Introduction
Ischemic heart disease (IHD) can cause congestive
heart failure and myocardial infarction (1). Among the treatment strategies
available for IHD, timely reperfusion of the occluded artery, such
as coronary bypass surgery, coronary angioplasty and thrombolysis,
is beneficial and can treat a wide range of myocardial injuries
(2). However, reperfusion can also
result in another myocardial ischemia/reperfusion (I/R) injury
(3), which can induce
cardiomyocyte death and remodeling, and result in irreversible
myocardial damage (4).
Cell apoptosis is a major morphologically
distinctive feature of programmed cell death, which is crucial to
numerous biological processes such as tumorigenesis,
differentiation, immunity, inflammation and cell growth (5). Autophagy participates in degrading
damaged cytoplasmic proteins and senescent organelles via the
lysosomal pathway (6). However,
there are complex interactions between apoptosis and autophagy
(7). Increasing evidence has
demonstrated that apoptosis and autophagy, which are two major
pathophysiological processes, serve a role in the pathogenesis of
myocardial I/R injury (8,9). However, the detailed mechanisms
underlying myocardial I/R injury are not completely understood.
Therefore, investigating the regulation of apoptosis and autophagy
may aid with the development of beneficial treatment strategies for
myocardial I/R injury.
MicroRNAs (miRNAs/miRs) are small endogenous
non-coding RNAs, 22–25 nucleotides in length, which can bind to the
target mRNA at the 3′-untranslated region (3′-UTR) to regulate gene
expression (10). miRs serve a
significant role in a number of human cardiovascular diseases,
including myocardial I/R injury, such as miR-21, which serves a
protective role in myocardial I/R injury (11,12).
Moreover, miRs are involved in the regulation of apoptosis,
autophagy, proliferation, differentiation and other biological
processes (13). Previous studies
have identified numerous miRs as critical regulators of myocardial
I/R injury. For example, miR-496 overexpression can protect
myocardial cells from apoptosis following hypoxia/reoxygenation
(H/R) treatment and promote cell proliferation (14). Moreover, miR-374a-5p displays a
protective effect on cardiac I/R injury in vitro and in
vivo (15), and miR-24-3p also
displays cardioprotective effects in myocardial I/R injury
(16). Increasing evidence has
suggested that miR-494 serves a pivotal role in cell apoptosis,
proliferation, tumorigenesis, metastasis and other processes
(17). Zhai et al (18) investigated the expressional
differences of miR-494 in rats with cerebral I/R injury. miR-494
upregulation inhibited human hepatocyte L02 cell apoptosis
following hypoxia/ischemia induction via activating the PI3K/AKT
signaling pathway (19). Wang
et al (20) reported that
miR-494 was involved in I/R-induced myocardium injury in
vivo. However, to the best of our knowledge, the role of
miR-494 in H/R-induced cardiomyocyte apoptosis and autophagy has
not been previously reported.
In previous years, as a nicotinamide adenine
dinucleotide-dependent histone deacetylation enzyme, silent
information regulator 1 (SIRT1) has become the focus of numerous
studies (21,22). SIRT1 can resist oxidative stress,
inhibit apoptosis and alleviate inflammatory reaction (23–25).
Moreover, Liu et al (26)
demonstrated that pancreatic cancer chemoresistance, invasion and
proliferation could be inhibited by miR-494 via SIRT1. Li et
al (27) reported that SIRT1
was a key factor in regulating gastric cancer cell autophagy via
the PI3K/AKT/mTOR signaling pathway. Increasing evidence has also
indicated that SIRT1 serves as a mediator for myocardial I/R injury
(28). However, the effects of
miR-494 and SIRT1 on myocardial I/R injury and their association
with apoptosis and autophagy require further investigation.
The present study used rat-derived H9c2 cells to
establish an in vitro H/R myocardial cell model to simulate
myocardial I/R injury. Subsequently, the functions and mechanisms
underlying miR-494 in H9c2 cell apoptosis and autophagy following
H/R were investigated.
Materials and methods
Cell culture and H/R treatment
H9c2 cells (Procell Life Science & Technology
Co., Ltd.) were maintained in DMEM (Beijing Solarbio Science &
Technology Co., Ltd.) supplemented with 100 mg/ml streptomycin, 100
U/ml penicillin, and 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. Mycoplasma contamination in
cell substrates was detected to confirm the availability of cells.
For control treatment, cells were cultured in DMEM with 5%
CO2 for 12 h. For H/R treatment, H9c2 cells were
cultured in glucose- and serum-free DMEM in a 94% N2, 5%
CO2 and 1% O2 atmosphere for 12 h at 37°C to
simulate hypoxia. Subsequently, the medium was replaced with DMEM
supplemented with 10% FBS in a 95% air and 5% CO2
atmosphere for 3 h at 37°C to simulate reoxygenation.
Cell transfection
H9c2 cells were seeded into 6-well plates
(3.5×105 cells/well) or 96-well plates
(1.5×104 cells/well). At 80% confluence, cells were
transfected with 50 nM miR-494 mimic
(5′-UGAAACAUACACGGGAAACCUCU-3′), 50 nM miR-494 mimic negative
control (NC; 5′-UUCUCCGAACGUGUCACGUTT-3′), 100 nM miR-494 inhibitor
(5′-AGAGGUUUCCCGUGUAUGUUUCA-3′), 100 nM miR-494 inhibitor NC
(5′-CAGUACUUUUGUGUAGUACAA-3′), 50 nM small interfering (si)RNA
targeting SIRT1 (siSIRT1; 5′-CCCUGUAAAGCUUUCAGAATT-3′) or 50 nM
siRNA negative control (5′-UUCUCCGAACGUGUCACGUTT-3′ for 4 h at
37°C; all purchased from Shanghai GenePharma Co., Ltd.) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 28 h post-transfection, cells were collected,
exposed to H/R treatment and used for subsequent experiments.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from H9c2 cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
RNA quality and concentration were detected using an ultraviolet
spectrophotometer. Total RNA was reverse transcribed into cDNA
using a Transcriptor cDNA Synthesis kit (Roche Applied Science).
The reverse transcription reactions were incubated for 40 min at
37°C and 5 min at 85°C. Subsequently, qPCR was performed using SYBR
Premix Ex Taq (Takara Bio, Inc.) on a StepOnePlus Realtime PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used for qPCR: 95°C for 30
sec; followed by 42 cycles of 95°C for 5 sec and 55°C for 35 sec.
The following primers were used for qPCR: miR-494 forward,
5′-CGCTGAAACATACACGGGAA-3′ and reverse, 5′-CAGTGCAGGGTCCGAGGTAT-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; SIRT1 forward,
5′-CTCCTCATTGTTATTGGGTCTTCTC-3′ and reverse,
5′-ACTCGCCACCTAACCTATGACAC-3′; and GAPDH forward,
5′-AGATCCCGCTAACATCAAATGG-3′ and reverse,
5′-GTTCACACCCATCACAAACATG-3′. miRNA and mRNA expression levels were
quantified using the 2−ΔΔCq method (29) and normalized to the internal
reference genes U6 and GAPDH, respectively.
Cell Counting Kit-8 (CCK-8) assay
H9c2 cells (1×106 cells/well) were
incubated with 10 µl CCK-8 reagent (Dojindo Molecular Technologies,
Inc.) for 1 h at 37°C. Absorbance was measured at a wavelength of
450 nm using a microplate reader. Subsequently, cell viability was
calculated. Cell viability (%) = (OD control group − OD treatment
group) / OD control group × 100%
Lactic dehydrogenase (LDH) and
superoxide dismutase (SOD) levels detection
To detect the release and activity of two specific
marker enzymes, LDH (cat. no. CK12) and SOD commercial kits (cat.
no. S311; both Dojindo Molecular Technologies, Inc.) were used
according to the manufacturer's protocol. Absorbance was measured
at a wavelength of 490 nm (LDH) or 450 nm (SOD) using a microplate
reader.
Early and late apoptosis detection via
flow cytometry
H9c2 cells were harvested with 0.25% trypsin and
washed with PBS. Following staining with 5 µl 7-Amino-Actinomycin D
and 5 µl PE Annexin V (cat. no. BD 559763; BD Biosciences) for 18
min in the dark at 37°C, early and late apoptosis was
examined using a FACSMelody flow cytometer (BD Biosciences) with
FlowJo software (version 7.6.1; FlowJollc).
TdT-mediated dUTP-biotin nick
end-labeling (TUNEL) staining
H9c2 cells were fixed for 30 min in 4%
paraformaldehyde at 37°C. Following washing three times with PBS
and permeabilization with 0.1% Triton X-100 for 15 min at 37°C,
cells were stained with 50 µl TUNEL mix (Roche Diagnostics) for 1 h
at room temperature. Subsequently, nuclei were labeled by
incubating cells with 5 µg/ml DAPI (Sigma-Aldrich; Merck KGaA) for
8 min in the dark. Following three washes with PBS, TUNEL-positive
apoptotic cells were observed in five randomly selected fields of
views using a fluorescent microscope (Olympus Corporation;
magnification, ×200).
Western blotting
Total protein was extracted from H9c2 cells using
RIPA buffer (Invitrogen; Thermo Fisher Scientific, Inc.).
Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific,
Inc.) was used to measure protein concentration. Equal amounts of
protein lysates (30 µg/lane) were separated via 10% SDS-PAGE and
transferred to PVDF membranes. Following blocking with 5% skim milk
for 1 h at 37°C, the membranes were incubated overnight at 4°C with
primary antibodies targeted against: Bcl-2 (cat. no. ab196495;
1:1,000; Abcam), Bax (cat. no. ab32503; 1:5,000; Abcam),
microtubule-associated proteins 1A/1B light chain 3 b2 (LC3B; cat.
no. ab192890; 1:2,000; Abcam), SIRT1 (cat. no. ab189494; 1:1,000;
Abcam), p62 (cat. no. ab109012; 1:8,000; Abcam), Beclin-1 (cat. no.
ab207612; 1:2,000; Abcam), phosphorylated (p)-PI3K (cat. no.
ab182651; 1:5,000; Abcam), p-AKT (cat. no. ab81283; 1:3,000;
Abcam), p-mTOR (cat. no. ab137133; 1:1,000; Abcam), PI3K (cat. no.
ab140307; 1:2,000; Abcam), AKT (cat. no. ab179463; 1:5,000; Abcam),
mTOR (cat. no. ab2732; 1:2,000; Abcam) and GAPDH (cat. no.
ab181602; 1:8,000; Abcam). Following washing, the membranes were
incubated with a horseradish peroxidase-conjugated goat anti-rabbit
IgG secondary antibody (cat. no. ab6721; 1:2,000; Abcam) or a
horseradish peroxidase-conjugated goat anti-mouse IgG secondary
antibody (cat. no. ab6728; 1:2,000; Abcam) at room temperature for
2.5 h. Protein bands were visualized using enhanced
chemiluminescence detection methods (Clarity; Bio-Rad Laboratories,
Inc.). Protein expression levels were semi-quantified using ImageJ
software (version 4.62; National Institutes of Health) with GAPDH
as the loading control.
Dual-luciferase reporter assay
TargetScan (version 7.2; www.targetscan.org/vert_72) was used to predict the
binding sites between SIRT1 mRNA and miR-494. A total of 354
transcripts containing 371 sites were predicted. The target gene
SIRT1 was selected due to its important role in myocardial I/R
injury (30). The wild-type (WT)
3′-UTR sequence of SIRT1 that can bind to miR-494 or the mutant
(MUT) 3′-UTR sequence was amplified and then cloned into a pGL3
vector (Promega Corporation). 293-T cells (3.5×105
cells/well; Procell Life Science & Technology Co., Ltd.) were
co-transfected with WT SIRT1 3′UTR or MUT SIRT1 3′-UTR (0.5 µg) and
miR-494 inhibitor, miR-494 mimic or the corresponding NCs (50 nM)
using Lipofectamine 2000. At 24 h post-transfection, the dual
luciferase reporter gene assay kit (Promega Corporation) was used
to measure luciferase activities according to the manufacturer's
protocol. Luciferase activity was normalized to Renilla
luciferase.
Statistical analysis
Data are presented as the mean ± SD. All experiments
were performed in triplicate. Comparisons among multiple groups
were analyzed using one-way ANOVA followed by a Tukey's post hoc
test. Statistical analyses were performed using SPSS software
(version 22.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of miR-494 on H/R-induced
cardiomyocyte injury
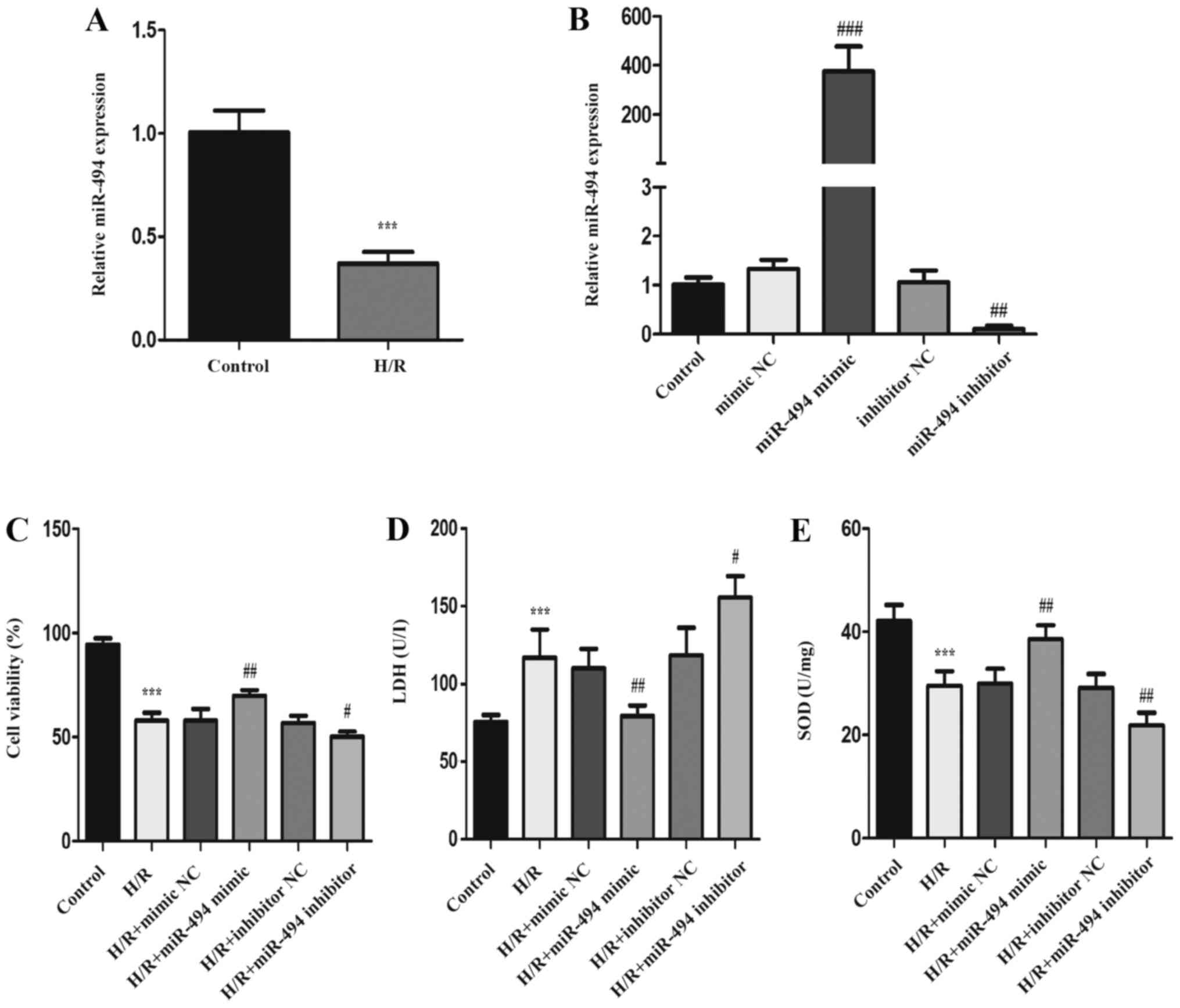
Following 12 h hypoxia and 3 h reoxygenation in H9c2
cells, the expression levels of miR-494 were measured to identify
the role of miR-494 in myocardial H/R injury. The RT-qPCR results
demonstrated that miR-494 expression levels were significantly
downregulated in H/R-treated H9c2 cells compared with control
cells. miR-494 mimic, miR-494 inhibitor and the corresponding NCs
were transfected into H9c2 cells. The RT-qPCR results demonstrated
that miR-494 expression was significantly increased in the miR-494
mimic group compared with the mimic NC group, and significantly
decreased in the miR-494 inhibitor group compared with inhibitor NC
groups (Fig. 1B). The CCK-8 assay
results indicated that, compared with the control group, the
percentage of viable cells was significantly reduced in the H/R
group. Compared with the corresponding NC groups, cell viability
was significantly increased in the miR-494 mimic group, but
significantly decreased in the miR-494 inhibitor group (Fig. 1C). Compared with the control group,
cells exposed to H/R displayed significantly elevated LDH release,
but significantly decreased SOD activity (Fig. 1D and E). In H/R-exposed cells
transfected with miR-494 mimic, LDH levels were significantly
decreased and SOD activity was significantly increased compared
with the mimic NC group. miR-494 inhibitor significantly increased
LDH release and reduced SOD activity following H/R stimulation
compared with the inhibitor NC group.
Effects of miR-494 on H/R-induced
cardiomyocyte apoptosis and autophagy
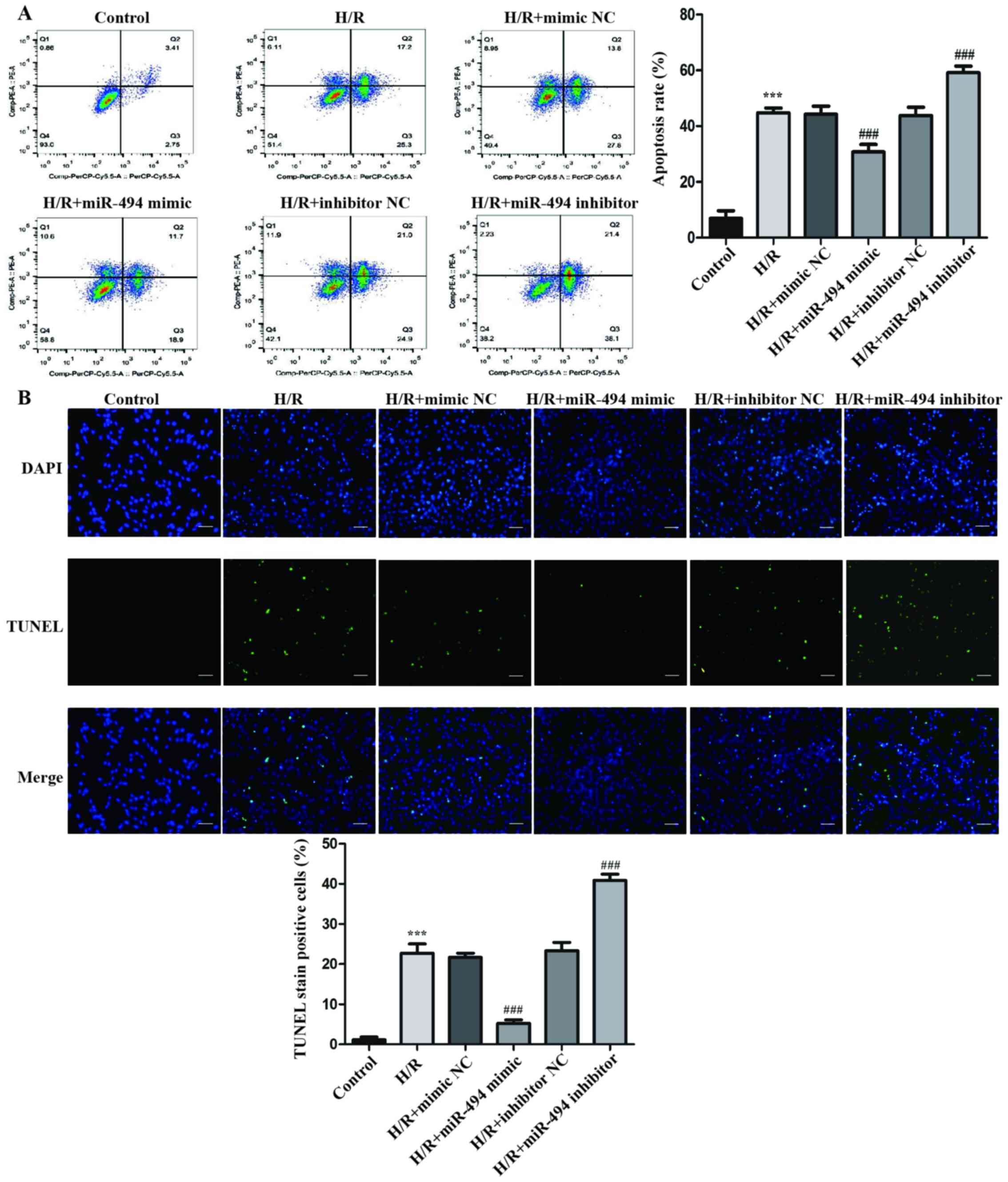
In H9c2 cells treated with H/R, the rate of
apoptosis was significantly higher compared with the control group
(Fig. 2A). Moreover, in
H/R-exposed cells, compared with the mimic NC group, the miR-494
mimic group displayed a significantly lower rate of apoptosis,
whereas the miR-494 inhibitor group displayed a significantly
higher rate of apoptosis compared with the inhibitor NC group.
Similar to the flow cytometry results, the
morphological alterations of H9c2 cells indicated by TUNEL staining
suggested that H/R significantly increased apoptosis compared with
the control group. DNA fragmentation and nuclear condensation are
unique morphological features of cell apoptosis following H/R
treatment (31). Moreover,
following H/R treatment, the miR-494 mimic group displayed a
significantly decreased number of cells with nuclear staining
compared with the mimic NC group, whereas the miR-494 inhibitor
group displayed a significantly increased number of TUNEL-positive
cells compared with the inhibitor NC group (Fig. 2B).
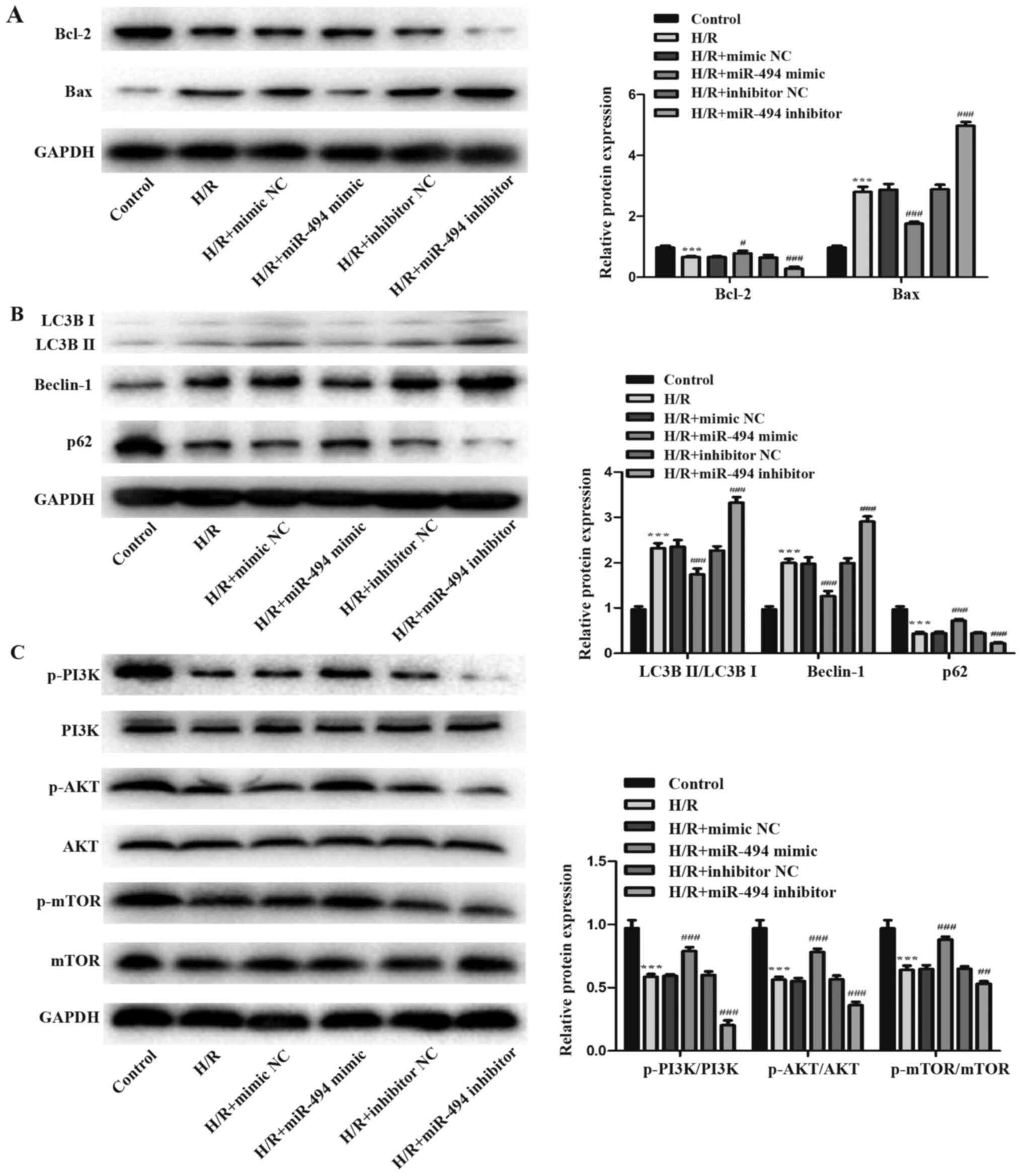
Western blotting was performed to detect the
expression levels of two apoptosis-related proteins, Bcl-2 and Bax.
The H/R group displayed significantly decreased Bcl-2 expression
levels and increased Bax expression levels compared with the
control group. H/R-mediated effects on apoptosis-related protein
expression levels were further enhanced in the miR-494 inhibitor
group, whereas miR-494 mimic partially reversed H/R-induced
alterations to apoptosis-related protein expression (Fig. 3A). Subsequently, the effects of
miR-494 on H/R-induced cell autophagy were evaluated. The
expression levels of autophagy-related proteins p62 and Beclin-1,
as well as the ratio of LC3BII/LC3BI were detected via western
blotting (Fig. 3B). The results
suggested that H/R stimulation activated autophagy, which was
demonstrated by a significant increase in Beclin-1 protein
expression levels and the ratio of LC3BII/LC3BI, and a significant
decrease in p62 protein expression levels in the H/R group compared
with the control group. However, miR-494 mimic significantly
decreased the expression levels of Beclin-1 and the ratio of
LC3BII/LC3BI, but elevated p62 protein expression levels in
H/R-treated cells compared with the mimic NC group. miR-494
inhibitor resulted in the opposite effects on cell autophagy
following H/R stimulation.
The present study further assessed the
phosphorylation of PI3K, AKT and mTOR following H/R injury combined
with miR-494 inhibitor or mimic transfection to investigate whether
miR-494 regulated the PI3K/AKT/mTOR signaling pathway in
H/R-induced apoptosis and autophagy in H9c2 cells. The western
blotting results suggested that H/R stimulation decreased PI3K, AKT
and mTOR phosphorylation, and significantly decreased the ratios of
p-PI3K/PI3K, p-AKT/AKT and p-mTOR/mTOR compared with the control
group. However, in H/R-treated cells, compared with the mimic NC
group, miR-494 mimic markedly enhanced the expression levels of
p-PI3K, p-AKT and p-mTOR, whereas miR-494 inhibitor markedly
decreased the expression levels of p-PI3K, p-AKT and p-mTOR levels
compared with the inhibitor NC group (Fig. 3C).
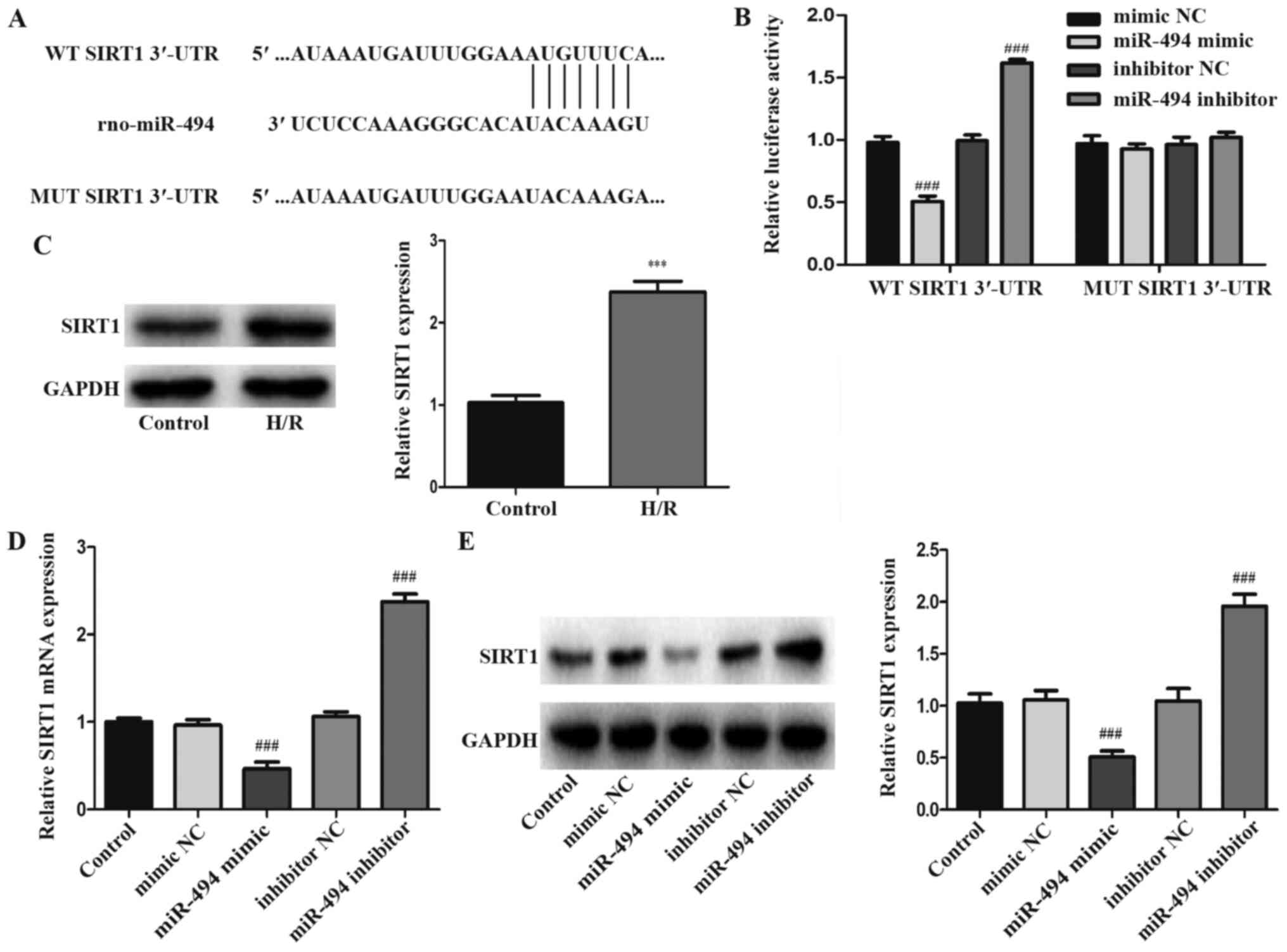
SIRT1 is a target gene of miR-494
TargetScan was used to predict the potential target
genes of miR-494. The prediction results demonstrated that the
3′-UTR of SIRT1 had a 7-nucleotide seed sequence complementary to
miR-494 (Fig. 4A). Compared with
mimic NC, miR-494 mimic significantly reduced the luciferase
activity of WT SIRT1 3′-UTR in 293-T cells. However, the luciferase
activity of MUT SIRT1 3′-UTR was not significantly altered by
miR-494 mimic compared with mimic NC. Following transfection with
miR-494 inhibitor, the luciferase activity of WT SIRT1 3′-UTR was
significantly increased compared with the inhibitor NC group.
Similarly to miR-494 mimic, miR-494 inhibitor did not significantly
alter the luciferase activity of MUT SIRT1 3′-UTR compared with
inhibitor NC (Fig. 4B).
Subsequently, the western blotting results demonstrated that SIRT1
expression in H/R-induced H9c2 cells was significantly higher
compared with control cells (Fig.
4C). To further confirm the interaction between miR-494 and
SIRT1, RT-qPCR and western blotting were performed to detect the
mRNA and protein expression levels of SIRT1, respectively. miR-494
mimic significantly decreased SIRT1 mRNA and protein expression
levels compared with the mimic NC group, whereas miR-494 inhibitor
displayed the opposite effect compared with the inhibitor NC group
(Fig. 4D and E).
SIRT1 regulates H/R-induced injury in
H9c2 cells
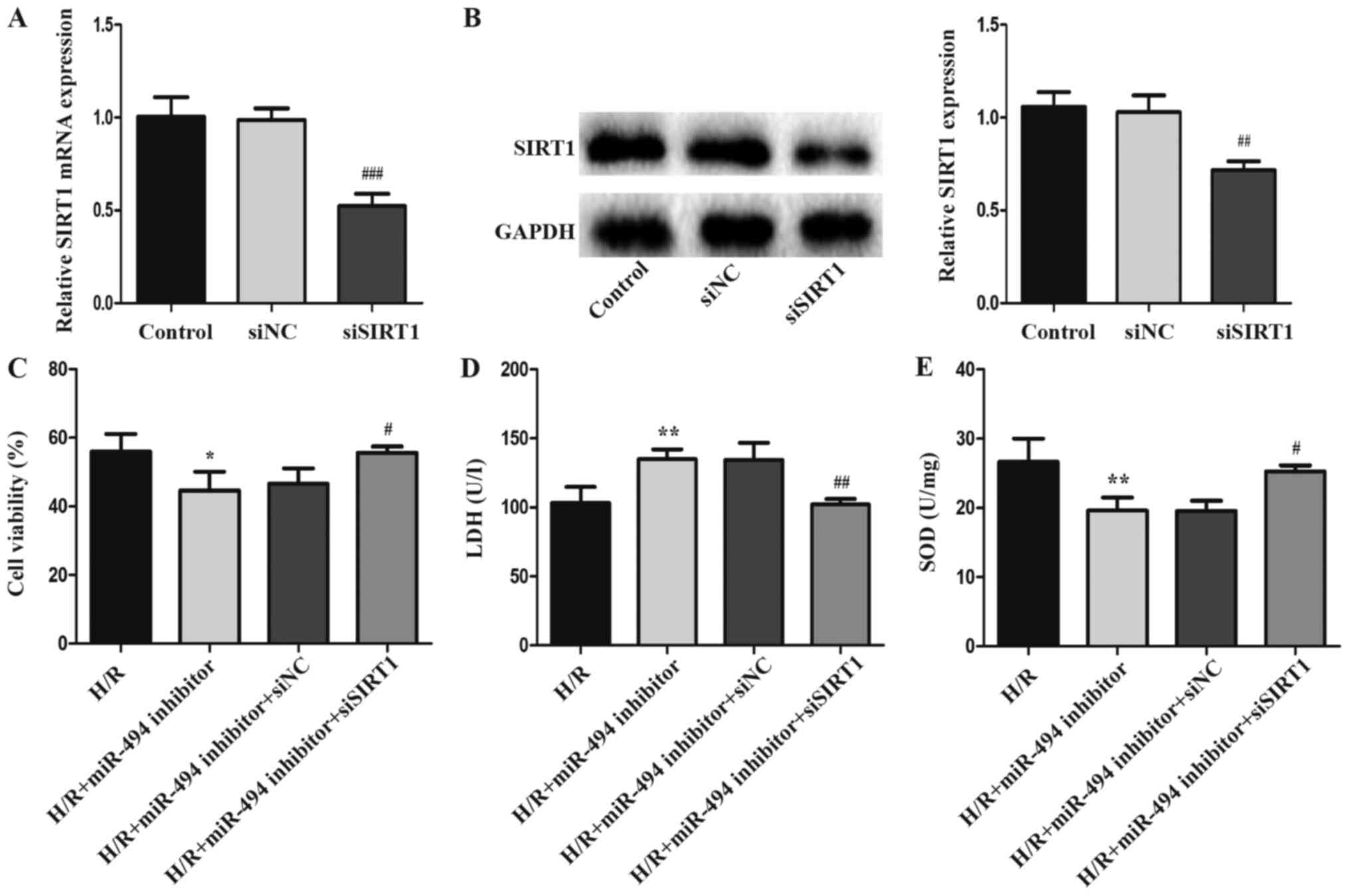
siSIRT1 was used to knock down SIRT1 expression in
H9c2 cells to investigate the involvement of SIRT1 in the
pathogenesis of H/R-induced injury (Fig. 5A and B). Compared with the siNC
group, the mRNA and protein expression levels of SIRT1 were
significantly downregulated following siSIRT1 transfection. LDH
release, cell viability and SOD activity levels were detected in
H/R-treated H9c2 cells following co-transfection with siSIRT1 and
miR-494 inhibitor. siSIRT1 significantly reduced H/R injury, as
demonstrated by inhibition of LDH activity, and increased SOD
activity and cell viability in the co-transfection group compared
with the miR-494 inhibitor group (Fig.
5C-E).
miR-494 regulates H/R-induced
cardiomyocyte apoptosis and autophagy via modulating SIRT1
expression
The effects of SIRT1 on apoptosis and autophagy were
further examined to investigate whether miR-494 served a role in
H/R-induced cardiomyocyte injury via regulating SIRT1.
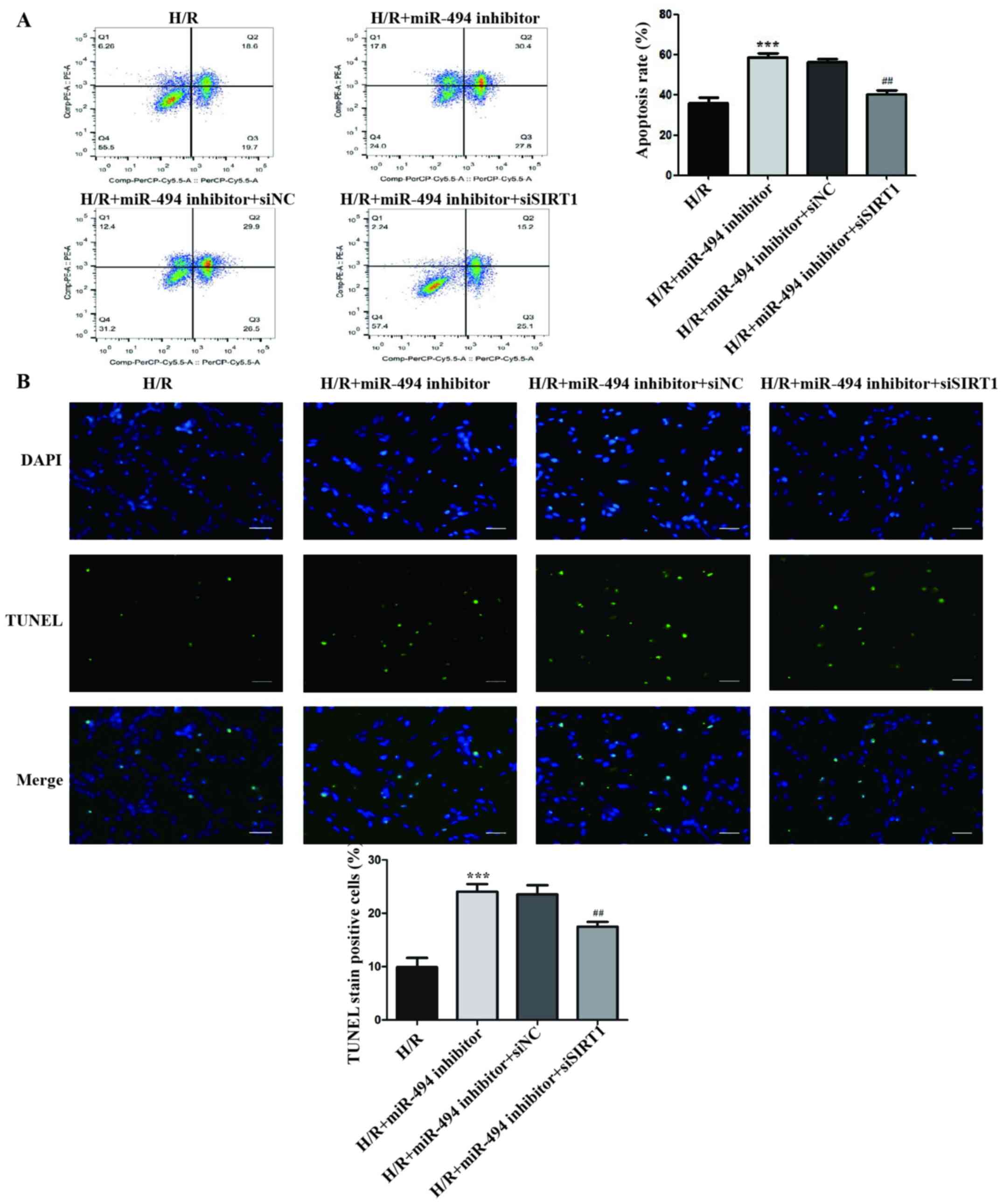
The flow cytometry assay results demonstrated that,
compared with the miR-494 inhibitor and siNC co-transfection group,
the apoptosis rate of H/R-treated cells was significantly decreased
in the miR-494 inhibitor and siSIRT1 co-transfection group
(Fig. 6A).
The results of the TUNEL staining assay indicated
that the ratio of TUNEL-positive cells was significantly increased
in H/R-treated cells in the miR-494 inhibitor group compared with
the H/R group, an effect that was partially reversed by
co-transfection with siSIRT1 transfection (Fig. 6B).
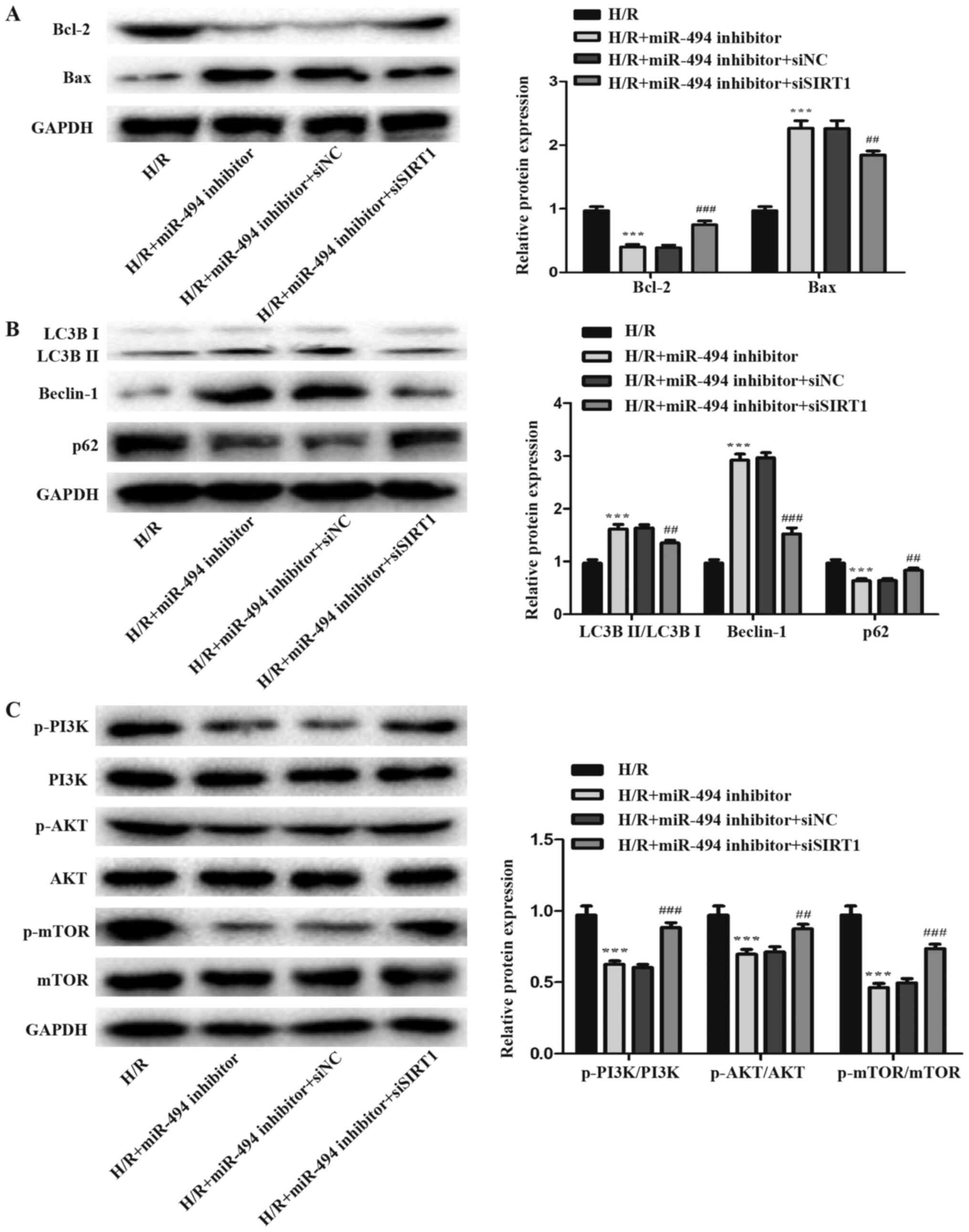
The levels of apoptosis- and autophagy-related
proteins were detected via western blotting. The results suggested
that siSIRT1 co-transfection attenuated H/R-induced H9c2 cell
apoptosis and autophagy compared with the miR-494 inhibitor and
siNC co-transfection group. Compared with miR-494 inhibitor
transfection alone, co-transfection with siSIRT1 significantly
reduced cell apoptosis in H/R-treated cells, as evidence by
decreased Bax expression levels and increased Bcl-2 expression
levels (Fig. 7A). Concurrently, in
H/R-treated cells, siSIRT1 co-transfection significantly decreased
Beclin-1 expression levels and the ratio of LC3BII/LC3BI, but
increased p62 expression levels compared with the miR-494 inhibitor
group (Fig. 7B).
miR-494 mediates effects on
H/R-induced cardiomyocyte apoptosis and autophagy via PI3K/AKT/mTOR
signaling
PI3K/AKT/mTOR is a crucial signaling pathway that
serves a key role in cardiac protection (32). The expression levels of p-PI3K,
p-AKT and p-mTOR were detected to further assess whether miR-494
regulated the activation of the PI3K/AKT/mTOR signaling pathway by
directly targeting SIRT1. miR-494 inhibitor significantly decreased
the expression levels of p-PI3K, p-AKT and p-mTOR in H/R-treated
cells. Moreover, the expression levels of p-PI3K, p-AKT and p-mTOR
were significantly increased in H/R-treated cells following
co-transfection with siSIRT1 compared with miR-494 inhibitor
transfection alone (Fig. 7C).
Discussion
Myocardial I/R injury has been associated with
cardiomyocyte death and remodeling, which can lead to adverse
cardiovascular outcomes, such as heart failure and death (33). During the pathological process of
myocardial I/R injury, autophagy and apoptosis can interact and
participate in the process (34).
The present study suggested that miR-494 attenuated cell injury
following H/R treatment by inhibiting apoptosis and autophagy in
H9c2 cells. Moreover, miR-494 regulated activation of the
PI3K/AKT/mTOR signaling pathway by directly targeting SIRT1.
Cardiomyocyte apoptosis exerts key effects on
myocardial I/R injury (35). In
addition, an increasing number of studies have suggested that low
level autophagy activation serves a protective role in I/R injury
by providing free amino acids and nucleotides, and clearing damaged
organelles (36,37). However, excessive autophagy
contributes to over self-digestion and degradation of numerous
cellular components, which further aggravates myocardial I/R injury
(38). The present study indicated
that apoptosis and autophagy were significantly increased in
H/R-treated H9c2 cells compared with control cells, which further
suggested that H/R treatment could aggravate myocardial I/R injury.
Moreover, the present study indicated that apoptosis and autophagy
exerted negative effects on H9c2 cells during H/R injury. A number
of miRs, such as miR-1, miR-195 and miR-320, could regulate
numerous signaling molecules to reduce the risk of myocardial
apoptosis and autophagy (39–41).
In the present study, significantly decreased expression levels of
miR-494 were detected in H/R-treated H9c2 cells compared with
control cells, which was similar to a previous study that reported
that miR-494 was associated with I/R-induced cardiac injury
(20). In addition, miR-494
overexpression decreased LDH release and apoptosis rates, but
increased SOD activity levels and cell viability in H/R-treated
H9c2 cells compared with mimic NC. Furthermore, miR-494
overexpression decreased the expression levels of Beclin-1 and the
ratio of LC3BII/LC3BI, but increased the ratio of Bcl-2/Bax and the
expression levels of p62 in H/R-treated H9c2 cells compared with
inhibitor NC. By contrast, miR-494 knockdown displayed the opposite
effects on H/R-treated H9c2 cells. Therefore, the results suggested
that miR-494 overexpression reduced H/R-induced cell apoptosis and
autophagy, indicating that miR-494 may serve a protective role
against myocardial I/R injury.
miRs function by degrading or inhibiting the
translation of target mRNAs (42).
The present study predicted and identified the target gene of
miR-494. Previous studies reported that miR-494 could target and
negatively regulate SIRT1 expression (43,44).
As an essential member of the seven categories of the sirtuin
protein family, SIRT1 is considered as a critical regulator of cell
apoptosis and autophagy (45).
Recently, numerous studies reported that SIRT1 was involved in
myocardial I/R injury (46,47).
Ding et al (48)
demonstrated that miR-29a inhibition could activate SIRT1, thus
preventing I/R injury by inhibiting oxidative stress. Hsu et
al (49) reported that SIRT1
attenuated oxidative stress and inhibited apoptosis by upregulating
cardioprotective molecules and downregulating proapoptotic
molecules during I/R. The present study indicated that SIRT1 was a
target gene of miR-494 using online tools and performing luciferase
reporter assays. The results demonstrated that miR-494
overexpression decreased SIRT1 expression levels compared with
mimic NC. Following co-transfection with siSIRT1 and miR-494
inhibitor, the aggravating effects of miR-494 inhibitor on
H/R-induced H9c2 cell apoptosis and autophagy were partially
reversed. Therefore, the results indicated that the effects of
miR-494 on autophagy and apoptosis were mediated via SIRT1.
As a significant intracellular signaling pathway,
the PI3K/AKT/mTOR signaling pathway is involved in the regulation
of multiple biological processes, including apoptosis, inflammation
and innate immunity (50).
Additionally, the PI3K/AKT/mTOR signaling pathway can regulate
myocardial I/R injury-induced effects (51,52).
Moreover, a previous study indicated that SIRT1 was involved in
regulating the activation of the PI3K/AKT/mTOR signaling pathway
and protecting cells against apoptosis (53). Zhang et al (54) also reported that SIRT1 was involved
in regulating cell cycle arrest via the PI3K/AKT/mTOR signaling
pathway. The present study demonstrated that, compared with
inhibitor NC, miR-494 inhibitor transfection inhibited the
PI3K/AKT/mTOR signaling pathway in H/R-treated H9c2 cells, which
was significantly reversed by siSIRT1 transfection. Collectively,
the results suggested that miR-494 could signal via the
SIRT1/PI3K/AKT/mTOR axis to inhibit H9c2 cell apoptosis and
autophagy during H/R injury. However, a rat model should be
established to verify the results of the present study.
In conclusion, the present study indicated that
miR-494 targeted SIRT1 to regulate the PI3K/AKT/mTOR signaling
pathway, which alleviated cell apoptosis and autophagy, thereby
protecting cardiomyocytes against I/R injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. U1404833) and the
Joint Construction Project of Medical Science and Technology
Research Plan of Henan Province (grant no. LHGJ20191110).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XM, YY and SN conceived and designed the study. DF,
KW, LH and JZ performed the experiments and collected the data. SN,
ZL, ZJ and QW analyzed and interpreted the data. SN performed the
experiments and drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
I/R
|
ischemia/reperfusion
|
|
miR-494
|
microRNA-494
|
|
H/R
|
hypoxia/reoxygenation
|
|
SIRT1
|
silent information regulator 1
|
|
IHD
|
ischemic heart disease
|
|
miRs
|
microRNAs
|
|
siSIRT1
|
small interfering RNA targeting
SIRT1
|
|
NC
|
negative control
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
CCK-8
|
Cell Counting Kit-8
|
|
LDH
|
lactic dehydrogenase
|
|
SOD
|
superoxide dismutase
|
|
WT
|
wild-type
|
|
MUT
|
mutant
|
References
|
1
|
Gupta R and Wood DA: Primary prevention of
ischaemic heart disease: Populations, individuals, and health
professionals. Lancet. 394:685–696. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nelson T, Garg P, Clayton RH and Lee J:
The role of cardiac MRI in the management of ventricular
arrhythmias in ischaemic and non-ischaemic dilated cardiomyopathy.
Arrhythm Electrophysiol Rev. 8:191–201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rader DJ: Lysosomal acid lipase deficiency
- A new therapy for a genetic lipid disease. N Engl J Med.
373:1071–1073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaczanowski S: Apoptosis: Its origin,
history, maintenance and the medical implications for cancer and
aging. Phys Biol. 13:0310012016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khalil H, Abd ElHady A, Elawdan KA,
Mohamed D, Mohamed DD, Abd El Maksoud AI, El-Chennawi FA, El-Fikiy
B and El-Sayed IH: The mechanical autophagy as a part of cellular
immunity; facts and features in treating the medical disorders.
Immunol Invest. Sep 29–2020.(Epub ahead of print). doi:
10.1080/08820139.2020.1828453. View Article : Google Scholar
|
|
7
|
Dong Y, Chen H, Gao J, Liu Y, Li J and
Wang J: Molecular machinery and interplay of apoptosis and
autophagy in coronary heart disease. J Mol Cell Cardiol. 136:27–41.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang A, Zhang H, Liang Z, Xu K, Qiu W,
Tian Y, Guo H, Jia J, Xing E, Chen R, et al: U0126 attenuates
ischemia/reperfusion-induced apoptosis and autophagy in myocardium
through MEK/ERK/EGR-1 pathway. Eur J Pharmacol. 788:280–285. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun MH, Chen XC, Han M, Yang YN, Gao XM,
Ma X, Huang Y, Li XM, Gai MT, Liu F, et al: Cardioprotective
effects of constitutively active MEK1 against
H2O2-induced apoptosis and autophagy in
cardiomyocytes via the ERK1/2 signaling pathway. Biochem Biophys
Res Commun. 512:125–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan H, Mischoulon D, Fava M and Otto MW:
Circulating microRNAs as biomarkers for depression: Many
candidates, few finalists. J Affect Disord. 233:68–78. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu K, Ma L, Zhou F, Yang Y, Hu HB, Wang L
and Zhong L: Identification of microRNAs related to myocardial
ischemic reperfusion injury. J Cell Physiol. 234:11380–11390. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Z, Wu S, Kong F, Cai X, Ye B, Shan P
and Huang W: MicroRNA-21 protects against cardiac
hypoxia/reoxygenation injury by inhibiting excessive autophagy in
H9c2 cells via the Akt/mTOR pathway. J Cell Mol Med. 21:467–474.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin Y and Ni S: miR-496 remedies hypoxia
reoxygenation-induced H9c2 cardiomyocyte apoptosis via
Hook3-targeted PI3k/Akt/mTOR signaling pathway activation. J Cell
Biochem. 121:698–712. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang ZQ, Xu W, Wu JL, Lu X and Chen XM:
MicroRNA-374a protects against myocardial ischemia-reperfusion
injury in mice by targeting the MAPK6 pathway. Life Sci.
232:1166192019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan H, Qi J, Fan BY, Zhang J, Su FF and
Wang HT: MicroRNA-24-3p Attenuates myocardial ischemia/reperfusion
injury by suppressing RIPK1 expression in mice. Cell Physiol
Biochem. 51:46–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu A, Lou L, Zhai J, Zhang D, Chai L, Nie
B, Zhu H, Gao Y, Shang H and Zhao M: miRNA expression profile and
effect of wenxin granule in rats with ligation-induced myocardial
infarction. Int J Genomics. 2017:21758712017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhai F, Zhang X, Guan Y, Yang X, Li Y,
Song G and Guan L: Expression profiles of microRNAs after focal
cerebral ischemia/reperfusion injury in rats. Neural Regen Res.
7:917–923. 2012.PubMed/NCBI
|
|
19
|
Sun G, Zhou Y, Li H, Guo Y, Shan J, Xia M,
Li Y, Li S, Long D and Feng L: Over-expression of microRNA-494
up-regulates hypoxia-inducible factor-1 alpha expression via
PI3K/Akt pathway and protects against hypoxia-induced apoptosis. J
Biomed Sci. 20:1002013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Zhang X, Ren XP, Chen J, Liu H,
Yang J, Medvedovic M, Hu Z and Fan GC: MicroRNA-494 targeting both
proapoptotic and antiapoptotic proteins protects against
ischemia/reperfusion-induced cardiac injury. Circulation.
122:1308–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng X, Tan J, Li M, Song S, Miao Y and
Zhang Q: Sirt1: Role under the condition of ischemia/hypoxia. Cell
Mol Neurobiol. 37:17–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan Y, Cruzat VF, Newsholme P, Cheng J,
Chen Y and Lu Y: Regulation of SIRT1 in aging: Roles in
mitochondrial function and biogenesis. Mech Ageing Dev. 155:10–21.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y
and Chen Z: Sirt1 inhibits oxidative stress in vascular endothelial
cells. Oxid Med Cell Longev. 2017:75439732017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han Y, Luo H, Wang H, Cai J and Zhang Y:
SIRT1 induces resistance to apoptosis in human granulosa cells by
activating the ERK pathway and inhibiting NF-kappaB signaling with
anti-inflammatory functions. Apoptosis. 22:1260–1272. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Wang P, Yang X, Wang W, Zhang J, He
Y, Zhang W, Jing T, Wang B and Lin R: SIRT1 inhibits inflammatory
response partly through regulation of NLRP3 inflammasome in
vascular endothelial cells. Mol Immunol. 77:148–156. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Li X, Zhu S, Zhang JG, Yang M, Qin
Q, Deng SC, Wang B, Tian K, Liu L, et al: Ectopic expression of
miR-494 inhibited the proliferation, invasion and chemoresistance
of pancreatic cancer by regulating SIRT1 and c-Myc. Gene Ther.
22:729–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, He C, Wang X, Wang H, Nan G and Fang
L: MicroRNA-183 affects the development of gastric cancer by
regulating autophagy via MALAT1-miR-183-SIRT1 axis and
PI3K/AKT/mTOR signals. Artif Cells Nanomed Biotechnol.
47:3163–3171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alves-Fernandes DK and Jasiulionis MG: The
role of SIRT1 on DNA damage response and epigenetic alterations in
cancer. Int J Mol Sci. 20:31532019. View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mao GX, Xu XG, Wang SY, Li HF, Zhang J,
Zhang ZS, Su HL, Chen SS, Xing WM, Wang YZ, et al: Salidroside
delays cellular senescence by stimulating mitochondrial biogenesis
partly through a miR-22/SIRT-1 pathway. Oxid Med Cell Longev.
2019:52760962019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeyad A, Hamad M, Amor H and Hammadeh ME:
Relationships between bacteriospermia, DNA integrity, nuclear
protamine alteration, sperm quality and ICSI outcome. Reprod Biol.
18:115–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G,
Liu J and Zhang J: Targeting PI3K/AKT/mTOR-mediated autophagy for
tumor therapy. Appl Microbiol Biotechnol. 104:575–587. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Diez ER, Altamirano LB, García IM, Mazzei
L, Prado NJ, Fornes MW, Carrión FD, Zumino AZ, Ferder L and Manucha
W: Heart remodeling and ischemia-reperfusion arrhythmias linked to
myocardial vitamin d receptors deficiency in obstructive
nephropathy are reversed by paricalcitol. J Cardiovasc Pharmacol
Ther. 20:211–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao L, Chen H, Wu Q and Xie K:
Hydrogen-rich saline alleviates inflammation and apoptosis in
myocardial I/R injury via PINK-mediated autophagy. Int J Mol Med.
44:1048–1062. 2019.PubMed/NCBI
|
|
35
|
Majtnerová P and Roušar T: An overview of
apoptosis assays detecting DNA fragmentation. Mol Biol Rep.
45:1469–1478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kasprowska-Liśkiewicz D: The cell on the
edge of life and death: Crosstalk between autophagy and apoptosis.
Postepy Hig Med Dosw. 71:825–841. 2017. View Article : Google Scholar
|
|
38
|
Doherty J and Baehrecke EH: Life, death
and autophagy. Nat Cell Biol. 20:1110–1117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhai C, Tang G, Peng L, Hu H, Qian G, Wang
S, Yao J, Zhang X, Fang Y, Yang S, et al: Inhibition of microRNA-1
attenuates hypoxia/re-oxygenation-induced apoptosis of
cardiomyocytes by directly targeting Bcl-2 but not GADD45Beta. Am J
Transl Res. 7:1952–1962. 2015.PubMed/NCBI
|
|
40
|
Gao CK, Liu H, Cui CJ, Liang ZG, Yao H and
Tian Y: Roles of MicroRNA-195 in cardiomyocyte apoptosis induced by
myocardial ischemia-reperfusion injury. J Genet. 95:99–108. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tian ZQ, Jiang H and Lu ZB: MiR-320
regulates cardiomyocyte apoptosis induced by ischemia-reperfusion
injury by targeting AKIP1. Cell Mol Biol Lett. 23:412018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan ZX and Yang J: The role of microRNAs
in regulating myocardial ischemia reperfusion injury. Saudi Med J.
36:787–793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu X, Zhang S, Zhao D, Zhang X, Xia C,
Wang T, Zhang M, Liu T, Huang W and Wu B: SIRT1 inhibits apoptosis
in in vivo and in vitro models of spinal cord injury via
microRNA-494. Int J Mol Med. 43:1758–1768. 2019.PubMed/NCBI
|
|
44
|
Tang Q, Len Q, Liu Z and Wang W:
Overexpression of miR-22 attenuates oxidative stress injury in
diabetic cardiomyopathy via Sirt 1. Cardiovasc Ther. Dec
29–2017.(Epub ahead of print). doi: 10.1111/1755-5922.12318.
|
|
45
|
Luo G, Jian Z, Zhu Y, Zhu Y, Chen B, Ma R,
Tang F and Xiao Y: Sirt1 promotes autophagy and inhibits apoptosis
to protect cardiomyocytes from hypoxic stress. Int J Mol Med.
43:2033–2043. 2019.PubMed/NCBI
|
|
46
|
Potenza MA, Sgarra L, Nacci C, Leo V, De
Salvia MA and Montagnani M: Activation of AMPK/SIRT1 axis is
required for adiponectin-mediated preconditioning on myocardial
ischemia-reperfusion (I/R) injury in rats. PLoS One.
14:e02106542019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang G, Hao F and Hu X: Downregulation of
microRNA-155 stimulates sevoflurane-mediated cardioprotection
against myocardial ischemia/reperfusion injury by binding to SIRT1
in mice. J Cell Biochem. 120:15494–15505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ding S, Liu D, Wang L, Wang G and Zhu Y:
Inhibiting MicroRNA-29a protects myocardial ischemia-reperfusion
injury by targeting SIRT1 and suppressing oxidative stress and
NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther.
372:128–135. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hsu CP, Zhai P, Yamamoto T, Maejima Y,
Matsushima S, Hariharan N, Shao D, Takagi H, Oka S and Sadoshima J:
Silent information regulator 1 protects the heart from
ischemia/reperfusion. Circulation. 122:2170–2182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
McKenna M, McGarrigle S and Pidgeon GP:
The next generation of PI3K-Akt-mTOR pathway inhibitors in breast
cancer cohorts. Biochim Biophys Acta Rev Cancer. 1870:185–197.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chi Y, Ma Q, Ding XQ, Qin X, Wang C and
Zhang J: Research on protective mechanism of ibuprofen in
myocardial ischemia-reperfusion injury in rats through the
PI3K/Akt/mTOR signaling pathway. Eur Rev Med Pharmacol Sci.
23:4465–4473. 2019.PubMed/NCBI
|
|
52
|
Li X, Hu X, Wang J, Xu W, Yi C, Ma R and
Jiang H: Inhibition of autophagy via activation of PI3K/Akt/mTOR
pathway contributes to the protection of hesperidin against
myocardial ischemia/reperfusion injury. Int J Mol Med.
42:1917–1924. 2018.PubMed/NCBI
|
|
53
|
Wang H, Liu H, Chen K, Xiao J, He K, Zhang
J and Xiang G: SIRT1 promotes tumorigenesis of hepatocellular
carcinoma through PI3K/PTEN/AKT signaling. Oncol Rep. 28:311–318.
2012.PubMed/NCBI
|
|
54
|
Zhang W, Zhang Y, Wang Z, Xu T, Huang C,
Yin W, Wang J, Xiong W, Lu W, Zheng H, et al:
Tris(2-chloroethyl)phosphate-induced cell growth arrest via
attenuation of SIRT1-independent PI3K/Akt/mTOR pathway. J Appl
Toxicol. 36:914–924. 2016. View Article : Google Scholar : PubMed/NCBI
|