Introduction
Cancer is a major life-threatening disease with a
high incidence and mortality worldwide (1,2). The
mechanisms of tumor formation are complex and diverse, and the
differential prognosis of cancer is associated with variations in
tumor proliferation, invasion and metastasis. The basic treatment
modalities for cancer are surgery, radiotherapy, chemotherapy and
targeted therapy. However, it is difficult to diagnose the majority
of cancers at an early stage; therefore, the curative effects of
these treatments remain limited, and there is an imminent need to
identify novel diagnostic and prognostic biomarkers and therapeutic
targets for cancer.
Angiogenesis is the formation of new blood vessels
from existing vessels. It is a pivotal process during cancer cell
proliferation, migration and differentiation. Since angiogenic
factors control tumor growth and metastasis (3), angiogenic regulation of this process
is pivotal for identifying novel cancer treatment strategies. The
latest research indicates that the dysregulated expression of
microRNAs (miRs) may lead to abnormal angiogenesis (4). miRs are a class of endogenous, small
and non-coding single-stranded RNA molecules containing ~22
nucleotides; they regulate the expression and function of multiple
genes. By binding to complementary sequences of the 3′-untranslated
region of the target mRNA, miRs inhibit or degrade the
post-transcriptional products of the target genes (4). Approximately 30–60% of human genes are
regulated by one or more miRs (5).
In addition, a previous study reported that miRs may be considered
a class of oncogenes or tumor suppressor genes (6). One study revealed that miRs in tumor
cells may affect the activity of endothelial cells via
non-cell-autonomous mechanisms and that miRs in endothelial cells
may regulate the cell-autonomous response (7). These observations provide evidence
that antiangiogenic treatment of tumors with miRs may inhibit the
growth of cancer.
This review describes miR biogenesis, regulation and
functions. The current research achievements in miRs biogenesis are
updated here. The role of angiogenesis is pivotal in the survival
of cancer cells. In this review, several critical endogenous
molecules and their receptors and their expressions, which promote
angiogenic activity in tumor metastasis, are expounded. Next, the
functions of miRs in regulating tumor angiogenesis are described in
detail. Particularly, the functions of exosomal delivery of miRs
are discussed. Tight junctions exist mainly between endothelial
cells. Innovatively, this review describes miRs' target on tight
junction-related proteins, which is helpful to the transendothelial
migration of tumor cells and tumor metastasis. Furthermore, the
functions of miRs in hypoxic environments are described.
Additionally, the latest advances in the role of various miRs and
their corresponding target genes involved in tumor angiogenesis are
updated. Various signaling pathways in different types of tumor
progression regulated by miRs are addressed.
In summary, this review demonstrates the potential
clinical value of miRs as biomarkers for diagnosing and monitoring
the response to therapy, as well as their ability to regulate tumor
angiogenesis and the mechanism underlying this regulation.
The current research achievements in miR
biogenesis
Although miRs are only 22 nucleotides long, they
serve an important role in gene expression by regulating various
target genes. miRs recruit an argonaute (AGO) protein complex to a
complementary target mRNA, which usually results in translation
repression or mRNA degradation (8).
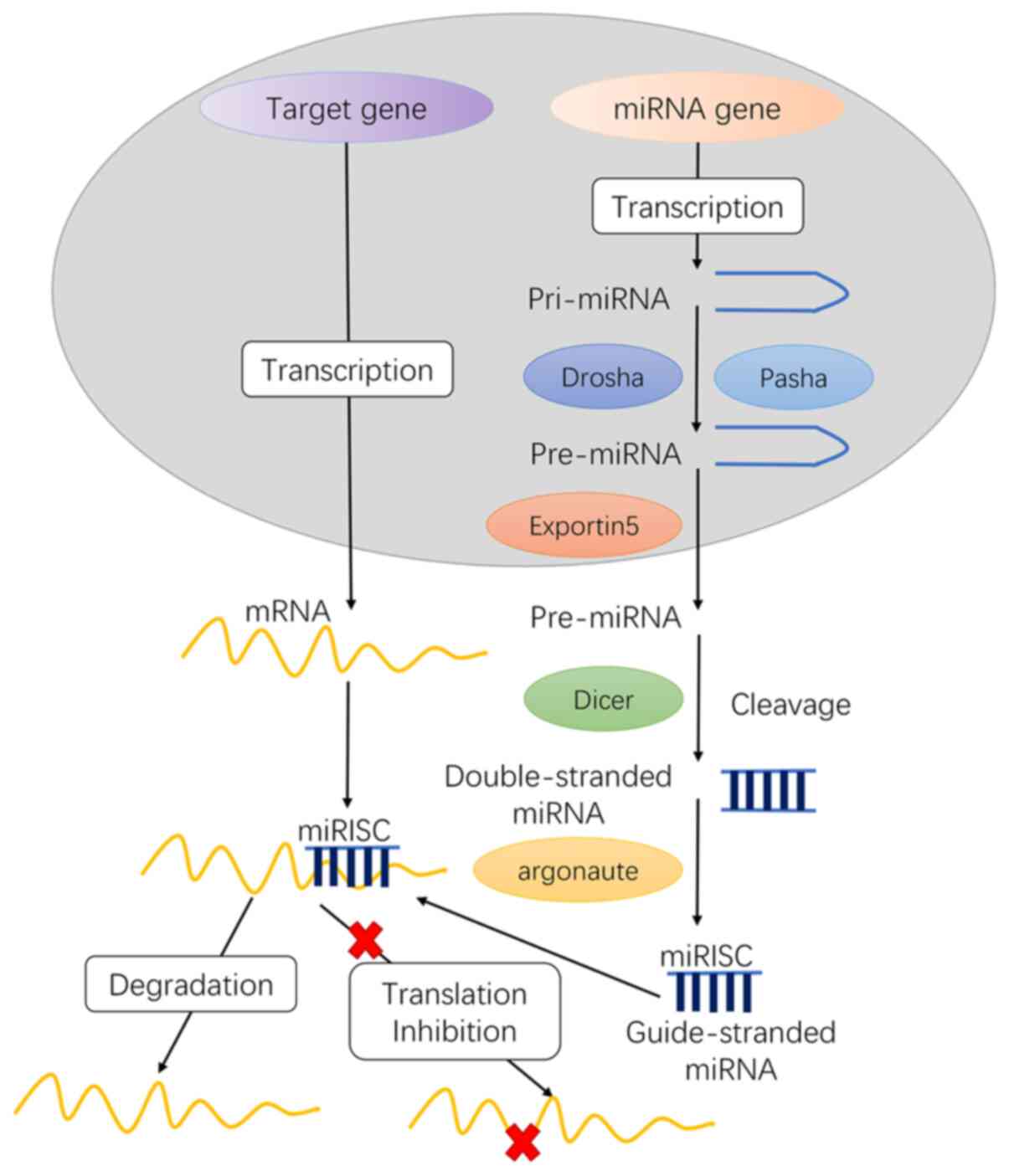
The conventional miR biogenesis pathway consists of two cleavage
events: Nuclear and cytoplasmic (9), which require approximately five
post-transcriptional processing steps to yield the functional
mature miRs as follows (Fig. 1): i)
transcription of initial miRs (pri-miRNA), ii) formation of
pre-miRs, iii) transport of pre-miRs from the nucleus to the
cytoplasm, iv) production of mature miRs and v) formation of the
miRNA-induced silencing complex (miRISC) core.
In the nucleus
The gene encoding miRs in the nucleus is transcribed
to generate a long primary transcript among the two RNA
polymerases, RNA pol II or III, which is called pri-miRNA.
Pri-miRNA has methylated guanine at the 5′ end and a polyadenine
base at the 3′ end. Furthermore, pri-miRNA contains sequences of
uridine residues, which terminate RNA pol III transcription.
Further processing of Pri-miRNA is mainly completed
in the 400–500 kDa microprocessor protein complex composed of
Drosha and Pasha. Pre-miRs are catalyzed by Drosha, a nuclease of
the RNase III family, while Pasha is a double-stranded RNA binding
protein that participates in substrate recognition by Drosha
(10). Drosha RNase III
endonuclease cleaves both strands of the stem at sites near the
base of the primary stem-loop, releasing ~60-70 nt stem-loop
intermediates, called miRNA precursors or pre-miRNA. The pre-miRNA
is transported into the cytoplasm by the translocator
RanGTP/Exportin-5.
MiRs residing in introns are known as mirtrons, and
are widespread in Drosophila, C. elegans and vertebrates (11). The transcription of mirtrons is
performed by spliceosome but not by Drosha. Next, the mirtron
precursor molecule released by the spliceosome in the form of a
lasso will be linearized under the action of a debranching enzyme.
Subsequently, they mimic the pre-miRNA structure and directly enter
the miRNA processing program and are transferred to the cytoplasm
to be treated by Dicer instead of being cleaved by Drosha.
In the cytoplasm
pre-miRNA is further matured by the RNase III
endonuclease Dicer in the cytoplasm (12). The loop structure is cut and
modified by Dicer, and miRNA: miRNA* complexes are formed in the
cytoplasm. Following helicase action, miRNA: miRNA* complexes
eventually generate mature, functional single-stranded miRNAs and
then combine with miRNA ribonucleoproteins, following which the
miRNA* is rapidly degraded.
Typically, one strand of this mature miRNA duplex,
termed the guide strand, can enter the RNA-induced silencing
complex (RISC), and the other strand is removed. The miRISC complex
contains Dicer, TRBP, PACT and Gemin3; however, the AGO factor
directly binds to the miRNA and can mediate the sequence matching
of the miRNA and the target mRNA to cause subsequent translational
inhibition.
The role of angiogenesis in cancer
Malignant tumors invade neighboring tissues and
metastasize through the bloodstream to distant organs. Therefore,
expanding the blood vasculature is pivotal in the survival of
cancer cells. Vascular endothelial growth factor (VEGF), a specific
mitogen of vascular endothelial cells, is overexpressed in numerous
malignant tumors (13). VEGF may
bind to its receptors (VEGFRs) expressed on the vascular
endothelial cells and promote endothelial cell division, migration
and proliferation, and induce capillary angiogenesis and growth
into tumor tissue (14,15). Platelet-derived growth factor (PDGF)
mainly originates from mesenchymal cells and participates in the
induction of angiogenesis (16).
The main functions of PDGF and its receptor, PDGFR, involve
autocrine stimulation of tumor cells, paracrine stimulation of
tumor stromal cells to promote angiogenesis, and regulation of the
interstitial fluid pressure to control the flow of drugs into and
out of the tumor (16).
CD31 is overexpressed on the surface of vascular
endothelial cells. Its distribution is associated with
angiogenesis, as well as the movement of endothelial cells
(17). CD31 acts as a bridge
between tumor and vascular endothelial cells to promote the
metastasis of malignant tumor cells (18). Therefore, CD31 is a useful marker
for evaluating tumor angiogenesis (18).
Endogenous molecules that promote angiogenic
activity serve several major roles in promoting tumor metastasis.
These include stimulating the growth and survival of endothelial
cells to ensure angiogenesis (19),
breaking the tight junctions of tumor vascular endothelial cells
and promoting tumor metastasis (15,20) to
ensure that the new tumor cells can obtain oxygen and nutrition to
grow rapidly and provide favorable conditions to transport cancer
cells for distant metastases (21,22).
The functions of miRs in regulating tumor
angiogenesis
Several studies have demonstrated that miRs may
affect cell proliferation, differentiation, metabolism and tumor
angiogenesis (6,14). A single miR can target several
mRNAs. In addition, aberrant miR expression can disrupt the
expression of several mRNAs and proteins involved in regulating
cell proliferation and apoptosis. Diverse miRs are involved in
tumor angiogenesis, affecting both anti- and pro-angiogenic
proteins (6). Here, the functions
of miRs in regulating tumor angiogenesis in the following sections
are reviewed.
Exosomal miRs and tumor
angiogenesis
Exosomes, the nanovesicles with diameters ranging
between 30 and 150 nm, are involved in cell-to-cell communication
and regulate various biological processes (23). In the tumor microenvironment,
exosomes released by different types of cells regulate tumor
survival, growth and facilitate tumor cell dissemination (24). Although miRs are one of the key
regulators of gene expression, exosomal miRs serve a dual role in
cancer. Exosomal delivery of miRs to recipient cancer or stromal
cells may induce cancer progression and metastasis (25). However, exosomes also facilitate
tumor initiation by affecting signaling pathways and inhibiting the
expression of tumor suppressors (25). A previous study demonstrated that
miRs are packaged and secreted by exosomes and are potential
biomarkers as well as important mediators of interactions among
different cells (23). Notably,
exosomal miRs are implicated in modulating endothelial cell
functions and angiogenesis in cancer (26,27).
High levels of miR-205 were observed in ovarian
cancer (OC) tissue and adjacent endothelial cells and were
associated with metastatic progression in patients with OC
(28). In addition, miR-205 was
markedly enriched in the serum and circulating exosomes of patients
with OC (28). miR-205 in exosomes
secreted by OC cells significantly promoted angiogenesis in
vitro and accelerated angiogenesis and tumor growth in
vivo via the phosphatase and tensin homolog (PTEN)-Akt pathway
(28). miR-141-3p present in small
extracellular vesicles (sEV) secreted by epithelial OC cells is an
important mediator of intercellular communication (29). Furthermore, it can regulate the
expression of VEGFR-2 and reactive oxygen species-dependent
activation of nuclear factor κ-B (NF-κB) signaling in endothelial
cells and promote endothelial cell angiogenesis (29). Another study based on in
vitro and in vivo experiments reported that miR-204-5p
promoted angiogenesis in OC via thrombospondin 1 (30).
The serum level of exosomal miR-21 is increased in
patients with hepatocellular carcinoma (HCC) making it a potential
biomarker for HCC (31). This
aforementioned study demonstrated that miR-21 derived from HCC
cells activated pyruvate dehydrogenase kinase 1/Akt signaling in
hepatic stellate cells. Cancer progression was promoted by
increased secretion of angiogenic cytokines, including VEGF, matrix
metalloproteinase (MMP)2, MMP9, basic fibroblast growth factor and
transforming growth factor (TGF)-β. Furthermore, decreased miR-451a
content was observed in serum-derived exosomes isolated from
patients with HCC. miR-451a suppressed human umbilical vein
endothelial cell (HUVEC) migration, tube formation and vascular
permeability. As the critical target of miR-451a, LPIN1
promoted apoptosis in HCC cell lines and HUVECs (32).
A previous study that comprehensively analyzed miR-9
expression demonstrated that it was overexpressed in glioma
specimens and cells and contributed toward their enhanced
proliferation, migration and invasion (33). Additionally, collagen alpha-1
(XVIII), thrombospondin 2 (THBS-2), protein patched
homolog 1 (PTCH1) and prolyl hydroxylase 3 (PHD3)
were verified as direct targets of miR-9 (33). miR-9 in glioma cell exosomes was
absorbed by vascular endothelial cells, thereby inducing
angiogenesis (33). Glioma stem
cells also contained exosomes overexpressing miR-26a, which
significantly increased proliferation and angiogenesis by
inhibiting PTEN (34).
Exosome-delivered miR-135b contribute significantly
toward angiogenesis in gastric cancer (GC). GC cells secrete
exosomal miR-135b that directly targets forkhead box O1
(FOXO1), suppresses the expression of the FOXO1
protein, and enhances the growth of blood vessels (35). Another study revealed that
miR-142-3p in EVs secreted by lung adenocarcinoma cells affected
endothelial and fibroblast cells and promoted angiogenesis via
inhibiting TGFβ receptor 1 (36).
This demonstrates a role for EVs in cell-cell communication. The
level of miR-501-3p is highly enriched in pancreatic ductal
adenocarcinoma (PDAC) tissues and tumor-associated
macrophage-derived exosomes. Treatment of M2 macrophages with
miR-501-3p activated the TGF-β signaling pathway and downregulated
TGF-β receptor 3 expression in PDAC cells. Furthermore, suppression
of miR-221-3p in exosomes secreted by macrophages inhibited tumor
formation and metastasis in vivo (37).
In colorectal carcinoma (CRC), colon cell-derived
EVs contain large amounts of miR-92a-3p and have a pro-angiogenic
function. Genes encoding Dickkopf-3 and claudin-11
were identified as targets of miR-92a-3p. In endothelial cells, the
ectopic expression of miR-92a-3p upregulated the expression of
genes associated with the cell cycle and mitosis while
downregulating the expression of genes associated with adhesion
(38). The study also demonstrated
that exosomal miR-1229 was highly enriched in the serum of patients
with CRC and was associated with tumor size, lymphatic metastasis,
TNM stage and poor survival. Notably, anti-miR-1229 therapy
inhibited tumor growth and angiogenesis in vivo. In
addition, HIPK2 was identified as a target of circulating
exosomal miR-1229 and influenced the angiogenesis of HUVECs via the
VEGF pathway (39).
Functions of miRs in regulating tumor
metastasis caused by tight junctions
Tight junctions exist mainly between endothelial
cells or between epithelial and endothelial cells. These junctions
make membranes of adjacent cells tightly connected and serve a role
in the paracellular permeability barrier and maintenance of cell
polarity (40,41). Disruption of tight junctions
enhances the permeability of vascular endothelial cells and
promotes the transendothelial migration of tumor cells, thereby
forming a protumor niche and promoting tumor metastasis (27).
Tight junctions are composed of a variety of
proteins. Previous studies have demonstrated that the abnormal
expression of claudin, occludin and zona occluden (ZO) is
associated with the proliferation, invasion and metastasis of tumor
cells (42,43). Claudin serves an important barrier
protective role to maintain the selective permeability of vascular
endothelial cells. The occludin protein generates a paracellular
block by binding to adjacent cells via the outer part of the cell
membrane (44). Zonula occludens-1
(ZO-1) is a central regulator of intercellular junctions in
endothelial cells; it regulates junctional tension and linkage of
vascular endothelial-cadherin junctions (45). Other proteins, including ZO-1/2/3,
cingulin and multi-PDZ domain protein 1, are skeleton proteins that
connect transmembrane proteins to the actin cytoskeleton. ZO-1 can
interact with a variety of tight junction proteins, regulate
intracellular and extracellular signal transduction pathways,
affect the function of tight cell junctions and regulate cell
permeability (27,45).
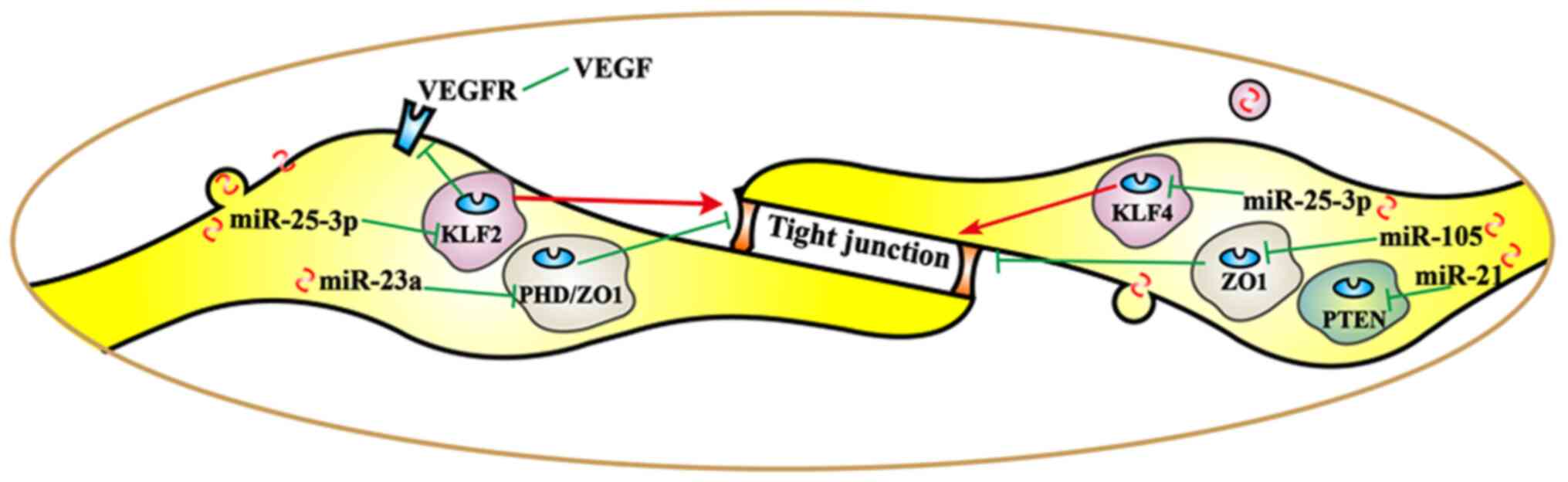
Different miRs exert different regulatory effects on
tight junction-related proteins (Fig.
2), thereby promoting tumor metastasis. By targeting
krüppel-like Factor 2 (KLF2/4), cancer-derived exosomal
miR-25-3p was transferred to vascular endothelial cells. miR-25-3p
regulated the expression of VEGFR2, ZO-1, occludin and claudin 5 in
endotheliocytes, and promoted pre-metastatic niche formation by
inducing vascular permeability and angiogenesis (27). Another study revealed that
overexpression of miR-25-3p enhanced the migration and invasion
ability of non-small cell lung cancer (NSCLC) cells (46). As one of the diagnostic markers in
lung cancer, the expression of miR-25 is associated with lymph node
metastasis, pathological stage and overall survival (47,48).
Angiogenesis, vascular permeability and cancer transendothelial
migration were enhanced by exosomal miR-23a by targeting PHD
and ZO-1 in lung cancer (26). Furthermore, miR-105 secreted by
metastatic breast cancer cells destroyed vascular endothelial
barriers to promote metastasis by targeting ZO-1 (49). Furthermore, the expression of miR-21
increased significantly in a tight junction barrier defect model;
therefore, miR-21 may regulate intestinal epithelial tight junction
permeability via the PTEN/phosphoinositide 3-kinase (PI3K)/Akt
signaling pathway (50).
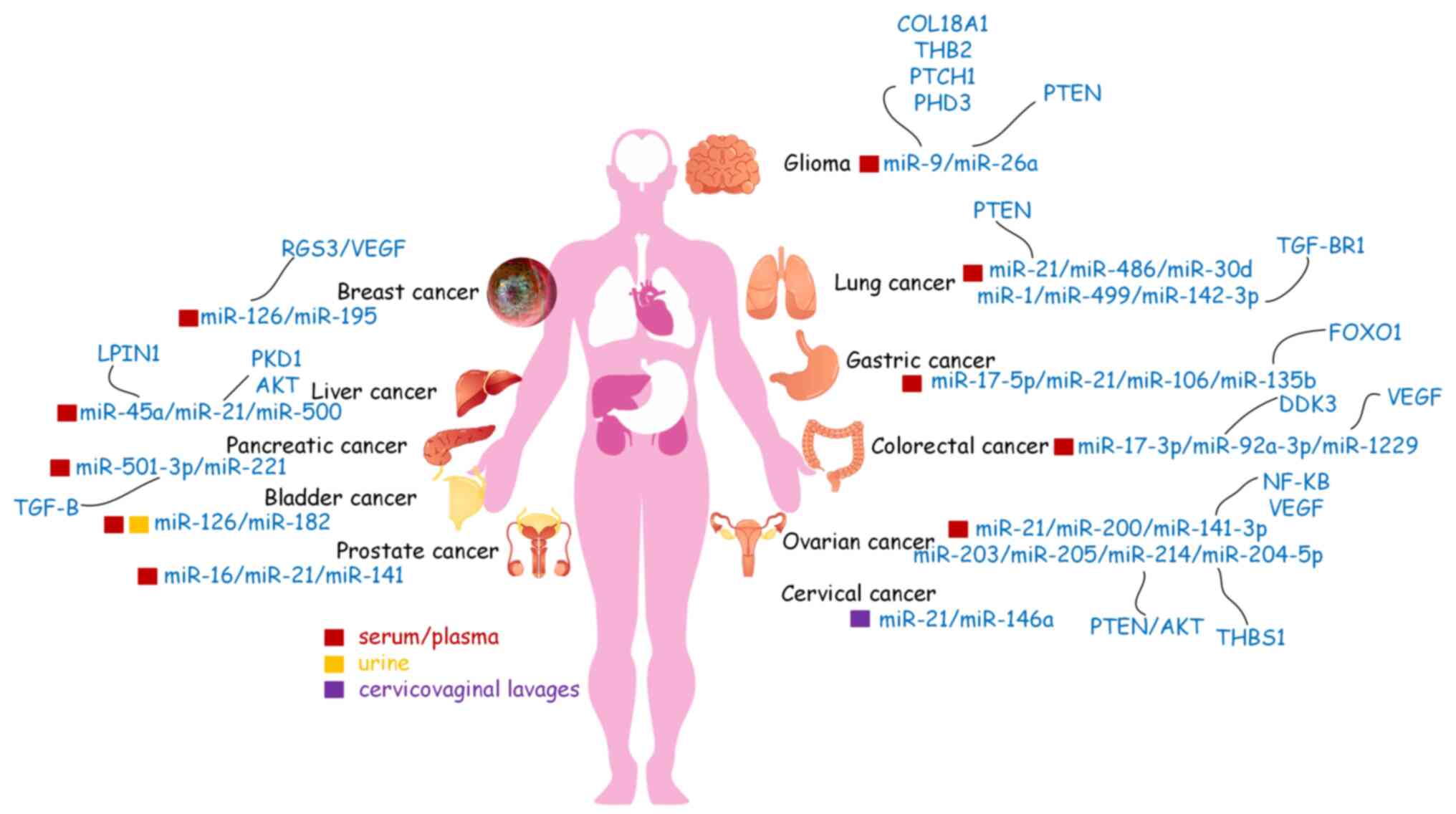 | Figure 2.Circulating microRNAs (miRs) may
serve as biomarkers in human body fluids for various types of
cancer. Liquid biopsy is a less invasive method that may detect
cancer biomarkers more frequently and efficiently than other
methods and is a good candidate method for future use in cancer
diagnosis. miRs, microRNAs; VEGF, vascular endothelial growth
factor; VEGFR, VEGF receptor; RGS3, regulator of G protein
signaling 3; PKD1, polycystin 1; LPIN1, lipin 1; TGF-β,
transforming growth factor-β; TGF-βR1, TGF-β receptor 1; COL18A1,
collagen type XVIII alpha 1 chain; THB2, truncated hemoglobin 2;
THBS1, thrombospondin 1; PTCH1, patched 1; PHD3, prolyl hydroxylase
3; PTEN, phosphatase and tensin homolog; FOXO1, forkhead box O1;
NF-κB, nuclear factor κ-B. |
Angiogenesis in a hypoxic
environment
Hypoxia-inducible factor (HIF) is a transcription
factor that serves a key role in the cellular response to hypoxia.
HIF is closely involved in tumorigenesis by regulating cell
survival, angiogenesis, metastasis, adaptation to the hypoxic tumor
microenvironment, and suppression of host immunity to drive cancer
progression (51). Hypoxia is a
pivotal factor required for activating angiogenesis as it induces
the expression and release of pro-angiogenic molecules, including
VEGF, PDGF and CD31, from the stroma and tumor cells (52).
Hypoxia-induced exosomes may influence macrophage
recruitment and promote M2-like polarization. By transferring
let-7a miR, hypoxic tumor exosomes enhanced oxidative
phosphorylation in bone marrow-derived macrophages and suppressed
the insulin-Akt-mammalian target of rapamycin (mTOR) signaling
pathway (53). Therefore, hypoxia
promotes secretion of exosomes from tumors that may modify the
immunological effects of infiltrating monocytes-macrophages,
thereby helping tumor to escape host immunity and grow (53).
miR-23a is significantly upregulated in exosomes
secreted by lung cancer under hypoxic conditions (26). Exosomal miR-23a directly inhibited
its target genes, PHD1 and PHD2, leading to the
accumulation of HIF-1α in endothelial cells, thereby enhancing
angiogenesis in hypoxic lung cancer cells. Furthermore, miR-21-5p
derived from hypoxia pre-challenged mesenchymal stem cell-derived
extracellular vesicles (MSC-EVs) promoted A549 cell growth and
mobility as well as macrophage M2 polarization. Knockdown of
miR-21-5p significantly inhibited lung cancer progression and
macrophage M2 polarization. Hypoxic MSC-EVs significantly enhanced
tumor growth, cancer cell proliferation, intra-tumoral angiogenesis
and macrophage M2 polarization, while downregulating PTEN,
programmed cell death protein 4 (PDCD4) and reversion-inducing
cysteine-rich protein with kazal motifs (RECK) gene expression via
miR-21-5p. By contrast, overexpression of PTEN, PDCD4 and RECK in
A549 cells significantly mitigated the antiapoptotic and
pro-metastatic effects of hypoxic MSC-EVs (54). Furthermore, miR-765, which targets
several protein-encoding genes involved in angiogenesis and
vasculogenic mimicry, is downregulated by hypoxia. The upregulation
of miR-574-5p enhanced angiogenesis, suppressed the expression of
tyrosine protein phosphatase non-receptor type 3, and enhanced
phosphorylation of p44/42 mitogen-activated protein kinases in GC
cells in hypoxic environments (55). As a hypoxia-specific miR
overexpressed in GC, miR-210 regulated HIF-1α expression,
facilitated the epithelial-mesenchymal transition (EMT) and
angiogenesis in response to hypoxia during tumorigenesis, and
inhibited chemoresistance, invasion and metastasis by targeting
homeobox protein Hox-A9 (Hoxa9) (56). Binding to hypoxia response elements
promoted metabolic switching to aerobic glycolysis (57). Low expression of miR-186 promoted
aerobic glycolysis, suppressed cell proliferation by downregulating
HIF-1α, and consequently affected programmed death ligand 1,
hexokinase 2 and platelet-type phosphofructokinase in GC (58).
Functions of miRs in regulating major
tumor angiogenesis pathways
A single miR may target multiple transcripts in
different types of cells, or an individual transcript may be
regulated by multiple miRs (59).
Angiogenesis serves a pivotal role in tumor growth, progression and
metastasis, which are complex processes involving essential
signaling pathways (60). miRs
participate in multiple aspects regulating vascular development and
the angiogenic response (61).
Essential signaling pathways, including VEGF and Notch, may affect
the angiogenic switch and carcinogenesis (62). VEGFA is directly targeted by
a diverse range of miRs in various types of cancer. For example,
miR-150-5p inhibits CRC cell proliferation, migration, invasion and
angiogenesis in vitro and in vivo by inactivating the
VEGFA/VEGFR2 signaling pathway (63). In summarize, all the known tumor
angiogenesis pathways regulated by miRs are basically in either of
two ways: i) promoting angiogenesis by targeting the negative
regulators of angiogenesis or ii) inhibiting vascularization by
targeting positive regulators. miR-29c, miR-942, miR-21, miR-526b,
miR-655, miR-632, miR-130b, miR-103a-3p, miR-382-5p and miR-26a are
reported to be pro-angiomiRs. They target various inhibitory
signaling pathway, including VEGF signaling pathway, PDCD4/c Jun
signaling pathway, NF-κB signaling pathways or PI3K/Akt
pathway-related molecules of angiogenesis and promote tube
formation. miR-181d-5p, miR-195, miR-126, miR-4306, miR-199b-5p,
miR-1249, MiR-150-5p/miR-193a-3p, miR-622, miR-7, miR-204,
miR-195-5p, miR-765, miR 335, miR-885-5p and miR-136 are termed
anti-angiomiRs, which inhibit capillary formation and angiogenesis
via the VGFA/VEGFR2 pathway, ERK signaling pathways, ALK1/Smad/Id1
pathway, GSK3β/β-catenin and Akt/mTOR pathway. In the following
sections, the angiogenesis pathways in different types of cancer
will be discussed and the findings are summarized in Table I.
 | Table I.Angiogenesis pathways in different
types of cancer. |
Table I.
Angiogenesis pathways in different
types of cancer.
| Non-coding
RNAs | Target |
Up-/down-regulation | Functions | Cancer type | (Refs.) |
|---|
| miR-29c | PVT1 | Upregulation | Promoted
angiogenesis VEGF pathway | NSCLC | (64) |
| miR-942 | BARX2 | Upregulation | Promoted cell
migration, invasion, metastasis and EMT | NSCLC | (65) |
| miR-181d-5p | CDKN3 | Downregulation | Suppressed
proliferation, invasion, migration, angiogenesis and EMT, and
increased cell apoptosis | NSCLC | (66) |
| miR-21 | PTEN | Upregulation | PI3K/Akt signaling
pathway | NSCLC | (67) |
| miR-195 | VEGF | Downregulation | Suppressed the
viability and migration and angiogenesis | SQCLC | (68) |
| miR-126 | RGS3/VEGF | Downregulation | Inhibited the
proliferation, migration, invasion and angiogenesis | Breast cancer | (69,70) |
| miR-4306 |
SIX1/Cdc42/VEGFA | Downregulation | Suppressed TNBC
cell proliferation, migration and invasion and abrogates
angiogenesis | Breast cancer | (71) |
| miR-526b and
miR-655 | PTEN | Upregulation | Upregulated the
angiogenesis and lymphangiogenesis markers, inhibited HIF-1α and
the PI3K/Akt pathway | Breast cancer | (72) |
| miR-199b-5p | ALK1 | Downregulation | Attenuated
ALK1/Smad/Id1 pathway | Breast cancer | (73) |
| miR-1249 | VEGF A/HMGA2 | Downregulation | Suppressed
colorectal cancer cell proliferation, migration, invasion, and
angiogenesis, regulate Akt/mTOR pathway and EMT | Colorectal
cancer | (74) |
|
miR-150-5p/miR-193a-3p | VEGFA | Downregulation | Inhibited cell
proliferation, migration, invasion and angiogenesis, VEGFA/VEGFR2
and Akt/mTOR signaling pathway | Colorectal
cancer | (63,75) |
| miR-622 | VEGFA | Downregulation | Suppressed the
CXCR4-VEGFA signaling axis | Colorectal
cancer | (76) |
| miR-7 | EGFR | Downregulation | ERK signaling
pathway | Colorectal
cancer | (77) |
| miR-204 | BIRC2 | Downregulation | Suppressed NF-κB
signaling pathways | Gastric cancer | (78) |
| miR-632 | TFF1 | Upregulation | Improved tube
formation and endothelialcell recruitment, accelerating
angiogenesis | Gastric cancer | (79) |
| miR-195-5p | PSAT1 | Downregulation | Inhibited
GSK3β/β-catenin signaling pathway | Ovarian cancer | (80) |
| miR-765 | VEGFA | Downregulation | Decreased the
VEGFA/Akt1/SRC-α axis | Ovarian cancer | (82) |
| miR-130b | TNF-α | Upregulation | Attenuated NF-κB
signaling and its downstream gene VEGFA | Prostate
cancer | (83) |
| miR-335 | EGR3 | Downregulation | Reduced the
activity of caspase-3 and inflammatory factor | Prostate
cancer | (84) |
| miR-103a-3p and
miR-382-5p | ZIC4 | Upregulation | Activated PI3K/Akt
signaling pathway | Glioma | (85) |
| miR-26a | PTEN | Upregulation | Activated the
PI3K/Akt signaling pathway | Glioma | (34) |
| miR-885-5p | AEG-1 | Downregulation | Inhibited cell
migration, invasion, proliferation, angiogenesis and EMT | Hepatocellular
carcinoma | (86) |
| miR-30e-5p | AEG-1 | Downregulation | Implicated in the
angiogenesis and metastasis | Squamous cell
cancer of the head and neck | (87) |
| miR-136 | MAP2K4 | Downregulation | Inhibited
angiogenesis and cell proliferation, and promoted apoptosis | Gallbladder
cancer | (88) |
| miR-21 | PDCD4 | Upregulation | Regulated
PDCD4/c-Jun signaling pathway | Renal cell
carcinoma | (89) |
Lung cancer
Angiogenesis serves an important role in the
progression of NSCLC. Plasmacytoma variant translocation 1 (PVT1)
is overexpressed in NSCLC and its upregulation is associated with
angiogenesis in NSCLC. One study demonstrated that PVT1
promoted angiogenesis by targeting the miR-29c/VEGF signaling
pathway in NSCLC (64). Another
study reported that miR-942 promoted NSCLC cell migration,
invasion, EMT-related metastasis and angiogenesis by directly
targeting BARX homeobox 2 (BARX2) in vitro and in
vivo (65). Furthermore, the
expression of cyclin-dependent kinase inhibitor 3 (CDKN3)
was increased, while miR-181d-5p was depleted in NSCLC, which
suppressed NSCLC cell proliferation, invasion, migration,
angiogenesis and the EMT, and increased apoptosis via the Akt
signaling pathway inactivation (66). It provided a clear understanding of
the mechanisms of the miR-181d-5p/CDKN3/Akt axis in NSCLC
progression and may serve as a prognostic marker for of NSCLC
treatments in the future. Another study demonstrated that miR-21
directly targeted PTEN and increase proliferation, migration
and tube formation in HUVEC cells (67). Increasing miR-21 induced the
accumulation of VEGF and HIF-1α in cells via the PI3K/Akt signaling
pathway (67). miR-21 may be
potentially involved in pathophysiology. In squamous cell lung
cancer (SQCLC), miR-195 acts as a tumor suppressor, inhibiting the
viability, growth and migration of SQCLC cells by targeting
VEGF and inhibiting angiogenesis in tumors (68). Additionally, miR-195 promotes
apoptosis and inhibits proliferation in various types of cancer. In
summary, the results may provide novel insights for developing
prognostic markers and efficacious strategies for clinical therapy
of NSCLC and SQCLC.
Breast cancer
The roles of angiogenesis in breast cancer tumour
growth and metastasis are well established. Breast cancer
metastasis requires the access of tumor cells into the blood
vessels. A previous study demonstrated that miR-126-3p expression
is downregulated in triple-negative breast cancer (TNBC) cells
(69). Regulator of G-protein
signaling 3 (RGS3) is a direct target of miR-126-3p in TNBC.
Overexpression of miR-126-3p inhibited the proliferation,
migration, invasion and angiogenesis of TNBC. Another study
reported that miR-126 regulated VEGF and was overexpressed in MCF7
cells, which was associated with a decrease in cell proliferation
(70). miR-126 may be regarded as a
promising therapy to increase breast cancer survival. Furthermore,
the upregulation of miR-4306 significantly suppressed TNBC cell
proliferation, migration and invasion, and abrogated angiogenesis
and lymphangiogenesis in vitro and in vivo (71). Mechanistic analyses indicated that
miR-4306 inactivated the signaling pathways mediated by its direct
targets, SIX1/Cdc42/VEGFA. Estrogen is of
paramount importance in breast cancer. However, there is no
preferred standard form of chemotherapy for TNBC. The study
verified that miR-4306 could promote cisplatin-induced apoptosis,
suggesting that a miR-4306 mimic combined with cisplatin treatment
in TNBC may represent a promising targeted therapy with high
specificity and limited toxicity (71). Notably, studies on breast cancer
have reported that the overexpression of miR-526b and miR-655
upregulates the angiogenesis and lymphangiogenesis markers,
including VEGFA, VEGFC, VEGFD, CD31 and LYVE1 (72); mechanistic research confirmed that
PTEN was a target of both miRs. PTEN inhibited HIF-1α and
the PI3K/Akt pathway, and dysregulation of these pathways via PTEN
resulted in VEGF-overexpression. Additionally,
miR-199b-5p-overexpression inhibited the mRNA and protein
expression of activin receptor-like kinase 1, and the formation of
capillary-like tubular structures in HUVECs. Furthermore, it
attenuated the induction of the activin receptor-like kinase
1/Smad/Id1 pathway in HUVECs (73).
These findings further establish the involvement of these miRNAs in
breast cancer metastasis and their potential as future breast
cancer biomarkers.
Colorectal cancer
VEGFA is directly targeted by miR-1249 and
suppresses CRC cell proliferation, migration, invasion and
angiogenesis (74). The study
verified that miR-1249 was a potentially effective target for
treating CRC, via the Akt/mTOR pathway and that the EMT process of
CRC cells was inhibited by targeting VEGFA and high-mobility
group AT-hook 2. Furthermore, a study reported that the gene
encoding VEGFA was directly targeted by miR-150-5p in CRC
(63). miR-150-5p (63) and miR-193a-3p (75) inhibited cell proliferation,
migration, invasion and angiogenesis, and inactivated the
VEGFA/VEGFR2 and Akt/mTOR signaling pathways (63). In addition, miR-622-overexpression
inhibited CRC microvessel density and angiogenesis in vitro
and in vivo, by suppressing the CXCR4-VEGFA signaling axis
(76). Another study reported that
the gene encoding EGFR was a target of miR-7 (77). In CRC tissues, the expression of
EGFR and microvascular density are increased, while miR-7
expression is decreased. With the overexpression of miR-7 and
silencing of EGFR, vasculogenic mimicry and density, cell
migration, and cell invasion were suppressed via the extracellular
signal-regulated kinase signaling pathway. Anti-angiogenesis
therapy is an important strategy of cancer treatment, the research
not only provides a novel insight into the mechanism of CRC
progression but also highlights miR-1249, miR-150-5p, miR-193a-3p,
miR-622 and miR-7 as potential biomarkers and therapeutic targets
for CRC.
Gastric cancer
A previous study reported that baculoviral IAP
repeat-containing 2 (BIRC2) was a target gene for miR-204
and that overexpressing miR-204 inhibited GC cell growth and
metastasis; furthermore, miR-204-overexpression suppressed the
tumor necrosis factor (TNF)-α-induced activation of the NF-κB
signaling pathways, which decreased tube formation in HUVECs,
leading to GC progression (78).
Another study reported that miR-632, which targets the gene
encoding trefoil factor 1 (TFF1), was overexpressed in GC
tissue and serum. miRs may regulate TFF1 expression and
secretion. Recombinant TFF1 reversed miR-632-mediated
angiogenesis, and downregulated TFF1-induced tube formation
and endothelial cell recruitment (79). As important regulators of gene
expression, miRs have not only been implicated in various signaling
pathways but also in anticancer therapy, and other biological
processes. They provide predictive information for patients with
early GC and have the potential to be applied in endoscopic
treatment.
OC
OC is one of the leading causes of cancer-related
mortalities among females. miRs have been proven to be vital to the
development and progression of OC, particularly in affecting
chemotherapy resistance and vasculogenic mimicry formation.
miR-195-5p directly targets phosphoserine aminotransferase 1
(PSAT1) and its expression is downregulated in OC tissues
(80). The GSK3β signaling pathway
is correlated with enhanced cell apoptosis and decreased
DDP-resistance to OC (81).
Overexpression of miR-195-5p or silencing PSAT1 decreased
glycogen synthase kinase3β (GSK3β) phosphorylation, decreased the
expression of HIF-1α, VEGF and β-catenin, and promoted apoptosis in
OC (80). Inhibition of the
GSK3β/β-catenin signaling pathway was involved in the angiogenesis
regulation process. The study revealed that miR-195-5p inhibited
angiogenesis, decreased chemotherapy resistance of OC to DDP, and
promoted OC cell apoptosis, demonstrating that miR-195-5p may serve
as a therapeutic target for OC treatment. In addition, miR-765
decreased the levels of VEGFA, Akt1 and SRC-α in SKOV3 OC cells,
and negatively regulated VEGFA by specific binding to its
3′-untranslated region. The miR-765 expression and VEGFA, Akt1 and
SRC-α levels were associated with the patient outcome. Furthermore,
miR-765 suppressed the formation of 3D channels-like structures
through modulation of negatively regulated VEGFA and downregulated
the VEGFA/Akt1/SRC-α axis in OC (82). miR-765 is a relevant type of
hypoxia-regulated miR and is involved in angiogenesis and the early
stages of vasculogenic mimicry formation.
Prostate cancer
Expression of miR-130b is significantly
downregulated in prostate cancer cell lines. Downregulation of
miR-130b significantly promoted the proliferation, invasion and
tubule formation in HUVECs, and regulated TNF-α directly. miR-130b
attenuated NF-κB signaling and its downstream gene, VEGFA, by
directly inhibiting TNF-α expression. VEGFA, in turn, decreased the
expression of miR-130b, thereby forming a negative feedback loop to
induce angiogenesis (83). The
miR-130b/TNF-α/NF-κB/VEGFA loop may be an effective therapeutic
target for future prostate cancer treatment. Additionally,
miR-335-overexpression decreased the expression of inflammatory
factors, and significantly inhibited the viability and formation of
regenerative tubes by prostate cancer cells. Silencing the early
growth response protein 3 (EGR3), a possible target of
miR-335, suppressed the proliferation and angiogenesis of DU145
cells, decreased the activity of caspase-3, and downregulated
interleukin (IL)-6, IL-8 and IL-1β inflammatory factor expression
(84). miR-335 may function as a
potential tumor suppressor of prostate cancer and may act as a
potential biomarker in treating patients with prostate cancer.
Glioma
In glioma, circ-DICER1 regulates the expression of
miR-103a-3p and miR-382-5p in glioma-exposed endothelial cells. The
MOV10, circ-DICER1, ZIC4 and Hsp90β proteins are upregulated in
glioma. Hsp90 promotes the viability, migration and tube formation
of glioma-exposed endothelial cells by activating the PI3K/Akt
signaling pathway (85). The study
provided novel mechanisms
(MOV10/circ-DICER1/miR-103a-3p/miR-382-5p/ZIC4 pathway) and their
vital roles in angiogenesis regulation in anti-angiogenesis
therapies for glioma. Glioma stem cells (GSCs) are involved in
cancer initiation and metastasis, potentially releasing exosomes
that mediate cellular communication by delivering miRs.
Furthermore, GSCs-derived exosomes regulate angiogenesis by
modulating microvessel endothelial cells. GSCs-derived exosomes
contain high levels of miR-26a, which activates the PI3K/Akt
signaling pathway by targeting PTEN (34). The study provided a novel
therapeutic RNA vehicle for glioma therapies.
Other cancers
Astrocyte elevated gene 1 (AEG1) is a direct
target of miR-885-5p. Silencing AEG1 inhibited the
expression of programmed death-ligand 1 and EGFR in HCC.
Overexpression of miR-885-5p significantly inhibited cell
migration, invasion, proliferation, angiogenesis and the EMT
(86). Furthermore, AEG1 was
a direct target of miR-30e-5p in squamous cell carcinoma of the
head and neck, which suppressed the migration of HUVECs, decreased
the expression of VEGF and HGF, and was implicated in angiogenesis
and metastasis (87). The miR
signature may potentially be effective in diagnosis, prognosis and
therapy in cancer. A previous study on gallbladder cancer validated
MAP2K4 as a target gene of miR-136 (88). miR-136 has been regarded as a tumor
suppressor and oncogene that exerts its effects by targeting
different genes in several cancer types. Overexpressed miR-136
inhibited angiogenesis and cell proliferation and promoted
apoptosis in vitro and in vivo. Furthermore, the
overexpression of dual-specificity mitogen-activated protein kinase
kinase 4, and activation of the c-Jun N-terminal kinase signaling
pathway, reversed the inhibitory effects of miR-136 on angiogenesis
and tumorigenicity of gallbladder cancer cells (88). Overexpression of miR-136 may possess
promising beneficial effects in therapeutic treatments for
gallbladder cancer. Furthermore, miR-21 expression promoted the
migration, invasion and angiogenic abilities of renal cell
carcinoma cells by directly targeting the programmed cell death
protein 4 (PDCD4)/c-Jun signaling pathway (89). The study illustrated the molecular
mechanism underlying renal cell carcinoma progression and provided
a promising target for miRs-based therapy.
miRs as biomarkers in patients with
cancer
Current research demonstrates that miRs in blood or
tissues may be used as potential biomarkers for tumor
classification, diagnosis and disease progression monitoring
(90). Several studies have
identified numerous upregulated oncogenic miRs and downregulated
tumor suppressor miRs in cancer (91–93).
miRs participate in the intercellular communication process and may
be used as diagnostic and prognostic biomarkers for cancer and as
potential therapeutic targets. Consistent with these roles, certain
miRs may function as oncogenes or tumor suppressors. miR expression
profiling may yield pivotal biomarkers for cancer diagnostics.
Therefore, herein, the miR biomarkers in body fluids for different
types of cancer are summarized.
Exosomes, which contain multiple proteins, RNAs, and
other molecules are secreted by a variety of viable cells. Certain
miR biomarkers of cancer were detected in exosomes present in body
fluids. Tumor-derived or tumor-related exosomes are important
mediators of regulating tumor development and progression.
Therefore, they may be utilized in the early diagnosis, treatment
efficacy evaluation and prognosis prediction. miRs are involved in
oncogenesis and demonstrate remarkable tissue specificity, a
characteristic that may be used to detect cancer and improve tumor
origin prediction (94,95). With the development of detection
methods, the majority of diagnostic expression profiling of miRs
has been conducted using tumor tissue samples. However, several
studies have reported the diagnostic and prognostic value of miRs
in human body fluids (Table II,
Fig. 3).
 | Table II.miR biomarkers for different types of
cancer in body fluids. |
Table II.
miR biomarkers for different types of
cancer in body fluids.
| Type of cancer | miR | Source | (Refs.) |
|---|
| Ovarian | miR-21, miR-141,
miR-200, miR-203, miR-205, miR-214 |
Serum/Plasma/Exosomes | (98,99) |
| Breast | miR-195 | Serum/Plasma | (100) |
| Prostate | miR-16, miR-21,
miR-141 | Serum/Plasma | (101) |
| Gastric | miR-17-5p, miR-21,
miR-106 | Serum/Plasma | (102) |
| Glioma | miR-21 | Serum/Plasma | (103) |
| Lung | miR-21, miR-486,
miR-30d, miR-1, miR-499 | Serum/Plasma | (104,105) |
| Liver | miR-500 | Serum/Plasma | (106) |
| Bladder | miR-126,
miR-182 |
Serum/Plasma/urine/Exosomes | (107,108) |
| Esophageal | miR-21 | Serum/Plasma | (109) |
| Cervical | miR-21,
miR-146a | cervicovaginal
lavages/Exosomes | (110) |
| Oral | miR-125,
miR-200a | Saliva | (111) |
| Colorectal | miR-17-3p,
miR-92 | Serum/Plasma | (112,113) |
Conclusions
Angiogenesis is essential for tumor growth and
metastasis. Several studies have made significant progress in
investigating the regulatory actions of miRs in terms of tumor
angiogenesis (6,14,96).
In the future, clinical breakthroughs are expected using miRs as
predictive biomarkers and miRs-based anti-angiogenic therapeutic
strategies; however, numerous challenges remain. Since a single miR
may regulate angiogenesis by targeting multiple miRs, multi-target
anti-angiogenesis drugs may form a novel therapeutic approach with
broad prospects for antitumor treatment. However, miR-targeting
approaches may affect normal and abnormal angiogenesis. Therefore,
it is important to identify and target miRs that may distinguish
angiogenic endothelial cells in the tumor vasculature from those in
normal tissues (7,97). This review aimed to improve the
understanding of the mechanisms of miRs in tumor angiogenesis. This
will guide further exploratory studies to understand the processes
of the tumor-associated neovasculature and may contribute toward
the development of miR-based therapies capable of delaying the
progression of angiogenesis-related diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by Beijing Municipal
Natural Science Foundation (grant no. 7214294).
Availability of data and materials
The data used to support the findings of this study
are available from the corresponding author upon reasonable
request.
Authors' contributions
QZ and WH conceived and designed the study. QZ
drafted the manuscript and supported the funding. WH provided
technical guidance and revised the paper. All authors read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lagacé F, Ghazawi FM, Le M, Rahme E, Savin
E, Zubarev A, Alakel A, Sasseville D, Moreau L, Meterissian S and
Litvinov IV: Analysis of incidence, mortality trends, and
geographic distribution of breast cancer patients in Canada. Breast
Cancer Res Treat. 178:683–691. 2019. View Article : Google Scholar
|
|
2
|
Ruppert AS, Dixon JG, Salles G, Wall A,
Cunningham D, Poeschel V, Haioun C, Tilly H, Ghesquieres H, Ziepert
M, et al: International prognostic indices in diffuse large B-cell
lymphoma: A comparison of IPI, R-IPI and NCCN-IPI. Blood.
135:2041–2048. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao
HL, Li H, Zhang SR, Xu JZ, Qi ZH, et al: Angiogenesis in pancreatic
cancer: Current research status and clinical implications.
Angiogenesis. 22:15–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orso F, Quirico L, Dettori D, Coppo R,
Virga F, Ferreira LC, Paoletti C, Baruffaldi D, Penna E and Taverna
D: Role of miRNAs in tumor and endothelial cell interactions during
tumor progression. Semin Cancer Biol. 60:214–224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bisgin H, Gong B, Wang Y and Tong W:
Evaluation of bioinformatics approaches for Next-Generation
sequencing analysis of microRNAs with a toxicogenomics study
design. Front Genet. 9:222018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Wang L, Chen C and Chu X: New
insights into the regulatory role of microRNA in tumor angiogenesis
and clinical implications. Mol Cancer. 17:222018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hansen TB: Detecting agotrons in ago
CLIPseq Data. Methods Mol Biol. 1823:221–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vishnoi A and Rani S: MiRNA biogenesis and
regulation of diseases: An overview. Methods Mol Biol. 1509:1–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Denli AM, Tops BB, Plasterk RH, Ketting RF
and Hannon GJ: Processing of primary microRNAs by the
Microprocessor complex. Nature. 432:231–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng L, Li F, Jiang Y, Yu H, Xie C, Shi Y
and Gong Q: Structural insights into a unique preference for 3′
terminal guanine of mirtron in Drosophila TUTase tailor. Nucleic
Acids Res. 47:495–508. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michlewski G and Cáceres JF:
Post-transcriptional control of miRNA biogenesis. RNA. 25:1–16.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goradel NH, Mohammadi N, Haghi-Aminjan H,
Farhood B, Negahdari B and Sahebkar A: Regulation of tumor
angiogenesis by microRNAs: State of the art. J Cell Physiol.
234:1099–1110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li R, Qi Y, Jiang M, Zhang T, Wang H, Wang
L and Han M: Primary tumor-secreted VEGF induces vascular
hyperpermeability in premetastatic lung via the occludin
phosphorylation/ubiquitination pathway. Mol Carcinog. 58:2316–2326.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J, Xie L and Guo W: PDGF/PDGFR effects
in osteosarcoma and the ‘add-on’ strategy. Clin Sarcoma Res.
8:152018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DeLisser HM, Christofidou-Solomidou M,
Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda
C, Merwin JR, Madri JA and Albelda SM: Involvement of endothelial
PECAM-1/CD31 in angiogenesis. Am J Pathol. 151:671–677.
1997.PubMed/NCBI
|
|
18
|
Fang L, He Y, Liu Y, Ding H, Tong Y, Hu L,
Wang C, Zhang Y, Zheng X and Huang P: Adjustment of microvessel
area by stromal area to improve survival prediction in non-small
cell lung cancer. J Cancer. 10:3397–3406. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hida K, Maishi N, Annan DA and Hida Y:
Contribution of tumor endothelial cells in cancer progression. Int
J Mol Sci. 19:12722018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Zhao HJ, Xia XR, Diao FY, Ma X,
Wang J, Gao L, Liu J, Gao C, Cui YG and Liu JY: Hypoxia-induced and
HIF1α-VEGF-mediated tight junction dysfunction in choriocarcinoma
cells: Implications for preeclampsia. Clin Chim Acta. 489:203–211.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Li JC, Yang H, Zhang X, Liu LL, Li
Y, Zeng TT, Zhu YH, Li XD, Li Y, et al: Expansion of cancer stem
cell pool initiates lung cancer recurrence before angiogenesis.
Proc Natl Acad Sci USA. 115:E8948–E8957. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okamoto T, Usuda H, Tanaka T, Wada K and
Shimaoka M: The functional implications of endothelial gap
junctions and cellular mechanics in vascular angiogenesis. Cancers.
11:2372019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang
G, Song J, Li Z, Zhang Z and Yuan W: Effect of exosomal miRNA on
cancer biology and clinical applications. Mol Cancer. 17:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomasetti M, Lee W, Santarelli L and
Neuzil J: Exosome-derived microRNAs in cancer metabolism: Possible
implications in cancer diagnostics and therapy. Exp Mol Med.
49:e2852017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC,
Tsai PH, Wu CY and Kuo PL: Hypoxic lung cancer-secreted exosomal
miR-23a increased angiogenesis and vascular permeability by
targeting prolyl hydroxylase and tight junction protein ZO-1.
Oncogene. 36:4929–4942. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J,
Zhou K, Liu X, Ren X, Wang F, et al: Cancer-derived exosomal
miR-25-3p promotes pre-metastatic niche formation by inducing
vascular permeability and angiogenesis. Nat Commun. 9:53952018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang
J and Wu X: Ovarian cancer cell-secreted exosomal miR-205 promotes
metastasis by inducing angiogenesis. Theranostics. 9:8206–8220.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masoumi-Dehghi S, Babashah S and
Sadeghizadeh M: MicroRNA-141-3p-containing small extracellular
vesicles derived from epithelial ovarian cancer cells promote
endothelial cell angiogenesis through activating the JAK/STAT3 and
NF-κB signaling pathways. J Cell Commun Signal. 14:233–244. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen X, Mangala LS, Mooberry L, Bayraktar
E, Dasari SK, Ma S, Ivan C, Court KA, Rodriguez-Aguayo C, Bayraktar
R, et al: Identifying and targeting angiogenesis-related microRNAs
in ovarian cancer. Oncogene. 38:6095–6108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou Y, Ren H, Dai B, Li J, Shang L, Huang
J and Shi X: Hepatocellular carcinoma-derived exosomal miRNA-21
contributes to tumor progression by converting hepatocyte stellate
cells to cancer-associated fibroblasts. J Exp Clin Cancer Res.
37:3242018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao S, Li J, Zhang G, Wang Q, Wu C, Zhang
Q, Wang H, Sun P, Xiang R and Yang S: Exosomal miR-451a functions
as a tumor suppressor in hepatocellular carcinoma by targeting
LPIN1. Cell Physiol Biochem. 53:19–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen X, Yang F, Zhang T, Wang W, Xi W, Li
Y, Zhang D, Huo Y, Zhang J, Yang A and Wang T: MiR-9 promotes
tumorigenesis and angiogenesis and is activated by MYC and OCT4 in
human glioma. J Exp Clin Cancer Res. 38:992019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang ZF, Liao F, Wu H and Dai J: Glioma
stem cells-derived exosomal miR-26a promotes angiogenesis of
microvessel endothelial cells in glioma. J Exp Clin Cancer Res.
38:2012019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng
T, Zhu K, Ning T, Fan Q, Ying G and Ba Y: MiR-135b delivered by
gastric tumor exosomes inhibits FOXO1 expression in endothelial
cells and promotes angiogenesis. Mol Ther. 27:1772–1783. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lawson J, Dickman C, Towle R, Jabalee J,
Javer A and Garnis C: Extracellular vesicle secretion of miR-142-3p
from lung adenocarcinoma cells induces tumor promoting changes in
the stroma through cell-cell communication. Mol Carcinog.
58:376–387. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan
J, Zou Y and Chen S: Macrophage-derived exosomal microRNA-501-3p
promotes progression of pancreatic ductal adenocarcinoma through
the TGFBR3-mediated TGF-β signaling pathway. J Exp Clin Cancer Res.
38:3102019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamada NO, Heishima K, Akao Y and Senda T:
Extracellular vesicles containing MicroRNA-92a-3p facilitate
partial Endothelial-Mesenchymal transition and angiogenesis in
endothelial cells. Int J Mol Sci. 20:44062019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu HY, Yu CH, Zhang HH, Zhang SZ, Yu WY,
Yang Y and Chen Q: Exosomal miR-1229 derived from colorectal cancer
cells promotes angiogenesis by targeting HIPK2. Int J Biol
Macromol. 132:470–477. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bazzoni G and Dejana E: Endothelial
cell-to-cell junctions: Molecular organization and role in vascular
homeostasis. Physiol Rev. 84:869–901. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lesage J, Suarez-Carmona M,
Neyrinck-Leglantier D, Grelet S, Blacher S, Hunziker W, Birembaut
P, Noël A, Nawrocki-Raby B, Gilles C and Polette M: Zonula
occludens-1/NF-κB/CXCL8: A new regulatory axis for tumor
angiogenesis. FASEB J. 31:1678–1688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bhat AA, Uppada S, Achkar IW, Hashem S,
Yadav SK, Shanmugakonar M, Al-Naemi HA, Haris M and Uddin S: Tight
junction proteins and signaling pathways in cancer and
inflammation: A functional crosstalk. Front Physiol. 9:19422019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chao YC, Pan SH, Yang SC, Yu SL, Che TF,
Lin CW, Tsai MS, Chang GC, Wu CH, Wu YY, et al: Claudin-1 is a
metastasis suppressor and correlates with clinical outcome in lung
adenocarcinoma. Am J Respir Crit Care Med. 179:123–133. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao L, Wang P, Liu Y, Ma J and Xue Y:
MiR-34c regulates the permeability of blood-tumor barrier via
MAZ-mediated expression changes of ZO-1, occludin, and claudin-5. J
Cell Physiol. 230:716–731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tornavaca O, Chia M, Dufton N, Almagro LO,
Conway DE, Randi AM, Schwartz MA, Matter K and Balda MS: ZO-1
controls endothelial adherens junctions, cell-cell tension,
angiogenesis, and barrier formation. J Cell Biol. 208:821–838.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu T, Hu H, Zhang T, Jiang L, Li X, Liu S,
Zheng C, Yan G, Chen W, Ning Y, et al: MiR-25 promotes cell
proliferation, migration, and invasion of Non-Small-Cell lung
cancer by targeting the LATS2/YAP signaling pathway. Oxid Med Cell
Longev. 2019:97197232019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu B and Sun X: MiR-25 promotes invasion
of human non-small cell lung cancer via CDH1. Bioengineered.
10:271–281. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu FX, Su YL, Zhang H, Kong JY, Yu H and
Qian BY: Prognostic implications for high expression of MiR-25 in
lung adenocarcinomas of female non-smokers. Asian Pac J Cancer
Prev. 15:1197–1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou W, Fong MY, Min Y, Somlo G, Liu L,
Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR, et al:
Cancer-secreted miR-105 destroys vascular endothelial barriers to
promote metastasis. Cancer Cell. 25:501–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang L, Shen J, Cheng J and Fan X:
MicroRNA-21 regulates intestinal epithelial tight junction
permeability. Cell Biochem Funct. 33:235–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cho HS, Han TS, Hur K and Ban HS: The
roles of Hypoxia-inducible factors and non-coding RNAs in
gastrointestinal cancer. Genes (Basel). 10:10082019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Salinas-Vera YM, Marchat LA,
Gallardo-Rincón D, Ruiz-García E, Astudillo-De La Vega H,
Echavarría-Zepeda R and López-Camarillo C: AngiomiRs: MicroRNAs
driving angiogenesis in cancer (Review). Int J Mol Med. 43:657–670.
2019.PubMed/NCBI
|
|
53
|
Park JE, Dutta B, Tse SW, Gupta N, Tan CF,
Low JK, Yeoh KW, Kon OL, Tam JP and Sze SK: Hypoxia-induced tumor
exosomes promote M2-like macrophage polarization of infiltrating
myeloid cells and microRNA-mediated metabolic shift. Oncogene.
38:5158–5173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y,
Zhao X and Lu C: Extracellular vesicles secreted by hypoxia
pre-challenged mesenchymal stem cells promote non-small cell lung
cancer cell growth and mobility as well as macrophage M2
polarization via miR-21-5p delivery. J Exp Clin Cancer Res.
38:622019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang S, Zhang R, Xu R, Shang J, He H and
Yang Q: MicroRNA-574-5p in gastric cancer cells promotes
angiogenesis by targeting protein tyrosine phosphatase non-receptor
type 3 (PTPN3). Gene. 733:1443832020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yu P, Fan S, Huang L, Yang L and Du Y:
MIR210 as a potential molecular target to block invasion and
metastasis of gastric cancer. Med Hypotheses. 84:209–212. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen F, Chen J, Yang L, Liu J, Zhang X,
Zhang Y, Tu Q, Yin D, Lin D, Wong PP, et al: Extracellular
vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated
macrophages regulates aerobic glycolysis of breast cancer cells.
Nat Cell Biol. 21:498–510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu L, Wang Y, Bai R, Yang K and Tian Z:
MiR-186 inhibited aerobic glycolysis in gastric cancer via HIF-1α
regulation. Oncogenesis. 6:e3182017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shivdasani RA: MicroRNAs: Regulators of
gene expression and cell differentiation. Blood. 108:3646–3653.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bielenberg DR and Zetter BR: The
contribution of angiogenesis to the process of metastasis. Cancer
J. 21:267–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Landskroner-Eiger S, Moneke I and Sessa
WC: MiRNAs as modulators of angiogenesis. Cold Spring Harb Perspect
Med. 3:a0066432013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dimova I, Popivanov G and Djonov V:
Angiogenesis in cancer-general pathways and their therapeutic
implications. J BUON. 19:15–21. 2014.PubMed/NCBI
|
|
63
|
Chen X, Xu X, Pan B, Zeng K, Xu M, Liu X,
He B, Pan Y, Sun H and Wang S: MiR-150-5p suppresses tumor
progression by targeting VEGFA in colorectal cancer. Aging (Albany
NY). 10:3421–3437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mao Z, Xu B, He L and Zhang G: PVT1
promotes angiogenesis by regulating miR-29c/Vascular endothelial
growth factor (VEGF) signaling pathway in non-small-cell lung
cancer (NSCLC). Med Sci Monit. 25:5418–5425. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang F, Shao C, Wei K, Jing X, Qin Z, Shi
Y, Shu Y and Shen H: MiR-942 promotes tumor migration, invasion,
and angiogenesis by regulating EMT via BARX2 in non-small-cell lung
cancer. J Cell Physiol. 234:23596–23607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gao LM, Zheng Y, Wang P, Zheng L, Zhang
WL, Di Y, Chen LL, Yin XB, Tian Q, Shi SS and Xu SF:
Tumor-suppressive effects of microRNA-181d-5p on non-small-cell
lung cancer through the CDKN3-mediated Akt signaling pathway in
vivo and in vitro. Am J Physiol Lung Cell Mol Physiol.
316:L918–L933. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang Y, Chen Z, Feng L, Jiang P, Li X and
Wang X: Ionizing Radiation-inducible microRNA-21 induces
angiogenesis by directly targeting PTEN. Asian Pac J Cancer Prev.
20:1587–1593. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu H, Chen Y, Li Y, Li C, Qin T, Bai M,
Zhang Z, Jia R, Su Y and Wang C: MiR-195 suppresses metastasis and
angiogenesis of squamous cell lung cancer by inhibiting the
expression of VEGF. Mol Med Rep. 20:2625–2632. 2019.PubMed/NCBI
|
|
69
|
Hong Z, Hong C, Ma B, Wang Q, Zhang X, Li
L, Wang C and Chen D: MicroRNA-126-3p inhibits the proliferation,
migration, invasion, and angiogenesis of triple-negative breast
cancer cells by targeting RGS3. Oncol Rep. 42:1569–1579.
2019.PubMed/NCBI
|
|
70
|
Alhasan L: MiR-126 modulates angiogenesis
in breast cancer by targeting VEGF-A-mRNA. Asian Pac J Cancer Prev.
20:193–197. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhao Z, Li L, Du P, Ma L, Zhang W, Zheng
L, Lan B, Zhang B, Ma F, Xu B, et al: Transcriptional
Downregulation of miR-4306 serves as a new therapeutic target for
triple negative breast cancer. Theranostics. 9:1401–1416. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hunter S, Nault B, Ugwuagbo KC, Maiti S
and Majumder M: Mir526b and Mir655 promote tumour associated
angiogenesis and lymphangiogenesis in breast cancer. Cancers
(Basel). 11:9382019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lin X, Qiu W, Xiao Y, Ma J, Xu F, Zhang K,
Gao Y, Chen Q, Li Y, Li H and Qian A: MiR-199b-5p suppresses tumor
angiogenesis mediated by vascular endothelial cells in breast
cancer by targeting ALK1. Front Genet. 10:13972019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chen X, Zeng K, Xu M, Liu X, Hu X, Xu T,
He B, Pan Y, Sun H and Wang S: P53-induced miR-1249 inhibits tumor
growth, metastasis, and angiogenesis by targeting VEGFA and HMGA2.
Cell Death Dis. 10:1312019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lin M, Zhang Z, Gao M, Yu H, Sheng H and
Huang J: MicroRNA-193a-3p suppresses the colorectal cancer cell
proliferation and progression through downregulating the PLAU
expression. Cancer Manag Res. 11:5353–5363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fang Y, Sun B, Wang J and Wang Y: MiR-622
inhibits angiogenesis by suppressing the CXCR4-VEGFA axis in
colorectal cancer. Gene. 699:37–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Fan X, Liu M, Tang H, Leng D, Hu S, Lu R,
Wan W and Yuan S: MicroRNA-7 exerts antiangiogenic effect on
colorectal cancer via ERK signaling. J Surg Res. 240:48–59. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen P, Guo H, Wu X, Li J, Duan X, Ba Q
and Wang H: Epigenetic silencing of microRNA-204 by Helicobacter
pylori augments the NF-κB signaling pathway in gastric cancer
development and progression. Carcinogenesis. 41:430–441. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shi Y, Huang X, Chen G, Wang Y, Liu Y, Xu
W, Tang S, Guleng B, Liu J and Ren J: MiR-632 promotes gastric
cancer progression by accelerating angiogenesis in a TFF1-dependent
manner. BMC Cancer. 19:142019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Dai J, Wei R, Zhang P and Kong B:
Overexpression of microRNA-195-5p reduces cisplatin resistance and
angiogenesis in ovarian cancer by inhibiting the PSAT1-dependent
GSK3β/β-catenin signaling pathway. J Transl Med. 17:1902019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lu J, Xu Y, Wei X, Zhao Z, Xue J and Liu
P: Emodin inhibits the epithelial to mesenchymal transition of
epithelial ovarian cancer cells via ILK/GSK-3β/Slug signaling
pathway. Biomed Res Int. 2016:62532802016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Salinas-Vera YM, Gallardo-Rincón D,
García-Vázquez R, Hernández-de la Cruz ON, Marchat LA,
González-Barrios JA, Ruíz-García E, Vázquez-Calzada C,
Contreras-Sanzón E, Resendiz-Hernández M, et al: HypoxamiRs
profiling identify miR-745 as a regulator of the early stages of
vasculogenic mimicry in SKOV3 ovarian cancer cells. Front Oncol.
9:3812019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mu HQ, He YH, Wang SB, Yang S, Wang YJ,
Nan CJ, Bao YF, Xie QP and Chen YH: MiR-130b/TNF-α/NF-κB/VEGFA loop
inhibits prostate cancer angiogenesis. Clin Transl Oncol.
22:111–121. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang P, Yang X, Wang L, Zhang D, Luo Q
and Wang B: Overexpressing miR-335 inhibits DU145 cell
proliferation by targeting early growth response 3 in prostate
cancer. Int J Oncol. 54:1981–1994. 2019.PubMed/NCBI
|
|
85
|
He Q, Zhao L, Liu X, Zheng J, Liu Y, Liu
L, Ma J, Cai H, Li Z and Xue Y: MOV10 binding circ-DICER1 regulates
the angiogenesis of glioma via miR-103a-3p/miR-382-5p mediated ZIC4
expression change. J Exp Clin Cancer Res. 38:92019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li C, Wang X and Song Q: MicroRNA 885-5p
inhibits hepatocellular carcinoma metastasis by repressing AEG1.
Onco Targets Ther. 13:981–988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang S, Li G, Liu C, Lu S, Jing Q, Chen
X, Zheng H, Ma H, Zhang D, Ren S, et al: MiR-30e-5p represses
angiogenesis and metastasis by directly targeting AEG-1 in squamous
cell carcinoma of the head and neck. Cancer Sci. 111:356–368. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Niu J, Li Z and Li F: Overexpressed
microRNA-136 works as a cancer suppressor in gallbladder cancer
through suppression of JNK signaling pathway via inhibition of
MAP2K4. Am J Physiol Gastrointest Liver Physiol. 317:G670–G681.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fan B, Jin Y, Zhang H, Zhao R, Sun M, Sun
M, Yuan X, Wang W, Wang X, Chen Z, et al: MicroRNA-21 contributes
to renal cell carcinoma cell invasiveness and angiogenesis via the
PDCD4/c-Jun (AP-1) signalling pathway. Int J Oncol. 56:178–192.
2020.PubMed/NCBI
|
|
90
|
Wang H, Peng R, Wang J, Qin Z and Xue L:
Circulating microRNAs as potential cancer biomarkers: The advantage
and disadvantage. Clin Epigenetics. 10:592018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ali Syeda Z, Langden SSS, Munkhzul C, Lee
M and Song SJ: Regulatory mechanism of MicroRNA expression in
cancer. Int J Mol Sci. 21:17232020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hammouz RY, Kołat D, Kałuzińska Ż,
Płuciennik E and Bednarek AK: MicroRNAs: Their role in metastasis,
angiogenesis, and the potential for biomarker utility in bladder
carcinomas. Cancers (Basel). 13:8912021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tipanee J, Di Matteo M, Tulalamba W,
Samara-Kuko E, Keirsse J, Van Ginderachter JA, Chuah MK and
VandenDriessche T: Validation of miR-20a as a tumor suppressor gene
in liver carcinoma using hepatocyte-specific hyperactive piggyBac
transposons. Mol Ther Nucleic Acids. 19:1309–1329. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Rosenfeld N, Aharonov R, Meiri E,
Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S,
Levy A, et al: MicroRNAs accurately identify cancer tissue origin.
Nat Biotechnol. 26:462–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Søkilde R, Vincent M, Møller AK, Hansen A,
Høiby PE, Blondal T, Nielsen BS, Daugaard G, Møller S and Litman T:
Efficient identification of miRNAs for classification of tumor
origin. J Mol Diagn. 16:106–115. 2014. View Article : Google Scholar
|
|
96
|
Yang Y, Guo Z, Chen W, Wang X, Cao M, Han
X, Zhang K, Teng B, Cao J, Wu W, et al: M2 macrophage-derived
exosomes promote angiogenesis and growth of pancreatic ductal
adenocarcinoma by targeting E2F2. Mol Ther. 29:1226–1238. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Caporali A and Emanueli C: MicroRNA
regulation in angiogenesis. Vascul Pharmacol. 55:79–86. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Szajnik M, Czystowska-Kuźmicz M, Elishaev
E and Whiteside TL: Biological markers of prognosis, response to
therapy and outcome in ovarian carcinoma. Expert Rev Mol Diagn.
16:811–826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Qattan A, Intabli H, Alkhayal W, Eltabache
C, Tweigieri T and Amer SB: Robust expression of tumor suppressor
miRNA's let-7 and miR-195 detected in plasma of Saudi female breast
cancer patients. BMC Cancer. 17:7992017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hu X, Fan J, Duan B, Zhang H, He Y, Duan P
and Li X: Single-molecule catalytic hairpin assembly for rapid and
direct quantification of circulating miRNA biomarkers. Anal Chim
Acta. 1042:109–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Tsujiura M, Ichikawa D, Komatsu S,
Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi
K, Fujiwara H, et al: Circulating microRNAs in plasma of patients
with gastric cancers. Br J Cancer. 102:1174–1179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhou Q, Liu J, Quan J, Liu W, Tan H and Li
W: MicroRNAs as potential biomarkers for the diagnosis of glioma: A
systematic review and meta-analysis. Cancer Sci. 109:2651–2659.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu
Y, Chen Y, Xu L, Zen K, Zhang C and Shen H: Serum microRNA
signatures identified in a genome-wide serum microRNA expression
profiling predict survival of non-small-cell lung cancer. J Clin
Oncol. 28:1721–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Rabinowits G, Gerçel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal microRNA: A diagnostic marker
for lung cancer. Clin Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yamamoto Y, Kosaka N, Tanaka M, Koizumi F,
Kanai Y, Mizutani T, Murakami Y, Kuroda M, Miyajima A, Kato T and
Ochiya T: MicroRNA-500 as a potential diagnostic marker for
hepatocellular carcinoma. Biomarkers. 14:529–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sabo AA, Birolo G, Naccarati A, Dragomir
MP, Aneli S, Allione A, Oderda M, Allasia M, Gontero P, Sacerdote
C, et al: Small Non-Coding RNA profiling in plasma extracellular
vesicles of bladder cancer patients by next-generation sequencing:
Expression levels of miR-126-3p and piR-5936 increase with higher
histologic grades. Cancers (Basel). 12:15072020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Xie F, Li Y, Wang M, Huang C, Tao D, Zheng
F, Zhang H, Zeng F, Xiao X and Jiang G: Circular RNA BCRC-3
suppresses bladder cancer proliferation through miR-182-5p/p27
axis. Mol Cancer. 17:1442018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tanaka Y, Kamohara H, Kinoshita K,
Kurashige J, Ishimoto T, Iwatsuki M, Watanabe M and Baba H:
Clinical impact of serum exosomal microRNA-21 as a clinical
biomarker in human esophageal squamous cell carcinoma. Cancer.
119:1159–1167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu J, Sun H, Wang X, Yu Q, Li S, Yu X and
Gong W: Increased exosomal microRNA-21 and microRNA-146a levels in
the cervicovaginal lavage specimens of patients with cervical
cancer. Int J Mol Sci. 15:758–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Park NJ, Zhou H, Elashoff D, Henson BS,
Kastratovic DA, Abemayor E and Wong DT: Salivary microRNA:
Discovery, characterization, and clinical utility for oral cancer
detection. Clin Cancer Res. 15:5473–5477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
El-Daly SM, Morsy SM, Medhat D, El-Bana
MA, Latif YA, Omara EA, Awadallah JR and Gamal-Eldeen AM: The
diagnostic efficacy of circulating miRNAs in monitoring the early
development of colitis-induced colorectal cancer. J Cell Biochem.
120:16668–16680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ng EK, Chong WW, Jin H, Lam EK, Shin VY,
Yu J, Poon TC, Ng SS and Sung JJ: Differential expression of
microRNAs in plasma of patients with colorectal cancer: A potential
marker for colorectal cancer screening. Gut. 58:1375–1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|