Introduction
Vascular endothelial cells (VECs), as a selective
barrier between tissues and the blood, serve a vital role in
maintaining normal vascular function. VEC dysfunction leads to the
development of atherosclerosis, arteriolosclerosis, infectious
arteritis, Raynaud's disease and thromboangiitis obliterans
(1). Lipopolysaccharide (LPS), a
bacterial endotoxin, is a common factor that induces VEC injury and
dysfunction by triggering an inflammatory reaction and releasing
inflammatory factors (2). For
example, LPS can significantly decline cell viability and promote
cell apoptosis in rat intestine epithelial cells via increasing the
levels of cytokines, including TNF-α, IL-6 and IL-10 (3). Moreover, LPS can markedly decreased
cell viability and increase apoptosis in murine chondrogenic ATDC5
cells, resulting from the release of several cytokines, including
TNF-α, IL-6 and monocyte chemoattractant protein-1 (4). In addition, the secretion of
inflammatory cytokines, such as TNF-α and IL-6, can be
significantly elevated by LPS in VECs in mice, resulting in
induction of cell apoptosis (5).
VEC apoptosis is related to the Bcl-2/Bax and NF-κB
signalling pathways (6). The key
role of Bcl-2 and caspases is apoptosis regulation (7). Bcl-2 regulates cellular apoptosis by
controlling the permeability of cellular mitochondria. Bcl-2 serves
as an antiapoptotic protein in the outer mitochondrial wall to
inhibit cytochrome c (Cyt-c) release. By contrast, Bax, a
proapoptotic protein, transfers from the cytosol to the
mitochondria to promote Cyt-c release from mitochondria, resulting
in apoptosis induction and DNA damage (8). Previous studies have demonstrated that
apoptosis induction by stimuli is associated with the Bcl-2/Bax
signalling pathway, and perfluorocarbon decreased blast
injury-induced cell damage by inhibiting the Bcl-2/Bax, NF-κB and
MAPK signalling pathways in A549 cells (9–13). The
caspase family are apoptosis-specific targets, and as
cysteine-aspartic acid proteases, caspase-3 and caspase-7 directly
induce apoptosis after sequential activation (14).
As a protein complex, NF-κB controls DNA
transcription, cytokine production, cell survival and apoptosis,
and dysregulation of NF-κB signalling can lead to inflammation,
autoimmune disease and cancer (15). NF-κB is translocated into the
nucleus to activate multiple target genes involved in proliferation
and apoptosis (16). In the early
stages of apoptosis, NF-κB protects against heat stress-induced
apoptosis in HUVECs (17). IκB-α
and IκB-β are major signal-responsive isoforms in the IκB family
(18). IκB-α is a critical
regulator of NF-κB and one of the first genes induced following
NF-κB activation (19). When
activated, IκB-α enters the nucleus to remove NF-κB from gene
promoters and transport NF-κB proteins back to the cytoplasm for
feedback regulation (20). IκB-β
activates NF-κB transcription factors by phosphorylation of IκB
inhibitors (21).
Procyanidin B2 (epicatechin-(4β-8)-epicatechin;
PB2), a phenolic compound, primarily exists in common fruits,
including red grapes, cocoa, lindera aggregate and cinnamon
(22). PB2 isolated from cinnamon
can significantly decline cell viability and accelerate apoptosis
in prostate cancer cells, and the proapoptotic effect of PB2 might
be associated with caspase-3 activity (23). PB2 is a bioactive component and a
potent antioxidant for restoring and maintaining homeostasis
(24). PB2 displays
anti-inflammatory and antiapoptotic effects via the regulation of
various cytokines and other inflammatory mediators (25). PB2 extracted from fruits and red
wine displays potential anti-inflammatory activities. For example,
PB2 dramatically inhibits NLR family pyrin domain containing 3
(NLRP3) inflammasome activation and further reduces subsequent
activation of caspase-1 or secretion of the inflammatory cytokine
IL-1β, resulting from inhibition of the activator protein-1
signalling pathway in HUVECs (26).
PB2 can suppress LPS-induced inflammation and apoptosis in human
type II alveolar epithelial cells, which is related to inhibition
of inflammatory cytokines, including TNF-α and IL-1β, as well as
activation of NF-κB and the NLRP3 inflammasome (27). However, the effects of PB2 on the
NF-κB or Bcl-2/Bax signalling pathways, and the expression of
related cytokines are not completely understood. The results of the
aforementioned studies suggest that PB2 displays protective effects
in HUVECs (26). Procyanidin
protects against glycated low-density lipoprotein-induced apoptosis
and LPS-induced acute gut injury via regulating the oxidative state
(28,29). However, the mechanism underlying the
anti-inflammatory and antiapoptotic effects of PB2 is not
completely understood. Therefore, the present study investigated
the protective effects of PB2 on LPS-induced cytotoxicity and
apoptosis in HUVECs, as well as the underlying mechanisms.
Materials and methods
Cell culture
HUVECs (Procell Life Science & Technology Co.
Ltd.) were cultured in DMEM supplemented with 10% FBS and 1%
streptomycin/penicillin (all purchased from Thermo Fisher
Scientific, Inc.) in a humidified incubator with 95% air and 5%
CO2 at 37°C. Cell culture medium was replaced every 2–3
days.
Drug preparation
PB2 (Push Bio-Technology) was dissolved in
endotoxin-free ultrapure water to make a 20 µg/ml stock solution.
LPS (Sigma-Aldrich; Merck KGaA) was dissolved in endotoxin-free
ultrapure water to make a 1 mg/ml stock solution. Pyrrolidine
dithiocarbamate (PDTC), a specific NF-κB inhibitor, was used as a
positive control for PB2. PDTC (Sigma-Aldrich; Merck KGaA) was also
dissolved in endotoxin-free ultrapure water to make a 4 µg/ml stock
solution. All stock solutions were stored at −20°C until use.
Cell treatment
HUVECs cultured as described above for 24 h were
divided into six groups: i) Vehicle control group, cells were
treated with free-serum medium for 24 h; ii) LPS group, cells were
incubated in serum-free medium for 12 h, followed by treatment with
1 µg/ml LPS for 12 h; iii) PB2 group, cells were incubated in
serum-free medium for 12 h followed by treatment with 5 µg/ml PB2
for 12 h; iv) PDTC group, cells were incubated in serum-free medium
for 12 h followed by treatment with 2 µg/ml PDTC for 12 h; v) LPS +
PB2 group, cells were treated with 5 µg/ml PB2 for 12 h, followed
by 1 µg/ml LPS for 12 h; and vi) LPS + PDTC group, cells were
treated with 2 µg/ml PDTC for 12 h, followed by 1 µg/ml LPS for 12
h. All treatments were performed at a constant temperature of
35°C.
Assessment of cell viability via the
cell counting Kit-8 (CCK-8) assay
The effects of LPS, PDTC and PB2 on HUVEC viability
were detected by performing the CCK-8 assay, as previously
described (30,31). Briefly, HUVECs were seeded
(5×104 cells/well) into 96-well plates and cultured in
DMEM containing 10% FBS for 24 h. Subsequently, cells were cultured
in serum-free DMEM medium for 12 h, and then treated with
serum-free medium (vehicle control), PDTC (2 µg/ml), PB2 (1.25–10
µg/ml) (32) or LPS (1 µg/ml) for
12 h. For combination treatment, cells were treated with PDTC (2
µg/ml) or PB2 (5 µg/ml) for 12 h, followed by treatment with LPS (1
µg/ml) for 12 h. Subsequently, 10 µl CCK-8 solution (Beijing
Solarbio Science & Technology Co., Ltd.) was added to each well
and incubated for 1 h. The absorbance was measured at a wavelength
of 450 nm using a SpectraMax M3 microplate reader (Molecular
Devices, LLC).
Analysis of HUVEC apoptosis via
Hoechst 33258 staining
Apoptotic cells were detected using a Hoechst 33258
staining kit (Beyotime Institute of Biotechnology) according the
manufacturer's protocol. Briefly, HUVECs were seeded
(1×105 cells/well) into 6-well plates and cultured in
DMEM for 12 h. Cells were then treated with serum-free medium
(control), LPS (1 µg/ml), PDTC (2 µg/ml) or PB2 (1.25–10 µg/ml) for
12 h. For combination treatment, cells were treated with PDTC (2
µg/ml) or PB2 (5 µg/ml) for 12 h, followed by treatment with LPS (1
µg/ml) for 12 h. Subsequently, cells were incubated with 4%
paraformaldehyde for 10 min at room temperature (25°C), washed with
PBS for 15 min and treated with 10 µg/ml Hoechst 33258 staining
solution (500 µl/well) for 5 min at room temperature (25°C).
Following washing with PBS for 15 min in the dark, the nuclear
morphology of apoptotic cells was observed using an AMG EVOS
fluorescent microscope (Thermo Fisher Scientific Inc.;
magnification, ×400). The percentage (%) of apoptotic cells was
calculated as the ratio of apoptotic cells to total cells.
Analysis of IL-1β, IL-6 and TNF-α mRNA
expression levels via reverse transcription-quantitative PCR
(RT-qPCR) in HUVECs
HUVECs were seeded (1×105 cells/well)
into flasks and cultured in DMEM for 24 h, followed by culture in
serum-free DMEM for 12 h. Subsequently, cells were treated with
serum-free medium (vehicle control), LPS (1 µg/ml), PB2 (5 µg/ml)
or PDTC (2 µg/ml) for 12 h. For combination treatment, cells were
treated with PB2 (5 µg/ml) or PDTC (2 µg/ml) for 12 h, washed with
PBS for 15 min at room temperature and treated with LPS (1 µg/ml)
for 12 h. Total RNA was extracted from cells using the RNAsimple
Total RNA kit (Tiangen Biotech Co., Ltd.) according to the
manufacturer's protocol. Total RNA (1 µg) was reverse transcribed
into cDNA using the TIANScript RT kit (Tiangen Biotech Co., Ltd.),
according to the manufacturer's protocol. Subsequently, qPCR was
performed using 1 µl cDNA in a 20-µl total reaction volume using
SuperReal PreMix Plus (SYBR Green Real Time PCR Mix; Tiangen
Biotech Co., Ltd.) and the 7900HT Fast Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for qPCR: 95°C for 10 min; 45
cycles (95°C for 15 sec and 60°C for 1 min per cycle). The primers
used for qPCR were designed using Primer Premier (version 5.0;
Premier Biosoft International): IL-1β forward,
5′-ATGATGGCTTATTACAGTGGCAA-3′ and reverse,
5′-GTCGGAGATTCGTAGCTGGA-3′ (amplicon size, 132 bp); IL-6 forward,
5′-ACTCACCTCTTCAGAACGAATTG-3′ and reverse,
5′-CCATCTTTGGAAGGTTCAGGTTG-3′ (amplicon size, 149 bp); TNF-α
forward, 5′-AGGACCAGCTAAGAGGGAGA-3′ and reverse,
5′-CCCGGATCATGCTTTCAGTG-3′ (amplicon size, 136 bp); and GAPDH
forward, 5′-CTCACCGGATGCACCAATGTT-3′ and reverse,
5′-CGCGTTGCTCACAATGTTCAT-3′ (amplicon size, 82 bp) (Sangon Biotech
Co., Ltd.). mRNA expression levels were normalized to the internal
reference gene GAPDH. The results were calculated using CFX Manager
software (version 3.1; Bio-Rad Laboratories, Inc.) and quantified
using the 2−∆∆Cq method (33).
Analysis of IL-1β, IL-6 and TNF-a
protein levels via ELISA in HUVECs
IL-1β, IL-6 and TNF-α protein levels in HUVECs were
measured using ELISA kits according to manufacturers' protocols.
Briefly, HUVECs were seeded (1×105 cells/well) into
6-well plates and cultured in DMEM for 12 h, followed by culture in
serum-free DMEM for 12 h. Subsequently, cells were treated with
serum-free medium (vehicle control), LPS (1 µg/ml), PB2 (5 µg/ml)
or PDTC (2 µg/ml) for 12 h. For combination treatment, cells were
treated with PB2 (5 µg/ml) or PDTC (2 µg/ml) for 12 h prior to
treatment with LPS (1 µg/ml) for 12 h. To harvest the cytokines
secreted by cells in each group, the cell suspensions were
centrifuged at 3,000 × g at room temperature (25°C) for 5 min. The
protein levels of IL-1β, IL-6, and TNF-a in the supernatants were
detected using the following ELISA kits (R&D Systems, Inc.):
Human IL-1β QuantiGlo ELISA kit (cat. no. QLB00B), human IL-6
Quantikine ELISA kit (cat. no. PD6050) and human TNF-α Quantikine
ELISA kit (cat. no. PDTA00D). The absorbance was measured at a
wavelength of 450 nm using a SpectraMax M3 microplate reader
(Molecular Devices, LLC).
Western blotting
HUVECs were seeded (1×105 cells/well)
into flasks and cultured in DMEM for 24 h, followed by culture in
serum-free DMEM for 12 h. Cells were treated with serum-free medium
(control), LPS (1 µg/ml), PB2 (5 µg/ml) or PDTC (2 µg/ml) for 12 h.
For combination treatment, cells were treated with PB2 (5 µg/ml) or
PDTC (2 µg/ml) for 12 h, washed with PBS for 15 min at room
temperature and then treated with LPS (1 µg/ml) for 12 h. The
culture medium was removed, and cells were washed with cold PBS.
Total protein was extracted from cells using RIPA lysis buffer
(0.5% NP-40, 50 mM Tris-HCl, 120 mM NaCl, 1 mM EDTA, 0.1 mM Na3VO4,
1 mM NaF, 1 mM PMSF; and 1 µg/ml leupeptin; pH 7.5). Cell lysates
were centrifuged at 10,000 × g for 20 min at 4°C. Protein
quantification was performed using a BCA assay, then proteins (~30
µg) were separated via 12% SDS-PAGE and electrotransferred to PVDF
membranes. Following blocking with 5% skimmed milk in TBS-0.1%
Tween 20 for 1 h at room temperature (25°C), the membranes were
incubated overnight at 4°C with primary antibodies (all 1:1,000;
Cell Signaling Technology, Inc.) diluted in primary antibody
dilution buffer (Beyotime Institute of Biotechnology) targeted
against Bcl-2 (cat. no. 4223), Bax (cat. no. 2774), cleaved
caspase-3 (cat. no. 9661), cleaved caspase-7 (cat. no. 8438),
cleaved caspase-9 (cat. no. 20750), phosphorylated (p)-IκB-α (cat.
no. 2859), total IκB-α (cat. no. 9242), p-IκB-β (cat. no. 4921),
total IκB-β (cat. no. 15519), p-NF-κB p65 (cat. no. 3033), total
NF-κB p65 (cat. no. 8242) and β-actin (cat. no. 4970S). Following
washing three times with TBST, the membranes were incubated at room
temperature with an anti-rabbit IgG, HRP-conjugated antibody
(1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.) for 1 h
with gentle agitation at room temperature. The membranes were
washed three times with TBST for 30 min. Subsequently, protein
bands were visualized using ECL western detection reagents (Thermo
Fisher Scientific, Inc.). Protein expression levels were
semi-quantified using ImageJ v1.8.0 software (National Institutes
of Health).
Analysis of nuclear translocation of
NF-κB p65 via immunofluorescence in HUVECs
HUVECs were seeded (1×105 cells/well)
into 6-well plates, cultured in DMEM for 24 h, followed by culture
in serum-free DMEM for 12 h. Cells were then treated with
serum-free medium (control), LPS (1 µg/ml), PB2 (5 µg/ml) or PDTC
(2 µg/ml). For combination treatment, cells were treated with PB2
(5 µg/ml) or PDTC (2 µg/ml) for 12 h, washed with PBS for 15 min at
room temperature and then treated with LPS (1 µg/ml) for 12 h.
Cells were washed with PBS for 10 min at room temperature and fixed
in 4% paraformaldehyde at 4°C for 30 min. Following washing three
times with PBS for 5 min each time, cells were incubated with 0.1%
Triton X-100 for 10 min at room temperature (25°C) and blocked with
1% BSA (Beyotime Institute of Biotechnology) for 30 min at room
temperature (25°C). Cells were incubated with an anti-NF-κB p65
rabbit monoclonal primary antibody (cat. no. 8801; 1:500; Cell
Signaling Technology, Inc.) at 4°C overnight. Subsequently, cells
were incubated with an HRP-conjugated goat anti-rabbit secondary
antibody (1:2,000; Cell Signaling Technology, Inc.) for 2 h at room
temperature (25°C). Following washing with PBS for 15 min, cells
were stained with DAPI for 10 min at room temperature (25°C) and
washed with PBS for 15 min. Stained cells were visualized using a
CKX41 inverted phase contrast fluorescence microscope (Olympus
Corporation; magnification, ×400).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 20.0; IBM Corp.). Data are presented as the mean
± SD. All experiments were repeated three times in triplicate.
Comparisons among multiple groups were analysed using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
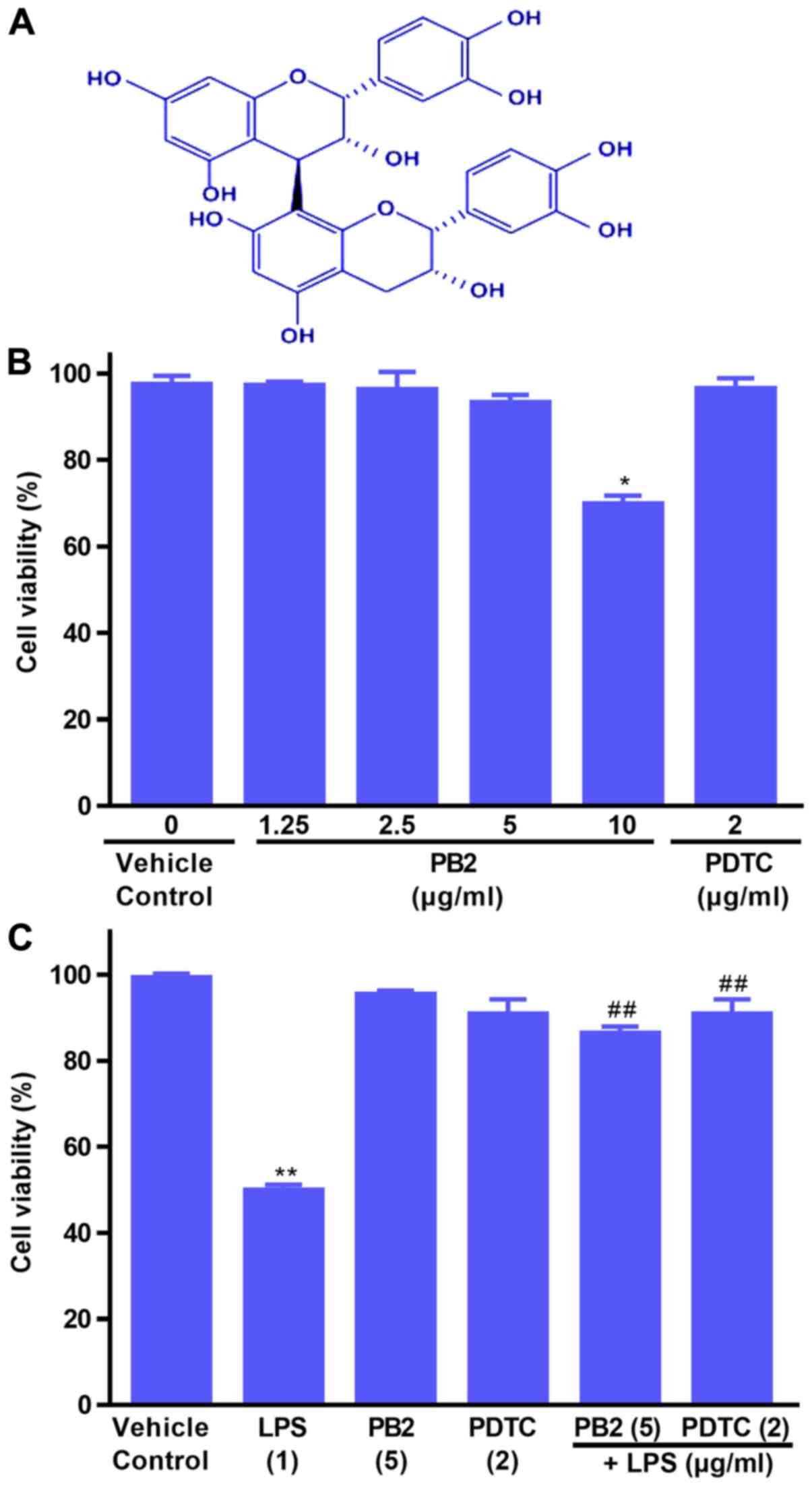
Protective effect of PB2 on
LPS-induced inhibition of HUVEC viability
The effects of PB2, PDTC and LPS on HUVEC viability
were evaluated by performing the CCK-8 assay (Fig. 1B and C). Compared with the vehicle
control group, PB2 (≤5 µg/ml) and PDTC (2 µg/ml) displayed no
significant inhibitory effect on cell viability, resulting in
>95% cell viability. However, PB2 (10 µg/ml) significantly
inhibited cell viability, resulting in 69.67±6.28% cell viability
compared with the vehicle control (98.46±3.23% cell viability).
Subsequently, the protective effects of PB2 and PDTC
on LPS-induced cytotoxicity were assessed. The results demonstrated
that pretreatment with PB2 or PDTC notably protected cells against
LPS-induced cytotoxicity in HUVECs (Fig. 1C). The results indicated that PB2
and PDTC effectively protected against LPS-induced inhibition of
HUVEC viability.
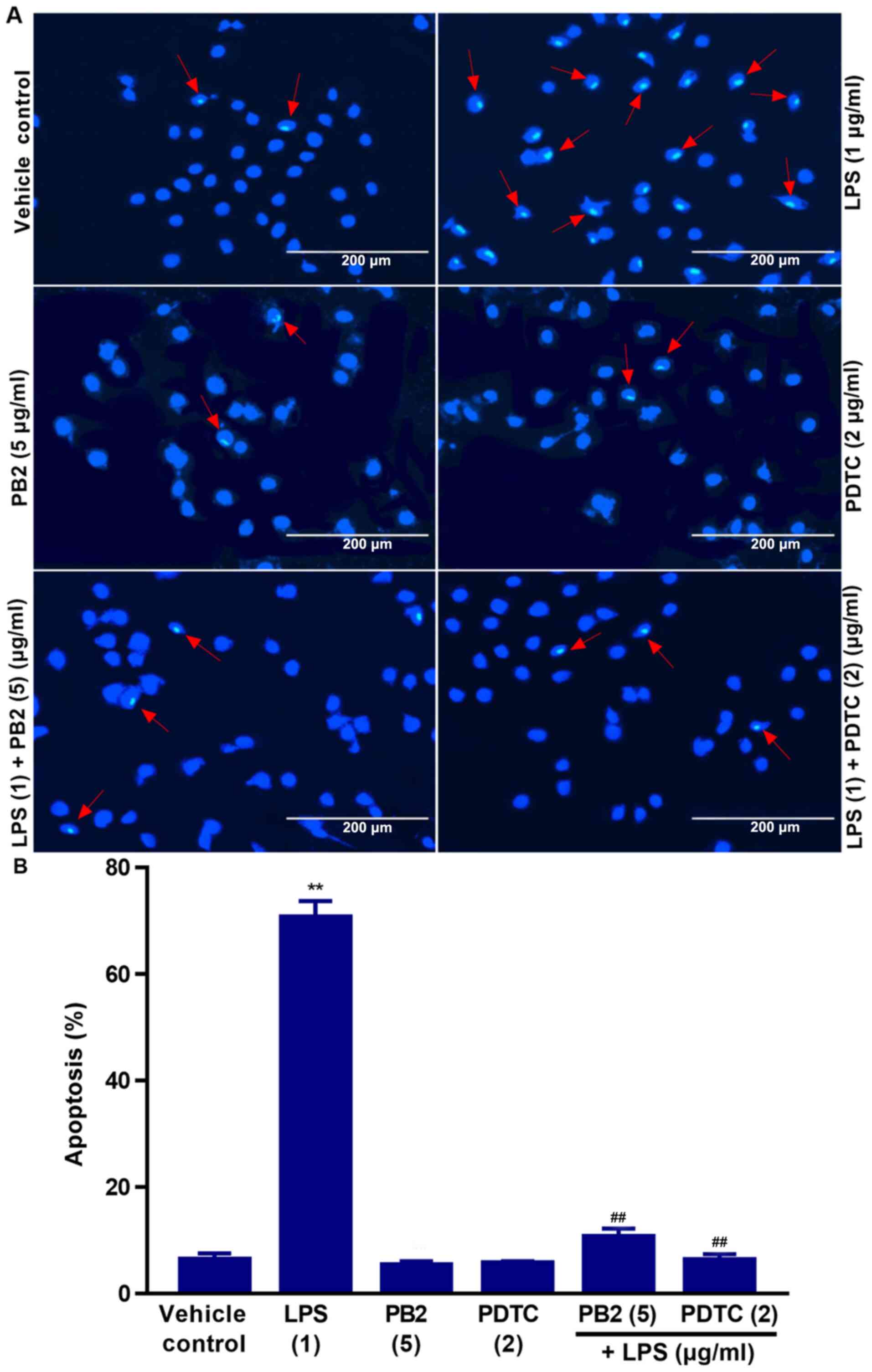
Effect of PB2 on LPS-induced HUVEC
apoptosis
Hoechst 33258 staining is used for the rapid
detection of cellular apoptosis by observing chromatin condensation
via fluorescence microscopy (34).
Therefore, the protective effect of PB2 on LPS-induced apoptosis
was assessed by performing Hoechst 33258 staining assays.
The vehicle control group displayed typical features
of HUVECs as demonstrated by normal blue fluorescence in the cell
nuclei (Fig. 2A). However,
apoptotic cells with condensation of nuclear chromatin and
fragmentation, which were stained white, were observed in
LPS-treated cells. The percentage of apoptotic cells was clearly
reduced in LPS-treated cells pretreated with PB2 or PDTC compared
with cells treated with LPS alone (Fig.
2B). However, PB2 or PDTC treatment alone displayed no
significant effect on HUVEC apoptosis compared with vehicle control
group. LPS significantly induced apoptosis (70.67±2.12% apoptotic
cells) compared with the vehicle control group (6.41±1.26%
apoptotic cells); however, PB2 or PDTC pretreatment notably
attenuated LPS-induced HUVEC apoptosis. The results indicated that
PB2 markedly inhibited LPS-induced HUVEC apoptosis.
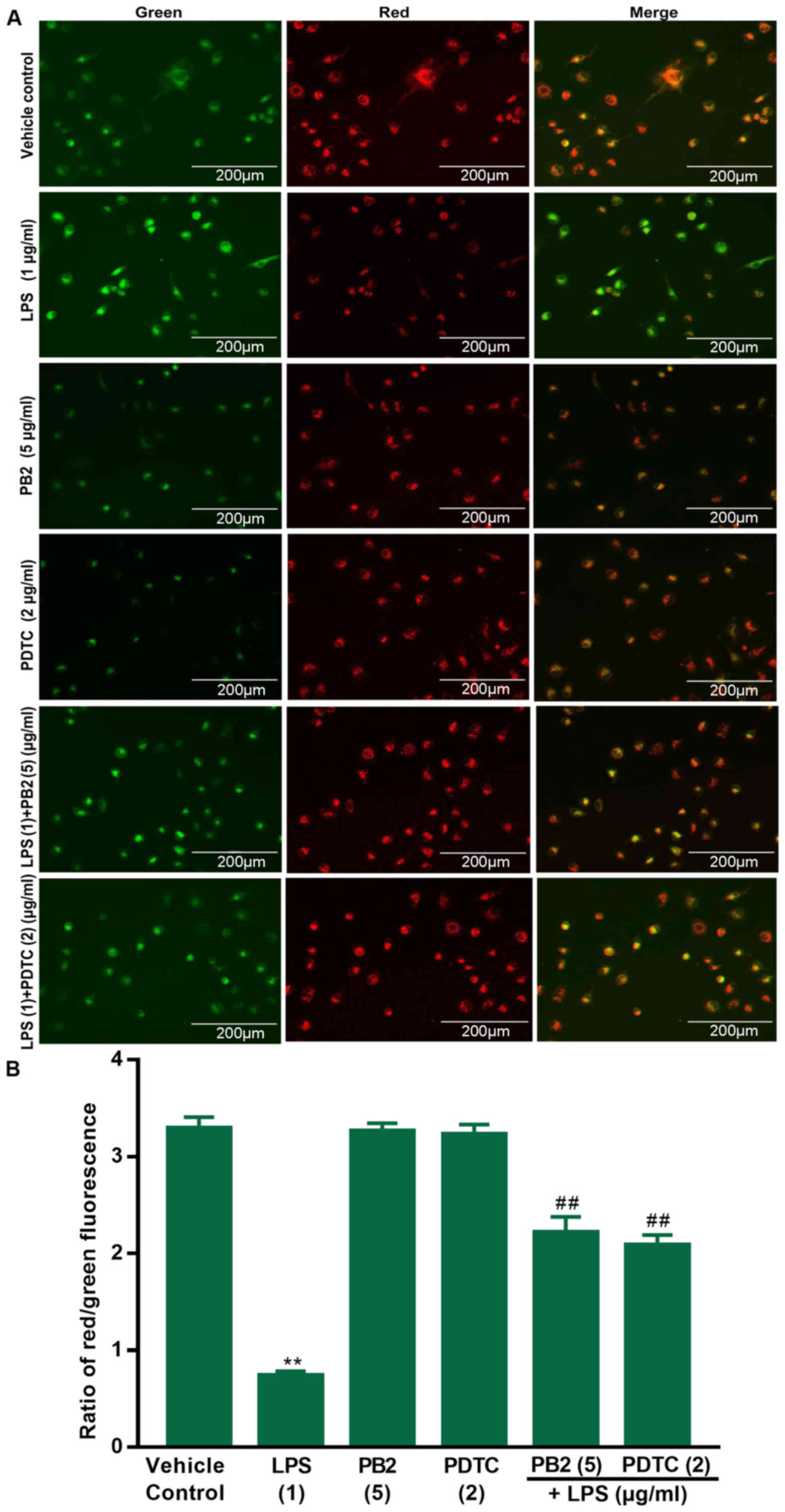
PB2 reverses LPS-induced reductions in
the mitochondrial membrane potential in HUVECs
A previous study demonstrated that declined
mitochondrial membrane potential is a hallmark of early apoptosis
(35). Therefore, the effects of
PB2 on LPS-induced reductions in the mitochondrial membrane
potential in HUVECs were assessed via JC-1 fluorescence. The red
fluorescence intensity and red/green ratio were significantly
reduced by LPS compared with the vehicle control group in HUVECs
(Fig. 3). The red fluorescence
intensity and ratio of red/green fluorescence were notably
increased in cells treated with PB2 or PDTC compared with the LPS
group. The results demonstrated that compared with the vehicle
control group, LPS significantly decreased the mitochondrial
membrane potential, but pretreatment with PB2 or PDTC reversed
LPS-mediated effects on the mitochondrial membrane potential in
HUVECs. Therefore, the results suggested that PB2-induced
inhibition of LPS-induced apoptosis might be mediated, at least in
part, via attenuating LPS-induced reductions in the mitochondrial
membrane potential in HUVECs.
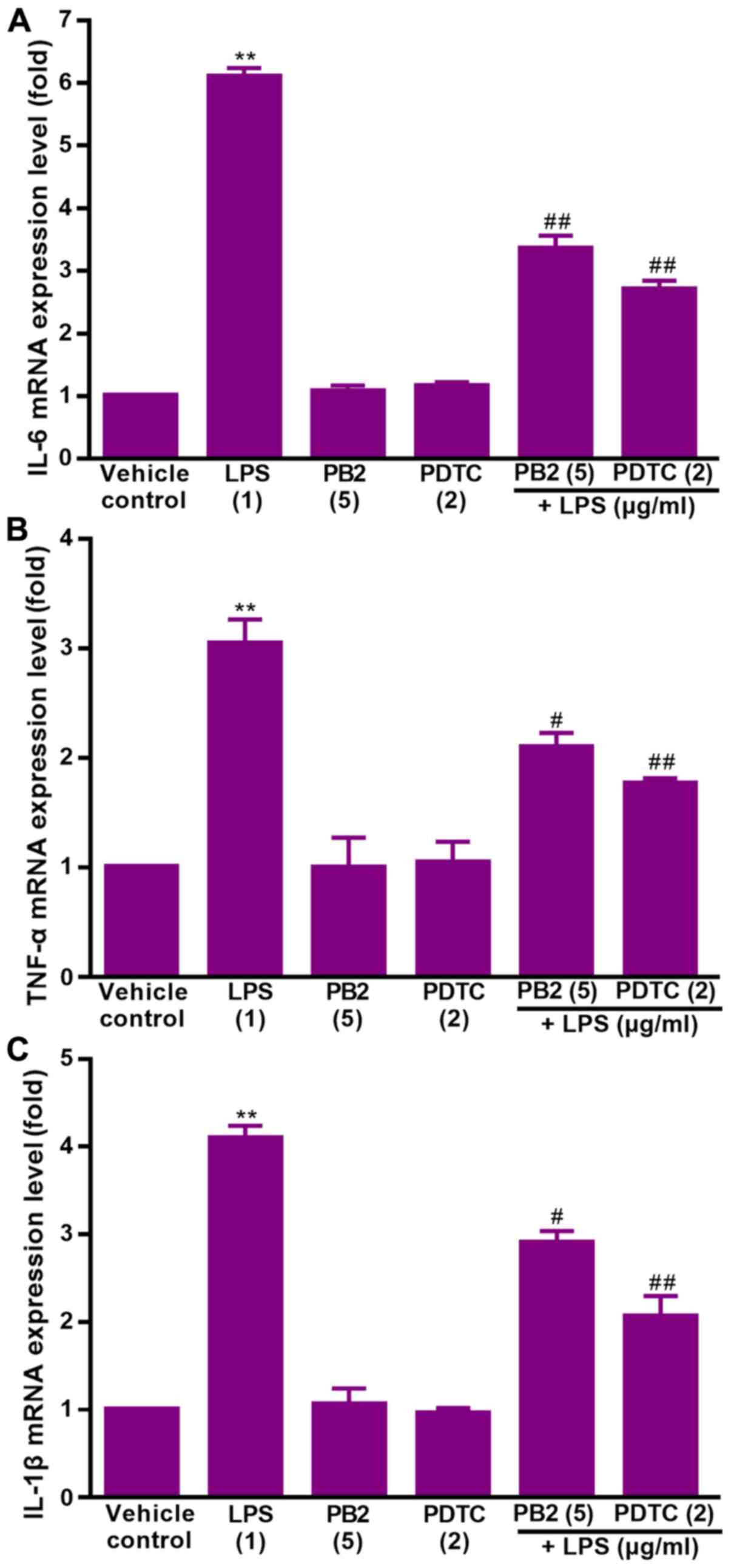
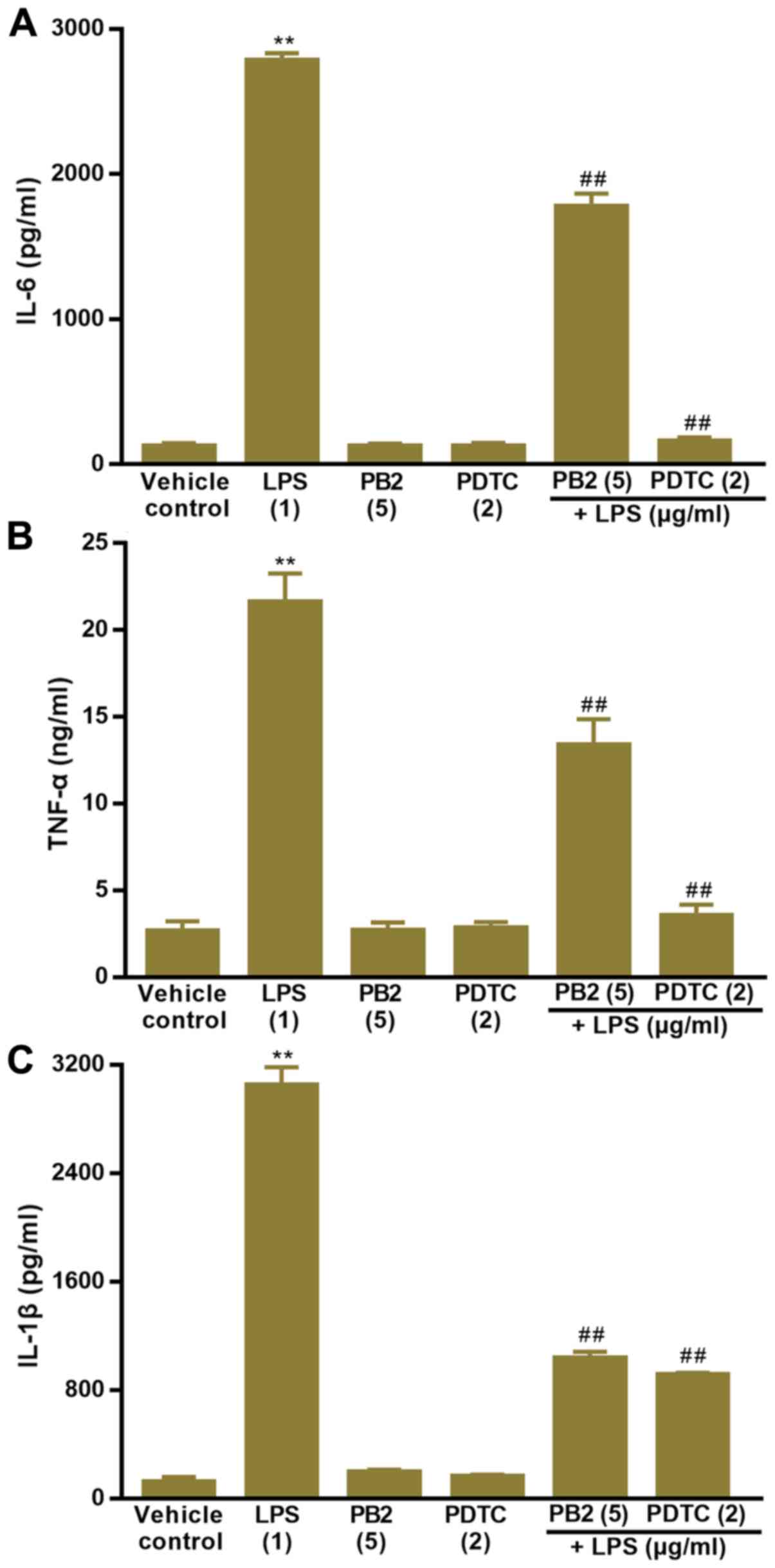
Effect of PB2 on LPS-induced
upregulation of IL-1β, IL-6 and TNF-α mRNA expression levels in
HUVECs
Key proinflammatory cytokines, including IL-1β, IL-6
and TNF-α, are involved in a variety of cellular activities, such
as proliferation, differentiation and apoptosis, and serve an
important role in the immune response to inflammation (36). LPS can stimulate the production of
IL-1β, IL-6, and TNF-α via different modes in human
monocytes/macrophages (37).
Therefore, the effect of PB2 on LPS-induced upregulation of IL-1β,
IL-6 and TNF-α mRNA expression levels was assessed.
The effect of PB2 on LPS-induced upregulation of
IL-1β, IL-6 and TNF-α mRNA expression levels in HUVECs was assessed
via RT-qPCR (Fig. 4). The results
demonstrated that IL-1β, IL-6 and TNF-α mRNA expression levels were
significantly increased by LPS compared with the vehicle control
group, but pretreatment of LPS-treated cells with PB2 or PDTC
notably reversed LPS-induced alterations to mRNA expression levels
in HUVECs.
Effect of PB2 on LPS-induced
upregulation of IL-1β, IL-6 and TNF-α protein levels in HUVECs
The effect of PB2 on LPS-induced increases in IL-1β,
IL-6 and TNF-α protein levels was assessed by performing ELISAs
(Fig. 5). The results also
demonstrated that IL-1β, IL-6 and TNF-α protein levels were
significantly increased by LPS compared with the vehicle control
group in HUVECs. However, pretreatment of LPS-treated cells with
PB2 or PDTC clearly decreased LPS-induced increases in the protein
levels of IL-1β, IL-6 and TNF-α in HUVECs.
Effect of PB2 on the Bcl-2/Bax
signalling pathway in HUVECs
The Bcl-2/Bax signalling pathway serves a critical
role in apoptosis (38). It was
hypothesized that the antiapoptotic effect of PB2 might be mediated
via regulating the Bcl-2/Bax signalling pathway. Therefore, the
effects of PB2 on Bcl-2, Bax, cleaved caspase-3, cleaved caspase-7
and cleaved caspase-9 expression levels in HUVECs were analysed via
western blotting (Fig. 6). The
protein expression levels of Bax, cleaved caspase-3, cleaved
caspase-7 and cleaved caspase-9 were significantly upregulated, but
Bcl-2 protein expression levels were significantly downregulated by
LPS compared with the vehicle control, PDTC or PB2 groups.
Moreover, pretreatment with PB2 notably reversed LPS-induced
elevations in the protein expression levels of Bax, cleaved
caspase-3, cleaved caspase-7 and cleaved caspase-9, but upregulated
the protein expression levels of Bcl-2. The results obtained from
the pretreatment with PDTC was similar to that of PB2. The results
suggested that the antiapoptotic effect of PB2 was related to the
Bcl-2/Bax signalling pathway in HUVECs.
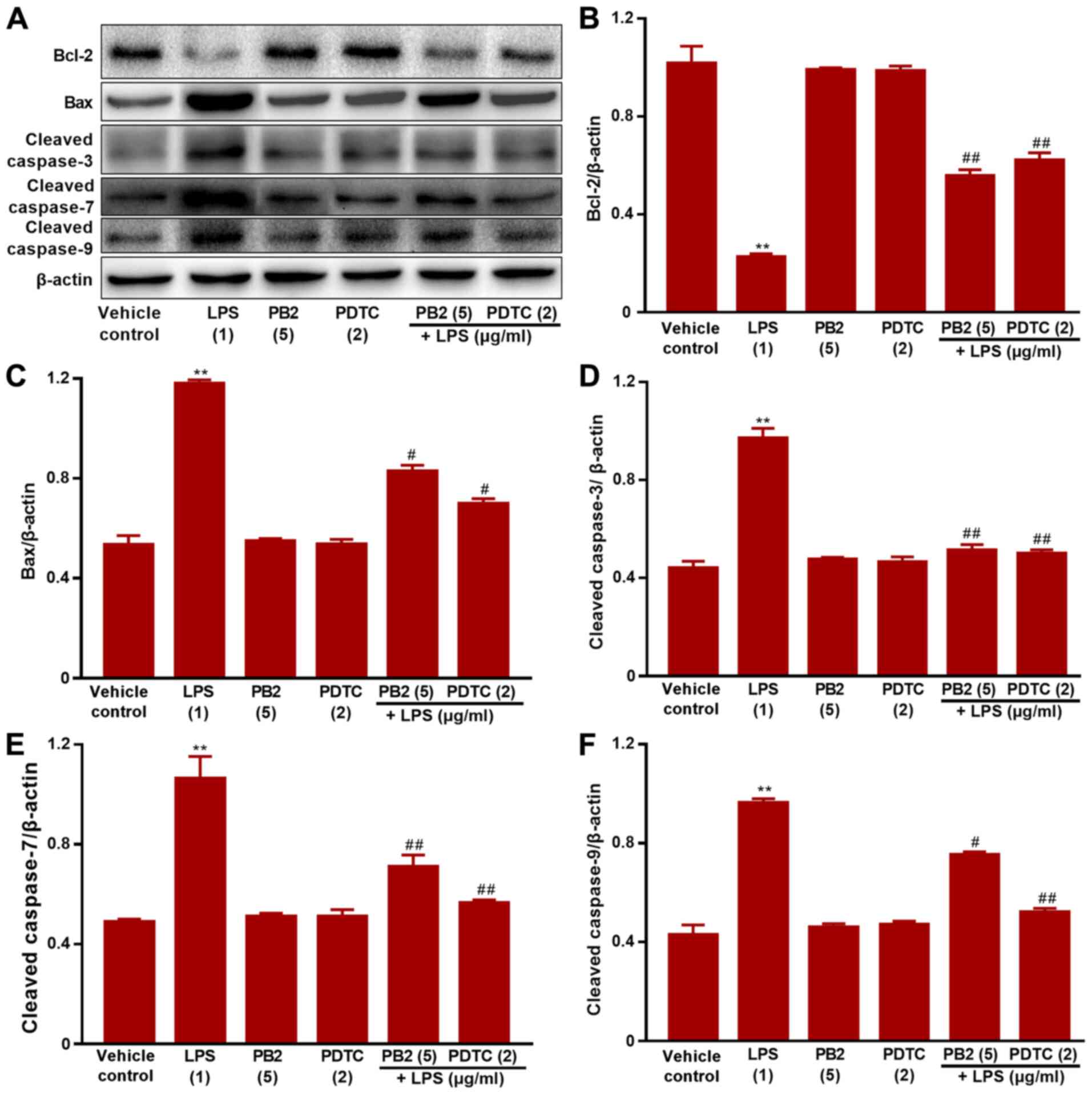 | Figure 6.Effects of LPS, PB2 and PDTC on
Bcl-2, Bax, cleaved caspase-3, cleaved caspase-7 and cleaved
caspase-9 protein expression levels in HUVECs. Cells were treated
with serum-free medium for 24 h in the vehicle control group; cells
were treated with serum-free medium for 12 h followed by LPS (1
µg/ml) for 12 h in the LPS group; cells were treated with
serum-free medium for 12 h followed by PB2 (5 µg/ml) for 12 h in
the PB2 group; cells were treated with serum-free medium for 12 h
followed by PDTC (2 µg/ml) for 12 h in the PDTC group; cells were
treated with PB2 (5 µg/ml) for 12 h followed by LPS (1 µg/ml) for
12 h in the LPS + PB2 group; cells were treated with PDTC (2 µg/ml)
for 12 h followed by LPS (1 µg/ml) for 12 h in the LPS + PDTC
group. Protein expression levels in HUVECs were (A) determined via
western blotting and semi-quantified for (B) Bcl-2, (C) Bax, (D)
cleaved caspase-3, (E) cleaved caspase-7 and (F) cleaved caspase-9.
β-actin was used as the loading control. Data are presented as the
mean ± SD of at least three independent experiments run in
triplicate (n=3). Data were analysed using one-way ANOVA followed
by Tukey's post hoc test. **P<0.01 vs. vehicle control;
#P<0.05, ##P<0.01 vs. LPS. LPS,
lipopolysaccharide; PB2, procyanidin B2; PDTC,
pyrrolidinedithiocarbamate ammonium; HUVEC, human umbilical vein
endothelial cell. |
Effect of PB2 on inhibition of the
NF-κB signalling pathway in HUVECs
The NF-κB signalling pathway serves a vital role in
regulating cell survival, apoptosis and cytokine production
(17). Activation of NF-κB involves
the phosphorylation and nuclear translocation of NF-κB p65 protein
(39). Therefore, the protein
expression levels of p-IκB-α, total IκB-α, p-IκB-β, total IκB-β,
p-NF-κB p65 and total NF-κB p65 in HUVECs were determined via
western blotting (Fig. 7). LPS
significantly upregulated the protein expression levels of p-NF-κB
p65, p-IκB-α and p-IκB-β compared with the vehicle control group in
HUVECs. Pretreatment of LPS-treated cells with PB2 clearly reversed
LPS-mediated alterations to p-NF-κB p65, p-IκB-α and p-IκB-β
protein expression levels in HUVECs. In addition, pretreatment of
LPS-treated cells with PDTC, a potent inhibitor of NF-κB, also
notably reversed LPS-mediated alterations to p-NF-κB p65, p-IκB-α
and p-IκB-β protein expression levels in HUVECs. The results
suggested that the antiapoptotic effect of PB2 might be mediated
via regulation of NF-κB signalling pathway-related proteins in
HUVECs.
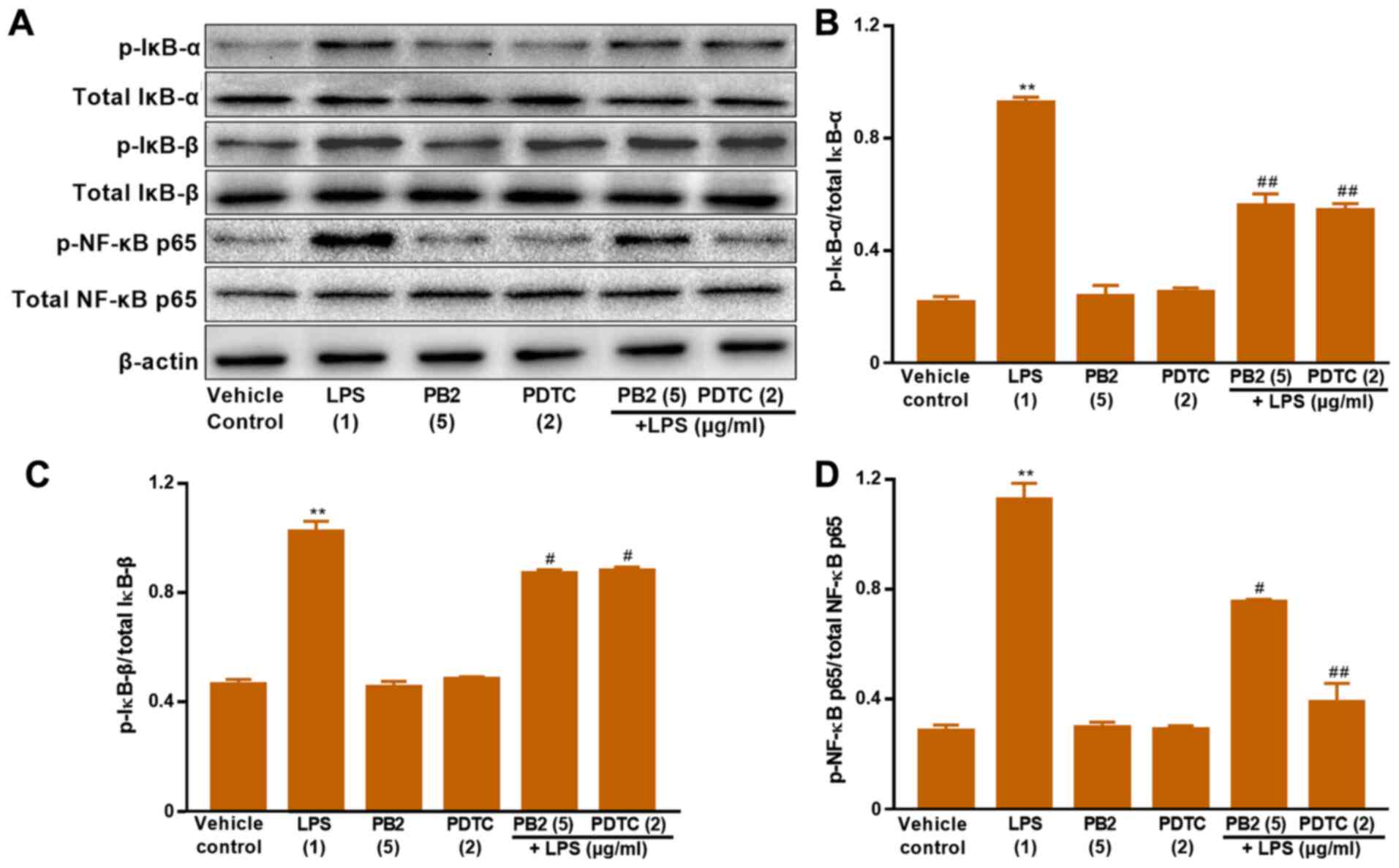 | Figure 7.Effects of LPS, PB2 and PDTC on
p-IκB-α, p-IκB-β, p-NF-κB p65 and total NF-κB p65 protein
expression levels in HUVECs. Cells were treated with serum-free
medium for 24 h in the vehicle control group; cells were treated
with serum-free medium for 12 h followed by LPS (1 µg/ml) for 12 h
in the LPS group; cells were treated with serum-free medium for 12
h followed by PB2 (5 µg/ml) for 12 h in the PB2 group; cells were
treated with serum-free medium for 12 h followed by PDTC (2 µg/ml)
for 12 h in the PDTC group; cells were treated with PB2 (5 µg/ml)
for 12 h followed by LPS (1 µg/ml) for 12 h in the LPS + PB2 group;
cells were treated with PDTC (2 µg/ml) for 12 h followed by LPS (1
µg/ml) for 12 h in the LPS + PDTC group. Protein expression levels
in HUVECs were (A) determined via western blotting and
semi-quantified for (B) p-IκB-α/total IκB-α, (C) p-IκB-β/total
IκB-β and (D) p-NF-κB p65/total NF-κB p65. β-actin was used as the
loading control. Data are presented as the mean ± SD of at least
three independent experiments run in triplicate (n=3). Data were
analysed using one-way ANOVA followed by Tukey's post hoc test.
**P<0.01 vs. vehicle control; #P<0.05,
##P<0.01 vs. LPS. LPS, lipopolysaccharide; PB2,
procyanidin B2; PDTC, pyrrolidinedithiocarbamate ammonium; p,
phosphorylated; HUVEC, human umbilical vein endothelial cell. |
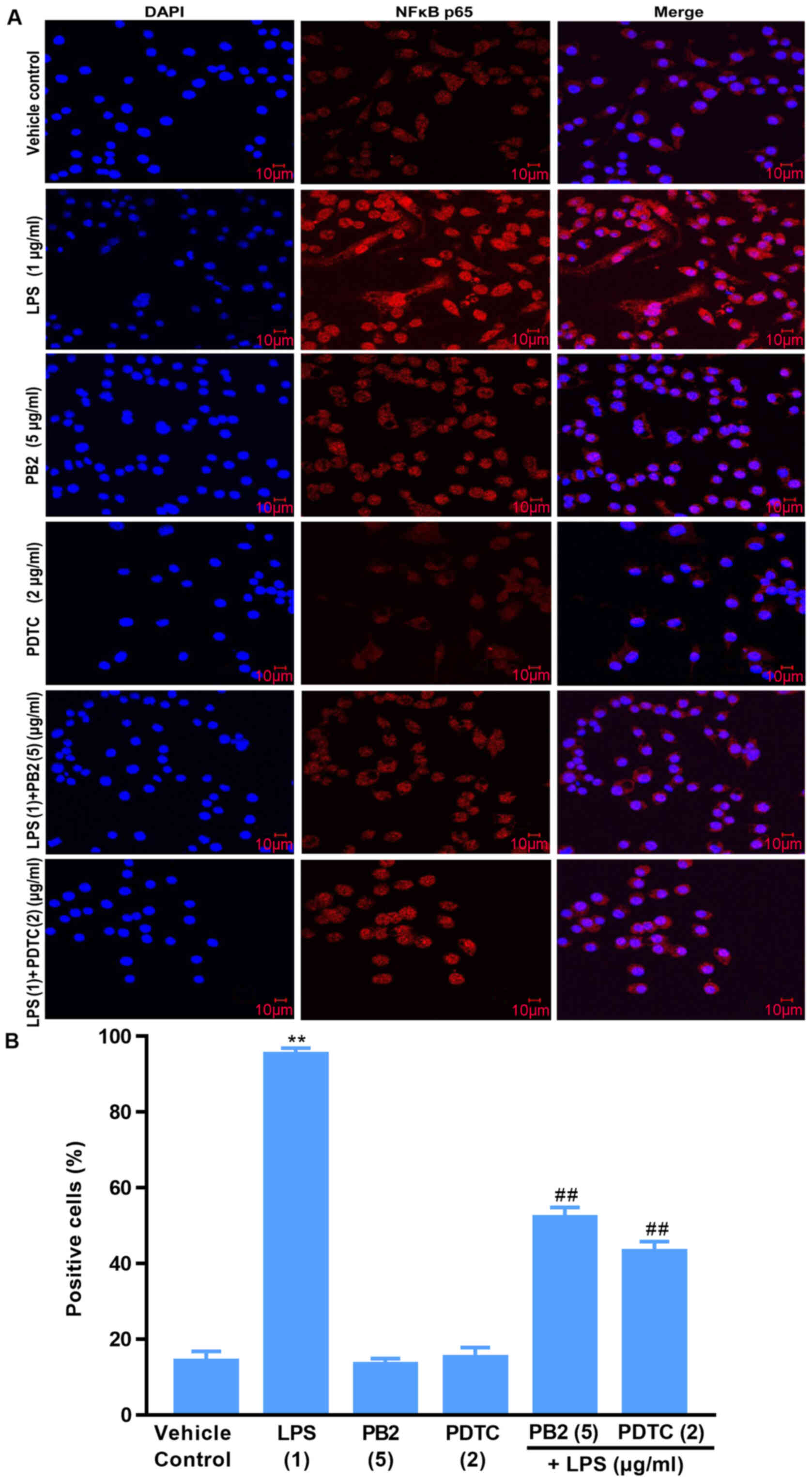
Effect of PB2 on the phosphorylation
and nuclear translocation of NF-κB p65 in HUVECs
Activation of NF-κB signalling is associated with
the translocation of the NF-κB p65 protein from the cytoplasm into
the nucleus, which is required for the release of cytokines and the
expression of apoptosis-related proteins (15). Thus, the localization of NF-κB p65
in HUVECs was investigated (Fig.
8). Compared with the vehicle control, PB2 and PDTC groups, LPS
induced significant translocation of NF-κB p65 from the cytoplasm
to the nucleus in HUVECs. However, pretreatment of LPS-treated
cells with PB2 or PDTC clearly inhibited LPS-induced nuclear
translocation of NF-κB p65 in HUVECs. The results demonstrated that
PB2 and PDTC effectively inhibited LPS-induced nuclear
translocation of NF-κB p65 in HUVECs, indicating that the
antiapoptotic effects of PB2 and PDTC might be mediated via
suppressing the NF-κB signalling pathway in HUVECs.
Discussion
The active ingredients from natural plants display a
variety of biological effects and thus have received increasing
attention for the development of novel therapeutics for various
diseases (40). Numerous studies
have reported that medicines extracted from natural plants
typically display fewer side effects compared with traditional
medicines (41–44). Procyanidins are widely present in
plants and are composed of a monomer, oligoprocyanidins and polymer
proeyanidins (45). PB2 displayed
potential pharmacological activity and a multitude of beneficial
effects, including scavenging free radicals, antioxidation,
anticancer, anti-inflammation, antiapoptosis and
antiatherosclerosis effects (31).
However, the molecular mechanism underlying the antiapoptotic
effects mediated by PB2 is not completely understood.
The present study demonstrated that pretreatment
with PB2 significantly attenuated LPS-mediated inhibition of cell
viability, although PB2 (≤5 µg/ml) did not display cytotoxic
effects on HUVECs. The results indicated the protective effect of
PB2 against LPS-induced cytotoxicity in HUVECs. Furthermore, PB2
markedly reduced the cell apoptotic ratio from >70% in the LPS
group to <10% in the LPS + PB2 group. The Hoechst 33258 staining
assay results indicated the protective effect of PB2 on
LPS-mediated inhibition of cell viability might be mediated via its
antiapoptotic effect in HUVECs. In addition, LPS significantly
decreased the mitochondrial membrane potential compared with the
vehicle control group, but PB2 reversed LPS-induced reductions in
the mitochondrial membrane potential in HUVECs. The results
suggested that the antiapoptotic effect of PB2 on LPS-induced
HUVECs was associated with regulation of the mitochondrial membrane
potential.
Key proinflammatory cytokines, such as IL-1β, IL-6
and TNF-α, serve an important role in the immune response to
inflammation and are closely related to a variety of cellular
activities including proliferation, differentiation and apoptosis
(36,46,47).
Therefore, the effects of PB2 on LPS-induced upregulation of IL-1β,
IL-6 and TNF-α mRNA and protein expression levels were assessed by
performing RT-qPCR and ELISAs, respectively. The results
demonstrated that compared with the vehicle control group, IL-1β,
IL-6 and TNF-α protein levels were significantly increased to
~3050, 2780 pg/ml and 21.57 ng/ml by LPS, respectively. The results
of the present study were consistent with a previous study that
reported that the production of IL-1β, IL-6, and TNF-α was
significantly increased by LPS in human monocytes/macrophages
(37). Interestingly, pretreatment
with PB2 or PDTC significantly decreased LPS-induced increases in
IL-1β, IL-6 and TNF-α mRNA and protein expression levels in HUVECs.
Therefore, the results of the present study demonstrated that PB2
and PDTC effectively inhibited LPS-induced upregulation of IL-1β,
IL-6 and TNF-α mRNA expression and protein levels.
Bcl-2 and Bax genes are the most important genes in
the regulation of apoptosis, and the Bcl-2/Bax signalling pathway
serves an important role in the process of cellular apoptosis
(7,8). The results of the present study
indicated that compared with the vehicle control group, LPS
significantly upregulated Bax, caspase-3, caspase-7 and caspase-9
expression levels, but markedly downregulated Bcl-2 protein
expression levels in HUVECs. Interestingly, pretreatment with PB2
notably downregulated LPS-mediated alterations to protein
expression levels in HUVECs. The results suggested that the
inhibitory effect of PB2 against LPS-induced apoptosis might be
associated with regulation of the Bax/Bcl-2 signalling pathway in
HUVECs.
NF-κB serves an important role in stress,
inflammation and immune response via control of DNA transcription,
cell survival, apoptosis and cytokine production (48). IKK is rapidly activated and is
responsible for the phosphorylation of IκB upon stimulation by
external signals or stress, targeting IκB to ubiquitin-mediated
protein degradation (49). IκB-α
and IκB-β are the major signal-responsive isoforms in the IκB
family that are responsible for promoting and terminating NF-κB
activation during persistent stimulation (18). Therefore, the effects of PB2 on
LPS-mediated alterations to IκB-α, IκB-β, p-NF-κB p65 and total
NF-κB p65 protein expression levels, as well as the localization of
NF-κB p65 in HUVECs were investigated. The results suggested that
PB2 may serve a crucial role in inhibiting LPS-induced upregulation
of IκB-α, IκB-β, p-NF-κB p65 expression levels, and nuclear
translocation of p-NF-κB p65.
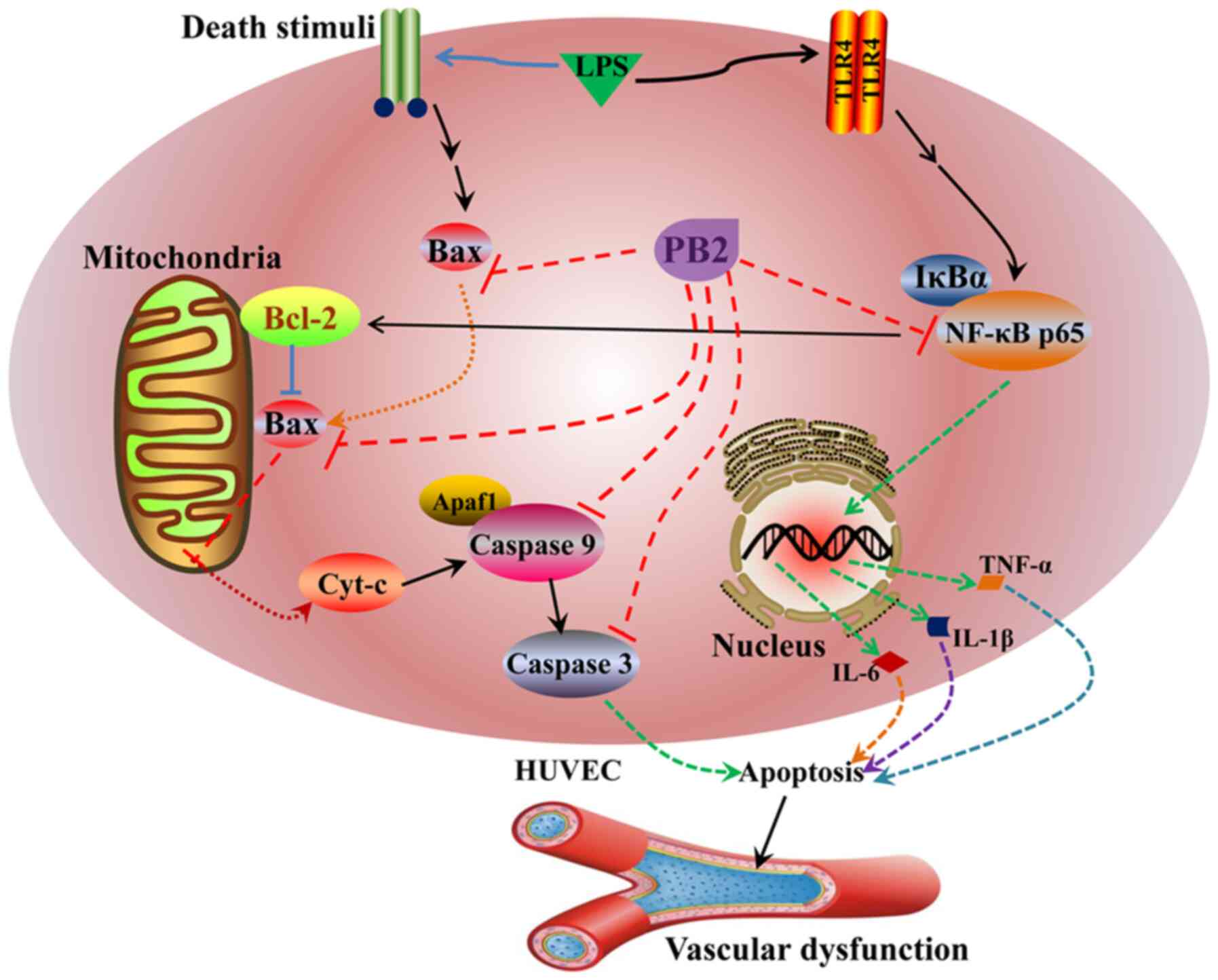
The potential mechanisms underlying the protective
effects of PB2 against LPS-induced cytotoxicity and apoptosis in
HUVECs identified in the present study are presented in Fig. 9. However, the molecular mechanisms
underlying the effects of PB2 are likely complicated, thus other
cellular signalling pathways require further investigation. The
present study provided novel insights into the protective effect of
PB2 against LPS in HUVECs, which might be important for the
pharmacological basis and future clinical application of PB2 for
the treatment of infectious vascular diseases. However, the in
vitro results of the present study require verification using
appropriate in vivo animal models and clinical trials.
In conclusion, the present study suggested that LPS
induced cytotoxicity and apoptosis in HUVECs by decreasing the
mitochondrial membrane potential and upregulating the mRNA
expression and protein levels of key proinflammatory cytokines,
including IL-1β, IL-6 and TNF-α. Moreover, compared with the
vehicle control group, LPS also significantly upregulated Bax,
cleaved caspase-3, cleaved caspase-7, cleaved caspase-9 and
p-NF-κB-p65 expression levels, but downregulated Bcl-2, p-IκB-α and
p-IκB-β protein expression levels, and promoted the translocation
of NF-κB p65 from the cytoplasm to nucleus in HUVECs.
Interestingly, PB2 attenuated LPS-induced cytotoxicity and
apoptosis, and reversed LPS-mediated reductions in the
mitochondrial membrane potential in HUVECs. PB2 also clearly
inhibited LPS-induced upregulation of Bax, cleaved caspase-3,
cleaved caspase-7, cleaved caspase-9, p-IκB-α, p-IκB-β and
p-NF-κB-p65 expression levels, and reversed LPS-induced
downregulation of Bcl-2 protein expression levels. Furthermore, PB2
inhibited LPS-induced NF-κB p65 translocation from the cytoplasm to
the nucleus in HUVECs. The possible molecular mechanism underlying
the protective effects of PB2 against LPS-induced cytotoxicity and
apoptosis in HUVECs might be mediated via inhibiting the Bcl-2/Bax
and NF-κB signalling pathways. Therefore, PB2 may serve as a novel
therapeutic agent for infectious vascular diseases.
Acknowledgements
Not applicable.
Funding
This study was supported by the Education Department
of Sichuan Province (grant nos. 13ZB0267 and 14ZA0143), the Joint
Research Fund of Technology Bureau of Luzhou City Government and
Luzhou Medical University (grant nos. 14JC0181 and 2013LZLY-J52)
and the Distinguished Professor Research Startup Funding (S. Cao)
from Southwest Medical University (grant no. 2015-RCYJ0002).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ and SSC designed the experiments and analysed the
data. DQS, FW, MHL, XFL, JL and ZZ performed the experiments. DQS
and SSC wrote the manuscript. All authors discussed the results.
DQS, JL, FW, SSC and XJ confirmed the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PB2
|
procyanidin B2
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
LPS
|
lipopolysaccharide
|
|
Cyt-c
|
cytochrome c
|
|
PDTC
|
pyrrolidinedithiocarbamate
ammonium
|
References
|
1
|
Gimbrone MJ and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yao Z, Mates JM, Cheplowitz AM, Hammer LP,
Maiseyeu A, Phillips GS, Wewers MD, Rajaram MV, Robinson JM,
Anderson CL and Ganesan LP: Blood-borne lipopolysaccharide is
rapidly eliminated by liver sinusoidal endothelial cells via
high-density lipoprotein. J Immunol. 197:2390–2399. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li L, Wan G, Han B and Zha ZW:
Echinacoside alleviated LPS-induced cell apoptosis and inflammation
in rat intestine epithelial cells by inhibiting the mTOR/STAT3
pathway. Biomed Pharmacother. 104:622–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang Z and Ren C: Emodin attenuates
apoptosis and inflammation induced by LPS through up-regulating
lncRNA TUG1 in murine chondrogenic ATDC5 cells. Biomed
Pharmacother. 103:897–902. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma H, Su L, He X and Miao J: Loss of
HMBOX1 promotes LPS-induced apoptosis and inhibits LPS-induced
autophagy of vascular endothelial cells in mouse. Apoptosis.
24:946–957. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohan S, Abdelwahab SI, Kamalidehghan B,
Syam S, May KS, Harmal NS, Shafifiyaz N, Hadi AH, Hashim NM,
Rahmani M, et al: Involvement of NF-κB and Bcl2/Bax signaling
pathways in the apoptosis of MCF7 cells induced by a xanthone
compound Pyranocycloartobiloxanthone A. Phytomedicine.
19:1007–1015. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lindsay J, Esposti MD and Gilmore AP:
Bcl-2 proteins and mitochondria-specificity in membrane targeting
for death. Biochim Biophys Acta. 1813:532–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chalah A and Khosravi-Far R: The
mitochondrial death pathway. Adv Exp Med Biol. 61:525–545.
2008.
|
|
9
|
Zhang Z, Liang Z, Li H, Li C, Yang Z, Li
Y, She D, Cao L, Wang W, Liu C and Chen L: Perfluorocarbon reduces
cell damage from blast injury by inhibiting signal paths of NF-κB,
MAPK and Bcl-2/Bax signaling pathway in A549 cells. PLoS One.
12:e01738842017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Yang X, Ge XH and Zhang FY:
Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved
caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid
hemorrhage mice. Biomed Pharmacother. 109:726–733. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin P, Zhou B, Yao HY and Guo YP: Effect
of carboplatin injection on Bcl-2 protein expression and apoptosis
induction in Raji cells. Eur J Histochem. 64:31342020. View Article : Google Scholar
|
|
12
|
Mao ZX, Xia W, Wang J, Chen T, Zeng Q, Xu
B, Li W, Chen X and Xu S: Perfluorooctane sulfonate induces
apoptosis in lung cancer A549 cells through reactive oxygen
species-mediated mitochondrion-dependent pathway. J Appl Toxicol.
33:1268–1276. 2013.PubMed/NCBI
|
|
13
|
Ke WW, Zhao XX and Lu ZM: Foeniculum
vulgare seed extract induces apoptosis in lung cancer cells partly
through the down-regulation of Bcl-2. Biomed Pharmacother.
135:1112132021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parrish AB, Freel CD and Kornbluth S:
Cellular mechanisms controlling caspase activation and function.
Cold Spring Harb Perspect Biol. 5:a0086722013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu DD, Zhang J, Deng W, Yip YL, Lung HL,
Tang CM, Law WT, Yang J, Lau VM, Shuen WH, et al: Significance of
NF-κB activation in immortalization of nasopharyngeal epithelial
cells. Int J Cancer. 138:1175–1185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Zhou G, Wang Z, Guo X, Xu Q, Huang
Q and Su L: NF-κB signaling is essential for resistance to heat
stress-induced early stage apoptosis in human umbilical vein
endothelial cells. Sci Rep. 5:1351472015.
|
|
18
|
Hoffmann A and Baltimore D: Circuitry of
nuclear factor KappaB signaling. Immunol Rev. 210:171–186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun SC, Ganchi PA, Ballard DW and Greene
WC: NF-kappa B controls expression of inhibitor I kappa B alpha:
Evidence for an inducible autoregulatory pathway. Science.
259:1912–1915. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arenzana-Seisdedos F, Thompson J,
Rodriguez MS, Bachelerie F, Thomas D and Hay RT: Inducible nuclear
expression of newly synthesized I kappa B alpha negatively
regulates DNA-binding and transcriptional activities of NF-kappa B.
Mol Cell Biol. 15:2689–2696. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar
|
|
22
|
Sakano K, Mizutani M, Murata M, Oikawa S,
Hiraku Y and Kawanishi S: Procyanidin B2 has anti-and pro-oxidant
effects on metal-mediated DNA damage. Free Radic Biol Med.
39:1041–1049. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gopalakrishnan S, Ediga HH, Reddy SS,
Reddy GB and Ismail A: Procyanidin-B2 enriched fraction of cinnamon
acts as a proteasome inhibitor and anti-proliferative agent in
human prostate cancer cells. IUBMB Life. 70:445–457. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chai WM, Lin MZ, Feng HL, Zou ZR and Wang
YX: Proanthocyanidins purified from fruit pericarp of Clausena
lansium (Lour.) Skeels as efficient tyrosinase inhibitors:
Structure evaluation, inhibitory activity and molecular mechanism.
Food Funct. 8:1043–1051. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu D: Effects of procyanidin on
cardiomyocyte apoptosis after myocardial ischemia reperfusion in
rats. BMC Cardiovasc Disord. 18:3512018. View Article : Google Scholar
|
|
26
|
Yang H, Xiao L, Yuan Y, Luo X, Jiang M, Ni
J and Ni JH: Procyanidin B2 inhibits NLRP3 inflammasome activation
in human vascular endothelial cells. Biochem Pharmacol. 92:599–606.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang Y, Wang X, Yang W and Gui S:
Procyanidin B2 suppresses Lipopolysaccharides-induced inflammation
and apoptosis in human type II alveolar epithelial cells and lung
fibroblasts. J Interferon Cytokine Res. 40:54–63. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li XL, Li BY, Cheng M, Yu F, Yin WB, Cai
Q, Zhang Z, Zhang JH, Wang JF, Zhou RH and Gao HQ: PIMT prevents
the apoptosis of endothelial cells in response to glycated low
density lipoproteins and protective effects of grape seed
procyanidin B2. PLoS One. 8:e699792013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu QJ, Wang YQ and Qi YX: The protective
effect of procyanidin against LPS-induced acute gut injury by the
regulations of oxidative state. Springerplus. 5:16452016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong F, Zhou X, Li C, Yan S, Deng X, Cao
Z, Li L, Tang B, Allen TD and Liu J: Dihydroartemisinin targets
VEGFR2 via the NF-κB pathway in endothelial cells to inhibit
angiogenesis. Cancer Biol Ther. 15:1479–1488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang JQ, Gao WB, Wang J, Ren QL, Chen JF,
Ma Q, Zhang ZJ and Xing BS: Critical role of FoxO1 in granulosa
cell apoptosis caused by oxidative stress and protective effects of
grape seed procyanidin B2. Oxid Med Cell Longev. 2016:61473452016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martinez-Micaelo N, González-Abuín N,
Pinent M, Ardévol N and Blay M: Procyanidin B2 inhibits
inflammasome-mediated IL-1β production in
lipopolysaccharide-stimulated macrophages. Mol Nutr Food Res.
59:262–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crews L and Masliah E: Molecular
mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol
Genet. 19:R12–R20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wlodkowic D, Telford W, Skommer J and
Darzynkiewicz Z: Apoptosis and beyond: Cytometry in studies of
programmed cell death. Methods Cell Biol. 103:55–98. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Troy CM and Jean YY: Caspases: Therapeutic
targets in neurologic disease. Neurotherapeutics. 12:42–48. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zou H, Li Y, Liu X and Wang X: An APAF-1
cytochrome c multimeric complex is a functional apoptosome
that activates procaspase-9. J Biol Chem. 274:11549–11556. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robertson JD, Orrenius S and Zhivotovsky
B: Review: Nuclear events in apoptosis. J Struct Biol. 129:346–358.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Christian F, Smith EL and Carmody RJ: The
regulation of NF-κB subunits by phosphorylation. Cells. 5:122016.
View Article : Google Scholar
|
|
40
|
Sellami M, Slimeni O, Pokrywka A, Kuvačić
G, D Hayes L, Milic M and Padulo J: Herbal medicine for sports: A
review. J Int Soc Sports Nut. 15:142018. View Article : Google Scholar
|
|
41
|
Shen CY, Jiang JG, Yang L, Wang DW and Zhu
W: Anti-ageing active ingredients from herbs and nutraceuticals
used in traditional Chinese medicine: Pharmacological mechanisms
and implications for drug discovery. Br J Pharmacol. 174:1395–1425.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang YS, Shen CY and Jiang JG:
Antidepressant active ingredients from herbs and nutraceuticals
used in TCM: Pharmacological mechanisms and prospects for drug
discovery. Pharmacol Res. 150:1041202019. View Article : Google Scholar
|
|
43
|
Hughes K, Ho R, Butaud JF, Filaire E,
Ranouille E, Berthon JY and Raharivelomanana P: A selection of
eleven plants used as traditional Polynesian cosmetics and their
development potential as anti-aging ingredients, hair growth
promoters and whitening products. J Ethnopharmacol. 245:1121592019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gordobil O, Olaizola P, Banales JM and
Labidi J: Lignins from Agroindustrial by-products as natural
ingredients for cosmetics: Chemical structure and in vitro
sunscreen and cytotoxic activities. Molecules. 25:11312020.
View Article : Google Scholar
|
|
45
|
Hollands WJ, Voorspoels S, Jacobs G, Aaby
K, Meisland A, Garcia-Villalba R, Tomas-Barberan F, Piskula MK,
Mawson D, Vovk I, et al: Development, validation and evaluation of
an analytical method for the determination of monomeric and
oligomeric procyanidins in apple extracts. J Chromatogr A.
1495:46–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wan FY and Lenardo MJ: The nuclear
signaling of NF-kappaB: Current knowledge, new insights, and future
perspectives. Cell Res. 20:24–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6:a0162952014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang HJ, Wang M, Wang L, Cheng BF, Lin XY
and Feng ZW: NF-κB regulates caspase-4 expression and sensitizes
neuroblastoma cells to Fas-induced apoptosis. PLoS One.
10:e01179532015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Verhoeven RJ, Tong S, Zhang G, Zong J,
Chen Y, Jin DY, Chen MR, Pan J and Chen H: NF-κB signaling
regulates expression of Epstein-Barr virus BART microRNAs and long
noncoding RNAs in nasopharyngeal carcinoma. J Virol. 90:6475–6488.
2016. View Article : Google Scholar : PubMed/NCBI
|