Introduction
Periodontitis is a chronic inflammatory disease, of
which the initiating factor, bacterial plaque, causes irreparable
damage to the tooth-supporting structures (1,2).
Tissue regeneration is currently the most effective therapeutic
method for severe periodontitis; however, the treatment effect is
limited (3). In 1992, Wise et
al (4) successfully cultured
rDFCs for the first time. Since, the stem cell properties of
self-renewal and multi-directional differentiation potential in
DFCs have been verified (5–8).
Bone morphogenetic protein (BMP) has been discovered to facilitate
the differentiation of stem cells into osteoblasts, which
subsequently increased osteogenesis (9,10).
Moreover, BMP9 induces cell differentiation (11–14).
Gene transfection using adenovirus vectors has previously
demonstrated efficacy (15). In a
previous study, TNF-α, a proinflammatory cytokine, lead to the
absorption and destruction of periodontal bone and collagen fibers
(16). Notably, Mukai et al
(17) discovered that TNF-α
suppressed BMP2-induced osteogenic differentiation.
Wnt signaling is involved in bone formation and bone
resorption via two major molecular signaling pathways: The
β-catenin-dependent canonical signaling pathway and the
Ca2+-dependent non-canonical Wnt pathway (18). Several studies have indicated that
the canonical Wnt signaling served a two-directional regulatory
role in osteogenic differentiation; for example, Qiu et al
(19) discovered that the
expression levels of osteogenesis-related factors alkaline
phosphatase (ALP) and Runt-related transcription factor 2 (RUNX2)
were upregulated in human mesenchymal stem cells via the activation
of the canonical Wnt signaling pathway; and Jansen et al
(20) conducted a tensile
experiment on human pre-osteoblasts and discovered that the
elevated concentrations of β-catenin at the early osteogenesis
stagewere markedly reduced at the moderate and advanced
osteogenesis stages, which subsequently induced the formation of a
large amount of mineralized tissues (20). These findings suggested that the
canonical Wnt signaling pathway may suppress osteogenesis at the
late differentiation stage. Additionally, numerous studies have
also reported that osteogenic induction or an inflammatory
microenvironment prompted canonical Wnt signaling to negatively
regulate cell osteogenic differentiation; for instance, Liu et
al (21) discovered that
suppressing the Wnt/β-catenin pathway and upregulating the
Wnt/Ca2+ non-canonical pathway enhanced the osteogenic
differentiation of periodontal ligament stem cells under
inflammation; and Xiang et al (22) further discovered that the Wnt5a
protein promoted osteogenic differentiation and interacted with the
BMP2 signaling pathway (22).
However, the Wnt canonical signaling pathway was reported to be
inhibited by Dickkopf 1 (DKK1) (23–25).
The Wnt/β-catenin pathway was also identified to be involved in
osteoclastogenesis, osteoclast differentiation and bone resorption
by influencing the expression levels of osteoprotegerin (24,25).
The present study aimed to determine the effects of
TNF-α on osteogenic differentiation and investigate the mechanisms
of the Wnt signaling pathway in BMP9 adenovirus (AdBMP9)-transduced
rDFCs.
Materials and methods
Cell culture and adenovirus-mediated
transfection
Sprague Dawley rats (n=10; age, 6–8 days old;
male:female, 1:1; weight, 20–30 g) were purchased from the
Experimental Animal Center of Chongqing Medical University
(Chongqing, China). The rats were housed in cages under a 12-h
light/dark cycle with free access to food and water, at a
temperature of 22–25°C and 50–60% humidity; the rats were monitored
every day. Rats were sacrificed with 35 mg/kg sodium pentobarbital
(1%) followed by decapitation then soaked in 20% ethanol for 10
min; respiratory arrest was confirmed following decapitation.
Dental sac tissues were obtained from the first and second
mandibular molars, and rDFCs were obtained as previously described
(6). rDFCs were cultured in DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 mg/ml streptomycin (Sigma-Aldrich; Merck KGaA), and
maintained at 37°C in a humidified atmosphere containing 5%
CO2.
Flow cytometric analysis and multi-lineage
differentiation were performed for the characterization of
mesenchymal stem cells, as previously described (6). The identification of rDFCs and the
determination of appropriate concentrations of TNF-α (10 ng/ml;
GenScript) and DKK1 (0.1 and 0.4 µg/ml; Sino Biological, Beijing
China) were determined based on previously published studies
(6,8).
At 80% confluence (passage 3), cells were digested
by 0.25% trypsin, re-suspended and re-cultured at 37°C in a
humidified atmosphere containing 5% CO2 for 24 h. AdBMP9
(MOI=100) and polybrene (1 µm/ml) were added to the adherent
culture according to previously described methods (6,14).
Following the addition of AdBMP9, cells were incubated for 4 h,
then 2 ml complete medium was added followed by incubation for 24 h
at 37°C in an atmosphere containing 5% CO2. The green
fluorescence was observed under a fluorescence microscope
(magnification, ×40) AdGFP(MOI=100) was used as the vector control.
Cells were subsequently collected for further experimentation.
Ad-BMP9 and Ad-GFP were provided by Dr Tong-Chuan He (Molecular
Oncology Laboratory, University of Chicago Medical Centre,
USA).
ALP staining
A total of 5×104 cells/well were plated
into 24-well plates and cultured in a conventional osteogenic
medium for 7 days at 37°C (Beyotime Institute of Biotechnology).
After transfection with AdBMP9, TNF-α (10 ng/ml, GenScript) and
DKK1 (0.1 or 0.4 µg/ml; Sino Biological Inc.) were added into the
medium and cultured at 37°C in an atmosphere containing 5%
CO2. The medium was refreshed every three days. At 7
days post-culture, cells were fixed with 4% paraformaldehyde at
room temperature for 2 h and washed with distilled, deionized
water. Stained cells were observed under a light microscope
(magnification, ×100). ALP staining was performed using an ALP
staining kit (Beyotime Institute of Biotechnology), according to
the manufacturer's protocol.
Alizarin Red S staining
Cells (5×104 cells/well) were plated into
24-well plates and cultured in a conventional osteogenic medium at
37°C for 14 days. TNF-α (10 ng/ml) and DKK1 (0.1 or 0.4 µg/ml) were
added into the medium and cultured at 37°C in an atmosphere
containing 5% CO2. The medium was refreshed every three
days. Following 14 days of culture, mineralized matrix nodules were
stained for calcium precipitation using Alizarin Red S. Briefly,
cells were fixed with 4% paraformaldehyde at 20°C for 15 min and
then washed with distilled, deionized water. Subsequently, the
cells were stained with Alizarin Red S (Beyotime Institute of
Biotechnology) at room temperature for 30 min, followed by three
washes with deionized water. Deionized water was added into each
well to prevent cells from drying before they were observed under a
light microscope (magnification, ×40).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA using the iScript™cDNA Synthesis kit (Bio-Rad
Laboratories, Inc.) according to the manufacturer's protocol.
Reverse transcription was performed using the following temperature
protocol: 85°C for 2 min and 37°C for 30 min. Subsequently, qPCR
was performed using SYBR-Green Master mix (Thermo Fisher
Scientific, Inc.). The sequences of the primers used for qPCR are
presented in Table I. Primers used
for qPCR amplification are presented in Table I, and β-actin was used as the
internal control. The following thermocycling conditions were used
for qPCR: 94°C for 2 min; followed by 35 cycles of denaturation at
95°C for 30 sec, annealing at 64°C for 30 sec, and extension 72°C
for 30 sec. Relative gene expression was calculated using the
2−ΔΔCq method (26) and
normalized to the internal reference gene β-actin.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| β-actin | F:
TGCTATGTTGCCCTAGACTTCG |
|
| R:
GTTGGCATAGAGGTCTTTACG |
| Collagen type 1
α1 | F:
CAAGAAGTCCCTGCTCCTCCA |
|
| R:
GGGAGGTCTTGGTGGTTTTGTAT |
| Cyclin D1 | F:
GTTCATTTCCAACCCACCCTC |
|
| R:
CGTTGTGCGGTAGCAGGAGA |
| Alkaline
phosphatase | F:
GGCACCTGCCTTACCAACTCT |
|
| R:
GTTGTGGTGTAGCTGGCCCTTA |
| Osteocalcin | F:
TTTCTGCTCACTCTGCTGACC |
|
| R:
CAGCACAACTCCTTCCTACCA |
| Runt-related
transcription factor 2 | F:
CAGCTGCTTAGACGCTGGATT |
|
| R:
AGGCGGGACACCTACTCTCATA |
| c-Myc | F:
CAGCTGCTTAGACGCTGGATT |
|
| R:
GTAGAAATACGGCTGCACCGA |
| β-catenin | F:
GAGCCTGCCATCTGTGCTCT |
|
| R:
ACGCAAAGGTGCATGATTTG |
|
Calcium/calmodulin-dependent protein
kinase type II α chain | F:
AACTGACCAGGCACAGACG |
|
| R:
CCCTAATGTCTTCCGCCTGC |
| Serine/threonine
protein kinase NLK | F:
TGGGCAACAACAGCCATATTT |
|
| R:
ATGGTGCGCCTTAACTGTAGC |
Western blotting
Cells were washed in precooled PBS twice and total
protein was extracted from cells using RIPA lysis buffer (Pierce;
Thermo Fisher Scientific, Inc.), containing protease inhibitor,
phosphatase inhibitor and 1 mM PMSF. The cells were incubated on
ice for 30 min and the lysate was subsequently collected in a 1.5
ml tube and centrifuged at 10,000 × g for 10 min at 4°C. The
supernatant was transferred into 1.5 ml new tubes, followed by the
addition of 5X loading buffer (volume 1:4), which was then boiled
for 5–8 min and stored at −20°C until use. Total protein was
quantified using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) and proteins (30 µg) were separated via
8% SDS-PAGE. The separated proteins were subsequently transferred
via the wet method (6,8,14) to
PVDF membranes and blocked with PBS containing 0.2% Tween-20 and 5%
non-fat milk at 4°C for 2 h. The membranes were then incubated
overnight at 4°C with the following primary antibodies overnight at
4°C: Anti-BMP9 (cat. no. ab35088; 1:1,000; Abcam), anti-β-catenin
(cat. no. ab6302; 1:1,000; Abcam), anti-phosphorylated (p)-glycogen
synthase kinase 3β (GSK3β; cat. no. ab131097; 1:1,000; Abcam),
anti-GSK3β (cat. no. ab93926; 1:1,000; Abcam),
anti-calcium/calmodulin dependent protein kinase II (CaMKII; cat.
no. ab52476; 1:1,000; Abcam), anti-nemo like kinase (NLK; cat. no.
ab26050; 1:1,000; Abcam) and anti-β-actin (cat. no. 4970; 1:1,000;
Cell Signaling Technology, Inc.). Following the primary antibody
incubation, the membranes were incubated at room temperature for 2
h with a HRP-conjugated goat anti-rabbit (cat. no. ab205718;
1:3,000; Abcam) and anti-mouse (cat. no. 58802; 1:3,000; Cell
Signaling Technology, Inc.). Protein bands were visualized using an
ECL chemiluminescent substrate kit (Beyotime Institute of
Biotechnology) and densitometric analysis was performed using
ImageJ software (version 1.52; National Institutes of Health).
Animal experimental design
The present study was approved by the Institutional
Animal Experimental Ethics Committee of Chongqing Medical
University (Chongqing, China). A total of 20 4-week-old Sprague
Dawley rats (male; weight, 200–250 g) were purchased from the
Experimental Animal Center of Chongqing Medical University. The
rats were housed in cages under a 12-h light/dark cycle with free
access to food and water, at 22–25°C and 50–60% humidity. Animal
health and behavior was monitored every day. The rats were randomly
divided into 4 groups (n=5 per group): i) AdBMP9 group; ii) AdBMP9
+ 0.1 µg/ml DKK1; iii) AdBMP9 + 0.4 µg/ml DKK1; and iv) AdBMP9 +
TNF-α and received implants respectively as detailed below. All
cells had been previously co-cultured for 7 days with
hydroxyapatite (40 mg/ml; Biomaterials Engineering Research Center
of Sichuan University), a scaffold material, at 37°C (14).
Rats were anaesthetized using an intraperitoneal
injection of 35 mg/kg sodium pentobarbital (1%). The periapical
alveolar bone of the first and second molars was exposed by surgery
and an osseous defect (2×3×3 mm) was created. Cells got
7-day-co-cultured with hydroxyapatite. The defect of was filled
with rDFCs-hydroxyapatite in accordance with the grouping of each
rat (4×106 cells; 20 mg; injection site, periapical
defect; age of rats at injection, 8 weeks) then covered with a
collagen membrane (5×5 mm; Bopei Biotech Co., Ltd). The wound was
closed with interrupted sutures. The 4th week following surgery was
chosen as the humane endpoint; although the rats were in a good
mental state and exhibited good locomotor activity, the scientific
goal of the research has been achieved, thus, it was of little
significance to continue the experiment. The rats were transferred
from the rearing room into the testing room at ≥30 min prior to the
initiation of the experiment. Subsequently, rats were anesthetized
with an intraperitoneal injection of 35 mg/kg sodium pentobarbital
(1%) and sacrificed by decapitation; respiratory arrest and death
were confirmed.
The alveolar bones were extracted and fixed in 4%
paraformaldehyde for 48 h at room temperature. Subsequently,
sections (1 mm3) were stained with hematoxylin for 5 min
at room temperature and eosin for 3 min at room temperature.
Stained sections were observed under a light microscope
(magnification, ×100).
Statistical analysis
Data are presented as the mean ± SD of ≥3
independent experiments. Statistical analysis was performed using
SPSS 21.0 software (IBM Corp.). Data from the different groups were
compared and analyzed using a one-way ANOVA, followed by a
Bonferroni's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
TNF-α suppresses the osteogenic
differentiation of AdBMP9-transduced rDFCs
rDFCs are polygonal or fusiform in morphology
(6,8,14).
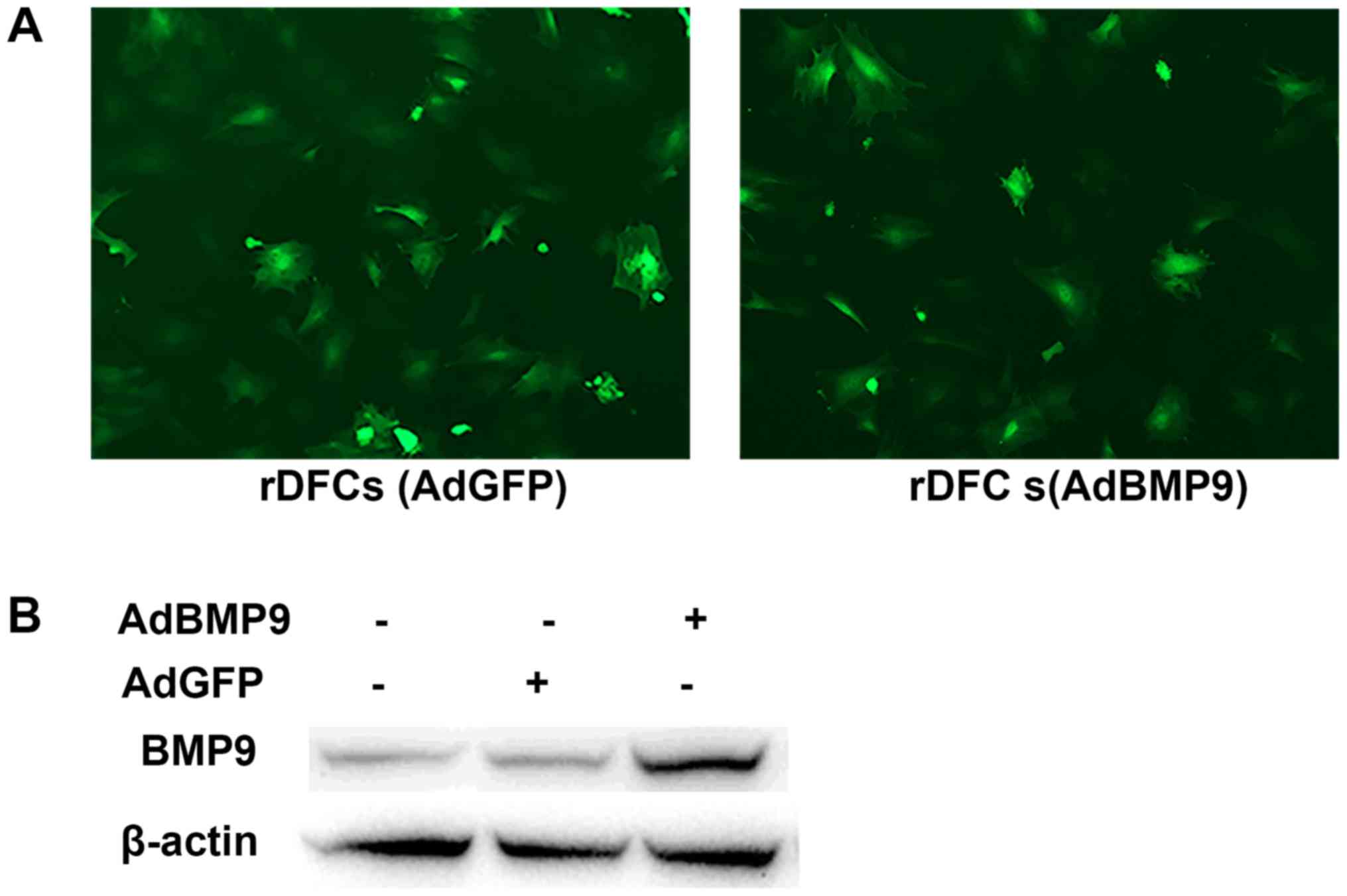
rDFCs were transfected with AdBMP9 or AdGFP and green fluorescence
was detected at 24 h post-transfection (Fig. 1A). In addition, following AdBMP9
transfection for 24 h, the expression levels of BMP9 were
upregulated compared with the AdGFP and blank groups (Fig. 1B). To determine the effect of TNF-α
on AdBMP9-induced osteogenic differentiation, rDFCs were
transfected with AdBMP9 prior to treatment with 10 ng/ml TNF-α,
which was selected as the stimulatory concentration for use in the
experiments (8). Cells were
collected 7- and 14-days post-transfection and analyzed via ALP
staining and Alizarin Red S staining, respectively. The results
revealed that TNF-α treatment not only the reduced osteogenic
medium-induced osteogenesis, but it also downregulated BMP9-induced
osteogenic differentiation (Fig.
2A). The expression levels of bone-related genes, ALP, collagen
type 1 α1 (COLI), and the late osteogenesis marker osteocalcin
(OCN), were further analyzed, in addition to the osteogenic
differentiation-related transcription factor, RUNX2. RT-qPCR
analysis discovered that the expression levels of these genes were
significantly upregulated in the rDFCs + AdBMP9 group compared with
the rDFCs group; however, these expression levels were
significantly downregulated in the rDFCs + AdBMP9 + TNF-α group
(Fig. 2B-E). These results
indicated that AdBMP9 may promote the osteogenic differentiation of
rDFCs, which may be suppressed by TNF-α.
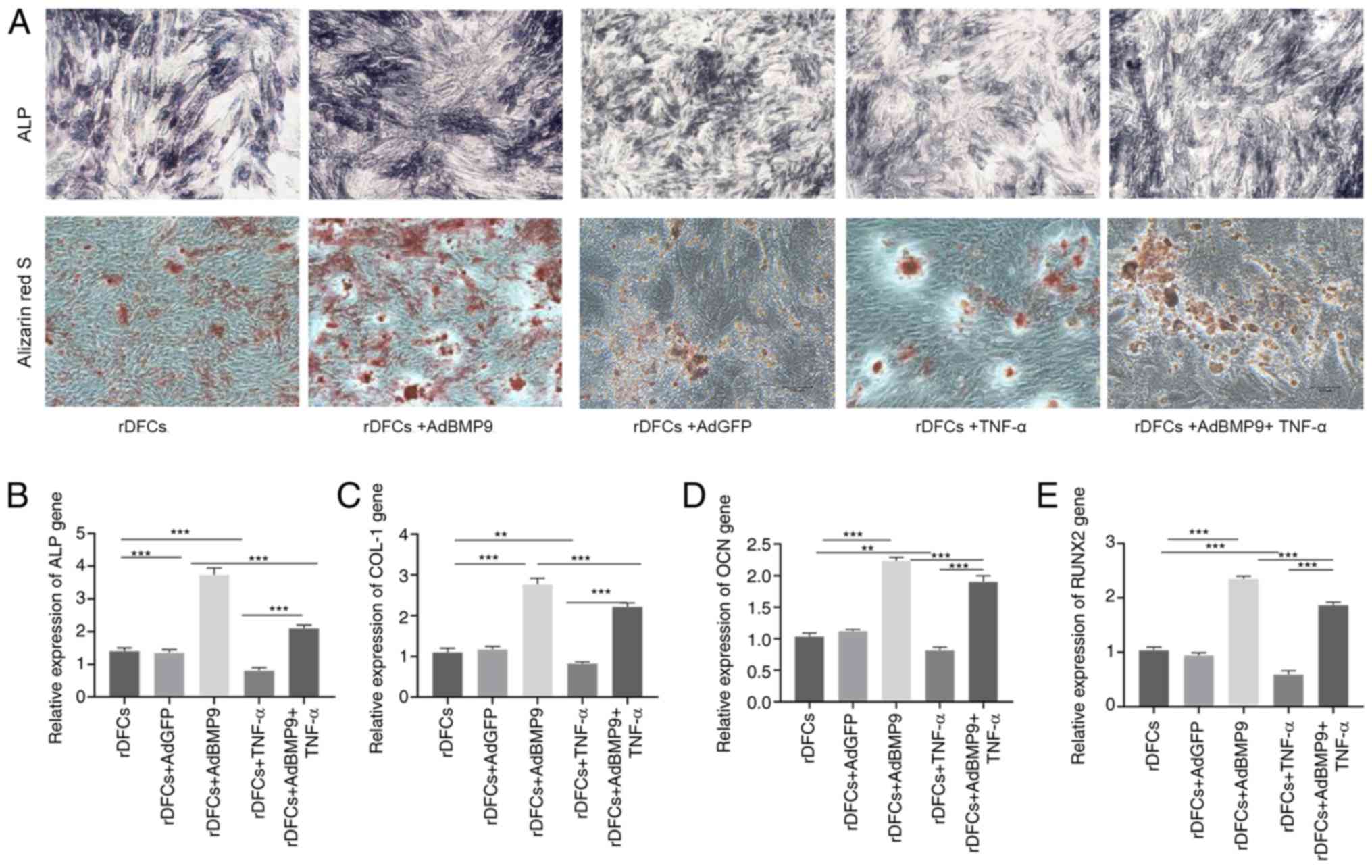 | Figure 2.TNF-α suppresses AdBMP9-induced
osteogenic differentiation of rDFCs. (A) ALP (magnification, ×40)
and Alizarin Red S staining (magnification, ×100) of cells were
performed on days 7 and 14, respectively. BMP9 enhanced ALP
activity and matrix mineralization and TNF-α reduced the effect of
osteogenesis. Reverse transcription-quantitative PCR was used to
analyze the expression levels of the osteogenic markers (B) ALP,
(C) COL1, (D) OCN and (E) RUNX2 on day 7 post-transfection. The
expression levels of these markers were upregulated by BMP9 but
downregulated by TNF-α. **P<0.01 and ***P<0.001. rDFCs, rat
follicle stem cells; Ad, adenovirus; BMP9, bone morphogenetic
protein 9; TNF-α, tumor necrosis factor α; ALP, alkaline
phosphatase; COL1, collagen type 1 α1; OCN, osteocalcin; RUNX2,
runt-related transcription factor 2. |
TNF-α regulates the Wnt signaling
pathway during the BMP9-induced osteogenic differentiation of
rDFCs
Wnt signaling has been discovered to be crucial for
BMP9-induced osteogenic differentiation (8,24).
AdGFP was used in preliminary western blotting experiments as the
vector control as a comparison to the effects of AdBMP9 (Fig. 3A and C). After TNF-α stimulation,
the expression levels of β-catenin and p-GSK3β in the TNF-α and
BMP9 + TNF-α groups were upregulated compared with the control and
BMP9 groups, respectively (Fig.
3B). The expression levels of CaMKII and NLK were also
downregulated following TNF-α stimulation in the TNF-α and BMP9 +
TNF-α groups compared with the control and BMP9 groups,
respectively (Fig. 3D). CaMKII and
NLK are parts of the non-canonical Wnt signaling pathway (21,23)
Thus, these results suggested that TNF-α may inhibit the
non-canonical Wnt signaling pathway. Cyclin D1 and c-Myc are
downstream genes in the canonical Wnt signaling pathway (27,28).
The RT-qPCR results demonstrated that the expression levels of
cyclin D1 and c-Myc were significantly upregulated in the TNF-α
stimulation group compared with the non-TNF-α stimulation group
(Fig. 3E), indicating that the
canonical Wnt signaling pathway of rDFCs may be activated by
TNF-α.
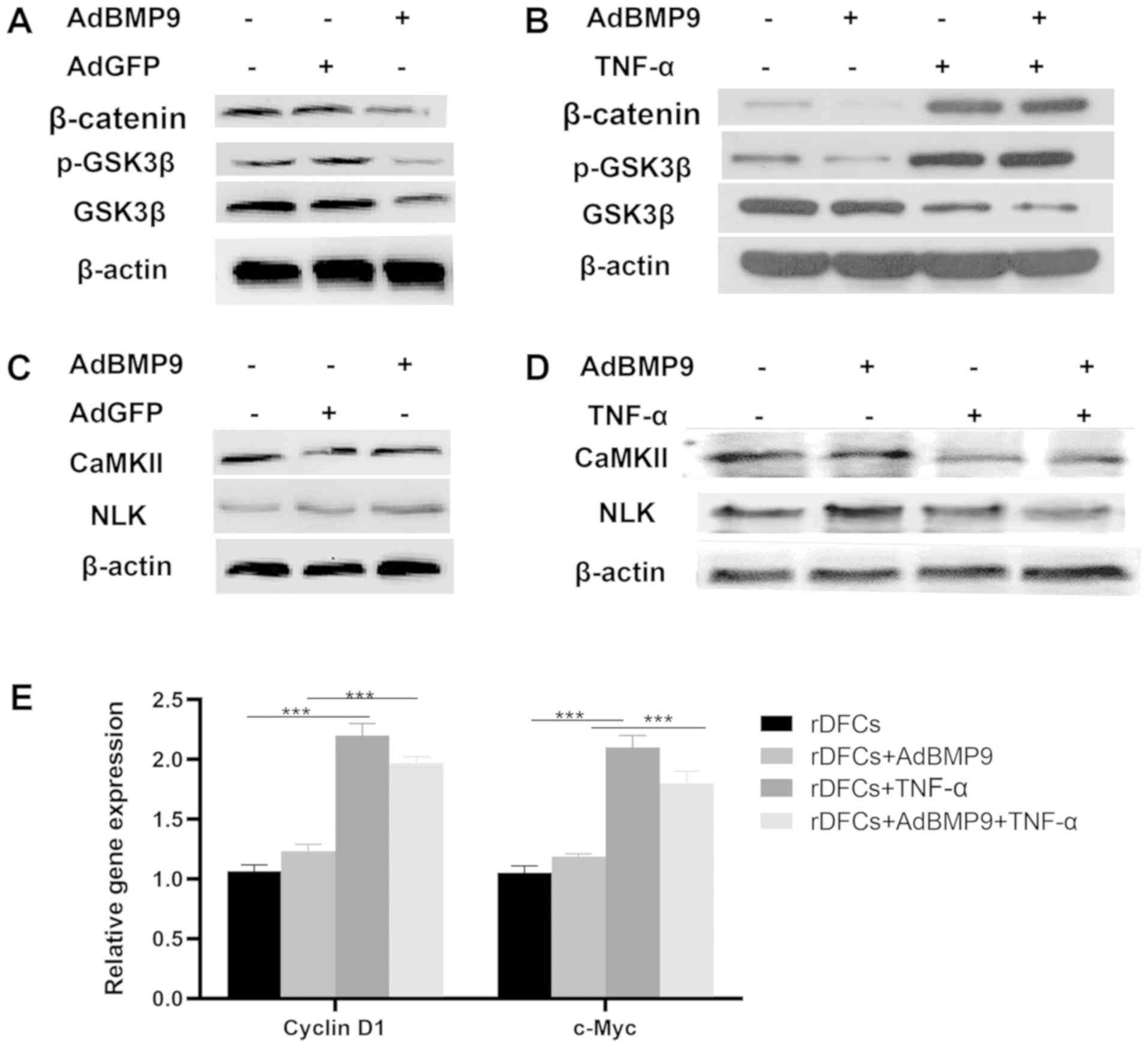 | Figure 3.TNF-α influences the Wnt signaling
pathways. (A) Western blotting was used to analyze the expression
levels of β-catenin, p-GSK3β and GSK3β in the blank, AdGFP and
AdBMP9 groups. (B) Western blotting revealed that TNF-α (10 ng/ml)
treatment upregulated the expression levels of β-catenin and
p-GSK3β. (C) Western blotting was used to analyze the expression
levels of CaMKII and NLK in the blank, AdGFP and AdBMP9 groups. (D)
Western blotting revealed that the expression levels of CaMKII and
NLK were downregulated by TNF-α (10 ng/ml) treatment. (E) Cyclin D1
and c-Myc expression levels were detected using reverse
transcription quantitative PCR. TNF-α upregulated the expression
levels of cyclin D1 and c-Myc. ***P<0.001. rDFCs, rat follicle
stem cells; Ad, adenovirus; BMP9, bone morphogenetic protein 9;
TNF-α, tumor necrosis factor α; GSK3β, glycogen synthase kinase 3β;
p-, phosphorylated; CaMKII, calcium/calmodulin-dependent protein
kinase type II α chain; NLK, serine/threonine protein kinase
NLK. |
Effect of DKK1 treatment on
AdBMP9-induced osteogenic differentiation of rDFCs
DKK1 is an inhibitor of the Wnt/β-catenin signaling
pathway (8,23). The present study selected 0.1 and
0.4 µg/ml DKK1 as the stimulatory concentrations to treat the rDFCs
with. Following the treatment with the higher concentration of DKK1
or TNF-α, AdBMP9-induced osteogenic differentiation was reduced
compared with the AdBMP9 group (Fig.
4A); however, the addition of low concentrations of DKK1
promoted osteogenesis. The RT-qPCR analysis revealed a similar
result; the expression levels of RUNX2, OCN and COLI were
significantly upregulated following the treatment with lose dose
DKK1 in AdBMP9-transduced rDFCs compared with the rDFCs + AdBMP9
group (Fig. 4E). However, TNF-α
treatment and high dose DKK1 treatment significantly downregulated
the expression levels of these genes compared with the rDFCs +
AdBMP9 group (Fig. 4E). In
addition, the expression levels of β-catenin, p-GSK3β and cyclin D1
were downregulated following the treatment with 0.1 and 0.4 µg/ml
DKK1 compared with the rDFC + AdBMP9 group (Fig. 4B and D). Thus, these findings
suggested that DKK1 may suppress the canonical Wnt/β-catenin
signaling pathway. Furthermore, following the treatment with 0.4
µg/ml DKK1, the expression levels of CaMKII and NLK were
upregulated compared with the rDFCs + AdBMP9 group, which suggested
the partial enhancement of the non-canonical Wnt signaling pathway
(Fig. 4C and D). Conversely, low
dose DKK1 (0.1 µg/ml) downregulated the expression levels of CaMKII
and NLK compared with the rDFCs + AdBMp9 group, which suggested the
inhibition of the non-canonical Wnt signaling pathway. The
abovementioned results suggested that high concentrations of DKK1
may activate non-canonical Wnt signaling and suppress canonical Wnt
signaling, whereas low concentrations of DKK1 may suppress both
canonical and non-canonical Wnt signaling in AdBMP9-transduced
rDFCs.
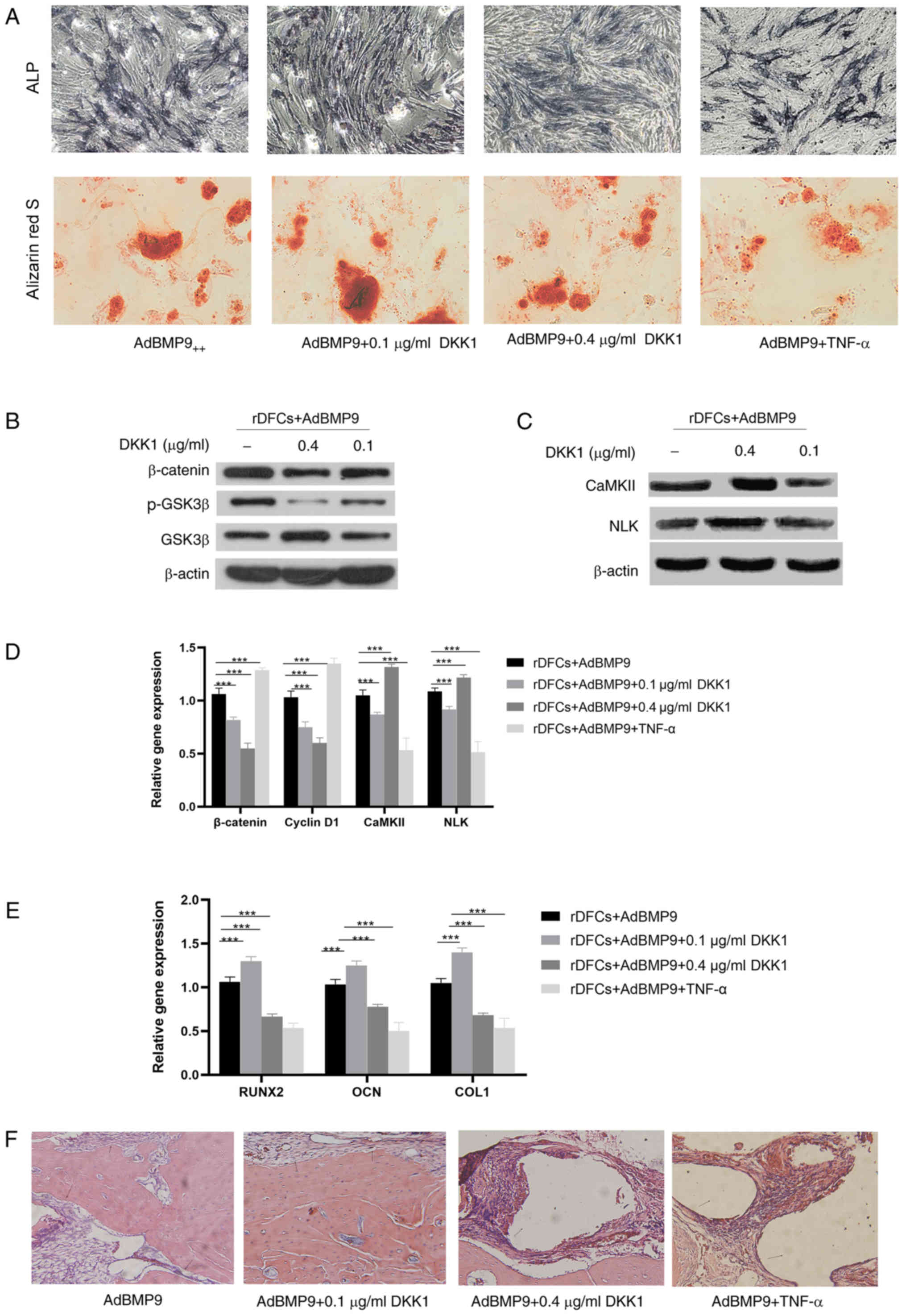 | Figure 4.Effect of DKK1 on AdBMP9-induced
osteogenic differentiation of rDFCs. (A) Alkaline phosphatase
staining (magnification, ×40) was performed on day 7
post-transfection and Alizarin Red S staining (magnification, ×100)
was performed on day 14 post-transfection. ALP staining intensity
was enhanced in BMP9 group and AdBMP9+DKK1 (0.1 µg/ml) group when
compared with the BMP9+TNF-α group and AdBMP9+DKK1 (0.4 µg/ml)
group. More calcified nodules were found in BMP9 group and AdBMP9+
DKK1 (0.1 µg/ml) group compared with the BMP9+TNF-α group and
AdBMP9+ DKK1 (0.4 µg/ml) group. (B) Effect of DKK1 on canonical Wnt
signaling in AdBMP9-transduced rDFCs. Western blotting revealed
that DKK1 downregulated the expression levels of β-catenin and
p-GSK3β. (C) Effect of DKK1 on non-canonical Wnt signaling in
AdBMP9-transduced rDFCs. The results revealed that 0.4 ng/ml DKK1
upregulated the expression levels of CaMKII and NLK, whereas 0.1
ng/ml DKK1 downregulated CaMKII expression levels. No obvious
differences were observed in NLK expression levels. (D) RT-qPCR was
used to analyze the mRNA expression levels of β-catenin, cyclin D1,
CaMKII and NLK. (E) RT-qPCR was used to determine mRNA expression
levels of the bone markers RUNX2, OCN and COLI. The expression
levels of these markers were upregulated following 0.1 ng/ml DKK1
treatment but downregulated with 0.4 ng/ml DKK1 and TNF-α (10
ng/ml). (F) Hematoxylin and eosin staining of sections of new bone
formation from Sprague Dawley rats (magnification, ×40).
***P<0.001. rDFCs, rat follicle stem cells; Ad, adenovirus;
BMP9, bone morphogenetic protein 9; TNF-α, tumor necrosis factor α;
DKK1, Dickkopf 1; GSK3β, glycogen synthase kinase 3β; p-,
phosphorylated; CaMKII, calcium/calmodulin-dependent protein kinase
type II α chain; NLK, serine/threonine protein kinase NLK; COL1,
collagen type 1 α1; OCN, osteocalcin; RUNX2, runt-related
transcription factor 2; RT-qPCR, reverse transcription-quantitative
PCR. |
Furthermore, the effect of restoring a bone defect
was evaluated in a Sprague Dawley rat model. H&E staining
demonstrated that TNF-α and high dose DKK1 treatment promoted a
small amount of bone formation from AdBMP9-transduced rDFCs.
Conversely, a mass of newly formed bone and numerous blood vessels
were observed in Sprague Dawley rats that were treated with
AdBMP9-transduced rDFCs and low dose DKK1 (Fig. 4F).
These results suggested that high concentrations of
DKK1 and TNF-α may act via the non-canonical and canonical Wnt
signaling pathways to downregulate bone formation, whereas low
concentrations of DKK-1 may promote BMP-9-induced osteogenic
differentiation via inhibition of the non-canonical and canonical
Wnt signaling pathways.
Discussion
rDFCs are the precursor cells of periodontal
ligament cells, cement cells and osteoblasts, which form
periodontal tissue at the late dental development stage via
cellular migration and differentiation (5–7).
AdBMP9 acts on cells as an exogenous factor to safely and
effectively transfect the target gene into the target cell
(8–13). The present study transfected cells
with an adenovirus to construct an AdBMP9-transduced rDFC
model.
TNF-α levels were reported to be markedly increased
in the gingival crevicular fluid of patients with periodontitis and
the expression levels of TNF-α have been closely associated with
the severity of periodontitis (29,30).
Previous studies have identified that TNF-α suppressed cell
osteogenic differentiation (8,21,31,32).
It was also discovered that high concentrations of TNF-α suppressed
the expression of osteogenesis-related factors, such as ALP, OCN
and RUNX2 in mesenchymal stem cells (33,34).
The effect of TNF-α on AdBMP-induced osteogenic
differentiation has been discovered to be associated with
periodontal tissue engineering (17,32,34).
Thus, the purpose of the present study was to investigate the
effect of TNF-α on the AdBMP9-induced osteogenic differentiation of
rDFCs. The results of the present study indicated that AdBMP9
promoted osteogenesis in rDFCs. However, the osteogenic effect was
markedly reduced following TNF-α stimulation, indicating that TNF-α
may suppress the AdBMP9-induced early and advanced stages of
osteogenesis in rDFCs. Similarly, some studied reported that TNF-α
prevented the osteogenic differentiation of cells under BMP2/7
induction. BMP2 and BMP9 belong to the same BMP family (12,35,36).
BMP9 has been proven to be one of the strongest osteogenic factors,
demonstrating an ability to repair and regenerate bone defects
(12,13). Consequently, BMP9 was also
suggested to potentially promote bone regeneration in TNF-α-induced
bone defects.
The present study preliminarily discovered that
TNF-α suppressed the osteogenic differentiation of rDFCs. However,
the regulatory molecular mechanisms of this pathway remain unclear.
A large number of previous studies have indicated that the Wnt
signaling pathways served an important role in the osteogenic
differentiation of stem cells (18,21,23,25,37,38).
Therefore, the present study analyzed the expression levels of
important proteins involved in the Wnt signaling pathways. The
experimental results revealed that following TNF-α interference,
the canonical Wnt/β-catenin signaling pathway was activated and the
Wnt/Ca2+ non-canonical signaling pathway was inhibited.
Previous studies have also suggested that the canonical
Wnt/β-catenin pathway may prevent the differentiation process of
certain stem cells, such as the odontoblast-like differentiation of
dental pulp stem cell and the osteogenic differentiation of adipose
derived stromal cells (37,39),
which is consistent with the results of the present study.
The primary mechanism of action of the canonical
Wnt/β-catenin signaling pathway is as follows: The Wnt protein
binds to the cell surface membrane Frizzled receptor, which
activates the Dishevelled receptor family, suppresses the
downstream Axin/GSK3β/adenomatous polyposis coli complex and thus,
represses the degradation of β-catenin (21,23).
Subsequently, β-catenin enters the cell nucleus, interacts with the
TCL/LEF transcription factor and promotes the expression of
specific genes (40,41). GSK3β is a protein kinase which
phosphorylates β-catenin, thus inducing its degradation through the
ubiquitin proteasome pathway (42). The results of the present study
revealed that compared with the AdBMP-transfected cells without
TNF-α stimulation, the TNF-α treatment upregulated the expression
levels of β-catenin and p-GSK3β. TNF-α upregulated expression
levels of cyclin D1 and c-Myc, which are downstream target genes of
the canonical Wnt signaling pathway.
Wnt signaling can be categorized into the canonical
and non-canonical Wnt/Ca2+ signaling pathway (23). However, to the best of our
knowledge, whether the non-canonical Wnt pathway is involved in the
osteogenic differentiation of stem cells following TNF-α
stimulation remained unclear. CaMKII and NLK are key proteins of
wnt non-canonical signaling pathway (23,43)
NLK translocates into the nucleus and suppress the regulatory
effect of the β-catenin/TCF/LEF polymer on gene transcription, thus
affecting cellular function (43,44).
The present study discovered that TNF-α treatment downregulated the
expression levels of CaMKII and NLK in AdBMP9-transduced rDFCs.
These results indicated that the Wnt/β-catenin signaling pathway
may be activated by TNF-α, whereas the non-canonical
Wnt/Ca2+ signaling pathway may be suppressed.
The aforementioned results of the present study
preliminarily verified that TNF-α may suppress the osteogenic
differentiation of rDFCs, which was closely associated with the
activation of the canonical Wnt/β-catenin signaling pathway and the
suppression of the Wnt/Ca2+ non-canonical signaling
pathway. To further verify the roles of the Wnt signaling pathways
in AdBMP9-indued osteogenic differentiation of rDFCs with TNF-α,
the Wnt canonical signaling inhibitor DKK1 was used. The results of
the western blotting experiments revealed that high concentrations
of DKK1 upregulated the expression levels of CaMKII and NLK, while
low concentrations of DKK1 downregulated the expression levels of
these proteins. β-catenin expression levels were downregulated by
DKK1 at both high and low concentrations; however, the inhibitory
effect was higher following high dose DKK1 treatment compared with
low dose DKK1. These results suggested that Wnt/β-catenin signaling
may be suppressed by both high and low concentrations of DKK1,
whereas the Wnt/Ca2+ non-canonical pathway may be
activated by high concentrations of DKK1 and suppressed by low
concentrations of DKK1. The RT-qPCR results revealed that low
concentrations of DKK1 significantly upregulated the expression
levels of RUNX2, OCN and COLI. Conversely, TNF-α treatment and high
concentrations of DKK1 downregulated the expression levels of these
genes. These results suggested that low concentrations of DKK1 may
promote AdBMP9-induced osteogenic differentiation.
These results highlighted the close association
between the canonical and Wnt/Ca2+ non-canonical
signaling pathways, suggesting that the two pathways may exert
their functions simultaneously to regulate stem cell
differentiation. Under an inflammatory microenvironment, the
dynamic balance between the canonical and non-canonical signaling
pathways is reportedly broken, which thereby affects the stem cell
function (21,25,31,34).
TNF-α has been reported as an important inflammatory
factor responsible for periodontal bone defects resulting from
periodontitis (17,32). In the present study, TNF-α
suppressed the AdBMP9-induced osteogenic differentiation of rDFCs
by activating the canonical Wnt signaling pathway and repressing
the non-canonical signaling pathway. The results indicated that
DKK1 also influenced canonical and non-canonical signaling
pathways, thereby affecting the osteogenic differentiation of
cells. These findings suggested that regulating the balance between
the Wnt canonical and non-canonical signaling pathways may be
promising for restoring the osteogenic differentiation of
rDFCs.
Collectively, the results of the present study
indicated that TNF-α may interact with the Wnt signaling pathway to
suppress osteogenesis. Thus, the enhanced promoting effect of BMP9
following treatment with low concentrations of DKK1 may be useful
for treating periodontitis bone absorption.
In conclusion, the results of the present study
provided novel evidence for studying stem cell function in relation
to TNF-α and DKK1, thus providing an opportunity for stem cells to
be applied in clinical studies in the future. However, the present
study did not investigate the effects of the feedback loop of TNF-α
and DKK1, which may provide an additional mechanism of crosstalk
between BMPs and Wnt signaling. Therefore, further scientific
research is required to determine the interaction between signaling
pathways in vivo and in vitro, which will enable the
optimization of therapies for bone tissue engineering.
Acknowledgements
The authors would like to thank Dr Tong-Chuan He
(Molecular Oncology Laboratory, Department of Surgery, The
University of Chicago Medical Center, Chicago, IL, USA) for
providing AdBMP9 and AdGFP. The authors would also like to thank Dr
Guo Ye (Chongqing Key Laboratory of Oral Diseases and Biomedical
Sciences, Chongqing Medical University, Chongqing, China) for
providing hydroxyapatite.
Funding
The study was funded by The Natural Science
Foundation of Chongqing, China (grant no. cstc2016jcyjA0243), The
Chongqing Municipal Health and Family Planning commission (grant
no. 2016MSXM049) and The Program for Innovation Team Building at
the Institutions of Higher Education in Chongqing in 2016 (grant
no. CXTDG201602006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL conceived the project; XL, GR, CC and CL designed
the experiments and wrote the manuscript; XY, XL, GR, LN and XJ
performed the experiments; and CL supervised the experiment and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures involving animals were
in accordance with the Guide for the Care and Use of Laboratory
Animals (45) and approved by the
Ethics Committee of Chongqing Medical University (approval no.
CQLA2019-0066; Chongqing, China). All animals were purchased from
the Experimental Animal Center of Chongqing Medical University
[license no. SCXK (Yu) 20012-0001].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:3141–1820. 2005. View Article : Google Scholar
|
|
2
|
Lim JC and Mitchell CH: Inflammation,
pain, and pressure-purinergic signaling in oral tissues. J Dent
Res. 91:1103–1109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kodama T, Minabe M, Sugiyama T, Mitarai E,
Fushimi H, Kitsugi D, Tsutsumi K and Katsuki M: Guided tissue
regeneration using a collagen barrier and bone swaging technique in
noncontained infrabony defects. Int J Periodontics Restorative
Dent. 33:805–812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wise GE, Lin F and Fan W: Culture and
characterization of dental follicle cells from rat molars. Cell
Tissue Res. 267:483–492. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo W, Chen L, Gong K, Ding B, Duan Y and
Jin Y: Heterogeneous dental follicle cells and the regeneration of
complex periodontal tissues. Tissue Eng Part A. 18:459–470. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li C, Yang X, He Y, Ye G, Li X, Zhang X,
Zhou L and Deng F: Bone morphogenetic protein-9 induces osteogenic
differentiation of rat dental follicle stem cells in P38 and ERK1/2
MAPK dependent manner. Int J Med Sci. 9:862–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao S, Pan F, Prpic V and Wise GE:
Differentiation of stem cells in the dental follicle. J Dent Res.
87:767–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Chen D, Jing X and Li C: Dkk1 and
TNF-alpha influence osteogenic differentiation of
adBMP9-infected-rDFCs. Oral Dis. 26:360–369. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carreira AC, Alves GG, Zambuzzi WF,
Sogayar MC and Granjeiro JM: Bone Morphogenetic Proteins:
Structure, biological function and therapeutic applications. Arch
Biochem Biophys. 561:64–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen D, Zhao M and Mundy GR: Bone
morphogenetic proteins. Growth Factors. 22:233–241. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng H, Jiang W, Phillips FM, Haydon RC,
Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, et al:
Osteogenic activity of the fourteen types of human bone
morphogenetic proteins (BMPs). J Bone Joint Surg Am.
85-A:1544–1552. 2003. View Article : Google Scholar
|
|
12
|
Kang Q, Sun MH, Cheng H, Peng Y, Montag
AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, et al:
Characterization of the distinct orthotopic bone-forming activity
of 14 BMPs using recombinant adenovirus-mediated gene delivery.
Gene Ther. 11:1312–1320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng Y, Kang Q, Cheng H, Li X, Sun MH,
Jiang W, Luu HH, Park JY, Haydon RC and He TC: Transcriptional
characterization of bone morphogenetic proteins (BMPs)-mediated
osteogenic signaling. J Cell Biochem. 90:1149–1165. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nie L, Yang X, Duan L, Huang E, Zhou PF,
Luo W, Zhang Y, Zeng X, Qiu Y, Cai T, et al: The healing of
alveolar bone defects with novel bio-implants composed of
Ad-BMP9-transfected rDFCs and CHA scaffolds. Sci Rep. 7:63732017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimelman Bleich N, Kallai I, Lieberman JR,
Schwarz EM, Pelled G and Gazit D: Gene therapy approaches to
regenerating bone. Adv Drug Deliver Rev. 64:1320–1330. 2012.
View Article : Google Scholar
|
|
16
|
Graves DT and Cochran D: The contribution
of interleukin-1 and tumor necrosis factor to periodontal tissue
destruction. J Periodontol. 74:391–401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mukai T, Otsuka F, Otani H, Yamashita M,
Takasugi K, Inagaki K, Yamamura M and Makino H: TNF-alpha inhibits
BMP-induced osteoblast differentiation through activating SAPK/JNK
signaling. Biochem Biophys Res Commun. 356:1004–1010. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maeda K, Takahashi N and Kobayashi Y:
Roles of Wnt signals in bone resorption during physiological and
pathological states. J Mol Med (Berl). 91:15–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu W, Andersen TE, Bollerslev J, Mandrup
S, Abdallah BM and Kassem M: Patients with high bone mass phenotype
exhibit enhanced osteoblast differentiation and inhibition of
adipogenesis of human mesenchymal stem cells. J Bone Miner Res.
22:1720–1731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jansen JH, Eijken M, Jahr H, Chiba H,
Verhaar JA, Van LJ and Weinans H: Stretch-induced inhibition of
Wnt/beta-catenin signaling in mineralizing osteoblasts. J Orthop
Re. 28:390–396. 2010. View Article : Google Scholar
|
|
21
|
Liu N, Shi HG, Zhang W and Gu B: The
crosstalk between canonical and noncanonical Wnt signaling pathway
in osteoblast differentiation of periodontal ligament stem cells in
inflammatory microenvironments. Zhonghua Kou Qiang Yi Xue Za Zhi.
51:673–679. 2016.(In Chinese). PubMed/NCBI
|
|
22
|
Xiang L, Chen M, He L, Cai B, Du Y, Zhang
X, Zhou C, Wang C, Mao JJ and Ling J: Wnt5a regulates dental
follicle stem/progenitor cells of the periodontium. Stem Cell Res
Ther. 5:1352014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lerner UH and Ohlsson C: The WNT system:
Background and its role in bone. J Intern Med. 277:630–649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salazar VS, Ohte S, Capelo LP, Gamer L and
Rosen V: Specification of osteoblast cell fate by canonical wnt
signaling requires Bmp2. Development. 143:4352–4367. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weivoda MM, Ruan M, Hachfeld CM, Pederson
L, Howe A, Davey RA, Zajac JD, Kobayashi Y, Williams BO, Westendorf
JJ, et al: Wnt signaling inhibits osteoclast differentiation by
activating canonical and noncanonical cAMP/PKA pathways. J Bone
Miner Res. 31:65–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shtutman M, Zhurinsky J, Simcha I,
Albanese C, D'Amico M, Pestell R and Ben-Ze'ev A: The cyclin D1
gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad
Sci USA. 96:5522–5527. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pelengaris S and Khan M: The many faces of
c-MYC. Arch Biochem Biophys. 416:129–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan J, Zhou L, Xue P, An Y, Luo L, Zhang
R, Wu G, Wang Y, Zhu H and Wang Q: Tumor necrosis factor-α
attenuates the osteogenic differentiation capacity of periodontal
ligament stem cells by Activating PERK Signaling. J Periodontal.
87:e159–e171. 2016. View Article : Google Scholar
|
|
30
|
Ding C, Ji X, Chen X, Xu Y and Zhong L:
TNF-α gene promoter polymorphisms contribute to periodontitis
susceptibility: Evidence from 46 studies. J Clin Periodontol.
41:748–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Tan GR, Yu M, Cai X, Zhou Y, Ding
H, Xie H, Qu F, Zhang R, Lam CU, et al: The effect of tumour
necrosis factor-α on periodontal ligament stem cell differentiation
and the related signaling pathways. Curr Stem Cell Res Ther.
11:593–602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang F, Jiang Y, Huang X, Liu Q, Zhang Y,
Luo W, Zhang F, Zhou P, Lin J and Zhang H: Pro-inflammatory
cytokine TNF-α attenuates BMP9-induced osteo/odontoblastic
differentiation of the stem cells of dental apical papilla (SCAPs).
Cell Physiol Biochem. 41:1725–1735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lacey DC, Simmons PJ, Graves SE and
Hamilton JA: Proinflammatory cytokines inhibit osteogenic
differentiation from stem cells: Implications for bone repair
during inflammation. Osteoarthri Cartilage. 17:735–742. 2009.
View Article : Google Scholar
|
|
34
|
Qin Z, Fang Z, Zhao L, Chen J, Li Y and
Liu G: High dose of TNF-α suppressed osteogenic differentiation of
human dental pulp stem cells by activating the Wnt/β-catenin
signaling. J Mol Histol. 46:409–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang RL, Yuan Y, Tu J, Zou GM and Li Q:
Opposing TNF-α/IL-1β-and BMP-2-activated MAPK signaling pathways
converge on Runx2 to regulate BMP-2-induced osteoblastic
differentiation. Cell Death Dis. 5:e11872014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jo JY, Jeong SI, Shin YM, Kang SS, Kim SE,
Jeong CM and Huh JB: Sequential delivery of BMP-2 and BMP-7 for
bone regeneration using a heparinized collagen membrane. Int J Oral
Max Surg. 44:921–928. 2015. View Article : Google Scholar
|
|
37
|
Cho HH, Kim YJ, Kim SJ, Kim JH, Bae YC, Ba
B and Jung JS: Endogenous Wnt signaling promotes proliferation and
suppresses osteogenic differentiation in human adipose derived
stromal cells. Tissue Eng. 12:111–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Li YP, Paulson C, Shao JZ, Zhang
X, Wu M and Chen W: Wnt and the Wnt signaling pathway in bone
development and disease. Front Biosci (Landmark Ed). 19:379–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Scheller EL, Chang J and Wang CY:
Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J
Den Res. 87:126–30. 2008. View Article : Google Scholar
|
|
40
|
Kim W, Kim M and Jho EH: Wnt/beta-catenin
signalling: from plasma membrane to nucleus. Biochem J. 450:9–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao C, Xiao G and Hu J: Regulation of
Wnt/β-catenin signaling by posttranslational modifications. Cell
Biosci. 4:132014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tejeda-Muñoz N and Robles-Flores M:
Glycogen synthase kinase 3 in Wnt signaling pathway and cancer.
IUBMB Life. 67:914–922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
De A: Wnt/Ca2+ signaling pathway: A brief
overview. Acta Biochim Biophys Sin (Shanghai). 43:745–756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rharass T, Lemcke H, Lantow M, Kuznetsov
SA, Weiss DG and Panáková D: Ca2+-mediated mitochondrial reactive
oxygen species metabolism augments Wnt/β-catenin pathway activation
to facilitate cell differentiation. J Biol Chem. 289:27937–29951.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals, Institute for Laboratory
Animal Research, Division on Earth and Life Studies, National
Research Council: Guide for the Care and Use of Laboratory Animals.
The National Academies Press; Washington, DC: 1998
|