Introduction
Lung cancer is one of the major diseases endangering
human health worldwide (1). The
incidence and mortality rates of lung cancer are high, particularly
non-small cell lung cancer (NSCLC) (2,3).
Invasion and metastasis are the main causes of poor prognosis and
high mortality rate in patients with lung cancer (4). Although great efforts, such as the
identification of novel biomarkers, establishment of modified
operations and development of specific drugs, have been made in the
clinical setting, the postoperative survival rate of patients has
not significantly improved (5,6).
Therefore, it is of great clinical significance to explore the
molecular mechanisms underlying the progression of NSCLC.
Glucose metabolism is one of the most distinguishing
characteristics between normal and tumorigenic cells (7). Tumor cells mainly rely on glycolysis
to obtain energy, even in aerobic environments; this is known as
the Warburg effect (8). A previous
study revealed that the growth and survival of tumor cells depend
on the transformation from anaerobic glycolysis to aerobic
glycolysis (9). Aerobic glycolysis
provides a material basis for the survival, growth and
proliferation of tumor cells (10).
Furthermore, a large amount of lactic acid causes damage to the
body, which is conducive to tumor tissues invading adjacent normal
tissues (11). In recent years, the
Warburg effect has garnered much attention in the study of tumor
development mechanisms (12,13).
Although the Warburg effect has been recognized in NSCLC (14–16),
the driving mechanism of aerobic glycolysis remains unknown.
The platelet isoform of phosphofructokinase (PFKP)
is a rate-limiting enzyme involved in glycolysis, which can cause
the conversion of fructose 6-phosphate to fructose 1,6-bisphosphate
(17). Enhanced activity of PFKP
can increase glycolysis, increase fatty acid oxidation, and promote
DNA biosynthesis and repair, thereby enhancing tumor cell growth,
migration and invasion (18,19).
It has been reported that PFKP expression is upregulated in various
types of cancer, including breast cancer (BC) (20), hepatocellular carcinoma (HCC)
(17) and renal carcinoma (21). However, to the best of our
knowledge, the specific regulatory relationship between PFKP and
NSCLC progression remains unclear.
The present study aimed to assess the effects of
PFKP on NSCLC progression, and further investigated the effects of
PFKP on cell proliferation, apoptosis, migration, invasion and
glycolysis. The present findings may provide a basis for novel
strategies for the treatment of NSCLC.
Materials and methods
The Cancer Genome Atlas (TCGA) data
analysis
The mRNA gene expression data were downloaded from
TCGA LUAD datasets (https://portal.gdc.cancer.gov/). Overall survival
information of all TCGA datasets was obtained from supplementary
data (22). For differentially
expressed gene (DEG) analysis, PFKP mRNA expression in cancer
tissues and paracancerous (normal) tissues was analyzed using the
DESeq2 R package (23); the
adjusted P-value for each gene was calculated using the
false-discovery rate (FDR) method. The cut-off to detect DEGs was
an FDR of <0.05 and an absolute log2-fold change of >1. For
overall survival analysis, data analysis was performed in R package
‘survival’ (24) using the
Kaplan-Meier curve method, and a log-rank test was used to compare
survival times between two groups.
Patient and tissue samples
In the present study, NSCLC tissues and
corresponding adjacent tissues (>2 cm away from the tumor edge)
were collected from 84 patients who underwent primary surgical
resection of NSCLC between January 2018 and December 2019 at
Shandong Provincial Hospital (Jinan, China). None of the patients
received radiotherapy or chemotherapy prior to surgical resection,
and the diagnosis of NSCLC was assessed by an experienced
pathologist. All tissue samples were stored at −80°C until RNA
extraction. The present study was approved by the Research Ethics
Committee of Shandong Provincial Hospital (approval no. 2020-181;
Jinan, China), and each patient provided written informed
consent.
Immunohistochemistry (IHC)
All tissue samples were fixed with 4% formaldehyde
solution for 24 h at room temperature and embedded in paraffin.
Paraffin-embedded tissues were cut into 4-µm sections, which were
routinely dehydrated, boiled in 0.01 M citrate buffer (pH 6.1) for
2 min for antigen retrieval and incubated with 3%
H2O2 at room temperature for 15 min. The
slides were then incubated with primary antibodies against PFKP
(1:150; cat. no. ab119796), hexokinase-2 (HK-2; 1:500; cat. no.
ab209847) and pyruvate kinase M2 (PKM2; 1:500; cat. no. ab137852)
(all Abcam) at 4°C overnight. Subsequently, the slides were
incubated with a secondary antibody labeled with horseradish
peroxidase (HRP) (1:2,000; cat. nos. ab205719 or ab205718; Abcam)
for 30 min at room temperature and 3′-diaminobenzidine reagent
(Beijing Solarbio Science & Technology Co., Ltd.) was added to
the slides for visualization. Finally, the sections were
dehydrated, cleared with xylene and fixed with resin. Two
experienced pathologists independently analyzed the IHC results.
Brown particles were considered positive expression of PFKP, HK-2
and PKM2.
Cell culture
Human bronchial epithelial cells (16HBE) and NSCLC
cells (H1299, H23 and A549) were purchased from American Type
Culture Collection. All cells were grown in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
cultured in an incubator containing 5% CO2 at 37°C.
Cell transfection
H1299 cells were divided into control (without
treatment), pVMV6-ENTRY [negative control (NC); cells were
transfected with pCMV6 empty plasmid] and pVMV6-PFKP
(overexpression of PFKP; cells were transfected with pCMV6-PFKP
plasmid) groups. H23 cells were divided into control (without
treatment), small interfering RNA (siRNA/si)-NC (NC; cells were
transfected with NC siRNAs), si1-PFKP (knockdown of PFKP; cells
were transfected with si1-PFKP) and si2-PFKP (knockdown of PFKP;
cells were transfected with si2-PFKP) groups. The plasmids (50 ng)
and siRNAs (50 nM) were transfected into cells (1×104
cells/well) using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The pCMV6-PFKP, pVMV6-ENTRY, siRNA and siRNA-NC were
purchased from OriGene Technologies, Inc. The sequences for the
siRNAs were as follows: si-NC sense, 5′-GGCUACGUCCAGGAGCGCAUU-3′
and antisense, 5′-UGCGCUCCUGGACGUAGCCUU-3′; si1-PFKP sense,
5′-GCUCCAUUCUUGGGACAAATT-3′ and antisense,
5′-AGAUUCUGGAAGGAACACCTT-3′; si2-PFKP sense,
5′-GGUGUUCCUUCCAGAAUCUTT-3′ and antisense,
5′-UUUGUCCCAAGAAUGGAGCTT-3′; and PFKP sense,
5′-GGACGCGGACGACTCCCGGGC-3′ and antisense,
5′-GTCAGACACTCCAGGGCTGCACATGTTCC-3′. A total of 48 h
post-transfection, follow-up experiments were conducted.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. cDNA was
synthesized using the PrimeScript RT reagent kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. RT-qPCR was performed using the SYBR Green PCR kit
(Takara Biotechnology Co., Ltd.) and was monitored using the CFX96
Touch Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.).
The reaction conditions were as follows: Pre-denaturation at 95°C
for 3 min, followed by 40 cycles of denaturation at 95°C for 15
sec, annealing at 58°C for 45 sec and extension at 72°C for 31 sec.
Relative mRNA expression was analyzed using the 2−ΔΔCq
method (25). GAPDH was used as an
endogenous control. The primers (Table
I) were synthesized by Invitrogen; Thermo Fisher Scientific,
Inc.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primers | Sequences
(5′-3′) |
|---|
| PFKP-F |
GGAGTGGAGTGGGCTGCTGGAG |
| PFKP-R |
CATGTCGGTGCCGCAGAAATCA |
| GAPDH -F |
TGTTGCCATCAATGACCCCTT |
| GAPDH -R |
CTCCACGACGTACTCAGCG |
Western blot analysis
Radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.) was used to extract total proteins from NSCLC
cells. A bicinchoninic acid protein assay kit (Beyotime Institute
of Biotechnology) was used to calculate protein concentration.
Total proteins (30 µg) were separated by SDS-PAGE on 10% gels and
were transferred to polyvinylidene fluoride membranes. After
blocking with 5% fat-free milk in TBS-0.1% Tween-20 for 1 h at room
temperature, the membranes were incubated with the following
primary antibodies at 4°C overnight: PFKP (cat. no. 12746),
N-cadherin (cat. no. 13116), E-cadherin (cat. no. 3195), vimentin
(cat. no. 5741), caspase-3 (cat. no. 9662) B-cell lymphoma-2
(Bcl-2; cat. no. 4223), hexokinase-2 (HK-2; cat. no. 2867), lactate
dehydrogenase A (LDHA; cat. no. 3582), GAPDH (cat. no. 5174) (all
1:1,000; all from Cell Signaling Technology, Inc.) and glucose
transporter-1 (Glut-1; cat. no. ab115730; 1:1,000; Abcam). GAPDH
was used as the internal control. Subsequently, the membranes were
incubated with HRP-conjugated goat anti-rabbit antibody (cat. no.
A0208; 1:4,000; Beyotime Institute of Biotechnology) for 1 h at
room temperature. Finally, an ECL western blotting substrate (cat.
no. 32106; Pierce; Thermo Fisher Scientific, Inc.) was used to
visualize protein bands and the specific bands were semi-quantified
using ImageJ v1.8.0 (National Institutes of Health).
Cell proliferation and colony
formation assays
Cell proliferation was measured by performing the
Cell Counting Kit-8 (CCK-8) assay (Beyotime Institute of
Biotechnology). Briefly, cells (2×103 cells) were seeded
on 96-well plates. After seeding for 0, 24, 48, 72 and 96 h, the
cells were incubated with CCK-8 solution (10 µl/ml) at 37°C for 2 h
in the dark. The optical density (OD) was measured at 450 nm using
a spectrophotometer (Thermo Fisher Scientific, Inc.).
For the colony formation assay, cells (500 cells)
were seeded on 6-well plates and maintained in RPMI-1640 medium
containing 10% FBS for 14 days. The medium was replaced every 3
days. Before acquiring the images under the light microscope
(Olympus Corporation; magnification, ×5), the cells were fixed with
4% formaldehyde for 15 min at room temperature and stained with
0.1% crystal violet for 20 min at room temperature. More than 50
cells in a colony were recorded as effective clones.
Wound healing assay
Cells were seeded on 6-well plates and cultured to
90–100% confluence. A 200-µl pipette tip was used to create wounds
across the cell monolayer. After incubation in a serum-free
RPMI-1640 medium for 48 h, the migration distance was observed. The
wound healing rate was calculated according to the following
formula: Wound healing rate (%) = (wound area at 0 h-wound area at
24 h)/wound area at 0 h ×100.
Transwell invasion assay
Transwell chambers (pore size, 8 µm; Corning, Inc.)
were used to investigate the invasive ability of the cells.
Briefly, a total of 200 µl cell suspension containing
1×105 cells was added to the upper chamber, which was
coated with Matrigel, and 600 µl medium containing 10% FBS was
added to the lower chamber. After incubation for 48 h in a
CO2 incubator at 37°C, cotton swabs were used to remove
cells from the upper surface. Subsequently, the cells were fixed
with 2% paraformaldehyde for 10 min at room temperature and stained
with 0.1% crystal violet for 10 min at room temperature. Finally,
the number of invasive cells was calculated in five random fields
under an inverted light microscope (Olympus Corporation;
magnification, ×100).
Flow cytometric analysis of
apoptosis
Cell apoptosis was detected by performing flow
cytometry using an Annexin V-FITC/PI apoptosis kit (BD
Biosciences). Briefly, cells were resuspended in binding buffer
(500 µl) and adjusted to 1×106 cells/ml, and Annexin V
(5 µl) and PI (5 µl) were added to the buffer. After incubation for
15 min at room temperature in the dark, the apoptotic rate was
analyzed by flow cytometry (Accuri C6 Plus; BD Biosciences).
Detection of glucose uptake, lactate
production and the adenosine triphosphate (ATP)/adenosine
diphosphate (ADP) ratio
The glucose test kit (cat. no. K686-100), lactate
assay kit (cat. no. K627-100) and ApoSENSOR™ ADP/ATP ratio
bioluminescence assay kit (cat. no. K255-200) were purchased from
BioVision, Inc. For the glucose uptake and lactate production
assays, cells (2.5×105 cells) were seeded in 6-well
plates. After culturing for 0 and 48 h, glucose uptake and lactate
production were measured according to the manufacturer's
instructions. Glucose uptake was referred to as the difference
between the initial (0 h) and final (48 h) glucose levels, and
lactate production was referred to as the difference between the
final (48 h) and initial (0 h) lactate levels. To detect the
ATP/ADP ratio, cells (1×104 cells) were seeded on a
luminometer plate and incubated with nucleotide-releasing buffer.
Subsequently, the ATP Monitoring Enzyme (1 µl) was added to the
luminometer plate. After incubation for 1 and 10 min, sample values
were recorded (the value recorded at 1 min was recorded as A,
whereas the value recorded at 10 min was recorded as B). Finally,
ADP-converting enzyme (1 µl) was added, and the sample values were
recorded (the value was recorded as C) after incubation for 2 min
at room temperature. The ATP/ADP ratio was calculated according to
the following formula: ATP/ADP ratio = A/(C-B).
Statistical analysis
Data are presented as the mean ± standard deviation.
GraphPad Prism 7.0 (GraphPad Software, Inc.) and SPSS 15.0
statistical software (SPSS, Inc.) were used for the statistical
analyses. The χ2 test was used to analyze the
association between PFKP expression and the clinicopathological
parameters of patients. The paired Student's t-test was used for
comparisons between the two groups. One-way analysis of variance
with Tukey's post-hoc test was used to compare multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Upregulation of PFKP expression in
NSCLC tissue and cells, and its association with
clinicopathological parameters
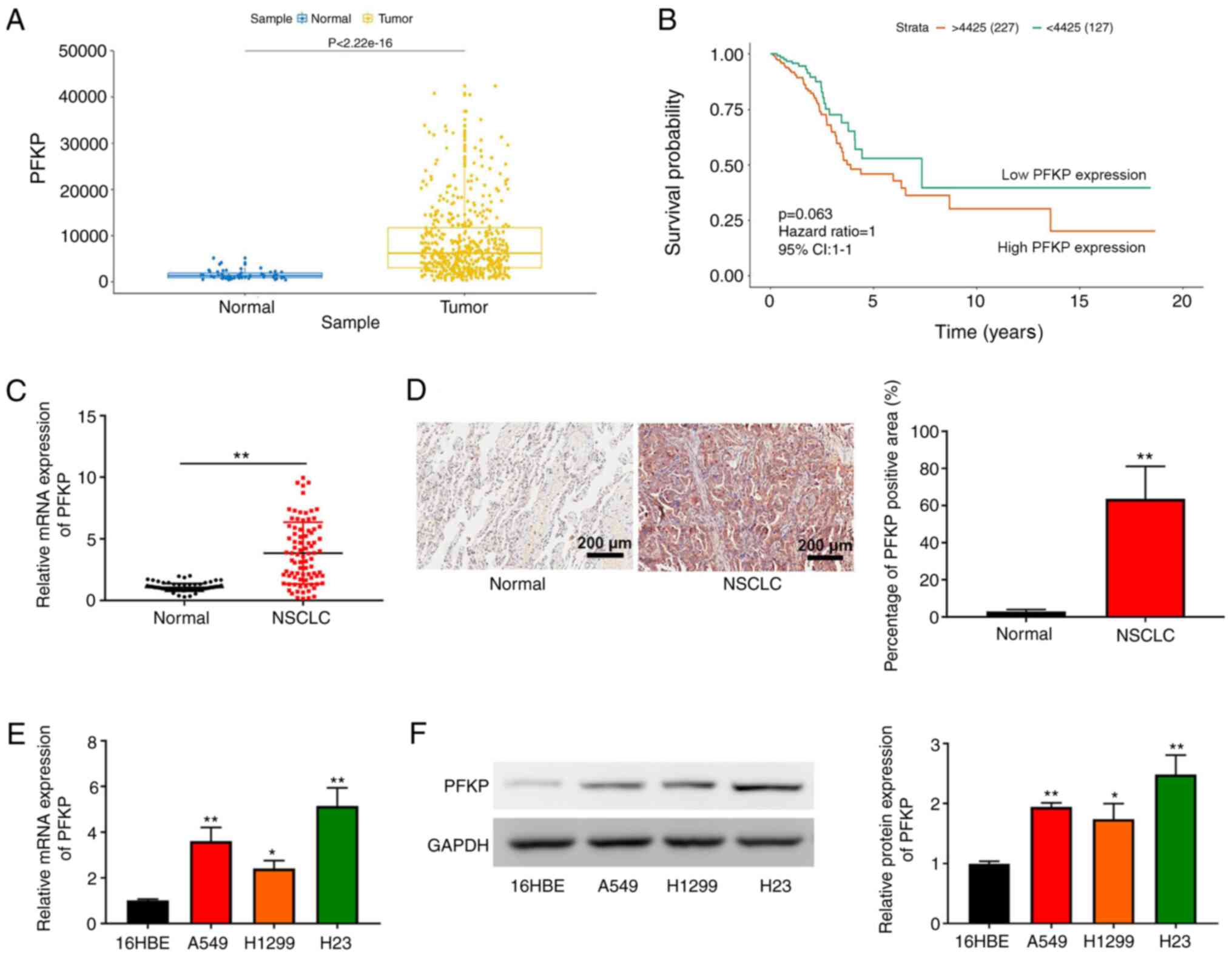
According to TCGA data, PFKP expression was higher
in NSCLC tissues compared with that in normal tissues
(P=2.22×10−16; Fig. 1A).
In addition, although overall survival did not exhibit a
significant difference, Kaplan-Meier survival analysis revealed
that the overall survival in patients with NSCLC and high PFKP
expression was shorter than that in patients with NSCLC and low
PFKP expression (P=0.063; Fig. 1B).
These data suggested that PFKP may be associated with poor
prognosis in NSCLC. The present study further examined PFKP
expression in NSCLC and corresponding adjacent tissues (normal)
using RT-qPCR and IHC. As shown in Fig.
1C and D, PFKP expression in NSCLC tissues was upregulated
compared with that in normal tissues (P<0.01). According to the
median mRNA expression of PFKP, patients with NSCLC were divided
into high expression and low expression groups. Subsequently, the
association between PFKP expression and patient clinicopathological
parameters was assessed. As shown in Table II, PFKP expression was associated
with lymph node metastasis and histological grade (both P<0.01),
but there was no significant association between PFKP expression
and other clinical characteristics (P>0.05). Furthermore, PFKP
expression was detected in 16HBE cells and NSCLC cells (H1299, H23
and A549) using RT-qPCR and western blotting. As shown in Fig. 1E and F, compared with those in 16HBE
cells, PFKP expression levels were upregulated in NSCLC cells
(P<0.05). Moreover, H23 cells expressed relatively high levels
of PFKP, whereas H1299 cells expressed relatively low levels of
PFKP; therefore, H1299 and H23 cells were used in subsequent
experiments.
 | Table II.Association of PFKP expression with
the clinicopathological parameters of patients. |
Table II.
Association of PFKP expression with
the clinicopathological parameters of patients.
|
|
| PFKP
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | No. of cases | High | Low | P-value |
|---|
| Sex |
|
|
| 0.182 |
|
Male | 50 | 28 | 22 |
|
|
Female | 34 | 14 | 20 |
|
| Age (years) |
|
|
| 0.382 |
|
<60 | 44 | 24 | 20 |
|
|
≥60 | 40 | 18 | 22 |
|
| Tumor size
(cm) |
|
|
| 0.374 |
|
<3 | 34 | 15 | 19 |
|
| ≥3 | 50 | 27 | 23 |
|
| Lymph node
metastasis |
|
|
| 0.009a |
| No | 40 | 14 | 26 |
|
|
Yes | 44 | 28 | 16 |
|
| Histological
grade |
|
|
|
<0.001b |
| I | 38 | 10 | 28 |
|
|
II–III | 46 | 32 | 14 |
|
PFKP promotes the proliferation of
NSCLC cells
To examine the effects of PFKP on NSCLC cells, H1299
cells were transfected with pCMV6-PFKP plasmid, and H23 cells were
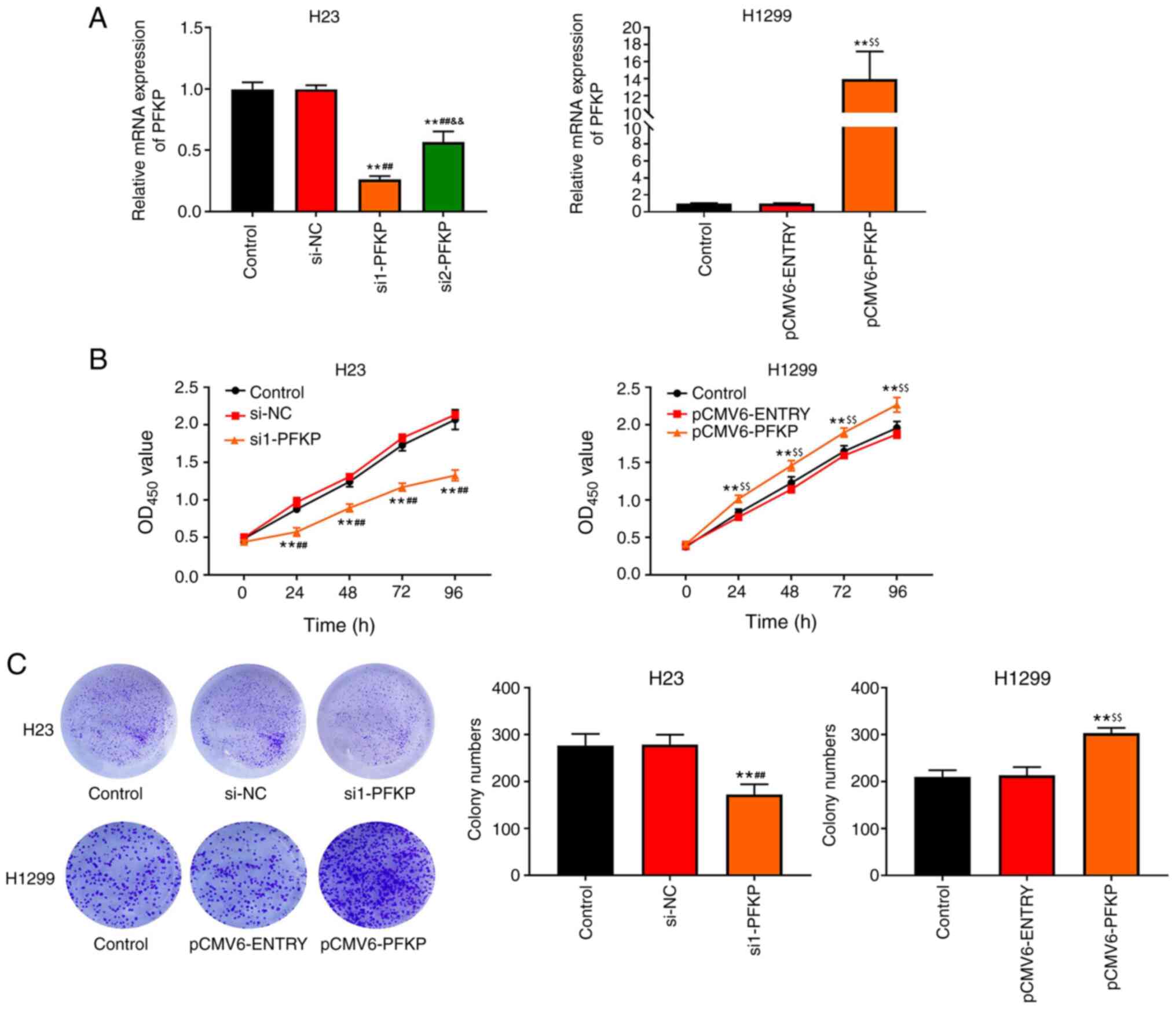
transfected with siRNA-PFKP. The results of RT-qPCR (Fig, 2A) revealed that the mRNA expression
levels of PFKP were downregulated in the si1-PFKP and si2-PFKP
groups (P<0.01), whereas the mRNA expression levels of PFKP were
upregulated in the pCMV6-PFKP group (P<0.01), compared with in
the control and si-NC or pCMV6-ENTRY groups. The results indicated
that transfection was successful. Moreover, the mRNA expression
levels of PFKP in the si1-PFKP group were significantly decreased
compared with the si2-PFKP group (P<0.01; Fig. 2A); therefore, si1-PFKP was used in
subsequent experiments. The effects of PFKP on the proliferation of
NSCLC cells were assessed using CCK-8 and colony formation assays.
As shown in Fig. 2B, the OD450
value in the si1-PFKP group was lower than that in the control and
si-NC groups (P<0.01); however, the OD450 value in the
pCMV6-PFKP group was higher than that in the control and
pCMV6-ENTRY groups (P<0.01). Moreover, similar results were
obtained from the colony formation assay (P<0.01; Fig. 2C).
PFKP promotes migration, invasion and
epithelial -mesenchymal transition (EMT) of NSCLC cells
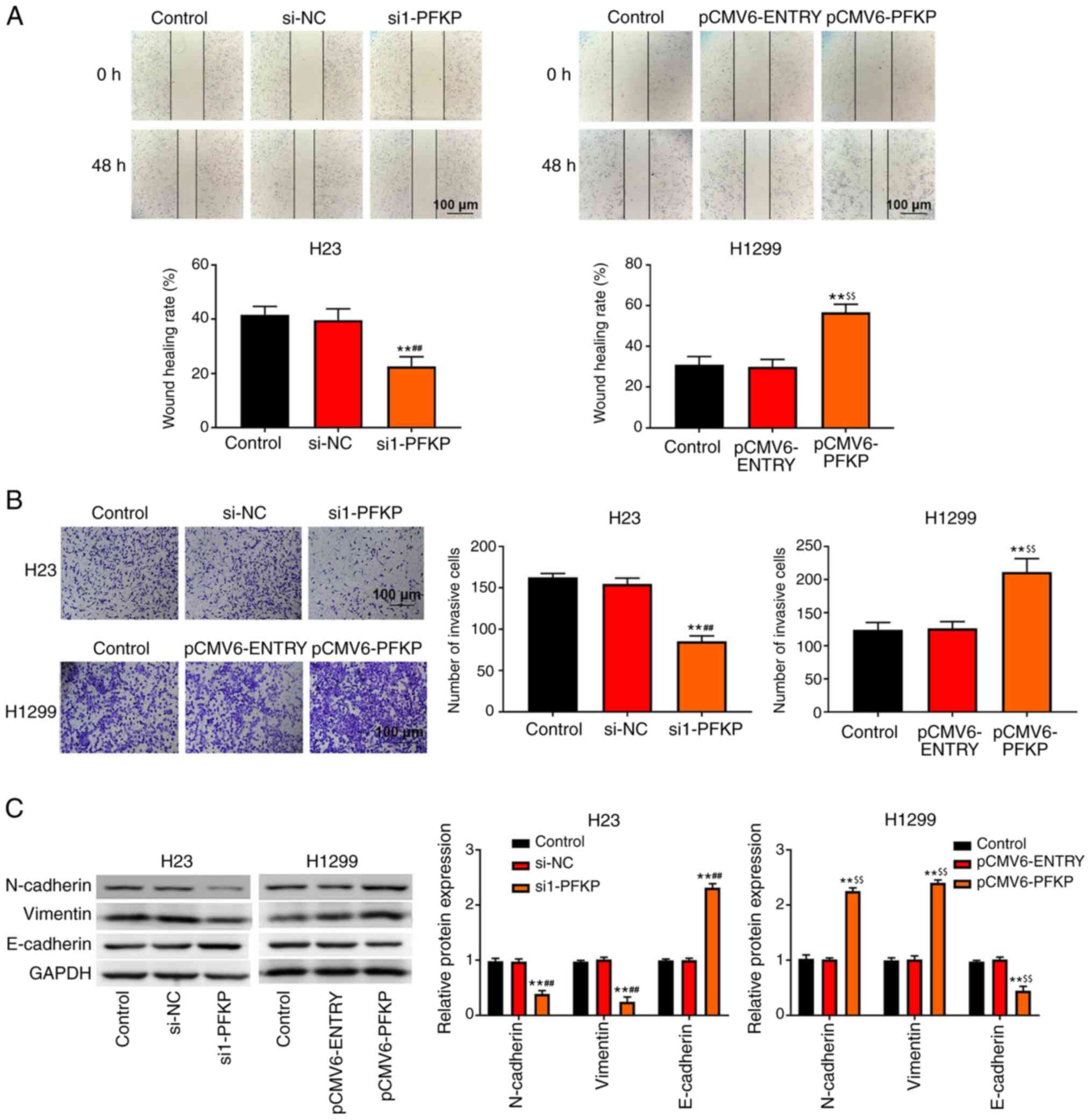
Transwell invasion and wound healing assays were
used to detect the invasion and migration of NSCLC cells. As shown
in Fig. 3A and B, overexpression of
PFKP increased the wound healing rate and number of invasive cells
among H1299 cells compared with those in the control and
pCMV6-ENTRY groups (P<0.01), whereas knockdown of PFKP decreased
the wound healing rate and number of invasive cells among H23 cells
compared with those in the control and si-NC groups (P<0.01). In
addition, western blot analysis (Fig.
3C) demonstrated that the protein expression levels of
N-cadherin and vimentin were significantly lower in the si1-PFKP
group than those in the control and si-NC groups (P<0.01),
whereas the protein expression levels of E-cadherin were increased
(P<0.01). When PFKP expression was upregulated, the protein
expression levels of N-cadherin and vimentin were increased
compared with those in the control and pCMV6-ENTRY groups
(P<0.01), whereas the protein expression levels of E-cadherin
were decreased (P<0.01).
PFKP inhibits apoptosis of NSCLC
cells
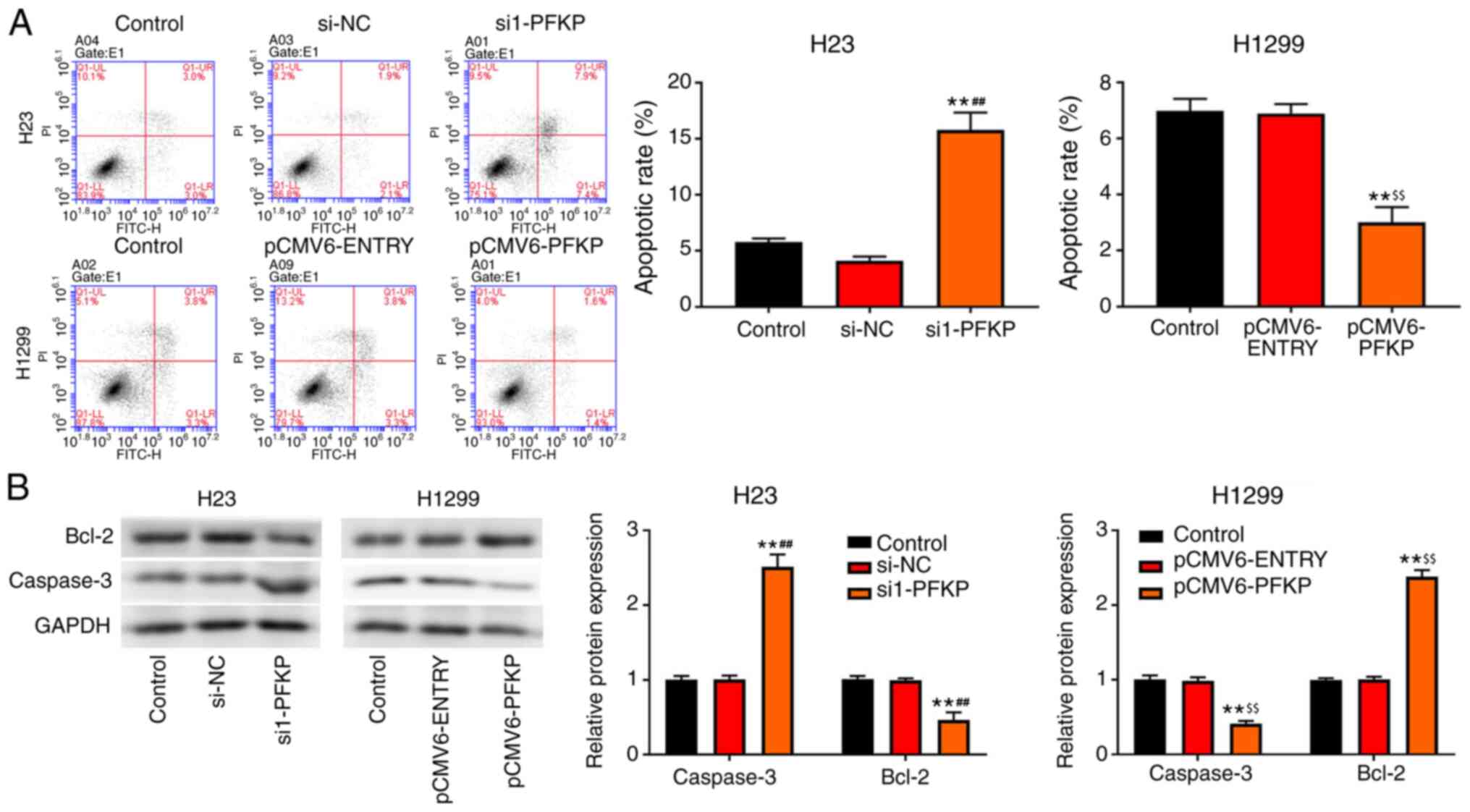
Apoptosis of H23 and H1299 cells was detected using
flow cytometry. As shown in Fig.
4A, overexpression of PFKP decreased the apoptotic rate in
H1299 cells compared with that in the control and pCMV6-ENTRY
groups (P<0.01), whereas an opposite trend in apoptotic rate was
observed in the si1-PFKP group of H23 cells. Moreover, the
expression levels of apoptosis-related proteins (caspase-3 and
Bcl-2) were detected using western blot analysis. As presented in
Fig. 4B, overexpression of PFKP
reduced the protein expression levels of caspase-3 and increased
the protein expression levels of Bcl-2 in H1299 cells compared with
those in the control and pCMV6-ENTRY groups (P<0.01), whereas
the opposite results were observed in the si1-PFKP group of H23
cells.
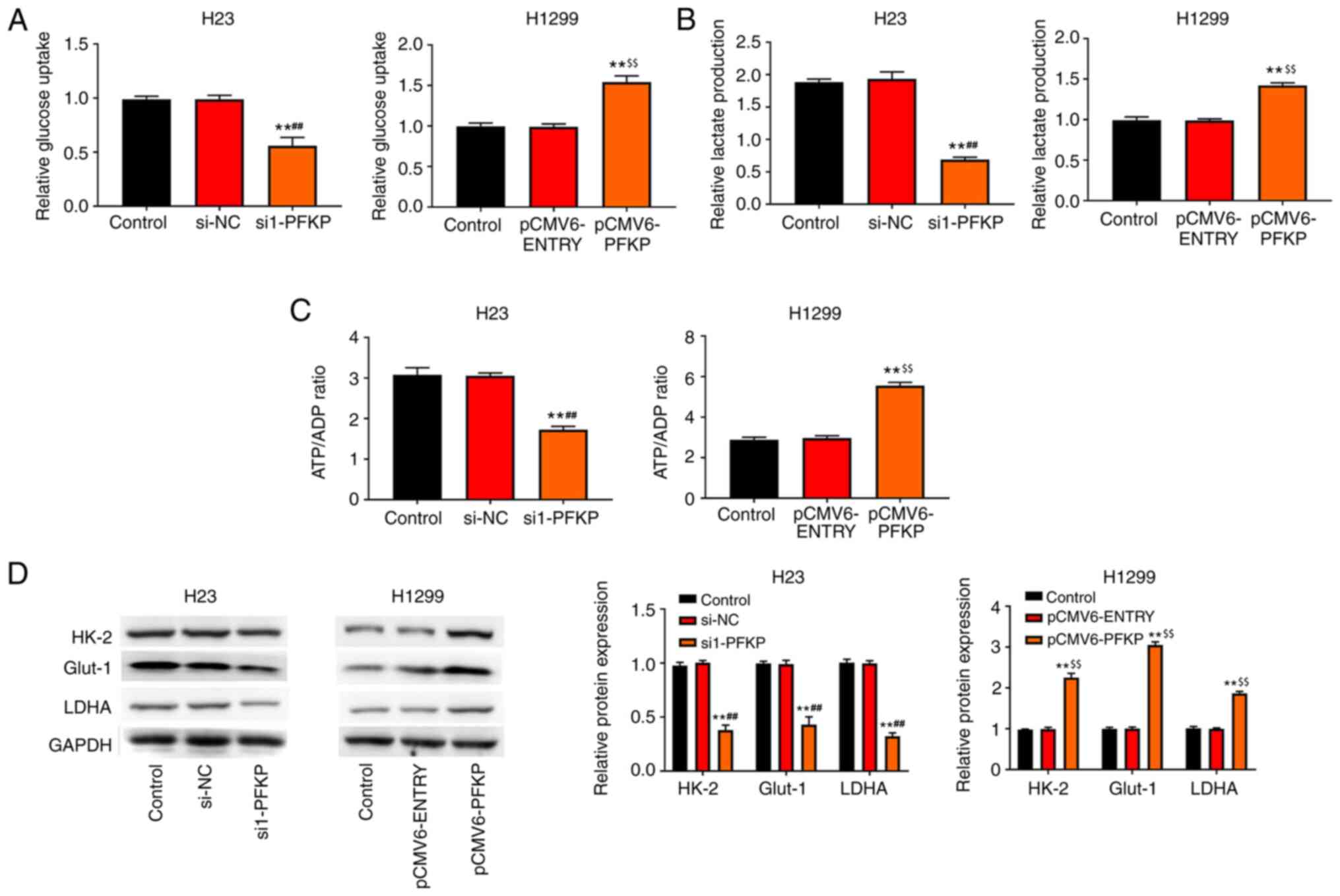
PFKP promotes glycolysis of NSCLC
cells
The present study also investigated the effect of
PFKP on glucose metabolism. The results revealed that
overexpression of PFKP enhanced glucose uptake, lactate production
and the ATP/ADP ratio in H1299 cells compared with those in the
control and pCMV6-ENTRY groups (P<0.01), whereas knockdown of
PFKP reduced glucose uptake, lactate production and the ATP/ADP
ratio in H23 cells compared with those in the control and si-NC
groups (P<0.01; Fig. 5A-C).
Moreover, the expression levels of glycolysis-associated enzymes
(HK-2, LDHA and Glut-1) were detected using western blot analysis.
As shown in Fig. 5D, overexpression
of PFKP increased the protein expression levels of HK-2, LDHA and
Glut-1 in H1299 cells compared with those in the control and
pCMV6-ENTRY groups (P<0.01), whereas knockdown of PFKP decreased
the protein expression levels of HK-2, LDHA and Glut-1 in H23 cells
compared with those in the control and si-NC groups
(P<0.01).
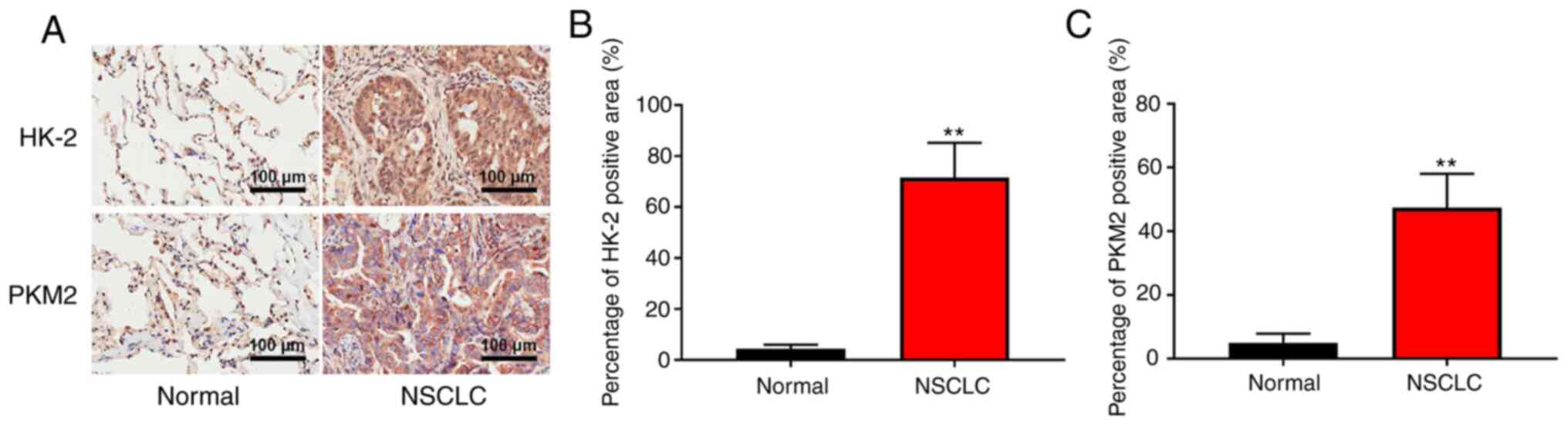
PFKP may increase the expression of
HK-2 and PKM2 and regulate glycolysis in NSCLC specimens
To further determine the clinical significance of
PFKP in glycolysis, the expression of glycolysis-associated enzymes
(HK-2 and PKM2) was detected in patient samples (NSCLC and
corresponding adjacent tissues) using IHC. As shown in Fig. 6, the expression levels of HK-2 and
PKM2 were significantly higher in NSCLC tissues than those in
normal tissues (P<0.01). These results suggested that PFKP may
increase the expression of HK-2 and PKM2, and regulate glycolysis
in NSCLC.
Discussion
NSCLC presents as fatal malignant tumors, and ≥65%
of patients with NSCLC exhibit cancer progression with local
advanced or metastatic features (26). PFKP is a second rate-limiting enzyme
that has an important role in glycolysis and affects tumor
progression (27). In the present
study, PFKP expression was revealed to be upregulated in NSCLC
tissues and cells, and was associated with lymph node metastasis
and histological grade. In addition, the present findings
demonstrated that overexpression of PFKP inhibited cell apoptosis,
and promoted proliferation, migration, invasion, EMT and glycolysis
in NSCLC cells, whereas knockdown of PFKP had the opposite
effect.
To develop novel treatment strategies for cancer,
accumulating evidence has demonstrated that biomarkers are
associated with cancer progression (28,29).
Previously, it has been reported that the expression of PFKP is
upregulated in most types of cancer and is associated with
prognosis (30). In addition, it
has been reported that the expression of PFKP in oral squamous cell
carcinoma tissues is higher than that in adjacent noncancerous
tissues, and that it is associated with differentiation and lymph
node metastasis (31). Lee et
al (30) suggested that PFKP
expression in human glioblastoma (GBM) specimens may be upregulated
compared with that in normal human brain tissue, and PFKP
expression was shown to be increased in primary GBM cells.
Consistent with these previous results, the present study
demonstrated that PFKP expression was upregulated in NSCLC tissues
and cells, and was associated with lymph node metastasis and
histological grade. These findings suggested that PFKP may have an
important role in the carcinogenesis of NSCLC.
Phosphofructokinase-1 (PFK-1) is a key rate-limiting
enzyme that has a critical role in the glycolytic pathway (32). PFK-1 has three isoforms: Liver
isoform of phosphofructokinase (PFKL), muscle isoform of
phosphofructokinase and PFKP (33).
It has been reported that knockdown of PFK-1 expression may inhibit
cancer cell proliferation and tumorigenicity (34). Yang et al (35) demonstrated that knockdown of PFKL
inhibited cell growth and colony formation in H460 and H1299 cells,
whereas overexpression of PFKL promoted cell growth and colony
formation. Moreover, a study by Wang et al (21) indicated that knockdown of PFKP
suppressed cell proliferation, promoted apoptosis and induced cell
cycle arrest in kidney cancer cells. In the present study,
overexpression of PFKP promoted cell proliferation, inhibited
apoptosis, reduced the protein expression of caspase-3 and
increased the protein expression of Bcl-2 in H1299 cells, whereas
knockdown of PFKP in H23 cells had the opposite effect. Taken
together, these findings indicated that PFKP may promote cell
proliferation and inhibit apoptosis of NSCLC cells.
Metastasis is the main cause of death in patients
with cancer (36). EMT is reported
to be an important cause of distal metastases of malignant tumors
(37). Accumulating evidence has
demonstrated that metastatic potential can be enhanced by
activating EMT in numerous types of cancer, including HCC (38), BC (39) and cervical cancer (40). The present study revealed that
overexpression of PFKP decreased the protein expression levels of
E-cadherin, and increased the protein expression levels of
N-cadherin and vimentin, whereas knockdown of PFKP had the opposite
effect. In addition, overexpression of PFKP increased the wound
healing rate and number of invasive cells among H1299 cells, but
knockdown of PFKP decreased the wound healing rate and number of
invasive cells among H23 cells. These results suggested that PFKP
may promote migration, invasion and EMT in NSCLC cells.
The Warburg effect serves a critical role in tumor
development (41). Aerobic
glycolysis is the main energy supply mode for tumor cells (42). During glycolysis, glucose is
converted to pyruvate to accumulate lactate, which is accompanied
by ATP production (43,44). Moreover, a number of enzymes
(including HK2, GLUT1, LDHA and PKM2) are involved in glycolysis
(45,46). Wang et al (21) reported that knockdown of PFKP
reduced glucose uptake and lactate production in kidney cancer
cells. In the present study, the results revealed that
overexpression of PFKP enhanced glucose uptake, lactate production
and the ATP/ADP ratio, and increased the protein expression of
HK-2, LDHA and Glut-1 in H1299 cells, whereas knockdown of PFKP
reduced glucose uptake, lactate production and the ATP/ADP ratio,
and decreased the protein expression of HK-2, LDHA and Glut-1 in
H23 cells. Moreover, the present results showed that the expression
levels of HK-2 and PKM2 in NSCLC tissues were higher than those in
normal tissues. These results suggested that PFKP may promote the
protein expression of HK-2, LDHA, Glut-1 and PKM2, and regulate
glycolysis in NSCLC.
In conclusion, PFKP expression in NSCLC tissues and
cells was upregulated, and was associated with lymph node
metastasis and histological grade. Moreover, PFKP inhibited cell
apoptosis, and promoted proliferation, migration, invasion and
glycolysis in NSCLC cells. Although Kaplan-Meier survival analysis
of TCGA data was performed in the present study, further studies,
such as Kaplan-Meier survival analysis of clinical data, are
required to investigate the prognostic value of PFKP. However, the
present study provides a deeper understanding of NSCLC progression,
which may provide a foundation for the treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ designed the study. FW and LL performed the
research, analyzed the data and wrote the paper. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong Provincial Hospital (grant no. 2020-181). All
patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADP
|
adenosine diphosphate
|
|
ATP
|
adenosine triphosphate
|
|
BC
|
breast cancer
|
|
Bcl-2
|
B-cell lymphoma-2
|
|
CCK-8
|
Cell Counting Kit-8
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FBS
|
fetal bovine serum
|
|
GBM
|
glioblastoma
|
|
Glut-1
|
glucose transporter-1
|
|
HCC
|
hepatocellular carcinoma
|
|
HK-2
|
hexokinase-2
|
|
HRP
|
horseradish peroxidase
|
|
IHC
|
immunohistochemistry
|
|
LDHA
|
lactate dehydrogenase A
|
|
NSCLC
|
non-small cell lung cancer
|
|
OD
|
optical density
|
|
PFK-1
|
phosphofructokinase-1
|
|
PFKL
|
liver isoform of
phosphofructokinase
|
|
PFKP
|
platelet isoform of
phosphofructokinase
|
|
PKM2
|
pyruvate kinase M2
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walker S: Updates in non-small cell lung
cancer. Clin J Oncol Nurs. 12:587–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shao Y, Liang B, Long L and Jiang SJ:
Diagnostic MicroRNA biomarker discovery for non-small-cell lung
cancer adenocarcinoma by integrative bioinformatics analysis.
Biomed Res Int. 2017:25630852017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu C, Zhu W, Qian J, He S, Wu C, Chen Y
and Shu Y: WT1 promotes invasion of NSCLC via suppression of CDH1.
J Thorac Oncol. 8:1163–1169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Mukherjee K, Vats A, Ghosh D and Pillai
SK: Data analysis and network study of non-small-cell lung cancer
biomarkers. Advances in Computational Intelligence. Sahana S and
Bhattacharjee V: Springer; Singapore: pp. 265–272. 2020, View Article : Google Scholar
|
|
6
|
Liao Y, Cao L, Wang F and Pang R:
miR-605-5p promotes invasion and proliferation by targeting TNFAIP3
in non-small-cell lung cancer. J Cell Biochem. 121:779–787. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Li X, Wu S, Xue M and Chen W: Long
non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase
2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci.
105:951–955. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Wang X, Zhang J, Lam EK, Shin VY,
Cheng AS, Yu J, Chan FK, Sung JJ and Jin HC: Warburg effect
revisited: An epigenetic link between glycolysis and gastric
carcinogenesis. Oncogene. 29:442–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meienhofer Mc, De Medicis E, Cognet M and
Kahn A: Regulation of genes for glycolytic enzymes in cultured rat
hepatoma cell lines. Eur J Biochem. 169:237–243. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brand K: Aerobic glycolysis by
proliferating Cells: Protection against oxidative stress at the
expense of energy yield. J Bioenerg Biomembr. 29:355–364. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gillies RJ, Robey I and Gatenby RA: Causes
and consequences of increased glucose metabolism of cancers. J Nucl
Med. 49 (Suppl 2):24S–42S. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lang N, Wang C, Zhao J, Shi F, Wu T and
Cao H: Long non-coding RNA BCYRN1 promotes glycolysis and tumor
progression by regulating the miR-149/PKM2 axis in non-small-cell
lung cancer. Mol Med Rep. 21:1509–1516. 2020.PubMed/NCBI
|
|
13
|
Gan J, Li S, Meng Y, Liao Y, Jiang M, Qi
L, Li Y and Bai Y: The influence of photodynamic therapy on the
Warburg effect in esophageal cancer cells. Lasers Med Sci.
35:1741–1750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Cheng D, Zhu M, Yu H, Pan Z, Liu L,
Geng Q, Pan H, Yan M and Yao M: OTUB2 stabilizes U2AF2 to promote
the Warburg effect and tumorigenesis via the AKT/mTOR signaling
pathway in non-small cell lung cancer. Theranostics. 9:179–195.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi J, Wang H, Feng W, Huang S, An J, Qiu
Y and Wu K: MicroRNA-130a targeting hypoxia-inducible factor 1
alpha suppresses cell metastasis and Warburg effect of NSCLC cells
under hypoxia. Life Sci. 255:1178262020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu T and Yin H: PDK1 promotes tumor cell
proliferation and migration by enhancing the Warburg effect in
non-small cell lung cancer. Oncol Rep. 37:193–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park YY, Kim SB, Han HD, Sohn BH, Kim JH,
Liang J, Lu Y, Rodriguez-Aguayo C, Lopez-Berestein G, Mills GB, et
al: Tat-activating regulatory DNA-binding protein regulates
glycolysis in hepatocellular carcinoma by regulating the platelet
isoform of phosphofructokinase through microRNA 520. Hepatology.
58:182–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang YM, Liu JK and Wong TY: The DNA
excision repair system of the highly radioresistant bacterium
Deinococcus radiodurans is facilitated by the pentose phosphate
pathway. Mol Microbiol. 48:1317–1323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Xu Q, Ji D, Wei Y, Chen H, Li T,
Wan B, Yuan L, Huang R and Chen G: Inhibition of pentose phosphate
pathway suppresses acute myelogenous leukemia. Tumour Biol.
37:6027–6034. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim NH, Cha YH, Lee J, Lee SH, Yang JH,
Yun JS, Cho ES, Zhang X, Nam M, Kim N, et al: Snail reprograms
glucose metabolism by repressing phosphofructokinase PFKP allowing
cancer cell survival under metabolic stress. Nat Commun.
8:143742017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Zhang P, Zhong J, Tan M, Ge J, Tao
L, Li Y, Zhu Y, Wu L, Qiu J and Tong X: The platelet isoform of
phosphofructokinase contributes to metabolic reprogramming and
maintains cell proliferation in clear cell renal cell carcinoma.
Oncotarget. 7:27142–27157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Lichtenberg T, Hoadley KA, Poisson
LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee
AV, et al: An integrated TCGA pan-cancer clinical data resource to
drive high-quality survival outcome analytics. Cell.
173:400–416.e11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Therneau T M and Grambsch P M: Modeling
Survival Data: Extending the Cox Model. Springer; New York, NY:
2000, View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mor I, Cheung EC and Vousden KH: Control
of glycolysis through regulation of PFK1: Old friends and recent
additions. Cold Spring Harb Symp Quant Biol. 76:211–216. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang C, Chen S, Wang Y, Liu X, Hu F, Sun J
and Yuan H: Lipase-triggered water-responsive ‘Pandora's Box’ for
cancer therapy: Toward induced neighboring effect and enhanced drug
penetration. Adv Mater. 30:e17064072018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang D, Zhao X, Zhang L, Wang Z and Wang
C: Identification of hub genes to regulate breast cancer metastasis
to brain by bioinformatics analyses. J Cell Biochem. 120:9522–9531.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JH, Liu R, Li J, Zhang C, Wang Y, Cai
Q, Qian X, Xia Y, Zheng Y, Piao Y, et al: Stabilization of
phosphofructokinase 1 platelet isoform by AKT promotes
tumorigenesis. Nat Commun. 8:9492017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen G, Liu H, Zhang Y, Liang J, Zhu Y,
Zhang M, Yu D, Wang C and Hou J: Silencing PFKP inhibits
starvation-induced autophagy, glycolysis, and epithelial
mesenchymal transition in oral squamous cell carcinoma. Exp Cell
Res. 370:46–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wegener G and Krause U: Different modes of
activating phosphofructokinase, a key regulatory enzyme of
glycolysis, in working vertebrate muscle. Biochem Soc Trans.
30:264–270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moon JS, Kim HE, Koh E, Park SH, Jin WJ,
Park BW, Park SW and Kim KS: Krüppel-like factor 4 (KLF4) activates
the transcription of the gene for the platelet isoform of
phosphofructokinase (PFKP) in breast cancer. J Biol Chem.
286:23808–23816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yi W, Clark PM, Mason DE, Keenan MC, Hill
C, Goddard WA III, Peters EC, Driggers EM and Hsieh-Wilson LC:
Phosphofructokinase 1 glycosylation regulates cell growth and
metabolism. Science. 337:975–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J, Li J, Le Y, Zhou C, Zhang S and
Gong Z: PFKL/miR-128 axis regulates glycolysis by inhibiting AKT
phosphorylation and predicts poor survival in lung cancer. Am J
Cancer Res. 6:473–485. 2016.PubMed/NCBI
|
|
36
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu
Y, Yao Y and Li D: The epithelial to mesenchymal transition (EMT)
and cancer stem cells: Implication for treatment resistance in
pancreatic cancer. Mol Cancer. 16:522017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma P, Tang WG, Hu JW, Hao Y, Xiong LK,
Wang M, Liu H, Bo WH and Yu KH: HSP4 triggers
epithelial-mesenchymal transition and promotes motility capacities
of hepatocellular carcinoma cells via activating AKT. Liver Int.
40:1211–1223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiao Y, Xie Q, Qin Q, Liang Y, Lin H and
Zeng D: Upregulation of SOX11 enhances tamoxifen resistance and
promotes epithelial-to-mesenchymal transition via slug in MCF-7
breast cancer cells. J Cell Physiol. 235:7295–7308. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang L, Yu Y, Xiong Z, Chen H, Tan B and
Hu H: Downregulation of SEMA4C inhibit epithelial-mesenchymal
transition (EMT) and the invasion and metastasis of cervical cancer
cells via inhibiting transforming growth factor-beta 1
(TGF-β1)-induced hela cells p38 mitogen-activated protein kinase
(MAPK) activation. Med Sci Monit. 26:e9181232020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo
F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z, et al:
ERK1/2-dependent phosphorylation and nuclear translocation of PKM2
promotes the Warburg effect. Nat Cell Biol. 14:1295–1304. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu L, Wang Y, Bai R, Yang K and Tian Z:
MiR-186 inhibited aerobic glycolysis in gastric cancer via HIF-1α
regulation. Oncogenesis. 6:e3182017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akram M: Mini-review on glycolysis and
cancer. J Cancer Educ. 28:454–457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ganapathy-Kanniappan S: Molecular
intricacies of aerobic glycolysis in cancer: Current insights into
the classic metabolic phenotype. Crit Rev Biochem Mol Biol.
53:667–682. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wan W, Peng K, Li M, Qin L, Tong Z, Yan J,
Shen B and Yu C: Histone demethylase JMJD1A promotes urinary
bladder cancer progression by enhancing glycolysis through
coactivation of hypoxia inducible factor 1α. Oncogene.
36:3868–3877. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu H, Zeng Y, Liu L, Gao Q, Jin S, Lan Q,
Lai W, Luo X, Wu H, Huang Y and Chu Z: PRL-3 improves colorectal
cancer cell proliferation and invasion through IL-8 mediated
glycolysis metabolism. Int J Oncol. 51:1271–1279. 2017. View Article : Google Scholar : PubMed/NCBI
|