Introduction
Lower back pain (LBP) is one of the predominant
factors contributing to dyskinesia and the second most common cause
of hospital visits, leading to a serious social and economic burden
worldwide (1,2). Symptomatic intervertebral disc
degeneration (IDD) is the most frequent cause of LBP (1,3).
Although several factors contribute to IDD, genetic factors are the
leading cause (4). The nucleus
pulposus (NP) is located in the center of the intervertebral disc
(ID) (5); it is the largest
avascular tissue in the body and lacks blood oxygenation, which
limits its self-repairing ability (6). Existing IDD treatments are not
satisfactory and cannot fully recover ID function (7). Non-coding RNA (ncRNA) generated by
gene back-splicing can regulate gene post-transcriptional
modification to modulate disease development (8). Frapin et al (9) described the pathological process of
IDD in detail and inferred that metabolic dysregulation of the
extracellular matrix (ECM) in the ID microenvironment was
predominantly involved in the pathogenesis of IDD. The various
types of IDD-related genes or protein expression disorders can
contribute to the synthesis and catabolic imbalance of ECM, giving
rise to the alteration of ID morphology, physics and mechanics,
leading to ID function loss, thereby triggering IDD (9–11).
Gene therapy uses viruses and other vectors to carry ncRNAs formed
by genes or genes to target ID, which can reverse or block the
pathological process of IDD and recover ID function at the genetic
level (10). In view of the
aforementioned reasons, gene-based diagnostic and treatment
strategies are critical measures for IDD management.
IDD-related genes or proteins can be divided into
protective factors and catabolic factors. The former includes
hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth
factor (VEGF), collagen type II (COL2), aggrecan (ACAN),
SRY-related high mobility group box 9 (SOX9) and a series of
antiapoptotic proteins, whereas the latter includes matrix
metalloproteinases (MMPs), disintegrin and ADAM metallopeptidases
with thrombospondin type 1 motifs (ADAMTSs), interleukin (IL)-1β,
tumor necrosis factor-α (TNF-α) and a number of proapoptotic
proteins (4,9–11).
Previous studies have suggested that ncRNAs,
including microRNAs (miRNAs/miRs) and circular RNAs (circRNAs),
serve a crucial role in the occurrence and progression of IDD
(12–17). In particular, circRNAs mediate NP
cell (NPC) apoptosis and regulate the expression of inflammatory
cytokines, MMP, ADAMTS, various apoptosis-related proteins and key
components of the ECM, such as COL2 and ACAN, which serve a role in
the pathogenesis of IDD (12–17).
The present article provides an up-to-date review of
circRNA characteristics, classification, biogenesis and function,
with particular emphasis on the potential future directions of
IDD-related research. Additionally, the limitations of current
research are also discussed.
Characteristics
CircRNAs are a type of ncRNA with high thermal
stability that were first discovered in plant-infecting virions in
1976 by Sanger et al (18).
Although circRNAs have been identified in different species
(19–22), these molecules were not initially
extensively studied. However, circRNAs were later found to exert a
previously unrecognized role in a wide spectrum of human diseases,
owing to the rapid development of next-generation sequencing
technology (23,24). Compared with linear RNAs,
covalently closed circRNAs have unique characteristics and
biological functions without 5′ to 3′ polarity or a polyadenylated
tail (25–27). They are predominantly located in
the cytoplasm, abundantly expressed, conserved, highly stable and
exonuclease-resistant (28–31).
In addition, circRNAs are expressed in a tissue- and time-specific
manner (31,32). They can also be carried in exosomes
and have potential applications as markers for disease diagnosis
(33,34). Direct back-splicing and exon
skipping are the main pathways of circRNA synthesis (23,27).
Classifications
Currently, seven types of circRNAs have been
identified according to the type and quantity of the parental gene
the circRNAs originate from. These include exonic circRNA
(ecircRNA), intronic circRNA (ciRNA), tRNA intronic circRNA
(tricRNA), exon-intron circRNA (eiciRNA), read-through circRNA
(rt-circRNA), fusion circRNA (f-circRNA) and mitochondria-encoded
circRNA (mecciRNA). The circularization of at least one intron or
one exon from a single gene gives rise to ciRNAs and ecircRNAs,
respectively, whereas the formation of eiciRNA is based on the
cyclization of at least an exon and an intron (24,26)
(Fig. 1). TricRNA is a special
type of circRNA synthesized through a pre-tRNA intron splicing
mechanism (35). Rt-circRNA is the
result of circularization of two exons from two different genes
(30). F-circRNA is synthesized
from the transcribed exons of several nuclear genes as a result of
chromosomal translocation (36).
Lastly, mecciRNA is produced from mitochondrial genes (37). EcircRNAs are the most common type
of circRNA.
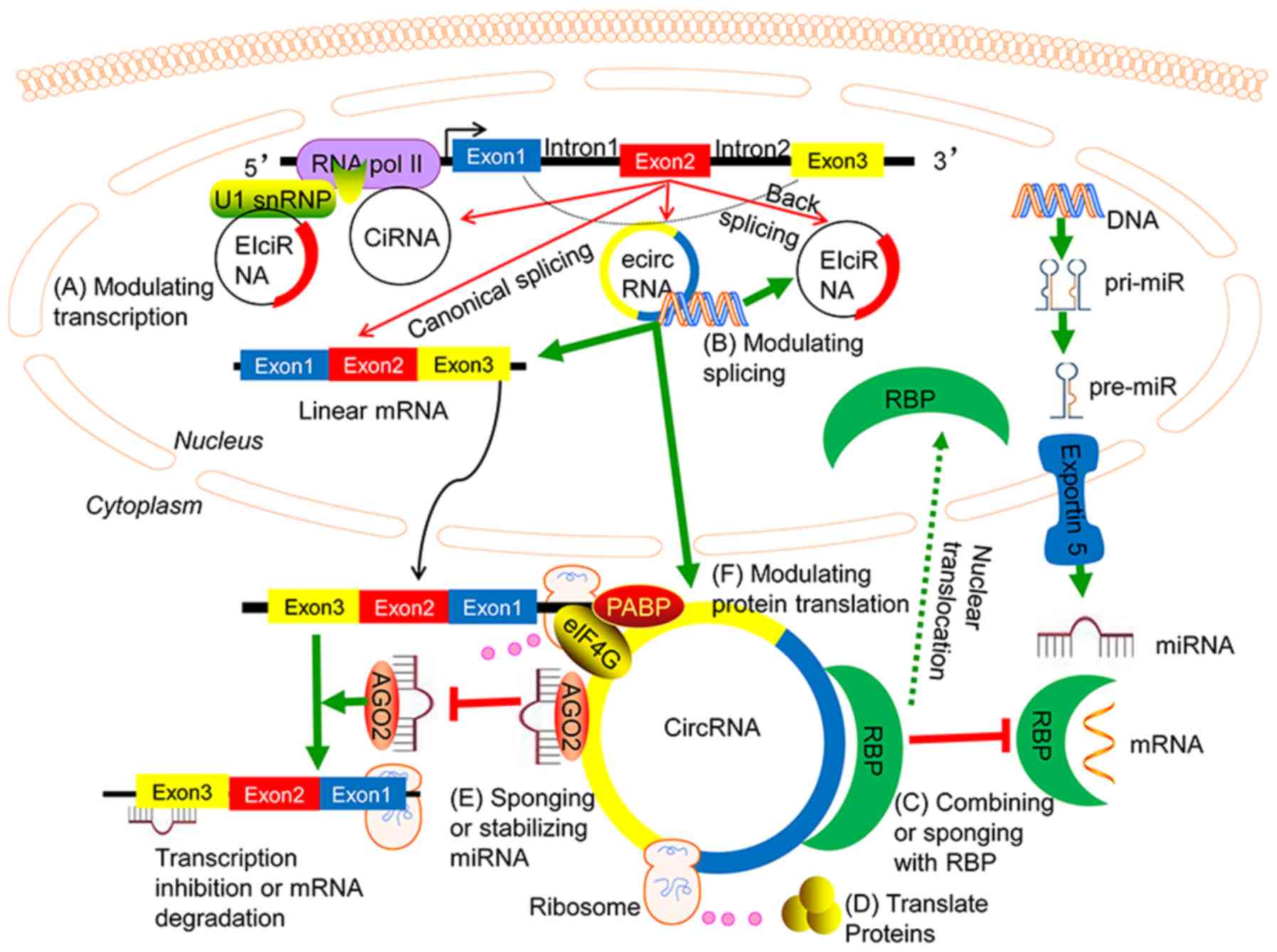 | Figure 1.Biogenesis and function of circRNAs.
In the nucleus, the circularization of at least one intron or one
exon from a single gene gives rise to ciRNAs and ecircRNAs,
respectively, whereas the formation of eiciRNA is based on the
cyclization of at least one exon and one intron. CirRNAs or
eiciRNA, mostly enriched in the nucleus, can modulate gene
transcription and alternative splicing. EcircRNAs, which are
primarily located in the cytoplasm, can modulate the expression of
their parental genes, sponge miRNAs, interact with or sponge RBP,
translate proteins and modulate protein translation. EcircRNAs
positively regulate the expression of their target genes via
sponging miRNAs to relieve the inhibitory effect of miRNAs on
target genes. CircRNA, circular RNA; RBP, RNA-binding protein;
ciRNA, intronic circRNA; ecircRNA, exonic circRNA; eiciRNA,
exon-intron circRNA; miRNA, microRNA. |
Biogenesis and functions
The biological functions of circRNAs depend on their
type and cellular location. In the nucleus, synthetic circRNAs can
modulate gene transcription (30,38)
and alternative splicing (39).
For example, eiciRNAs circ-EIF3J and circ-PAIP2 are largely
localized in the nucleus and can interact with U1 small nuclear
ribonucleoprotein and RNA polymerase (Pol) II to promote the
transcription of their host genes (38). The ciRNA ci-ankrd52 positively
regulates the function of RNA Pol II to modulate the transcription
of its parental gene (ANKRD52), predominantly converging to the
cell nucleus (39). Increased
synthesis of circ-Mbl may repress the transcription of its parental
gene (40).
After synthesis, ecircRNAs are transferred from the
nucleus to the cytoplasm. As shown in Fig. 1, the synthesis of mature miRNAs
involves a range of processing steps. First, miRNA genes are
transcribed into pri-miRNAs, and then processed into pre-miRNAs in
the nucleus, pre-miRNAs are then transported into the cytoplasm via
the nuclear export protein Exportin5 to produce mature miRNAs
(41). Subsequently, mature miRNAs
directly interact with the 3′UTRs of target mRNAs, thereby
inhibiting mRNA translation or degrading mRNA (42). EcircRNAs that are primarily located
in the cytoplasm can modulate the expression of their parental
genes (43,44), sponge miRNAs by acting as
competitive endogenous RNAs (ceRNAs) (25,31,43–46),
attach miRNAs (47,48), interact with or sponge RNA-binding
proteins (RBPs) (25,31,49–52),
encode proteins (25,31,43,53–55)
and modulate protein translation (56–58)
(Fig. 1).
The crosstalk between circRNAs and miRNAs is
complex. CircRNAs commonly inhibit miRNA expression through a
‘sponging’ mechanism (25,31,41–44).
Additionally, circ-CSNK1G3 and ci-RS-7 positively regulate the
levels of miR-181b/d and miR-7, respectively (45,46).
Conversely, miR-200b negatively modulates the expression and
function of circRNA-000839 (59).
Whether miRNA, in turn, can regulate circRNA expression remains
unknown. Moreover, the possibility that circRNAs also interact with
each other has yet to be demonstrated. Furthermore, cytoplasmic
circRNAs also modulate parental gene expression. For example, F-box
and WD repeat domain-containing 7 (FBXW7), a well-known tumor
suppressor gene, encodes the FBXW7 protein and circ-FBXW7.
Circ-FBXW7 regulates the mRNA and protein levels of FBXW7 to
repress the expression of c-Myc in a miRNA-dependent manner
(41). Another study suggests that
circ-filamin-binding LIM protein 1 (FBLIM1) positively regulates
the expression of the FBLIM1 gene by sponging miR-346 (42).
CircRNAs regulate the activity and function of
proteins in several ways, including via sponging, as a protein
scaffold, by encoding proteins and modulating protein translation
(Fig. 1). Circ-forkhead box O3
(Foxo3) can bind to both P21 and cyclin-dependent kinase 2 (CDK2)
to generate ternary complexes that enhance the inhibitory effect of
P21 on CDK2 (47). Circ-Foxo3 also
functions as a protein scaffold, stabilizing Foxo3 protein
expression by interacting with mouse double minute 2 (Mdm2) and P53
and inhibiting Mdm2-induced Foxo3 ubiquitination (50). Additionally, circ-Foxo3 inhibits
the nuclear translocation of transcription factors, including
HIF-1α, thereby inhibiting their functions (49). Circ-ZKSCAN1 sponges fragile X
mental retardation protein, preventing it from binding to its
downstream target mRNA, cell cycle and apoptosis regulator 1,
thereby attenuating the malignant biological behavior of
hepatocellular carcinoma (HCC) through the Wnt signaling pathway
(48). RBPs also regulate the
formation of circRNAs. For example, RNA-binding motif protein 3
(RBM3) increases stearoyl-CoA desaturase-circ-RNA 2 synthesis to
promote HCC cell proliferation (60), and Quaking 5 may promote the
synthesis of circ-ZKSCAN1 (48).
In addition, some circRNAs function through the proteins they
encode. Circ-β-catenin encodes a 370-amino-acid β-catenin, which
inhibits glycogen synthase kinase 3β (GSK3β)-mediated β-catenin
degradation and activates the Wnt/β-catenin pathway in HCC
(51). Circ-protein phosphatase 1
regulatory subunit 12A (PPP1R12A)-73aa (a protein encoded by
circ-PPP1R12A), but not circPPP1R12A itself, accelerates colon
cancer growth and metastasis (52). Moreover, circ-FBXW7 encodes the
FBXW7 185-amino-acid protein (FBXW7-185aa), and the synergistic
action of FBXW7 and FBXW7-185aa stabilizes c-Myc and promotes
oncogenesis and tumor progression (42). Lastly, partial circRNAs modulate
protein translation in a protein-dependent manner. CircRHOT1
regulates the translation of nuclear receptor subfamily 2 group F
member 6 (NR2F6) by activating Tat-interacting protein of 60 kDa to
the Nr2f6 gene promoter (54). Circ-YY1-associated protein 1 (YAP)
regulates the initiation efficiency of YAP protein translation by
interacting with Yap mRNA and the proteins eIF4G and PABP
(55). More recently, Sun et
al (58) reported that
circMYBL2 could facilitate fms-related receptor tyrosine kinase 3
(FLT3) protein translation efficiency by recruiting polypyrimidine
tract-binding protein 1 to bind to Flt3 mRNA.
Recently, Chen et al (47) suggested that ~90% of circRNAs have
an independent regulatory role in cell proliferation, compared with
their linear counterparts. Nevertheless, certain circRNAs have
functions that are similar to linear RNAs (61,62).
Peroxisome proliferator-activated receptors and their associated
circRNA, circ-5379-6, both suppress tumor progression (61). Yao et al (62) also reported that circ-ZKSCAN1 and
its parental gene both inhibited cell growth through distinct
signaling pathways.
Specificroles of circRNA in IDD
Biofunctions of circRNA in IDD
A growing body of evidence suggests that circRNAs
are extensively involved in a multitude of chronic diseases,
including osteoarthritis (63) and
cancer (64), as well as
cardiovascular (65),
neurodegenerative (66,67) and immunological (68,69)
diseases. However, the role of circRNA in IDD remains unclear. To
the best of our knowledge, only nine upregulated and nine
downregulated circRNAs have been identified in degenerative NP
samples compared with normal NP samples, and the functions of 12 of
these dysregulated circRNAs remain fairly poorly understood
(70,71). Using microarray data from the Gene
Expression Omnibus (GEO) database, Zhu et al (72) predicted three circRNA-mediated
regulatory pathways in IDD, namely,
circRNA-102348/miR-185-5p/TGFB1/FOS,
circRNA-102399/miR-302a-3p/HIF1A and
circRNA-100086/miR-509-3p/MAPK1. However, further investigation is
needed to elucidate their potential role in IDD (72). Moreover, the detailed biofunctions
of circRNAs that have been identified are listed in Table I and Fig. 2. The upregulated circRNAs except
circ-0004099 contribute to the occurrence and progression of IDD,
whereas circ-0004099 and all downregulated circRNAs function as IDD
repressors. CircRNAs act as IDD repressors or enhancers through the
regulation of several pathological processes, including NPC
apoptosis, proliferation, mitophagy and senescence, as well as the
dysregulation of MMP, ADAMTS, inflammatory cytokines and ECM
expression.
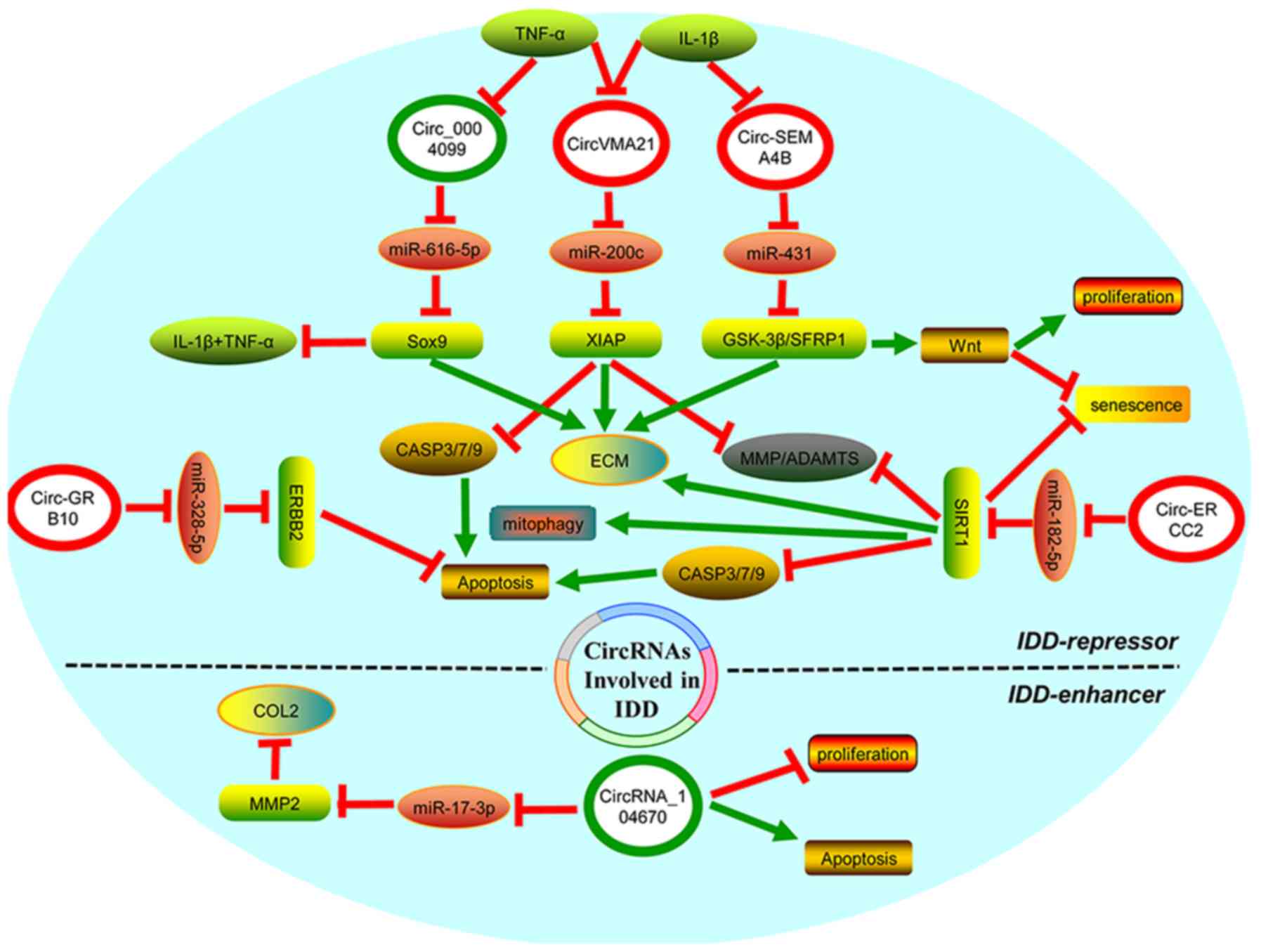 | Figure 2.CircRNAs are involved in the
regulation of IDD. In the upper half of the ellipse, circ-SEMA4B,
circ-GRB10, circ-VMA21 and circ-ERCC2 were downregulated, whereas
circ-0004099 was upregulated in IDD (green circle). All of them
function as an IDD-repressor by regulating different miRNA-mRNA
pathways, of which circ-SEMA4B, circ-0004099, circ-VMA21 and
circ-ERCC2 promote the synthesis of key components of the ECM;
circ-GRB10, circ-VMA21 and circ-ERCC2 suppress NPC apoptosis;
circ-VMA21 and circ-ERCC2 suppress NPC senescence; circ-SEMA4B
enhances NPC proliferation; circ-VMA21 and circ-ERCC2 suppress MMP
or ADAMTS expression; circ-ERCC2 facilitates mitophagy; and
circ-0004099 inhibits TNF-α and IL-1β secretion. Additionally,
TNF-α can inhibit circ-0004099 expression, IL-1β can inhibit
circ-SEMA4B expression, and both of them elevate the expression of
circ-VMA21. In the lower half of the ellipse, circRNA-104670 was
upregulated in IDD (green circle), which not only represses NPC
proliferation and synthesis of ECM components, but also promotes
NPC apoptosis and MMP2 expression via sponging miR-17-3p, thereby
its acts as an IDD enhancer. CircRNA, circular RNA; IDD,
intervertebral disc degeneration; circ-VMA21, circ-vacuolar ATPase
assembly factor 21; circ-SEMA4B, circ-semaphorin 4B; circ-ERCC2,
circ-excision repair cross-complementation group 2; circ-GRB10,
circ-growth factor receptor bound protein 10; miR, microRNA; ECM,
extracellular matrix; MMP, matrix metalloproteinase; NPC, nucleus
pulposus cell; ADAMTS, ADAM metallopeptidases with thrombospondin
type 1 motifs; TNF-α, tumor necrosis factor-α; IL-1β, interleukin
1β. |
 | Table I.List of circRNAs involved in IDD. |
Table I.
List of circRNAs involved in IDD.
| Author, year | circRNA | Condition | Expression | Treatment | Spinal type | Pathway | Investigated
process | ceRNA
mechanism | (Refs.) |
|---|
| Guo et al,
2018 | circ-GRB10 | Fracture vs. IDD
disease | Downregulated in
IDD disease | N/A | Lumbar |
circ-GRB10/miR-328-5p/ERBB2 | Inhibition of NPC
apoptosis | IDD repressor | (13) |
| Cheng et al,
2018 | circ-VMA21 | Fracture or
scoliosis vs. degenerative spinal disease | Downregulated in
IDD disease | TNF-α (5 ng/ml) +
IL-1β (5 ng/ml) | Lumbar |
circ-VMA21/miR-200c/XIAP/CASPs/ECM | NPC apoptosis
inhibition; ECM degradation; MMP3/13; and ADAMTS4/5 expression | IDD repressor | (12) |
| Wang et al,
2018a | circ_0004099 | Fracture or
scoliosis vs. degenerative spinal disease | Upregulated in IDD
disease | TNF-α (50
ng/ml) | Lumbar |
TNF-α/MAPK/NF-κB/circ_0004099/miR-616-5p/Sox9/ECM/TNF-α
and IL-1β | Sox9 activation;
ECM synthesis; and TNF-α/ IL-1β secretion | IDD repressor | (14) |
| Wang et al,
2018b | circ-SEMA4B | Mild vs. severe IDD
disease | Downregulated in
IDD disease | IL-1β (10
ng/ml) | Lumbar |
IL-1β/circ-SEMA4B/miR-431/GSK-3β/SFRP1/Wnt
pathway | ECM generation; NPC
proliferation; and suppression of NPC senescence | IDD repressor | (16) |
| Xie et al,
2019 | circ-ERCC2 | Cervical
spondylotic myelopathy vs. Hirayama disease | Downregulated in
IDD disease | TBHP (100 µM) | Cervical |
circ-ERCC2/miR-182-5p/SIRT1 | Inhibition of NPC
apoptosis; senescence; mitophagy; COL2; degradation and MMP13
expression | IDD repressor | (17) |
| Song et al,
2018 | circRNA_104670 | Cervical
spondylotic myelopathy vs. Hirayama disease | Upregulated in IDD
disease |
| Cervical |
circRNA_104670/miR-17-3p/MMP2 | Enhancement of NPCs
apoptosis and MMP2 expression; suppression of NPC proliferation and
COL2 synthesis | IDD enhancer | (15) |
The five described IDD repressors have diverse
functions: i) Circ-semaphorin 4B (SEMA4B) enhances NPC
proliferation; ii) circ-SEMA4B and circ-excision repair
cross-complementation group 2 (ERCC2) suppress NPC senescence; iii)
circ-growth factor receptor bound protein 10 (GRB10), circ-vacuolar
ATPase assembly factor VMA21 (VMA21) and circ-ERCC2 suppress NPC
apoptosis; iv) circ-SEMA4B, circ-0004099, circ-VMA21 and circ-ERCC2
promote ECM synthesis; v) circ-VMA21 and circ-ERCC2 suppress MMP or
ADAMTS expression; vi) circ-ERCC2 facilitates mitophagy; and vii)
circ-0004099 inhibits IC secretion (12–17).
The only known IDD enhancer, circRNA-104670, not only represses NPC
proliferation and the synthesis of ECM components, but also
promotes NPC apoptosis and MMP2 expression (15). These abnormally expressed circRNAs
mediate pathological processes through several signaling pathways,
including apoptosis-related pathways and ECM-related pathways.
Circ-VMA21-mediated IDD
repression
Circ-VMA21 was the first identified IDD-related
circRNA (12), providing
invaluable insight into the modulation of IDD pathogenesis by
circRNAs. Circ-VMA21 is downregulated in degenerative NP samples
from patients with IDD compared with NP samples from controls
(12). Similarly, the expression
of circ-VMA21 is reduced in NPCs treated with both TNF-α and IL-1β
(12). The progression of IDD is
associated with the aberrant expression of circ-VMA21 in
degenerative and normal NP samples. More specifically, circ-VMA21
blocked the progression of IDD, and its downregulation limited its
protective effect. Circ-VMA21 serves a protective role in human
NPCs and rat NP tissues predominantly through apoptosis-related
pathways and the ECM components metabolism-related pathways.
Circ-VMA21 positively modulates X linked
inhibitor-of-apoptosis protein (XIAP) and represses the expression
of caspase (CASP) family members (CASP-3, CASP-7 and CASP-9), as
well as a number of degrading metabolic enzymes (MMP-3, MMP-13,
ADAMTS-4 and ADAMTS-5). Circ-VMA21 also promotes the expression of
ECM components, including COL2 and ACAN, by sponging miR-200c
(12,73). Luciferase reporter and RNA
pull-down assays confirmed that circ-VMA21 has five effective
binding sites in miR-200c (12,73).
Pfirrmann classification is the most common method
used for the evaluation of IDD severity, according to magnetic
resonance imaging grade (74).
Circ-VMA21 markedly decreased the Pfirrmann grade of IDD following
injection into rat IDs. Altogether, these studies suggested that
the circ-VMA21/miR-200c/XIAP axis may be involved in the regulation
of IDD pathogenesis, providing novel therapeutic targets for
IDD.
IDD repressor circ-GRB10
Lan et al (75) analyzed the microarray data of human
lumbar IDD and uploaded it into the GEO database. Our previous
study identified three abnormally expressed circRNAs by analyzing
circRNA microarray data from the GEO database, of which two were
upregulated (circ-FAM169A and circ-SETD2) and one was downregulated
(circ-GRB10) (13). The expression
of circ-GRB10 was downregulated in 20 degenerative NP samples from
patients with IDD undergoing discectomy compared with 20
nondegenerative NP samples from patients with fresh traumatic
lumbar fracture (13).
Mechanistically, the circ-GRB10-mediated pathological process of
IDD is miR-328-5p-dependent. Functionally, circ-GRB10 acts as an
IDD repressor of NPC apoptosis under nutrient deprivation
conditions by sequestering miR-328-5p and promoting Erb-B2 receptor
tyrosine kinase 2 (ERBB2) expression in NPCs. Thus, circ-GRB10
downregulation could decrease NPC survival, leading to IDD onset
and progression.
IDD repressor circ-0004099
Wang et al (14) categorized patients with IDD
according to the Pfirrmann classification criteria (74). Patients with Pfirrmann grade I/II
were assigned to a nondegenerative group, whereas those with
Pfirrmann grade IV/V constituted the degeneration group. The
authors collected six degenerative NP samples from the patients of
the degenerative group who were undergoing spinal surgery and six
nondegenerative NP samples from the patients of the nondegenerative
group with vertebral fracture or scoliosis (13,14).
Using circRNA microarray, circRNA expression profiles in the twelve
samples were examined (14).
Circ-0004099 was the most frequently upregulated circRNA in the
degenerative samples (14).
Moreover, circ-0004099 expression was increased in NPCs in a dose
and time-dependent manner following treatment with TNF-α. It was
also demonstrated that this effect was mediated by MAPK and the
NF-κB signaling pathways (14).
Notably, circ-0004099 overexpression enhanced (rather than
repressed) the expression of Sox9 and ECM proteins, such as ACAN
and COL2. Circ-004099 also repressed (rather than enhanced)
proinflammatory cytokine secretion (TNF-α, IL-1β and prostaglandin
E2), and these changes were reversed by miR-616-5p mimic 14). Sox9
is a chondrocyte-specific transcription factor that promotes COL2
and ACAN synthesis (76,77). Wang et al (14) also confirmed that Sox9 was the
direct target of miR-616-5p. In addition, luciferase reporter, RNA
immunoprecipitation and RNA pull-down assays indicated that
circ-0004099 could bind to miR-616-5p (14). Collectively, these research results
reveal that the circ-0004099/miR-616-5p/Sox9 axis might play a
protective role in IDD.
IDD repressor circ-SEMA4B
Consistent with Guo's method (13), Wang et al (16) also analyzed the same circRNA
microarray data that was downloaded from the GEO database.
Circ-SEMA4B expression was the most significantly downregulated
circRNA in 45 IDD specimens and had a negative association with IDD
severity, as bard on the Pfirrmann grade (16). Notably, circ-SEMA4B promoted the
synthesis of ECM components and NPC proliferation, while inhibiting
NPC senescence under IL-1β stimulation. It was also demonstrated
that IL-1β exerted these effects by inhibiting circ-SEMA4B
expression (16).
Previous studies have demonstrated that the Wnt
signaling pathway serves an important role in the regulation of NPC
proliferation and senescence (78,79).
For instance, circ-SEMA4B regulates the activation of the Wnt
signaling pathway by sponging miR-431, the upstream regulator of
two well-known Wnt signaling pathway inhibitors, GSK-3β and
secreted frizzled-related protein 1 (SFRP1) (16,80,81).
Collectively, results from these studies suggest that circ-SEMA4B
may be associated with the prognosis of patients with IDD and
inhibits IDD development by regulating the miR-431/GSK-3β/SFRP1
axis.
IDD repressor circ-ERCC2
Xie et al (17) analyzed Song's (15) microarray analysis of circRNAs and
Lan's (75) microarray dataset
(GSE67566) and demonstrated that circ-ERCC2 was the most frequently
downregulated circRNA in degenerative NP tissue (15,74).
Functional analyses also suggested that circ-ERCC2 modulated
tert-butyl hydroperoxide-induced NPC apoptosis (through CASP-3,
CASP-7 and CASP-9), mitophagy (through PTEN-induced kinase 1,
parkin, p62, and LC3II/I) and ECM structure (MMP13 and COL2) in
vitro and in vivo (17). Fluorescence in situ
hybridization and dual-luciferase assays demonstrated that
circ-ERCC2 could bind to miR-182-5p (17).
Previous studies have indicated that silent mating
type information regulator 2 homolog 1 (SIRT1) plays a significant
role in mitophagy and apoptosis (82–84).
SIRT1-small interfering (si)RNA inhibits NPC apoptosis and
senescence. Moreover, this effect is suppressed by circ-ERCC2 and
miR-182-5p inhibitor, suggesting that circERCC2 exerts a protective
effect on NPCs that is dependent on miR-182-5p (17).
IDD enhancer circRNA_104670
CircRNA-104670 is related to cervical IDD and is
upregulated ~4.5-fold in degenerative tissues from patients with
cervical spondylotic myelopathy compared with normal tissues from
patients with Hirayama disease (15). Furthermore, circRNA-104670 may
represent a diagnostic marker for IDD. In a previous study,
receiver operating characteristic curve analysis indicated that the
area under the curve value of circRNA_104670 was 0.96 and the
expression of circRNA_104670 was positively associated with
Pfirrmann grade (15).
Functionally, circRNA-104670 increased NPC apoptosis and suppressed
NPC proliferation. Moreover, this circRNA also promoted MMP2 and
repressed COL2 expression by sequestering miR-17-3p (15). Mice injected with circRNA_104670
siRNA presented lower Pfirrmann grades (15). Thus, circRNA-104670 may act as an
IDD enhancer that regulates miR-17-3p and MMP2, leading to IDD
progression.
Current limitations and future
directions
All of the research on IDD-related circRNAs has
shortcomings. Although a universal approach to NPC culture with
inflammatory cytokine treatment and ID microenvironment stimulation
has been developed, it does not account for the fact that different
circRNAs have diverse affinities for various inflammatory
cytokines. However, as highlighted in Table I, none of the investigations
explain why they used IL-1β or TNF-α or both. Recently, Shen et
al (63) approached this
problem by detecting the expression of circRNAs in the cells
stimulated with IL-1β, TNF-α or both.
As the NP is a hypoxic environment, the potential
role of HIF-1α in IDD has been reviewed previously. In the
development of IDD, HIF-1α activation is involved in the regulation
of IDD-related gene or protein expression (9). Thus, HIF-1α is a crucial
transcriptional regulator of IDD. Increasing evidence suggests that
HIF-1α is a target of ncRNAs in several diseases (26,85,86).
Nevertheless, hypoxia-related circRNA pathways in IDD are still
poorly characterized.
Exosomes serve an important role in numerous
physiological and pathological processes in various diseases
(33,87,88).
Various factors, including HIF-1α, ncRNAs and proteins, among
others, are present in exosomes (89). Thus, circRNAs contained within
exosomes could serve as markers for diseases (33,87,88).
Importantly, whether HIF-1α affects MMP and the ECM remains unclear
(9). Nevertheless, we cannot rule
out the possibility of the existence of the circRNA/HIF-1α/MMP/ECM
axis in the exosomes of IDD. Further research is needed to
elucidate this.
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (AKT)/mechanistic target of rapamycin (mTOR) axis regulates
numerous biological events, including cell proliferation,
apoptosis, metastasis and metabolism (90). Growing evidence also indicates that
circRNA-mediated regulation of the PI3K/AKT/mTOR axis serves an
essential role in the pathogenesis of several diseases, such as
hepatocellular carcinoma and kidney cancer (85,91,92).
However, whether this holds true in IDD remains unclear. Recently,
bioinformatics analysis predicted that circRNAs could regulate
autophagy signaling pathways in IDD via sponging miRNAs (93). So, the role of circRNA-mediated
autophagy in IDD cannot be ruled out and is a worthwhile research
direction.
Conclusions
In conclusion, circRNAs function as ceRNAs to
regulate the pathological process of IDD in a miRNA-dependent
manner. However, circRNAs also have a number of other functions
aside from their role as a ceRNA that have not been reported on in
IDD. Whether circRNAs can sponge or interact with RBP, encode
proteins, modulate protein translation and gene expression in the
context of IDD should also be addressed. Thus, understanding the
biological role of circRNAs and their underlying molecular
mechanism in the context of IDD would provide further insight into
disease prevention strategies and contribute to the development of
therapeutic targets for IDD.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
National Natural Science Foundation of China (grant nos. 31670983
and 31900967) and The Natural Science Foundation of Tianjin City
(grant no. 19JCQNJC09300).
Availability of data and materials
Not applicable.
Authors' contributions
YL, SZ and PP contributed to the concept and the
design of the review. YL wrote the manuscript. XW helped draft the
manuscript and drew the figures. LD provided significant
suggestions for the study. ZH searched the literature and collated
important reference information. BX critically reviewed the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vos T, Abajobir AA, Abate KH, Abbafati C,
Abbas KM, Abd-Allah F, Abdulkadre RS, Abdulle AM, Abebo TA, Abera
SF, et al: Global, regional, and national incidence, prevalence,
and years lived with disability for 328 diseases and injuries for
195 countries, 1990–2016: A systematic analysis for the global
burden of disease study 2016. Lancet. 390:3057–1259. 2017.
View Article : Google Scholar
|
|
2
|
Deyo RA and Tsui-Wu YJ: Descriptive
epidemiology of low-back pain and its related medical care in the
United States. Spine (Phila Pa 1976). 12:264–268. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Battié MC, Videman T and Parent E: Lumbar
disc degeneration: Epidemiology and genetic influences. Spine
(Phila Pa 1976). 29:2679–2690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Humzah MD and Soames RW: Human
intervertebral disc: Structure and function. Anat Rec. 220:337–356.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakai D and Andersson GB: Stem cell
therapy for intervertebral disc regeneration: Obstacles and
solutions. Nat Rev Rheumatol. 11:243–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jacobs WC, van der Gaag NA, Kruyt MC,
Tuschel A, de Kleuver M, Peul WC, Verbout AJ and Oner FC: Total
disc replacement for chronic discogenic low back pain: A cochrane
review. Spine (Phila Pa 1976). 38:24–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skvortsova K, Iovino N and Bogdanović O:
Functions and mechanisms of epigenetic inheritance in animals. Nat
Rev Mol Cell Biol. 19:774–790. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frapin L, Clouet J, Delplace V, Fusellier
M, Guicheux J and Le Visage C: Lessons learned from intervertebral
disc pathophysiology to guide rational design of sequential
delivery systems for therapeutic biological factors. Advanced Drug
Delivery Reviews. 149-150:49–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fontana G, See E and Pandit A: Current
trends in biologics delivery to restore intervertebral disc
anabolism. Adv Drug Deliv Rev. 84:146–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vergroesen PP, Kingma I, Emanuel KS,
Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH and Smit TH:
Mechanics and biology in intervertebral disc degeneration: A
vicious circle. Osteoarthritis Cartilage. 23:1057–1070. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng X, Zhang L, Zhang K, Zhang G, Hu Y,
Sun X, Zhao C, Li H, Li YM and Zhao J: Circular RNA VMA21 protects
against intervertebral disc degeneration through targeting miR-200c
and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis.
77:770–779. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo W, Zhang B, Mu K, Feng SQ, Dong ZY,
Ning GZ, Li HR, Liu S, Zhao L, Li Y, et al: Circular RNA GRB10 as a
competitive endogenous RNA regulating nucleus pulposus cells death
in degenerative intervertebral disk. Cell Death Dis. 9:3192018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, He P, Pan H, Long J, Wang J, Li Z,
Liu H, Jiang W and Zheng Z: Circular RNA circ-4099 is induced by
TNF-α and regulates ECM synthesis by blocking miR-616-5p inhibition
of Sox9 in intervertebral disc degeneration. Exp Mol Med.
50:272018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song J, Wang HL, Song KH, Ding ZW, Wang
HL, Ma XS, Lu FZ, Xia XL, Wang YW, Fei-Zou and Jiang JY:
CircularRNA_104670 plays a critical role in intervertebral disc
degeneration by functioning as a ceRNA. Exp Mol Med. 50:942018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Wang B, Zou M, Li J, Lü G, Zhang
Q, Liu F and Lu C: CircSEMA4B targets miR-431 modulating
IL-1β-induced degradative changes in nucleus pulposus cells in
intervertebral disc degeneration via Wnt pathway. Biochim Biophys
Acta Mol Basis Dis. 1864:3754–3768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie L, Huang W, Fang Z, Ding F, Zou F, Ma
X, Tao J, Guo J, Xia X, Wang H, et al: CircERCC2 ameliorated
intervertebral disc degeneration by regulating mitophagy and
apoptosis through miR-182-5p/SIRT1 axis. Cell Death Dis.
10:7512019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grabowski PJ, Zaug AJ and Cech TR: The
intervening sequence of the ribosomal RNA precursor is converted to
a circular RNA in isolated nuclei of Tetrahymena. Cell. 23:467–476.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nigro JM, Cho KR, Fearon ER, Kern SE,
Ruppert JM, Oliner JD, Kinzler KW and Vogelstein B: Scrambled
exons. Cell. 64:607–613. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Capel B, Swain A, Nicolis S, Hacker A,
Walter M, Koopman P, Goodfellow P and Lovell-Badge R: Circular
transcripts of the testis-determining gene Sry in adult mouse
testis. Cell. 73:1019–1030. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z
and Sharpless NE: Expression of linear and novel circular forms of
an INK4/ARF associated non-coding RNA correlates with
atherosclerosis risk. PLoS Genet. 6:e10012332010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morris KV and Mattick JS: The rise of
regulatory RNA. Nat Rev Genet. 15:423–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeck WR and Sharpless NE: Detecting and
characterizing circular RNAs. Nat Biotechnol. 32:453–461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Yang L and Chen LL: The biogenesis,
functions, and challenges of circular RNAs. Mol Cell. 71:428–442.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boeckel JN, Jaé N, Heumüller AW, Chen W,
Boon RA, Stellos K, Zeiher AM, John D, Uchida S and Dimmeler S:
Identification and characterization of hypoxia-regulated
endothelial circular RNA. Circ Res. 117:884–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vicens Q and Westhof E: Biogenesis of
circular RNAs. Cell. 159:13–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suzuki H, Zuo Y, Wang J, Zhang MQ,
Malhotra A and Mayeda A: Characterization of RNase R-digested
cellular RNA source that consists of lariat and circular RNAs from
pre-mRNA splicing. Nucleic Acids Res. 34:e632006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao
L, Zhang Y, Wu YM, Dhanasekaran SM, Engelke CG, Cao X, et al: The
Landscape of circular RNA in cancer. Cell. 176:869–881.e13. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patop IL, Wüst S and Kadener S: Past,
present, and future of circRNAs. EMBO J. 38:e1008362019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang
W, Wang G, Wu P, Wang H, Jiang L, et al: Exosomal circRNAs:
Biogenesis, effect and application in human diseases. Mol Cancer.
18:1162019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J,
Chen D, Gu J, He X and Huang S: Circular RNA is enriched and stable
in exosomes: A promising biomarker for cancer diagnosis. Cell Res.
25:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmidt CA, Giusto JD, Bao A, Hopper AK
and Matera AG: Molecular determinants of metazoan tricRNA
biogenesis. Nucleic Acids Res. 47:6452–6465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guarnerio J, Bezzi M, Jeong JC, Paffenholz
SV, Berry K, Naldini MM, Lo-Coco F, Tay Y, Beck AH and Pandolfi PP:
Oncogenic role of fusion-circRNAs derived from cancer-associated
chromosomal translocations. Cell. 165:289–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu X, Wang X, Li J, Hu S, Deng Y, Yin H,
Bao X, Zhang QC, Wang G, Wang B, et al: Identification of mecciRNAs
and their roles in mitochondrial entry of proteins. Sci China Life
Sci. Jan 21–2020.(Epub ahead of print).
|
|
38
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: circRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Graves P and Zeng Y: Biogenesis of
mammalian MicroRNAs: A global view. Genomics Proteomics
Bioinformatics. 10:239–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bartel DP: MiRNAs: Target recognition and
regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ye F, Gao G, Zou Y, Zheng S, Zhang L, Ou
X, Xie X and Tang H: circFBXW7 inhibits malignant progression by
sponging miR-197-3p and encoding a 185-aa protein in
triple-negative breast cancer. Mol Ther Nucleic Acids. 18:88–98.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bai N, Peng E, Qiu X, Lyu N, Zhang Z, Tao
Y, Li X and Wang Z: circFBLIM1 act as a ceRNA to promote
hepatocellular cancer progression by sponging miR-346. J Exp Clin
Cancer Res. 37:1722018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang PF, Wei CY, Huang XY, Peng R, Yang
X, Lu JC, Zhang C, Gao C, Cai JB, Gao PT, et al: Circular RNA
circTRIM33-12 acts as the sponge of MicroRNA-191 to suppress
hepatocellular carcinoma progression. Mol Cancer. 18:1052019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen S, Huang V, Xu X, Livingstone J,
Soares F, Jeon J, Zeng Y, Hua JT, Petricca J, Guo H, et al:
Widespread and functional RNA circularization in localized prostate
cancer. Cell. 176:831–843.e22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Piwecka M, Glazar P, Hernandez-Miranda LR,
Memczak S, Wolf SA, Rybak-Wolf A, Filipchyk A, Klironomos F, Cerda
Jara CA, Fenske P, et al: Loss of a mammalian circular RNA locus
causes miRNA deregulation and affects brain function. Science.
357:eaam85262017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu YJ, Zheng B, Luo GJ, Ma XK, Lu XY, Lin
XM, Yang S, Zhao Q, Wu T, Li ZX, et al: Circular RNAs negatively
regulate cancer stem cells by physically binding FMRP against CCAR1
complex in hepatocellular carcinoma. Theranostics. 9:3526–3540.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Du WW, Yang W, Chen Y, Wu ZK, Foster FS,
Yang Z, Li X and Yang BB: Foxo3 circular RNA promotes cardiac
senescence by modulating multiple factors associated with stress
and senescence responses. Eur Heart J. 38:1402–1412.
2017.PubMed/NCBI
|
|
52
|
Du WW, Fang L, Yang W, Wu N, Awan FM, Yang
Z and Yang BB: Induction of tumor apoptosis through a circular RNA
enhancing Foxo3 activity. Cell Death Differ. 24:357–370. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liang WC, Wong CW, Liang PP, Shi M, Cao Y,
Rao ST, Tsui SK, Waye MM, Zhang Q, Fu WM and Zhang JF: Translation
of the circular RNA circβ-catenin promotes liver cancer cell growth
through activation of the Wnt pathway. Genome Biol. 20:842019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zheng X, Chen L, Zhou Y, Wang Q, Zheng Z,
Xu B, Wu C, Zhou Q, Hu W, Wu C and Jiang J: A novel protein encoded
by a circular RNA circPPP1R12A promotes tumor pathogenesis and
metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer.
18:472019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao
F, Huang N, Yang X, Zhao K, Zhou H, et al: Novel role of FBXW7
circular RNA in repressing glioma tumorigenesis. J Natl Cancer
Inst. 110:304–315. 2018. View Article : Google Scholar
|
|
56
|
Wang L, Long H, Zheng Q, Bo X, Xiao X and
Li B: Circular RNA circRHOT1 promotes hepatocellular carcinoma
progression by initiation of NR2F6 expression. Mol Cancer.
18:1192019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu N, Yuan Z, Du KY, Fang L, Lyu J, Zhang
C, He A, Eshaghi E, Zeng K, Ma J, et al: Translation of
yes-associated protein (YAP) was antagonized by its circular RNA
via suppressing the assembly of the translation initiation
machinery. Cell Death Differ. 26:2758–2773. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun YM, Wang WT, Zeng ZC, Chen TQ, Han C,
Pan Q, Huang W, Fang K, Sun LY, Zhou YF, et al: CircMYBL2, a
circRNA from, MYBL2 regulates FLT3 translation by recruiting PTBP1
to promote FLP3-ITD AML progression. Blood. 134:1533–1546. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang BG, Li JS, Liu YF and Xu Q:
MicroRNA-200b suppresses the invasion and migration of
hepatocellular carcinoma by downregulating RhoA and circRNA_000839.
Tumour Biol. 39:10104283177195772017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dong W, Dai ZH, Liu FC, Guo XG, Ge CM,
Ding J, Liu H and Yang F: The RNA-binding protein RBM3 promotes
cell proliferation in hepatocellular carcinoma by regulating
circular RNA SCD-circRNA 2 production. EBioMedicine. 45:155–167.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang N, Li G, Li X, Xu L and Chen M:
Circ5379-6, a circular form of tumor suppressor PPARα, participates
in the inhibition of hepatocellular carcinoma tumorigenesis and
metastasis. Am J Transl Res. 10:3493–3503. 2018.PubMed/NCBI
|
|
62
|
Yao Z, Luo J, Hu K, Lin J, Huang H, Wang
Q, Zhang P, Xiong Z, He C, Huang Z, et al: ZKSCAN1 gene and its
related circular RNA (circZKSCAN1) both inhibit hepatocellular
carcinoma cell growth, migration, and invasion but through
different signaling pathways. Mol Oncol. 11:422–437. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shen S, Wu Y, Chen J, Xie Z, Huang K, Wang
G, Yang Y, Ni W, Chen Z, Shi P, et al: CircSERPINE2 protects
against osteoarthritis by targeting miR-1271 and ETS-related gene.
Ann Rheum Dis. 78:826–836. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shang Q, Yang Z, Jia R and Ge S: The novel
roles of circRNAs in human cancer. Mol Cancer. 18:62019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Aufiero S, Reckman YJ, Pinto YM and
Creemers EE: Circular RNAs open a new chapter in cardiovascular
biology. Nat Rev Cardiol. 16:503–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Akhter R: Circular RNA and Alzheimer's
disease. Adv Exp Med Biol. 1087:239–243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Floris G, Zhang L, Follesa P and Sun T:
Regulatory role of circular RNAs and neurological disorders. Mol
Neurobiol. 54:5156–5165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen X, Yang T, Wang W, Xi W, Zhang T, Li
Q, Yang A and Wang T: Circular RNAs in immune responses and immune
diseases. Theranostics. 9:588–607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang L, Fu J and Zhou Y: Circular RNAs and
their emerging roles in immune regulation. Front Immunol.
9:29772018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zou F, Ding Z, Jiang J, Lu F, Xia X and Ma
X: Confirmation and preliminary analysis of circRNAs potentially
involved in human intervertebral disc degeneration. Mol Med Rep.
16:9173–9180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang S, Sun J, Yang H, Zou W, Zheng B,
Chen Y, Guo Y and Shi J: Profiling and bioinformatics analysis of
differentially expressed circular RNAs in human intervertebral disc
degeneration. Acta Biochim Biophys Sin (Shanghai). 51:571–579.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhu J, Zhang X, Gao W, Hu H, Wang X and
Hao D: lncRNA/circRNA-miRNA-mRNA ceRNA network in lumbar
intervertebral disc degeneration. Mol Med Rep. 20:3160–3174.
2019.PubMed/NCBI
|
|
73
|
Collison J: Degenerative disc disease:
Circular RNA reduces cell death in IVD disease. Nat Rev Rheumatol.
14:1232018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila PA 1976).
26:1873–1878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lan PH, Liu ZH, Pei YJ, Wu ZG, Yu Y, Yang
YF, Liu X, Che L, Ma CJ, Xie YK, et al: Landscape of RNAs in human
lumbar disc degeneration. Oncotarget. 7:63166–63176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sekiya I, Tsuji K, Koopman P, Watanabe H,
Yamada Y, Shinomiya K, Nifuji A and Noda M: SOX9 enhances aggrecan
gene promoter/enhancer activity and is up-regulated by retinoic
acid in a cartilage-derived cell line, TC6. J Biol Chem.
275:10738–10744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lefebvre V, Huang W, Harley VR, Goodfellow
PN and de Crombrugghe B: SOX9 is a potent activator of the
chondrocyte-specific enhancer of the pro alpha1(II) collagen gene.
Mol Cell Biol. 17:2336–2346. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hiyama A, Sakai D, Risbud MV, Tanaka M,
Arai F, Abe K and Mochida J: Enhancement of intervertebral disc
cell senescence by WNT/β-catenin signaling induced matrix
metalloproteinase expression. Arthritis Rheum. 62:3036–3047. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hiyama A, Sakai D, Tanaka M, Arai F,
Nakajima D, Abe K and Mochida J: The relationship between the
Wnt/β-catenin and TGF-β/BMP signals in the intervertebral disc
cell. J Cell Physiol. 226:1139–1148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Guezguez B, Almakadi M, Benoit YD,
Shapovalova Z, Rahmig S, Fiebig-Comyn A, Casado FL, Tanasijevic B,
Bresolin S, Masetti R, et al: GSK3 deficiencies in hematopoietic
stem cells initiate pre-neoplastic state that is predictive of
clinical outcomes of human acute leukemia. Cancer Cell. 29:61–74.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tao J, Abudoukelimu M, Ma YT, Yang YN, Li
XM, Chen BD, Liu F, He CH and Li HY: Secreted frizzled related
protein 1 protects H9C2 cells from hypoxia/re-oxygenation injury by
blocking the Wnt signaling pathway. Lipids Health Dis. 15:722016.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Guo J, Shao M, Lu F, Jiang J and Xia X:
Role of Sirt1 plays in nucleus pulposus cells and intervertebral
disc degeneration. Spine (Phila Pa 1976). 42:E757–E766. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang D, Hu Z, Hao J, He B, Gan Q, Zhong X,
Zhang X, Shen J, Fang J and Jiang W: SIRT1 inhibits apoptosis of
degenerative human disc nucleus pulposus cells through activation
of Akt pathway. Age (Dordr). 35:1741–1753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yao ZQ, Zhang X, Zhen Y, He XY, Zhao S, Li
XF, Yang B, Gao F, Guo FY, Fu L, et al: A novel small-molecule
activator of Sirtuin-1 induces autophagic cell death/mitophagy as a
potential therapeutic strategy in glioblastoma. Cell Death Dis.
9:7672018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wei Y, Chen X, Liang C, Ling Y, Yang X, Ye
X, Zhang H, Yang P, Cui X, Ren Y, et al: A noncoding regulatory
RNAs network driven by Circ-CDYL acts specifically in the early
stages hepatocellular carcinoma. Hepatology. 71:130–147. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ren S, Liu J, Feng Y, Li Z, He L, Li L,
Cao X, Wang Z and Zhang Y: Knockdown of circDENND4C inhibits
glycolysis, migration and invasion by up-regulating miR-200b/c in
breast cancer under hypoxia. J Exp Clin Cancer Res. 38:3882019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ren GL, Zhu J, Li J and Meng XM: Noncoding
RNAs in acute kidney injury. J Cell Physiol. 234:2266–2276. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chaichian S, Shafabakhsh R, Mirhashemi SM,
Moazzami B and Asemi Z: Circular RNAs: A novel biomarker for
cervical cancer. J Cell Physiol. 235:718–724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Braicu C, Tomuleasa C, Monroig P, Cucuianu
A, Berindan-Neagoe I and Calin GA: Exosomes as divine messengers:
Are they the hermes of modern molecular oncology? Cell Death
Differ. 22:34–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liu Y, Hou J, Zhang M, Seleh-Zo E, Wang J,
Cao B and An X: circ-016910 sponges miR-574-5p to regulate cell
physiology and milk synthesis via MAPK and PI3K/AKT-mTOR pathways
in GMECs. J Cell Physiol. 235:4198–4216. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen T, Yu Q, Xin L and Guo L: Circular
RNA circC3P1 restrains kidney cancer cell activity by regulating
miR-21/PTEN axis and inactivating PI3K/AKT and NF-kB pathways. J
Cell Physiol. 235:4001–4010. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xu G, Liu C, Jiang J, Liang T, Yu C, Qin
Z, Zhang Z, Lu Z and Zhan X: A novel mechanism of intervertebral
disc degeneration: Imbalance between autophagy and apoptosis.
Epigenomics. Apr 14–2020.(Epub ahead of print). View Article : Google Scholar
|
















