Introduction
The liver is an important organ for lipid
metabolism. In individuals with atherosclerosis (AS), lipid levels
increase excessively, exceeding the liver metabolic capacity and
damaging liver function and structure, thereby causing hepatic
steatosis (1). According to
multiple studies, hepatic steatosis is not only an indicator of AS
but also an early regulatory factor that promotes the development
of AS (2,3). Indeed, both AS and hepatic steatosis,
which is a lipid-storage disease, involve ongoing inflammatory
responses and disturbances in lipid metabolism.
Urotensin II (UII), the most potent vasoconstrictor
peptide, is involved in the pathophysiology of numerous disorders,
including heart failure, essential hypertension, renal disease,
diabetes and liver cirrhosis (4).
By binding to its receptor G protein-coupled receptor 14 (GPR14),
UII participates in the regulation of multiple signaling pathways,
such as inflammation, cell proliferation and lipid metabolism
(5,6). Therefore, UII/GPR14 is regarded as a
specific target for the treatment of AS and hepatic steatosis.
The MAPK pathway is an important signal transduction
pathway occurring from the cell surface to the nucleus (7). Among the components of the MAPK
pathway, Erk is involved in the regulation of cell proliferation
and differentiation, JNK is the key molecule involved in
stress-induced cell signal transduction and p38 MAPK (p38) mediates
the occurrence of cell inflammation and apoptosis (8). Therefore, UII/GPR14 mediates the
activation of MAPK signaling to induce vasoconstriction and
vasodilation, as well as cell proliferation and migration (4,9).
However, the mechanism via which UII/GPR14 regulates MAPK
activation in AS and its association with fatty liver remains
unknown.
The peptide compound urantide is a type of UII
receptor antagonist. Our previous studies have confirmed that
urantide exerts protective effects on the thoracic aorta in rats
with AS (10,11). However, to the best of our
knowledge, researchers have not yet determined whether urantide
also exerts a protective effect on the livers of rats with AS. The
current study used the AS rat model to study the protective effect
of urantide on liver injury and to investigate the regulatory
effect of UII/GPR14 on MAPK signaling in urantide-treated AS rats
with hepatic steatosis. Therefore, the present results may provide
experimental evidence for the clinical application of urantide.
Materials and methods
Animal experiments
Urantide (peptide purity >95%) was synthesized by
ChinaPeptides Co., Ltd., and has the following amino acid sequence:
Glu-Thr-Pro-Asp-Cys-Phe-Trp-Lys-Tyr-Cys-Val.
All animals were maintained in accordance with the
guidelines of the Certification and Accreditation of the People's
Republic of China general requirements for quality and competence
of laboratory animal institutions (GB/T 27416-2014) regarding
animal care. A total of 45 male Wistar rats (age, 4 weeks; weight,
180–200 g) were purchased from Vital River Laboratory Animal
Technology, Beijing, China [License no. SCXK (Jing)-2016-0011] and
housed at a constant temperature of 22±2°C with a humidity of
40–60% and under a 12-h light/dark cycle, with free access to food
and water. The rats were randomly divided into three groups, with
15 rats per group: The control group was fed basal feed daily,
while the model group (AS) and urantide group were injected with
150 U/kg vitamin D3 (Animal Pharmaceutical Huasheng
Technology, Harbin, China) for 3 continuous days to damage the
arterial intima (12) and were fed
a high-fat diet (Beijing Keao Xieli Feed Co., Ltd.), which was
composed of 80.8% basal feed, 10% hog fat, 3.5% cholesterol, 0.5%
sodium cholate, 5% refined sugar and 0.2% propylthiouracil. The AS
rat model was established using previously described methods
(13). Then, 4 weeks later, 5 rats
from each group were randomly sacrificed using an intraperitoneal
injection of 150 mg/kg pentobarbital sodium [Fortune (Tianjin)
Chemical Reagent Co., Ltd.] to detect whether the AS rat model was
successfully replicated. Following successful modeling, the
urantide group was injected with 30 µg/kg urantide, while the
control and model groups were injected with 30 µg/kg normal saline
daily for 2 continuous weeks. In the present study, the dosage of
urantide was based on two preliminary studies by Zhao et al
(10,11). After 6 weeks of treatment, all rats
were fasted for 1 day, and the body weight of each rat was
measured. Then, the rats were sacrificed using an intraperitoneal
injection of 150 mg/kg pentobarbital sodium, and 5–8 ml blood per
rat was harvested from the aorta pectoralis. The liver weight of
each rat was measured to calculate the liver index with the
following formula: Liver index = liver weight/body weight × 100%.
The tissues were collected and immediately frozen at −196°C.
Assessment of aortic atherosclerosis
and hepatic histology
Sections of the aorta pectoralis and liver tissues
were harvested, fixed with 10% formalin at 4°C overnight,
dehydrated in 70–100% ethanol and finally embedded in paraffin.
Then, paraffin sections were sliced into 5-µm thick sections and
used for H&E staining, immunohistochemistry and
immunofluorescence. H&E (Nanchang Yulu Experimental Equipment
Co., Ltd.) was performed according to the manufacturer's
instructions. Pathological changes in the aorta pectoralis and
liver tissues were observed in ten microscopic fields at ×400
magnification using a light microscope (Olympus Corporation).
The presence of positive granules in the
atherosclerotic plaques and liver cells were visualized using
immunohistochemical staining for UII and GPR14, and
immunofluorescence staining for phosphorylated (p)-Erk1/2 and
p-JNK. Immunohistochemistry was performed using the SP kit (cat.
no. SP-9002; OriGene Technologies, Inc.) according to the
manufacturer's instructions. Briefly, sections were blocked with 3%
H2O2 (cat. no. SP-9002; OriGene Technologies,
Inc.) at 37°C for 30 min and then incubated with 1:100 dilutions of
anti-UII antibody (cat. no. sc-52299; Santa Cruz Biotechnology,
Inc.) and anti-GPR14 antibody (cat. no. sc-514460; Santa Cruz
Biotechnology, Inc.) at 4°C overnight. Sections were then incubated
with biotin-labeled goat anti-mouse IgG antibody (cat. no. SP-9002;
OriGene Technologies, Inc.) at 1:1,000 at 37°C for 30 min,
incubated with 100 µl horseradish enzyme labeled streptomycin (cat.
no. SP-9002; OriGene Technologies, Inc.) at 37°C for 15 min,
incubated with 100 µl DAB (cat. no. ZLI-9017; OriGene Technologies,
Inc.) at 37°C for 5 min, and mounted with neutral balsam. For
immunofluorescence staining, the isolated paraffinized liver
sections were dewaxed with xylene, and subjected to antigen
retrieval with the EDTA antigen repair solution (cat. no. ZLI-9071;
OriGene Technologies, Inc.) at 100°C for 2 min. The sections were
then rinsed with TBS-0.1% Tween-20 (TBST) at 37°C for 5 min,
blocked with 10% goat serum (Beijing Solarbio Science &
Technology Co., Ltd.) in PBS-0.1% Tween-20 at 37°C for 1 h and
incubated with 1:100 dilutions of anti-p-Erk1/2 (cat. no. 4370;
Cell Signaling Technology, Inc.) and anti-p-JNK antibodies (cat.
no. 9255; Cell Signaling Technology, Inc.) at 4°C overnight.
Sections were then incubated with FITC-labeled
anti-species-specific antibodies (cat. nos. A0562 and A0568;
Beyotime Institute of Biotechnology) in the dark at 37°C for 1 h,
and stained with a DAPI solution in the dark at 37°C for 45 min.
Sections were then mounted with antifade mounting medium (Beyotime
Institute of Biotechnology). Then, immunohistochemical and
immunofluorescence staining were observed in ten microscopic fields
at ×400 magnification using a fluorescence microscope (Olympus
Corporation). Digital quantification (Image-Pro Plus 6.0; Media
Cybernetics, Inc.) was performed in a blinded manner.
Measurement of blood lipid levels,
hepatic lipid levels and liver function
The plasma was centrifuged at 1,500 × g for 10 min
at 4°C, and the serum was stored at −20°C. An automatic biochemical
analyzer was used to detect rat serum levels of calcium
(Ca2+), total cholesterol (TC), triglyceride (TG),
high-density lipoprotein (HDL) and low-density lipoprotein (LDL),
which were determined to assess the extent of AS in rats. The
automatic biochemical analyzer was also used to measure alanine
transaminase (ALT), aspartate aminotransferase (AST), γ glutamyl
transferase (GGT), lactate dehydrogenase (LDH-L), alkaline
phosphatase (ALP), total bilirubin (TBIL), direct bilirubin (DBIL)
and indirect bilirubin (IBIL), which were determined to assess
liver function in rats. The measurements were made on the automatic
biochemical analyzer (BS-480; Shenzhen Mindray Bio-Medical
Electronics Co., Ltd.) at the 266 PLA Hospital (Hebei, China).
Liver tissues (1 g) were homogenized and extracted with 10 ml
ice-cold dehydrated alcohol and then centrifuged at 1,500 × g for
10 min at 4°C. The hepatic levels of TC and TG were measured using
Ultraviolet-Visible Spectrophotometry kits (cat. nos. BC1980 and
BC0620; Beijing Solarbio Science & Technology Co., Ltd.)
according to the manufacturer's protocol.
Quantitation of gene expression
Total RNA was isolated from the rat livers using
TRIzol® reagent (Tiangen Biotech Co., Ltd.) according to
the manufacturer's protocol. The mRNA concentrations were measured
using a Nano Drop spectrophotometer (Thermo Fisher Scientific,
Inc.). The appropriate quantity of cDNA templates (~0.5 mg) was
generated with a Fast Quant RT kit (Tiangen Biotech Co., Ltd.) at
42°C for 15 min and 95°C for 3 min, according to the manufacturer's
protocol. Reverse transcription-quantitative (RT-q)PCR was
performed using a SuperReal PreMix Plus kit (Tiangen Biotech Co.,
Ltd.) at 95°C for 15 min; followed by 40 cycles of 95°C for 10 sec
and 60°C for 32 sec, according to the manufacturer's protocol
(14). All samples from 6 rats per
group were measured in triplicate, and the mean value was used for
the comparative analysis. Quantitative measurements were calculated
using the 2−∆∆Cq method (15). β-actin served as the housekeeping
gene for the comparison of the gene expression data. The primer
sequences used for RT-qPCR analyses were synthesized by Takara
Biotechnology Co., Ltd. and are listed in Table I.
 | Table I.Primer sequences used to measure mRNA
expression via reverse transcription-quantitative PCR. |
Table I.
Primer sequences used to measure mRNA
expression via reverse transcription-quantitative PCR.
| Genes | Primer sequences
(5′→3′) |
|---|
| UII | F:
GGAGGAGCTGGAGAGGACTG |
|
| R:
GAGTCTCGGCACTGGGATCT |
| GPR14 | F:
AATGGCTCTAGGGTCCTCCT |
|
| R:
AACAGCCTCTGTGATGGACA |
| β-actin | F:
CAGGCATTGCTGACAGGATG |
|
| R:
TGCTGATCCACATCTGCTGG |
Western blotting
The total protein was isolated from livers of 3 rats
in each group using RIPA lysis buffer (Beijing Solarbio Science
& Technology Co., Ltd.), and the protein concentrations were
evaluated using a BCA protein assay kit (Beijing Solarbio Science
& Technology Co., Ltd.). The samples were boiled at 100°C for 7
min, and then 45 µg protein/lane was separated using SDS-PAGE (10%
gel) and transferred to a PVDF membrane. The PVDF membrane was
incubated with 5% fat-free milk powder dissolved in a TBS solution
containing 0.1% Tween-20 (TBST) for 1 h at room temperature and
then exposed to the following primary antibodies: Anti-UII (1:800),
anti-GPR14 (1:800), anti-p-Erk1/2 (1:1,000), anti-Erk1/2 (1:1,000;
cat. no. 4695; Cell Signaling Technology, Inc.), anti-p-p38
(1:1,000; cat. no. 4511; Cell Signaling Technology, Inc.), anti-p38
(1:1,000; cat. no. 8690; Cell Signaling Technology, Inc.), anti-JNK
(1:1,000; cat. no. 9252; Cell Signaling Technology, Inc.),
anti-p-JNK (1:1,000) and β-actin (1:1,000; cat. no. 4970; Cell
Signaling Technology, Inc.) at 4°C overnight. The PVDF membrane was
rinsed with TBST and incubated with horseradish peroxidase-labeled
goat anti-rabbit or anti-mouse secondary antibodies (1:5,000; cat.
nos. 074-1506 and 074-1806; KPL, Inc.) for 1 h at room temperature.
The immunoreactive bands were visualized using the ECL western
blotting substrate (Beijing Solarbio Science & Technology Co.,
Ltd.). A grayscale analysis of the target protein bands was
performed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.).
Statistical analysis
The experimental data were statistically analyzed
using SPSS 20.0 software (IBM Corp.) and are presented as the mean
± SEM of ≥3 independent experiments. One-way ANOVA was used to
compare the parameters among all groups followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference. Statistical charts were produced using
GraphPad Prism version 7.0 software (GraphPad Software, Inc.).
Results
Urantide prevents atherosclerotic
development and hepatic steatosis in AS rats
The present study examined the pathological changes
in the thoracic aorta and liver of rats in each group to determine
whether the AS rat model was successfully established, and to
assess the effects of vitamin D3 and a high-fat diet on
the livers of AS rats. As shown in Fig.
1, in the control group, the thoracic aortic membrane had a
clear boundary between the vascular tunica externa, media and
intima. The vascular endothelial cells of the thoracic aorta were
arranged in an orderly manner, and the elastic fiber layer
structure and vascular smooth muscle cells were intact in the
tunica media. In the AS model group, the thoracic aorta exhibited
marked calcification and inflammatory cell infiltration, vascular
endothelial cells were significantly damaged, elastic fibers were
broken and disintegrated, and vascular smooth muscle cells were
atrophied in the tunica media. All AS rats presented typical AS
pathological characteristics. Following treatment with urantide,
the vascular endothelial cells were notably recovered, and
calcification and inflammatory cell infiltration were significantly
decreased. Moreover, the elastic fibers and vascular smooth muscle
cells in the tunica media were restored to normal.
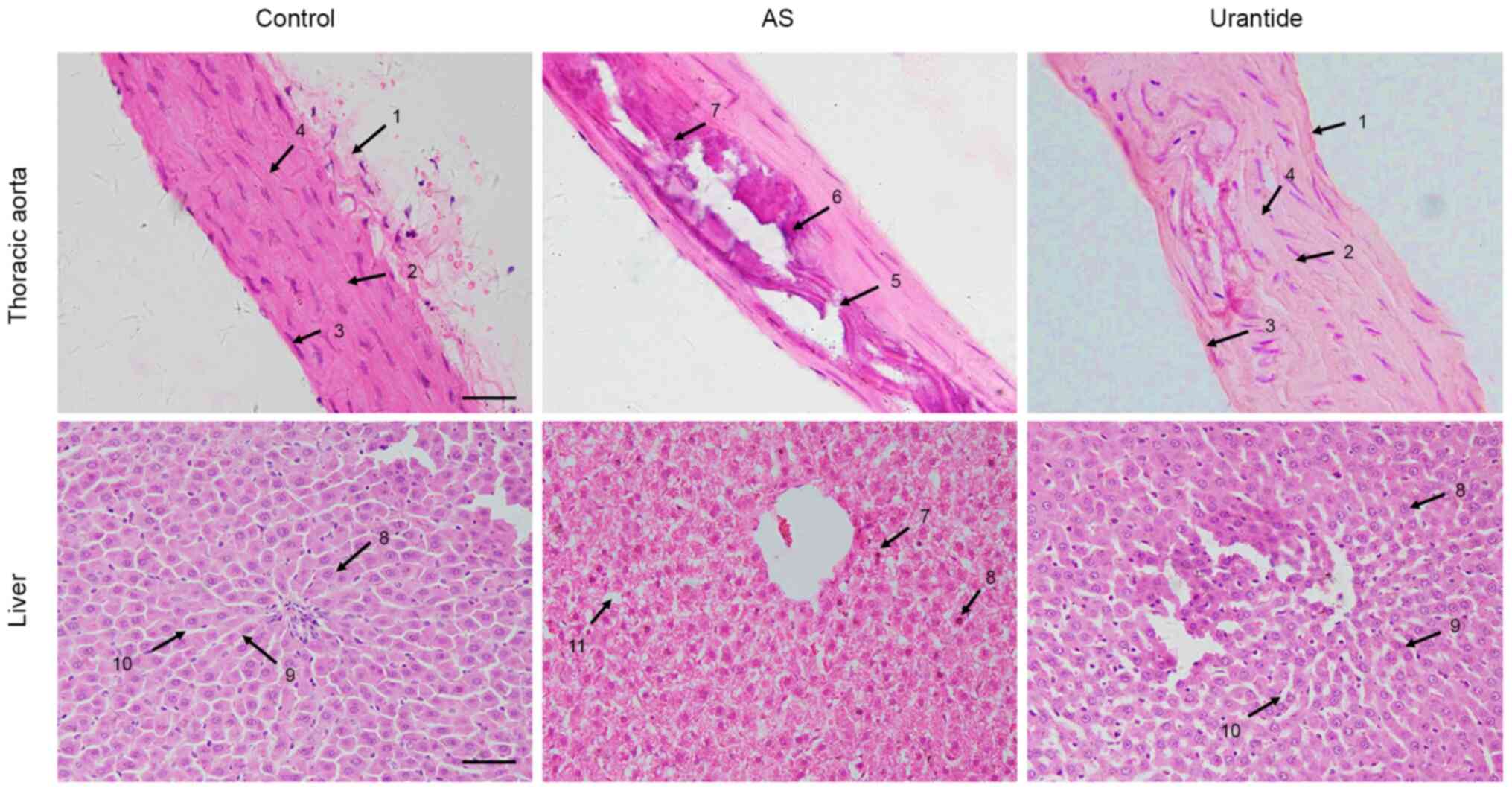 | Figure 1.Morphological characteristics of the
thoracic aorta and liver tissues from rats in each group. Arrows 1,
vascular tunica externa; 2, vascular tunica media; 3, vascular
tunica intima; 4, elastic fibers; 5, broken elastic fibers in the
vascular tunica media associated with atherosclerosis; 6,
calcification associated with AS; 7, inflammatory cell
infiltration; 8, hepatocytes; 9, hepatic plate; 10, hepatic
sinusoid; 11, steatosis in hepatocytes. H&E staining; scale
bar, 20 µm. AS, atherosclerosis. |
Correspondingly, in the control group, the
morphology of hepatocytes and the structure of hepatic plates were
normal, and hepatic sinusoids displayed a uniform and regular
arrangement. In the AS model group, the hepatocytes were obviously
swollen and deformed, the arrangement of hepatic sinusoids was
disordered, and the hepatic plates did not have a clear boundary.
Inflammatory cell infiltration and vacuole-like lipid droplets were
observed in the cytoplasm, and some nuclei were shifted to one side
of the cells, which indicated typical hepatic steatosis. In the
urantide group, the morphology of hepatocytes was restored to
normal, and the hepatic sinusoids were arranged uniformly and
regularly (Fig. 1). Based on these
results, it was suggested that vitamin D3 combined with
a high-fat diet successfully induced AS and hepatic steatosis in
rats, and urantide ameliorated the AS-related pathological changes
and hepatic steatosis.
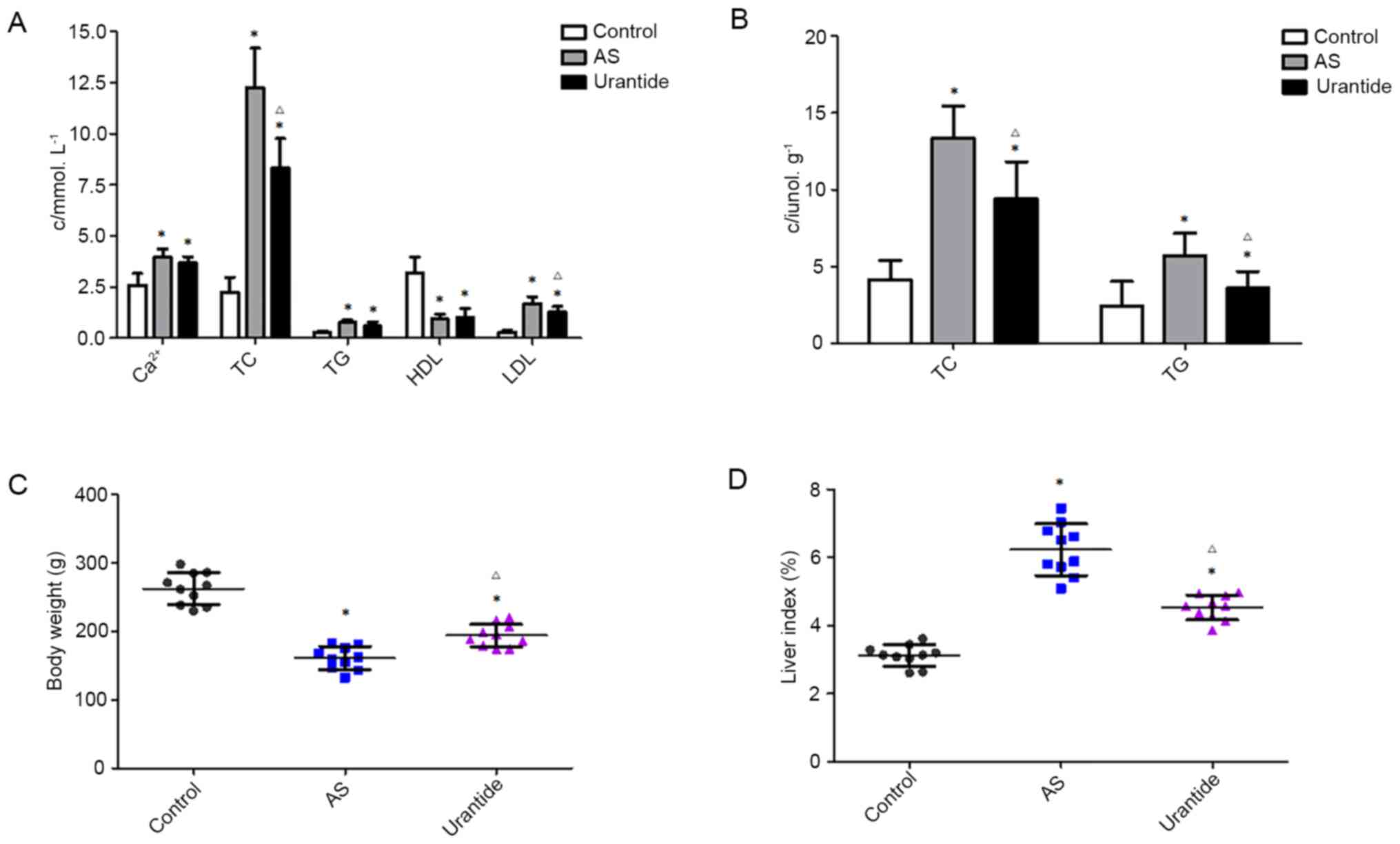
Urantide restores the Ca2+,
blood and hepatic lipid levels, body weight and liver index in rats
with AS
Vitamin D3 combined with a high-fat diet
induces AS and hepatic steatosis, resulting in lipid deposition in
the blood and liver (12). The
current study measured the serum Ca2+ and blood lipid
levels to investigate the antihyperlipidemic effects of urantide.
Compared with the levels in the control group, the serum levels of
Ca2+, TC, TG and LDL in the AS model group were
significantly increased, and the HDL levels were decreased.
Furthermore, the TC and LDL levels in the urantide group were
significantly decreased, and no changes in the levels of
Ca2+, TG and HDL were observed, compared with the model
group. Thus, urantide improved TC and LDL levels in rats with AS
(Fig. 2A). The hepatic lipid levels
and the liver index were also measured to examine the effects of
urantide on hepatic steatosis. Compared with the levels in the
control group, the hepatic levels of TC and TG in the AS model
group were significantly increased, while the levels in the
urantide group were significantly decreased (Fig. 2B).
The body weight was significantly decreased, and the
liver index was significantly increased in the AS model group
compared with the control group. Following treatment with urantide,
the body weight and liver index in the urantide groups were both
significantly recovered compared with the model group (Fig. 2C and D). Therefore, urantide
effectively alleviated hepatic steatosis in rats with AS
Urantide improves the liver function
of rats with AS
Liver function was assessed by measuring biochemical
indexes to investigate the degree of liver injury in rats with AS,
according to a method described by Lu et al (16). Compared with the levels in the
control group, the serum levels of ALT, AST, GGT, LDH-L, ALP, TBIL
and IBIL in the AS model group were significantly increased
(Tables II and III). Following treatment with urantide,
the ALT, AST, GGT, LDH-L and IBIL levels in the urantide group were
significantly decreased compared with the levels in the AS model
group. Furthermore, the levels of DBIL showed no significant
changes in the rats from any group (Tables II and III). In conclusion, rats with AS
exhibited liver dysfunction, and urantide improved liver function,
particularly hepatic synthesis, storage and metabolic functions, in
rats with AS.
 | Table II.Serum ALT, AST, GGT, LDH-L and ALP
levels in rats from each group. |
Table II.
Serum ALT, AST, GGT, LDH-L and ALP
levels in rats from each group.
| Parameter | Control | AS | Urantide |
|---|
| ALT, U/l | 46.18±8.66 |
81.82±19.34a |
60.62±16.04b |
| AST, U/l |
88.03±14.79 |
148.20±50.41a |
112.00±52.07b |
| GGT, U/l |
0.47±0.16 |
3.07±0.54a |
2.30±0.67a,b |
| LDH-L, U/l | 177.80±52.20 |
393.70±119.00a |
172.80±29.33b |
| ALP, U/l | 151.10±35.51 |
276.50±26.09a |
209.10±45.43a |
 | Table III.Serum TBIL, DBIL and IBIL levels in
rats from each group. |
Table III.
Serum TBIL, DBIL and IBIL levels in
rats from each group.
| Parameter | Control | AS | Urantide |
|---|
| TBIL, µmol/l | 0.60±0.26 |
1.39±0.44a |
0.76±0.49b |
| DBIL, µmol/l | 0.25±0.08 | 0.37±0.15 | 0.47±0.16 |
| IBIL, µmol/l | 0.18±0.06 |
0.63±0.16a |
0.23±0.12b |
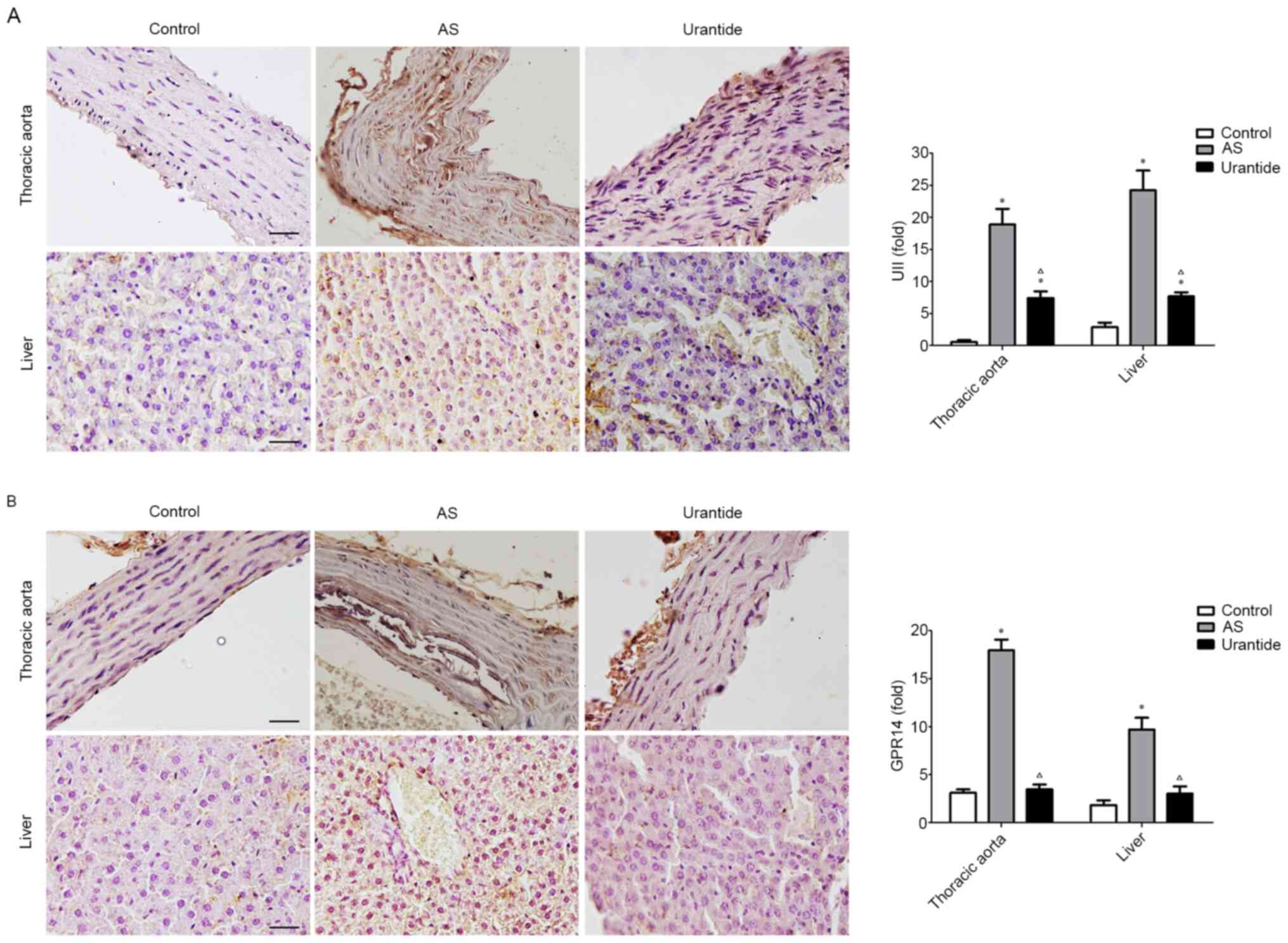
Expression levels of UII- and GPR14-positive
particles are significantly upregulated in the thoracic aortas and
livers of rats with AS. Immunohistochemical staining identified
significantly higher expression levels of UII- and GPR14-positive
particles in the thoracic aortas of rats in the AS model group
compared with in the control group, and those indexes were
significantly lower in the urantide group compared with the model
group (Fig. 3A and B). Consistent
with these findings, the expression levels of UII- and
GPR14-positive particles in the livers of rats in the model group
were also significantly increased compared with the control group,
and urantide significantly decreased those indexes in the livers of
rats with AS. Based on these results, the UII/GPR14 system may
serve an important role in hepatic steatosis in rats with AS, and
urantide-mediated inhibition of the UII/GPR14 system effectively
improves hepatic steatosis.
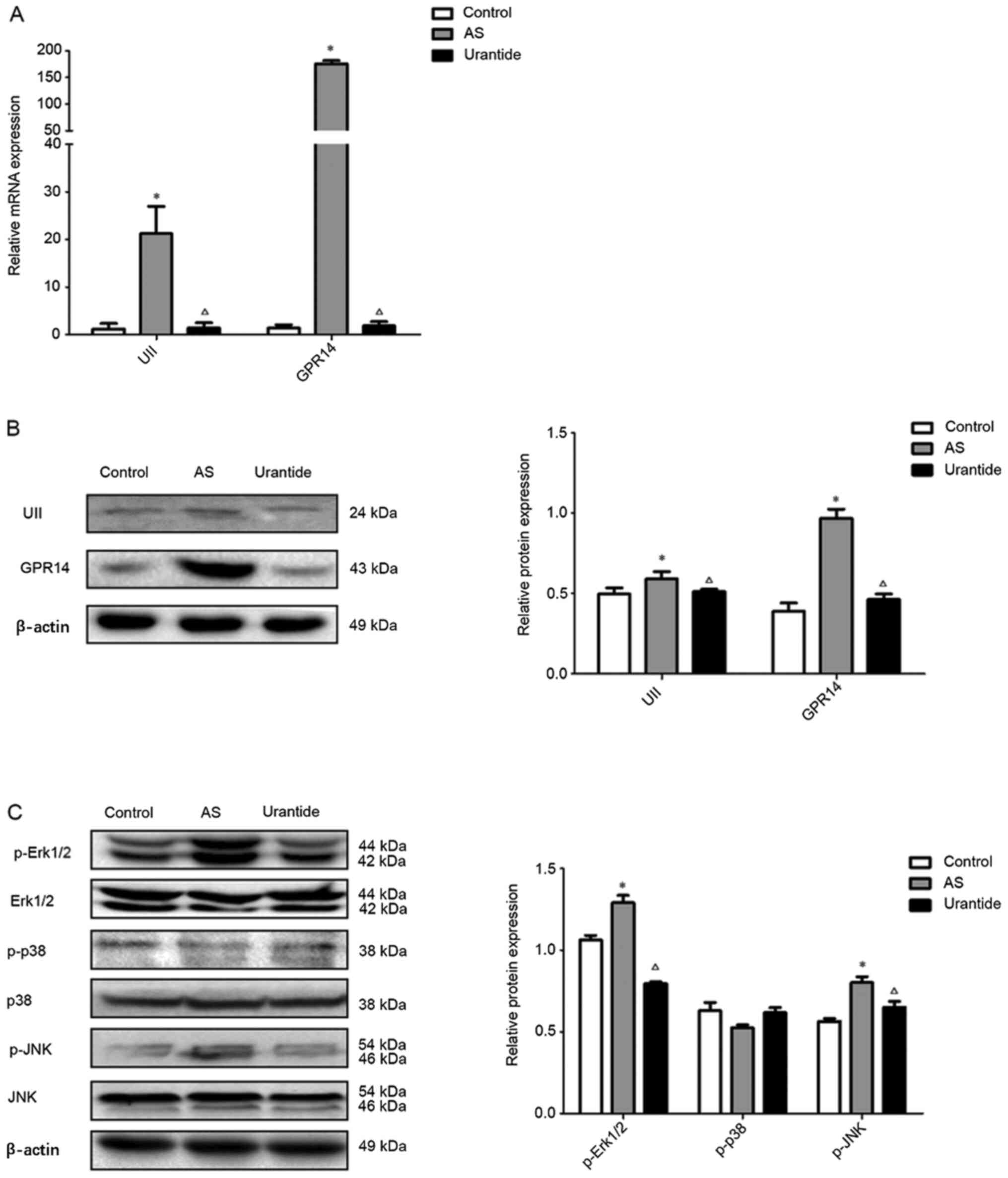
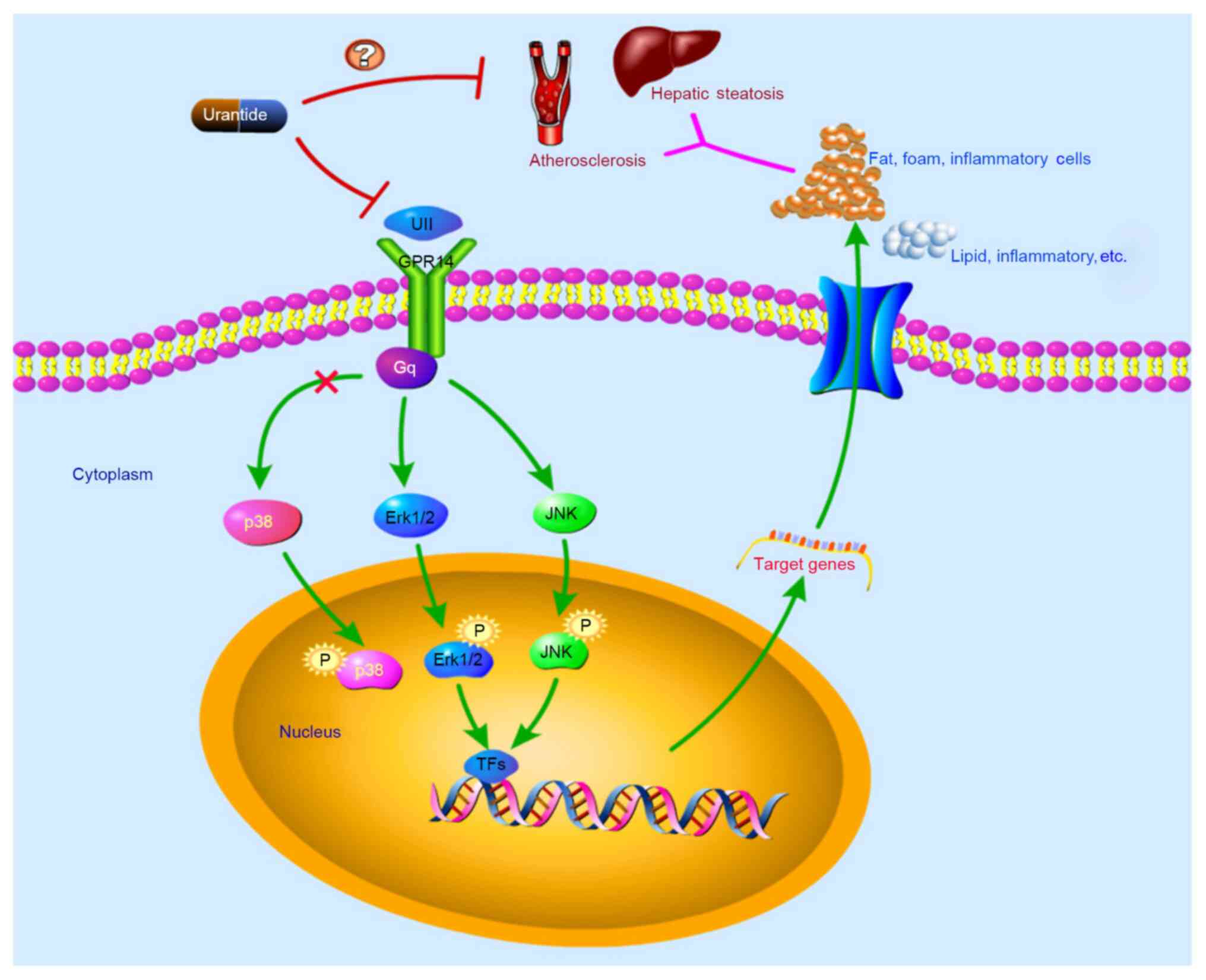
Urantide inhibits the UII/GPR14/MAPK
pathway in the livers of rats with AS
The present study measured the mRNA and protein
expression levels of UII and GPR14, as well as intermediates in the
MAPK pathway, in the liver tissues of the rats in order to examine
the molecular mechanism via which UII/GPR14 affects hepatic
steatosis in AS rats. Significantly higher mRNA and protein
expression levels of UII and GPR14 were detected in the liver
tissues of rats in the model group compared with in the control
group, and significantly lower mRNA and protein expression levels
of UII and GPR14 were observed in the urantide group compared with
in the model group (Fig. 4A and B).
Consistent with these findings, compared with the control group,
expression levels of the p-Erk1/2 and p-JNK proteins were
significantly increased in the liver tissues of rats in the model
group, and expression levels of the p-Erk1/2 and p-JNK proteins
were significantly decreased in the urantide group compared with
the model group (Fig. 4C).
Moreover, the expression levels of the Erk1/2, p-p38, p38 and JNK
proteins were not markedly changed in the liver tissues of rats in
the various groups (Fig. 4C). Thus,
hepatic steatosis in rats with AS was closely associated with the
Erk1/2 and JNK pathways, and urantide significantly inhibited the
activity of the Erk1/2 and JNK pathways. Therefore, urantide may
effectively improve hepatic steatosis in rats with AS by regulating
the Erk1/2 and JNK pathways.
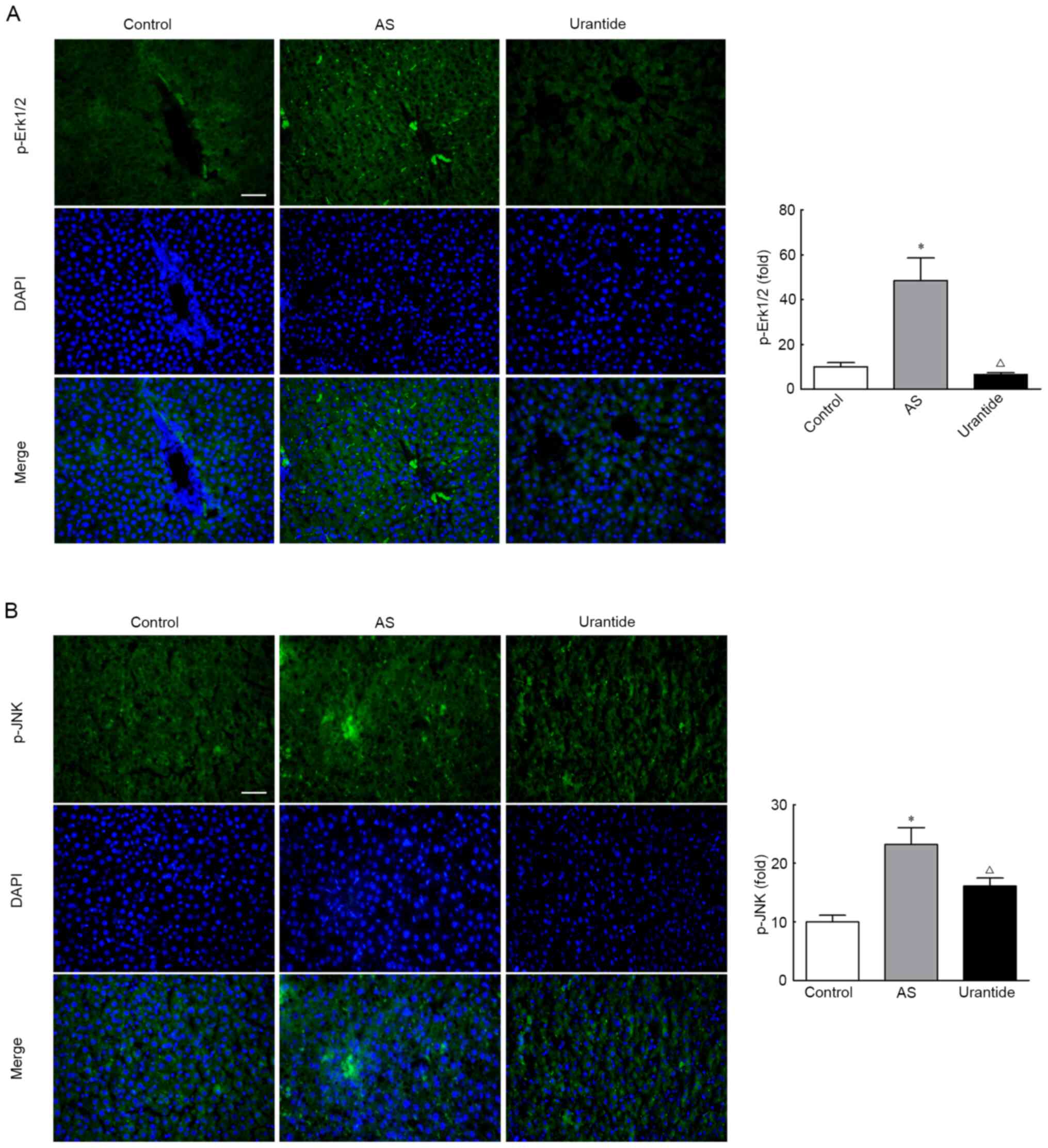
Localization of p-Erk1/2 and p-JNK
protein expression in the livers of rats
The localization of UII and GPR14 was further
analyzed to determine whether the UII/GPR14 system directly or
indirectly affects the Erk1/2 and JNK pathways. These proteins were
mainly expressed in perivascular and necrotic hepatocytes, as
detected using immunohistochemistry. Consistent with these
findings, p-Erk1/2 and p-JNK displayed similar localization
patterns, as detected using immunofluorescence staining. Moreover,
urantide significantly decreased the expression levels of p-Erk1/2
and p-JNK-positive particles in the livers of rats with AS
(Fig. 5A and B). Based on these
results, it was suggested that the MAPK/Erk/JNK pathway was
substantially activated in the livers of rats with AS. Moreover,
the expression levels of p-Erk1/2 and p-JNK are gradually restored
in rats with AS treated with urantide.
Discussion
AS is a process marked by chronic inflammation of
the arterial wall due to infiltration of lipids, macrophages and
other inflammatory mediators (1,10). An
increasing number of adverse cardiovascular disease events have
been shown to be associated with non-alcoholic fatty liver disease,
including dyslipidemia, inflammation and liver dysfunction
(17). Non-alcoholic fatty liver
disease is either an independent primary disease or a pathological
manifestation of other systemic diseases, such as AS, in which
non-alcoholic fatty liver synergistically promotes AS occurrence
and development (18,19). An AS rat model was established in
the current study, and the incidence of hepatic steatosis in rats
was increased by feeding the rats a high-fat diet to enhance blood
and hepatic lipid levels. Concurrently, the effect of
urantide-mediated alleviation of AS was associated with a decrease
in hepatic steatosis. Then, liver index and function were tested to
investigate the degree of liver injury in rats with AS. The results
demonstrated that rats with AS had liver dysfunction, according to
Lu et al (16), and urantide
restored the liver function in rats with AS and decreased liver
injury, resulting in protective effects on the livers of rats with
AS. Furthermore, the expression levels of UII and GPR14 in the
livers of AS rats were significantly increased, which was
consistent with the results observed in the thoracic aorta. Thus,
it was suggested that the UII/GPR14 system may serve an important
role in hepatic steatosis in rats with AS, and the protective
mechanism of urantide may involve inhibiting the expression of UII
and its receptor GPR14 in the livers of rats with AS.
The somatostatin-like cyclic peptide UII was first
isolated from the urophysis of bony fish (20), and the UII/UT system is involved in
the pathogenic mechanism that promotes AS occurrence and
development (10,21). Furthermore, the UII/UT system
promotes inflammation and accelerates foam cell formation (22,23).
However, to the best of our knowledge, the role of the UII/UT
system in AS-associated liver injury has not been previously
reported. According to Sun et al (24), UII/GPR14 signaling is involved in
CCl4-induced liver injury by inducing inflammation and oxidative
stress. In addition, the UII/UT system induces acute liver failure
by triggering an inflammatory cascade (25,26).
To further examine the regulatory molecular
mechanism of UII/GPR14 in hepatic steatosis and the pathogenesis of
AS, the phosphorylation of MAPK signaling molecules was examined in
the livers of rats from various groups. The binding of UII to its
receptor GPR14 has been shown to increase the phosphorylation of
Erk and promote the contraction and proliferation of vascular
smooth muscle cells, and thus participate in the occurrence and
development of AS (3,9,27).
After phosphorylation, Erk is transferred from the cytoplasm to the
nucleus where it mediates the activation of transcription factors,
such as ETS domain-containing protein, activating transcription
factor, amino acid permease 1, c-Fos and c-Jun, and participates in
the development of hepatic steatosis (28). The phosphorylation of JNK may also
serve a role in AS inflammatory reactions (29). In addition, p38 MAPK is involved in
inflammatory signaling and is activated in response to oxidative
stress, cytokines and growth factors, all of which are abundantly
present in AS and aortic valve sclerotic lesions (30). As demonstrated in the current study,
UII and GPR14 expression levels were significantly increased in the
livers of rats with AS, and the corresponding levels of
phosphorylated Erk1/2 and JNK were significantly increased.
Interestingly, the data from the present study revealed that p38
phosphorylation was not significantly increased in the livers of
rats with AS. Thus, p38 may not serve an important role in hepatic
steatosis, and a similar result has been reported by Kardakaris
et al (31). Therefore, it
was suggested that the molecular mechanism via which hepatic
steatosis occurs in rats with AS is via UII/GPR14-mediated
increases in Erk1/2 and JNK phosphorylation in the liver, which
activates the Erk/JNK signaling pathway and promotes the occurrence
of hepatic steatosis in rats with AS.
Urantide is the strongest known UII receptor
antagonist, and the antagonist effect is 50–100 times higher
compared with other compounds such as endothelin-1 (32,33).
The present study examined the literature but found few reports
describing the association of urantide with AS. At present, to the
best of our knowledge, only the authors' research team has provided
a small amount of evidence indicating that urantide blocks the
effects of UII on promoting the occurrence and development of AS
(10,11). Concurrently, as shown in our
previous studies using an AS rat model, urantide exerted protective
effects on myocardial collagen metabolism disorder (34) and kidney injury (35), and the survival rate of rats with AS
was also greatly improved by injecting with 30 µg/kg urantide. As
to whether human patients are suitable for this dose, it is
necessary to determine the best dose for patients by converting the
equivalent dose between animals and humans. Nevertheless, the study
of urantide is still in the basic research stage and has not yet
been used in clinical trials, and its safety needs to be verified
in future studies.
The detailed mechanism via which urantide affects AS
remains unknown. The present study examined the effect of urantide
on signaling molecules in the Erk1/2 and JNK pathways in the rat
liver to further evaluate the mechanism via which urantide
regulates Erk1/2 and JNK signaling in AS rats with hepatic
steatosis. The expression levels of UII and GPR14 in the livers of
the urantide group were significantly decreased, and levels of
Erk1/2 and JNK phosphorylation were reduced. The immunofluorescence
staining further revealed a marked decrease in the number of
p-Erk1/2- and p-JNK-positive cells in the liver after treatment
with urantide. These findings confirmed the hypothesis that by
inhibiting UII/GPR14 expression, urantide reduces the release of
pathogenic factors after injury to liver function and the
development of hepatic steatosis in rats with AS, thus ameliorating
the AS-related pathological changes in rats. Collectively, it was
suggested that urantide inhibited the phosphorylation of Erk1/2 and
JNK by blocking UII binding to its receptor GPR14 (Fig. 6), thereby alleviating liver function
damage and hepatic steatosis in rats.
In conclusion, the treatment of AS rats with
urantide ameliorated hepatic steatosis and significantly improved
lipid deposition and liver functions. The underlying molecular
mechanism may be associated with the inhibition of the UII/UT
system by urantide. The present study examined the relationship
between the MAPK pathway and the UII/GPR14 system in hepatic
steatosis in rats with AS. It was identified that urantide
inhibited the activation of Erk1/2 and JNK, thereby alleviating
hepatic steatosis and AS lesions. However, it was not determined
whether the UII/GPR14 system directly regulates the MAPK pathway
and how it regulates lipid metabolism. The main limitation of this
study is the lack of validation of the downstream genes regulated
by the MAPK pathway that affect lipid metabolism. Therefore, in
further studies of urantide, the mechanisms via which urantide
protects the liver to restrain AS development will be further
clarified. The present study does provide novel perspectives and an
experimental basis for the clinical use of urantide to treat
AS.
Acknowledgements
Not applicable.
Funding
The study was supported by the Natural Science
Foundation of Hebei Province of China (grant no. H2020406011), the
Youth Science and Technology Research Project of Health and Family
Planning Commission in Hebei Province (grant no. 20200183), Hebei
Provincial Department of Education Key Project (grant no.
ZD2019098), Chengde Medical University Natural Science Youth Fund
(grant no. 201922) and Fund for Key Discipline Construction
Projects in Hebei Province [grant no. Hebei (2013) 4].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JZ and HPC conceived and designed the study. HPC,
YXL and LDX performed the experiments. HPC and YXL analyzed the
data. HPC and YXL wrote the paper. JZ, HPC and YXL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal procedures were ethically approved
(approval no. CDMULAC-20171218009) by the Experimental Animal Care
and Use Committee of Chengde Medical University (Chengde, China)
and were conducted in accordance with the National Institutes of
Health Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gaudio E, Nobili V, Franchitto A, Onori P
and Carpino G: Nonalcoholic fatty liver disease and
atherosclerosis. Intern Emerg Med. 7 (Suppl 3):S297–S305. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guth S, Windler E, Leise U and Bamberger
CM: Ultrasound Diagnosis of Hepatic Steatosis as a Surrogate for
Atherosclerosis. Ultrasound Int Open. 2:E27–E31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kapuria D, Takyar VK, Etzion O, Surana P,
O'Keefe JH and Koh C: Association of Hepatic Steatosis With
Subclinical Atherosclerosis: Systematic Review and Meta-Analysis.
Hepatol Commun. 2:873–883. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ross B, McKendy K and Giaid A: Role of
urotensin II in health and disease. Am J Physiol Regul Integr Comp
Physiol. 298:R1156–R1172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brancaccio D, Limatola A, Campiglia P,
Gomez-Monterrey I, Novellino E, Grieco P and Carotenuto A: Urantide
conformation and interaction with the urotensin-II receptor. Arch
Pharm (Weinheim). 347:185–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Svistunov AA, Tarasov VV, Shakhmardanova
SA, Sologova SS, Bagaturiya ET, Chubarev VN, Galenko-Yaroshevsky
PA, Avila-Rodriguez MF, Barreto GE and Aliev G: Urotensin II:
Molecular Mechanisms of Biological Activity. Curr Protein Pept Sci.
19:924–934. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bigeard J and Hirt H: Nuclear Signaling of
Plant MAPKs. Front Plant Sci. 9:4692018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keshet Y and Seger R: The MAP kinase
signaling cascades: A system of hundreds of components regulates a
diverse array of physiological functions. Methods Mol Biol.
661:3–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ziltener P, Mueller C, Haenig B, Scherz MW
and Nayler O: Urotensin II mediates ERK1/2 phosphorylation and
proliferation in GPR14-transfected cell lines. J Recept Signal
Transduct Res. 22:155–168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao J, Yu QX, Kong W, Gao HC, Sun B, Xie
YQ and Ren LQ: The urotensin II receptor antagonist, urantide,
protects against atherosclerosis in rats. Exp Ther Med.
5:1765–1769. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao J, Xie LD, Song CJ, Mao XX, Yu HR, Yu
QX, Ren LQ, Shi Y, Xie YQ, Li Y, et al: Urantide improves
atherosclerosis by controlling C-reactive protein, monocyte
chemotactic protein-1 and transforming growth factor-β expression
in rats. Exp Ther Med. 7:1647–1652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gou SH, Liu BJ, Han XF, Wang L, Zhong C,
Liang S, Liu H, Qiang Y, Zhang Y and Ni JM: Anti-atherosclerotic
effect of Fermentum Rubrum and Gynostemma pentaphyllum mixture in
high-fat emulsion- and vitamin D3-induced atherosclerotic rats. J
Chin Med Assoc. 81:398–408. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang ZY and Yang PY, Almofti MR, Yu YL,
Rui YC and Yang PY: Comparative analysis of the proteome of left
ventricular heart of arteriosclerosis in rat. Life Sci.
75:3103–3115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Li X, Luo R, Wang W, Wang T and Tang
H: Detection of KIT genotype in pigs by TaqMan MGB real-time
quantitative polymerase chain reaction. DNA Cell Biol. 37:457–464.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu YW, Zhu YC, Zhang L, Li P, Yang J and
Wen XD: Ilexgenin A enhances the effects of simvastatin on
non-alcoholic fatty liver disease without changes in simvastatin
pharmacokinetics. Chin J Nat Med. 16:436–445. 2018.PubMed/NCBI
|
|
17
|
Targher G, Bertolini L, Padovani R,
Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G and Arcaro G:
Relations between carotid artery wall thickness and liver histology
in subjects with nonalcoholic fatty liver disease. Diabetes Care.
29:1325–1330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Minno MN, Di Minno A, Ambrosino P,
Songia P, Tremoli E and Poggio P: Aortic valve sclerosis as a
marker of atherosclerosis: Novel insights from hepatic steatosis.
Int J Cardiol. 217:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SB, Park GM, Lee JY, Lee BU, Park JH,
Kim BG, Jung SW, Jeong ID, Bang SJ, Shin JW, et al: Association
between non-alcoholic fatty liver disease and subclinical coronary
atherosclerosis: An observational cohort study. J Hepatol.
68:1018–1024. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pearson D, Shively JE, Clark BR, Geschwind
II, Barkley M, Nishioka RS and Bern HA: Urotensin II: A
somatostatin-like peptide in the caudal neurosecretory system of
fishes. Proc Natl Acad Sci USA. 77:5021–5024. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gabunia K, Jain S, England RN and Autieri
MV: Anti-inflammatory cytokine interleukin-19 inhibits smooth
muscle cell migration and activation of cytoskeletal regulators of
VSMC motility. Am J Physiol Cell Physiol. 300:C896–C906. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Segain JP, Rolli-Derkinderen M, Gervois N,
Raingeard de la Blétière D, Loirand G and Pacaud P: Urotensin II is
a new chemotactic factor for UT receptor-expressing monocytes. J
Immunol. 179:901–909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Watanabe T, Suguro T, Kanome T, Sakamoto
Y, Kodate S, Hagiwara T, Hongo S, Hirano T, Adachi M and Miyazaki
A: Human urotensin II accelerates foam cell formation in human
monocyte-derived macrophages. Hypertension. 46:738–744. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun H, Zhang L and Shen D: Urantide
protects CCl4-induced liver injury via inhibiting GPR14 signal in
mice. Biotechnol Biotechnol Equip. 31:156–161. 2017. View Article : Google Scholar
|
|
25
|
Liang DY, Liu LM, Ye CG, Zhao L, Yu FP,
Gao DY and Wang YY, Yang ZW and Wang YY: Inhibition of UII/UTR
system relieves acute inflammation of liver through preventing
activation of NF-κB pathway in ALF mice. PLoS One. 8:e648952013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu LM, Zhao L, Liang DY, Yu FP, Ye CG, Tu
WJ and Zhu T: Effects of urotensin-II on cytokines in early acute
liver failure in mice. World J Gastroenterol. 21:3239–3244. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watanabe T, Kanome T, Miyazaki A and
Katagiri T: Human urotensin II as a link between hypertension and
coronary artery disease. Hypertens Res. 29:375–387. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cseh B, Doma E and Baccarini M: ‘RAF’
neighborhood: Protein-protein interaction in the Raf/Mek/Erk
pathway. FEBS Lett. 588:2398–2406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hwang HJ, Jung TW, Hong HC, Seo JA, Kim
SG, Kim NH, Choi KM, Choi DS, Baik SH and Yoo HJ: LECT2 induces
atherosclerotic inflammatory reaction via CD209 receptor-mediated
JNK phosphorylation in human endothelial cells. Metabolism.
64:1175–1182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reustle A and Torzewski M: Role of p38
MAPK in atherosclerosis and aortic valve sclerosis. Int J Mol Sci.
19:37612018. View Article : Google Scholar
|
|
31
|
Kardakaris R, Gareus R, Xanthoulea S and
Pasparakis M: Endothelial and macrophage-specific deficiency of
P38α MAPK does not affect the pathogenesis of atherosclerosis in
ApoE-/- mice. PLoS One. 6:e210552011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leprince J, Chatenet D, Dubessy C,
Fournier A, Pfeiffer B, Scalbert E, Renard P, Pacaud P, Oulyadi H,
Ségalas-Milazzo I, et al: Structure-activity relationships of
urotensin II and URP. Peptides. 29:658–673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guidolin D, Albertin G and Ribatti D:
Urotensin-II as an angiogenic factor. Peptides. 31:1219–1224. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang T, Sun X, Cui H, Liu K and Zhao J:
The peptide compound urantide regulates collagen metabolism in
atherosclerotic rat hearts and inhibits the JAK2/STAT3 pathway. Mol
Med Rep. 21:1097–1106. 2020.PubMed/NCBI
|
|
35
|
Wang T, Xie YQ, Miao GX, Cui HP, Liu K, Li
Y, Li Y and Zhao J: Urotensin receptor antagonist urantide improves
atherosclerosis-related kidney injury by inhibiting JAK2/STAT3
signaling pathway in rats. Life Sci. 247:1174212020. View Article : Google Scholar : PubMed/NCBI
|