Introduction
Inflammation, which is one of the physical barriers
of innate immunity, is the protective response by which the body
eliminates harmful stimuli, such as damaged cells, pathogens and
irritants (1). However, the
prolonged inflammatory response of immune cells to noxious stimuli
leads to chronic inflammation, which causes various diseases,
including rheumatoid arthritis, type 2 diabetes, cancer, cirrhosis,
Alzheimer's disease and neurological diseases (2). Macrophages, which play a pivotal role
in the innate immune system, release various inflammatory cytokines
and mediators to protect the body from external harmful factors
(3). However, excessive amounts of
inflammatory cytokines, such as tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and IL-6, and inflammatory mediators, such as
nitric oxide (NO), prostaglandin E2, inducible NO
synthase (iNOS) and cyclooxygenase-2 (COX-2), have been linked to
pathophysiological events and chronic inflammatory diseases
(4). Therefore, the control of
excessive inflammatory responses is important for the prevention of
chronic inflammatory diseases, and drugs that suppress excessive
inflammatory responses are constantly being developed.
Currently used anti-inflammatory drugs, such as
non-steroidal anti-inflammatory drugs (NSAIDs), may cause serious
side effects. Therefore, numerous researchers have focused on the
development of anti-inflammatory drugs from plant sources that are
considered to be safe, effective, biocompatible and cost-effective
alternatives (2). Berries are
fruits rich in nutritive compounds, such as minerals, vitamins and
dietary fiber. They also contain polyphenolic compounds that have
strong anti-inflammatory properties (5). Honeyberry (Lonicera caerulea)
has long been used as a traditional medicine in China, Japan and
northern Russia (6). Honeyberry
fruits have been reported to have strong antioxidant effects due to
their ascorbic acid content and the presence of phenolic compounds,
including anthocyanins, flavonoids and low-molecular-weight
phenolic acids (7). In addition, a
study found that honeyberry fruits had the strongest antioxidant
property among 12 types of colored berries from northern China
(8). A number of studies have
shown the various effects of honeyberry fruits, which include
anti-inflammatory (9,10), hepatoprotective (11), lipid and glucose
metabolism-enhancing (12),
anti-hyperthyroidism (13),
anti-obesity (14) and anticancer
effects (15,16). These fruits contain a variety of
polyphenolic compounds, including protocatechuic acid, caffeic
acid, chlorogenic acid, coumaric acid and ferulic acid, which have
a close association with anti-inflammatory effects (17,18).
In addition, the anti-inflammatory activity of honeyberry cultivars
has been shown to depend on the levels of polyphenolic compounds
they contain (19). Much research
has been carried out into the pharmacological activity of
honeyberry; however, these studies have mainly focused on
honeyberry fruits, and reports of the pharmacological activities of
its leaves and branches are limited. The leaves and branches of
plants are important for the development of natural medicines due
to the presence of high levels of functional substances with
various biological activities; therefore, pharmacological studies
on the leaves and branches of honeyberry are recommended. Notably,
the leaves of chokeberry and mulberry have higher contents of
functional substances and higher levels of antioxidant activity
than the fruits (20,21). Thus, in the present study, the
anti-inflammatory activity of honeyberry leaves and branches was
evaluated, and their mechanisms of action were investigated.
Materials and methods
Chemical reagents
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), N-acetyl-L-cysteine (NAC), SB203580 (p38 inhibitor),
lipopolysaccharide (LPS) and 2′,7′-dichlorofluorescein diacetate
(DCFH-DA) were purchased from Sigma-Aldrich (Merck KGaA). The
control, HO-1 (cat. no. sc-35555) and ATF3 (cat. no. sc-29758)
small interfering RNAs (siRNAs) were purchased from Santa Cruz
Biotechnology, Inc.
Sample preparation
Honeyberry (FMCLC-20190506-01) was provided by
Forest Medicinal Resources Research Center, Korea. The leaves,
branches or fruits of honeyberry were extracted according to a
previously described method (22).
Briefly, leaves, branches or fruits of honeyberry (20 g) were
extracted by shaking in 70% ethanol (400 ml) at room temperature
for 72 h. The ethanol-soluble fraction was then filtered,
concentrated to a volume of ~120 ml using a vacuum evaporator and
freeze-dried. The ethanol extracts were kept in a freezer (−80°C)
until use. The extracts from the leaves (HBL), branches (HBB) or
fruits (HBF) of honeyberry were dissolved in dimethyl sulfoxide
(DMSO) when used to treat cells. DMSO was used as a control and the
final DMSO concentration in the cell culture was ≤0.1% (v/v).
Determination of total phenolic
content
Total phenolic content was measured using the
Folin-Ciocalteu assay (23).
Briefly, 0.5 ml HBL, HBB or HBF (50 mg/ml) was mixed with 0.5 ml 2N
Folin-Ciocalteu reagent (cat. no. 47641; Sigma-Aldrich; Merck KGaA)
at room temperature for 5 min, followed by the addition of 2 ml 7%
sodium carbonate (w/v). The mixtures were incubated for 90 min at
room temperature. The absorbance of the mixture was then measured
at 750 nm using a UV/visible spectrophotometer (Xma-3000PC; Human
Corporation).
Determination of total flavonoid
content
Total flavonoid content was measured according to a
previously described method with some modifications (23). Briefly, 20 µl HBL, HBB or HBF (50
mg/ml), 80 µl distilled water and 6 µl 5% NaNO2 were
mixed and incubated for 5 min at room temperature. Next, 12 µl 10%
AlCl3·6H2O was added and the mixture was
incubated at room temperature. After 6 min, 40 µl 1 M NaOH was
added and the mixture was incubated for 11 min at room temperature.
Finally, the absorbance was measured at 510 nm using a UV/visible
spectrophotometer (Xma-3000PC).
Cell culture
RAW264.7 cells purchased from the American Type
Culture Collection were cultured according to a previously
described method (22). The cells
were maintained in DMEM/F-12 1:1 (Hyclone Laboratories Inc.)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C under
a humidified atmosphere of 5% CO2.
Cell viability assay
MTT assay for measuring cell viability was performed
according to the method described in a previous study (22). Briefly, the cells were plated on a
96-well plate at a density of 3×103 cells/well, followed
by treatment with HBL and HBB (0, 100 or 200 µg/ml) at 37°C for 24
h. Next, the cells were treated with 50 µl MTT solution (1 mg/ml)
at 37°C for 4 h. After 4 h, the cell culture supernatant was
removed and DMSO was added to dissolve the resulting crystals. The
formation of formazan was evaluated by measuring the absorbance at
570 nm using a UV/visible spectrophotometer (Xma-3000PC).
Measurement of NO production
The Griess assay was performed to measure NO
production, following a previously described method (22). Briefly, after plating RAW264.7
cells (1×105 cells/well) on a 12-well plate for 24 h,
the cells were pretreated with 100 and/or 200 µg/ml HBL, HBB and
HBF, and 100 µg/ml ibuprofen at 37°C for 4 h and then co-treated
with LPS (1 µg/ml) at 37°C for 20 h. Then, 100 µl cell culture
supernatant was mixed with 100 µl Griess reagent (Sigma-Aldrich;
Merck KGaA) at room temperature for 15 min, and the absorbance was
measured at 540 nm using UV/visible spectrophotometer
(Xma-3000PC).
Measurement of reactive oxygen species
(ROS)
The levels of ROS produced by HBL- and HBB-treated
RAW264.7 cells were measured with DCFH-DA staining. Briefly,
RAW264.7 cells plated on a 96-well plate (3×103
cells/well) for 24 h were treated with 200 µg/ml HBL and HBB at
37°C for 24 h. The culture medium was then removed, and the cells
were treated with DCFH-DA (10 µM) for 20 min at 37°C in the dark.
After washing the cells three times with 1X phosphate-buffered
saline (PBS) and the fluorescence intensity was measured at an
excitation wavelength of 488 nm and emission wavelength of 525 nm
using a UV/visible spectrophotometer (Xma-3000PC).
Isolation of nuclear fraction
Nuclear protein was extracted from the treated
RAW264.7 cells using a Nuclear Extract kit (Active Motif, Inc.)
according to the manufacturer's protocols. The nuclear protein was
stored at −80°C prior to further analysis.
SDS-PAGE and western blotting
Western blot analysis was performed according to a
previously described method (22).
RAW264.7 cells were pretreated with 100 and 200 µg/ml HBL and HBB
at 37°C for 4 h and then co-treated with LPS (1 µg/ml) at 37°C for
30 min or 1 h. In addition, RAW264.7 cells were pretreated with
SB203580 (20 µM; p38 inhibitor) or NAC (10 mM; ROS scavenger) at
37°C for 2 h and then co-treated with 200 µg/ml HBL and HBB at 37°C
for 20 min. After treatment, RAW264.7 cells were washed three times
with cold 1X PBS, and the protein was extracted using
radioimmunoprecipitation buffer containing 50 mM Tris
Base/Tris-HCl, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% sodium
dodecyl sulfate and 1% Nonidet P-40 substitute (Boston BioProducts)
containing protease and phosphatase inhibitor cocktails
(Sigma-Aldrich; Merck KGaA) at 4°C for 30 min. The protein
concentration was quantified using a BCA protein assay (Thermo
Fisher Scientific, Inc.). Briefly, 5 µl cell lysate was mixed with
45 µl distilled water and 1 ml BCA protein assay solution (Reagent
A:B=50:1, v/v), and the mixture was incubated at 37°C for 30 min.
The absorbance was then measured at 562 nm using a UV/visible
spectrophotometer (Xma-3000PC). The protein (30 µg/lane) was
separated with 10% SDS-PAGE and transferred to a PVDF membrane
(Bio-Rad Laboratories, Inc.). After blocking the PVDF membrane
using 5% non-fat dry milk in Tris-buffered saline containing 0.05%
Tween-20 (TBS-T) with stirring at room temperature for 1 h, the
PVDF membrane was incubated overnight at 4°C with the following
primary antibodies in 0.05% TBS-T containing 5% non-fat dry milk:
IκB-α (1:1,000; cat. no. 4814), p65 (1:1,000; cat. no. 8242),
phospho-ERK1/2 (1:1,000; cat. no. 4377), ERK1/2 (1:1,000; cat. no.
9102), phospho-p38 (1:1,000; cat. no. 4511), p38 (1:1,000; cat. no.
9212), heme oxygenase 1 (HO-1; 1:1,000; cat. no. 70081), nuclear
factor erythroid 2-related factor 2 (Nrf2; 1:1,000; cat. no.
12721), β-actin (1:1,000; cat. no. 5172) and TATA-box-binding
protein (TBP; 1:1,000; cat. no. 8515) all from Cell Signaling
Technology, Inc., and activating transcription factor-3 (ATF-3;
1:1,000; cat. no. sc-188) from Santa Cruz Biotechnology, Inc. After
treatment with the primary antibodies, the PVDF membrane was washed
three times with 0.05% TBS-T, and then incubated with the following
secondary antibodies in 0.05% TBS-T containing 5% non-fat dry milk
for 1 h at room temperature: Anti-rabbit immunoglobulin G (IgG),
horseradish peroxidase (HRP)-linked antibody (1:1,000; cat. no.
7074) and anti-mouse IgG, HRP-linked antibody (1:1,000; cat. no.
7076), both from Cell Signaling Technology, Inc. Chemiluminescence
was detected with Amersham ECL western blotting reagent (Cytiva)
and visualized using a LI-COR C-DiGit Blot Scanner (Li-COR
Biosciences). The density of the western blot bands was calculated
using the software UN-SCAN-IT gel version 5.1 (Silk Scientific,
Inc.).
Reverse transcriptase-polymerase chain
reaction (RT-q)PCR
RT-PCR was performed according to a previously
described method (22). After
treatment, total RNA was extracted from the cells using a RNeasy
Mini kit (Qiagen Sciences, Inc.), and cDNA was synthesized from
total RNA (1 µg) using a Verso cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. PCR was
performed using a PCR Master Mix (Promega Corporation) and mouse
primers for iNOS, COX-2, IL-1β, IL-6 and GAPDH as follows: iNOS:
Forward, 5′-ttgtgcatcgacctaggctggaa-3′ and reverse,
5′-gacctttcgcattagcatggaagc-3′; COX-2: Forward,
5′-gtactggctcatgctggacga-3′ and reverse,
5′-caccatacactgccaggtcagcaa-3′; IL-1β: Forward,
5′-ggcaggcagtatcactcatt-3′ and reverse, 5′-cccaaggccacaggtattt-3′;
IL-6: Forward, 5-gaggataccactcccaacagacc-3 and reverse,
5-aagtgcatcatcgttgttcataca-3; TNF-α: Forward,
5′-tggaactggcagaagaggca-3′ and reverse, 5′-tgctcctccacttggtggtt-3′;
GAPDH: Forward, 5′-ggactgtggtcatgagcccttcca-3′ and reverse,
5′-actcacggcaaattcaacggcac-3′. PCR reaction conditions were as
follows: 5 min at 94°C for denaturation, 30 cycles of 30 sec at
94°C for denaturation, 1 min at 55°C for annealing and 1 min at
72°C for elongation and final extension for 10 min at 72°C. The PCR
results were visualized using 1% agarose gel electrophoresis and
Safe Shine Green (10,000 X; Biosesang). The density of the mRNA
bands was calculated using the software UN-SCAN-IT gel version
5.1.
siRNA transfection
RAW264.7 cells were seeded in 6-well plates
(1×105 cells/well) and incubated at 37°C for 24 h. The
cells were then transfected with control, HO-1 or ATF3 siRNA at
37°C for 48 h at a concentration of 100 nM using TransIT-TKO
transfection reagent (Mirus Bio, LLC) according to the
manufacturer's instructions. After 48 h, RAW264.7 cells were
pretreated with 200 µg/ml HBL and HBB were treated at 37°C for 4 h
and then co-treated with LPS (1 µg/ml) at 37°C for 20 h. Following
treatment, mRNA expression levels of HO-1, ATF3, iNOS or IL-1β were
analyzed using RT-qPCR.
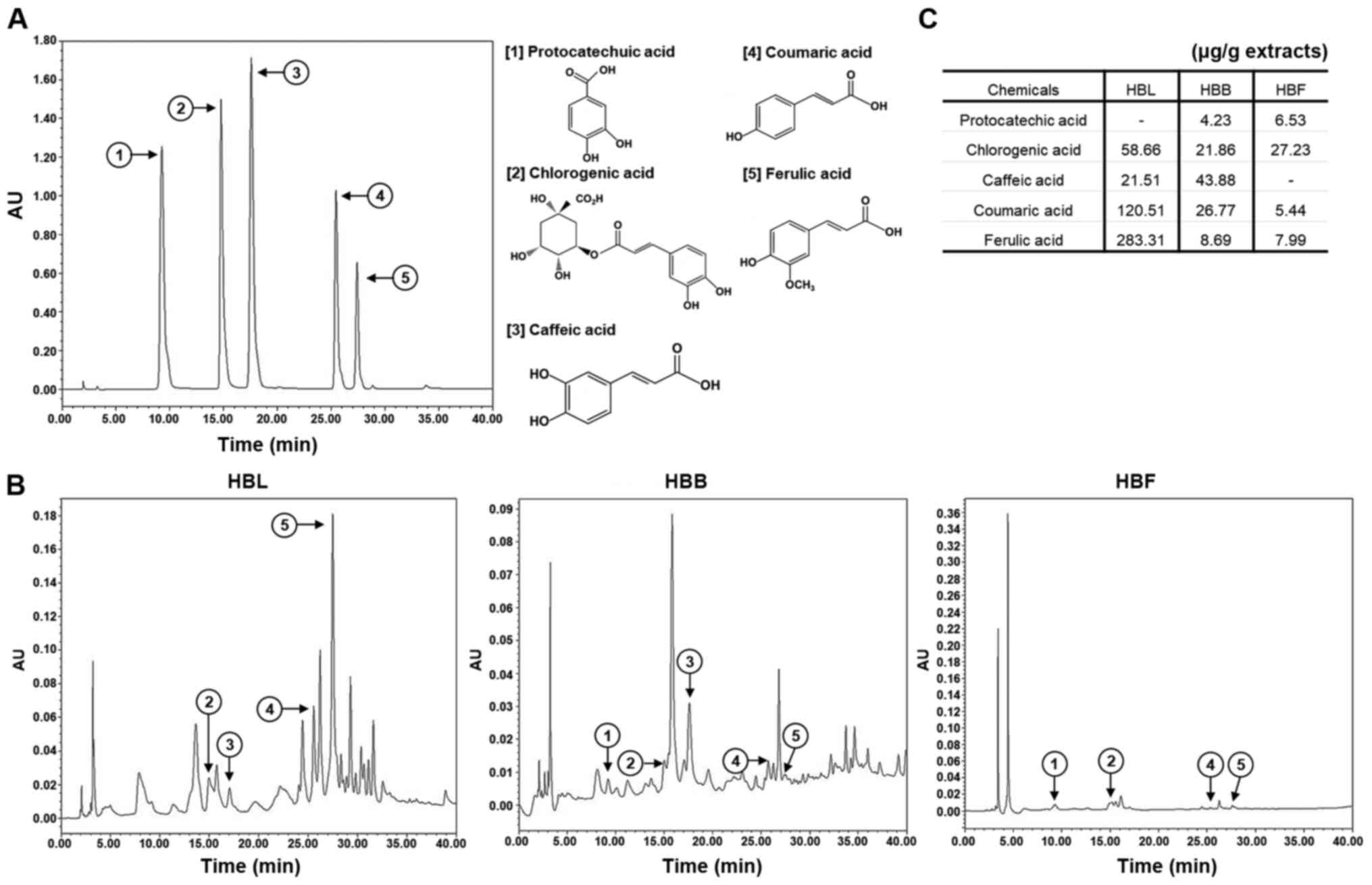
High-performance liquid chromatography
(HPLC) analysis of HBL, HBB and HBF
An analysis of the anti-inflammatory compounds in
HBL, HBB and HBF was performed using a Waters 1525 HPLC system with
Waters 2487-dual λ absorbance detector (Waters Corporation). The
column was equipped with a SunFire™ C18 column (250×4.6 mm; Waters
Corporation), and the binary mobile phase consisted of water
(solvent A) and acetonitrile containing 1% acetic acid (solvent B).
The flow rate was kept constant at 1.0 ml/min at 37°C for a total
run time of 40 min. The mobile phase was programmed consecutively
in a linear gradient as follows: 0–5 min (10% A); 5–10 min (10→15%
A); 10–15 min (15% A), and 15–40 min (15→40% A). The injection
volume of the extract was 10 µl and the elution was monitored at
254 nm. The anti-inflammatory compounds of HBL, HBB and HBF were
identified by comparison with the chromatograms of analytical
standards, including protocatechuic acid, chlorogenic acid, caffeic
acid, coumaric acid and ferulic acid.
Statistical analysis
All experiments were performed in triplicate. Data
are presented as the mean ± standard error of the mean. Data
analysis was carried out using SPSS software (version 19.0; IBM
Corp.). One-way analysis of variance followed by Tukey's honestly
significant difference test was used to compare differences among
groups. P<0.05 was considered to indicate a significant
difference.
Results
HBL and HBB suppress the
overproduction of inflammatory cytokines and mediators in
LPS-stimulated RAW264.7 cells
RAW264.7 cells were pretreated with HBL, HBB or HBF
for 4 h to ensure that the extracts were sufficiently absorbed by
the cells, and then co-treated with LPS for 20 h so that
inflammatory mediators were expressed by LPS in sufficient
quantities for detection (22).
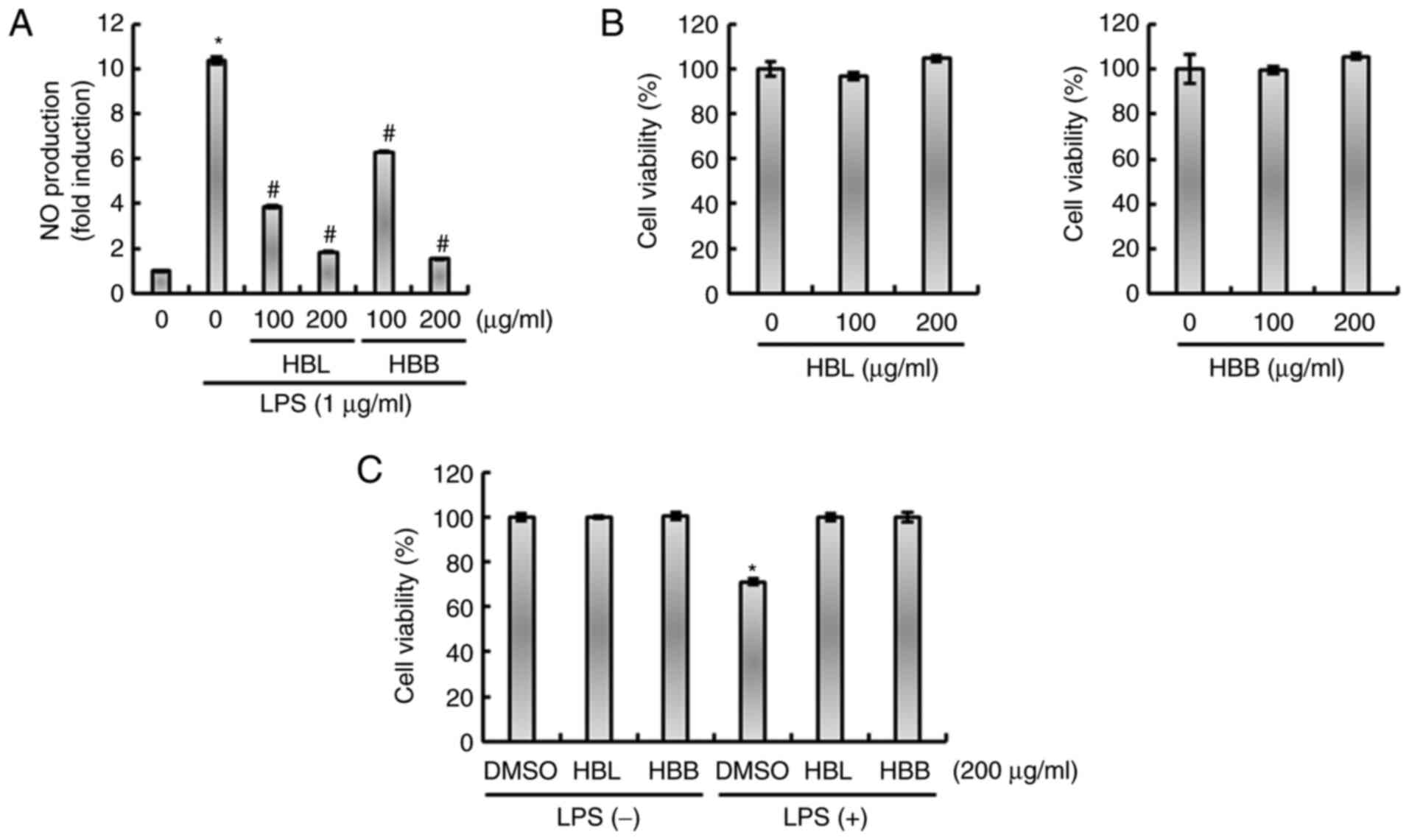
HBL and HBB significantly reduced the LPS-mediated production of NO
(Fig. 1A). The results of the MTT
assay demonstrated that HBL and HBB were not toxic to RAW264.7
cells (Fig. 1B), which indicates
that the inhibition of NO production by HBL and HBB was not due to
the cytotoxicity of the extracts. The effects of HBL and HBB on
cell viability in the presence of LPS were also evaluated. As shown
in Fig. 1C, treatment with LPS
alone decreased cell viability, while HBL and HBB attenuated the
LPS-induced reduction in cell viability. The ability of HBL and HBB
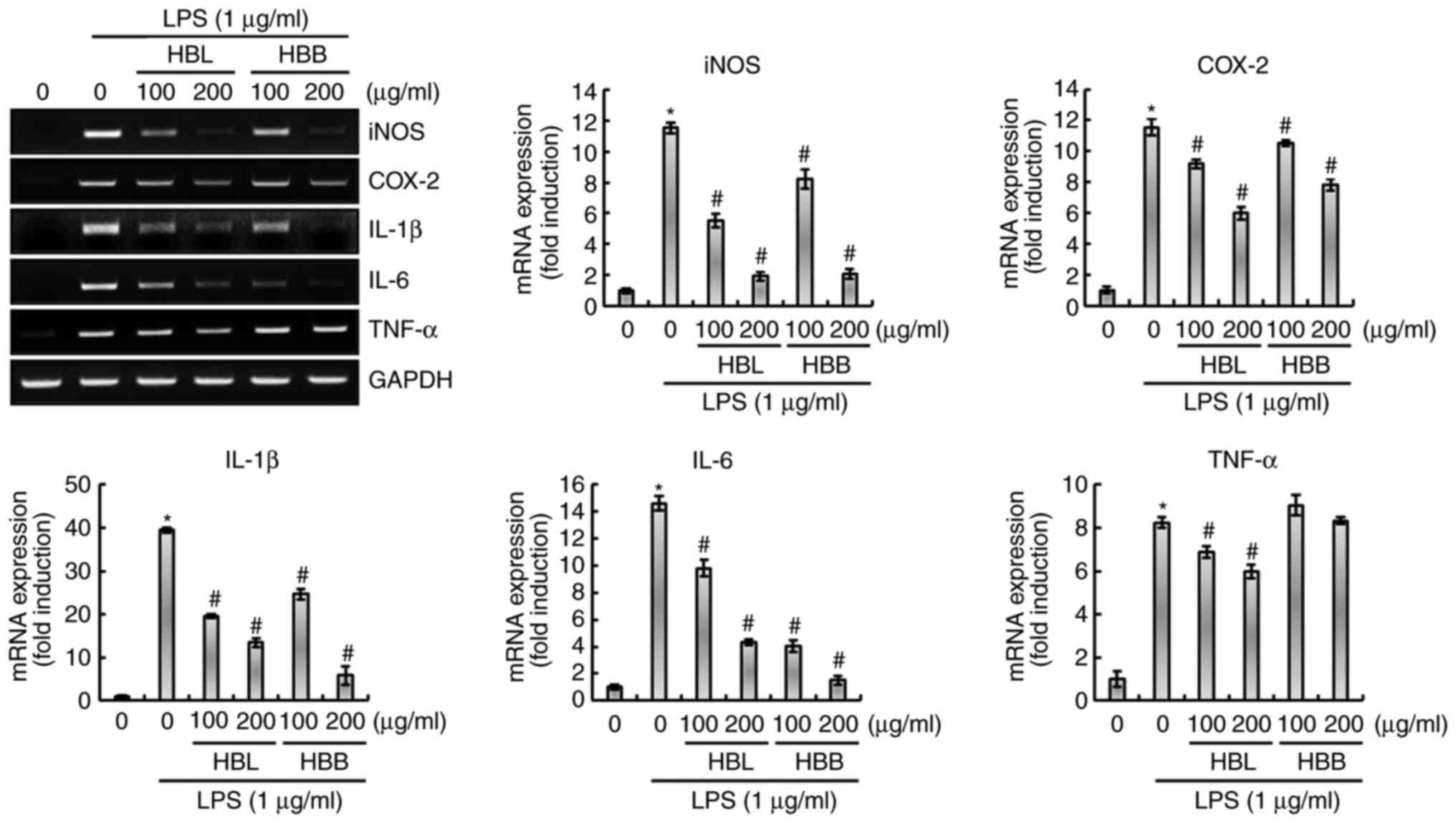
to block the LPS-induced overexpression of inflammatory mediators,
namely iNOS and COX-2, and inflammatory cytokines, namely IL-1β,
IL-6 and TNF-α, in RAW264.7 cells was also investigated. As shown
in Fig. 2, the upregulation of
iNOS, COX-2, IL-1β, IL-6 and TNF-α was observed in LPS-treated
RAW264.7 cells, which was significantly inhibited by HBL.
Similarly, HBB significantly attenuated the expression of iNOS,
COX-2, IL-1β and IL-6, but not TNF-α in the LPS-induced cells.
Previous studies have reported the anti-inflammatory activity of
HBF (9,10). Therefore, the anti-inflammatory
activities of HBL, HBB and HBF were compared in the present study.
As shown in Fig. 3A and B, the
inhibitory effect of HBL on the production of NO and expression of
iNOS and IL-1β was higher than that of HBB at a concentration of
100 µg/ml. Moreover, HBF did not significantly inhibit the
LPS-induced production of NO and expression of iNOS and IL-1β
(Fig. 3A and B). We hypothesized
that the differences in the activities of the extracts may be
associated with the levels of polyphenols and flavonoids they
contain. Therefore, the total polyphenol and flavonoid contents of
HBL, HBB and HBF were compared. As shown in Fig. 3C, the total polyphenol and
flavonoid contents were reduced in the order HBL > HBB > HBF,
which was the same as the order of their inhibitory activities
against the production of inflammatory mediators. This result
indicates that the difference in the inhibitory activities of HBL,
HBB and HBF against the production of inflammatory mediators is
likely due to differences in the total polyphenol and flavonoid
contents of the extracts. Furthermore, the anti-inflammatory
activities of HBL and HBB were compared with that of ibuprofen, an
NSAID. As shown in Fig. 3D, the
inhibitory activity of HBB against NO production was lower than
that of ibuprofen, whereas the inhibitory activity of HBL was
comparable with that of ibuprofen.
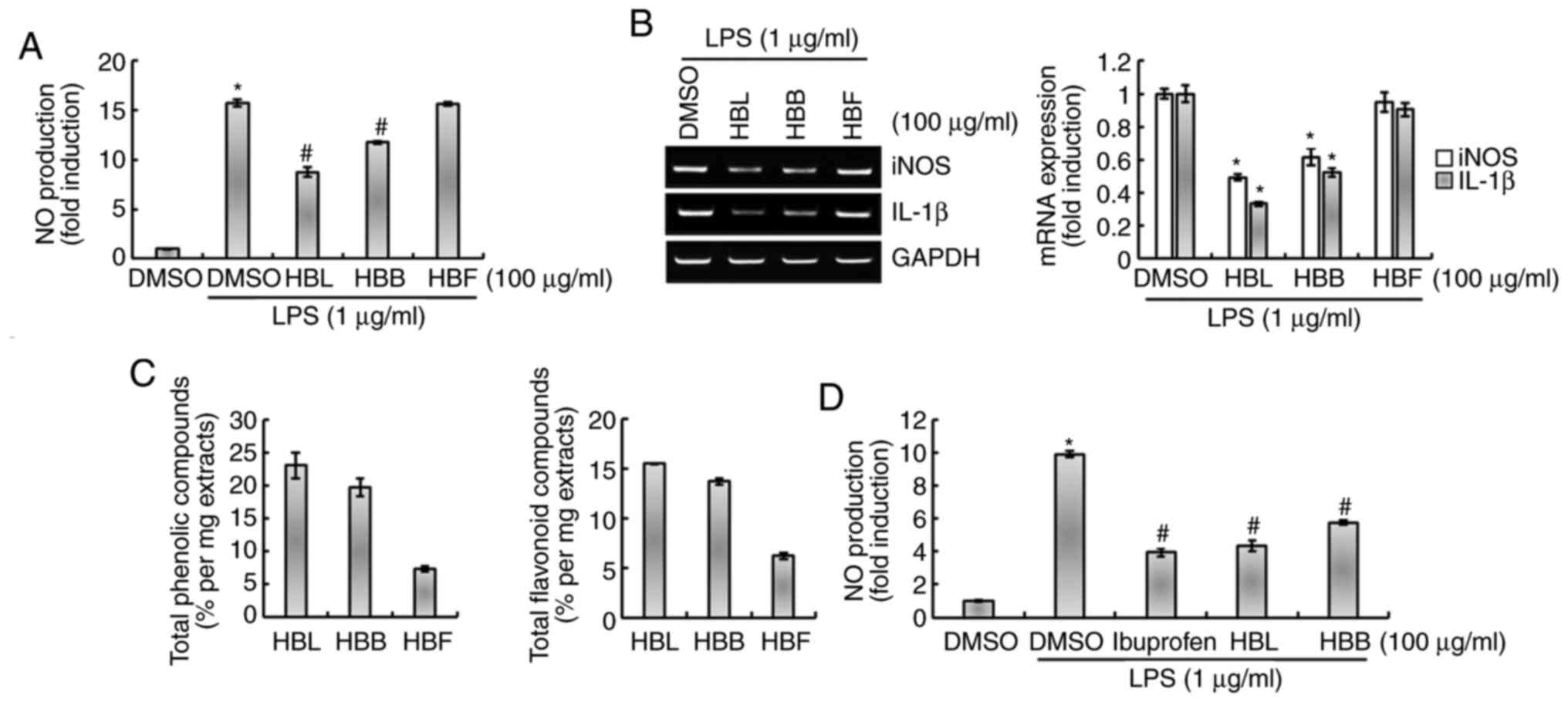 | Figure 3.Comparison of the inhibitory effects
of HBL, HBB and HBF on the LPS-induced production of NO, iNOS and
IL-1β in RAW264.7 cells. (A) RAW264.7 cells were pretreated with
100 µg/ml HBL, HBB or HBF for 4 h and then co-treated with LPS (1
µg/ml) for 20 h. The concentration of NO in the cell culture medium
was measured by Griess assay. (B) Total RNA was extracted after the
treatment. GAPDH was used as internal control for analysis using
reverse transcription-polymerase chain reaction. *P<0.05 vs.
cells treated with LPS alone. (C) Contents of total phenolic and
flavonoid compounds in HBL, HBB and HBF were analyzed. (D) RAW264.7
cells were pretreated with 100 µg/ml ibuprofen, HBL or HBB for 4 h
and then co-treated with LPS (1 µg/ml) for 20 h. The concentration
of NO in the cell culture medium was measured by Griess assay.
*P<0.05 vs. untreated cells and #P<0.05 vs. cells
treated with LPS alone. HBL, honeyberry leaf extract; HBB,
honeyberry bark extract; HBF, honeyberry flower extract; LPS,
lipopolysaccharide; NO, nitric oxide; iNOS, inducible NO synthase;
IL-1β, interleukin 1β; DMSO, dimethylsulfoxide. |
Effects of HBL and HBB on the
regulation of NF-κB and mitogen-activated protein kinase (MAPK)
signaling pathways in LPS-stimulated RAW264.7 cells
The ability of HBL and HBB to inhibit the
LPS-mediated activation of NF-κB was investigated. As shown in
Fig. 4A, HBL and HBB did not
suppress the LPS-induced degradation of IκB-α; however, HBL and HBB
significantly attenuated the LPS-induced nuclear accumulation of
NF-κB p65 (Fig. 4B). These results
indicate that HBL and HBB may inhibit the activation of NF-κB
signaling independently of IκB-α degradation. Whether HBL and HBB
inhibit the LPS-induced activation of the MAPK signaling pathway
was also investigated. As shown in Fig. 4A, LPS increased the phosphorylation
levels of p38 and ERK1/2, which was not inhibited by HBL. However,
HBB significantly attenuated the LPS-induced phosphorylation of p38
but not that of ERK1/2. These results indicate that HBL did not
inhibit the LPS-induced activation of the MAPK signaling pathway,
whereas HBB inhibited the activation of p38.
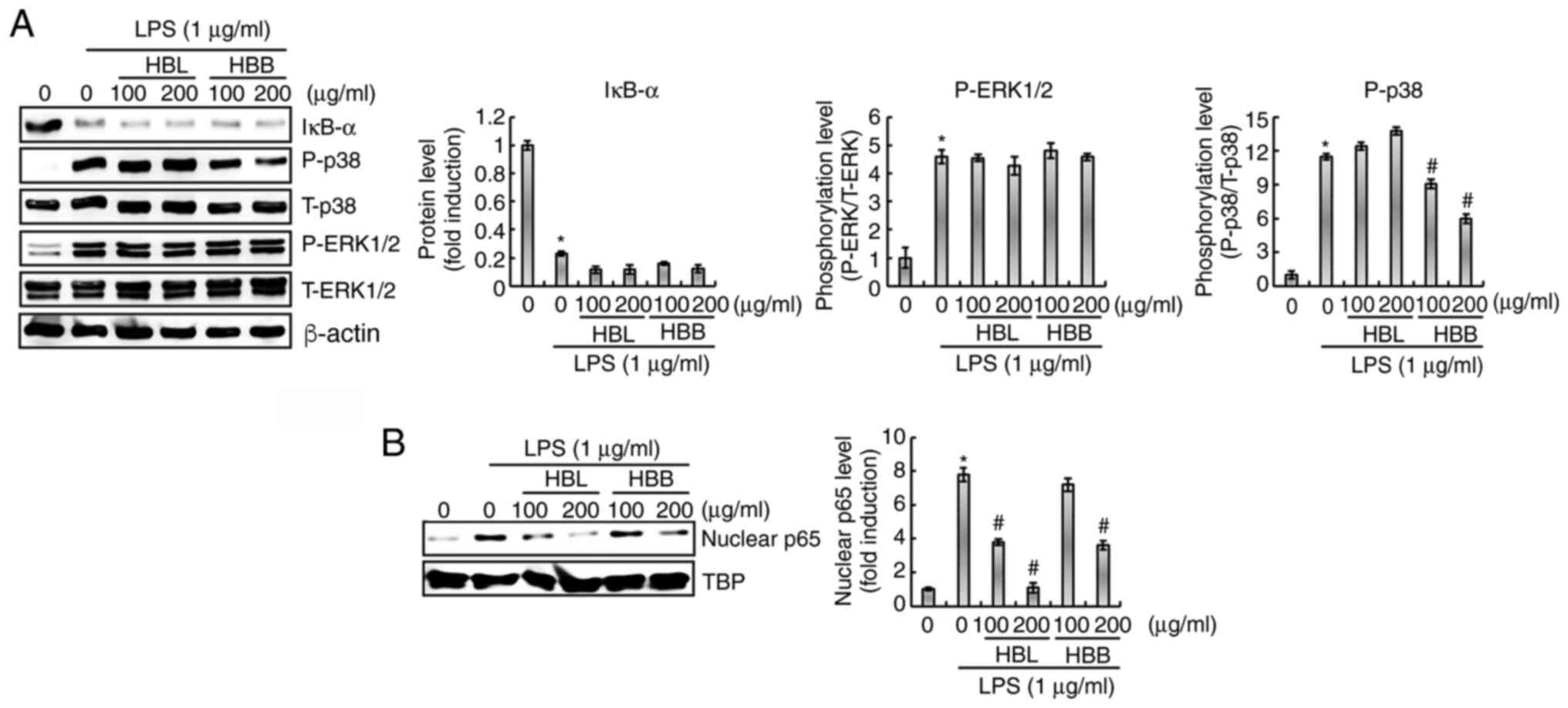 | Figure 4.Inhibitory effects of HBL and HBB on
NF-κB and MAPK activation in LPS-stimulated RAW264.7 cells. (A)
RAW264.7 cells were pretreated with 100 and 200 µg/ml HBL and HBB
for 4 h and then co-treated with LPS (1 µg/ml) for 30 min. (B)
RAW264.7 cells were pretreated with 100 and 200 µg/ml HBL and HBB
and then co-treated with LPS (1 µg/ml) for 1 h. After the
treatment, nuclear protein was prepared. The cell lysates were
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and western blotting was performed using antibodies
against IκB-α, P-p38, p38, P-ERK1/2, ERK1/2 and p65. Actin or TBP
was used as the internal control for western blot analysis.
*P<0.05 vs. untreated cells and #P<0.05 vs. cells
treated with LPS alone. HBL, honeyberry leaf extract; HBB,
honeyberry bark extract; NF-κB, nuclear factor-κB; MAPK,
mitogen-activated protein kinase; LPS, lipopolysaccharide; P,
phosphorylated; T, total; TBP, TATA-box-binding protein. |
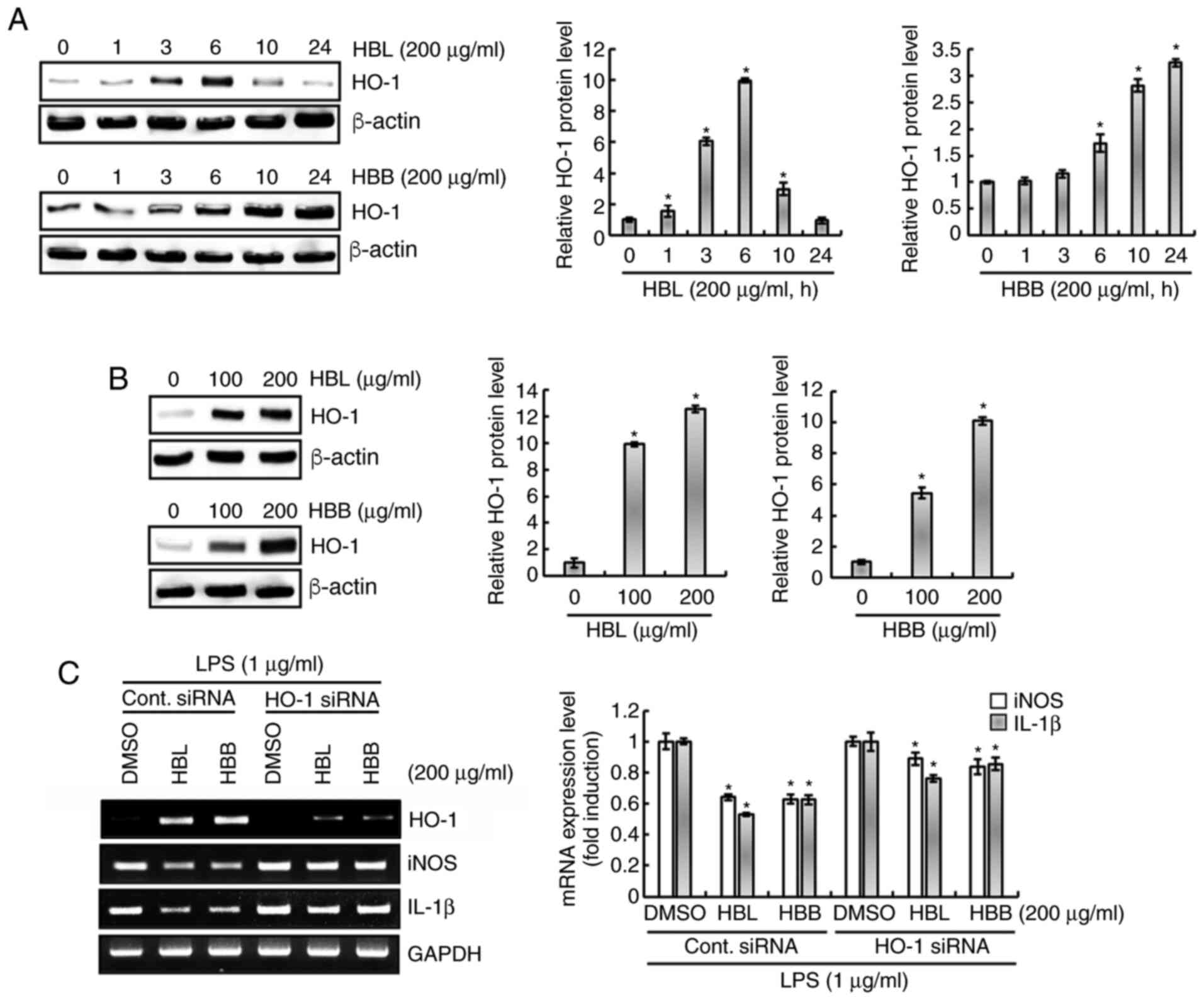
Effect of HBL and HBB on HO-1
expression in RAW264.7 cells
As shown in Fig.
5A, HBL significantly increased the expression of HO-1 protein
from 1 h after treatment. The expression level of HO-1 was observed
to be highest at 6 h of HBL treatment, and gradually decreased when
the treatment duration was >6 h. HBB started to significantly
increase the expression level of HO-1 protein from 6 h of
treatment, and the level continued to increase until 24 h after
treatment. The effect of different HBL and HBB concentrations on
HO-1 protein expression levels were also examined. As the previous
results confirmed that the HO-1 expression induced by HBL and HBB
was highest at 6 and 10 h after the start of treatment,
respectively, RAW264.7 cells were treated with HBL and HBB for 6
and 10 h. As shown in Fig. 5B, HBL
and HBB concentration-dependently increased the HO-1 protein level.
Furthermore, whether the increase in HO-1 protein level induced by
HBL and HBB was associated with the LPS-mediated overexpression of
iNOS and IL-1β was evaluated. The results demonstrated that the
knockdown of HO-1 by transfection with HO-1 siRNA blocked the HBL-
and HBB-induced downregulation of iNOS and IL-1β in LPS-stimulated
RAW264.7 cells (Fig. 5C). These
results indicate that HBL and HBB inhibited the overexpression of
pro-inflammatory mediators through the upregulation of the HO-1
protein expression level.
HBL- and HBB-induced increase in HO-1
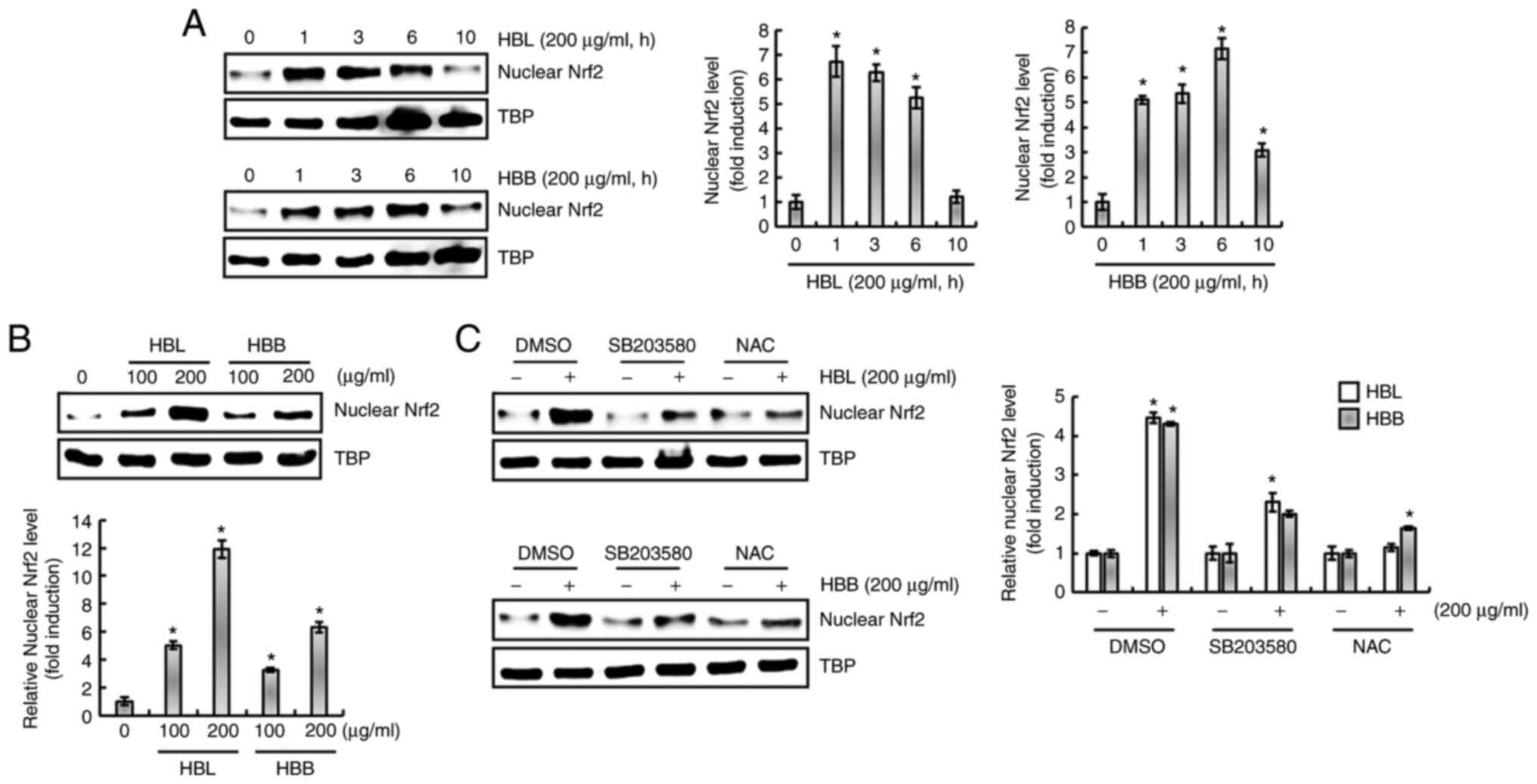
protein level is dependent on ROS/p38 activation
It has been reported that ROS increase the
expression of HO-1 and are upstream of p38 (24). Isoegomaketone has been shown to
inhibit the production of pro-inflammatory mediators through the
activation of a ROS/p38/HO-1 pathway (24). Thus, the effect of ROS/p38
signaling on the HBL- and HBB-mediated expression of HO-1 was
investigated. As shown in Fig. 6A,
the inhibition of p38 by SB203580 and of ROS by NAC suppressed the
HBL- and HBB-induced expression of HO-1. Thus, whether HBL and HBB
induce the production of ROS was investigated. As shown in Fig. 6B, ROS production was observed in
HBL- and HBB-treated RAW264.7 cells. In addition, HBL and HBB
increased the phosphorylation level of p38 (Fig. 6C), and the inhibition of ROS by NAC
attenuated the HBL and HBB-induced p38 phosphorylation (Fig. 6D). These results indicate that HBL
and HBB induced HO-1 expression through the ROS-dependent
activation of p38.
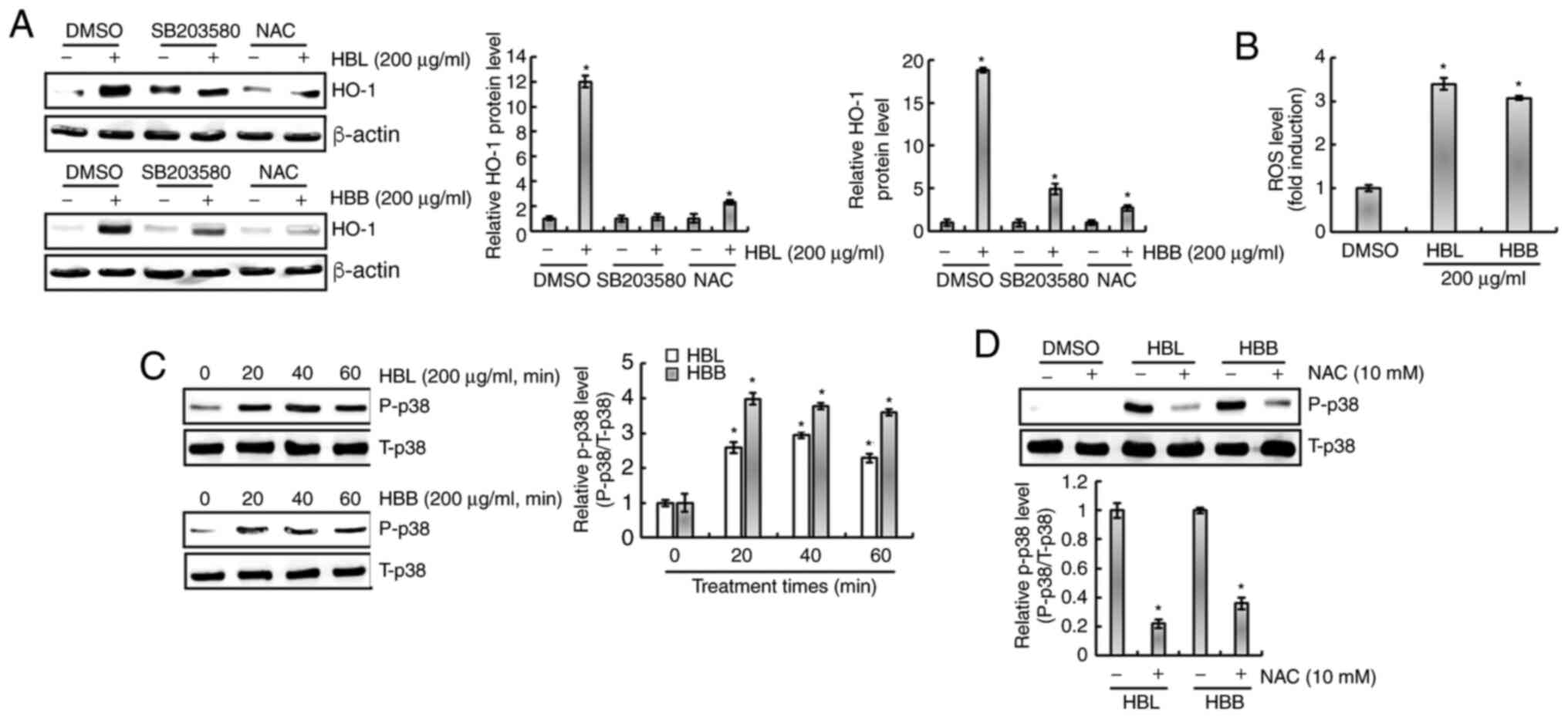 | Figure 6.Effects of p38 and ROS on the changes
in HO-1 expression mediated by HBL and HBB. (A) RAW264.7 cells were
pretreated with SB203580 (20 µM, p38 inhibitor) or NAC (10 mM, ROS
scavenger) for 2 h and then co-treated with HBL (200 µg/ml) for 6 h
or HBB (200 µg/ml) for 10 h. (B) RAW264.7 cells were treated with
200 µg/ml HBL or HBB for 24 h and then the ROS level was measured
using 2′,7′-dichlorofluorescein diacetate staining. (C) RAW264.7
cells were treated with 200 µg/ml HBL and HBB for the indicated
times. (D) RAW264.7 cells were pre-treated with NAC (10 mM) for 2 h
and then co-treated with 200 µg/ml HBL and HBB for 20 min. The cell
lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and western blotting was performed using antibodies
against HO-1, P-p38 and p38. Actin was used as an internal control
for western blot analysis. *P<0.05 vs. cells without HBL or HBB
treatment. ROS, reactive oxygen species; HO-1, heme oxygenase 1;
HBL, honeyberry leaf extract; HBB, honeyberry bark extract; NAC,
N-acetyl-L-cysteine; P, phosphorylated; T, total; DMSO,
dimethylsulfoxide. |
HBL- and HBB-induced increase in HO-1
protein level is dependent on ROS/p38/Nrf2 activation
The nuclear accumulation of Nrf2 is associated with
the upregulation of HO-1 expression (22,24).
Therefore, whether HBL and HBB influence the nuclear accumulation
of Nrf2 was investigated. As shown in Fig. 7A, the nuclear accumulation of Nrf2
significantly increased after 1 h of HBL and HBB treatment. In
addition, HBL and HBB induced a concentration-dependent increase in
the nuclear accumulation of Nrf2 (Fig.
7B). Furthermore, it was observed that the inhibition of p38 by
SB203580 and the inhibition of ROS by NAC blocked the nuclear
accumulation of Nrf2 induced by HBL and HBB (Fig. 7C). These results indicate that HBL
and HBB induced the upregulation of HO-1 protein through the
activation of ROS, p38 and Nrf2.
Upregulation of ATF3 contributes to
the inhibition of the production of pro-inflammatory mediators by
HBL and HBB
There is evidence to suggest that ATF3 inhibits the
inflammatory response (25). Thus,
the effect of ATF3 on the inhibition of pro-inflammatory mediators
by HBL and HBB was investigated. As shown in Fig. 8A, HBL and HBB rapidly increased the
expression of ATF3. In addition, HBL and HBB dose-dependently
increased the ATF3 protein expression level (Fig. 8B). The knockdown of ATF3 by
transfection with ATF3 siRNA was observed to attenuate the HBL- and
HBB-induced downregulation of iNOS and IL-1β (Fig. 8C). These results indicate that the
upregulation of ATF3 by HBL and HBB may be associated with the
downregulation of pro-inflammatory mediators.
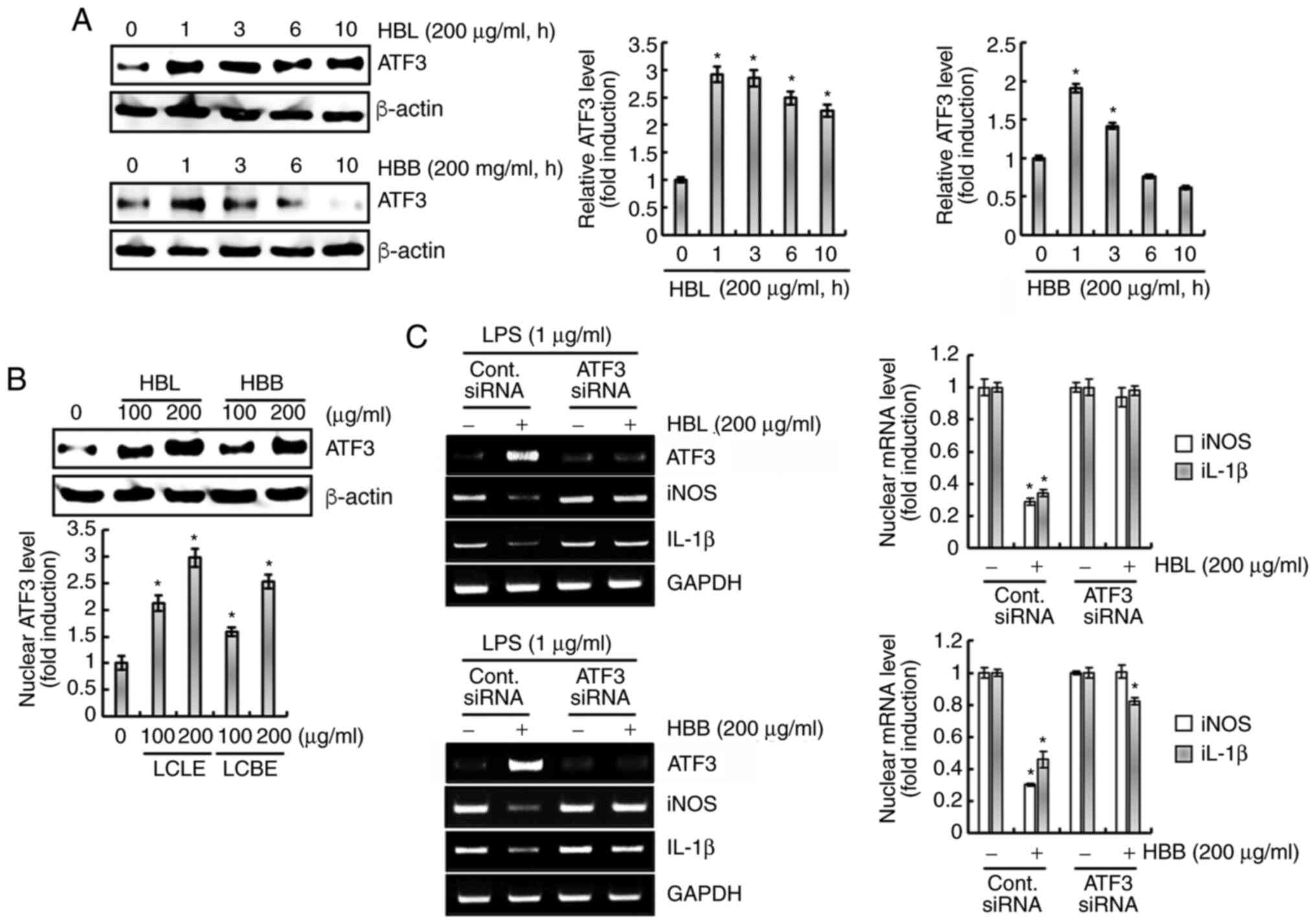 | Figure 8.Effects of HBL and HBB on ATF3
expression. (A) RAW264.7 cells were treated with 200 µg/ml HBL and
HBB for the indicated times. (B) RAW264.7 cells were treated with
100 and 200 µg/ml HBL and HBB for 1 h. For western blot analysis,
the cell lysates were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and western blotting was
performed using an antibody against ATF3. Actin was used as an
internal control for western blot analysis. (C) RAW264.7 cells were
transfected with control or ATF3 siRNA for 48 h. After
transfection, the cells were pretreated with 200 µg/ml HBL and HBB
for 4 h and then co-treated with LPS (1 µg/ml) for 20 h. After the
treatment, total RNA was extracted. *P<0.05 vs. cells without
HBL and HBB treatment. HBL, honeyberry leaf extract; HBB,
honeyberry bark extract; ATF3, activating transcription factor-3;
siRNA, small interfering RNA; LPS, lipopolysaccharide; iNOS,
inducible nitric oxide synthase; IL-1β, interleukin 1β. |
Analysis of bioactive compounds in
HBL, HBB and HBF
Standardization of materials is essential for the
development of functional products. Thus, the levels of certain
polyphenolic compounds in HBL, HBB and HBF were analyzed, as
honeyberry has been reported to contain polyphenolic compounds such
as protocatechuic acid, caffeic acid, chlorogenic acid, coumaric
acid and ferulic acid (17,18).
As shown in Fig. 9, chlorogenic
acid (58.66 µg/g extract), caffeic acid (21.51 µg/g extract),
coumaric acid (120.51 µg/g extract) and ferulic acid (283.31 µg/g
extracts) were detected in HBL. In HBB, protocatechuic acid (4.23
µg/g extract), chlorogenic acid (21.86 µg/g extract), caffeic acid
(43.88 µg/g extract), coumaric acid (26.77 µg/g extract) and
ferulic acid (8.69 µg/g extract) were detected. HBF contained
protocatechuic acid (6.53 µg/g extract), chlorogenic acid (27.23
µg/g extract), coumaric acid (5.44 µg/g extract) and ferulic acid
(7.99 µg/g extract). The anti-inflammatory activities of these
polyphenolic compounds have been reported in previous studies
(26–30). In addition, it has been reported
that ferulic acid, coumaric acid, chlorogenic acid, and caffeic
acid are able to activate the Nrf2/HO-1 signaling pathway (31–34).
Discussion
Although inflammation is a beneficial response that
helps to protect the body from external pathogens, it is considered
that excessive inflammatory responses should be controlled to
prevent the development of chronic inflammation-associated diseases
(1,2). The development of new
anti-inflammatory agents is desirable due to the side effects and
treatment failures associated with currently available
anti-inflammatory drugs (35).
There is growing evidence that various plants and plant-derived
compounds have therapeutic anti-inflammatory effects with few or no
side effects (35,36). Thus, in the present study, the
anti-inflammatory activity of HBL and HBB derived from honeyberry
were evaluated and their potential molecular mechanisms of action
were elucidated.
According to previous reports, HBF inhibits the
production of pro-inflammatory mediators during the LPS-induced
inflammatory response (6,9). However, in the present study, it was
observed that HBF (100 µg/ml) did not inhibit the LPS-induced
overproduction of pro-inflammatory mediators, namely NO, iNOS, and
IL-1β, whereas HBL and HBB significantly suppressed the
overproduction of these mediators at the same concentration.
Polyphenolic compounds are associated with lower risk of
degenerative and chronic diseases than other dietary components and
are known to have anti-inflammatory properties (37). The present study found that the
levels of polyphenolic compounds in the extracts decreased in the
order HBL > HBB > HBF. This result indicates that the
difference in the inhibitory activities of HBL, HBB and HBF against
the production of inflammatory mediators may be associated with the
levels of polyphenolic compounds they contain. We also hypothesize
that the differences in the inhibitory effect of HBF on the
production of pro-inflammatory mediators in different studies may
be due to variations in the growth environment of honeyberry. This
is because the secondary metabolites of plants are regulated by the
environment in which the plants are grown (38).
NF-κB is an important transcription factor involved
in the inflammatory response (39). Inflammatory stimuli, such as LPS,
activate NF-κB by inducing cytosolic IκB-α degradation and the
subsequent accumulation of p65 in the nucleus, which leads to the
upregulation of pro-inflammatory mediators (39). Interestingly, the present study
demonstrated that HBL and HBB did not inhibit the LPS-induced
cytosolic degradation of IκB-α but attenuated the increase in the
nuclear level of p65 protein. There is evidence that p65
phosphorylation is essential for the nuclear accumulation of p65
and transcriptional activation (40). There are various phosphorylation
sites of p65, among which the phosphorylation of serine 529 has
been reported to be involved in the nuclear accumulation of p65
(40). Therefore, we hypothesize
that the inhibitory effects of HBL and HBB on p65 nuclear
accumulation are caused by the inhibition of p65 phosphorylation at
serine 529. However, this hypothesis requires elucidation through
further study.
MAPK is also involved in the upregulation of
pro-inflammatory mediators (41,42).
In the present study, HBL did not inhibit the LPS-induced
phosphorylation of ERK1/2 and p38. However, HBB suppressed
LPS-induced p38 phosphorylation but not ERK1/2 phosphorylation. As
p38 MAPK is considered a potential molecular target for the
development of anti-inflammatory drugs, p38 inhibitors are
currently being developed. The anti-inflammatory activities of
these p38 inhibitors have been confirmed clinically (41,42).
Therefore, the data in the present study indicate that the
inhibition of p38 activation by HBB was associated with the
downregulation of pro-inflammatory mediators.
The present study also indicated that HBL and HBB
upregulated the HO-1 protein expression level through the
ROS/p38-dependent activation of Nrf2, and that HO-1 knockdown
inhibited the downregulation of inflammatory mediators by HBL and
HBB. It has previously been shown that ROS induce the expression of
HO-1 through the p38-dependent activation of Nrf2 (24,43),
which can inhibit the inflammatory process. Therefore, Nrf2
activators are considered potential therapeutic agents for the
treatment of various human diseases, including inflammatory
diseases (44). Furthermore, Nrf2
activation-dependent responses are an effective means of preventing
and treating inflammation, the most effective of which is the
expression of HO-1 (45,46). Nrf2 is a redox-sensitive
transcription factor that is known to regulate the expression of
antioxidant enzymes such as HO-1 (47). In the absence of stimulation, Nrf2
binds to Keap1 and is present in the cytoplasm in an inactive form.
However, ROS induces the translocation of Nrf2 to the nucleus
through the degradation of Keap1. Nuclear Nrf2 binds to the
antioxidant response element and induces the transcription of HO-1
(48). On the basis of these
previous studies (47,48), we hypothesize that ROS produced by
HBL and HBB may induce Nrf2 translocation into the nucleus through
the degradation of Keap1, contributing the expression of HO-1.
Thus, the present data may provide evidence of a link between
ROS/p38/Nrf2/HO-1 signaling and the anti-inflammatory activity of
HBL and HBB.
Finally, it was observed that the expression of ATF3
was increased in cells treated with HBL and HBB. It has been
reported that ATF3 plays an important role in innate immunity
(49) and that its expression
mitigates the production of various pro-inflammatory mediators
(50). In an in vivo study,
ATF3 knock-out mice died sooner than wild-type mice when injected
with a high dose of LPS (49).
Previous studies have demonstrated that ATF3 mitigates inflammatory
responses by inhibiting the overproduction of pro-inflammatory
mediators (49–51). In the present study, ATF3 knockdown
completely blocked the HBL- and HBB-induced downregulation of
inflammatory mediators, which indicates that ATF3 may be a major
mediator of the anti-inflammatory activity of HBL and HBB, although
HBL and HBB can also inhibit the production of inflammatory
mediators through various signaling pathways such as MAPK, NF-kB,
and Nrf2/HO-1. It has been reported that a deficiency of ATF3
increases the production of inflammatory mediators via the
inhibition of Nrf2/HO-1 signaling (52). Thus, the effect of the HBL- and
HBB-induced expression of ATF3 on Nrf2/HO-1 signaling requires
further elucidation. In addition, the mechanisms through which HBL
and HBB promote the expression of ATF3 need to be investigated.
In summary, the present study demonstrated that HBL
and HBB inhibited LPS-induced NF-κB activation by blocking the
nuclear accumulation of p65, increasing HO-1 expression through
ROS/p38/Nrf2 activation, and increasing ATF3 expression.
Furthermore, HBB inhibited LPS-induced p38 phosphorylation. The
regulatory effects of HBL and HBB on these signaling pathways may
be associated with their anti-inflammatory activities. These
findings suggest that HBL and HBB may have great potential as
anti-inflammatory drugs and should be further explored for
development as natural anti-inflammatory drugs. However, the study
is limited as it involved only cell-based experiments. Therefore,
preclinical studies using animal models are required to validate
the anti-inflammatory activities of HBL and HBB prior to their
development as natural anti-inflammatory drugs.
Acknowledgements
Not applicable.
Funding
This study was supported by Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Education (grant nos.
NRF-2019R1D1A3A03103685 and NRF-2018R1A6A1A03024862).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JBJ directed and designed the study. MYA, HJE, HJS,
NGG and GHP performed the experiments. HJE and NGG analyzed the
bioactive compounds using HPLC. HJS and GHP drafted the manuscript.
JBJ corrected the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATF3
|
activating transcription factor-3
|
|
COX-2
|
cyclooxygenase-2
|
|
DCFH-DA
|
2′,7′-dichlorofluorescein
diacetate
|
|
HO-1
|
heme oxygenase-1
|
|
HBB
|
honeyberry branch extract
|
|
HBF
|
honeyberry fruit extract
|
|
HBL
|
honeyberry leaf extract
|
|
iNOS
|
inducible nitric oxide synthase
|
|
IL-1β
|
interleukin-1β
|
|
IL-6
|
interleukin-6
|
|
LPS
|
lipopolysaccharide
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
NF-κB
|
nuclear factor-κB
|
|
NO
|
nitric oxide
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
References
|
1
|
Ferrero-Miliani L, Nielsen OH, Andersen PS
and Girardin SE: Chronic inflammation: Importance of NOD2 and NALP3
in interleukin-1beta generation. Clin Exp Immunol. 147:227–235.
2007.PubMed/NCBI
|
|
2
|
Xue N, Wu X, Wu L, Li L and Wang F:
Antinociceptive and anti-inflammatory effect of naringenin in
different nociceptive and inflammatory mice models. Life Sci.
217:148–154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sieweke MH and Allen JE: Beyond stem
cells: Self-renewal of differentiated macrophages. Science.
342:12429742013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medzhitov R: Inflammation 2010: New
adventures of an old flame. Cell. 140:771–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mazzoni L, Perez-Lopez P, Giampieri F,
Alvarez-Suarez JM, Gasparrini M, Forbes-Hernandez TY, Quiles JL,
Mezzetti B and Battino M: The genetic aspects of berries: From
field to health. J Sci Food Agric. 96:365–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee YS, Cho IJ, Kim JW, Lee SK, Ku SK and
Lee HJ: Evaluation of in vitro anti-oxidant and anti-inflammatory
activities of Korean and Chinese Lonicera caerulea. Nutr Res
Pract. 12:486–493. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Svarcova I, Heinrich J and Valentova K:
Berry fruits as a source of biologically active compounds: The case
of Lonicera caerulea. Biomed Pap Med Fac Univ Palacky
Olomouc Czech Rupub. 151:163–174. 2017. View Article : Google Scholar
|
|
8
|
Chen L, Xin X, Yuan Q, Su D and Liu W:
Phytochemical properties and antioxidant capacities of various
colored berries. J Sci Food Agric. 94:180–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin XH, Ohgami K, Shiratori K, Suzuki Y,
Koyama Y, Yoshida K, Ilieva I, Tanaka T, Onoe K and Ohno S: Effects
of blue honeysuckle (Lonicera caerulea L.) extract on
lipopolysaccharide-induced inflammation in vitro and in vivo. Exp
Eye Res. 82:860–867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zdarilova A, Rajnochova Svobodova A,
Chytilova K, Simanek V and Ulrichova J: Polyphenolic fraction of
Lonicera caerulea L. fruits reduces oxidative stress and
inflammatory markers induced by lipopolysaccharide in gingival
fibroblasts. Food Chem Toxicol. 48:1555–1561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palikova I, Valentova K, Oborna I and
Ulrichova J: Protectivity of blue honeysuckle extract against
oxidative human endothelial cells and rat hepatocyte damage. J
Agric Food Chem. 57:6584–6589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jurgonski A, Juskiewicz J and Zdunczyk Z:
An anthocyanin-rich extract from Kamchatka honeysuckle increases
enzymatic activity within the gut and ameliorates abnormal lipid
and glucose metabolism in rats. Nutrition. 29:898–902. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SI, Lee YJ, Choi SH, Park SJ, Song CH
and Ku SK: Therapeutic effects of blue honeysuckle on lesions of
hyperthyroidism in rats. Am J Chin Med. 44:1441–1456. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JW, Lee YS, Seol DJ, Cho IJ, Ku SK,
Choi JS and Lee HJ: Anti-obesity and fatty liver-preventing
activities of Lonicera caerulea in high-fat diet-fed mice.
Int J Mol Med. 42:3047–3064. 2018.PubMed/NCBI
|
|
15
|
Zhou L, Wang H, Yi J, Yang B, Li M, He D,
Yang W, Zhang Y and Ni H: Anti-tumor properties of anthocyanins
from Lonicera caerulea ‘Beilei’ fruit on human
hepatocellular carcinoma: In vitro and in vivo study. Biomed
Pharmacother. 104:520–529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pace E, Jiang Y, Clemens A, Crossman T and
Rupasinghe HPV: Impact of thermal degradation of
cyanidin-3-O-glucoside of haskap Berry on cytotoxicity of
hepatocellular carcinoma HepG2 and breast cancer MDA-MB-231 cells.
Antioxidants (Basel). 7:E242018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jurikova T, Rop O, Mlcek J, Sochor J,
Balla S, Szekeres L, Hegedusova A, Hubalek J, Adam V and Kizek R:
Phenolic profile of edible honeysuckle berries (genus Lonicera) and
their biological effects. Molecules. 17:61–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vasantha Rupasinghe HP, Arumuggam N,
Amararathna M and De Silva ABKH: The potential health benefits of
haskap (Lonicera caerulea L.): Role of
cyaniding-3-O-glucoside. J Funct Foods. 44:24–39. 2018. View Article : Google Scholar
|
|
19
|
Rupasinghe HP, Boehm MM, Sekhon-Loodu S,
Parmar I, Bors B and Jamieson AR: Anti-inflammatory activity of
haskap cultivars is polyphenols-dependent. Biomolecules.
5:1079–1098. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szopa A, Kokotkiewicz A, Kubica P,
Banaszczak P, Wojtanowska-Krośniak A, Krośniak M, Marzec-Wróblewska
U, Badura A, Zagrodzki P, Bucinski A, et al: Comparative analysis
of different groups of phenolic compounds in fruit and leaf
extracts of Aronia sp.: A. melanocarpa, A. arbutifolia, and
A. ×prunifolia and their antioxidant activities. Eur Food
Res Technol. 243:1645–1657. 2017. View Article : Google Scholar
|
|
21
|
Anchalee R, Ubonrat S, Arpathsra S, Simona
B and Susanna B: Rapid evaluation of phenolic compounds and
antioxidant activity of mulberry leaf tea during storage using
electronic tongue coupled with chemometrics. J Berry Res.
9:563–574. 2019. View Article : Google Scholar
|
|
22
|
Kim HN, Park GH, Park SB, Kim JD, Eo HJ,
Son HJ, Song JH and Jeong JB: Sageretia thea inhibits inflammation
through suppression of NF-κB and MAPK and activation of Nrf2/HO-1
signaling pathways in RAW264.7 cells. Am J Chin Med. 47:385–403.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park YS, Jung ST, Kang SG, Heo BK,
Arancibia-Avila P, Toledo F, Drzewiecki J, Namiesnik J and
Gorinstein S: Antioxidants and proteins in ethylene-treated
kiwifruits. Food Chem. 107:640–648. 2008. View Article : Google Scholar
|
|
24
|
Jin CH, So YK, Han SN and Kim JB:
Isoegomaketone upregulates heme oxygenase-1 in RAW264.7 cells via
ROS/p38 MAPK/Nrf2 pathway. Biomol Ther (Seoul). 24:510–516. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon JW, Kwon HK, Shin HJ, Choi YM, Anwar
MA and Choi S: Activating transcription factor 3 represses
inflammatory responses by binding to the p65 subunit of NF-kB. Sci
Rep. 5:144702015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Juman S, Yasui N, Ikeda K, Ueda A,
Sakanaka M, Negishi H and Miki T: Caffeic acid phenethyl ester
suppresses the production of pro-inflammatory cytokines in
hypertrophic adipocytes through lipopolysaccharide-stimulated
macrophages. Biol Pharm Bull. 35:1941–1946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SH, Park SY, Park YL, Myung DS, Rew JS
and Joo YE: Chlorogenic acid suppresses lipopolysaccharide-induced
nitric oxide and interleukin-1β expression by inhibiting JAK2/STAT3
activation in RAW264.7 cells. Mol Med Rep. 16:9224–9232. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee M, Rho HS and Choi K:
Anti-inflammatory effects of a p-coumaric acid and kojic acid
derivative in LPS-stimulated RAW264.7 macrophage cells. Biotechnol
Bioproc E. 24:653–657. 2019. View Article : Google Scholar
|
|
29
|
Sakai S, Ochiai H, Nakajima K and Terasawa
K: Inhibitory effect of ferulic acid on macrophage inflammatory
protein-2 production in a murine macrophage cell line, RAW264.7.
Cytokine. 9:242–248. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Zhou J, Fu S, Wang C and Zhou B:
Preventive effects of protocatechuic acid on LPS-induced
inflammatory response in human gingival fibroblasts via activating
PPAR-γ. Inflammation. 38:1080–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen JJ, Deng JS, Huang CC, Li PY, Liang
YC, Chou CY and Huang GJ: p-Coumaric-acid-containing Adenostemma
lavenia ameliorates acute lung injury by activating
AMPK/Nrf2/HO-1 signaling and improving the anti-oxidant response.
Am J Chin Med. 47:1483–1506. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han D, Gu X, Gao J, Wang Z, Liu G, Barkema
HW and Han B: Chlorogenic acid promotes the Nrf2/HO-1
anti-oxidative pathway by activating p21Waf1/Cip1 to
resist dexamethasone-induced apoptosis in osteoblastic cells. Free
Radic Biol Med. 137:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma Z, Hong Q, Wang Y, Liang Q, Tan H, Xiao
C, Tang X, Shao S, Zhou S and Gao Y: Ferulic acid induces heme
oxygenase-1 via activation of ERK1/2 and Nrf2. Drug Discov Ther.
5:299–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang SY, Pyo MC, Nam MH and Lee KW:
ERK/Nrf2 pathway activation by caffeic acid in HepG2 cells
alleviates its hepatocellular damage caused by
t-butylhydroperoxide-induced oxidative stress. BMC Complement
Altern Med. 19:1392019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Musialik K, Szulińska M, Hen K, Skrypnik D
and Bogdański P: The relation between osteoprotegerin, inflammatory
processes, and atherosclerosis in patients with metabolic syndrome.
Eur Rev Med Pharmacol Sci. 21:4379–4385. 2017.PubMed/NCBI
|
|
36
|
Oguntibeju OO: Medicinal plants with
anti-inflammatory activities from selected countries and regions of
Africa. J Inflamm Res. 11:307–317. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leyva-Jiménez FJ, Lozano-Sánchez J,
Cádiz-Gurrea ML, Arráez-Román D and Segura-Carretero A: Functional
ingredients based on nutritional phenolics. A case study against
inflammation: Lippia genus. Nutrients. 11:16462019. View Article : Google Scholar
|
|
38
|
Yang L, Wen KS, Ruan X, Zhao YX, Wei F and
Wang Q: Response of plant secondary metabolites to environmental
factors. Molecules. 23:7622018. View Article : Google Scholar
|
|
39
|
Chen JW, Chen YH, Lin FY, Chen YL and Lin
SJ: Ginkgo Biloba extract inhibits tumor necrosis
factor-alpha-induced reactive oxygen species generation,
transcription factor activation, and cell adhesion molecule
expression in human aortic endothelial cells. Arterioscler Thromb
Vasc Biol. 23:1559–1566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maguire O, O'Loughlin K and Minderman H:
Simultaneous assessment of NF-kB/p65 phosphorylation and nuclear
localization using imaging flow cytometry. J Immunol Methods.
423:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Frazier WJ, Xue J, Luce WA and Liu Y: MAPK
signaling drives inflammation in LPS-stimulated cardiomyocytes: The
route of crosstalk to G-protein-coupled receptors. PLoS One.
7:e500712012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu X, Peyton KJ, Shebib AR, Wang H and
Durante W: Compound C stimulates heme oxygenase-1 gene expression
via the Nrf2-ARE pathway to preserve human endothelial cell
survival. Biochem Pharmacol. 82:371–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim J, Cha YN and Surh YJ: A protective
role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in
inflammatory disorders. Mutat Res. 690:12–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park SY, Park DJ, Kim YH, Kim Y, Kim SG,
Shon KJ, Choi YW and Lee SJ: Upregulation of heme oxygenase-1 via
PI3K/Akt and Nrf-2 signaling pathways mediates the
anti-inflammatory activity of schisandrin in Porphyromonas
gingivalis LPS-stimulated macrophages. Immunol Lett.
139:93–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ren J, Li L, Wang Y, Zhai J, Chen G and Hu
K: Gambogic acid induces heme oxygenase-1 through Nrf2 signaling
pathway and inhibits NF-kB and MAPK activation to reduce
inflammation in LPS-activated RAW264.7 cells. Biomed Pharmacother.
109:555–562. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lim DW, Choi HJ, Park SD, Kim H, Yu GR,
Kim JE and Park WH: Activation of the Nrf2/HO-1 pathway by
Amomum villosum extract suppresses LPS-induced oxidative
stress in vitro and ex vivo. Evid Based Complement Alternat Med.
2020:28378532020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Habtemariam S: The Nrf2/HO-1 axis as
targets for flavanones: Neuroprotection by pinocembrin, naringenin,
and eriodictyol. Oxid Med Cell Longev. 2019:47249202019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thompson MR, Xu D and Williams BR: ATF3
transcription factor and its emerging roles in immunity and cancer.
J Mol Med (Berl). 87:1053–1060. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hai T, Wolford CC and Chang YS: ATF3, a
hub of the cellular adaptive-response network, in the pathogenesis
of diseases: Is modulation of inflammation a unifying component.
Gene Expr. 15:1–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takii R, Inouye S, Fujimoto M, Nakamura T,
Shinkawa T, Prakasam R, Tan K, Hayashida N, Ichikawa H, Hai T and
Nakai A: Heat shock transcription factor 1 inhibits expression of
IL-6 through activating transcription factor 3. J Immunol.
184:1041–1048. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rao J, Qian X, Li G, Pan X, Zhang C, Zhang
F, Zhai Y, Wang X and Lu L: ATF3-mediated NRF2/HO-1 signaling
regulates TLR4 innate immune responses in mouse liver
ischemia/reperfusion injury. Am J Transplant. 15:76–87. 2015.
View Article : Google Scholar : PubMed/NCBI
|