Coronavirus disease 2019 (COVID-19), caused by
severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), was
initially viewed as a primarily respiratory disease, leading to
viral pneumonia in some cases. However, it is now recognized as a
complex disease affecting various body systems (1). Among these effects, an accumulating
body of research links severe COVID-19 to new-onset mental illness
(2–5).
The data on whether mental illness affects the
severity of COVID-19 are mixed. Studies indicate that the
prevalence of COVID-19 among patients with serious mental illness
(SMI) is either lower than (6) or
similar to that among patients without a history of mental illness
(7). However, patients with SMI are
reported to have a slightly higher risk for severe clinical
outcomes following infection with COVID-19 compared with those who
do not have a history of mental illness (7). These findings suggest that COVID-19
and SMI might be reciprocal risk factors, raising the question of
which pathways might link these two factors. A disadvantageous
effect of SMIs on COVID-19 outcomes has been attributed primarily
to medication non-adherence (7,8) and
cardiovascular comorbidities (7,9).
Another potential explanation is that the shared attribute of
increased inflammation may constitute the link between SMI and
severe COVID-19 (10). Sedentary
habits and low physical activity during COVID-19 quarantine have
been associated with an inflammatory state and a negative impact on
mental health (11–13).
Severe COVID-19 comprises the presence of pneumonia,
severe acute respiratory distress syndrome, microvascular
thrombosis and/or cytokine storms, all of which involve underlying
inflammation (14). Numerous
proinflammatory cytokines reportedly associated with severe
COVID-19 (15) are also linked with
SMIs, including schizophrenia, depression and bipolar affective
disorder (16–19). Several recent reports have described
new-onset depression in patients with COVID-19, accompanied by
increased levels of interleukin-6, a key molecule in the cytokine
storm (4,10,20).
These findings suggest that SMI-associated factors that could
affect COVID-19 outcomes and vice versa should be investigated for
their association with the inflammatory response. Thus far, the
systematic evaluation of candidate factors is lacking (7).
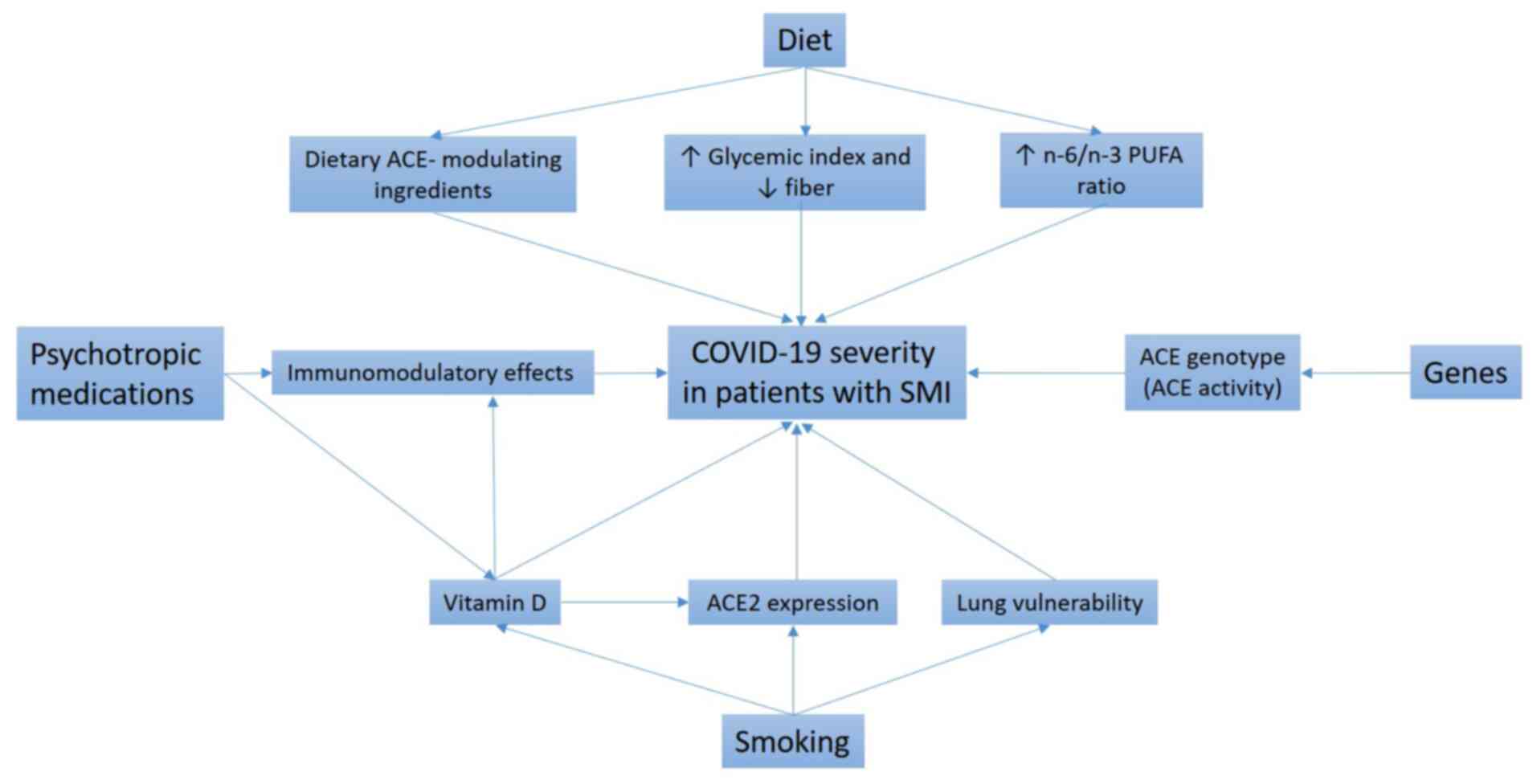
In the present review, some of these candidate
factors are critically discussed (Fig.
1). The initial focus is on psychotropic medication use,
unhealthy dietary patterns and excessive smoking as potential
extrinsic factors in existing mental illness that might heighten
the risk of severe COVID-19. Next, the role of
angiotensin-converting enzyme (ACE) insertion/deletion (I/D) gene
polymorphisms as possible intrinsic factors in the development of
both SMIs and COVID-19 is addressed. In addition, the possibility
of using genetics-based methods to predict the new-onset SMI risk
among patients experiencing severe COVID-19 is examined. Finally,
some therapeutic options that might be relevant to the prevention
of SMI onset following severe COVID-19 are proposed.
Previous studies have suggested that psychotropic
medications could affect inflammatory processes. Various
antipsychotics, antidepressants and antiepileptics have been shown
to favor the anti-inflammatory state by increasing levels of
anti-inflammatory cytokines and decreasing those of proinflammatory
cytokines (21–24).
Clinical trials have shown that aspirin, estrogens,
N-acetylcysteine, minocycline, pregnenolone, celecoxib and n-3
polyunsaturated fatty acids (PUFAs) have antipsychotic effects
(25). Other studies have indicated
that by promoting an enhanced anti-inflammatory state, specific
atypical antipsychotic medications may dysregulate innate and
adaptive immune responses (Fig. 1),
thereby increasing susceptibility to respiratory infections,
including COVID-19 (26,27). In a preclinical study, the treatment
of healthy mice with low-dose risperidone diminished the secretion
of several important proinflammatory cytokines. In addition,
risperidone inhibited an antibody response in the animals following
vaccination with Pneumovax23® (26). The latter observation is alarming,
given increasing concerns about secondary bacterial pneumonia,
including pneumococcal pneumonia, during COVID-19 (22). Furthermore, in a large group of
individuals with schizophrenia-spectrum disorders (n=6,309), among
whom 102 tested positive for COVID-19, those treated with clozapine
had a ~3-fold increased risk for COVID-19 infection compared with
those taking other antipsychotic medications (27). Several adverse effects of clozapine,
including diabetes, obesity and hypersalivation (leading to
aspiration pneumonia), have been proposed to contribute to the
mechanism by which clozapine treatment affects COVID-19 risk
(28). Moreover, clozapine
treatment affects the innate immune system, leading to transient
eosinophilia, cytokine release and fever during early treatment,
and to neutropenia and agranulocytosis in a small minority of
patients. Recent data also imply a link between clozapine treatment
and adaptive immunity. Specifically, patients treated with
clozapine experienced a significant reduction in all three classes
of circulating immunoglobulins (M, A and G) compared with those
treated with alternative antipsychotics (29).
The use of antipsychotics has also been reported to
be associated with reduced physical activity, possibly because of
the side effects of antipsychotics, which include extrapyramidal
symptoms and fatigue (30,31). However, physical activity supports
immune function in viral respiratory infections by triggering the
release of stress hormones, namely catecholamines and
glucocorticoids, which are responsible for dampening local
inflammation, and by promoting the secretion of anti-inflammatory
cytokines (11,12). Studies have demonstrated that
physical activity also offers benefits for mental well-being and
may prevent symptoms associated with depression and anxiety during
COVID-19 quarantine (11,32). One large study of people from the
general population in Italy (n=2,524) revealed that reduced total
physical activity during quarantine had a negative effect on
Psychological General Well-Being Index scores (11). Furthermore, a cross-national study
involving people living in Germany, Italy, Russia and Spain
indicated that individuals with symptoms of depression were at risk
of a worsening psychological state during the COVID-19 pandemic and
that physical activity counteracted this negative effect (32). The favorable effects of physical
activity on psychological health have been attributed to
stimulation of the cholinergic, dopaminergic and serotonergic
neurotransmitter systems, endogenous opioid release and the
expression of several trophic factors, including brain-derived
neurotrophic factor (13,33).
Nonsteroidal anti-inflammatory medications, various
pro-inflammatory cytokine inhibitors, statins, n-3 PUFAs,
pioglitazone, minocyclin and modafinil have been indicated to exert
antidepressant effects (34).
Certain antidepressant medications from the selective serotonin
reuptake inhibitor class, such as fluvoxamine, may exert favorable
effects on patients with COVID-19 because of their immunomodulatory
action. A preliminary clinical trial of fluvoxamine compared with
placebo showed that adult patients with symptomatic COVID-19
treated with fluvoxamine had a lower likelihood of clinical
deterioration and serious adverse events over 15 days (35). A protective effect of fluvoxamine in
COVID-19 might arise from the ability of the drug to stimulate σ-1
receptor activity. The σ-1 receptor is an important endoplasmic
reticulum chaperone protein with various cellular functions,
including regulation of cytokine production (36). In addition, fluvoxamine accumulates
in lysosomes, endosomes and biological membranes, where it
interferes with the endosomal pathway and intracellular membrane
trafficking crucial for viral infection (37).
Antiepileptics are regularly used to treat bipolar
affective disorder, and are also prescribed to patients with
schizophrenia at a lower rate, estimated at 20% (38,39).
To the best of our knowledge, no studies have indicated a possible
link between antiepileptic use and COVID-19 among patients with
SMI. However, the use of antiepileptics has been reported to have
an association with vitamin D deficiency (Fig. 1), which has been recognized as an
important modulator of innate and adaptive immune responses
(40). Several mechanisms have been
proposed to explain the link between antiepileptic use and vitamin
D deficiency (41–43). Certain antiepileptic drugs, for
example, carbamazepine, phenobarbital, phenytoin, primidone and
topiramate, induce the hepatic cytochrome system, leading to
increased catabolism of the active form of vitamin D
(1,25-dihydroxyvitamin D) (43).
Furthermore, antiepileptic drugs may bind to the steroid and
xenobiotic receptor transcription factor, potentially interacting
with the vitamin D-responsive element for vitamin D-24-hydroxylase
and thereby activating the enzyme (41,43).
Several studies have convincingly shown an
association between vitamin D deficiency and COVID-19 severity
(44,45). Recently conducted interventional
clinical studies have further emphasized the importance of vitamin
D in the prevention of severe COVID-19 outcomes (46,47).
Several mechanisms have been proposed for the protective effects of
vitamin D in COVID-19 (Fig. 1),
including immunomodulatory activity, the enhancement of defensin
and interferon α expression, and initiation of local
pro-regenerative processes (48,49).
The vitamin D-mediated reduction of proinflammatory cytokine
hypersecretion is an important protective effect because of its
role in preventing the development of a cytokine storm (50,51).
In addition, vitamin D deficiency is associated with accelerated
thrombogenesis and a consequent increase in thrombotic episodes,
which are frequently observed in patients with severe COVID-19
(52,53).
Dietary intake represents another important
modulator of the inflammatory response (Fig. 1). Numerous studies have indicated
that the dietary patterns of patients with SMI are characterized by
a high intake of saturated fat and calories and a high n-6/n-3 PUFA
ratio (54–59). These increases in saturated fat and
the n-6/n-3 PUFA ratio are associated with an overall increase in
the production of pro-inflammatory cytokines and an overreactive
inflammatory response (60,61). Furthermore, the consumption of
monounsaturated fatty acids, fiber, fruit and vegetables, which
exert potent anti-inflammatory effects, is often low among patients
with SMI (54–59).
A number of PUFAs act as natural ligands of
peroxisome proliferator-activated receptors (PPARs) and sterol
regulatory element-binding protein (SREBP) transcription factors,
which regulate lipid and glucose metabolism, and overall energy
homeostasis (62–64). Thus, an unbalanced n-6/n-3 PUFA
ratio in patients with SMI could indirectly contribute to diabetes,
dyslipidemia and obesity risk via PPAR and SREBP transcriptional
activation. This speculation may be relevant to COVID-19 because a
greater risk of severe outcomes has been reported among individuals
with diabetes, dyslipidemia and/or obesity (65,66).
A dietary deficiency of n-3 PUFAs can lead to
changes in the phospholipid fatty acid composition of membranes,
and thereby induce changes in the collective physicochemical
properties of the bilayer, such as flexibility and fluidity
(67). These modifications can
affect the function of membrane proteins that mediate the action of
insulin, such as glucose transporter type 4, and lead to insulin
resistance, diabetes and dyslipidemia (67–69).
In addition, in vitro studies have indicated that membrane
n-3 PUFA levels influence the therapeutic efficiency of
psychotropic drugs, as n-3 PUFAs facilitate the intercalation of
the drugs between acidic glycerophospholipids (68).
The estimated prevalence of smoking among people
with SMI is 50–80% worldwide, which is significantly higher than
that in the general population, and people with SMI are also more
likely to smoke heavily, considered as ≥30 cigarettes or 1.5 packs
daily (83–85). The reported prevalence of smoking
among patients with schizophrenia is higher than that in patients
with bipolar disorder or depression (86,87).
Several mechanisms have been proposed to explain the link between
cigarette smoke and inflammation, such as an imbalanced ratio of
pro-inflammatory and anti-inflammatory cytokines, impairment of the
innate immune response and increased oxidative stress (88,89).
Studies of patients with COVID-19 have identified
cigarette smoking as an important risk factor for severe outcomes
(90–92) (Fig.
1). The elevated rates of severe COVID-19 in individuals who
smoke may be attributable to diseases associated with smoking, for
example, chronic obstructive pulmonary disease, diabetes and
cardiovascular disease (90,92).
Furthermore, cigarette smoke has been proposed to increase
expression of the ACE2 receptor in the bronchial epithelium
(Fig. 1). This receptor is the
mediator of SARS-CoV-2 entry into host cells (90,91).
However, considering the anti-inflammatory properties of ACE2,
certain studies have claimed that the upregulation of ACE2 by
smoking may exert a protective effect against COVID-19 rather than
a harmful one (93,94). One possible explanation for this is
that the ACE2-mediated cleavage of detrimental Ang II becomes more
efficient and subsequently increases the production of angiotensins
1–7 (Ang 1–7), which show anti-inflammatory and proregenerative
activity (71,95). Moreover, the nitric oxide produced
during smoking promotes the maintenance of airway dilation and
filtration prior to its entry into the lungs (94,96)
and also inhibits the replication of SARS-CoV-2 in vitro
(94,97).
Among people with SMI, smoking is likely to interact
with factors modulating vulnerability to COVID-19, such as the use
of psychotropic medications and maintenance of an unhealthy dietary
pattern (98–101). For instance, smoking has been
shown to increase the clearance of specific antipsychotics, namely
clozapine and olanzapine, and antidepressants, namely fluvoxamine,
duloxetine, mirtazapine and trazodone, by inducing their metabolism
in the liver, suggesting that smokers may be at risk of
undertreatment (98,101). In addition, nicotine has been
reported to have inhibitory effects on the activity and
concentration of various antiepileptic medications, including
lamotrigine, carbamazepine, diphenylhydantoin, phenobarbital and
topiramate in animals (98), while
cigarette smoking was shown to reduce serum levels of lamotrigine
in a clinical study (99).
Certain studies have indicated a link between
smoking and an unhealthy or proinflammatory dietary pattern among
patients with SMI (57,102,103). For instance, smokers with
schizophrenia are reported to be more likely to consume salt and
saturated fat, and less likely to follow a high-fiber and
low-calorie diet compared with nonsmokers (102). In addition, among individuals with
depression, current smokers reported consuming more high-fat foods
compared with never smokers and more fast-food fats compared with
former and never smokers (103).
Furthermore, several reports suggest a poorer diet accompanied by
lower levels of physical activity among smokers with bipolar
disorder compared with healthy individuals (104–106).
Smoking has been identified as an important factor
contributing to vitamin D deficiency (Fig. 1) among patients with SMI (107,108). A suggested mechanism for the link
between smoking and vitamin D deficiency is that cigarette smoke
decreases the production of 1,25-dihydroxyvitamin D in lung
epithelial cells (109,110). In addition, various cigarette
smoke extracts have been demonstrated to inhibit translocation of
the vitamin D receptor from the nucleus to microsome in human
alveolar basal epithelial cell line (111).
Results of a meta-analysis that included a large
number of participants (n=10,223) in case-control studies indicated
that the ACE-DD homozygous genotype was associated with an elevated
risk of depression in a Caucasian and mixed ethnic group consisting
of several European populations (German, British, Belgian, Finish
and Israeli) and Asian populations (Japanese and Chinese) (119). Several studies have indicated that
the ACE-I/D polymorphism might be associated with an elevated risk
of schizophrenia, although the results were conflicting (120–122) and not confirmed in a meta-analysis
(123). A greater risk for
schizophrenia was observed among individuals carrying the ACE-D
allele (ACE-DD homozygous and ACE-ID heterozygous) in the Turkish
and Iranian populations (120,121), and among those carrying the ACE-I
allele (ACE-II homozygous and ACE-ID heterozygous) in the Spanish
population (122). Furthermore, a
greater severity of schizophrenia, based on Positive and Negative
Syndrome Scale psychopathology evaluations (124–126) and improved response to specific
antidepressants, was detected among individuals who were ACE-DD
homozygous and ACE-D carriers (127,128).
A number of studies have assessed the relevance of
the ACE-I/D polymorphism in COVID-19-associated deaths. During the
first wave of the pandemic, Delanghe et al (129) compared the D-allele frequency of
the ACE gene in 25 European countries with the prevalence and
mortality rates of COVID-19. The authors concluded that the
prevalence of COVID-19 and mortality were negatively correlated
with frequency of the ACE-D allele, indicating that this allele
might be protective. The protective effects of this allele against
COVID-19 prevalence and mortality were supported by a further study
from the same research team, in which COVID-19 risk and ACE-I/D
polymorphism were assessed in 33 countries from Europe, North
Africa and the Middle East (130).
In that study, the authors also assessed whether COVID-19 infection
was correlated with additional immune system-associated human
plasma protein polymorphisms, including the F and S alleles of
complement C3, the C282Y mutation of homeostatic iron regulator
(HFE), the Hp1 and Hp2 alleles of haptoglobin, and the DBP1 and
DBP2 alleles of vitamin D-binding protein; however, no significant
associations were detected. The ACE-D allele results in higher ACE
activity, favoring a more intense inflammatory response through
increased production of Ang II. According to the principle of
classical competitive inhibition, such findings may be explained in
part by the competition between large amounts of Ang II, which is
the ACE2 substrate, and SARS-CoV-2 in binding to the ACE2 receptor.
However, a meta-analysis of ACE-D allele distribution in various
European countries revealed high frequencies in Spain, Italy and
the United Kingdom (131), which
are among those most severely affected by COVID-19. Thus, the ACE-D
allele may be a harmful rather than a protective factor in
COVID-19.
Given the association of ACE-DD with the highest ACE
activity and consequently high production levels of proinflammatory
and profibrotic Ang II, Bellone and Calvisi (132) examined the correlation of
COVID-19-associated deaths with ACE-DD and ACE-II genotypes in 25
European countries. The authors detected a significant positive
correlation between ACE-DD frequency and COVID-19-associated
deaths. By contrast, the ACE-II genotype frequency was inversely
correlated with COVID-19-associated deaths, and no correlation was
found between ACE-ID and COVID-19-associated mortality. The
relevance of ACE-I/D polymorphism to COVID-19 severity was
evaluated in a global meta-analysis including a high number of
participants (n=48,758) from 30 countries. The authors investigated
COVID-19 recovery and mortality rates according to the ACE-I/D
allele frequency ratio (133).
They found that an increased ACE-I/D allele ratio was associated
with an increased rate of recovery, but identified no significant
association between mortality rate and the ACE-I/D ratio.
The disadvantageous effects in COVID-19 of the
ACE-DD homozygous genotype and, by implication, high ACE activity
(118), is supported by recent
clinical studies showing a lower rate of severe disease and lower
all-cause mortality among patients with COVID-19 whose hypertension
was being treated with ACE inhibitors (134–137). In addition, evidence suggests that
an abrupt suspension of ACE inhibitors in patients with COVID-19
and cardiovascular disease may result in clinical deterioration and
worse outcomes (136,138,139). In addition, the results of some
preclinical studies suggested that ACE inhibition shows promise in
the mitigation of psychotic symptomatology and cognitive deficits
(140–142). Furthermore, human studies have
indicated that ACE inhibitors may have favorable effects on
cognitive deficits in schizophrenia, possibly by modulating the
cleavage of specific neuropeptides, such as substance P and
neurotensin (141,142).
SMI and severe COVID-19 appear to have an increased
inflammatory response in common. Numerous extrinsic and intrinsic
factors may act as modulators of inflammatory processes among
patients with SMI. Extrinsic factors, such as an unhealthy dietary
pattern and excessive smoking, could contribute to the etiology of
severe COVID-19 in people with SMI, as in the general population,
but are potentially modifiable through lifestyle changes. In
addition, physical activity, which has been reduced during COVID-19
quarantine, is relevant to the ability of the immune system to
defend against viral infection, as well as to psychological health
and well-being. However, the etiopathogenesis of severe COVID-19
may be heterogeneous among patients with SMI because of
interactions among these factors, antipsychotic medications and
genetic polymorphisms, such as ACE-I/D. Within the RAS, ACE
simultaneously promotes the inflammatory reponse and counteracts
the activity of ACE2, the host receptor that mediates SARS-CoV-2
cell entry. Several studies support the relevance of the functional
ACE-I/D polymorphism in both SMI and severe COVID-19. For this
reason, genotyping individuals with severe COVID-19 for the ACE-I/D
polymorphism might offer predictive utility for COVID-19 outcomes
and the risk of new-onset, COVID-19-associated SMI. Furthermore,
ACE inhibitors exhibit promise for the mitigation of psychotic
symptomatology and cognitive deficits, and their positive effects
in severe COVID-19 accompanied by hypertension and other
cardiovascular comorbidities have been reported. Thus, the
administration of ACE inhibitors to patients with severe COVID-19
might be of benefit in the mitigation of severe disease and
prevention of new-onset SMI secondary to COVID-19 disease.
Not applicable.
This study was supported by grants from the
University of Rijeka, Croatia (grant nos. 17.07.2.1.10 and
uniri-biomed-18-251). The university had no further role in the
study design; data collection, analysis or interpretation; or the
decision to submit this paper for publication.
Not applicable.
SN and HJ designed the study and wrote the
manuscript. VP, DK and ABT wrote and drafted the manuscript. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Roberts CM, Levi M, McKee M, Schilling R,
Lim WS and Grocott MPW: COVID-19: A complex multisystem disorder.
Br J Anaesth. 125:238–242. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chacko M, Job A, Caston F III, George P,
Yacoub A and Cáceda R: COVID-19-induced psychosis and suicidal
behavior: Case report. SN Compr Clin Med. 26:1–5. 2020.PubMed/NCBI
|
|
3
|
Ferrando SJ, Klepacz L, Lynch S, Tavakkoli
M, Dornbush R, Baharani R, Smolin Y and Bartell A: COVID-19
psychosis: A potential new neuropsychiatric condition triggered by
novel coronavirus infection and the inflammatory response?
Psychosomatics. 6:551–555. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mazza MG, De Lorenzo R, Conte C, Poletti
S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F,
Rovere-Querini P, et al: Anxiety and depression in COVID-19
survivors: Role of inflammatory and clinical predictors. Brain
Behav Immun. 89:594–600. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anjum S, Ullah R, Rana MS, Khan HA, Memon
FS, Ahmed Y, Jabeen S and Faryal R: COVID-19 pandemic: A serious
threat for public mental health globally. Psychiatr Danub.
32:245–250. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Khoury F, Cuenca M, Niel P and Masson
VD: Low prevalence of SARS-CoV-2 among patients presenting at a
Parisian psychiatry University Hospital Group. Eur J Psychiatry.
Sep 24–2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee SW, Yang JM, Moon SY, Yoo IK, Ha EK,
Kim SY, Park UM, Choi S, Lee SH, Ahn YM, et al: Association between
mental illness and COVID-19 susceptibility and clinical outcomes in
South Korea: A nationwide cohort study. Lancet Psychiatry.
7:1025–1031. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DiMatteo MR, Lepper HS and Croghan TW:
Depression is a risk factor for noncompliance with medical
treatment: Meta-analysis of the effects of anxiety and depression
on patient adherence. Arch Intern Med. 160:2101–2107. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicholson A, Kuper H and Hemingway H:
Depression as an aetiologic and prognostic factor in coronary heart
disease: A meta-analysis of 6362 events among 146 538 participants
in 54 observational studies. Eur Heart J. 27:2763–2774. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alpert O, Begun L, Garren P and Solhkhah
R: Cytokine storm induced new onset depression in patients with
COVID-19. A new look into the association between depression and
cytokines-two case reports. Brain Behav Immun Health. 9:1001732020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maugeri G, Castrogiovanni P, Battaglia G,
Pippi R, D'Agata V, Palma A, Di Rosa M and Musumeci G: The impact
of physical activity on psychological health during Covid-19
pandemic in Italy. Heliyon. 6:e043152020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ravalli S and Musumeci G: Coronavirus
outbreak in Italy: Physiological benefits of home-based exercise
during pandemic. J Funct Morphol Kinesiol. 5:312020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maugeri G and Musumeci G: Adapted physical
activity to ensure the physical and psychological well-being of
COVID-19 patients. J Funct Morphol Kinesiol. 6:132021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weir EK, Thenappan T, Bhargava M and Chen
Y: Does vitamin D deficiency increase the severity of COVID-19?
Clin Med (Lond). 20:e107–e108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leisman DE, Ronner L, Pinotti R, Taylor
MD, Sinha P, Calfee CS, Hirayama AV, Mastroiani F, Turtle CJ,
Harhay MO, et al: Cytokine elevation in severe and critical
COVID-19: A rapid systematic review, meta-analysis, and comparison
with other inflammatory syndromes. Lancet Respir Med. 8:1233–1244.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldstein BI, Kemp DE, Soczynska JK and
McIntyre RS: Inflammation and the phenomenology, pathophysiology,
comorbidity, and treatment of bipolar disorder: A systematic review
of the literature. J Clin Psychiatry. 70:1078–1090. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Farooq RK, Asghar K, Kanwal S and
Zulqernain A: Role of inflammatory cytokines in depression: Focus
on interleukin-1β. Biomed Rep. 6:15–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Himmerich H, Patsalos O, Lichtblau N,
Ibrahim MAA and Dalton B: Cytokine research in depression:
Principles, challenges, and open questions. Front Psychiatry.
10:302019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Momtazmanesh S, Zare-Shahabadi A and
Rezaei N: Cytokine alterations in schizophrenia: An updated review.
Front Psychiatry. 10:8922019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Lu H, Zeng H, Zhang S, Du Q,
Jiang T and Du B: The differential psychological distress of
populations affected by the COVID-19 pandemic. Brain Behav Immun.
87:49–50. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stienen MN, Haghikia A, Dambach H, Thöne
J, Wiemann M, Gold R, Chan A, Dermietzel R, Faustmann PM, Hinkerohe
D and Prochnow N: Anti-inflammatory effects of the anticonvulsant
drug levetiracetam on electrophysiological properties of astroglia
are mediated via TGFβ1 regulation. Br J Pharmacol. 162:491–507.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoşgörler F, Keleş D,
Tanrıverdi-Akhisaroğlu S, İnanç Ş, Akhisaroğlu M, Cankurt Ü,
Aydoğdu Z, Uçar AD, Çetinayak O, Oktay G and Arda SG:
Anti-inflammatory and Anti-apoptotic effect of valproic acid and
doxycycline independent from MMP inhibition in early radiation
damage. Balkan Med J. 33:488–495. 2013. View Article : Google Scholar
|
|
23
|
Al-Amin MM, Nasir Uddin MM and Mahmud Reza
H: Effects of antipsychotics on the inflammatory response system of
patients with schizophrenia in peripheral blood mononuclear cell
cultures. Clin Psychopharmacol Neurosci. 11:144–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fourrier C, Sampson E, Mills NT and Baune
BT: Anti-inflammatory treatment of depression: Study protocol for a
randomised controlled trial of vortioxetine augmented with
celecoxib or placebo. Trials. 19:4472018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho M, Lee TY, Kwak YB, Yoon YB, Kim M and
Kwon JS: Adjunctive use of anti-inflammatory drugs for
schizophrenia: A meta-analytic investigation of randomized
controlled trials. Aust N Z J Psychiatry. 53:742–759. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
May M, Slitzky M, Rostama B, Barlow D and
Houseknecht KL: Antipsychotic-induced immune dysfunction: A
consideration for COVID-19 risk. Brain Behav Immun Health.
6:1000972020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Govind R, Fonseca de Freitas D, Pritchard
M, Hayes RD and MacCabe JH: Clozapine treatment and risk of
COVID-19 infection: Retrospective cohort study. Br J Psychiatry.
27:1–7. 2020.PubMed/NCBI
|
|
28
|
Gurrera RJ and Perry NL:
Clozapine-associated aspiration pneumonia: Case series and review
of the literature: Reply. Psychosomatics. 60:1032019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ponsford M, Castle D, Tahir T, Robinson R,
Wade W, Steven R, Bramhall K, Moody M, Carne E, Ford C, et al:
Clozapine is associated with secondary antibody deficiency. Br J
Psychiatry. 214:1–7. 2018.PubMed/NCBI
|
|
30
|
Vancampfort D, Probst M, Daenen A, Damme
TV, De Hert M, Rosenbaum S and Bruyninckx D: Impact of
antipsychotic medication on physical activity and physical fitness
in adolescents: An exploratory study. Psychiatry Res. 242:192–197.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perez-Cruzado D, Cuesta-Vargas A,
Vera-Garcia E and Mayoral-Cleries F: Medication and physical
activity and physical fitness in severe mental illness. Psychiatry
Res. 267:19–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brailovskaia J, Cosci F, Mansueto G,
Miragall M, Herrero R, Baños RM, Krasavtseva Y, Kochetkov Y and
Margraf J: The association between depression symptoms,
psychological burden caused by Covid-19 and physical activity: An
investigation in Germany, Italy, Russia, and Spain. Psychiatry Res.
295:1135962021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Phillips C: Brain-Derived neurotrophic
factor, depression, and physical activity: Making the neuroplastic
connection. Neural Plast. 2017:72601302017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Köhler-Forsberg O, N Lydholm C, Hjorthøj
C, Nordentoft M, Mors O and Benros ME: Efficacy of
anti-inflammatory treatment on major depressive disorder or
depressive symptoms: Meta-analysis of clinical trials. Acta
Psychiatr Scand. 139:404–419. 2019. View Article : Google Scholar
|
|
35
|
Lenze EJ, Mattar C, Zorumski CF, Stevens
A, Schweiger J, Nicol GE, Miller JP, Yang L, Yingling M, Avidan MS
and Reiersen AM: Fluvoxamine vs placebo and clinical deterioration
in outpatients with symptomatic COVID-19: A randomized clinical
trial. JAMA. 324:2292–2300. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ishima T, Fujita Y and Hashimoto K:
Interaction of new antidepressants with sigma-1 receptor chaperones
and their potentiation of neurite outgrowth in PC12 cells. Eur J
Pharmac. 727:167–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Homolak J and Kodvanj I: Widely available
lysosome targeting agents should be considered as potential therapy
for COVID-19. Int J Antimicrob Agents. 56:1060442020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nadkarni S and Devinsky O: Psychotropic
effects of antiepileptic drugs. Epilepsy Curr. 5:176–181. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grunze HC: The effectiveness of
anticonvulsants in psychiatric disorders. Dialogues Clin Neurosci.
10:77–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aranow C: Vitamin D and the immune system.
J Investig Med. 59:881–886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Menon B and Harinarayan CV: The effect of
anti epileptic drug therapy on serum 25-hydroxyvitamin D and
parameters of calcium and bone metabolism-a longitudinal study.
Seizure. 19:153–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Teagarden DL, Meador KJ and Loring DW: Low
vitamin D levels are common in patients with epilepsy. Epilepsy
Res. 108:1352–1356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chaudhuri JR, Mridula KR, Rathnakishore C,
Balaraju B and Bandaru VS: Association of 25-Hydroxyvitamin D
Deficiency in pediatric epileptic patients. Iran J Child Neurol.
11:48–56. 2017.PubMed/NCBI
|
|
44
|
Mariani J, Giménez VMM, Bergam I, Tajer C,
Antonietti L, Inserra F, Ferder L and Manucha W: Association
between vitamin D deficiency and COVID-19 incidence, complications,
and mortality in 46 countries: An ecological study. Health Secur.
19:302–308. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jain A, Chaurasia R, Sengar NS, Singh M,
Mahor S and Narain S: Analysis of vitamin D level among
asymptomatic and critically ill COVID-19 patients and its
correlation with inflammatory markers. Sci Rep. 10:201912020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Entrenas Castillo M, Entrenas Costa LM,
Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R and
Quesada Gomez JM: ‘Effect of calcifediol treatment and best
available therapy versus best available therapy on intensive care
unit admission and mortality among patients hospitalized for
COVID-19: A pilot randomized clinical study’. J Steroid Biochem Mol
Biol. 203:1057512020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tan CW, Ho LP, Kalimuddin S, Cherng BPZ,
Teh YE, Thien SY, Wong HM, Tern PJW, Chandran M, Chay JWM, et al:
Cohort study to evaluate the effect of vitamin D, magnesium, and
vitamin B12 in combination on progression to severe
outcomes in older patients with coronavirus (COVID-19). Nutrition.
79-80:1110172020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jakovac H: COVID-19 and vitamin D-Is there
a link and an opportunity for intervention? Am J Physiol Endocrinol
Metab. 318:E5892020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ahmed F: A network-based analysis reveals
the mechanism underlying vitamin D in suppressing cytokine storm
and virus in SARS-CoV-2 infection. Front Immunol. 11:5904592020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Aygun H: Vitamin D can prevent COVID-19
infection-induced multiple organ damage. Naunyn Schmiedebergs Arch
Pharmacol. 393:1157–1160. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kumar R, Rathi H, Haq A, Wimalawansa SJ
and Sharma A: Putative roles of vitamin D in modulating immune
response and immunopathology associated with COVID-19. Virus Res.
292:1982352021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Verdoia M, Pergolini P, Nardin M, Rolla R,
Negro F, Kedhi E, Suryapranata H, Marcolongo M, Carriero A and De
Luca G; Novara Atherosclerosis Study Group (NAS), : Vitamin D
levels and platelet reactivity in diabetic patients receiving dual
antiplatelet therapy. Vascul Pharmacol. 120:1065642019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zabetakis I, Lordan R, Norton C and
Tsoupras A: COVID-19: The inflammation link and the role of
nutrition in potential mitigation. Nutrients. 12:14662020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
McCreadie RG; Scottish Schizophrenia
Lifestyle Group, : Diet, smoking and cardiovascular risk in people
with schizophrenia: Descriptive study. Br J Psychiatry.
183:534–539. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Henderson DC, Borba CP, Daley TB, Boxill
R, Nguyen DD, Culhane MA, Louie P, Cather C, Eden Evins A,
Freudenreich O, et al: Dietary intake profile of patients with
schizophrenia. Ann Clin Psychiatry. 18:99–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Amani R: Is dietary pattern of
schizophrenia patients different from healthy subjects? BMC
Psychiatry. 7:152007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dipasquale S, Pariante CM, Dazzan P,
Aguglia E, McGuire P and Mondelli V: The dietary pattern of
patients with schizophrenia: A systematic review. J Psychiatr Res.
47:197–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Firth J, Stubbs B, Teasdale SB, Ward PB,
Veronese N, Shivappa N, Hebert JR, Berk M, Yung AR and Sarris J:
Diet as a hot topic in psychiatry: A population-scale study of
nutritional intake and inflammatory potential in severe mental
illness. World Psychiatry. 17:365–367. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Teasdale SB, Ward PB, Samaras K, Firth J,
Stubbs B, Tripodi E and Burrows TL: Dietary intake of people with
severe mental illness: Systematic review and meta-analysis. Br J
Psychiatry. 214:251–259. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Milanski M, Degasperi G, Coope A, Morari
J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK,
et al: Saturated fatty acids produce an inflammatory response
predominantly through the activation of TLR4 signaling in
hypothalamus: Implications for the pathogenesis of obesity. J
Neurosci. 29:359–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Patterson E, Wall R, Fitzgerald GF, Ross
RP and Stanton C: Health implications of high dietary omega-6
polyunsaturated Fatty acids. J Nutr Metab. 2012:5394262012.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jump DB, Botolin D, Wang Y, Xu J and
Christian B: Fatty acids and gene transcription. Food Nutr Res. 50
(Suppl 2):5–12. 2006. View Article : Google Scholar
|
|
63
|
Stienstra R, Duval C, Müller M and Kersten
S: PPARs, obesity, and inflammation. PPAR Res. 2007:959742007.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Muralikumar S, Vetrivel U, Narayanasamy A
and N Das U: Probing the intermolecular interactions of PPARγ-LBD
with polyunsaturated fatty acids and their anti-inflammatory
metabolites to infer most potential binding moieties. Lipids Health
Dis. 16:172017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Choi GJ, Kim HM and Kang H: The potential
role of dyslipidemia in COVID-19 severity: An umbrella review of
systematic reviews. J Lipid Atheroscler. 9:435–448. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Perona JS: Membrane lipid alterations in
the metabolic syndrome and the role of dietary oils. Biochim
Biophys Acta Biomembr. 1859 (9 Pt B). 1690–1703. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Oruch R, Lund A, Pryme IF and Holmsen H:
An intercalation mechanism as a mode of action exerted by
psychotropic drugs: Results of altered phospholipid substrate
availabilities in membranes? J Chem Biol. 3:67–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Leguisamo NM, Lehnen AM, Machado UF,
Okamoto MM, Markoski MM, Pinto GH and Schaan BD: GLUT4 content
decreases along with insulin resistance and high levels of
inflammatory markers in rats with metabolic syndrome. Cardiovasc
Diabetol. 11:1002012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bousquet J, Anto JM, Iaccarino G,
Czarlewski W, Haahtela T, Anto A, Akdis CA, Blain H, Canonica GW,
Cardona V, et al: Is diet partly responsible for differences in
COVID-19 death rates between and within countries? Clin Transl
Allergy. 10:162020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jakovac H: COVID-19: Is the ACE2 just a
foe? Am J Physiol Lung Cell Mol Physiol. 318:L1025–L1026. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Warner FJ, Rajapaksha H, Shackel N and
Herath CB: ACE2: From protection of liver disease to propagation of
COVID-19. Clin Sci (Lond). 134:3137–3158. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gohil K, Samson R, Dastager S and Dharne
M: Probiotics in the prophylaxis of COVID-19: Something is better
than nothing. 3 Biotech. 11:12021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Karl JP: Gut Microbiota-targeted
interventions for reducing the incidence, duration, and severity of
respiratory tract infections in healthy non-elderly adults. Mil
Med. 186:e310–e318. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hamida RS, Shami A, Ali MA, Almohawes ZN,
Mohammed AE and Bin-Meferij MM: Kefir: A protective dietary
supplementation against viral infection. Biomed Pharmacother.
133:1109742021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Abdulah DM and Hassan AB: Relation of
dietary factors with infection and mortality rates of COVID-19
across the World. J Nutr Health Aging. 24:1011–1018. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Falagas ME and Kompoti M: Obesity and
infection. Lancet Infect Dis. 6:438–446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Karlsson EA and Beck MA: The burden of
obesity on infectious disease. Exp Biol Med (Maywood).
235:1412–1424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li P, Yin YL, Li D, Kim SW and Wu G: Amino
acids and immune function. Br J Nutr. 98:237–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jenkins DJA, Srichaikul KK, Kendall CWC
and Sievenpiper JL: Bean, fruit, and vegetable fiber, but not
cereal fiber are associated with reduced mortality in Japan. Am J
Clin Nutr. 111:941–943. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gill SK, Rossi M, Bajka B and Whelan K:
Dietary fibre in gastrointestinal health and disease. Nat Rev
Gastroenterol Hepatol. 18:101–116. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yao T, Chen MH and Lindemann SR:
Structurally complex carbohydrates maintain diversity in
gut-derived microbial consortia under high dilution pressure. FEMS
Microbiol Ecol. 96:fiaa1582020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Vanable PA, Carey MP, Carey KB and Maisto
SA: Smoking among psychiatric outpatients: Relationship to
substance use, diagnosis, and illness severity. Psychol Addict
Behav. 17:259–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
de Leon J and Diaz FJ: A meta-analysis of
worldwide studies demonstrates an association between schizophrenia
and tobacco smoking behaviors. Schizophr Res. 76:135–157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gilbody S, Peckham E, Bailey D, Arundel C,
Heron P, Crosland S, Fairhurst C, Hewitt C and Li J; members of the
SCIMITAR+ collaborative, : Smoking cessation in severe mental
illness: Combined long-term quit rates from the UK SCIMITAR trials
programme. Br J Psychiatry. 18:95–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Thomson D, Berk M, Dodd S, Rapado-Castro
M, Quirk SE, Ellegaard PK, Berk L and Dean OM: Tobacco use in
bipolar disorder. Clin Psychopharmacol Neurosci. 13:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li XH, An FR, Ungvari GS, Ng CH, Chiu HFK,
Wu PP, Jin X and Xiang YT: Prevalence of smoking in patients with
bipolar disorder, major depressive disorder and schizophrenia and
their relationships with quality of life. Sci Rep. 7:84302017.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Arnson Y, Shoenfeld Y and Amital H:
Effects of tobacco smoke on immunity, inflammation and
autoimmunity. J Autoimmun. 34:J258–J265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lee J, Taneja V and Vassallo R: Cigarette
smoking and inflammation: Cellular and molecular mechanisms. J Dent
Res. 91:142–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cattaruzza MS, Zagà V, Gallus S, D'Argenio
P and Gorini G: Tobacco smoking and COVID-19 pandemic: Old and new
issues. A summary of the evidence from the scientific literature.
Acta Biomed. 91:106–112. 2020.PubMed/NCBI
|
|
91
|
Polverino F: Cigarette smoking and
COVID-19: A complex interaction. Am J Respir Crit Care Med.
202:471–472. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Reddy RK, Charles WN, Sklavounos A, Dutt
A, Seed PT and Khajuria A: The effect of smoking on COVID-19
severity: A systematic review and meta-analysis. J Med Virol.
93:1045–1056. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lombardi C, Roca E, Ventura L and Cottini
M: Smoking and COVID-19, the paradox to discover: An Italian
retrospective, observational study in hospitalized and
non-hospitalized patients. Med Hypotheses. 146:1103912021.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Usman MS, Siddiqi TJ, Khan MS, Patel UK,
Shahid I, Ahmed J, Kalra A and Michos ED: Is there a smoker's
paradox in COVID-19? BMJ Evid Based Med. Aug 11–2020.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Edmonston DL, South AM, Sparks MA and
Cohen JB: Coronavirus disease 2019 and hypertension: The role of
angiotensin-converting enzyme 2 and the renin-angiotensin system.
Adv Chronic Kidney Dis. 27:404–411. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Vleeming W, Rambali B and Opperhuizen A:
The role of nitric oxide in cigarette smoking and nicotine
addiction. Nicotine Tob Res. 4:341–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Akerström S, Mousavi-Jazi M, Klingström J,
Leijon M, Lundkvist A and Mirazimi A: Nitric oxide inhibits the
replication cycle of severe acute respiratory syndrome coronavirus.
J Virol. 79:1966–1969. 2005. View Article : Google Scholar
|
|
98
|
Czuczwar M, Kiś J, Czuczwar P, Wielosz M
and Turski W: Nicotine diminishes anticonvulsant activity of
antiepileptic drugs in mice. Pol J Pharmacol. 55:799–802.
2003.PubMed/NCBI
|
|
99
|
Reinsberger C, Dorn T and Krämer G:
Smoking reduces serum levels of lamotrigine. Seizure. 17:651–653.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tsuda Y, Saruwatari J and Yasui-Furukori
N: Meta-analysis: The effects of smoking on the disposition of two
commonly used antipsychotic agents, olanzapine and clozapine. BMJ
Open. 4:e0042162014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Oliveira P, Ribeiro J, Donato H and
Madeira N: Smoking and antidepressants pharmacokinetics: A
systematic review. Ann Gen Psychiatry. 16:172017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bobes J, Arango C, Garcia-Garcia M and
Rejas J: Healthy lifestyle habits and 10-year cardiovascular risk
in schizophrenia spectrum disorders: An analysis of the impact of
smoking tobacco in the CLAMORS schizophrenia cohort. Schizophr Res.
119:101–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chao AM, White MA, Grilo CM and Sinha R:
Examining the effects of cigarette smoking on food cravings and
intake, depressive symptoms, and stress. Eat Behav. 24:61–65. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Alsuwaidan MT, Kucyi A, Law CW and
McIntyre RS: Exercise and bipolar disorder: A review of
neurobiological mediators. Neuromolecular Med. 11:328–336. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sylvia LG, Ametrano RM and Nierenberg AA:
Exercise treatment for bipolar disorder: Potential mechanisms of
action mediated through increased neurogenesis and decreased
allostatic load. Psychother Psychosom. 79:87–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kay-Lambkin FJ, Thornton L, Lappin JM,
Hanstock T, Sylvia L, Jacka F, Baker AL, Berk M, Mitchell PB,
Callister R, et al: Study protocol for a systematic review of
evidence for lifestyle interventions targeting smoking, sleep,
alcohol/other drug use, physical activity, and healthy diet in
people with bipolar disorder. Syst Rev. 5:1062016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Cuomo A, Giordano N, Goracci A and
Fagiolini A: Depression and vitamin D deficiency: Causality,
assessment, and clinical practice implications. Neuropsychiatry
(London). 7:606–614. 2017.
|
|
108
|
Cuomo A, Maina G, Bolognesi S, Rosso G,
Beccarini Crescenzi B, Zanobini F, Goracci A, Facchi E, Favaretto
E, Baldini I, et al: Prevalence and correlates of vitamin D
deficiency in a sample of 290 inpatients with mental Illness. Front
Psychiatry. 10:1672019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hansdottir S, Monick MM, Lovan N, Powers
LS and Hunninghake GW: Smoking disrupts vitamin D metabolism in the
lungs. Am J Respir Crit Care Med. 181:A14252010.
|
|
110
|
Lange NE, Sparrow D, Vokonas P and
Litonjua AA: Vitamin D deficiency, smoking, and lung function in
the normative aging study. Am J Respir Crit Care Med. 186:616–621.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Uh ST, Koo SM, Kim YK, Kim KU, Park SW,
Jang AS, Kim DJ, Kim YH and Park CS: Inhibition of vitamin D
receptor translocation by cigarette smoking extracts. Tuberc Respir
Dis (Seoul). 73:258–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gejman PV, Sanders AR and Duan J: The role
of genetics in the etiology of schizophrenia. Psychiatr Clin North
Am. 33:35–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kerner B: Genetics of bipolar disorder.
Appl Clin Genet. 7:33–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shadrina M, Bondarenko EA and Slominsky
PA: Genetics factors in major depression disease. Front Psychiatry.
9:3342018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kaser A: Genetic risk of severe covid-19.
N Engl J Med. 383:1590–1591. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Pairo-Castineira E, Clohisey S, Klaric L,
Bretherick AD, Rawlik K, Pasko D, Walker S, Parkinson N, Fourman
MH, Russell CD, et al: Genetic mechanisms of critical illness in
Covid-19. Nature. 591:92–98. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zeberg H and Pääbo S: The major genetic
risk factor for severe COVID-19 is inherited from Neanderthals.
Nature. 587:610–612. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Rigat B, Hubert C, Alhenc-Gelas F, Cambien
F, Corvol P and Soubrier F: An insertion/deletion polymorphism in
the angiotensin I-converting enzyme gene accounting for half the
variance of serum enzyme levels. J Clin Invest. 86:1343–1346. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wu Y, Wang X, Shen X, Tan Z and Yuan Y:
The I/D polymorphism of angiotensin-converting enzyme gene in major
depressive disorder and therapeutic outcome: A case-control study
and meta-analysis. J Affect Disord. 136:971–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Kucukali CI, Aydin M, Ozkok E, Bilge E,
Zengin A, Cakir U and Kara I: Angiotensin-converting enzyme
polymorphism in schizophrenia, bipolar disorders, and their
first-degree relatives. Psychiatr Genet. 20:14–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Mazaheri H and Saadat M: Association
between insertion/deletion polymorphism in angiotension converting
enzyme and susceptibility to schizophrenia. Iran J Public Health.
44:369–373. 2015.PubMed/NCBI
|
|
122
|
Crescenti A, Gassó P, Mas S, Abellana R,
Deulofeu R, Parellada E, Bernardo M and Lafuente A:
Insertion/deletion polymorphism of the angiotensin-converting
enzyme gene is associated with schizophrenia in a Spanish
population. Psychiatry Res. 165:175–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Song GG and Lee YH: The insertion/deletion
polymorphism in the angiotensin-converting enzyme and
susceptibility to schizophrenia or Parkinson's disease: A
meta-analysis. J Renin Angiotensin Aldosterone Syst. 16:434–442.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Nadalin S, Buretić-Tomljanović A, Rubeša
G, Jonovska S, Tomljanović D and Ristić S: Angiotensin-converting
enzyme gene insertion/deletion polymorphism is not associated with
schizophrenia in a Croatian population. Psychiatr Genet.
22:267–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Hui L, Wu JQ, Zhang X, Lv J, Du WL, Kou
CG, Yu YQ, Lv MH, Chen DC and Zhang XY: Association between the
angiotensin-converting enzyme gene insertion/deletion polymorphism
and first-episode patients with schizophrenia in a Chinese Han
population. Hum Psychopharmacol. 29:274–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hui L, Wu JQ, Ye MJ, Zheng K, He JC, Zhang
X, Liu JH, Tian HJ, Gong BH, Chen DC, et al: Association of
angiotensin-converting enzyme gene polymorphism with schizophrenia
and depressive symptom severity in a Chinese population. Hum
Psychopharmacol. 30:100–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Baghai TC, Schule C, Zwanzger P, Minov C,
Zill P, Ella R, Eser D, Oezer S, Bondy B and Rupprecht R:
Hypothalamic-pituitary-adrenocortical axis dysregulation in
patients with major depression is influenced by the
insertion/deletion polymorphism in the angiotensin I-converting
enzyme gene. Neurosci Lett. 328:299–303. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bahramali E, Firouzabadi N, Yavarian I,
Shayesteh MR, Erfani N, Shoushtari AA and Asadpour R: Influence of
ACE gene on differential response to sertraline versus fluoxetine
in patients with major depression: A randomized controlled trial.
Eur J Clin Pharmacol. 72:1059–1064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Delanghe JR, Speeckaert MM and De Buyzere
M: The host's angiotensin-converting enzyme polymorphism may
explain epidemiological findings in COVID-19 infections. Clin Chim
Acta. 505:192–193. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Delanghe JR, Speeckaert MM and De Buyzere
ML: COVID-19 infections are also affected by human ACE1 D/I
polymorphism. Clin Chem Lab Med. 58:1125–1126. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zajc Petranović M, Skarić-Jurić T, Smolej
Narančić N, Tomas Z, Krajačić P, Miličić J, Barbalić M and
Tomek-Roksandić S: Angiotensin-converting enzyme deletion allele is
beneficial for the longevity of Europeans. Age (Dordr). 34:583–595.
2012. View Article : Google Scholar
|
|
132
|
Bellone M and Calvisi SL: ACE
polymorphisms and COVID-19-related mortality in Europe. J Mol Med
(Berl). 98:1505–1509. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Hatami N, Ahi S, Sadeghinikoo A,
Foroughian M, Javdani F, Kalani N, Fereydoni M, Keshavarz P and
Hosseini A: Worldwide ACE (I/D) polymorphism may affect COVID-19
recovery rate: An ecological meta-regression. Endocrine.
68:479–484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Jakovac H: COVID-19 and hypertension: Is
the HSP60 culprit for the severe course and worse outcome? Am J
Physiol Heart Circ Physiol. 319:H793–H796. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Meng J, Xiao G, Zhang J, He X, Ou M, Bi J,
Yang R, Di W, Wang Z, Li Z, et al: Renin-angiotensin system
inhibitors improve the clinical outcomes of COVID-19 patients with
hypertension. Emerg Microbes Infect. 9:757–760. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Pirola CJ and Sookoian S: Estimation of
Renin-Angiotensin-Aldosterone-System (RAAS)-Inhibitor effect on
COVID-19 outcome: A Meta-analysis. J Infect. 81:276–281. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhong Y, Zhao L, Wu G, Hu C, Wu C, Xu M,
Dong H, Zhang Q, Wang G, Yu B, et al: Impact of renin-angiotensin
system inhibitors use on mortality in severe COVID-19 patients with
hypertension: A retrospective observational study. J Int Med Res.
48:3000605209791512020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Cetinkal G, Kocas BB, Ser OS, Kilci H,
Yildiz SS, Ozcan SN, Verdi Y, Altinay M and Kilickesmez K: The
association between chronic use of renin-angiotensin-aldosterone
system blockers and in-hospital adverse events among covid-19
patients with hypertension. Sisli Etfal Hastan Tip Bul. 54:399–404.
2020.PubMed/NCBI
|
|
139
|
Lee MMY, Docherty KF, Sattar N, Mehta N,
Kalra A, Nowacki AS, Solomon SD, Vaduganathan M, Petrie MC, Jhund
PS and McMurray JJV: Renin-angiotensin system blockers, risk of
SARS-CoV-2 infection and outcomes from CoViD-19: Systematic review
and meta-analysis. Eur Heart J Cardiovasc Pharmacother pvaa138.
2020.(Epub ahead of prin). View Article : Google Scholar
|
|
140
|
Niederhofer H: Angiotensin converting
enzyme inhibitors/estrogen/cortisol: Maybe an additional option in
the treatment of psychiatrically disordered patients? Med
Hypotheses. 70:703–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Gadelha A, Vendramini AM, Yonamine CM,
Nering M, Berberian A, Suiama MA, Oliveira V, Lima-Landman MT,
Breen G, Bressan RA, et al: Convergent evidences from human and
animal studies implicate angiotensin I-converting enzyme activity
in cognitive performance in schizophrenia. Transl Psychiatry.
5:e6912015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wincewicz D and Braszko JJ: Validation of
brain angiotensin system blockade as a novel drug target in
pharmacological treatment of neuropsychiatric disorders.
Pharmacopsychiatry. 50:233–247. 2017. View Article : Google Scholar : PubMed/NCBI
|