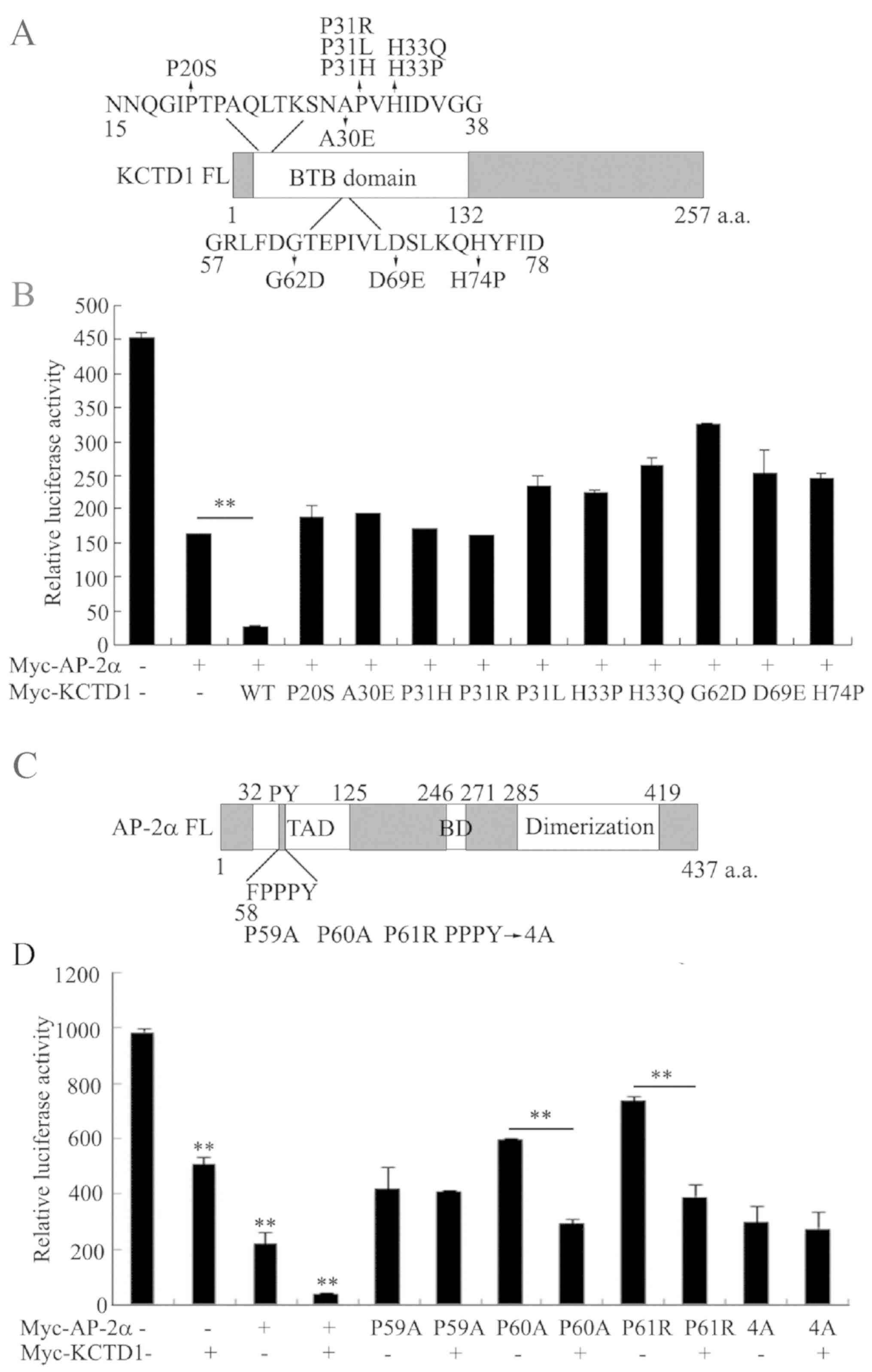
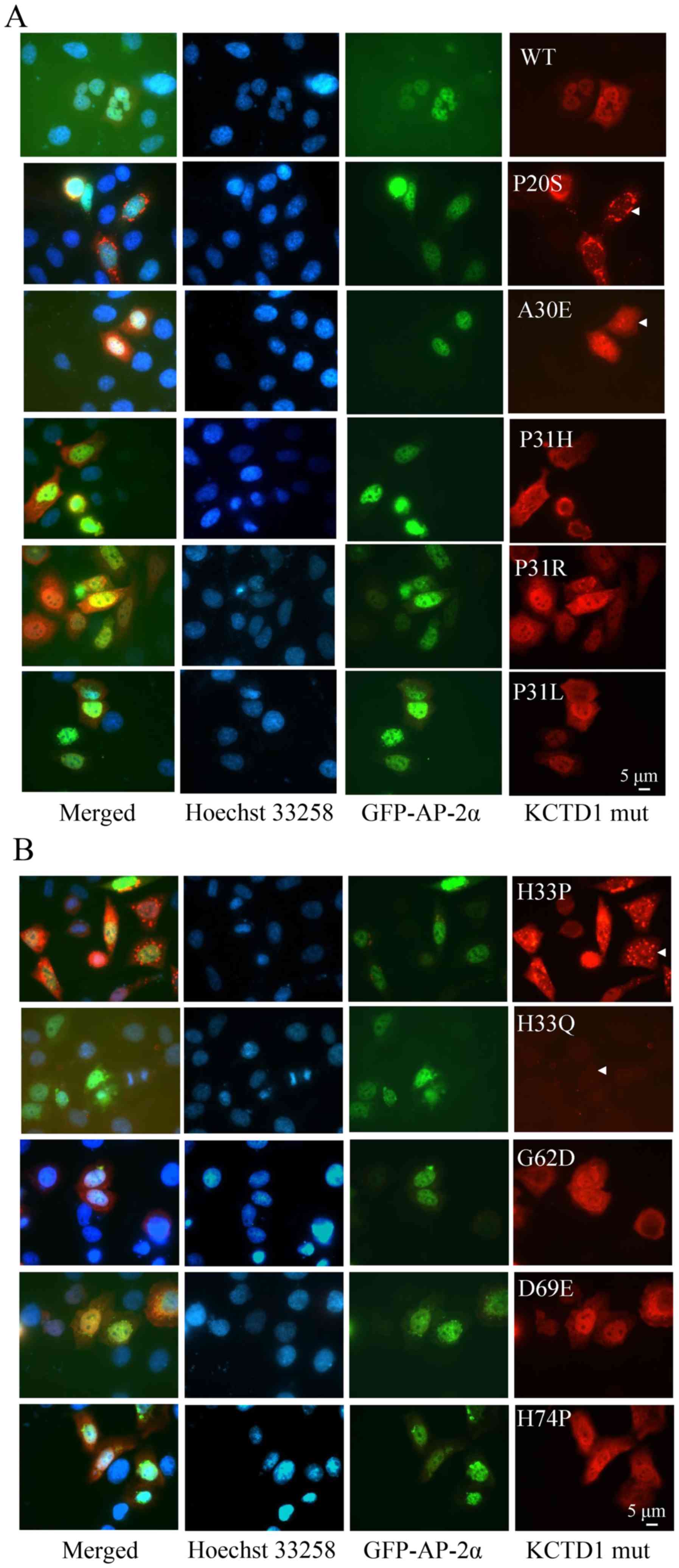
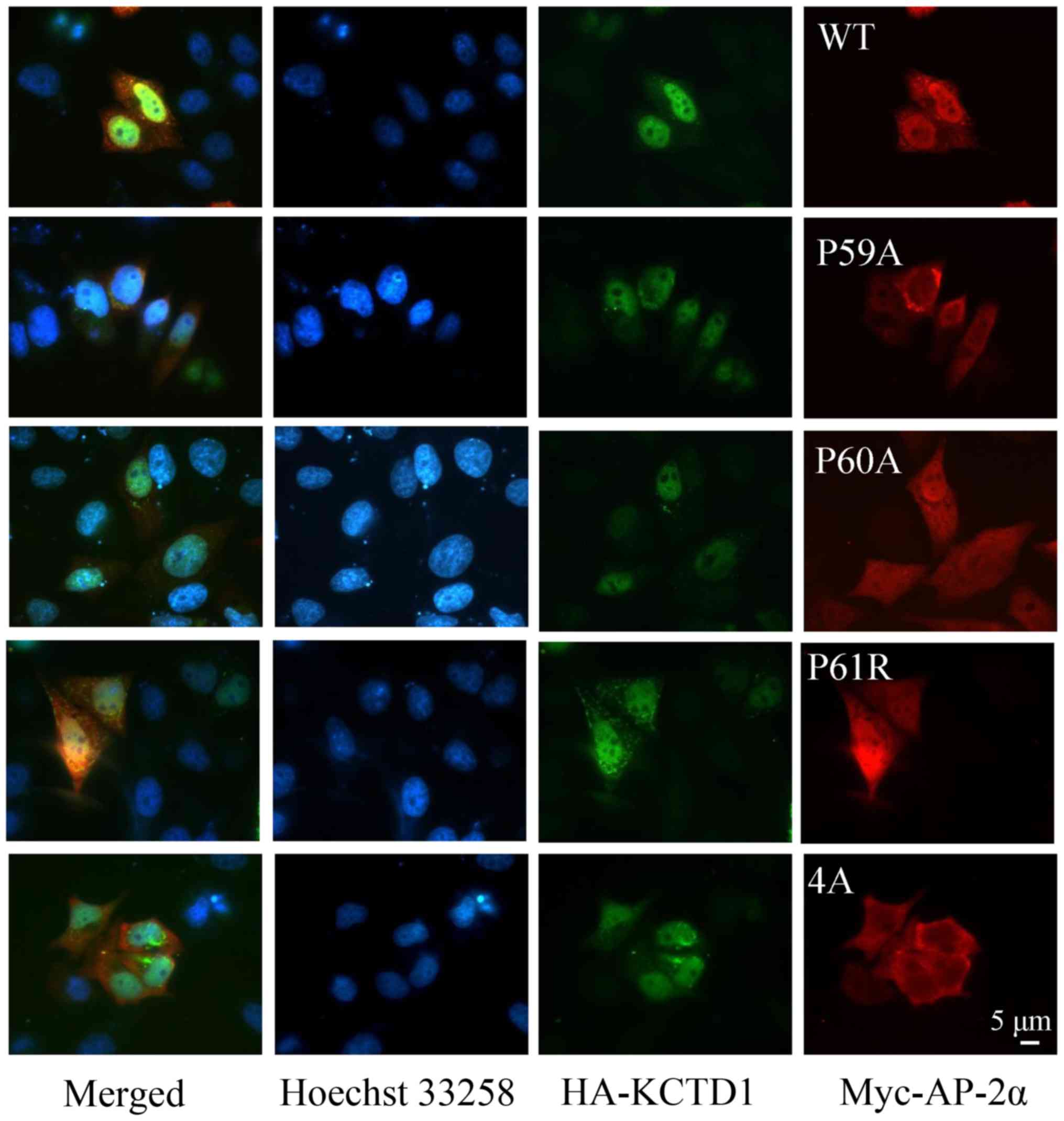
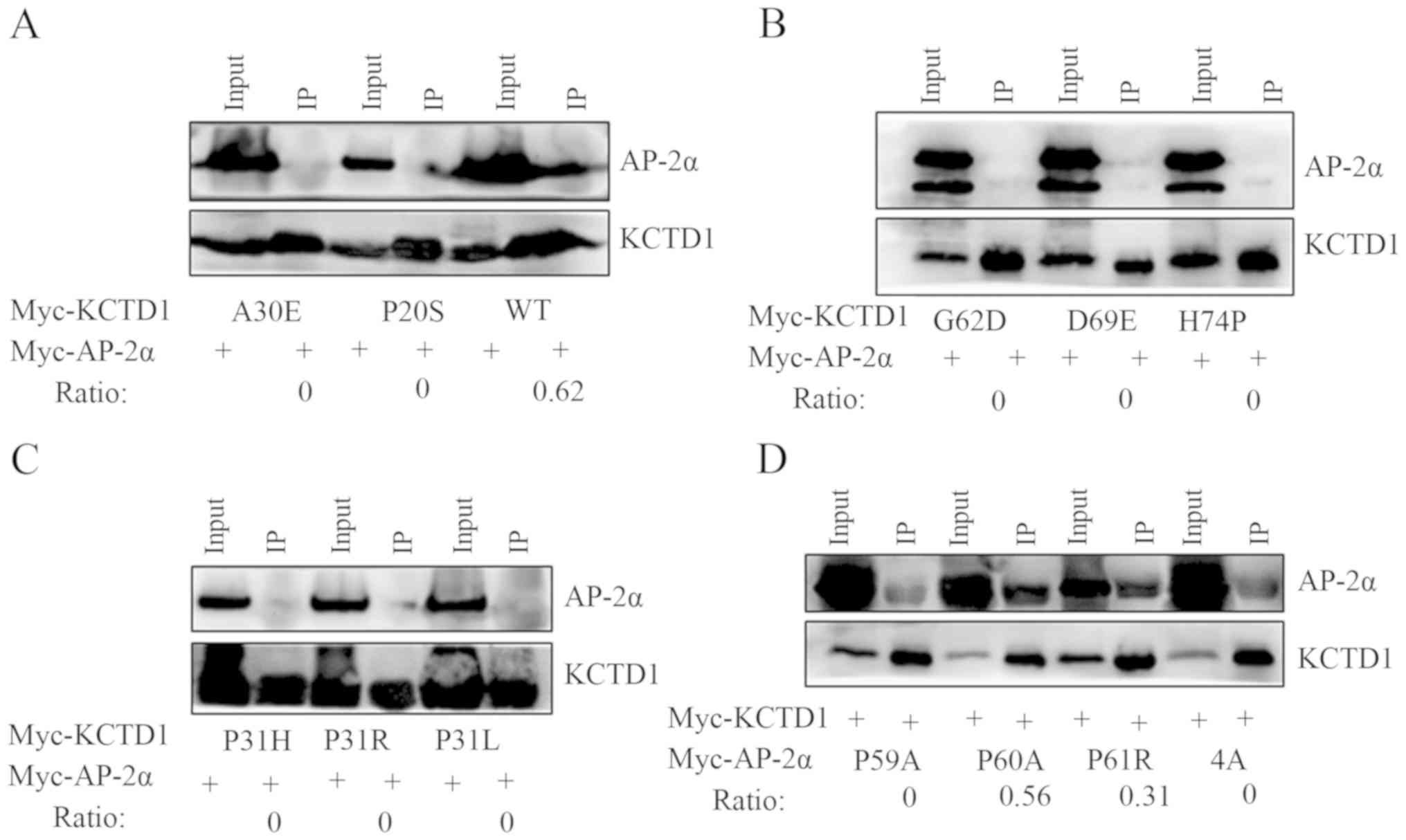
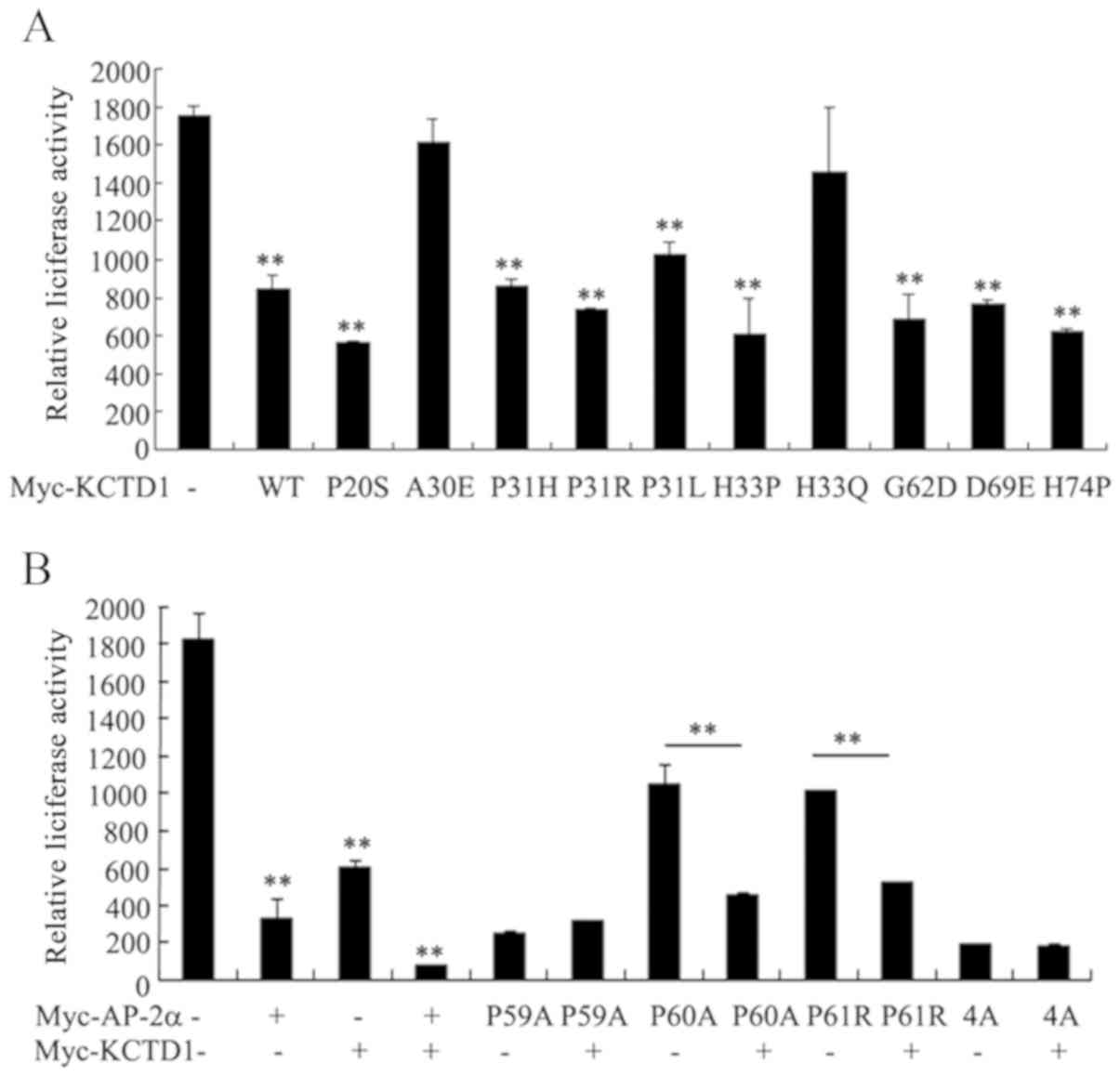
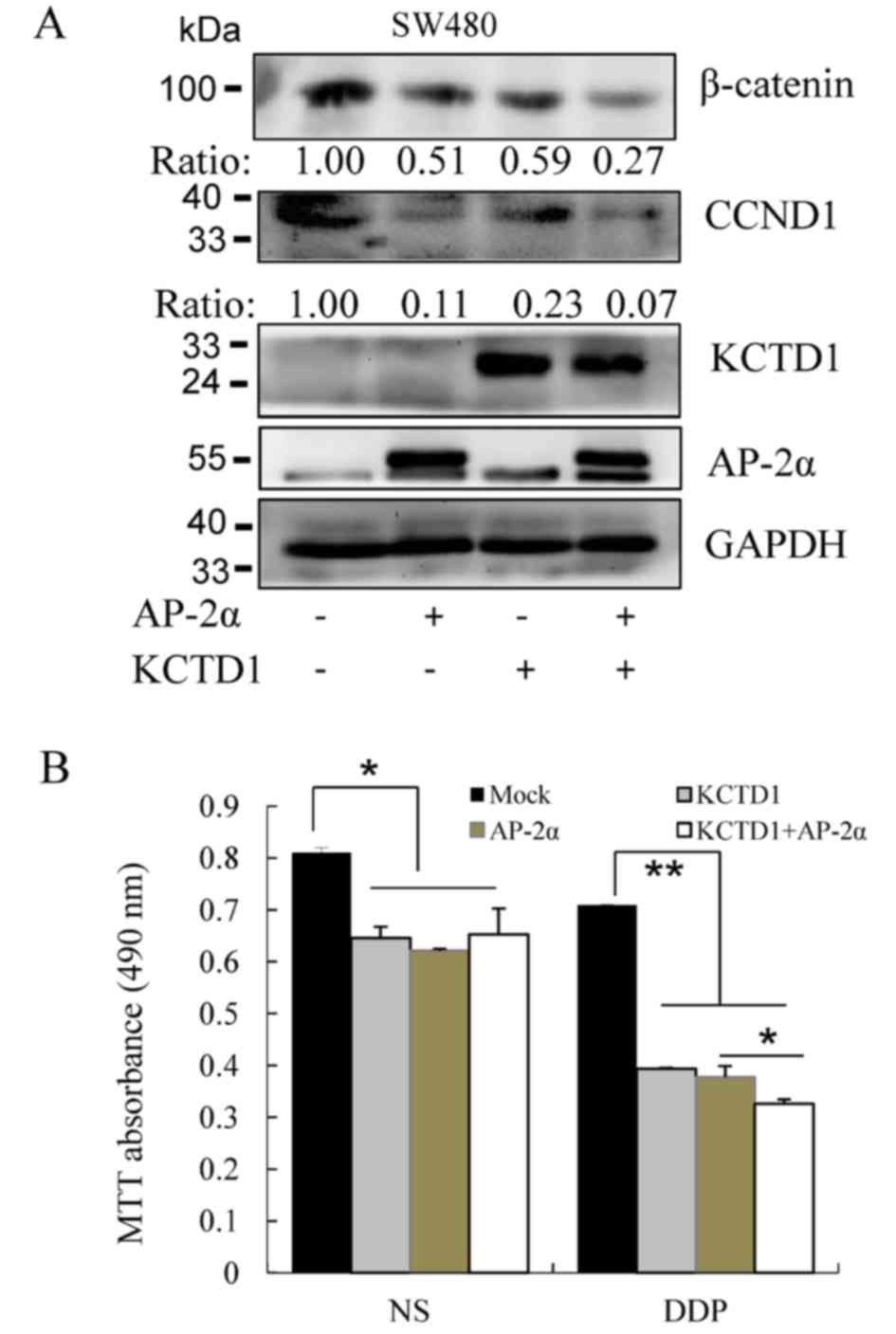
|
1
|
Finlay AY and Marks R: An hereditary
syndrome of lumpy scalp, odd ears and rudimentary nipples. Br J
Dermatol. 99:423–430. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Steensel MA, Celli J, van Bokhoven JH
and Brunner HG: Probing the gene expression database for candidate
genes. Eur J Hum Genet. 7:910–919. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marneros AG, Beck AE, Turner EH, McMillin
MJ, Edwards MJ, Field M, de Macena Sobreira NL, Perez ABA, Fortes
JAR, Lampe AK, et al: Mutations in KCTD1 cause scalp-ear-nipple
syndrome. Am J Hum Genet. 92:621–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Hagopian-Donaldson S, Serbedzija
G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA and
Williams T: Neural tube, skeletal and body wall defects in mice
lacking transcription factor AP-2. Nature. 381:238–241. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahituv N, Erven A, Fuchs H, Guy K,
Ashery-Padan R, Williams T, de Angelis MH, Avraham KB and Steel KP:
An ENU-induced mutation in AP-2alpha leads to middle ear and ocular
defects in doarad mice. Mamm Genome. 15:424–432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sletten LJ and Pierpont ME: Familial
occurrence of patent ductus arteriosus. Am J Med Genet. 57:27–30.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao F, Weismann CG, Satoda M, Pierpont
ME, Sweeney E, Thompson EM and Gelb BD: Novel TFAP2B mutations that
cause char syndrome provide a genotype-phenotype correlation. Am J
Hum Genet. 69:695–703. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Sheridan R and Williams T: Analysis
of TFAP2A mutations in branchio-oculo-facial syndrome indicates
functional complexity within the AP-2α DNA-binding domain. Hum Mol
Genet. 22:3195–3206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Milunsky JM, Maher TA, Zhao G, Roberts AE,
Stalker HJ, Zori RT, Burch MN, Clemens M, Mulliken JB, Smith R and
Lin AE: TFAP2A mutations result in branchio-oculo-facial syndrome.
Am J Hum Genet. 82:1171–1177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milunsky JM, Maher TM, Zhao G, Wang Z,
Mulliken JB, Chitayat D, Clemens M, Stalker HJ, Bauer M, Burch M,
et al: Genotype-phenotype analysis of the branchio-oculo-facial
syndrome. Am J Med Genet A 155A. 22–32. 2011. View Article : Google Scholar
|
|
11
|
Dumitrescu AV, Milunsky JM, Longmuir SQ
and Drack AV: A family with branchio-oculo-facial syndrome with
primarily ocular involvement associated with mutation of the TFAP2A
gene. Ophthalmic Genet. 33:100–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomeer HG, Crins TT, Kamsteeg EJ,
Buijsman W, Cruysberg JR, Knoers NV and Cremers CWRJ: Clinical
presentation and the presence of hearing impairment in
branchio-oculo-facial syndrome: A new mutation in the TFAP2A gene.
Ann Otol Rhinol Laryngol. 119:806–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding X, Luo C, Zhou J, Zhong Y, Hu X, Zhou
F, Ren K, Gan L, He A, Zhu J, et al: The interaction of KCTD1 with
transcription factor AP-2alpha inhibits its transactivation. J Cell
Biochem. 106:285–295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zarelli VE and Dawid IB: Inhibition of
neural crest formation by Kctd15 involves regulation of
transcription factor AP-2. Proc Natl Acad Sci USA. 110:2870–2875.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heffer A, Marquart GD, Aquilina-Beck A,
Saleem N, Burgess HA and Dawid IB: Generation and characterization
of Kctd15 mutations in zebrafish. PLoS One. 12:e01891622017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dutta S and Dawid IB: Kctd15 inhibits
neural crest formation by attenuating Wnt/beta-catenin signaling
output. Development. 137:3013–3018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q and Dashwood RH: Activator protein
2alpha associates with adenomatous polyposis coli/beta-catenin and
inhibits beta-catenin/T-cell factor transcriptional activity in
colorectal cancer cells. J Biol Chem. 279:45669–45675. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Chen C, Wang F, Huang W, Liang Z,
Xiao Y, Wei K, Wan Z, Hu X, Xiang S, et al: KCTD1 suppresses
canonical Wnt signaling pathway by enhancing β-catenin degradation.
PLoS One. 9:e943432014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding X, Fan C, Zhou J, Zhong Y, Liu R, Ren
K, Hu X, Luo C, Xiao S, Wang Y, et al: GAS41 interacts with
transcription factor AP-2beta and stimulates AP-2beta-mediated
transactivation. Nucleic Acids Res. 34:2570–2578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding X, Yang Z, Zhou F, Wang F, Li X, Chen
C, Li X, Hu X, Xiang S and Zhang J: Transcription factor AP-2α
regulates acute myeloid leukemia cell proliferation by influencing
hoxa gene expression. Int J Biochem Cell Biol. 45:1647–1656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding XF, Luo C, Ren KQ and Zhang J, Zhou
JL, Hu X, Liu RS, Wang Y, Gao X and Zhang J: Characterization and
expression of a human KCTD1 gene containing the BTB domain, which
mediates transcriptional repression and homomeric interactions. DNA
Cell Biol. 27:257–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: From human mutations to treatments.
Nat Med. 19:179–192. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gay A and Towler DA: Wnt signaling in
cardiovascular disease: Opportunities and challenges. Curr Opin
Lipidol. 28:387–396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ng L, Kaur P, Bunnag N, Suresh J, Sung
ICH, Tan QH, Gruber J and Tolwinski NS: WNT signaling in disease.
Cells. 8:8262019. View Article : Google Scholar
|
|
26
|
Li X, Peng H, Schultz DC, Lopez-Guisa JM,
Rauscher FJ III and Marmorstein R: Structure-function studies of
the BTB/POZ transcriptional repression domain from the
promyelocytic leukemia zinc finger oncoprotein. Cancer Res.
59:5275–5282. 1999.PubMed/NCBI
|
|
27
|
Fedele M, Benvenuto G, Pero R, Majello B,
Battista S, Lembo F, Vollono E, Day PM, Santoro M, Lania L, et al:
A novel member of the BTB/POZ family, PATZ, associates with the
RNF4 RING finger protein and acts as a transcriptional repressor. J
Biol Chem. 275:7894–7901. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoatlin ME, Zhi Y, Ball H, Silvey K,
Melnick A, Stone S, Arai S, Hawe N, Owen G, Zelent A and Licht JD:
A novel BTB/POZ transcriptional repressor protein interacts with
the fanconi anemia group C protein and PLZF. Blood. 94:3737–3747.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelly KF and Daniel JM: POZ for
effect--POZ-ZF transcription factors in cancer and development.
Trends Cell Biol. 16:578–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kojima S, Hatano M, Okada S, Fukuda T,
Toyama Y, Yuasa S, Ito H and Tokuhisa T: Testicular germ cell
apoptosis in Bcl6-deficient mice. Development. 128:57–65.
2001.PubMed/NCBI
|
|
31
|
Shaffer AL, Yu X, He Y, Boldrick J, Chan
EP and Staudt LM: BCL-6 represses genes that function in lymphocyte
differentiation, inflammation, and cell cycle control. Immunity.
13:199–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barna M, Hawe N, Niswander L and Pandolfi
PP: Plzf regulates limb and axial skeletal patterning. Nat Genet.
25:166–172. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Costoya JA, Hobbs RM, Barna M, Cattoretti
G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ and Pandolfi PP:
Essential role of Plzf in maintenance of spermatogonial stem cells.
Nat Genet. 36:653–659. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang C, Hatzi K and Melnick A:
Lineage-specific functions of Bcl-6 in immunity and inflammation
are mediated by distinct biochemical mechanisms. Nat Immunol.
14:380–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahmad KF, Engel CK and Prive GG: Crystal
structure of the BTB domain from PLZF. Proc Natl Acad Sci USA.
95:12123–12128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carey RM, Balcz BA, Lopez-Coviella I and
Slack BE: Inhibition of dynamin-dependent endocytosis increases
shedding of the amyloid precursor protein ectodomain and reduces
generation of amyloid beta protein. BMC Cell Biol. 6:302005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Char F: Peculiar facies with short
philtrum, duck-bill lips, ptosis and low-set ears-a new syndrome?
Birth Defects Orig Artic Ser. 14:303–305. 1978.PubMed/NCBI
|
|
38
|
Marston DJ, Roh M, Mikels AJ, Nusse R and
Goldstein B: Wnt signaling during caenorhabditis elegans embryonic
development. Methods Mol Biol. 469:103–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Munoz-Descalzo S, Hadjantonakis AK and
Arias AM: Wnt/ß-catenin signalling and the dynamics of fate
decisions in early mouse embryos and embryonic stem (ES) cells.
Semin Cell Dev Biol. 47-48:101–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang B, Tian T, Kalland KH, Ke X and Qu Y:
Targeting Wnt/β-catenin signaling for cancer immunotherapy. Trends
Pharmacol Sci. 39:648–658. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang W, Smits R, Hao H and He C:
Wnt/β-catenin signaling in liver cancers. Cancers (Basel).
11:9262019. View Article : Google Scholar
|
|
42
|
Jung YS and Park JI: Wnt signaling in
cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and
the destruction complex. Exp Mol Med. 52:183–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nishisho I, Nakamura Y, Miyoshi Y, Miki Y,
Ando H, Horii A, Koyama K, Utsunomiya J, Baba S and Hedge P:
Mutations of chromosome 5q21 genes in FAP and colorectal cancer
patients. Science. 253:665–669. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakamura Y, Nishisho I, Kinzler KW,
Vogelstein B, Miyoshi Y, Miki Y, Ando H, Horii A and Nagase H:
Mutations of the adenomatous polyposis coli gene in familial
polyposis coli patients and sporadic colorectal tumors. Princess
Takamatsu Symp. 22:285–292. 1991.PubMed/NCBI
|
|
45
|
Suraweera N, Robinson J, Volikos E,
Guenther T, Talbot I, Tomlinson I and Silver A: Mutations within
Wnt pathway genes in sporadic colorectal cancers and cell lines.
Int J Cancer. 119:1837–1842. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Colnot S: Focusing on beta-catenin
activating mutations to refine liver tumor profiling. Hepatology.
64:1850–1852. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fujikura K, Akita M, Ajiki T, Fukumoto T,
Itoh T and Zen Y: Recurrent mutations in APC and CTNNB1 and
activated Wnt/β-catenin signaling in intraductal papillary
neoplasms of the bile duct: A whole exome sequencing study. Am J
Surg Pathol. 42:1674–1685. 2018. View Article : Google Scholar : PubMed/NCBI
|