Introduction
Acute lung injury (ALI) is a condition in which
progressive hypoxemia and respiratory distress are caused by
non-cardiogenic factors, including hyperoxia and infection
(1). Exposure to hyperoxia can
induce ALI, which is a key risk factor for the occurrence and
development of bronchopulmonary dysplasia (BPD). Hyperoxia-induced
lung injury may result in atelectasis, poor lung compliance and
susceptibility to infection as a consequence of surfactant
deficiency, mucociliary dysfunction and histological damage
(2). Experimental models have
demonstrated that hyperoxia can disrupt alveolar and microvascular
development, and thereby cause alveolar simplification (3). Similarly, exposure to hyperoxia at
birth is known to increase the risk of BPD (4). Markers of oxidative stress have been
shown to be associated with the development of lung disease
(5). Between the years 2007–2011
and 2012–2015, the incidence of BPD among preterm infants in 11
high-income countries exhibited a significant increase, from 23.3
to 27.5% (6).
The oxidative stress triggered by reactive oxygen
species (ROS) contributes to ALI by causing pulmonary parenchymal
damage (4). Nuclear
factor-erythroid 2-related factor 2 (Nrf2) is a member of the cap
‘n’ collar family of transcription factors, and the Nrf2-kelch-like
ECH-associated protein 1 (Keap1)/antioxidant response element (ARE)
signaling pathway has been shown to regulate antioxidant proteases,
scavenge ROS, maintain intracellular redox homeostasis, and
regulate apoptosis and anti-inflammatory responses (7). NAD(P)H quinone oxidoreductase 1 (NQO1)
is a phase II stress response protein that regulates the production
of ROS and is able to alleviate oxidative stress injury induced by
the exposure of respiratory epithelial cells to hyperoxia (8). However, the relationships among Nrf2,
NQO1 and hyperoxia-induced lung injury remain unclear.
Reparative responses to lung epithelial lesions in
infants with BPD are dependent on type II alveolar epithelial cells
(AECIIs) (9); however, AECIIs tend
to degenerate in primary culture. The A549 cell line is derived
from human alveolar basal epithelium adenocarcinoma, is suitable
for gene transfection, and has characteristics similar to those of
AECIIs; therefore, A549 cells are often used in the study of
pulmonary antioxidation mechanisms (10). Since previous studies have used A549
cells to investigate the pathogenesis of BPD in premature infants
(11,12), A549 cells exposed to hyperoxia were
used in the present study as a model to investigate the molecular
processes that contribute toward BPD in premature infants.
In the present study, the expression of Nrf2, Keap1
and NQO1 in A549 cells was investigated under exposure to hyperoxia
and with small interfering RNA (siRNA) transfection, and their
associations with cellular apoptosis were elucidated. Thus, the aim
of the study was to provide insights into the pathogenesis of BPD
in premature infants.
Materials and methods
Cell line
A549 cells were obtained from The Chinese Academy of
Sciences (Shanghai, China) and cultured at the Cell Laboratory
Center, Shanghai Ssmdata Medical Information Technology Company
(Shanghai, China).
Reagents
Three pairs of 21-base siRNAs were designed by
Suzhou GenePharma Co., Ltd. based on the human Nrf2 gene
sequence in GenBank (National Institutes of Health) using standard
design principles (Table I).
Lipofectamine® 2000, TRIzol®, fetal bovine
serum (FBS) and Dulbecco's modified Eagle's medium (DMEM) were
purchased from Thermo Fisher Scientific, Inc., and the PrimeScript
RT reagent kit was obtained from Takara Bio, Inc.
 | Table I.Primer and siRNA sequences. |
Table I.
Primer and siRNA sequences.
| siRNA or gene | Primer | Sequence
(5′-3′) |
|---|
| siRNA-1 | Sense |
AUACUUCUCGACUUACUCCAA |
|
| Antisense |
GGAGUAAGUCGAGAAGUAUUU |
| siRNA-2 | Sense |
AAACGUAGCCGAAGAAACCUC |
|
| Antisense |
GGUUUCUUCGGCUACGUUUCA |
| siRNA-3 | Sense |
AAUAUUAAGACACUGUAACUC |
|
| Antisense |
GUUACAGUGUCUUAAUAUUGA |
| NC | Sense |
UUCUCCGAACGUGUCACGUTT |
|
| Antisense |
ACGUGACACGUUCGGAGAATT |
| Nrf2 | Sense |
ATGGATTTGATTGACATACTTT |
|
| Antisense |
ACTGAGCCTGATTAGTAGCAAT |
| Keap1 | Sense |
TGCGCTGCGAGTCCGAGGTCTTC |
|
| Antisense |
TCGAAGATCTTGACCAGGTAGT |
| NQO1 | Sense |
ACATATAGCATTGGGCACACTC |
|
| Antisense |
TCATTAAGAATCCTGCCTGGAAGT |
| GAPDH | Sense |
CATCACTGCCACCCAGAAGACTG |
|
| Antisense |
ATGCCAGTGAGCTTCCCGTTCAG |
Cell culture and grouping of A549
cells
A549 cells (5×104/well) were inoculated
into a 24-well culture plate with 10% FBS, DMEM, supplemented with
100 U/ml penicillin at 37°C the day before transfection, and grown
to 40–70% confluence within 24 h. The siRNA (100 nM) was mixed
gently with 50 µl serum-free DMEM, and 1 µl Lipofectamine 2000 was
mixed with 50 µl DMEM at 25°C for 5 min. The siRNA and
Lipofectamine reagents were then mixed and added to the culture
plate containing the cells at 37°C for 12 h. The cells were divided
into the following four groups: Normoxic without transfection
(group I), hyperoxia-exposed without transfection (group II),
normoxic with transfection (group III) and hyperoxia-exposed with
transfection (group IV). After transfection for 24 h, the
hyperoxia-exposed groups (II and IV) were exposed to 95%
O2 and 5% CO2 for 24 h while the normoxic
groups (I and IV) were incubated with 5% CO2 in air for
24 h.
Nrf2 siRNA screening
Cells were in inoculated into a 6-well culture plate
at a density of 4×105 cells/well and transfected with
one of three siRNAs (100 nM) targeting Nrf2 expression (siRNA-1,
siRNA-2 or siRNA-3) or a negative control siRNA (100 nM) using
Lipofectamine 2000. The cells were cultured in an incubator at 37°C
with 5% CO2 for 12 h and collected to determine the
transfection efficiency. The siRNA with the highest efficiency for
the repression of Nrf2 expression (siRNA-1) was used for subsequent
experiments.
Immunofluorescence and confocal laser
scanning microscopy
The A549 cells transfected with siRNA-1 were fixed
with 4% paraformaldehyde at −20°C for 20 min and then treated with
0.3% Triton-X100 for membrane permeabilization. Subsequently, 3%
bovine serum albumin (Thermo Fisher Scientific, Inc.) was added,
and the cells were blocked in an incubator at 37°C for 1 h. Then,
the cells were washed with phosphate-buffered saline (PBS) and
incubated overnight at 4°C with Nrf2 (cat. no. ab137550; 1:1,000
Abcam) according to the manufacturer's instructions. After
incubation for 1 h at 37°C, the cells were washed in PBS and then
incubated in the dark with diluted goat-anti-rabbit IgG (cat. no.
ab150077; 1:10,000; Abcam) secondary antibody at 37°C for 1 h. The
cells were then mounted on slides and the nuclei were stained with
4′,6-diamidino-2-phenylindole at 25°C for 5 min. All samples were
analyzed using confocal laser scanning microscopy.
Reverse transcription-qPCR
(RT-qPCR)
A549 cells from each group were collected and total
RNA was extracted from them using TRIzol. The total RNA was reverse
transcribed into cDNA using a PrimeScript RT reagent kit (Takara
Bio, Inc.) at 42°C for 15 min and 85°C for 5 sec. The cDNA was then
subjected to fluorogenic qPCR. Differences in gene expression
between groups were compared using a relative quantitation method
with GAPDH as the internal reference gene. The samples were
pre-amplified at 95°C for 15 min, followed by 40 cycles of qPCR at
95°C for 20 sec and 60°C for 45 sec. Relative target gene mRNA
expression was calculated using the 2−ΔΔCt method
(13). The sequences of the primers
used are shown in Table I.
Western blotting
A549 cells from each group were lysed and total
protein was extracted using radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology). The bicinchoninic acid
method was used for total protein quantification. The proteins (30
µg) were separated using SDS-PAGE on 7.5% gels (Beyotime Institute
of Biotechnology), transferred to polyvinylidene fluoride membranes
and blocked with PBS containing 5% (w/v) skimmed milk powder for 2
h at 25°C. The membranes were then incubated with Nrf2 (cat no.
ab137550; Abcam), Keap1 (cat. no. ab139729; Abcam), NQO1 (cat no.
ab2346; Abcam) and GAPDH (cat. no. ab9485; Abcam) primary
antibodies diluted 1:1,000 overnight at 4°C followed by horseradish
peroxidase-conjugated goat-anti-rabbit (cat no. ab6721; Abcam)
secondary antibodies diluted 1:5,000 for 1 h at 25°C. Next, the
membranes were washed with 150 mM NaCl and 50 mM Tris-Cl at 25°C
three times. Finally, the bands were visualized using Pierce
enhanced chemiluminescence western blotting substrate (Thermo
Fisher Scientific, Inc.) was added, and the blots were scanned
using a Bio-Rad Gel Doc XR+ gel documentation system (Bio-Rad
Laboratories, Inc.). Bio-Rad Image Lab Software (version 5.1;
Bio-Rad Laboratories, Inc.) was used for densitometric
analysis.
Flow cytometry
Cell apoptosis in groups I, II and IV was detected
using flow cytometry. Briefly, A549 cells (3×105
cells/well) from each group were inoculated into 6-well culture
plates and cultured at 37°C for 48 h, then collected for the
detection of apoptosis. After removal of the culture medium, the
cells were digested with trypsin, centrifuged at 1,000 × g for 5
min at 25°C, then resuspended in PBS. The cells (1×105)
were centrifuged again at 1,000 × g for 5 min at 25°C. After
resuspension, the cells were incubated in the dark with 5 µl
Annexin V-FITC at 4°C for 15 min and then with 5 µl propidium
iodide staining solution in the dark at 4°C for 5 min. Unstained
cells were used as the negative control. The flow cytometry data
were acquired using an Attune NxT flow cytometer (Thermo Fisher
Scientific, Inc.) and analyzed using FlowJo (version 10; FlowJo
LLC).
Statistical analysis
All statistical analyses were conducted using SPSS
20.0 software (IBM Corp.) and the results are presented as the mean
± standard deviation. Results were analyzed by one-way ANOVA.
Pairwise comparisons using Bonferroni correction were performed if
one-way ANOVA indicated a significant difference. P<0.05 was
considered to indicate a statistically significant difference.
Results
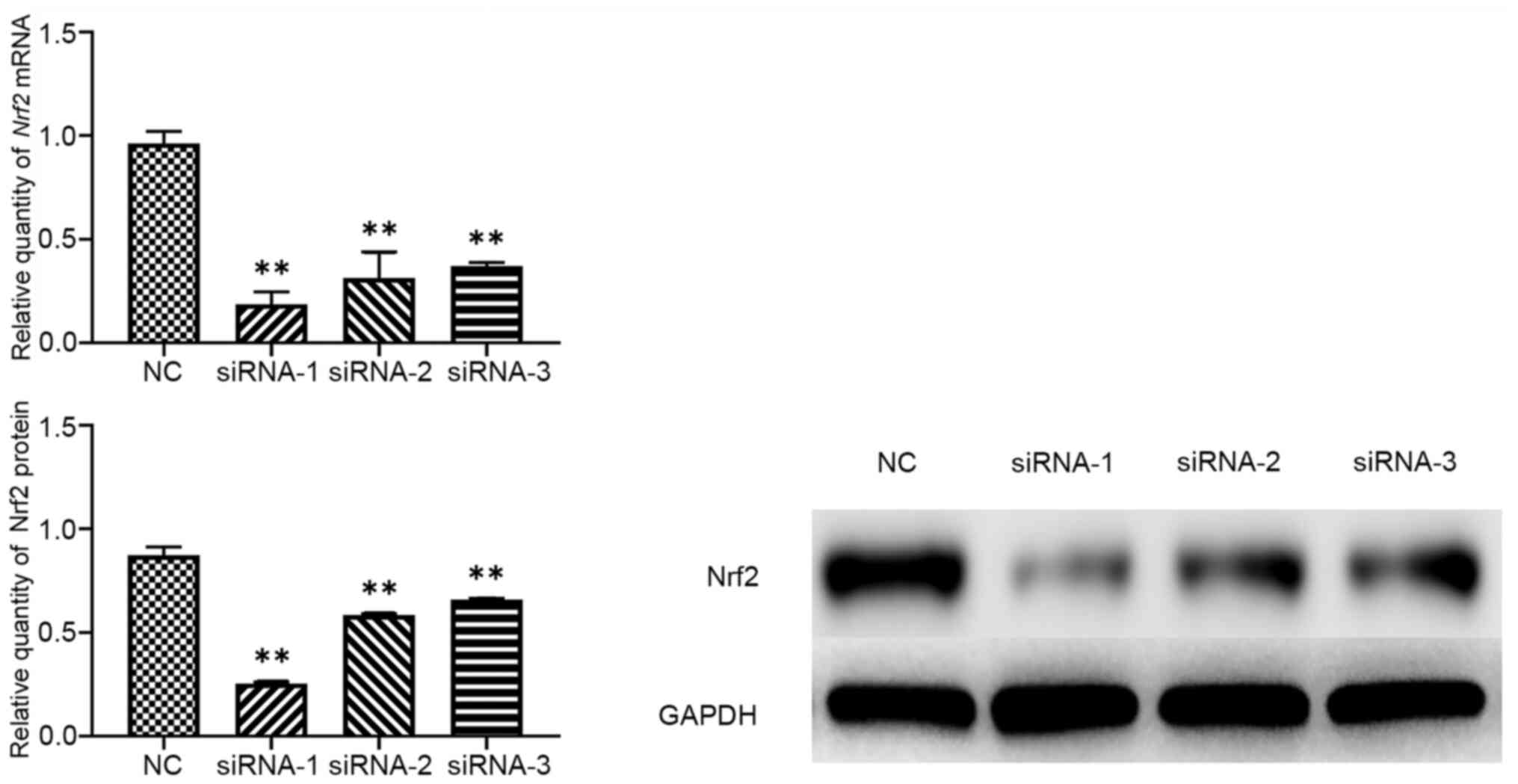
Nrf2 siRNA efficiency
The extent by which Nrf2 was downregulated following
transfection with three different siRNAs was investigated using
RT-qPCR and western blotting. Nrf2 expression was significantly
downregulated by Nrf2 siRNA-1, −2 and −3, with siRNA-1 displaying
the highest inhibition efficiency (80.57% for Nrf2 siRNA;
Table II, Fig. 1). Therefore, siRNA-1 was used in
subsequent experiments.
 | Table II.Nrf2 mRNA suppression
following siRNA transfection. |
Table II.
Nrf2 mRNA suppression
following siRNA transfection.
| siRNA | Nrf2
expression |
|---|
| siRNA-1 | 0.1871±0.0592 |
| siRNA-2 | 0.3135±0.1262 |
| siRNA-3 | 0.3703±0.0182 |
| NC | 0.9634±0.0574 |
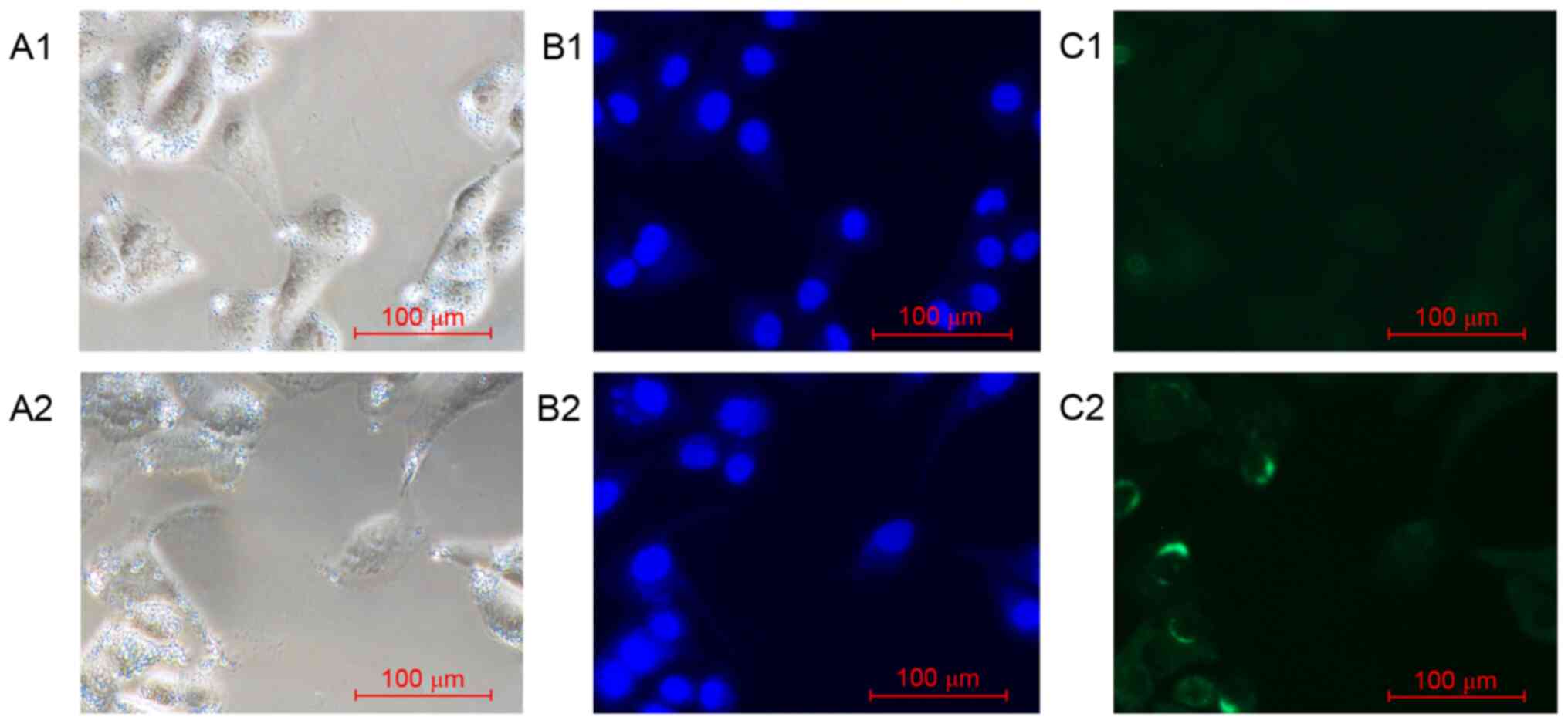
Nrf2 protein expression and
distribution in A549 cells
The expression and distribution of Nrf2 in A549
cells incubated under two different conditions were examined using
immunofluorescence. Nrf2 was preferentially distributed throughout
the cytoplasm of A549 cells under normoxic conditions (group I).
However, the expression of Nrf2 protein was downregulated following
transfection with Nrf2 siRNA (group III; Fig. 2).
Hyperoxia upregulates Nrf2, Keap1 and
NQO1 in A549 cells
To determine the effects of hyperoxia on Nrf2, Keap1
and NQO1, their expression levels were measured in A549 cells
incubated under hyperoxic and normoxic conditions. Relative
Nrf2, Keap1 and NQO1 mRNA expression levels in the
cells exposed to hyperoxia without siRNA transfection (group II;
4.553±0.498, 3.299±0.483 and 5.866±0.582, respectively) were
significantly higher compared with those in untransfected cells
under normoxic conditions (group I; F=65.310–209.249, P<0.01;
Fig. 3). Similarly, relative Nrf2,
Keap1 and NQO1 protein expression levels were significantly higher
in cells exposed to hyperoxia without transfection (group II;
1.118±0.143, 1.217±0.070 and 1.064±0.053, respectively) compared
with those in untransfected cells under normoxic conditions (group
I; F=49.103–96.875, P<0.01; Fig.
4). Therefore, it appears that hyperoxia upregulates Nrf2,
Keap1 and NQO1 expression.
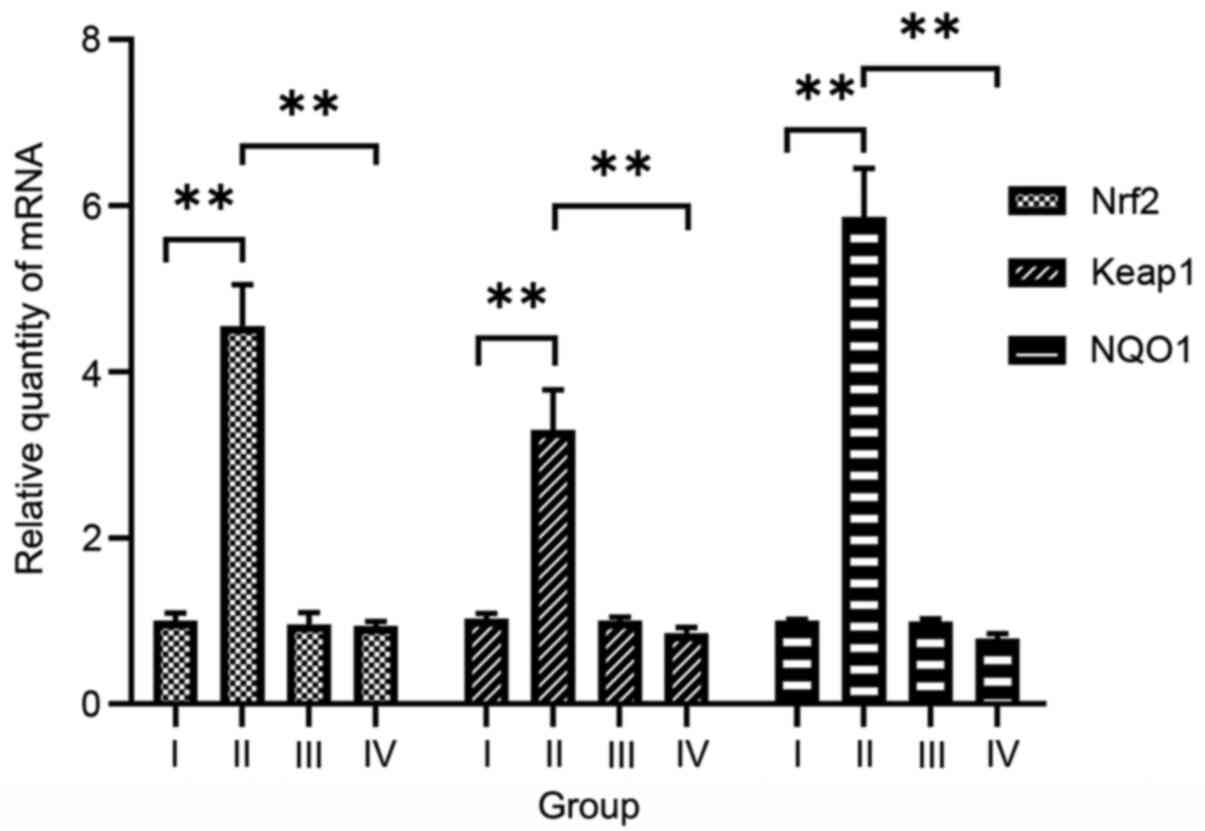 | Figure 3.Effects of hyperoxia and Nrf2 siRNA
on Nrf2, Keap1 and NQO1 mRNA expression in A549
cells. Reverse transcription-quantitative polymerase chain reaction
demonstrated that Nrf2, Keap1 and NQO1 mRNA
expression levels were significantly higher in the hyperoxia
without Nrf2 siRNA group than in the normoxia without Nrf2 siRNA
group, but significantly lower in the hyperoxia after Nrf2 siRNA
transfection group than in the hyperoxia without Nrf2 siRNA group.
Data are presented as the mean ± SD. **P< 0.01. Group I,
normoxia without siRNA; group II, hyperoxia without siRNA; group
III, normoxia after Nrf2 siRNA transfection; group IV, hyperoxia
after Nrf2 siRNA transfection; Nrf2, nuclear factor-erythroid
2-related factor 2; siRNA, small interfering RNA; Keap1, kelch-like
ECH-associated protein 1; NQO1, NAD(P)H quinone oxidoreductase 1
enzyme. |
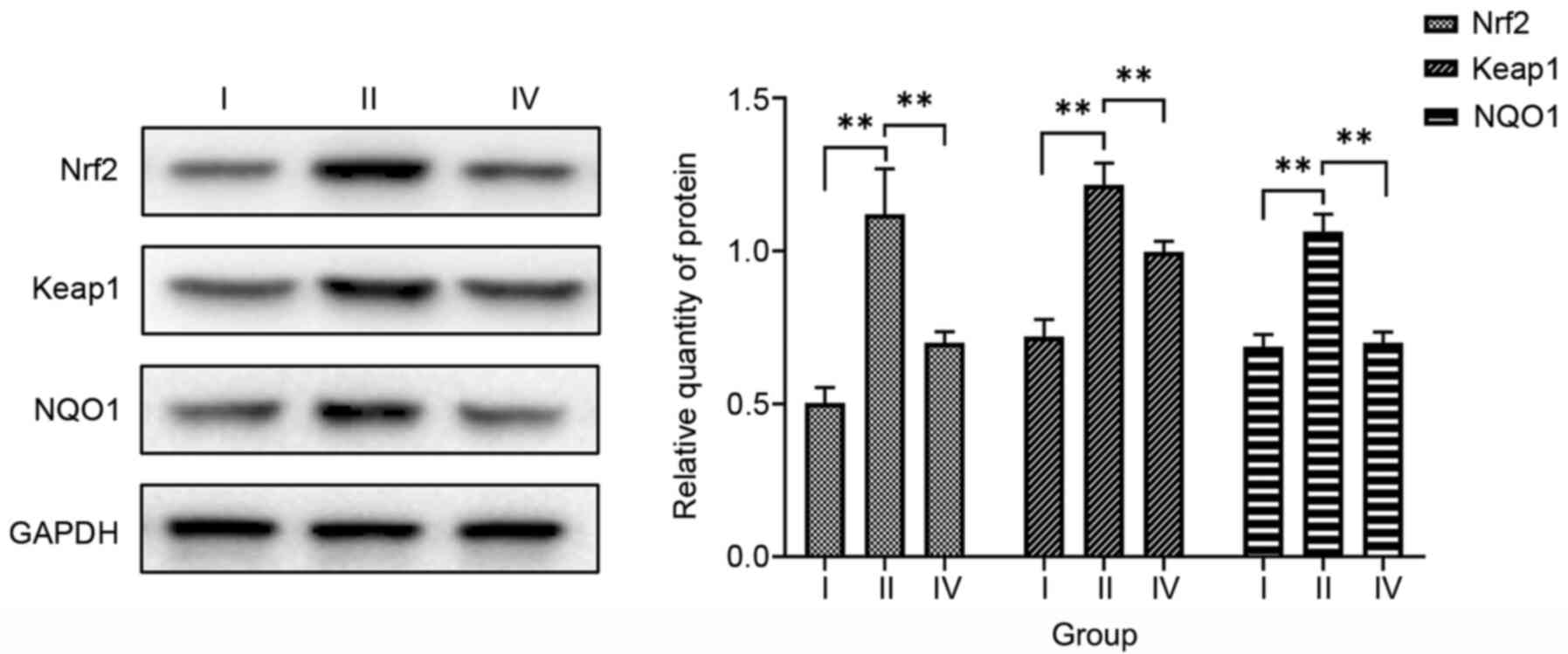 | Figure 4.Effects of hyperoxia and Nrf2 siRNA
on Nrf2, Keap1 and NQO1 protein expression. Western blotting
demonstrated that Nrf2, Keap1 and NQO1 protein expression levels
were significantly higher in the hyperoxia without Nrf2 siRNA group
than in the normoxia without Nrf2 siRNA group, but were
significantly lower in the hyperoxia after Nrf2 siRNA transfection
group than in the hyperoxia without Nrf2 siRNA group. Data are
presented as the mean ± SD. **P<0.01. Group I, normoxia without
siRNA; group II, hyperoxia without siRNA; group IV, hyperoxia after
Nrf2 siRNA transfection; Nrf2, nuclear factor-erythroid 2-related
factor 2; siRNA, small interfering RNA; Keap1, kelch-like
ECH-associated protein 1; NQO1, NAD(P)H quinone oxidoreductase 1
enzyme. |
Nrf2 siRNA downregulates Nrf2, Keap1
and NQO1 in A549 cells
The effects of Nrf2 siRNA on Nrf2, Keap1 and NQO1
expression were examined in A549 cells exposed to hyperoxia.
Relative Nrf2, Keap1 and NQO1 mRNA expression levels
in cells exposed to hyperoxia after transfection (group IV;
0.937±0.057, 0.854±0.067 and 0.789±0.058, respectively) were
significantly lower compared with those in untransfected cells
exposed to hyperoxia (group II; F=75.337–226.208, P<0.01;
Fig. 3). Similarly, relative
Nrf2, Keap1 and NQO1 protein expression levels were
significantly lower in hyperoxia-exposed cells after Nrf2 siRNA
transfection (group IV; 0.703±0.036, 0.996±0.036 and 0.701±0.037,
respectively) compared with those in untransfected cells exposed to
hyperoxia (group II; F=23.600–93.816, P<0.01; Fig. 4). These results indicate that Nrf2
siRNA downregulates Nrf2, Keap1 and NQO1 in cells exposed to
hyperoxia.
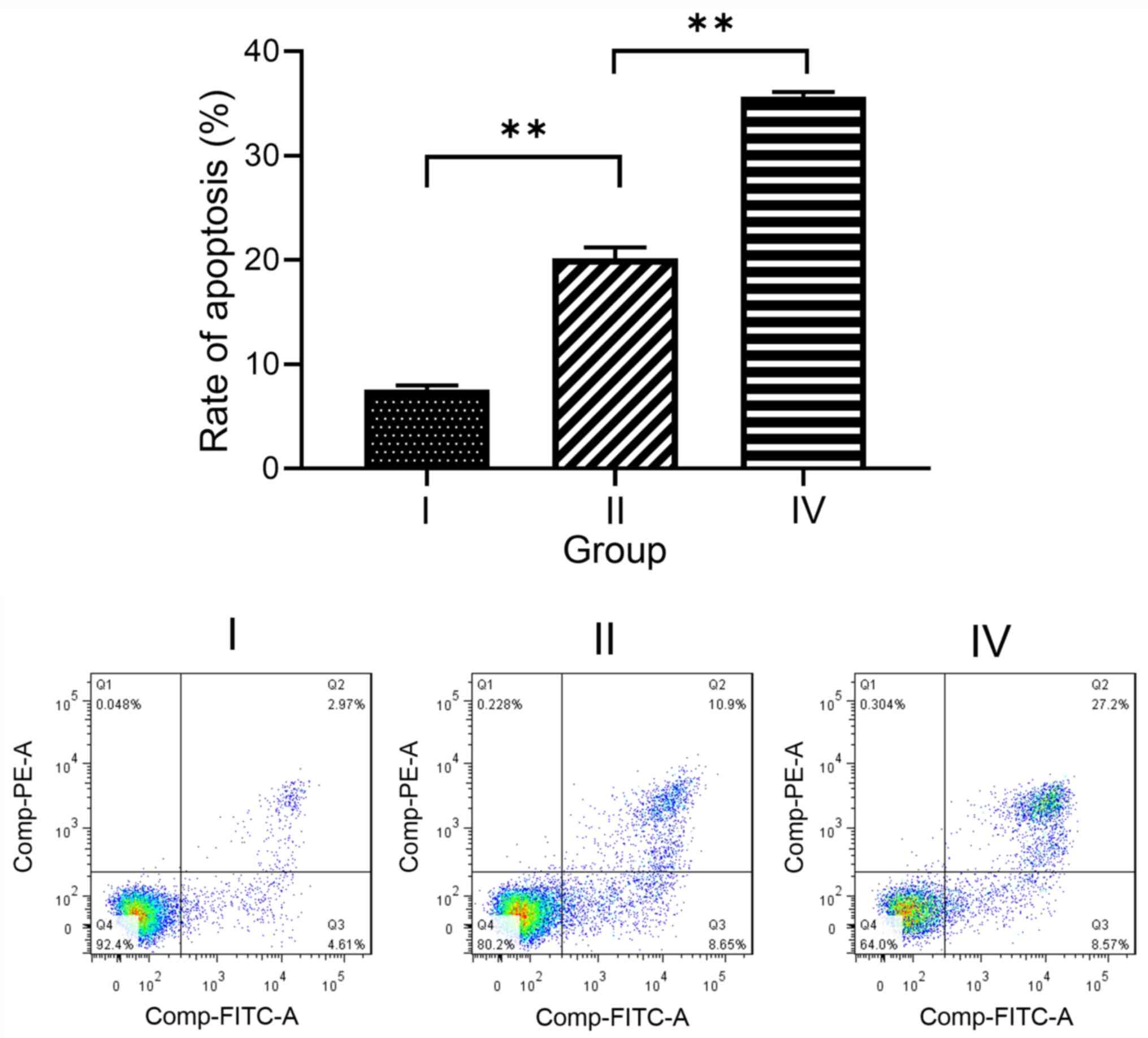
Effects of hyperoxia and Nrf2 siRNA on
cell apoptosis
Finally, the effects of hyperoxia and Nrf2 siRNA on
apoptosis in A549 cells were investigated. In untransfected cells,
apoptosis following exposure to hyperoxia (group II; 20.15±1.08%)
was significantly higher compared with that without hyperoxia
exposure (group I; 7.59±0.39%; F=357.466, P<0.01). Furthermore,
the rate of apoptosis was significantly higher in cells exposed to
hyperoxia after transfection with Nrf2 siRNA (group IV;
35.64±0.49%) than in untransfected cells exposed to hyperoxia
(group II; F=510.221, P<0.01; Fig.
5). Together, these findings indicate that Nrf2, Keap1 and NQO1
may protect against hyperoxia-induced lung injury via the
inhibition of apoptosis.
Discussion
Improvements in perinatal care have increased the
survival rates of premature infants and thus have also increased
the incidence of BPD (14).
Multiple factors serve roles in the etiology of BPD, including
hyperoxia, postnatal infection and ventilator-induced lung injury.
However, the degree of prematurity and exposure to hyperoxia are
the most important predisposing factors for BPD in neonates
(15,16). The specific pathogenesis of ALI
following hyperoxia exposure has not yet been fully defined. In the
present study, transfection with Nrf2 siRNA was used to advance our
understanding of the pathogenesis of ALI and help to improve the
prevention and management of BPD.
The results of the present study demonstrated that
relative Nrf2, Keap1 and NQO1 expression levels were significantly
higher in untransfected cells exposed to hyperoxia than in
untransfected cells under normoxic conditions, as was the rate of
apoptosis. These findings indicate that the oxidative stress caused
by hyperoxia leads to cell injury and apoptosis, and suggest that
the Nrf2-Keap1-ARE-NQO1 signaling pathway may play an essential
role in hyperoxia-induced ALI in addition to serving as a key
endogenous antioxidant defense mechanism. Hyperoxia is known to
result in oxidative stress and cell apoptosis, and to serve an
important role in the development of BPD in premature infants
(11). Therefore, the upregulation
of genes in antioxidative signaling pathways, such as the
Nrf2-Keap1-ARE-NQO1 pathway, may reduce hyperoxia-induced lung
injury in preterm infants.
siRNAs are double-stranded RNAs, 20–25 base pairs in
length, that provide RNA interference (17), and can be used to explore the
mechanism of BPD (18). In the
present study, siRNA was used to interfere with Nrf2 expression,
and it was found that Nrf2 siRNA significantly decreased Keap1 and
NQO1 expression under hyperoxic conditions, and increased the
susceptibility of A549 cells to hyperoxia-induced damage, as
demonstrated by the aggravation of apoptosis. These findings
suggest that Nrf2 exerts regulatory effects on Keap1 and NQO1,
which may be involved in cellular apoptosis during the occurrence
of hyperoxia-induced ALI.
Nrf2 contains a highly conserved basic region
leucine zipper and induces the transcription of numerous
cytoprotective genes via signal transduction (19). In the present study, hyperoxia
significantly increased Nrf2 expression and the rate of apoptosis,
whereas Nrf2 siRNA significantly decreased Nrf2 expression and
downregulated the expression of its downstream mediators Keap1 and
NQO1. Furthermore, when the A549 cells were transfected with Nrf2
siRNA, they were more susceptible to hyperoxia-induced damage and
exhibited an increased rate of apoptosis. These findings indicate
that Nrf2 plays a key role in oxidative stress reactions and
suggest that the self-protective mechanisms of lung cells exposed
to hyperoxia are associated with Nrf2-Keap1-ARE-NQO1 signaling.
Nrf2 is a key genetic determinant of ALI
pathogenesis (20), with
Nrf2-knockout mice displaying increased susceptibility to
hyperoxia-induced damage and exacerbated ALI compared with
wild-type mice (21,22). Previous studies have shown that Nrf2
and its downstream effectors are significantly upregulated in the
lung tissues of premature mice exposed to hyperoxia, and confer
protection against BPD (23,24).
Moreover, hyperoxia induces a BPD-like phenotype for which
mortality rates, arrested lung development, apoptosis,
inflammation, and structural protein and membrane lipid oxidation
are more severe in Nrf2−/− neonatal mice compared with
Nrf2+/+ neonatal mice (25). In addition to its ability to provide
enhanced antioxidative effects, Nrf2 also displays strong
anti-inflammatory activity (26,27).
Therefore, Nrf2 appears to be a promising focus for the prevention
and treatment of BPD owing to its ability to alleviate oxidative
stress reactions through multiple mechanisms.
Keap1 is a cysteine-rich protein that acts as a
redox damage sensor, whereas ARE is a cis-acting enhancer in
a Nrf2 target gene cluster (28).
The ARE consensus sequence has been identified in the promoter
region of numerous genes that encode phase II detoxification
enzymes (29). In the present
study, it was found that Nrf2 was primarily localized in the
cytoplasm of untransfected normoxic A549 cells. Moreover, relative
Keap1 expression was significantly increased under hyperoxic
conditions, and decreased following transfection with Nrf2 siRNA.
Under physiological conditions, Keap1 traps and ubiquitinates Nrf2
in the cytoplasm, leading to its rapid degradation by the
ubiquitin-proteasome system (30,31).
In addition, broad complex-tramtrack-bric-a-brac and cap ‘n’ collar
homology1 forms a heterodimer with small musculo-aponeurotic
fibrosarcoma (sMAF) protein and prevents Nrf2 from binding to the
ARE (32). However, hyperoxia
modifies the reactive cysteine residues of Keap1, preventing it
from targeting Nrf2 for ubiquitination and degradation.
Consequently, Nrf2 is translocated into the nucleus and forms a
heterodimer with sMAF (33), which
recognizes and binds to the ARE, and activates a series of
antioxidant enzymes such as NQO1 (34). Therefore, it appears that Keap1 and
ARE may serve critical roles in redox homeostasis during
hyperoxia-induced lung injury.
NQO1 is an antioxidant enzyme activated by
cytoprotective Nrf2-Keap1-ARE target gene products (35). This enzyme catalyzes the
two-electron reduction of quinone compounds to generate less
reactive hydroquinones. In the present study, NQO1 expression was
significantly upregulated in untransfected cells exposed to
hyperoxia. This upregulation was accompanied by apoptosis,
indicating the involvement of NQO1 in hyperoxia-induced ALI.
Moreover, transfection with Nrf2 siRNA significantly decreased the
expression of NQO1 under hyperoxic conditions and further increased
the rate of apoptosis, suggesting that downregulation of the
Nrf2-Keap1-ARE-NQO1 pathway exacerbates hyperoxia-induced ALI. In
addition, the hyperoxia-induced cellular apoptosis exhibited a
negative association with Nrf2 and NQO1 expression, suggesting that
high Nrf2 and NQO1 expression in the lung tissue may strengthen its
antioxidant defenses and reduce the injury induced by oxidative
stress and apoptosis.
A previous study demonstrated the significant
upregulation of NQO1 expression in transgenic mice carrying the
human CYP1A1-Luc promoter upon exposure to hyperoxia, and suggested
that these mice are less susceptible than wild-type mice to
hyperoxia-induced ALI and alveolar simplification (36). In another study, miR-494 was shown
to negatively regulate NQO1 and block the Nrf2 signaling pathway,
resulting in the acceleration of ALI in rats with sepsis-associated
acute respiratory distress syndrome (37). Furthermore, in cells exposed to
hyperoxia, oxidative stress has been shown to increase the
expression of NQO1 and regulate ROS generation, thereby preventing
cells and tissues from undergoing hyperoxia-induced lung injury
(38). The findings of the present
study indicate that hyperoxia-induced activation of the
Nrf2-Keap1-ARE-NQO1 signaling pathway is Nrf2-dependent and
protects against hyperoxia-induced lung injury via the inhibition
of apoptosis. However, further studies are required to fully
elucidate the relationship between the Nrf2-Keap1-ARE-NQO1
signaling pathway and the duration of hyperoxia exposure, as well
as the threshold of its protective effect. In addition, the effect
of upregulation of the Nrf2-Keap1-ARE-NQO1 pathway on apoptosis
should be explored to confirm the conclusions of the present
study.
In summary, the present study demonstrated that the
Nrf2-Keap1-ARE-NQO1 signaling pathway protects against the
hyperoxia-induced injury of lung cells by inhibiting apoptosis. The
protective effects of the Nrf2-Keap1-ARE-NQO1 signaling observed in
the in vitro model provide insights into the pathogenesis of
hyperoxia-induced ALI and indicate that drugs that induce Nrf2 and
NQO1 expression may be promising agents for the prevention and
treatment of BPD.
Acknowledgements
The authors would like to thank the Cell Laboratory
Centre, Shanghai Ssmdata Medical Information Technology Company for
assistance with the experiments.
Funding
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81571467).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BWW, CC and XHG designed the study. BWW and CC
managed the experiments, analyzed the data and drafted the
manuscript. CC, XHG, XYZ and XYC interpreted the results and
revised the manuscript. The authors agree to be accountable for the
version published. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BPD
|
bronchopulmonary dysplasia
|
|
ALI
|
acute lung injury
|
|
Nrf2
|
nuclear factor-erythroid 2-related
factor 2
|
|
Keap1
|
kelch-like ECH-associated protein
1
|
|
ARE
|
antioxidant response element
|
|
NQO1
|
NAD(P)H quinone oxidoreductase 1
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
ROS
|
reactive oxygen species
|
|
PBS
|
phosphate-buffered saline
|
|
AECII
|
alveolar epithelial cell type II
|
|
siRNA
|
small interfering RNA
|
|
sMAF
|
small musculo-aponeurotic
fibrosarcoma
|
References
|
1
|
Xie JL, Lin MB and Hou Q: [Recent advances
in the study of Nrf2 and inflammatory respiratory diseases. Yao Xue
Xue Bao. 50:1080–1087. 2015.(In Chinese). PubMed/NCBI
|
|
2
|
Damiani E, Donati A and Girardis M: Oxygen
in the critically ill: Friend or foe? Curr Opin Anaesthesiol.
31:129–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Chu X, Weng B, Gong X and Cai C:
An innovative model of bronchopulmonary dysplasia in premature
infants. Front Pediatr. 8:2712020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalikkot Thekkeveedu R, Guaman MC and
Shivanna B: Bronchopulmonary dysplasia: A review of pathogenesis
and pathophysiology. Respir Med. 132:170–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wai KC, Kohn MA, Ballard RA, Truog WE,
Black DM, Asselin JM, Ballard PL, Rogers EE and Keller RL; Trial of
Late Surfactant (TOLSURF) Study Group, : Early cumulative
supplemental oxygen predicts bronchopulmonary dysplasia in high
risk extremely low gestational age newborns. J Pediatr.
177:97–102.e2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lui K, Lee SK, Kusuda S, Adams M, Vento M,
Reichman B, Darlow BA, Lehtonen L, Modi N, Norman M, et al
International Network for Evaluation of Outcomes (iNeo) of neonates
Investigators, : Trends in outcomes for neonates born very preterm
and very low birth weight in 11 high-income countries. J Pediatr.
215:32–40.e14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abreu CC, Cardozo LF and Mafra D: Could
physical exercises modulate Nrf2-Keap1 pathway in chronic kidney
disease? Med Hypotheses. 84:44–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Courcot E, Leclerc J, Lafitte JJ, Mensier
E, Jaillard S, Gosset P, Shirali P, Pottier N, Broly F and
Lo-Guidice JM: Xenobiotic metabolism and disposition in human lung
cell models: Comparison with in vivo expression profiles. Drug
Metab Dispos. 40:1953–1965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yee M, Domm W, Gelein R, Bentley KL,
Kottmann RM, Sime PJ, Lawrence BP and O'Reilly MA: Alternative
progenitor lineages regenerate the adult lung depleted of alveolar
epithelial type 2 cells. Am J Respir Cell Mol Biol. 56:453–464.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forred BJ, Daugaard DR, Titus BK, Wood RR,
Floen MJ, Booze ML and Vitiello PF: Detoxification of mitochondrial
oxidants and apoptotic signaling are facilitated by thioredoxin-2
and peroxiredoxin-3 during hyperoxic injury. PLoS One.
12:e01687772017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai C, Qiu J, Qiu G, Chen Y, Song Z, Li J
and Gong X: Long non-coding RNA MALAT1 protects preterm infants
with bronchopulmonary dysplasia by inhibiting cell apoptosis. BMC
Pulm Med. 17:1992017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kunzmann S, Ottensmeier B, Speer CP and
Fehrholz M: Effect of progesterone on Smad signaling and
TGF-β/Smad-regulated genes in lung epithelial cells. PLoS One.
13:e02006612018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stoll BJ, Hansen NI, Bell EF, Walsh MC,
Carlo WA, Shankaran S, Laptook AR, Sánchez PJ, Van Meurs KP,
Wyckoff M, et al: Eunice kennedy shriver national institute of
child health and human development neonatal research network:
trends in care practices, morbidity, and mortality of extremely
preterm neonates, 1993–2012. JAMA. 314:1039–1051. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai YX, Fang F, Jiang JL and Xu F:
Extrinsic calcitonin gene-related peptide inhibits
hyperoxia-induced alveolar epithelial type II cells apoptosis,
oxidative stress, and reactive oxygen species (ROS) production by
enhancing Notch 1 and homocysteine-induced endoplasmic reticulum
protein (HERP) expression. Med Sci Monit. 23:5774–5782. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lal CV and Ambalavanan N: Genetic
predisposition to bronchopulmonary dysplasia. Semin Perinatol.
39:584–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bernstein E, Caudy AA, Hammond SM and
Hannon GJ: Role for a bidentate ribonuclease in the initiation step
of RNA interference. Nature. 409:363–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu H, Gao C, Tang W and Zhang T: Effect of
glucose regulated protein 78 gene silencing on hyperoxia-induced
apoptosis in alveolar epithelial cells. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 30:1247–1250. 2014.(In Chinese). PubMed/NCBI
|
|
19
|
Yamamoto M, Kensler TW and Motohashi H:
The KEAP1-NRF2 System: A thiol-based sensor-effector apparatus for
maintaining redox homeostasis. Physiol Rev. 98:1169–1203. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho HY, Jedlicka AE, Gladwell W, Marzec J,
McCaw ZR, Bienstock RJ and Kleeberger SR: Association of Nrf2
polymorphism haplotypes with acute lung injury phenotypes in inbred
strains of mice. Antioxid Redox Signal. 22:325–338. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KH, Kwun MJ, Han CW, Ha KT, Choi JY
and Joo M: Suppression of lung inflammation in an LPS-induced acute
lung injury model by the fruit hull of Gleditsia sinensis. BMC
Complement Altern Med. 14:4022014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho HY, Miller-DeGraff L,
Blankenship-Paris T, Wang X, Bell DA, Lih F, Deterding L, Panduri
V, Morgan DL, Yamamoto M, et al: Sulforaphane enriched
transcriptome of lung mitochondrial energy metabolism and provided
pulmonary injury protection via Nrf2 in mice. Toxicol Appl
Pharmacol. 364:29–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Wall SB, Ren C, Velten M, Hill CL,
Locy ML, Rogers LK and Tipple TE: Thioredoxin reductase inhibition
attenuates neonatal hyperoxic lung injury and enhances nuclear
factor E2-related factor 2 activation. Am J Respir Cell Mol Biol.
55:419–428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Chu X, Gong X, Zhou H and Cai C:
The expression of miR-125b in Nrf2-silenced A549 cells exposed to
hyperoxia and its relationship with apoptosis. J Cell Mol Med.
24:965–972. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho HY and Kleeberger SR: Association of
Nrf2 with airway pathogenesis: Lessons learned from genetic mouse
models. Arch Toxicol. 89:1931–1957. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sussan TE, Gajghate S, Chatterjee S,
Mandke P, McCormick S, Sudini K, Kumar S, Breysse PN, Diette GB,
Sidhaye VK, et al: Nrf2 reduces allergic asthma in mice through
enhanced airway epithelial cytoprotective function. Am J Physiol
Lung Cell Mol Physiol. 309:L27–L36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobayashi EH, Suzuki T, Funayama R,
Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi
H, Nakayama K, et al: Nrf2 suppresses macrophage inflammatory
response by blocking proinflammatory cytokine transcription. Nat
Commun. 7:116242016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raghunath A, Sundarraj K, Nagarajan R,
Arfuso F, Bian J, Kumar AP, Sethi G and Perumal E: Antioxidant
response elements: Discovery, classes, regulation and potential
applications. Redox Biol. 17:297–314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiu L, Wang M, Zhu Y, Xiang Y and Zhang Y:
A naturally-occurring dominant-negative inhibitor of Keap1
competitively against its negative regulation of Nrf2. Int J Mol
Sci. 19:192018. View Article : Google Scholar
|
|
30
|
Iso T, Suzuki T, Baird L and Yamamoto M:
Absolute amounts and status of the Nrf2-Keap1-Cul3 complex within
cells. Mol Cell Biol. 36:3100–3112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sekine H, Okazaki K, Ota N, Shima H, Katoh
Y, Suzuki N, Igarashi K, Ito M, Motohashi H and Yamamoto M: The
mediator subunit MED16 transduces NRF2-activating signals into
antioxidant gene expression. Mol Cell Biol. 36:407–420. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H, Zhou L, Davies KJA and Forman HJ:
Silencing Bach1 alters aging-related changes in the expression of
Nrf2-regulated genes in primary human bronchial epithelial cells.
Arch Biochem Biophys. 672:1080742019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Otsuki A, Suzuki M, Katsuoka F, Tsuchida
K, Suda H, Morita M, Shimizu R and Yamamoto M: Unique cistrome
defined as CsMBE is strictly required for Nrf2-sMaf heterodimer
function in cytoprotection. Free Radic Biol Med. 91:45–57. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu MC, Ji JA, Jiang ZY and You QD: The
Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic
target: an update. Med Res Rev. 36:924–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jung JS, Lee SY, Kim DH and Kim HS:
Protopanaxatriol ginsenoside Rh1 upregulates phase II antioxidant
enzyme gene expression in rat primary astrocytes: involvement of
MAP kinases and Nrf2/ARE signaling. Biomol Ther (Seoul). 24:33–39.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang W, Maturu P, Liang YW, Wang L,
Lingappan K and Couroucli X: Hyperoxia-mediated transcriptional
activation of cytochrome P4501A1 (CYP1A1) and decreased
susceptibility to oxygen-mediated lung injury in newborn mice.
Biochem Biophys Res Commun. 495:408–413. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ling Y, Li ZZ, Zhang JF, Zheng XW, Lei ZQ,
Chen RY and Feng JH: MicroRNA-494 inhibition alleviates acute lung
injury through Nrf2 signaling pathway via NQO1 in sepsis-associated
acute respiratory distress syndrome. Life Sci. 210:1–8. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development, oxidative
stress response and diseases: An evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016. View Article : Google Scholar : PubMed/NCBI
|