Introduction
Immunoglobulin A (IgA) nephropathy (IgAN) is the
most prevalent form of primary glomerulonephritis and frequently
causes end-stage renal disease, to the extent that 30–40% of
patients with biopsy-proven IgAN will progress to end-stage renal
disease in 20 years (1). IgAN is
characterized by highly heterogeneous clinical features, ranging
from asymptomatic microscopic hematuria to a rapidly progressive
form of glomerulonephritis (2).
Renal biopsy is the initial method of IgAN diagnosis and disease
assessment (1). Because of its
invasiveness, renal biopsy is difficult, and repeated procedures
pose considerable inconveniences for patients. IgA1 is the main
subclass of IgA; however, the sensitivity and specificity of serum
IgA1 and common biomarkers (e.g. serum creatinine, glomerular
filtration rate, and proteinuria) are insufficient for IgAN
diagnosis. Thus, there is an urgent need to investigate the
mechanism underlying the development of IgAN, and to screen for
disease biomarkers and targets of IgAN for diagnosis and therapy
(2,3).
Currently, multiple biomarkers for IgAN are known,
such as proteins and microRNAs (miRNAs/miRs) in blood and urine
(3). miRNAs constitute a class of
non-coding RNAs that regulate gene expression by binding to
complementary sequences in the coding portion or 3′ untranslated
regions (UTRs) of mRNAs (4).
miRNAs have been shown to exhibit functional dysregulation in
diseases such as cancer, cardiopathy, and nephropathy (5). miRNAs are not easily degraded by
RNase and are stable in blood (6);
therefore, they have previously been considered to be good
biomarkers for diagnosis. Previously, urinary levels of miR-146a,
miR-155, miR-200a, miR-200b and miR-429 were shown to be associated
with clinical and histological severity of IgAN (7,8).
miR-148b and miR-let7b are known to promote the accumulation of
IgA1 in IgAN, and are potential serum biomarkers for the detection
of primary IgAN (9–11). However, there is not much research
into the association between miRNAs and IgAN in blood, and the
roles of various miRNAs remain unclear in the pathogenesis of
IgAN.
Peripheral blood mononuclear cells (PBMCs) are known
to participate in IgA deposition and IgAN development, and previous
studies have shown that miRNAs in PBMCs could promote the
progression of IgAN (9,10,12,13).
The miRNA expression profile of PBMCs in patients with IgAN has
been studied by using a miRNA microarray, and miR-148b was found to
be a potential promoter of IgAN (9). In the present study, small RNAs from
PBMCs were sequenced to identify novel miRNAs associated with IgAN.
Furthermore, the potential roles of differentially expressed miRNAs
were investigated through bioinformatics analysis. In total, 44
differentially expressed miRNAs were identified, and this study is
the first to report an association for most of these miRNAs with
IgAN, indicating that these miRNAs may serve as biomarkers or
targets for IgAN.
Materials and methods
Patients
In this study, 10 healthy participants and 10
patients with IgAN were enrolled during the period from January
2018 to December 2018 in The Second Affiliated Hospital of
Guangzhou University of Chinese Medicine. The admission criteria of
IgAN nephropathy patients were as follows: i) IgAN was confirmed by
renal biopsy; ii) normal renal function (blood creatinine, blood
urea nitrogen); iii) ≥18 years old; iv) no previous hormone,
immunosuppressant or kidney transplantation treatments; v) the
patients did not experience secondary IgAN such as purpura
nephritis, lupus nephritis and hepatitis B-related nephritis. The
exclusion criteria for healthy participants were as follows: i)
Those with chronic diseases, such as coronary heart disease,
hypertension, acute and chronic cerebrovascular disease or
diabetes; ii) those with infectious diseases and fever; iii) those
with mental illnesses; iv) those with any metabolic syndromes. The
research protocol was approved by the Medical Ethical Committee of
Guangdong Provincial Hospital of Chinese Medicine, and all patients
provided written informed consent. All patients were diagnosed with
IgAN by an experienced pathologist, and the clinical features are
shown in Table I.
 | Table I.Demographic and baseline clinical
data of the healthy participants and patients with IgAN. |
Table I.
Demographic and baseline clinical
data of the healthy participants and patients with IgAN.
| Characteristic | IgAN | Healthy
control |
|---|
| Number of
cases | 10 | 10 |
| Sex,
male:female | 5:5 | 5:5 |
| Age, years | 29.30±4.24 |
27.2±9.31 |
| IgA, g/l |
3.53±1.11 |
2.36±0.52 |
| Serum creatinine,
µmol/l |
74.40±20.04 |
80.8±14.48 |
| GFR, ml/min/1.73
m2 | 108.26±19.52 | 102.01±10.56 |
PBMC small RNA isolation
Human PBMC isolation buffer (TBD sciences; Tianjin
Haoyang Biological Products Technology Co. Ltd.) was used to
isolate PBMCs from 2 ml peripheral blood samples of patients, in
accordance with the manufacturer's protocol. TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to isolate
total RNA from PBMCs, in accordance with the manufacturer's
protocol. RNA molecules of 18–40 nucleotides in length were
collected for the analysis of small RNAs.
Small RNA sequencing and
differentially expressed miRNA screening
Small RNA library preparation was performed using
the NEB Next® Multiplex Small RNA Library Prep Set for
Illumina® (New England BioLabs, Inc.). Briefly, RNA
molecules were ligated with a 3′ RNA adapter, followed by ligation
with a 5′ adapter. Subsequently, adapter-ligated RNAs were
subjected to reverse transcription into cDNA at 50°C for 60 min and
amplified. PCR was performed using the following thermocycling
conditions: Initial denaturation at 94°C for 30 sec; followed by 15
cycles of denaturation at 94°C for 15 sec, annellation at 62°C for
30 sec, extension at 70°C for 15 sec; and a final extension at 70°C
for 5 min. The PCR products were then size-selected by
polyacrylamide gel electrophoresis, in accordance with the
manufacturer's instructions. Purified library products were
evaluated using the Agilent 2200 TapeStation (Agilent Technologies,
Inc.) and diluted to 2 pM for cluster generation in situ on the
HiSeq 2500 single-end flow cell (Illumina, Inc.), followed by
sequencing on the HiSeq 2500 at 1×50 base pairs.
For miRNA analysis, clean reads were mapped to hg19.
miRNAs and Piwi-interacting (pi)RNAs were identified using miRBase
version 21 (www.mirbase.org) (14) and pirnabank (pirnabank.ibab.ac.in)
(15), respectively. Transfer
(t)RNA, ribosomal (r)RNA, small nuclear (sn)RNA and small nuclear
non-coding (sno)RNA data were from Rfam12.1 (rfam.xfam.org)
(16). The software edgeR
(17) was used to compare the
expression of miRNAs between PBMCs from healthy participants and
those from patients with IgAN and to calculate the P-value. miRNA
reads were normalized to miRNA reads per million reads, and the
average values were examined. miRNAs with a fold change ≥2 and
P<0.05 were considered to be indicate a statistically
significant difference in the expression profile (Table SI).
Kyoto Encyclopedia of Genes and
Genomes (KEGG), Gene Ontology (GO) and miRNA-mRNA regulatory
networks analyses
KEGG and GO functional annotations were used to
highlight the most overrepresented GO terms and KEGG pathways that
were closely associated with top 10 differentially expressed
miRNAs. KEGG pathways analysis was based on the KEGG database
(http://www.genome.jp/) (18) to obtain the signal transduction and
disease pathway annotation information for the candidate target
genes. The P-value was calculated by a Fisher's exact test, and the
signal transduction and disease pathways with statistical
significance were compared with the background were obtained with
P<0.05 as the significant threshold. GO functional analysis was
based on the GO resource (http://geneontology.org) (19) to provide molecular function,
biological process and cellular component annotation for candidate
target genes. P<0.05 was considered to indicate a statistically
significant difference of the enrichment degree of GO annotation.
miRNA-target gene network analysis was performed using the mirWalk
program (http://mirwalk.umm.uni-heidelberg.de/) (20).
miRNA binding sites prediction on the
C1GALT1 3′ UTR
The potential miRNAs that may regulate C1GALT1 were
predicted using TargetScan (http://www.targetscan.org) (21) and miRDB (http://mirdb.org) (22).
Results
Small RNA sequencing and analysis in
healthy participants and patients with IgAN
To study the aberrant expression of miRNAs in the
PBMCs of patients with IgAN, small RNAs were isolated and cDNA
libraries were prepared. The cDNA libraries were sequenced, and the
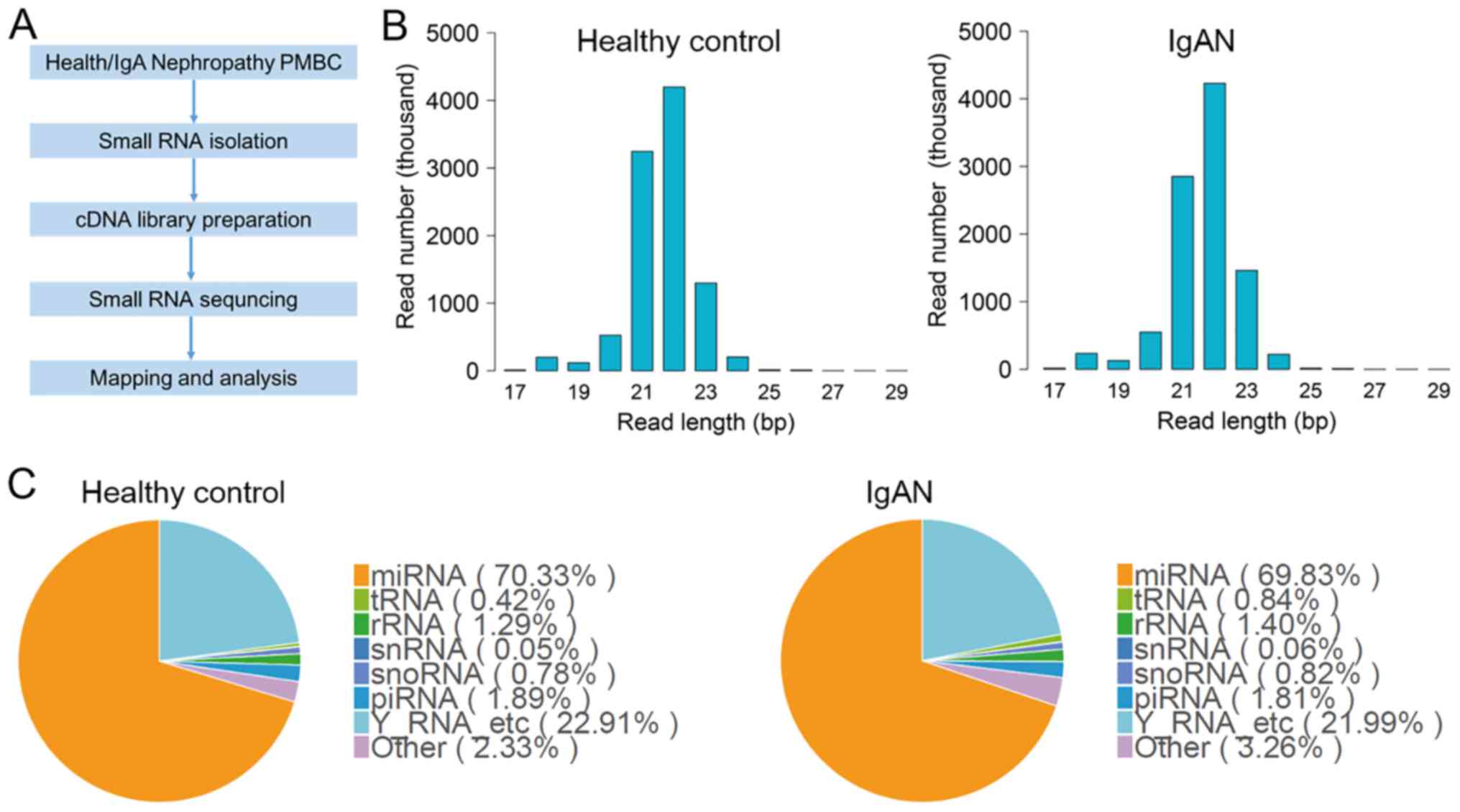
data were analyzed following the workflow shown in Fig. 1A. Small RNAs isolated from the
PBMCs of healthy participants and patients with IgAN were primarily
composed of 18–24 bp sequences (Fig.
1B). The numbers of 22 and 21 bp nucleotides were highest in
these cells, which indicated that mature miRNAs were the most
abundant class of small RNAs in PBMCs (Fig. 1B). To annotate the RNA species in
PBMCs, the experimental sequences were mapped to databases
containing human miRNAs, rRNAs, tRNAs, snRNAs and snoRNAs. Fig. 1C shows that miRNAs, tRNAs, rRNAs,
snRNAs, snoRNAs, piRNAs and Y RNAs were detected in PBMCs in this
study. miRNAs were the most common RNA species in both healthy
participants (70.33% of small RNAs) and patients with IgAN (69.83%
of small RNAs); these levels were not significantly different
between groups.
Significantly differentially expressed
miRNAs in PBMCs of patients with IgAN
To identify miRNAs associated with the development
of IgAN, miRNAs that were significantly differentially expressed
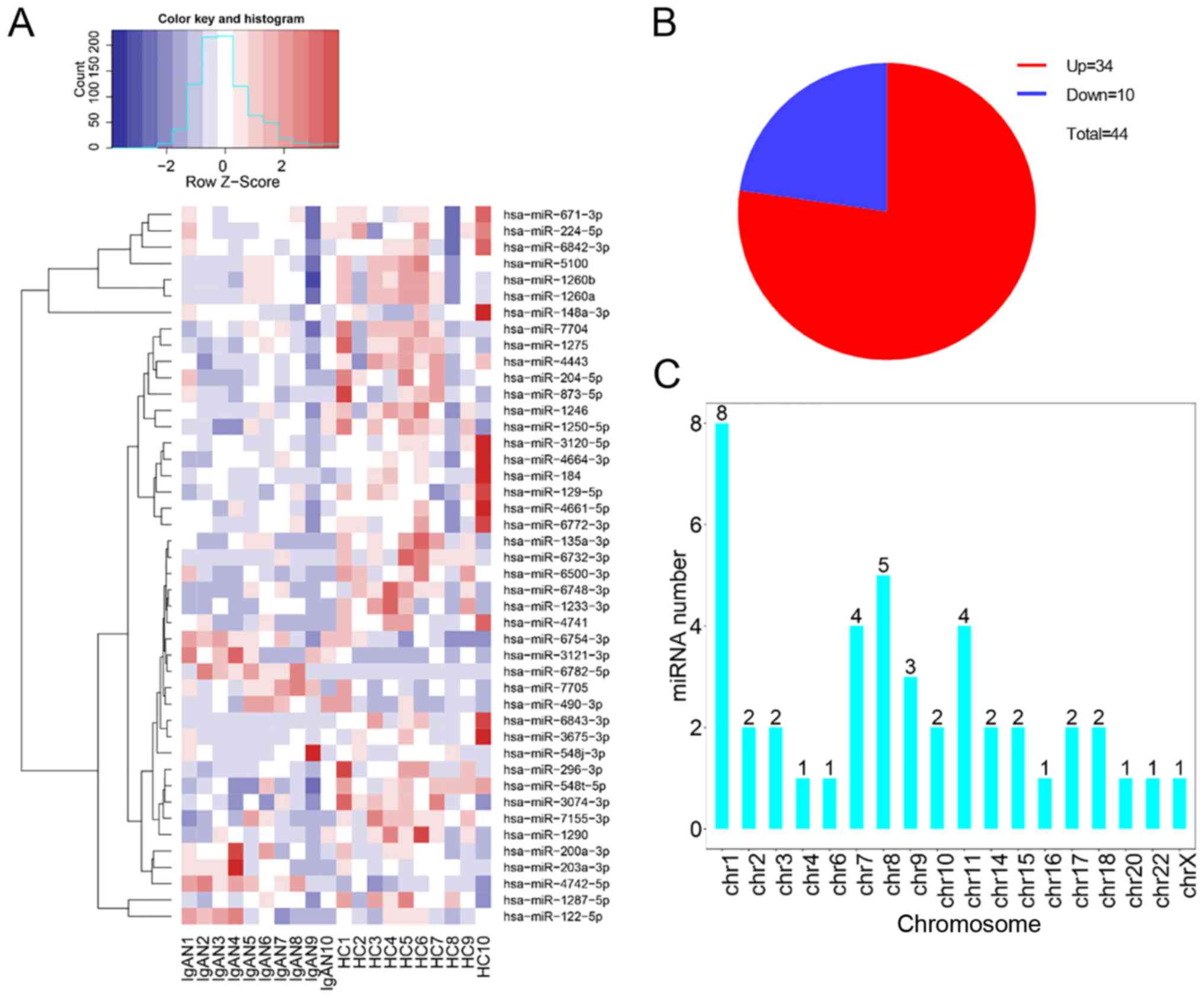
were assessed (P<0.05, fold change ≥2 or ≤0.5). As shown in
Fig. 2A and B, there were 44
differentially expressed miRNAs identified: A total of 34 miRNAs
were upregulated and 10 miRNAs were downregulated in PBMCs from
patients with IgAN, compared with PBMCs from healthy participants.
The chromosomal distribution of these differentially expressed
miRNAs showed that most originated from chromosomes 1, 7, 8 and 11.
In particular, chromosome 1 had 8 miRNAs, which constituted the
highest number (Fig. 2C).
Functional prediction for upregulated
genes
To elucidate the potential roles of differentially
expressed miRNAs in IgAN, the top 10 upregulated miRNAs were
initially identified. Among them, miR-6843-3p showed the most
significant upregulation in patients with IgAN; miR-671-3p and
hsa-miR-5100 both showed high abundance in healthy participants and
patients with IgAN (Table II).
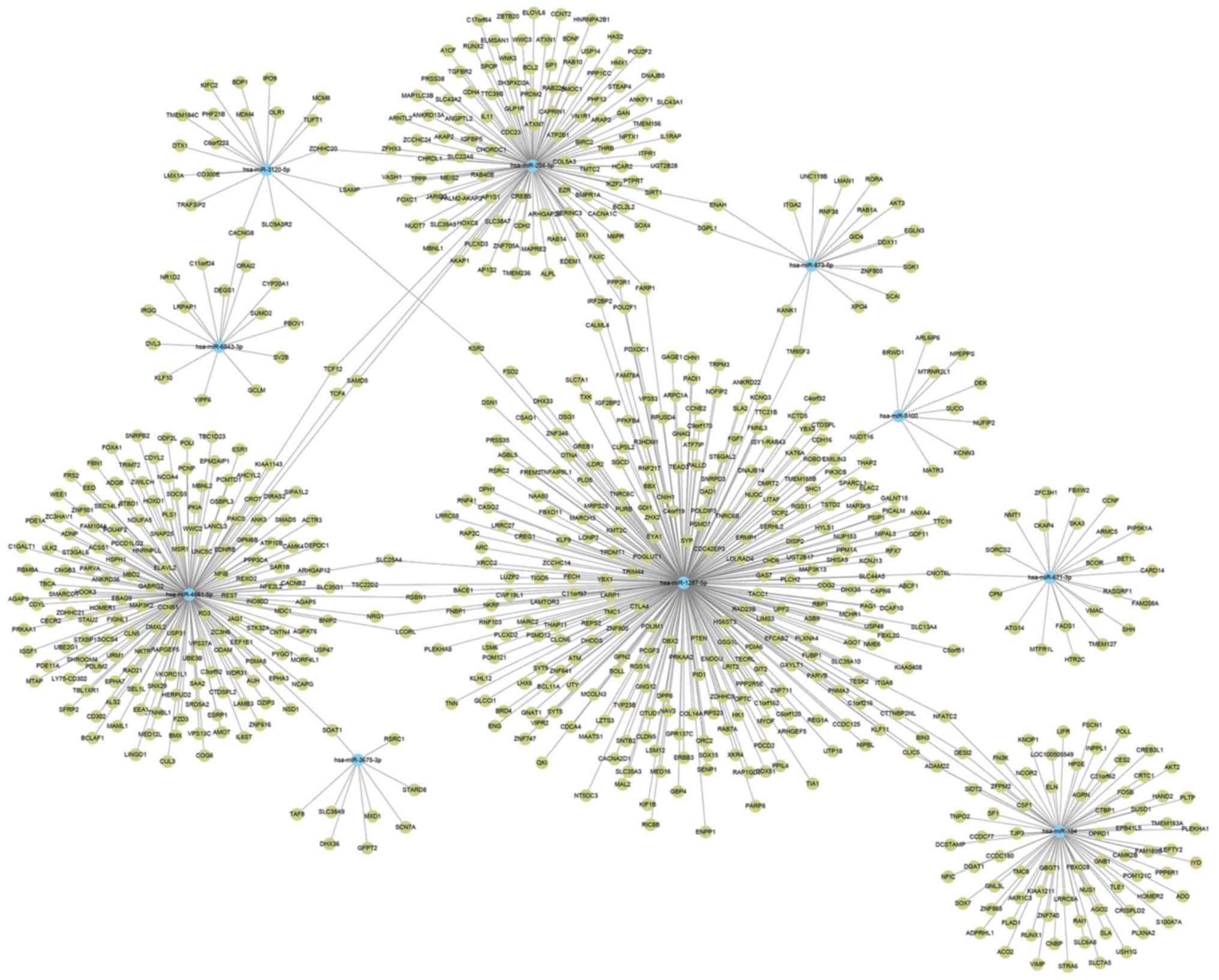
The 3′ UTRs of mRNAs are targeted by miRNAs, and the mRNA
stabilities are reduced or translation efficiency is inhibited. In
this study, from the top 10 upregulated miRNAs, >800 potential
target genes were identified; subsequently, an interaction network
of miRNAs and mRNAs was constructed by mirWalk program (Fig. 3). miR-1287-5p had the largest
subnetwork, which included 397 potential target genes. KEGG pathway
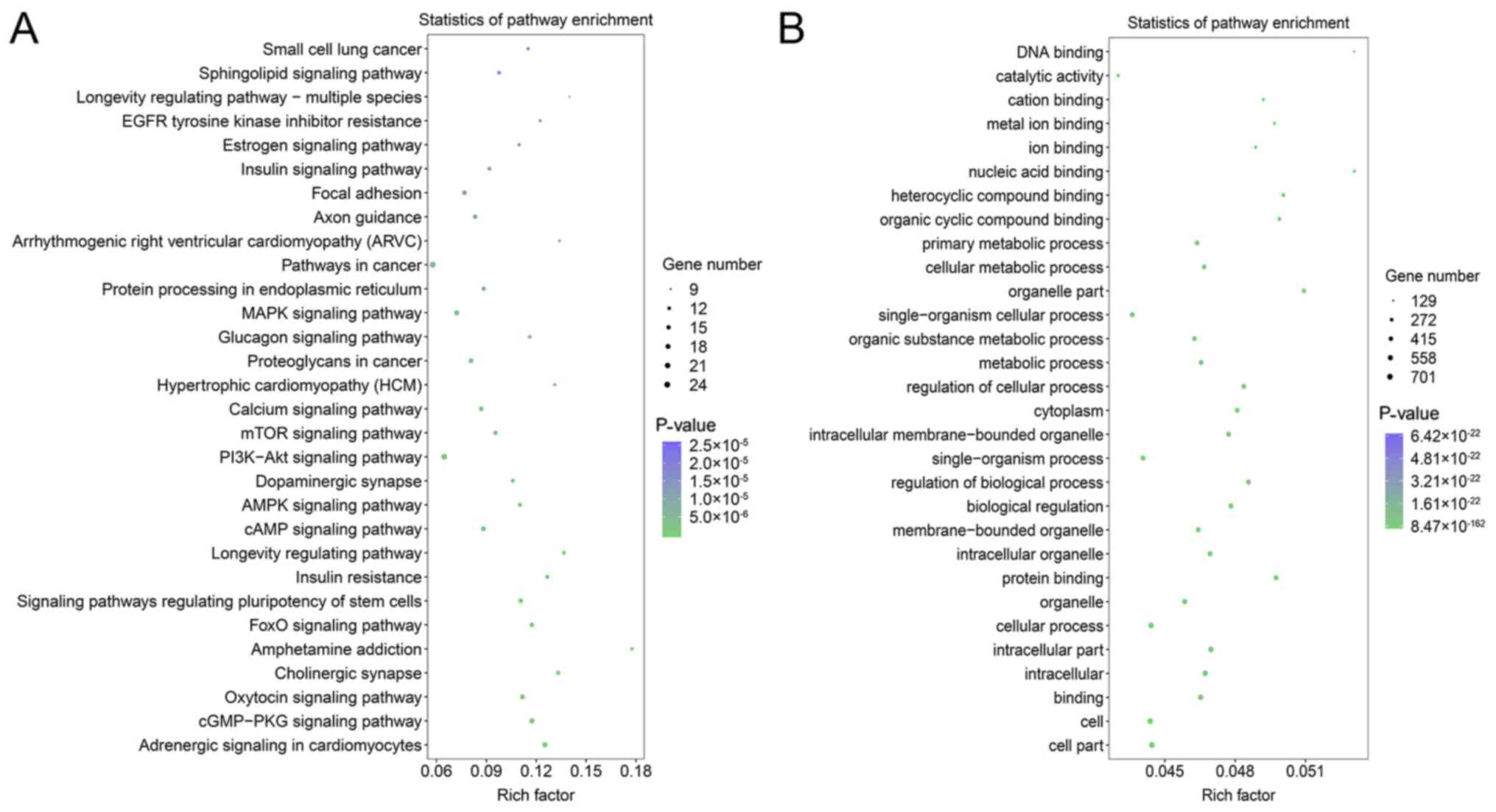
analyses of enriched target genes were conducted, which revealed
that ‘pathways in cancer’, the ‘PI3K-Akt signaling pathway’, the
‘MAPK signaling pathway’, the ‘cGMP-PKG signaling pathway’, the
‘oxytocin signaling pathway’ and the ‘adrenergic signaling in
cardiomyocytes’ were likely targets of the identified miRNAs
(Fig. 4A). GO term analyses showed
that most genes were enriched in ‘binding’ and ‘protein binding’ in
the molecular function component. In the cellular component, genes
were mainly enriched in the ‘organelle’, ‘cytoplasm’ and
‘membrane-bounded organelle’ (Fig.
4B). In the biological component, genes were mainly enriched in
‘cellular process’, ‘biological regulation’, ‘regulation of
biological process’ and ‘single-organism process’ (Fig. 4B).
 | Table II.Top 10 upregulated miRNAs in small
RNA sequencing analysis. |
Table II.
Top 10 upregulated miRNAs in small
RNA sequencing analysis.
| Order | miRNA | IgAN RPM | Healthy control
RPM | log2 (fold
change) | P-value |
|---|
| 1 |
hsa-miR-6843-3p | 0.054 | 0.399 | 2.8854 | 0.000744547 |
| 2 |
hsa-miR-3675-3p | 0.079 | 0.349 | 2.1433 | 0.00775704 |
| 3 |
hsa-miR-3120-5p | 0.673 | 2.901 | 2.1079 | 0.000941252 |
| 4 | hsa-miR-873-5p | 0.786 | 3.282 | 2.062 | 0.005967363 |
| 5 | hsa-miR-5100 | 28.728 | 113.291 | 1.9795 | 0.000493526 |
| 6 |
hsa-miR-4661-5p | 1.323 | 4.252 | 1.6843 | 0.006082614 |
| 7 | hsa-miR-204-5p | 0.717 | 2.122 | 1.5654 | 0.005360923 |
| 8 | hsa-miR-184 | 0.57 | 1.687 | 1.5654 | 0.007100943 |
| 9 | hsa-miR-671-3p | 51.661 | 140.942 | 1.448 | 0.006420685 |
| 10 |
hsa-miR-1287-5p | 7.066 | 15.25 | 1.1098 | 0.009113073 |
Functional prediction for
downregulated genes
The top 10 downregulated miRNAs are listed in
Table III; miR-6782-5p showed
the most significant downregulation in patients with IgAN, and
miR-122-5p showed the highest abundance. The target genes of the 10
downregulated miRNAs were predicted, and an interaction network of
miRNAs and mRNAs was constructed (Fig.
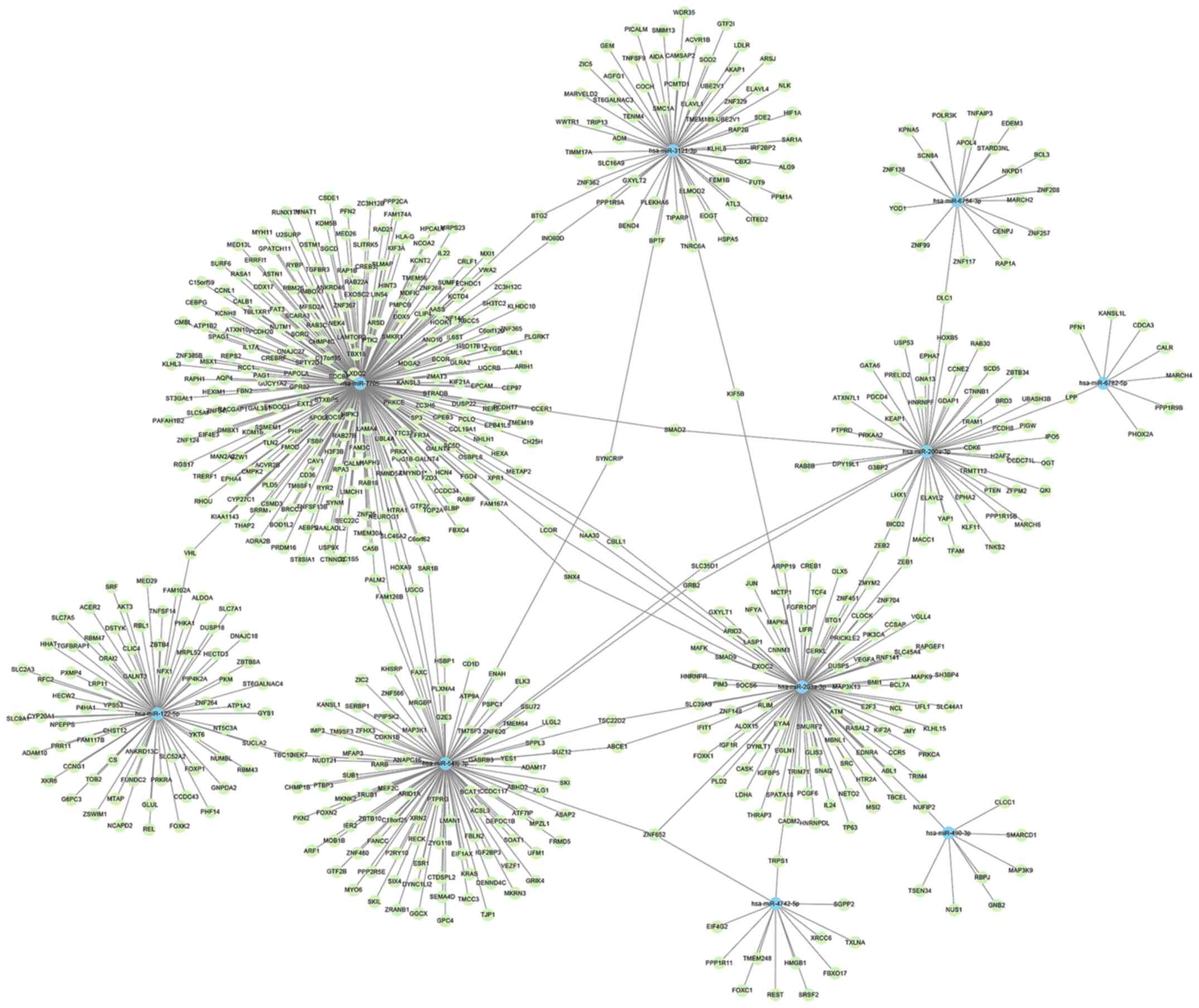
5). This demonstrated that miR-7705 had the largest subnetwork,
which included 242 potential target genes. To identify the
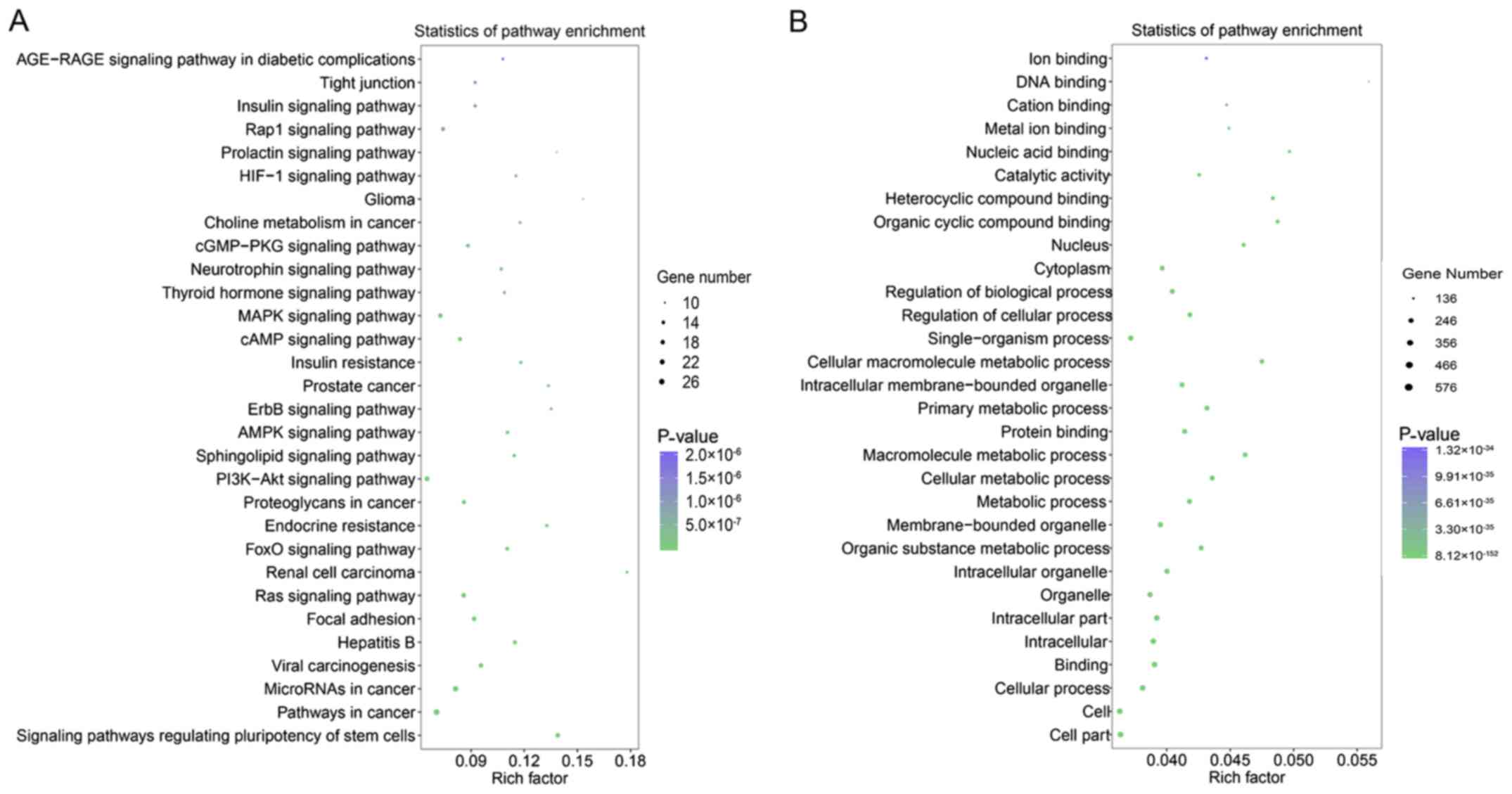
potential roles of the downregulated miRNAs, GO and KEGG enrichment
analyses were performed. The ‘PI3K-Akt signaling pathway’,
‘microRNAs in cancer’, ‘pathways in cancer’, ‘Ras signaling
pathway’ and ‘MAPK signaling pathway’ were identified as likely
targets of the miRNAs (Fig. 6A).
GO analysis showed that most genes in the molecular function group
were involved in ‘organic cyclic compound binding’ and ‘protein
binding’. Within the cellular component, ‘organelle’, ‘cytoplasm’,
and ‘nucleus’ had the largest groups of enriched genes (Fig. 6B). In the biological component,
‘cellular process’, ‘single-organism process’, ‘metabolic process’,
‘regulation of biological process’ and ‘primary metabolic process’
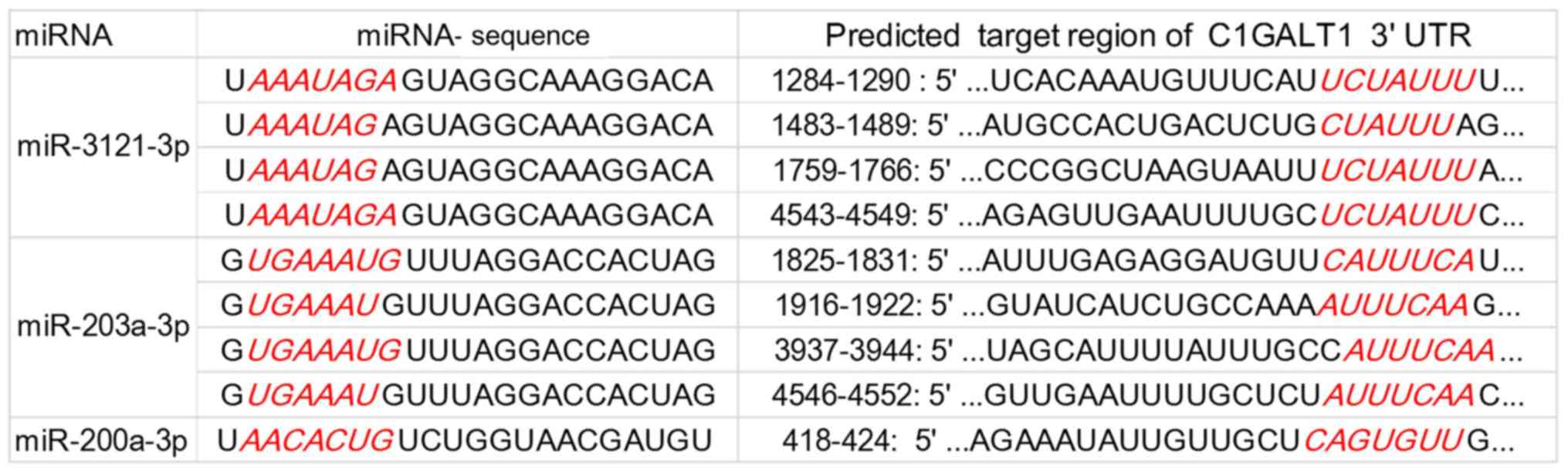
were the top 5 enriched groups of target genes (Fig. 6B). Because aberrant
O-glycosylation, which is catalyzed by core 1 synthase,
glycoprotein-N-acetylgalactosamine 3-β-galactosyltransferase 1
(C1GALT1), is critical for IgA accumulation, potential miRNAs that
can regulate it were predicted using TargetScan and miRDB. As shown
in Fig. 7, the C1GALT1 3′ UTR
possesses binding regions for miR-3121-3p (4 regions), miR-203a-3p
(4 regions), and miR-200a-3p (1 region).
 | Table III.Top 10 downregulated miRNAs in small
RNA sequencing analysis. |
Table III.
Top 10 downregulated miRNAs in small
RNA sequencing analysis.
| Order | miRNA | IgAN RPM | Healthy control
RPM | log2 (fold
change) | P-value |
|---|
| 1 |
hsa-miR-6782-5p | 0.149 | 0 | −3.8972 | 0.000324358 |
| 2 |
hsa-miR-3121-3p | 0.152 | 0.029 | −2.3899 | 0.005884351 |
| 3 |
hsa-miR-203a-3p | 1.039 | 0.306 | −1.7636 | 0.015739813 |
| 4 |
hsa-miR-548j-3p | 0.54 | 0.182 | −1.569 | 0.024266267 |
| 5 | hsa-miR-122-5p | 19.627 | 6.864 | −1.5157 | 0.009925759 |
| 6 | hsa-miR-490-3p | 0.324 | 0.117 | −1.4695 | 0.013063963 |
| 7 |
hsa-miR-6754-3p | 0.295 | 0.115 | −1.3591 | 0.020103511 |
| 8 |
hsa-miR-200a-3p | 2.488 | 1.091 | −1.1893 | 0.026159717 |
| 9 |
hsa-miR-4742-5p | 1.616 | 0.734 | −1.1386 | 0.00109596 |
| 10 | hsa-miR-7705 | 0.32 | 0.154 | −1.0551 | 0.042978753 |
Discussion
The exact pathogenesis of IgAN is not well-defined,
although it is characterized by the aberrant glycosylation and
deposition of IgA in mesangial areas. Previous studies found that
abnormal immune regulation is closely associated with the
development of IgAN (3,9,12,13).
Proliferating cell nuclear antigen expression increased in PBMCs,
and the percentage of circulating B lymphocytes (CD19) increased in
patients with IgAN (12,23), indicating abnormal proliferation of
PBMC in the development of IgAN. PMBCs from patients with IgAN
produced more IgA than PBMCs from healthy controls (13). Interleukin 4, mainly produced by T
cells, can induce IgA synthesis in PBMCs (13). Degradation of C1GALT1 by
miR-148b mimics can attenuate IgA glycosylation in PBMCs (9). The results of these studies indicate
that abnormally regulated PBMCs proliferation along with IgAN
production and glycosylation have important roles in IgA
accumulation and IgAN development.
miRNAs play important roles in cell behaviors such
as cell proliferation, migration and apoptosis by regulating gene
expression (5). miRNAs are
ubiquitous and abundant in body fluids and cells; importantly, they
are also stable (24). Therefore,
miRNAs can be good biomarkers and drug targets (5,25).
Previously, several studies have demonstrated that miRNAs could be
involved in IgAN development, including miR-148b, let7b, miR-146a,
miR-155, miR-200a, miR-200b and miR-429 (7–10).
However, the role of miRNA expression in the pathogenesis of IgAN
has not been well explored. To identify additional functional
miRNAs, small RNA sequencing was performed; this analysis showed
that miRNAs constituted nearly 70% of small RNAs in PBMCs from both
healthy participants and patients with IgAN. Furthermore, 44
differentially expressed miRNAs were identified, of which 34 were
upregulated and 10 were downregulated. Among them, miR-148a-3p,
miR-184, and miR-200a were previously reported to be associated
with IgAN (26,27), the other 41 were newly found to be
associated with IgAN. In addition, miR-135a is involved in podocyte
injury, and miR-203 is known to promote diabetic nephropathy
(28,29). To the best of the authors'
knowledge, this is the first report that the remaining miRNAs are
potentially associated with kidney disease.
Among the top 10 upregulated miRNAs, miR-6843-3p
exhibited the greatest fold change, followed by miR-3675-3p and
miR-3120-5p. miR-671-3p showed the most abundant expression in both
healthy participants and patients with IgAN, followed by miR-5100
and miR-1287-5p. KEGG pathway analysis showed enriched target genes
in the PI3K-Akt signaling pathway and MAPK pathways, which were
reportedly involved in IgAN (30,31),
indicating a potential role in IgAN for the top 10 upregulated
miRNAs in PBMCs in this study.
Among the top downregulated miRNAs, miR-6872-5p
exhibited the greatest fold change, followed by miR-3121-3p and
miR-203a-3p. miR-122-5p showed the most abundant expression in both
healthy participants and patients with IgAN, followed by
miR-200a-3p and miR-4742-5p. Previously, miRNAs were shown to
promote cell proliferation and the production of aberrant
glycosylated IgA1 in IgAN (9,12).
C1GALT1, which is a critical enzyme involved in IgA glycosylation,
may be regulated by miRNAs, such as miR-148b (9). Among the members of the miR-200
family, the intrarenal expression of miR-200c was downregulated in
renal tissue; however, miR-200a and miR-200b were upregulated
(7). In the present study,
miR-200a-3p was identified in PBMCs, and 4 potential binding sites
were predicted. Moreover, miR-3121-3p and miR-203a-3p, the top 2
downregulated miRNAs, were newly identified and predicted to
regulate C1GALT1 expression. miR-200a-3p and miR-203a-3p
were previously demonstrated to inhibit cell proliferation
(28,32,33).
KEGG pathway analysis of downregulated miRNA target genes suggested
that the PI3K-Akt signaling pathway, RAS signaling pathway and MAPK
signaling pathways may also be involved in the role of miRNAs in
IgAN by regulating cell proliferation or gene expression involved
in IgAN development. These findings suggested that differentially
expressed miRNAs may be involved in the progress of IgAN by
controlling PBMC biological behaviors and IgA deposition through
direct targeting of genes or indirect regulation of various
signaling pathways.
In conclusion, the present study identified 44
differentially expressed miRNAs associated with IgAN, of which
numerous associations were novel. In addition, these miRNAs may
provide biomarkers and drug targets for IgAN diagnosis and
therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Planning Project of Guangdong Province (grant no.
2014A020221082), The Specific Research Fund For TCM Science and
Technology of Guangdong Provincial Hospital of Chinese Medicine
(grant no. YN2014WBR203) and the Famous Master of Chinese Medicine
Construction Project of WangQi's Academic Experience Office (grant
no. E43713).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW and NY designed the study and wrote the initial
draft of the manuscript. ZW, YuL, LW, YaL, ZY, XL and XZ performed
the experiments and contributed to the collection, analysis and
interpretation of data, and assisted in the preparation of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethical
Committee of Guangdong Provincial Hospital of Chinese Medicine and
was conducted in accordance with the ethical standards of the
Declaration of Helsinki. All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IgAN
|
IgA nephropathy
|
|
PBMC
|
peripheral blood mononuclear cells
|
References
|
1
|
Lai KN, Tang SC, Schena FP, Novak J,
Tomino Y, Fogo AB and Glassock RJ: IgA nephropathy. Nat Rev Dis
Primers. 2:33782016. View Article : Google Scholar
|
|
2
|
Suzuki H, Kiryluk K, Novak J, Moldoveanu
Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG
and Julian BA: The pathophysiology of IgA nephropathy. J Am Soc
Nephrol. 22:1795–1803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schena FP and Cox SN: Biomarkers and
precision medicine in IgA nephropathy. Semin Nephrol. 38:521–530.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szeto CC and Li PK: MicroRNAs in IgA
nephropathy. Nat Rev Nephrol. 10:249–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM
and Zhang GZ: Biological functions of microRNAs: A review. J
Physiol Biochem. 67:129–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang G, Kwan BC, Lai FM, Choi PC, Chow KM,
Li PK and Szeto CC: Intrarenal expression of microRNAs in patients
with IgA nephropathy. Lab Invest. 90:98–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang G, Kwan BC, Lai FM, Chow KM, Li PK
and Szeto CC: Elevated levels of miR-146a and miR-155 in kidney
biopsy and urine from patients with IgA nephropathy. Dis Markers.
30:171–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Serino G, Sallustio F, Cox SN, Pesce F and
Schena FP: Abnormal miR-148b expression promotes aberrant
glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol.
23:814–824. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Serino G, Sallustio F, Curci C, Cox SN,
Pesce F, De Palma G and Schena FP: Role of let-7b in the regulation
of N-acetylgalactosaminyltransferase 2 in IgA nephropathy. Nephrol
Dial Transplant. 30:1132–1139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Serino G, Pesce F, Sallustio F, De Palma
G, Cox SN, Curci C, Zaza G, Lai KN, Leung JC, Tang SC, et al: In a
retrospective international study, circulating miR-148b and let-7b
were found to be serum markers for detecting primary IgA
nephropathy. Kidney Int. 89:683–692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu S, Bao H, Xu X, Zhou X, Qin W, Zeng C
and Liu Z: Increased miR-374b promotes cell proliferation and the
production of aberrant glycosylated IgA1 in B cells of IgA
nephropathy. FEBS Lett 589B. B4019–B4025. 2015. View Article : Google Scholar
|
|
13
|
Yano N, Endoh M, Miyazaki M, Yamauchi F,
Nomoto Y and Sakai H: Altered production of IgE and IgA induced by
IL-4 in peripheral blood mononuclear cells from patients with IgA
nephropathy. Clin Exp Immunol. 88:295–300. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kozomara A, Birgaoanu M and
Griffiths-Jones S: miRBase: From microRNA sequences to function.
Nucleic Acids Res. 47:D155–D162. 2018. View Article : Google Scholar
|
|
15
|
Sai Lakshmi S and Agrawal S: piRNABank: A
web resource on classified and clustered Piwi-interacting RNAs.
Nucleic Acids Res. 36:D173–D177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalvari I, Argasinska J, Quinones-Olvera
N, Nawrocki EP, Rivas E, Eddy SR, Bateman A, Finn RD and Petrov AI:
Rfam 13.0: Shifting to a genome-centric resource for non-coding RNA
families. Nucleic Acids Res. 46:D335–D342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
The Gene Ontology Consortium, . The gene
ontology resource: 20 years and still GOing strong. Nucleic Acids
Res. 47:D330–D338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sticht C, De La Torre C, Parveen A and
Gretz N: miRWalk: An online resource for prediction of microRNA
binding sites. PLoS One. 13:e02062392018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar
|
|
22
|
Wang X: miRDB: A microRNA target
prediction and functional annotation database with a wiki
interface. RNA. 14:1012–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura T, Ebihara I, Takasaki Y, Tomino
Y and Koide H: Increased expression of proliferating cell nuclear
antigen mRNA in peripheral blood mononuclear cells from patients
with IgA nephropathy. Am J Med Sci. 302:214–219. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang H, Gong F, Zhang S, Zhang CY, Zen K
and Chen X: The origin, function, and diagnostic potential of
extracellular microRNAs in human body fluids. Wiley Interdiscip Rev
RNA. 5:285–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Chen J and Sen S: MicroRNA as
biomarkers and diagnostics. J Cell Physiol. 231:25–30. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan K, Chen J, Li W, Chen Y, Sui W, Zhang
Y and Dai Y: Genome-wide analysis of microRNAs expression profiling
in patients with primary IgA nephropathy. Genome. 56:161–169. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J, Zhang H, Wang W, Zhu M, Qi LW, Wang
T, Cheng W, Zhu J, Shan X, Huang Z, et al: Plasma microRNA
signature of patients with IgA nephropathy. Gene. 649:80–86. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu ZM, Zheng HY, Chen LH, Li YL, Wang Q,
Liao CF and Li XW: Low expression of miR-203 promoted diabetic
nephropathy via increasing TLR4. Eur Rev Med Pharmacol Sci.
22:5627–5634. 2018.PubMed/NCBI
|
|
29
|
Yang X, Wu D, Du H, Nie F, Pang X and Xu
Y: MicroRNA-135a is involved in podocyte injury in a transient
receptor potential channel 1-dependent manner. Int J Mol Med.
40:1511–1519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cox SN, Sallustio F, Serino G, Pontrelli
P, Verrienti R, Pesce F, Torres DD, Ancona N, Stifanelli P, Zaza G
and Schena FP: Altered modulation of WNT-beta-catenin and PI3K/Akt
pathways in IgA nephropathy. Kidney Int. 78:396–407. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin TJ, Yang SS, Hua KF, Tsai YL, Lin SH
and Ka SM: SPAK plays a pathogenic role in IgA nephropathy through
the activation of NF-κB/MAPKs signaling pathway. Free Radic Biol
Med. 99:214–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Jiang F, Song H, Li X, Xian J and
Gu X: MicroRNA-200a-3p suppresses tumor proliferation and induces
apoptosis by targeting SPAG9 in renal cell carcinoma. Biochem
Biophys Res Commun. 470:620–626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Z, Zhao Z, Yang Y, Luo M, Zhang M,
Wang X, Liu L, Hou N, Guo Q, Song T, et al: MiR-99b-5p and
miR-203a-3p function as tumor suppressors by targeting IGF-1R in
gastric cancer. Sci Rep. 8:101192018. View Article : Google Scholar : PubMed/NCBI
|