Introduction
Cervical cancer is the second most common cancer
among women worldwide. High-risk human papillomavirus (HPV) 16 and
HPV 18 cause over 70% of cervical cancers (1). These cancers can be prevented with
vaccines and, if diagnosed early, are highly curable. However, the
outcome of patients with advanced cervical cancers remains poor due
to recurrence, metastasis, and resistance to radiation and
chemotherapy (2). Therefore, the
discovery of promising anticancer agents for the prevention and
treatment of cervical cancer is necessary.
Cancer cells can proliferate abnormally by avoiding
apoptosis and cell cycle arrest (3,4).
Infection with HPV leads to the expression of oncogenes E6 and E7.
E6 binds to p53 and disrupts its function through degradation,
resulting in resistance to apoptosis. E7 inhibits another tumor
suppressor, retinoblastoma 1 (RB1), thereby releasing E2F
transcription factors that induce the expression of cell cycle
regulators that stimulate cell proliferation (5,6).
Accordingly, recovery of apoptosis and cell cycle arrest are
important strategies for the treatment of cervical cancer.
It has been recently reported that the recurrence
and radio/chemotherapy resistance of cervical cancer are due to the
presence of cancer stem cells (CSCs) (7,8). CSCs
cause genetic heterogeneity in cervical carcinoma, thereby lowering
the effects of anticancer therapies and promoting metastasis to
other tissues (9,10). Several potential markers of cervical
CSCs have been identified such as Sox2, ALDH1A1, CD133,
integrin-α6, Nanog and Oct4 (11).
They upregulate cancer stem-like features including self-renewal,
tumorigenicity, and radio/chemo-resistance. For this reason,
targeting CSCs can contribute to a better therapeutic outcome for
cervical cancer.
Natural products have been used as medicine since
ancient times and are often found to have several advantages in
vivo, such as higher solubility and metabolic stability
(12,13). Moreover, natural components isolated
from plants and with known biological activities have been
developed as therapeutic agents for various human diseases. For
example, the first microtubule stabilizing agent that was isolated
from the bark of the tree Taxus brevifolia, paclitaxel has
been used in the treatment of many types of solid tumors including
breast and ovarian cancers (14).
Besides, metformin that is widely used as the first-line medication
for the treatment of type 2 diabetes was originally found in the
plant Galega officinalis, known as French lilac (15). Citrus fruits are known to contain a
variety of flavonoids, including hesperidin and naringenin, as
their main ingredients (16).
Citrus unshiu Markovich peel (CECU) has been used as a
traditional medicine in China and Korea. It possesses antioxidant,
anti-allergic, and anti-inflammatory effects, as well as cancer
cell apoptotic and metastasis inhibitory activities (17–21).
The ethanol extract of CECU has been shown to induce apoptosis of
bladder cancer cells by inactivating the PI3K/AKT pathway (19). Water extract of the natural product
showed anticancer effects against breast cancer cells through AMPK
activation and ROS-mediated apoptosis (20,21).
However, there have been no studies on the effects of CECU extracts
on cervical cancer cells. Moreover, previous studies have focused
on investigating the anticancer effects of ethanol and water
extracts of CECU (19–21). To further identify its anticancer
activity, we obtained a non-aqueous fraction that is expected to
contain non-polar substances of CECU. In this study, we
investigated the anticancer activity and the molecular mechanisms
involved in apoptosis-inducing and cancer stemness-inhibiting
effects of the chloroform extract of CECU in HeLa human cervical
carcinoma cells.
Materials and methods
Reagents and antibodies
Dulbecco's modified Eagle's medium (DMEM) was
obtained from Corning Cellgro. Fetal bovine serum (FBS),
DMEM/nutrient mixture F-12 (F12), B-27 serum-free supplement,
L-glutamine, epidermal growth factor (EGF), basic fibroblast growth
factor (bFGF), and penicillin/streptomycin were purchased from
Gibco; Thermo Fisher Scientific, Inc. Heparin,
3-[4,5-dimethylthiazol-2-yl]2,5-diphenyl tetrazolium bromide (MTT),
hesperidin, hesperetin, gallic acid, and crystal violet were
purchased from Sigma-Aldrich; Merck KGaA.
Penicillin-streptomycin-amphotericin B was obtained from Lonza,
Inc. Antibodies against p53 (53 kDa; cat. no. 2524), phospho-AKT
(Ser473, 60 kDa; cat. no. 4060), AKT (60 kDa; cat. no. 9272),
phospho-ERK1/2 (Thr202/Tyr204, 42,44 kDa; cat. no. 9101), ERK1/2
(42,44 kDa; cat. no. 9102), Bcl-2 (28 kDa; cat. no. 2872), Bcl-XL
(30 kDa; cat. no. 2764), Bax (20 kDa; cat. no. 2772), cleaved
caspase-3 (17,19 kDa; cat. no. 9661), cleaved caspase-9 (37 kDa;
cat. no. 9501), cleaved caspase-8 (10 kDa; cat. no. 9748), PARP
(89,116 kDa; cat. no. 9542), DR5 (40,48 kDa; cat. no. 8074), Fas
(40–50 kDa; cat. no. 4233), cyclin B1 (55 kDa; cat. no. 12231),
cyclin D1 (36 kDa; cat. no. 55506), CD133 (133 kDa; cat. no.
64326), Sox2 (35 kDa; cat. no. 3579), Oct4 (45 kDa; cat. no. 2750),
Nanog (42 kDa; cat. no. 3580), integrin-α6 (125,150 kDa; cat. no.
3750), ALDH1A1 (55 kDa; cat. no. 12035), rabbit IgG (cat. no.
7074), and mouse IgG (cat. no. 7076) were purchased from Cell
Signaling Technology. Antibody against p21 (21 kDa; cat. no.
sc-397) was obtained Santa Cruz Biotechnology, Inc.. Antibodies
against β-actin (42 kDa; cat. no. ab6276) and Bad (22 kDa; cat. no.
ab62465) were purchased from Abcam.
Preparation of CECU
Dried C. unshiu Markovich peels were
purchased from Yeong-cheon Herbal Wholesale Market. A voucher
specimen (NCB-CECU-2018) was deposited in the Department of
Pharmaceutical Engineering and Biotechnology, Sun Moon University.
It was extracted with 100% ethanol for 24 h and concentrated in
vacuo. The extract was partitioned between chloroform and
distilled water in a 1:1 ratio to obtain the chloroform fraction.
CECU was prepared at a concentration of 100 mg/ml using DMSO.
Cell culture
HeLa, CaSki, and SiHa human cervical cancer cell
lines were purchased from the Korean Cell Line Bank. Cells were
grown in DMEM containing 10% FBS and 1%
penicillin-streptomycin-amphotericin B and maintained at 37°C in a
humidified 5% CO2 incubator (Thermo Fisher Scientific,
Inc.). 267B1 human normal prostate epithelial cells were kindly
provided by the Anticancer Agent Research Center in Korea Research
Institute of Bioscience and Biotechnology and were grown in
RPMI-1640 supplemented with 10% FBS and 1% antibiotics.
HeLa-derived cancer stem-like cells were cultured in DMEM/F12
supplemented with 1X B-27, 5 µg/ml heparin, 2 mM L-glutamine, 20
ng/ml bFGF, 20 ng/ml EGF, and 1% penicillin/streptomycin.
Total polyphenol content
The total polyphenol content of CECU was estimated
using Folin-Ciocalteu reagent (Sigma-Aldrich; Merck KGaA). CECU
(100 µl) and Folin-Ciocalteu reagent (60 µl) were mixed for 5 min,
and then 600 µl of 2% Na2CO3 was added. After
incubation in the dark for 2 h, the absorbance was measured at 750
nm using a multimode microplate reader (BioTek, Inc.). A
calibration curve of gallic acid was constructed and linearity was
obtained in the range of 0–0.9 mg/ml. The total phenolic content in
CECU was expressed as milligram of gallic acid equivalent (mg GAE/g
extract) using the standard curve.
HPLC analysis
HPLC was performed to identify the content of
hesperidin and heperetin in CECU. The reference compounds were
diluted with MeOH to a concentration of 0.5 mg/ml, and CECU was
prepared to a concentration of 20 mg/ml. The prepared samples (10
µl) were injected and analyzed by HPLC-PDA (Shimadzu LC-2030C;
SPD-M20A Detector) using a reverse phase C18 column
[Mightysil-RP-18 GP, 250×4.6 mm (5 µm); Kanto Chemical] with oven
temperature of 40°C at 288 nm. The binary mobile phases were
composed of solvent A (0.025% trifluoroacetic acid in HPLC-grade
water) and solvent B (100% acetonitrile). The flow rate of the
mobile phase was maintained at 1 ml/min for the 35 min gradient
program. The program used was as follows: 5% B to 100% B (linear
gradient, 0–25 min), 100% B (25–27 min), 100% B to 5% B (27–32
min), and 5% B (32–35 min).
Cell proliferation assay
HeLa, SiHa, CaSki and 267B1 cells (2×103
cells/well) were seeded in a 96-well culture plate. After a 24 h
incubation, cells were treated with various concentrations of CECU.
After incubation for 72 h, 50 µl of MTT solution (2 mg/ml) was
added to each well. Cells were incubated for 3 h and then dissolved
in 100 µl of DMSO per well. Absorbance was measured at a wavelength
of 540 nm using a microplate reader (BioTek). The IC50
values from the obtained data were analyzed using the curve-fitting
program GraphPad Prism 5 (GraphPad Software).
Colony formation assay
HeLa cells (3×102 cells/well) were seeded
in a 6-well culture plate and treated with CECU. Cells were grown
for 13 days, and formed colonies were fixed with 3.7% formaldehyde
for 10 min. After washing with PBS, the colonies were stained with
1 ml of 0.5% crystal violet for 20 min. Stained colonies were
washed with PBS, and the number of colonies was counted.
Wound healing assay
HeLa cells (15×104 cells/well) were
seeded in a 24-well culture plate. After incubation for 24 h, cells
were scratched using a 10 µl of pipette tip, washed with PBS, and
treated with CECU in a medium containing 1% FBS. After a 72-h
incubation, images were obtained under a ×40 optical microscope
(Olympus). The number of cells that migrated into the gap was
counted and results were presented as a percentage of control.
Migration assay
Cell migration was also assayed using a Transwell
chamber system with polycarbonate filter inserts with a pore size
of 8.0 µm (Corning Costar). The lower side of the filter was coated
with 10 µl gelatin (1 mg/ml), and HeLa cells (1×105
cells/well) were placed in the upper chamber of the filter. CECU
was added to the lower chamber filled with a medium containing 1%
FBS, and the chamber was incubated at 37°C for 24 h. The cells were
subsequently fixed with methanol and stained with hematoxylin and
eosin. Images were obtained under a ×100 optical microscope
(Olympus), and the total number of cells that migrated the lower
chamber of the filter was counted. Results were presented as a
percentage of control.
Cell cycle analysis
Cell cycle analysis was performed using a Muse™ cell
cycle kit (Merck Millipore) according to the manufacturer's
instructions. Briefly, HeLa cells (3×105 cells/dish)
were seeded in a 60-mm culture dish and treated with CECU for 24 h.
The cells were collected, washed with PBS, and fixed with cold 70%
ethanol. After overnight storage at −20°C, ethanol was removed, and
the cells were washed with PBS. Further, 200 µl of Muse cell cycle
reagent was added and reacted in the dark for 30 min. The
percentage of cells in G0/G1, S and G2/M phases was then calculated
using Muse cell analyzer and Muse analysis software
(MuseSoft_V1.8.0.3; Luminex Corporation). The Muse cell cycle
software module displays the data in two plots: A dot plot
displaying DNA Content Index vs. Cell Size Index for setting the
gate and a histogram displaying DNA Content Index vs. Count for
assessing the percentage of cells in each phase.
Apoptosis analysis
HeLa cells (5×105 cells/dish) were placed
in a 60-mm culture dish and treated with CECU for 24 h. The cells
were harvested, washed with PBS, and stained with Annexin V-FITC
and PI according to the manufacturer's instructions (Invitrogen;
Thermo Fisher Scientific, Inc.). Stained cells were analyzed by
flow cytometry (Cyto FLEX; Beckman Coulter).
ROS analysis
HeLa cells (1×105 cells/well) were seeded
in a 96-black well culture plate and treated with CECU for 30 min.
Cells were then incubated with 10 µM of
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma-Aldrich;
Merck KGaA) for 20 min and washed with PBS. The fluorescence
intensity of DCF was detected using a multimode microplate reader
(BioTek) at excitation and emission wavelengths of 495 and 529 nm,
respectively.
DAPI staining
HeLa cells (5×104 cells/well) were seeded
in a 24-well culture plate and treated with CECU for 24 h. Cells
were fixed with 3.7% formaldehyde for 10 min. Nuclei were stained
with 4 µg/ml of 4,6-diamidine-2-phenylindole dihydrochloride (DAPI)
for 30 min and washed with PBS. The nuclear morphology of cells was
captured using a fluorescence microscope (Korea Lab Tech).
ATP-monitoring luminescence assay
ATPlite Luminescence Assay System (PerkinElmer) was
used to quantitatively evaluate the proliferation of HeLa cancer
stem-like cells. Cells (3×103 cells/well) were seeded in
a 96-white well culture plate using serum-free media with EGF and
bFGF and treated with CECU, hesperidin, and hesperetin for 7 days.
Following the addition of 50 µl of substrate solution to each well,
the culture plate was shaken for 5 min and incubated in the dark
for 10 min. Luminescence was detected using a multimode microplate
reader (BioTek).
Tumorsphere-forming assay
HeLa cancer stem-like cells were cultured in
Dulbeccos modified Eagles medium/nutrient mixture F-12 (DMEM/F12;
Gibco; Thermo Fisher Scientific, Inc.) containing 1X B-27
serum-free supplement (Gibco; Thermo Fisher Scientific, Inc.), 5
µg/ml heparin (Sigma-Aldrich; Merck KGaA), 2 mM L-glutamine (Gibco;
Thermo Fisher Scientific, Inc.), 20 ng/ml epidermal growth factor
(EGF; Gibco; Thermo Fisher Scientific, Inc.), 20 ng/ml basic
fibroblast growth factor (bFGF; Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). The serum-free media with EGF and bFGF were
added to the cells twice a week. Cultured tumorspheres were passed
every 7 days by dissociating with Accutase (Millipore). To evaluate
the effect of CECU on the tumorsphere-forming ability of HeLa
cancer stem-like cells, the cells (5×102 cells/well)
were seeded in a 96-well culture plate using serum-free media with
EGF and bFGF (200 µl/well) and treated with CECU (0, 4, 8, 16, 31
and 63 µg/ml). After incubation for 7 days without changing the
media, the number of tumorspheres that are >75 µm in diameter
was counted under a ×200 optical microscope (Olympus). Results were
presented as a percentage of control.
Western blot analysis
Cells were lysed using RIPA buffer (Sigma-Aldrich;
Merck KGaA) supplemented with a protease inhibitor cocktail (Roche
Diagnostics), on ice. Extract protein concentrations were
determined using a BCA Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Equal amounts of cell lysate (40 µg/lane) were
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and the separated proteins were
transferred to polyvinylidene difluoride (PVDF) membranes (EMD
Millipore) using standard electroblotting procedures. Blots were
blocked in Tris-buffered saline with Tween-20 (TBST) containing 5%
skim milk at room temperature for 1 h and immunolabeled with
primary antibodies against p53 (dilution 1:2,000), p21 (dilution
1:500), phospho-AKT (dilution 1:2,000), AKT (dilution 1:2,000),
phospho-ERK1/2 (dilution 1:2,000), ERK1/2 (dilution 1:2,000), Bcl-2
(dilution 1:2,000), Bcl-XL (dilution 1:2,000), Bad (dilution
1:2,000), Bax (dilution 1:2,000), cleaved caspase-3 (dilution
1:2,000), cleaved caspase-9 (dilution 1:2,000), cleaved caspase-8
(dilution 1:2,000), PARP (dilution 1:2,000), DR5 (dilution
1:2,000), Fas (dilution 1:2,000), cyclin B1 (dilution 1:2,000),
cyclin D1 (dilution 1:2,000), CD133 (dilution 1:2,000), Sox2
(dilution 1:2,000), Oct4 (dilution 1:2,000), Nanog (dilution
1:2,000), integrin-α6 (dilution 1:2,000), ALDH1A1 (dilution
1:2,000), and β-actin (dilution 1:10,000) overnight at 4°C. After
washing with TBST three times, membranes were incubated with
horseradish peroxidase-conjugated anti-rabbit (dilution 1:3,000) or
anti-mouse (dilution 1:3,000) secondary antibody for 1 h at room
temperature. Immunolabeling was detected using an enhanced
chemiluminescence (ECL) kit (Bio-Rad Laboratories, Inc.) according
to the manufacturer's instructions. The band density was analyzed
using ImageJ software (version 1.5; NIH).
Statistical analysis
Results are expressed as the mean ± standard
deviation (SD). Differences among groups were analyzed using
analysis of variance (ANOVA) with SPSS statistics package (SPSS
9.0; SPSS, Inc.). Post hoc analysis was carried out using Tukey's
test. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of the composition of
CECU
Polyphenols have shown numerous biological
activities resulting in the prevention and treatment of human
diseases, including cancers (22).
To evaluate the phytochemical composition of CECU, the total
phenolic content was determined using the Folin-Ciocalteu method.
CECU contained 8.3 mg GAE/g of polyphenols.
Hesperidin and its aglycone, hesperetin, are the
main bioactive phytochemicals found in citrus species (16). To determine the content of these
ingredients in CECU, the reference compounds and CECU were
subjected to HPLC analysis. The detection wavelength for the
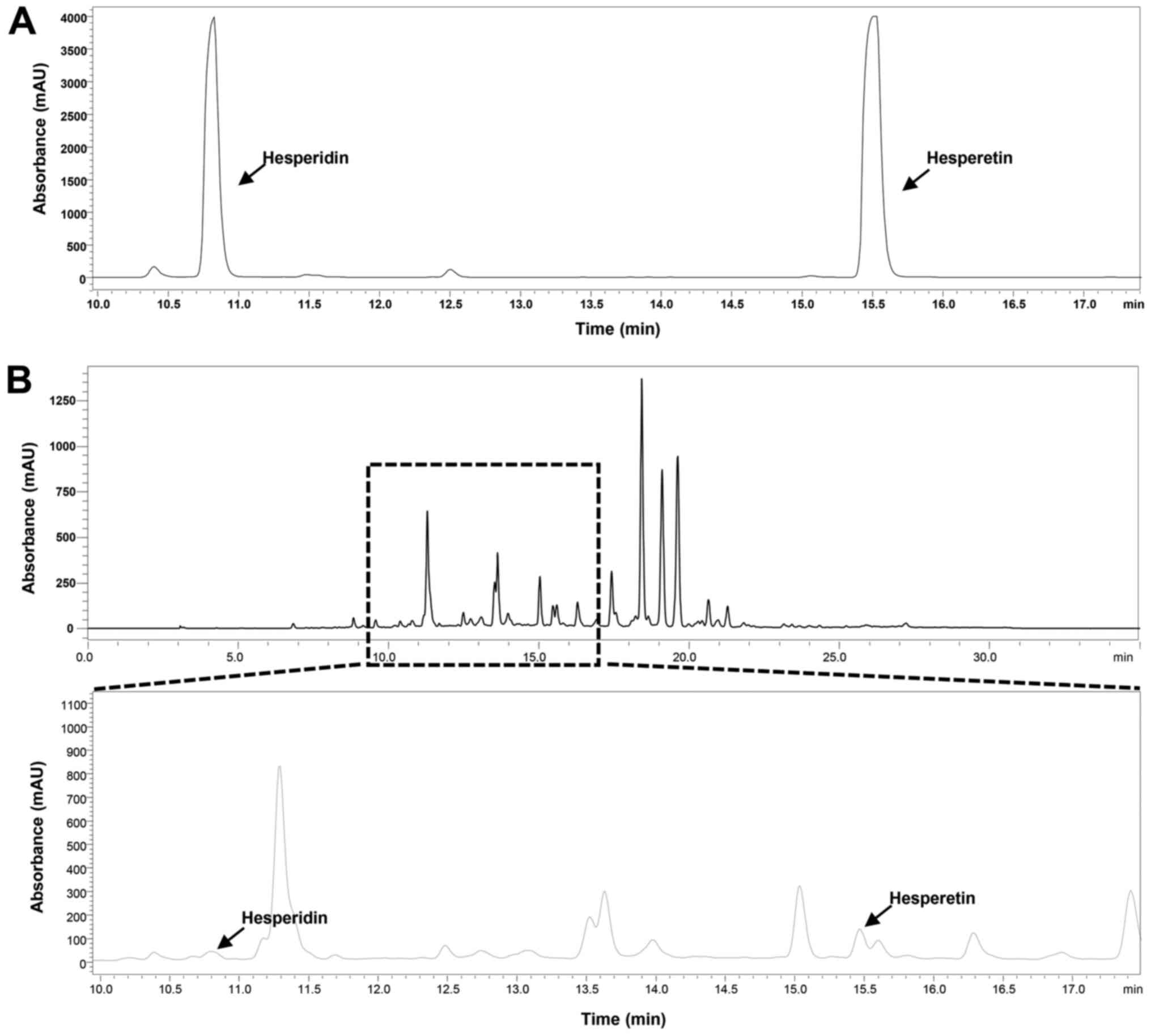
compounds was 288 nm. The HPLC chromatogram of CECU detected
hesperidin and hesperetin at retention times of 10.82 and 15.53
min, respectively (Fig. 1). The
estimated content of hesperidin and hesperetin in CECU was 0.739
and 1.641%, respectively.
Effects of CECU on the proliferation
and migration of HeLa cells
To examine whether CECU affects the proliferation of
cervical cancer cells, three different cervical cancer cell lines
were treated with CECU (0–500 µg/ml) for 72 h. Cell proliferation
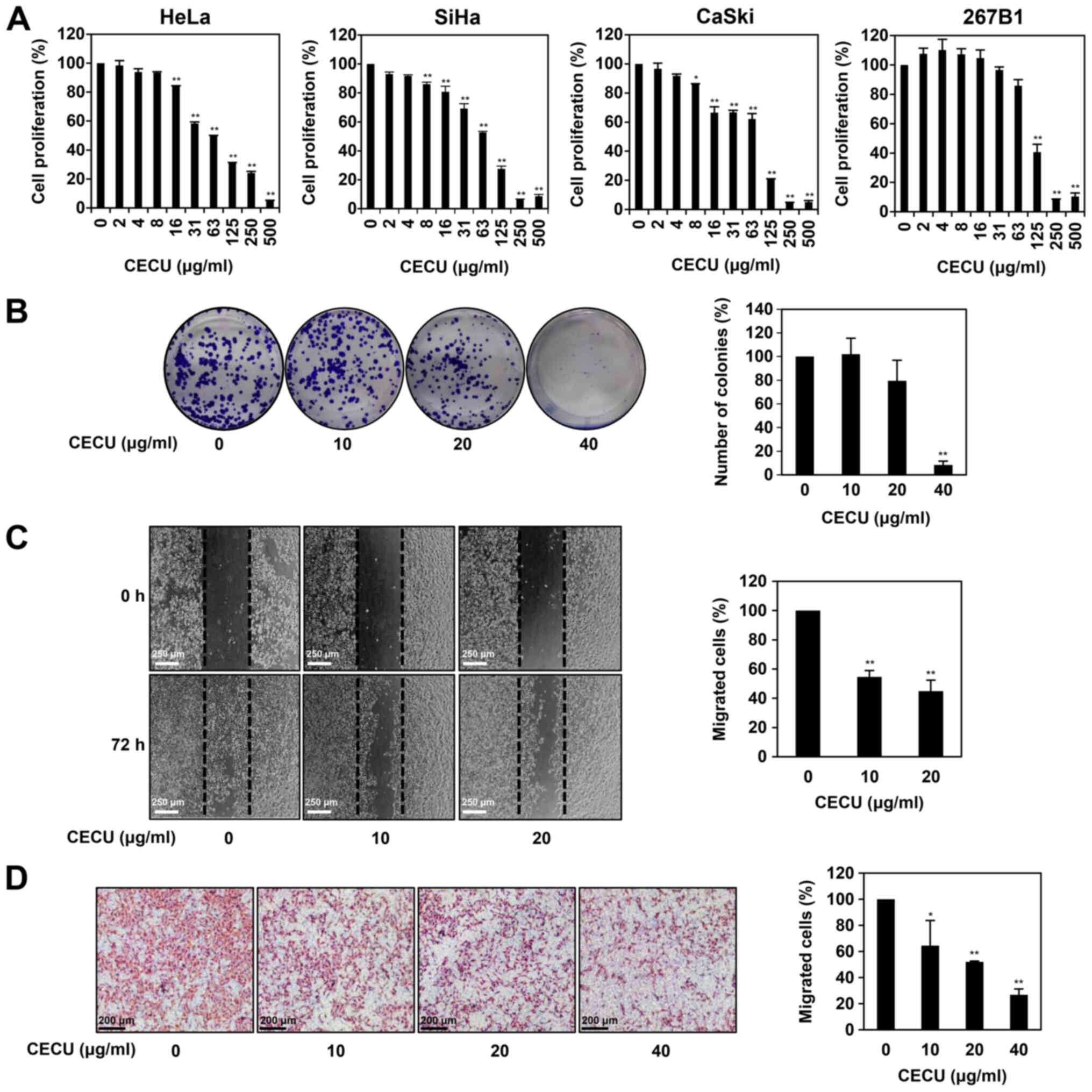
was then evaluated by the MTT assay. As shown in Fig. 2A, CECU treatment inhibited the
proliferation of HeLa, SiHa and CaSki cells in a dose-dependent
manner, with IC50 values of 58.95, 73.41 and 69.63
µg/ml, respectively. Notably, CECU showed the highest inhibitory
effect on the proliferation of HeLa cells. We further evaluated the
effect of CECU on the proliferation of 267B1 human normal prostate
epithelial cells. CECU inhibited the proliferation of 267B1 cells
with an IC50 value of 114.7 µg/ml, indicating that CECU
suppresses the proliferation of cervical cancer cells more
sensitively compared to normal cells (Fig. 2A). Based on these results, we
further assessed the inhibitory effects of CECU on the
proliferative and migratory abilities of HeLa cells at
concentrations ranging from 10–80 µg/ml.
Next, we evaluated the effect of CECU on the colony
formation of HeLa cells. Treatment with CECU suppressed the
clonogenic proliferation of HeLa cells in a dose-dependent manner
(Fig. 2B). In particular, the
colony-forming ability of the cells was remarkably decreased at 40
µg/ml of CECU.
To confirm the effect of CECU on the migration
ability of HeLa cells, a monolayer wound healing assay was
performed. Wound closure by HeLa cell migration was observed after
72 h of incubation. Treatment with CECU (10 and 20 µg/ml)
significantly reduced the migration of HeLa cells compared with the
untreated control (Fig. 2C).
We further examined the effect of CECU on the
migration of HeLa cells using Transwell chamber inserts. As shown
in Fig. 2D, CECU treatment (10, 20
and 40 µg/ml) led to significant reduction of cell migration in
HeLa cells. These results demonstrate that CECU effectively
inhibits the proliferation and migration of cervical cancer
cells.
Effects of CECU on cell cycle
distribution and apoptosis in HeLa cells
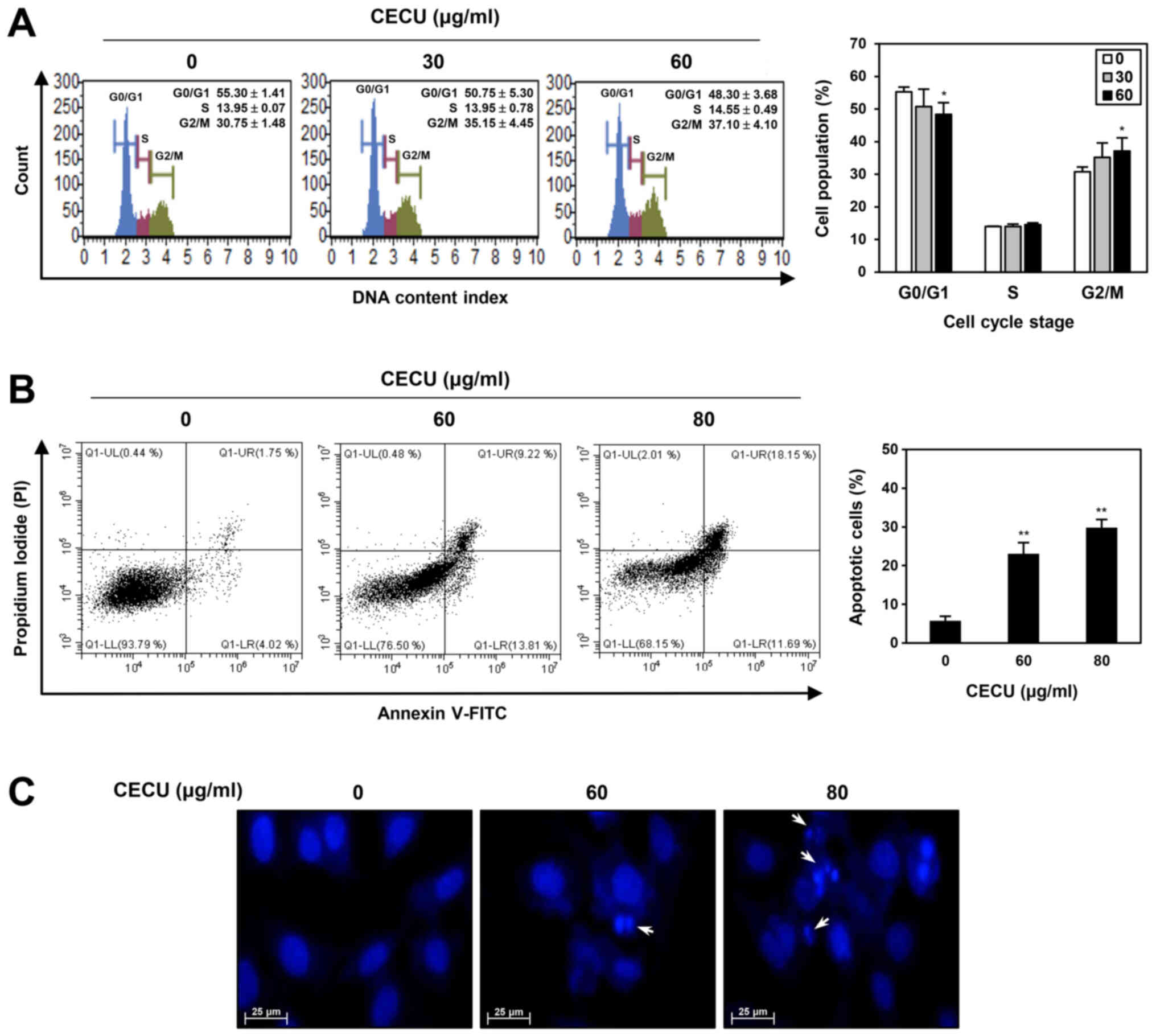
To evaluate whether CECU inhibits the proliferation
of HeLa cells by regulating the cell cycle, we examined the effect
of CECU on cell cycle distribution using a Muse cell analyzer.
Compared with the untreated control cells, treatment with CECU
increased the G2/M phase cell population, while decreasing the
G0/G1 phase cell population (Fig.
3A). These data indicate that CECU caused G2/M phase arrest in
HeLa cells.
To further investigate whether the CECU-induced
proliferation inhibition is associated with apoptosis induction,
CECU-treated HeLa cells were stained with Annexin V-FITC and PI and
then analyzed by flow cytometry. After treatment with CECU, the
proportion of early and late apoptotic cells increased compared to
that of untreated control cells (Fig.
3B). Consequently, CECU exhibited a dose-dependent
apoptosis-inducing effect in HeLa cells.
To confirm whether CECU causes morphological changes
related to apoptosis in HeLa cells, DAPI staining was performed. As
shown in Fig. 3C, CECU treatment
resulted in nuclear condensation and fragmentation. These results
demonstrate that CECU inhibits the proliferation of HeLa cells
through the induction of cell cycle arrest and apoptosis.
Effects of CECU on apoptosis-related
pathways in HeLa cells
The PI3K/AKT and the Ras/MEK/ERK pathways contribute
to the survival and proliferation of cervical cancer cells
(23). To elucidate the molecular
mechanism by which CECU inhibits the proliferation of HeLa cells,
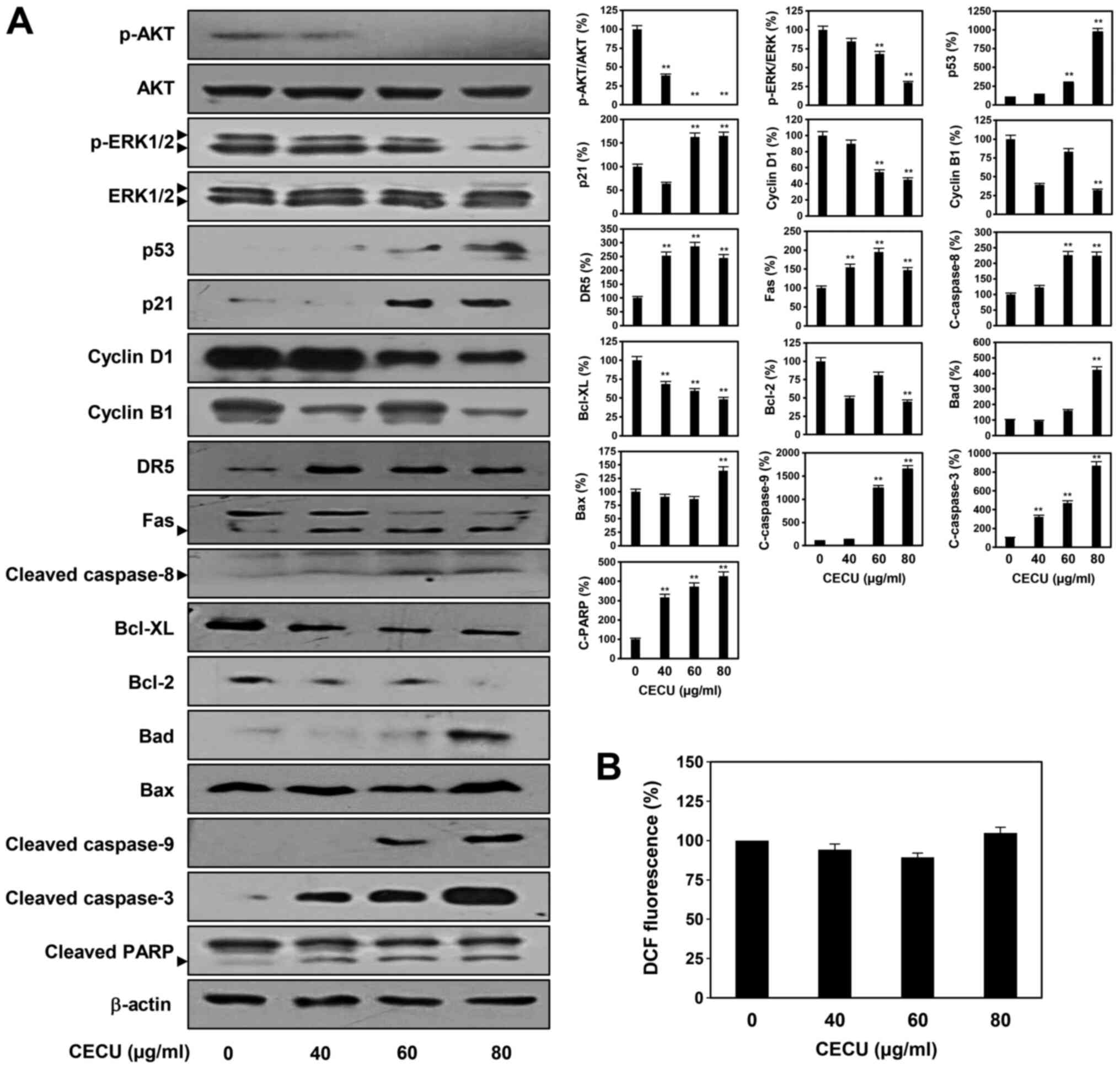
we first examined whether CECU regulates the activation of AKT and
ERK, the key effectors of these signaling pathways. Treatment with
CECU led to a significant downregulation of AKT and ERK1/2
phosphorylation without affecting total protein levels in HeLa
cells (Fig. 4A).
Activation of the tumor suppressor protein p53
arrests the cell cycle at the G2/M phase. Cell cycle arrest by p53
is mainly mediated by the transcriptional activation of p21/WAF1
(24). Thus, we examined the effect
of CECU on the expression of p53 and p21. Results showed that
treatment with CECU markedly elevated the expression of p53 and p21
in HeLa cells (Fig. 4A). Moreover,
CECU decreased the expression levels of cyclin B1 and cyclin D1,
which are implicated in the regulation of G2/M phase transition
(Fig. 4A).
Cell apoptosis can be induced either through death
receptor-mediated extrinsic pathways or mitochondria-mediated
intrinsic pathways (25,26). We therefore assessed whether CECU
affects these apoptotic pathways in HeLa cells. Treatment with CECU
clearly increased the expression levels of death receptors, DR5 and
Fas as well as their downstream apoptosis effector, the active form
of caspase-8 (Fig. 4A).
Furthermore, among the Bcl-2 family members that are involved in
the intrinsic apoptotic pathway, the expression of anti-apoptotic
proteins such as Bcl-XL and Bcl-2 were downregulated by CECU
treatment, whereas the levels of pro-apoptotic proteins including
Bad and Bax were upregulated (Fig.
4A). Consequently, the expression of the downstream apoptosis
effector, caspase-9 was activated. By regulating the extrinsic and
intrinsic apoptotic pathways, CECU triggered the activation of the
critical executioner of apoptosis caspase-3 and its substrate,
PARP.
Reactive oxygen species (ROS) play an important role
in the induction of apoptosis (27). Thus, we further evaluated whether
the apoptosis-inducing effect of CECU is mediated by ROS in HeLa
cells. Treatment with CECU did not cause a significant change in
ROS generation, indicating that the apoptosis-inducing effect of
CECU was ROS-independent (Fig. 4B).
Taken together, these findings suggest that the inhibition of HeLa
cell proliferation by CECU may be associated with the inactivation
of AKT and ERK signaling, upregulation of p53 and p21,
downregulation of cyclin B1 and cyclin D1, and activation of
ROS-independent apoptotic pathways.
Effects of CECU on cancer stem-like
features of HeLa cells
To assess the potential of CECU in suppressing
cervical CSCs, we investigated the effects of CECU on the cancer
stem-like properties of HeLa cells. The CSC population in HeLa
cells was enriched through spheroid culture using serum-free media
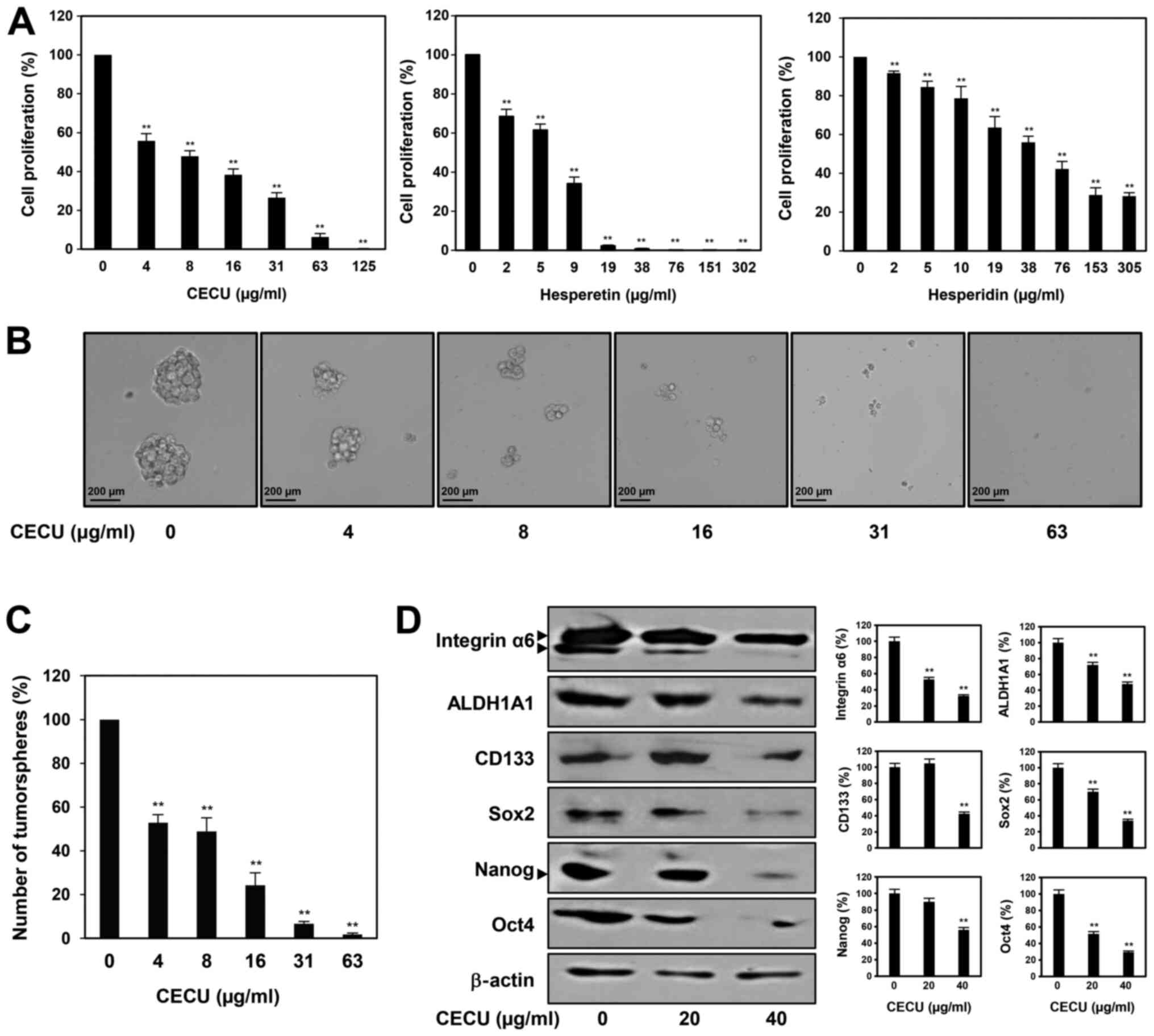
with EGF and bFGF (28,29). As shown in Fig. 5A, treatment with CECU inhibited the
proliferation of HeLa cancer stem-like cells in a dose-dependent
manner. We also evaluated whether hesperidin and hesperetin are the
possible active ingredients contributing to the antiproliferative
activity of CECU against cervical CSCs. The two compounds
suppressed the proliferation of HeLa cancer stem-like cells in a
dose-dependent manner (Fig. 5A).
Notably, hesperetin showed a better proliferation inhibitory effect
compared to hesperidin in these cells. In addition, the clonogenic
proliferation of HeLa cancer stem-like cells was remarkably
suppressed by treatment with CECU (Fig.
5B and C). CECU treatment reduced the size and number of
tumorspheres.
We further examined whether CECU regulates the
expression of key stemness-related markers in cervical CSCs.
Treatment with CECU significantly decreased the expression levels
of stemness regulators including Sox2, Nanog, Oct4, ALDH1A1,
integrin-α6 and CD133, in HeLa cancer stem-like cells (Fig. 5D). These results suggest that CECU
has therapeutic potential to eliminate cervical CSCs.
Discussion
Cervical cancer is one of the leading gynecological
malignancies worldwide. Although chemotherapy is the main approach
for the treatment of cervical cancer, it often causes many side
effects, and cancer cells can become chemo-resistant (1,2).
Natural products are a good source of new, potent, and selective
anticancer agents (12,13). Accumulating evidence has shown that
a variety of non-polar compounds found in the chloroform extracts
of natural products possess potent anticancer activities (30,31).
In this study, we assessed, for the first time, the anticancer
activity, and the underlying molecular mechanism of the chloroform
extract of CECU, in HeLa human cervical cancer cells. CECU
effectively inhibited the proliferation and migration of HeLa
cells, even at concentrations that do not affect normal cells. The
anticancer effect of CECU was mediated by induction of cell cycle
arrest at the G2/M phase via upregulation of p53 and p21 expression
and downregulation of cyclin B1 and cyclin D1 expression as well as
activation of death receptor-mediated extrinsic and
mitochondria-mediated intrinsic apoptotic pathways. However, CECU
did not increase intracellular ROS generation in HeLa cells,
suggesting that it induces apoptosis of cervical cancer cells in a
ROS independent manner. Furthermore, the proliferation inhibition
of HeLa cells by CECU was mediated by the inactivation of AKT and
ERK signaling. Therefore, CECU can be used as a complementary and
alternative medicine for the prevention and treatment of cervical
cancer.
Several recent studies have confirmed the
apoptosis-promoting effect of CECU in different cancer cells. The
ethanol extract of C. unshiu Markovich peel inhibited T24
bladder cancer cell proliferation by activating intrinsic and
extrinsic apoptotic pathways via ROS-mediated inactivation of
PI3K/AKT signaling (19). The water
extract of C. unshiu Markovich peel induced apoptosis in
MCF-7 and MDA-MB-231 breast cancer cells by activating both, the
extrinsic and intrinsic apoptotic pathways through ROS-dependent
activation of AMPK signaling (20,21).
The ethanol and water extracts showed cancer cell proliferation
inhibitory activities at concentrations ranging from 200–800 µg/ml
and 250–1,500 µg/ml, respectively. It should be noted that the
chloroform extract of C. unshiu Markovich peel, exhibits
antiproliferative effects at concentrations ranging from 10–80
µg/ml and activates apoptosis in a ROS independent manner in HeLa
cervical cancer cells, unlike the ethanol and water extracts.
Citrus species, including C. unshiu Markovich, are known to
contain various flavonoids such as naringin, hesperidin, and its
aglycone hesperetin (16). These
flavonoids have diverse biological activities, such as
anti-inflammatory, anticancer, anti-obesity, antioxidant,
antimicrobial, and anti-mutagenic properties (32,33).
Although we confirmed the presence of hesperidin and hesperetin in
CECU by HPLC analysis, the non-aqueous extract may contain various
non-polar bioactive substances different from the ingredients found
in the ethanol and water extracts. Accordingly, differences in
effective concentrations and mechanisms of action are expected to
be due to differences in the composition of the extracts.
Moreover, for the first time, we evaluated the
potential of CECU in suppressing the cancer stem-like features of
HeLa cells. Cancer stem cells (CSCs), a small population of cancer
cells with a capacity for self-renewal and differentiation
potential, have been considered as a promising therapeutic target
for cancer (34). CSCs contribute
to multiple tumor malignancies, such as tumor metastasis and
recurrence, chemotherapy and radiotherapy resistance, and genetic
heterogeneity (10,11). In our present study, cervical CSCs
were cultured in 3D spheroid culture condition, which is known to
better represent the in vivo cellular environment (35). Our results showed that CECU potently
inhibited the proliferation and tumorsphere-forming ability of HeLa
cancer stem-like cells. In addition, hesperidin and hesperetin
suppressed the proliferation of HeLa CSCs, suggesting that these
two compounds might be the possible active ingredients contributing
to the antiproliferative activity of CECU against cervical CSCs.
However, further identification of other active compounds in CECU
is required to clearly understand the anticancer mechanism of CECU
in cervical cancer cells. The biological characteristics of
cervical CSCs are regulated by several key stemness-related
biomarkers. Transcription factors, including Sox2, Oct4 and Nanog,
play a critical role in the regulation of cervical CSC
proliferation and maintenance (36,37).
CD133 and integrin-α6 are cell surface markers of cervical CSCs,
which are related to self-renewal, tumorigenesis, and resistance to
radiation therapy (38,39). The activity of aldehyde
dehydrogenase 1A1 (ALDH1A1) is associated with drug detoxification
by aldehyde oxidation. ALDH1A1 eliminates oxidative stress and thus
enhances the resistance of cervical CSCs to chemotherapeutic drugs
(40,41). Our results showed that CECU
significantly suppressed the expression of cancer stemness
regulators, including Sox2, Nanog, Oct4, ALDH1A1, integrin-α6 and
CD133, in HeLa cancer stem-like cells. Therefore, CECU may have
therapeutic potential to eradicate cervical CSCs. Taken together,
our findings provide a new perspective on the anticancer activity
and mechanism of action of the non-aqueous extract of CECU against
cervical cancer cells. However, further in vivo experiments
are required to be performed to support the therapeutic efficacy of
CECU against cervical cancer.
Acknowledgements
Not applicable.
Funding
The current study was supported by Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
NRF-2016R1D1A1B03932956) and the NRF grant funded by the Ministry
of Science and ICT (grant no. NRF-2019R1A2C1009033). This work was
also supported by the Brain Korea 21 Plus Project, Republic of
Korea.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJJ conceived and designed the experiments. YSC and
JMH performed the experiments and analyzed the data. YSC and HJJ
wrote the paper. HJJ and YJK interpreted the data and revised the
paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schiffman M, Castle PE, Jeronimo J,
Rodriguez AC and Wacholder S: Human papillomavirus and cervical
cancer. Lancet. 370:890–907. 2007. View Article : Google Scholar
|
|
2
|
Liontos M, Kyriazoglou A, Dimitriadis I,
Dimopoulos MA and Bamias A: Systemic therapy in cervical cancer: 30
years in review. Crit Rev Oncol Hematol. 137:9–17. 2019. View Article : Google Scholar
|
|
3
|
Pfeffer CM and Singh AT: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:E4482018.
View Article : Google Scholar
|
|
4
|
Otto T and Sicinski P: Cell cycle proteins
as promising targets in cancer therapy. Nat Rev Cancer. 17:93–115.
2017. View Article : Google Scholar
|
|
5
|
Hu Z, Ding W, Zhu D, Yu L, Jiang X, Wang
X, Zhang C, Wang L, Ji T, Liu D, et al: TALEN-mediated targeting of
HPV oncogenes ameliorates HPV-related cervical malignancy. J Clin
Invest. 125:425–436. 2015. View
Article : Google Scholar
|
|
6
|
Arroyo M, Bagchi S and Raychaudhuri P:
Association of the human papillomavirus type 16 E7 protein with the
S-phase-specific E2F-cyclin A complex. Mol Cell Biol. 13:6537–6546.
1993. View Article : Google Scholar
|
|
7
|
Ayob AZ and Ramasamy TS: Cancer stem cells
as key drivers of tumour progression. J Biomed Sci. 25:202018.
View Article : Google Scholar
|
|
8
|
Feng D, Peng C, Li C, Zhou Y, Li M, Ling
B, Wei H and Tian Z: Identification and characterization of cancer
stem-like cells from primary carcinoma of the cervix uteri. Oncol
Rep. 22:1129–1134. 2009.
|
|
9
|
Cooke SL, Temple J, Macarthur S, Zahra MA,
Tan LT, Crawford RA, Ng CK, Jimenez-Linan M, Sala E and Brenton JD:
Intra-tumour genetic heterogeneity and poor chemoradiotherapy
response in cervical cancer. Br J Cancer. 104:361–368. 2011.
View Article : Google Scholar
|
|
10
|
Ortiz-Sánchez E, Santiago-López L,
Cruz-Domínguez VB, Toledo-Guzmán ME, Hernández-Cueto D,
Muñiz-Hernández S, Garrido E, Cantú De León D and García-Carrancá
A: Characterization of cervical cancer stem cell-like cells:
Phenotyping, stemness, and human papilloma virus co-receptor
expression. Oncotarget. 7:31943–31954. 2016. View Article : Google Scholar
|
|
11
|
Huang R and Rofstad EK: Cancer stem cells
(CSCs), cervical CSCs and targeted therapies. Oncotarget.
8:35351–35367. 2017. View Article : Google Scholar
|
|
12
|
Lichota A and Gwozdzinski K: Anticancer
activity of natural compounds from plant and marine environment.
Int J Mol Sci. 19:E35332018. View Article : Google Scholar
|
|
13
|
Roy M, Mukherjee A, Sarkar R, Mukherjee S
and Biswas J: In search of natural remediation for cervical cancer.
Anticancer Agents Med Chem. 15:57–65. 2015. View Article : Google Scholar
|
|
14
|
Yang CH and Horwitz SB: Taxol®:
The first microtubule stabilizing agent. Int J Mol Sci.
18:17332017. View Article : Google Scholar
|
|
15
|
McCreight LJ, Bailey CJ and Pearson ER:
Metformin and the gastrointestinal tract. Diabetologia. 59:426–435.
2016. View Article : Google Scholar
|
|
16
|
Kanaze FI, Bounartzi MI, Georgarakis M and
Niopas I: Pharmacokinetics of the citrus flavanone aglycones
hesperetin and naringenin after single oral administration in human
subjects. Eur J Clin Nutr. 61:472–477. 2007. View Article : Google Scholar
|
|
17
|
Min KY, Kim HJ, Lee KA, Kim KT and Paik
HD: Antimicrobial activity of acid-hydrolyzed Citrus unshiu
peel extract in milk. J Dairy Sci. 97:1955–1960. 2014. View Article : Google Scholar
|
|
18
|
Oh YC, Cho WK, Jeong YH, Im GY, Yang MC,
Hwang YH and Ma JY: Anti-inflammatory effect of Citrus
Unshiu peel in LPS-stimulated RAW 264.7 macrophage cells. Am J
Chin Med. 40:611–629. 2012. View Article : Google Scholar
|
|
19
|
Ahn KI, Choi EO, Kwon DH, HwangBo H, Kim
MY, Kim HJ, Ji SY, Hong SH, Jeong JW, Park C, et al: Induction of
apoptosis by ethanol extract of Citrus unshiu Markovich peel
in human bladder cancer T24 cells through ROS-mediated inactivation
of the PI3K/Akt pathway. Biosci Trends. 11:565–573. 2017.
View Article : Google Scholar
|
|
20
|
Kim MY, Choi EO, HwangBo H, Kwon DH, Ahn
KI, Kim HJ, Ji SY, Hong SH, Jeong JW, Kim GY, et al: Reactive
oxygen species-dependent apoptosis induction by water extract of
Citrus unshiu peel in MDA-MB-231 human breast carcinoma
cells. Nutr Res Pract. 12:129–134. 2018. View Article : Google Scholar
|
|
21
|
Kim MY, Bo HH, Choi EO, Kwon DH, Kim HJ,
Ahn KI, Ji SY, Jeong JW, Park SH, Hong SH, et al: Induction of
apoptosis by Citrus unshiu peel in human breast cancer MCF-7
cells: Involvement of ROS-dependent activation of AMPK. Biol Pharm
Bull. 41:713–721. 2018. View Article : Google Scholar
|
|
22
|
Li AN, Li S, Zhang YJ, Xu XR, Chen YM and
Li HB: Resources and biological activities of natural polyphenols.
Nutrients. 6:6020–6047. 2014. View Article : Google Scholar
|
|
23
|
Li S, Ma YM, Zheng PS and Zhang P: GDF15
promotes the proliferation of cervical cancer cells by
phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J Exp
Clin Cancer Res. 37:802018. View Article : Google Scholar
|
|
24
|
Al Bitar S and Gali-Muhtasib H: The role
of the cyclin dependent kinase inhibitor p21cip1/waf1 in targeting
cancer: Molecular mechanisms and novel therapeutics. Cancers
(Basel). 11:E14752019. View Article : Google Scholar
|
|
25
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar
|
|
26
|
Llambi F and Green DR: Apoptosis and
oncogenesis: Give and take in the BCL-2 family. Curr Opin Genet
Dev. 21:12–20. 2011. View Article : Google Scholar
|
|
27
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016. View Article : Google Scholar
|
|
28
|
Shin HJ, Han JM, Choi YS and Jung HJ:
Pterostilbene suppresses both cancer cells and cancer stem-like
cells in cervical cancer with superior bioavailability to
resveratrol. Molecules. 25:E2282020. View Article : Google Scholar
|
|
29
|
Jung N, Kwon HJ and Jung HJ:
Downregulation of mitochondrial UQCRB inhibits cancer stem
cell-like properties in glioblastoma. Int J Oncol. 52:241–251.
2018.
|
|
30
|
Vafaee K, Dehghani S, Tahmasvand R, Saeed
Abadi F, Irian S and Salimi M: Potent antitumor property of
Allium bakhtiaricum extracts. BMC Complement Altern Med.
19:1162019. View Article : Google Scholar
|
|
31
|
Yan Z, Feng J, Peng J, Lai Z, Zhang L, Jin
Y, Yang H, Chen W and Lin J: Chloroform extract of Hedyotis
diffusa Willd inhibits viability of human colorectal cancer
cells via suppression of AKT and ERK signaling pathways. Oncol
Lett. 14:7923–7930. 2017.
|
|
32
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5:e472016. View Article : Google Scholar
|
|
33
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: An updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–331. 2015. View Article : Google Scholar
|
|
34
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F, et al: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5:82020. View Article : Google Scholar
|
|
35
|
Bielecka ZF, Maliszewska-Olejniczak K,
Safir IJ, Szczylik C and Czarnecka AM: Three-dimensional cell
culture model utilization in cancer stem cell research. Biol Rev
Camb Philos Soc. 92:1505–1520. 2017. View Article : Google Scholar
|
|
36
|
Liu XF, Yang WT, Xu R, Liu JT and Zheng
PS: Cervical cancer cells with positive Sox2 expression exhibit the
properties of cancer stem cells. PLoS One. 9:e870922014. View Article : Google Scholar
|
|
37
|
Wang YD, Cai N, Wu XL, Cao HZ, Xie LL and
Zheng PS: OCT4 promotes tumorigenesis and inhibits apoptosis of
cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis.
4:e7602013. View Article : Google Scholar
|
|
38
|
Javed S, Sharma BK, Sood S, Sharma S,
Bagga R, Bhattacharyya S, Rayat CS, Dhaliwal L and Srinivasan R:
Significance of CD133 positive cells in four novel HPV-16 positive
cervical cancer-derived cell lines and biopsies of invasive
cervical cancer. BMC Cancer. 18:3572018. View Article : Google Scholar
|
|
39
|
Krebsbach PH and Villa-Diaz LG: The role
of integrin α6 (CD49f) in stem cells: More than a conserved
biomarker. Stem Cells Dev. 26:1090–1099. 2017. View Article : Google Scholar
|
|
40
|
Organista-Nava J, Gómez-Gómez Y,
Garibay-Cerdenares OL, Leyva-Vázquez MA and Illades-Aguiar B:
Cervical cancer stem cell-associated genes: Prognostic implications
in cervical cancer. Oncol Lett. 18:7–14. 2019.
|
|
41
|
Tomita H, Tanaka K, Tanaka T and Hara A:
Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget.
7:11018–11032. 2016. View Article : Google Scholar
|