Introduction
Cerebral ischemia and the hypoxia that arises from
this condition are the direct causes of stroke and other cerebral
diseases (1). Over recent years,
mild hypothermia therapy and stem cell therapy have been introduced
as a treatment option for patients suffering from these conditions
(2). For example, randomized,
double-blind, Phase III clinical trials have been carried out by
two European groups to investigate the potential effects of
hypothermia therapy over 6 months, demonstrating that treatment
involving mild hypothermia resulted in good neurological recovery
(3,4). Additionally, experiments of cerebral
infarction in a rat model demonstrated that mild hypothermia
treatment can decrease the area of infarction and achieve good
levels of recovery in terms of nerve function (5). In addition, there have been several
successful cases of stem cell therapy and mild hypothermia combined
with stem cell therapy for stroke in infants (6–8).
A previous study has suggested that basic fibroblast
growth factor (bFGF) and epidermal growth factor (EGF) can promote
the transformation of bone marrow-derived mesenchymal stem cells
(MSCs) or adipose MSCs into neural cells (9). Glial cells are primary cells that can
be isolated and cultured from the cerebral cortex; these represent
a heterogeneous mixture of cell types that have the ability to
proliferate and differentiate (10). The development of new induction
methods to improve the efficiency of glial cell transformation into
neural stem cells, MSCs would allow us to produce greater numbers
of specific cells for neural repair treatments.
Previous studies injected the brains of rats with
MSCs and achieved good levels of repair in the central nervous
system (11,12). In addition, a range of efficient
methods have now been described to facilitate the acquisition of
mesenchymal stem cells; these cells are important as they have the
ability to differentiate into neural cells (13,14).
However, a previous study investigated the transformation of rat
primary glial cells into MSCs (15). The present study hypothesized that
treatment with mild hypothermia or growth factors may promote the
differentiation of glial cells into MSCs, thus supplementing nerve
cells that died due to hypoxia. The aim of the present study was to
use a rat model to investigate the effects of cytokine induction
and anaerobic mild hypothermia on the differentiation of primary
glial cells.
Materials and methods
Primary isolation of rat glial
cells
All animal procedures were approved by the Ethics
Committee of Jiangxi Provincial People's Hospital Affiliated to
Nanchang University (approval no. 2017BBG70066; Nanchang, China).
All experiments involving animals were carried out in accordance
with the Guide for the Care and Use of Laboratory Animals published
by the National Institutes of Health (16). A total of ten specific pathogen-free
neonatal Sprague-Dawley rats (1–3 days postnatal; 6–8 g;
male:female, 1:1) were provided by the Animal Center of Jiangxi
Provincial People's Hospital Affiliated to Nanchang University. The
animals were housed in a specific pathogen-free environment at a
temperature of 23±2°C, with 45–65% humidity, 12-h light/dark
cycles, and free access to food and water. The animals were
sacrificed by cervical dislocation and immersed in 75% alcohol for
2–3 min on an ultra-clean table. D-Hank's solution (Gibco; Thermo
Fisher Scientific, Inc.), containing a mixture of penicillin
(10,000 U/ml) and streptomycin (10 mg/ml), was added to four petri
dishes (labeled 1–4). First, the sterilized rats were transferred
to dish number 1. Their heads were removed and placed in dish
number 2. The skulls were then separated at the brainstem, and the
brain was quickly transferred to dish number 3. The cortex was then
freed and the meninges on the cortex were fully removed with
tweezers and transferred to dish number 4. The cortical tissue was
then rinsed carefully in D-Hank's solution and then transferred to
a vial containing penicillin (10,000 U/ml) and D-Hank's solution.
The tissue suspension was then cut into tissue fragments that were
~1 mm3 in size and then transferred to a centrifuge
tube. An equivalent volume of 0.25% trypsin was added to the
centrifuge tube, the contents were mixed carefully and then allowed
to digest at 37°C for 15 min. The digestion was terminated by
adding three equivalent volumes of complete culture medium
including DF12 (cat. no. 1859228; Gibco; Thermo Fisher Scientific,
Inc.) and 20% fetal bovine serum (FBS; cat. no. 04-007-1A;
Biological Industries). The cell suspension was then mixed by
gentle blowing with a suction tube and filtered with a 200-mesh,
and the isolated cells were centrifuged at 850 × g for 3 min at
room temperature. The supernatant was discarded and the cell
precipitation was suspended in DF12 medium with 20% FBS. Finally,
the cells were cultured with 5% CO2 at 37°C until the
first passage prior to subsequent experimentation.
Hypoxia, mild hypothermia and growth
factor treatments
Cells at 70% confluency were divided into eight
groups for culture in normal culture medium (DF12 with 20% FBS) as
follows: Control-normal (astrocytes cultured at 37°C with 5%
CO2); control-hypothermia (33°C, 5% CO2, 12
h); control-hypoxia (37°C, 95% N2, 5% CO2, 12
h); control-hypothermia + hypoxia; bFGF + EGF-normal; bFGF +
EGF-hypothermia (33°C, 5% CO2, 12 h); bFGF + EGF-hypoxia
(37°C, 95% N2, 5% CO2, 12 h); and bFGF +
EGF-hypothermia + hypoxia. Cells in the bFGF + EGF groups received
10 ng/ml bFGF (cat. no. P1020-500; Novoprotein) and 20 ng/ml EGF
(cat. no. REGFP-05011; Cyagen Biosciences, Inc.) for 7 days.
Hypothermia involved a reduction in the culture temperature from
37°C to 33°C for 12 h. To mimic a hypoxic environment, cells were
cultured in 95% N2 + 5% CO2 for 12 h. The
cells were treated for a total of 7 days and collected for
subsequent experiments.
Immunocytochemical staining
Cells were fixed in 4% paraformaldehyde at room
temperature for 1 h. Following antigen retrieval by heating in a
microwave in Tris/EDTA (pH 9.0), 3% hydrogen peroxide was used to
block endogenous peroxidase activity for 15 min at room
temperature. After non-specific blocking in 5% BSA (HyClone; GE
Healthcare Life Science) at room temperature for 2 h, the cells
were incubated overnight at 4°C with primary antibodies against
nestin (cat. no. OM264981; OmniAb; 1:250), glial fibrillary acidic
protein (GFAP; cat. no. ab33922; Abcam; 1:500), neuronal nuclei
(NeuN; cat. no. ab177487; Abcam; 1:500), Musashi-1 (cat. no.
bs-20241r; BIOSS; 1:250) and neuron specific enolase (NSE; cat. no.
bs-10445r; BIOSS; 1:500). The following morning, the cells were
washed with PBS and then incubated with an HRP-conjugated goat
anti-rabbit IgG H&L secondary antibody (cat. no. ab6721; Abcam;
1:500) at 37°C for 30 min. Subsequently, the cells were washed with
PBS, and stained with 3,3′-diaminobenzidine for 5–10 min at room
temperature. Cells were then re-washed in PBS for 1 min and stained
with hematoxylin for 3 min at room temperature; this staining was
then converted to a blue color using ethanol hydrochloride.
Finally, cells were observed by light microscopy. ImagePro Plus
(version 6.0; Media Cybernetics, Inc.) was used to measure the mean
optical density of positive cells within three randomly selected
fields of view at high magnification (×200).
Flow cytometry
Instrument parameters were adjusted by fluorescence
homology control. Cells were washed twice with PBS and digested
with 0.25% trypsin containing 0.02% EDTA. The cell suspension was
then transferred into a 10-ml centrifuge tube. Cells were collected
from each treatment group by centrifugation at 850 × g for 3 min at
room temperature. Next, 1 ml of PBS solution was added to each tube
and centrifuged at 1,700 × g for 1 min at room temperature. The
supernatant was discarded, and 5×105 cells were
collected in 300 ml PBS. Subsequently, 300 µl 2X binding buffer and
5 µl of the following antibodies were added to each tube: Mouse
anti-CD90-FITC (cat. no. 561973; BD Biosciences; 1:100), mouse
anti-CD29-PE (cat. no. 102207; BioLegend, Inc.; 1:100), mouse
anti-CD44-PE-cy7 (cat. no. ab4679; Abcam; 1:100) and mouse
anti-CD105-APC (cat. no. 17-1051-80; eBioscience; Thermo Fisher
Scientific, Inc.; 1:100). The tubes were mixed carefully and
incubated at room temperature in the dark for 10 min. Multichannel
fluorescence counting was then performed on a FACSCalibur flow
cytometer (BD Biosciences). The combinations of CD105/CD90 and
CD29/CD44 were used in the flow cytometry experiments. Data were
analyzed using FlowJo software (version 7.6; FlowJo, LLC).
Western blotting
Cells from each treatment group were mixed with RIPA
solution (Beyotime Institute of Biotechnology) and incubated at 4°C
for 30 min to create a lysed suspension containing protein extract.
The extracts were then centrifuged at 8,500 × g for 10 min at 4°C;
the supernatant, containing the total protein extract, was retained
for analysis. Next, a bicinchoninic acid kit (Beyotime Institute of
Biotechnology) was used to determine the concentration of each
protein extract. Proteins (20 µg) were then separated by 10%
SDS-PAGE, and then transferred to PVDF membranes. After blocking in
5% skimmed milk at room temperature for 2 h, membranes were then
incubated overnight at 4°C with a range of primary antibodies,
including: Nestin (cat. no. OM264981; OMNIAB; 1:250), GFAP (cat.
no. ab33922; Abcam; 1:500), NeuN (cat. no. ab177487; Abcam; 1:500),
Musashi-1 (cat. no. bs-20241r: BIOSS; 1:250), NSE (cat. no.
bs-10445r; BIOSS; 1:500) and GAPDH (cat. no. ab8245; Abcam;
1:1,000). The following morning, the membranes were washed with PBS
and incubated with an HRP goat anti-rabbit IgG H&L secondary
antibody (cat. no. ab6721; Abcam; 1:500) or HRP goat anti-mouse IgG
H&L secondary antibody (cat. no. ab6789; Abcam; 1:500) at room
temperature for 1–2 h. The membranes were then developed using ECL
exposure solution (cat. no. SW2010-1; Beijing Solarbio Science
& Technology Co., Ltd.). Quantity one software (version 4.6;
Bio-Rad Laboratories, Inc.) was used to analyze the gray value of
specific bands of interest.
Statistical analysis
Data are presented as the mean ± SD of six repeats.
Comparisons among multiple groups were analyzed using two-way ANOVA
followed by a Bonferroni post-hoc test. All data were analyzed
using SPSS (version 19.0; IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference
Results
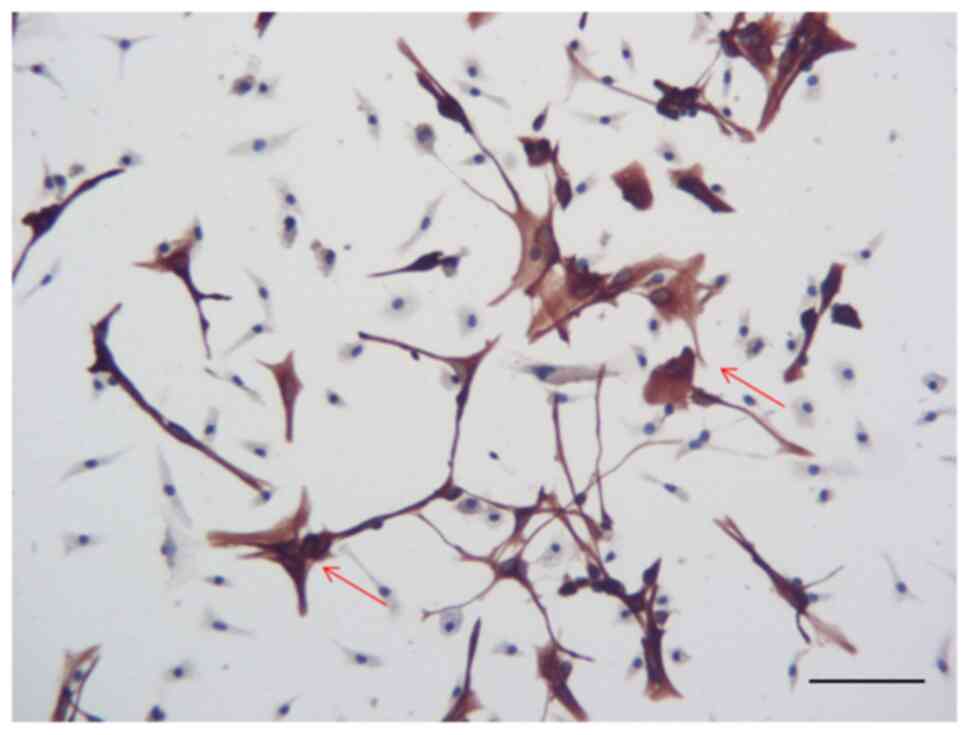
Glial cell identification
Data relating to the immunohistochemical detection
of GFAP are shown in Fig. 1.
Primary cultured glial cells expressed GFAP, indicating that these
cells exhibited low levels of differentiation. Some of these cells
expressed strong immunofluorescence for GFAP; these cells were
polygonal in shape and were bifurcated, thus indicating that they
were astrocytes. However, ~40% cells in the staining were
GFAP− cells, which were small in size. These cells may
not be in healthy conditions.
Identification of differentiation
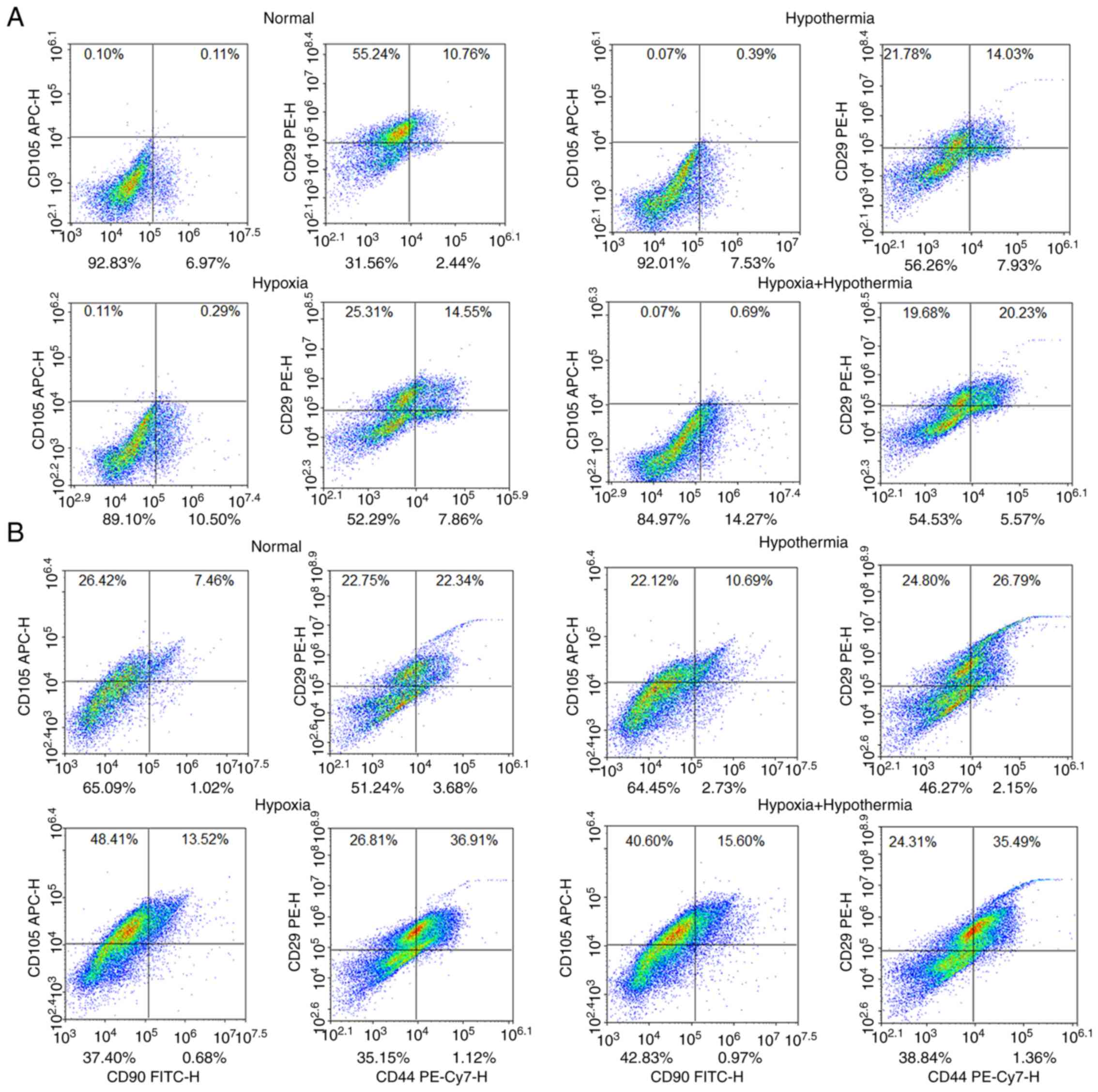
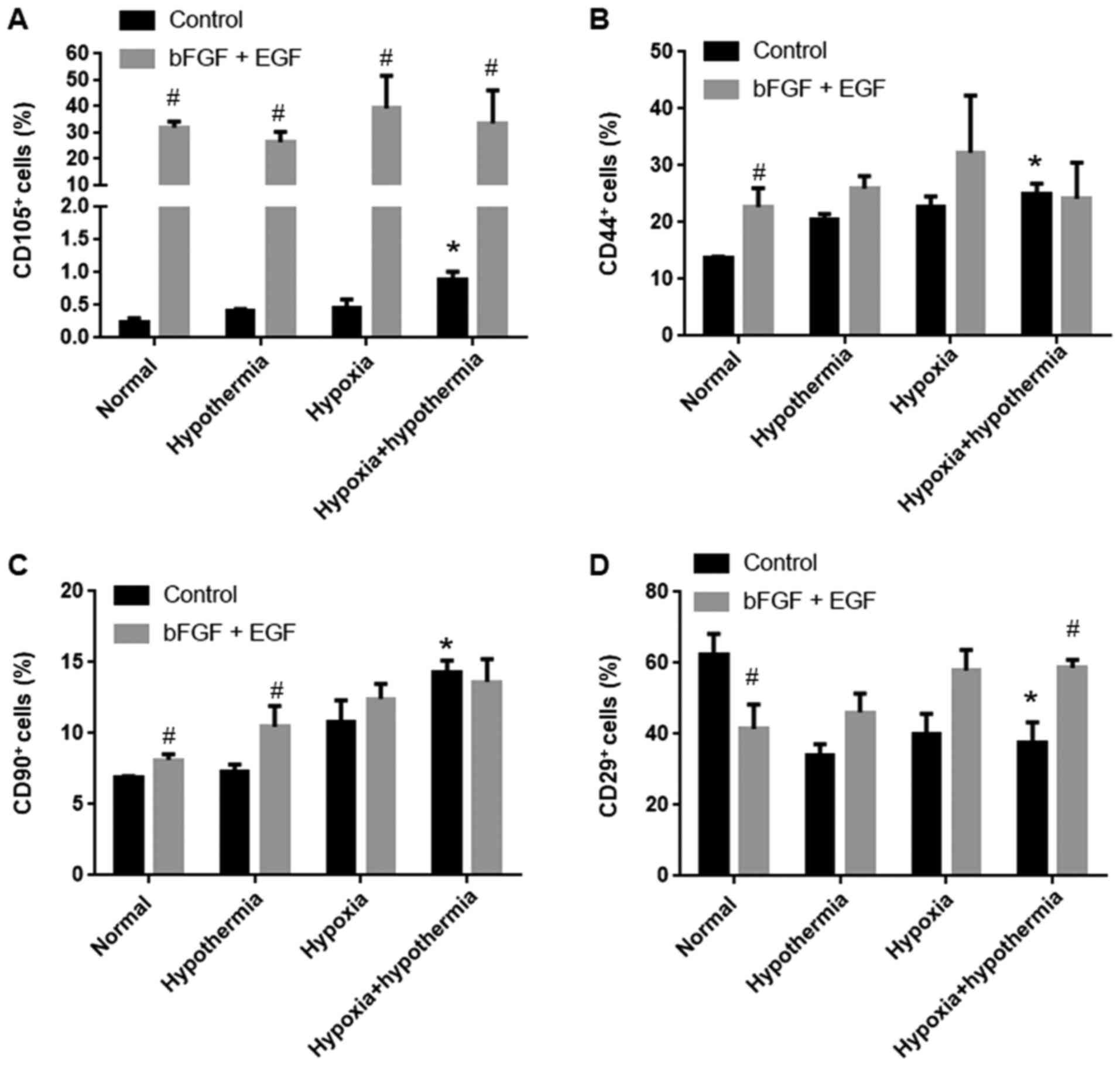
clusters by flow cytometry
Flow cytometry was used to detect clusters of
differentiated cells (CD44, CD29, CD90 and CD105) associated with
MSCs. Under normal conditions, bFGF and EGF treatment increased the
proportion of CD105+ cells from <1 to ~30%
(P<0.05; Figs. 2 and 3A), and also significantly increased the
proportion of CD44+ and CD90+ cells compared
with the control group (P<0.05; Figs. 2, 3B and
C). However, the proportion of CD29+ cells
significantly decreased from ~60 to ~40% following bFGF and EGF
treatment compared with the control group under normal conditions
(P<0.05; Figs. 2 and 3D). The combined treatment of anaerobic
mild hypothermia, bFGF and EGF led to an increase in the proportion
of CD29+ cells to ~60% compared with the control group
(P<0.05; Figs. 2 and 3D). Additionally, anaerobic mild
hypothermia treatment increased the proportion of CD44+,
CD90+ and CD105+ cells compared with the
normal group (P<0.05; Figs. 2
and 3A-C), although anaerobic mild
hypothermia treatment with bFGF and EGF did not show a synergistic
effect with regards to increasing the proportion of
CD44+ and CD90+ clusters compared with the
normal group (P>0.05; Figs. 2
and 3A-C).
Growth factor and anaerobic mild
hypothermia treatment decreases the expression levels of NSE, NeuN
and GFAP, but promotes the expression levels of nestin and
Musashi-1
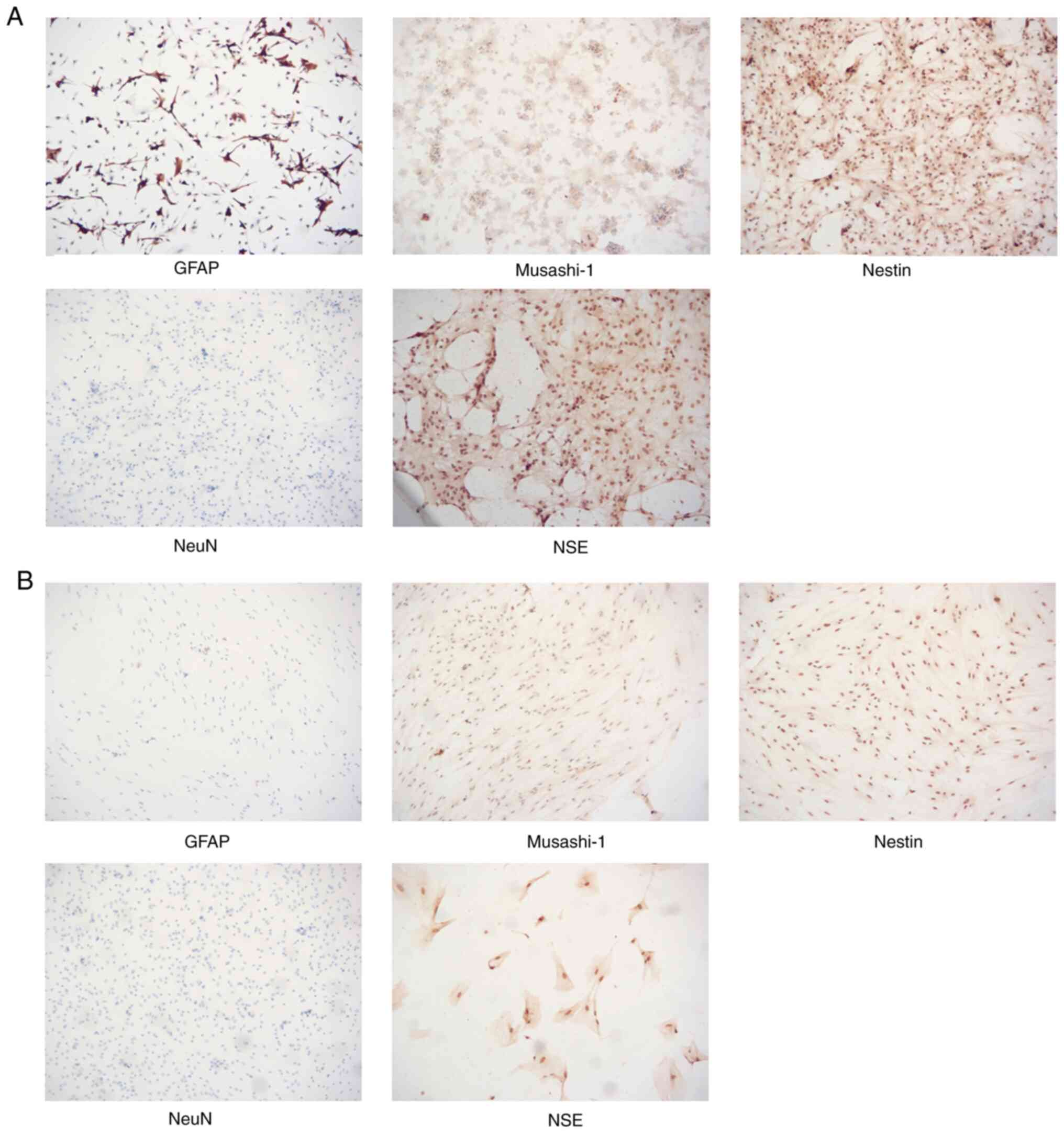
Cells from the control-normal group and the bFGF +
EGF-hypoxia + hypothermia group were tested by immunocytochemistry
to determine the expression levels of a range of marker genes that
are specific for neural cells, including GFAP, nestin, musashi-1,
NSE and NeuN (Fig. 4). Compared
with normal cultured glial cells, cells that were treated with
anaerobic mild hypothermia, bFGF and EGF demonstrated a decrease in
the proportions of GFAP+, NSE+ and
NeuN+ cells, but an increase in the proportions of
Musashi-1+ and Nestin+ cells; additionally,
these cells tended to grow flat on the wall of culture dishes with
fewer clusters (Fig. 4).
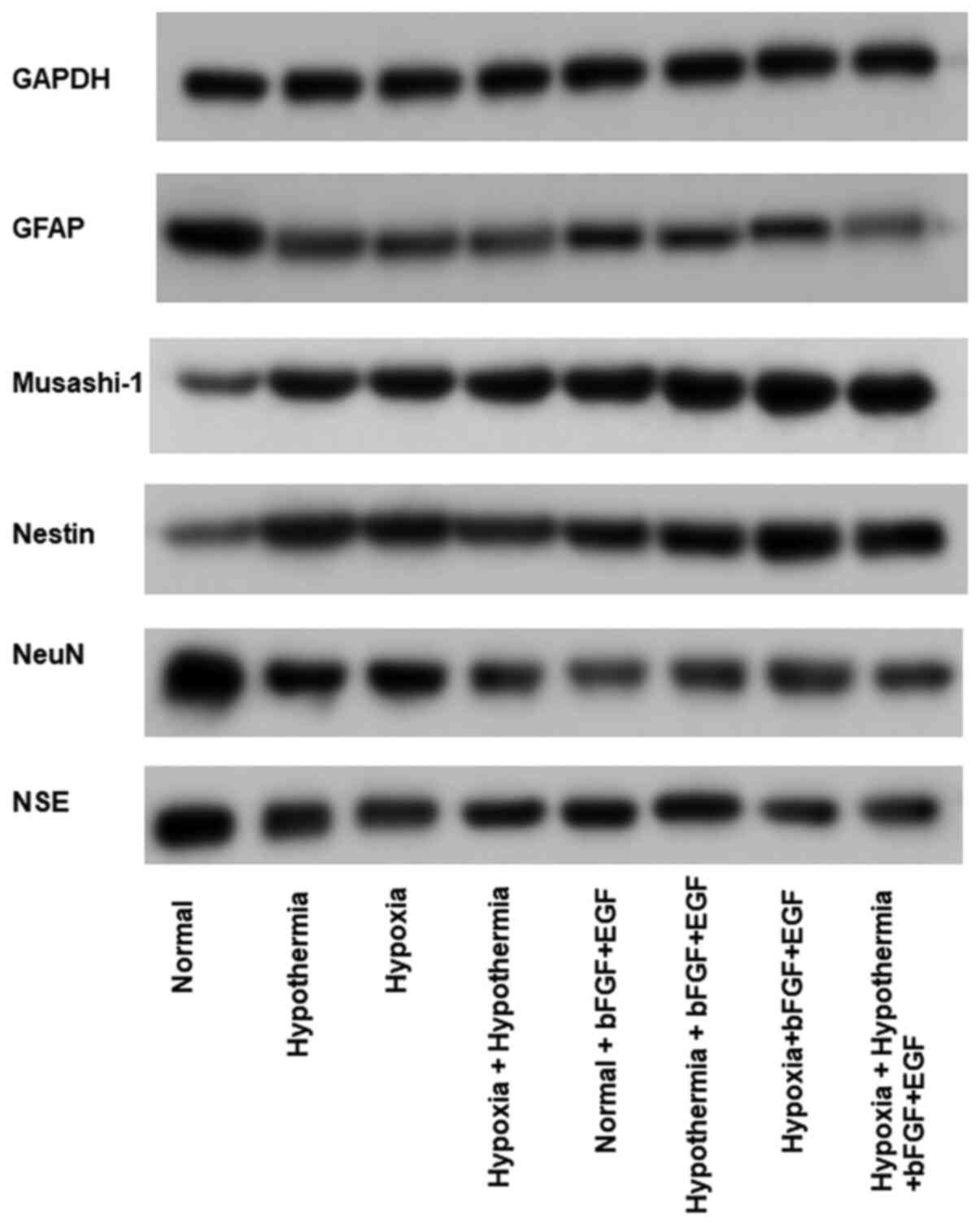
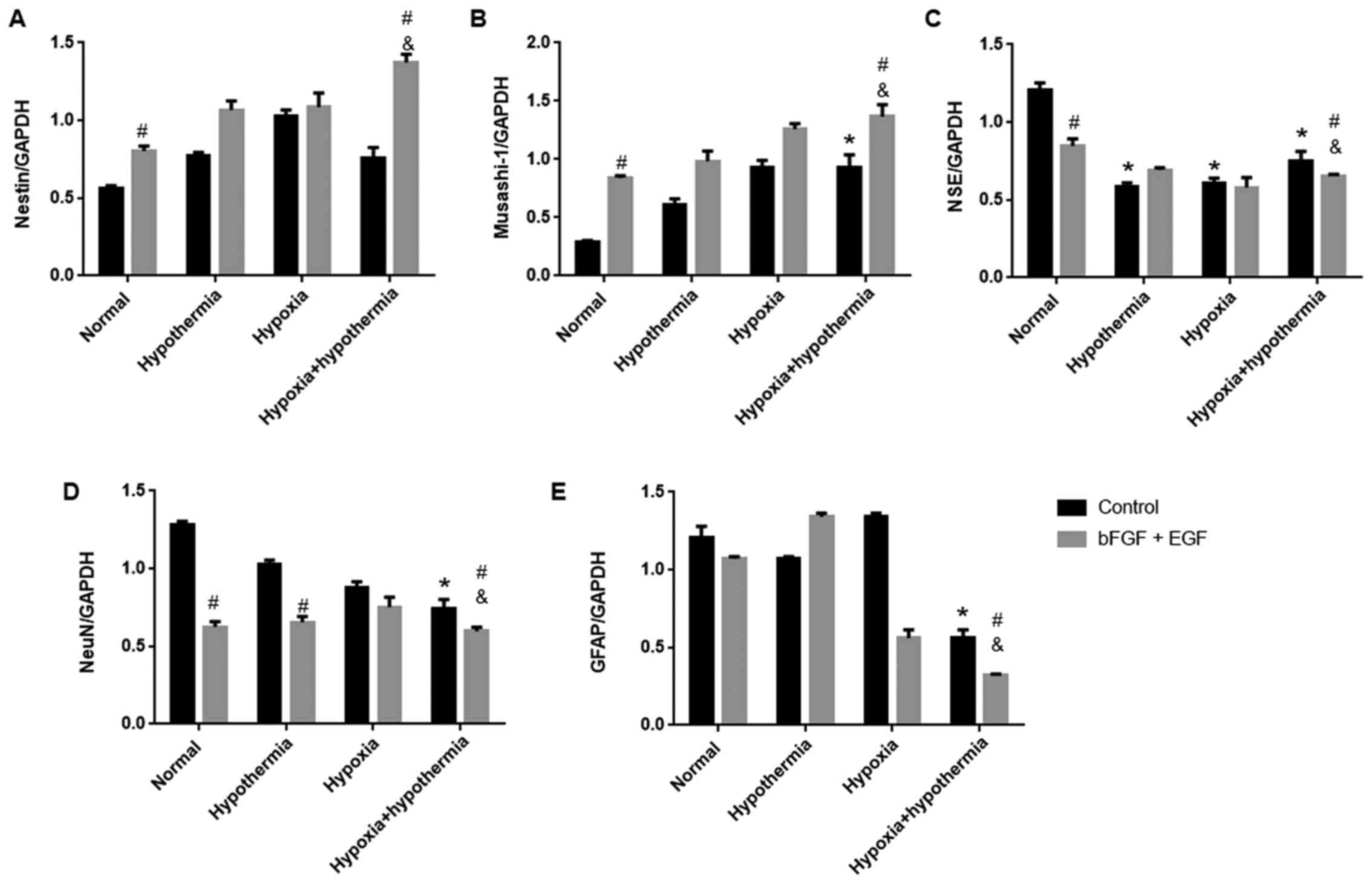
Western blotting was performed to detect specific
cellular markers. Growth factor treatment (bFGF and EGF) led to an
increase in the expression levels of nestin and Musashi-1, and a
decrease in the expression levels of NSE and NeuN under normal
conditions compared with control cells (Figs. 5 and 6). Anaerobic mild hypothermia treatment
increased the expression levels of Musashi-1, but decreased the
expression levels of NSE and NeuN in glial cells compared with the
normal group. Additionally, anaerobic mild hypothermia treatment
significantly decreased GFAP expression compared with the
control-normal group (P<0.05; Figs.
5 and 6). Additionally, the
combination of growth factors and anaerobic mild hypothermia
treatments had synergistic effects on the inhibition of GFAP when
compared with the bFGF + EGF + normal group (P<0.05; Figs. 5 and 6).
Discussion
According to the classical biological view, cell
differentiation is unidirectional; in normal tissues, stem cells
can differentiate into a range of different cell types, such as
neurons and glial cells (4).
Although cells can be induced to differentiate into stem cells by a
range of artificial techniques (17), it appears that under normal
physiological conditions, differentiated cells cannot revert back
to stem cells. However, this view has been challenged. A previous
study found that glial cells in dental pulp can undergo a form of
‘reverse differentiation’ and revert back into stem cells (15). This discovery not only challenged
the traditional concept, but also created a new option for the
treatment of disease. The current study identified differences
between growth factor treatment and mild hypothermia treatment with
regards to the induction of glial cell differentiation, and that
the combination of growth factor treatment and mild hypothermia
treatment could improve the proportion of stem cell-like cells
derived from a glial cell population.
Neuronal lineage markers and CD molecules were
selected as indicators to evaluate the differentiation of glial
cells under a range of different treatments involving growth
factors, anaerobic conditions and mild hypothermic conditions.
Nestin is known to be predominantly expressed in neural stem cells,
but not in mature neurons; therefore, nestin is an effective marker
for neural stem cells (18).
Neuroblasts (neuroprogenitors) are monopotent stem cells with a
certain potential for differentiation; these cells differentiate
mainly into neurons, astrocytes and oligodendrocytes (19). GFAP, an established marker for
neuroblasts, is also expressed in astrocytes (20) and is therefore commonly used to
identify neuroblasts containing nestin. Musashi-1, an RNA-binding
protein, is mainly expressed in mitotic neural stem cells and is
therefore used to identify neural stem cells and neuroblasts
(21). NeuN antigen is a
nucleoprotein that serves a vital role in neurons (22); the positive expression of NeuN
antigen indicates that neurons are no longer undergoing mitotic
events (23). NSE is a cytoplasmic
protein that is mainly expressed in mature neurons and also
represents an effective marker for differentiated neurons (24). The detection of CD molecules is an
effective method with which to identify MSCs (25). According to the International
Society for Cellular Therapy, the simplest identification criterion
for human MSCs is the presence of adherent human cells that exhibit
positivity for CD90/Thy1 and CD105/Endoglin, as determined by flow
cytometry (26,27). However, this simple criterion cannot
be readily applied to the CDs of non-human MSCs. For example, MSCs
from mouse adipose tissue can be CD105−, and MSCs from
rat bone marrow can be CD90− (28). Therefore, the identification of
mouse-derived MSCs should also consider positivity for CD29 and
CD44 (29,30).
The indicators selected for the present study
provide information from two different perspectives. One
perspective is to reflect the growth of MSCs in the nervous system
by detecting the positivity of MSC transforming clusters on the
surface of glial cells in response to different treatments. The
other perspective is to measure the expression levels of genetic
and protein markers in glial cells in response to different
treatment conditions. The central nervous system also contains
MSCs; when a large number of neural cells die, MSCs proliferate and
then differentiate to help repair the nervous system injury
(31). This is the theoretical
basis of stem cell therapy, in which MSCs are injected into the
brain. Growth factor induction is a common and effective way of
promoting this proliferation and to increase the proportion of MSCs
produced (29). In order to
identify MSCs in an accurate manner, it is important to maintain a
high ratio of positivity in specific CD clusters (32). In the present study,
CD105+ cells accounted for <0.5% of all glial cells
in the normal state; this increased to ~1% after hypoxia and mild
hypothermia treatments, thus indicating that mild hypothermia
treatments can slightly increase the proportion of MSCs after
hypoxia. Additionally, the present study demonstrated that this
treatment increased the proportions of cells that were positive for
CD105 and CD90 (to 30 and 20%, respectively), although the
proportion of cells that were positive for CD44 and CD90 did not
change significantly. This indicated that growth factors may induce
the proliferation of cells that are positive for both CD105 and
CD29. However, these cells may not be MSCs; instead, these cells
may be somatic cells exhibiting greater levels of differentiation.
The proportion of MSCs that were positive for CD90 was ~15%. This
is in accordance with the present immunocytochemistry results,
which demonstrated that glial cells in the group receiving growth
factors adopted a morphology that was more similar to
fibroblasts.
During normal body function, different generations
of cells must undergo processes that turn mature cells into stem
cells (33). Although research
relating to the induction of stem cells by acid has been questioned
recently (34), there is no
essential difference between the basic concept of this research and
stem cell induction. The present study demonstrated that changes in
the cellular environment may induce mature cells to become stem
cells, although the identity of these specific environmental
factors remains unknown. The results of the present study
demonstrated that hypoxic mild hypothermia treatment or growth
factor treatment upregulated the expression levels of nestin and
Musashi-1 in differentiated neurons and downregulated the
expression levels of NSE and NeuN in mature neurons. Hypoxia
treatment, combined with mild hypothermia and growth factor
treatment, further enhanced this effect. Additionally, the results
of the present study demonstrated that the relative expression
levels of GFAP were inhibited by mild hypothermia or growth factor
treatment after hypoxia treatment. It is possible that neural stem
cells are positive for GFAP, nestin and Musashi-1, whereas
neuroblasts are positive for nestin and Musashi-1 (35). The observed decrease in GFAP
expression may indicate that neural stem cells that are derived
from MSCs have the ability to rapidly differentiate into nerve
cells.
Previous studies have demonstrated that it is
difficult to identify MSCs from various rat tissues using surface
markers alone (36,37). In the present study, nestin and
Musashi-1 were selected as biomarkers for MSCs (38) and the induction of MSC-like
transformation in rat primary glial cells by hypoxia, mild
hypothermia and growth factors from the morphological aspect was
verified. Moreover, CD29 and CD44 positivity supported the MSC-like
characteristics, at least to some extent. However, these biomarkers
and surface markers are not sufficient to prove the specific
features of MSCs. Future work should aim to stimulate transformed
cells into different types of neurons (39), which could provide further support
for the hypothesis of the current study.
In conclusion, the results of the present study
suggested that treatments involving bFGF and EGF, and anaerobic
mild hypothermia may promote the transformation of glial cells into
MSC-like cells, and that these effects were optimized when the two
treatments were combined.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW, WZ and GH performed the experiments and analyzed
the data. HW and CS designed the study and wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jiangxi Provincial People's Hospital Affiliated to
Nanchang University (Nanchang, China; approval no.
2017BBG70066).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee RHC, Lee MHH, Wu CYC, Silva ACE,
Possoit HE, Hsieh TH, Minagar A and Lin HW: Cerebral ischemia and
neuroregeneration. Neural Regen Res. 13:373–385. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun YJ, Zhang ZY, Fan B and Li GY:
Neuroprotection by therapeutic hypothermia. Front Neurosci.
13:5862019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wassink G, Gunn ER, Drury PP, Bennet L and
Gunn AJ: The mechanisms and treatment of asphyxial encephalopathy.
Front Neurosci. 8:402014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shankaran S: Outcomes of hypoxic-ischemic
encephalopathy in neonates treated with hypothermia. Clin
Perinatol. 41:149–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Darwazeh R and Yan Y: Mild hypothermia as
a treatment for central nervous system injuries: Positive or
negative effects. Neural Regen Res. 8:2677–2686. 2013.PubMed/NCBI
|
|
6
|
Park WS, Sung SI, Ahn SY, Yoo HS, Sung DK,
Im GH, Choi SJ and Chang YS: Hypothermia augments neuroprotective
activity of mesenchymal stem cells for neonatal hypoxic-ischemic
encephalopathy. PLoS One. 10:e01208932015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Savitz SI, Cramer SC, Wechsler L and
Consortium S: Stem cells as an emerging paradigm in stroke 3:
Enhancing the development of clinical trials. Stroke. 45:634–639.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cox CS Jr, Hetz RA, Liao GP, Aertker BM,
Ewing-Cobbs L, Juranek J, Savitz SI, Jackson ML,
Romanowska-Pawliczek AM, Triolo F, et al: Treatment of severe adult
traumatic brain injury using bone marrow mononuclear cells. Stem
Cells. 35:1065–1079. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ullah I, Subbarao RB and Rho GJ: Human
mesenchymal stem cells-current trends and future prospective.
Biosci Rep. 35:e001912015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kriegstein A and Alvarez-Buylla A: The
glial nature of embryonic and adult neural stem cells. Annu Rev
Neurosci. 32:149–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Velthoven CT, Kavelaars A, van Bel F
and Heijnen CJ: Repeated mesenchymal stem cell treatment after
neonatal hypoxia-ischemia has distinct effects on formation and
maturation of new neurons and oligodendrocytes leading to
restoration of damage, corticospinal motor tract activity, and
sensorimotor function. J Neurosci. 30:9603–9611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parolini O, Alviano F, Bergwerf I,
Boraschi D, De Bari C, De Waele P, Dominici M, Evangelista M, Falk
W, Hennerbichler S, et al: Toward cell therapy using
placenta-derived cells: Disease mechanisms, cell biology,
preclinical studies, and regulatory aspects at the round table.
Stem Cells Dev. 19:143–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L and Ho C: Mesenchymal stem cell
preparation and transfection-free ferumoxytol labeling for MRI cell
tracking. Curr Protoc Stem Cell Biol. 43:2B.7.1–2B.7.14. 2017.
View Article : Google Scholar
|
|
14
|
Liu L, Tseng L, Ye Q, Wu YL, Bain DJ and
Ho C: A new method for preparing mesenchymal stem cells and
labeling with ferumoxytol for cell tracking by MRI. Sci Rep.
6:262712016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo L, He Y, Wang X, Key B, Lee BH, Li H
and Ye Q: Potential roles of dental pulp stem cells in neural
regeneration and repair. Stem Cells Int. 2018:17312892018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. National Academies
Press; Washington, DC: 2011
|
|
17
|
Donovan PJ and de Miguel MP: Turning germ
cells into stem cells. Curr Opin Genet Dev. 13:463–471. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Homem CC, Repic M and Knoblich JA:
Proliferation control in neural stem and progenitor cells. Nat Rev
Neurosci. 16:647–659. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jefferis GS and Livet J: Sparse and
combinatorial neuron labelling. Curr Opin Neurobiol. 22:101–110.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song Z, Shen F, Zhang Z, Wu S and Zhu G:
Calpain inhibition ameliorates depression-like behaviors by
reducing inflammation and promoting synaptic protein expression in
the hippocampus. Neuropharmacology. 174:1081752020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mirsadeghi S, Shahbazi E, Hemmesi K,
Nemati S, Baharvand H, Mirnajafi-Zadeh J and Kiani S: Development
of membrane ion channels during neural differentiation from human
embryonic stem cells. Biochem Biophys Res Commun. 491:166–172.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu G, Wang Y, Li J and Wang J: Chronic
treatment with ginsenoside Rg1 promotes memory and hippocampal
long-term potentiation in middle-aged mice. Neuroscience.
292:81–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gusel'nikova VV and Korzhevskiy DE: NeuN
As a neuronal nuclear antigen and neuron differentiation marker.
Acta Naturae. 7:42–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sarnat HB: Clinical neuropathology
practice guide 5–2013: Markers of neuronal maturation. Clin
Neuropathol. 32:340–369. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pittenger MF, Discher DE, Peault BM,
Phinney DG, Hare JM and Caplan AI: Mesenchymal stem cell
perspective: Cell biology to clinical progress. NPJ Regen Med.
4:222019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lavezzi AM, Corna MF and Matturri L:
Neuronal nuclear antigen (NeuN): A useful marker of neuronal
immaturity in sudden unexplained perinatal death. J Neurol Sci.
329:45–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galipeau J and Krampera M: The challenge
of defining mesenchymal stromal cell potency assays and their
potential use as release criteria. Cytotherapy. 17:125–127. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lv FJ, Tuan RS, Cheung KM and Leung VY:
Concise review: The surface markers and identity of human
mesenchymal stem cells. Stem Cells. 32:1408–1419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu H, Guo ZK, Jiang XX, Li H, Wang XY,
Yao HY, Zhang Y and Mao N: A protocol for isolation and culture of
mesenchymal stem cells from mouse compact bone. Nat Protoc.
5:550–560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sági B, Maraghechi P, Urbán VS, Hegyi B,
Szigeti A, Fajka-Boja R, Kudlik G, Német K, Monostori E, Gócza E
and Uher F: Positional identity of murine mesenchymal stem cells
resident in different organs is determined in the postsegmentation
mesoderm. Stem Cells Dev. 21:814–828. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nandoe Tewarie RS, Hurtado A, Bartels RH,
Grotenhuis A and Oudega M: Stem cell-based therapies for spinal
cord injury. J Spinal Cord Med. 32:105–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maleki M, Ghanbarvand F, Reza Behvarz M,
Ejtemaei M and Ghadirkhomi E: Comparison of mesenchymal stem cell
markers in multiple human adult stem cells. Int J Stem Cells.
7:118–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shyh-Chang N and Ng HH: The metabolic
programming of stem cells. Genes Dev. 31:336–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim EM, Manzar G and Zavazava N: Induced
pluripotent stem cell-derived gamete-associated proteins incite
rejection of induced pluripotent stem cells in syngeneic mice.
Immunology. 151:191–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Campbell JG, Miller DC, Cundiff DD, Feng Q
and Litofsky NS: Neural stem/progenitor cells react to non-glial
cns neoplasms. SpringerPlus. 4:532015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sullivan MO, Gordon-Evans WJ, Fredericks
LP, Kiefer K, Conzemius MG and Griffon DJ: Comparison of
mesenchymal stem cell surface markers from bone marrow aspirates
and adipose stromal vascular fraction sites. Front Vet Sci.
2:822016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin CS, Xin ZC, Dai J and Lue TF: Commonly
used mesenchymal stem cell markers and tracking labels: Limitations
and challenges. Histol Histopathol. 28:1109–1116. 2013.PubMed/NCBI
|
|
38
|
Xie L, Zeng X, Hu J and Chen Q:
Characterization of nestin, a selective marker for bone marrow
derived mesenchymal stem cells. Stem Cells Int. 2015:7620982015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khalil W, Tiraihi T, Soleimani M,
Baheiraei N and Zibara K: Conversion of neural stem cells into
functional neuron-like cells by MicroRNA-218: Differential
expression of functionality genes. Neurotox Res. 38:707–722. 2020.
View Article : Google Scholar : PubMed/NCBI
|