Introduction
Various factors, such as the genetic background and
biological environment, are suggested to induce vasoconstriction of
the pulmonary arteries and cell proliferation in pulmonary
vasculature in many forms of pulmonary hypertension (PH) (1–3). This
leads to stenosis and obstruction of the vascular lumen, resulting
in an increase in the pulmonary arterial (PA) pressure (4). In accordance with the increase in
pressure, the right heart function decreases, leading to right
heart failure, which eventually becomes a prognostic factor in
these patients (5,6).
Metabolic remodeling has been described not only in
pulmonary artery vascular cells but also in the right ventricle
cardiomyocytes of pulmonary arterial hypertension (PAH) (7). The metabolic alterations include
mitochondrial inactivation, which leads to the suppression of
glucose oxidation through the tricarboxylic acid (TCA) cycle and
upregulation of normoxic glycolysis (8,9).
Fatty acid (FA) oxidation is suggested to be the
major source of energy production, especially in the left
ventricle. Indeed, it is responsible for 60–90% of ATP synthesis
(10). Although the details of FA
metabolism in the hypertrophied RV in PH remain unclear, our recent
studies using positron emission tomography (PET) and
123I-β-methyl iodophenyl pentadecanoic acid (BMIPP)
uptake imaging have revealed the accumulation of not only glucose
but also FA in the RV of patients with chronic thromboembolic
pulmonary hypertension (CTEPH) (11,12).
However, these studies only showed the accumulation of FA in the
cytoplasm, and whether or not FA oxidation actually occurs through
the TCA cycle in mitochondria has remained unclear.
FA oxidation in the hypertrophied RV in PH is still
controversial. An increase in FA oxidation in the RV was shown in
the PA banding model (13).
However, a reduction in FA oxidation was noted in both the adaptive
and maladaptive RV in the monocrotaline rat model and the PA
banding model (14–16). In addition, impaired FA transport
into the stressed myocardium has been demonstrated in a PAH model
with right heart failure, which was developed by treatment with a
vascular endothelial growth factor receptor blocker (SUGEN5416),
followed by exposure to chronic hypoxia (Su/Hx) (17).
Metabolomics can comprehensively analyze metabolites
induced in vivo by the action of proteins. This analysis can
reveal the activity of the protein, allowing for the direct
monitoring of life phenomena (18).
In the present study, we investigated the metabolic remodeling of
glucose and FA in the RV of a rat model of PAH (Su/Hx model) via a
metabolome analysis.
Materials and methods
Animals
Five-week-old male wild-type Sprague-Dawley rats
weighing 100–150 g were purchased from CLEA Japan. All animal
studies were approved by the Review Board for Animal Experiments of
Chiba University (no. 30-126) and were therefore performed in
accordance with the ethical standards laid down in the principles
of the NIH Guide for the Care and Use of Laboratory Animals (NIH
Publication, 8th edition, 2011).
Rat model of severe PAH (Su/Hx
model)
PH was induced in rats with a single 20 mg/kg
subcutaneous injection of a vascular endothelial growth factor
receptor antagonist (SU5416; R&D Systems), followed by 3 weeks
of exposure to hypoxic conditions (10% O2) and then 5
weeks of exposure to normoxic conditions, as previously reported
(19). Hemodynamic measurements
were performed, and the RV was harvested for the measurement of
metabolites. All rats were housed in standard cages and had free
access to food and water
Hemodynamic measurements
Rats were intraperitoneally anesthetized with
pentobarbital sodium (30 mg/kg). A polyethylene tube catheter
[outer diameter (OD): 1.0 mm; Hibiki] was inserted into the RV
through the right jugular vein to measure the RV systolic pressure
(RVSP). Signals were monitored with a physiological transducer (NEC
Sanei), an amplifier system (NEC Sanei), and recorder (Nihon
Kohden). After the hemodynamic measurements, the rats were
euthanized with pentobarbital sodium (150 mg/kg). The heart was
harvested for the measurement of metabolites, and then the weights
of the RV and left ventricle + septum (LV+S) were measured to
determine the RV/LV+S ratio.
Metabolite extraction
C-SCOPE
Metabolite extraction was conducted at Human
Metabolome Technologies (HMT). Approximately 32 mg of the frozen RV
tissue was plunged into 750 µl of 50% acetonitrile/Milli-Q water
containing internal standards (H3304-1002; HMT) at 0°C in order to
inactivate enzymes. The tissue was homogenized three times at 3,500
rpm for 1 min using a tissue homogenizer (Micro Smash MS100R; Tomy
Digital Biology Co., Ltd.) and then the homogenate was centrifuged
at 2,300 × g and 4°C for 5 min. Subsequently, 400 µl of upper
aqueous layer was centrifugally filtered through a Millipore 5-kDa
cutoff filter at 9,100 × g and 4°C for 120 min to remove proteins.
The filtrate was centrifugally concentrated and re-suspended in 50
µl of Milli-Q water for a CE-MS analysis.
LC-MS
Metabolite extraction and the metabolome analysis
were conducted at HMT. Briefly, approximately 35 mg of the frozen
RV tissue was plunged into 500 µl of 1% formic acid/acetonitrile
containing internal standard solution (H3304-1002; HMT) at 0°C. The
tissue was homogenized three times at 1,500 rpm for 120 sec using a
tissue homogenizer (Micro Smash MS100R; Tomy Digital Biology Co.,
Ltd.) and then the homogenate was centrifuged at 2,300 × g and 4°C
for 5 min and the mixture was homogenized again after adding 167 µl
of Milli-Q water. Then the homogenate was centrifuged at 2,300 × g
and 4°C for 5 min. The supernatant was then mixed with 500 µl of 1%
formic acid/acetonitrile and 167 µl of Milli-Q water, and the
solution was filtrated through a 3-kDa cutoff filter (NANOCEP 3K
OMEGA; PALL Corporation) to remove macromolecules and further
filtrated using a hybrid SPE phospholipid cartridge (55261-U;
Supelco) to remove phospholipids. The filtrate was desiccated and
then re-suspended in 100 µl of isopropanol/Milli-Q water for the
LC-TOFMS analysis.
Metabolome analyses
C-SCOPE
The metabolome analyses were conducted with the
C-SCOPE package of HMT using capillary electrophoresis
time-of-flight mass spectrometry (CE-TOFMS) for the cation analysis
and CE-tandem mass spectrometry (CE-MS/MS) for the anion analysis,
based on previously described methods (20–23).
Briefly, the CE-TOFMS analysis was carried out using an Agilent CE
capillary electrophoresis system equipped with an Agilent 6210
time-of-flight mass spectrometer (Agilent Technologies). The
systems were controlled by the Agilent G2201AA ChemStation software
program (version B.03.01 for CE; Agilent Technologies) and
connected by a fused silica capillary (50 µm i.d. ×80 cm total
length) with a commercial electrophoresis buffer (H3301-1001 and
I3302-1023 for the cation and anion analyses, respectively, HMT) as
the electrolyte. The spectrometer scanned from m/z 50 to 1,000
(20). Peaks were extracted using
MasterHands, an automatic integration software program (Keio
University) (21) and MassHunter
Quantitative Analysis B.04.00 (Agilent Technologies) in order to
obtain peak information, including m/z, peak area, and migration
time (MT). Signal peaks were annotated according to the HMT
metabolite database, based on their m/z values with the MTs. The
concentrations of metabolites were calculated by normalizing the
peak area of each metabolite with respect to the area of the
internal standard and using standard curves with three-point
calibrations. A hierarchical cluster analysis (HCA) and principal
component analysis (PCA) were performed using HMT's proprietary
software programs, PeakStat and SampleStat, respectively. The
detected metabolites were plotted on metabolic pathway maps using
the VANTED software program (23).
LC-MS
The metabolome analyses were conducted using the
LC-MS package of HMT, based on previously described methods
(21–23). Briefly, an LC-TOFMS analysis was
carried out using an Agilent LC System (Agilent 1200 series RRLC
system SL) equipped with an Agilent 6230 TOFMS (Agilent
Technologies). The systems were controlled by the Agilent G2201AA
ChemStation software program (version B.03.01; Agilent
Technologies) equipped with an ODS column (2×50 mm, 2 µm) (21). Peaks were extracted using
MasterHands, an automatic integration software program (Keio
University) in order to obtain peak information, including m/z,
peak area, and retention time (RT) (22). Signal peaks corresponding to
isotopomers, adduct ions, and other product ions of known
metabolites were excluded, and the remaining peaks were annotated
according to the HMT metabolite database, based on their m/z values
with the RTs determined by TOFMS. The areas of the annotated peaks
were then normalized based on internal standard levels and sample
amounts, in order to obtain the relative levels of each metabolite.
The HCA and PCA were performed using HMT's proprietary software
programs, PeakStat and SampleStat, respectively. Detected
metabolites were plotted on metabolic pathway maps using the VANTED
software program (23).
Statistical analyses
The RV afterload was compared between the groups
using Student's t-test. In the metabolome analysis, the mean
metabolite concentrations were compared between the groups by the
Brunner-Munzel test (24). P values
of <0.05 were considered to indicate statistical significance.
The SampleStat software program (ver. 3.14; HMT) was used to
perform the metabolome analysis. This program does not support
scatter/dot plots. Thus, a box-and-whisker plot was used to present
these results. For the other statistical analyses, the
JMP® (SAS Institute, Inc.) and R software programs
(https://cran.r-project.org/src/base/R-4/R-4.0.3.tar.gz)
were used. For the hierarchical clustering analysis, the Pearson
correlation coefficient was used to evaluate the similarity between
metabolite profiles. Ward's method was employed for hierarchical
clustering.
Results
The evaluation of the RV overload in
Su/Hx and control rats
To evaluate the RV afterload, all animals were
subjected to hemodynamic measurements and dissected at the end of
the experimental period. The RV hemodynamics were measured by
closed chased cannulation of the jugular vein. The RVSP and RV/LV+S
in Su/Hx and untreated control rats are presented in Table I. The RVSP in Su/Hx rats was
significantly higher than that in untreated controls (55.3±5.9 vs.
21.8±4.3 mmHg, P=0.001), as was the RV/LV+S (0.53±0.17 vs.
0.19±0.02, P=0.030) (Table I).
 | Table I.Evaluation of RV overload in Su/Hx
and control rats. |
Table I.
Evaluation of RV overload in Su/Hx
and control rats.
| Rat group | Control-1 | Control-2 | Control-3 | Su/Hx-1 | Su/Hx-2 | Su/Hx-3 | Control | Su/Hx | P-value |
|---|
| BW | 440 | 600 | 565 | 374 | 505 | 442 | 535±84 | 440±66 | 0.199 |
| RVSP | 26.7 | 20 | 18.8 | 61.8 | 53.9 | 50.3 | 21.8±4.3 | 55.3±5.9 | 0.001 |
| RV/LV+S | 0.2 | 0.21 | 0.17 | 0.725 | 0.42 | 0.43 | 0.19±0.02 | 0.53±0.17 | 0.030 |
The cation and anion analyses by
CE-TOFMS and CE-MS/MS
Metabolome analyses were conducted using CE-TOFMS
for the cation analysis and CE-MS/MS for the anion analysis based
on previously described methods (20–23).
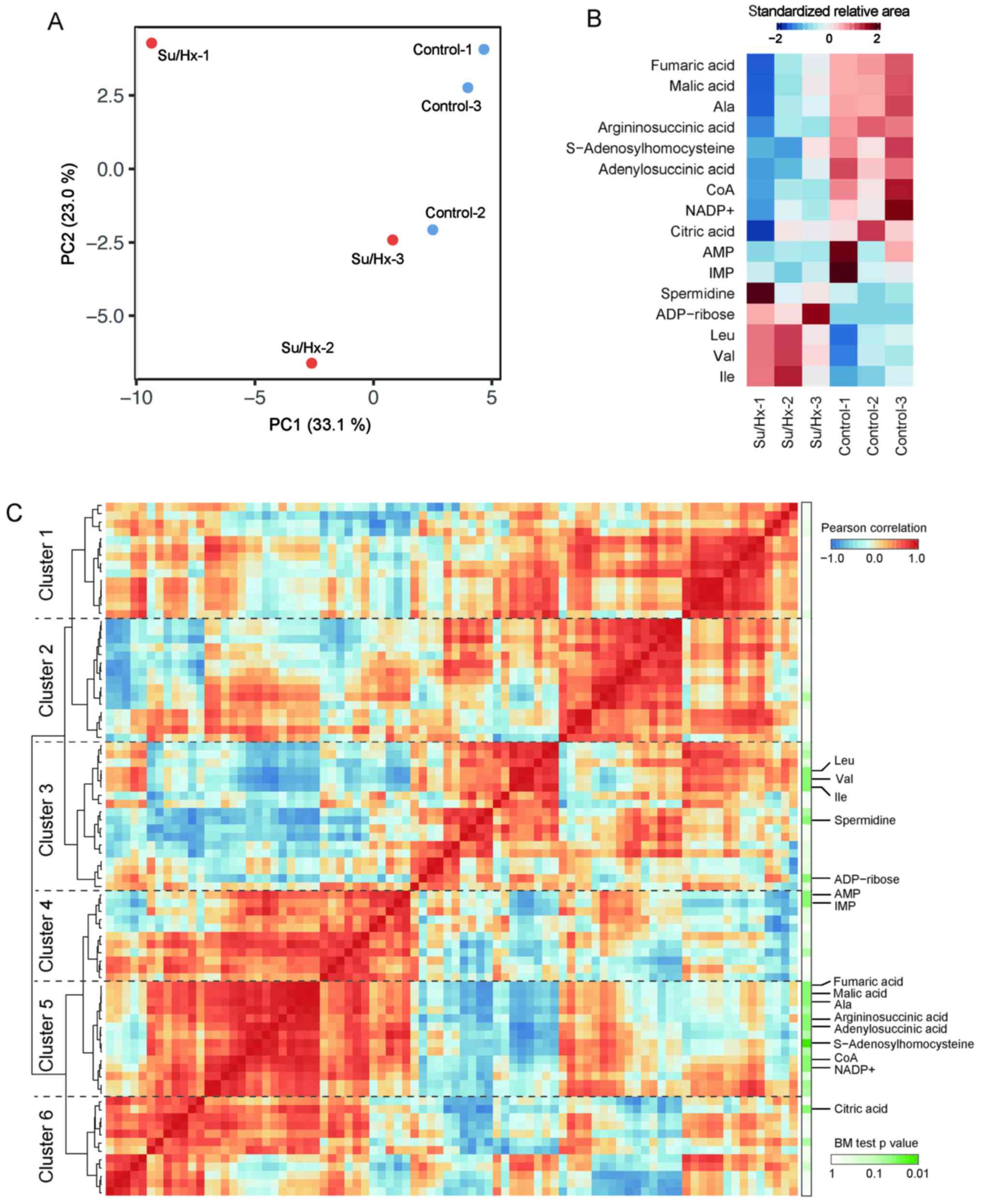
Fig. 1A shows that the principal
component analysis (PCA) completely separated the metabolic
profiles of Su/Hx and control rats in CE-TOFMS and CE-MS/MS. The
principal component (PC) 1 in Fig.
1A was interpreted as treatment condition separation (Su/Hx vs.
control rats). Among 84 features obtained by CE-TOFMS and CE-MS/MS,
16 compounds significantly differed between Su/Hx and control rats
(Fig. 1B; Brunner-Munzel test:
P<0.05). Branched-chain amino acids (BCAAs), including
isoleucine, leucine and valine were increased in Su/Hx rats, while
the levels of these metabolites, including citric acid, malic acid,
fumaric acid, adenylosuccinic acid and argininosuccinic acid
showed, a decreasing trend in Su/Hx rats. The level of alanine, a
TCA cycle-related amino acid, was lower in the Su/Hx rats than in
the control rats.
We identified six major clusters of metabolites
using a hierarchical clustering analysis (HCA) based on the Pearson
correlation coefficient (Fig. 1C).
Since malic acid, fumaric acid, adenylosuccinic acid,
argininosuccinic acid and alanine were included in the same cluster
(Cluster 5 in Fig. 1C), together
with CoA, NADPH, and NADP+, cofactors in anabolic reactions, the
metabolic pathway related to these metabolites seemed to be
downregulated in Su/Hx rats. On the other hand, BCAAs were in
another cluster (Cluster 3 in Fig.
1C), which showed a strong negative correlation with Cluster 1,
indicating that BCAAs might be regulated in a coordinated manner in
Su/Hx rats.
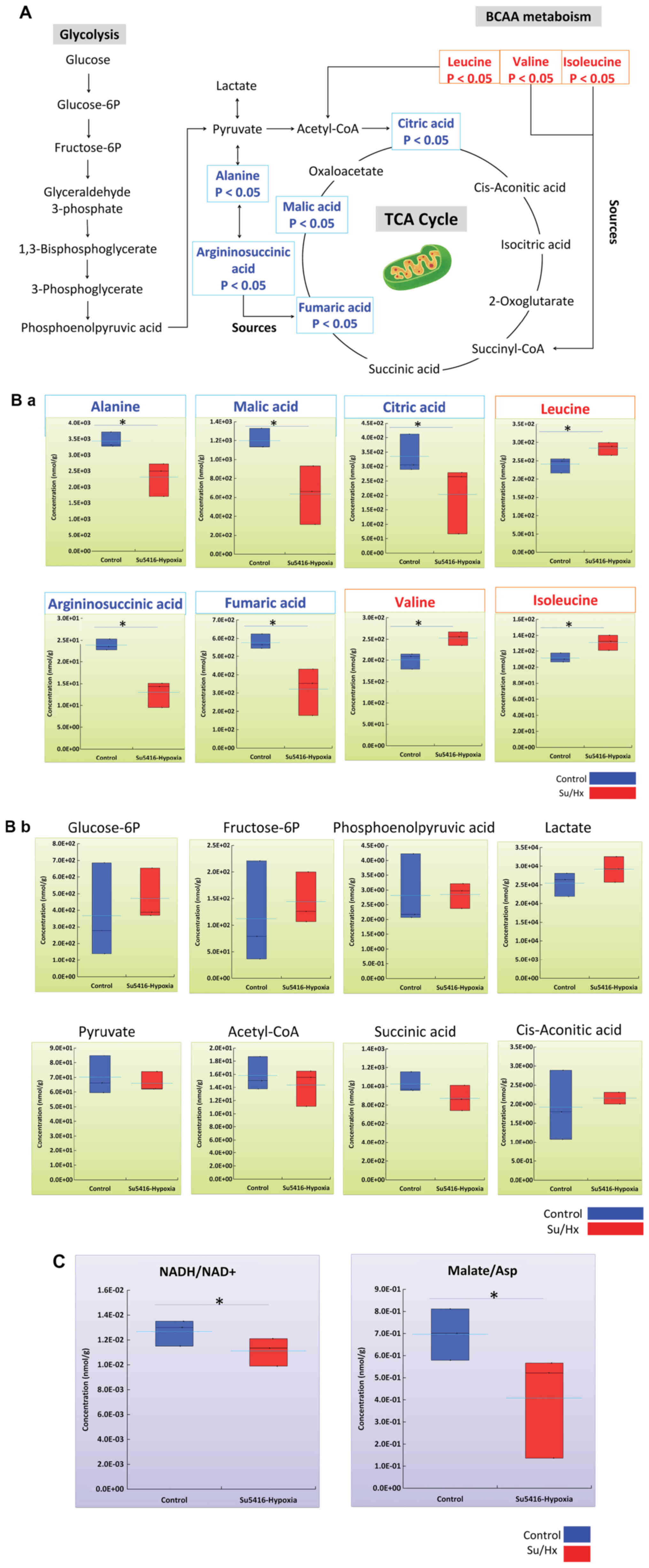
A metabolic map of glycolysis and the
TCA cycle in the metabolic pathway of Su/Hx and control rats
To understand the relationships among metabolites
that are differentially expressed in Su/Hx rats, we then mapped the
metabolites to metabolic pathway maps. The glycolysis pathway map
showed no statistically significant differences in any of the
intermediates (Fig. 2A and B).
These results suggested that the glycolytic metabolic pathway did
not differ between the groups.
The TCA cycle map further showed a decreasing trend
in the levels of alanine, argininosuccinic acid and intermediates,
as well as fumaric acid, malic acid, and citric acid, in the TCA
cycle in Su/Hx rats in comparison to control rats (Fig. 2A and B). These results indicated
that the TCA cycle in the RV of Su/Hx rats is less active than that
in controls.
As noted in the previous section, all BCAAs were
increased in Su/Hx rats in comparison to controls (Fig. 2A and B). BCAAs are amino acids with
aliphatic side chains with branching (binding of two or more other
carbon atoms to any carbon atom). Proteinogenic amino acids include
three types of BCAA: Leucine, isoleucine, and valine. These three
types of BCAAs are essential amino acids in humans. The branched
chain α-keto acid dehydrogenase complex (BCKDH) is involved in the
cleavage of BCAAs, whereby the BCAA is converted to an acyl CoA
derivative, which is subsequently converted to acetyl CoA or
succinyl CoA before finally being incorporated into the TCA cycle
(Fig. 2A and B). Total BCAA is
calculated as the total amount of leucine, isoleucine, and valine,
and was found to be significantly increased in Su/Hx rats in
comparison to controls (Su/Hx vs. Control; P<0.05) (Fig. 2B).
Several metabolic parameters were calculated based
on metabolite measurements in CE-TOFMS and CE-MS/MS to assess the
metabolic balance in each pathway. Among them, we observed a number
of notable trends.
The NADH to NAD+ Ratio (NADH/NAD+): NAD+ is used as
a cofactor necessary for the catalytic activity of the enzyme in
many reactions, including glycolysis, citric acid cycle, and
β-oxidation of fatty acids. Then, NADH produced as a reactant plays
a role in the electron transfer system in ATP production via
oxidative phosphorylation. The NADH/NAD+ ratio is known to decrease
during stagnation of central carbon metabolism under hypoxia. The
ratio showed a significant decrease in Su/Hx rats in comparison to
controls (Su/Hx vs. Control; P<0.05) (Fig. 2C).
The Malate to Aspartic acid Ratio (Malate/Asp):
Malic acid is produced from oxaloacetate with a reaction from NADH
to NAD+ in the cytoplasm. Conversely, in the mitochondria, it is
converted to oxaloacetate in the reaction from NAD+ to NADH.
Oxaloacetate is converted to Asp, which is capable of passing from
the inner mitochondrial membrane to the cytoplasm, and in the
cytoplasm, Asp is converted to oxaloacetate. This cycle is called
as the malate-aspartate shuttle, and is a mechanism for
transporting NADH, which is required for ATP production by
oxidative phosphorylation, from the cytoplasm to the inner
mitochondrial membrane. Thus, the Malic acid/Asp ratio is an
indirect indicator of the NADH/NAD + ratio and energy status. There
was a significant decrease in Su/Hx rats in comparison to controls
(Su/Hx vs. Control; P<0.05) (Fig.
2C).
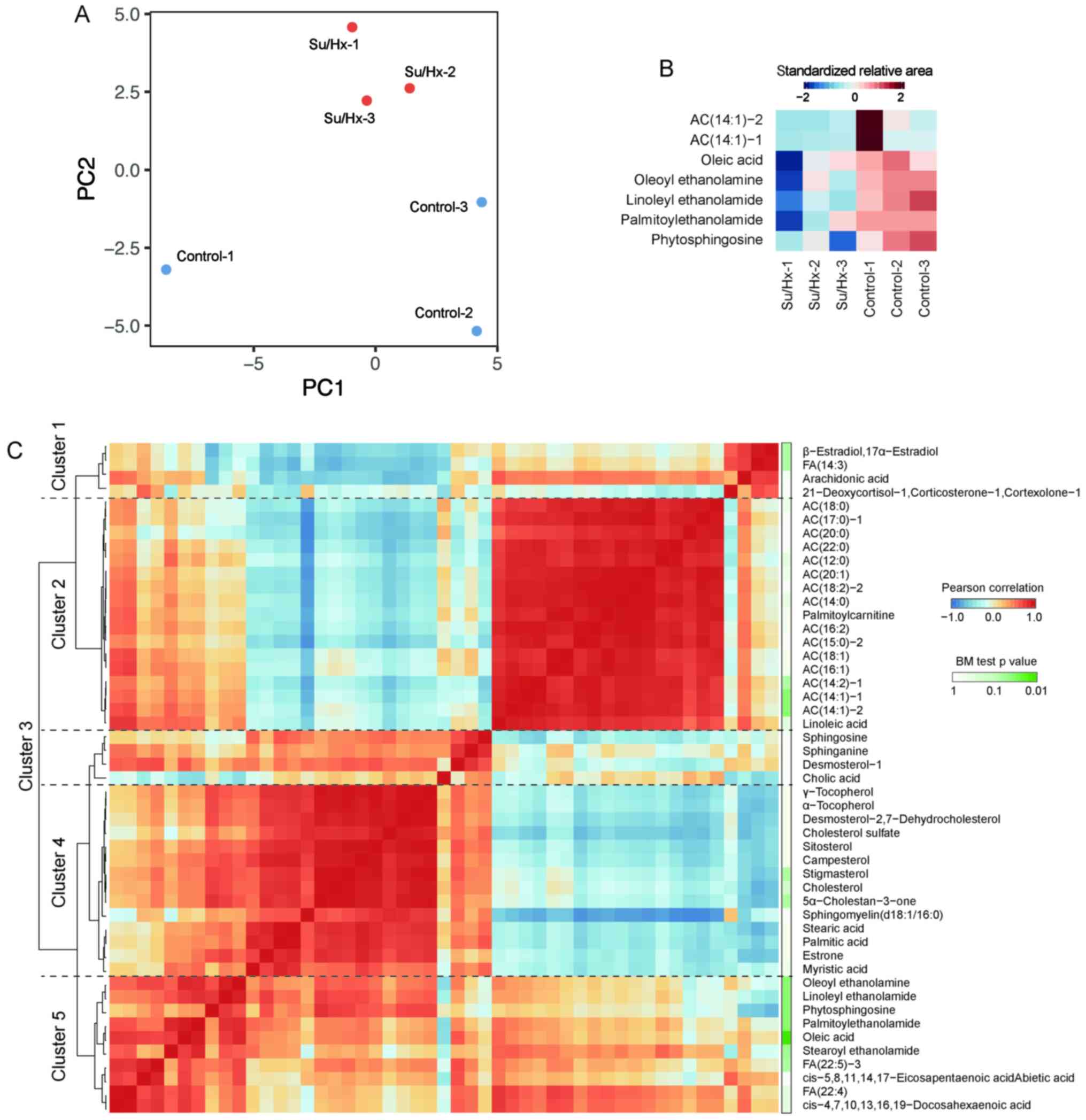
The HCA in LC-TOFMS
Metabolome analyses were conducted using LC-TOFMS
for long-chain FAs and long-chain acylcarnitines. FA is known to be
an important substrates for energy production and acylcarnitines,
as a transporter of FA from the cytosol to mitochondria and to be
essential for the entry of FA into β-oxidation (25). The PCA completely separated the
metabolic profiles of long-chain FAs and long-chain acylcarnitines
of Su/Hx and control rats (Fig.
3A). Among 49 features obtained with LC-TOFMS, 7 compounds
showed significant differences between Su/Hx and control rats
(Fig. 3B; Brunner-Munzel test:
P<0.05). The levels of oleoyl ethanolamine, linoleyl
ethanolamide, palmitoylethanolamide and phytosphingosine, in
addition to two acylcarnitines, AC(14:1)-1 and AC(14:1)-2, were
significantly lower in Su/Hx rats than in control rats. The
long-chain FAs and long-chain acylcarnitines were grouped into five
clusters by the HCA, based on the Pearson correlation coefficient
(Fig. 3C). The biggest cluster,
Cluster 2, contains all of long-chain acylcarnitines indicating the
common regulation. Since Oleoyl ethanolamine, linoleyl
ethanolamide, palmitoylethanolamide and phytosphingosine were
located adjacent to each other in Cluster 5, it was suggested that
these metabolites were involved in similar pathways.
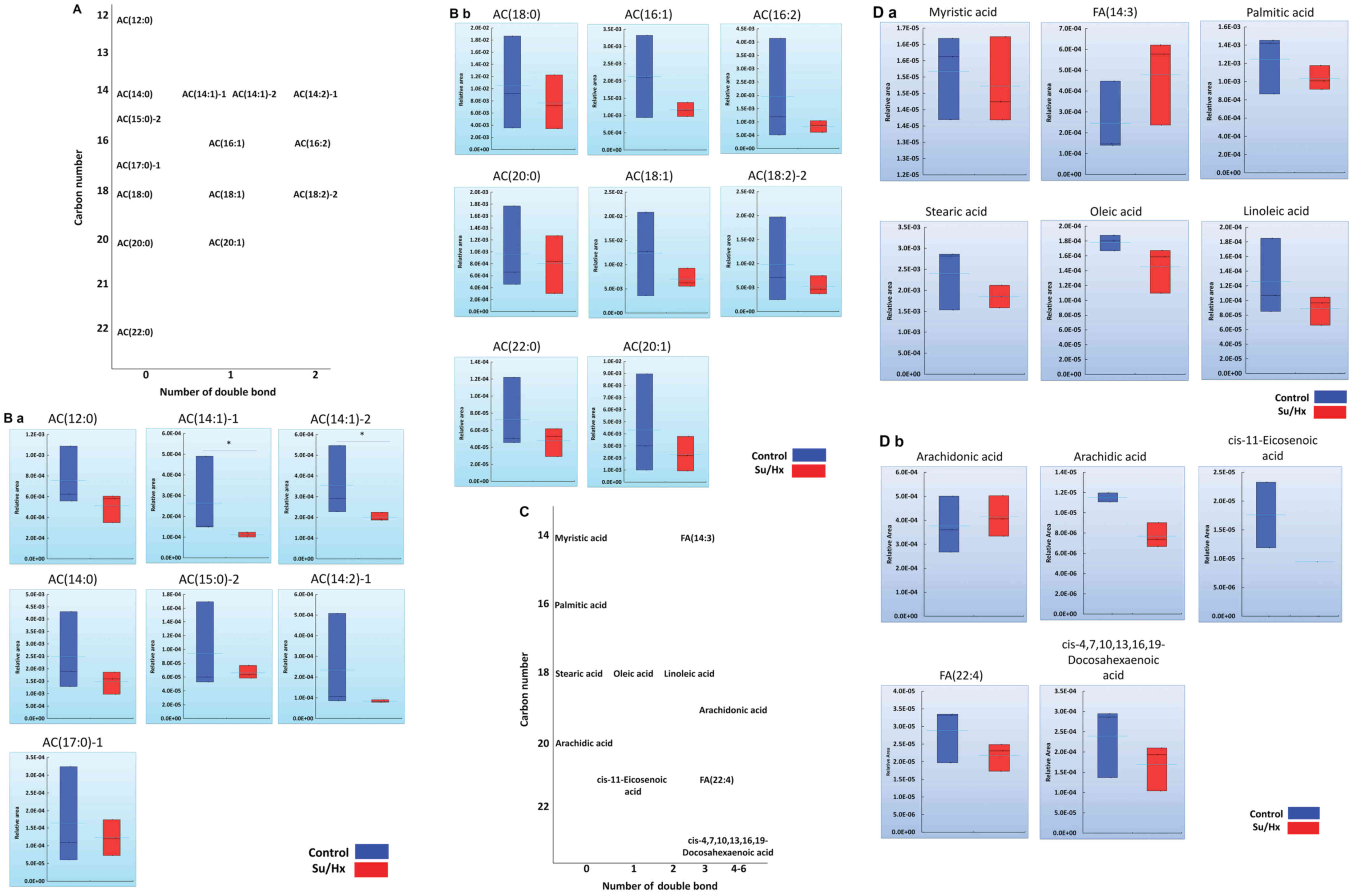
Long-chain acylcarnitines and FA
profiles in Su/Hx and control rats
Among 15 long-chain acylcarnitines, FA metabolomics
revealed statistically significant differences in AC(14:1)-1 and
AC(14:1)-2 in Su/Hx rats in comparison to control rats and a
decreasing trend in the levels of other long-chain acylcarnitines
(Fig. 4A and B). Long-chain FAs are
hydrolyzed to acyl-CoAs by mitochondrial acyl-CoA synthase, after
which carnitine palmitoyltransferase 1 (CPT1) converts the
acyl-CoAs to long-chain acylcarnitines (26). No marked differences in the
cytoplasm concentrations of long-chain FAs were noted between Su/Hx
and control rats (Fig. 4C and D).
Despite this lack of a marked difference in FAs, the decrease in
the levels of long-chain acylcarnitines in the RV of Su/Hx rats
might reflect dysregulated β-oxidation.
Discussion
The current study showed that although there was
almost no difference in the glycolytic metabolic pathway (Fig. 2A and B), the TCA cycle in the RV of
Su/Hx rats was less active in comparison to controls (Fig. 2A and B). In addition, the levels of
long-chain acylcarnitines in the RV of Su/Hx rats tended to be
lower than those in controls (Fig. 4A
and B). These results suggested that the glycolytic and fatty
acid metabolic pathway might be dysregulated and supported that the
disordered RV in Su/Hx rats was the result of multilevel failure of
FA metabolism (17). However, no
marked differences in the concentration of long-chain FAs were
noted between the Su/Hx and control rats.
It is true that the TCA cycle was less active
because of a decreasing trend in the levels of fumaric acid, malic
acid and citric acid. However, it is also important that
cis-aconitic acid and succinic acid showed almost no difference
between the groups. As shown in Figs.
1B and 2, the current analysis
demonstrated that there was a decrease in the level of
adenylosuccinic acid and argininosuccinic acid, which were involved
in the metabolic pathway of alanine, aspartate and glutamate, and
which are transformed into fumaric acid (27). It is hypothesized that the decrease
in the level of fumaric acid might be attributable to the
expression levels of adenylosuccinic acid and argininosuccinic
acid.
Our recent studies using 123I-BMIPP
uptake imaging revealed the FA accumulation in the RV of patients
with CTEPH, although whether or not the accumulated FAs actually
function as a substrate for ATP synthesis through the TCA cycle in
mitochondria remained unclear (11,12).
One possible explanation for these discrepant results between the
present and previous studies may be differences in the adaptability
of the RV to an increased afterload. In patients with CTEPH,
successful thromboendarterectomy is able to reverse the RV function
and reduce the increased FA accumulation in the RV, suggesting that
there is some degree of adaptive capacity in response to the
altered FA metabolism in the RV of patients with CTEPH. In
contrast, in patients with a severely impaired RV with a low
ejection fraction, the RV might be maladaptive to a severely
increased afterload. Indeed, the decreased accumulation of FA has
been demonstrated in the severely hypertrophied RV with a reduced
systolic function in patients with PH, suggesting that a
maladaptive RV may possess metabolic alterations, including
decreased FA β-oxidation (28).
Bogaard et al (29) showed that, in the RV myocardium of
the Su/Hx rat model, fatal RV failure, including myocardial
apoptosis, fibrosis and a decreased RV capillary density,
developed, whereas no such failure was noted in a rat model with PA
banding that induced only chronic progressive RV pressure overload.
The Su/Hx rat model, which is an established model of
angioproliferative PH, is suggested to induce RV alterations, in
addition to changes due to isolated RV pressure overload alone,
which may include FA metabolic remodeling. These are several
possible reasons for the difference in the FA kinetics in the RV
between our Su/Hx model and actual patients with CTEPH.
The present study showed that there were decreases
in the levels of downstream TCA cycle intermediates, including
fumaric acid and malic acid. These metabolites were categorized
into the same cluster in the HCA analysis based on CE-TOFMS and
CE-MS/MS data (Fig. 1C). The
quantitative metabolome analysis revealed decreased levels of
fumaric acid (Su/Hx vs. Control; P<0.05) and malic acid (Su/Hx
vs. Control; P<0.05) and increased levels of leucine (Su/Hx vs.
Control; P<0.05), valine (Su/Hx vs. Control; P<0.05) and
isoleucine (Su/Hx vs. Control; P<0.05) (absolute values;
Fig. 2A and B). Although valine and
isoleucine, which were subsequently converted to succinyl CoA,
increased in Su/Hx rats, decreasing trends were noted in the levels
of downstream TCA cycle intermediates after succinyl CoA (Fig. 2A and B). Our findings suggested that
the flow from BCAAs to the TCA cycle may be suppressed.
The quantitative metabolome analysis showed that the
levels of metabolites such as adenylosuccinic acid and
argininosuccinic acid were significantly lower in Su/Hx rats than
in controls (Su/Hx vs. Control; P<0.050, P<0.05). Recently,
it was shown that argininosuccinic acid is synthesized directly
from fumaric acid (30). The
reduction in the level of arginosuccinic acid may be associated
with a decrease in the level of fumaric acid, an intermediate in
the TCA cycle. Arginosuccinic acid is a metabolic intermediate of
the urea cycle, and the results of the present study suggested that
the TCA cycle and the urea cycle may be suppressed in the RV of
Su/Hx rats. However, whether or not adenylosuccinic acid is
involved in the progression of RV remodeling remains unclear.
In the PCA based on LC-TOFMS data, Control-1 was
plotted in separate locations despite being prepared in the same
experiment (Fig. 3A). We must
therefore consider the possibility that some outliers were included
in the sample. In normal controls, metabolites targeted in the
LC-TOFMS analysis may be easily altered in certain environmental
conditions. In Su/Hx rats, however, it should be meaningful that
each one had almost the same metabolic background (Fig. 3A).
Several recent studies have explored the utility of
plasma metabolites as biomarkers for PH (31,32).
Lewis et al showed that some plasma metabolites were useful
as biomarkers reflecting RV and pulmonary vascular dysfunction in
patients with PAH (31). Rhodes
et al (32) demonstrated a
relationship between the metabolic profile and the outcomes of
patients with PAH. Both studies also detected increased levels of
TCA cycle intermediates, suggesting that glucose oxidation was
upregulated in patients with PAH. In the present study, however,
conflicting results were confirmed in the RV of Su/Hx PAH rats. It
is suspected that plasma metabolites can, to some extent, reflect
the pathophysiological features of organ-specific disease. However,
plasma metabolites also reflect metabolites from all types of cells
exposed to the PH-induced microenvironment. Although the
metabolites detected in the current study cannot be used as PH
biomarkers, they may directly reflect the metabolism of the
dysfunctional RV in PAH.
It is generally acknowledged that, in the condition
with hypoxia, there is an increase in glycolysis, leading to a
decrease in glucose oxidation through the tricarboxylic acid (TCA)
cycle. Only the hypoxic condition can induce the increase of
pulmonary arterial pressure in rat models. Therefore, the metabolic
remodeling as an increase in glycolysis is supposed to occur in RV.
However, it remains uncertain whether the metabolic remodeling in
RV are changed according to the condition with normoxia or
hypoxia.
The current study, there are some limitations.
First, the total number of rats was extremely small. Because
metabolome analyses including C-SCOPE and LC-MS cost a fortune, but
our budget was limited. Second, there was the lack of mitochondrial
staining experiments. We tried to confirm differences of
mitochondrial form between control and Su/Hx rats RV cardiomyocytes
by using MitoTracker Red, which is a red-fluorescent dye that
stains mitochondria. However, it was impossible to confirm
mitochondrial formation by using RV tissue. To detect mitochondria
clearly, cultured single layered cells from RV should be needed.
However, it was impossible to isolate live RV cardiomyocytes from
the tissues. Third, in the current study, we analyzed only the RV,
not LV. Because right heart failure eventually becomes a prognostic
factor in patients with PH (5,6). It
remains uncertain if LV has metabolic remodeling as RV.
In conclusion, the current study showed that the TCA
cycle was less activated because of a decreasing trend in the
expression of fumaric acid, malic acid, and citric acid, which
might be attributable to the expression levels of adenylosuccinic
acid and argininosuccinic acid, and suggested that dysregulated
BCAA metabolism and a decrease in FA oxidation might contribute to
a reduction in TCA cycle reactions.
Acknowledgements
Not applicable.
Funding
The present study was supported by research grants
from the Respiratory Failure Research Group (H26-Intractable
diseases-General-076) from the Ministry of Health, Labour and
Welfare, Japan; a grant to the Pulmonary Hypertension Research
Group (grant no. 15ek0109127h0001) from the Japan Agency for
Medical Research and Development (AMED); a Grant-in-Aid for
Scientific Research (JSPS KAKENHI grant no. 17H04181,
19H03664).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS, EK, HS, AN, HM, RS, TJS, NT and KT contributed
to the study conception and design. Material preparation, data
collection and analysis were performed by SS, EK, HS, AN, HM, RS,
TJS, NT and KT. The first draft of the manuscript was written by SS
and all authors reviewed on previous versions of the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal studies have been approved by the Review
Board for Animal Experiments of Chiba University (no. 30-126) and
have therefore been performed in accordance with the ethical
standards laid down in the 1964 Declaration of Helsinki and its
later amendments.
Patient consent for publication
Not applicable.
Competing interests
Dr Sakao has received honoraria for lectures from
Nippon Shinyaku Co., Ltd.; Bayer Yakuhin, Ltd.; Actelion
Pharmaceuticals, Ltd.; and Pfizer. Tanabe has received honoraria
for lectures from Actelion Pharmaceuticals, Nippon Shinyaku Co.,
Ltd., Astellas and Pfizer and research grant support from Actelion
Pharmaceuticals. Dr Tatsumi has received honoraria for lectures
from Glaxo Smith Kline, Nippon Shinyaku Co., Ltd., and Actelion
Pharmaceutical Ltd. and research grant support from Ono
Pharmaceuticals, Ltd., Actelion Pharmaceuticals, Ltd. and Teijin
Limited Teijin Ltd.
Glossary
Abbreviations
Abbreviations:
|
BCAAs
|
branched-chain amino acids
|
|
BMIPP
|
123I-β-methyl iodophenyl
pentadecanoic acid
|
|
CTEPH
|
chronic thrombombolic pulmonary
hypertension
|
|
FA
|
fatty acid
|
|
HCA
|
Hierarchical cluster analysis
|
|
PA
|
pulmonary arterial
|
|
PAH
|
pulmonary arterial hypertension
|
|
PCA
|
principal component analysis
|
|
PH
|
pulmonary hypertension
|
|
RV
|
right ventricle
|
|
RVSP
|
RV systolic pressure
|
|
TCA cycle
|
the tricarboxylic acid cycle
|
References
|
1
|
Davie NJ, Schermuly RT, Weissmann N,
Grimminger F and Ghofrani HA: The science of endothelin-1 and
endothelin receptor antagonists in the management of pulmonary
arterial hypertension: Current understanding and future studies.
Eur J Clin Inves. 39 (Suppl):38–49. 2009. View Article : Google Scholar
|
|
2
|
Humbert M, Morrell NW, Archer SL, Stenmark
KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O,
Voelkel NF and Rabinovitch M: Cellular and molecular pathobiology
of pulmonary arterial hypertension. J Am Coll Cardiol. 43 (12 Suppl
S):13S–24S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oka M, Homma N, Taraseviciene-Stewart L,
Morris KG, Kraskauskas D, Burns N, Voelkel NF and McMurtry IF: Rho
kinase-mediated vasoconstriction is important in severe occlusive
pulmonary arterial hypertension in rats. Circ Res. 100:923–929.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakao S, Voelkel NF, Tanabe N and Tatsumi
K: Determinants of an elevated pulmonary arterial pressure in
patients with pulmonary arterial hypertension. Respir Res.
16:842015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van de Veerdonk MC, Kind T, Marcus JT,
Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM,
Westerhof N and Vonk-Noordegraaf A: Progressive right ventricular
dysfunction in patients with pulmonary arterial hypertension
responding to therapy. J Am Coll Cardiol. 58:2511–2519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freed BH, Gomberg-Maitland M, Chandra S,
Mor-Avi V, Rich S, Archer SL, Jamison EB Jr, Lang RM and Patel AR:
Late gadolinium enhancement cardiovascular magnetic resonance
predicts clinical worsening in patients with pulmonary
hypertension. J Cardiovasc Magn Reson. 14:112012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paulin R and Michelakis ED: The metabolic
theory of pulmonary arterial hypertension. Circ Res. 115:148–164.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Archer SL, Gomberg-Maitland M, Maitland
ML, Rich S, Garcia JG and Weir EK: Mitochondrial metabolism, redox
signaling, and fusion: A mitochondria-ROS-HIF-1alpha-Kv1.5
O2-sensing pathway at the intersection of pulmonary hypertension
and cancer. Am J Physiol Heart Circ Physiol. 294:H570–H578. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piao L, Fang YH, Cadete VJ, Wietholt C,
Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, et
al: The inhibition of pyruvate dehydrogenase kinase improves
impaired cardiac function and electrical remodeling in two models
of right ventricular hypertrophy: Resuscitating the hibernating
right ventricle. J Mol Med (Berl). 88:47–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stanley WC, Lopaschuk GD, Hall JL and
McCormack JG: Regulation of myocardial carbohydrate metabolism
under normal and ischaemic conditions. Potential for
pharmacological interventions. Cardiovasc Res. 33:243–257. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakao S, Miyauchi H, Voelkel NF, Sugiura
T, Tanabe N, Kobayashi Y and Tatsumi K: Increased right ventricular
fatty acid accumulation in chronic thromboembolic pulmonary
hypertension. Ann Am Thorac Soc. 12:1465–1472. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakao S, Daimon M, Voelkel NF, Miyauchi H,
Jujo T, Sugiura T, Ishida K, Tanabe N, Kobayashi Y and Tatsumi K:
Right ventricular sugars and fats in chronic thromboembolic
pulmonary hypertension. Int J Cardiol. 219:143–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang YH, Piao L, Hong Z, Toth PT, Marsboom
G, Bache-Wiig P, Rehman J and Archer SL: Therapeutic inhibition of
fatty acid oxidation in right ventricular hypertrophy: Exploiting
Randle's cycle. J Mol Med (Berl). 90:31–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buermans HP, Redout EM, Schiel AE, Musters
RJ, Zuidwijk M, Eijk PP, van Hardeveld C, Kasanmoentalib S, Visser
FC, Ylstra B and Simonides WS: Microarray analysis reveals pivotal
divergent mRNA expression profiles early in the development of
either compensated ventricular hypertrophy or heart failure.
Physiol Genomics. 21:314–323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faber MJ, Dalinghaus M, Lankhuizen IM,
Bezstarosti K, Dekkers DH, Duncker DJ, Helbing WA and Lamers JM:
Proteomic changes in the pressure overloaded right ventricle after
6 weeks in young rats: Correlations with the degree of hypertrophy.
Proteomics. 5:2519–2530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Faber MJ, Dalinghaus M, Lankhuizen IM,
Bezstarosti K, Verhoeven AJ, Duncker DJ, Helbing WA and Lamers JM:
Time dependent changes in cytoplasmic proteins of the right
ventricle during prolonged pressure overload. J Mol Cell Cardiol.
43:197–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomez-Arroyo J, Mizuno S, Szczepanek K,
Van Tassell B, Natarajan R, dos Remedios CG, Drake JI, Farkas L,
Kraskauskas D, Wijesinghe DS, et al: Metabolic gene remodeling and
mitochondrial dysfunction in failing right ventricular hypertrophy
secondary to pulmonary arterial hypertension. Circ Heart Fail.
6:136–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wishart DS: Current progress in
computational metabolomics. Brief Bioinform. 8:279–293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kato F, Sakao S, Takeuchi T, Suzuki T,
Nishimura R, Yasuda T, Tanabe N and Tatsumi K: Endothelial
cell-related autophagic pathways in Sugen/hypoxia-exposed pulmonary
arterial hypertensive rats. Am J Physiol Lung Cell Mol Physiol.
313:L899–L915. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohashi Y, Hirayama A, Ishikawa T, Nakamura
S, Shimizu K, Ueno Y, Tomita M and Soga T: Depiction of metabolome
changes in histidine-starved Escherichia coli by CE-TOFMS. Mol
Biosyst. 4:135–147. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ooga T, Sato H, Nagashima A, Sasaki K,
Tomita M, Soga T and Ohashi Y: Metabolomic anatomy of an animal
model revealing homeostatic imbalances in dyslipidaemia. Mol
Biosyst. 7:1217–1223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sugimoto M, Wong DT, Hirayama A, Soga T
and Tomita M: Capillary electrophoresis mass spectrometry-based
saliva metabolomics identified oral, breast and pancreatic
cancer-specific profiles. Metabolomics. 6:78–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Junker BH, Klukas C and Schreiber F:
VANTED: A system for advanced data analysis and visualization in
the context of biological networks. BMC Bioinformatics. 7:1092006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brunner E and Munzel U: The nonparametric
Behrens-Fisher problem: Asymptotic theory and a small-sample
approximation. Biom J. 42:17–25. 2000. View Article : Google Scholar
|
|
25
|
Håugaa H, Thorgersen EB, Pharo A, Boberg
KM, Foss A, Line PD, Sanengen T, Almaas R, Grindheim G, Pischke SE,
et al: Early bedside detection of ischemia and rejection in liver
transplants by microdialysis. Liver Transpl. 18:839–849. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramsay RR, Gandour RD and van der Leij FR:
Molecular enzymology of carnitine transfer and transport. Biochim
Biophys Acta. 1546:21–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kyoto Encyclopedia of Genes and Genomes
(KEGG), . Alanine, aspartate and glutamate metabolism - Mus
musculus (mouse). https://www.genome.jp/kegg-bin/show_pathway?org_name=mmu&mapno=00250&mapscale=&show_description=hideMarch
8–2018
|
|
28
|
Kim Y, Goto H, Kobayashi K, Sawada Y,
Miyake Y, Fujiwara G, Chiba H, Okada T and Nishimura T: Detection
of impaired fatty acid metabolism in right ventricular hypertrophy:
Assessment by I-123 beta-methyl iodophenyl pentadecanoic acid
(BMIPP) myocardial single-photon emission computed tomography. Ann
Nucl Med. 11:207–212. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bogaard HJ, Natarajan R, Henderson SC,
Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM and
Voelkel NF: Chronic pulmonary artery pressure elevation is
insufficient to explain right heart failure. Circulation.
120:1951–1960. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adam J, Yang M, Bauerschmidt C, Kitagawa
M, O'Flaherty L, Maheswaran P, Özkan G, Sahgal N, Baban D, Kato K,
et al: A role for cytosolic fumarate hydratase in urea cycle
metabolism and renal neoplasia. Cell Rep. 3:1440–1448. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lewis GD, Ngo D, Hemnes AR, Farrell L,
Domos C, Pappagianopoulos PP, Dhakal BP, Souza A, Shi X, Pugh ME,
et al: Metabolic profiling of right ventricular-pulmonary vascular
function reveals circulating biomarkers of pulmonary hypertension.
J Am Coll Cardiol. 67:174–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rhodes CJ, Ghataorhe P, Wharton J,
Rue-Albrecht KC, Hadinnapola C, Watson G, Bleda M, Haimel M,
Coghlan G, Corris PA, et al: Plasma metabolomics implicates
modified Transfer RNAs and altered bioenergetics in the outcomes of
pulmonary arterial hypertension. Circulation. 135:460–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|