Introduction
Rheumatoid arthritis (RA) is a chronic disease
characterized by persistent synovitis involving multiple joints,
systemic inflammation and autoantibodies, especially rheumatoid
factor (RF) and anti-cyclic citrullinated peptide (anti-CCP), that
can eventually lead to cartilage, bone damage and disability
(1). A study from the United
Kingdom shows that RA is more prevalent in women than in men
(2). Studies have identified that
genetic factors, environmental factors and lifestyle are high-risk
factors for RA, such as smoking, drinking and poor diet (3,4).
However, the clear pathogenesis remains to be elucidated. Early
diagnosis of RA is critical for the active treatment of
irreversible damage to joints or organs and the control of chronic
inflammation through the widespread use of conventional drugs and
disease-modifying anti-rheumatic drugs (5). However, some RA patients whose RF and
anti-CCP are both negative could be misdiagnosed and the belated
treatment may cause deterioration of clinical outcome (6). Therefore, it is necessary to discover
novel biomarkers to improve the diagnosis and prognosis of RA
patients.
Circular RNA (circRNA) was originally found in
pathogens and characterized by single-stranded covalent closure and
has been considered an aberrant splicing byproduct of endogenous
RNA (7,8). circRNAs with unique ‘head-to-tail’
splice junctions possess the characteristics of wide expression,
high conservation and stability and tissue- or cell-specific
expression, which determine their important noncoding functions
(9,10). A number of biological functions of
circRNAs have been demonstrated to act as microRNAs (miRNA) sponges
(11), interact with a number of
different RBPs to act as protein sponges to enhance protein
function or recruit proteins to specific sites (12,13)
and regulate the transcription of maternal genes (14). In addition, circRNAs can perform
cap-independent translation (15,16).
Based on the above characteristics, circRNA has been verified to be
involved in the development and progression of a number of types of
cancer, such as cervical and gastric cancer (17,18) or
participating in the regulation of autoimmune disease (19,20).
In our previous study, circPTPN22 was screened as a
potential marker in the peripheral blood mononuclear cells (PBMCs)
of systemic lupus erythematosus (SLE) patients by high-throughput
RNA sequencing (21). The parental
gene protein tyrosine phosphatase nonreceptor 22 (PTPN22) of
circPTPN22, located on chromosome 1P13, is a member of the protein
tyrosine phosphatase family, encoding lymphoid protein tyrosine
phosphatase (22). It is well known
that PTPN22 is closely related to the occurrence and development of
autoimmune diseases, such as RA, SLE and type 1 diabetes, by
modulating signaling pathways through antigen and innate immune
receptors. Also, it serves roles in lymphocyte development and
activation, establishment of tolerance and innate immune cell
mediated host defense and immunoregulation (23,24).
Though circPTPN22 was demonstrated to be a potential biomarker for
SLE diagnosis in our previous study (21), whether circPTPN22 also presents a
diagnostic value in RA patients remains to be elucidated.
To this end, the present study detected the
differentially expressed circRNAs in PBMCs of RA patients and HC
controls with high-throughput RNA sequencing and found that the
differentially expressed circRNAs in RA patients indeed included
circPTPN22. Further experiments verified the downregulated
circPTPN22 expression in RA patients and the statistical analysis
revealed the possibility of circPTPN22 as a diagnostic biomarker to
distinguish RA patients from HC controls and even from SLE
patients. In addition, the expression level of circPTPN22 was
markedly related to the classification of RA and the levels of
autoantibodies in RA patients.
Materials and methods
Patients
A total of 46 patients with RA aged 28–76 years old
were recruited from the First Affiliated Hospital of Third Military
Medical University (Chongqing, China) between March 2019 and
November 2020. RA disease activity was defined by the disease
activity score 28 (DAS28) (25). In
addition, the present study included 47 healthy controls (HCs) aged
27–76 years, who were free from autoimmune or inflammatory diseases
and unrelated to the patients, after receiving a medical
examination at the First Affiliated Hospital of Third Military
Medical University. As an autoimmune disease control, 45 SLE
patients diagnosed by the American College of Rheumatology revised
criteria (26) were also enrolled
from the same hospital at the research stage. The clinical
characteristics of the participants in the validation phase are
presented in Table I. The present
study was approved and supervised by the ethical committee of The
First Affiliated Hospital of Third Military Medical University
(Hefei, China; approval no. KY2019119). Written informed consent
was obtained from all subjects prior to participation.
 | Table I.Clinical characteristics of the study
population in the validation phase. |
Table I.
Clinical characteristics of the study
population in the validation phase.
| Index | RA | Health control | SLE |
|---|
| Sex
(male/female) | 4/38 | 5/39 | 5/40 |
| Age (years) | 56.47±12.07 | 54.57±14.27 | 53±9.87 |
| Duration
(years) | 15.10±10.67 | N/A | N/A |
| TNF-α (ng/l) | 14.96±6.14 | N/A | N/A |
| IL-6 (pg/ml) | 21.21±19.17 | N/A | N/A |
| C3 (g/l) | 1.07±0.20 | N/A | N/A |
| C4 (g/l) | 0.21±0.07 | N/A | N/A |
| RA-IgG (IU/ml) | 201.103±91.77 | N/A | N/A |
| RA-IgM (IU/ml) | 289.58±166.40 | N/A | N/A |
| RA-IgA (IU/ml) | 327.21±112.02 | N/A | N/A |
| IgA (g/l) | 4.59±3.88 | N/A | N/A |
| IgG (g/l) | 14.01±4.84 | N/A | N/A |
| IgM (g/l) | 2.02±0.71 | N/A | N/A |
| IgE (g/l) | 37.515±19.49 | N/A | N/A |
| RF (IU/ml) | 530.827±378.19 | N/A | N/A |
| Anti-CCP
(U/ml) | 477.97±207.23 | N/A | N/A |
| TJC | 13.96±8.05 | N/A | N/A |
| SJC | 10.12±6.72 | N/A | N/A |
| ESR (mm/h) | 68.08±27.91 | N/A | N/A |
| CRP (mg/l) | 51.27±29.36 | N/A | N/A |
| DAS28 score | 5.59±1.39 | N/A | N/A |
| SLEDAI | N/A | N/A | 9.64±4.66 |
PBMC preparation and total RNA
extraction
Whole blood (~6 ml) was obtained from each subject
before the experiment and stored in an ethylenediaminetetraacetic
acid anticoagulant tube. Fresh PBMCs from each donor blood were
isolated by Ficoll-Hypaque (Beijing Solarbio Science &
Technology Co., Ltd.) density-gradient centrifugation (6,500 × g
for 20 min) at room temperature. Then, total RNA was extracted by
using TRIzol (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Subsequently, RNA concentration and purity
were measured by a NanoDrop spectrophotometer (Invitrogen; Thermo
Fisher Scientific, Inc.) and 1% agarose gel. RNA was stored in
−80°C for further experiments.
High-throughput RNA sequencing
RNA with RNA integrity number >8.0 was used for
the rRNA depletion library (VAHTSTM Total RNA-seq (H/M/R); Agilent
Technologies, Inc.) according to the manufacturer's instructions.
Whole transcriptome sequencing was carried out by NovelBio Corp.
Laboratory using a Hiseq XTEN Sequencer (Illumina, Inc.). Prior to
read mapping, clean reads were obtained from the raw reads by
removing the adaptor sequences (reads with >5% ambiguous bases
(noted as N) and low-quality reads containing >20% bases with
qualities of <13). Then, the clean reads were aligned to the
human reference genome sequence GRCh38 (NCBI) using the MapSplice
program (netlab.uky.edu/p/bioinfo/MapSplice) V2.1.6 (27). The RNA-seq data have been submitted
to the NCBI Gene Expression Omnibus (GEO), under GEO accession
number GSE169080 and GSE169082 (ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169080 and
ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169082).
Computational bioinformatics
analysis
HTseq (huber.embl.de/HTSeq; V2.05) was used to count genes
and the reads per kilo base per million mapped reads method was
used to normalize the gene expression level (28). To identify differentially expressed
genes, the DEseq (29) algorithm
was used to identify the differentially expressed genes under the
criteria of fold change >2 and false discovery rate <0.05.
Gene Ontology (GO; http://www.geneontology.org/) was used to clarify the
biological significance of differentially expressed genes and Kyoto
Encyclopedia of Genes and Genomes (KEGG; www.genome.jp/kegg/) enrichment analysis and gene set
enrichment analysis (GSEA) were performed to identify important
pathways.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA from PBMCs was reverse-transcribed into
cDNA using a Prime-Script RT reagent kit with gDNA Eraser according
to the manufacturer's protocol (Takara Bio, Inc.). RT-qPCR was
performed using cDNA and SYBR Premix Ex Taq II (Takara
Biotechnology Co., Ltd.) using CFX96 Thermal Cycler equipment
(Bio-Rad Laboratories, Inc.) following the manufacturer's
instructions. The conditions were: 95°C for 30 sec, followed by 39
cycles of 95°C for 15 sec and 60°C for 30 sec. The primers used for
quantitative real-time PCR were as follows: circPTPN22: F:
5′-AATTCTCACCAAATGTTCCCA-3′, R: 5′-AAGGTACATCATGGTCTGGC-3′; GAPDH:
F: 5′-GGAGTCCACTGGCGTCTTC-3′, R: 5′-GCTGATGATCTTGAGGCTGTTG-3′. The
levels of GAPDH were used to normalize the relative expression
levels of circRNA.
The forward primers of microRNA and U6 used in the
present study were synthesized by Tiangen Biotech Co., Ltd. and U6
small nuclear RNA was used as an endogenous control for miRNA
normalization. The universal reverse primer was provided in the kit
(Tiangen Biotech Co., Ltd.). Then, Ct values were obtained and
calculated them by the 2−∆∆Cq method (30) for each sample. Each sample was
repeated more than three times independently.
Competing endogenous (Ce)RNA
regulatory network construction and hub gene identification
RNA pairs were predicted using the databases of
RNAhybrid (bibiserv.techfak.uni-bielefeld.de/rnahybrid)
(31) and miRanda (http://www.microrna.org/microrna/home.do). Based on
the predicted binding sites of differentially expressed genes, a
coexpression network of circRNA-miRNA-mRNA was established using
Cytoscape 3.7.2 (32). By setting
the interaction confidence score at the highest level of 0.9, the
protein-protein interaction (PPI) network was constructed by the
STRING database (https://string-db.org/). Cytoscape was used to
visualize the PPI network and the hub genes were identified by the
11 topological analysis methods on the CytoHubba Plug-in (Degree
≥36) (33).
Statistical analysis
Fisher's exact test and χ2 test were
applied to identify the significant GO categories and pathway
analysis. One-way ANOVA and nonparametric tests, corrected for
multiple comparisons using Tukey's test, were used to evaluate the
expression of normally distributed parameters and skewed
distribution parameters among the three groups. In correlation
analysis, after testing by the one-sample Kolmogorov-Smirnov test,
the data were analyzed by Pearson correlation test in case of a
normal distribution among groups; otherwise, the data were analyzed
by Spearman rank correlation among groups. Receiver operating
characteristic (ROC) curves were used to analyze the sensitivity,
specificity and area under the curve (AUC) to indicate the
diagnostic value with the 95% CI. All the data were analyzed using
SPSS statistical software (version 26.0; IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression profiling of circRNA and
mRNA in PBMCs of patients with RA
To understand the expression profiles of circRNAs
and mRNAs in RA, RNA sequence analysis was applied to the PBMCs of
4 patients with RA and 3 healthy controls. A total of 25,646
circRNAs were detected by RNA-Seq and were widely distributed on
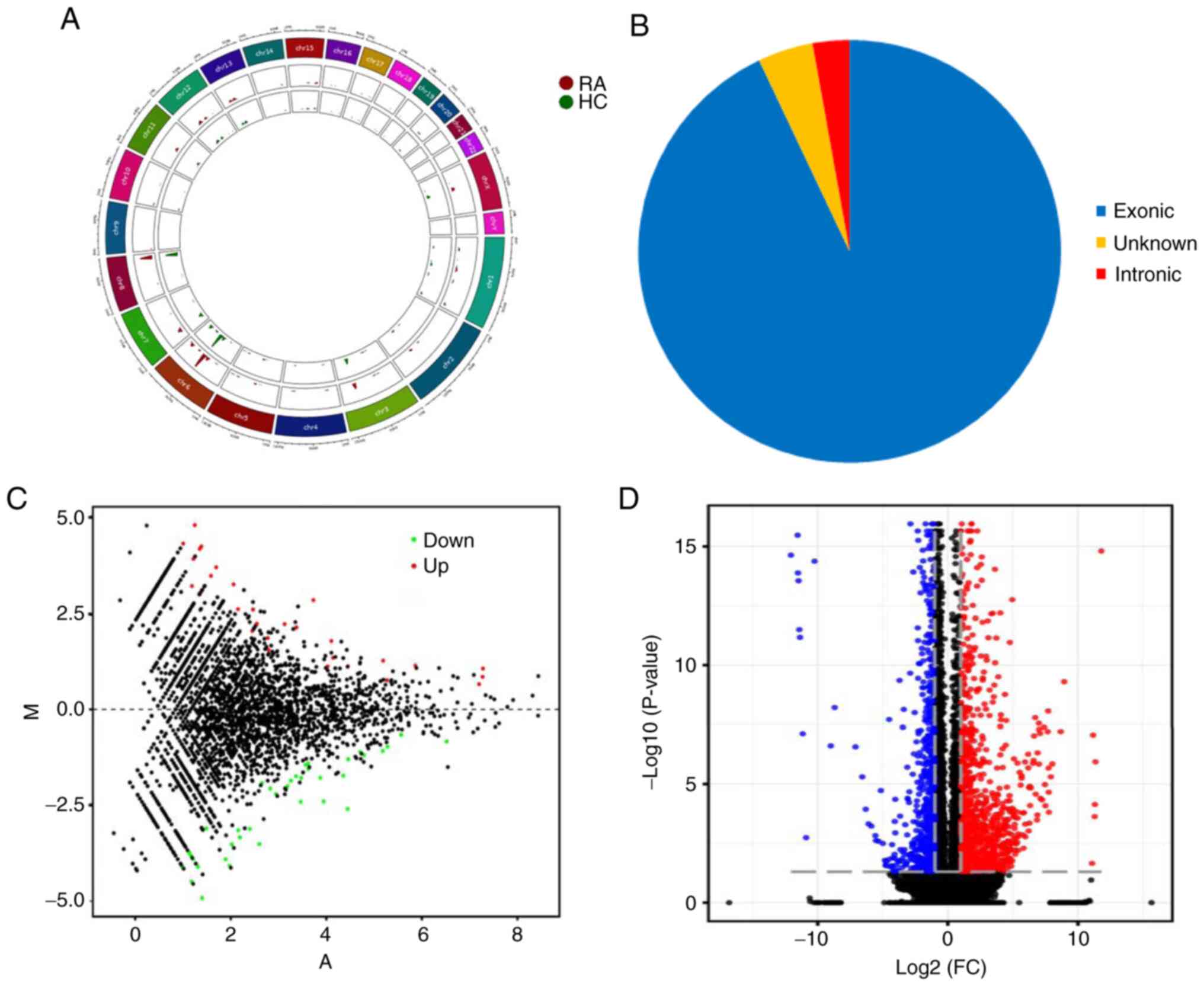
all chromosomes, including sex chromosomes X and Y (Fig. 1A). There were 71 differentially
expressed circRNAs between RA patients and HCs, of which 66
circRNAs were derived from exons (Fig.
1B). A violin plot demonstrated approximately equivalent
distributions of normalized intensities for all the datasets in the
tested subjects (Fig. S1).
Compared with healthy controls, 41 and 30 circRNAs were upregulated
and downregulated in RA patients, respectively. MA plots fully
demonstrate the relationship between gene abundance and the
significantly differentially expressed circRNAs with cutoff
criteria of fold change >2.0 and P<0.05 in PBMCs of RA
(Fig. 1C). In addition, 924 mRNAs
were differentially expressed using the same cutoff criteria,
including 597 upregulated and 327 downregulated mRNAs. A volcano
plot demonstrated the variance among differentially expressed genes
between RA and healthy controls (Fig.
1D).
Network construction of circRNA-miRNA
interactions and GO and KEGG pathway analysis for the
differentially expressed genes
To investigate the function of circRNAs in RA, the
present study predicted the differentially expressed miRNAs of
their potential role through specific base-paired and constructed
circRNA-miRNA networks of the top five up- and downregulated
circRNAs (Fig. 2A). Additionally,
the functions of differentially expressed mRNAs were investigated
using GO analyses, sequencing the top 20 GO terms according to the
P-value of enrichment in the biological process (BP), cellular
component (CC) and molecular function (MF) categories (Fig. 2B). In the BP category, enriched
terms included cytoplasmic mRNA processing body assembly,
regulation of NF-κB transcription factor activity and nuclear
transcription. The degree of MF was significantly enriched in
poly(A)-specific ribonuclease activity and syntaxin-1 binding. The
genes in the CC group were significantly enriched in the cytoplasm
and cytoplasmic side of the mitochondrial outer membrane. The
specific term information is shown in Table SI. Furthermore, gene set enrichment
analysis (GSEA) was conducted. These functional analyses therefore
identified relevant metabolic pathways that are important in the
development of autoimmune disease. The analysis results
demonstrated that the Toll-like receptor signaling pathway and MAPK
signaling pathway were the significantly enriched pathways
(Fig. 2C and D), which contained
the core enrichment genes for the related pathways, such as JUN,
NF-κB, TLR2 and NFκBIA.
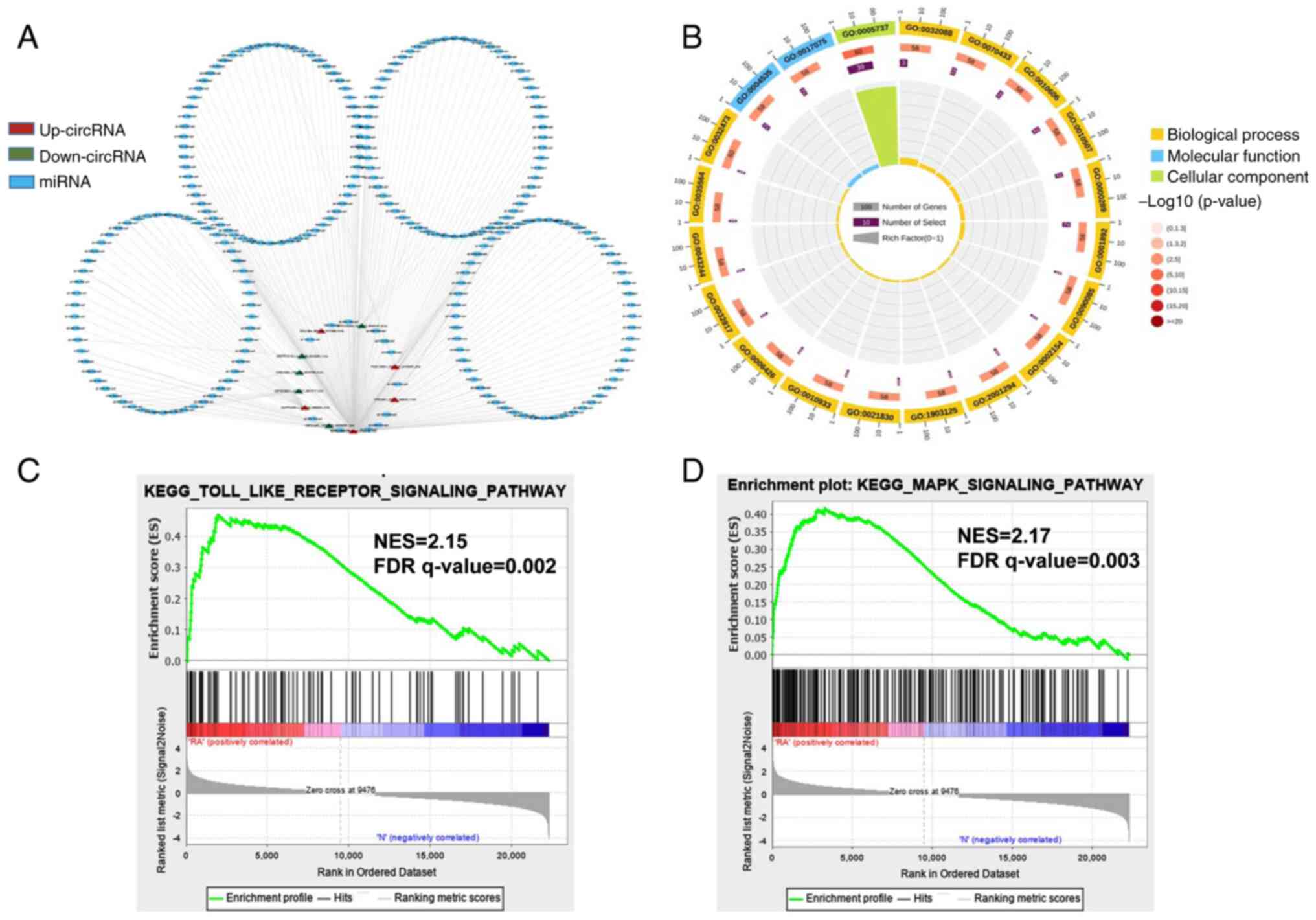 | Figure 2.Network construction of circRNA-miRNA
interactions and GO and KEGG pathway analysis for the
differentially expressed genes. (A) Construction of the
circRNA-miRNA regulatory network. The circRNA-miRNA regulatory
network included 10 circRNAs and 251 miRNAs (red, upregulated
circRNAs; green, downregulated circRNAs; blue, miRNA). (B) The top
20 significantly enriched GO annotations in BP, MF and CC placed in
three columns in the circle diagram. The outermost layer was a GO
ID and specific GO term information is shown in Table SI. The inner circles from outside
to inside corresponded to the number of genes sequenced by their
enrichment scores (−log10 of the P-values), number of
selected genes and rich factor, respectively. The coloured dots
represent the enriched P-values, yellow represents BP, blue
represents MF and green represents CC. Gene set enrichment analysis
was performed to determine the differential expression of mRNAs for
the (C) Toll-like receptor and (D) MAPK signaling pathways.
circRNA, circular RNA; miRNA, microRNA; GO, Gene Ontology; KEGG,
Kyoto Encyclopedia of Genes and Genomes; BP, biological process;
MF, molecular function; CC, cellular component; NES, Normalized
enrichment scores; FDR, false discovery rate. |
circPTPN22 is a common differentially
expressed circRNA among RA and SLE patients and acts as a potential
diagnostic biomarker for RA
To screen out circRNAs simultaneously associated
with both RA and SLE, clustering was used to identify common
differentially expressed circRNAs by referring to our previous
study that identified 128 aberrantly expressed circRNAs in SLE
patients compared with HCs (21).
Calculating the intersection of differentially expressed circRNAs
between RA patients and SLE patients compared with healthy
controls, 21 common differentially expressed circRNAs were
obtained. A Venn diagram of this clustering demonstrated that these
circRNAs consisted of 8 significantly upregulated and 13
downregulated circRNAs overlapping between the foregoing
comparisons (Fig. 3A). Notably,
circPTPN22 was included in the 13 downregulated circRNAs among RA
and SLE patients (detailed in Table
SII).
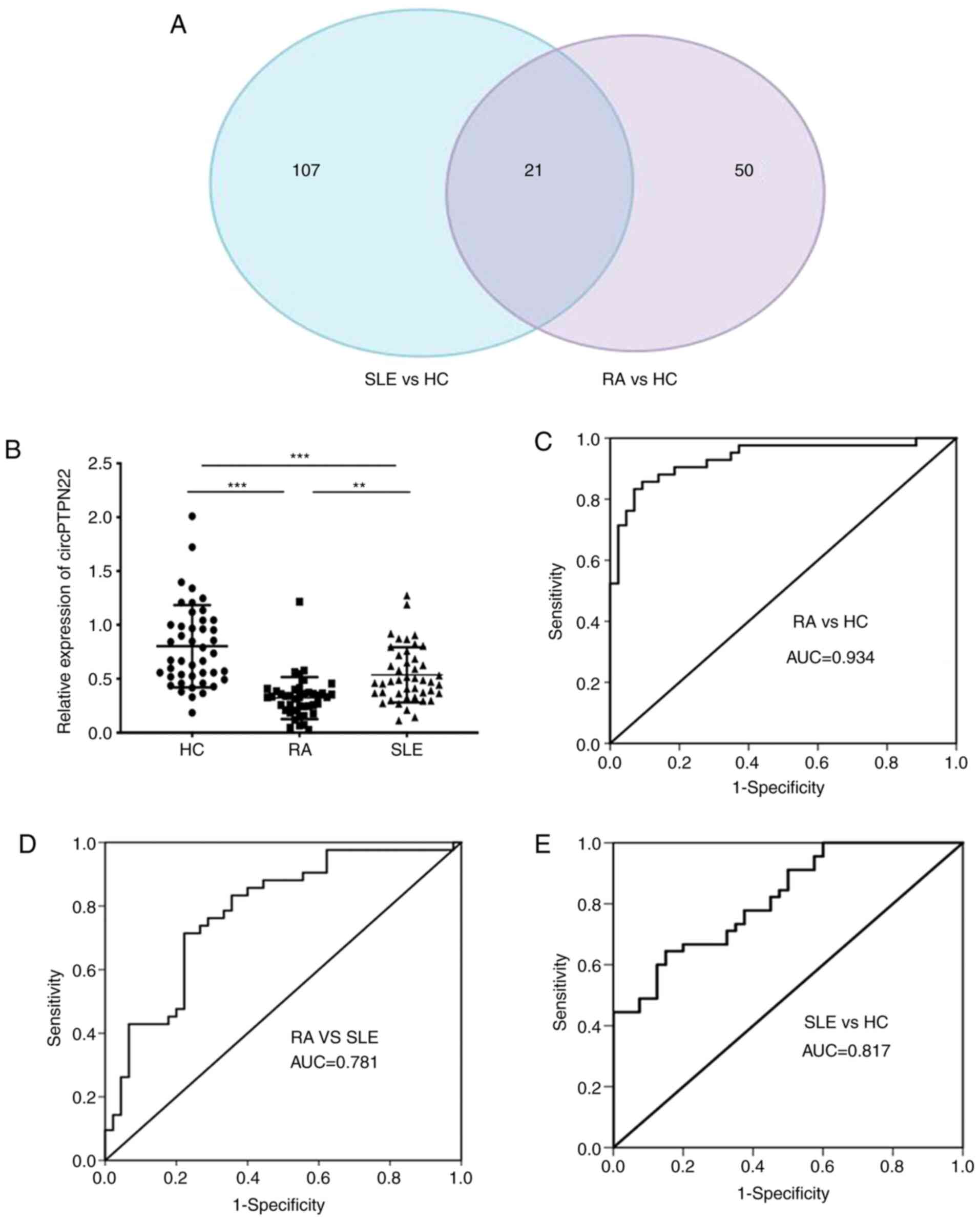 | Figure 3.circPTPN22 expression as a potential
biomarker for RA diagnosis. (A) Venn diagram showing the overlap of
21 significantly differentially expressed circRNAs in RA and SLE
compared with HCs. There were 128 significantly dysregulated
circRNAs in PBMCs of SLE patients vs. HCs (blue area) and 71
significantly dysregulated circRNAs in PBMCs of RA patients vs. HCs
(purple area). (B) The expression levels of circPTPN22 in PBMCs of
42 RA patients, 45 SLE patients and 44 HC were determined by
reverse transcription-quantitative PCR. ROC curve analysis of the
sensitivity and specificity of (C) circPTPN22 in RA patients and
HCs, (D) circPTPN22 in PBMCs of RA patients and SLE and (E)
circPTPN22 in PBMCs of SLE patients and HCs. **P<0.01,
***P<0.001. circRNA, circular RNA; RA, rheumatoid arthritis;
SLE, systemic lupus erythematosus; HC, healthy controls; ROC,
receiver operating characteristic; PBMCs, peripheral blood
mononuclear cells; AUC, area under the curve. |
To verify whether the expression level of circPTPN22
in RA was consistent with the sequencing data, RT-qPCR was
performed using an independent set of samples from 42 RA patients,
44 healthy controls and 45 SLE patients as an autoimmune disease
control. The results demonstrated that the average expression level
of circPTPN22 was significantly downregulated in the PBMCs of RA
and SLE patients compared to healthy controls; In addition, the
circPTPN22 level was even lower in RA patients than in SLE patients
(Fig. 3B). Similarly, the
expression of the parental gene PTPN22 of circPTPN22 was
also examined in RA patients, HCs and SLE patients. The results
demonstrated that compared with the HC group, the expression level
of PTPN22 in PBMCs in RA and SLE patients was significantly
downregulated and the expression level of PTPN22 in PBMCs in
RA patients was lower than that in SLE patients (Fig. S2A). In addition, Pearson's
correlation test demonstrated that the expression levels of
circPTPN22 and PTPN22 in the PBMCs of RA patients were
positively correlated (Fig. S2B).
Furthermore, receiver operating characteristic (ROC) curve analysis
was used to validate the potential diagnostic biomarker of
circPTPN22 in RA. The AUC values indicated that circPTPN22 in PBMCs
could distinguish RA patients from HCs (Fig. 3C) and even from SLE patients
(Fig. 3D), with the ability to
discriminate SLE patients from HCs (Fig. 3E), as expected.
Correlation between circPTPN22
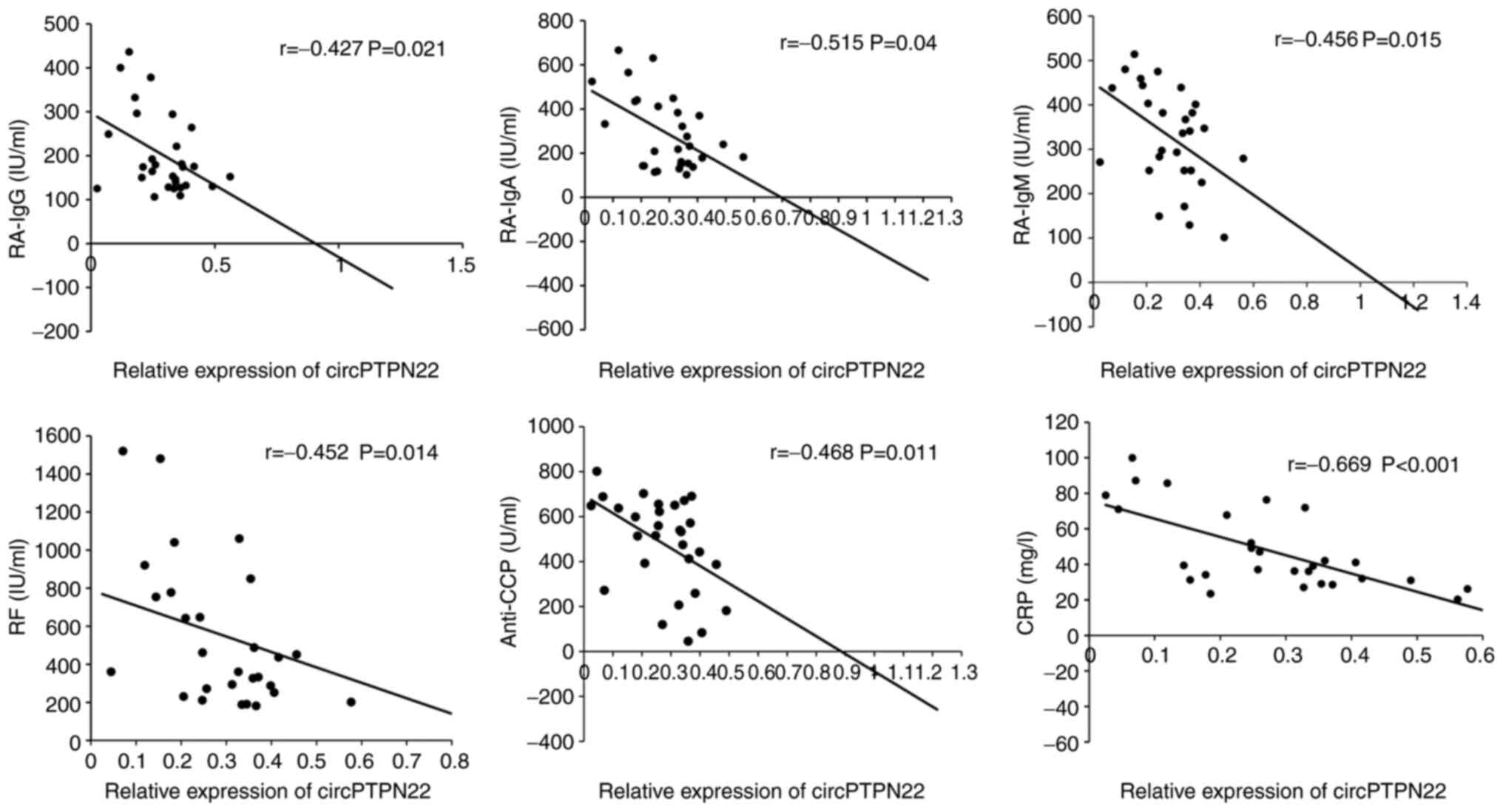
expression and RA clinical characteristics
To determine whether circPTPN22 in PBMCs of RA
patients was a potentially relevant biomarker of RA severity, the
correlations between circPTPN22 levels and RA clinical features
were examined, including duration, TNF-α, IL-6, C3, C4, RA-IgG,
RA-IgM, RA-IgA, IgA, IgG, IgM, IgE, anti-CCP, TJC, ESR, CRP and
DAS28. The results demonstrated that the circPTPN22 level was
negatively associated with RA-IgG, RA-IgA, RA-IgM, anti-CCP, RF and
CRP levels in RA (Fig. 4); however,
the circPTPN22 level did not correlate with other indicators of RA
severity (data not shown). Therefore, circPTPN22 could at least
partly reflect the severity of the disease.
RT-qPCR validation of the
circPTP22-miRNA-mRNA network
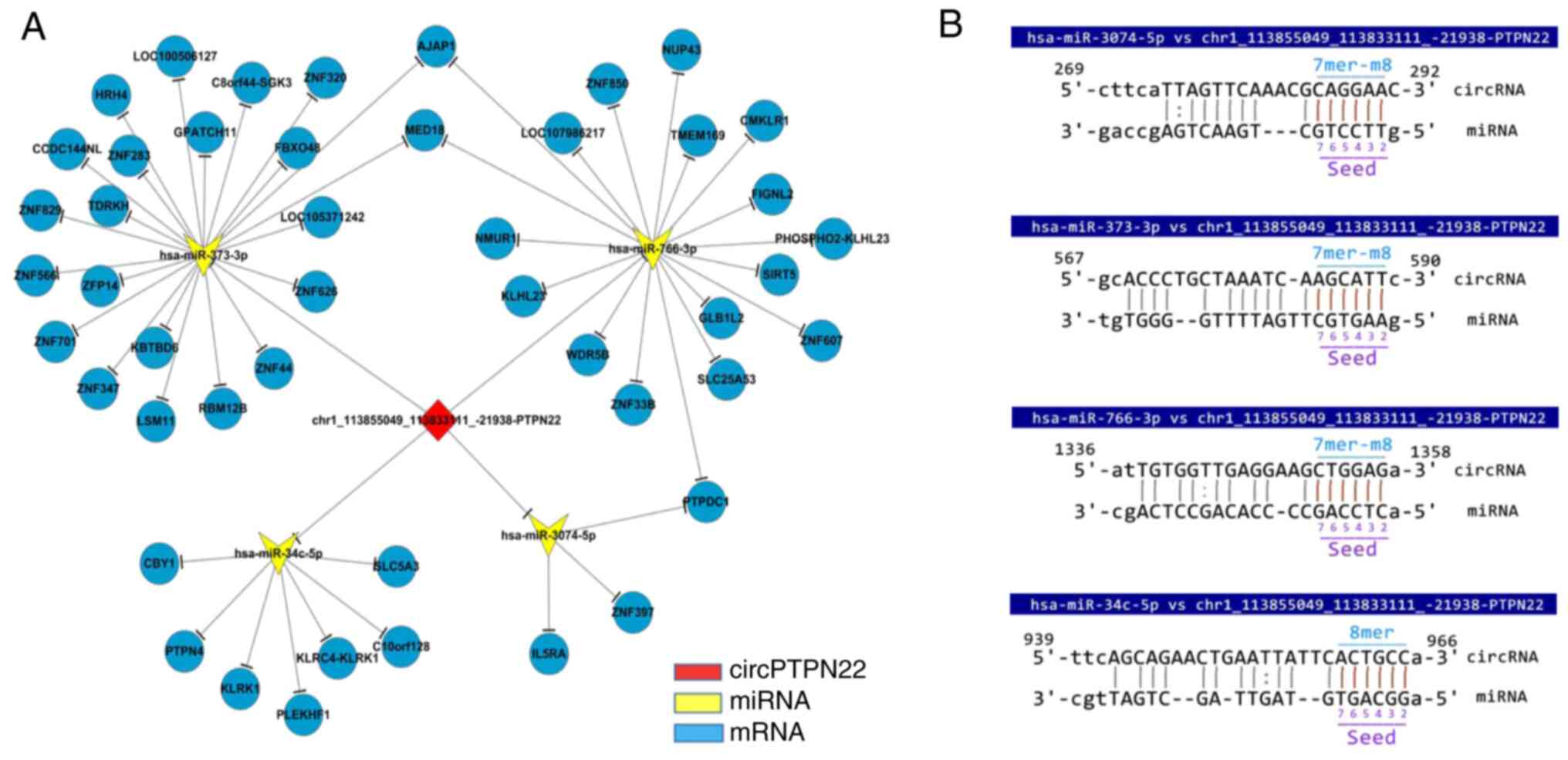
Based on the prediction software, differentially
expressed microRNAs matched with circPTPN22 were predicted to
construct the interaction network (Fig.
5A). The predicted target mRNAs selected from the RNA-seq data
were associated with inflammation. Using Miranda software, it was
found that the sequence of circPTPN22 had potential binding sites
with the seed regions of the four putative target miRNAs (Fig. 5B).
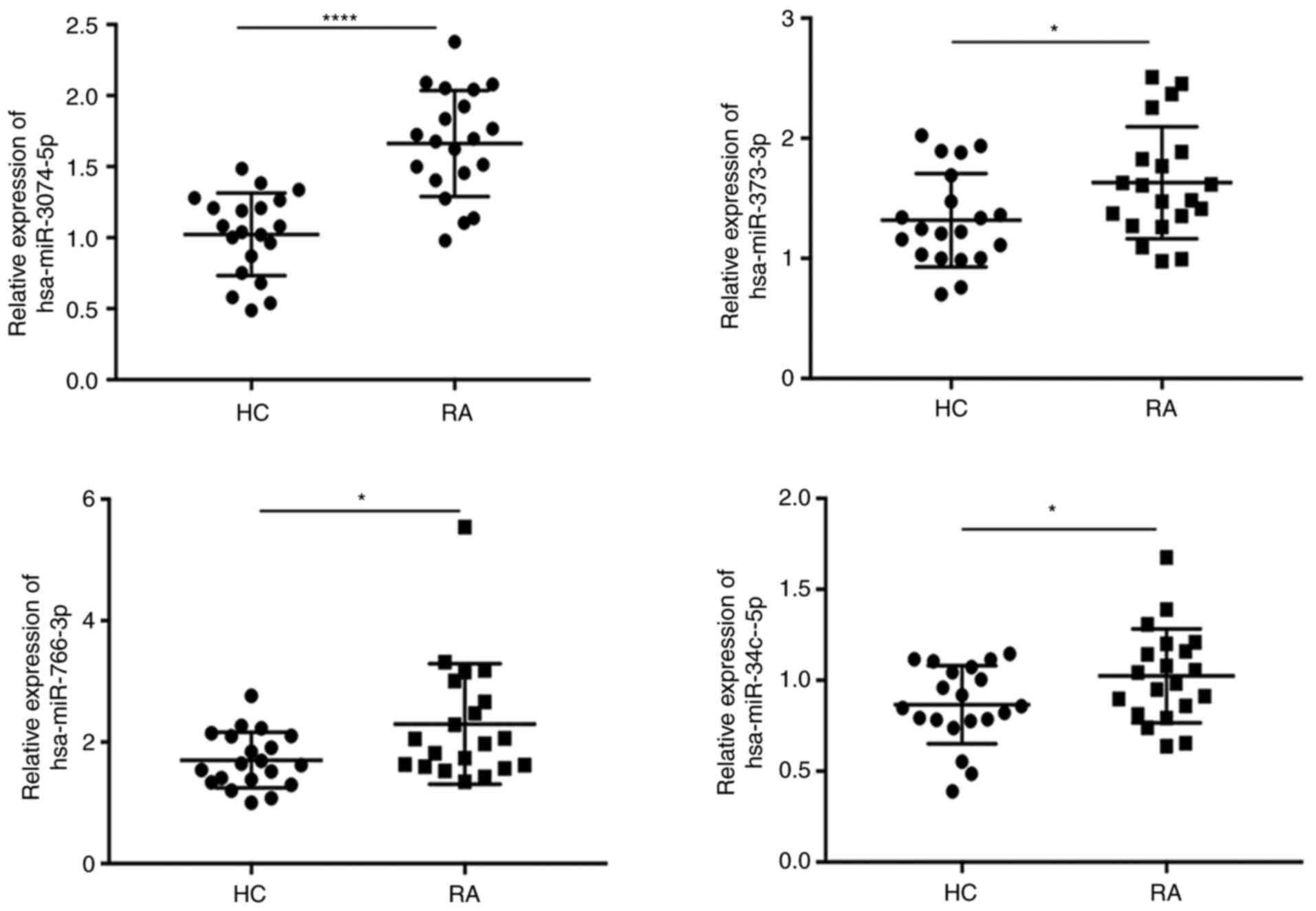
To confirm whether circPTPN22 is involved in the
development and progression of RA disease through a sponge
mechanism, RT-qPCR was conducted to validate the expression levels
of miRNA in the PBMCs of RA and HC. The results demonstrated that
hsa-miR-3074-5p, hsa-miR-373-3p, hsa-miR-766-3p and hsa-miR-34c-5p
were upregulated in the PBMCs of the RA group compared to the HC
group (Fig. 6), which was
consistent with the RNA-seq results.
Construction of the PPI network and
identification of hub genes
First, the differentially expressed mRNA genes were
constructed into a PPI network using the STRING database, with the
maximum interaction confidence set to 0.9. A total of 246 nodes and
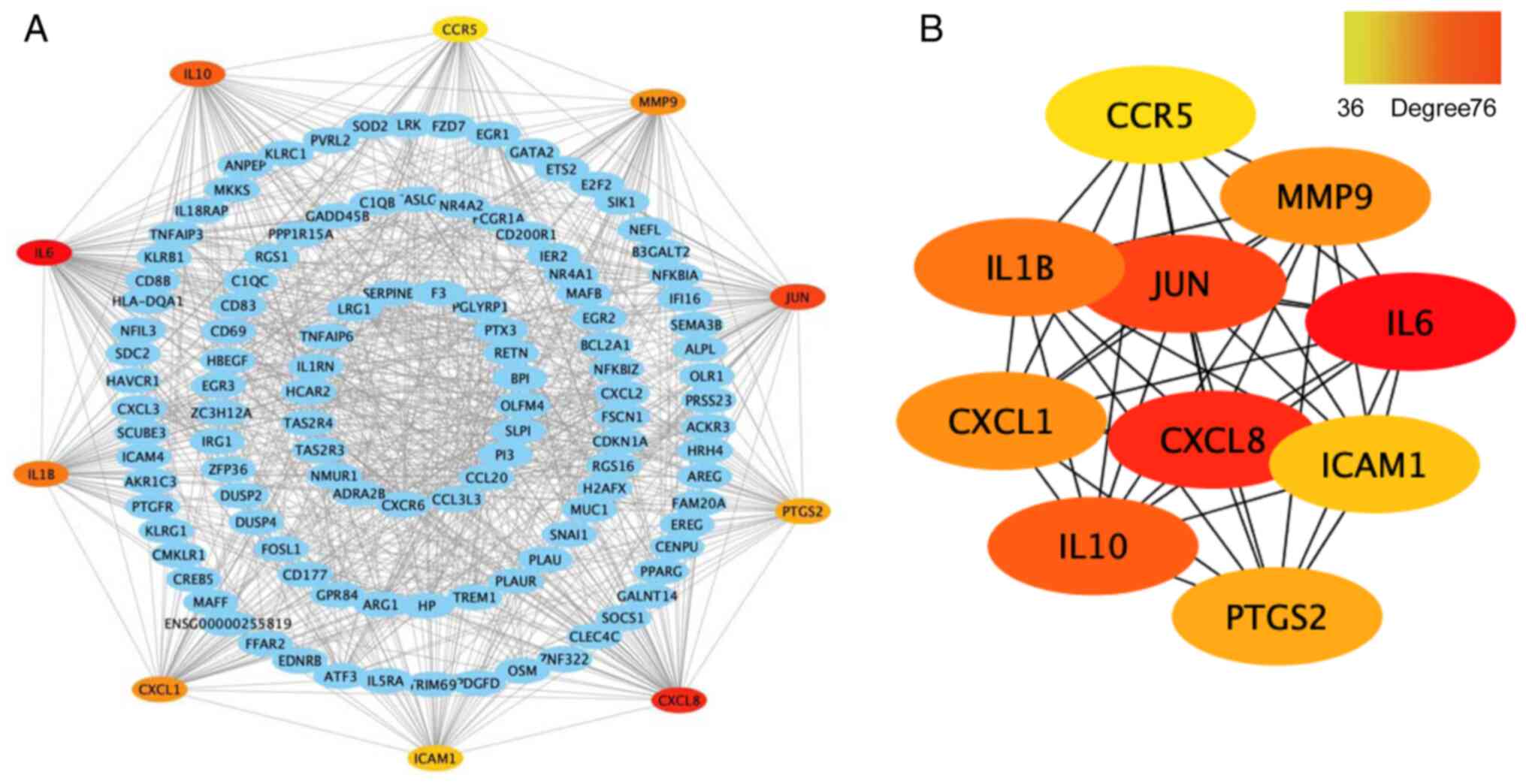
998 edges were involved in the PPI network (Fig. 7A). To find the key nodes in the
network, the 11 topological analysis methods available on the
Cytoscape plug in CytoHubba were used to rank all the nodes by
their network features (33). Then,
the top 10 genes were identified (Fig.
7B). The results demonstrated that IL6 was the most prominent
gene with connectivity degree=76, followed by IL-8 (CXCL8;
degree=63), transcription factor AP-1 (JUN; degree=56), IL-10
(degree=53), IL-1β (degree=51), MMP9 (degree=48), growth-regulated
α protein (CXCL1; degree=48), prostaglandin G/H synthase 2 (PTGS2;
degree=42), intercellular adhesion molecule 1 (ICAM1; degree=41)
and C-C chemokine receptor type 5 (CCR5; degree=36). Since gene
products are the core of the PPI network, these hub genes were
considered potential therapeutic targets and some genes have been
proven to serve a role in RA (34).
Discussion
RA is a common complex autoimmune disease involving
multiple joints and systemic inflammation. Early diagnosis is the
key event for RA treatment; nevertheless, a number of patients with
negative RF and/or anti-CCP are easily misdiagnosed at an early
stage (1,6). It has been found that circRNAs are
important members of the ncRNA family. Compared with microRNAs and
lncRNAs, circRNAs are more widely expressed and more stable in
mammalian cells (8,9), which indicates that circRNAs may be
potential diagnostic markers for a number of diseases. The
development of high-throughput sequencing and bioinformatics
analysis has increased our understanding of the important role of
circRNAs in the pathogenesis and development of human disease.
To explore new biomarkers for RA, the present study
investigated the expression profile of circRNAs in PBMCs in RA
patients through high-throughput RNA sequencing. A total of 71
circRNAs were found to be significantly dysregulated (41
upregulated and 30 downregulated) in RA patients compared with
healthy controls. Based on a previous study (21), 21 differentially expressed circRNAs
shared in both RA and SLE PBMCs were found, which notably included
circPTPN22, which has been identified as a potential diagnostic
biomarker in SLE (21).
The pathogenesis of RA is commonly attributed to the
infiltration of T cells into the synovial membrane and T cells are
the main culprits in inducing inflammation (35). In addition, the loss of tolerance to
autoantigens may be another cause of RA (36). Despite these biological factors,
other predisposing factors, such as genetic components, have been
reported as high-risk factors for RA (3). Due to the complex clinical
manifestations of RA, a single experimental indication cannot
provide an accurate diagnosis of RA. Therefore, the present study
evaluated the clinical diagnostic value of circPTPN22 in RA by
using ROC curves and found the potential role of a biomarker for
the diagnosis of RA. The results also demonstrated that the
circPTPN22 expression levels were negatively correlated with
RA-IgG, RA-IgA, RA-IgM, anti-CCP, RF and CRP levels in RA PBMCs but
not correlated with TNF-α, IL-6, C3, C4, IgA, IgG, IgM, IgE, TJC,
ESR or DAS28. These results further indicate that circPTPN22 could
be a promising novel diagnostic biomarker for RA.
circRNAs have been shown to regulate the level of
target miRNAs through molecular sponge mechanisms and then regulate
the expression of corresponding target genes of miRNAs (11). To explore the molecular mechanism of
circPTPN22 in RA, a ceRNA interaction network was constructed
through Cytoscape. A total of four putative targets of miRNAs were
predicted and their roles in RA are unclear. GO analysis
demonstrated that the differentially expressed genes for
‘transcription factor NF-κB activity’, ‘nuclear-transcribe’,
‘poly(A)-specific ribonuclease activity’ and ‘syntaxin-1 binding’
are key checkpoints of effector function in RA. In addition, gene
set enrichment analysis demonstrated that they were involved in
‘TLR signaling pathway’ and ‘MAPK signaling pathway’. PTPN22 has
been reported to be involved in a variety of autoimmune diseases
and modulates signaling through antigens and innate immune
receptors (23). Additionally, it
serves roles in lymphocyte development and activation,
establishment of tolerance and innate immune cell-mediated host
defence and immunoregulation (23).
However, whether circPTPN22 also participates in autoimmune
diseases through the same or similar mechanism has not yet been
reported.
Few circRNA disorders have previously been reported
in RA patients. Due to different sample and database comparison
methods, the results of these data may be inconsistent. Ouyang
et al (37) identified 12
circRNAs that were differentially expressed in patients with RA and
healthy controls using microarray profiling of circRNAs. Their
RT-qPCR analysis demonstrated increased expression of
circRNA_104871, circRNA_003524, circRNA_101873 and circRNA_103047
in PBMCs from RA. The present study suggested that the five
increased circRNAs had a significant value in RA diagnosis by ROC
curve analysis. In addition, Zheng et al (38) screened 584 circRNAs differentially
expressed in PBMCs of RA patients compared with HCs by circRNA
microarray and they demonstrated that the expression levels of
hsa_circRNA_104194, hsa_circRNA_104593, hsa_circRNA_103334,
hsa_circRNA_101407 and hsa_circRNA_102594 were consistent with the
results from the microarray analysis by RT-qPCR validation. Yang
et al (39) found 71
markedly dysregulated circRNAs using RNA-seq. Their results
indicated that hsa_circ_0000396 and hsa_circ_0130438 were
downregulated in an RA group compared with a healthy group. The ROC
curve indicated the diagnostic value of both circRNAs for RA. In
Wen et al (40), a total of
165 circRNAs and 63 miRNAs were differentially expressed between RA
patients and healthy individuals according to RNA-seq. They
suggested that the expression of hsa_circ_0001200,
hsa_circ_0001566, hsa_circ_0003972 and hsa_circ_0008360 in PBMCs
from RA patients might serve as potential biomarkers for the
diagnosis of RA. Although the above studies investigated the RNA
expression profile in RA PBMC by vary sequencing methods, most did
not provide raw RNA-seq dataset, or some papers only present top 10
circRNAs, or only randomly selected six circRNAs for further
RT-qPCR validation. Therefore, the authors of the present study
could not find how a number of circRNAs are the same and how a
number of circRNAs differ among these studies. The present study
demonstrated significant differences in circPTPN22 expression
between RA patients, SLE patients and HCs in three validation
cohorts. Although circPTPN22 expression in RA is lower compared
with that in SLE patients, circPTPN22 expression in both RA and SLE
patients was significantly lower compared with that in HCs,
suggesting that circPTPN22 could be a common diagnostic circRNA for
autoimmune diseases such as RA, SLE and other possible immune
diseases. To verify that circPTPN22 can reliably distinguish RA
patients from SLE patients and HCs, a larger sample size is
needed.
In conclusion, circPTPN22 may be associated with the
classification of RA and involved in the pathogenesis of the RA
process through ceRNA mechanisms. In our previous study, circPTPN22
was verified as a potential biomarker of SLE and was predicted to
translate a truncated form of PTPN22 (21) and it is possible that the truncated
PTPN22 would interact with the full-length PTPN22 protein directly
or indirectly. Since the PTPN22 gene codes for a tyrosine
phosphatase, with a potential function in the regulation of T-cell
and B-cell activation, it is of clear interest for the study of the
etiology of RA to know whether circPTPN22 also participates in
autoimmune diseases through regulating its parental gene
PTPN22. However, the detailed and specific mechanisms of
circPTPN22 potentially involved in RA pathogenesis remain to be
clarified in the future.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Shifei Li and Dr
Qijun Wu (Department of Dermatology, The First Affiliated Hospital
of Third Military Medical University, Chongqing, China) for helping
them discuss the revision of the manuscript.
Funding
The present study was supported by grants from the
National Key Research and Development Project (grant no.
2016YFA0502203), the Natural Science Foundation of China (grant no.
81773316) and the Key Research and Development Project of Anhui
Province (grant no. 202004j07020002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZJ performed the main experiments in this study. ZZ,
QM and YZ performed the clinical data collection and statistical
analysis. ZJ and ZZ confirm the authenticity of all the raw data.
BN interpreted the clinical data of patients. MZ and JT designed
the study and drafted the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the First Affiliated Hospital of Third Military
Medical University (Chongqing, China) and the reference ethical
number is KY2019119. Written informed consent was obtained from all
subjects prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smolen JS, Aletaha D and McInnes IB:
Rheumatoid arthritis. Lancet. 388:2023–2038. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Symmons D, Turner G, Webb R, Asten P,
Barrett E, Lunt M, Scott D and Silman A: The prevalence of
rheumatoid arthritis in the United Kingdom: New estimates for a new
century. Rheumatology (Oxford). 41:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okada Y, Wu D, Trynka G, Raj T, Terao C,
Ikari K, Kochi Y, Ohmura K, Suzuki A, Yoshida S, et al: Genetics of
rheumatoid arthritis contributes to biology and drug discovery.
Nature. 506:376–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugiyama D, Nishimura K, Tamaki K, Tsuji
G, Nakazawa T, Morinobu A and Kumagai S: Impact of smoking as a
risk factor for developing rheumatoid arthritis: A meta-analysis of
observational studies. Ann Rheum Dis. 69:70–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nisar MK and Ostor AJ: Disease remission
the goal of therapy in rheumatoid arthritis. Practitioner.
254:17–21. 2010.PubMed/NCBI
|
|
6
|
van Venrooij WJ, van Beers JJ and Pruijn
GJ: Anti-CCP antibody, a marker for the early detection of
rheumatoid arthritis. Ann N Y Acad Sci. 1143:268–285. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cocquerelle C, Mascrez B, Hétuin D and
Bailleul B: Mis-splicing yields circular RNA molecules. FASEB J.
7:155–160. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abdelmohsen K, Panda AC, Munk R,
Grammatikakis I, Dudekula DB, De S, Kim J, Noh JH, Kim KM,
Martindale JL and Gorospe M: Identification of HuR target circular
RNAs uncovers suppression of PABPN1 translation by circPABPN1. RNA
Biol. 14:361–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen N, Zhao G, Yan X, Lv Z, Yin H, Zhang
S, Song W, Li X, Li L, Du Z, et al: A novel FLI1 exonic circular
RNA promotes metastasis in breast cancer by coordinately regulating
TET1 and DNMT1. Genome Biol. 19:2182018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang M, Huang N, Yang X, Luo J, Yan S,
Xiao F, Chen W, Gao X, Zhao K, Zhou H, et al: A novel protein
encoded by the circular form of the SHPRH gene suppresses glioma
tumorigenesis. Oncogene. 37:1805–1814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Kong R, Wu C, Wang S, Liu Z and
Liu S, Li S, Chen T, Mao C and Liu S: Circ-MALAT1 functions as both
an mRNA translation brake and a microRNA sponge to promote
Self-Renewal of hepatocellular cancer stem cells. Adv Sci (Weinh).
7:19009492020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mao Y, Zhang L and Li Y: circEIF4G2
modulates the malignant features of cervical cancer via the
miR218/HOXA1 pathway. Mol Med Rep. 19:3714–3722. 2019.PubMed/NCBI
|
|
18
|
Zhang H, Zhu L, Bai M, Liu Y, Zhan Y, Deng
T, Yang H, Sun W, Wang X, Zhu K, et al: Exosomal circRNA derived
from gastric tumor promotes white adipose browning by targeting the
miR-133/PRDM16 pathway. Int J Cancer. 144:2501–2515. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo G, Wang H, Ye L, Shi X, Yan K, Lin K,
Huang Q, Li B, Lin Q, Zhu L, et al: Hsa_circ_0000479 as a novel
diagnostic biomarker of systemic lupus erythematosus. Front
Immunol. 10:22812019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong S, Ouyang Q, Zhu D, Huang Q, Zhao J,
Fan M, Cai Y and Yang M: Hsa_circ_0088036 promotes the
proliferation and migration of fibroblast-like synoviocytes by
sponging miR-140-3p and upregulating SIRT 1 expression in
rheumatoid arthritis. Mol Immunol. 125:131–139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miao Q, Zhong Z, Jiang Z, Lin Y, Ni B,
Yang W and Tang J: RNA-seq of circular RNAs identified circPTPN22
as a potential new activity indicator in systemic lupus
erythematosus. Lupus. 28:520–528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cohen S, Dadi H, Shaoul E, Sharfe N and
Roifman CM: Cloning and characterization of a lymphoid-specific,
inducible human protein tyrosine phosphatase, Lyp. Blood.
93:2013–2024. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bottini N and Peterson EJ: Tyrosine
phosphatase PTPN22: Multifunctional regulator of immune signaling,
development, and disease. Annu Rev Immunol. 32:83–119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Svensson MN, Doody KM, Schmiedel BJ,
Bhattacharyya S, Panwar B, Wiede F, Yang S, Santelli E, Wu DJ,
Sacchetti C, et al: Reduced expression of phosphatase PTPN2
promotes pathogenic conversion of Tregs in autoimmunity. J Clin
Invest. 129:1193–1210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prevoo ML, van't Hof MA, Kuper HH, van
Leeuwen MA, van de Putte LB and van Riel PL: Modified disease
activity scores that include twenty-eight-joint counts. Development
and validation in a prospective longitudinal study of patients with
rheumatoid arthritis. Arthritis Rheum. 38:44–48. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hochberg MC: Updating the American College
of Rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40:17251997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang K, Singh D, Zeng Z, Coleman SJ, Huang
Y, Savich GL, He X, Mieczkowski P, Grimm SA, Perou CM, et al:
MapSplice: Accurate mapping of RNA-seq reads for splice junction
discovery. Nucleic Acids Res. 38:e1782010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anders S, Pyl PT and Huber W: HTSeq-a
Python framework to work with high-throughput sequencing data.
Bioinformatics. 31:166–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anders S and Huber W: Differential
expression analysis for sequence count data. Genome Biol.
11:R1062010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rehmsmeier M, Steffen P, Hochsmann M and
Giegerich R: Fast and effective prediction of microRNA/target
duplexes. RNA. 10:1507–1517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: cytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol. 8 (Suppl 4):S112014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bie Y, Ge W, Yang Z, Cheng X, Zhao Z, Li
S, Wang W, Wang Y, Zhao X, Yin Z and Li Y: The crucial role of
CXCL8 and Its receptors in colorectal liver metastasis. Dis
Markers. 2019:80234602019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smolen JS and Steiner G: Therapeutic
strategies for rheumatoid arthritis. Nat Rev Drug Discov.
2:473–488. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choy E: Understanding the dynamics:
Pathways involved in the pathogenesis of rheumatoid arthritis.
Rheumatology (Oxford). 51 (Suppl 5):v3–v11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ouyang Q, Wu J, Jiang Z, Zhao J, Wang R,
Lou A, Zhu D, Shi GP and Yang M: Microarray expression profile of
circular rnas in peripheral blood mononuclear cells from rheumatoid
arthritis patients. Cell Physiol Biochem. 42:651–659. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng F, Yu X, Huang J and Dai Y: Circular
RNA expression profiles of peripheral blood mononuclear cells in
rheumatoid arthritis patients, based on microarray chip technology.
Mol Med Rep. 16:8029–8036. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang X, Li J, Wu Y, Ni B and Zhang B:
Aberrant dysregulated circular RNAs in the peripheral blood
mononuclear cells of patients with rheumatoid arthritis revealed by
RNA sequencing: Novel diagnostic markers for RA. Scand J Clin Lab
Invest. 79:551–559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wen J, Liu J, Zhang P, Jiang H, Xin L, Wan
L, Sun Y, Huang D, Sun Y, Long Y, et al: RNA-seq reveals the
circular RNA and miRNA expression profile of peripheral blood
mononuclear cells in patients with rheumatoid arthritis. Biosci
Rep. 40:BSR201931602020. View Article : Google Scholar : PubMed/NCBI
|