Introduction
Atherosclerosis has become a common disease, which
increasingly threatens human health. According to the World Health
Organization, an estimated 17.5 million people died of
cardiovascular disease in 2012, of which 7.4 million died of
ischemic heart disease and 6.7 million died of stroke (1). Atherosclerosis is the fundamental
cause of a series of diseases, such as myocardial infarction,
stroke and gangrene (2). Its
pathogenesis is complex. With the development of research, the
currently recognized mechanisms include lipid metabolism disorder,
inflammatory response, oxidative stress and so on (3,4).
Vascular endothelial cells serve a key role in the pathogenesis of
atherosclerosis by regulating vascular tension, platelet adhesion,
inflammation and fibrinolysis (5).
Oxidized low-density lipoprotein (ox-LDL) is widely recognized to
participate in the occurrence and development of atherosclerosis
through inducing oxidative chain reaction and endothelial
dysfunction (6). At the onset of
atherosclerosis, LDL is deposited on vascular endothelial cells to
be oxidized to form ox-LDL, which is then absorbed by macrophages
to form foam cells. During this process, many inflammatory factors
are released, leading to aggravated progression of atherosclerosis
and the formation of atherosclerotic plaques (7). Therefore, the study of ox-LDL-mediated
endothelial cell dysfunction can further clarify the pathogenesis
of atherosclerosis and provide a more theoretical basis for the
treatment and prevention of atherosclerosis.
Long non-coding RNAs (lncRNAs) belong to non-coding
RNAs without protein-coding function and comprise >200
nucleotides (8). Although they
cannot encode proteins, they are involved in many biological
processes as regulators, such as proliferation, apoptosis,
migration and invasion (9). The
metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was
initially identified as a tumor-related lncRNA, which can control
the proliferation and metastasis of lung adenocarcinoma (10). Previous studies have shown that the
expression of MALAT1 is increased in endothelial cells induced by
ox-LDL and its expression level can reflect the damage degree of
endothelial cells (11,12).
Sacubitril/valsartan (S/V) is the first angiotensin
receptor neprilysin inhibitor drug, which is comprises two
components, neprilysin inhibitor sacubitril and angiotensin II
receptor antagonist (ARB) valsartan (13). S/V is more effective than the
classic renin-angiotensin system blockers (including ARBs and
angiotensin-converting-enzyme inhibitors in the treatment of
congestive heart failure (14).
Myocardial ischemia caused by coronary atherosclerosis is an
important cause of heart failure (15). However, whether S/V has an
anti-atherosclerotic effect remains controversial. The protective
effect of S/V on endothelial cells induced by ox-LDL has not been
studied. Therefore, the present study first established an
ox-LDL-induced injury model of human umbilical vein endothelial
cells (HUVECs) and then explored the effects of S/V on MALAT1
expression, inflammation, apoptosis and other indicators and
clarified the protective effect of S/V on endothelial cells and its
underlying mechanism.
Materials and methods
Cell culture and intervention
HUVECs were purchased from Shanghai Zhongqiaoxinzhou
Biotechnology Co., Ltd. and grown in endothelial cell culture
medium supplemented with 1% endothelial growth factor and 5% fetal
bovine serum (Shanghai Zhongqiaoxinzhou Biotechnology Co., Ltd).
The cells were maintained at 37°C in a humidified atmosphere
containing 5% CO2. Subsequently, the cells were exposed
to 80 µg/ml ox-LDL (Peking Union-Biology Co., Ltd.) for 72 h or
pretreated with S/V (Novartis International AG) for 2 h and then
exposed to 80 µg/ml ox-LDL for another 72 h.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was determined using a CCK-8 assay
(Wanleibio Co., Ltd.). The cells were prepared as a single-cell
suspension (3×104 cells/ml) and seeded into 96-well
plates. After 72 h of incubation under different conditions, 10 µl
of CCK-8 solution was added into each well, followed by the
incubation at 37°C for another 2 h. Subsequently, 10 µl termination
solution was added into each well. The absorbance at a wavelength
of 450 nm was determined by a microplate reader (BioTek
Instruments, Inc.).
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was extracted from cells using TriPure
reagent (BioTeke Corporation) according to the manufacturer's
protocols. The number of cells in each group was ~1×106.
Purified RNA was reversely transcribed into cDNA using super M-MLV
reverse transcriptase kit (BioTeke Corporation) according to the
manufacturer's protocols. RT-qPCR was conducted on an Exicycler 96
(Bioneer Corporation) using 2X Power Taq PCR MasterMix kit (BioTeke
Corporation) according to the manufacturer's protocols. The
reaction volume was 20 µl. RT was performed as follows: 70°C for 5
min, 42°C for 60 min and 80°C for 10 min. The thermocycling
conditions of PCR amplification consisted of initial denaturation
at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C
for 30 sec, annealing at 60°C for 30 sec and elongation at 72°C for
30 sec. GAPDH was used as a housekeeping gene. The relative
expression of the target gene were calculated with the
2−ΔΔCq method (16).
Each experiment was replicated three times. The primer sequences
were as follows: MALAT1 forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and
reverse, 5′-ACGUGACACGUUCGGAGAATT-3′; GAPDH forward,
5′-TCAAGAAGGTGGTGAAGCAGG-3′ and reverse,
5′-TCAAAGGTGGAGGAGTGGGT-3′.
ELISA
The levels of interleukin IL-1β (cat. no. WLE03),
IL-6 (cat. no. WLE04) and TNF-α (cat. no. WLE05) were determined by
ELISA. Following cell stimulation, the supernatant was collected
and tested according to the manufacturer's instructions (Wanleibio
Co., Ltd.).
Flow cytometry
After the cells were harvested and resuspended, cell
apoptosis was determined with the Annexin V-FITC/PI Apoptosis
Detection kit (Wanleibio Co., Ltd.). The cells were incubated with
5 µl Annexin V-FITC and 10 µl PI in the dark for 15 min at room
temperature. The apoptotic rate was analyzed by a flow cytometer
(NovoCyte; ACEA Bioscience, Inc.; Agilent Technologies, Inc.).
NovoExpress 13.0 software (Agilent Technologies, Inc.) was used for
analysis. The percentage of early + late apoptotic cells was the
apoptotic rate.
Measurement of nitric oxide (NO)
production
HUVECs were divided into various groups according to
different treatments and the supernatant of each group was
collected. The content of NO in the supernatant was detected by the
NO detection kit (Nanjing Jiancheng Bioengineering Inc.) according
to the manufacturer's instructions.
Western blotting analysis
Total proteins were extracted from HUVECs of
different groups using a total protein extraction kit (Wanleibio
Co., Ltd.) according to the manufacturer's instructions and the
protein concentration was determined using a BCA kit (Wanleibio
Co., Ltd.). Briefly, equal amounts of proteins (40 µg) were
subjected to SDS-PAGE on 10% gels and transferred onto a PVDF
membrane (EMD Millipore). The membranes were blocked with 5%
skimmed milk in TBST with 0.15% Tween-20 at room temperature for 1
h, followed by the incubation with different primary antibodies as
follows: Intercellular cell adhesion molecule (ICAM)-1 (cat. no.
WL02268; 1:500; Wanleibio Co., Ltd.), vascular cell adhesion
molecule (VCAM)-1 (cat. no. A0279; 1:2,000; ABclonal Biotech Co.,
Ltd.), endothelin (ET)-1 (cat. no. WL07780; 1:500; Wanleibio Co.,
Ltd.), p65 (cat. no. WL01980; 1:500; Wanleibio Co., Ltd.), p-p65
(cat. no. WL01980; 1:500; Wanleibio Co., Ltd.), Toll-like receptor
4 (TLR4; cat. no. WL00196; 1:500; Wanleibio Co., Ltd.), caspase-3
(cat. no. WL02117; 1:500; Wanleibio Co., Ltd.), Bcl-2 (cat. no.
WL01556; 1:500; Wanleibio Co., Ltd.), Bax (cat. no. WL01637; 1:500;
Wanleibio Co., Ltd.) and GAPDH (cat. no. WL01114; 1:500; Wanleibio
Co., Ltd.) at 4°C overnight. Membranes were washed with TBST three
times (10 min ×3). Subsequently, the membranes were incubated with
HRP-conjugated secondary antibody (cat. no. WLA023; 1:5,000;
Wanleibio Co., Ltd.) at room temperature for 1 h and washed with
TBST three times (10 min ×3). Immunoreactive bands were visualized
using an ECL detection system (Wanleibio Co., Ltd.) and quantified
by Gel-Pro analyzer software (Media Cybernetics, Inc.).
Statistical analysis
All data were expressed as mean ± standard
deviation. SPSS 21.0 software (IBM Corp.) was used for statistics.
Groups of data were compared by one-way ANOVA with post hoc
analysis using Tukey's test for pairwise comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
S/V increases the viability of HUVECs
induced by ox-LDL
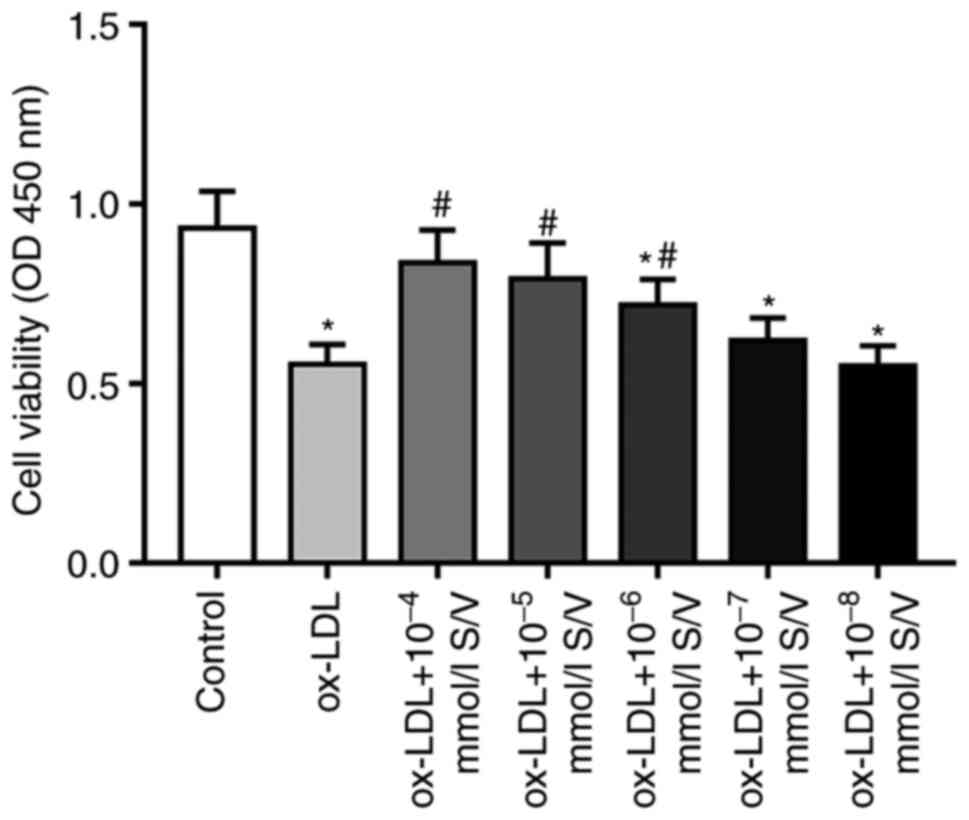
The present study first used a CCK-8 assay to assess
the effect of S/V on the viability of HUVECs induced by ox-LDL.
Compared with the control group, the cell viability of HUVECs
exposed to 80 µg/ml ox-LDL alone was significantly decreased, while
pretreatments with different concentrations (10−4,
10−5, 10−6, 10−7 and
10−8 mmol/l) of S/V increased the cell viability and the
most significant increase was found in the group of 10−4
mmol/l S/V (Fig. 1). Therefore,
cells were pretreated with 10−4 mmol/l S/V in subsequent
experiments.
S/V reduces the expression of MALAT1
in HUVECs induced by ox-LDL
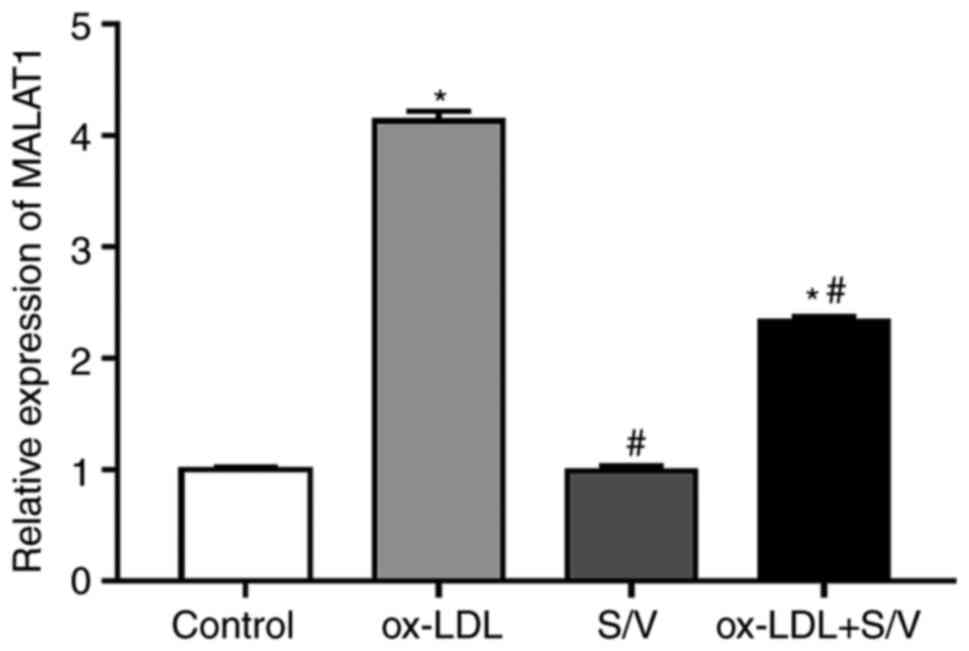
The expression of MALAT1 was detected by RT-qPCR.
Compared with the control group, the expression of MALAT1 in the
ox-LDL group was significantly increased (P<0.05). Pretreatment
with S/V significantly reduced the expression of MALAT1 in HUVECs
induced by ox-LDL (P<0.05; Fig.
2). However, treatment with S/V alone did not affect the
expression of MALAT1.
S/V alleviates ox-LDL-induced
inflammation of HUVECs
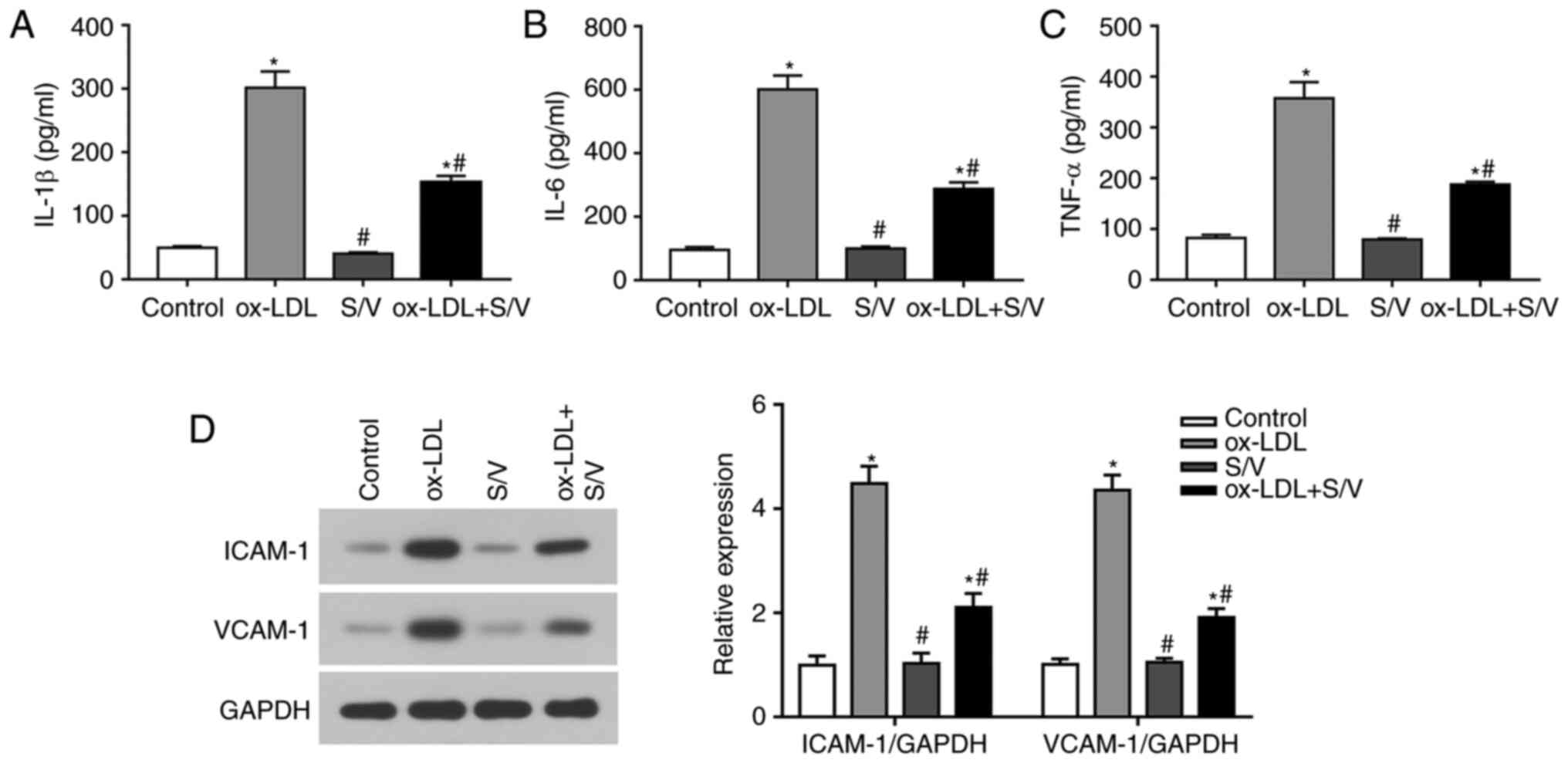
The levels of IL-1β, IL-6 and TNF-α were detected by
ELISA. Compared with the control group, the levels of IL-1β, IL-6
and TNF-α in the ox-LDL group were significantly increased
(P<0.05), while the levels of IL-1β, IL-6 and TNF-α in the
ox-LDL+S/V group were significantly decreased compared with the
ox-LDL group (P<0.05) (Fig.
3A-C). The expressions of adhesion molecules at the protein
level were detected using western blotting analysis. Compared with
the control group, the expressions of VCAM-1 and ICAM-1 in the
ox-LDL group were significantly increased (P<0.05), while the
expressions of VCAM-1 and ICAM-1 in the ox-LDL+S/V group were
significantly decreased compared with the ox-LDL group (P<0.05;
Fig. 3D). These results indicated
that S/V alleviated ox-LDL-induced inflammation of HUVECs.
S/V inhibits ox-LDL-induced apoptosis
of HUVECs
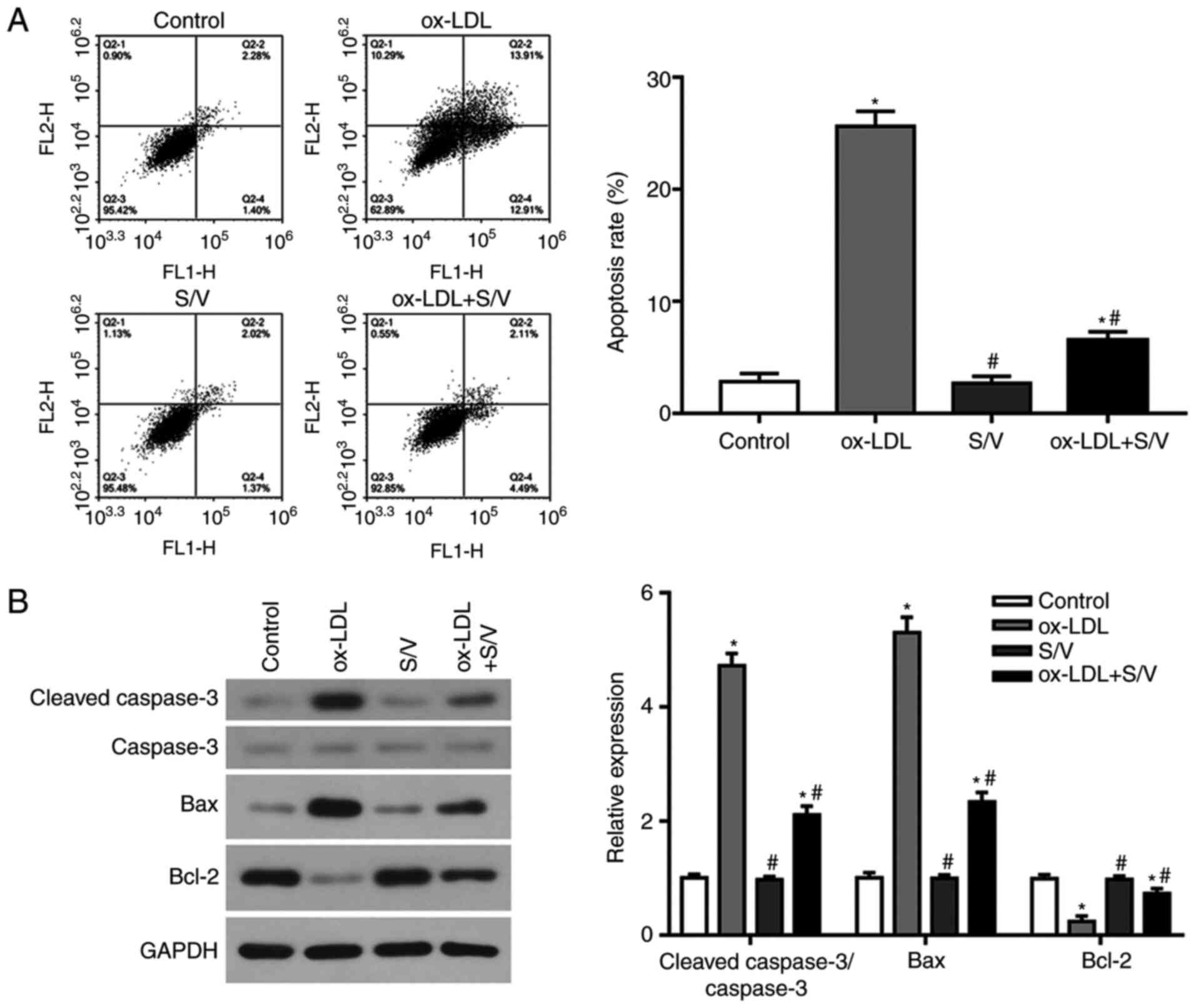
The apoptotic rate of each group was detected by
flow cytometry. Compared with the control group, the apoptotic rate
of the ox-LDL group was significantly increased (P<0.05), while
the apoptotic rate of the ox-LDL+S/V group was significantly
decreased compared with the ox-LDL group (P<0.05; Fig. 4A). The expression levels of
apoptosis-related proteins, caspase-3, Bax and Bcl-2 were detected
at the protein level using western blotting analysis. Compared with
the control group, the expressions of pro-apoptotic proteins,
cleaved-caspase-3 and Bax in the ox-LDL group were significantly
increased, while the expression of anti-apoptotic protein Bcl-2 was
significantly decreased (P<0.05). Furthermore, compared with the
ox-LDL group, the expressions of cleaved-caspase-3 and Bax were
significantly decreased and the expression of Bcl-2 was
significantly increased in the ox-LDL+S/V group (P<0.05;
Fig. 4B). These results suggested
that S/V could inhibit the apoptosis of HUVECs induced by
ox-LDL.
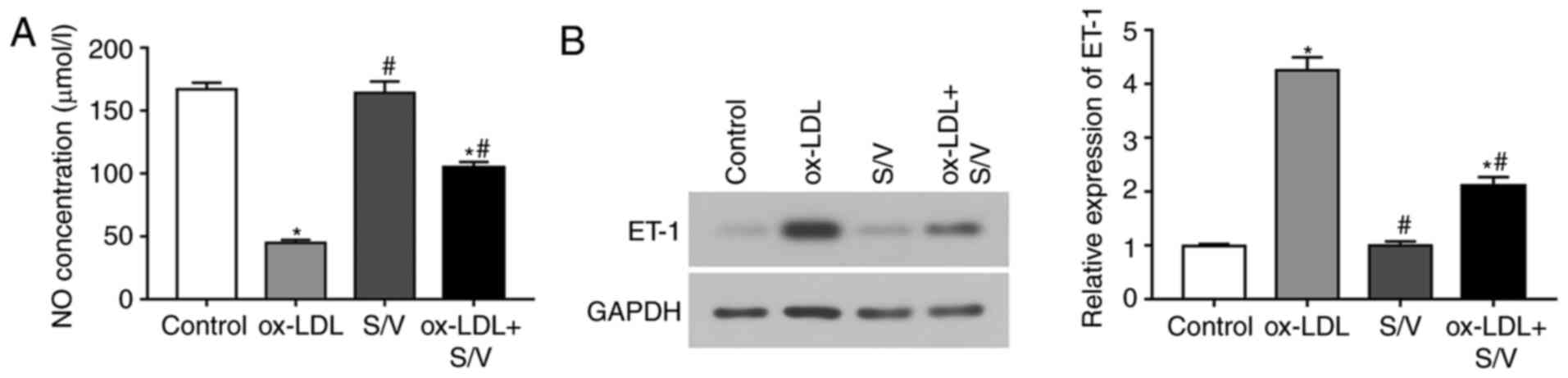
S/V increases NO release and decreases
endothelin 1 (ET-1) expression in ox-LDL-induced HUVECs
To examine whether S/V could promote the production
and release of NO in HUVECs, the content of NO was measured under
different treatment conditions. Compared with the control group,
the NO concentration of the ox-LDL group was significantly
decreased (P<0.05), while the NO concentration of the ox-LDL+S/V
group was significantly increased compared with the ox-LDL group
(P<0.05; Fig. 5A). ET-1
expression was detected by western blotting analysis. Compared with
the control group, the expression of ET-1 in the ox-LDL group was
significantly increased, while the expression of ET-1 in the
ox-LDL+S/V group was significantly decreased compared with the
ox-LDL group (P<0.05; Fig. 5B).
These results indicated that S/V alleviated ox-LDL-induced
endothelial dysfunction.
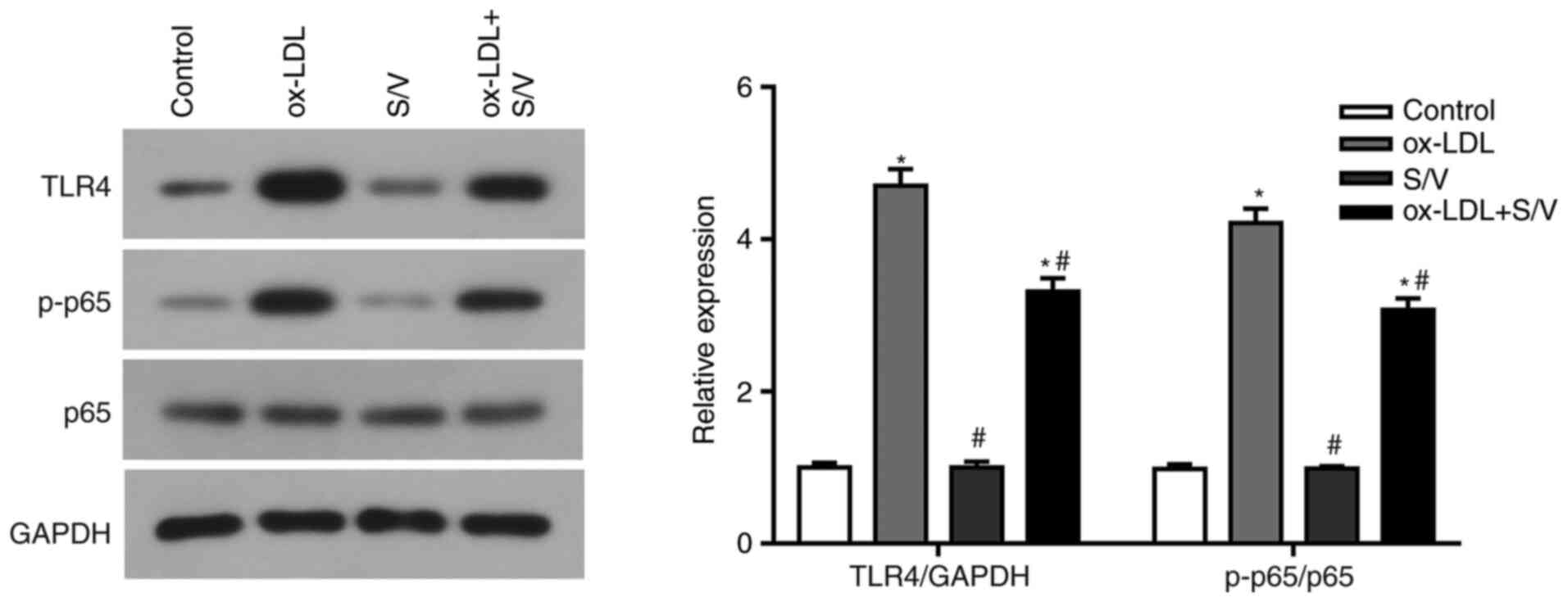
S/V inhibits TLR4/NF-κB signaling
pathway in ox-LDL-induced HUVECs
The present study found that S/V alleviated the
inflammation and apoptosis of HUVECs induced by ox-LDL, while the
specific mechanism remained unclear. TLR4/NF-κB signaling pathway
has been found to regulate endothelial cell inflammation and
apoptosis (17,18). Based on these studies, the present
study examined whether S/V alleviated inflammation and apoptosis by
inhibiting the TLR4/NF-κB signaling pathway. Compared with the
control group, the expressions of TLR4 and p-p65 in the ox-LDL
group were significantly increased (P<0.05), while the
expressions of TLR4 and p-p65 in the ox-LDL+S/V group were
significantly decreased compared with the ox-LDL group (P<0.05;
Fig. 6). These results suggested
that S/V reduced ox-LDL-induced endothelial cell injury by
suppressing the TLR4/NF-κB signaling pathway.
Discussion
As a drug with dual targets, S/V has been fully
affirmed in the treatment of heart failure and is considered a
major breakthrough in the field of heart failure treatment in
recent years (19). The PARADIGM-HF
study has shown that compared with enalapril, S/V significantly
reduces the major composite endpoint of hospitalization or
cardiovascular mortality due to heart failure, as well as
cardiovascular mortality and the hospitalization rate of heart
failure (14). S/V also shows great
potential in the treatment of hypertension, diabetes and other
diseases (20,21). Endothelial dysfunction serves a key
role in the formation of early atherosclerosis, including many
complex processes, such as inflammatory response and cell apoptosis
(5). Seki et al (22) found that S/V can improve endothelial
dysfunction in spontaneously hypertensive rats. The present study
investigated the role of S/V in ox-LDL-induced endothelial cell
injury and elucidated its possible mechanism.
In general, the stated does of S/V in patients is
100 mg twice daily and the target maintenance dose is 200 mg twice
daily (14,23). The absorption and metabolism of
drugs in the body is a very complicated process. In in vitro
experiments, there is no influence from neuroendocrine, immune and
other complex factors. Therefore, the drug concentration in
vitro does not correspond to the drug concentration in
vivo or in patients. In the present study, in order to select
the appropriate concentration, the drug was first made into
different concentration gradients (10−4,
10−5, 10−6, 10−7 and
10−8 mmol/l). Then CCK-8 was used to assess the effect
of S/V on the viability of HUVECs induced by ox-LDL. The results
showed that 10−4 mmol/l S/V had the best intervention
effect. Therefore, cells were pretreated with 10−4
mmol/l S/V in subsequent experiments.
A number of studies have confirmed that lncRNA
MALAT1 is upregulated in ox-LDL-induced endothelial cells and
participates in ox-LDL-induced endothelial dysfunction. Wang et
al (11) reported that MALAT1
enhances the expression of Beclin-1 by combining miR-216a-5p and
promotes autophagy to protect the endothelial cells. Tang et
al (12) demonstrated that
MALAT1 protects the endothelial cells from ox-LDL-induced
endothelial dysfunction partly through competing with miR-22-3p for
endogenous RNA. Based on the above-mentioned studies, it was
hypothesized that MALAT1 could be used as an indicator of
endothelial function. The present study found that the expression
of MALAT1 was increased in ox-LDL-induced HUVECs, which was
consistent with previous findings. In addition, compared with the
ox-LDL group, pretreatment with S/V significantly reduced the
expression of MALAT1 in HUVECs induced by ox-LDL. Taken together,
it was confirmed that S/V could improve endothelial function.
Ox-LDL is a key component of hyperlipidemia, which
can induce endothelial inflammation and apoptosis by enhancing the
oxidative stress of endothelial cells (24). Ox-LDL-induced inflammation is the
leading cause of endothelial dysfunction (25). Atherosclerosis is a persistent
inflammatory response, which can be activated by ox-LDL aggregation
on the arterial wall (26). The
increased expressions of inflammatory cytokines, including
inflammatory factors and cell adhesion molecules, can advance the
adhesion between monocytes and vascular endothelial cells (27). Macrophages are subsequently
activated and these macrophages absorb lipoproteins, leading to
foam cell formation (28). The
present study found that the levels of IL-1β, IL-6, TNF-α, VCAM-1
and ICAM-1 were increased in endothelial cells after ox-LDL
stimulation. When HUVECs were pretreated with S/V, the levels of
these pro-inflammatory factors were decreased compared with the
ox-LDL group, indicating that S/V could alleviate the inflammatory
response induced by ox-LDL.
Endothelial cell apoptosis can increase the
permeability of endothelial monolayer by reducing the number of
endothelial cells, thus promoting lipid migration and deposition
(29). Then, monocytes and smooth
muscle cells migrate to the endothelium, engulf large amounts of
lipids, form foam cells, further damage blood vessels and promote
plaque formation (29). Meanwhile,
the growth factors and cytokines secreted by infiltrating white
cells also affect the proliferation of smooth muscle cells
(30). Ox-LDL is a carrier of
oxygen-free radicals, which can produce toxic effects on vascular
cells, promote their apoptosis and cause vascular endothelial
damage (31). In the present study,
the results of flow cytometry showed that the apoptotic rate of the
ox-LDL group was significantly increased compared with the control
group and S/V pretreatment could significantly reduce the apoptotic
rate. Western blotting analysis showed that S/V pretreatment could
reverse the upregulation of pro-apoptotic proteins,
cleaved-caspase-3 and Bax and promoted the expression of
anti-apoptotic protein Bcl-2. This finding was consistent with the
results of flow cytometry. These results suggested that S/V could
inhibit the apoptosis of HUVECs induced by ox-LDL.
Endothelium-derived NO is an important regulator of
endothelial function, which serves an important role in the
regulation of vascular homeostasis. Its regulatory role is mainly
achieved by regulating vascular tension and blood pressure,
inhibiting vascular smooth muscle proliferation and migration,
suppressing platelet aggregation and constraining monocyte and
platelet adhesion (32). ET-1 is a
factor secreted by the vascular endothelium that has the opposite
effect of NO (33). ET-1 can
activate the exchange of Na+/H+ and
Na+/Ca+ in vascular smooth muscle fibers,
increase intracellular Ca + concentration, induce
vascular smooth muscle contraction and cause ischemia and hypoxia
(34). As important indicators of
endothelial function, NO and ET-1 serve an important role in
maintaining vascular tension and cardiovascular system homeostasis.
In the present study, HUVECs exposed to ox-LDL showed increased
expression of ET-1 and decreased level of NO. However, pretreatment
with S/V could reduce the ET-1 expression and increase the NO
level, indicating that S/V could reduce the injury of endothelial
cells induced by ox-LDL.
The present study identified the role of S/V in
ox-LDL-induced inflammation and apoptosis in HUVECs and further
studied its underlying mechanism. TLRs are the most important
pattern recognition receptors in the natural immune system and TLR4
is an important member of the TLR family (35). After binding to its ligand, TLR4 can
promote the expressions of IL-1β, IL-6, TNF-α, ICAM-1 and other
inflammatory factors through NF-κB and other signal transduction
pathways, enhance the immune-inflammatory response and induce the
apoptosis of target cells (36).
NF-κB is an important downstream signaling molecule of TLR4
(24,37), which exists in the cytoplasm as a
dimer (p65/p50) and usually binds to its inhibitory protein IκB.
When ox-LDL binds to TLR4 and activates downstream signaling
molecules, IκB kinase is activated (38). Consequently, IκB protein is
phosphorylated, ubiquitinated and then degraded and cytoplasmic p65
is released (39). Then
phosphorylated p65 enters the nucleus and combines with target
genes on the nucleus to generate a large number of inflammatory
factors, which in turn act on the receptors of endothelial cells to
induce apoptosis (40). In the
present study, compared with the ox-LDL group, S/V pretreatment
reduced the expressions of TLR4 and p-p65 in HUVECs induced by
ox-LDL, indicating that S/V could suppress the TLR4/NF-κB signaling
pathway. However, there are several limitations to the present
study. S/V is a new drug composed of two drugs, sacubitril and
valsartan. At present, there are few studies on the effect of S/V
on endothelial cells. The purpose of the present study was only to
initially explore whether S/V had beneficial effects on endothelial
cells, so groups of each drug alone were not added to the study.
Therefore, it is unclear whether the beneficial effects of the drug
were caused by sacubitril, valsartan, or both. The present study
was conducted in vitro only and it is unclear whether S/V
has the same beneficial effect on endothelial cells in vivo.
Thus, further research is still required.
In conclusion, the present study found that S/V
could downregulate the expression of MALAT1, inhibit inflammation
and apoptosis and improve endothelial function in ox-LDL-induced
HUVECs by suppressing the TLR4/NF-κB signaling pathway. Therefore,
S/V might be used as a promising therapeutic strategy for the
prevention and treatment of atherosclerosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
WB and XQ designed the study, analyzed the data and
wrote the manuscript. TH, XC, and XS performed the experiments and
prepared the figures. CM, YD, CR and LD prepared the figures and
analyzed the data. WL and XQ performed critical revision of the
manuscript and supervised the study. WB and XQ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mendis S, Davis S and Norrving B:
Organizational update: the world health organization global status
report on noncommunicable diseases 2014; one more landmark step in
the combat against stroke and vascular disease. Stroke.
46:e121–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hansson GK: Inflammatory mechanisms in
atherosclerosis. J Thromb Haemost. 7 (Suppl 1):S328–S331. 2009.
View Article : Google Scholar
|
|
3
|
Moore KJ and Tabas I: Macrophages in the
pathogenesis of atherosclerosis. Cell. 145:341–355. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Förstermann U, Xia N and Li H: Roles of
vascular oxidative stress and nitric oxide in the pathogenesis of
atherosclerosis. Circ Res. 120:713–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gimbrone MA and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han QA, Yan C, Wang L, Li G, Xu Y and Xia
X: Urolithin A attenuates ox-LDL-induced endothelial dysfunction
partly by modulating microRNA-27 and ERK/PPAR-γ pathway. Mol Nutr
Food Res. 60:1933–1943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan X, Wang J, Hou J, Lin C, Bensoussan A,
Chang D, Liu J and Wang B: Berberine alleviates ox-LDL induced
inflammatory factors by up-regulation of autophagy via AMPK/mTOR
signaling pathway. J Transl Med. 13:922015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boon RA, Jaé N, Holdt L and Dimmeler S:
Long noncoding RNAs: From clinical genetics to therapeutic targets.
J Am Coll Cardiol. 67:1214–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tano K, Mizuno R, Okada T, Rakwal R,
Shibato J, Masuo Y, Ijiri K and Akimitsu N: MALAT-1 enhances cell
motility of lung adenocarcinoma cells by influencing the expression
of motility-related genes. FEBS Lett. 584:4575–4580. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang K, Yang C, Shi J and Gao T:
Ox-LDL-induced lncRNA MALAT1 promotes autophagy in human umbilical
vein endothelial cells by sponging miR-216a-5p and regulating
Beclin-1 expression. Eur J Pharmacol. 858:1723382019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang Y, Jin X, Xiang Y, Chen Y, Shen CX,
Zhang YC and Li YG: The lncRNA MALAT1 protects the endothelium
against ox-LDL-induced dysfunction via upregulating the expression
of the miR-22-3p target genes CXCR2 and AKT. FEBS Lett.
589:3189–3196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu J, Noe A, Chandra P, Al-Fayoumi S,
Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF,
Jeng AY, et al: Pharmacokinetics and pharmacodynamics of LCZ696, a
novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi).
J Clin Pharmacol. 50:401–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McMurray JJ, Packer M, Desai AS, Gong J,
Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg
K, et al: Angiotensin-neprilysin inhibition versus enalapril in
heart failure. N Engl J Med. 371:993–1004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pagliaro BR, Cannata F, Stefanini GG and
Bolognese L: Myocardial ischemia and coronary disease in heart
failure. Heart Fail Rev. 25:53–65. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng J, Liu Q, Hu N, Zheng F, Zhang X, Ni
Y and Liu J: Downregulation of hsa_circ_0068087 ameliorates
TLR4/NF-κB/NLRP3 inflammasome-mediated inflammation and endothelial
cell dysfunction in high glucose conditioned by sponging miR-197.
Gene. 709:1–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan CX, Xu M, Huang SH, Wu QQ, Yuan Y,
Deng W and Tang QZ: Baicalein protects against endothelial cell
injury by inhibiting the TLR4/NF-κB signaling pathway. Mol Med Rep.
17:3085–3091. 2018.PubMed/NCBI
|
|
19
|
Proudfoot C, Studer R, Rajput T, Jindal R,
Agrawal R, Corda S and Senni M: Real-world effectiveness and safety
of sacubitril/valsartan in heart failure: A systematic review. Int
J Cardiol. Feb 3–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruilope LM, Dukat A, Böhm M, Lacourcière
Y, Gong J and Lefkowitz MP: Blood-pressure reduction with LCZ696, a
novel dual-acting inhibitor of the angiotensin II receptor and
neprilysin: A randomised, double-blind, placebo-controlled, active
comparator study. Lancet. 375:1255–1266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seferovic JP, Claggett B, Seidelmann SB,
Seely EW, Packer M, Zile MR, Rouleau JL, Swedberg K, Lefkowitz M,
Shi VC, et al: Effect of sacubitril/valsartan versus enalapril on
glycaemic control in patients with heart failure and diabetes: A
post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes
Endocrinol. 5:333–340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seki T, Goto K, Kansui Y, Ohtsubo T,
Matsumura K and Kitazono T: Angiotensin II receptor-neprilysin
inhibitor sacubitril/valsartan improves endothelial dysfunction in
spontaneously hypertensive rats. J Am Heart Assoc. 6:e0066172017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kido K, Bianco C, Caccamo M, Fang W and
Sokos G: Evaluating sacubitril/valsartan dose dependence on
clinical outcomes in patients with heart failure with reduced
ejection fraction. Ann Pharmacother. Dec 31–2020.(Epub ahead of
print). View Article : Google Scholar
|
|
24
|
Zhu L, Gong X, Gong J, Xuan Y, Fu T, Ni S,
Xu L and Ji N: Notoginsenoside R1 upregulates miR-221-3p expression
to alleviate ox-LDL-induced apoptosis, inflammation, and oxidative
stress by inhibiting the TLR4/NF-κB pathway in HUVECs. Braz J Med
Biol Res. 53:e93462020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li W, Li Y, Zhao Y and Ren L: The
protective effects of aloperine against ox-LDL-induced endothelial
dysfunction and inflammation in HUVECs. Artif Cells Nanomed
Biotechnol. 48:107–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
García de Tena J: Inflammation,
atherosclerosis, and coronary artery disease. N Engl J Med.
353:429–430. 2005. View Article : Google Scholar
|
|
27
|
Yang X, Wan M, Cheng Z, Wang Z and Wu Q:
Tofacitinib inhibits ox-LDL-induced adhesion of THP-1 monocytes to
endothelial cells. Artif Cells Nanomed Biotechnol. 47:2775–2782.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng C, Chen Q, Fan M, Guo J, Liu Y, Ji T,
Zhu J and Zhao X: Platelet-derived microparticles promote
phagocytosis of oxidized low-density lipoprotein by macrophages,
potentially enhancing foam cell formation. Ann Transl Med.
7:4772019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choy JC, Granville DJ, Hunt DW and McManus
BM: Endothelial cell apoptosis: Biochemical characteristics and
potential implications for atherosclerosis. J Mol Cell Cardiol.
33:1673–1690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin C and Liu Z: In atherogenesis, the
apoptosis of endothelial cell itself could directly induce
over-proliferation of smooth muscle cells. Med Hypotheses.
68:275–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Wang L, Xu J, Kong X and Zou L:
Up-regulated miR-106b inhibits ox-LDL-induced endothelial cell
apoptosis in atherosclerosis. Braz J Med Biol Res. 53:e89602020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moncada S and Higgs EA: The discovery of
nitric oxide and its role in vascular biology. Br J Pharmacol. 147
(Suppl 1):S193–S201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bourque SL, Davidge ST and Adams MA: The
interaction between endothelin-1 and nitric oxide in the
vasculature: New perspectives. Am J Physiol Regul Integr Comp
Physiol. 300:R1288–R1295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Penna C, Rastaldo R, Mancardi D, Cappello
S, Pagliaro P, Westerhof N and Losano G: Effect of endothelins on
the cardiovascular system. J Cardiovasc Med (Hagerstown).
7:645–652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moghimpour Bijani F, Vallejo JG and Rezaei
N: Toll-like receptor signaling pathways in cardiovascular
diseases: Challenges and opportunities. Int Rev Immunol.
31:379–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao L, Li M, Sun K, Su S, Geng T and Sun
H: Hippophae rhamnoides polysaccharides protect IPEC-J2 cells from
LPS-induced inflammation, apoptosis and barrier dysfunction in
vitro via inhibiting TLR4/NF-κB signaling pathway. Int J Biol
Macromol. 155:1202–1215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang G and Ghosh S: Toll-like
receptor-mediated NF-kappaB activation: A phylogenetically
conserved paradigm in innate immunity. J Clin Invest. 107:13–19.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang M, Xue Y, Chen H, Meng L, Chen B,
Gong H, Zhao Y and Qi R: Resveratrol Inhibits MMP3 and MMP9
Expression and Secretion by Suppressing TLR4/NF-κB/STAT3 Activation
in Ox-LDL-Treated HUVECs. Oxid Med Cell Longev.
2019:90131692019.PubMed/NCBI
|
|
39
|
Yu XH, Zheng XL and Tang CK: Nuclear
Factor-κB activation as a pathological mechanism of lipid
metabolism and atherosclerosis. Adv Clin Chem. 70:1–30. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhong X, Zhang L, Li Y, Li P, Li J and
Cheng G: Kaempferol alleviates ox-LDL-induced apoptosis by
up-regulation of miR-26a-5p via inhibiting TLR4/NF-κB pathway in
human endothelial cells. Biomed Pharmacother. 108:1783–1789. 2018.
View Article : Google Scholar : PubMed/NCBI
|