Introduction
Pulmonary artery hypertension (PAH) is a major
complication associated with chronic obstructive pulmonary disease
(COPD), which is one of the most common health problems worldwide
(1,2). PAH is frequently observed in patients
with advanced COPD and is considered as a predictor of poor
outcomes (3). PAH in COPD is caused
by the remodeling of pulmonary arteries, which is characterized by
the intimal proliferation of poorly differentiated smooth muscle
cells and the deposition of elastic and collagen fibers (4). To date, long-term oxygen therapy is
the most effective treatment strategy for patients with COPD
complicated by PAH and hypoxia as it can slow or reverse the
progression of the disease (5).
Conventional vasodilators are not used because of the potential
harmful influences of gas exchange, due to inhibition of hypoxic
pulmonary vasoconstriction and their lack of efficacy after
long-term treatment (6). Therefore,
the development of novel drugs and therapeutic strategies for PAH
is important.
In China, patients with COPD and PAH often turn to
alternative and complementary treatments, which have been reported
to be effective and safe (7). In
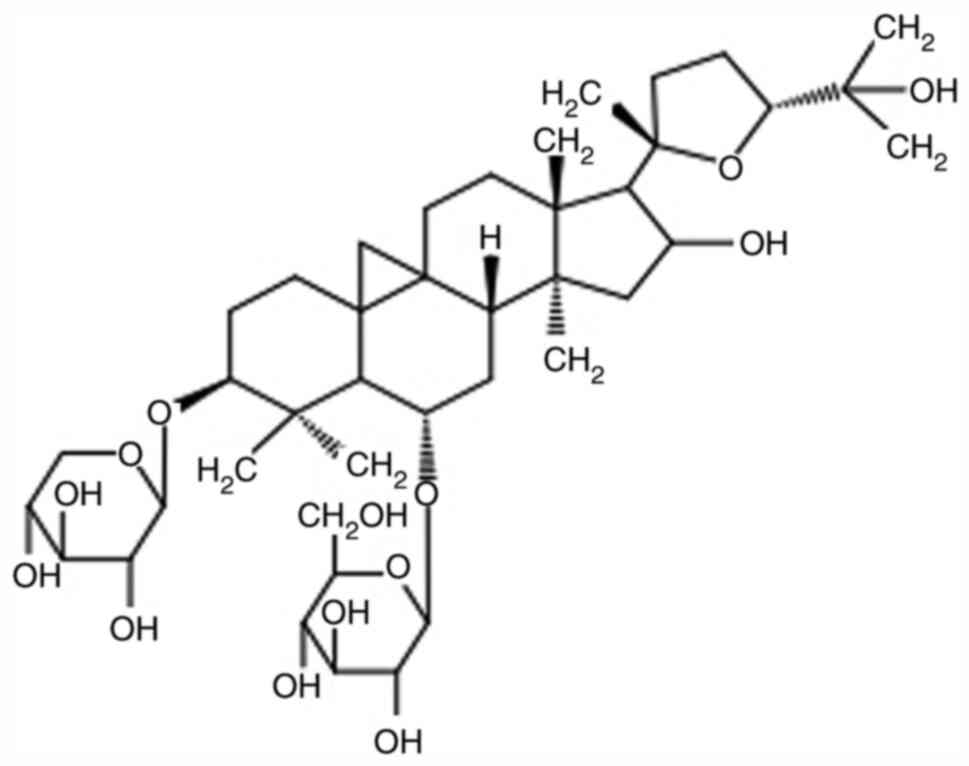
the Chinese Pharmacopeia, Astragaloside IV (AS-IV;
3-O-β-D-xylopyranosyl-6-O-β-D-glucopyranosyl cycloastragenol;
Fig. 1) is the major biologically
active compound in Huangqi (Radix Astragali Mongolici), a
Chinese herbal remedy widely used for the clinical treatment of
vascular diseases, such as essential hypertension and PAH (8,9).
Recently, a study in vitro experiments have confirmed that
AS-IV can stimulate human umbilical vein endothelial cell
proliferation and the development of tube-like structures (10). Furthermore, AS-IV can suppress
platelet-derived growth factor-BB-induced vascular smooth muscle
cell proliferation and migration, potentially via inhibition of the
p38MAPK signaling pathway (11,12).
The results indicated that AS-IV may serve an important therapeutic
role in diseases caused by abnormal vascular function.
The pathogenesis of PAH is relatively complex and is
not completely understood (13).
Pulmonary vascular remodeling is an important marker of the degree
of severity and progression in PAH, which is primarily due to the
imbalance between pulmonary artery smooth muscle cell (PASMC)
proliferation and apoptosis (14,15).
Notch signaling, a highly evolutionarily conserved signaling
pathway, serves an important role in regulating cell fate
proliferation, differentiation and apoptosis (16,17).
Notch-3 targets hes family bHLH transcription factor 5 (Hes-5),
which is expressed exclusively in smooth muscle cells (SMCs) in
adults and might be associated with SMC identity, maturation and
proliferation (18,19). In vitro, PASMCs from patients
with PAH display higher mRNA and protein expression levels of
Notch-3 and Hes-5 compared with healthy controls (20). Moreover, Notch-3 knockout mice
display a lack of PASMCs; however, treatment with the γ-secretase
inhibitor,
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycinet-butyl
ester (DAPT), which blocks Notch receptor cleavage, attenuates
hypoxia-induced PAH in mice (21).
The aforementioned studies indicated that Notch signaling is
associated with the development of PAH, favoring a vascular
proliferative phenotype. In the present study, hypoxia-induced PAH
was established in vitro and in vivo to investigate
the regulatory activity of AS-IV in pulmonary vascular remodeling
and to explore the underlying mechanisms.
Materials and methods
Reagents and antibodies
High purity AS-IV (95.8% by high-performance liquid
chromatography; analytical grade) was obtained from the National
Institutes for Food and Drug Control. AS-IV was dissolved in DMSO
and the final DMSO concentration did not exceed 0.1%. DAPT and MTT
were purchased from Sigma-Aldrich (Merck KGaA). TRIzol®
Reagent, Super-Script II reverse transcriptase and Hot Master Taq
DNA Polymerase were obtained from Takara Bio, Inc. The SYBR-Green I
assay kit was from Roche Diagnostics.
Animal model and treatment groups
A total of 40 male Sprague-Dawley rats were obtained
from the Center for Experimental Animals, Central South University
(license no. 20-010). Male, 12-week-old Sprague-Dawley rats
(weight, 210±10 g) were fed a standard diet and water. The
temperature and humidity were set at 21-23°C and 40–60%,
respectively. A 12-h light/dark cycle was used. All animals were
acclimatized in the metabolic cages for a week prior to
experiments. All animal experiments were approved by the Animal
Care and Use Committee of Central South University. The rats were
randomly assigned to the following four groups (n=10 per group): i)
normoxia (N); ii) hypoxia (H); iii) treatment (T); and iv) DAPT
(DAPT).
Rats in the N group were exposed to fractional
inspired oxygen at 21%. Rats in the H, T and DAPT groups were
maintained in a Poor Oxygen Controller chamber (10% O2
for 8 h/day) for 6 weeks. Anhydrous calcium chloride was used to
maintain <60% humidity (20).
Sodium hydroxide was applied for carbon dioxide absorption.
At the beginning of the third week of hypoxia, rats
in the T and DAPT groups were administered 2 mg/kg AS-IV
intragastrically once daily for 42 days. At the beginning, the
second, and the fourth week after hypoxia, rats in the DAPT group
were subcutaneously injected with 10 mg/kg DAPT three times
daily.
Measurement of pulmonary arterial
pressure
After six weeks of hypoxic exposure, rats were
weighed and anesthetized with an intraperitoneal injection of 40
mg/kg sodium pentobarbital. A micro-catheter (inner diameter, 0.9
mm) was gradually inserted via the right external jugular vein into
the pulmonary artery. Following a 30 min equilibration period, mean
pulmonary arterial blood pressure (mPAP) was collected and analyzed
using a BL-420F biological and functional information collection
system (Biolap 420F; Chengdu TaiMeng Technology Co.). The right
lung was frozen in liquid nitrogen, fixed with 10% formalin for 48
h and subsequently used for histology and IHC analyses. RV
hypertrophy was assessed in the right and left ventricles, and the
septum weight ratio was calculated according to the following
formula: (RV weight/LV weight + S weight), where S is the
septum.
Histology and microscopy
Formalin-fixed lung tissue was embedded in paraffin
and cut into 5-µm thick sections. Subsequently, tissue sections
were stained with hematoxylin and eosin (H&E) as previously
described (22). Stained sections
were observed in four randomly selected fields of view using an
SZX7 light microscope (magnification, ×200; Olympus Corporation)
and analyzed using Image-Pro Plus software 6.0 (Media Cybernetics,
Inc.). The results were evaluated according to elastic fiber
staining, which is indicated by black or dark blue staining. The
distance between inner and outer elastic fibers was calculated in
each field of view and the average of three measurements was
calculated to determine the thickness of vessel walls.
Immunohistochemistry
For immunohistochemistry, lung sections (5-µm thick)
were deparaffinized in xylene, rehydrated using a graduated alcohol
concentration series, then washed with PBS (pH 7.2–7.4). Following
antigen retrieval at 100°C and blocking with 5% BSA at room
temperature for 1 h, the sections were incubated overnight at 4°C
with a rabbit anti-PCNA antibody (1:200, cat. no. 13110; Cell
Signaling Technology, Inc.) and parallel control samples were
treated with PBS. Subsequently, sections were incubated with
HRP-conjugated anti-rabbit IgG secondary antibody (1:200, cat. no.
31460; Invitrogen; Thermo Fisher Scientific, Inc.) for 2 h at room
temperature. Sections were visualized using DAB and counterstained
using hematoxylin. Positive staining was indicated by brown and
yellow. The positive staining area in pulmonary vessels were
observed under a light microscope (magnification, ×400; Nikon
Corporation). The integrated optical density (IOD) of PCNA in the
pulmonary arteriole was examined using Image-Pro Plus 4.5 software
(Media Cybernetics, Inc.), and the ratio of the IOD to the area of
the arteriole was calculated to assess the expression of PCNA. The
number of PCNA-positive pulmonary vessels was considered as an
index of cell proliferation.
Cell culture
PASMCs were isolated from the pulmonary arteries of
each rat, as previously described (23). Briefly, the endothelia were removed
from isolated pulmonary arteries using a sterile cotton swab,
gently digested with 0.2% collagenase and incubated with PBS
supplemented with 0.1% BSA (cat. no. P3688, Sigma-Aldrich; Merck
KGaA) at 37°C for 2 h. Digested PASMCs were incubated in DMEM (cat.
no. D6046; Sigma-Aldrich; Merck KGaA) supplemented with 20% FBS
(cat. no. 12003, Sigma-Aldrich, Merck) at 37°C with 5%
CO2 for 5–7 days. Primary cell cultures at passage 3–5
were used for subsequent experiments. To determine cell purity,
PASMCs were subjected to α-smooth muscle actin (α-SMA)
immunofluorescence staining.
α-SMA immunofluorescence. PASMCs were fixed in 4%
formaldehyde for 15 min at 25°C, then incubated in 12% normal goat
serum (Vector Laboratories, Inc.) for 30 min at 25°C. The cells
were then incubated with the primary rabbit anti-α-smooth muscle
actin antibody (cat. no. 19245S; 1:200; Cell Signaling Technology,
Inc.) overnight at 4°C. Next, cells were incubated with an goat
anti-rabbit IgG secondary antibody (cat. no. 31460; 1:200;
Invitrogen; Thermo Fisher Scientific, Inc.) for 2 h at 37°C.
Propidium iodide (2.0 µmol/l; cat. no. 4087S; Cell Signaling
Technology, Inc.) was used to stain the cell nuclei for 1 h at
37°C. The images were observed using a DMI3000B fluorescence
microscope (Leica).
Cells were exposed to normoxia (21% O2
and 5% CO2) or hypoxia (3% O2 and 5%
CO2 balanced with 92% N2) for 6, 12, 24 or 48
h. PASMCs were divided into the following four groups: i) N; ii) H;
iii) T; and iv) DAPT. PASMCs in the N group were cultured in
normoxic conditions for 48 h and PASMCs in the H group were
cultured in hypoxic conditions for 48 h. PASMCs in the T group were
cultured in serum-free medium supplemented with 5, 10 or 20 µmol/l
AS-IV for 48 h under hypoxic conditions. Cells in the DAPT group
were pretreated with 5 mmol/l DAPT, the Notch signaling pathway
inhibitor, for 1 h, then cultured in medium supplemented with 20
µmol/l AS-IV under hypoxic conditions for 48 h.
Cell viability assay
PASMCs were subjected to cell cycle arrest for 24 h.
Subsequently, cells were transferred to PBS containing 5% FBS for
48 h at room temperature under normoxic or hypoxic conditions.
Cells were pretreated with DAPT for 1 h, then treated with AS-IV
under hypoxic conditions at 37°C for 48 h. Subsequently, PASMCs
were cultured in medium containing 0.5% MTT for 4 h at 25°C. DMSO
was used to dissolve the purple formazan for 10 min at 37°C. The
absorbance was measured at a wavelength of 540 nm using a
spectrophotometer.
Flow cytometry
Pulmonary vascular remodeling in rats is
characterized by increased vascular smooth muscle and endothelial
cell proliferation (24). To assess
cell cycle distribution, cells (5×105) were seeded into
glass dishes, digested by trypsinization and fixed with 75%
ethanol. Before cell cycle analysis, the ethanol-fixed cells were
centrifuged at 1,000 × g for 10 min and washed three times by
resuspending the cells in PBS at room temperature. The cells were
stained with 500 µl FxCycle™ propidium iodide (PI)/RNase staining
solution (Thermo Fisher Scientific, Inc.) at room temperature in
the dark for 30 min. The stained samples were then transferred to
new sterile flow cytometry tubes and maintained on ice until the
samples were analysed by flow cytometry using a BD FACSCanto™ II
flow cytometer (BD Biosciences), and cell cycle distribution was
determined using ModFit LT software 3.1(Verity Software House). The
cell proliferation index was calculated according to the following
formula: Proliferation index (%)=(S +
G2/M)/(G0/G1 + S +
G2/M) ×100.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from rat distal pulmonary
vessels and PASMCs using a TRIzol™ reagent (cat. no. 15596018;
Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. RNA purity was measured as the A260/A280
ratio using a Multiskan Sky Microplate spectrophotometer (cat. no.
51119570; Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA
(1,000 ng) was reverse transcribed into cDNA using the RevertAid
First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.).
Reverse transcription was performed using the following temperature
protocol: 37°C for 1 h and 94°C for 5 min. All primers used were
designed using the Primer Express™ software v.3.0.1 (Thermo Fisher
Scientific, Inc.) and are listed in Table SI. The reverse-transcribed cDNA was
then subjected to PCR using Taq DNA polymerase (cat. no. 10342020;
Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, qPCR was
performed using the SYBR-GreenER PCR kit. The following
thermocycling conditions were used for qPCR: Initial denaturation
at 94°C for 20 sec; followed by 45 cycles of 60°C for 30 sec and
72°C for 60 sec; and a cooling step at 4°C. mRNA expression levels
were quantified using the 2−ΔΔCq method (25) and normalized to the internal
reference gene GAPDH.
Western blotting
Western blotting was performed as previously
described (26). Briefly, total
proteins were extracted from PASMCs and homogenized lung tissue
samples using a lysis buffer containing protease (Beyotime
Institute of Biotechnology) and phosphatase inhibitors (Cell
Signaling Technology, Inc.). Protein concentrations were measured
using the BCA method. Proteins (50 µg) were separated via 10%
SDS-PAGE and electrophoretically transferred to equilibrated PVDF
membranes using semi-dry transfer. Following blocking with 5%
skimmed milk for 3 h at room temperature, the membranes were
incubated overnight at 4°C with primary antibodies targeted
against: GAPDH, Notch-3, Jagged-1, Hes-5 and PCNA (Table SII). Subsequently, the membranes
were incubated with anti-rabbit IgG HRP-conjugated antibody (1:200,
cat. no. 31470; Invitrogen; Thermo Fisher Scientific, Inc.) or
anti-mouse IgG HRP-conjugated antibody (1:800, cat. no. A-11077;
Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature for
1 h. Protein bands were visualized using an enhanced
chemiluminescence kit (cat. no. 32132X3; Invitrogen; Thermo Fisher
Scientific, Inc.). GAPDH was used as the loading control. Detection
was performed using the LI-COR Odyssey Scanning Infrared
Fluorescence Imaging system (LI-COR Bioscience).
Statistical analysis
All data are presented as the mean ± SD of three
experiments, and all statistical analyses were performed using
GraphPad Prism 7.05 (GraphPad Software, Inc.). Differences among
groups were analyzed using one-way analysis of variance (ANOVA)
followed by Tukey's post hoc test. P<0.05 were considered to
indicate a statistically significant difference.
Results
AS-IV can alleviate hypoxia-induced
pulmonary hypertension and vascular remodeling
To examine the effect of AS-IV on experimental PAH,
a rat model of hypoxia-induced PAH was established. Consistent with
a previous study (20), the results
demonstrated that hypoxia-induced PAH model rats displayed
significantly elevated mPAP levels, RV/LV+S ratios and percentage
wall thickness (WT) compared with the N group (Fig. 2A-C). Moreover, compared with the N
group, the H group displayed a notable increase in the thickness of
the smooth muscle layer, as determined by H&E staining
(Fig. 2D).
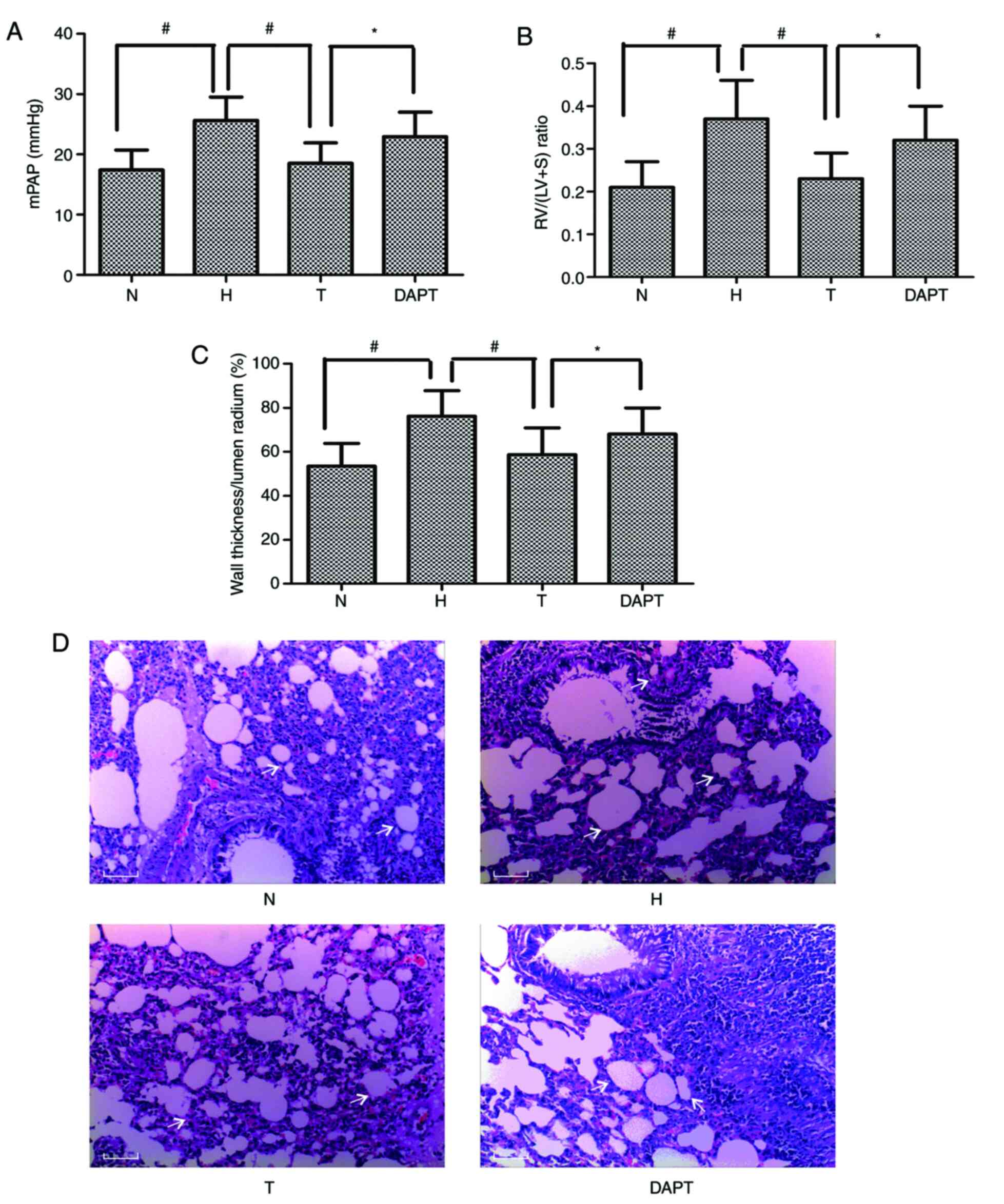 | Figure 2.Astragaloside IV attenuates chronic
hypoxia-induced pulmonary hypertension and pulmonary vascular
remodeling. Alterations in (A) mPAP, (B) the RV/LV+S ratio and (C)
percentage wall thickness. (D) Hematoxylin and eosin staining of
pulmonary arterioles. The white arrow refers to the wall and lumen
of pulmonary vessels. Scale bar, 5 µl. Magnification, ×200.
*P<0.05; #P<0.01. mPAP, mean pulmonary arterial
blood pressure; RV/LV+S, right ventricle/left ventricle + septum;
N, normoxia; H, hypoxia; T, treatment; DAPT,
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycinet-butyl
ester. |
Hypoxia-induced effects were significantly
alleviated by treatment with AS-IV, whereas pretreatment with DAPT
significantly inhibited the effects of AS-IV in alleviating
hypoxia-induced responses, including alterations to mPAP, RV/LV+S
ratios and the percentage WT. The results indicated that AS-IV
inhibited the progression of hypoxia-induced pulmonary hypertension
by pulmonary vascular remodeling via the Notch signaling
pathway.
AS-IV inhibits hypoxia-induced PASMC
proliferation
PASMCs displayed a typical ‘hill and valley’
appearance and were positive for α-smooth muscle actin (Fig. 3A). To assess the effects of AS-IV on
hypoxia-stimulated PASMCs, cell viability was measured by
performing an MTT assay. The results indicated that hypoxia
exposure significantly increased PASMC viability compared with the
N group. PASMC viability was increased in a dose- and
time-dependent manner following treatment with AS-IV. Compared with
the H group, the most significant inhibitory effects on cell
viability were observed in cells treated with 20 µmol/l AS-IV for
48 h (Fig. 3B and C). Furthermore,
PASMC proliferation was significantly increased under hypoxic
conditions, compared with normoxia. However, this increase in
proliferation under hypoxic conditions was abrogated following
treatment AS-IV with 20 µmol/l for 48 h. DAPT pretreatment restored
cell proliferation in AS-IV-treated cells (Fig. 3D).
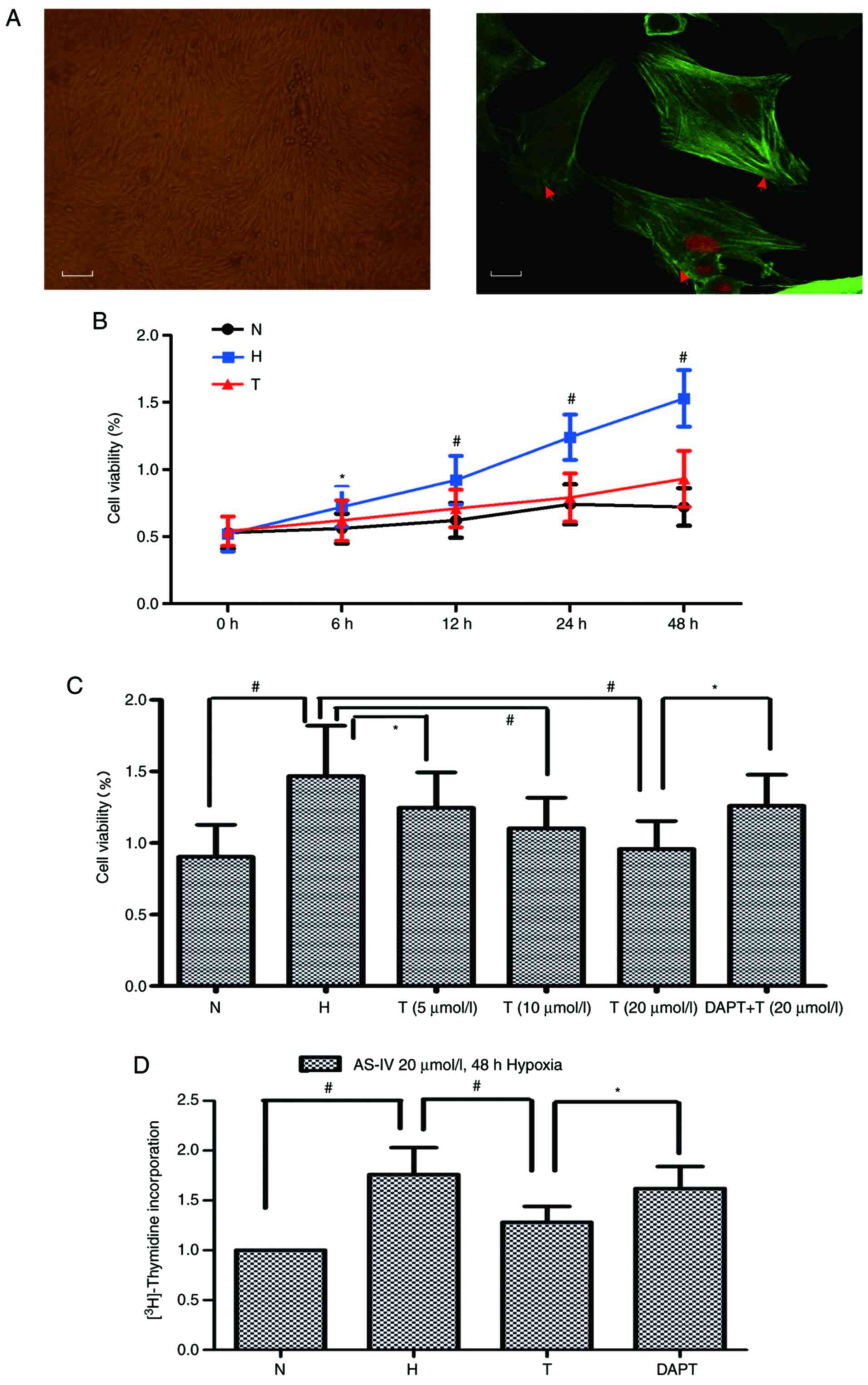 | Figure 3.AS-IV inhibits hypoxia-induced PASMC
proliferation. (A) Typical ‘hill and valley’ appearance of PASMCs.
Scale bar, 10 µl. Magnification, ×100. Immunofluorescence detection
of α-smooth muscle actin. The red arrow indicates skeleton protein
in the cytoplasm of pulmonary vascular smooth muscle cells. Scale
bar, 2.5 µl. Magnification, ×400. (B) PASMCs were treated with
AS-IV (20 µmol/l) under hypoxic conditions (3% O2) for
6, 12, 24 or 48 h. Cell viability was assessed by performing the
MTT assay. (C) PASMCs were pretreated with DAPT (5 mmol/l) for 1 h,
then treated with AS-IV (5–20 µmol/l) under hypoxic conditions for
48 h. Cell viability was assessed by performing the MTT assay. (D)
PASMCs were pretreated with DAPT (5 mmol/l) for 1 h, then treated
with AS-IV (20 µmol/l) under hypoxic conditions (3% O2)
for 48 h. *P<0.05; #P<0.01. AS-IV, Astragaloside
IV; PASMC, pulmonary artery smooth muscle cell; DAPT,
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycinet-butyl
ester; N, normoxia; H, hypoxia; T, treatment. |
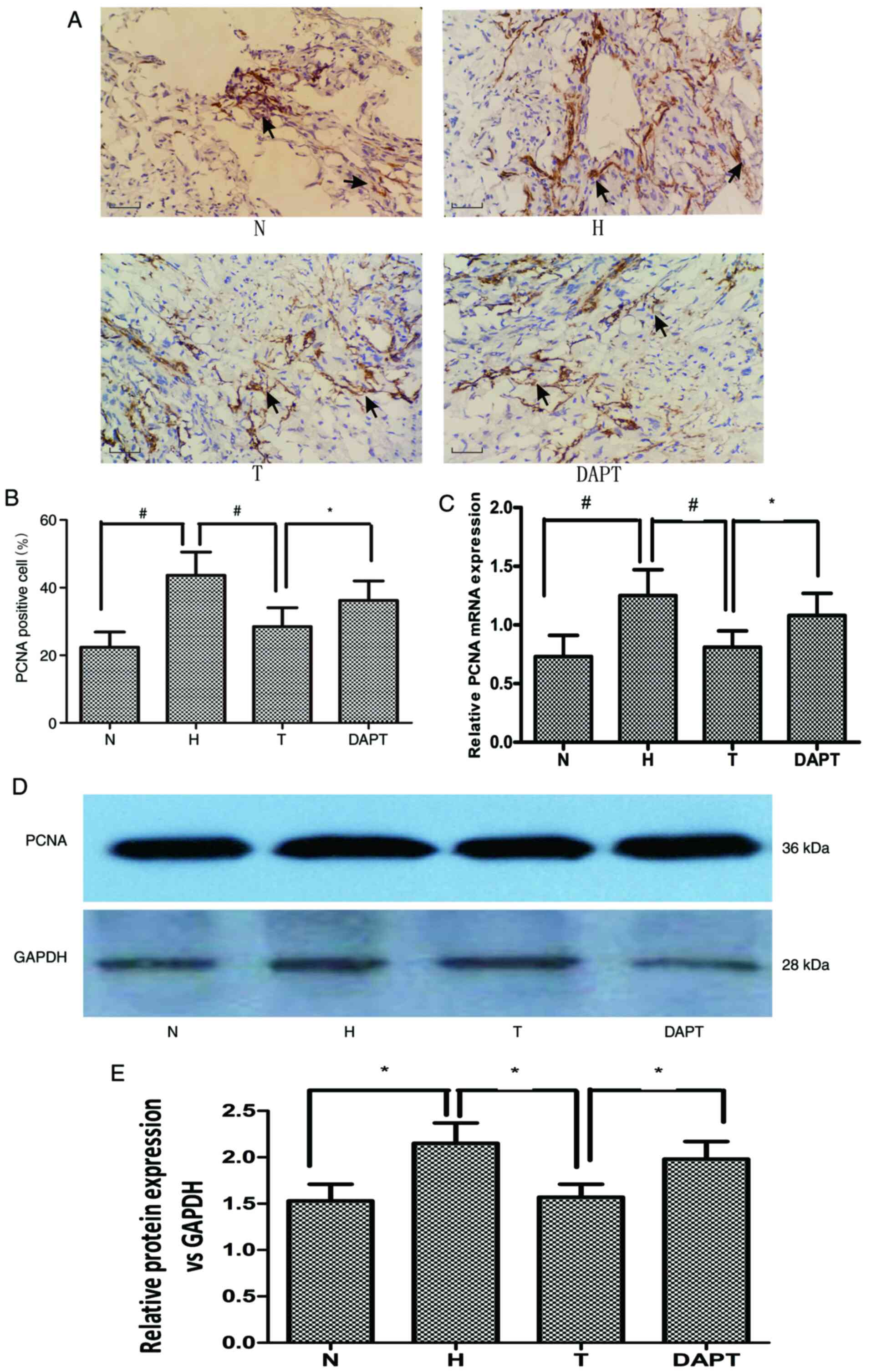
PCNA serves important role in cell proliferation and
its levels can be used as a cell proliferation index (27). Compared with the N group, the H
group displayed significantly increased PCNA expression in
pulmonary vascular tissue and PASMCs (Fig. 4), which was significantly reversed
by treatment with AS-IV. However, DAPT pretreatment restored PCNA
expression levels that were repressed by AS-IV in hypoxia-treated
pulmonary vascular tissue and PASMCs. Collectively, the results
suggested that hypoxia-induced PASMC proliferation was inhibited by
treatment with AS-IV via activation of Notch signaling.
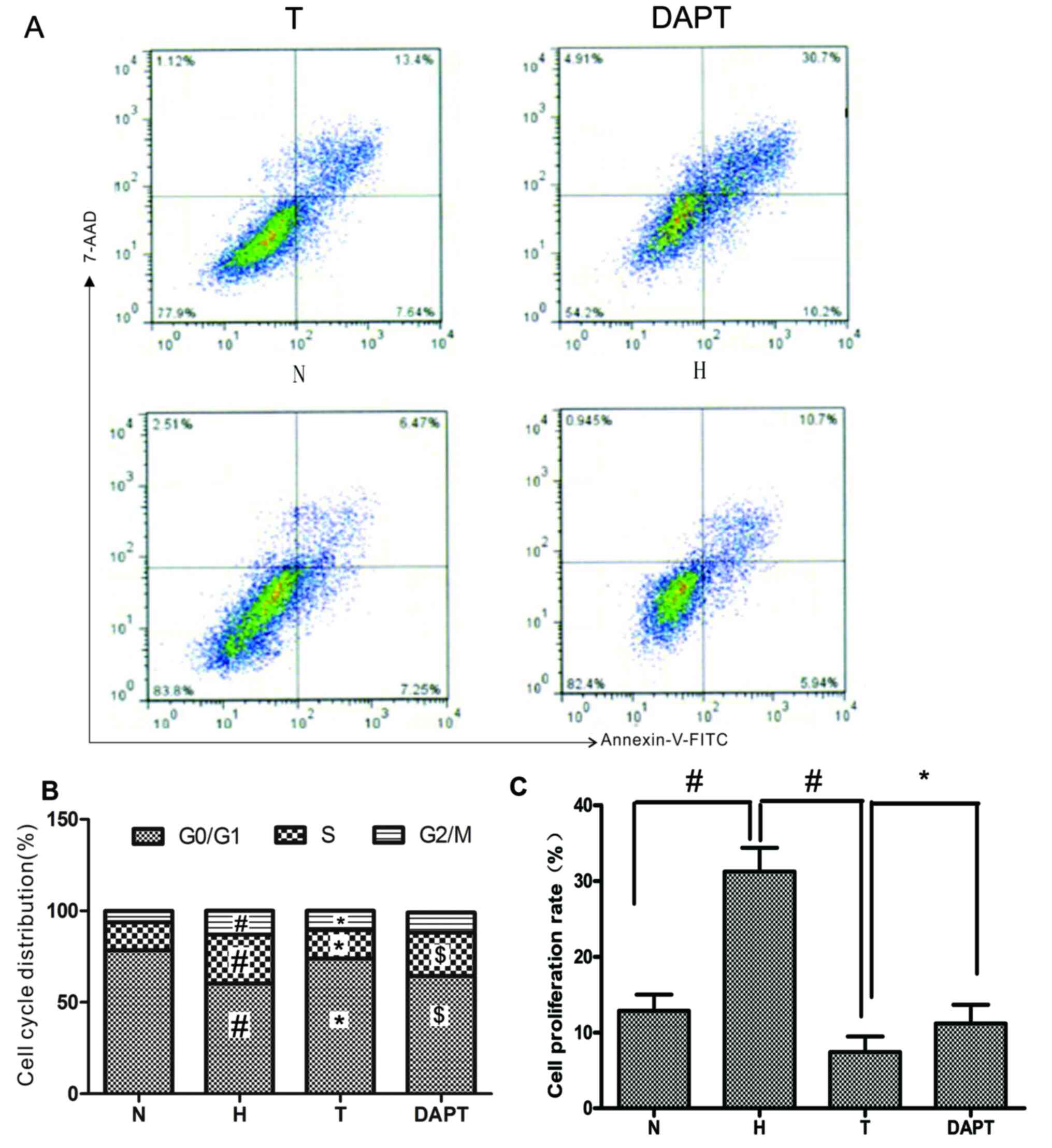
Effect of AS-IV on cell cycle
progression
To further investigate the mechanism underlying the
effects of AS-IV on hypoxia-stimulated PASMC proliferation, whether
AS-IV affected cell cycle progression was examined by performing
flow cytometry (Fig. 5). Compared
with the N group, the H group displayed a markedly increased the
cell proliferation rate and cell cycle arrest in the S and
G2/M phases. By contrast, the cell proliferation rate
was inhibited by treatment with AS-IV, which notably reduced the
proportion of cells entering the S and G2/M phases
compared with the H group. Moreover, DAPT pretreatment promoted
cell cycle progression, increasing the proportion of cells entering
the S and G2/M phases compared with the T group. The
results suggested that AS-IV displayed an important effect on the
cell cycle, inhibiting PASMC proliferation via Notch signaling.
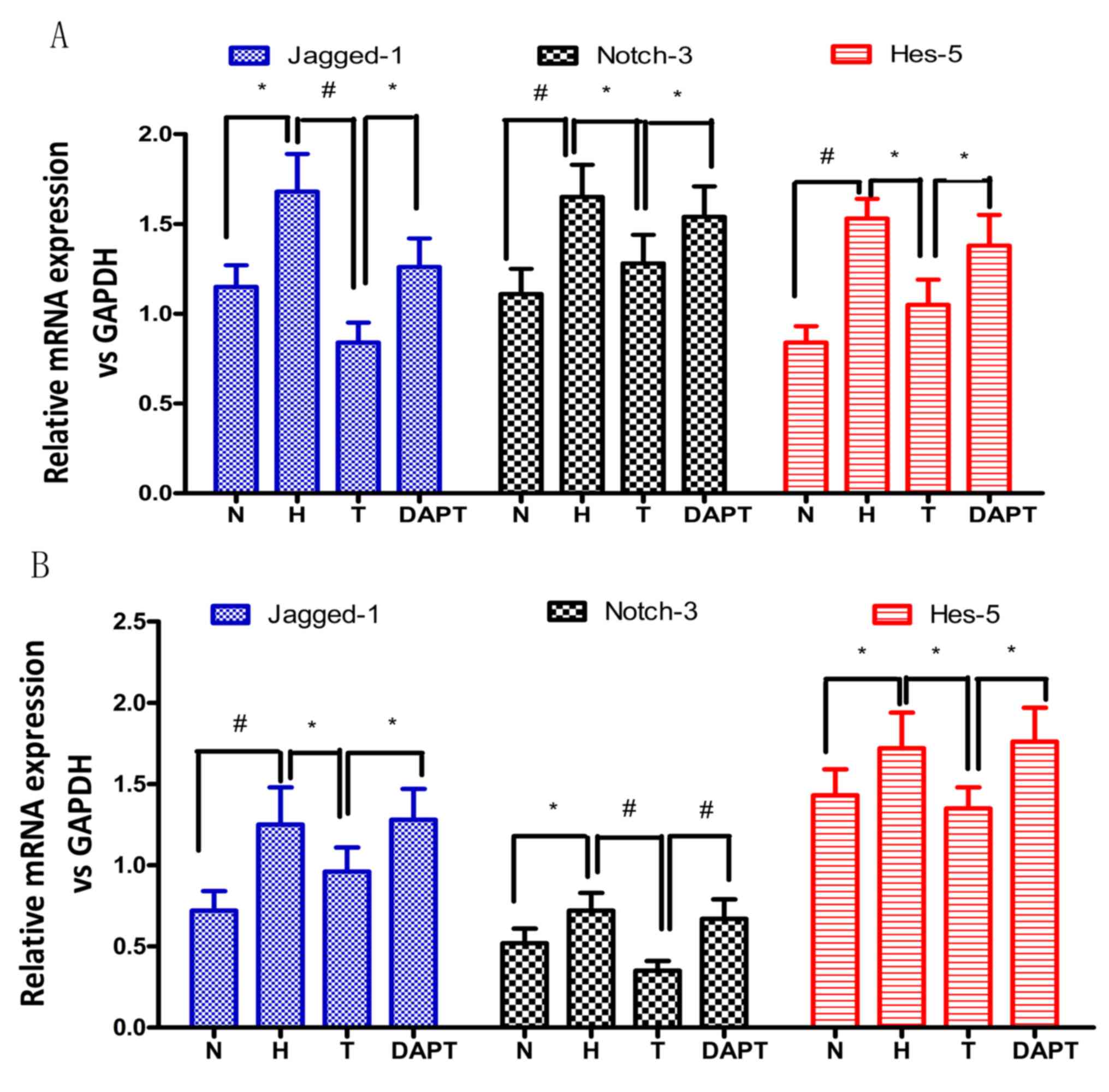
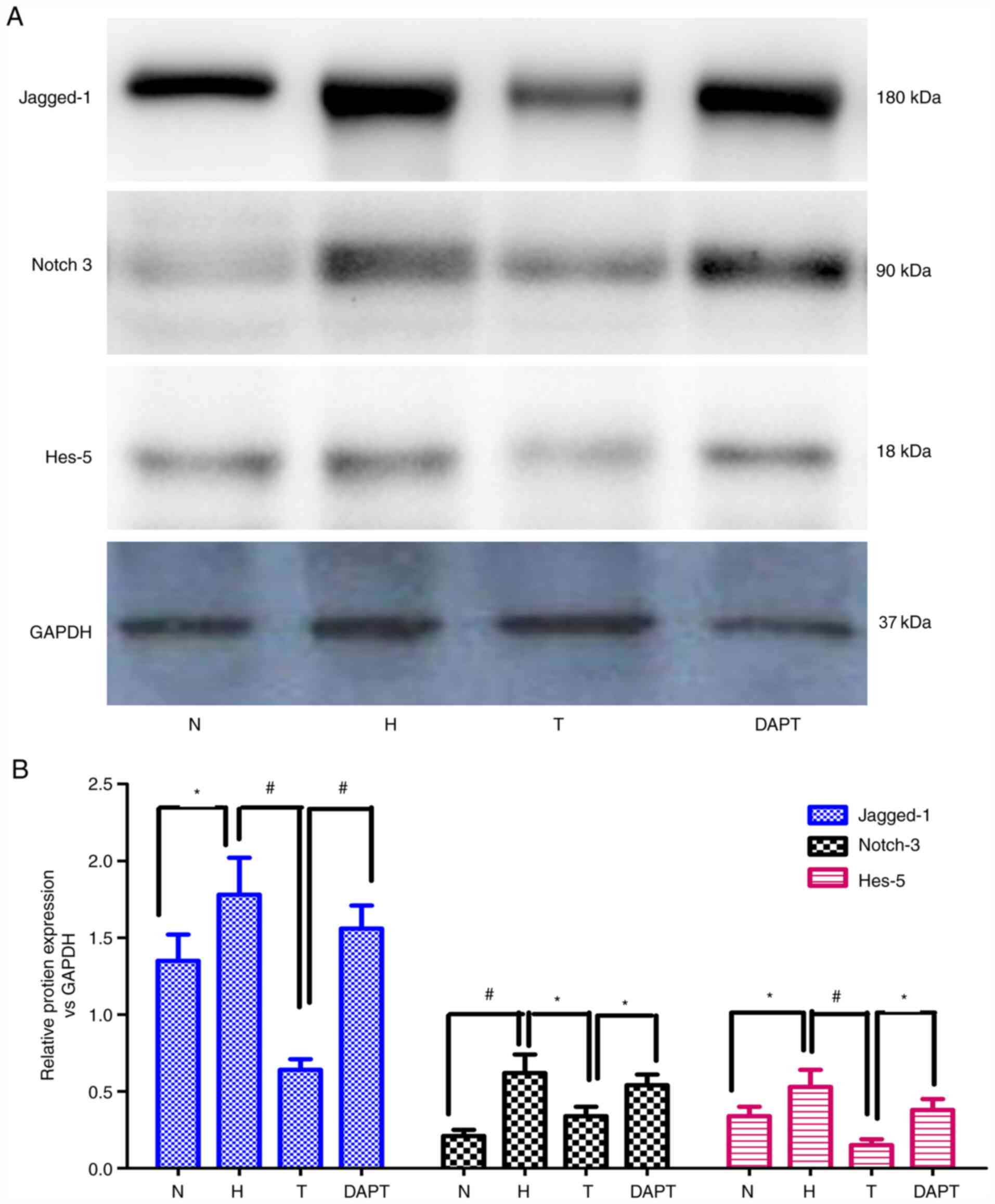
AS-IV reduces Jagged-1, Notch-3 and
Hes-5 expression
Subsequently, whether the Notch signaling pathway
was regulated at the transcriptional level during AS-IV-mediated
attenuation of hypoxic pulmonary vascular remodeling was
investigated. The mRNA and protein expression levels of Jagged-1,
Notch-3 and Hes-5 in rat lung tissues and PASMCs were detected via
RT-qPCR and western blotting, respectively (Figs. 6–8).
Jagged-1, Notch-3 and Hes-5 mRNA and protein expression levels were
significantly upregulated in hypoxia-treated PAH model rats and
PASMCs compared with the corresponding N groups. Treatment with
AS-IV significantly inhibited hypoxia-induced upregulation of
Jagged-1, Notch-3 and Hes-5 mRNA and protein expression levels in
hypoxia-treated PAH model rats and PASMCs, whereas DAPT
pretreatment significantly reversed AS-IV-mediated restoration of
expression levels. The results indicated that AS-IV regulated the
expression of Jagged-1, Notch-3 and Hes-5 during hypoxic pulmonary
vascular remodeling in vivo and in vitro, indicating
that AS-IV attenuated hypoxic pulmonary vascular remodeling via the
Notch signaling pathway.
Discussion
The major finding of the present study was that
AS-IV, a major biologically active compound extracted from Huangqi
(Radix Astragali Mongolici), alleviated and partially
reversed hypoxia-induced pulmonary vascular remodeling. Treatment
with AS-IV reversed hypoxia-induced increases in the mPAP,
ventricular hypertrophy, thickness of pulmonary arteriole media and
cell proliferation in vivo and in vitro. Furthermore,
the results indicated that the therapeutic effects of AS-IV on
pulmonary vascular remodeling were associated with the Notch
signaling pathway.
Pharmacological and clinical practice research has
demonstrated that Astragalus displays a wide range of
clinical effects, including immune regulation, cardiovascular
protection, anti-inflammatory, hepatoprotective, antidiabetic,
anticancer and neuroprotection (28,29).
As one of the primary active ingredients of Astragalus, AS-IV is
regarded as the factor for quality evaluation of Astragalus
in the Chinese Pharmacopeia, and has been reported to display
cardioprotective (30) and
anti-inflammatory effects via regulation of the NF-κB and activator
protein 1 signaling pathways (31).
In the present study, AS-IV inhibited hypoxia-induced elevation of
mPAP, RV/LV+S ratios, pulmonary arteriole wall thickening and PCNA
expression levels. Therefore, the results indicated that AS-IV
attenuated hypoxia-induced pulmonary vascular remodeling.
In arterial disease, vascular smooth muscle cells
are typically static and remain in the G0/G1
phase of the cell cycle (10).
Vascular smooth muscle cell proliferation serves an important role
in chronic hypoxia-induced PAH (32). Therefore, the present study aimed to
determine whether AS-IV could exert an ameliorative effect on
pulmonary vascular remodeling via inhibition of PASMC
proliferation. It has been previously reported that AS-IV displays
an antiproliferation effect of on angiotensin II-stimulated
vascular smooth muscle cells via regulation of CDK2 activity,
indicating that it displays ameliorative effects on vascular
disease (33). Consistent with the
aforementioned studies, the present study demonstrated that AS-IV
inhibited hypoxia-induced increases in PASMC viability in a
dose-dependent manner via regulating the expression of PCNA. In
addition, AS-IV treatment suppressed cell cycle progression by
inducing cell cycle arrest at the G0/G1 phase
in hypoxia-treated PASMCs. Collectively, the results suggested that
AS-IV inhibited cell cycle progression during PASMC proliferation,
thereby reversing vascular remodeling and reducing pulmonary artery
medial thickening in response to hypoxic conditions.
Several previous studies have reported that AS-IV
serves an important role in cell proliferation, migration and
differentiation via various signaling pathways, including p38MAPK
(34), Wnt (35), JAK2/STAT3 and ERK1/2 (36) signaling. In the present study, the
results indicated that DAPT, an inhibitor of Notch signaling,
significantly attenuated the effect of AS-IV treatment on pulmonary
vascular remodeling and PASMC proliferation under hypoxic
conditions. Moreover, the results indicated that AS-IV ameliorated
hypoxia-induced pulmonary vascular remodeling and PASMC
proliferation, at least in part, by regulating Notch signaling.
However, further investigations are required to identify the
mechanisms underlying AS-IV-mediated regulation of Jagged-1,
Notch-3 and Hes-5 expression in hypoxia pulmonary vascular
remodeling in vivo and in vitro.
Notch receptors and ligands expressed in pulmonary
arterial vessels contribute to the regulation of endothelial cell
and vascular smooth muscle cell proliferation and differentiation
(19,37). Upregulation of Notch-3 receptor and
ligand expression in PASMCs advances the development of pulmonary
vascular remodeling in animal and clinical research (21), whereas DAPT can alleviate the
development and reverse the progression of pulmonary vascular
remodeling in animal experiments (38). The present study indicated that
upregulated expression of the Jagged-1/Notch-3/Hes-5 axis was
associated with the progression of hypoxia-induced pulmonary
vascular remodeling in vivo and in vitro, which was
consistent with the results of a previous study (39). Furthermore, the results indicated
that AS-IV attenuated hypoxia-induced upregulated expression of the
Jagged-1/Notch-3/Hes-5 axis in vivo and in vitro,
which was consistent with a previous study that reported that DAPT
can reverse the development of pulmonary vascular remodeling
(40). Collectively, the
aforementioned results indicated that AS-IV-mediated inhibitory
effects on hypoxia-induced pulmonary vascular remodeling were
associated with suppression of the Notch signaling pathway. In
summary, the results of the present study suggested that AS-IV
might display beneficial effects in reversing the progression of
pulmonary vascular remodeling in PAH.
In conclusion, the present study indicated that
regulation of the Notch signaling pathway might be important for
hypoxia-induced pulmonary vascular remodeling. Moreover, the
results indicated that AS-IV alleviated hypoxia-induced pulmonary
vascular remodeling in vitro and in vivo. Together
with the results of a previous study (41), the present study suggested that the
effects of AS-IV might be mediated via downregulation of
Jagged-1/Notch-3/Hes-5 expression. Therefore, the present study
indicated that AS-IV might display important therapeutic functions
as part of the Radix Astragali Mongolici extract for the
prevention and treatment of cardiovascular disorders, such as
PAH.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81673858, 81704062
and 30500644), the Project of Natural Science Foundation of Hunan
Province (grant no. 2020JJ8073) and the Program for National Center
for Clinical Medicine for Geriatric Diseases (Ministry of Science
and Technology; grant no. 2017-07-1007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XF and CZ performed the experiments. GZ and JY
designed the study. YY performed the statistical analyses. DW and
QC analyzed and interpreted the data. QC and XF drafted the
manuscript. GZ and JY critically revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sakao S: Chronic obstructive pulmonary
disease and the early stage of cor pulmonale: A perspective in
treatment with pulmonary arterial hypertension-approved drugs.
Respir Investig. 57:325–329. 2019. View Article : Google Scholar
|
|
2
|
Samareh Fekri M, Torabi M, Azizi Shoul S
and Mirzaee M: Prevalence and predictors associated with severe
pulmonary hypertension in COPD. Am J Emerg Med. 36:277–280. 2018.
View Article : Google Scholar
|
|
3
|
Rowan SC, Keane MP, Gaine S and McLoughlin
P: Hypoxic pulmonary hypertension in chronic lung diseases: Novel
vasoconstrictor pathways. Lancet Respir Med. 4:225–236. 2016.
View Article : Google Scholar
|
|
4
|
Bunel V, Guyard A, Dauriat G, Danel C,
Montani D, Gauvain C, Thabut G, Humbert M, Castier Y, Dorfmüller P
and Mal H: Pulmonary arterial histologic lesions in patients with
COPD with severe pulmonary hypertension. Chest. 156:33–44. 2019.
View Article : Google Scholar
|
|
5
|
Sauler M, Fares WH and Trow TK: Standard
nonspecific therapies in the management of pulmonary arterial
hypertension. Clin Chest Med. 34:799–810. 2013. View Article : Google Scholar
|
|
6
|
Shimoda LA, Yun X and Sikka G: Revisiting
the role of hypoxia-inducible factors in pulmonary hypertension.
Curr Opin Physiol. 7:33–40. 2019. View Article : Google Scholar
|
|
7
|
Liu S, Shergis J, Chen X, Yu X, Guo X,
Zhang AL, Lu C and Xue CC: Chinese herbal medicine (weijing
decoction) combined with pharmacotherapy for the treatment of acute
exacerbations of chronic obstructive pulmonary disease. Evid Based
Complement Alternat Med. 2014:2570122014. View Article : Google Scholar
|
|
8
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of Astragaloside IV: A literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar
|
|
9
|
Yuan X, Sun S, Wang S and Sun Y: Effects
of astragaloside IV on IFN-gamma level and prolonged airway
dysfunction in a murine model of chronic asthma. Planta Med.
77:328–333. 2011. View Article : Google Scholar
|
|
10
|
Leng B, Tang F, Lu M, Zhang Z, Wang H and
Zhang Y: Astragaloside IV improves vascular endothelial dysfunction
by inhibiting the TLR4/NF-κB signaling pathway. Life Sci.
209:111–121. 2018. View Article : Google Scholar
|
|
11
|
Chen Z, Cai Y, Zhang W, Liu X and Liu S:
Astragaloside IV inhibits platelet-derived growth
factor-BB-stimulated proliferation and migration of vascular smooth
muscle cells via the inhibition of p38 MAPK signaling. Exp Ther
Med. 8:1253–1258. 2014. View Article : Google Scholar
|
|
12
|
Song Z, Wei D, Chen Y, Chen L, Bian Y,
Shen Y, Chen J and Pan Y: Association of astragaloside IV-inhibited
autophagy and mineralization in vascular smooth muscle cells with
lncRNA H19 and DUSP5-mediated ERK signaling. Toxicol Appl
Pharmacol. 364:45–54. 2019. View Article : Google Scholar
|
|
13
|
Spiekerkoetter E, Goncharova EA,
Guignabert C, Stenmark K, Kwapiszewska G, Rabinovitch M, Voelkel N,
Bogaard HJ, Graham B, Pullamsetti SS and Kuebler WM: Hot topics in
the mechanisms of pulmonary arterial hypertension disease:
Cancer-like pathobiology, the role of the adventitia, systemic
involvement, and right ventricular failure. Pulm Circ.
9:20458940198897752019. View Article : Google Scholar
|
|
14
|
Wang X, Xiao D, Ma C, Zhang L, Duan Q,
Zheng X, Mao M, Zhu D and Li Q: The effect of honokiol on pulmonary
artery endothelium cell autophagy mediated by cyclophilin A in
hypoxic pulmonary arterial hypertension. J Pharmacol Sci.
139:158–165. 2019. View Article : Google Scholar
|
|
15
|
Wang S, Cao W, Gao S, Nie X, Zheng X, Xing
Y, Chen Y, Bao H and Zhu D: TUG1 regulates pulmonary arterial
smooth muscle cell proliferation in pulmonary arterial
hypertension. Can J Cardiol. 35:1534–1545. 2019. View Article : Google Scholar
|
|
16
|
Borggrefe T, Lauth M, Zwijsen A,
Huylebroeck D, Oswald F and Giaimo BD: The Notch intracellular
domain integrates signals from Wnt, Hedgehog, TGFβ/BMP and hypoxia
pathways. Biochim Biophys Acta. 1863:303–313. 2016. View Article : Google Scholar
|
|
17
|
Bigas A and Espinosa L: The multiple
usages of Notch signaling in development, cell differentiation and
cancer. Curr Opin Cell Biol. 55:1–7. 2018. View Article : Google Scholar
|
|
18
|
Wang Y, Dai S, Cheng X, Prado E, Yan L, Hu
J, He Q, Lv Y, Lv Y and Du L: Notch3 signaling activation in smooth
muscle cells promotes extrauterine growth restriction-induced
pulmonary hypertension. Nutr Metab Cardiovasc Dis. 29:639–651.
2019. View Article : Google Scholar
|
|
19
|
Harrison OJ, Visan AC, Moorjani N, Modi A,
Salhiyyah K, Torrens C, Ohri S and Cagampang FR: Defective NOTCH
signaling drives increased vascular smooth muscle cell apoptosis
and contractile differentiation in bicuspid aortic valve
aortopathy: A review of the evidence and future directions. Trends
Cardiovasc Med. 29:61–68. 2019. View Article : Google Scholar
|
|
20
|
Yu YR, Mao L, Piantadosi CA and Gunn MD:
CCR2 deficiency, dysregulation of Notch signaling, and spontaneous
pulmonary arterial hypertension. Am J Respir Cell Mol Biol.
48:647–654. 2013. View Article : Google Scholar
|
|
21
|
Song Y, Zhang Y, Jiang H, Zhu Y, Liu L,
Feng W, Yang L, Wang Y and Li M: Activation of Notch3 promotes
pulmonary arterial smooth muscle cells proliferation via
Hes1/p27Kip1 signaling pathway. FEBS Open Bio. 5:656–660. 2015.
View Article : Google Scholar
|
|
22
|
Chen X, Yao JM, Fang X, Zhang C, Yang YS,
Hu CP, Chen Q and Zhong GW: Hypoxia promotes pulmonary vascular
remodeling via HIF-1α to regulate mitochondrial dynamics. J Geriatr
Cardiol. 16:855–871. 2019.
|
|
23
|
Yu X, Li T, Liu X, Yu H, Hao Z, Chen Y,
Zhang C, Liu Y, Li Q, Mao M and Zhu D: Modulation of pulmonary
vascular remodeling in hypoxia: Role of 15-LOX-2/15-HETE-MAPKs
pathway. Cell Physiol Biochem. 35:2079–2097. 2015. View Article : Google Scholar
|
|
24
|
Crnkovic S, Marsh LM, El Agha E,
Voswinckel R, Ghanim B, Klepetko W, Stacher-Priehse E, Olschewski
H, Bloch W, Bellusci S, et al: Resident cell lineages are preserved
in pulmonary vascular remodeling. J Pathol. 244:485–498. 2018.
View Article : Google Scholar
|
|
25
|
Yan J, Chen R, Liu P and Gu Y:
Docosahexaenoic acid inhibits development of hypoxic pulmonary
hypertension: In vitro and in vivo studies. Int J Cardiol.
168:4111–4116. 2013. View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Roels S, Tilmant K, Van Daele A, Van Marck
E and Ducatelle R: Proliferation, DNA ploidy, p53 overexpression
and nuclear DNA fragmentation in six equine melanocytic tumours. J
Vet Med A Physiol Pathol Clin Med. 47:439–48. 2000. View Article : Google Scholar
|
|
28
|
Heidebrecht F, Heidebrecht A, Schulz I,
Behrens SE and Bader A: Improved semiquantitative western blot
technique with increased quantification range. J Immunol Methods.
345:40–48. 2009. View Article : Google Scholar
|
|
29
|
Yang C, Mo Y, Xu E, Wen H, Wei R, Li S,
Zheng J, Li W, Le B, Chen Y, et al: Astragaloside IV ameliorates
motor deficits and dopaminergic neuron degeneration via inhibiting
neuroinflammation and oxidative stress in a Parkinson's disease
mouse model. Int Immunopharmacol. 75:1056512019. View Article : Google Scholar
|
|
30
|
Du J, Liu J, Zhen J, Yang ST, Zheng EL and
Leng JY: Astragaloside IV protects cardiomyocytes from
hypoxia-induced injury by down-regulation of lncRNA GAS5. Biomed
Pharmacother. 116:1090282019. View Article : Google Scholar
|
|
31
|
Liu ZH, Liu HB and Wang J: Astragaloside
IV protects against the pathological cardiac hypertrophy in mice.
Biomed Pharmacother. 97:1468–1478. 2018. View Article : Google Scholar
|
|
32
|
Yao Y, Li H, Da X, He Z, Tang B, Li Y, Hu
C, Xu C, Chen Q and Wang QK: SUMOylation of Vps34 by SUMO1 promotes
phenotypic switching of vascular smooth muscle cells by activating
autophagy in pulmonary arterial hypertension. Pulm Pharmacol Ther.
55:38–49. 2019. View Article : Google Scholar
|
|
33
|
Zhang DQ, Li JS, Zhang YM, Gao F and Dai
RZ: Astragaloside IV inhibits Angiotensin II-stimulated
proliferation of rat vascular smooth muscle cells via the
regulation of CDK2 activity. Life Sci. 105–109. 2018. View Article : Google Scholar
|
|
34
|
Gu L, Tao X, Xu Y, Han X, Qi Y, Xu L, Yin
L and Peng J: Dioscin alleviates BDL- and DMN-induced hepatic
fibrosis via Sirt1/Nrf2-mediated inhibition of p38MAPK pathway.
Toxicol Appl Pharmacol. 2921:19–29. 2016. View Article : Google Scholar
|
|
35
|
Liu D, Chen L, Zhao H, Vaziri ND, Ma SC
and Zhao YY: Small molecules from natural products targeting the
Wnt/β-catenin pathway as a therapeutic strategy. Biomed
Pharmacother. 117:1089902019. View Article : Google Scholar
|
|
36
|
Wang SG, Xu Y, Chen JD, Yang CH and Chen
XH: Astragaloside IV stimulates angiogenesis and increases nitric
oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules.
18:12809–12819. 2013. View Article : Google Scholar
|
|
37
|
Kostina A, Semenova D, Kostina D, Uspensky
V, Kostareva A and Malashicheva A: Human aortic endothelial cells
have osteogenic Notch-dependent properties in co-culture with
aortic smooth muscle cells. Biochem Biophys Res Commun.
514:462–468. 2019. View Article : Google Scholar
|
|
38
|
Yamamura H, Yamamura A, Ko EA, Pohl NM,
Smith KA, Zeifman A, Powell FL, Thistlethwaite PA and Yuan JX:
Activation of Notch signaling by short-term treatment with Jagged-1
enhances store-operated Ca(2+) entry in human pulmonary arterial
smooth muscle cells. Am J Physiol Cell Physiol. 306:C871–C878.
2014. View Article : Google Scholar
|
|
39
|
Zhang X, Chen J, Xu P and Tian X:
Protective effects of Astragaloside IV against hypoxic pulmonary
hypertension. Medchemcomm. 9:1715–1721. 2018. View Article : Google Scholar
|
|
40
|
Wang W, Liu J, Ma A, Miao R, Jin Y, Zhang
H, Xu K, Wang C and Wang J: mTORC1 is involved in hypoxia-induced
pulmonary hypertension through the activation of Notch3. J Cell
Physiol. 229:2117–2125. 2014. View Article : Google Scholar
|
|
41
|
Liang C, Ni GX, Shi XL, Jia L and Wang YL:
Astragaloside IV regulates the HIF/VEGF/Notch signaling pathway
through miRNA-210 to promote angiogenesis after ischemic stroke.
Restor Neurol Neurosci. 38:271–282. 2020.
|