Introduction
Diabetes is a chronic disease with worldwide
distribution that occurs when insufficient insulin is produced by
the pancreas, or insulin utilization in the body is defective
(1). At present, there are ~463
million (9.3%) adults ranging from 20–79 years old who have been
diagnosed with diabetes, of which 90% have type 2 diabetes mellitus
(T2DM) (2). The two main mechanisms
that result in T2DM are insulin resistance (IR) and insulin
secretory dysfunction (3). Skeletal
muscle is the most essential tissue that maintains glucose
homeostasis in the body (4). Under
normal conditions, skeletal muscle is responsible for 80% of the
insulin-induced glucose uptake and utilization of the whole body
(5). During IR, the expression of
proteins involved in GLUT4 translocation machinery in the muscle
was reported to impact glucose uptake (6). Insulin in the muscle closely
coordinates GLUT4 with insulin receptor substrate 1 (IRS1), PI3K
and AKT (7).
Nicotinamide (NAM) is the precursor of nicotinamide
adenine dinucleotide (NAD+), which is widely involved in
the regulation of energy metabolism, physiological rhythms and the
cellular redox state (8). NAM has
been reported to induce adverse effects; its 50% lethal dose (LD50)
was reported to be 2.5 g/kg via oral administration and 2.05 g/kg
via intraperitoneal administration in mice, with slightly higher
LD50s in rats (9). NAM can be
transformed into N1-methylnicotinamide (MNAM) after being
methylated by nicotinamide N-methyltransferase (10). MNAM is the urinary excretory form of
NAM and one of the catabolic metabolites of NAM, suggesting that
MNAM is soluble in water and easy to be excluded with urine
(9). MNAM possesses several
protective physiological properties, including antithrombotic and
anti-inflammatory effects, as well as providing protection in the
vascular system (10). Previous
studies have reported that MNAM can reduce fasting blood glucose
(FBG) and fasting insulin (FINS) levels in mice fed with a
saturated-fat diet (11). MNAM can
also improve IR, which has the potential to be useful in the
prevention or treatment of T2DM (12). However, the mechanisms via which
MNAM affects IR are not fully understood.
Sirtuin 1 (SIRT1) is an NAD+-dependent
deacetylase that is involved in insulin signal transduction in the
regulation of glycolipid metabolism (13). MNAM was reported to regulate
nutritional metabolism in liver via SIRT1 (11). One of the downstream regulators of
SIRT1 is peroxisome proliferator-activated receptor γ
coactivator-1α (PGC-1α) (14).
PGC-1α is a transcriptional coactivator that regulates
mitochondrial biosynthesis and respiration, and is reported to be
involved in a wide range of metabolic pathways (15). A saturated-fat diet has been
reported to induce IR and decrease PGC-1α expression in muscle
tissue (16). It has been
hypothesized that enhanced PGC-1α activity may protect the body
from lipid-induced IR (17). The
aim of the present study was to establish a mouse model of T2DM to
observe the effects of MNAM on IR and glucose metabolism.
Additionally, an in vitro IR model was established in muscle
cells to further explore the effects of MNAM on IR in skeletal
muscle and the regulatory mechanism of the SIRT1/PGC-1α signaling
pathway.
Materials and methods
Establishment of T2DM mouse model
A total of 20 specific pathogen-free-grade C57BL/6
mice and 30 ob/ob mice (males; weight, 20±5 g; age, 6–8 weeks) were
purchased from Beijing HFK Bio-Technology Co., Ltd. The animal
experiments were conducted in the Shengjing Hospital of China
Medical University. All mice were fed the standard laboratory diet
for four weeks. Body weights and FBG were measured weekly. When the
FBG of the ob/ob mice was >13.8 mmol/l, and the FBG and body
weights were higher than those of C57BL/6 mice, the T2DM model was
considered to be established successfully (18). The experimental protocols were
approved by the Animal Ethics Committee of Shengjing Hospital of
China Medical University (approval no. 2016PS340K).
Experimental animal grouping and
treatment
Mice were randomly divided into the following groups
(10 mice/group): C57BL/6 mice fed normal diet (control group);
C57BL/6 mice fed MNAM (Sigma-Aldrich; Merck KGaA) at a high dose
(1%; CMNAMH group); ob/ob T2DM model mice fed normal diet (DM
group); T2DM mice fed with MNAM at a low dose (0.3%; DMNAML group);
and T2DM mice fed with MNAM at a high dose (1%; DMNAMH group). Mice
in each group were fed with their respective diets continuously for
8 weeks. The body weights and FBG were measured every 2 weeks after
fasting for 12 h. Blood was collected from the tip of the tail, and
FBG was measured using a CONTOUR®PLUS Blood Glucose
Monitoring System 7600P (Bayer AG). Finally, mice were anesthetized
with 1% pentobarbital sodium (45 mg/kg) and sacrificed via
exsanguination, with blood collected from the abdominal aorta.
Determination of IR and sensitivity
indices
An insulin ELISA kit (cat. no. CEA448Mu; Wuhan USCN
Business Co., Ltd.) was used to detect the FINS level in the serum
from each group according to the manufacturer's instructions. The
serum was isolated from the blood through centrifugation at 200 × g
for 10 min at 4°C. The IR indices were calculated according to the
results and the equations of FBG and FINS as follows: Homeostatic
model assessment for insulin resistance (HOMA-IR): HOMA-IR = [FBG
(mmol/l) × FINS (mIU/l)]/22.5 (19); Quantitative insulin resistance check
index (QUICKI): QUICKI = 1/[log FBG (mg/dl) + log FINS (mIU/l)]
(20).
Determination of triglyceride (TG)
content
A TG ELISA kit (cat. no. CEB687Ge; Wuhan USCN
Business Co., Ltd.) was used to determine the TG content in
gastrocnemius muscle according to the manufacturer's protocols.
Samples were assayed using a model 680 microplate reader (Bio-Rad
Laboratories, Inc.); the TG content was calculated according to a
standard curve.
In vitro experiments
C2C12 cells, an immortalized mouse myoblast cell
line originally obtained by Yaffe and Saxel (21), were used for the in vitro
experiments. C2C12 cells were purchased from the American Type
Culture Collection and cultured in high-glucose DMEM (HyClone;
Cytiva) supplemented with 10% FBS (HyClone; Cytiva). When the cells
reached a logarithmic growth stage, the DMEM was supplemented with
2% horse serum (Sigma-Aldrich; Merck KGaA) to induce
differentiation. Cells were collected when 90% cells were
differentiated into myotubes. The experimental groups included a
blank control group (control group), cells supplemented with 0.75
mM palmitic acid (PA) to establish an IR muscle cell model (PA
group), cells supplemented with 0.75 mM PA + 1 mM MNAM (PM group),
and cells supplemented with 0.75 mM PA + 1 mM MNAM + 30 µM SIRT1
inhibitor, EX527 (Sigma-Aldrich; Merck KGaA) (PME group). Cells
were incubated at 37°C in 5% CO2 for 16 h, and then 100
nmol/l insulin was added 10 min before the cells were
harvested.
Detection of residual glucose
content
Following treatment of cells under each of the
aforementioned conditions, 2 µl supernatant was taken and added to
98 µl deionized water. According to the manufacturer's instructions
(cat. no. BC2500; Beijing Solarbio Science & Technology Co.,
Ltd.), the glucose content was calculated by detecting the
absorbance at 505 nm using a microplate reader.
Reverse transcription-quantitative
(RT-q)PCR
The relative mRNA levels of SIRT1 and PGC-1α in
skeletal muscle and C2C12 cells were determined using RT-qPCR.
Primers were designed using DNASTAR v8.1 (www.dnastar.com) according to the sequence information
published in GenBank (Table I).
Total RNAs from tissues or cells were extracted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
First-strand cDNA was synthesized using a PrimeScript RT kit (cat.
no. RR047A; Takara Bio, Inc.), and qPCR was conducted using
PrimeScript Master Mix (cat. no. RR036A; Takara Bio, Inc.). The
reaction conditions used in the experiment were as follows:
Pre-denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C
for 5 sec and 60°C for 20 sec. The relative mRNA expression levels
were calculated according to the 2−ΔΔCq method (22). GAPDH was used as the internal
reference.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Primer sequence
(5′→3′) |
|---|
| GAPDH | F:
TGTGTCCGTCGTGGATCTGA |
|
| R:
TTGCTGTTGAAGTCGCAGGAG |
| SIRT1 | F:
ACAGTGAGAAAATGCTGGC |
|
| R:
GCCACTGTCACTGTTACTGC |
| PGC-1α | F:
AGCAGAAAGCAATTGAAGAG |
|
| R:
AGGTGTAACGGTAGGTGATG |
Western blotting
Mouse gastrocnemius muscle tissues or C2C12 cells
were processed to form suspensions using RIPA lysis buffer (Santa
Cruz Biotechnology, Inc.) containing 1% PMSF (Beyotime Institute of
Biotechnology) to extract the total protein. The solutions were
centrifuged at 10,000 × g for 15 min, and the protein concentration
of the supernatant was determined using the BCA method. Then, 30 µg
of each sample was separated via 12% SDS-PAGE, blotted onto PVDF
membranes and blocked with 5% skim milk at room temperature for 1
h. The membranes were then washed three times with TBS-0.1%
Tween-20 (TBST), and primary antibodies against IRS1 (1:1,000; cat.
no. 2382; Cell Signaling Technology, Inc.), phosphorylated (p)-IRS1
(1:1,000; cat. no. 2385; Cell Signaling Technology, Inc.), PI3K
(1:1,000; cat. no. 4255; Cell Signaling Technology, Inc.), p-PI3K
(1:1,000; cat. no. 4228; Cell Signaling Technology, Inc.), AKT
(1:1,000; cat. no. 4691; Cell Signaling Technology, Inc.), p-AKT
(1:1,000; cat. no. 4060; Cell Signaling Technology, Inc.), GLUT4
(1:1,000; cat. no. 2213S; Cell Signaling Technology, Inc.), SIRT1
(1:1,000; cat. no. 2496; Cell Signaling Technology, Inc.), PGC-1α
(1:1,000; cat. no. 2178; Cell Signaling Technology, Inc.) and GAPDH
(1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.) were
incubated with the membrane for 2 h at room temperature. After
being washed three times with TBST, the membranes were incubated
with an anti-rabbit HRP-labeled secondary antibody (1:1,000; cat.
no. 7074; Cell Signaling Technology, Inc.) or anti-mouse
HRP-labeled secondary antibody (1:1,000; cat. no. 7076S; Cell
Signaling Technology, Inc.) for 1 h at room temperature. The
protein bands were visualized using an ECL chemiluminescence
detection kit (EMD Millipore) under a gel imaging system, and
ImageJ software (v1.6.0; National Institutes of Health) was used to
perform densitometric analysis.
Statistical analysis
All data were statistically analyzed using SPSS
(version 22.0; IBM Corp.) and expressed as the mean ± SD. One-way
analysis of variance (ANOVA) with Tukey's multiple comparison post
hoc test were used to compare the data among groups, and P<0.05
was considered to indicate a statistically significant difference.
GraphPad Prism 8.0 (GraphPad Software, Inc.) and SPSS was used to
draw the graphs.
Results
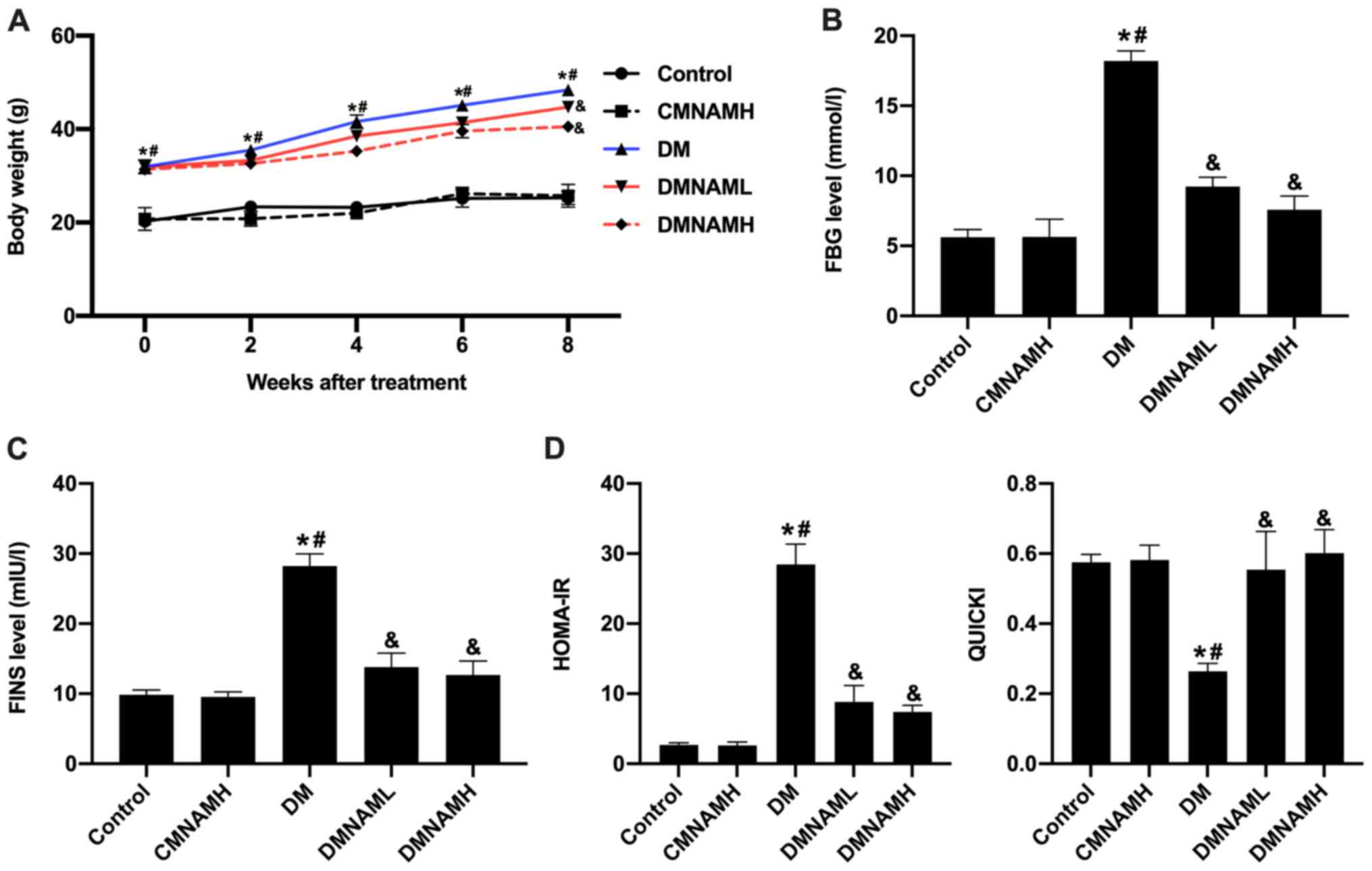
MNAM improves IR in T2DM mice
The body weights of mice in the DM group were
increased significantly throughout the 8-week feeding period
compared with the control group (Fig.
1A), and the levels of FBG (Fig.
1B) and FINS (Fig. 1C) were
also elevated. When different concentrations of MNAM were added to
the diet, the body weight gain of ob/ob mice was attenuated
(Fig. 1A), as were the increases in
FBG and FINS levels (Fig. 1B and
C). These results suggested that MNAM may reduce the body
weights, FBG and FINS of T2DM mice. Furthermore, IR was analyzed
according to the HOMA-IR and QUICKI equations. The results showed
that mice in the DM group exhibited the highest HOMA-IR and the
lowest QUICKI values (Fig. 1D),
compared with the results observed in the control and CMNAMH
groups. The HOMA-IR levels of ob/ob mice fed with different
concentrations of MNAM were decreased, whereas the QUICKI indexes
were increased (P<0.05 vs. DM group). These results indicated
that MNAM could improve IR in T2DM mice.
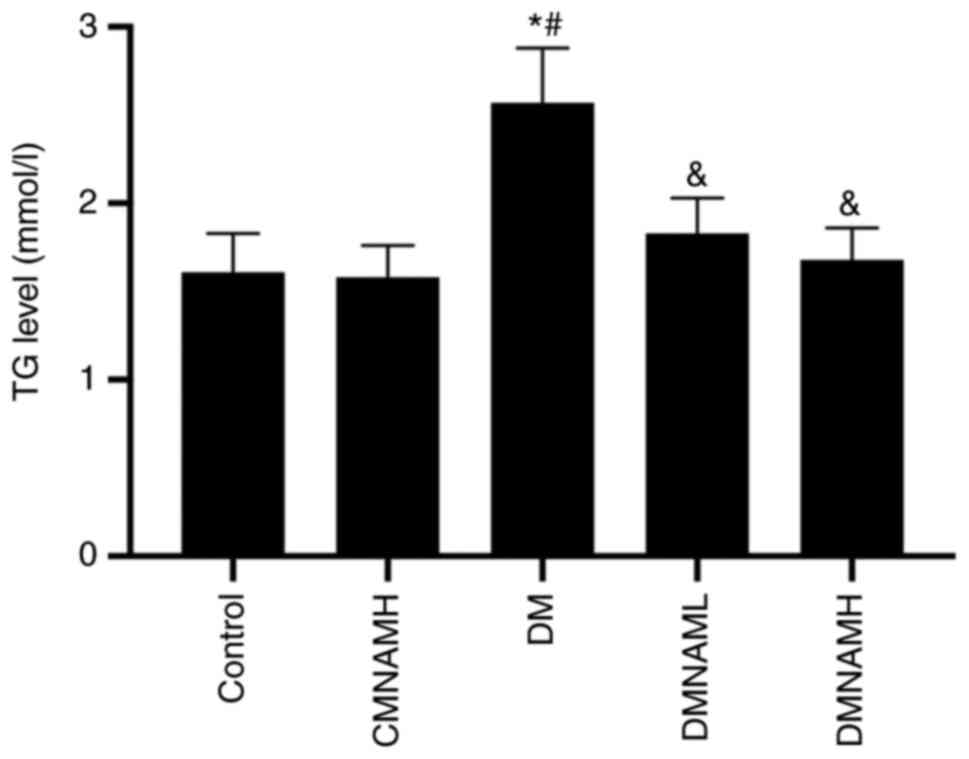
MNAM reduces the TG content in the
skeletal muscle of T2DM mice
It was shown that the highest TG content was
observed in ob/ob mice in DM group (Fig. 2). The TG content in the DM group was
significantly increased compared with the control group.
Interestingly, both of DMNAML and DMNAMH were shown to
significantly reduce the TG content of skeletal muscle in T2DM mice
(P<0.05 vs. DM). Moreover, no statistical differences were found
in the TG levels between the DMNAML and DMANAML groups. These
results suggest that MNAM could significantly decrease the TG level
in T2DM mice skeletal muscle tissue.
Effects of MNAM on the insulin
signaling pathway in skeletal muscle
In the DM group, no significant changes were
observed in IRS1 expression; however, the phosphorylation of IRS1
was downregulated compared with control group, which was
accompanied by the downregulation of the phosphorylation of PI3K
and AKT (Fig. 3A). Moreover, the
decease of GLUT4 expression in skeletal muscle in the DM group was
also observed compared with the control group (Fig. 3B). When MNAM was applied, the
activities of IRS1, PI3K and AKT were increased, in addition to the
expression of GLUT4.
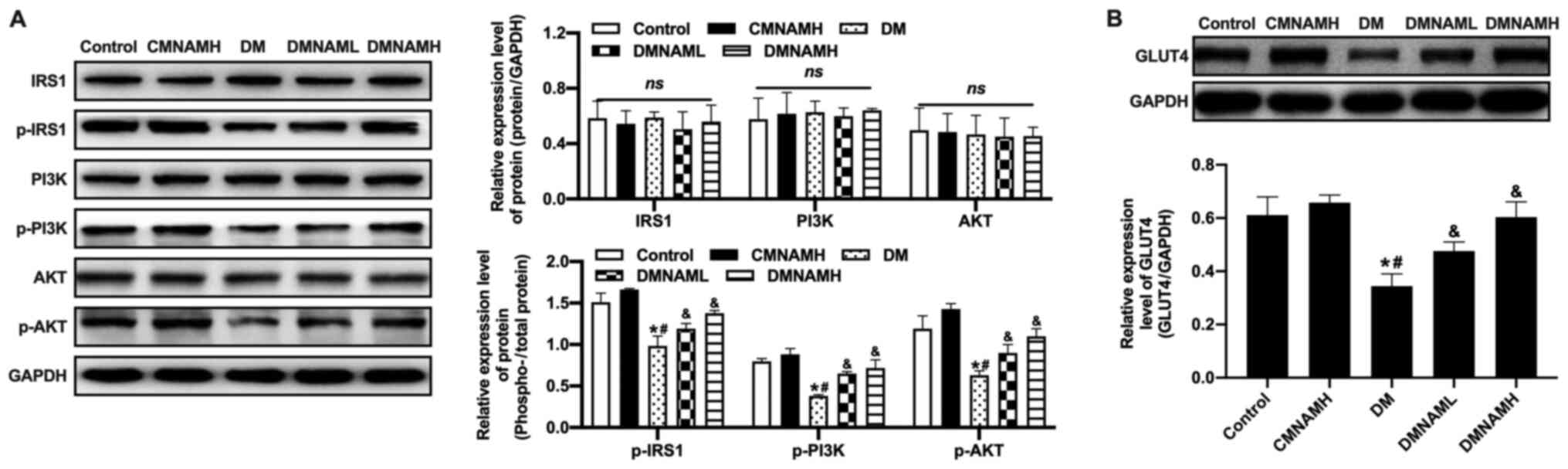 | Figure 3.Effect of MNAM on the insulin
signaling pathway in skeletal muscle. (A) Western blotting was used
to detect the levels of p-IRS1/IRS1, p-PI3K/PI3K and p-AKT/AKT. (B)
Western blotting was used to determine the protein expression of
GLUT4. *P<0.05 vs. control; #P<0.05 vs. CMNAMH;
&P<0.05 vs. DM. DM, diabetes mellitus; MNAM,
N1-methylnicotinamide; CMNAMH, control treated with high dose of
MNAM; DMNAML, DM group treated with low dose of MNAM; DMNAMH, DM
group treated with high dose of MNAM; IRS1, insulin receptor
substrate 1; p, phosphorylated; GLUT4, glucose transporter 4; ns,
not significant. |
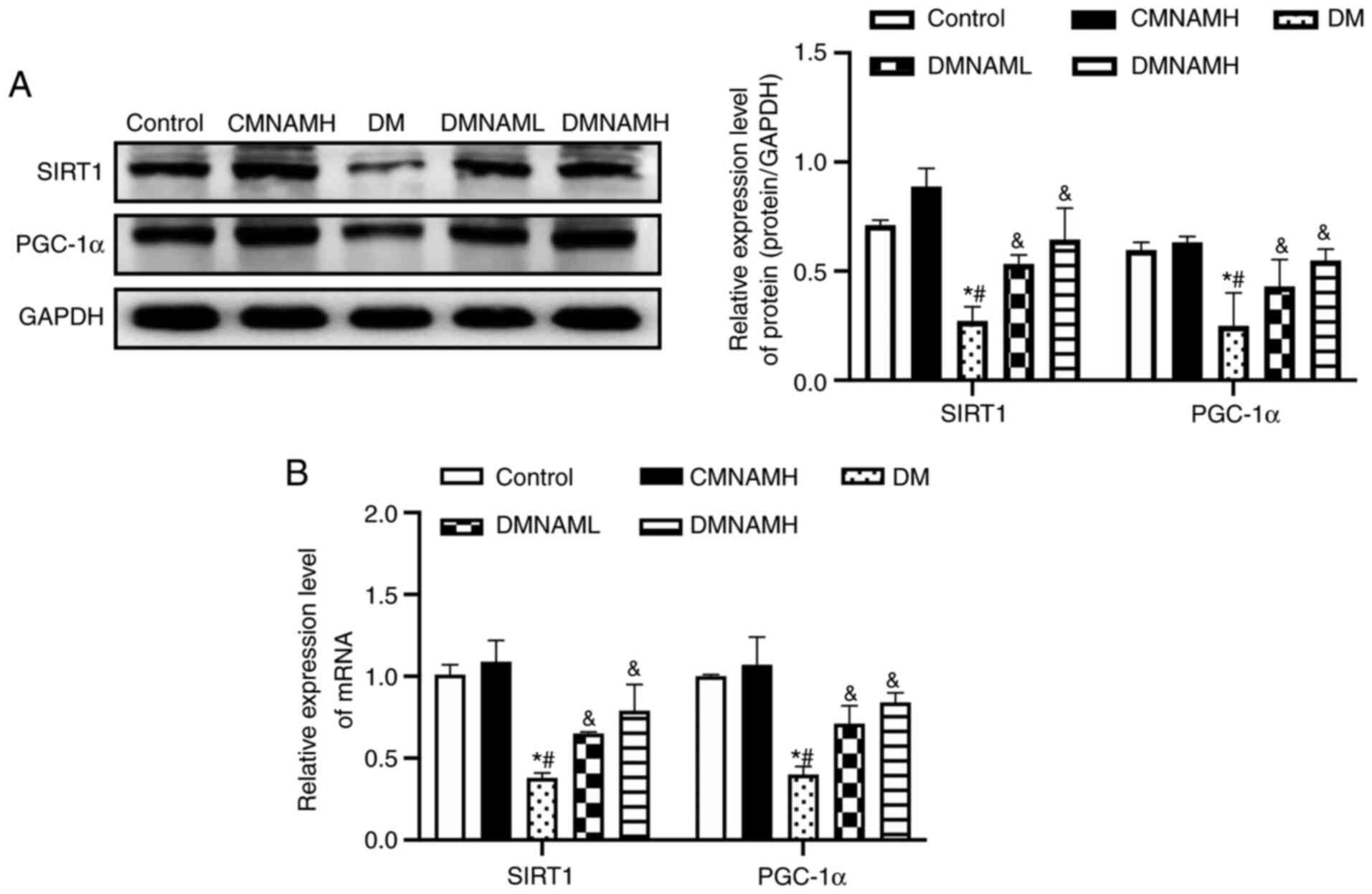
MNAM activates the SIRT1/PGC-1α
pathway in the skeletal muscle of T2DM mice
In the DM group, the protein (Fig. 4A) and mRNA (Fig. 4B) expression levels of SIRT1 were
decreased compared with control group, with the expression of its
downstream regulator, PGC-1α, also inhibited. After being fed with
1% MNAM, the expression levels of SIRT1 and PGC-1α mRNA and protein
were significantly elevated in the skeletal muscle of ob/ob
mice.
Effects of inhibiting SIRT1 on the
effects of MNAM in insulin-resistant myocytes
The regulatory mechanisms of the SIRT1/PGC-1α
pathway in IR were further investigated in vitro. An in
vitro IR model was established by exposing C2C12 myoblasts to
PA. It was observed that the expression levels of SIRT1 and PGC-1α
protein (Fig. 5A) and mRNA
(Fig. 5B) in the PA-exposed group
were decreased compared with the control, which was consistent with
the results obtained in vivo. MNAM application activated the
SIRT1/PGC-1α signaling pathway, increased glucose consumption
(Fig. 5C), promoted the
phosphorylation of IRS1, PI3K and AKT, and increased GLUT4
expression in muscle cells (Fig.
5D), suggestive of attenuated IR. When EX527, a SIRT1
inhibitor, was administered to C2C12 cells, glucose consumption was
inhibited, as the residual glucose content was significantly
increased (P<0.05 vs. PM), PGC-1α expression was decreased, the
phosphorylation levels of IRS1, PI3K and AKT were inhibited, and
the expression of GLUT4 in muscle cells was downregulated (Fig. 5E), suggesting that MNAM may improve
IR by activating the SIRT1/PGC-1α signaling pathway.
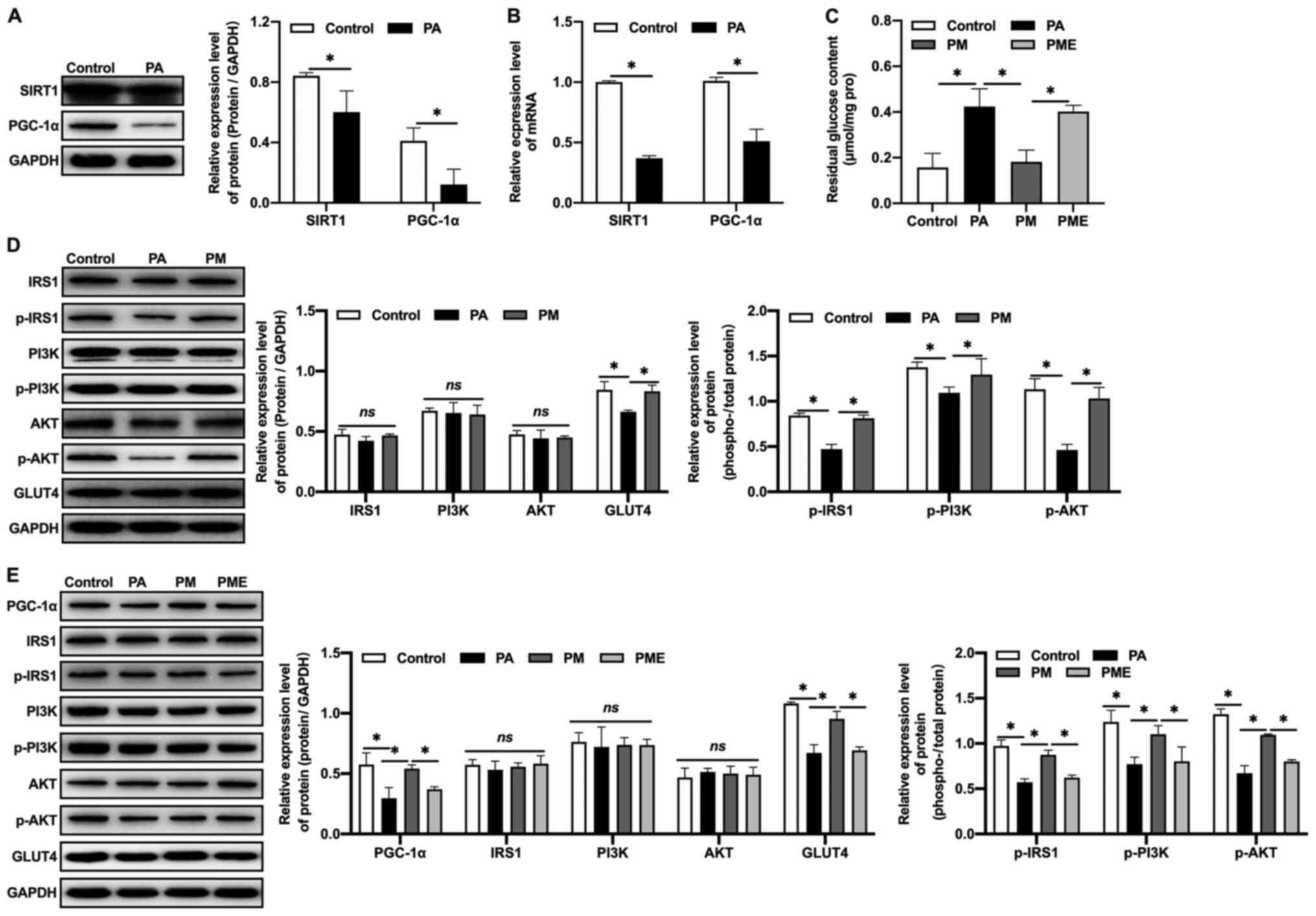 | Figure 5.Effects of inhibiting SIRT1 on
MNAM-induced improvements in insulin resistance in
insulin-resistant myocytes. (A) Western blotting was used to detect
the protein expression levels of SIRT1 and PGC-1α. (B) Reverse
transcription-quantitative PCR was used to quantify the mRNA
expression of SIRT1 and PGC-1α. (C) Residual glucose content. (D)
Western blotting was used to determine the levels of p-IRS1/IRS1,
p-PI3K/PI3K and p-AKT/AKT. (E) Western blotting was used to detect
the levels of PGC-1α, p-IRS1/IRS1, p-PI3K/PI3K, p-AKT/AKT and GLUT4
following SIRT1 inhibition. *P<0.05. PA, palmitic acid; MNAM,
N1-methylnicotinamide; PM, PA + MNAM; PME, PA + MNAM + EX527;
SIRT1, sirtuin 1; PGC-1α, peroxisome proliferator-activated
receptor γ coactivator-1α; IRS1, insulin receptor substrate 1; p,
phosphorylated; GLUT4, glucose transporter 4; ns, not
significant. |
Discussion
The leading causes of T2DM are IR and islet β cell
dysfunction (23). IR typically
occurs prior to islet β cell injury. When islet β cells can no
longer compensate for IR by increasing insulin secretion,
impairments in glucose tolerance occur and gradually develops into
DM (24). In the present study, a
mouse model of T2DM was established according to the protocols
described by Hong et al (11), who fed cohorts of wild-type C57BL/6J
mice with a saturated-fat diet supplemented with different doses of
MNAM (0.3% and 1%) for several weeks without adverse effects. Our
previous study showed that MNAM could improve hepatic insulin
sensitivity by activating SIRT1 and inhibiting FOXO1 acetylation
(12). In the present study, it was
demonstrated that MNAM could reduce body weight increases in ob/ob
T2DM mice and decrease the levels of FBG and FINS in serum.
Moreover, MNAM regulated insulin signal transduction, reduced lipid
deposition and promoted glucose utilization in skeletal muscle,
resulting in the attenuation of IR in the skeletal muscle of T2DM
mice. The mechanism of action was suggested to involve activation
of the SIRT1/PGC-1α pathway. The present and aforementioned
previous studies indicated that MNAM performed similar functions in
different tissues in T2DM mice.
Skeletal muscle is the most essential tissue for
maintaining glucose homeostasis in the body, and is responsible for
80% of insulin-induced glucose uptake and utilization under normal
conditions (25). One of the main
effects of IR in skeletal muscle is to increase the lipid content
of muscle cells (26). When the
levels of free fatty acids exceed the oxidation capability of
tissues, excessive fatty acids are deposited in skeletal muscle as
TGs, which affects the occurrence and development of IR (26). In the present study, it was found
that the TG content in gastrocnemius muscle in the DM group was
notably elevated compared with in the other four groups. It was
further observed that MNAM intervention decreased the TG content in
gastrocnemius muscles of ob/ob mice to normal levels, indicating
that MNAM attenuated the IR of skeletal muscle by improving lipid
metabolism in skeletal muscle.
When insulin is bound to membrane receptors located
on skeletal muscle cells, insulin signal cascade reactions are
triggered, catalyzing the phosphorylation of IRS1 (27). p-IRS1 immediately combines with PI3K
and subsequently induces its downstream factor, AKT (28). As the uninduced PI3K signaling
pathway regulates the metabolism of glucose, fat and protein, the
decreased induction of IRS1 signaling may promote IR (29). GLUT4 is a downstream regulator of
PI3K and a key protein that controls glucose uptake and glycogen
metabolism (30). GLUT4 expression
is decreased in patients with T2DM, which reduces the uptake and
utilization of glucose (31).
Therefore, the IRS1/PI3K/AKT/GLUT4 signaling pathway serves an
important role in insulin signal transduction in skeletal muscle.
Any disruption within this pathway may reduce the sensitivity of
skeletal muscle to insulin, leading to dysfunctional glucose uptake
and utilization, and reduced glucose tolerance.
It was found that the phosphorylation levels of
IRS1, PI3K, and AKT in skeletal muscle of DM group mice were
decreased, and GLUT4 expression was downregulated, compared with
control group mice, suggesting that there was abnormal insulin
signal transduction in skeletal muscle in T2DM mice. However, the
levels of p-IRS1, p-PI3K, p-AKT and GLUT4 were increased in T2DM
mice that were administered MNAM, suggesting that MNAM improved IR
by restoring insulin signal transduction in skeletal muscle.
SIRT1 plays a vital role in glucose homeostasis and
energy metabolism; previous studies reported that SIRT1 can improve
IR in the liver, skeletal muscle and adipose tissues, and protect
pancreatic β cells (32,33). Muscle biopsies from patients with
T2DM showed decreased expression of SIRT1, and overexpression of
SIRT1 could reduce IR in skeletal muscle cells (34). SIRT1 in skeletal muscle can activate
the insulin signaling pathway, and specifically increase the
phosphorylation of PI3K/AKT (35).
It was reported that SIRT1 in skeletal muscle also affects the
phosphorylation of IRS1 and GLUT4 recruitment, and can regulate
glycolipid metabolism via direct or indirect participation in
insulin signal transduction (36,37).
In the present study, it was reported that the
levels of p-IRS1, p-PI3K, p-AKT and GLUT4, as well as glucose
uptake, were significantly decreased in skeletal muscle tissue and
muscle cells during IR. It was also demonstrated that MNAM
intervention could increase the phosphorylation levels of IRS1,
PI3K and AKT, the expression of GLUT4, and glucose uptake and
utilization. When SIRT1 was inhibited, the expression of downstream
PGC-1α was also inhibited, as well the phosphorylation levels of
IRS1, PI3K and AKT, and the expression of GLUT4. The residual
glucose content was increased, indicating that the glucose
consumption of muscle cells was decreased. It was further suggested
that MNAM modulated the insulin signaling pathway, improved IR and
increased glucose utilization in skeletal muscle and muscle cells,
which was attenuated by inhibition of SIRT1. The results indicated
that MNAM regulated IR in skeletal muscle via the SIRT1/PGC-1α
signaling pathway.
In conclusion, the results suggested that MNAM
reduced body weight gain in obese T2DM mice, decreased FBG and FINS
levels, and regulated insulin signal transduction in skeletal
muscle, reducing lipid build-up and promoting glucose utilization,
and resulting in the improvement of IR in the skeletal muscle of
T2DM mice. These improvements in functions may occur due to the
MNAM-regulated activation of the SIRT1/PGC-1α signaling
pathway.
Acknowledgements
Not applicable.
Funding
This work was supported by Young Scientists of the
National Science Foundation of China (grant no. 81600644).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL designed the study. YC performed the animal
experiments. JZ performed the cell experiments. YC and CL analyzed
and interpreted the data. YC and PL performed the western blotting
and RT-qPCR analyses. YC was a major contributor to the writing of
the manuscript. YC and LL confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Animal Ethics Committee of Shengjing Hospital of China Medical
University (approval no. 2016PS340K).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barnett R: Type 2 diabetes. Lancet.
394:5572019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al: Global and regional diabetes prevalence
estimates for 2019 and projections for 2030 and 2045: Results from
the international diabetes federation diabetes atlas, 9(th)
edition. Diabetes Res Clin Pract. 157:1078432019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weyer C, Tataranni PA, Bogardus C and
Pratley RE: Insulin resistance and insulin secretory dysfunction
are independent predictors of worsening of glucose tolerance during
each stage of type 2 diabetes development. Diabetes Care. 24:89–94.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hernandez-Carretero A, Weber N, LaBarge
SA, Peterka V, Doan NYT, Schenk S and Osborn O: Cysteine- and
glycine-rich protein 3 regulates glucose homeostasis in skeletal
muscle. Am J Physiol Endocrinol Metab. 315:E267–E78. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amati F: Revisiting the
diacylglycerol-induced insulin resistance hypothesis. Obes Rev. 13
(Suppl 2):S40–S50. 2012. View Article : Google Scholar
|
|
6
|
Esteves JV, Enguita FJ and Machado UF:
MicroRNAs-mediated regulation of skeletal muscle GLUT4 expression
and translocation in insulin resistance. J Diabetes Res.
2017:72679102017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo X, Sun W, Luo G, Wu L, Xu G, Hou D,
Hou Y, Guo X, Mu X, Qin L and Liu T: Panax notoginseng saponins
alleviate skeletal muscle insulin resistance by regulating the
IRS1-PI3K-AKT signaling pathway and GLUT4 expression. FEBS Open
Bio. 9:1008–1019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nikas IP, Paschou SA and Ryu HS: The role
of nicotinamide in cancer chemoprevention and therapy.
Biomolecules. 10:4772020. View Article : Google Scholar
|
|
9
|
Knip M, Douek IF, Moore WP, Gillmor HA,
McLean AE, Bingley PJ and Gale EA; European Nicotinamide Diabetes
Intervention Trial Group, : Safety of high-dose nicotinamide: A
review. Diabetologia. 43:1337–1345. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nejabati HR, Mihanfar A, Pezeshkian M,
Fattahi A, Latifi Z, Safaie N, Valiloo M, Jodati AR and Nouri M:
N1-methylnicotinamide (MNAM) as a guardian of cardiovascular
system. J Cell Physiol. 233:6386–6394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong S, Moreno-Navarrete JM, Wei X,
Kikukawa Y, Tzameli I, Prasad D, Lee Y, Asara JM, Fernandez-Real
JM, Maratos-Flier E and Pissios P: Nicotinamide N-methyltransferase
regulates hepatic nutrient metabolism through Sirt1 protein
stabilization. Nat Med. 21:887–894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Chen Y, Liu C, Li L and Li P:
N(1)-methylnicotinamide improves hepatic insulin sensitivity via
activation of SIRT1 and inhibition of FOXO1 acetylation. J Diabetes
Res. 2020:10801522020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu C, Zeng Y, Tang Z, Wang C, He Y, Feng X
and Zhou L: Astragalus polysaccharides affect insulin resistance by
regulating the hepatic SIRT1-PGC-1α/PPARα-FGF21 signaling pathway
in male Sprague Dawley rats undergoing catch-up growth. Mol Med
Rep. 12:6451–6460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Waldman M, Nudelman V, Shainberg A,
Abraham NG, Kornwoski R, Aravot D, Arad M and Hochhauser E: PARP-1
inhibition protects the diabetic heart through activation of
SIRT1-PGC-1α axis. Exp Cell Res. 373:112–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng CF, Ku HC and Lin H: PGC-1α as a
pivotal factor in lipid and metabolic regulation. Int J Mol Sci.
19:34472018. View Article : Google Scholar
|
|
16
|
Guilford BL, Parson JC, Grote CW, Vick SN,
Ryals JM and Wright DE: Increased FNDC5 is associated with insulin
resistance in high fat-fed mice. Physiol Rep. 5:e133192017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lagouge M, Argmann C, Gerhart-Hines Z,
Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P,
Elliott P, et al: Resveratrol improves mitochondrial function and
protects against metabolic disease by activating SIRT1 and
PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Drel VR, Mashtalir N, Ilnytska O, Shin J,
Li F, Lyzogubov VV and Obrosova IG: The leptin-deficient (ob/ob)
mouse: A new animal model of peripheral neuropathy of type 2
diabetes and obesity. Diabetes. 55:3335–3343. 2007. View Article : Google Scholar
|
|
19
|
Tang Q, Li X, Song P and Xu L: Optimal
cut-off values for the homeostasis model assessment of insulin
resistance (HOMA-IR) and pre-diabetes screening: Developments in
research and prospects for the future. Drug Discov Ther. 9:380–385.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shojaeian Z, Sadeghi R and Latifnejad
Roudsari R: Calcium and vitamin D supplementation effects on
metabolic factors, menstrual cycles and follicular responses in
women with polycystic ocvary syndrome: A systematic review and
meta-analysis. Caspian J Intern Med. 10:359–369. 2019.PubMed/NCBI
|
|
21
|
Yaffe D and Saxel O: Serial passaging and
differentiation of myogenic cells isolated from dystrophic mouse
muscle. Nature. 270:725–727. 1977. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin JJ, Lee EK, Park TJ and Kim W:
Damage-associated molecular patterns and their pathological
relevance in diabetes mellitus. Ageing Res Rev. 24:66–76. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ortega Á, Berná G, Rojas A, Martín F and
Soria B: Gene-diet interactions in type 2 diabetes: The chicken and
egg debate. Int J Mol Sci. 18:11882017. View Article : Google Scholar
|
|
25
|
Carnagarin R, Dharmarajan AM and Dass CR:
Molecular aspects of glucose homeostasis in skeletal muscle-A focus
on the molecular mechanisms of insulin resistance. Mol Cell
Endocrinol. 417:52–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beaudry KM and Devries MC: Sex-based
differences in hepatic and skeletal muscle triglyceride storage and
metabolism (1). Appl Physiol Nutr Metab. 44:805–813. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu N, Fang X, Zhao D, Mu Q, Zuo J, Ma Y,
Zhang Y, Mo F, Zhang D, Jiang G, et al: Anti-diabetic effects of
Jiang Tang Xiao Ke granule via PI3K/Akt signalling pathway in type
2 diabetes KKAy mice. PLoS One. 12:e01689802017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao Y, Zhang M, Zhang R, You L, Li T and
Liu RH: Whole grain brown rice extrudate ameliorates the symptoms
of diabetes by activating the IRS1/PI3K/AKT insulin pathway in
db/db mice. J Agric Food Chem. 67:11657–11664. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang X, Liu G, Guo J and Su Z: The
PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci.
14:1483–1496. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Richter EA and Hargreaves M: Exercise,
GLUT4, and skeletal muscle glucose uptake. Physiol Rev.
93:993–1017. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piątkiewicz P, Bernat-Karpińska M, Miłek
T, Rabijewski M and Rosiak E: NK cell count and glucotransporter 4
(GLUT4) expression in subjects with type 2 diabetes and colon
cancer. Diabetol Metab Syndr. 8:382016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu L, Chen JF, Shuai X, Xu Y, Ding Y,
Zhang J, Yang W, Liang X, Su D and Yan C: Artesunate protects
pancreatic beta cells against cytokine-induced damage via SIRT1
inhibiting NF-κB activation. J Endocrinol Invest. 39:83–91. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou S, Tang X and Chen HZ: Sirtuins and
insulin resistance. Front Endocrinol (Lausanne). 9:7482018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kitada M, Ogura Y, Monno I and Koya D:
Sirtuins and type 2 diabetes: Role in inflammation, oxidative
stress, and mitochondrial function. Front Endocrinol (Lausanne).
10:1872019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee D and Goldberg AL: SIRT1 protein, by
blocking the activities of transcription factors FoxO1 and FoxO3,
inhibits muscle atrophy and promotes muscle growth. J Biol Chem.
288:30515–30526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sin TK, Yu AP, Yung BY, Yip SP, Chan LW,
Wong CS, Rudd JA and Siu PM: Effects of long-term
resveratrol-induced SIRT1 activation on insulin and apoptotic
signalling in aged skeletal muscle. Acta Diabetol. 52:1063–1075.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manna P, Achari AE and Jain SK:
1,25(OH)(2)-vitamin D(3) upregulates glucose uptake mediated by
SIRT1/IRS1/GLUT4 signaling cascade in C2C12 myotubes. Mol Cell
Biochem. 444:103–108. 2018. View Article : Google Scholar : PubMed/NCBI
|