Introduction
Polycystic ovary syndrome (PCOS) is a complex,
progressive, refractory gynecological endocrine disease (1) that clinically manifests as
hyperandrogenism with menstrual disorders, infertility, obesity and
hemorrhoids (2,3). In addition, PCOS is often linked with
various complications, including insulin (INS) resistance,
endometrial cancer, hypertension, metabolic syndrome and
cardiovascular disease (4–6). PCOS is also a concern for patients of
childbearing age because the condition not only causes abnormal
menstruation, but also ovulatory dysfunction and infertility
(7). Therefore, understanding the
pathogenesis of PCOS and developing treatment options for patients
with PCOS is of great importance (1,8).
Epidemiological surveys from the Netherlands and
other countries indicate that the incidence of PCOS in patients of
childbearing age is 10–15% and as high as 90% in patients with
clinical anovulatory infertility (1,9).
Anovulatory sterility caused by PCOS represents a challenge both in
the fields of gynecological endocrinology and reproductive
healthcare. However, the molecular mechanisms underlying PCOS
pathogenesis remain unclear. In addition, several therapeutic
options are based on hormones and metformin drugs, with serious
side effects and unsatisfactory results (10). A meta-analysis conducted by Li
et al (11) revealed that
metformin might lead to nausea, diarrhea and abdominal cramping in
the treatment of PCOS. Moreover, a study from the Cholesterol
Treatment Trialists' Collaboration demonstrated that high dose
statin caused some adverse effects, such as headaches, muscle pain
and myolysis (12).
Cryptotanshinone (CRY) is a monomeric compound
derived from tanshinone with antibacterial, anti-inflammatory,
antioxidant, antiaging and cooling effects. In Chinese traditional
medicine, CRY is believed to have curative effects against angina,
coronary heart disease and myocardial injury (13,14).
Previous studies suggested that CRY might be used for the treatment
of PCOS (15,16). Yu et al (15) demonstrated that CRY could improve
ovarian morphology and decrease levels of luteinizing hormone (LH),
testosterone (T) and androstenedione in PCOS model rats. In
addition, Huang et al (17)
also reported that CRY could reverse androgen excess and ovarian
INS resistance (IR) in mice. However, whether CRY can reverse
ovulatory dysfunction caused by PCOS remains unclear.
Our previous study suggested an association between
high-mobility group box 1 protein (HMGB1) and IR in PCOS (8). A previous study demonstrated that
inhibition of HMGB1 could improve IR in PCOS by suppressing the
toll-like receptor (TLR)4/NF-κB signaling pathway (18). Therefore, it was hypothesized that
CRY may serve a therapeutic role in PCOS by regulating the
HMGB1/TLR4/NF-κB signaling pathway. The aim of the present study
was to determine whether CRY can inhibit the reproductive disorders
caused by PCOS in a rat model. Moreover, in vivo and in
vitro experiments were carried out in order to evaluate the
associations between CRY and HMGB1, as well as the TLR4/NF-κB
signaling pathway. The effects of CRY treatment on reproductive
hormone levels, ovarian quotiety and inflammatory factors were also
assessed. The present findings may provide an experimental basis
for future research and clinical applications for ovarian tissue
repair.
Materials and methods
Animals
A total of 60 female Sprague-Dawley rats (age, 85
days; weight, 170–200 g) were supplied by Shanghai Laboratory
Animal Center, Co. Ltd. The rats were housed at a controlled
temperature of 25±2°C in standard cages with 55±15% humidity and a
12-h dark/light cycle. The animals had free access to water and
food. All rats were allowed to acclimatize in their new environment
for a period of 7 days before experimentation. All methodological
procedures were performed under strict conformity with the
guidelines of the Chinese Ministry of Science and Technology for
the Care and Use of Laboratory Animals. The experimental protocol
was approved by The Animal Care and Experiment Review Board of
Shanghai Traditional Chinese Medicine Hospital (Shanghai, China)
(approval no. 20190103).
Reagents
CRY (>98% purity; cat. no. C5624), INS (cat. no.
I9278) and the glucose and sucrose assay kit (cat. no. MAK013) were
purchased from Sigma-Aldrich; Merck KGaA. Human chorionic
gonadotropin (HCG; cat. no. 161008; Hangzhou Animal Medicine
Factory). ELISA kits for LH (cat. no. ab108651),
follicle-stimulating hormone (FSH) (cat. no. ab108641),
testosterone (cat. no. ab178663) and tumor necrosis factor (TNF)-α
(cat. no. ab181421) and the glucose oxidase assay kit (cat. no.
ab138884) were purchased from Abcam. Bovine serum albumin (BSA;
cat. no. SW3015) and pregnant mare serum gonadotrophin (PMSG; cat.
no. P9970) were purchased from Beijing Solarbio Science &
Technology Co., Ltd. The EnVision+ Detection system,
(Peroxidase/DAB; rabbit/mouse; cat. no. K406511-2) was purchased
from Dako; Agilent Technologies, Inc. Lipofectamine®
RNAiMAX reagent (cat. no. 13-778-075) was purchased from
Invitrogen; Thermo Fisher Scientific, Inc.
Animal model establishment and drug
treatments
A total of 60 rats were randomly divided into five
groups (n=12 in each group): i) Control group, consisting of normal
rats receiving an intragastric administration of saline for 3
weeks; ii) PCOS group, consisting of rats with PCOS receiving an
intragastric administration of saline for 3 weeks; iii) PCOS+HMGB1
group, consisting of rats with PCOS intraperitoneally injected with
80 µg/kg HMGB1 daily and an intragastric administration of saline
for 3 weeks; iv) PCOS + CRY group, including rats with PCOS
receiving an intragastric administration of CRY for 3 weeks and v)
PCOS + HMGB1 + CRY group, including rats with PCOS
intraperitoneally injected with 80 µg/kg HMGB1 daily and an
intragastric administration of CRY for 3 weeks. CRY was dissolved
in normal saline and administered orally once daily (27 mg/kg)
(19).
The rat model of PCOS was established according to
the protocol of a previous study (20), with some modifications (the dose of
INS used in the present study was lower compared with the the
previous study). Briefly, 48 rats were injected subcutaneously with
HCG (3.0 IU/day) and INS twice daily for 22 days. INS was increased
from 0.5 to 3.0 U/day from day 1 to day 11, and was maintained at
3.0 U/day until the 22nd day (Table
I). The remaining 12 rats (control group) were injected
subcutaneously with an equal volume of normal saline twice daily
for 22 days. Daily drinking water was replaced with 5% dextrose
solution at the beginning of the experiment. All rats were weighed
once a week, and a vaginal smear was performed daily until the 23rd
day. The loss of rat normal estrous cycle indicated successful
establishment of the PCOS model.
 | Table I.Schedule of INS injection. |
Table I.
Schedule of INS injection.
| Day | INS dose
(U/day) |
|---|
| 1-2 | 0.5 |
| 3-4 | 1.0 |
| 5-6 | 1.5 |
| 7-8 | 2.0 |
| 9-10 | 2.5 |
| 11-22 | 3.0 |
Determination of Lee's index, body
mass index (BMI) and ovarian quotiety
Both Lee's index and BMI were calculated at the end
of the experiment. The length of rats was measured from the nose to
the anus (21). Lee's index was
calculated as weight1/3 ×103/length, and the
BMI as weight/length2, where the length is given in cm
and the weight in g. In addition, ovarian quotiety was calculated
as follows: Ovarian weight/body weight (mg/100 g).
Specimen collection
At the end of the experimental period, rats were
fasted overnight then anesthetized with intraperitoneal injection
of 55 mg/kg sodium pentobarbitone. A volume of 5–7 ml blood was
then collected from the abdominal aorta and centrifuged at 1,500 ×
g for 20 min at 4°C to obtain serum. The rats were sacrificed using
250 mg/kg pentobarbital sodium and animal death was confirmed by
exsanguination. Ovarian tissue was dissected on ice and weighed.
For subsequent analysis, one-half of each sample was fixed in 4%
paraformaldehyde solution for 24 h at 25°C, while the remaining
tissue was stored at −80°C.
Determination of LH, LH/FSH ratio, T
and TNF-α levels
The serum levels of LH, T, FSH and TNF-α were
determined using ELISA kits, according to the manufacturer's
protocol. Absorbance was spectrophotometrically determined at 450
nm.
Histomorphometry analysis
Fixed tissues were dehydrated in ascending ethanol
concentrations (50, 70, 80 and 90%) for 30 sec and 100% ethanol
three times for 30 sec, then washed in xylene. The samples were
then embedded in paraffin, and cut to 4-µm thickness. The sections
were subjected to picric acid staining for 30 min at room
temperature and hematoxylin-eosin staining for 15 min at room
temperature, respectively. Histology and histomorphometry of the
stained slides were investigated using a Leica DM5000 fluorescence
microscope (Leica Microsystems, Inc.; magnification, ×100).
Immunohistochemical analysis
Ovarian tissue was fixed in 4% paraformaldehyde for
24 h at 4°C, then embedded in paraffin and cut into 4-µm thick
sections. The sections were then blocked for 30 min at 25°C in a
solution of 0.5% BSA, then incubated with anti-HMGB1 antibody
(1:200; cat. no. ab227168; Abcam) at 4°C overnight. Subsequently,
the sections were incubated with a biotinylated secondary antibody
(1:300; cat. no. ab7176; Abcam) for 30 min at 25°C. The
visualization of the immune complexes was carried out using the
Dako EnVision+ Detection System kit, according to the
manufacturer's protocol. The slides were counterstained using
hematoxylin at 25°C for 5 min, then observed under a Leica DM5000
fluorescence microscope (Leica Microsystems, Inc.; magnification,
×100) (22).
Isolation and culture of rat ovarian
granulosa cells (GCs)
For the isolation of the GCs, six immature (age,
21–25 days) female rats were administered a subcutaneous injection
of PMSG (150 IU/kg), and their ovaries collected after 48 h. The
follicular puncture approach (23)
was then used to isolate ovarian GCs, which were then pooled. The
cells were filtered, then centrifuged at 200 × g for 5 min at 4°C.
Following centrifugation, the supernatant was discarded and the GCs
were resuspended in Dulbecco's modified Eagles medium (Thermo
Fisher Scientific, Inc.) supplemented with 0.05 mg/ml gentamicin,
transferrin, selenium and INS. The isolated GCs were seeded into a
6-well cell culture plate (5×104/ml) and incubated in a
5% CO2 atmosphere at 37°C in a humidified atmosphere for
24 h.
Establishment of the IR models of
GCs
To establish IR, the GCs were treated with 1 µmol/l
dexamethasone for 72 h. The cultured media was harvested for
measurement of glucose concentrations using a commercial glucose
and sucrose assay kit.
Cell transfection
A commercially synthesized HMGB1-specific small
interfering (si) RNA (Shanghai GeneChem Co. Ltd.) was used for
HMGB1 silencing, together with AllStars scrambled siRNA as the
negative control (NC; cat. no. 1027280; Qiagen GmbH). The siRNA
sequences were as follows: si-HMGB1#1, 5′-GGACAAGGCCCGTTATGAA-3′;
si-HMGB1#2, 5′-UCUUGACCACAGAUCUUAA-3′; and si-HMGB1#3,
5′-GGCUGACAAGGCUCGUUAU-3′. The resultant siRNA was purified,
quantified and suspended in water at a concentration of 0.5 µg/µ.
siRNA (0.5 µl) was combined with 0.5 µl Lipofectamine®
RNAiMAX reagent (50 nM; Thermo Fisher Scientific, Inc.) for 20 min
before subsequent experimentation. GCs in the logarithmic growth
phase were trypsinized and inoculated into a 6-well cell culture
plate (3–5×104 cells/ml). When confluence reached
90–95%, GCs were allocated to the following groups: i) Control,
untransfected cells; ii) si-NC, cells transfected with plasmid
containing non-targeting siRNA; iii) si-HMGB1#1, cells transfected
with HMGB1 siRNA-1 recombinant plasmid; iv) si-HMGB1#2, cells
transfected with HMGB1 siRNA-2 recombinant plasmid; and v)
si-HMGB1#3, cells transfected with HMGB1 siRNA-3 recombinant
plasmid. At 24 h post-transfection, transfection efficiency was
evaluated by reverse transcription-quantitative (RT-q) PCR and
western blot analysis. si-HMGB1#2 displayed the highest silencing
efficiency and was therefore used to transfect INS-resistant GCs
for further analysis.
Cell treatment and viability
assay
GCs were divided into five groups: i) Control group,
untreated GCs; ii) IR group, INS-resistant GCs; iii) IR + NC group,
INS-resistant GCs transfected with si-NC; iv) IR + si-HMGB1,
INS-resistant GCs transfected with si-HMGB1; and v) IR + CRY group,
INS-resistant GCs treated with 300 nmol/l CRY (17). An MTT assay was used to assess the
viability of GCs in each group. Cells were cultured at a seed
density of 1×104 cells/well in 96-well plates. A final
concentration of 0.2 mg/ml MTT reagent (Sigma-Aldrich; Merck KGaA)
was added to each well, and the cells were incubated for 4 h at
37°C with 5% CO2. DMSO was used to dissolve the formazan
crystals for 20 min. Cell viability was calculated after measuring
the absorbance at 490 nm with a Safire 2 microplate reader (Tecan
Group, Ltd.).
RNA isolation and RT-qPCR
TRIzol® reagent (cat. no. 10-296-010;
Invitrogen; Thermo Fisher Scientific, Inc.) was used for the
extraction of total RNA from cells. Total RNA was reverse
transcribed into cDNA using the GoScript™ Reverse Transcription
System (Promega Corporation), according to the manufacturer's
protocol for 60 min at 42°C. RT-qPCR was performed to determine the
mRNA expression levels of HMGB1, TLR4 and NF-κB/p65 using
SYBR-Green PCR master mix (Thermo Fisher Scientific, Inc.). The
thermocycling conditions used for qPCR were as follows: 50°C UNG
activation step; denaturation for 30 sec at 95°C; followed by 40
cycles of denaturation for 5 sec at 95°C and annealing for 30 sec
at 60°C. The mRNA expression levels relative to β-actin were
obtained using the 2−ΔΔCq method (24). Primer sequences used in the present
study are provided in Table
II.
 | Table II.Primer sequences for reverse
transcription-quantitative PCR. |
Table II.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer sequence,
5′-3′ |
|---|
| HMGB1 |
|
Forward |
TGAGGGACAAAAGCCACTCC |
|
Reverse |
TTGGGAGGGCGGAGAATCA |
| TLR4 |
|
Forward |
TTGGCTTAGAAAACCAAGGTGG |
|
Reverse |
ATTGAGCTTCCCGCCTGAAA |
|
NF-κB/p65 |
|
Forward |
GGACACCGCAGCCCCATTA |
|
Reverse |
CACCCCTTAGTTTCACCGCA |
| β-actin |
|
Forward |
CTAGCCACGAGAGAGCGAAG |
|
Reverse |
TGTACATCTGGGCCTACGGA |
Western blot analysis
Cells were lysed on ice for 30 min using RIPA
protein lysate (cat. no. ab7937; Abcam) and centrifuged at 11,500 ×
g for 20 min at 4°C. The concentration of total protein in the
collected supernatant was measured using a BCA Protein Assay kit
(cat. no. 23227; Thermo Fisher Scientific, Inc.). A total of 50 µg
protein was purified by 12% SDS-PAGE, then transferred onto PVDF
membranes. The membranes were blocked in 8% skimmed milk for 2 h at
25°C, then incubated with primary antibodies specific for GAPDH
(1:5,000; cat. no. ab8245; Abcam), HMGB1 (1:1,000; cat. no.
ab227168; Abcam), TLR4 (1:1,000; cat. no. ab22048; Abcam) and
NF-κB/p65 (1:1,000; cat. no. ab16502; Abcam) overnight at 4°C. The
membranes were then washed with Tris-buffered saline containing
0.05% Tween-20 (TBST) and probed with a HRP-conjugated secondary
antibody (1:5,000; cat. no. ab7097; Abcam) for 1 h at 25°C. Signals
were detected with ECL reagent (cat. no. WBKLS0050; EMD Millipore).
Band intensities were measured using ImageJ software (version 1.47;
National Institutes of Health).
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three experiments. Multigroup comparisons
were analyzed using one-way ANOVA followed by Bonferroni's post hoc
test. Statistical analysis was conducted using GraphPad Prism
software version 8.0.1 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of CRY treatment on rat body
weight, ovarian quotiety, Lee's index and BMI
Compared with the control group, the body weights of
rats in the PCOS group, PCOS + HMGB1 group and PCOS + HMGB1 + CRY
group were significantly increased. Notably, total body weight in
PCOS + CRY rats was only marginally increased compared with the
control group. Moreover, treatment with CRY significantly decreased
body weight, compared with rats with PCOS (P<0.0001; Fig. 1A).
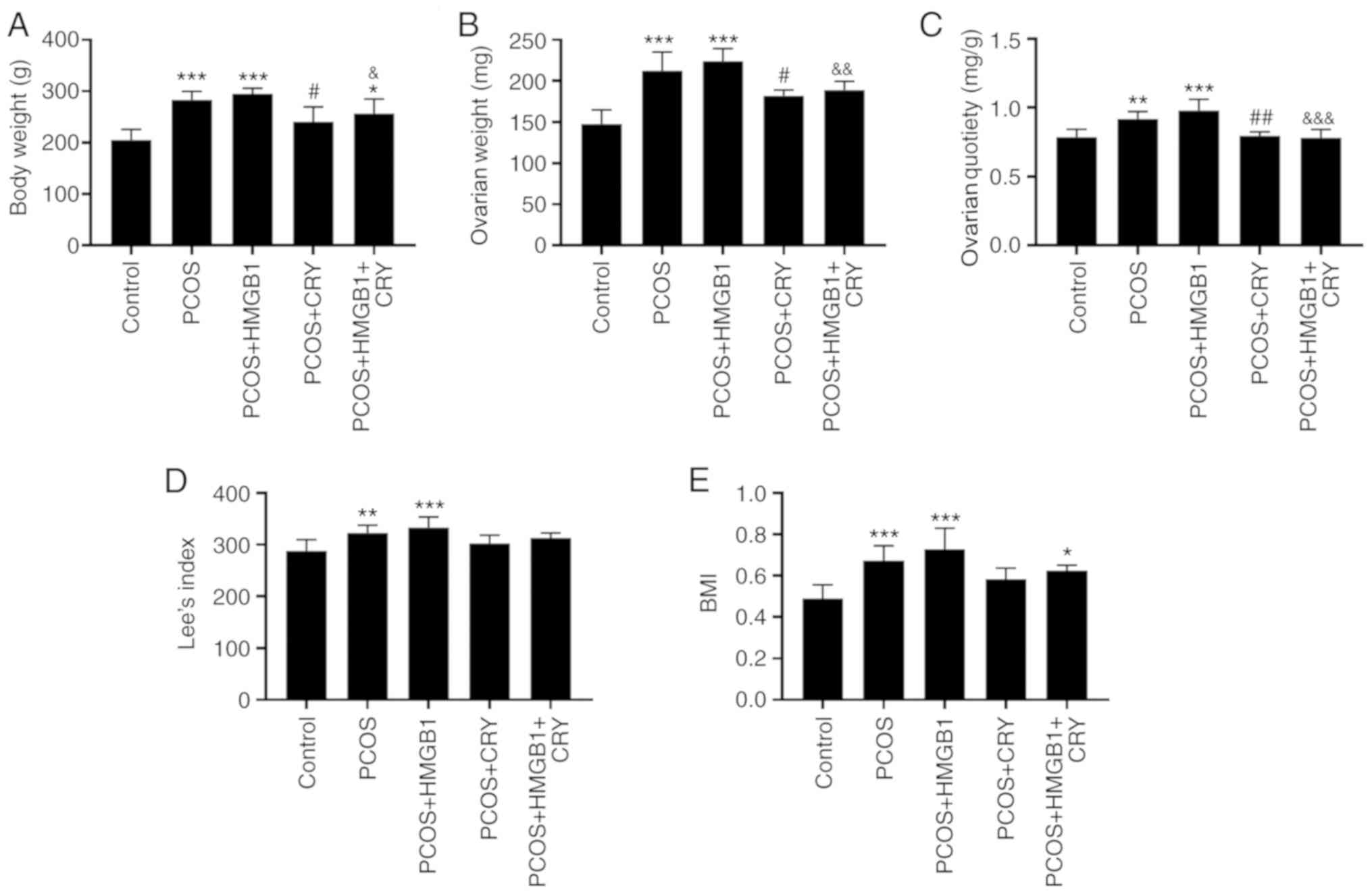 | Figure 1.Effects of CRY and HMGB1 on (A)
ovarian weight, (B) body weight, (C) ovaries quotiety, (D) Lee's
index and (E) BMI in rats with human chorionic gonadotropin and
insulin-induced PCOS. Data are presented as the mean ± SEM.
*P<0.05, **P<0.01, ***P<0.005 vs. control;
#P<0.05, ##P<0.01 vs. PCOS;
&P<0.05, &&P<0.01,
&&&P<0.005 vs. PCOS + HMGB1. CRY,
cryptotanshinone; PCOS, polycystic ovary syndrome; HMGB1,
high-mobility group box 1; BMI, body mass index. |
Rats in the PCOS and PCOS + HMGB1 groups had
significantly heavier ovaries compared with control rats. The
ovarian weight for rats in the PCOS + CRY group were significantly
decreased compared with the PCOS group (P=0.0193), and were
significantly decreased in the PCOS + HMGB1 + CRY group compared
with PCOS + HMGB1 rats (P=0.0052) (Fig. 1B). Additionally, the ovarian
quotiety in the PCOS and the PCOS + HMGB1 groups was significantly
increased relative to the control group (P=0.0043 and P<0.0001).
However, CRY treatment significantly reduced ovarian quotiety
compared with untreated groups (PCOS group, P=0.0086; PCOS + HMGB1
group, P<0.0001; Fig. 1C).
Lee's index and BMI were also significantly increased in the PCOS
and PCOS + HMGB1 groups compared with controls (PCOS group,
P=0.0095 and P=0.0007; PCOS + HMGB1 group, P=0.0006 and
P<0.0001; Fig. 1D and E). The
results indicated that CRY may serve a potential role in the
treatment of PCOS, as indicated by alterations in phenotype.
Effects of CRY on serum levels of
hormonal and inflammatory factors in rats with PCOS
Serum LH, LH/FSH ratio, T and TNF-α concentrations
in the PCOS and PCOS + HMGB1 groups were significantly increased
compared with the control group. Moreover, LH, LH/FSH ratio, T and
TNF-α concentrations of rats in the PCOS + CRY group were
significantly decreased compared with the PCOS group (P<0.0001,
P=0.0152, P=0.0051 and P=0.0008). Moreover, LH, LH/FSH ratio, T and
TNF-α concentrations of rats in the PCOS + HMGB1 + CRY group were
significantly decreased compared with the PCOS + HMGB1 group
(P<0.0001, P=0.0334, P=0.0002 and P=0.0002; Fig. 2). The results suggested that CRY
might display a therapeutic effect against PCOS.
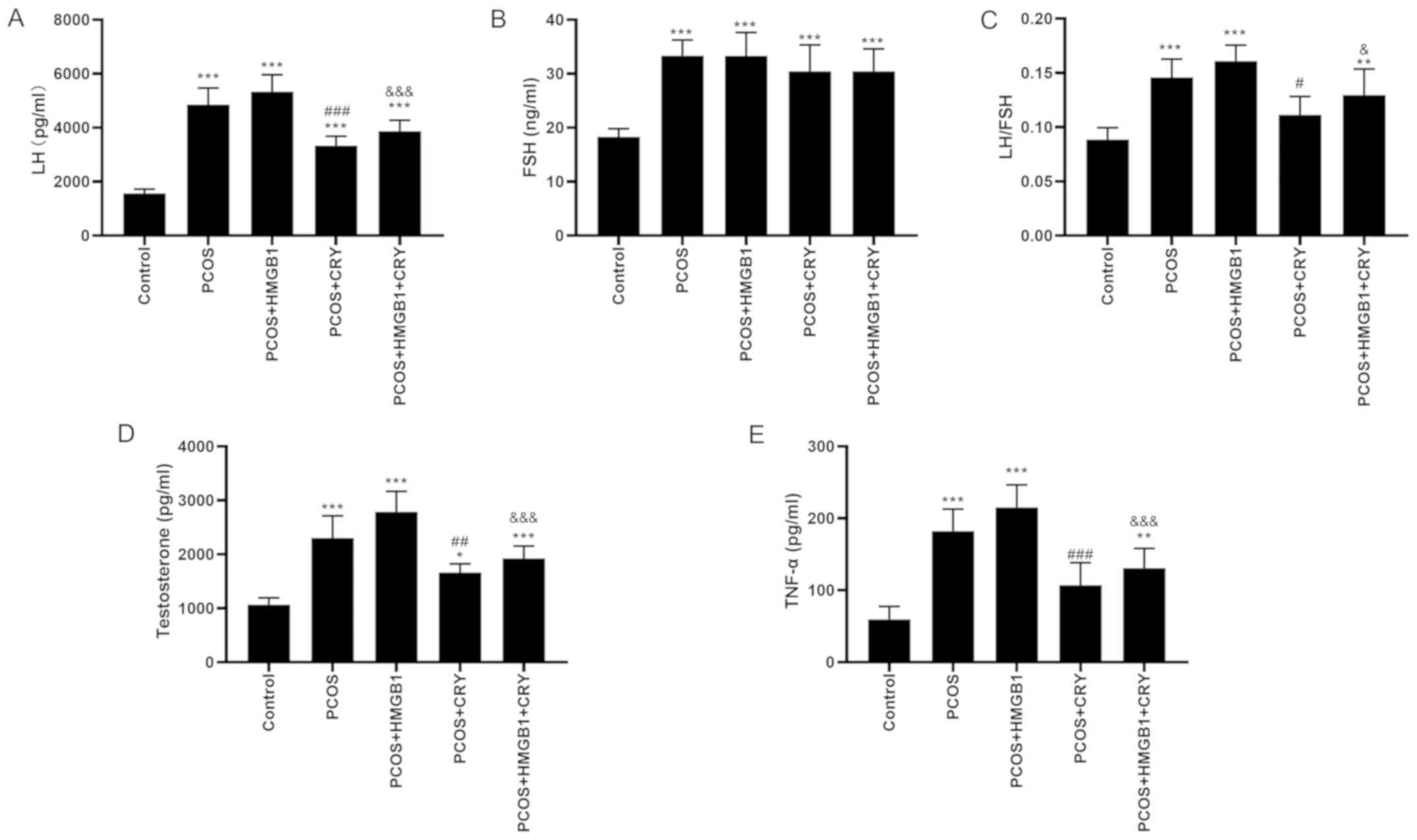 | Figure 2.Effect of CRY on serum levels of (A)
LH, (B) FSH, (C) LH/FSH ratio, (D) testosterone and (E) TNF-α. Data
are presented as the mean ± SEM. *P<0.05, **P<0.01,
***P<0.005 vs. control; #P<0.05,
##P<0.01, ###P<0.005 vs. PCOS;
&P<0.05, &&&P<0.005 vs.
PCOS + HMGB1. CRY, cryptotanshinone; PCOS, polycystic ovary
syndrome; LH, luteinizing hormone; FSH, follicle-stimulating
hormone; TNF-α, tumor necrosis factor-α; HMGB1, high-mobility group
box 1. |
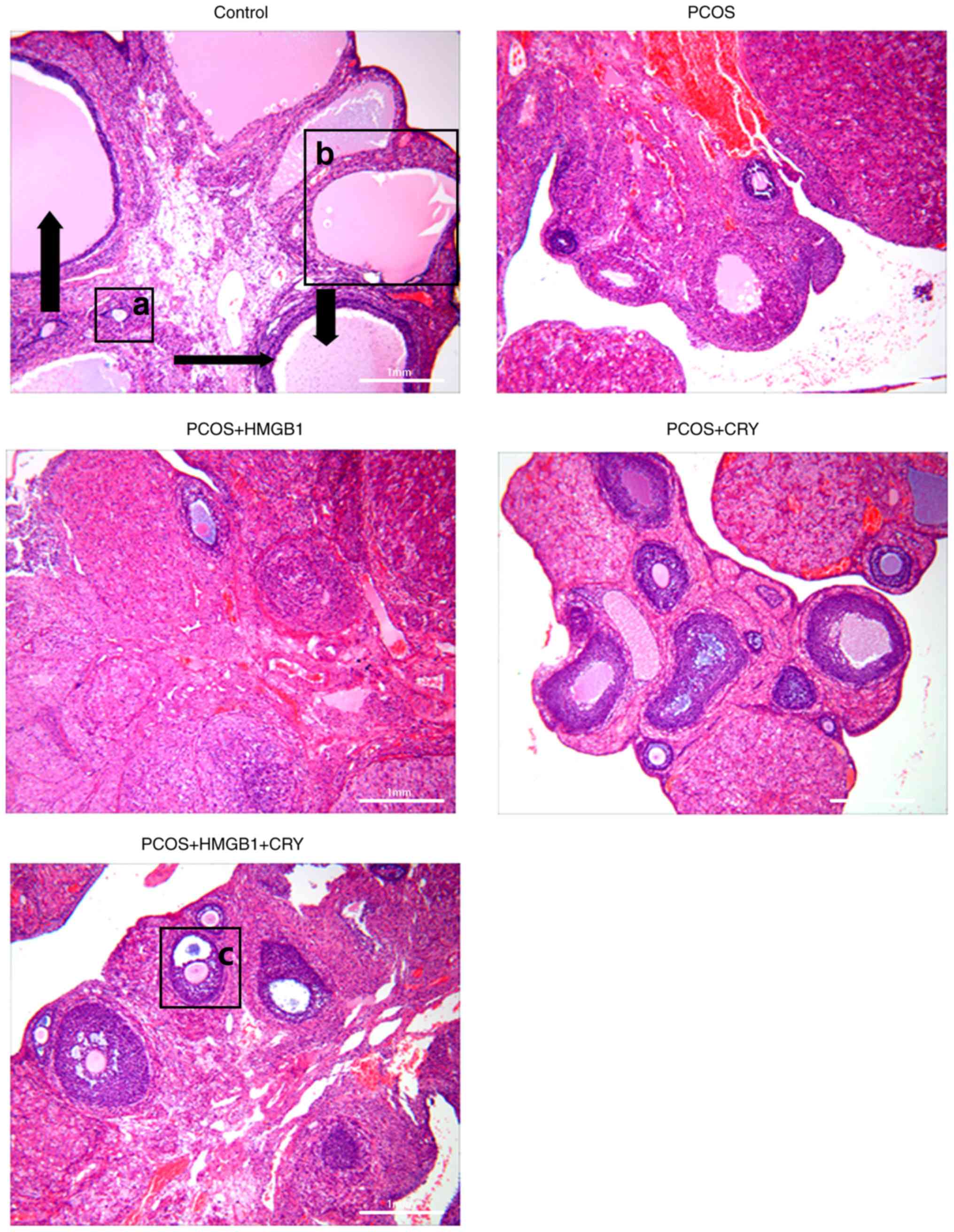
Pathological morphology
No structural pathological changes were observed in
the control group after examination under a light microscope. In
particular, the corpora lutea and follicles (6±3/field) were in
diverse developmental stages (such as primordial follicles, primary
follicles and secondary follicles). The tissues were stained with
hematoxylin and normal GC layers (6–8 layers) were counted.
Structural pathological changes were observed in the PCOS and PCOS
+ HMGB1 groups. Moreover, compared with the control group,
increased numbers (13±5/field) of cystic follicles, which were
large and filled with fluid, were recorded, and deteriorated GC
layers (0–3) were observed in the PCOS and PCOS + HMGB1 groups. In
addition, the numbers of corpora lutea in the PCOS and PCOS + HMGB1
groups were reduced, compared with the control group. Following CRY
treatment, the pathological morphology of ovaries in the PCOS and
PCOS + HMGB1 groups was reversed, increased GC layers (3–5 layers)
and decreased cystic follicles (7±2/field) were observed, compared
with the PCOS and PCOS + HMGB1 groups (Fig. 3). The pathological morphology
results indicated that CRY altered the pathological morphology of
PCOS.
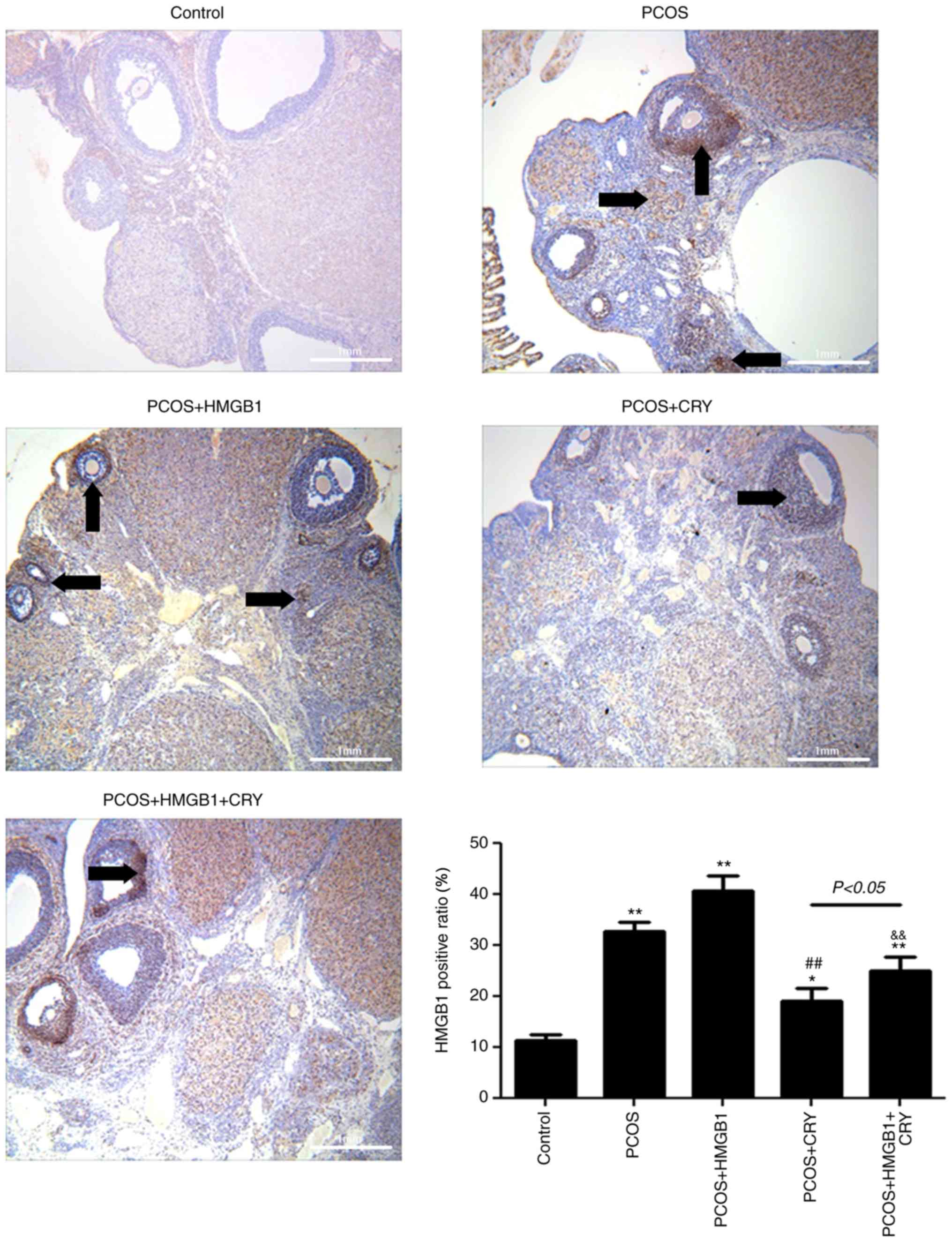
Immunohistochemical staining
analysis
The HMGB1 positive ratio in the PCOS and PCOS +
HMGB1 groups was significantly increased compared with the control
group (P=0.0092 and P=0.0061). However, following treatment with
CRY, the HMGB1 positivity in the PCOS or PCOS + HMGB1 groups was
significantly reduced ((P=0.0080 and P=0.0051). Additionally, the
HMGB1 positive ratio in the PCOS + CRY group was significantly
lower compared with the PCOS + HMGB1 + CRY group (P=0.0381;
Fig. 4). The results indicated
that the therapeutic function of CRY in PCOS might be associated
with regulation of HMGB1.
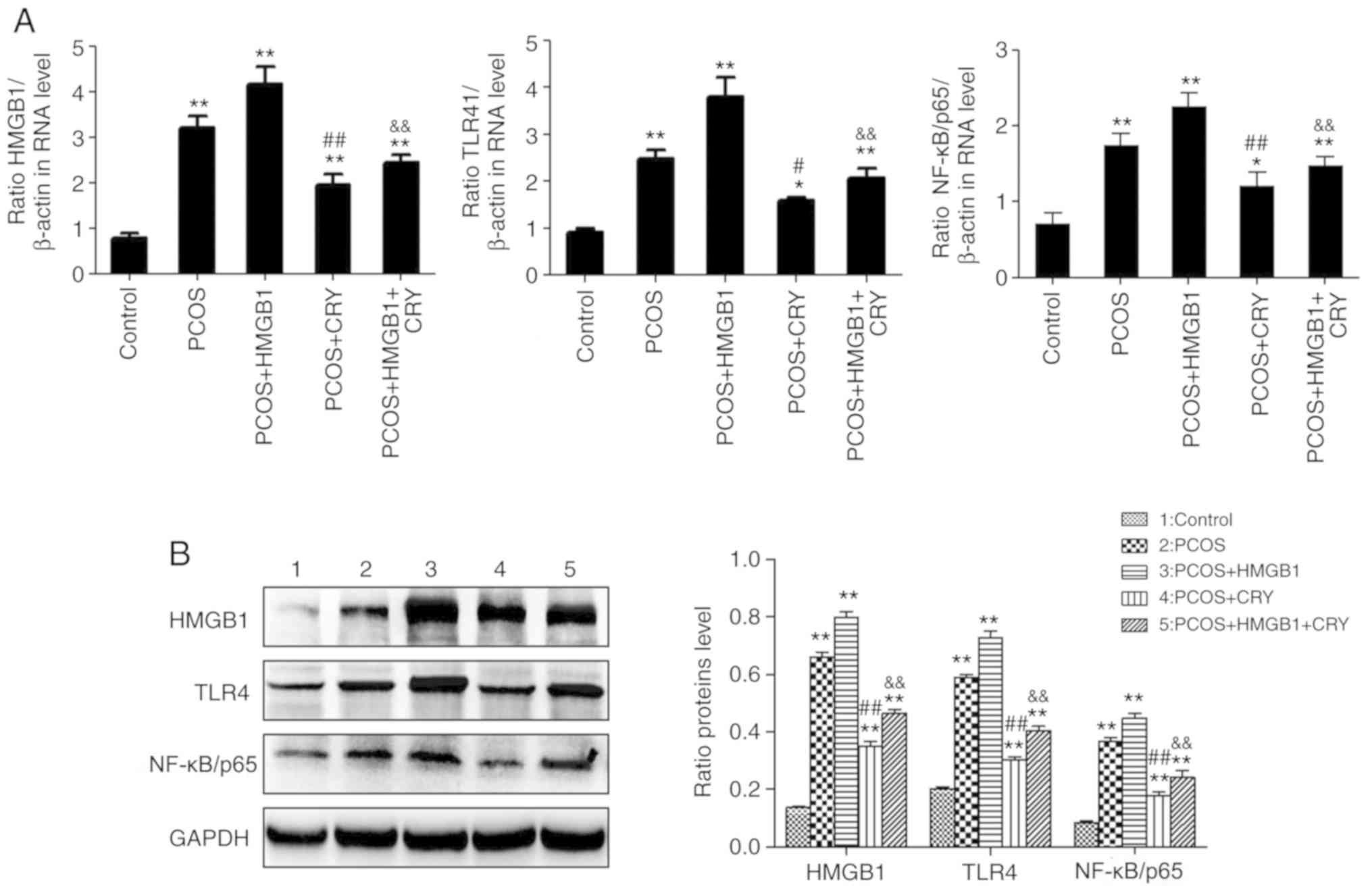
Effects of CRY on the expression of
HMGB1, TLR4 and NF-κB/p65 in the ovarian tissues
Western blotting and RT-qPCR were used to evaluate
the expression levels of HMGB1, TLR4 and NF-κB/p65. Consistent with
immunohistochemistry staining, the expressions of HMGB1, TLR4 and
NF-κB/p65 in the PCOS and the PCOS + HMGB1 groups significantly
increased, compared with the control group (PCOS group: P=0.0072,
P=0.0079 and P=0.0096; PCOS + HMGB1 group: P=0.0051, P=0.0059 and
P=0.0066). Additionally, the expression of these three proteins in
the PCOS + HMGB1 + CRY group was significantly lower compared with
the PCOS + HMGB1 group (P=0.0067, P=0.0073 and P=0.0065), and the
expression of these three proteins in the PCOS + CRY group was
significantly lower compared with the PCOS group (P=0.0058,
P=0.0063 and P=0.0060). The results suggested that CRY treatment
could reduce the expression of HMGB1, TLR4 and NF-κB/p65 in rats
with PCOS compared with the PCOS and PCOS + CRY groups. In
addition, CRY treatment downregulated the expression of HMGB1, TLR4
and NF-κB/p65 in rats with PCOS and HMGB1 compared with the PCOS +
HMGB1 and PCOS + HMGB1 + CRY groups (Fig. 5).
CRY inhibits proliferation of GCs and
regulates TNF-α and HMGB1 expression in vitro
GCs were transfected with three si-RNAs targeting
HMGB1, and HMGB1 mRNA and protein expression levels were evaluated.
Compared with si-NC and untransfected cells, mRNA and protein
expression of HMGB1 in the si-HMGB1#1, si-HMGB1#2, and si-HMGB1#3
groups was significantly decreased (P=0.0061, P=0.0031 and
P=0.0052), particularly in the si-HMGB1#2 group (Fig. 6A and B). Therefore, the HMGB1
siRNA-2 was used in subsequent experimentation. The viability of
GCs in different groups was determined using an MTT assay at 0, 24
and 48 h. Cell viability in the IR and IR + si-NC groups was
notably decreased compared with the control group (Fig. 6C). The viability of IR-GCs treated
with CRY or transfected with si-HMGB1 was higher compared with
untreated cells. In addition, TNF-α and HMGB1 concentrations in the
serum of IR and IR + si-NC rats were significantly increased,
compared with the control group (P=0.0087 and P=0.0091; Fig. 6D). Moreover, compared with the IR
group, TNF-α and HMGB1 concentrations in the IR + si-HMGB1 and IR +
CRY groups were significantly reduced (P=0.0086 and P=0.0074). The
results suggested that CRY improved the viability of IR-GCs by
regulating the production of HMGB1.
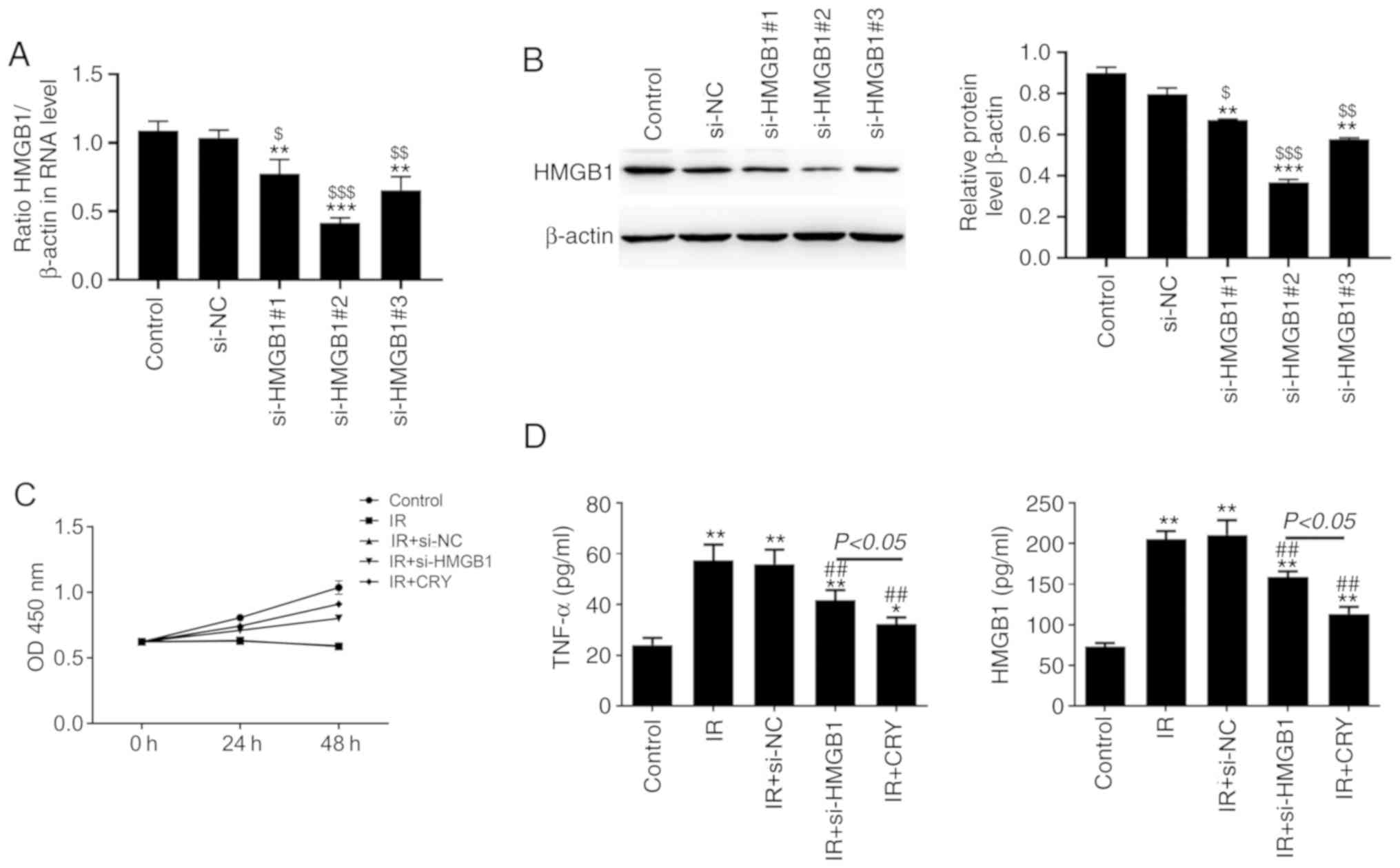 | Figure 6.Effect of CRY on GC proliferation,
and regulation of TNF-α and HMGB1 expression in vitro. (A)
mRNA and (B) protein expression of HMGB1 in GCs transfected with
siHMBG1. (C) Cell viability of insulin-resistant GCs following CRY
treatment or siHMGB1 transfection. (D) Concentration of TNF-α and
HMGB1 in culture medium. Data are presented as the mean ± SEM.
*P<0.05, **P<0.01, ***P<0.005 vs. control;
$P<0.05, $$P<0.01,
$$$P<0.05 vs. si-NC, ##P<0.01 vs. IR.
CRY, cryptotanshinone; IR, insulin resistance; GC, granulosa cell;
HMGB1, high-mobility group box 1; TNF-α, tumor necrosis factor-α;
si, small interfering; OD, optical density; si-NC, si-negative
control. |
Effects of CRY on the expression of
HMGB1, TLR4 and NF-κB/p65 in GCs
HMGB1, TLR4 and NF-κB/p65 expression levels were
analyzed using RT-qPCR and western blotting. The expression of
HMGB1, TLR4 and NF-κB/p65 in the IR and IR + si-NC groups were
significantly increased (IR group, P=0.0068, P=0.0063 and P=0.0076;
IR + si-NC group, P=0.0065, P=0.0069 and P=0.0079), compared with
the control group. Additionally, the expression of HMGB1, TLR4 and
NF-κB/p65 in both IR + si-HMGB1 and IR + CRY groups were
significantly lower compared with the IR group (IR + si-HMGB group,
P=0.0098, P=0.0093 and P=0.0486; IR + CRY group, P=0.0095, P=0.0089
and P=0.0339). The effect of CRY treatment on HMGB1, TLR4 and
NF-κB/p65 expression levels was also notably greater compared with
si-HMGB1 transfection (Fig. 7).
The results demonstrated that CRY treatment downregulated the
expression of HMGB1, TLR4 and NF-κB/p65.
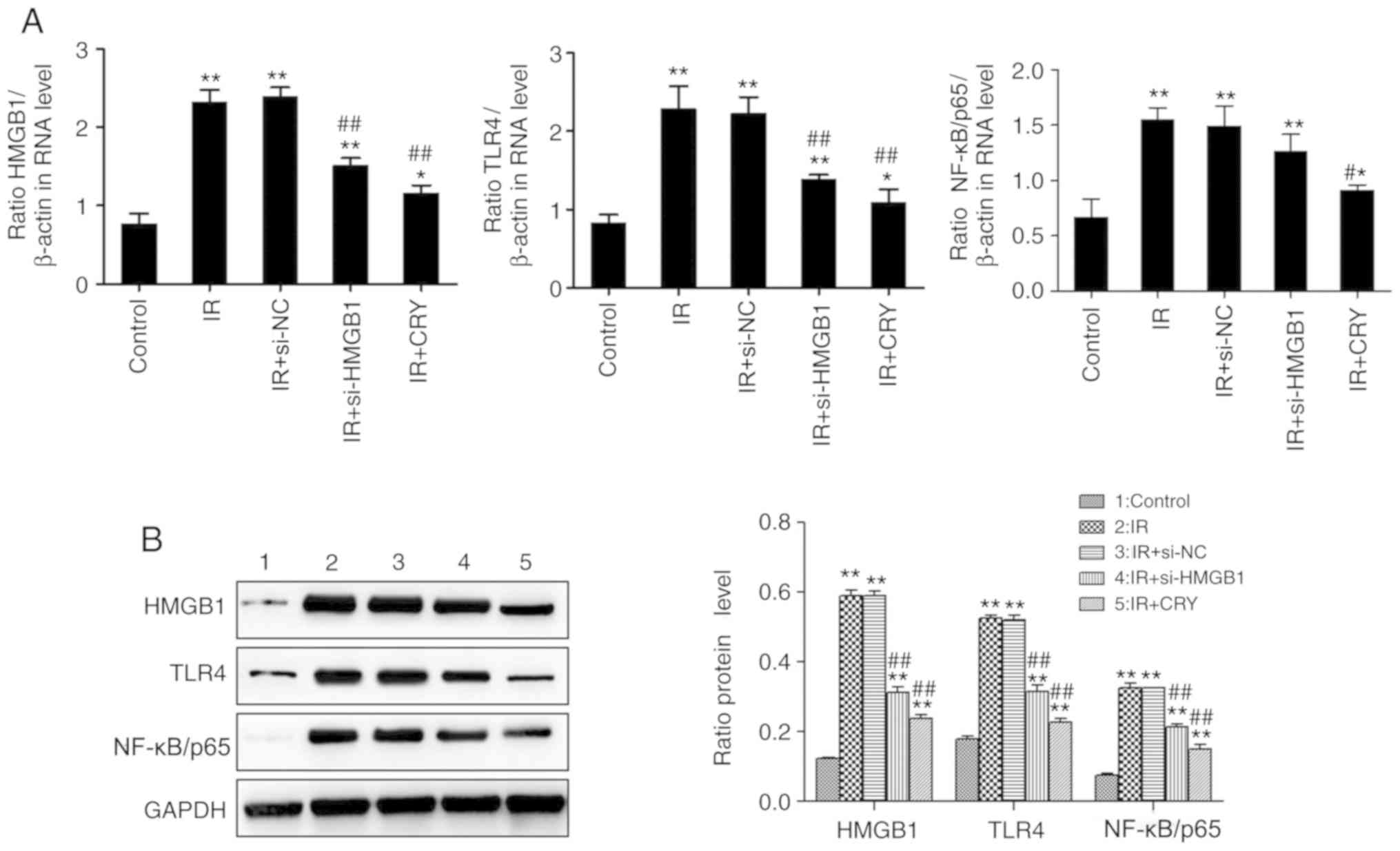 | Figure 7.Effects of CRY on HMGB1, TLR4 and
NF-κB/p65 expression in ovarian granulosa cells. (A) mRNA
expression levels. (B) Protein expression levels. Data are
presented as the mean ± SEM. *P<0.05, **P<0.01 vs. control;
#P<0.05, ##P<0.01 vs. IR. CRY,
cryptotanshinone; IR, insulin resistance; HMGB1, high-mobility
group box 1; TLR4, toll-like receptor 4; NF-κB, nuclear factor-κB;
si, small interfering; si-NC, si-negative control. |
Discussion
PCOS is one of the most common endocrinological
disorders and is a major cause of anovulatory infertility. PCOS
represents a public health concern due to its detrimental effects
on women's health and quality of life (25). Thus, there is an urgent need to
develop effective therapeutic strategies for patients with PCOS
(26,27).
Previous studies have suggested that CRY might be
used for the treatment of PCOS (17,28).
However, the mechanism through which CRY might reverse ovulatory
dysfunction is only partially understood. In our previous study,
HMGB1 was upregulated in the peripheral blood of patients with PCOS
and INS resistance, and HMGB1 upregulation was correlated with the
pathogenesis of PCOS (8). Thus,
the aim of the present study was to determine whether CRY could
serve a therapeutic role in PCOS through HMGB1. Several
PCOS-related indicators, such as ovarian quotiety, BMI and hormone
levels were assessed, suggesting that treatment with HMGB1 could
aggravate PCOS, which was consistent with our previous study.
High levels of LH, T and TNF-α are often found in
the blood of patients with PCOS, and increased LH/FSH ratio have
been reported as a causal factor of PCOS (29,30).
In the present study, serum LH, T and TNF-α levels, as well as the
LH/FSH ratio were significantly increased in all rats with PCOS,
including those treated with HMGB1, compared with the control
group. Moreover, CRY treatment decreased the body weight and
ovarian quotiety and notably reduced serum LH, LH/FSH ratio, T and
TNF-α levels in all PCOS rat groups. These findings suggested that
CRY might have a therapeutic effect against PCOS. In addition,
pathomorphological analysis further confirmed that CRY could
reverse the ovulation disorders seen in PCOS.
Chronic inflammation plays an important role in the
development of PCOS (31,32). The TLR4/NF-κB pathway has distinct
functions during the stress reaction and inflammation (33). Several studies have demonstrated
that HMGB1 could trigger the TLR4 signaling pathway and NF-κB
activation, thereby inducing the expression of inflammatory factors
(34,35). Jiang et al (18) suggested that inhibition of HMGB1
could improve IR in PCOS through the suppression of the TLR4/NF-κB
pathway. To determine whether CRY could reverse PCOS by regulating
HMGB1/TLR4/NF-κB signaling, the expression of these IR-related
markers were evaluated in ovarian tissue and GCs. HMGB1, TLR4 and
NF-κB/p65 expression was upregulated in the ovarian tissue from
rats with PCOS, including those treated with HMGB1, consistent with
a study conducted by Koc et al (36). In contrast, CRY treatment resulted
in HMGB1, TLR4 and NF-κB/p65 downregulation, compared with PCOS
model rats.
Furthermore, si-HMGB1 silencing experiments were
carried out in INS-resistant GCs in order to evaluate the effect of
HMGB1 on cell viability and expression of IR-related markers in
rats with PCOS. In the present study, immature rats were selected
for the isolation of GCs. Compared with GCs from mature rats, the
GCs in immature rats had not been exposed to endogenous
gonadotropins. Therefore, a large number of GCs could be obtained
in the same stage after inducing estrus of immature rats by PMSG.
Both transfection with si-HMGB1 and CRY treatment resulted in
improved viability in INS-resistant GCs. Several studies have
suggested that HMGB1 could promote apoptosis of GCs (36,37).
Another previous study demonstrated that HMGB1 silencing
significantly reduced GC apoptosis (8). Moreover, in the present study, the
concentration of HMGB1 was measured in the GC culture supernatant
significantly decreased following CRY and si-HMGB1 treatment,
compared with untreated or untransfected cells, further confirming
that the protective effects of CRY might be mediated by regulating
HMGB1. Furthermore, the results of RT-qPCR and western blotting in
INS-resistant GCs were consistent with those in ovarian tissues,
suggesting that CRY treatment could reduce the expression of HMGB1,
TLR4 and NF-κB/p65 in both PCOS model and INS-resistant GCs.
Collectively, the present findings suggest that CRY
might attenuate PCOS, at least in part, by decreasing the serum
levels of LH, LH/FSH ratio, T and TNF-α through HMGB1, TLR4 and
NF-κB/p65 downregulation in ovarian tissue. However, the current
study presents some limitations. Although CRY can decrease the
expression of HMGB1, TLR4 and NF-κB/p65 in ovarian tissues from
rats with PCOS, the underlying mechanism remains unclear. Moreover,
the protective effects of CRY on IR-GCs were not confirmed through
validations experiments other than cell viability detection. In
summary, the present study demonstrated that CRY treatment may
effectively improve PCOS by lowering the serum LH, LH/FSH ratio, T
and TNF-α levels and decreasing the expression of HMGB1, TLR4 and
NF-κB/p65 in ovarian tissues and GCs. Thus, CRY could represent a
potential treatment option for PCOS. Nevertheless, further studies
and clinical trials are required to confirm the efficacy and safety
of this approach. Furthermore, the present study also provided a
novel theoretical basis for the treatment of PCOS through HMGB1,
TLR4 and NF-κB/p65.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Natural
Science Foundation (grant no. 81804136), The Shanghai Natural
Science Foundation (grant no. 17ZR1427900) and The Shanghai
Municipal Commission Foundation of Health and Family Planning
(grant no. 201540087).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GH, LW, ZS, CQ, LY and YY performed the experiments
and analyzed data. YY, CQ and LY drafted and revised the
manuscript. XN designed the study. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All methodological procedures were performed under
strict conformity with the guidelines of the Chinese Ministry of
Science and Technology for the Care and Use of Laboratory Animals.
The experimental protocol was approved by The Animal Care and
Experiment Review Board of Shanghai Traditional Chinese Medicine
Hospital (Shanghai, China) (approval no. 20190103).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRY
|
cryptotanshinone
|
|
PCOS
|
polycystic ovary syndrome
|
|
HCG
|
human chorionic gonadotropin
|
|
INS
|
insulin
|
|
LH
|
luteinizing hormone
|
|
FSH
|
follicle-stimulating hormone
|
|
TNF-α
|
tumor necrosis factor-α
|
|
HMGB1
|
high-mobility group box 1
|
References
|
1
|
Witchel SF, Recabarren SE, González F,
Diamanti-Kandarakis E, Cheang KI, Duleba AJ, Legro RS, Homburg R,
Pasquali R, Lobo RA, et al: Emerging concepts about prenatal
genesis, aberrant metabolism and treatment paradigms in polycystic
ovary syndrome. Endocrine. 42:526–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rojas J, Chávez M, Olivar L, Rojas M,
Morillo J, Mejías J, Calvo M and Bermúdez V: Polycystic ovary
syndrome, insulin resistance, and obesity: Navigating the
pathophysiologic labyrinth. Int J Reprod Med. 2014:7190502014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fauser BC, Tarlatzis BC, Rebar RW, Legro
RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, et
al: Consensus on women's health aspects of polycystic ovary
syndrome (PCOS): The amsterdam ESHRE/ASRM-sponsored 3rd PCOS
consensus workshop group. Fertil Steril. 97:28–38.e25. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan H, Zhu G, Wang F, Wang X, Guo H and
Shen M: Interaction between common variants of FTO and MC4R is
associated with risk of PCOS. Reprod Biol Endocrinol. 13:552015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goodarzi MO, Dumesic DA, Chazenbalk G and
Azziz R: Polycystic ovary syndrome: Etiology, pathogenesis and
diagnosis. Nat Rev Endocrinol. 7:219–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ollila MM, Piltonen T, Puukka K, Ruokonen
A, Järvelin MR, Tapanainen JS, Franks S and Morin-Papunen L: Weight
gain and dyslipidemia in early adulthood associate with polycystic
ovary syndrome: Prospective cohort study. J Clin Endocrinol Metab.
101:739–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diamanti-Kandarakis E: Polycystic ovarian
syndrome: Pathophysiology, molecular aspects and clinical
implications. Expert Rev Mol Med. 10:e32008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ni XR, Sun ZJ, Hu GH and Wang RH: High
concentration of insulin promotes apoptosis of primary cultured rat
ovarian granulosa cells via its increase in extracellular HMGB1.
Reprod Sci. 22:271–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Velásquez E: Chronic complications of
polycystic ovary syndrome. Review. Invest Clin. 43:205–213.
2002.(In Spanish). PubMed/NCBI
|
|
10
|
Huang Y, Sun J, Wang X, Tao X, Wang H and
Tan W: Asymptomatic chronic gastritis decreases metformin tolerance
in patients with type 2 diabetes. J Clin Pharm Ther. 40:461–465.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li XJ, Yu YX, Liu CQ, Zhang W, Zhang HJ,
Yan B, Wang LY, Yang SY and Zhang SH: Metformin vs.
thiazolidinediones for treatment of clinical, hormonal and
metabolic characteristics of polycystic ovary syndrome: A
meta-analysis. Clin Endocrinol (Oxf). 74:332–339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cholesterol Treatment Trialists' (CTT)
Collaboration, ; Baigent C, Blackwell L, Emberson J, Holland LE,
Reith C, Bhala N, Peto R, Barnes EH, Keech A, et al: Efficacy and
safety of more intensive lowering of LDL cholesterol: A
meta-analysis of data from 170,000 participants in 26 randomised
trials. Lancet. 376:1670–1681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji XY, Tan BK and Zhu YZ: Salvia
miltiorrhiza and ischemic diseases. Acta Pharmacol Sin.
21:1089–1094. 2000.PubMed/NCBI
|
|
14
|
Wang X, Morris-Natschke SL and Lee KH: New
developments in the chemistry and biology of the bioactive
constituents of Tanshen. Med Res Rev. 27:133–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu J, Zhai D, Hao L, Zhang D, Bai L, Cai Z
and Yu C: Cryptotanshinone reverses reproductive and metabolic
disturbances in PCOS model rats via regulating the expression of
CYP17 and AR. Evid Based Complement Alternat Med. 2014:6707432014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ong M, Peng J, Jin X and Qu X: Chinese
herbal medicine for the optimal management of polycystic ovary
syndrome. Am J Chin Med. 45:405–422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang Y, Li W, Wang CC, Wu X and Zheng J:
Cryptotanshinone reverses ovarian insulin resistance in mice
through activation of insulin signaling and the regulation of
glucose transporters and hormone synthesizing enzymes. Fertil
Steril. 102:589–596.e584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang B, Xue M, Xu D, Song Y and Zhu S:
Upregulation of microRNA-204 improves insulin resistance of
polycystic ovarian syndrome via inhibition of HMGB1 and the
inactivation of the TLR4/NF-κB pathway. Cell Cycle. 19:697–710.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang X, Zhang Y, Wu X, Bae CS, Hou L,
Kuang H, Wang Y and Stener-Victorin E: Cryptotanshinone reverses
reproductive and metabolic disturbances in prenatally androgenized
rats via regulation of ovarian signaling mechanisms and androgen
synthesis. Am J Physiol Regul Integr Comp Physiol. 300:R869–R875.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poretsky L, Clemons J and Bogovich K:
Hyperinsulinemia and human chorionic gonadotropin synergistically
promote the growth of ovarian follicular cysts in rats. Metabolism.
41:903–910. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ngo HT, Hetland RB, Sabaredzovic A, Haug
LS and Steffensen IL: In utero exposure to perfluorooctanoate
(PFOA) or perfluorooctane sulfonate (PFOS) did not increase body
weight or intestinal tumorigenesis in multiple intestinal neoplasia
(Min/+) mice. Environ Res. 132:251–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mangano C, Scarano A, Perrotti V, Iezzi G
and Piattelli A: Maxillary sinus augmentation with a porous
synthetic hydroxyapatite and bovine-derived hydroxyapatite: A
comparative clinical and histologic study. Int J Oral Maxillofac
Implants. 22:980–986. 2007.PubMed/NCBI
|
|
23
|
deMoura MD, Chamoun D, Resnick CE and
Adashi EY: Insulin-like growth factor (IGF)-I stimulates IGF-I and
type 1 IGF receptor expression in cultured rat granulosa cells:
Autocrine regulation of the intrafollicular IGF-I system.
Endocrine. 13:103–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
March WA, Moore VM, Willson KJ, Phillips
DIW, Norman RJ and Davies MJ: The prevalence of polycystic ovary
syndrome in a community sample assessed under contrasting
diagnostic criteria. Hum Reprod. 25:544–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azziz R, Marin C, Hoq L, Badamgarav E and
Song P: Health care-related economic burden of the polycystic ovary
syndrome during the reproductive life span. J Clin Endocrinol
Metab. 90:4650–4658. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barthelmess EK and Naz RK: Polycystic
ovary syndrome: Current status and future perspective. Front Biosci
(Elite Ed). 6:104–119. 2014.PubMed/NCBI
|
|
28
|
Xia Y, Zhao P, Huang H, Xie Y, Lu R and Li
D: Cryptotanshinone reverses reproductive disturbances in rats with
dehydroepiandrosterone-induced polycystic ovary syndrome. Am J
Transl Res. 9:2447–2456. 2017.PubMed/NCBI
|
|
29
|
Rosenfield RL and Ehrmann DA: The
pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of
PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev.
37:467–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nestler JE, Powers LP, Matt DW, Steingold
KA, Plymate SR, Rittmaster RS, Clore JN and Blackard WG: A direct
effect of hyperinsulinemia on serum sex hormone-binding globulin
levels in obese women with the polycystic ovary syndrome. J Clin
Endocrinol Metab. 72:83–89. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shorakae S, Ranasinha S, Abell S, Lambert
G, Lambert E, de Courten B and Teede H: Inter-related effects of
insulin resistance, hyperandrogenism, sympathetic dysfunction and
chronic inflammation in PCOS. Clin Endocrinol (Oxf). 89:628–633.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu M, Gao J, Zhang Y, Li P, Wang H, Ren X
and Li C: Serum levels of TSP-1, NF-κB and TGF-β1 in polycystic
ovarian syndrome (PCOS) patients in northern China suggest PCOS is
associated with chronic inflammation. Clin Endocrinol (Oxf).
83:913–922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Xue C, Xu Q, Zhang Y, Li H, Li F,
Liu Y and Guo C: Caprylic acid suppresses inflammation via
TLR4/NF-κB signaling and improves atherosclerosis in ApoE-deficient
mice. Nutr Metab (Lond). 16:402019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li G, Wu X, Yang L, He Y, Liu Y, Jin X and
Yuan H: TLR4-mediated NF-κB signaling pathway mediates
HMGB1-induced pancreatic injury in mice with severe acute
pancreatitis. Int J Mol Med. 37:99–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Osuka K, Watanabe Y, Usuda N, Iwami K,
Miyachi S and Takayasu M: Expression of high mobility group B1 and
toll-like receptor-nuclear factor κB signaling pathway in chronic
subdural hematomas. PLoS One. 15:e02336432020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koc O, Ozdemirici S, Acet M, Soyturk U and
Aydin S: Nuclear factor-κB expression in the endometrium of normal
and overweight women with polycystic ovary syndrome. J Obstet
Gynaecol. 37:924–930. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim SW, Lim CM, Kim JB, Shin JH, Lee S,
Lee M and Lee JK: Extracellular HMGB1 released by NMDA treatment
confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway.
Neurotox Res. 20:159–169. 2011. View Article : Google Scholar : PubMed/NCBI
|