Introduction
Colorectal cancer (CRC) is one of the most common
cancer types and is the second cause of cancer-associated mortality
worldwide, causing about 600,000 deaths per year (1). Despite progress in diagnostic and
therapeutic techniques, the overall survival rate of patients with
CRC remains poor, due to diagnosis occurring at a later stage,
where metastasis has developed (2,3).
Therefore, it is important to gain a greater understanding of the
underlying molecular mechanisms involved in CRC, which will
contribute to the early diagnosis and effective treatment of the
disease.
S100 calcium binding protein A16 (S100A16) is a
member of the S100 protein family, which is comprised of acidic
proteins with an EF-hand Ca2+ binding motif (4). S100 proteins serve a variety of
regulatory functions in cellular processes, such as motility,
differentiation, cell proliferation, contraction, transcription,
cell cycle processes and secretion (5). The abnormal protein expression of S100
has been exhibited in several types of tumour and is considered to
be a potential marker of cancer (6,7). As
the most recent member of the S100 family, S100A16 has been
implicated in numerous human pathophysiological processes, such as
inflammation, adipogenesis, osteoporosis and tumour progression
(8–10). In addition, previous studies have
reported that the function of S100A16 in cancer is complex
(11–14). For instance, S100A16 demonstrates a
variable effect in tumour cells of different tissues. Several
studies have reported that S100A16 is associated with tumour
progression in bladder, lung and breast cancer (11–13).
However, an additional study reported the tumour-suppressive
function of S100A16 in oral squamous cell carcinoma (14). Moreover, the abnormal expression of
S100A16 is involved in CRC progression. It has also been shown that
the low membrane expression of S100A16 is associated with a poor
prognosis in patients with CRC, potentially serving as a suppressor
of cancer (15). However, the
specific modulatory role of S100A16 in CRC is yet to be
elucidated.
The aim of the present study was to investigate
whether S100A16 could inhibit the progression of CRC by regulating
JNK/p38 MAPK signalling pathway, which may provide insight into the
role of S100A16 in CRC as a potential prognostic marker of patients
with CRC.
Materials and methods
Data collection and analysis
The data of 45 colorectal adenocarcinoma and 24
normal colorectal tissues were downloaded from the GSE20916
microarray dataset (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE20916)
(16). The mRNA expression values
of S100A16 in colorectal adenocarcinoma and normal colorectal
tissues were analyzed on the Oncomine platform (https://www.oncomine.org/), which collects publicly
available cancer microarray data. The term ‘S100A16’ was searched
on Oncomine. ‘Cancer type’ and ‘analysis type’ were then defined as
‘colorectal cancer’ and ‘cancer vs. normal analysis’, respectively.
The cut-off of P-value and fold change were 0.05 and 2,
respectively. The data were also analysed with an unpaired-t test
between two groups using GraphPad Prism 6 (GraphPad Software,
Inc.).
Tissue samples and microarrays
A total of 10 CRC samples and paired colorectal
normal tissues were obtained from patients (age range, 31–71 years;
6 male and 4 female patients) admitted to the Department of
Gastrointestinal Surgery, Nanfang Hospital, Southern Medical
University between January 2015 and June 2019. All patients
provided written informed consent and the study protocol was
approved by the Nanfang Hospital Institutional Review Board
(approval no. NFEC-2017-147). In addition, microarrays of CRC
tissue comprising 100 cases (60 cases of CRC samples with matched,
normal colorectal tissue samples; 40 cases were CRC samples alone.)
were purchased from Shanghai Outdo Biotech Co., Ltd. All tumours
were diagnosed as colorectal adenocarcinoma and all specimens were
used for routine pathological processing. Out of all 110 specimens
used in the present study, 104 had complete clinicopathological
features and complete follow-up data.
Cell culture and treatment
Human CRC cell lines, including SW480, SW620, HCT116
and Lovo, were obtained from The Cell Bank of Type Culture
Collection of Chinese Academy of Sciences and identified by the
American Type Culture Collection. RKO cells were obtained from the
American Type Culture Collection. All cells were cultured in medium
containing 90% DMEM (HyClone; Cytiva) and 10% FBS (HyClone; Cytiva)
in a 5% CO2 atmosphere at 37°C.
To determine the impact of the JNK or p38 MAPK
pathway, 10 µM JNK inhibitor SP600125 (cat. no. 8177; Cell
Signaling Technology, Inc.) or 10 µM p38 inhibitor SB203580 (cat.
no. 5633; Cell Signaling Technology, Inc.) were applied to HCT116
cells and incubated at 37°C with 5% CO2 for 48 h.
Immunohistochemistry (IHC)
staining
Tissue samples were fixed with 4% paraformaldehyde
for 2 days at room temperature and embedded in paraffin, after
which 4-µm were cut and mounted onto slides. Slides were incubated
at 56°C, deparaffinised in xylene and dehydrated in a graded series
of alcohol. Heat-induced antigen retrieval was carried out with
sodium citrate (pH 6.0) in a microwaving at 100°C for 30 min, IHC
staining was performed as previously described (17). Briefly, endogenous peroxidase
activity was inhibited using 3% hydrogen peroxide for 10 min at
room temperature. After blocking with 5% goat serum (cat. no.
BMS0050; Abbkine Scientific Co., Ltd.) diluted with PBS at room
temperature for 30 min, sections were incubated with anti-S100A16
antibodies (cat. no. 11456-1-AP; 1:300; ProteinTech Group, Inc.) at
4°C overnight. Samples were then incubated with HRP-conjugated
secondary antibodies (cat. no. ab5879; Abcam) at room temperature
for 30 min. The resulting protein was presented as a brown pigment
using the standard diaminodianiline method (cat. no. SAP 9101;
OriGene Technologies, Inc.). Slides were subsequently
double-stained with haematoxylin at room temperature for 20 sec,
after which immunostaining was assessed using an Olympus BX-53
microscope (Olympus Corporation; magnification, ×100-400). The
degree of IHC staining was evaluated as previously described (0, no
staining; 1, weak staining; 2, moderate staining; and 3, strong
staining) (18). Scores of 0–1 were
defined as low expression, and scores of 2–3 were defined as high
expression. The immunoreactivity score was determined as follows:
Immunoreactivity score = the percentage of positive staining × the
staining intensity score.
Overexpression and knockdown of
S100A16
Based on the S100A16 gene sequence in GenBank
(NM_080388; http://www.ncbi.nlm.nih.gov/nuccore), primers
specifically matching S100A16 mRNA were constructed, which were
amplified, cut by HindIII and BamHI and then
sub-cloned into pcDNA3.1 vector (cat. no. P0157; www.miaolingbio.com) or GV287-EGFP lentiviral vector
(Shanghai GeneChem Co., Ltd.) to overexpress S100A16 after
screening. The empty lentiviral vector was used as a negative
control.
To overexpress S100A16, colon cancer cells were
transfected with 5 nM S100A16 pcDNA3.1(+) vector using
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturers instructions for two days at
37°C. After 48 h of transfection, the expression of S100A16 was
determined via western blotting, and the transfected cells were
collected for subsequent experiments in vitro.
For lentivirus transduction, 7.5 µg
GV287-EGFP-S100A16 (lenti-S100A16) lentiviral vector (Shanghai
GeneChem Co., Ltd.), mixed with 5 µg pMD2.G (cat. no. p2062;
www.miaolingbio.com) and 10 µg psPAX2
(cat. no. p2061; www.miaolingbio.com), were co-transfected into 293T
cells (The Cell Bank of Type Culture Collection of Chinese Academy
of Sciences) with Lipofectamine® 2000 reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturers
instructions at 37°C with 5% CO2 for 48 h. The viral
supernatants were collected, centrifuged at 4000 × g for 10 min at
4°C, then filtered through 0.45-µm PVDF membranes. The virus
concentration was determined by reverse transcription-quantitative
PCR to measure the number of integrated copies of 293T cells.
Lentivirus (lentivirus-S100A16) were then used to infect colon
cancer cells using 5 µg/ml polybrene (Hanbio Biotechnology Co.,
Ltd.) with an MOI of 5. The infected cells were cultured with
puromycin (5 µg/ml) for 14 days at 37°C to establish stably
infected cells for mouse xenografts.
To knockdown S100A16, CRC cells were transfected
with 50 nM S100A16 small interfering RNA (siRNA/siS100A16; Shanghai
GenePharma Co., Ltd.) using Lipofectamine 2000 reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturers
instructions for 2 days at 37°C. The following sequences were
utilized: si-S100A16#1, sense, 5′-GCAUCAGCUUCGAUGAGUATT-3′ and
antisense, 5′-UACUCAUCGAAGCUGAUGCTT-3′; si-S100A16#2, sense,
5′-CCAGAACCUGGAUGCCAAUTT-3′ and antisense,
5′-AUUGGCAUCCAGGUUCUGGTT-3′; si-S100A16#3, sense,
5′-GCUGGAGAAGGCAGUCAUUTT-3′ and antisense,
5′-AAUGACUGCCUUCUCCAGCTT-3′; negative control, sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. At 48 h following transfection, the
expression of S100A16 was determined via western blotting, and the
transfected cells were collected for subsequent experiments.
Cell proliferation assay
After S100A16 siRNA was transfected into SW480 and
HCT116 cells or following S100A16 pcDNA3.1(+) vector transfection
into Lovo cells, CRC cells (1×103 cells/well) were
seeded into 96-well plates. Cells were cultured for 24, 48, 72, 96
and 120 h, after which 10 µl Cell Counting Kit-8 (CCK-8) solution
(Dojindo Molecular Technologies, Inc.) was added and incubated in a
5% CO2 atmosphere at 37°C for an additional 4 h. The kit
was used according to the manufacturers instructions. Optical
density was measured at 450 nm using an automatic enzyme
standard.
Cell migration and invasion
assays
Cell migration and invasion assays were performed
using a Transwell system (Corning, Inc.). For cell invasion, an 8
µm-pore polycarbonate membrane was coated with Matrigel (BD
Biosciences) at 37°C for 5 h. Cells at density of
2.5×104 (migration) and 5×104 (invasion) per
well were suspended in 500 µl serum-free medium and subsequently
added to the upper chamber. A total of 600 µl medium containing 10%
FBS was added to the lower chamber. After 24 (migration) or 48 h
(invasion) incubation at 37°C, migrated or invaded cells in the
lower chambers were stained with 0.1% crystal violet at room
temperature for 30 min and counted using light microscopy
(magnification, ×100).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the harvested cells
using TRIzol® (Thermo Fisher Scientific, Inc.) according
to the manufacturers protocol. First-Strand cDNA Synthesis was
carried out by reverse-transcription using PrimeScript™ RT Master
Mix (Takara) and qPCR was performed with a LightCycler
480® System (Roche Diagnostics) using SYBR- Green mix
(Takara Bio, Inc.), according to the manufacturers instructions.
The thermocycling conditions were as follows: i) 94°C for 5 min;
ii) 40 amplification cycles at 94°C for 30 sec, 57°C for 30 sec and
72°C for 30 sec; and iii) 72°C for 5 min. The primer sequences used
for PCR were as follows: i) S100A16 forward,
5′-TTTTGTCCATCTCCTTTCACCA-3′ and reverse,
5′-CAGGCAAATCAGACTCCCTTC-3′; ii) S100A4 forward,
5′-GTACTCGGGCAAAGAGGGTG-3′ and reverse, 5′-TTGTCCCTGTTGCTGTCCAA-3′;
iii) S100A14 forward, 5′-TGCTCTAGAATGGGACAGTGTCGGTCAGCC-3′ and
reverse, 5′-CGCGGATCCTCAGTGCCCCCGGA CAGGCCT-3′; and iv) GAPDH (the
internal reference) forward, 5′-CTGGGCTACACTGAGCACC-3′ and reverse,
5′-AAGTGGTCGTTGAGGGCAATG-3′. Relative gene expression was
determined using the 2−ΔΔCq method (19).
Western blotting
Cultured cells were lysed in RIPA buffer containing
1X protease cocktail inhibitor (Sigma-Aldrich; Merck KGaA). Protein
concentrations were determined using a BCA protein assay kit
(Thermo Fisher Scientific, Inc.). A total of 30 µg proteins from
different groups were then separated on 10–12% SDS-polyacrylamide
gels for electrophoresis. Separated proteins were transferred to
PVDF membranes (EMD Millipore). After being blocked with non-fat
milk for 1 h at room temperature, the membranes were incubated with
the following primary antibodies at 4°C overnight: anti-rabbit
S100A4 (cat. no. 16105-1-AP; 1:500; ProteinTech Group, Inc.),
anti-rabbit S100A14 (cat. no. 10489-1-AP; 1:500; ProteinTech Group,
Inc.), anti-rabbit S100A16 (cat. no. 11456-1-AP; 1:500; ProteinTech
Group, Inc.) anti-phosphorylated (p)-rabbit JNK (cat. no. 9255;
Cell Signaling Technology, Inc.), anti-rabbit JNK (cat. no. 9252;
Cell Signaling Technology, Inc.), anti-p-rabbit ERK1/2 (cat. no.
4370; Cell Signaling Technology, Inc.), anti-rabbit ERK1/2 (cat.
no. 4695; Cell Signaling Technology, Inc.), anti-p-rabbit p38 MAPK
(cat. no. 9216; Cell Signaling Technology, Inc.), anti-rabbit p38
MAPK (cat. no. 9212; Cell Signaling Technology, Inc.), anti-rabbit
vimentin (cat. no. 12826; Cell Signaling Technology, Inc.),
anti-mouse E-cadherin (cat. no. 14472; Cell Signaling Technology,
Inc.), anti-mouse N-cadherin (cat. no. 14215; Cell Signaling
Technology, Inc.) and anti-mouse GAPDH (cat. no. 51332; Cell
Signaling Technology, Inc.). After incubation with horseradish
peroxidase-conjugated secondary antibodies (cat. nos. 7074 and
7076; Cell Signaling Technology, Inc.) for 1 h at room temperature,
immunoreactive proteins were visualized using chemiluminescence
detection reagents (EMD Millipore) using the FluorChem E system
(ProteinSimple). Blots were semi-quantified using AlphaView SA
software (ProteinSimple; version 3.4.0.0). The grayscale values of
protein bands were normalized to that of GAPDH.
Mouse xenografts of CRC cells
A total of 10 BALB/c nude male mice (weight, 12–14
g; age, 4–6 weeks) were purchased from Hunan SJA Laboratory Animal
Co., Ltd. The mice were fed with common feed and sterile water
ad libitum and housed in 21–23°C with 54–56% humidity and a
12-h light/dark cycle. Lovo cells (2×106) were infected
with Lenti-Vector or Lenti-S100A16 (S100A16 overexpression vector)
and were injected subcutaneously into the flanks of mice to
construct the xenograft model (n=5/group). Tumour volume was
measured and recorded every three days. At 21 days after injection,
all mice were euthanized via cervical dislocation. The mice were
considered to be dead when the heart and breathing stopped. The
tumour xenografts were removed to determine their size and weight.
The largest tumour diameter was 1.5 cm. All experimental procedures
were conducted in accordance with the ethical standards of the
Ethical Committee of Nanfang Hospital, Southern Medical University.
Study approval was additionally gained from the Institutional
Animal Care and Use Committee of Southern Medical University
(approval no. L2018160).
Statistical analysis
GraphPad Prism 6 (GraphPad Software, Inc.) and SPSS
22.0 (IBM Corp.) software was used to assess the statistical
significance of differences between groups. Data were analysed
using an unpaired or a paired two-tailed Students t-test for two
groups, or one-way ANOVA followed by Tukeys post hoc test for
multiple groups. Two-way ANOVA with Bonferronis correction was used
for statistical analyses between different treatments, different
cell cohorts or different time points. χ2 or Fishers
exact tests were used to compare the differences in age, sex,
pathological differentiation, T classification, N classification
and American Joint Committee on Cancer (AJCC) stages (Table I). Data are presented as the mean ±
SD from a minimum of three experimental repeats. P<0.05 was
considered to indicate a statistically significant difference.
 | Table I.Association between
clinicopathological features and S100A16 expression in 104 patients
with colorectal cancer. |
Table I.
Association between
clinicopathological features and S100A16 expression in 104 patients
with colorectal cancer.
|
|
| S100A16
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | n | Low (n=71) | High (n=33) | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.280 | 0.596 |
|
≤50 | 19 | 12 | 7 |
|
|
|
>50 | 85 | 59 | 26 |
|
|
| Sex |
|
|
| 0.000 | 0.987 |
|
Female | 44 | 30 | 14 |
|
|
|
Male | 60 | 41 | 19 |
|
|
| Pathological
differentiation |
|
|
| 6.638 | 0.036 |
| I
(high) | 11 | 7 | 4 |
|
|
| II
(moderate) | 49 | 28 | 21 |
|
|
| III
(poor) | 44 | 36 | 8 |
|
|
| T
classification |
|
|
| 0.133 | 0.715 |
| T1 +
T2 | 6 | 5 | 1 |
|
|
| T3 +
T4 | 98 | 66 | 32 |
|
|
| N
classification |
|
|
| 4.477 | 0.034 |
| N0 | 60 | 36 | 24 |
|
|
| N1 +
N2 | 44 | 35 | 9 |
|
|
| AJCC stage |
|
|
| 3.311 | 0.069 |
| I +
II | 59 | 36 | 23 |
|
|
| III +
IV | 45 | 35 | 10 |
|
|
Results
S100A16 is decreased in human CRC
tissues
To identify the potential role of S100A16 in CRC,
gene expression profiles were analysed from Oncomine microarray
datasets (16). The results
revealed that S100A16 mRNA was significantly downregulated in
colorectal adenocarcinoma compared with normal tissues (Fig. 1A). The expression of S100A16 was
subsequently determined in 70 CRC samples (clinical samples from
Nanfang Hospital, n=10; paired samples in CRC tissue microarrays
purchased from Shanghai Outdo Biotech Co., Ltd., n=60) and paired
normal adjacent tissues. As presented in Fig. 1B, IHC analysis demonstrated that
S100A16 exhibited weaker staining in CRC tumour tissues compared
with normal tissues. In addition, S100A16 protein was localized to
the cell membrane and cytoplasm of CRC cells. To confirm the
expression of S100A16 in CRC, CRC microarrays were screened
alongside the CRC samples obtained in the present study. The
results demonstrated that S100A16 was decreased in CRC tissues
compared with normal colon tissues (Fig. 1C).
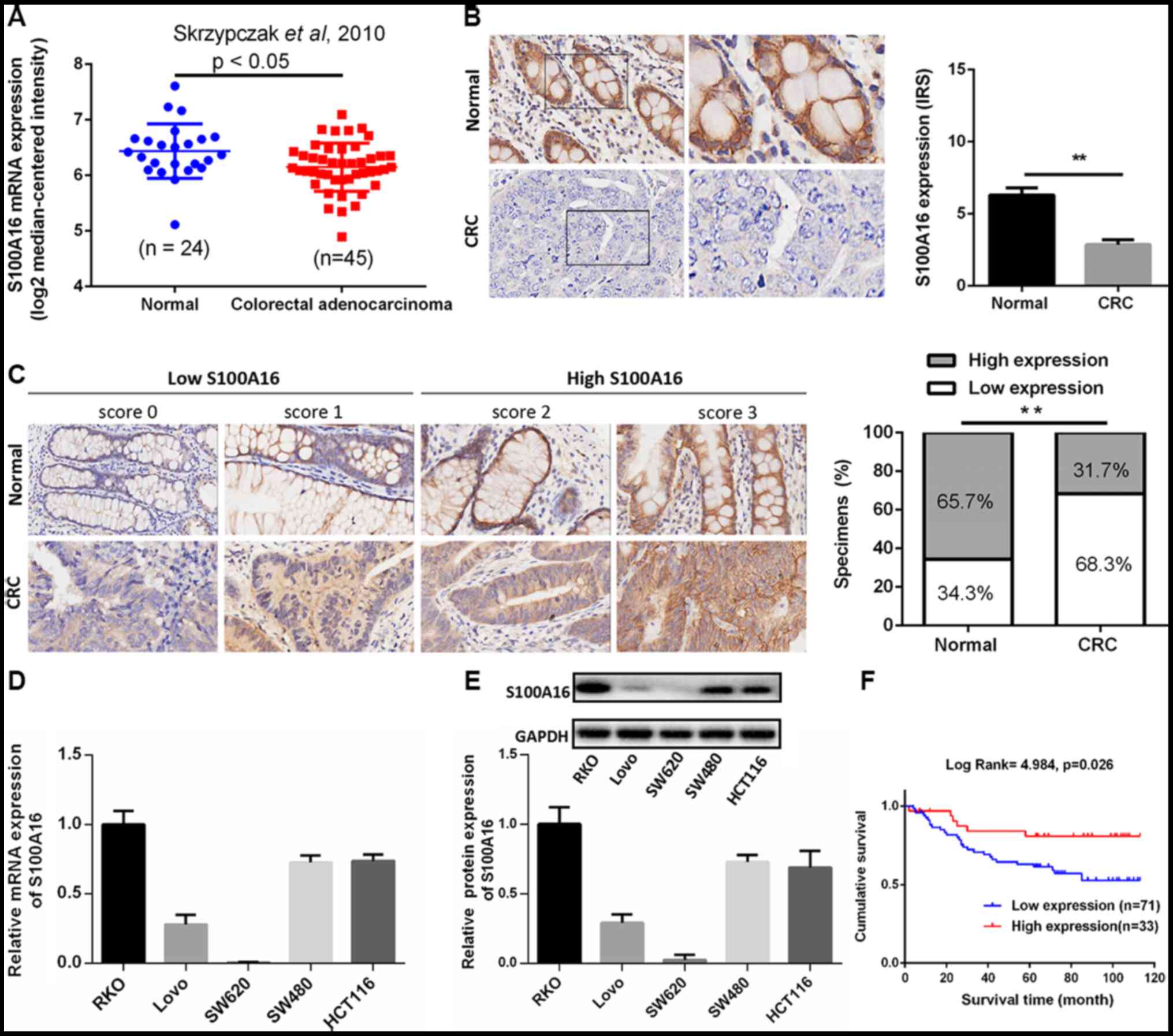 | Figure 1.S100A16 is decreased in human
colorectal cancer. (A)Analysis of S100A16 expression in normal
colorectal tissues and colorectal adenocarcinoma tissues in the
Skrzypczak in Oncomine microarray dataset, as assessed using an
unpaired-t test. P<0.05. (B) Representative images of S100A16
protein expression in CRC tumour tissues and paired normal adjacent
tissues, as assessed via immunohistochemistry. Magnification, ×400.
**P<0.01, paired Students t-test. (C) Representative images of
difference scores indicating S100A16 protein expression in CRC
tumour tissues and paired normal adjacent tissues (magnification,
×400). The proportion of S100A16-expressing in CRC tissue samples
was also analysed. **P<0.01, χ2 test. S100A16 (D)
mRNA and (E) protein expression in CRC cell lines, as determined
via reverse transcription-quantitative PCR and western blotting,
respectively. (F) Kaplan-Meier analysis of the survival rates in
patients with CRC with S100A16 low or high expression. CRC,
colorectal cancer; S100A16, S100 calcium binding protein A16, IRS,
immunoreactive score. |
The expression levels of S100A16 mRNA and protein in
five CRC cell lines were examined via RT-qPCR and western blotting,
respectively. The results indicated that S100A16 mRNA and protein
expression levels were markedly decreased in Lovo and SW620 CRC
cell lines (Fig. 1D and E). Among
the CRC cell lines assessed in the present study, HCT116 and SW480
cells exhibited higher expression levels of S100A16, and as such,
were selected to perform loss-of-function experiments. Lovo cells
exhibiting lower expression levels of S100A16 were selected for
gain-of-function experiments. As SW620 cells did not express an
appropriate level of S100A16, they were not selected for further
study.
S100A16 is negatively associated with
CRC progression and positively associated with patient
survival
To investigate the clinical importance of S100A16 in
CRC, the relationship between patient clinicopathological features
and S100A16 expression was analysed. Patients with CRC were
classified into low and high S100A16 expression groups based on
immunostaining scores (16). The
results suggested that the expression of S100A16 was closely
associated with pathological differentiation (P=0.036) and N
classification (P=0.034). However, no association was identified
between age, sex, T classification and AJCC stage (Table I). Furthermore, patients in the low
S100A16 expression group demonstrated poor prognosis, with a lower
survival time (Fig. 1F). The
results indicated that S100A16 may be involved in CRC development
and could serve as a potential therapeutic target for CRC.
S100A16 inhibits CRC cell
proliferation, migration and invasion
To determine whether S100A16 affects CRC cell
proliferation, migration and invasion, siRNAs targeting S100A16
were transfected into HCT116 and SW480 cells. The results
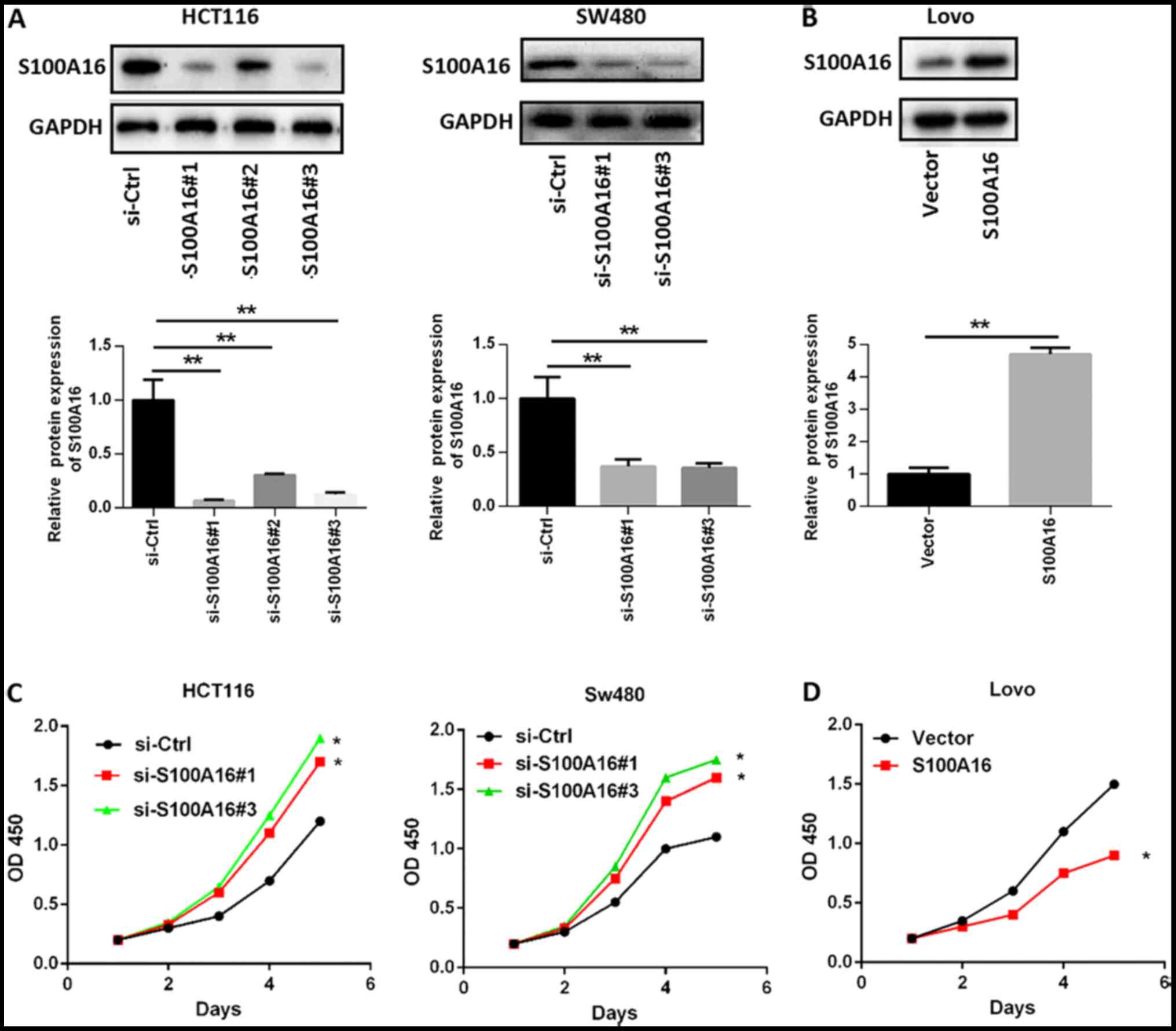
demonstrated that S100A16 protein expression was significantly
decreased following siRNA transfection. si-S100A16#1, si-S100A16#2,
and si-S100A16#3 were all efficiently in silencing S100A16; thus,
only two siRNAs were arbitrarily selected for subsequent
experiments (Fig. 2A). However, no
significant differences were identified in cells transfected with
S100A4 and S100A14 siRNA, indicating that S100A16 knockdown was
specific to S100A16 (Fig. S1A and
B). Subsequently, the S100A16 overexpression vector was
transfected into Lovo cells for S100A16 upregulation (Fig. 2B). CCK-8 assays were then performed
to determine cell proliferation. The results indicated that
proliferation was increased in S100A16-silenced HCT116 and SW480
cells, but decreased in S100A16-overexpressing Lovo cells, compared
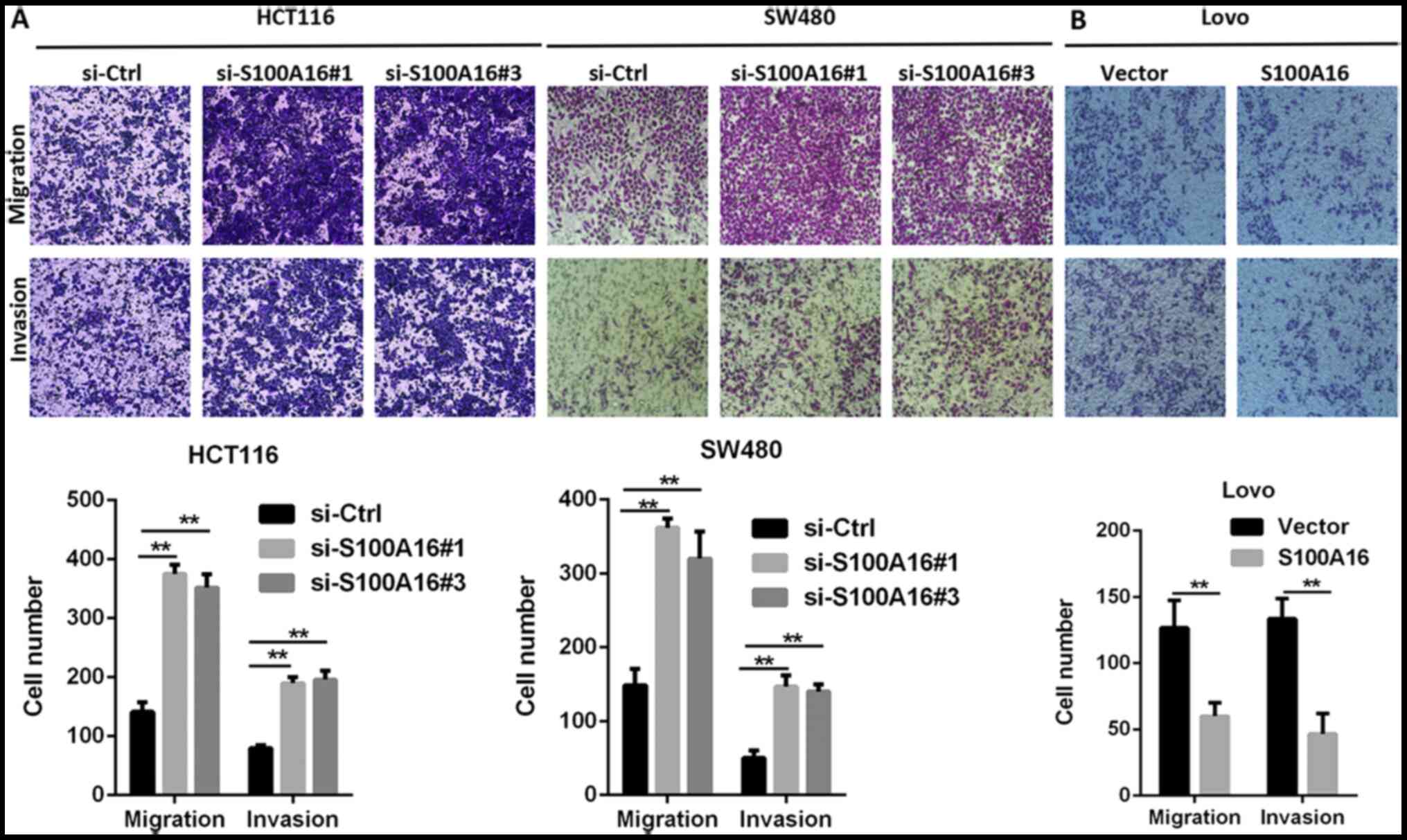
with the corresponding control groups (Fig. 2C and D). The results of Transwell
assays demonstrated that S100A16 knockdown promoted the migration
and invasion of HCT116 and SW480 cells, whereas S100A16
overexpression had the opposite effect in Lovo cells (Fig. 3A and B).
S100A16 knockdown activates the
JNK/p38 MAPK signalling pathway to promote epithelial-mesenchymal
transition (EMT)
The signalling pathways associated with the
S100A16-mediated suppression of CRC cell proliferation, migration
and invasion were assessed in the current study. Previous reports
have revealed that S100A16 affects the JNK/p38 MAPK signalling
pathway (9,20), which serves an important role in CRC
(21,22). Thus, western blotting was performed
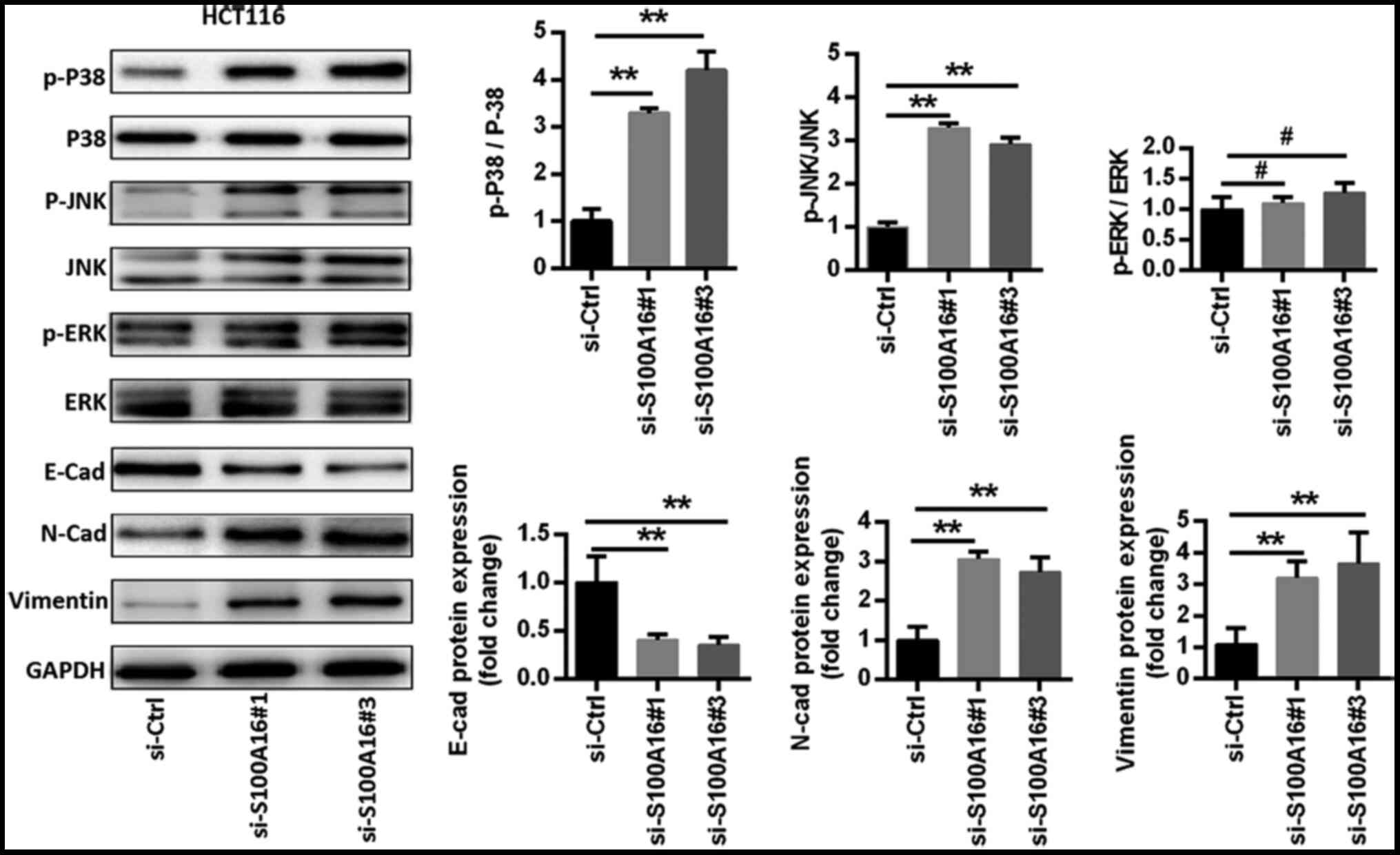
to assess the factors associated with the MAPK signalling pathway
in S100A16-inhibited HCT116 cells. The results identified that p38,
ERK and JNK phosphorylation levels were increased in HCT116 cells
following S100A16 knockdown. However, ERK phosphorylation levels
were not as high as those observed for p38 and JNK (Fig. 4). Additionally, S100A16 knockdown
increased the expression levels of the mesenchymal markers
N-cadherin and vimentin, as well as decreased the expression of the
epithelial marker E-cadherin (Fig.
4).
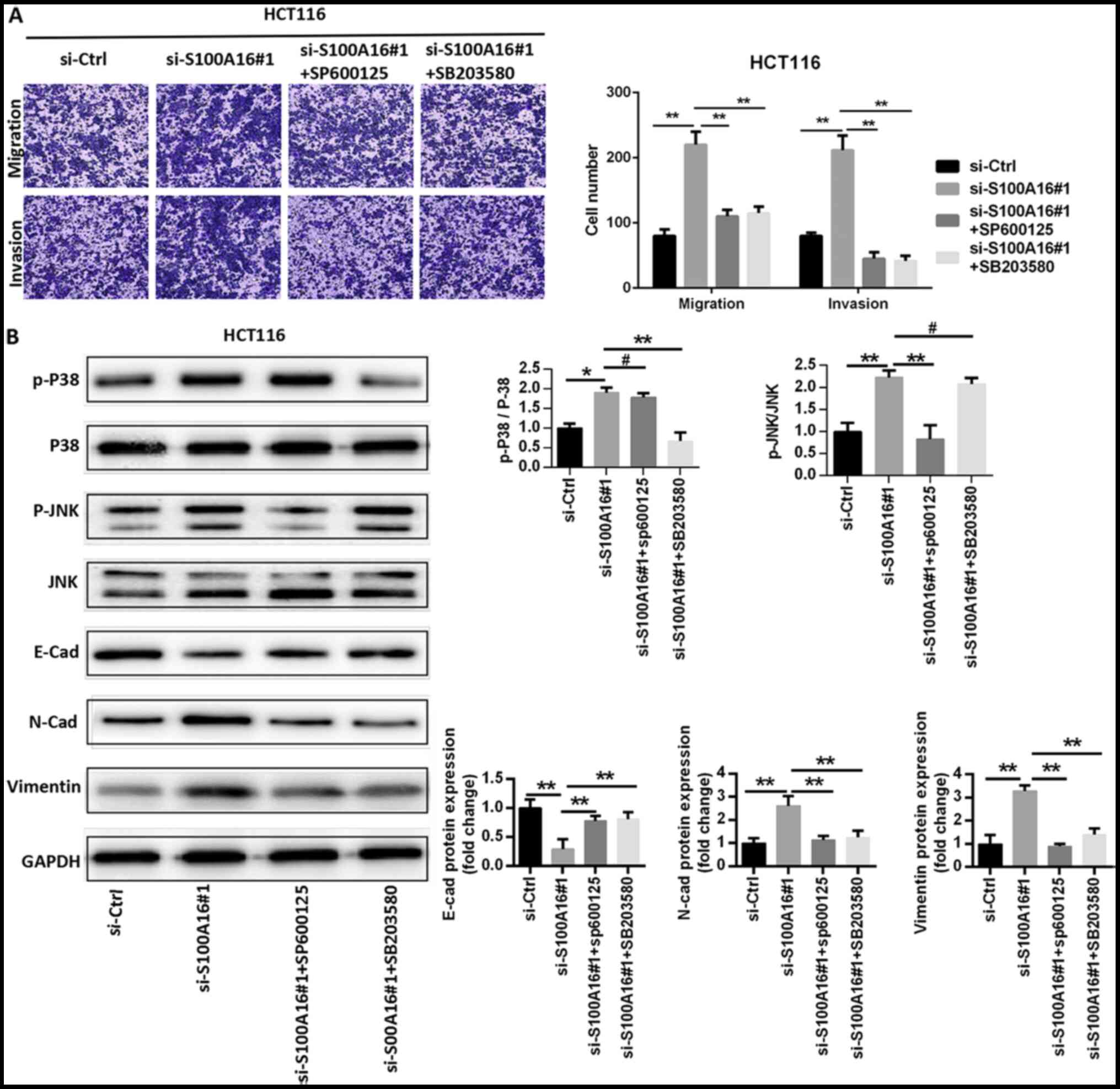
To confirm that the MAPK signalling pathway served a
role in S100A16-modulated CRC cellular activity, the JNK inhibitor
SP600125 and the p38 inhibitor SB203580 were administered to HCT116
cells. SP600125 and SB203580 significantly repressed the increase
in HCT116 cell migration and invasion caused by S100A16 silencing
(Fig. 5A). The results of western
blotting suggested that SP600125 and SB203580 treatments
significantly inhibited the S100A16 knockdown-mediated activation
of JNK and p38 in HCT116 cells. SP600125 and SB203580 also
suppressed the augmented protein expression levels of N-cadherin
and vimentin, and reversed the effect of S100A16 silencing on the
protein expression of E-cadherin in HCT116 cells (Fig. 5B). The results suggested that
S100A16 suppressed EMT and that S100A16 may exert its effects via
the JNK/p38 MAPK signalling pathway.
S100A16 elevation suppresses tumour
growth in vivo
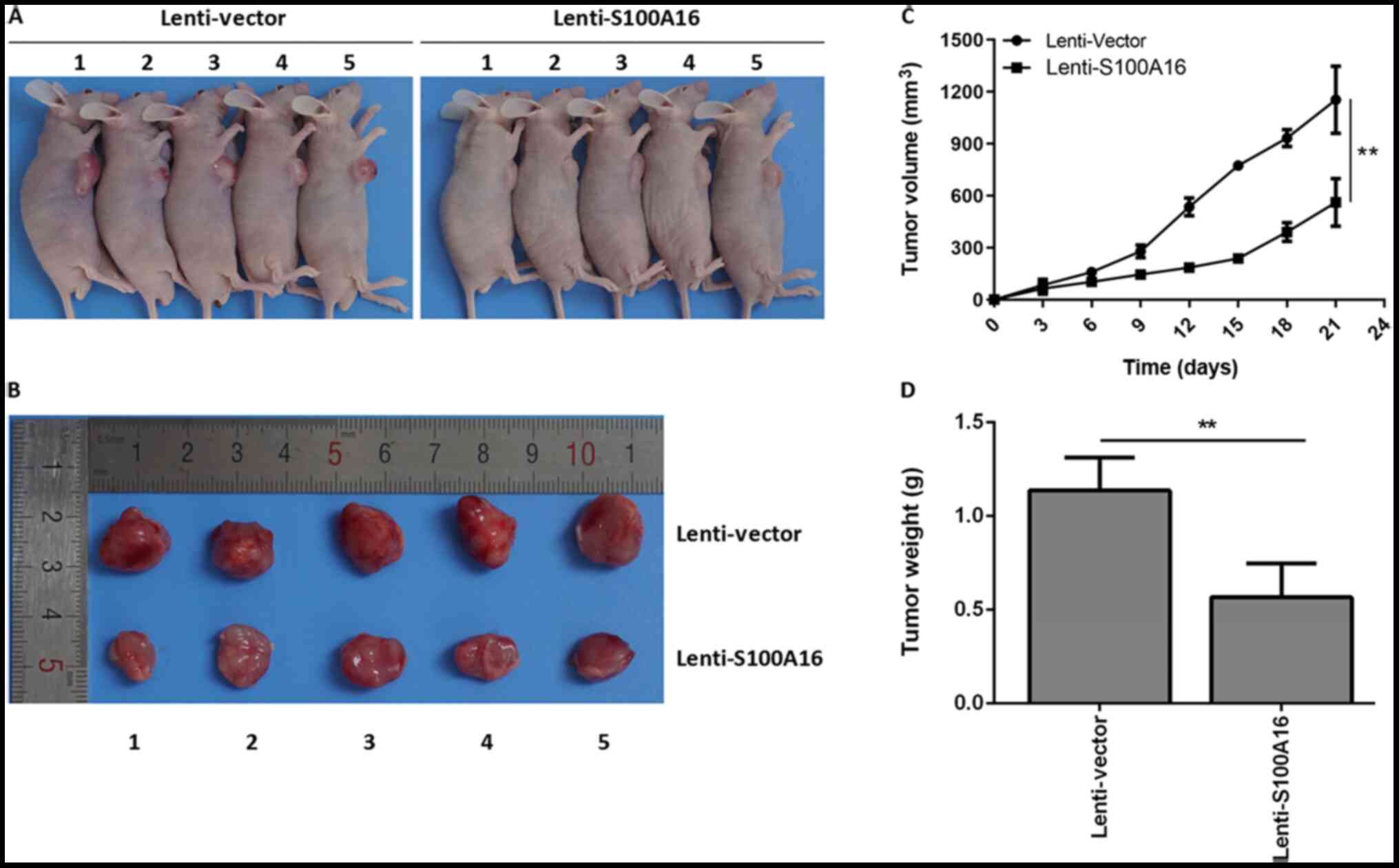
To further determine the effects of S100A16 on
colorectal cancer in vivo, tumour-bearing mice were
constructed. Lenti-Vector and Lenti-S100A16 were transfected into
Lovo cells to elevate S100A6 expression (Fig. S1C). The largest tumour diameter
observed was 1.5 cm. It was determined that the size of tumours in
mice of the S100A16 overexpression group were markedly decreased
compared with the control group (Fig.
6A and B). Additionally, S100A16 overexpression mice exhibited
significantly decreased tumour volumes and weights relative to the
control mice (Fig. 6C and D). The
results indicated that S100A16 overexpression inhibited CRC tumours
in vivo.
Discussion
The S100 family comprises 21 identified
Ca2+ binding proteins, several of which are upregulated
in various tumours, serving vital roles in tumour progression
(4,23). Among these proteins, S100A6 promotes
the proliferation and migration of CRC cells (24). Additionally, S100A11 promotes CRC
aggressiveness by modulating the TGFβ/Smad signalling pathway
(25). Previous studies have
reported that several S100 members are upregulated in CRC,
including S100A2, S100A3, S100A4 and S100A8, and function as tumour
promoters (26–29). In addition, low and high expression
levels of S100A14 and S100A4, respectively, are associated with
increased CRC metastasis (30).
S100A14 interacts with S100A16 and regulates its expression in
human cancer cells (31).
Furthermore, S100A16 expression is decreased in CRC, which is
associated with shorter overall survival times in patients with CRC
(15). However, the role of S100A16
in CRC is yet to be fully elucidated. Therefore, the aim of the
present study was to determine whether S100A16 serves a functional
role in CRC progression.
The present study analysed the expression of S100A16
in the Oncomine microarray dataset, which revealed that S100A16 was
downregulated in CRC. The expression of S100A16 was further
examined in CRC tissue samples and paired adjacent normal tissues
via IHC analysis. The results indicated that S100A16 expression was
significantly decreased in CRC tumour tissues. The association
between patient clinicopathological characteristics and S100A16
expression was subsequently determined. The results demonstrated
that S100A16 expression was significantly associated with
pathological differentiation and N classification. Furthermore,
decreased S100A16 expression levels were significantly associated
with a poor patient prognosis, which is in line with the results of
a previous study (15).
Previous studies have revealed that high levels of
S100A16 are associated with increased proliferation, migration and
invasion (13,23,32).
Additionally, S100A16 has been found to affect metastasis (20). However, the effect of S100A16 on the
biological activity of CRC cells remains undetermined. The present
study therefore investigated the proliferation, migration and
invasion of CRC cells in vitro. The results demonstrated
that S100A16 knockdown promoted the proliferation, migration and
invasion of HCT116 and SW480 cells. By contrast, following S100A16
overexpression, the proliferation, migration and invasion of Lovo
cells were decreased. These results indicated that S100A16 may
serve as a tumour suppressor in CRC.
Since it has been reported that S100A16 interacts
with the MAPK signalling pathway (9,20),
which regulates cell proliferation and migration, it was
hypothesized that the MAPK signalling pathway may be involved in
S100A16-mediated CRC. In the present study, markers of the MAPK
signalling pathway were assessed following S100A16 knockdown. It
was identified that S100A16 knockdown activated the JNK/p38 MAPK
signalling pathway. EMT is a physiological process that increases
the migration and invasion of cells, as well as induces tumour
metastasis and development (1,33,34).
Additionally, EMT is regulated by S100A16 in breast cancer
(35). Therefore, the present study
assessed EMT markers to investigate the underlying mechanism of CRC
progression using MAPK inhibitors following S100A16 knockdown. The
results indicated that S100A16 knockdown promoted CRC progression
partially via the JNK/P38 MAPK pathway and its subsequent EMT.
However, the detailed mechanism via which S100A16 affects the
JNK/P38 MAPK pathway was not fully assessed in the present study
and may involve additional factors, such as transcription target
genes or downstream effectors.
In conclusion, the present study investigated the
potential function of S100A16 in CRC, the results of which provided
insights into the tumour-suppressive role of S100A16 in CRC cells.
S100A16 may serve as a prognostic indicator of CRC, potentially
providing a reference for the early diagnosis of CRC, as well as a
novel therapeutic target. However, the current study had several
limitations. Additional experiments are therefore required to
reveal the detailed underlying mechanisms of S100A16 in CRC
regulation and should be the focus of future research.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81970451, 81500398
and 81470790), the Natural Science Foundation of Guangdong Province
(grant nos. 2019A1515010667, 2015A030310480 and 2015A030313295) and
the Guangdong Gastrointestinal Disease Research Center (grant no.
2017B02029003).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
SO conceived and designed the experiments, performed
the experiments, analysed the data, contributed
reagents/materials/analytical tools, wrote the manuscript, prepared
figures and tables and reviewed the manuscript. YL, JS, JT, YY, FW,
WW, JF and FX performed the experiments, analysed the data,
contributed reagents/materials/analysis tools and prepared figures
and tables. LB conceived and designed the experiments, as well as
drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent for
publication
All patients provided written informed consent and
the study protocol for the use of clinical samples was approved by
The Nanfang Hospital Institutional Review Board (approval no.
NFEC-2017-147). Approval for animal studies was additionally gained
from The Institutional Animal Care and Use Committee of Southern
Medical University (approval no. L2018160).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keane MG and Johnson GJ: Early diagnosis
improves survival in colorectal cancer. Practitioner. 256:15–18, 2.
2012.PubMed/NCBI
|
|
4
|
Sturchler E, Cox JA, Durussel I, Weibel M
and Heizmann CW: S100A16, a novel calcium-binding protein of the
EF-hand superfamily. J Biol Chem. 281:38905–38917. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donato R: S100: A multigenic family of
calcium-modulated proteins of the EF-hand type with intracellular
and extracellular functional roles. Int J Biochem Cell Biol.
33:637–668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sedaghat F and Notopoulos A: S100 protein
family and its application in clinical practice. Hippokratia.
12:198–204. 2008.PubMed/NCBI
|
|
7
|
Heizmann CW: The multifunctional S100
protein family. Methods Mol Biol. 172:69–80. 2002.PubMed/NCBI
|
|
8
|
Domínguez B, Pardo BG, Noia M, Millán A,
Gómez-Tato A, Martínez P, Leiro J and Lamas J: Microarray analysis
of the inflammatory and immune responses in head kidney turbot
leucocytes treated with resveratrol. Int Immunopharmacol.
15:588–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li D, Zhang R, Zhu W, Xue Y, Zhang Y,
Huang Q, Liu M and Liu Y: S100A16 inhibits osteogenesis but
stimulates adipogenesis. Mol Biol Rep. 40:3465–3473. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Babini E, Bertini I, Borsi V, Calderone V,
Hu X, Luchinat C and Parigi G: Structural characterization of human
S100A16, a low-affinity calcium binder. J Biol Inorg Chem.
16:243–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Zhu X, Li A, Yang S, Qiao R and
Zhang J: S100A16 regulated by Snail promotes the chemoresistance of
nonmuscle invasive bladder cancer through the AKT/Bcl-2 pathway.
Cancer Manag Res. 11:2449–2456. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen, Luo L and Liang C: Aberrant S100A16
expression might be an independent prognostic indicator of
unfavorable survival in non-small cell lung adenocarcinoma. PLoS
One. 13:e1974022018.
|
|
13
|
Zhou W, Pan H, Xia T, Xue J, Cheng L, Fan
P, Zhang Y, Zhu W, Xue Y, Liu X, et al: Up-regulation of S100A16
expression promotes epithelial-mesenchymal transition via Notch1
pathway in breast cancer. J Biomed Sci. 21:972014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sapkota D, Bruland O, Parajuli H, Osman
TA, Teh MT, Johannessen AC and Costea DE: S100A16 promotes
differentiation and contributes to a less aggressive tumor
phenotype in oral squamous cell carcinoma. BMC Cancer. 15:6312015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun X, Wang T, Zhang C, Ning K, Guan ZR,
Chen SX, Hong TT and Hua D: S100A16 is a prognostic marker for
colorectal cancer. J Surg Oncol. 117:275–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skrzypczak M, Goryca K, Rubel T, Paziewska
A, Mikula M, Jarosz D, Pachlewski J, Oledzki J and Ostrowski J:
Modeling oncogenic signaling in colon tumors by multidirectional
analyses of microarray data directed for maximization of analytical
reliability. PLoS One. 5:52010. View Article : Google Scholar
|
|
17
|
Shi J, Sun S, Liao Y, Tang J, Xu X, Qin B,
Qin C, Peng L, Luo M, Bai L, et al: Advanced oxidation protein
products induce G1 phase arrest in intestinal epithelial cells via
a RAGE/CD36-JNK-p27kip1 mediated pathway. Redox Biol.
25:1011962019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang J, Liao Y, He S, Shi J, Peng L, Xu X,
Xie F, Diao N, Huang J, Xie Q, et al: Autocrine parathyroid
hormone-like hormone promotes intrahepatic cholangiocarcinoma cell
proliferation via increased ERK/JNK-ATF2-cyclinD1 signaling. J
Transl Med. 15:2382017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu W, Xue Y, Liang C, Zhang R, Zhang Z,
Li H, Su D, Liang X, Zhang Y, Huang Q, et al: S100A16 promotes cell
proliferation and metastasis via AKT and ERK cell signaling
pathways in human prostate cancer. Tumour Biol. 37:12241–12250.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang MH, Zhao L, Wang L, Ou-Yang W, Hu SS,
Li WL, Ai ML, Wang YQ, Han Y, Li TT, et al: Nuclear lncRNA HOXD-AS1
suppresses colorectal carcinoma growth and metastasis via
inhibiting HOXD3-induced integrin β3 transcriptional activating and
MAPK/AKT signalling. Mol Cancer. 18:312019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schroyer AL, Stimes NW, Abi Saab WF and
Chadee DN: MLK3 phosphorylation by ERK1/2 is required for oxidative
stress-induced invasion of colorectal cancer cells. Oncogene.
37:1031–1040. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marenholz I and Heizmann CW: S100A16, a
ubiquitously expressed EF-hand protein which is up-regulated in
tumors. Biochem Biophys Res Commun. 313:237–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duan L, Wu R, Zou Z, Wang H, Ye L, Li H,
Yuan S, Li X, Zha H, Sun H, et al: S100A6 stimulates proliferation
and migration of colorectal carcinoma cells through activation of
the MAPK pathways. Int J Oncol. 44:781–790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Niu Y, Shao Z, Wang H, Yang J, Zhang F,
Luo Y, Xu L, Ding Y and Zhao L: LASP1-S100A11 axis promotes
colorectal cancer aggressiveness by modulating TGFβ/Smad signaling.
Sci Rep. 6:261122016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masuda T, Ishikawa T, Mogushi K, Okazaki
S, Ishiguro M, Iida S, Mizushima H, Tanaka H, Uetake H and Sugihara
K: Overexpression of the S100A2 protein as a prognostic marker for
patients with stage II and III colorectal cancer. Int J Oncol.
48:975–982. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu B, Sun WY, Zhi CY, Lu TC, Gao HM, Zhou
JH, Yan WQ and Gao HC: Role of S100A3 in human colorectal cancer
and the anticancer effect of cantharidinate. Exp Ther Med.
6:1499–1503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dahlmann M, Okhrimenko A, Marcinkowski P,
Osterland M, Herrmann P, Smith J, Heizmann CW, Schlag PM and Stein
U: RAGE mediates S100A4-induced cell motility via MAPK/ERK and
hypoxia signaling and is a prognostic biomarker for human
colorectal cancer metastasis. Oncotarget. 5:3220–3233. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zha H, Sun H, Li X, Duan L, Li A, Gu Y,
Zeng Z, Zhao J, Xie J, Yuan S, et al: S100A8 facilitates the
migration of colorectal cancer cells through regulating macrophages
in the inflammatory microenvironment. Oncol Rep. 36:279–290. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HY, Zhang JY, Cui JT, Tan XH, Li WM,
Gu J and Lu YY: Expression status of S100A14 and S100A4 correlates
with metastatic potential and clinical outcome in colorectal cancer
after surgery. Oncol Rep. 23:45–52. 2010.PubMed/NCBI
|
|
31
|
Sapkota D, Costea DE, Ibrahim SO,
Johannessen AC and Bruland O: S100A14 interacts with S100A16 and
regulates its expression in human cancer cells. PLoS One.
8:e760582013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kobayashi M, Nagashio R, Saito K,
Aguilar-Bonavides C, Ryuge S, Katono K, Igawa S, Tsuchiya B, Jiang
SX, Ichinoe M, et al: Prognostic significance of S100A16
subcellular localization in lung adenocarcinoma. Hum Pathol.
74:148–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zeindl-Eberhart E, Brandl L, Liebmann S,
Ormanns S, Scheel SK, Brabletz T, Kirchner T and Jung A:
Epithelial-mesenchymal transition induces
endoplasmic-reticulum-stress response in human colorectal tumor
cells. PLoS One. 9:e873862014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka M, Ichikawa-Tomikawa N, Shishito N,
Nishiura K, Miura T, Hozumi A, Chiba H, Yoshida S, Ohtake T and
Sugino T: Co-expression of S100A14 and S100A16 correlates with a
poor prognosis in human breast cancer and promotes cancer cell
invasion. BMC Cancer. 15:532015. View Article : Google Scholar : PubMed/NCBI
|