Introduction
It is commonly known that acute and chronic wounds
pose serious and unresolved treatment issues that increase
morbidity and mortality, thus leading to increased healthcare
costs. Bioinspired approaches derived from insights into mechanisms
of healing can help to find solutions that improve skin repair.
Initial steps in the direction of finding practical possibilities
in this respect have been taken by exposing organ cultured human
skin and wounded adult newts to plant agglutinins, such as
phytohemagglutinin or concanavalin A (1,2).
Endogenous lectins in the skin, which are involved in healing
pathways, would be superior candidates over exogenous reagents.
Indeed, the skin is home to a variety of glycan receptors,
including C-type lectins and galactoside-binding lectins, or
galectins.
Galectins are a family of β-sheet proteins
synthesized on free ribosomes that exert various context-specific
activities both intracellularly and following non-conventional
secretion that do not involve the endo reticulum-Golgi route
(3–12). First detected (as Mac-2 antigen) in
the keratinocytes of murine skin (13) and also human skin (SL66) fibroblasts
(14,15), galectin-3 (Gal-3) has become a focus
of research in this type of lectin in dermatology (16–23).
In addition to its ability to bridge cell surface counterreceptors,
thus reducing the rate of their dynamic endocytosis, Gal-3 is able
to trigger changes in gene expression profiles by outside-in
signaling, not only in human skin fibroblasts, but also in other
types of cells (24–26). This evidence has prompted further
exploration beyond Gal-3 to obtain a full view on galectin
representation in skin. Since the presence of galectins has been
described in human skin as a network (27), our previous studies examined their
expression profiles in pig and rat skin during wound healing
(28,29). Based on the presence of several
galectins in the skin and emerging insights into their regulation
during wound healing, together with their known multifunctionality,
it may be hypothesized that galectins may mechanistically be
involved in this regenerative process.
In our previous study, this hypothesis was tested
for six galectins in vitro, demonstrating their capacity to
convert dermal fibroblasts into myofibroblasts and to remodel the
extracellular matrix (ECM) (30).
Moreover, these observations were dependent on galectin type and
provided evidence that Gal-1 could reduce the area of an excisional
wound in vivo in rats (30).
In the present study, expression profiling of wound-healing-related
genes was carried out in human dermal fibroblasts in vitro
following exposure to Gal-1 and −3. Moreover, immunocytochemical
analysis of keratinocytes cultured on an ECM substratum derived
from the galectin-treated fibroblasts, histology of rat skin
wounds, including collagen staining, and measurements of tensile
strength of an incisional wound were also conducted in vivo.
The present findings pointed to a potential beneficial role for
human Gal-3 in the healing of skin incisions.
Materials and methods
Galectins
Human wild-type (WT) Gal-1 and −3 were obtained by
recombinant production and purified by affinity chromatography on
lactosylated Sepharose 4B prepared by ligand conjugation after
activation of resin by divinyl sulfone. In the case His-tagged
Gal-1 (E71Q) mutant (mutant that lost lectin activity by the
site-directed mutagenesis), Ni-CAMTM HC (Sigma-Aldrich; Merck KGaA)
was used. This was followed by the removal of any
lipopolysaccharide contamination and desorption of bound
(His-tagged) proteins by histidine (31,32).
Product analysis was performed using one- and two-dimensional gel
electrophoresis, gel filtration and mass spectrometry, as well as
carbohydrate-inhibitable hemagglutination and solid-phase assays in
order to ascertain β-galactoside binding (or its loss) (33,34).
Human dermal fibroblast (HDF) and
human interfollicular keratinocyte (HIK) primary culture
HDFs and HIKs were isolated from the skin of healthy
donors who underwent routine aesthetic surgery at the Department of
Aesthetic Surgery, Third Faculty of Medicine, Charles University
(Prague, Czech Republic). Written informed consent was obtained
from all donors, in agreement with the Declaration of Helsinki and
with approval from The Ethics Committee of University Hospital
Kralovske Vinohrady and Third Faculty of Medicine, Charles
University (approval no. 100/1947/2005). HDF cultures were expanded
in Dulbecco's modified Eagle medium (DMEM) supplemented with 10%
FBS (both from Biochrom, Ltd.) and penicillin (100
U/ml)/streptomycin (100 µg/ml; both from Biochrom, Ltd.), while
HIKs were cultured in a mixture of DMEM and F12 (BioConcept AG)
medium (3:1 vol:vol) containing 10% FBS that was further enriched
with insulin (0.12 U/ml; Novo Nordisk A/S), cholera toxin (1 nM;
Sigma-Aldrich; Merck KGaA), hydrocortisone (0.4 µg/ml;
Sigma-Aldrich; Merck KGaA) and epidermal growth factor (10 ng/ml;
Sigma-Aldrich; Merck KGaA) at 37°C with 5% CO2, as
described previously (35).
Isolation of RNA and reverse
transcription (RT)
Following 48-h treatment with Gal-1 (300 ng/ml) and
Gal-3 (600 ng/ml), total cellular RNA was isolated from HDFs using
an RNeasy® Mini kit (Qiagen GmbH) according to the
manufacturer's instructions. RNA quantification was performed with
a NanoDrop™ 1000 Spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.), after which 250 ng total RNA was
subjected to 1% agarose gel electrophoresis to confirm its
integrity. The RNA samples were stored at −80°C. For analytical
profiling, RNA was reverse transcribed using the RT2
First Strand kit (Qiagen GmbH) following the manufacturer's
instructions.
RT-quantitative (RT-q)PCR
RT2 Profiler PCR Array for Human Wound
Healing (Qiagen GmbH) was carried out using SYBR-Green as the
reporter dye, according to the manufacturer's instructions. The RT
array included reference genes, a control for excluding the
presence of genomic DNA, three reverse-transcription controls and
set of 84 wound-repair-related genes. Three positive RNA controls
were also present. The sequences of forward and reverse primers
were designed and supplied by Qiagen GmbH. Two biological
replicates were used for each sample group in two independent
experimental batches and mRNA profiling was performed at two time
points (24 and 48 h of culture). PCR was performed on an Applied
Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher
Scientific, Inc.). The thermocycling conditions were set as
follows: Initial denaturation step (10 min, 95°C) was followed by
40 cycles each consisting of a denaturation step for 15 sec at 95°C
and annealing for 1 min at 60°C. The obtained data were analyzed
using the 2−ΔΔCq method (36), with the average expression of four
housekeeping genes used as a reference (β-actin, β2-microglobulin,
GAPDH and hypoxanthine phosphoribosyltransferase 1) to obtain
relative expression values for each gene. The analysis was carried
out using limma (version 3.42.2) (37). To detect differentially transcribed
genes following treatment with Gal-1, −3 and TGF-β1 (30 ng/ml;
PeproTech EC Ltd.), groups were individually compared with the
untreated control using a linear model and a moderated t-test.
Genes with >2-fold up- or downregulation relative to the
untreated control were considered statistically significant. Data
and overlaps of differentially transcribed genes were visualized
using gplots (version 3.0.3; http://github.com/talgalili/gplots) and Vennerable
(version 3.1.0.9000; http://github.com/js229/Vennerable) packages in R
version 3.6.3 (https://www.r-project.org).
Culture of HIKs on ECM produced by
galectin-treated HDFs
HDFs were seeded at a density of 2,000
cells/cm2 and cultured for 10 days in the absence or
presence of Gal-1 (WT or E71Q mutant) or Gal-3. Sterile solutions
containing either no additive or Gal-1 (WT or E71Q mutant) at 300
ng/ml and Gal-3 at 600 ng/ml, which were determined to be the most
effective concentrations based on a previous experiment (30), were prepared in DMEM containing 10%
FBS and antibiotics. ECM scaffolds produced by the cell
preparations cultured in parallel on the surface of glass
microscope slide cover slips were tested as substrate for the HIKs.
Cells were removed from cover slips by osmotic shock (exposure to
sterile distilled water for 60 min), then surfaces were covered
with keratinocyte medium (mixture of DMEM and F12; 3:1) and cover
slips were incubated for 24 h. HIKs were seeded at a density of
10,000 cells/cm2 and kept for 7 days at 5%
CO2 and 37°C prior to immunocytochemistry.
Immunocytochemistry of cultured
cells
The tested specimens were fixed with 2% buffered
paraformaldehyde (pH 7.2) for 5 min at room temperature and washed
with phosphate-buffered saline (PBS; pH 7.2). Cells were
permeabilized by exposure to Triton X-100 (Sigma-Aldrich; Merck
KGaA), and sites for antigen-independent binding of antibodies were
blocked by incubation with porcine serum albumin (diluted in PBS,
1:30; Dako; Agilent Technologies, Inc.) for 30 min at room
temperature. Commercial antibodies were used at concentrations
recommended by the suppliers (Table
I). Incubations with primary and secondary antibodies were
performed for 90 and 45 min at room temperature, respectively.
Nuclear DNA was stained using DAPI for 1 min at room temperature.
All specimens were mounted to Vectashield (Vector Laboratories,
Inc.) and examined using an Eclipse 90i microscope equipped with
filter blocks for the three types of dyes (Nikon Corporation), as
well as a Cool-1300Q CCD camera (Vosskühler) and a
computer-assisted image analysis system LUCIA 5.1 (Laboratory
Imaging).
 | Table I.Antibodies used for
immunocytochemistry and lectin histochemistry. |
Table I.
Antibodies used for
immunocytochemistry and lectin histochemistry.
| A, Primary
antibodies |
|---|
|
|---|
| Name | Host | Supplier | Cat. no. | Dilution |
|---|
| α-smooth muscle
actin | Mouse
monoclonal | Dako (Agilent
Technologies, Inc.) | M0851 | 1:50 |
| Vimentin | Mouse
monoclonal | Dako (Agilent
Technologies, Inc.) | M0725 | 1:200 |
| Tenascin | Mouse
monoclonal | Sigma-Aldrich
(Merck KGaA) | T3413 | 1:200 |
| Fibronectin | Rabbit
polyclonal | Dako (Agilent
Technologies, Inc.) | A0245 | 1:1,000 |
| Ki67 | Rabbit
polyclonal | EMD Millipore | AB9260 | 1:200 |
|
| B, Secondary
antibodies |
|
| Name | Host |
Supplier | Cat.
no. |
Dilution |
|
| Anti-mouse;
TRITC | Goat | Sigma-Aldrich
(Merck KGaA) | T5393 | 1:30 |
| Anti-rabbit;
FITC | Swine | Dako (Agilent
Technologies, Inc.) | F0205 | 1:100 |
Cell counting
In vitro experiments were repeated twice to
assess the expression of keratin-19 (K-19; marker of low-degree
keratinocyte differentiation) and Ki67 (marker of proliferation).
Staining (1 min at room temperature) of nuclear DNA with DAPI
(Sigma-Aldrich; Merck KGaA) was performed so that the total numbers
of HIKs per three visualization fields (magnification, ×200) of
each biological replicate could be counted, then positive
expression of Ki67 and K-19 in cell populations was quantitated.
The data are given as a percentage of the total number of counted
cells. The aforementioned imaging system described for
immunocytochemistry was also utilized for this assay.
Animal model
This study was approved by the Ethical Committee of
the Faculty of Medicine of the Pavol Jozef Šafárik University
(Košice, Slovak Republic) and by the State Veterinary
Administration of the Slovak Republic. It was performed as
described previously (30). A total
of 96 male Sprague-Dawley rats (age, 1 years old; weight, 507±48 g)
were included in the study. Animals were housed in plexiglass cages
(22–24°C, 50–70% relative humidity, 12/12 h light/dark cycles) with
free access to food and water. Surgery was performed under general
anesthesia induced by intramuscular administration of 40 mg/kg
ketamine, 15 mg/kg xylazine and 5 mg/kg tramadol (38,39).
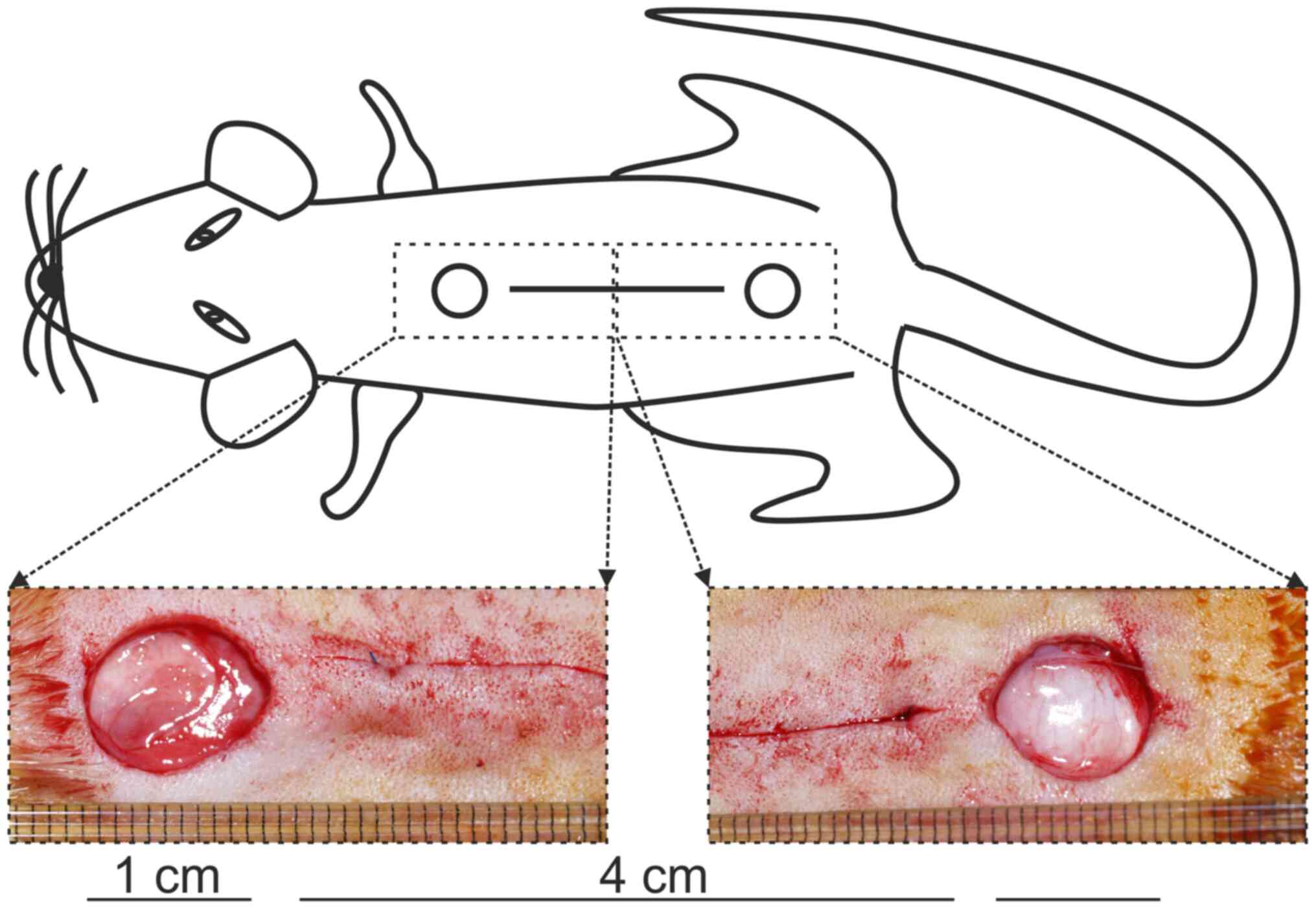
Under strict aseptic conditions, two 1-cm, round, full-thickness,
excisional skin wounds and one 4-cm, full-thickness skin incision
were inflicted to the back of each rat at the position depicted in
Fig. 1. The incision was
subsequently sutured using intradermal running suture. In each
group, 6 rats were sacrificed on day 7 and 21 post-surgery,
respectively. Allocation of rats to treatment groups is shown in
Table II.
 | Table II.Allocation of rats in treatment
groups. |
Table II.
Allocation of rats in treatment
groups.
|
|
| Gal-1 treatment
group, n | Gal-1(E71Q)
treatment group, n | Gal-3 treatment
group |
|---|
|
|
|
|
|
|
|---|
| Time/group | Untreated control,
n | 10 μg/ml | 20 μg/ml | 10 μg/ml | 20 μg/ml | 10 μg/ml | 20 μg/ml |
|---|
| 7 days | 12 | 6 | 6 | 6 | 6 | 6 | 6 |
| 21 days | 12 | 6 | 6 | 6 | 6 | 6 | 6 |
Wound treatment
In vivo experiments were performed in
parallel under identical conditions, first on an exploratory level
(n=48; data not shown) with galectin concentrations of 10 μg/ml
(lyophilized protein containing K/Na-phosphate salts as buffer
substances dissolved in physiological saline solution), then
systematically (n=48) with galectin concentrations of 20 μg/ml
applied topically on the wound surface (using an eye dropper)
during the first 3 post-operative days (three times a day).
Histology
Specimens of wounds were removed from rats
sacrificed by cervical dislocation following ether anesthesia
(using a vaporizer) at the two given time points and routinely
processed for classical histological staining performed at room
temperature (fixation in 4% buffered formaldehyde for 10 min,
dehydration using a series of solutions with increasing
concentration of ethanol, paraffin embedding, sectioning).
Deparaffinized sections (5 µm thick) were stained with Van Gieson's
solution (non-specific collagen staining) and also with hematoxylin
for 10 min and eosin at 4 min, according to a previous study
(30). Sections were examined under
an Olympus BX51 microscope equipped with an Olympus DP73 CCD camera
(Olympus Corporation).
Immunohistochemistry
A second set of specimens of wounds was
cryoprotected using Tissue-Tek (Sakura Finetek Europe B.V.) and
frozen in liquid nitrogen. Tissue sections (~10 µm thickness) were
first mounted on the surface of poly-l-lysine-treated glass slides
(Sigma-Aldrich; Merck KGaA), then fixed at room temperature using
2% (w/v) paraformaldehyde in PBS for 10 min. Non-specific binding
of the secondary antibody was blocked at room temperature with
normal swine serum (DakoCytomation; Agilent Technologies, Inc.)
diluted with PBS (1:30) for 30 min.
Solutions of commercially available primary and
secondary antibodies are shown in Table
I. Incubation with primary and secondary antibodies was
performed for 90 and 45 min (at room temperature), respectively.
Nuclear DNA was stained using DAPI for 1 min at room temperature.
Controls for specificity of the immunohistochemical detection were
as follows: i) Replacement of the target-specific antibody by an
irrelevant antibody (in the case of monoclonal antibodies of the
same isotype); and ii) omission of the incubation step with the
primary antibody to exclude antigen-independent signal generation.
Specimens were mounted using Vectashield (Vector Laboratories,
Inc.) and examined under an Eclipse 90i microscope (Nikon
Corporation), as aforementioned.
Semi-quantitative scoring of
histological sections
The status of re-epithelialization and extent of the
presence of polymorphonuclear leukocytes (PMNL), fibroblasts, newly
formed vessels and collagen were assessed according to a
semi-quantitative scale system, as defined in Table III (40).
 | Table III.Scale used for the semi-quantitative
evaluation of histological sections. |
Table III.
Scale used for the semi-quantitative
evaluation of histological sections.
| Scale |
Epithelialization | PMNL | Fibroblasts | Luminized
vessels | Collagen |
|---|
| 0 | Thickness of cut
edges | Absent | Absent | Absent | Absent |
| 1 | Migration of cells
(<50%) | Mild-ST | Mild-ST | Mild-SCT | Mild-GT |
| 2 | Migration of cells
(≥50%) | Mild-DL/GT | Mild-GT | Mild-GT | Moderate-GT |
| 3 | Bridging the
excision | Moderate-DL/GT | Moderate-GT | Moderate-GT | Marked-GT |
| 4 | Complete
keratinization | Marked-DL/GT | Marked-GT | Marked-GT | Organized-GT |
Wound tensile strength (TS)
Measurement of force to rupture an incisional wound
was facilitated by equipment assembled in our laboratory using
commercial devices (41). Briefly,
an adequately shaped horizontal arm was used to pull at one side of
a specimen, while the opposite side was fixed to a sensor tip of a
force meter unit (Omega Engineering, Inc.). The moving arm was
driven by a high-precision stepper motor MDI-17 (Intelligent Motion
Systems Inc.) through a linear slider. The technique for
determining the TS had been described in detail previously
(42). Briefly, following
euthanasia, two 1-cm-wide skin strips were removed from each
incisional wound and placed lengthwise between the clamps of the TS
testing device. Pulling was performed perpendicularly to the
original direction of the incision. The maximal strength of rupture
was measured for each specimen. TS (g/mm2) was
calculated as TS=MRS/A, where MRS is the maximal rupture strength
(g) and A is the wound area (mm2).
Statistical analysis
Continuous data are presented as the mean ± SD.
Categorical data are presented as the median. Data obtained from
cell counting were compared using one-way ANOVA followed by the
Tukey-Kramer post hoc test. Two-way ANOVA followed by Tukey's post
hoc test was used to compare the effects of treatment modalities
and time on wound TS. The Kruskal-Wallis test with multiple
comparison (Dunn's test) was applied to compare non-parametric
semi-quantitative data. The aforementioned statistical analyses
were performed in SPSS v22 software (IBM Corp.). RT-qPCR data were
analyzed using a moderated t-test in limma (version 3.42.2)
(37) R version 3.6.3 (https://www.r-project.org) and Bioconductor version
3.1.0 software packages (http://www.bioconductor.org). P<0.05 was considered
to indicate a statistically significant difference.
Results
In vitro study
The influence of administration of the two galectins
on biochemical and cellular features was tested in vitro in
two experimental settings.
HDF gene expression profiling
In order to determine whether Gal-1 and −3 could
affect HDF gene expression in vitro, the effective
concentration required to induce conversion to myofibroblasts or
ECM redesign (30), the Qiagen
RT2 Profiler PCR Array for Human Wound Healing system
was used to monitor expression levels of 84 selected genes relevant
for wound healing. Technically, the sets of experiments recommended
for stringent quality control completely satisfied the standards of
this commercial system (data not shown). Indeed, Gal-1 led to
significant changes of signal intensity relative to the control
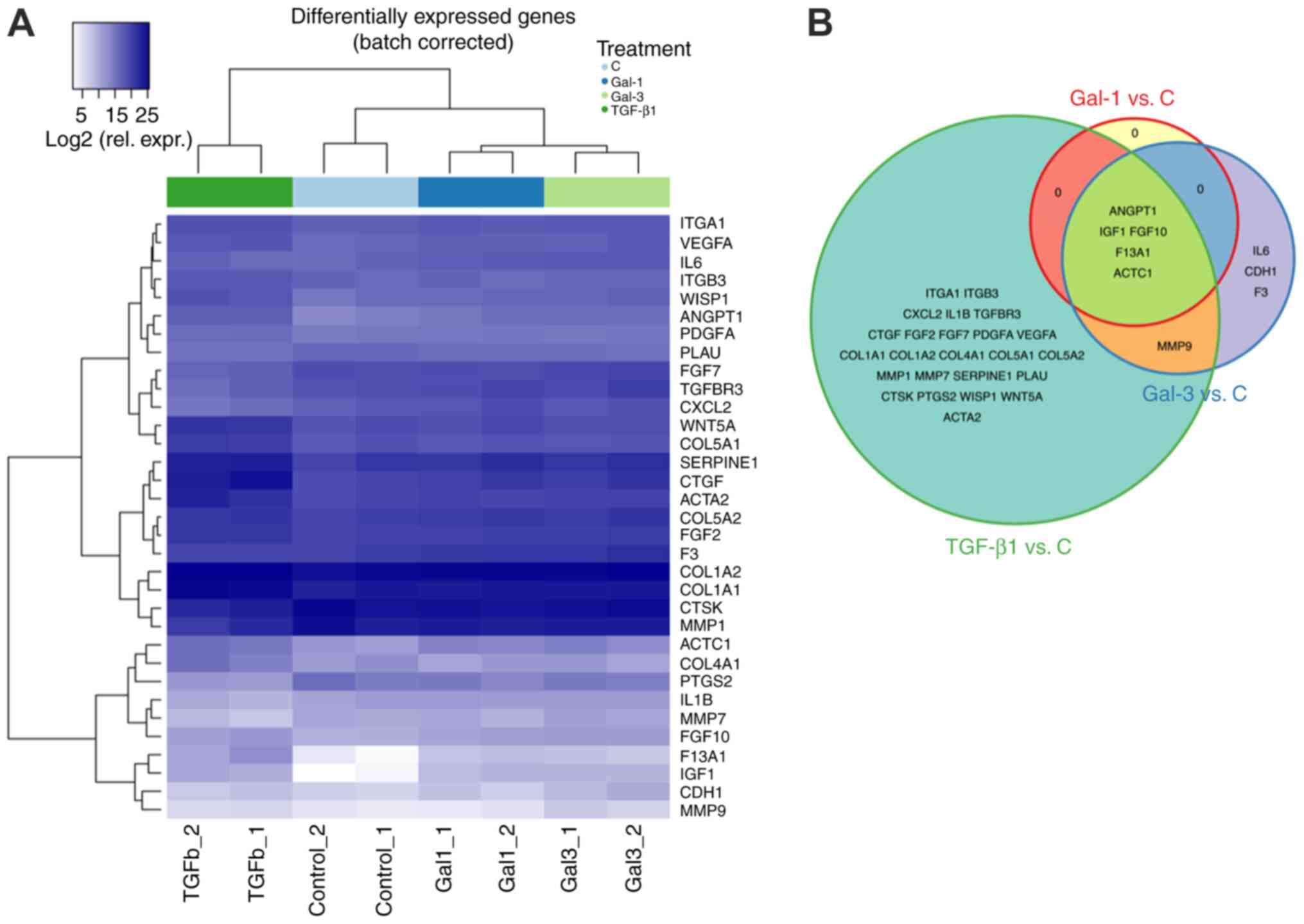
(log2 fold change, 1.62–6.00; Fig. 2A). Genes with increased expression
are presented in Table SI. Gal-3
treatment also caused deviations from the respective control from
1.14 to 6.28 log2 fold change upregulation relative to
the control (Fig. 2A). There was
considerable overlap between the lists of upregulated genes for
Gal-1 and −3, including ANGPT1, IGF1, FGF10, F13A1 and
ACTC1 (Fig. 2B). In order to
ascertain common responsiveness of cell populations to a known
effector of fibroblast activation, TGF-β1 was tested in parallel as
a positive control (Table SI). Of
note, a full overlap of profiles between TGF-β1 and a Gal-1 was
observed, whereas Gal-3 treatment resulted in uniquely regulated
genes overlapping neither Gal-1 nor TGF-β1 profiles, such as
IL6, CDH1 and F3 (Fig.
2B).
HIK immunocytochemistry
The low-level fibronectin staining at day 10
following HIK seeding demonstrated that the original
three-dimensional structure of the ECM was completely replaced by a
confluent layer of epithelial cells (Fig. 3A). The presence of fibronectin was
restricted to small intracellular granules. K-19 was present in
typically small cells, and a relative increase in the red signal
was seen in the Gal-1-treated group, compared with the control,
Gal-1(E71Q) mutant and Gal-3 conditions (Fig. 3A and B). Of note, the
loss-of-glycan-binding Gal-1 (Gal-1E71Q) single-site mutant did not
trigger the pronounced effect of WT Gal-1 (Fig. 3A and B). As a measure of the growth
fraction of cell populations, the Ki67 antigen status was assessed.
Ki67-Positive HIKs were generally seen, most frequently for Gal-3
(Fig. 3B). Double staining
suggested that K-19-positive cells did not present the Ki67 antigen
(see insert in Fig. 3B), an
observation made independent of the experimental condition.
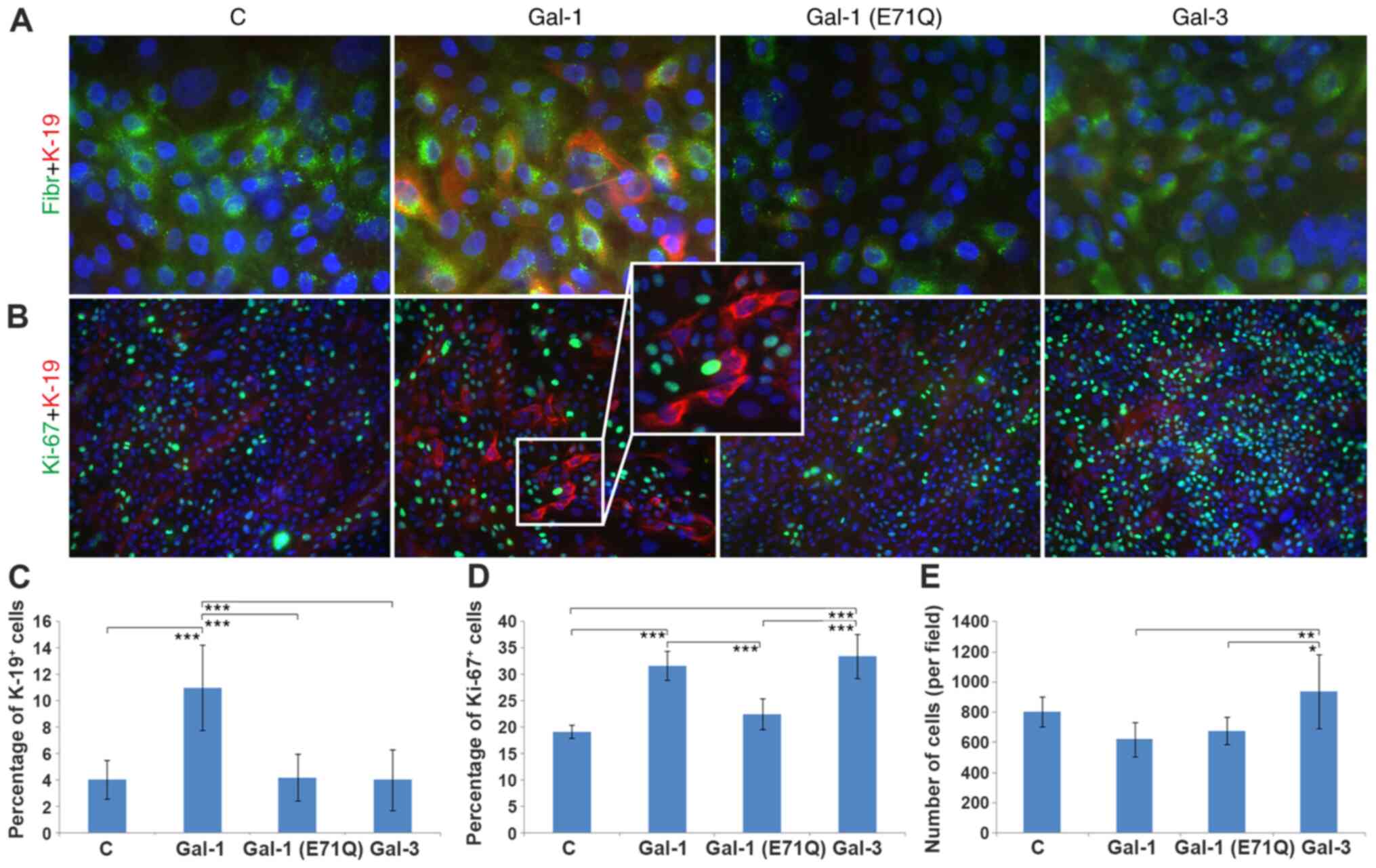 | Figure 3.Characteristics of human
interfollicular keratinocytes grown on an ECM produced by untreated
or galectin-treated human dermal fibroblast cultures. Nuclei are
counterstained with DAPI. (A) Cells were immunostained for the
presence of Fibr (green signal) and K-19 (red signal).
Magnification, ×600. (B) Cells positive for Ki67 (green signal) and
K-19 (red signal). Magnification, ×200. Insert magnification, ×400.
(see insert, magnification 400×). Quantification of the studied
parameters. (C) Frequency of K-19+ keratinocytes. (D)
Frequency of Ki67+ cells. (E) Total number of cells in
cultures using ECM from each group. *P<0.05, **P<0.01,
***P<0.001. Gal, galectin; C, control; Fibr, fibronectin; ECM,
extracellular matrix. |
Quantification of the observed parameters is
presented in Fig. 3C-E. The
increase in the positive expression of K-19 in Gal-1-treated cells
was significant when compared with the control group, the
Gal-1(E71Q) mutant- and Gal-3-treated cell populations (Fig. 3C). Positive expression of Ki67 was
increased significantly in the Gal-1- and Gal-3-treated groups
compared with the control and Gal-1(E71Q) mutant-treated cells, but
not relative to each other (Fig.
3D). The total number of cells counted for each condition
reached the most statistically significant level (among test
groups) for Gal-3-exposed cells (Fig.
3E).
In vivo study
The effect of the two galectins on wound healing was
tested on rats using two basic models of skin repair (open excision
and sutured incision). During the post-surgical period, all animals
remained healthy and did not show symptoms of infection. Initial
experiments using concentrations of 10 μg/ml galectins showed no
activity (data not shown). Thus, subsequent experiments were
performed with 20 μg/ml concentrations (equal molar concentration
due to similar molecular weights of Gal-1 homodimer and monomeric
chimera-type Gal-3).
Histology of open excision wounds
The results of the extended histological analysis
using immunohistochemistry and Van Gieson staining (top and middle
panel) and of the semi-quantitative evaluation of distinct
parameters of the wounds at the two time points (bottom panel) are
shown in Fig. 4. At day 7, the
newly formed granulation tissue (GT) was rich in fibronectin
(Fig. 4A), populated by
fibroblasts, and also notably vascularized. The inset in the
photomicrograph of a section of a specimen of the Gal-1-treated
group illustrates the already known capacity of this protein to
generate SMA-positive myofibroblasts (Fig. 4A). When quantitated, the numbers of
fibroblasts and luminized vessels were increased in both
lectin-treated groups (Fig. 4C). An
increase in collagen score was seen at this time point for Gal-3
(Fig. 4C).
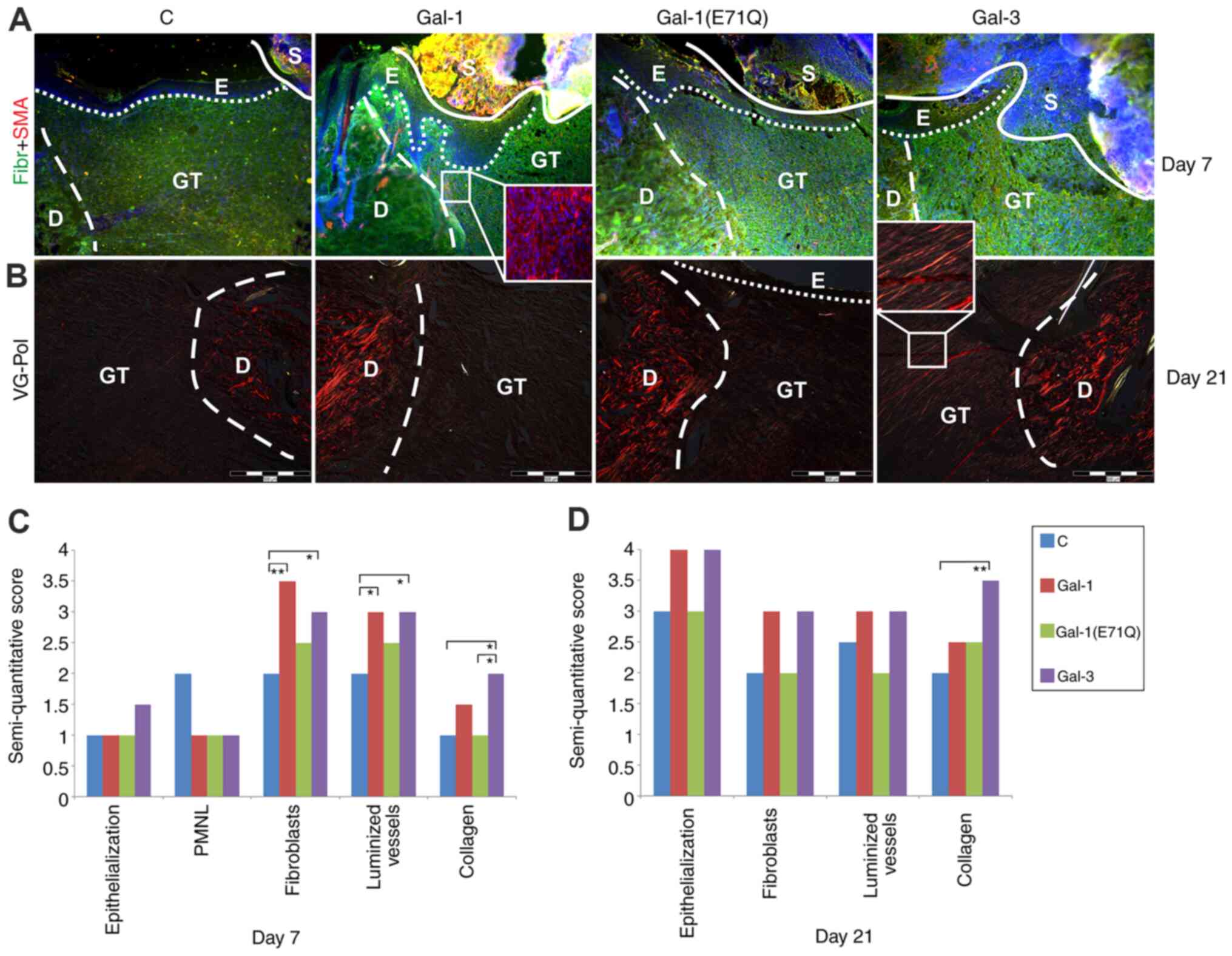 | Figure 4.Characteristics of open wounds
following treatment with tested galectins. Untreated group was used
as C. Nuclei are counterstained with DAPI. (A) Sections of
excisional wounds immunostained for Fibr (green signal) and SMA
(red signal) seven days after surgery, presenting well-formed
fibronectin-rich GT with SMA+ vessels and
re-epithelialization beneath scab can be seen in all groups, only
Gal-1-treated wounds are characterized by increased number of
SMA-positive myofibroblast-like cells. Magnification, ×100. Insert
magnification, ×200. (B) VG-stained wounds monitored under
polarized light 21 days after surgery, wounds treated with Gal-3
exhibited collagen organized into polarized light-reflecting
fibers. Magnification, ×100. Insert magnification, ×200.
Semi-quantitative analysis of histological parameters/changes. Data
are presented as the median in two separate graphs for (C) day 7
and (D) day 21. PMNL was not evaluated at day 21. *P<0.05,
**P<0.01. Gal, galectin; C, control; SMA, α-smooth muscle actin;
Fibr, fibronectin; VG-Pol, Van Gieson-polarized light; D, dermis;
E, epidermis; GT, granulation tissue; S, scab. |
At day 21, the level of fibronectin in the
granulation tissue had leveled off, and no myofibroblasts were
present in the granulation tissue of any group (data not shown). In
contrast, the contents of collagen had further increased, as the
Van Gieson staining in granulation tissue revealed (Fig. 4B and D). Inspection of such stained
specimens under polarized light showed a marked effect of Gal-3 on
collagen type-1 formation (Fig.
4B). Median values for scores of the measured characteristics
reflect these microscopical observations, as they also indicate a
tendency for an increase by presence of Gal-1 and −3 of the scores
on re-epithelialization and number of fibroblasts (Fig. 4D).
TS of sutured incision wounds
Following the systematic determination of TS values
of incisional wounds (Fig. 5), the
results of the statistical analysis of these experimental series
using the two-way ANOVA are summarized in Table IV. Data analysis using one-way
ANOVA is presented in Fig. 5A and
B.
 | Table IV.Results from the statistical
comparison of wound tensile strength using two-way ANOVA. |
Table IV.
Results from the statistical
comparison of wound tensile strength using two-way ANOVA.
| A, Overall
results |
|---|
|
|---|
| Group | P-value | 95% CI |
|---|
| Time (7 days vs. 21
days) | <0.001 | N/A |
| Group (C vs. Gal-1
vs. Gal-1(E71Q) vs. Gal-3) | <0.001 | N/A |
| Time vs. Group | 0.797 | N/A |
|
| B, Time
comparison |
|
| Group | P-value | 95% CI |
|
| 7 days vs. 21
days | 0.0001 | 62.3995 to
74.7000 |
|
| C, Treatment
comparison |
|
| Group | P-value | 95% CI |
|
| C vs. Gal-1 | 0.8815 | −18.5813 to
5.5063 |
| C vs.
Gal-1(E71Q) | 10.000 | −17.1219 to
6.9657 |
| C vs. Gal-3 | 0.0003 | −31.8079 to
−7.1180 |
| Gal-1 vs.
Gal-1(E71Q) | 10.000 | −9.8777 to
12.7964 |
| Gal-1 vs.
Gal-3 | 0.0214 | −24.5819 to
−1.2690 |
| Gal-3 vs.
Gal-1(E71Q) | 0.0075 | 2.7284 to
26.0412 |
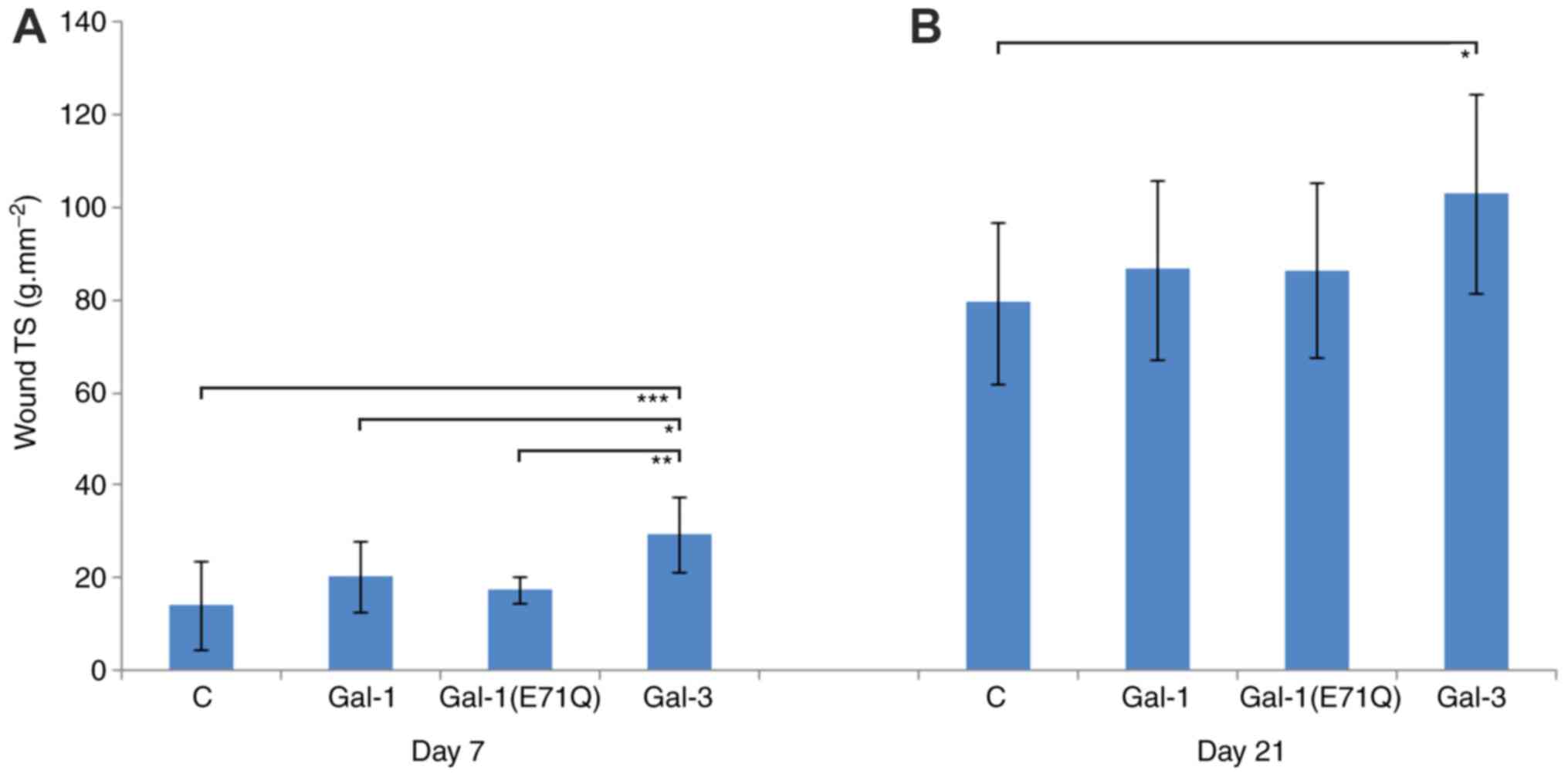
As expected, a significant increase of wound TS was
observed between day 7 and 21. The data from the Gal-3-treated
group significantly differed from those of all other groups. At day
7, TS in the Gal-3 group reached 29.6±8.2 g/mm2,
compared with 14.3±9.6 g/mm2 for the control, 20.2±7.6
g/mm2 for Gal-1 and 17.4±2.8 g/mm2 for
Gal-1(E71Q) (Fig. 5A).
At day 21 after surgery, the Gal-3 group continued
to exhibit a significantly increased TS when compared with the
untreated control (Gal-3, 103.1±21.4 g/mm2 vs. control,
79.5±17.5 g/mm2), the Gal-1 group (86.7±19.4
g/mm2) and the Gal-1(E71Q) mutant (86.5±18.8
g/mm2) (Fig. 5B). Thus,
TS is a parameter separating galectin activity in incisional skin
wound healing.
Discussion
The data of the present study provided further
evidence of the favorable effect of Gal-1 and −3 on excisional/open
and incisional/sutured skin wound healing. Similar to TGF-β1, the
positive control, both galectins acted as molecular switches for
the expression of genes related to the repair of skin wounds. Genes
for IGF1, FGF10 and ANGPT1 were among the most
upregulated genes, all of which have previously been implicated in
healing processes (43–45). Multiplex cytokine assays of human
skin fibroblasts after Gal-3 exposure have demonstrated the
increased concentrations of interleukin-6, granulocyte-macrophage
colony-stimulating factor, C-X-C motif chemokine ligand 8 and
matrix metalloproteinase (MMP)-3, an overall
pro-inflammatory/degradative signature (24), in osteoarthritic chondrocytes
(25,46). On the other hand, the increase in
the expression of MMP-9, cleaving the Ala62-Tyr63 bond in Gal-3's
N-terminal stalk (47), can
indicate a regulatory loop to put restrictions to prolonged Gal-3
activity for Gal-3-induced neutrophil activation by neutralizing
contacts between lectin domains (48).
The ECM of the HDFs is then subject to a remodeling
by Gal-3 that in turn induces proliferation of keratinocytes,
extending our previous data on responses to Gal-1 presence
(30). In detail, we observed
significantly upregulated CDH1 (49) and MMP9 (50) transcripts in Gal-3 treated
fibroblasts, which supports the role of lectins in the
re-epithelialization of wounds. Reduced Gal-3 expression has been
implicated in defective skin wound repair in patients with diabetes
by two mechanisms: i) Inverse correlation between Gal-3 and
advanced glycation end products; and ii) decreased Gal-3 in chronic
human wounds leading to delayed re-epithelialization as seen also
in Gal-3 knockout mice (20,21).
Of note, Gal-1 has been shown to accelerate cellular migration and
proliferation (51), and augment
skin wound repair in normal and diabetic mice (deficient for this
galectin) (52). In parallel, these
processes may also contribute to the stability of wound closure,
which prompted us to proceed with the animal study. In the present
study, the pro-fibrotic effect of Gal-3 was demonstrated, resulting
in wound tensile strength increase and improved collagen
organization (characteristic reflective appearance of collagen
fibers under the polarized light) of healing skin incisions and
excisions, respectively. Molecular analysis further revealed
significantly increased transcripts of IL6 and F3
genes in Gal-3-treated cells, more so than Gal-1-treated cells. In
particular, IL-6 and TGF-β1 play important roles in liver fibrosis
and/or structural changes in human tendon, a wound healing-like
processes characterized by the accumulation and turnover of ECM
(53–55). On the other hand, the F3 gene
product (tissue factor), exerts potent pro-angiogenic activity
(56), which may also contribute to
wound healing improvement in Gal-3-treated rats.
When envisioning to further pursue this route of
experimental testing, two aspects should be noted. Firstly,
galectins are expressed as a network beyond Gal-1 and −3 with
possibility for context-dependent functional antagonism and
cooperation (26,57–60).
For example, our previous study reported an additive effect of
TGF-β1 (10 µg/ml) with Gal-1 (200 or 300 ng/ml) on inducing
expression of SMA in HDF cultures (30). Further studies should investigate
Gal-mixtures (also including other types of proteins such as
TGF-β1), as they likely occur in situ, to attain the optimal
efficacy, and the present study provides a solid foundation for
this. Secondly, galectin function arises from their modular
structure. Therefore, their protein architecture has become the
subject of redesign using engineering, for example by creating
homo-oligomers from natural dimers (61–64),
in order to optimize a favorable activity. Of note, whether and how
a Gal-1-like (homodimeric) Gal-3 or a Gal-3-like (monomeric, in
solution) Gal-1 will influence in vitro and in vivo
aspects of wound healing remains to be determined.
In summary, the present findings provided further
evidence that galectins should be listed within the group of
efficient wound healing modulators at several key steps of skin
repair, including ECM formation/reorganization and
re-epithelialization (65,66). This conclusion is incentive for
further work. From a clinical perspective, the present data makes a
strong case for directing further efforts to treat incisional and
excisional wounds differently. In fact, Gal-1 seems to play a role
in wound contraction, whereas Gal-3 seems to be a skin scar
inductor. However, understanding the activity profile of each
galectin in vitro and in acute and chronic wound repair,
along with the potential of administration of mixtures, including
custom-made variants in experimental in vitro and in
vivo testing (67,68), will be important when investigating
the potential applications of these endogenous effectors in the
treatment of skin lesions.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr B Dvořánková, Ms
I Burdová, Ms H Stýblová (all, Institute of Anatomy, First Faculty
of Medicine, Charles University) and Ms M Majnušová (Department of
Forensic Medicine, P.J. Šafárik University in Košice) for their
expert technical assistance, and Dr B Friday, Dr A Leddoz and Dr
AWL Nose for inspiring discussions.
Funding
This study was supported by Slovak Research and
Development Agency (grant no. APVV-14-0731), Slovak Grant Agency
(grant nos. VEGA-1/0319/20 and VEGA-1/0561/18), Charles University
(grant nos. PROGRES Q28 and Q37), Operational Programme Research,
Development and Education (Center for Tumor Ecology, Research of
the Cancer Microenvironment Supporting Cancer Growth and Spread;
grant no. CZ.02.1.01/0.0/0.0/16_019/0000785), Research and
Development for Innovations Operational Program (grant no.
CZ.1.05/2.1.00/19.0400; co-financed by the European Union Regional
Development Fund and State Budget of Czech Republic), Medical
University Science Park in Košice supported by Operational
Programme Research & Innovation (grant nos. ITMS2014 and
313011D103), and COST (European Cooperation in Science and
Technology) Action (InnoGly-INNOvation with Glycans, grant no.
CA18103).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PG, HJG, FS, JL and KS acquired funding and made
substantial contributions to conception and design of the study.
PG, TV, IK, MČ, JJ, MJ, VP, LU, MK, MN and JM performed the
experiments. FS and JL supervised the animal study. HJG and KS
validated the obtained data. PG and HJG wrote the original draft.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The use of clinical samples was approved by The
Ethics Committee of University Hospital Kralovske Vinohrady and
Third Faculty of Medicine, Charles University (approval no.
100/1947/2005). Human cells were isolated and cultured with
informed consent from the donors. The animal study was approved by
the Ethics Committee of the Faculty of Medicine of the P. J.
Šafárik University and by the State Veterinary Administration of
the Slovak Republic (approval nos. Ro-580/10-221a and
Ro-2617/15-221a).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gaylarde PM and Sarkany I: Cell migration
and DNA synthesis in organ culture of human skin. Br J Dermatol.
92:375–380. 1975. View Article : Google Scholar
|
|
2
|
Donaldson DJ and Mason JM: Inhibition of
epidermal cell migration by concanavalin A in skin wounds of the
adult newt. J Exp Zool. 200:55–64. 1977. View Article : Google Scholar
|
|
3
|
Harrison FL and Chesterton CJ: Factors
mediating cell-cell recognition and adhesion. Galaptins, a recently
discovered class of bridging molecules. FEBS Lett. 122:157–165.
1980. View Article : Google Scholar
|
|
4
|
Barondes SH: Galectins: A personal
overview. Trends Glycosci Glycotechnol. 9:1–7. 1997. View Article : Google Scholar
|
|
5
|
Hughes RC: Secretion of the galectin
family of mammalian carbohydrate-binding proteins. Biochim Biophys
Acta. 1473:172–185. 1999. View Article : Google Scholar
|
|
6
|
Liu FT, Patterson RJ and Wang JL:
Intracellular functions of galectins. Biochim Biophys Acta.
1572:263–273. 2002. View Article : Google Scholar
|
|
7
|
Thiemann S and Baum LG: Galectins and
immune responses-just how do they do those things they do? Annu Rev
Immunol. 34:243–264. 2016. View Article : Google Scholar
|
|
8
|
Kaltner H, Toegel S, Caballero GG, Manning
JC, Ledeen RW and Gabius HJ: Galectins: Their network and roles in
immunity/tumor growth control. Histochem Cell Biol. 147:239–256.
2017. View Article : Google Scholar
|
|
9
|
Sato S: Cytosolic galectins and their
release and roles as carbohydrate-binding proteins in host-pathogen
interaction. Trends Glycosci Glycotechnol. 30:SE199–SE209. 2018.
View Article : Google Scholar
|
|
10
|
de Jong CGHM, Gabius HJ and Baron W: The
emerging role of galectins in (re)myelination and its potential for
developing new approaches to treat multiple sclerosis. Cell Mol
Life Sci. 77:1289–1317. 2020. View Article : Google Scholar
|
|
11
|
García Caballero G, Kaltner H, Kutzner TJ,
Ludwig AK, Manning JC, Schmidt S, Sinowatz F and Gabius HJ: How
galectins have become multifunctional proteins. Histol Histopathol.
35:509–539. 2020.
|
|
12
|
Kutzner TJ, Higuero AM, Süssmair M, Kopitz
J, Hingar M, Díez-Revuelta N, Caballero GG, Kaltner H, Lindner I,
Abad-Rodríguez J, et al: How presence of a signal peptide affects
human galectins-1 and −4: Clues to explain common absence of a
leader sequence among adhesion/growth-regulatory galectins. Biochim
Biophys Acta Gen Subj. 1864:1294492020. View Article : Google Scholar
|
|
13
|
Flotte TJ, Springer TA and Thorbecke GJ:
Dendritic cell and macrophage staining by monoclonal antibodies in
tissue sections and epidermal sheets. Am J Pathol. 111:112–124.
1983.
|
|
14
|
Roff CF, Rosevear PR, Wang JL and Barker
R: Identification of carbohydrate-binding proteins from mouse and
human fibroblasts. Biochem J. 211:625–629. 1983. View Article : Google Scholar
|
|
15
|
Cowles EA, Moutsatsos IK, Wang JL and
Anderson RL: Expression of carbohydrate binding protein 35 in human
fibroblasts: Comparisons between cells with different proliferative
capacities. Exp Gerontol. 24:577–585. 1989. View Article : Google Scholar
|
|
16
|
Wollenberg A, de la Salle H, Hanau D, Liu
FT and Bieber T: Human keratinocytes release the endogenous
beta-galactoside-binding soluble lectin immunoglobulin E
(IgE-binding protein) which binds to Langerhans cells where it
modulates their binding capacity for IgE glycoforms. J Exp Med.
178:777–785. 1993. View Article : Google Scholar
|
|
17
|
Konstantinov KN, Shames B, Izuno G and Liu
FT: Expression of epsilon BP, a beta-galactoside-binding soluble
lectin, in normal and neoplastic epidermis. Exp Dermatol. 3:9–16.
1994. View Article : Google Scholar
|
|
18
|
Holiková Z, Smetana K Jr, Bartunková J,
Dvoránková B, Kaltner H and Gabius HJ: Human epidermal langerhans
cells are selectively recognized by galectin-3 but not by
galectin-1. Folia Biol (Praha). 46:195–198. 2000.
|
|
19
|
Larsen L, Chen HY, Saegusa J and Liu FT:
Galectin-3 and the skin. J Dermatol Sci. 64:85–91. 2011. View Article : Google Scholar
|
|
20
|
Pepe D, Elliott CG, Forbes TL and Hamilton
DW: Detection of galectin-3 and localization of advanced glycation
end products (AGE) in human chronic skin wounds. Histol
Histopathol. 29:251–258. 2014.
|
|
21
|
Walker JT, Elliott CG, Forbes TL and
Hamilton DW: Genetic deletion of galectin-3 does not impair
full-thickness excisional skin healing. J Invest Dermatol.
136:1042–1050. 2016. View Article : Google Scholar
|
|
22
|
McLeod K, Walker JT and Hamilton DW:
Galectin-3 regulation of wound healing and fibrotic processes:
Insights for chronic skin wound therapeutics. J Cell Commun Signal.
12:281–287. 2018. View Article : Google Scholar
|
|
23
|
Wu NL and Liu FT: The expression and
function of galectins in skin physiology and pathology. Exp
Dermatol. 27:217–226. 2018. View Article : Google Scholar
|
|
24
|
Filer A, Bik M, Parsonage GN, Fitton J,
Trebilcock E, Howlett K, Cook M, Raza K, Simmons DL, Thomas AM, et
al: Galectin-3 induces a distinctive pattern of cytokine and
chemokine production in rheumatoid synovial fibroblasts via
selective signaling pathways. Arthritis Rheum. 60:1604–1614. 2009.
View Article : Google Scholar
|
|
25
|
Weinmann D, Schlangen K, André S, Schmidt
S, Walzer SM, Kubista B, Windhager R, Toegel S and Gabius HJ:
Galectin-3 induces a pro-degradative/inflammatory gene signature in
human chondrocytes, teaming up with galectin-1 in osteoarthritis
pathogenesis. Sci Rep. 6:391122016. View Article : Google Scholar
|
|
26
|
Weinmann D, Kenn M, Schmidt S, Schmidt K,
Walzer SM, Kubista B, Windhager R, Schreiner W, Toegel S and Gabius
HJ: Galectin-8 induces functional disease markers in human
osteoarthritis and cooperates with galectins-1 and −3. Cell Mol
Life Sci. 75:4187–4205. 2018. View Article : Google Scholar
|
|
27
|
Cada Z, Smetana K Jr, Lacina L, Plzáková
Z, Stork J, Kaltner H, Russwurm R, Lensch M, André S and Gabius HJ:
Immunohistochemical fingerprinting of the network of seven
adhesion/growth-regulatory lectins in human skin and detection of
distinct tumor-associated alterations. Folia Biol (Praha).
55:145–152. 2009.
|
|
28
|
Klíma J, Lacina L, Dvorankova B, Herrmann
D, Carnwath JW, Niemann H, Kaltner H, André S, Motlík J, Gabius HJ
and Smetana K Jr: Differential regulation of galectin
expression/reactivity during wound healing in porcine skin and in
cultures of epidermal cells with functional impact on migration.
Physiol Res. 58:873–884. 2009.
|
|
29
|
Gál P, Vasilenko T, Kostelniková M,
Jakubco J, Kovác I, Sabol F, André S, Kaltner H, Gabius HJ and
Smetana K Jr: Open wound healing in vivo: Monitoring binding and
presence of adhesion/growth-regulatory galectins in rat skin during
the course of complete re-epithelialization. Acta Histochem
Cytochem. 44:191–199. 2011. View Article : Google Scholar
|
|
30
|
Dvoránková B, Szabo P, Lacina L, Gal P,
Uhrova J, Zima T, Kaltner H, André S, Gabius HJ, Sykova E and
Smetana K Jr: Human galectins induce conversion of dermal
fibroblasts into myofibroblasts and production of extracellular
matrix: Potential application in tissue engineering and wound
repair. Cells Tissues Organs. 194:469–480. 2011. View Article : Google Scholar
|
|
31
|
Gabius HJ: Influence of type of linkage
and spacer on the interaction of beta-galactoside-binding proteins
with immobilized affinity ligands. Anal Biochem. 189:91–94. 1990.
View Article : Google Scholar
|
|
32
|
Sarter K, André S, Kaltner H, Lensch M,
Schulze C, Urbonaviciute V, Schett G, Herrmann M and Gabius HJ:
Detection and chromatographic removal of lipopolysaccharide in
preparations of multifunctional galectins. Biochem Biophys Res
Commun. 379:155–159. 2009. View Article : Google Scholar
|
|
33
|
Kopitz J, Vértesy S, André S, Fiedler S,
Schnölzer M and Gabius HJ: Human chimera-type galectin-3: Defining
the critical tail length for high-affinity glycoprotein/cell
surface binding and functional competition with galectin-1 in
neuroblastoma cell growth regulation. Biochimie. 104:90–99. 2014.
View Article : Google Scholar
|
|
34
|
García Caballero G, Kaltner H, Michalak M,
Shilova N, Yegres M, André S, Ludwig AK, Manning JC, Schmidt S,
Schnölzer M, et al: Chicken GRIFIN: A homodimeric member of the
galectin network with canonical properties and a unique expression
profile. Biochimie. 128-129:34–47. 2016. View Article : Google Scholar
|
|
35
|
Rheinwald JG and Green H: Serial
cultivation of strains of human epidermal keratinocytes: The
formation of keratinizing colonies from single cells. Cell.
6:331–343. 1975. View Article : Google Scholar
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar
|
|
38
|
Perzelova V, Sabol F, Vasilenko T, Novotný
M, Kováč I, Slezák M, Ďurkáč J, Hollý M, Pilátová M, Szabo P, et
al: Pharmacological activation of estrogen receptors-α and -β
differentially modulates keratinocyte differentiation with
functional impact on wound healing. Int J Mol Med. 37:21–28. 2016.
View Article : Google Scholar
|
|
39
|
Kovac I, Melegova N, Coma M, Takáč P,
Kováčová K, Hollý M, Ďurkáč J, Urban L, Gurbáľová M, Švajdlenka E,
et al: Aesculus hippocastanum L. extract does not induce fibroblast
to myofibroblast conversion but increases extracellular matrix
production in vitro leading to increased wound tensile strength in
rats. Molecules. 25:19172020. View Article : Google Scholar
|
|
40
|
Kovac I, Durkac J, Holly M, Jakubčová K,
Peržeľová V, Mučaji P, Švajdlenka E, Sabol F, Legáth J, Belák J, et
al: Plantago lanceolata L. water extract induces transition of
fibroblasts into myofibroblasts and increases tensile strength of
healing skin wounds. J Pharm Pharmacol. 67:117–125. 2015.
View Article : Google Scholar
|
|
41
|
Sabol F, Vasilenko T, Novotny M, Tomori Z,
Bobrov N, Zivčák J, Hudák R and Gál P: Intradermal running suture
versus 3M™ Vetbond™ tissue adhesive for wound closure in rodents: A
biomechanical and histological study. Eur Surg Res. 45:321–326.
2010. View Article : Google Scholar
|
|
42
|
Gál P, Toporcer T, Vidinsky B, Hudak R,
Zivcak J and Sabo J: Simple interrupted percutaneous suture versus
intradermal running suture for wound tensile strength measurement
in rats: A technical note. Eur Surg Res. 43:61–65. 2009. View Article : Google Scholar
|
|
43
|
Koshizuka S, Kanazawa K, Kobayashi N,
Takazawa I, Waki Y, Shibusawa H and Shumiya S: The beneficial
effects of recombinant human insulin-like growth factor-I (IGF-I)
on wound healing in severely wounded senescent mice. Surg Today.
27:946–952. 1997. View Article : Google Scholar
|
|
44
|
Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT,
Hong HJ, Yoo OJ and Koh GY: COMP-angiopoietin-1 promotes wound
healing through enhanced angiogenesis, lymphangiogenesis, and blood
flow in a diabetic mouse model. Proc Natl Acad Sci USA.
103:4946–4951. 2006. View Article : Google Scholar
|
|
45
|
Quirinia A and Viidik A: The effect of
recombinant basic fibroblast growth factor (bFGF) in fibrin
adhesive vehicle on the healing of ischaemic and normal incisional
skin wounds. Scand J Plast Reconstr Surg Hand Surg. 32:9–18. 1998.
View Article : Google Scholar
|
|
46
|
Toegel S, Weinmann D, André S, Walzer SM,
Bilban M, Schmidt S, Chiari C, Windhager R, Krall C, Bennani-Baiti
IM and Gabius HJ: Galectin-1 couples glycobiology to inflammation
in osteoarthritis through the activation of an NF-κB-regulated gene
network. J Immunol. 196:1910–1921. 2016. View Article : Google Scholar
|
|
47
|
Ochieng J, Fridman R, Nangia-Makker P,
Kleiner DE, Liotta LA, Stetler-Stevenson WG and Raz A: Galectin-3
is a novel substrate for human matrix metalloproteinases-2 and −9.
Biochemistry. 33:14109–14114. 1994. View Article : Google Scholar
|
|
48
|
Sundqvist M, Welin A, Elmwall J, Osla V,
Nilsson UJ, Leffler H, Bylund J and Karlsson A: Galectin-3 type-C
self-association on neutrophil surfaces: The carbohydrate
recognition domain regulates cell function. J Leukoc Biol.
103:341–353. 2018. View Article : Google Scholar
|
|
49
|
Advedissian T, Proux-Gillardeaux V, Nkosi
R, Peyret G, Nguyen T, Poirier F, Viguier M and Deshayes F:
E-cadherin dynamics is regulated by galectin-7 at epithelial cell
surface. Sci Rep. 7:170862017. View Article : Google Scholar
|
|
50
|
Kyriakides TR, Wulsin D, Skokos EA,
Fleckman P, Pirrone A, Shipley JM, Senior RM and Bornstein P: Mice
that lack matrix metalloproteinase-9 display delayed wound healing
associated with delayed reepithelization and disordered collagen
fibrillogenesis. Matrix Biol. 28:65–73. 2009. View Article : Google Scholar
|
|
51
|
Kim MH, Wu WH, Choi JH, Kim J, Jun JH, Ko
Y and Lee JH: Galectin-1 from conditioned medium of
three-dimensional culture of adipose-derived stem cells accelerates
migration and proliferation of human keratinocytes and fibroblasts.
Wound Repair Regen. 26 (Suppl 1):S9–S18. 2018. View Article : Google Scholar
|
|
52
|
Liu W, Hsu DK, Chen HY, Yang RY, Carraway
KL III, Isseroff RR and Liu FT: Galectin-3 regulates intracellular
trafficking of EGFR through Alix and promotes keratinocyte
migration. J Invest Dermatol. 132:2828–2837. 2012. View Article : Google Scholar
|
|
53
|
Kershenobich Stalnikowitz D and Weissbrod
AB: Liver fibrosis and inflammation. A review. Ann Hepatol.
2:159–163. 2003. View Article : Google Scholar
|
|
54
|
Kjaer M, Langberg H, Heinemeier K, Bayer
ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR and
Magnusson SP: From mechanical loading to collagen synthesis,
structural changes and function in human tendon. Scand J Med Sci
Sports. 19:500–510. 2009. View Article : Google Scholar
|
|
55
|
Kjaer M, Magnusson P, Krogsgaard M, Boysen
Møller J, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen
S, Esmarck B and Langberg H: Extracellular matrix adaptation of
tendon and skeletal muscle to exercise. J Anat. 208:445–450. 2006.
View Article : Google Scholar
|
|
56
|
Pena E, de la Torre R, Arderiu G, Slevin M
and Badimon L: mCRP triggers angiogenesis by inducing F3
transcription and TF signalling in microvascular endothelial cells.
Thromb Haemost. 117:357–370. 2017. View Article : Google Scholar
|
|
57
|
Kopitz J, von Reitzenstein C, André S,
Kaltner H, Uhl J, Ehemann V, Cantz M and Gabius HJ: Negative
regulation of neuroblastoma cell growth by carbohydrate-dependent
surface binding of galectin-1 and functional divergence from
galectin-3. J Biol Chem. 276:35917–35923. 2001. View Article : Google Scholar
|
|
58
|
Manning JC, García Caballero G, Knospe C,
Kaltner H and Gabius HJ: Network analysis of
adhesion/growth-regulatory galectins and their binding sites in
adult chicken retina and choroid. J Anat. 231:23–37. 2017.
View Article : Google Scholar
|
|
59
|
García Caballero G, Schmidt S, Schnölzer
M, Schlötzer-Schrehardt U, Knospe C, Ludwig AK, Manning JC,
Muschler P, Kaltner H, Kopitz J and Gabius HJ: Chicken GRIFIN:
Binding partners, developmental course of localization and
activation of its lens-specific gene expression by L-Maf/Pax6. Cell
Tissue Res. 375:665–683. 2019. View Article : Google Scholar
|
|
60
|
García Caballero G, Schmidt S, Manning JC,
Michalak M, Schlötzer-Schrehardt U, Ludwig AK, Kaltner H, Sinowatz
F, Schnölzer M, Kopitz J and Gabius HJ: Chicken lens development:
Complete signature of expression of galectins during embryogenesis
and evidence for their complex formation with α-, β-, δ- and
τ-crystallins, N-CAM, and N-cadherin obtained by affinity
chromatography. Cell Tissue Res. 379:13–35. 2020. View Article : Google Scholar
|
|
61
|
Gabius HJ: How to crack the sugar code.
Folia Biol (Praha). 63:121–131. 2017.
|
|
62
|
Kopitz J, Xiao Q, Ludwig AK, Romero A,
Michalak M, Sherman SE, Zhou X, Dazen C, Vértesy S, Kaltner H, et
al: Reaction of a programmable glycan presentation of
glycodendrimersomes and cells with engineered human lectins to show
the sugar functionality of the cell surface. Angew Chem Int Ed
Engl. 56:14677–14681. 2017. View Article : Google Scholar
|
|
63
|
Ludwig AK, Michalak M, Xiao Q, Gilles U,
Medrano FJ, Ma H, FitzGerald FG, Hasley WD, Melendez-Davila A, Liu
M, et al: Design-functionality relationships for
adhesion/growth-regulatory galectins. Proc Natl Acad Sci USA.
116:2837–2842. 2019. View Article : Google Scholar
|
|
64
|
Ludwig AK, Kaltner H, Kopitz J and Gabius
HJ: Lectinology 4.0: Altering modular (ga)lectin display for
functional analysis and biomedical applications. Biochim Biophys
Acta Gen Subj. 1863:935–940. 2019. View Article : Google Scholar
|
|
65
|
Stone RC, Pastar I, Ojeh N, Chen V, Liu S,
Garzon KI and Tomic-Canic M: Epithelial-mesenchymal transition in
tissue repair and fibrosis. Cell Tissue Res. 365:495–506. 2016.
View Article : Google Scholar
|
|
66
|
Haensel D and Dai X:
Epithelial-to-mesenchymal transition in cutaneous wound healing:
Where we are and where we are heading. Dev Dyn. 247:473–480. 2018.
View Article : Google Scholar
|
|
67
|
Smetana K Jr, André S, Kaltner H, Kopitz J
and Gabius HJ: Context-dependent multifunctionality of galectin-1:
A challenge for defining the lectin as therapeutic target. Expert
Opin Ther Targets. 17:379–392. 2013. View Article : Google Scholar
|
|
68
|
Romero A and Gabius HJ: Galectin-3: Is
this member of a large family of multifunctional lectins (already)
a therapeutic target? Expert Opin Ther Targets. 23:819–828. 2019.
View Article : Google Scholar
|