Introduction
Osteosarcoma accounts <1% of malignancies
overall, with an incidence of ~5 cases per million in individuals
<19 years of age in the USA. However, it is the most widely
diagnosed primary malignant bone tumor, particularly among children
and young people. Males are prone to the onset of osteosarcoma, and
the ratio of male to female incidence is ~3:2 (1). Osteosarcoma is thought to arise from
mesenchymal primitive bone-forming cells, and is characterized by
the sustainable production of malignant osteogenesis. In addition,
the production of pro-angiogenic factors in the malignant
development of osteosarcoma has also been suggested by a previous
study, which concluded that osteosarcoma had a strong tendency to
metastasize early and was associated with poor prognosis (2). Thus, the degree of osteosarcoma
malignancy is extremely high, and increased tumor invasiveness and
vascularity are associated with metastatic potential and poor
prognosis.
The main current treatment strategy for patients
with newly diagnosed osteosarcoma includes neoadjuvant
chemotherapy, followed by surgical removal of the primary tumor and
all metastatic lesions with clinical manifestations, as well as
postoperative adjuvant chemotherapy (3). The three-drug chemotherapy regimen of
cisplatin, doxorubicin and methotrexate constitutes the primary
option for backbone treatment, and the overall 5-year survival rate
in America for osteosarcoma has increased to 60–70% in patients
receiving the three-drug regimen (4). Recently, biologic agents, such as
muramyl tripeptide and IFN-α-2b, and additional cytotoxic
chemotherapy, such as ifosfamide, have been introduced into
clinical trials. However, these have failed to significantly
improve the survival of young patients with osteosarcoma (5,6).
Therefore, an improved understanding of the underlying mechanisms
of tumor progression and angiogenesis in osteosarcoma is required
in order to identify and develop more effective therapies.
Dysregulation in nuclear factor-κB (NF-κB) signaling
is associated with excessive cellular proliferation and
developmental signals during tumorigenesis. Indeed, this pathway
has been reported to be involved in inflammatory proliferation and
differentiation of osteosarcoma cells (7). NF-κB can also regulate the generation
of proinflammatory and proangiogenic cytokines around cancer cells
(8). Thus, it was previously
suggested that NF-κB could serve a causative role in osteosarcoma
progression (9). Punicalagin is an
antioxidant ellagitannin found in pomegranate juice with known
anti-proliferation or anti-angiogenesis properties against many
cancer cell lines, including leukemia, glioma and prostate cancer
cells (10). The present study
aimed to examine the detailed function of the NF-κB pathway in
osteosarcoma, and to determine whether punicalagin can inhibit the
NF-κB pathway to suppress inflammation and osteosarcoma
tumorigenicity. Combined treatment targeting the NF-κB pathway may
represent a novel and promising strategy to significantly enhance
the therapeutic activity of routine anticancer drugs against
osteosarcoma.
Materials and methods
Reagents and cell lines
A 50 mM stock solution consisting of 5 mg
punicalagin (Sigma-Aldrich; Merck KGaA) in 1 ml DMSO were prepared.
The stock was diluted to the desired concentrations with culture
medium to give a water-soluble fraction, in which DMSO
concentration did not exceed 0.2% in the highest punicalagin
concentrations applied. The three human osteosarcoma cell lines
(U2OS, MG63 and SaOS2) and one normal osteoblast cell line
(hFOB1.19) were purchased from American Type Culture Collection and
cultured according to the instructions. All of the cell lines were
grown in Dulbecco's modified Eagle medium supplemented with 10% FBS
(both from Invitrogen; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere with 5% CO2 at 37°C. Following
treatment, culture medium was prepared serum-free and collected in
24-h cultures. Phorbol myristate acetate (PMA; Sigma-Aldrich; Merck
KGaA), an activator of the NF-κB pathway, was added to cells
together with punicalagin and incubated for 45 min in in a
humidified atmosphere with 5% CO2 at 37°C.
Cell proliferation assay
Cell proliferation was examined using Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.). Cells were
seeded at a density of 1×103 cells per well in 96-well
plates, then treated with different concentrations of punicalagin
or DMSO as control. Following 1–3 days of culture, 10 µl CCK-8
reagent was added to each well and cells were cultured for 1 h. A
microplate reader (Bio-Rad Laboratories, Inc.) was used to measure
the absorbance at 450 nm.
Cell invasion assay
Transwell migration assays were performed with
8.0-µm pore polycarbonate filter inserts (Corning, Inc.) coated
with Matrigel™ (BD Biosciences) at room temperature for 1 h before
use. Briefly, osteosarcoma cells in 100 µM punicalagin contained or
vehicle medium supplemented with 1% FBS were placed in the top
chamber at a density of 1×104 cells/well. In the bottom
chamber, complete medium with 10% FBS was used as a positive
control. After 48 h of incubation, the migrated cells were fixed
with 4% paraformaldehyde for 20 min and stained with 1% crystal
violet for 10 min at room temperature. Images were captured with a
light microscope at ×400 magnification and the migrated cells were
counted manually and averaged from 5 high-power fields.
Cell apoptosis analysis
To investigate early and late apoptotic cells,
annexin V-FITC and propidium iodide (PI) double staining was
performed. The APOAF annexin V apoptosis kit (Sigma-Aldrich; Merck
KGaA) was used for annexin V staining, according to the
manufacturer's protocol. All samples were quantified using a Canto
II flow cytometer (BD Biosciences) and analyzed with FlowJo version
7.6 software (TreeStar). Early apoptotic cells were defined as
FITChighPIlow cells and late apoptotic cells
were defined as FITChighPIhigh. Additionally,
FITClowPIlow represented healthy cells and
FITClowPIhigh accounted for cells debris that
was eliminated.
Western blotting assay
The cells or tissues were homogenized in RIPA buffer
(Beyotime Institute of Biotechnology). A BCA protein assay kit
(Thermo Fisher Scientific, Inc.) was used to determine the protein
concentration in lysates and conditioned medium. Equal amounts of
protein (15 µg) were loaded per lane and separated via SDS-PAGE
(10% gel), then transferred to a PVDF membrane (Bio-Rad
Laboratories, Inc.). The PVDF membrane was blocked with 5% skimmed
milk in TBS + 0.1% Tween®−20 buffer on a shaker for 1 h
at room temperature. The membrane was then incubated in 4°C with
the following primary antibodies overnight: Anti-phosphorylated
(phosphor)-inhibitor of κBα (IκBα; Ser32; cat. no. 2859), anti-IκBα
(cat. no. 9242), anti-phospho-mammalian target of rapamycin (mTOR;
Ser2448, cat. no. 2971), anti-mTOR (cat. no. 2983), anti-p65 (cat.
no. 8242), anti-histone 2A family member X (H2AX, cat. no. 7631)
(all 1:1,000; all from Cell Signaling Technology Biological
Reagents Co., Ltd.), anti-β-actin (cat. no. sc-130656; 1:2,000),
anti-interleukin (IL)-6 (cat. no. sc-130326; 1:500), and anti-IL-8
(cat. no. sc-8427; 1:500; all purchased from Santa Cruz
Biotechnology, Inc.). After washing, the membrane was incubated
with horseradish peroxide-conjugated secondary antibody (cat. no
7074; 1:1,000 Cell Signaling Technology Biological Reagents Co.,
Ltd.) for 1 h on the shaker at room temperature. The membrane was
then incubated with chemiluminescence reagent (GE Healthcare Life)
for 5 min at room temperature. The relative quantity of the protein
was measured using ImageJ software v1.51 (National Institutes of
Health).
Tumor xenografts
The in vivo experiment protocol was approved
by the Institutional Animal Care and Use Committee at The Second
Affiliated Hospital of Air Force Medical University and followed
the Chinese national standards: Laboratory animal welfare ethics
review guidelines for the humane and customary care and use of
experimental animals. A total of 20 female 6–8-week-old, 18 g,
Balb/c nude mice (n=5 per group) were purchased from Model Animal
Research Center of Nanjing University. Mice were housed at 20–24°C
with an average humidity of 40% and a 12-h light/dark cycle and
received food and water ad libitum. Mice were then injected
with osteosarcoma cells near the back of the neck at a density of
2×107 cells in 200 µl PBS. Mice were anesthetized by
inhalation using 2.0–2.5% sevoflurane during injection and
measurement. After 1 week of tumor cell inoculation, 5 mg/kg
punicalagin in saline or an equal volume (300 µl) saline as vehicle
(control) was injected intraperitoneally once a week for a total of
7 weeks, and the mouse health and behavior were monitored daily for
8 weeks. No death was observed prior to sacrifice. The tumor size
was measured with a sliding caliper twice a week, and the tumor
volume was calculated using the formula: Size, mm3
=[tumor length × (tumor width)2]/2. When volume was
>500 mm3, the experiment was stopped and the mice
were sacrificed using CO2 asphyxiation with a flow rate
≤50% of the chamber volume per min, followed by cervical
dislocation. Tumors were then harvested, weighed and snap-frozen in
liquid nitrogen and stored at −80°C for subsequent use
immunohistochemistry assays.
Immunohistochemistry assay
Solid tumors were fixed with 10% formaldehyde for 48
h at room temperature and embedded in paraffin. Tissue slides were
blocked with 1% BSA (Beyotime Institute of Biotechnology) in PBS
for blocking for 1 h at room temperature. To identify infiltrating
blood vessels, immunohistochemistry was carried out on 5-µm
deparaffinized sections using an anti-CD31 antibody (cat. no.
77699; 1:150; Cell Signaling Technology Biological Reagents Co.,
Ltd.) at 4°C overnight, and then peroxide-conjugated secondary
antibodies (cat. no. ab6721; 1:500; Abcam) for 1 h at room
temperature with ABC Staining kits (Thermo Fisher Scientific, Inc.)
were applied for generating chromogenic signals. Images were
captured with a light microscope at magnification, ×100.
Statistical analysis
Data are presented as the mean ± standard error of
the mean (SEM), unless otherwise stated. Analysis of two
independent groups was performed using unpaired Student's t-test.
One-way ANOVA followed by Bonferroni correction was used for
multiple comparisons between groups. Statistical analysis was
carried out using the GraphPad software v5.0 (GraphPad, Inc.). Each
experiment was performed in triplicate. P<0.05 was considered to
indicate a statistically significant difference.
Results
Punicalagin treatment inhibits the
proliferation of human osteosarcoma cell lines
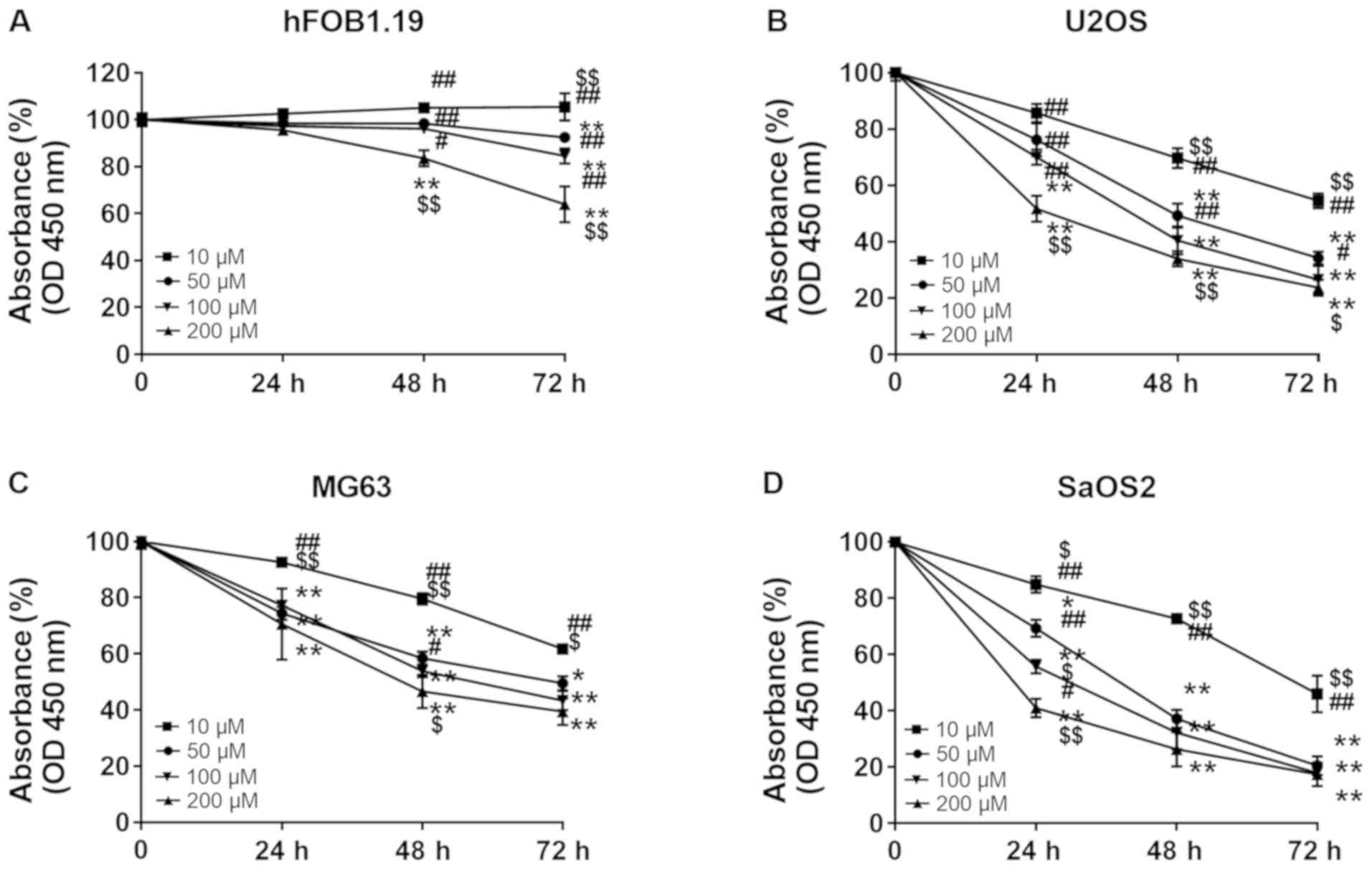
In order to investigate whether punicalagin
treatment could affect the viability malignant cells, hFOB1.19
osteoblast cells and U2OS, MG63 and SaOS2 osteosarcoma cells were
treated with increasing concentrations of punicalagin (10, 50, 100
and 200 µM) for 24–72 h. Cell viability was then evaluated using
CCK-8 assays (Fig. 1). Cell
proliferation was suspended after 24 h but the viability of
osteoblast cells did not significantly decrease at punicalagin
concentrations <100 µM or incubation time <48 h. Overall, the
viability of human osteosarcoma cell lines was decreased in a
concentration- and time-dependent manner. In 2 of the 3 (MG2 and
SaOS2) osteosarcoma cell lines, the decrease in cell viability was
not further exacerbated following prolonged treatment >48 h and
concentrations >100 µM. Thus, a concentration of 100 µM was
selected for subsequent experimentation.
Punicalagin increases apoptosis of
human osteosarcoma cell lines
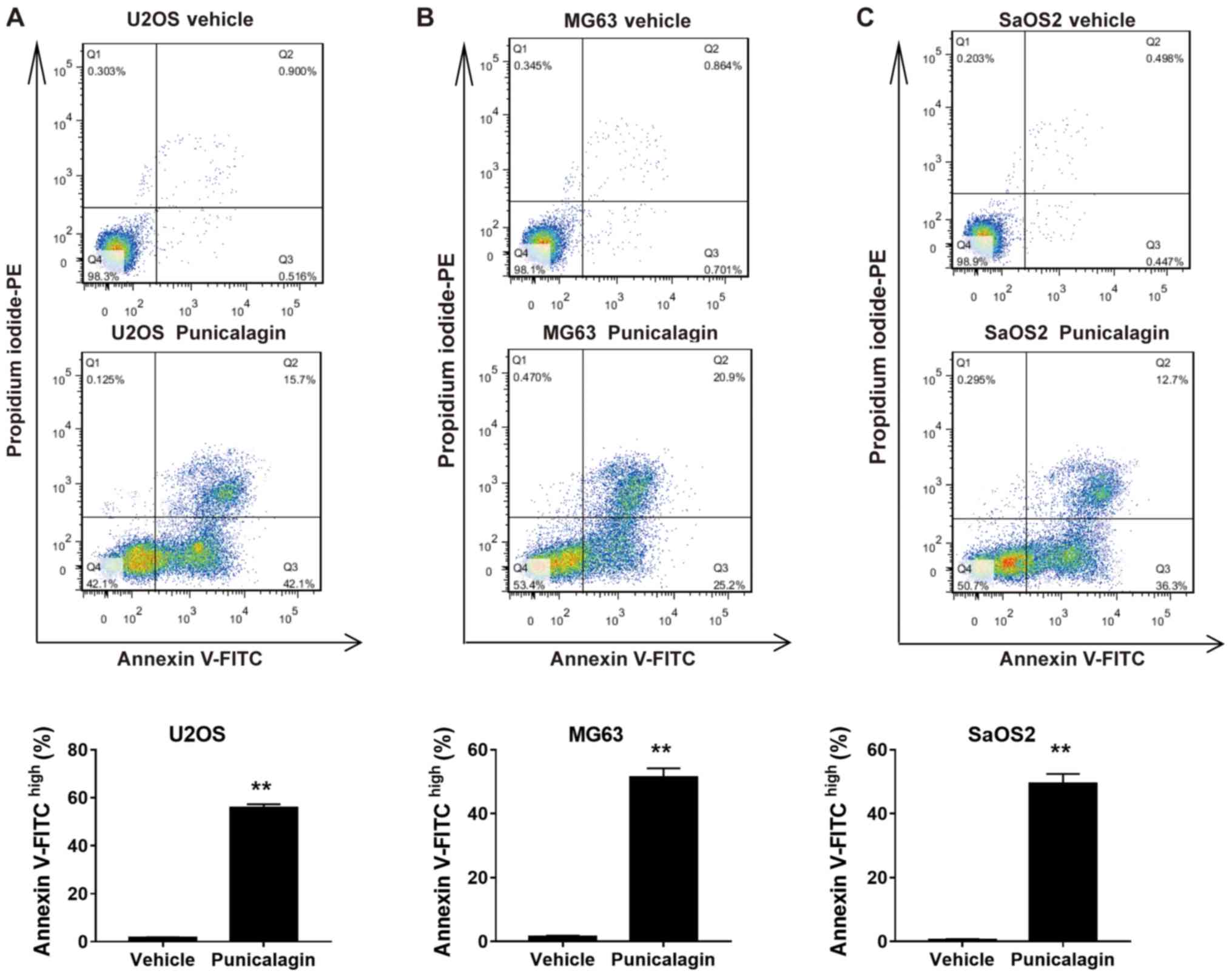
In order to determine whether the decrease in cell
viability following treatment with a moderate concentration of
punicalagin was due to increased apoptosis, annexin V-FITC and PI
double staining was used to assess the frequency of early
(FITChighPIlow) and late
(FITChighPIhigh) apoptotic cells in U2OS,
MG63 and SaOS2 cell lines. Treatment with 100 µM punicalagin for 48
h increased the cumulative percentage of early and late apoptotic
tumor cells (Fig. 2).
Punicalagin suppresses the invasion of
human osteosarcoma cells
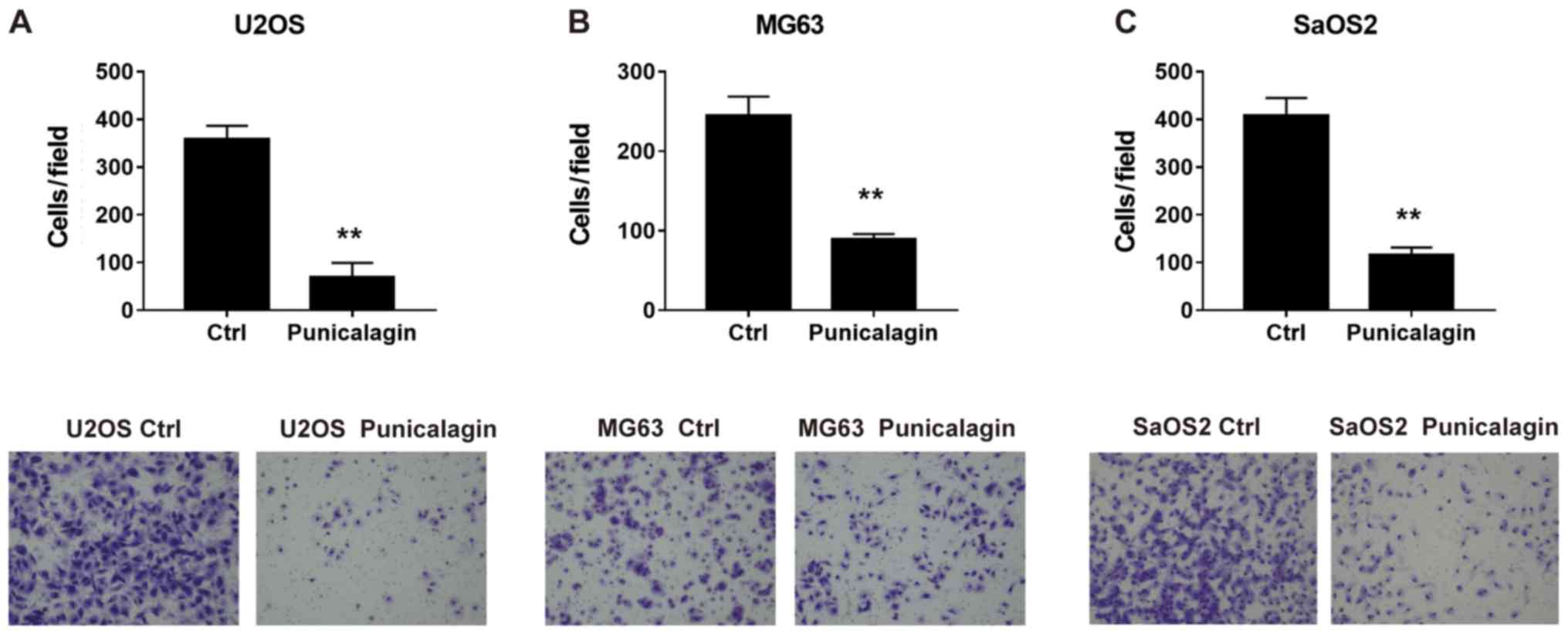
To determine whether punicalagin could suppress the
invasiveness of osteosarcoma cells, a Transwell Matrigel™ invasion
assay was performed using U2OS, MG63 and SaOS2 cells in the
presence or absence of punicalagin. Following treatment with
punicalagin for 24 h, fewer migrated tumor cells were detected,
suggesting that cell migration was inhibited in the presence of
punicalagin (Fig. 3).
Punicalagin downregulates the NF-κB,
but not mTOR signaling pathway in osteosarcoma cell lines
AKT signaling regulates cell survival. As one of the
main downstream mediators, mTOR signaling is essential for cell
proliferation, and suppressed mTOR signaling is associated with
apoptosis induction by various stimuli, delayed cell cycle
progression and cell proliferation (11). The levels of phospho-mTOR/mTOR
expression in osteosarcoma cells lysates were not altered by
punicalagin treatment, but compared with those of the control group
the levels of pIκBα/IκBα, p65, IL-6, IL-8 were significantly
reduced by punicalagin treatment (Fig.
4).
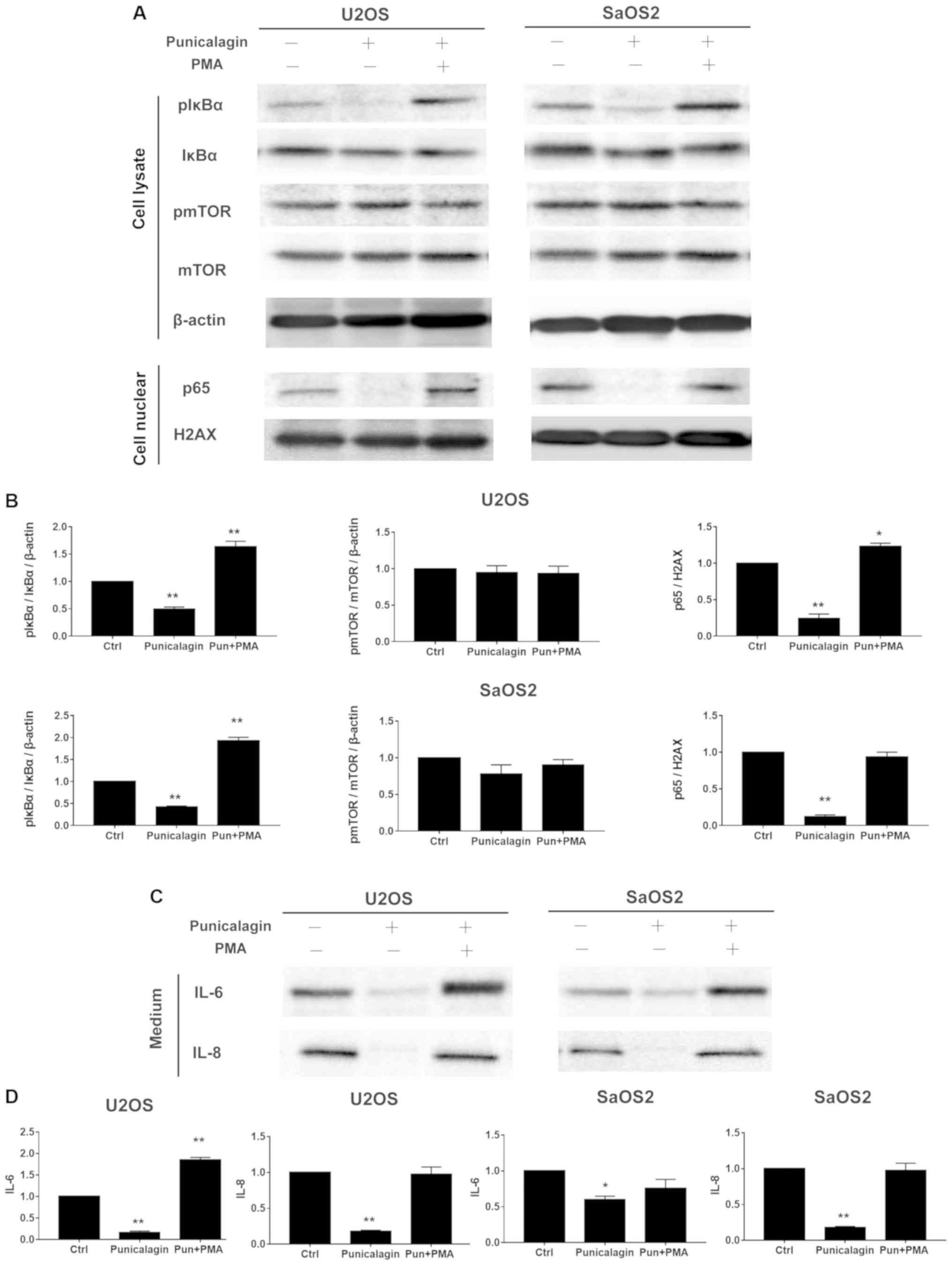 | Figure 4.Punicalagin regulates the NF-κB
pathway in human osteosarcoma cells. (A) Total cell extracts or
nuclear fractions were collected and subjected to western blot
analysis using anti-mTOR, pmTOR (Ser2448), IκBα, pIκBα (Ser32),
β-actin, nuclear p65 and H2AX. (B) Quantification of the results
shown in (A). (C) U2OS and SaOS2 cells were also treated with
punicalagin solution. The conditioned medium with or without NF-κB
signaling activator, PMA and punicalagin treatment were used to
determine the levels of IL-6 and IL-8. (D) Quantification of the
results shown in (C). *P<0.05 and **P<0.01 vs. control group.
Ctrl, control; p, phosphorylated; IL, interleukin; mTOR,
mechanistic target of rapamycin kinase; NFκB; nuclear factor κB;
IκBα, inhibitor of κBα, H2AX, histone 2A family member X; PMA,
phorbol myristate acetate; Pun, punicalagin. |
NF-κB is a key regulator of inflammatory immune
responses, including cytokine production (12). During tumorigenesis, these
cytokine-associated chemotactic effects are required for the
initiation of tumor-associated inflammation and neovascularization
(13). Osteosarcoma cells were
treated with saline (vehicle) or 100 µM punicalagin alone for 48 h,
following which, changes in the activated levels of NF-κB
represented by pIκBα/IκBα and p65, and its downstream inflammatory
factors, including IL-6, IL-8 were examined (Fig. 4). Punicalagin affected the stable
expression of NF-κB in U2OS and SaOS2 cells. The expression levels
of phosphor-IκBα, nuclear p65 and IL-6, IL-8 significantly
decreased in U2OS and SaOS2 cells compared with untreated cells. To
further evaluate the effect of punicalagin on the NF-κB pathway,
200 nM PMA, an activator of the NF-κB pathway, was added and cells
were incubated for 45 min. Following the addition of PMA, the
downregulation of IL-6 and IL-8 levels observed in punicalagin
pre-treated osteosarcoma cells was reversed.
Punicalagin attenuates proliferation
and angiogenesis of osteosarcoma cells in a murine tumor xenograft
model
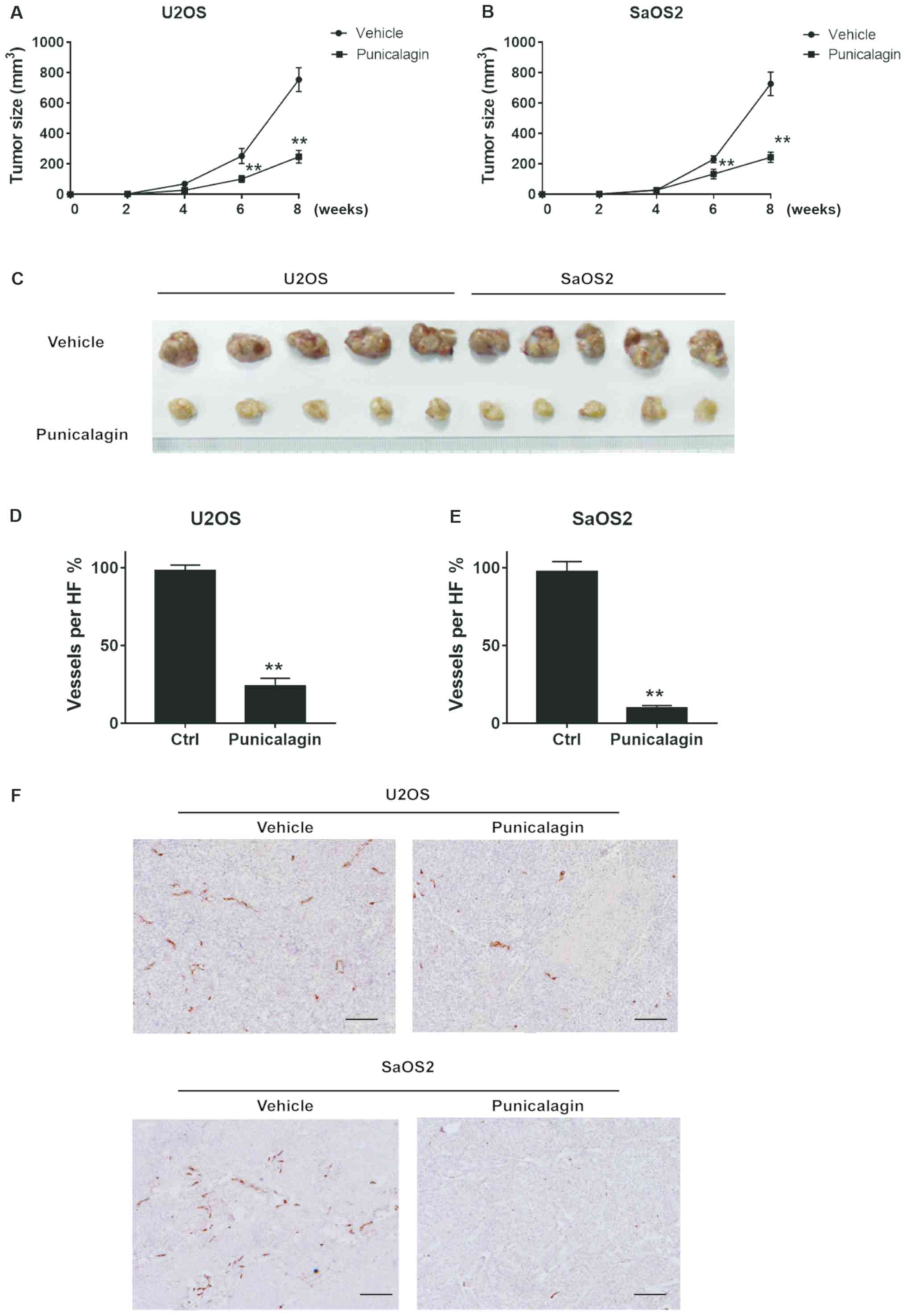
Proliferation and migration of osteosarcoma cells
were significantly attenuated in a xenograft model in mice. Tumors
typically had a length of 11–18 mm and a width of 8–12 mm in the
vehicle group. By contrast, punicalagin treatment significantly
decreased tumor growth, to a length of 7–11 mm and width of 6–8 mm
(Fig. 5A-C). Thus, punicalagin
injection resulted in slower malignant growth of human osteosarcoma
cells in the mouse model in vivo. Staining of blood vessels
with CD31 antibodies was then used to evaluate angiogenesis in
malignant tissues of xenograft mice. CD31 staining represented the
wall of the blood vessel of osteosarcoma core sections that were
used in a rat subcutaneous model, as described in a previous study
(14). The mean density of blood
vessels in the punicalagin-treated was significantly reduced,
compared with that in the vehicle group, which suggested that that
punicalagin could inhibit tumor angiogenesis (Fig. 5D-F).
Discussion
The present study suggested that punicalagin
treatment in osteosarcoma cells significantly decreased tumor cell
viability and induced cell apoptosis. Punicalagin can inhibit
proliferation and survival of osteosarcoma cells in a
concentration- and time-dependent manner. These results were also
replicated in a xenograft model, in which impaired angiogenesis was
also observed following injection of punicalagin. The molecular
mechanisms were further investigated using biological methods,
which demonstrated that the therapeutic effects of punicalagin were
associated with downregulation of NF-κB but not mTOR signaling.
Osteosarcoma is the most commonly diagnosed primary
solid bone malignancy. Metastasis is the main cause of death in
patients with osteosarcoma, and treatment options remain
unsatisfactory. The incidence of osteosarcoma in the general
population is 2–3 per million per year. However, annual incidence
is around 1.2–7.6 per million per year in people younger than 24
years of age worldwide (15).
Although major efforts have been made to establish the potential
pathognomonic driver mutations in young patients, only sporadic
mutations have detected in great majority of cases. Similar to the
majority of common types of human cancer, osteosarcoma exhibits a
high degree of mutational diversity. This diversity is driven by
complex rearrangement processes, chromothripsis and chromothripsic
amplification that predominate in osteosarcoma, although the
underlying causes remain unknown (16). Currently, early detection, quick
confirmation or targeted therapeutic strategies by molecular
biology are not available in clinical practice. Therefore, the
present study aimed to investigate the efficacy of a potential
anti-cancer compound, punicalagin, in osteosarcoma cells.
Although they represent the main available treatment
in osteosarcoma, chemotherapeutics are also toxic to normal tissue,
and can lead to myelosuppression, opportunistic infection, heart
damage and other adverse reactions, thereby decreasing the patient
survival rate and quality of life (17). Therefore, new agents with fewer
side effects and improved therapeutic advantages are required.
Block et al (18) suggested
that a phytochemical-rich diet, which includes compounds such as
polyphenols, salicylates, phytosterols, saponins, glucosinolates,
protease inhibitors, monoterpenes, terpenes, lectins, was
associated with decreased risk of cancer. Punicalagin is one of the
most abundant polyphenols in pomegranate. In addition, increasing
evidence suggests that punicalagin inhibits tumor invasion and
metastasis of various types of cancer, such as cervical (19), ovarian (20), colon (21) and lung cancer (22) as well as antioxidants in chronic
inflammation (23). To the best of
our knowledge, the present study was the first to identify that
punicalagin could significantly inhibit osteosarcoma cell
proliferation and invasion, induce apoptosis, and decrease
angiogenesis. Thus, the results of the present study may provide
insight into future therapeutic strategies against
osteosarcoma.
However, as previously shown in both rats and
humans, the poor bioavailability of punicalagin represents a
considerable limitation to pharmaceutical research on its potential
therapeutic effects in vivo (24). The low bioavailability of ellagic
acid generated from punicalagin is due to its hydrophilic structure
and large molecular weight, which limits its absorption by simple
diffusion, including oral administration (25). In addition, extremely low lipid
solubility further restricts its permeability through the
lipophilic layer of the gastrointestinal tract (26). Furthermore, punicalagin can be
metabolized into the bioavailable but relatively poor antioxidant,
hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora
in healthy humans (27). Based on
animal studies, the serum concentration after absorption of
punicalagin in rodents was ~30 µM (28), which is lower than the
concentration used in vitro in the present study. Thus, the
multifaceted therapeutic benefits of punicalagin observed in the
present study may be difficult to fully replicate in patients.
Furthermore, a relatively high concentration of punicalagin may
result in non-specific effects due to the biological differences
between osteoblasts and osteosarcoma cells. However, the
development of novel punicalagin derivatives, compound preparation
and administration methods may overcome these limitations in the
future.
The precise mechanisms through which punicalagin
inhibits osteosarcoma invasion and angiogenesis, as well as its
regulation, are not well understood. Several previous studies
demonstrated that multiple signaling pathways, including the MAPK
(29), β-catenin (19), TGF-β1 (30), AKT, and JNK (31) pathways, were modulated by
punicalagin administration. Furthermore, Adams et al
(32) suggested that punicalagin
decreased phosphorylation of the p65 subunit and binding of NF-κB
about 3.6-fold in colon cancer. In nerve cells, chronic
neuroinflammation and oxidative stress were dramatically diminished
by punicalagin via NF-κB inhibition (33). Furthermore, vascular endothelial
growth factor, an NF-κB transcriptional target gene, was
downregulated by punicalagin, thereby decreasing angiogenesis in
the tumor environment (34,35).
The in vitro and in vivo results of the present study
were consistent with previous reports, and demonstrated the
therapeutic potential of punicalagin against mesodermal illness
likes osteosarcoma, through modulation of NF-κB activity.
In general, NF-κB signaling controls many cellular
processes, including immune responses, immune cell proliferation
and viability, lymphogenesis and B cell maturation (36). The NF-κB pathway is also involved
in the regulation of skeletal muscle cell differentiation (37). Recently, activation of NF-κB was
demonstrated to increase glucose uptake and glycolytic flux in
sarcoma cells, which suggested that NF-κB played a crucial role in
the development of osteosarcoma malignancies (38). Consistently, Gong et al
(39) found at least 75%
osteosarcoma tissues from patients showed positive stain of
activated NF-κB pathways and patients whose osteosarcoma with
active NF-κB had short median overall survival time as compared
with patients whose osteosarcoma had inactive NF-κB. Expression of
metastasis-associated proteins, angiogenesis, cell invasion and
metastasis have also been linked to NF-ĸB activation in
osteosarcoma (8). Liao et
al (40) used short hairpin
RNA to knockdown NF-ĸB expression, which abolished cell invasion
and metastasis in osteosarcoma. In another previous study, the
NF-ĸB inhibitor QNZ suppressed NF-ĸB activation, which resulted in
downregulation of proteins associated with metastasis, cell
migration and cell invasion in osteosarcoma cells (41). The present study further
demonstrated that NF-κB is an important transcription factor during
pathogenesis of osteosarcoma, and that punicalagin was involved in
modulating the expression of molecules downstream of NF-κ B, such
as IL-6, and IL-8.
A previous study demonstrated that IL-6 and IL-8
genes were directly regulated by the NF-κB pathway (42) and that IL-6 and IL-8 levels
increased with NF-κB overexpression during chronic inflammation in
bone and joint tissues (43). IL-6
and IL-8 activation promotes an inflammatory microenvironment
during malignant progression (44)
and these cytokine-associated chemotactic effects are required for
the initiation of tumor-associated inflammation and
neovascularization (45). The
present study indicated that punicalagin decreased IL-6 as well as
IL-8 production by osteosarcoma cells, which was consistent with
angiogenesis inhibition in xenograft models. Thus, these findings
further elucidate the mechanisms underlying the preventive and
therapeutic potential of punicalagin against osteosarcoma.
Although a previous study suggested that pomegranate
extract, including a large amount of active punicalagin, had a
strong anti-aging effect through the mTOR pathway (46), the present study failed to confirm
this finding. Thus, the present results highlight the significance
of punicalagin as a promising tumor suppressor in osteosarcoma by
targeting NF-κB, but not mTOR pathway. Further characterization of
this compound will provide a new insight into punicalagin-mediated
suppression of osteosarcoma genesis and development.
In conclusion, punicalagin treatment inhibited
osteosarcoma growth, including proliferation, invasion and
angiogenesis through NF-κB suppression. Further in-depth and
long-term studies are required in order to establish the
therapeutic target of the NF-κB signaling pathway in
punicalagin-induced cell survival and inhibition of invasive
abilities, as well as excessive angiogenesis. Nonetheless, the
results of our present study provide preliminary evidence to
support punicalagin, a phytochemical used in herbal medicine, as a
novel and effective candidate for the systemic treatment and/or
chemoenhancement of osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HW designed and directed the study, and analyzed and
interpreted the data. TH performed the experiments and wrote the
manuscript. XZ performed the literature search, analyzed the data
and designed the figures. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Animal Ethics
Committee of The Second Affiliated Hospital of Air Force Medical
University (approval no. 201904-11).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NF-κB
|
nuclear factor-κB
|
|
PMA
|
phorbol myristate acetate
|
|
mTOR
|
mammalian target of rapamycin
|
|
Ctrl
|
control
|
References
|
1
|
Damron TA, Ward WG and Stewart A:
Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National cancer
data base report. Clin Orthop Relat Res. 459:40–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.PubMed/NCBI
|
|
4
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma (M)//. Jaffe N, Bruland OS and Bielack S: Pediatric
and adolescent osteosarcoma Boston, MA: Springer US; pp. 3–13.
2010
|
|
5
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bielack SS, Smeland S, Whelan JS, Marina
N, Jovic G, Hook JM, Krailo MD, Gebhardt M, Pápai Z, Meyer J, et
al: Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance
pegylated interferon Alfa-2b versus MAP alone in patients with
resectable high-grade osteosarcoma and good histologic response to
preoperative MAP: First results of the EURAMOS-1 good response
randomized controlled trial. J Clin Oncol. 33:2279–2287. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang J, Wang Z, Tang E, Fan Z, McCauley
L, Franceschi R, Guan K, Krebsbach HP and Wang C: Inhibition of
osteoblastic bone formation by nuclear factor-kappaB. Nat Med.
15:682–689. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Avnet S, Di Pompo G, Chano T, Errani C,
Ibrahim-Hashim A, Gillie RJ, Donati DM and Baldini N:
Cancer-associated mesenchymal stroma fosters the stemness of
osteosarcoma cells in response to intratumoral acidosis via NF-κB
activation. Int J Cancer. 140:1331–1345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mongre RK, Sodhi SS, Ghosh M, Kim JH, Kim
N, Sharma N and Jeong DK: A new paradigm to mitigate osteosarcoma
by regulation of MicroRNAs and suppression of the NF-κB signaling
cascade. Dev Reprod. 18:197–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang QL, Xie XB, Wang J, Chen Q, Han AJ,
Zou CY, Yin JQ, Liu DW, Liang Y, Zhao ZQ, et al: Glycogen synthase
kinase-3β, NF-κB signaling, and tumorigenesis of human
osteosarcoma. J Natl Cancer Inst. 104:749–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Testa JR and Tsichlis PN: AKT signaling in
normal and malignant cells. Oncogene. 24:7391–7393. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:170232017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Germano G, Allavena P and Mantovani A:
Cytokines as a key component of cancer-related inflammation.
Cytokine. 43:374–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng N, Gao S, Guo X, Wang G, Cheng C, Li
M and Liu K: Silencing of VEGF inhibits human osteosarcoma
angiogenesis and promotes cell apoptosis via VEGF/PI3K/AKT
signaling pathway. Am J Transl Res. 8:10052016.PubMed/NCBI
|
|
15
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Behjati S, Tarpey PS, Haase K, Ye H, Young
MD, Alexandrov LB, Farndon SJ, Collord G, Wedge DC, Martincorena I,
et al: Recurrent mutation of IGF signalling genes and distinct
patterns of genomic rearrangement in osteosarcoma. Nat Commun.
8:159362017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang B and Zhang Y, Li R, Li J, Lu X and
Zhang Y: The efficacy and safety comparison of first-line
chemotherapeutic agents (high-dose methotrexate, doxorubicin,
cisplatin, and ifosfamide) for osteosarcoma: A network
meta-analysis. J Orthop Surg Res. 15:512020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Block G, Patterson B and Subar A: Fruit,
vegetables, and cancer prevention: A review of the epidemiological
evidence. Nutr Cancer. 18:1–29. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang J, Li B, Hong S, Liu C, Min J, Hu M,
Li Y, Liu Y and Hong L: Punicalagin suppresses the proliferation
and invasion of cervical cancer cells through inhibition of the
β-catenin pathway. Mol Med Rep. 16:1439–1444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang JM, Min J, Li BS, Hong SS, Liu C, Hu
M, Li Y, Yang J and Hong L: Therapeutic effects of punicalagin
against ovarian carcinoma cells in association with β-Catenin
signaling inhibition. Int J Gynecol Cancer. 26:1557–1563. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Omar U, Aloqbi A, Yousr M and Howell NK:
Effect of punicalagin on human colon cancer caco-cells. Malaysian J
Nutri. 22:125–136. 2016.
|
|
22
|
Li Y, Yang F, Zheng W, Hu M, Wang J, Ma S,
Deng Y, Luo Y, Ye T and Yin W: Punica granatum (pomegranate) leaves
extract induces apoptosis through mitochondrial intrinsic pathway
and inhibits migration and invasion in non-small cell lung cancer
in vitro. Biomed Pharmacother. 80:227–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aloqbi A, Omar U, Yousr M, Grace M, Lila
MA and Howell N: Antioxidant activity of pomegranate juice and
punicalagin. Nat Sci. 8:235–246. 2016.
|
|
24
|
Mertens-Talcott SU, Jilma-Stohlawetz P,
Rios J, Hingorani L and Derendorf H: Absorption, metabolism, and
antioxidant effects of pomegranate (Punica granatum L.) polyphenols
after ingestion of a standardized extract in healthy human
volunteers. J Agric Food Chem. 54:8956–8961. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vora A, Londhe V and Pandita N: Herbosomes
enhance the in vivo antioxidant activity and bioavailability of
punicalagins from standardized pomegranate extract. J Funct Foods.
12:540–548. 2015. View Article : Google Scholar
|
|
26
|
Seeram NP, Lee R and Heber D:
Bioavailability of ellagic acid in human plasma after consumption
of ellagitannins from pomegranate (Punica granatum L.) juice. Clin
Chim Acta. 348:63–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cerdá B, Espín JC, Parra S, Martínez P and
Tomás-Barberán FA: The potent in vitro antioxidant ellagitannins
from pomegranate juice are metabolised into bioavailable but poor
antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the
colonic microflora of healthy humans. Eur J Nutr. 43:205–220. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cerdá B, Llorach R, Cerón JJ, Espín JC and
Tomás-Barberán FA: Evaluation of the bioavailability and metabolism
in the rat of punicalagin, an antioxidant polyphenol from
pomegranate juice. Eur J Nutr. 42:18–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chu G, Zhang W, Chen M, Yang H and Yuan Z:
Punicalagin inhibits RANKL-induced osteoclastogenesis by
suppressing NF-κB and MAPK signaling pathways. Int J Clin Exp Med.
11:6571–6582. 2018.
|
|
30
|
Tang J, Liu C, Min J, Hu M, Li Y and Hong
L: Potential therapeutic role of punicalagin against
mechanical-trauma-induced stress urinary incontinence via
upregulation of Nrf2 and TGF-β1 signaling. Int Urogynecol J.
28:947–955. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwatake M, Okamoto K, Tanaka T and Tsukuba
T: Punicalagin attenuates osteoclast differentiation by impairing
NFATc1 expression and blocking Akt-and JNK-dependent pathways. Mol
Cell Biochem. 407:161–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adams LS, Seeram NP, Aggarwal BB, Takada
Y, Sand D and Heber D: Pomegranate juice, total pomegranate
ellagitannins, and punicalagin suppress inflammatory cell signaling
in colon cancer cells. J Agric Food Chem. 54:980–985. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim YE, Hwang CJ, Lee HP, Kim CS, Son DJ,
Ham YW, Hellström M, Han SB, Kim HS, Park EK and Hong JT:
Inhibitory effect of punicalagin on lipopolysaccharide-induced
neuroinflammation, oxidative stress and memory impairment via
inhibition of nuclear factor-kappaB. Neuropharmacology. 117:21–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Naugler WE and Karin M: NF-kappaB and
cancer-identifying targets and mechanisms. Curr Opin Genet Dev.
18:19–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toi M, Bando H, Ramachandran C, Melnick
SJ, Imai A, Fife RS, Carr RE, Oikawa T and Lansky EP: Preliminary
studies on the anti-angiogenic potential of pomegranate fractions
in vitro and in vivo. Angiogenesis. 6:121–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bakkar N and Guttridge DC: NF-kappaB
signaling: A tale of two pathways in skeletal myogenesis. Physiol
Rev. 90:495–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bakkar N, Wang J, Ladner KJ, Wang H,
Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD and
Guttridge DC: IKK/NF-kappaB regulates skeletal myogenesis via a
signaling switch to inhibit differentiation and promote
mitochondrial biogenesis. J Cell Biol. 180:787–802. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Londhe P, Yu PY, Ijiri Y, Ladner KJ,
Fenger JM, London C, Houghton PJ and Guttridge DC: Classical NF-κB
metabolically reprograms sarcoma cells through regulation of
hexokinase 2. Front Oncol. 8:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gong T, Su X, Xia Q, Wang J and Kan S:
Expression of NF-κB and PTEN in osteosarcoma and its clinical
significance. Oncol Lett. 14:6744–6748. 2017.PubMed/NCBI
|
|
40
|
Liao CL, Lin JH, Lien JC, Hsu SC, Chueh
FS, Yu CC, Wu PP, Huang YP, Lin JG and Chung JG: The crude extract
of Corni Fructus inhibits the migration and invasion of U-2 OS
human osteosarcoma cells through the inhibition of matrix
metalloproteinase-2/-9 by MAPK signaling. Environ Toxicol.
30:53–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pan PJ, Tsai JJ and Liu YC: Amentoflavone
inhibits metastatic potential through suppression of ERK/NF-κB
activation in osteosarcoma U2OS cells. Anticancer Res.
37:4911–4918. 2017.PubMed/NCBI
|
|
42
|
Hoesel B and Schmid JA: The complexity of
NF-kappaB signaling in inflammation and cancer. Mol Cancer.
12:862013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lv F, Song LJ, Wang XH, Qiu F and Li XF:
The role of Act1, a NF-κB-activating protein, in IL-6 and IL-8
levels induced by IL-17 stimulation in SW982 cells. Pharm Biol.
51:1444–1450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Karin M: NF-κB as a critical link between
inflammation and cancer. Cold Spring Harb Perspect Biol.
1:a0001412009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sparmann A and Bar-Sagi D: Ras-induced
interleukin-8 expression plays a critical role in tumor growth and
angiogenesis. Cancer Cell. 6:447–458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Syed DN, Chamcheu JC, Adhami VM and
Mukhtar H: Pomegranate extracts and cancer prevention: Molecular
and cellular activities. Anticancer Agents Med Chem. 13:1149–1161.
2013. View Article : Google Scholar : PubMed/NCBI
|