, Lin ST, Chyau CC and
Peng RY: Improved hepatoprotective effect of liposome-encapsulated
astaxanthin in lipopolysaccharide-induced acute hepatotoxicity. Int
J Mol Sci. 17:E11282016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wu YC, Huang HH, Wu YJ, Manousakas I, Yang
CC and Kuo SM: Therapeutic and protective effects of liposomal
encapsulation of astaxanthin in mice with alcoholic liver fibrosis.
Int J Mol Sci. 20:E40572019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Anarjan N, Tan CP, Nehdi IA and Ling TC:
Colloidal astaxanthin: Preparation, characterisation and
bioavailability evaluation. Food Chem. 135:1303–1309. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang Y, Kim B and Lee JY: Astaxanthin
structure, metabolism, and health benefits. J Hum Nutr Food Sci.
1:10032013.
|
|
73
|
Kamezaki C, Nakashima A, Yamada A, Uenishi
S, Ishibashi H, Shibuya N, Hama S, Hosoi S, Yamashita E and Kogure
K: Synergistic antioxidative effect of astaxanthin and tocotrienol
by co-encapsulated in liposomes. J Clin Biochem Nutr. 59:100–106.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ishikawa M, Hirai S, Yoshida T, Shibuya N,
Hama S, Takahashi Y, Fukuta T, Tanaka T, Hosoi S and Kogure K:
Carotenoid stereochemistry affects antioxidative activity of
liposomes co-encapsulating astaxanthin and tocotrienol. Chem Pharm
Bull (Tokyo). 66:714–720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kittikaiwan P, Powthongsook S, Pavasant P
and Shotipruk A: Encapsulation of Haematococcus pluvialis
using chitosan for astaxanthin stability enhancement. Carbohydr
Polym. 70:378–385. 2007. View Article : Google Scholar
|
|
76
|
Wang Q, Zhao Y, Guan L, Zhang Y, Dang Q,
Dong P, Li J and Liang X: Preparation of astaxanthin-loaded
DNA/chitosan nanoparticles for improved cellular uptake and
antioxidation capability. Food Chem. 227:9–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liu C, Liu Z, Sun X, Zhang S, Wang S, Feng
F, Wang D and Xu Y: Fabrication and characterization of
β-lactoglobulin-based nanocomplexes composed of chitosan
oligosaccharides as vehicles for delivery of astaxanthin. J Agric
Food Chem. 66:6717–6726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chereddy KK, Vandermeulen G and Préat V:
PLGA based drug delivery systems: Promising carriers for wound
healing activity. Wound Repair Regen. 24:223–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang J and Peng CA: Enhanced
proliferation and differentiation of mesenchymal stem cells by
astaxanthin-encapsulated polymeric micelles. PLoS One.
14:e02167552019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Dias V, Junn E and Mouradian MM: The role
of oxidative stress in Parkinsons disease. J Parkinsons Dis.
3:461–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kim SH and Kim H: Inhibitory effect of
astaxanthin on oxidative stress-induced mitochondrial dysfunction-A
mini-review. Nutrients. 10:E11372018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Starkov AA: The role of mitochondria in
reactive oxygen species metabolism and signaling. Ann NY Acad Sci.
1147:37–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Siemen D and Ziemer M: What is the nature
of the mitochondrial permeability transition pore and what is it
not? IUBMB Life. 65:255–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Valenti MT, Garbin U, Pasini A, Zanatta M,
Stranieri C, Manfro S, Zucal C and Dalle Carbonare L: Role of
ox-PAPCs in the differentiation of mesenchymal stem cells (MSCs)
and Runx2 and PPARγ2 expression in MSCs-like of osteoporotic
patients. PLoS One. 6:e203632011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sobenin IA, Sazonova MA, Postnov AY,
Bobryshev YV and Orekhov AN: Mitochondrial mutations are associated
with atherosclerotic lesions in the human aorta. Clin Dev Immunol.
2012:8324642012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kluge MA, Fetterman JL and Vita JA:
Mitochondria and endothelial function. Circ Res. 112:1171–1188.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Apostolova N and Victor VM: Molecular
strategies for targeting antioxidants to mitochondria: Therapeutic
implications. Antioxid Redox Signal. 22:686–729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wolf AM, Asoh S, Hiranuma H, Ohsawa I, Iio
K, Satou A, Ishikura M and Ohta S: Astaxanthin protects
mitochondrial redox state and functional integrity against
oxidative stress. J Nutr Biochem. 21:381–389. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Park JS, Mathison BD, Hayek MG, Zhang J,
Reinhart GA and Chew BP: Astaxanthin modulates age-associated
mitochondrial dysfunction in healthy dogs. J Anim Sci. 91:268–275.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Song X, Wang B, Lin S, Jing L, Mao C, Xu
P, Lv C, Liu W and Zuo J: Astaxanthin inhibits apoptosis in
alveolar epithelial cells type II in vivo and in vitro through the
ROS-dependent mitochondrial signalling pathway. J Cell Mol Med.
18:2198–2212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Fan CD, Sun JY, Fu XT, Hou YJ, Li Y, Yang
MF, Fu XY and Sun BL: Astaxanthin attenuates homocysteine-induced
cardiotoxicity in vitro and in vivo by inhibiting mitochondrial
dysfunction and oxidative damage. Front Physiol. 8:10412017.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Reid MB, Shoji T, Moody MR and Entman ML:
Reactive oxygen in skeletal muscle. II. Extracellular release of
free radicals. J Appl Physiol (1985). 73:1805–1809. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Aoi W, Naito Y, Sakuma K, Kuchide M,
Tokuda H, Maoka T, Toyokuni S, Oka S, Yasuhara M and Yoshikawa T:
Astaxanthin limits exercise-induced skeletal and cardiac muscle
damage in mice. Antioxid Redox Signal. 5:139–144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
McGarry JD and Brown NF: The mitochondrial
carnitine palmitoyltransferase system. From concept to molecular
analysis. Eur J Biochem. 244:1–14. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ikeuchi M, Koyama T, Takahashi J and
Yazawa K: Effects of astaxanthin supplementation on
exercise-induced fatigue in mice. Biol Pharm Bull. 29:2106–2110.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Belviranli M, Okudan N and Lamprecht M:
Well-Known Antioxidants and Newcomers in Sport Nutrition: Coenzyme
Q10. Quercetin, Resveratrol, Pterostilbene, Pycnogenol and
Astaxanthin. Antioxidants in Sport Nutrition. CRC Press/Taylor and
Francis; Boca Raton, FL: 2015
|
|
97
|
Yaghooti H, Mohammadtaghvaei N and
Mahboobnia K: Effects of palmitate and astaxanthin on cell
viability and proinflammatory characteristics of mesenchymal stem
cells. Int Immunopharmacol. 68:164–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Domazetovic V, Marcucci G, Pierucci F,
Bruno G, Di Cesare Mannelli L, Ghelardini C, Brandi ML, Iantomasi
T, Meacci E and Vincenzini MT: Blueberry juice protects osteocytes
and bone precursor cells against oxidative stress partly through
SIRT1. FEBS Open Bio. 9:1082–1096. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Balci Yuce H, Lektemur Alpan A, Gevrek F
and Toker H: Investigation of the effect of astaxanthin on alveolar
bone loss in experimental periodontitis. J Periodontal Res.
53:131–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hwang YH, Kim KJ, Kim SJ, Mun SK, Hong SG,
Son YJ and Yee ST: Suppression effect of astaxanthin on osteoclast
formation in vitro and bone loss in vivo. Int J Mol Sci.
19:E9122018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
El-Baz FK, Saleh DO, Abdel Jaleel GA,
Hussein RA and Hassan A: Heamatococcus pluvialis ameliorates
bone loss in experimentally-induced osteoporosis in rats via the
regulation of OPG/RANKL pathway. Biomed Pharmacother.
116:1090172019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Valenti MT, Dalle Carbonare L and Mottes
M: Osteogenic Differentiation in Healthy and Pathological
Conditions. Int J Mol Sci. 18:412016. View Article : Google Scholar
|
|
103
|
Kimble L, Mathison B and Chew BP:
Astaxanthin mediates inflammatory biomarkers associated with
arthritis in human chondrosarcoma cells induced with interleukin-1
beta. FASEB J. 27 (Suppl 1):6382013.
|
|
104
|
Chen WP, Xiong Y, Shi YX, Hu PF, Bao JP
and Wu LD: Astaxanthin reduces matrix metalloproteinase expression
in human chondrocytes. Int Immunopharmacol. 19:174–177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Huang LJ and Chen WP: Astaxanthin
ameliorates cartilage damage in experimental osteoarthritis. Mod
Rheumatol. 25:768–771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Booth FW, Roberts CK and Laye MJ: Lack of
exercise is a major cause of chronic diseases. Compr Physiol.
2:1143–1211. 2012.PubMed/NCBI
|