Introduction
Tropomyosin receptor kinases (TrkA, TrkB, and TrkC)
encoded by the NTRK1-3 gene family are activated by neurotrophins
(1). Trk family proteins were found
to be expressed only in cells of the nervous system and identified
to be involved in neuronal development and function (2). Various studies have revealed that
overexpression of TrkB not only plays a critical role in cancer
cell proliferation and metastasis/invasion (3,4) but is
also associated with poor clinical outcomes in patients with
various cancers (5). The activation
of TrkB stimulated by brain-derived neurotrophic factor (BDNF)
enhances the resistance of head and neck squamous cell carcinoma to
cisplatin through the upregulation of multidrug resistance 1 (MDR1)
and X-linked inhibitor of apoptosis protein (XIAP) (6,7).
Furthermore, high expression of TrkB and TrkC contributes to tumor
proliferation, invasion, and inhibition of apoptosis in colon
cancer (8,9). However, the expressional changes,
exact roles, and downstream targets of TrkB and TrkC that promote
epithelial-mesenchymal transition in drug-resistant colon cancer
cells remain unclear.
Human homeobox (HOX) transcription factors regulated
by Hox genes play critical roles in embryonic development and
differentiation (10,11). Deregulated Hox families (HOXA, HOXB,
HOXC and HOXD) have been reported in many cancers, such as breast,
ovary, colon, prostate, and lung (10,12,13).
HOXC6 is not only overexpressed in numerous cancers but also plays
an important role in cancer progression and the survival of cancer
cells (14–16). Higher HOXC6 expression is associated
with poor prognosis of colon cancer patients and contributes to
enhanced cell viability and colony formation of colon cancer cells
in vitro (17). Furthermore,
overexpression of HOXC6 results in upregulated MDR1 expression
through the activation of the promoter activity in colon cancer
cells, causing resistance to paclitaxel (18). Although TrkB- or HOXC6-mediated
signaling pathways prevent the apoptosis of cancer cells and
enhance anticancer drug resistance, the relationship between TrkB/C
and HOXC6 in drug-resistant cancer cells is largely unknown.
A disintegrin and metalloproteinase proteins (ADAMs)
are tetraspanin-transmembrane proteins that play critical roles in
cell adhesion, cell migration, and related signaling pathways
(19). In colon cancer cells,
ADAM10 induces liver metastasis through the cleavage of endogenous
L1-cell adhesion molecule (L1-CAM) (20). We have also reported that ADAM10- or
ADAM17-mediated lactate production stimulated by a Toll-like
receptor 4 (TLR4) ligand increases the invasiveness and mesenchymal
characteristics of colon cancer cells (21). ADAM8 is detected in most cancer
tissues, and the serum level of ADAM8 in lung cancer patients is
higher than that in the normal group (22). In addition, higher ADAM8 in
astrocytoma plays a critical role in tumor cell migration and
invasion (23). Furthermore, the
upregulation of ADAM8 is associated with brain metastasis of breast
cancer through the activation of matrix metalloproteinase 9 (MMP9)
(24,25). Although patients with ADAM8-positive
colon tumors show a worse prognosis and a lower rate of 5-year
disease-free survival (26), no
studies have examined the role of ADAM8 in drug-resistant colon
cancer cells.
An increase in TrkB or ADAM expression levels
triggers various downstream signaling pathways, including the
phosphorylation of extracellular-signal-regulated kinase (ERK)
(27,28). Furthermore, the upregulation of
HOXC6 promotes the proliferation and migration of glioblastoma
cells through the activation of mitogen-activated protein kinase
(MAPK) (16). Based on these
results, we investigated whether TrkB/C- or HOXC6-induced signaling
regulates ADAM8 expression to enhance the migratory and invasive
abilities of drug-resistant colon cancer cells, which have been
established in previous reports (23–26).
We also examined whether the TrkB/C- or HOXC6-mediated regulation
of ADAM8 levels in drug-resistant colon cancer involves the
modulation of ERK activation.
Materials and methods
Cell lines and reagents
Human colorectal carcinoma cell lines HCT116 and
HCT8 were obtained from the American Type Culture Collection. These
cells were cultured in Roswell Park Memorial Institute (RPMI) 1640
media (Corning Inc.) containing 10% fetal bovine serum (FBS;
RMBIO), glutamine, and antibiotics and were incubated at 37°C in 5%
CO2. Oxaliplatin (Ox) and 5-fluorouracil (5-Fu) were
purchased from Sigma-Aldrich; Merck KGaA. The selective tropomyosin
receptor kinase (TrK) inhibitor CH7057288 and PD98059 (inhibitor of
the MEK/ERK pathway) were purchased from Selleck Chemicals.
GI254023X (ADAM10 inhibitor) and Marimastat (ADAM17 inhibitor) were
purchased from TOCRIS Biosciences.
Establishment of drug-resistant cell
lines
To generate colorectal cancer cell lines with stable
chronic resistance to Ox or 5-Fu, HCT116 and HCT8 cells were
initially exposed to 1 µM of Ox or 5-Fu in RPMI-1640 medium plus
10% FBS, as previously described with slight modifications
(29). When the cells that survived
Ox or 5-Fu treatment reached 70–90% confluency, they were
subcultured twice a week to confirm their viability. Then, the dose
of Ox or 5-Fu was doubled in each surviving population and
sequentially increased to 50 µM. All resistant cell lines were
maintained and experimented with the presence of 20 µM Ox or 5-Fu
in RPMI-1640 medium supplemented with 10% FBS. Finally, the
authenticity of the drug-resistant sublines was verified by short
tandem repeat profiling according to the ANSI Standard (ASN-0002)
from the ATCC Standards Development Organization.
Reverse transcription-quantitative
PCR
Total RNA from cells was extracted using an RNeasy
Mini Kit (Qiagen), according to the supplier's instructions. cDNA
was synthesized from 2 µg of purified total RNA using an
Accupower® RT PreMix (Bioneer) and oligo(dT) primer
(Bioneer). To evaluate miRNA levels, total RNA was isolated from
cells using a miRNeasy Mini Kit (Qiagen). cDNA was synthesized with
a Mir-XTM miRNA First-Strand Synthesis Kit (Clontech). The mRNA and
miRNA levels were quantified using SYBR-Green (Takara), an ABI7300
real-time PCR system (Applied Biosystems), and specific primer sets
(Table I). β-actin was used as an
internal control for mRNA expression.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
|
| Primers (5–3) |
|---|
|
|
|
|---|
| Target | Forward | Reverse |
|---|
| TrkA |
AACCTCACCATCGTGAAGAGT |
TGAAGGAGAGATTCAGGCGAC |
| TrkB |
ACCCGAAACAAACTGACGAGT |
AGCATGTAAATGGATTGCCCA |
| TrkC |
GCCAGTATCAACATCACGGAC |
AGCCGGTTACTTGACAGGTTT |
| HOXC4 |
GAGCGCCAGTATAGCTGCAC |
GCGACTGTGATTTCTCGGGG |
| HOXC6 |
ACAGACCTCAATCGCTCAGGA |
AGGGGTAAATCTGGATACTGGC |
| ADAM8 |
GAGGGTGAGCTACGTCCTTG |
CAGCCGTATAGGTCTCTGTGT |
| ADAM10 |
ATGGGAGGTCAGTATGGGAATC |
ACTGCTCTTTTGGCACGCT |
| ADAM12 |
AACCTCGCTGCAAAGAATGTG |
CTCTGAAACTCTCGGTTGTCTG |
| ADAM17 |
GACTCTAGGGTTCTAGCCCAC |
GGAGACTGCAAACGTGAAACAT |
| β-actin |
ATCCACGAAACTACCTTCAA |
ATCCACACGGAGTACTTGC |
Western blot analysis
Harvested cells were lysed with NP-40 buffer (Elpis
Biotech) supplemented with a protease inhibitor cocktail and
phosphatase inhibitors (Sigma-Aldrich; Merck KGaA). Protein
concentrations were determined using a bicinchoninic acid (BCA)
Protein Assay Kit (Pierce), and equal amounts of proteins (10
µg/sample) were subjected to sodium dodecyl sulfate-polyacrylamide
gel electrophoresis. The proteins were then transferred onto
nitrocellulose membranes (Millipore Corp.). The membranes were
blocked with 5% non-fat skim milk and probed with primary
antibodies. The expression levels of the target proteins were
determined using a Chemiluminescence Kit (Advansta Corp.) and an
Amersham Imager 600 (GE Healthcare Life Sciences). The following
primary antibodies were used: TrkB (#4603), TrkC (#3376),
phospho-ERK1/2 (Thr202/Tyr204; #9101), ERK1/2
(#9102), MMP2 (#4022), MMP9 (#3852), E-cadherin (#3195), N-cadherin
(#13116), Snail (#3879), and β-actin (#4967) (Cell Signaling
Technology); phospho-TrkB (Tyr817; #NBP2-67578), ADAM8
(#NB600-1393), and HOXC4 (#NBP2-56195) (Novus Biologicals);
phospho-TrkC (Tyr518; #PA5-40271) (Thermo Fisher
Scientific); and HOXC4 (#sc-81965), HOXC6 (#sc-376330),
phospho-MEK1/2 (Ser218/Ser222; #sc-7995), and
MEK1/2 (#sc-436) (Santa Cruz Biotechnology). The expression levels
of β-actin were measured as a control. Densitometry for
quantifications of the bands was performed using ImageJ 1.38
software (National Institutes of Health). Relative intensity of
bands was calculated by ImageJ and expressed as relative values to
β-actin and/or total protein.
Small interfering RNA (siRNA) or micro
RNA (miRNA) transfection
Human ADAM8-siRNA (5′-GCATCATCGTCTACCGCAATT-3′),
HOXC6-siRNA (5′-CUCGUUCUCGGCUUGUCUA-3′), and negative control siRNA
(cat. no. SN-1001-CFG) were obtained from Bioneer. Transfection
with 200 nM siRNA using Lipofectamine RNAiMAX Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) was performed according to the
supplier's instructions. The cells were used for subsequent
experiments at 48 h after transfection.
Cell viability assay with Cell
Counting Kit-8 (CCK-8)
The viability of the cell treated with Trk inhibitor
or transfected with siRNA was measured using a CCK-8 (Enzo Life
Sciences) according to the supplier's protocol. Briefly, the
chemoresistant cells, which were pre-treated with 2 µM CH7057288 or
200 nM HOXC6-siRNA, were seeded into 96-well plates
(2×104 cells/well) and then incubated for 24 h. For
comparison, control cells exposed to DMSO or control siRNA were
cultured with same media. After treatment, the cells were stained
with 10 µl of CCK-8 dye in 90 µl of culture medium for 2 h at 37°C.
The absorbance was measured at 450 nm.
Migration and invasion assays
According to the supplier's instructions, the
migratory and invasive activities of cancer cells were determined
using a CytoSelect™ Tumor Transendothelial Migration Assay Kit
(Cell Biolabs, Inc.) and CultreCoat 96-well Medium BME Cell
Invasion Assay Kit (R&D Systems), respectively. The relative
fluorescence units (RFUs) of migrated cells were determined by a
microplate reader. Briefly, drug-resistant colon cancer cells
(5×105/well) were incubated for 48 h to form a monolayer
on the upper surface of the membrane inside the insert and then
stained with cell tracking solution (CytoTracker™) to detect
migration. After culture for 24 h, fluorescence of lysate derived
from cells inside insert were measured with a fluorescence plate
reader at 480/520 nm (Perkin Elmer Wallac 1420 VICTOR™; Perkin
Elmer). The relative invasion of cells was also compared with the
fluorescence intensity of calcein-AM stained invading cells
measured by a microplate reader (Perkin Elmer Wallac 1420 VICTOR™).
Harvested migrating cells were suspended in calcein-AM/cell
dissociation solution and then stock solution was diluted serially.
Fluorescence of cell lysates was read at 480/520 nm. Relative
invasiveness of chemoresistant colon cancer cells was analyzed
using standard curve.
Measurement of gelatinase
activity
The gelatinase activity of colon cancer cells was
detected using the Gelatinase (Gelatin Zymography) Assay Kit
(BioVision, Inc.), according to the supplier's instructions.
Briefly, fresh cells (2×106/sample) were homogenized
with cell lysis buffer and incubated for 5 min on ice. The
supernatants collected by centrifugation were used to measure the
amount of protein using a BCA Protein Assay Kit (Pierce), mixed
with a gelatinase substrate, and then the fluorescence at Ex/Em
490/520 nm was measured.
Statistical analysis
Student's t-test and one-way analysis of variance
using SPSS version 24.0 statistical software (IBM Corp.) were used
for all statistical analyses. Bonferroni post hoc analysis was
performed following ANOVA for multiple comparisons. Data are
presented as the mean ± standard deviation (SD). Differences were
determined to be statistically significant at P<0.05 and highly
significant at P<0.005, respectively.
Results
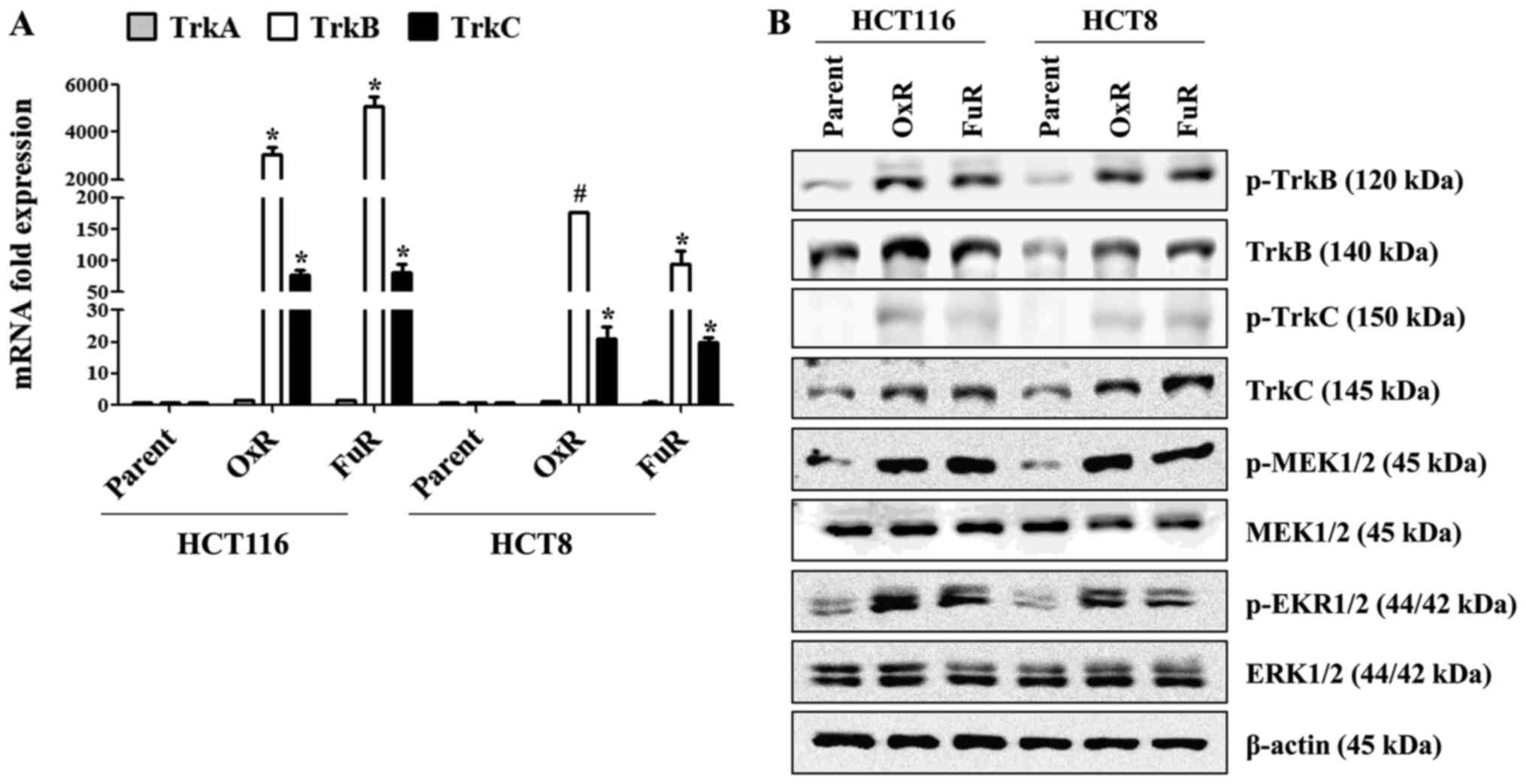
The expression levels of
phosphorylated TrkB/C, HOXC6 and ADAM8 are significantly increased
in chemoresistant colon cancer cells
The expression of TrkB in colon cancer is higher
than the level in normal tissue (8), and the activation of Trk receptors
triggers various downstream signaling pathways, including MEK/ERK
(27). Based on previous reports,
we first investigated whether the Trk family proteins are
overexpressed, resulting in MEK/ERK activation in drug-resistant
colon cancer cells. In Ox-resistant (OxR) and 5-Fu-resistant (FuR)
colon cancer cells (HCT116_OxR, HCT116_FuR, HCT8_OxR and HCT8_FuR),
the mRNA expression of TrkB and TrkC but not TrkA was enhanced than
that of parent cancer cells (Fig.
1A). Immunoblotting assays also revealed that the
phosphorylation of TrkB/C or activation of MEK and ERK in
chemoresistant colon cancer cells was higher than that of the
parent cancer cells (Figs. 1B and
S1). We also examined whether
HOXC6 and ADAM8 expression are upregulated in drug-resistant colon
cancer cells. Although the mRNA expression of HOXC4 was prominently
increased in HCT116_OxR and HCT8_OxR cells (Fig. 2A) and HOXC6 mRNA and protein
expression were markedly increased in all chemoresistant colon
cancer cell lines, the slightly upregulated HOXC4 protein was
detected using two different antibodies only in HCT8_FuR cells
(Fig. 2B). Among the upregulated
ADAM family proteins, ADAM8 mRNA expression was significantly
higher than other ADAM family members (Fig. 2C). Chemoresistant colon cancer cells
upregulated the expression of ADAM8, MMP2 and MMP9 and mesenchymal
markers, N-cadherin and Snail, whereas the expression of E-cadherin
was downregulated (Fig. 2D). The
migratory or invasive activity of HCT116_OxR, HCT116_FuR, HCT8_OxR
and HCT8_FuR cells was significantly enhanced (Fig. 2E), and the potential contribution of
MMP2 and MMP9 to the metastatic activity was detected through
gelatinase activity assays (Fig.
2F). These results suggest that the expression levels of
TrkB/C, HOXC6 and ADAM8, which are well-known regulators of cell
migration, might influence drug-resistant colon cancer cell
metastasis.
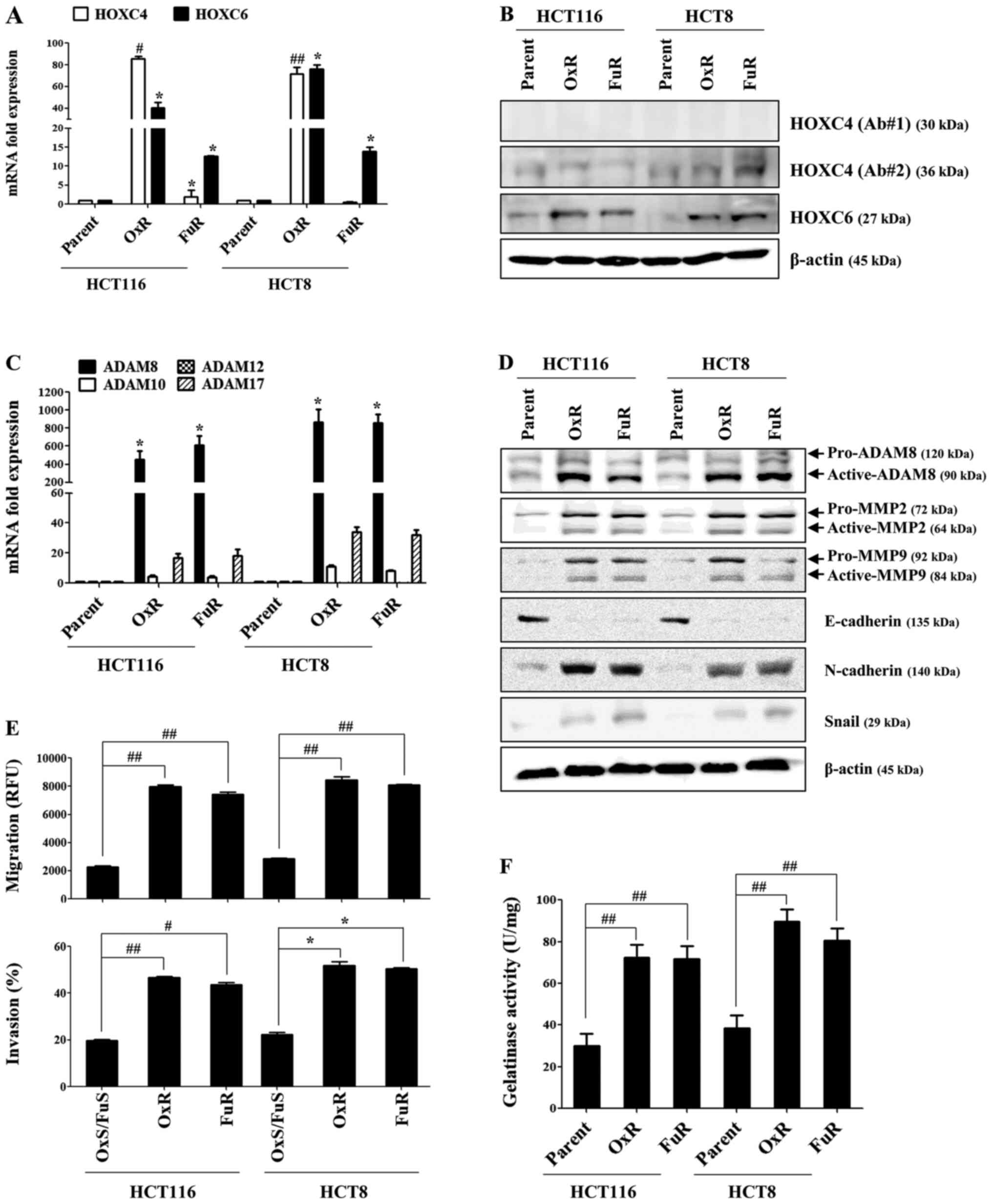 | Figure 2.Expressions of HOXC6 and ADAM8 are
significantly increased in chemoresistant colon cancer cells. OxR
or FuR colon cancer and parent cells were seeded into 6-well plates
(1.5×105/well) and cultured for 24 h. (A and C)
Quantitative real-time PCR was performed to determine the relative
expression of HOXC4, HOXC6, ADAM8, ADAM10, ADAM12 and ADAM17. (B
and D) The total protein of each group was subjected to western
blot analysis with the indicated antibodies. β-actin served as an
internal control. (E) The migration and invasion of cells were
detected using tumor transendothelial migration and BME cell
invasion assay kits, respectively. (F) The gelatinase activity of
cells was measured using a gelatinase assay kit. Data are presented
as the mean ± SD of three independent experiments. *P<0.05,
#P<0.005 and ##P<0.001 vs. resistant
cells. HOX, homeobox; ADAM, A disintegrin and metalloproteinase
domain-containing 8; OxR, oxaliplatin resistant; FuR,
5-Fu-resistant; p, phosphorylated. |
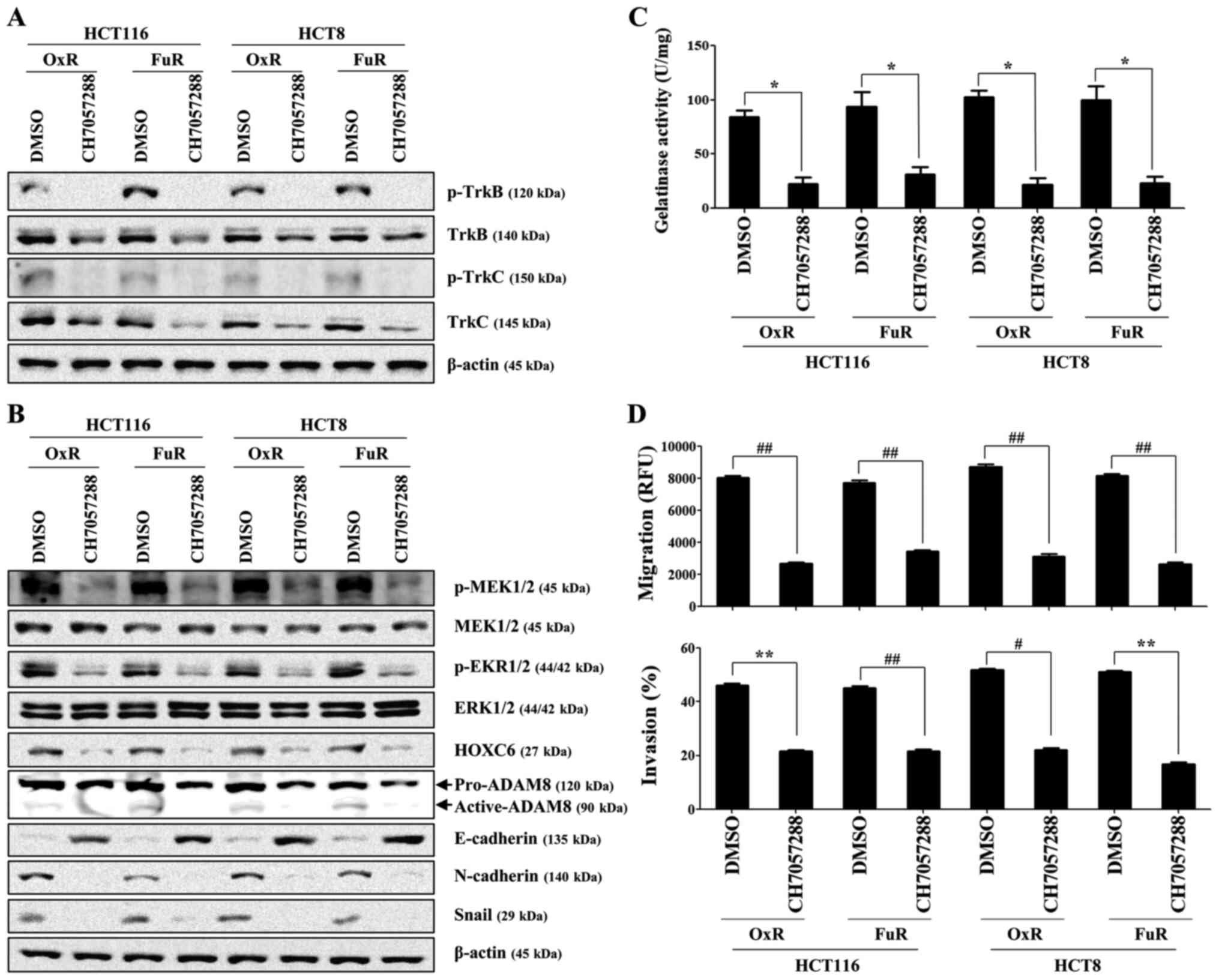
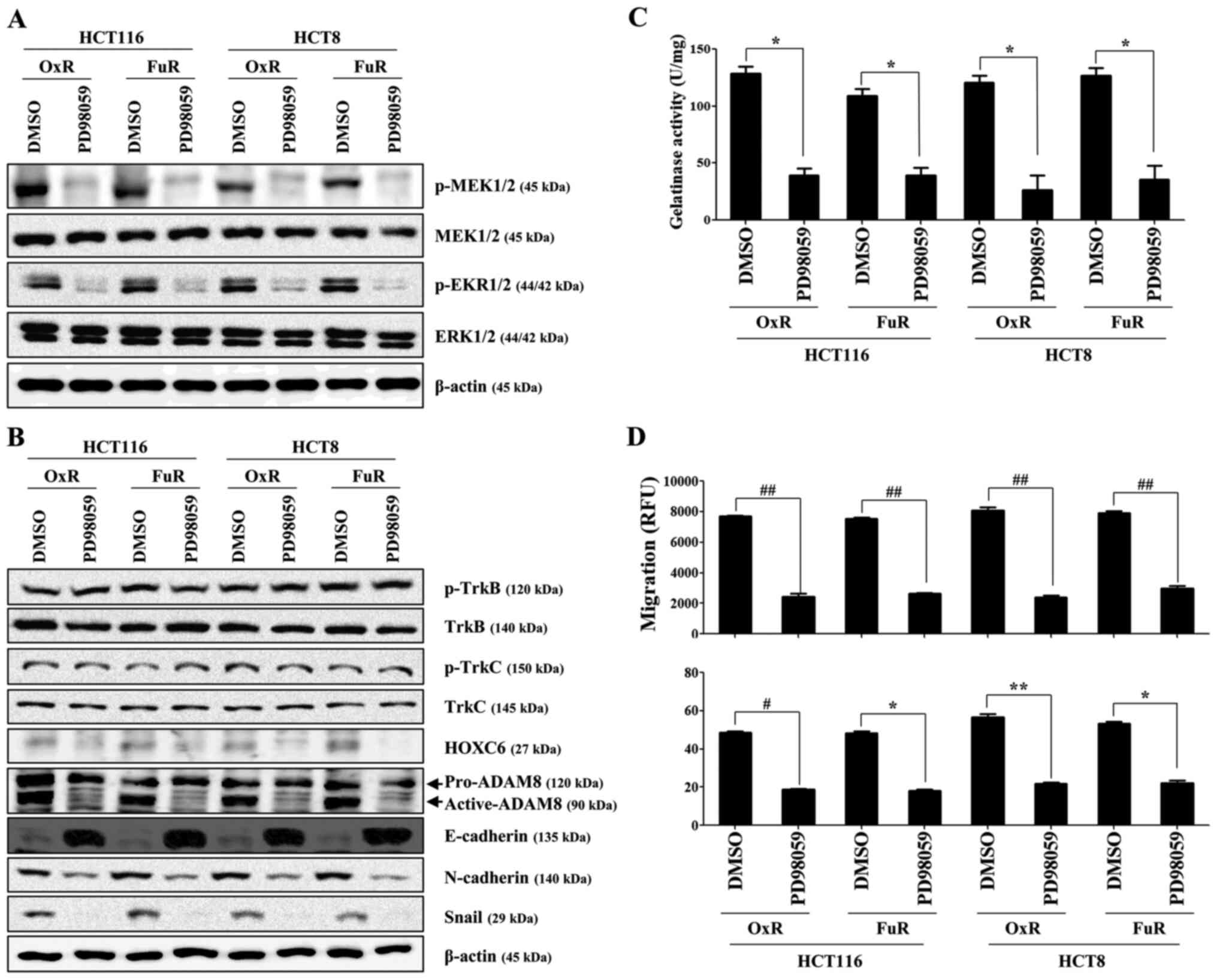
TrkB/C-mediated ERK activation
regulates HOXC6 and ADAM8 activity during the metastasis of
chemoresistant colon cancer cells
We next investigated whether the activation of
TrkB/C and related downstream target molecules control the
migration of drug-resistant colon cancer cells. After confirming
the concentration of Trk inhibitor used in this study had no
influence on cell survival and proliferation (Fig. S2A and B), we examined the role of
TrkB/C-mediated signaling pathways in the promoting of HOXC6 and
ADAM8 expression. Pharmacological inhibition of Trk using a
selective TrK inhibitor (CH7057288) prominently suppressed the
expression of both total and phosphorylated TrkB/C (Figs. 3A and S3), phosphorylation of MEK/ERK (Fig. 3B), expression of HOXC6 (Fig. 3B), and level of ADAM8 (Fig. 3B). Pretreatment with CH7057288
effectively prevented the migratory and invasive activity of
chemoresistant colon cancer cells and the action of gelatinase by
inhibiting MMP (Fig. 3C and D).
Specific inhibition of ERK (Fig.
4A), which is a downstream target of TrkB/C, using PD98059
reduced the expression of HOXC6 and ADAM8 and the induction of
mesenchymal markers in chemoresistant colon cancer cells, whereas
treatment with PD98059 failed to attenuate the expression of
phosphorylated TrkB and TrkC (Fig.
4B). Furthermore, PD98059 treatment significantly prevented the
migration and invasion of drug-resistant colon cancer cells through
the inhibition of MMP activation (Fig.
4C and D). These results suggest that TrkB/C-induced ERK
signaling plays an essential role in the activation of HOXC6 and
ADAM8 during the metastasis of chemoresistant colon cancer
cells.
A HOXC6-mediated ADAM8 activation
cascade regulates the metastasis of drug-resistant colon cancer
cells
We finally investigated the ability of HOXC6 and
ADAM8 to control the migratory activity of drug-resistant colon
cancer cells. We first confirmed that targeted downregulation of
HOXC6 using siRNA had no role in the viability of
drug-resistant colon cancer cells (Fig. S2A and B). Gene silencing of
HOXC6 with siRNA efficiently inhibited the expression of
ADAM8, MMP2, and MMP9 and the upregulation of N-cadherin and snail,
but did not influence the activation of the TrkB-mediated ERK
signaling pathway (Fig. 5A). In
addition, targeted inhibition of HOXC6 prominently suppressed the
migration, invasion, and gelatinase activity of chemoresistant
colon cancer cells (Fig. 5B and C).
Although the downregulation of ADAM8 with siRNA failed to reduce
the expression level of TrkB/C and related downstream signaling
molecules, such as ERK and HOXC6 (Fig.
6A and B), the metastatic activity of chemoresistant colon
cancer cells was significantly decreased by suppressing the action
of MMP (Fig. 6C and D). However,
the suppression of ADAM10 and ADAM17 using specific inhibitors had
no effect on the ADAM8-induced metastatic activity of
chemoresistant colon cancer cells (Fig.
6C and D). These results suggest that TrkB/C activation and its
downstream ERK-mediated HOXC6 signaling is one of the regulatory
pathways that enhance the metastasis of drug-resistant colon cancer
cells through the stimulation of ADAM8/MMP activity.
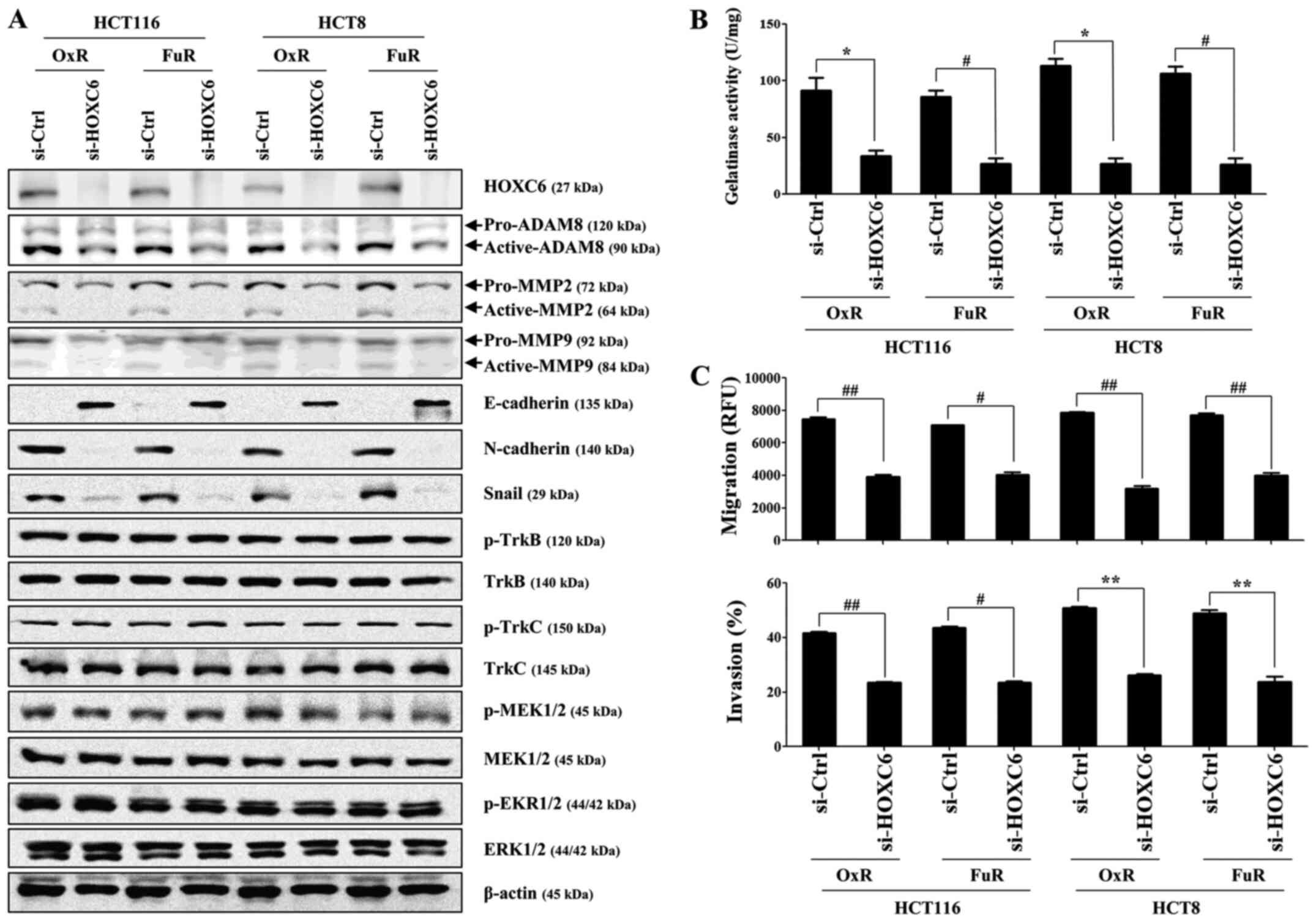 | Figure 5.HOXC6 knockdown in OxR or FuR colon
cancer cells decreases cell invasion and downregulates ADAM8
activation and gelatinase activity. OxR or FuR colon cancer cells
were seeded into 6-well plates (1.5×105/well) and grown
overnight. Cells were transfected with small interfering RNA
against HOXC6 or control for 48 h. (A) Total cell lysates were
immunoblotted with the indicated antibodies. β-actin served as an
internal control. (B) The gelatinase activity of cells was measured
by the gelatinase assay kit. (C) The migration and invasion of
cells was detected using tumor transendothelial migration and the
BME cell invasion assay kits, respectively. Data are presented as
the mean ± SD of the three independent experiments. *P<0.05,
**P<0.01, #P<0.005 and ##P<0.001 as
indicated. HOX, homeobox; OxR, oxaliplatin resistant; FuR,
5-Fu-resistant; ADAM, A disintegrin and metalloproteinase
domain-containing 8; Trk, tropomyosin receptor kinase; si, small
interfering RNA; ctrl, control; p, phosphorylated. |
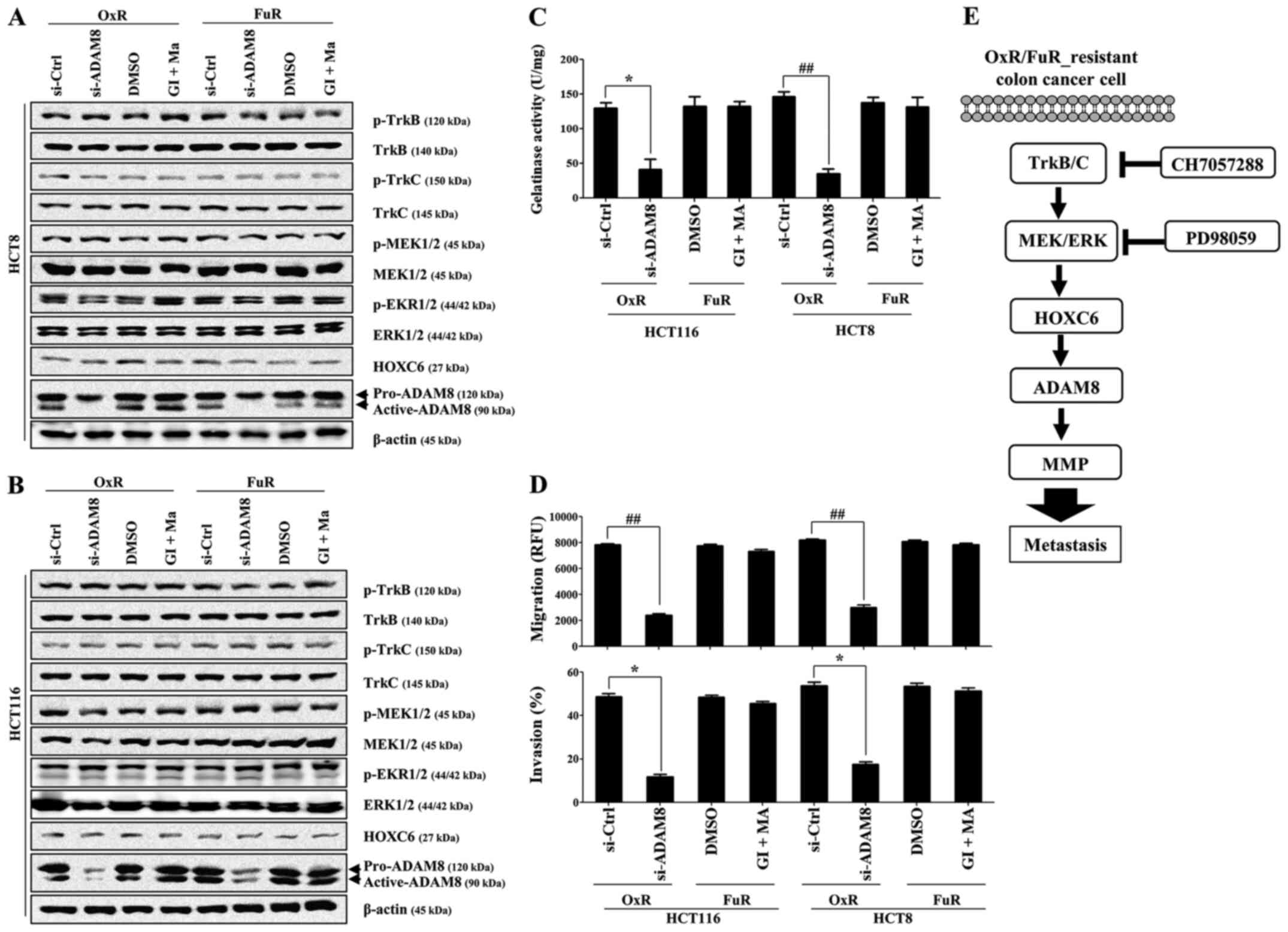 | Figure 6.Activation of ADAM8 but not ADAM10 or
ADAM17 regulates the invasion and gelatinase activity of metastatic
drug-resistant colon cancer cells. OxR or FuR colon cancer cells
were seeded into 6-well plates (1.5×105/well) and grown
overnight. Cells were transfected with small interfering RNA
against ADAM8 or control for 48 h, or treated with GI (10 µM) and
MA (50 nM) for 24 h. (A and B) Total cell lysates were
immunoblotted with the indicated antibodies. β-actin served as an
internal control. (C) The gelatinase activity of cells was measured
using the gelatinase assay kit. (D) The migration and invasion of
cells were detected using tumor transendothelial migration and BME
cell invasion assay kits, respectively. Data are presented as the
mean ± SD of three independent experiments. *P<0.05 and
##P<0.001 as indicated. (E) Schematic diagram of the
intracellular signaling pathway in drug-resistant human colon
cancer cells. ADAM8 expression contributed to the increased
resistance and migratory activity of chemoresistant colon cancer
cells through the activation of the TrkB/C-HOXC6 axis. ADAM, A
disintegrin and metalloproteinase domain-containing 8; OxR,
oxaliplatin resistant; FuR, 5-Fu-resistant; GI, ADAM10 inhibitor
GI254023X; MA, ADAM17 inhibitor marimastat; Trk, tropomyosin
receptor kinase; HOX, homeobox; si, small interfering RNA; Ctrl,
control; p, phosphorylated. |
Discussion
Pharmacological inhibition of Trk significantly
suppressed the migratory and invasive activity of drug-resistant
colon cancer cells through the blocking the metalloproteinase
activity. The inhibition of TrkB in lung squamous cell carcinoma
not only prevents tumor cell invasion but also enhances the
sensitivity to epidermal growth factor receptor (EGFR) inhibitors
(30). The overexpression of TrkB/C
and ADAM8 is also correlated with increased tumor invasion and poor
prognosis of colon cancer patients (8,26).
Therefore, it is important to understand the potential relationship
between ADAM and TrkB/C in drug-resistant colon cancer cells. The
overexpression of HOXC6 not only regulates chemoresistance through
the upregulation of multidrug resistance protein 1 (MDR1) (18) but also triggers the metastasis of
gastric cancer by activating MMP9 (31). Although higher HOXC6 expression is
detected in metastatic colorectal cancer tissues than that of the
primary cancer region (32), the
exact function and relationship with other signaling pathways,
particularly in drug-resistant colon cancer cells, are largely
unknown. In this study, we have shown that TrkB/C-mediated MEK/ERK
activation contributed to the stimulation of the HOXC6-mediated
ADAM8 expression, resulting in the enhanced migratory and invasive
activity of drug-resistant colon cancer cells. These results
suggest that the TrkB/C-mediated HOXC6/ADAM8 signaling pathway is
one of the regulatory factors that promote the metastasis of
chemotherapy-resistant cancer cells (Fig. 6E).
Recent studies have shown that estrogen-induced BDNF
in astrocytes activates the TrkB-AKT/ERK pathway, triggering the
brain metastasis of triple-negative breast cancer (33). BDNF binding to its receptor,
especially TrkB, plays a critical role in promoting proliferation,
survival, and migration in various cancers, including colorectal
cancer (34). BDNF-TrkB signaling
pathway is well known for connection with nervous system
development. However, the role or even expression of TrkB/C in
chemoresistant colon cancer cells is still unknown. The mRNA
expression of TrkB/C in drug-resistant HCT116 cells was
significantly higher than that of chemoresistant HCT8 cells.
Furthermore, we observed that the protein expression of Trk family
has changed in cell-type dependent manner. These results suggest
that several regulatory mechanisms at each step, such as mRNA and
post-translational stage, work to control the expression of Trk
family in colon cancer cells. It is necessary to investigate what
controlling processes adjust the level of TrkB family in
drug-resistant colon cancer cells in further study. Targeted
inhibition of TrkB/C with CH7057288 suppressed the activation of
MEK/ERK and downstream signaling pathways in this study. In
addition, inhibiting the phosphorylation of ERK with PD98059
effectively prevented the expression of HOXC6 and ADAM8 and the
MMP-mediated migratory activity of the chemoresistant colon cancer
cells. These results suggest that TrkB/C-mediated ERK activation
plays an important role in the regulation of HOXC6 and ADAM8
activity.
The aberrant expression of HOX family genes is
reported in various cancers, including colorectal cancer (17). HOXA9 expression is significantly
upregulated in colon cancer tissues than in non-cancer areas and is
closely related to increased lymph node metastasis (35). In addition, upregulation of HOXC6
triggers MMP9 gene expression to promote the cell invasion
of gastric cancer (31). However,
the mechanism that induces HOXC6 expression and its downstream
targets to enhance cancer metastasis remains unknown. Although
HOXC6 expression induces MAPK activation to promote the metastasis
of glioblastoma cells (16), we
report for the first time that TrkB/C-mediated ERK activation may
be one of the critical regulatory signaling pathways that trigger
HOXC6 expression in this study. Furthermore, pharmacological
inhibition of TrkB/C and ERK activation markedly reduced HOXC6
expression, but HOXC6 downregulation using siRNA did not affect the
TrkB/C-mediated ERK signaling pathway in chemoresistant colon
cancer cells. Despite upregulation of HOXC4 mRNA level, HOXC4
protein was barely detected in drug-resistant colon cancers. We can
predict the various reasons why HOXC4 protein is downregulated in
chemoresistant colon cancer cells, such as post-translational
modification and miRNA-mediated regulation. The roles and
controlling mechanisms of HOXC4 in drug-resistant colon cancer
cells need to be investigated in future study. These results
suggest that the TrkB/C-ERK pathway in drug-resistant colon cancer
cells plays a critical role in the activation of HOXC6 and
downstream signaling pathways.
Cancer cell metastasis requires several processes;
these include proteolytic activity against adhesion molecules,
penetration of the basement membrane by matrix remodeling, and
angiogenesis (36,37). ADAM8-induced metalloprotease
activation facilitates matrix remodeling, resulting in enhanced
metastasis of breast cancer cells into the brain (25). A high level of ADAM8 expression in
astrocytoma is closely related to increased tumor invasiveness
through the activation of protease activity (23). The overexpression of ADAM10 and
ADAM17 are connected to cancer cell proliferation, invasion, and
the development of drug resistance (38,39).
We have also reported that the downregulation of ADAM10 and ADAM17
by pretreatment with 2-deoxy-D-glucose (2-DG) sensitizes
chemoresistant colon cancer cells to 5-Fu (40). Although exposure lipopolysaccharide
(LPS) to TLR4 in colon cancer cells enhances the ADAM10- and
ADAM17-induced migratory activity (21), we have reported that the levels of
TLR4 in drug-resistant colon cancer cells are significantly
decreased in recent study (29).
Based on these results, we expected that different member of ADAM
family might involve in the metastatic activity of drug-resistant
colon cancer cells. Targeted inhibition of ADAM8 in drug-resistant
HCT8 and HCT116 cells effectively prevented their migratory
activity by suppressing MMP. However, pharmacological inhibition of
ADAM10 and ADAM17 failed to suppress the activation of the
TrkB/C-ERK-HOXC6 signaling pathway. In addition, pretreatment with
GI254023X (ADAM10 inhibitor, 10 µM) and Marimastat (ADAM17
inhibitor, 50 nM) had no effect on ADAM8 expression and the
migratory or invasive activity of drug-resistant colon cancer
cells. ADAM8 stimulates the ERK signaling pathway, which activates
MMP to enhance the invasiveness of pancreatic ductal adenocarcinoma
(28). However, we have shown that
ADAM8 activation is dependent on TrkB/C-ERK-mediated HOXC6
activation during the metastasis of drug-resistant colon cancer
cells in this study. Since ADAM8 has an influence on the expression
of MMP9 in breast cancer (25) and
HOXC6 expression triggers metastatic activity of gastric cancer
cells through the activation of MMP9 (31), we investigated the relationship
between ADAM8 with HOXC6 and regulatory role in migration of
chemoresistant colon cancer cells. In this study, targeted
downregulation of HOXC6 using siRNA prevented the ADAM8 expression
as well as MMP-mediated invasive activity. Furthermore, gene
silencing of ADAM8 using siRNA significantly blocked the MMP
activity, resulting in inhibiting the migration. Based on these
results, ADAM8 might be one of the potential downstream targets in
HOXC6 signaling pathway to promote the MMP9 activity for inducing
cancer cell metastasis.
Taken together, our results suggest that the
activation of TrkB/C followed by the stimulation of the downstream
MEK/ERK signaling pathway promotes HOXC6-mediated ADAM8 activation
to induce metastasis in chemoresistant colon cancer cells.
Therefore, screening the levels of TrkB/C, HOXC6, and ADAM8 protein
may be a new diagnostic measure to detect metastatic cancer cells
in advanced colon cancer patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The current study was supported by the Basic Science
Research Program of the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
NRF-2018R1D1A1B07040382) and the National Research Foundation of
Korea funded by the Korean government Ministry of Science and ICT
(NRF-2018R1C1B6002381).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
GBP and SC performed the experiments and analyzed
the data. DK and YSY conceived and designed the present study, and
wrote the manuscript. GBP and DK contributed to study design,
coordinated the research and critically reviewed the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chao MV: Neurotrophins and their
receptors: A convergence point for many signalling pathways. Nat
Rev Neurosci. 4:299–309. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang EJ and Reichardt LF: Neurotrophins:
Roles in neuronal development and function. Annu Rev Neurosci.
24:677–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douma S, Van Laar T, Zevenhoven J,
Meuwissen R, Van Garderen E and Peeper DS: Suppression of anoikis
and induction of metastasis by the neurotrophic receptor TrkB.
Nature. 430:1034–1039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kupferman ME, Jiffar T, El-Naggar A,
Yilmaz T, Zhou G, Xie T, Feng L, Wang J, Holsinger FC, Yu D, et al:
TrkB induces EMT and has a key role in invasion of head and neck
squamous cell carcinoma. Oncogene. 29:2047–2059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Desmet CJ and Peeper DS: The neurotrophic
receptor TrkB: A drug target in anti-cancer therapy? Cell Mol Life
Sci. 63:755–759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yilmaz T, Jiffar T, de la Garza G, Lin H,
Milas Z, Takahashi Y, Hanna E, MacIntyre T, Brown JL, Myers JN, et
al: Theraputic targeting of Trk supresses tumor proliferation and
enhances cisplatin activity in HNSCC. Cancer Biol Ther. 10:644–653.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee J, Jiffar T and Kupferman ME: A novel
role for BDNF-TrkB in the regulation of chemotherapy resistance in
head and neck squamous cell carcinoma. PLoS One. 7:e302462012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu Y, Zhang S, Wang X, Yang Z and Ou G:
Overexpression of TrkB promotes the progression of colon cancer.
APMIS. 118:188–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blondy S, Christou N, David V, Verdier M,
Jauberteau MO, Mathonnet M and Perraud A: Neurotrophins and their
involvement in digestive cancers. Cell Death Dis. 10:1232019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abate-Shen C: Deregulated homeobox gene
expression in cancer: Cause or consequence? Nat Rev Cancer.
2:777–785. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Friedmann Y, Daniel CA, Strickland P and
Daniel CW: Hox genes in normal and neoplastic mouse mammary gland.
Cancer Res. 54:5981–5985. 1994.PubMed/NCBI
|
|
12
|
Bhatlekar S, Fields JZ and Boman BM: HOX
genes and their role in the development of human cancers. J Mol Med
(Berl). 92:811–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhatlekar S, Fields JZ and Boman BM: Role
of HOX genes in stem cell differentiation and cancer. Stem Cells
Int. 2018:35694932018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang SL, Chan TC, Chen TJ, Lee SW, Lin LC
and Win KT: HOXC6 overexpression is associated with Ki-67
expression and poor survival in NPC patients. J Cancer.
8:1647–1654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramachandran S, Liu P, Young AN, Yin-Goen
Q, Lim SD, Laycock N, Amin MB, Carney JK, Marshall FF, Petros JA,
et al: Loss of HOXC6 expression induces apoptosis in prostate
cancer cells. Oncogene. 24:188–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang P, Kang W, Pan Y, Zhao X and Duan L:
Overexpression of HOXC6 promotes cell proliferation and migration
via MAPK signaling and predicts a poor prognosis in glioblastoma.
Cancer Manag Res. 11:8167–8179. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji M, Feng Q, He G, Yang L, Tang W, Lao X,
Zhu D, Lin Q, Xu P, Wei Y, et al: Silencing homeobox C6 inhibits
colorectal cancer cell proliferation. Oncotarget. 7:29216–29227.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KJ, Moon SM, Kim SA, Kang KW, Yoon JH
and Ahn SG: Transcriptional regulation of MDR-1 by HOXC6 in
multidrug-resistant cells. Oncogene. 32:3339–3349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reiss K and Saftig P: The ‘a disintegrin
and metalloprotease’ (ADAM) family of sheddases: Physiological and
cellular functions. Semin Cell Dev Biol. 20:126–137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gavert N, Sheffer M, Raveh S, Spaderna S,
Shtutman M, Brabletz T, Barany F, Paty P, Notterman D, Domany E, et
al: Expression of L1-CAM and ADAM10 in human colon cancer cells
induces metastasis. Cancer Res. 67:7703–7712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park GB and Kim D: TLR4-mediated
galectin-1 production triggers epithelial-mesenchymal transition in
colon cancer cells through ADAM10- and ADAM17-associated lactate
production. Mol Cell Biochem. 425:191–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishikawa N, Daigo Y, Yasui W, Inai K,
Nishimura H, Tsuchiya E, Kohno N and Nakamura Y: ADAM8 as a novel
serological and histochemical marker for lung cancer. Clin Cancer
Res. 10:8363–8370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wildeboer D, Naus S, Amy Sang QX, Bartsch
JW and Pagenstecher A: Metalloproteinase disintegrins ADAM8 and
ADAM19 are highly regulated in human primary brain tumors and their
expression levels and activities are associated with invasiveness.
J Neuropathol Exp Neurol. 65:516–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Romagnoli M, Mineva ND, Polmear M, Conrad
C, Srinivasan S, Loussouarn D, Barillé-Nion S, Georgakoudi I, Dagg
Á, McDermott EW, et al: ADAM8 expression in invasive breast cancer
promotes tumor dissemination and metastasis. EMBO Mol Med.
6:278–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Conrad C, Götte M, Schlomann U, Roessler
M, Pagenstecher A, Anderson P, Preston J, Pruessmeyer J, Ludwig A,
Li R, et al: ADAM8 expression in breast cancer derived brain
metastases: Functional implications on MMP-9 expression and
transendothelial migration in breast cancer cells. Int J Cancer.
142:779–791. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Z, Bai Y, Huo L, Chen H, Huang J, Li
J, Fan X, Yang Z, Wang L and Wang J: Expression of A disintegrin
and metalloprotease 8 is associated with cell growth and poor
survival in colorectal cancer. BMC Cancer. 14:5682014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng L, Liu B, Ji R, Jiang X, Yan X and
Xin Y: Targeting the BDNF/TrkB pathway for the treatment of tumors.
Oncol Lett. 17:2031–2039. 2019.PubMed/NCBI
|
|
28
|
Schlomann U, Koller G, Conrad C, Ferdous
T, Golfi P, Garcia AM, Höfling S, Parsons M, Costa P, Soper R, et
al: ADAM8 as a drug target in pancreatic cancer. Nat Commun.
6:61752015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park GB, Jeong JY and Kim D: Modified
TLR-mediated downregulation of miR-125b-5p enhances CD248
(endosialin)-induced metastasis and drug resistance in colorectal
cancer cells. Mol Carcinog. 59:154–167. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gomez DR, Byers LA, Nilsson M, Diao L,
Wang J, Li L, Tong P, Hofstad M, Saigal B, Wistuba I, et al:
Integrative proteomic and transcriptomic analysis provides evidence
for TrkB (NTRK2) as a therapeutic target in combination with
tyrosine kinase inhibitors for non-small cell lung cancer.
Oncotarget. 9:14268–14284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen SW, Zhang Q, Xu ZF, Wang HP, Shi Y,
Xu F, Zhang WJ, Wang P and Li Y: HOXC6 promotes gastric cancer cell
invasion by upregulating the expression of MMP9. Mol Med Rep.
14:3261–3268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang DD, Xu Y, Tu YL, Tan XL, Zhu ZM, Han
MM, Dou CQ, Zeng JP, Tan JW, Du JD, et al: Comparison analysis in
synchronous and metachronous metastatic colorectal cancer based on
microarray expression profile. Hepatogastroenterology.
61:2215–2218. 2014.PubMed/NCBI
|
|
33
|
Contreras-Zárate MJ, Day NL, Ormond DR,
Borges VF, Tobet S, Gril B, Steeg PS and Cittelly DM: Estradiol
induces BDNF/TrkB signaling in triple-negative breast cancer to
promote brain metastases. Oncogene. 38:4685–4699. 2019. View Article : Google Scholar
|
|
34
|
Meldolesi J: Neurotrophin Trk receptors:
New targets for cancer therapy. Rev Physiol Biochem Pharmacol.
174:67–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Watanabe Y, Saito M, Saito K, Matsumoto Y,
Kanke Y, Onozawa H, Hayase S, Sakamoto W, Ishigame T, Momma T, et
al: Upregulated HOXA9 expression is associated with lymph node
metastasis in colorectal cancer. Oncol Lett. 15:2756–2762.
2018.PubMed/NCBI
|
|
36
|
López-Otín C and Hunter T: The regulatory
crosstalk between kinases and proteases in cancer. Nat Rev Cancer.
10:278–292. 2010. View Article : Google Scholar
|
|
37
|
Mason SD and Joyce JA: Proteolytic
networks in cancer. Trends Cell Biol. 21:228–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fu L, Liu N, Han Y, Xie C, Li Q and Wang
E: ADAM10 regulates proliferation, invasion, and chemoresistance of
bladder cancer cells. Tumour Biol. 35:9263–9268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang XJ, Feng CW and Li M: ADAM17 mediates
hypoxia-induced drug resistance in hepatocellular carcinoma cells
through activation of EGFR/PI3K/Akt pathway. Mol Cell Biochem.
380:57–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park GB, Chung YH and Kim D:
2-Deoxy-D-glucose suppresses the migration and reverses the drug
resistance of colon cancer cells through ADAM expression
regulation. Anticancer Drugs. 28:410–420. 2017. View Article : Google Scholar : PubMed/NCBI
|