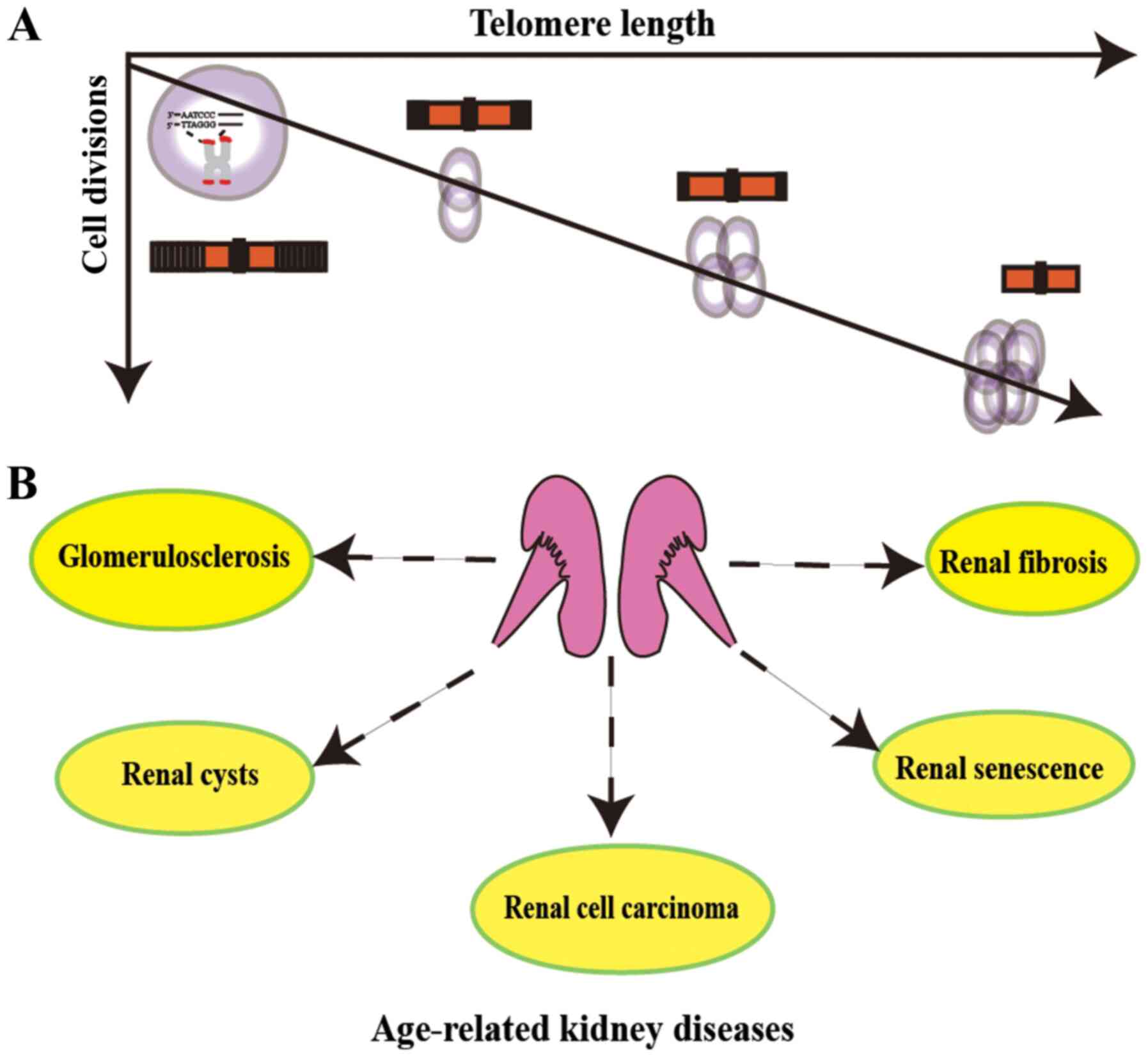
Telomeres have been reported to serve an essential
role in the renal ageing process, as they are an age-related
component (1). Telomeres are
composed of telomere proteins and telomere DNA. Telomere proteins
interact with telomere DNA to resist external attacks on the ends
of chromosomes and maintain the stability of chromosomes (2). When cells continue to live for a very
short period of time, telomere structure and function are
disrupted, resulting in genomic instability and increased risk of
disease (3). Ageing is a
significant risk factor for kidney diseases, such as chronic kidney
disease, and epidemiological studies have identified that elderly
individuals are predisposed to diverse fluid and electrolyte
abnormalities induced by renal diseases (4). The kidneys are significantly affected
by profound anatomical and functional changes induced by
senescence, and these changes have been discovered to lead to a
decreased glomerular filtration rate, reduced urine concentration
and dilution abilities, diminished urinary acidification and
impaired potassium clearance (5).
Numerous studies have demonstrated that cells within the human
kidney cortex undergo telomeric shortening over time (6,7).
Therefore, determining the multiple signalling pathways, such as
p53/p21 and p16INK4a, associated with telomeres and age-related
kidney diseases may be important for the treatment of
ageing-induced kidney diseases (8).
Telomeres consist of specific short repetitive
nucleotide sequences (5′-TTAGGG-3′) (Fig. 1) (3). A protein complex termed shelterin,
which is made up of telomere protective proteins, is located at the
end of chromosomes and maintains telomere structural integrity
(2). Shelterin complexes can form
telomere-loop (T-loop) by binding specifically to telomeres.
Similar to shelterin protein structure function, the T-loop can
protect chromosomal ends from end-to-end fusion and degradation
(9,10). When a telomere can shorten no more,
several cellular signals such as DNA damage response, inflammatory
and p53/p21 pathways are activated (11). The reverse transcriptase telomerase
is an RNA-dependent DNA polymerase with two primary components:
Reverse transcriptase and RNA (3,12). The
RNA component of the telomerase sequence is complementary to the
telomere template TTAGGG repeat, and the reverse transcriptase
component helps to add six-base-pair units to the ends of
chromosomes in repetitive cycles (2,12). In
this manner, telomerase helps to maintain telomere length (TL) in
human stem cells, reproductive cells and cancer cells (13). Telomerase is activated throughout
embryogenesis and in numerous types of cancer, but remains inactive
during tissue differentiation (14–16).
In addition, telomerase is inactivated in the majority of types of
mature human cells, which allows telomeres to shorten with every
cycle of cell division and eventually lead to chromosomal
instability. However, significant differences exist between humans
and mice/rodents such as the function of telomerase in cancer and
how the state of senescence is reached. For example, by comparing
structural and functional changes in ageing rat kidneys in
vivo and in vitro, a previous study revealed that rat
kidney telomeres did not significantly shorten in aging kidneys,
which may contribute to the age-related pathology (17).
While changes are known to occur in the kidneys
during the ageing process, the roles of telomeres and telomerase in
renal diseases, including renal cell carcinoma (RCC), chronic
kidney disease (CKD), glomerulosclerosis, acute kidney injury (AKI)
and renal cysts, have gained increasing attention in recent years
(Fig. 1) (18,19).
Thus, to further understand the causes of renal disease during
ageing, the present review aimed to summarise the association
between different types of renal disease and related molecular
signalling pathways to facilitate the elucidation of novel
therapeutic targets for kidney disease.
During the ageing process, the renal parenchyma
becomes thinner, which is primarily due to cortical tissue
regression, while no significant changes are observed in the
thickness of the renal medulla (20). Several changes also occur in the
glomerulus that affect the glomerular filtration rate, urine
concentration and dilution abilities, and urinary acidification,
and some tubulointerstitial alterations have also been noted in
ageing kidneys (21). As the
understanding of the roles of telomeres and telomerase in renal
physiology continues to improve, researchers are actively
attempting to elucidate their effects on renal disorders and
related diseases (22).
Previously, telomerase deficiency was revealed to
reduce the proliferative capacities of glomerular, tubular and
interstitial cells; however, telomerase activity differs between
humans and mice (23). TL is
genetically determined, with the average TL and rate of telomere
shortening varying among species. For example, humans are born with
shorter telomeres than mice, but mouse telomeres shorten 100 times
faster than those of humans (24).
It was reported that telomeric shortening limits the recovery
capacity following AKI (25).
Changes in the kidneys have also been found to be accompanied by
vascular changes and blood pressure increases during the ageing
process (26). However, although
numerous morphological changes, such as the roughness of kidney
surface, are observed in ageing organs, none are specifically
attributed to kidney pathogeneses. Verzola et al (27) reported that patients with type II
diabetic nephropathy had accelerated renal and proximal tubule cell
senescence. However, while kidney cell senescence may be induced by
telomere shortening, whether it is affected by telomeric loss
remains unknown. Aged patients with advanced CKD are known to
exhibit numerous age-associated conditions, including myophagism,
vascular calcification, premature vascular disease and osteoporosis
(28). These age-associated
diseases may therefore share mechanisms involved in the ageing
process, such as telomere shortening, mitochondrial dysfunction and
DNA damage response. Melk et al (8) reported that upregulated
p16INK4a expression could directly affect the prognosis
of ageing patients with kidney diseases. As changes in kidney
function can be quantified in longitudinal studies more readily
compared with other organs, kidneys are often used as a model to
research the effects on organs during ageing.
In recent years, RCC has become the most common type
of kidney cancer, accounting for >90% of all cases of kidney
cancer (29). As RCCs are often
diagnosed at an advanced stage, patients with RCC have a poor
survival rate, <8% (30). TL is
known to gradually shorten with age, which may lead to chromosomal
instability and subsequent tumorigenesis (31). While some previous studies have
demonstrated that a shortened TL promoted poor outcomes for
patients with RCC (32), others
studies have failed to elucidate such a relationship (33,34).
However, Morais and Dias (31)
suggested that TL may serve a dual role in RCC, for instance, short
telomere length can increase RCC risk in late carcinogenesis, while
long telomere length appears to be associated with tumor prognosis
in the early stages. Therefore, an increasing number of researchers
have also investigated methods, such as reverse transcription PCR,
TRF-Southern blotting and fluorescence in situ
hybridization, for measuring TL to predict the survival of patients
with RCC (4,35,36).
Svenson et al (37) used
reverse transcription PCR to analyze the TL in the blood cells of
patients with kidney cancer instead of the kidney cortex or tumour
tissue, and indicated that measuring the TL in the blood may be
useful in predicting patient survival. Therefore, as a biomarker of
ageing-related diseases, TL should be further studied to elucidate
its impact on other tissues (38).
Repressor-activator protein 1 (RAP1) and protection
of telomeres protein 1 (POT1) are essential members of the
shelterin complex, the expression of which is crucial for telomere
maintenance (3,39). Pal et al (40) analysed the gene expression profiles
of RAP1 and POT1 in 65 samples of RCC tumour and adjacent normal
renal parenchymal tissues. The RAP1 mRNA expression was found to be
significantly increased with the grade and subtype of RCC, whereas
POT1 expression levels were upregulated in tumour tissues compared
with the corresponding normal renal tissues, but POT1 expression
was not associated with grades, stages and subtypes of RCC
(33,39).
Telomeres have been reported to serve an important
role in the prognosis of patients with cancer, and the level of
telomerase activity has been discovered to be maintained in 85% of
malignancies, including RCC (12);
however, how telomerase activity is maintained in RCC remains
unknown. Human telomerase reverse transcriptase (TERT) is an active
component of telomerase that is mainly responsible for its
catalytic activity and is known to participate in the maintenance
of stem cells, enhancement of DNA repair and promotion of late
carcinogenesis (2). Dahse et
al (41) demonstrated that the
PCR detection of telomerase activity could be used to predict
tumour malignancy in patients with RCC. The reported detection of
telomerase activity in the majority of RCCs may imply that
telomerase activation may be a critical step in the process of RCC
(42), and measuring telomerase
activity may provide additional information regarding tumour
progression and therapeutic strategies.
Numerous further studies are required to elucidate
the role of telomeres in the carcinogenesis of RCC. Although an
association between telomere-related proteins and the RCC grade and
subtype has been well established, the related molecular pathways
activated by telomere-related proteins in kidney diseases have not
been fully elucidated.
CKD is a major public health problem characterized
by poor outcomes, especially with the increasing ageing population
(43). Kidney function has been
found to decline due to the development of ageing-associated
glomerulosclerosis, which is the most common pathological finding
in patients with CKD (44). Ageing
presents a risk factor for the development of CKD, which is defined
by an estimated glomerular filtration rate of <60 ml/min per
1.73 m2 (45). The
reciprocal relationship between TL and CKD risk has been proven by
numerous previous studies (46–48),
and while telomere shortening was found to be associated with
ageing-related CKD, the interaction is offset by the cellular
telomere repair process (4).
Telomerase maintains the length and structure of telomeres and
extends the cellular life span, and its activity has been suggested
to be potentially associated with CKD (49). Despite telomerase activity gradually
decreasing with cellular division, telomerase activation holds
substantial promise for CKD treatment in the clinical setting
(4), as numerous studies have
proven the relationship between telomerase activity and CKD stages
(50). For example, Kidir et
al (49) investigated and
followed-up patients with stage 5D CKD and used the TRAP assay to
measure telomerase activity in peripheral blood mononuclear cells
(PBMCs); the authors concluded that telomerase activity in PBMCs
was increased in patients with CKD at risk of advanced stage.
However, the study also indicated that telomerase gene
polymorphisms may be more related to CKD risks than telomerase
activity and TL. It was shown that during the progression of CKD,
telomeric RNA component (TERC) rs12696304 and TERT rs2736100
polymorphisms served greater roles in primary glomerulonephritis,
end-stage renal disease (ESRD) and CKD in females compared with TL
and telomerase activity (48).
Moreover, these germline variants were not affected by ageing,
diseases or other pathological and physiological environmental
elements (48,51). In ESRD, patients exhibited loss of
thymic function and telomere attrition, which inhibited the
cellular immune system (52).
Telomerase activation and telomere attrition are usually
accompanied by activation of the immune system and the release of
inflammatory factors that could be used as biomarkers for the
treatment of CKD (53). In addition
to inflammation, numerous genes and molecular mechanisms, such as
the klotho gene (54) and oxidative
stress, have been found to be activated by telomerase activation
and telomere attrition during the progression of CKD (55).
Despite the identification of several molecular
targets related to telomere dysfunction, numerous therapeutic
trials, such as the target telomerase drugs GX301 and GV1001, are
not helpful for CKD in older patients. Therefore, telomeres and
telomerase activity should be further considered as personalized
medicine targets for patients with CKD.
Fibrosis can occur in a variety of organs, such as
the kidneys, lungs and other major organs under similar fibrosis
pathological conditions (56) The
main pathological changes underlying fibrosis are the increased
accumulation of fibrous connective tissue in organs and decreased
numbers of parenchymal cells (57).
The deterioration of fibrosis can lead to the destruction of organ
structure and functional decline or even failure, which
significantly threatens human health (58). Kidney fibrosis is the main
pathological feature of chronic kidney failure and manifests as
tissue structure disorganisation (59,60).
Piñeiro-Hermida et al (61)
revealed an association between the development of idiopathic
pulmonary fibrosis and telomere dysfunction and telomerase
mutations. Likewise, renal fibrosis has been found to be also
influenced by cellular ageing-related mechanisms, such as the
decline in renal function induced by telomere shortening (62). Cianciolo et al (63) demonstrated that the glomerular
filtration rate in humans and dogs decreased over time and while
telomere shortening was not definitively the underlying factor, it
had an ability to induce ageing-related disease, such as CKD. The
length of telomeres is shorten with each cell division, and
telomere dysfunction can lead to cell cycle arrest (64). Therefore, telomere
dysfunction-induced renal cells with fibrosis are often accompanied
by the activation of cell cycle arrest signals (65). Yang et al (66) demonstrated that
G2/M-arrested proximal tubular cells activated by JNK
signalling to prevent fibrosis. Thus, telomeres and related
molecules may be implicated in renal fibrosis and serve as useful
diagnostic biomarkers and therapeutic targets.
AKI has become a public health concern because it is
associated with high mortality rates. AKI is characterized by a
decreased glomerular filtration rate that consequentially results
in the deterioration of renal function and ultimately, a
considerable percentage of patients requiring dialysis (67). The elderly are particularly
susceptible to AKI and demonstrate poor prognoses, which is <16%
(68). Epel et al (69) identified the relationship between
dynamic telomerase activity and acute stressors, and concluded that
telomerase activity could be used as a molecular marker for AKI.
These data confirmed observations, such as renal regeneration, that
telomerase may be a molecular marker in numerous injury models and
suggested that the telomere shortening process may be advanced by a
single renal insult and potentially accelerated by repeated
injuries (61,70). In addition, shortened telomeres were
indicated to potentially contribute to increased renal injury and
decreased renal recovery following insult (71). Numerous other factors associated
with ageing were also indicated to be increased, including reactive
oxygen species generation, cell cycle arrest and mitochondrial
dysfunction (72,73). Cisplatin (CDDP) is an effective
chemotherapeutic agent that is commonly used to treat cancer and
has been shown to induce renal toxicity (74). However, the effect of CDDP on AKI in
the elderly with shortened telomeres requires further
investigation. Shin et al (75) demonstrated that older animals
(20-week-old rats) with shortened telomeres treated with CDDP
continually had high expression levels of biological markers, such
as urinary kidney injury model-1, TIMP metallopeptidase inhibitor 1
and VEGF, which may be helpful in the early diagnosis of elderly
patients, who may have shorter telomeres, with CDDP-induced AKI. In
the elderly with AKI, telomeres may be used as a molecular maker to
evaluate kidney function. The ageing of organs has been identified
as the main factor leading to the exacerbation/deterioration of
kidney structure during the process of AKI, and cell cycle arrest
was discovered to occur not only renal fibrosis, but also in AKI
(76). With each cell cycle,
telomere repeats are lost because telomerase is not activated in
adult human cells. Since the DNA polymerase cannot replicate linear
chromosomes from the ends, telomeres are critically shortened
(77). In addition, renal
ischaemia-reperfusion injury was found to lead to more significant
impairment of renal function, and was increased in
fourth-generation telomerase-deficient
(G4Terc−/−) mice compared with both wild-type and
G1Terc−/− mice (78). In addition, another previous study
reported that critically short telomeres upregulated the expression
levels of the cell cycle inhibitors p21 and p16INK4a,
and increased renal cell apoptosis accompanied activated
ataxia-telangiectasia (79). These
data also demonstrated significantly reduced proliferative
capacities of tubular, glomerular and interstitial cells. As
age-related renal diseases significantly contribute to morbidity by
~18.7%, strategies for preventing these diseases may markedly
improve healthy ageing (22).
Therefore, renal regeneration following AKI therapy is promising,
as telomere shortening can increase cellular senescence and
apoptosis, thereby limiting the regenerative capacity in response
to injury (80).
Renal cysts occur in nephrons and have been
discovered to lead to end-stage renal failure due to progressive
tubular cystic expansion and loss of typical renal structure and
function (81,82). Renal cysts, a condition in which the
kidneys are filled with fluid-containing cysts replacing much of
the normal renal structure, cause progressive kidney enlargement
over time and eventually leads to uremia (83). In addition, fluid within the cysts
is derived from glomerular filtrate and transepithelial fluid
secretion (84). Renal cysts were
also discovered to occur in the development of long-term CKDs and
other types of organ dysfunction, such as pancreas and ovary
(85,86). For example, Yaghoubian et al
(87) observed that patients with
abdominal aortic aneurysms had increased renal cyst incidence
rates, indicating that renal cysts may be associated with other
types of disease. In addition, previous research on polycystic
kidney disease (PKD) identified numerous of the molecular
mechanisms that were closely associated with the occurrence of
renal cysts, such as genetic mutations in hepatocyte nuclear
factor-1β and polycystin-1/2 (88–90),
abnormal renin-angiotensin-aldosterone system activation (91), altered intracellular calcium
signalling (82,92), MET oncogene mutations (93), vasopressin receptor upregulation
(94), mTOR pathway activation
(95,96), c-Myc-derived apoptosis (97), endothelin-1 receptor upregulation
(98), TGF-β1 and apelin pathway
activation (99), VEGF gene
variants (100) and dysfunction of
the innate immune system (101).
According to the results of NCBI literature search, there are a few
studies evaluating the relationship between telomere and PKD
(102–104). Although there is not sufficient
evidence to prove the direct relationship between telomeres or
telomerase and renal cysts, the association between telomere
dysfunction-induced tumours and PKD was confirmed in our previous
study; RNA-Seq and single-sample gene set enrichment analysis was
used to identify the gene signatures and pathways. The results have
shown that mutant p53 and telomere dysfunction increased the
development of PKD (105). In
regard to these data, further research should be performed to
explore the combination of mutant p53-target and telomere drugs,
such as GRN163L and APR-246 in the future (106,107). The application of this combination
for renal cyst treatment could enhance renal function and relieve
the pain caused by abnormally functioning kidneys.
Due to the ageing population, ageing-related renal
diseases have attracted increasing attention. Extensive studies
have revealed that telomeres and telomerase serve important roles
in not only normal nephrogenesis, but also in renal cysts,
fibrosis, regeneration after AKI, RCC and various CKDs (Fig. 1). Thus, future research should focus
on whether telomeres and renal diseases exert a cause or effect
role to improve the research and treatment of kidney disease. It
should be noted that telomere dysfunction is not always required
for the induction of ageing-related renal diseases, and could be
due to cumulative environmental stress. Although numerous studies
have proven that telomere dysfunction is involved in various types
of renal disease in the elderly and the role of telomere
maintenance in age-related renal diseases, it is currently unknown
whether telomere homeostasis has beneficial effects on the ageing
kidney. Thus, further evaluation of the impact of telomere dynamics
on the ageing kidney is required. In conclusion, the study of
telomeres and telomerase in relation to kidney diseases is only in
its infancy and further research questions, such as drug
development targeting telomerase will need to be addressed in the
future.
Not applicable.
This work was supported by the Academic promotion
programme of Shandong First Medical University (grant no.
2019QL016) and the Innovation Project of Shandong Academy of
Medical Sciences.
Not applicable.
HL and BW were major contributors in writing the
manuscript. DL and JL corrected the English writing. YL and JD read
and approved the final manuscript. All authors (HL, BW, DL, JL, YL
and JD) read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Shay JW and Wright WE: Hallmarks of
telomeres in ageing research. J Pathol. 211:114–123. 2007.
View Article : Google Scholar
|
|
2
|
Pańczyszyn A, Boniewska-Bernacka E and Goc
A: The role of telomeres and telomerase in the senescence of
postmitotic cells. DNA Repair (Amst). 95:1029562020. View Article : Google Scholar
|
|
3
|
Smith EM, Pendlebury DF and Nandakumar J:
Structural biology of telomeres and telomerase. Cell Mol Life Sci.
77:61–79. 2020. View Article : Google Scholar
|
|
4
|
Ameh OI, Okpechi IG, Dandara C and Kengne
AP: Association between telomere length, chronic kidney disease,
and renal traits: A systematic review. OMICS. 21:143–155. 2017.
View Article : Google Scholar
|
|
5
|
Bolignano D, Mattace-Raso F, Sijbrands EJ
and Zoccali C: The aging kidney revisited: A systematic review.
Ageing Res Rev. 14:65–80. 2014. View Article : Google Scholar
|
|
6
|
Sitaram RT, Cairney CJ, Grabowski P, Keith
WN, Hallberg B, Ljungberg B and Roos G: The PTEN regulator DJ-1 is
associated with hTERT expression in clear cell renal cell
carcinoma. Int J Cancer. 125:783–790. 2009. View Article : Google Scholar
|
|
7
|
Svenson U, Gronlund E, Soderstrom I,
Sitaram RT, Ljungberg B and Roos G: Telomere length in relation to
immunological parameters in patients with renal cell carcinoma.
PLoS One. 8:e555432013. View Article : Google Scholar
|
|
8
|
Melk A, Schmidt BM, Takeuchi O, Sawitzki
B, Rayner DC and Halloran PF: Expression of p16INK4a and other cell
cycle regulator and senescence associated genes in aging human
kidney. Kidney Int. 65:510–520. 2004. View Article : Google Scholar
|
|
9
|
de Lange T: A loopy view of telomere
evolution. Front Genet. 6:3212015. View Article : Google Scholar
|
|
10
|
Wood AM, Laster K, Rice EL and Kosak ST: A
beginning of the end: New insights into the functional organization
of telomeres. Nucleus. 6:172–178. 2015. View Article : Google Scholar
|
|
11
|
de Lange T: Protection of mammalian
telomeres. Oncogene. 21:532–540. 2002. View Article : Google Scholar
|
|
12
|
Nagpal N and Agarwal S: Telomerase RNA
processing: Implications for human health and disease. Stem Cells.
2020.(Epub ahead of print). View Article : Google Scholar
|
|
13
|
Serakinci N, Graakjaer J and Kolvraa S:
Telomere stability and telomerase in mesenchymal stem cells.
Biochimie. 90:33–40. 2008. View Article : Google Scholar
|
|
14
|
Blagoev KB: Cell Proliferation in the
presence of telomerase. PLoS One. 4:e26222009. View Article : Google Scholar
|
|
15
|
Cong YS, Wright WE and Shay JW: Human
telomerase and its regulation. Microbiol Mol Biol Rev. 66:407–425,
table of contents. 2002. View Article : Google Scholar
|
|
16
|
Buseman CM, Wright WE and Shay JW: Is
telomerase a viable target in cancer? Mutat Res. 730:90–97. 2012.
View Article : Google Scholar
|
|
17
|
Melk A, Kittikowit W, Sandhu I, Halloran
KM, Grimm P, Schmidt BMW and Halloran PF: Cell senescence in rat
kidneys in vivo increases with growth and age despite lack of
telomere shortening. Kidney Int. 63:2134–2143. 2003. View Article : Google Scholar
|
|
18
|
Li Y and Lerman LO: Cellular senescence: A
new player in kidney injury. Hypertension. 76:1069–1075. 2020.
View Article : Google Scholar
|
|
19
|
Kato S, Shiels PG, McGuinness D, Lindholm
B, Stenvinkel P, Nordfors L, Qureshi AR, Yuzawa Y, Matsuo S and
Maruyama S: Telomere attrition and elongation after chronic
dialysis initiation in patients with end-stage renal disease. Blood
Purif. 41:25–33. 2016. View Article : Google Scholar
|
|
20
|
Perico N, Remuzzi G and Benigni A: Aging
and the kidney. Curr Opin Nephrol Hypertens. 20:312–317. 2011.
View Article : Google Scholar
|
|
21
|
Abdel-Rahman EM and Okusa MD: Effects of
aging on renal function and regenerative capacity. Nephron Clin
Pract. 127:15–20. 2014. View Article : Google Scholar
|
|
22
|
Wills LP and Schnellmann RG: Telomeres and
telomerase in renal health. J Am Soc Nephrol. 22:39–41. 2011.
View Article : Google Scholar
|
|
23
|
Blasco MA, Lee HW, Hande MP, Samper E,
Lansdorp PM, DePinho RA and Greider CW: Telomere shortening and
tumor formation by mouse cells lacking telomerase RNA. Cell.
91:25–34. 1997. View Article : Google Scholar
|
|
24
|
Wright WE and Shay JW: Telomere dynamics
in cancer progression and prevention: Fundamental differences in
human and mouse telomere biology. Nat Med. 6:849–851. 2000.
View Article : Google Scholar
|
|
25
|
Kloda K, Domanski L, Kwiatkowska E,
Borowiecka E, Safranow K, Drozd A, Ciechanowicz A,
Maciejewska-Karłowska A, Sawczuk M, Pawlik A and Ciechanowski K:
hTERT, BICD1 and chromosome 18 polymorphisms associated with
telomere length affect kidney allograft function after
transplantation. Kidney Blood Press Res. 40:111–120. 2015.
View Article : Google Scholar
|
|
26
|
O'Hare AM, Bertenthal D, Walter LC, Garg
AX, Covinsky K, Kaufman JS, Rodriguez RA and Allon M: When to refer
patients with chronic kidney disease for vascular access surgery:
Should age be a consideration? Kidney Int. 71:555–561. 2007.
View Article : Google Scholar
|
|
27
|
Verzola D, Gandolfo MT, Gaetani G,
Ferraris A, Mangerini R, Ferrario F, Villaggio B, Gianiorio F,
Tosetti F, Weiss U, et al: Accelerated senescence in the kidneys of
patients with type 2 diabetic nephropathy. Am J Physiol Renal
Physiol. 295:F1563–F1573. 2008. View Article : Google Scholar
|
|
28
|
Lindeman RD, Tobin J and Shock NW:
Longitudinal studies on the rate of decline in renal function with
age. J Am Geriatr Soc. 33:278–285. 1985. View Article : Google Scholar
|
|
29
|
Chow WH, Dong LM and Devesa SS:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar
|
|
30
|
Klatte T, Rossi SH and Stewart GD:
Prognostic factors and prognostic models for renal cell carcinoma:
A literature review. World J Urol. 36:1943–1952. 2018. View Article : Google Scholar
|
|
31
|
Morais M, Dias F, Teixeira AL and Medeiros
R: Telomere length in renal cell carcinoma: The Jekyll and Hyde
biomarker of ageing of the kidney. Cancer Manag Res. 12:1669–1679.
2020. View Article : Google Scholar
|
|
32
|
Pal D, Sharma U, Singh SK, Kakkar N and
Prasad R: Inhibition of hTERT expression by MAP kinase inhibitor
induces cell death in renal cell carcinoma. Urol Oncol. 35:401–408.
2017. View Article : Google Scholar
|
|
33
|
Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH,
Shay JW, Luo S, Hong WK and Spitz MR: Telomere dysfunction: A
potential cancer predisposition factor. J Natl Cancer Inst.
95:1211–1218. 2003. View Article : Google Scholar
|
|
34
|
Mehle C, Ljungberg B and Roos G: Telomere
shortening in renal cell carcinoma. Cancer Res. 54:236–241.
1994.
|
|
35
|
Gisselsson D, Gorunova L, Hoglund M,
Mandahl N and Elfving P: Telomere shortening and mitotic
dysfunction generate cytogenetic heterogeneity in a subgroup of
renal cell carcinomas. Br J Cancer. 91:327–332. 2004. View Article : Google Scholar
|
|
36
|
Carrozza F, Santoni M, Piva F, Cheng L,
Lopez-Beltran A, Scarpelli M, Montironi R, Battelli N and Tamberi
S: Emerging immunotherapeutic strategies targeting telomerases in
genitourinary tumors. Crit Rev Oncol Hematol. 131:1–6. 2018.
View Article : Google Scholar
|
|
37
|
Svenson U, Ljungberg B and Roos G:
Telomere length in peripheral blood predicts survival in clear cell
renal cell carcinoma. Cancer Res. 69:2896–2901. 2009. View Article : Google Scholar
|
|
38
|
Demanelis K, Jasmine F, Chen LS, Chernoff
M, Tong L, Delgado D, Zhang C, Shinkle J, Sabarinathan M, Lin H, et
al: Determinants of telomere length across human tissues. Science.
369:eaaz68762020. View Article : Google Scholar
|
|
39
|
Aramburu T, Plucinsky S and Skordalakes E:
POT1-TPP1 telomere length regulation and disease. Comput Struct
Biotechnol J. 18:1939–1946. 2020. View Article : Google Scholar
|
|
40
|
Pal D, Singh SK, Kakkar N and Prasad R:
Expression of telomere binding proteins (RAP1 and POT1) in renal
cell carcinoma and their correlation with clinicopathological
parameters. Indian J Clin Biochem. 32:301–305. 2017. View Article : Google Scholar
|
|
41
|
Dahse R, Fiedler W, Junker K, Schlichter
A, Schubert J and Claussen U: Telomerase activity and telomere
lengths: Alterations in renal cell carcinomas. Kidney Int.
56:1289–1290. 1999. View Article : Google Scholar
|
|
42
|
Sugimura K, Yoshida N, Hisatomi H,
Nakatani T and Ikemoto S: Telomerase activity in human renal cell
carcinoma. BJU Int. 83:693–697. 1999. View Article : Google Scholar
|
|
43
|
Tonelli M and Riella MC: Chronic kidney
disease and the aging population. Kidney Int. 85:487–491. 2014.
View Article : Google Scholar
|
|
44
|
Kremers WK, Denic A, Lieske JC, Alexander
MP, Kaushik V, Elsherbiny HE, Chakkera HA, Poggio ED and Rule AD:
Distinguishing age-related from disease-related glomerulosclerosis
on kidney biopsy: The aging kidney anatomy study. Nephrol Dial
Transplant. 30:2034–2039. 2015. View Article : Google Scholar
|
|
45
|
Nitta K, Okada K, Yanai M and Takahashi S:
Aging and chronic kidney disease. Kidney Blood Press Res.
38:109–120. 2013. View Article : Google Scholar
|
|
46
|
Cañadas-Garre M, Anderson K, Cappa R,
Skelly R, Smyth LJ, McKnight AJ and Maxwell AP: Genetic
susceptibility to chronic kidney disease-some more pieces for the
heritability puzzle. Front Genet. 10:4532019. View Article : Google Scholar
|
|
47
|
Tonelli M and Riella M: Chronic kidney
disease and the aging population. J Cross Cult Gerontol.
29:231–237. 2014. View Article : Google Scholar
|
|
48
|
Mazidi M, Rezaie P, Covic A, Malyszko J,
Rysz J, Kengne AP and Banach M: Telomere attrition, kidney
function, and prevalent chronic kidney disease in the United
States. Oncotarget. 8:80175–80181. 2017. View Article : Google Scholar
|
|
49
|
Kidir V, Aynali A, Altuntas A, Inal S,
Aridogan B and Sezer MT: Telomerase activity in patients with stage
2–5D chronic kidney disease. Nefrologia. 37:592–597. 2017.(In
English and Spanish). View Article : Google Scholar
|
|
50
|
Tsirpanlis G, Chatzipanagiotou S, Boufidou
F, Kordinas V, Alevyzaki F, Zoga M, Kyritsis I, Stamatelou K,
Triantafyllis G and Nicolaou C: Telomerase activity is decreased in
peripheral blood mononuclear cells of hemodialysis patients. Am J
Nephrol. 26:91–96. 2006. View Article : Google Scholar
|
|
51
|
Sell DR and Monnier VM: End-stage renal
disease and diabetes catalyze the formation of a pentose-derived
crosslink from aging human collagen. J Clin Invest. 85:380–384.
1990. View Article : Google Scholar
|
|
52
|
Wong LS, van der Harst P, de Boer RA, Codd
V, Huzen J, Samani NJ, Hillege HL, Voors AA, van Gilst WH, Jaarsma
T and van Veldhuisen DJ: Renal dysfunction is associated with
shorter telomere length in heart failure. Clin Res Cardiol.
98:629–634. 2009. View Article : Google Scholar
|
|
53
|
Kooman JP, Dekker MJ, Usvyat LA, Kotanko
P, van der Sande FM, Schalkwijk CG, Shiels PG and Stenvinkel P:
Inflammation and premature aging in advanced chronic kidney
disease. Am J Physiol Renal Physiol. 313:F938–F950. 2017.
View Article : Google Scholar
|
|
54
|
Hu MC, Kuro-o M and Moe OW: Klotho and
chronic kidney disease. Contrib Nephrol. 180:47–63. 2013.
View Article : Google Scholar
|
|
55
|
Vlassara H, Torreggiani M, Post JB, Zheng
F, Uribarri J and Striker GE: Role of oxidants/inflammation in
declining renal function in chronic kidney disease and normal
aging. Kidney Int Suppl. S3-11:2009.
|
|
56
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012. View Article : Google Scholar
|
|
57
|
Rockey DC, Bell PD and Hill JA: Fibrosis-a
common pathway to organ injury and failure. N Engl J Med.
372:1138–1149. 2015. View Article : Google Scholar
|
|
58
|
Vaglio A, Salvarani C and Buzio C:
Retroperitoneal fibrosis. Lancet. 367:241–251. 2006. View Article : Google Scholar
|
|
59
|
Sziksz E, Pap D, Lippai R, Béres NJ,
Fekete A, Szabó AJ and Vannay Á: Fibrosis related inflammatory
mediators: Role of the IL-10 cytokine family. Mediators Inflamm.
2015:7646412015. View Article : Google Scholar
|
|
60
|
Nowakowski ACH: Cystic fibrosis kidney
disease: 10 Tips for clinicians. Front Med (Lausanne). 5:2422018.
View Article : Google Scholar
|
|
61
|
Piñeiro-Hermida S, Autilio C, Martínez P,
Bosch F, Pérez-Gil J and Blasco MA: Telomerase treatment prevents
lung profibrotic pathologies associated with physiological aging. J
Cell Biol. 219:e2020021202020. View Article : Google Scholar
|
|
62
|
Sangaralingham SJ, Wang BH, Huang L, Kumfu
S, Ichiki T, Krum H and Burnett JC Jr: Cardiorenal fibrosis and
dysfunction in aging: Imbalance in mediators and regulators of
collagen. Peptides. 76:108–114. 2016. View Article : Google Scholar
|
|
63
|
Cianciolo RE, Benali SL and Aresu L: Aging
in the canine kidney. Vet Pathol. 53:299–308. 2016. View Article : Google Scholar
|
|
64
|
Naesens M: Replicative senescence in
kidney aging, renal disease, and renal transplantation. Discov Med.
11:65–75. 2011.
|
|
65
|
Turner KJ, Vasu V and Griffin DK: Telomere
biology and human phenotype. Cells. 8:732019. View Article : Google Scholar
|
|
66
|
Yang L, Besschetnova TY, Brooks CR, Shah
JV and Bonventre JV: Epithelial cell cycle arrest in G2/M mediates
kidney fibrosis after injury. Nat Med. 16:535–543, 531p following
143. 2010. View Article : Google Scholar
|
|
67
|
Ronco C, Bellomo R and Kellum JA: Acute
kidney injury. Lancet. 394:1949–1964. 2019. View Article : Google Scholar
|
|
68
|
Ishani A, Xue JL, Himmelfarb J, Eggers PW,
Kimmel PL, Molitoris BA and Collins AJ: Acute kidney injury
increases risk of ESRD among elderly. J Am Soc Nephrol. 20:223–228.
2009. View Article : Google Scholar
|
|
69
|
Epel ES, Lin J, Dhabhar FS, Wolkowitz OM,
Puterman E, Karan L and Blackburn EH: Dynamics of telomerase
activity in response to acute psychological stress. Brain Behav
Immun. 24:531–539. 2010. View Article : Google Scholar
|
|
70
|
Kiecolt-Glaser JK and Glaser R:
Psychological stress, telomeres, and telomerase. Brain Behav Immun.
24:529–530. 2010. View Article : Google Scholar
|
|
71
|
Cheng H, Fan X, Lawson WE, Paueksakon P
and Harris RC: Telomerase deficiency delays renal recovery in mice
after ischemia-reperfusion injury by impairing autophagy. Kidney
Int. 88:85–94. 2015. View Article : Google Scholar
|
|
72
|
Zhan M, Brooks C, Liu F, Sun L and Dong Z:
Mitochondrial dynamics: Regulatory mechanisms and emerging role in
renal pathophysiology. Kidney Int. 83:568–581. 2013. View Article : Google Scholar
|
|
73
|
Lane RK, Hilsabeck T and Rea SL: The role
of mitochondrial dysfunction in age-related diseases. Biochim
Biophys Acta. 1847:1387–1400. 2015. View Article : Google Scholar
|
|
74
|
Volarevic V, Djokovic B, Jankovic MG,
Harrell CR, Fellabaum C, Djonov V and Arsenijevic N: Molecular
mechanisms of cisplatin-induced nephrotoxicity: A balance on the
knife edge between renoprotection and tumor toxicity. J Biomed Sci.
26:252019. View Article : Google Scholar
|
|
75
|
Shin YJ, Kim TH, Won AJ, Jung JY, Kwack
SJ, Kacew S, Chung KH, Lee BM and Kim HS: Age-related differences
in kidney injury biomarkers induced by cisplatin. Environ Toxicol
Pharmacol. 37:1028–1039. 2014. View Article : Google Scholar
|
|
76
|
Abdel-Kader K and Palevsky PM: Acute
kidney injury in the elderly. Clin Geriatr Med. 25:331–358. 2009.
View Article : Google Scholar
|
|
77
|
Aubert G and Lansdorp PM: Telomeres and
aging. Physiol Rev. 88:557–579. 2008. View Article : Google Scholar
|
|
78
|
Westhoff JH, Schildhorn C, Jacobi C, Hömme
M, Hartner A, Braun H, Kryzer C, Wang C, von Zglinicki T, Kränzlin
B, et al: Telomere shortening reduces regenerative capacity after
acute kidney injury. J Am Soc Nephrol. 21:327–336. 2010. View Article : Google Scholar
|
|
79
|
Schmitt R and Cantley LG: The impact of
aging on kidney repair. Am J Physiol Renal Physiol.
294:F1265–F1272. 2008. View Article : Google Scholar
|
|
80
|
Andrade L, Rodrigues CE, Gomes SA and
Noronha IL: Acute kidney injury as a condition of renal senescence.
Cell Transplant. 27:739–753. 2018. View Article : Google Scholar
|
|
81
|
Pei Y: Diagnostic approach in autosomal
dominant polycystic kidney disease. Clin J Am Soc Nephrol.
1:1108–1114. 2006. View Article : Google Scholar
|
|
82
|
Mangolini A, de Stephanis L and Aguiari G:
Role of calcium in polycystic kidney disease: From signaling to
pathology. World J Nephrol. 5:76–83. 2016. View Article : Google Scholar
|
|
83
|
Perumareddi P and Trelka DP: Autosomal
dominant polycystic kidney disease. Prim Care. 47:673–689. 2020.
View Article : Google Scholar
|
|
84
|
McConnachie DJ, Stow JL and Mallett AJ:
Ciliopathies and the kidney: A review. Am J Kidney Dis. Oct
8–2020.(Epub ahead of print). View Article : Google Scholar
|
|
85
|
Rangan GK, Tchan MC, Tong A, Wong AT and
Nankivell BJ: Recent advances in autosomal-dominant polycystic
kidney disease. Intern Med J. 46:883–892. 2016. View Article : Google Scholar
|
|
86
|
Iliuta IA, Kitchlu A and Pei Y:
Methodological issues in clinical trials of polycystic kidney
disease: A focused review. J Nephrol. 30:363–371. 2017. View Article : Google Scholar
|
|
87
|
Yaghoubian A, de Virgilio C, White RA and
Sarkisyan G: Increased incidence of renal cysts in patients with
abdominal aortic aneurysms: A common pathogenesis? Ann Vasc Surg.
20:787–791. 2006. View Article : Google Scholar
|
|
88
|
Kolatsi-Joannou M, Bingham C, Ellard S,
Bulman MP, Allen LI, Hattersley AT and Woolf AS: Hepatocyte nuclear
factor-1beta: A new kindred with renal cysts and diabetes and gene
expression in normal human development. J Am Soc Nephrol.
12:2175–2180. 2001.
|
|
89
|
Ta MH, Schwensen KG, Liuwantara D, Huso
DL, Watnick T and Rangan GK: Constitutive renal Rel/nuclear
factor-κB expression in lewis polycystic kidney disease rats. World
J Nephrol. 5:339–357. 2016. View Article : Google Scholar
|
|
90
|
Bergmann C, von Bothmer J, Ortiz Bruchle
N, Venghaus A, Frank V, Fehrenbach H, Hampel T, Pape L, Buske A,
Jonsson J, et al: Mutations in multiple PKD genes may explain early
and severe polycystic kidney disease. J Am Soc Nephrol.
22:2047–2056. 2011. View Article : Google Scholar
|
|
91
|
Hian CK, Lee CL and Thomas W:
Renin-angiotensin-aldosterone system antagonism and polycystic
kidney disease progression. Nephron. 134:59–63. 2016. View Article : Google Scholar
|
|
92
|
Chapman AB: Autosomal dominant polycystic
kidney disease: Time for a change? J Am Soc Nephrol. 18:1399–1407.
2007. View Article : Google Scholar
|
|
93
|
Zhang W, Tan AY, Blumenfeld J, Liu G,
Michaeel A, Zhang T, Robinson BD, Salvatore SP, Kapur S, Donahue S,
et al: Papillary renal cell carcinoma with a somatic mutation in
MET in a patient with autosomal dominant polycystic kidney disease.
Cancer Genet. 209:11–20. 2016. View Article : Google Scholar
|
|
94
|
Gansevoort RT, Arici M, Benzing T, Birn H,
Capasso G, Covic A, Devuyst O, Drechsler C, Eckardt KU, Emma F, et
al: Recommendations for the use of tolvaptan in autosomal dominant
polycystic kidney disease: A position statement on behalf of the
ERA-EDTA working groups on inherited kidney disorders and european
renal best practice. Nephrol Dial Transplant. 31:337–348. 2016.
View Article : Google Scholar
|
|
95
|
Riwanto M, Kapoor S, Rodriguez D,
Edenhofer I, Segerer S and Wuthrich RP: Inhibition of aerobic
glycolysis attenuates disease progression in polycystic kidney
disease. PLoS One. 11:e01466542016. View Article : Google Scholar
|
|
96
|
Ta MH, Schwensen KG, Foster S, Korgaonkar
M, Ozimek-Kulik JE, Phillips JK, Peduto A and Rangan GK: Effects of
TORC1 inhibition during the early and established phases of
polycystic kidney disease. PLoS One. 11:e01641932016. View Article : Google Scholar
|
|
97
|
Couillard M, Guillaume R, Tanji N, D'Agati
V and Trudel M: c-myc-induced apoptosis in polycystic kidney
disease is independent of FasL/Fas interaction. Cancer Res.
62:2210–2214. 2002.
|
|
98
|
Raina R, Lou L, Berger B, Vogt B, Sao-Mai
Do A, Cunningham R, Vasavada P, Herrmann K, Dell K and Simonson M:
Relationship of urinary endothelin-1 with estimated glomerular
filtration rate in autosomal dominant polycystic kidney disease: A
pilot cross-sectional analysis. BMC Nephrol. 17:222016. View Article : Google Scholar
|
|
99
|
Kocer D, Karakukcu C, Ozturk F, Eroglu E
and Kocyigit I: Evaluation of fibrosis markers: Apelin and
transforming growth factor-β1 in autosomal dominant polycystic
kidney disease patients. Ther Apher Dial. 20:517–522. 2016.
View Article : Google Scholar
|
|
100
|
Martins DP, Souza MA, Baitello ME,
Nogueira V, Oliveira CI, Pinhel MA, Caldas HC, Filho MA and Souza
DR: Vascular endothelial growth factor as an angiogenesis biomarker
for the progression of autosomal dominant polycystic kidney
disease. Genet Mol Res. 15:2016. View Article : Google Scholar
|
|
101
|
Peda JD, Salah SM, Wallace DP, Fields PE,
Grantham CJ, Fields TA and Swenson-Fields KI: Autocrine IL-10
activation of the STAT3 pathway is required for pathological
macrophage differentiation in polycystic kidney disease. Dis Model
Mech. 9:1051–1061. 2016. View Article : Google Scholar
|
|
102
|
de Melo AS, Dias SV, Cavalli Rde C,
Cardoso VC, Bettiol H, Barbieri MA, Ferriani RA and Vieira CS:
Pathogenesis of polycystic ovary syndrome: Multifactorial
assessment from the foetal stage to menopause. Reproduction.
150:R11–R24. 2015. View Article : Google Scholar
|
|
103
|
Borie R, Kannengiesser C, Dupin C, Debray
MP, Cazes A and Crestani B: Impact of genetic factors on fibrosing
interstitial lung diseases. Incidence and clinical presentation in
adults. Presse Med. 49:1040242020. View Article : Google Scholar
|
|
104
|
Ballew BJ and Savage SA: Updates on the
biology and management of dyskeratosis congenita and related
telomere biology disorders. Expert Rev Hematol. 6:327–337. 2013.
View Article : Google Scholar
|
|
105
|
Li H, Zhang Y, Dan J, Zhou R, Li C, Li R,
Wu X, Singh SK, Chang JT, Yang J and Luo Y: p53 mutation regulates
PKD genes and results in co-occurrence of PKD and tumorigenesis.
Cancer Biol Med. 16:79–102. 2019. View Article : Google Scholar
|
|
106
|
Duffy MJ, Synnott NC, O'Grady S and Crown
J: Targeting p53 for the treatment of cancer. Semin Cancer Biol.
Jul 30–2020.(Epub ahead of print). View Article : Google Scholar
|
|
107
|
Sugarman ET, Zhang G and Shay JW: In
perspective: An update on telomere targeting in cancer. Mol
Carcinog. 58:1581–1588. 2019. View Article : Google Scholar
|