Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed malignancy and the second leading cause of cancer-related
mortality worldwide, resulting in an estimated 1.8 million new
cases and 881,000 deaths in 2018 (1). Despite advances in curative surgical
resection and chemotherapy, the 5-year survival rate is ~65%
(2). Moreover, the recurrence rates
of stage I–III and stage IV CRCs are 30 and 65%, respectively
(3), and most patients with
metastatic CRC cannot be treated (4). Therefore, a major challenge in the
treatment of CRC is identifying effective and low toxicity
chemotherapeutic agents.
Resveratrol (RSV) is a stilbenoid
(3,4′,5-trihydroxy-trans-stilbene) found in the skin of red grapes
and other fruits; thus, red wine is the primary source of RSV
(5). RSV has attracted considerable
attention in the last two decades due to its numerous proposed
health benefits, including anticancer (6–8),
chemopreventive and chemotherapeutic (9), cardio-neuro-protective (10) and antidiabetic (11) effects. RSV is a prospective
multitarget anticancer agent, which displays therapeutic potential
in all three stages of carcinogenesis (initiation, promotion and
progression) (12). RSV in
combination with 5-fuorouracil (5-FU) increased the inhibitory
effects of 5-FU on CRC cells (13).
Moreover, RSV in combination with oxaliplatin synergistically
suppressed CRC cell proliferation (14). RSV also displays low renal and
hepatic toxicity both in vivo and in vitro (15,16).
Oxidative stress serves an important role in
colorectal carcinogenesis (17).
Reactive oxygen species (ROS), a group of highly reactive ions and
molecules that are generated by mitochondria and participate in
redox signalling pathways, influence the regulation of cell
function, including proliferation (18). Excessive ROS generation or the
failure of oxidant-scavenging systems in cancer cells can destroy
the balance between Bcl-2 and Bax, and induce mitochondrial
oxidative damage, leading to the release of cytochrome c,
which is required for the activation of caspases (19). Therefore, stimulation of
mitochondrial ROS production may serve as a potential anticancer
strategy. However, the modulatory mechanisms underlying RSV in
human cancer cells are not completely understood, and relevant
results reported in certain scientific literatures are
controversial. For example, RSV suppresses reactive superoxide
species, including ROS, in the mitochondria (20), whereas various RSV-treated cancer
cells display elevated ROS generation, which induces extensive
apoptosis (21–23).
In view of the aforementioned data, the aim of the
present study was to investigate whether pharmacological
concentrations of RSV could induce the apoptosis of HCT116 and
SW620 human CRC cell lines and to explore the possible underlying
mechanisms.
Materials and methods
Cell culture
Human CRC cells HCT116 and SW620 were purchased from
American Type Culture Collection and maintained in the Department
of Pathology of Southern Medical University (Guangzhou, China).
Cells were cultured in RPMI-1640 (Biological Industries)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
in a humidified incubator containing 5% CO2 at 37°C. The
culture medium was changed every other day.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was assessed by performing the CCK-8
assay (Dojindo Molecular Technologies, Inc.). CRC cells were seeded
(1×104 cells) into 96-well plates with 100 µl medium per
well and cultured for 24 h. Cells were cultured with different
concentrations of RSV (0, 2, 4, 8, 16, 32, 64, 125, 250 or 500
µg/ml; Sino Biological, Inc.) at 37°C for 48 h. Different doses of
RSV were added. After 48 h, 10 µl CCK-8 buffer was added to each
well and incubated at 37°C for 2 h in the dark. Absorbance was
measured at a wavelength of 450 nm using a microplate autoreader
(Bio-Rad Laboratories, Inc.).
Cell apoptosis assay
To assess cell apoptosis, CRC cells were incubated
with RSV (0, 6 or 12 µg/ml) at 37°C for 48 h. The control group was
treated with 0.2% DMSO. Cells (1×105) were harvested and
washed with cold PBS. Cells were resuspended in 500 µl binding
buffer supplemented with 5 μl FITC-Annexin V and 5 µl PI (Annexin
V-FITC/PI Apoptosis Detection Kit; cat. no. KGA106; Nanjing KeyGen
Biotech Co., Ltd.) and incubated in the dark at room temperature
for 15 min. Flow cytometry was performed to measure apoptotic cells
using a FACSAria™ flow cytometer (BD Biosciences) and FlowJo
software (version 7.6; FlowJo LLC). Cell apoptosis was calculated
as a sum of early and late apoptotic cells.
Investigation of intracellular
ROS
Intracellular ROS levels were measured by performing
2′,7′-dichlorofluorescein-diacetate (DCFH-DA; Beyotime Institute of
Biotechnology) staining according to the manufacturer's
instructions. CRC cells at a density of 1×105/ml were
exposed to RSV (0, 6 or 12 µg/ml) at 37°C for 48 h, washed with PBS
and incubated with 10 µM DCFH-DA at 37°C for 30 min. Subsequently,
flow cytometry was performed to measure fluorescence intensity in
the FITC channel.
Western blotting
Total protein was isolated from cells using
radioimmunoprecipitation assay lysis buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology) on ice. Cell lysates were
sonicated and centrifuged at 10,000 × g for 15 min at 4°C. Total
protein was quantified by performing a bicinchoninic acid assay.
Proteins (40 µg/lane) were separated via 12% SDS-PAGE and
transferred to 0.45 µm PVDF membranes. After washing with PBS,
membranes were blocked with 5% milk at room temperature for 1 h.
Subsequently, the membranes were incubated overnight at 4°C with
primary antibodies targeted against: Bax (1:500; cat. no. ab182734;
Abcam), Bcl-2 (1:1,000; cat. no. 26593-1-AP; ProteinTech Group,
Inc.), cytochrome c (1:1,000; cat. no. BSM-52050R; BIOSS),
cleaved caspase-3 (1:1,000; cat. no. AF1150; Beyotime Institute of
Biotechnology), caspase-3 (1:1,000; cat. no. AF0081; Beyotime
Institute of Biotechnology), cleaved caspase-9 (1:1,000; cat. no.
PB0285; Boster Biological Technology), caspase-9 (1:1,000; cat. no.
bs-0049R; BIOSS) and β-actin (1:2,000; cat. no. 20536-1-AP;
ProteinTech Group, Inc.). Following primary incubation, the
membranes were incubated with a HRP conjugated secondary goat anti
rabbit antibody (1:100,000; cat. no. FD0128) or HRP conjugated goat
anti mouse antibody (1:100,000; cat. no. FD0142; both purchased
from Hangzhou Fude Biological Technology Co., Ltd.) for 1 h at room
temperature. Following washing with PBS with 0.1% Tween, protein
bands were visualized using enhanced chemiluminescence (cat. no.
36208ES60; Shanghai Yeasen Biotechnology Co., Ltd.) and
photographed using a Tanon-5200 image analyser (Tanon Science and
Technology Co., Ltd.). Protein expression levels were
semi-quantified with β-actin as the loading control.
Semi-quantitative analysis of protein signals were measured using
ImageJ software (version 1.52; National Institutes of Health).
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± standard deviation. Statistical
analyses were performed using SPSS software (version 26.0; IBM
Corp.). The unpaired Student's t-test was used to analyse
comparisons between two groups. Comparisons among multiple groups
were analysed using one-way ANOVA followed by Dunnett's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
RSV inhibits CRC cell viability
The CCK-8 assay was performed to assess the
potential therapeutic effect of RSV on CRC. HCT116 and SW620 cells
were treated with different concentrations of RSV (0, 2, 4, 8, 16,
32, 64, 125, 250 or 500 µg/ml) for 48 h (Fig. 1). Exposure to RSV significantly
reduced HCT116 and SW620 cell viability in a dose-dependent manner
compared with the 0 µg/ml RSV group. The dose-dependent decrease in
SW620 cell viability was more obvious in the lower concentration
range (≤32 µg/ml) compared with the higher concentration range
(>32 µg/ml). By contrast, the dose-dependent decrease in HCT116
cell viability was more obvious in the higher concentration range
(>32 µg/ml) compared with the lower concentration range (≤32
µg/ml). At 48 h, the 50% maximal inhibition concentration
(IC50) values of RSV for HCT116 and SW620 cells were
43.54 and 51.75 µg/ml, respectively. For subsequent experiments,
the 1/8 and 1/4 IC50 value doses (6 and 12 µg/ml,
respectively) were selected.
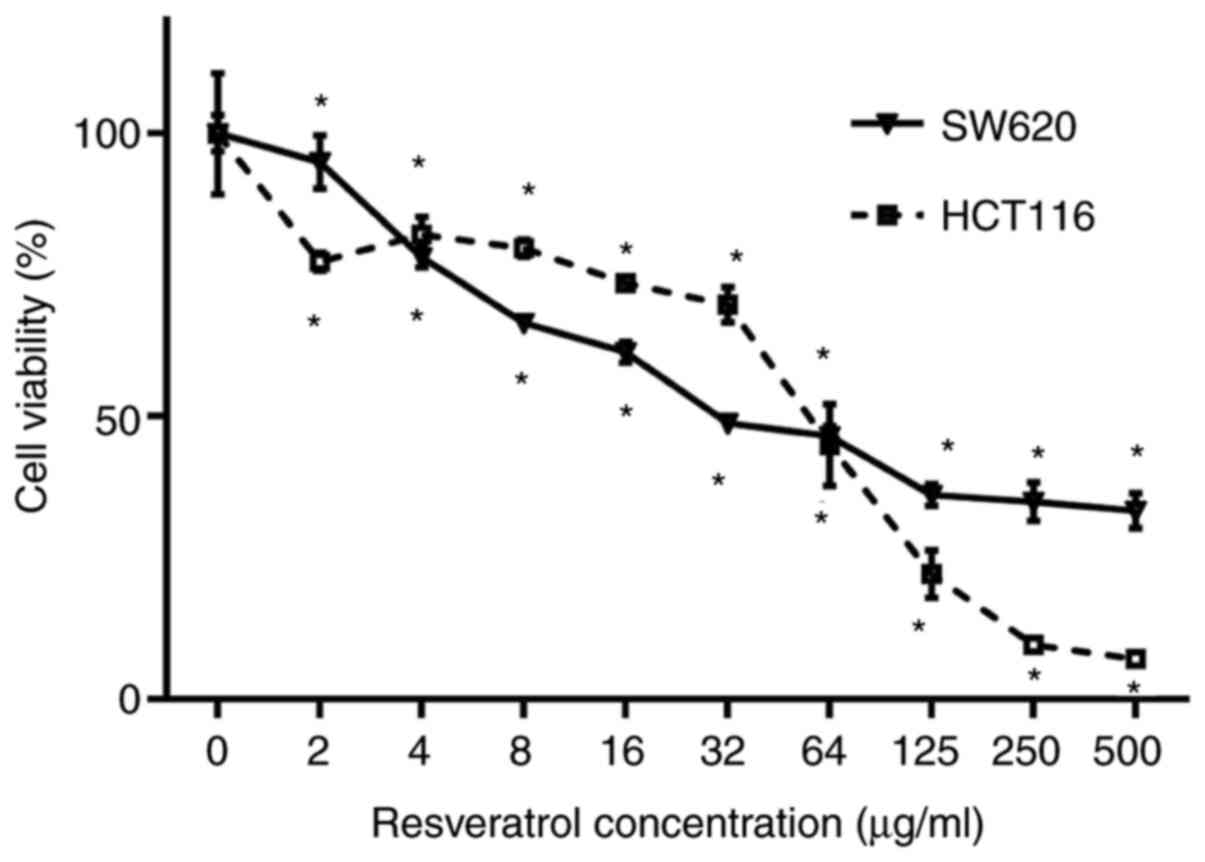 | Figure 1.RSV inhibits SW620 and HCT116 cell
viability. SW620 and HCT116 cells were treated with RSV (0, 2, 4,
8, 16, 32, 64, 125, 250 or 500 µg/ml) in 96-well plates for 48 h.
The IC50 of RSV for SW620 and HCT116 cells at 48 h were
51.75 and 43.54 µg/ml, respectively. *P<0.05 vs. 0 µg/ml RSV.
RSV, resveratrol. |
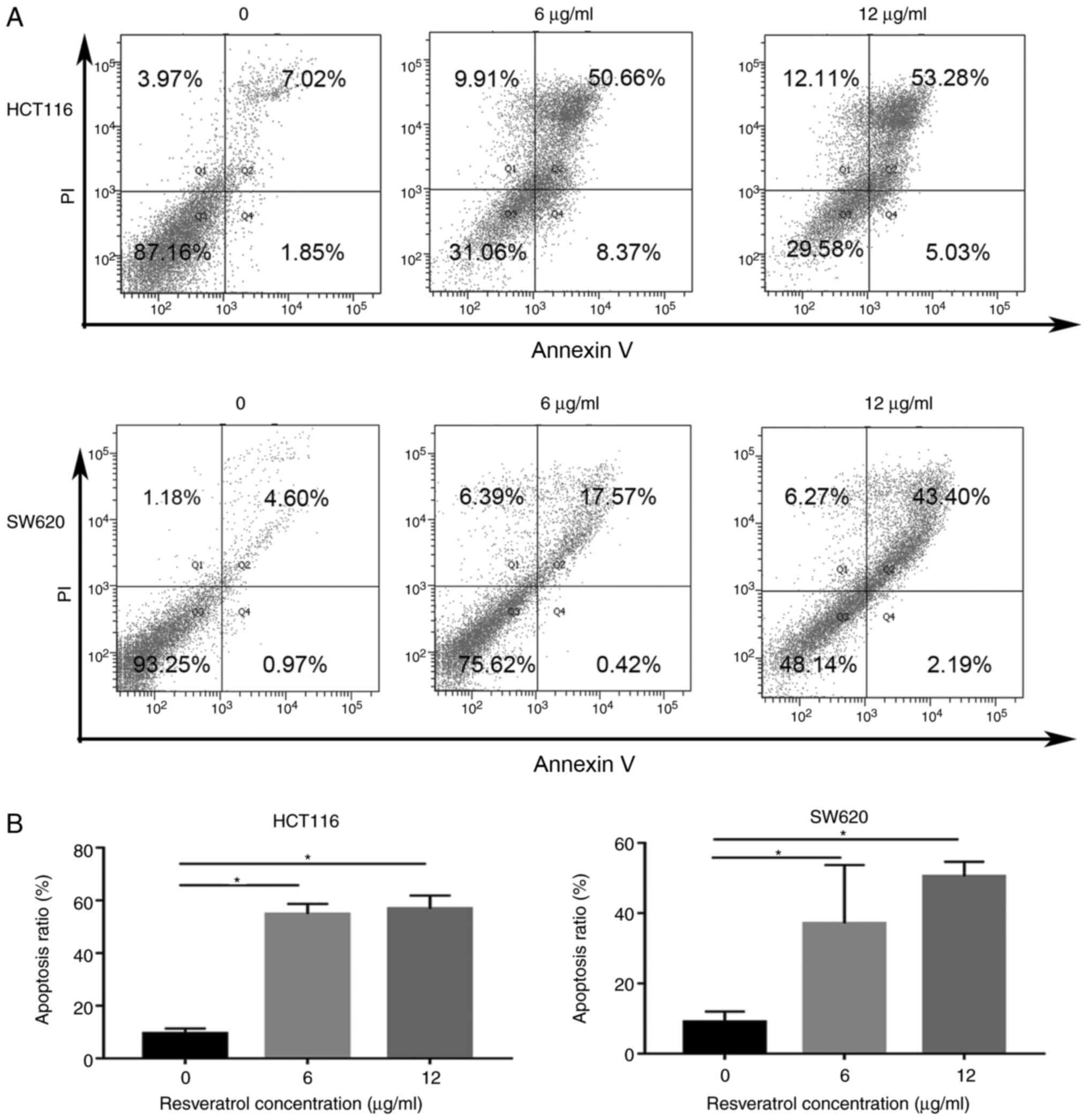
RSV induces extensive CRC cell
apoptosis
Annexin V/PI double staining and flow cytometry were
performed to evaluate apoptosis in RSV-treated CRC cells (Fig. 2). In HCT116 cells, apoptosis was
significantly increased in the 6 (54.82±3.866%) and 12 µg/ml
(56.84±5.087%) RSV groups compared with the control group
(9.42±1.935%). Similarly, the proportion of apoptotic SW620 cells
in the control group was 9.023±2.991%, which was significantly
increased to 37.13±16.59% and 50.46±4.225% in the 6 and 12 µg/ml
RSV groups.
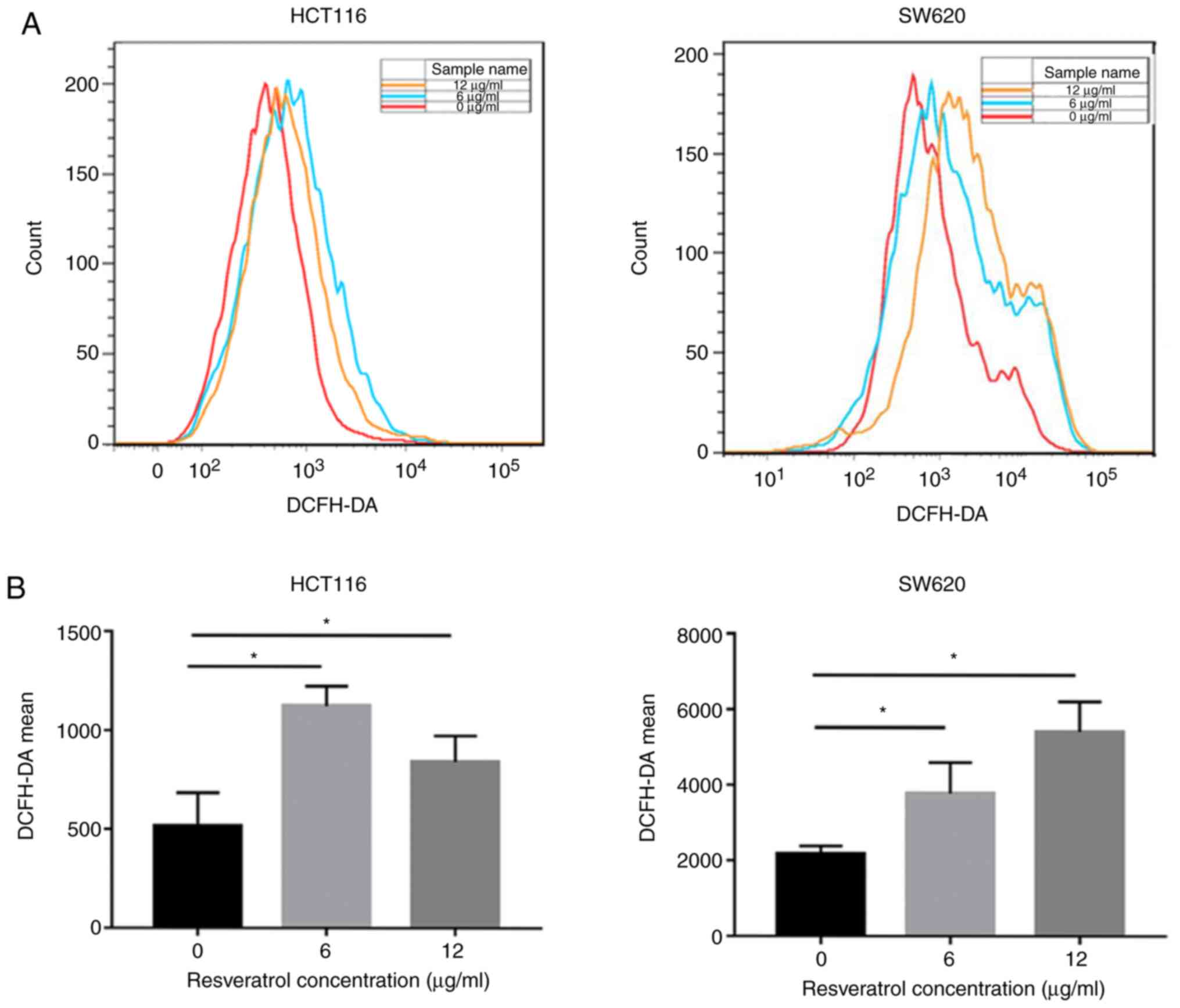
RSV enhances ROS generation in CRC
cells
Whether RSV enhanced ROS accumulation in CRC cells
was assessed via flow cytometry (Fig.
3). In HCT116 cells, ROS generation was significantly increased
in the 6 (1,124±99.05) and 12 µg/ml (841±132.1) RSV groups compared
with the control group (514.7±168.3). Similarly, in SW620 cells,
ROS generation was significantly increased in the 6 (3,767±828.5)
and 12 µg/ml (5,412±792.4) RSV groups compared with the control
group (2,173±208.2). The results suggested that RSV remarkably
enhanced ROS generation in CRC cells.
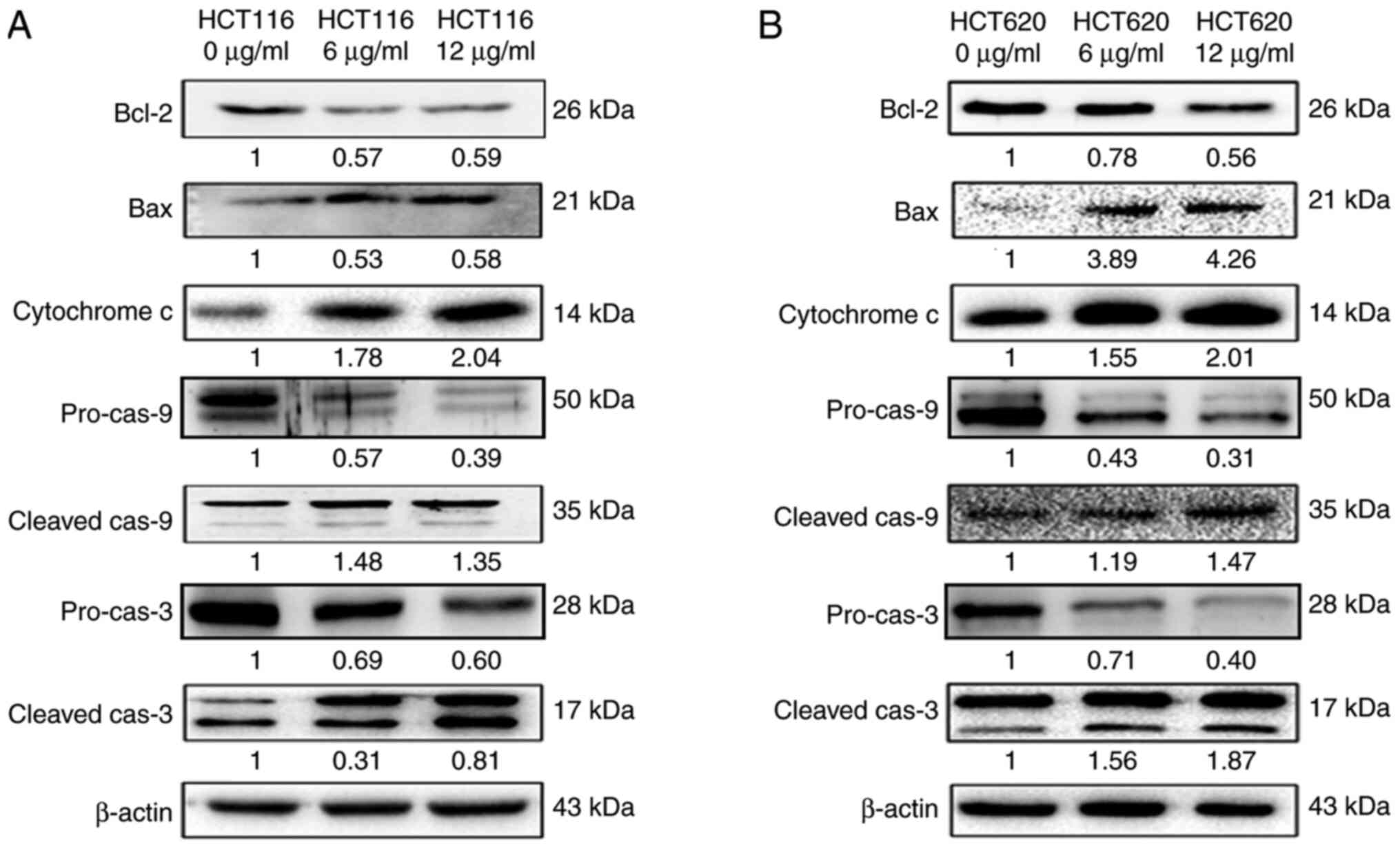
RSV triggers the mitochondrial pathway
in CRC cells
HCT116 and SW620 cells treated with 0, 6 and 12
µg/ml RSV for 48 h were subjected to western blotting to assess the
expression levels of proteins involved in the mitochondrial
apoptotic pathway (Fig. 4). The
protein expression levels of Bax and Bcl-2 in CRC cells were
analysed because Bcl-2 family proteins serve an essential
regulatory role in the mitochondrial pathway (24). The expression level of Bax was
upregulated, whereas the expression level of Bcl-2 was
downregulated in the 6 and 12 µg/ml RSV groups compared with the
control group. Moreover, the expression levels of cytochrome
c, cleaved caspase-9 and cleaved caspase-3 were increased in
the 6 and 12 µg/ml RSV groups compared with the control group. The
results indicated that the activation of the mitochondrial pathway
by oxidative stress is the underlying mechanism by which RSV kills
CRC cells, as illustrated in Fig.
5.
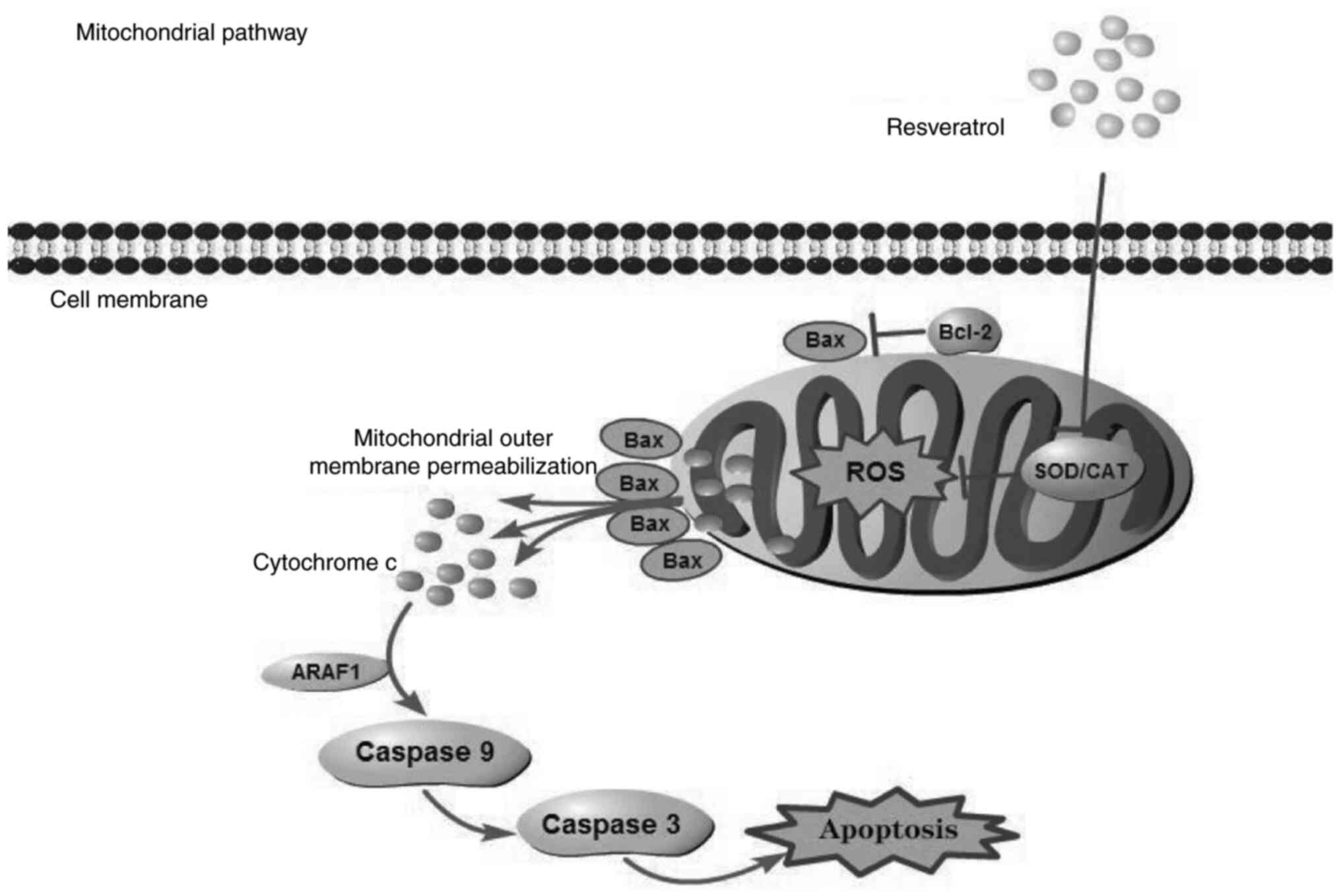 | Figure 5.Summary of RSV-induced cytotoxicity.
RSV induced intracellular ROS accumulation, which resulted in
mitochondrial outer membrane permeabilization. Subsequently,
cytochrome c was released to the cytoplasm and combined with
ARAF1, which activated caspase cascades, leading to cell apoptosis.
RSV, resveratrol; ROS, reactive oxygen species; ARAF1, A-Raf
proto-oncogene, serine/threonine kinase; SOD, superoxidedismutase;
CAT, catalase. |
Discussion
Phenolic compounds possess potential inhibitory
effects on cancer invasion and metastasis (25,26).
The anticancer effect of RSV has been associated with its
proliferation-inhibiting and apoptosis-inducing activities,
antioxidant properties and inhibition of stress induction in
multiple types of cancer, such as breast and prostate cancer
(27–29). However, the functions and mechanism
underlying RSV in CRC are not completely understood. In the present
study, the results indicated that the anticancer effects of RSV on
CRC cell lines were related to activation of the mitochondrial
apoptotic pathway via enhanced ROS generation.
Apoptosis is an important means of eliminating most
tumorigenic cells for tissue homeostasis (30). Further understanding apoptosis will
provide molecular basis for novel targeted therapies that can
induce cancer cell death or sensitise cancer cells to other
established chemotherapeutic agents (31). In the present study, the
Annexin-V/PI double staining results indicated that RSV
significantly increased apoptosis in CRC cell lines compared with
the control group. The dynamic balance between proliferation and
apoptosis is tightly regulated by specific signalling pathways
(32). The apoptotic pathways
include extrinsic (cytoplasmic) and intrinsic (mitochondrial)
pathways (31). The former is
triggered via the Fas death receptor whereas the latter is
triggered via cellular stress, including DNA damage, oxidative
stress and growth factor deprivation, resulting in mitochondrial
depolarisation and the release of cytochrome c from the
mitochondria to the cytosol (24).
Both pathways converge to a common pathway involved in the
activation of the caspase cascade. Proapoptotic caspases, including
caspase-3/8/9, serve an important role in mediating the apoptosis
signalling pathway (33). Caspase-3
can be activated by caspase-8 or caspase-9, which are important
markers of the extrinsic and intrinsic pathways, respectively
(34). One of the key regulators of
the intrinsic pathway is the Bcl-2 family of proteins, which
includes proapoptotic members, such as Bax, and antiapoptotic
members, such as Bcl-2 (35). The
Bcl-2 family members regulate apoptosis by controlling the release
of cytochrome c, and a direct interaction between Bcl-2 and
Bax has been observed in individual mitochondria (36). Cytochrome c released from the
mitochondria binds to apoptosis protease-activating factor 1 to
activate caspase-9 and caspase-3, which induce the terminal events
in cell apoptosis (37). The
western blotting results of the present study indicated that RSV
increased the expression levels of cytochrome c, Bax,
cleaved caspase-9 and cleaved caspase-3, but decreased the
expression of Bcl-2 compared with the control group. The results
indicated that RSV induced CRC cell apoptosis by activating the
intrinsic apoptotic pathway.
Elevated ROS generation is related to RSV-induced
apoptosis in numerous cancer cells (38,39).
ROS overproduction is the early step involved in the mitochondrial
apoptotic pathway (40). In the
present study, RSV increased ROS generation in HCT116 and SW620
cells compared with the control group; thus, indicating that
apoptosis in RSV-treated cells may be increased by oxidation. ROS
accumulation in cancer cells can induce genomic damage, such as
double-strand breaks and chromosome damage, accompanied by
apoptosis (41). Moreover,
excessive ROS leads to mitochondrial swelling, the permeabilization
of outer mitochondrial membrane and the release of cytochrome
c. Therefore, a pro-oxidant action serves an important role
in the anticancer effect of RSV.
The present study had several limitations. First,
only the anticancer effect of RSV on CRC cells was investigated in
the present study, but the toxicity of RSV on normal cell lines
remains unclear. Secondly, the results indicated that RSV
dose-dependently inhibited CRC cell viability by performing the
CCK-8 assay. However, RSV did not display dose-dependent effects on
cell apoptosis and intracellular ROS among the three groups (0, 6
and 12 µg/ml).
In conclusion, the present study suggested that
RSV-induced apoptosis in CRC cells involved activation of the
ROS-mediated mitochondrial pathway. Therefore, RSV may serve as a
potential therapeutic agent for patients with CRC.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81772918 and 81972277), the
Guangdong Provincial Natural Science Foundation of China (grant no.
2017A030313896) and the Guangzhou Science and Technology Project
(grant no. 201804010319).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ conceived and designed the study, and wrote the
manuscript. YF, YY, GZ, YX, JS, HW, FF, ZW, SJ and YL performed the
experiments. YF analysed the data and revised the manuscript. YY
helped with the writing of the manuscript. All authors discussed
the results, and read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
RSV
|
resveratrol
|
|
CCK
|
Cell Counting Kit
|
|
ROS
|
reactive oxygen species
|
|
5-FU
|
5-fuorouracil
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Stok EP, Spaander MCW, Grünhagen
DJ, Verhoef C and Kuipers EJ: Surveillance after curative treatment
for colorectal cancer. Nat Rev Clin Oncol. 14:297–315. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loree JM and Kopetz S: Recent developments
in the treatment of metastatic colorectal cancer. Ther Adv Med
Oncol. 9:551–564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huminiecki L and Horbańczuk J: The
functional genomic studies of resveratrol in respect to its
anti-cancer effects. Biotechnol Adv. 36:1699–1708. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panaro MA, Carofiglio V, Acquafredda A,
Cavallo P and Cianciulli A: Anti-inflammatory effects of
resveratrol occur via inhibition of lipopolysaccharide-induced
NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br J
Nutr. 108:1623–1632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garvin S, Ollinger K and Dabrosin C:
Resveratrol induces apoptosis and inhibits angiogenesis in human
breast cancer xenografts in vivo. Cancer Lett. 231:113–122. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu J, Liu D, Niu H, Zhu G, Xu Y, Ye D, Li
J and Zhang Q: Resveratrol reverses doxorubicin resistance by
inhibiting epithelial-mesenchymal transition (EMT) through
modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin
Cancer Res. 36:192017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
et al: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sung MM, Hamza SM and Dyck JR: Myocardial
metabolism in diabetic cardiomyopathy: Potential therapeutic
targets. Antioxid Redox Signal. 22:1606–1630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Athar M, Back JH, Tang X, Kim KH,
Kopelovich L, Bickers DR and Kim AL: Resveratrol: A review of
preclinical studies for human cancer prevention. Toxicol Appl
Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buhrmann C, Yazdi M, Popper B, Shayan P,
Goel A, Aggarwal BB and Shakibaei M: Resveratrol Chemosensitizes
TNF-β-Induced Survival of 5-FU-Treated Colorectal Cancer Cells.
Nutrients. 10:102018. View Article : Google Scholar
|
|
14
|
Kaminski BM, Weigert A, Scherzberg MC, Ley
S, Gilbert B, Brecht K, Brüne B, Steinhilber D, Stein J and
Ulrich-Rückert S: Resveratrol-induced potentiation of the antitumor
effects of oxaliplatin is accompanied by an altered cytokine
profile of human monocyte-derived macrophages. Apoptosis.
19:1136–1147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crowell JA, Korytko PJ, Morrissey RL,
Booth TD and Levine BS: Resveratrol-associated renal toxicity.
Toxicol Sci. 82:614–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Farghali H, Kgalalelo Kemelo M, Wojnarová
L and Kutinová Canová N: In vitro and in vivo experimental
hepatotoxic models in liver research: Applications to the
assessment of potential hepatoprotective drugs. Physiol Res. 65
(Suppl 4):S417–S425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perše M: Oxidative stress in the
pathogenesis of colorectal cancer: Cause or consequence? BioMed Res
Int. 2013:7257102013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sabharwal SS and Schumacker PT:
Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles'
heel? Nat Rev Cancer. 14:709–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
NavaneethaKrishnan S, Rosales JL and Lee
KY: Loss of Cdk5 in breast cancer cells promotes ROS-mediated cell
death through dysregulation of the mitochondrial permeability
transition pore. Oncogene. 37:1788–1804. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar S, Stokes J III, Singh UP,
Scissum-Gunn K, Singh R, Manne U and Mishra MK: Prolonged exposure
of resveratrol induces reactive superoxide species-independent
apoptosis in murine prostate cells. Tumour Biol.
39:10104283177150392017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng X, Jia B, Tian XT, Song X, Wu ML,
Kong QY, Li H and Liu J: Correlation of reactive oxygen species
levels with resveratrol sensitivities of anaplastic thyroid cancer
cells. Oxid Med Cell Longev. 2018:62354172018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santandreu FM, Valle A, Oliver J and Roca
P: Resveratrol potentiates the cytotoxic oxidative stress induced
by chemotherapy in human colon cancer cells. Cell Physiol Biochem.
28:219–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng L, Yan B, Chen K, Jiang Z, Zhou C,
Cao J, Qian W, Li J, Sun L, Ma J, et al: Resveratrol-induced
downregulation of NAF-1 enhances the sensitivity of pancreatic
cancer cells to gemcitabine via the ROS/Nrf2 signaling pathways.
Oxid Med Cell Longev. 2018:94820182018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ashkenazi A, Fairbrother WJ, Leverson JD
and Souers AJ: From basic apoptosis discoveries to advanced
selective BCL-2 family inhibitors. Nat Rev Drug Discov. 16:273–284.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weng CJ and Yen GC: Chemopreventive
effects of dietary phytochemicals against cancer invasion and
metastasis: Phenolic acids, monophenol, polyphenol, and their
derivatives. Cancer Treat Rev. 38:76–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sliva D: Suppression of cancer
invasiveness by dietary compounds. Mini Rev Med Chem. 8:677–688.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan X, Zhao Y, Cheng T, Zheng A, Ge A,
Zang L, Xu K and Tang B: Monitoring NAD(P)H by an ultrasensitive
fluorescent probe to reveal reductive stress induced by natural
antioxidants in HepG2 cells under hypoxia. Chem Sci (Camb).
10:8179–8186. 2019. View Article : Google Scholar
|
|
28
|
Wu H, Chen L, Zhu F, Han X, Sun L and Chen
K: The cytotoxicity effect of resveratrol: cell cycle arrest and
induced apoptosis of breast cancer 4T1 cells. Toxins (Basel).
11:112019. View Article : Google Scholar
|
|
29
|
Khusbu FY, Zhou X, Roy M, Chen FZ, Cao Q
and Chen HC: Resveratrol induces depletion of TRAF6 and suppresses
prostate cancer cell proliferation and migration. Int J Biochem
Cell Biol. 118:1056442020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ashkenazi A: Directing cancer cells to
self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug
Discov. 7:1001–1012. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schellenberg B, Wang P, Keeble JA,
Rodriguez-Enriquez R, Walker S, Owens TW, Foster F, Tanianis-Hughes
J, Brennan K, Streuli CH, et al: Bax exists in a dynamic
equilibrium between the cytosol and mitochondria to control
apoptotic priming. Mol Cell. 49:959–971. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the Nomenclature Committee on Cell
Death 2012. Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reed JC: Bcl-2 and the regulation of
programmed cell death. J Cell Biol. 124:1–6. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mahajan NP, Linder K, Berry G, Gordon GW,
Heim R and Herman B: Bcl-2 and Bax interactions in mitochondria
probed with green fluorescent protein and fluorescence resonance
energy transfer. Nat Biotechnol. 16:547–552. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hussain AR, Uddin S, Bu R, Khan OS, Ahmed
SO, Ahmed M and Al-Kuraya KS: Resveratrol suppresses constitutive
activation of AKT via generation of ROS and induces apoptosis in
diffuse large B cell lymphoma cell lines. PLoS One. 6:e247032011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Z, Li W, Meng X and Jia B:
Resveratrol induces gastric cancer cell apoptosis via reactive
oxygen species, but independent of sirtuin1. Clin Exp Pharmacol
Physiol. 39:227–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu S, Sun Z, Chu P, Li H, Ahsan A, Zhou
Z, Zhang Z, Sun B, Wu J, Xi Y, et al: EGCG protects against
homocysteine-induced human umbilical vein endothelial cells
apoptosis by modulating mitochondrial-dependent apoptotic signaling
and PI3K/Akt/eNOS signaling pathways. Apoptosis. 22:672–680. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu L, Trimarchi JR, Smith PJ and Keefe
DL: Mitochondrial dysfunction leads to telomere attrition and
genomic instability. Aging Cell. 1:40–46. 2002. View Article : Google Scholar : PubMed/NCBI
|