Introduction
Sepsis results in life-threatening organ dysfunction
that is characterized by overwhelming systemic inflammation
(1). Lung inflammation, as a type
of acute lung injury (ALI), is often induced in severe sepsis
(2). Despite improvements in
intensive care management and multidisciplinary treatments, the
mortality rate of sepsis was 17% and was 26% for severe sepsis
during 1979–2015 worldwide (3–7).
Therefore, the pathogenesis of sepsis-induced lung injury requires
further investigation to identify new effective therapeutics.
Previous studies have indicated that sepsis-induced
lung injury is primarily associated with systemic inflammatory
response syndrome, including the activation of inflammatory
cytokines and inflammatory signaling pathways (8–10).
Numerous inflammatory signaling pathways serve an important role in
the pathogenesis of sepsis-induced lung injury, including the MAPK
and NF-κB signaling pathways. The MAPK and NF-κB signaling pathways
induce the excessive release of inflammatory cytokines, including
TNF-α, IL-1β and other associated cytokines, which contribute to
lung dysfunction and mortality in sepsis-induced lung injury
(11–13). p38/MAPK is a key member of the MAPK
signaling pathway that serves an essential role in cell
proliferation and survival, and can be activated by various
inflammatory mediators and environmental stress (14). Previous studies have reported that
activation of the p38/MAPK signaling pathway is involved in the
development of ALI/acute respiratory distress syndrome (ARDS).
These studies indicated that pharmacological targeting of the
p38/MAPK signaling pathway could decrease the expression levels of
inflammation factors and attenuate ALI/ARDS (15,16).
Taurine (2-aminoethanesulfonic acid) is a type of
intracellular free amino acid. As a cysteine derivative, taurine is
highly expressed in all mammalian tissues and has important
physiological effects on all cells in the body, including
endogenous antioxidant, detoxification, osmoregulation,
anti-inflammatory and membrane stability effects (17–19).
In addition, previous studies have indicated that taurine could
protect against lung injury by modulating oxidative stress,
reducing cell apoptosis and decreasing the expression levels of
inflammatory factors (20–22). Therefore, taurine may serve as a
novel candidate for treating sepsis-induced ALI; however, the
underlying molecular mechanism remains unclear.
The aim of the present study was to investigate the
protective effects of taurine on sepsis-induced ALI, as well as the
underlying molecular mechanism. In particular, the effects of
taurine on lung tissue pathological changes, respiratory function,
malondialdehyde (MDA) levels, superoxide dismutase (SOD) activity
and inflammatory factor concentrations were evaluated in a Sprague
Dawley (SD) cecal ligation and puncture (CLP)-induced septic ALI
rat model. Furthermore, the roles of the p38/MAPK and NF-κB
signaling pathways in the pathogenesis of sepsis-induced ALI were
investigated.
Materials and methods
Ethics statement
The present study was conducted according to the
Guide for the Care and Use of Experimental Animals established by
the National Society for Medical Research (23). The present study was approved by the
Ethics Committee of Nanjing Medical University (approval no. SYXK
2019-0042). All surgeries on the rats were conducted under
anesthesia (sodium pentobarbital), and every effort was made to
alleviate pain.
Animal model and study design
In total, 168 SD rats (male; age, 8–10 weeks;
weight, 220–250 g) were provided by the Laboratory Animal Centre of
Nanjing Medical University. All rats were acclimatized for 7 days
prior to the experiment, fed with standard chow (Global Diet;
Shanghai, China) and provided with ad libitum access to
water in standard shoebox cages. The rats were housed in a normal
atmosphere under 12-h light/dark cycles at 20–25°C with 50–60%
humidity. The surgical procedure to establish the CLP-induced
septic rat model was performed as previously described (24). Briefly, all rats were fasted and
anesthetized by the intraperitoneal injection of 50 mg/kg sodium
pentobarbital (Sigma-Aldrich; Merck KGaA). Subsequently, the middle
abdominal incision site was shaved and the cecum was exposed via a
2-cm abdominal midline incision. The cecum was mobilized, ligated
and punctured with a 21-gauge needle. Some of the fecal matter was
squeezed through the puncture wound, then the bowel was
repositioned and the abdomen was sutured. The animals were
resuscitated with a subcutaneous sterile saline immediately after
CLP surgery. The sham-operated control rats underwent the same
procedure, but without ligation or puncture of the cecum. No
mortality was observed in the rats after CLP was induced. At 3 days
post-CLP, rats were anesthetized by the intraperitoneal injection
of 50 mg/kg sodium pentobarbital (Sigma-Aldrich; Merck KGaA) and
blood samples were collected via cardiac puncture using a 1.5 ml
tube. Subsequently, the anesthetized rats were sacrificed by
cervical dislocation. The lung tissue samples were collected for
further analysis. Death was confirmed by monitoring cessation of
the heartbeat and pupil dilation.
To investigate the protective effects of taurine on
sepsis-induced lung injury, SD rats were randomly assigned to the
following three groups (n=24 per group): i) Control, rats received
an intraperitoneal injection of normal saline (1 ml/kg; 200 µl);
ii) ALI, CLP-induced septic ALI model rats received an
intraperitoneal injection of normal saline (1 ml/kg; 200 µl); iii)
ALI + taurine, CLP-induced septic ALI model rats received an
intraperitoneal injection of taurine for 3 days (200 mg/kg/day in
200 µl normal saline; Sigma-Aldrich; Merck KGaA). To clarify the
molecular mechanisms underlying taurine-mediated protection of lung
tissue following CLP induction, rats were randomly assigned to the
following four groups (n=24 per group): i) Control, rats received
an intraperitoneal injection of normal saline (1 ml/kg; 200 µl);
ii) ALI, CLP-induced septic ALI model rats received an
intraperitoneal injection of normal saline (1 ml/kg; 200 µl); iii)
ALI + taurine, CLP-induced septic ALI model rats received an
intraperitoneal injection of taurine for 3 days (200 mg/kg/day in
200 µl normal saline; Sigma-Aldrich; Merck KGaA); and iv) ALI +
SB203580, CLP-induced septic ALI model rats received an
intraperitoneal injection of SB203580 (10 mg/kg in 200 µl normal
saline; Sigma-Aldrich; Merck KGaA). The dose selection and route of
administration of taurine was based on our previous study (14).
Respiratory function detection
Prior to sacrifice, arterial blood gas content was
examined using i-STAT® G7+ cartridges and an i-STAT
portable clinical analyzer (Abbott Point of Care Inc.). The
cartridges were recovered immediately before use to avoid
compression. For arterial blood gas analysis, arterial blood gas
was sampled from the left ventricle of each rat, added into the
sample well and allowed to fill by passive movement to the
indicated level (volume, 80–100 ml). The cap on the sample well was
closed and the cartridge was inserted into the analyzer. The values
of partial pressure (Pa) of oxygen (PaO2) and carbon
dioxide (PaCO2) were recorded after the calibration and
analysis cycle.
ELISA
Part of right lung tissue was cut into pieces and
mechanically disrupted into a homogenate with RIPA buffer (Beyotime
Institute of Biotechnology) containing protease inhibitor cocktail
(Roche Diagnostics GmbH). Then, the samples were centrifuged at
12,000 × g for 30 min at 4°C and the supernatants were collected.
The concentrations of IL-1β and TNF-α in the supernatants were
measured using commercially available ELISA kits according to the
manufacturer's instructions. The IL-1β (cat. no. ELR-IL1b) and
TNF-α (cat. no. ELR-TNFa1) ELISA kits were purchased from Ray
Biotech, Inc.
MDA level and SOD activity
detection
The level of MDA is an index of membrane lipid
peroxidation, and its level reflects the lipid peroxidation rate
and strength. Moreover, MDA levels can be used as a marker to
indicate the degree of tissue peroxidation damage. SOD serves an
important role in the balance of oxidation and antioxidation. SOD
can remove superoxide anion free radicals and protect cells from
damage (25). The activity of SOD
and the levels of MDA were measured in the lung tissue sample
supernatants as previously described (25). The SOD (cat. no. A001-1-2) and MDA
(cat. no. A003-1-1) kits were purchased from Nanjing Jiancheng
Bioengineering Institute, and were used according to the
manufacturer's protocol. Briefly, the right lung tissue of each rat
was dissected and homogenized with three volumes of ice-cold 1.15%
potassium chloride (ratio of tissue to potassium chloride was 1:3).
To determine MDA levels, the reaction mixture (0.2 ml 8.0% SDS, 1.5
ml 20% acetic acid and 1.5 ml 0.8% aqueous thiobarbituric acid) was
added to 0.1 ml homogenized tissue and adjusted to pH 3.5. Then,
the final volume was adjusted to 4.0 ml with distilled water.
Subsequently, 5.0 ml N-butanol and pyridine mixture [15:1 (v/v)]
was added. The mixture was shaken vigorously and centrifuged at
1,200 × g for 10 min at 4°C. Finally, the absorbance of the organic
layer was measured at a wavelength of 532 nm using a Spectronic UV
120 spectrophotometer (Thermo Fisher Scientific, Inc.). The MDA
levels are presented as nmol/mg protein.
Xanthine and xanthine oxidase are used to generate
superoxide radicals that react with p-iodonitrotetrazlium violet
(INT) to form a red formazan dye, which can be measured with a
Spectronic UV 120 spectrophotometer (Thermo Fisher Scientific,
Inc.) at a wavelength of 505 nm to determine SOD activity (26). SOD activity is presented as U/mg
protein. The assay mixture contained 0.01 M phosphate buffer,
3-cyclohexilamino-1-propanesulfonic acid (CAPS) buffer solution (50
mM CAPS and 0.94 mM EDTA in a saturated solution of sodium
hydroxide; pH 10.2), substrate solution (0.05 mM xanthine and 0.025
mM INT) and 80 µl xanthine oxidase.
Histological examination
After fixing with 4% neutral phosphate-buffered
paraformaldehyde for 16 h at room temperature, the lower left lung
was embedded in paraffin and sectioned into 5-µm thick slices. To
determine the integrity of the histology structure and the collagen
deposition condition, the slices were stained with hematoxylin and
eosin (H&E; Beyotime Institute of Biotechnology) according to
the manufacturer's instructions. Stained sections were observed
using a light microscope (Olympus Corporation) and scored by two
pathologists who were blinded to the samples and had expertise in
lung pathology. The lung injury score was determined using the
Tanino Method (27). In brief,
morphological alterations in the lungs were scored based on the
extent of pathology on a scale of 0–4 by assessing interstitial
edema, alveolar edema, hemorrhage and neutrophil infiltration. The
following scoring values were used: 0, no reaction in the alveolar
walls; 1, diffuse reaction in the alveolar walls, but without
thickening of the interstitium; 2, diffuse presence of the
inflammatory cells in the alveolar walls with a slight thickening
of the interstitium; 3, moderate interstitial thickening
accompanied by inflammatory cell infiltrates; and 4, interstitial
thickening involving >1/2 of the microscopic field. The results
are expressed as the average of the values from 30 microscopic
fields.
Lung wet/dry ratio determination
After sacrifice, the left upper lung tissue (~1 g)
was dissected, cleansed of blood or liquid with absorbent paper,
weighed to obtain the ‘wet’ weight and then heated in an 80°C
thermostatic oven for 48 h. Subsequently, the tissue was weighed
again to obtain the ‘dry’ weight. The ratio of wet to dry lung was
calculated to assess tissue edema.
Western blotting
Western blotting was performed as previously
described (28). Proteins were
extracted from lung tissues using RIPA (Beyotime Institute of
Biotechnology) containing protease inhibitor cocktail (Roche
Diagnostics GmbH) for 30 min on ice. Protein concentrations were
determined using the BCA Protein Assay Kit (Beyotime Institute of
Biotechnology). All the protein samples were boiled for 10 min in
equal volumes of 5X SDS buffer. Subsequently, proteins (20 µg) were
separated via 12% SDS-PAGE and transferred to PVDF membranes (Roche
Diagnostics GmbH) using a semi-dry transfer apparatus (Bio-Rad
Laboratories, Inc.). After blocking with 5% skimmed milk in
PBS-Tween-20 (PBST; PBS with 0.5% Tween-20) at room temperature for
1 h and washing three times with PBST, the membranes were incubated
overnight at 4°C with the following primary antibodies (all
purchased from Abcam): Rabbit anti-occludin (1:1,000; cat. no.
ab216327), rabbit anti-E-cadherin (1:10,000; cat. no. ab76319),
rabbit anti-p-p65 (1:1,000; cat. no. ab194726), rabbit anti-p65
(1:1,000; cat. no. ab16502), rabbit anti-p-p38 (1:1,000; cat. no.
ab47363), rabbit anti-p38 (1:3,000; cat. no. ab170099) and mouse
anti-β-actin (1:3,000; cat. no. ab7817). After three washes with
PBST, the membranes were incubated with a horseradish
peroxidase-conjugated goat anti-rabbit or anti-mouse secondary
antibody (1:5,000; cat. nos. BM2006 or BA1050, respectively; Wuhan
Boster Biological Technology, Ltd.) for 1 h at 37°C.
Immunocomplexes were visualized using an enhanced chemiluminescence
system (Thermo Fisher Scientific, Inc.) and an Odyssey Scanning
System (LI-COR Biosciences). Protein expression was semi-quantified
using ImageJ software (version 3.0; National Institutes of Health)
with β-actin as the loading control.
Statistical analysis
All experiments were repeated at least three times
with nearly identical results. Statistical analyses were performed
using SPSS software (version 21.0; IBM Corp.). Data are presented
as the mean ± standard deviation. Parametric data were analyzed
using one-way ANOVA followed by Tukey's post hoc test. Data with a
non-parametric distribution were analyzed using the Kruskal-Wallis
test followed by Dunn's post hoc test. P<0.05 was considered to
indicate a significant difference.
Results
Taurine suppresses sepsis-induced ALI
in rats
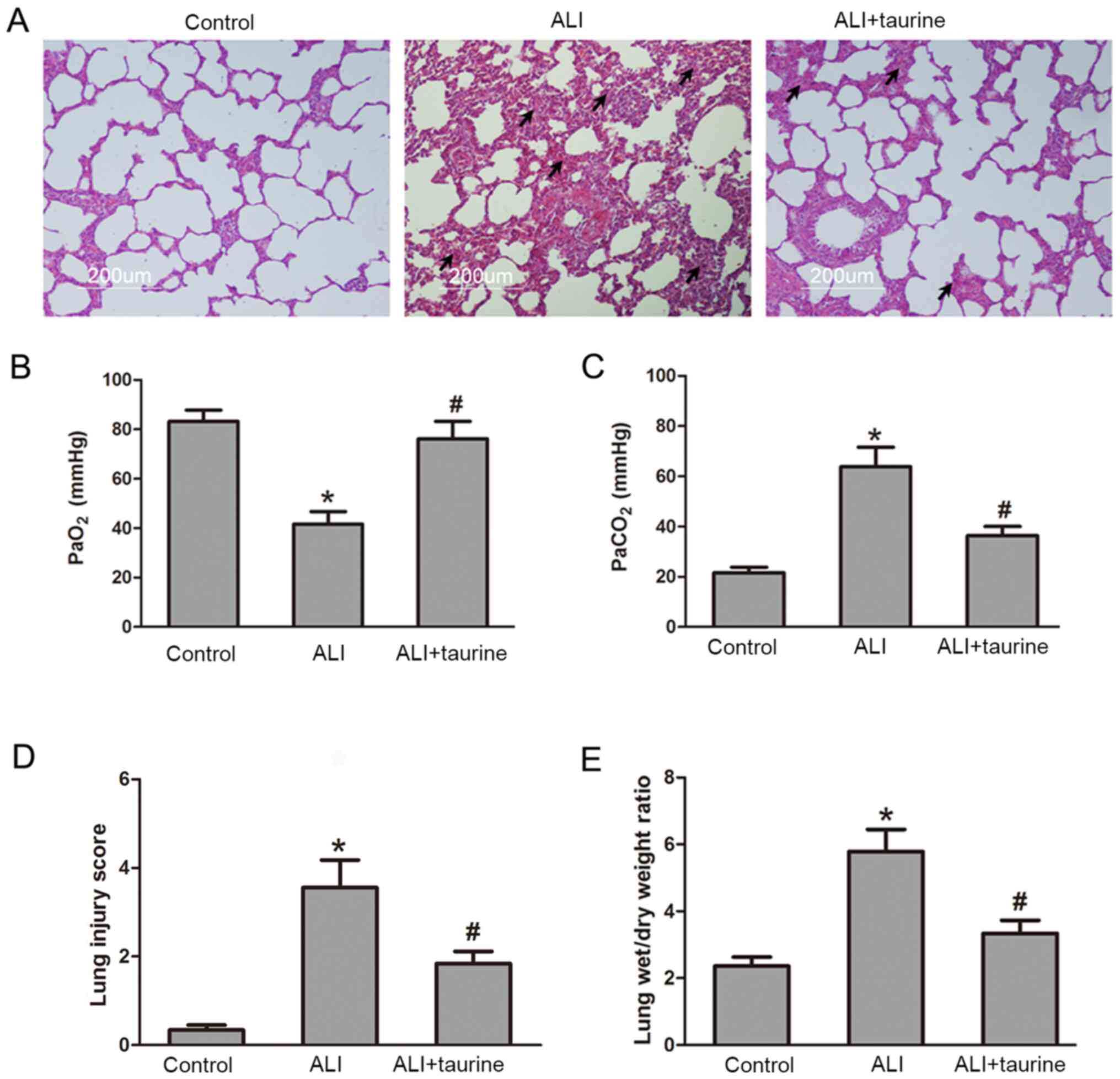
The lung is particularly susceptible to acute injury
in CLP-induced polymicrobial sepsis (29). To determine the protective effect of
taurine on sepsis-induced lung injury, a series of lung sections
obtained from the rats in each group were stained with H&E
(Fig. 1A). The tissue sections
obtained from the control rats were histologically normal and
displayed no inflammation or epithelial damage. After CLP-induced
sepsis, the lung tissue sections from rats in the ALI group
displayed extensive cellular thickening of the interalveolar septa
and interstitial edema, and infiltration of inflammatory cells.
However, inflammatory cell infiltration and edema were notably
ameliorated in the lungs following treatment with taurine in ALI
rats. As shown in Fig. 1B and C,
the respiratory function test results indicated that
PaO2 levels in the ALI group were significantly
decreased, but PaCO2 levels were significantly increased
compared with those in the control group. Taurine treatment
significantly increased PaO2 levels and decreased
PaCO2 levels in CLP-induced septic ALI model rats. The
lung injury score was also evaluated using histological sections
and a semi-quantitative scale (Fig.
1D). The lung injury score following CLP was significantly
increased compared with that in the control group, whereas the
score was significantly decreased in CLP-induced septic ALI model
rats following treatment with taurine. In addition, after lung
injury, sepsis resulted in an increase in lung weight owing to
inflammatory and fibrotic components. Compared with that in the
control group, the lung wet/dry ratio in the ALI group was
significantly increased (Fig. 1E).
By contrast, the lung wet/dry ratio was significantly decreased in
the ALI + taurine group compared with that in the ALI group. These
results indicated that taurine protected the lung tissue and
attenuated sepsis-induced injury.
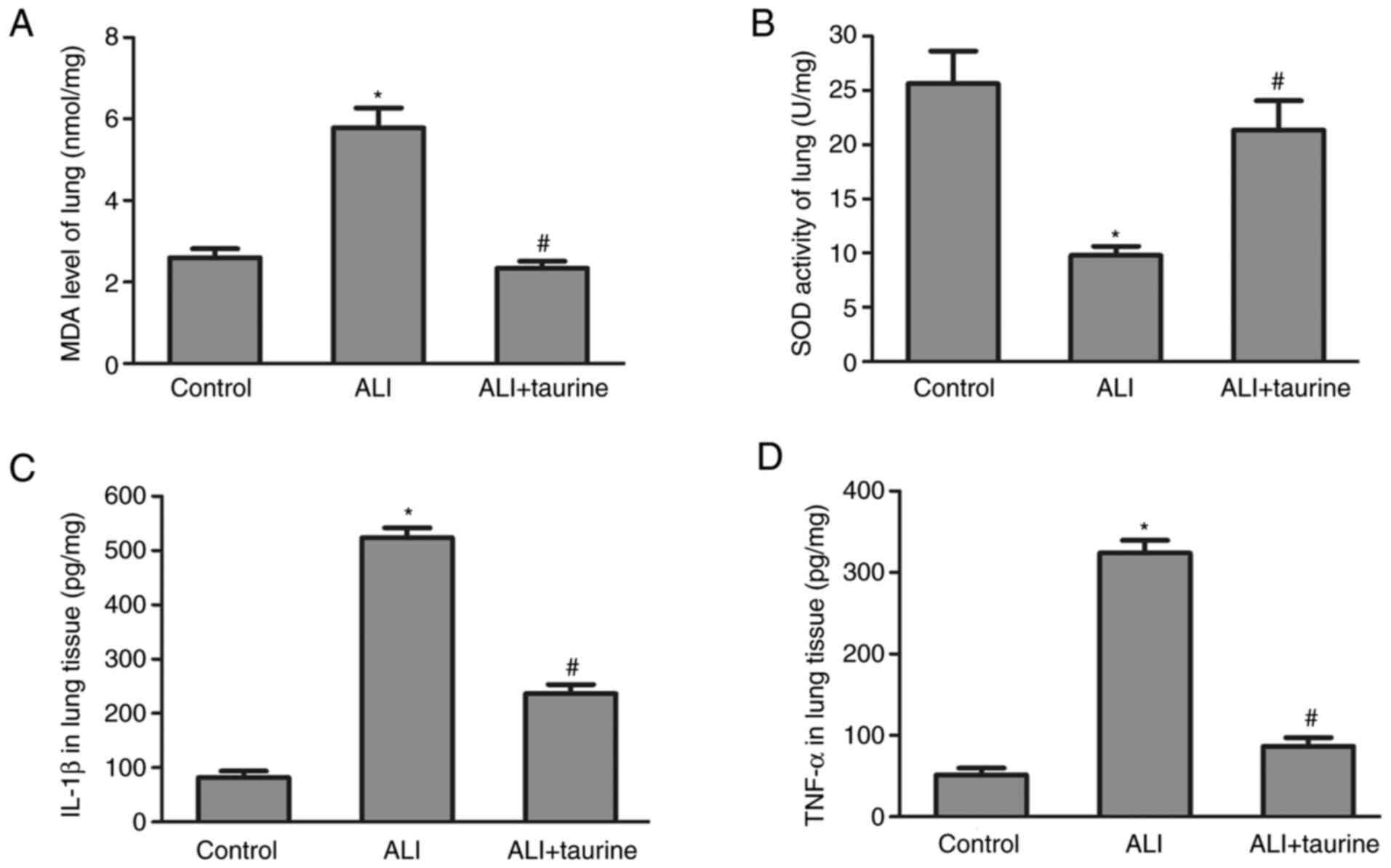
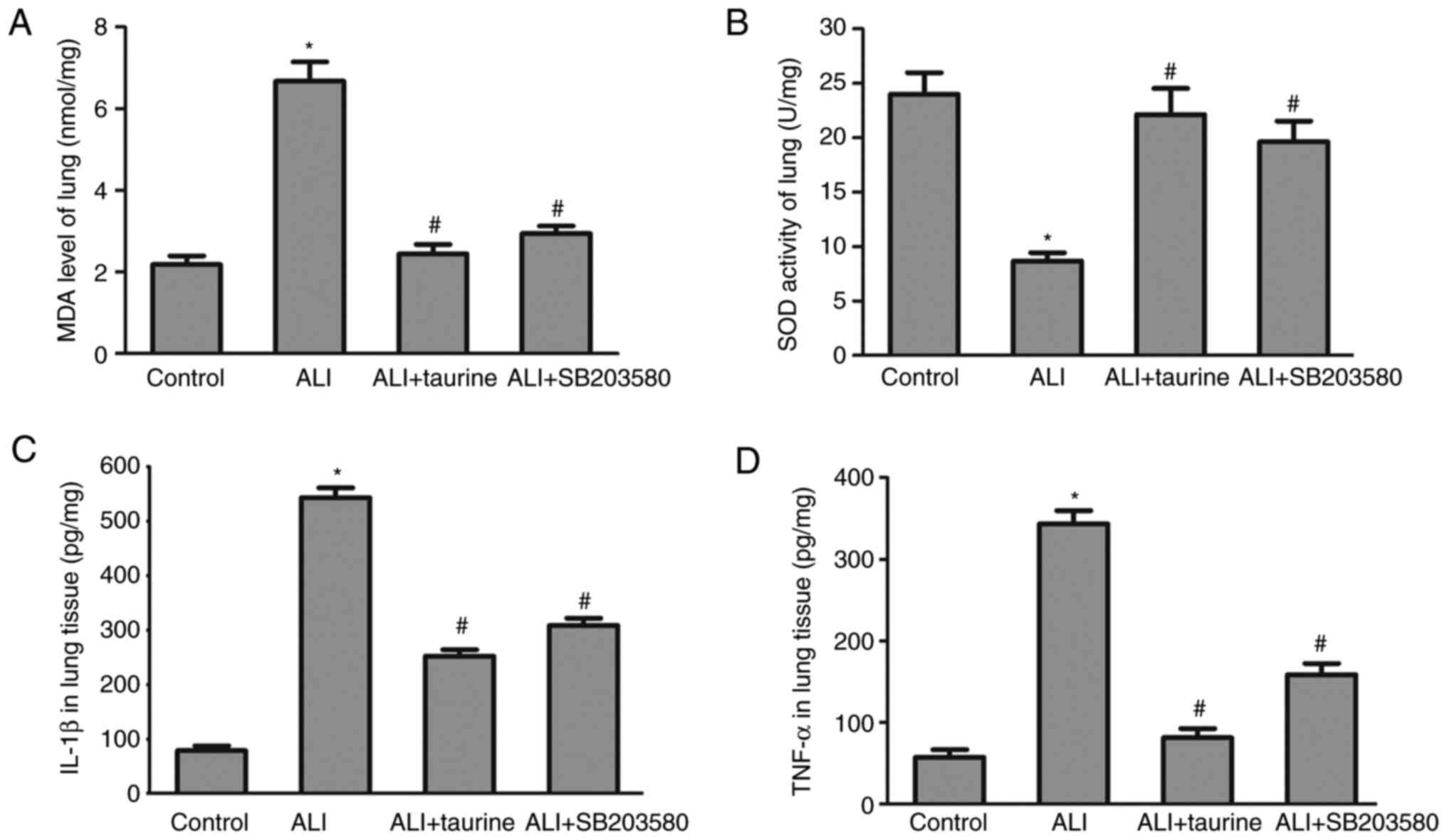
MDA levels and SOD activity in lung
tissues following taurine treatment
MDA levels and SOD activity in the lung tissues of
each group were detected to assess the effects of taurine on
oxidative stress. A significant increase in MDA levels (Fig. 2A) and a decrease in SOD activity
(Fig. 2B) was observed in the lung
tissue of the ALI group compared with that of the control group.
The results indicated that sepsis induced membrane lipid
peroxidation and superoxide toxicity in the lung tissue, whereas
taurine treatment significantly reversed ALI-mediated effects,
suggesting that taurine may serve a protective role against
oxidative stress.
Taurine downregulates TNF-α and IL-1β
concentrations in lung tissue
The inflammatory response is induced in sepsis
(20). Therefore, at 3 days
post-CLP, IL-1β and TNF-α concentrations in the lung tissues of
septic rats were measured by performing ELISAs. The results
demonstrated that the concentrations of IL-1β and TNF-α were
significantly elevated in the lung tissues of CLP-induced septic
ALI model rats compared with those in the control group (Fig. 2C and D). However, the concentrations
of IL-6 and TNF-α were significantly decreased in the taurine
treatment group compared with those in the ALI group. The results
indicated that taurine treatment inhibited the inflammatory
response in sepsis-induced lung injury.
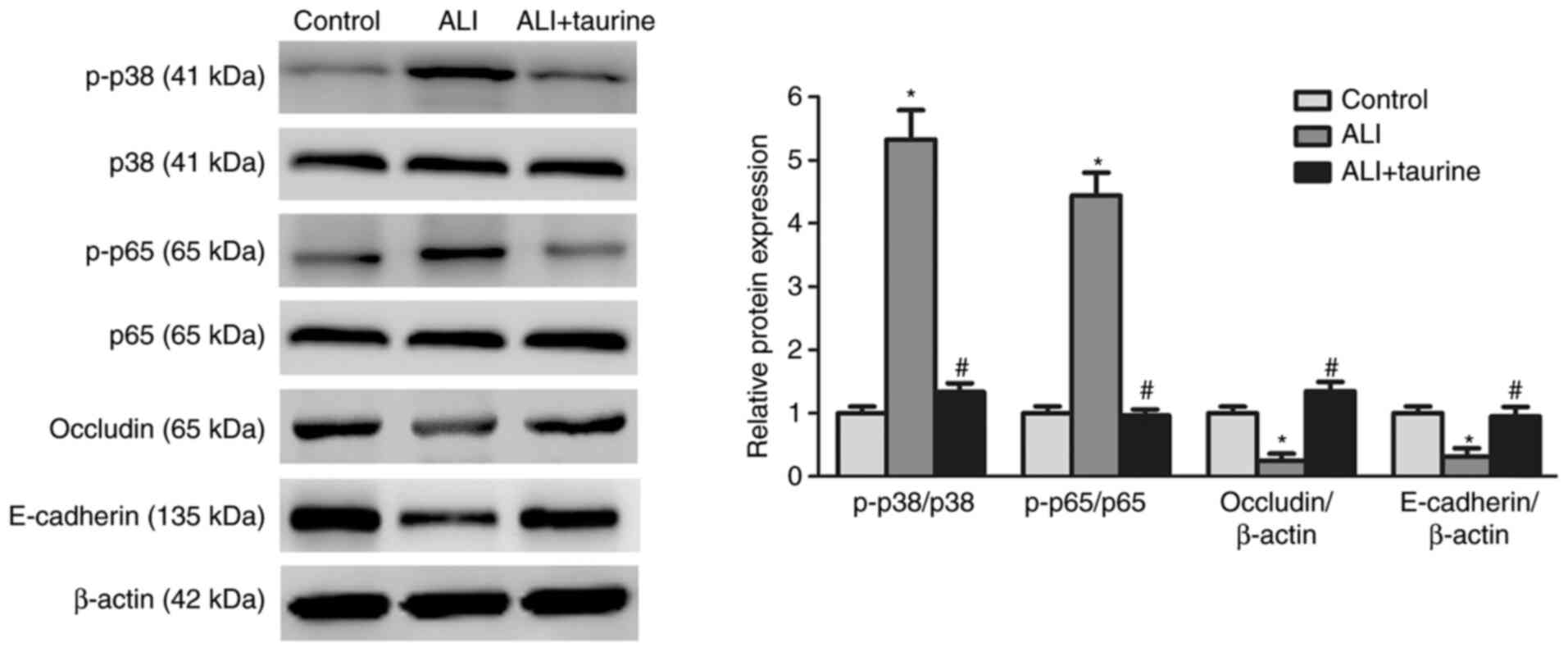
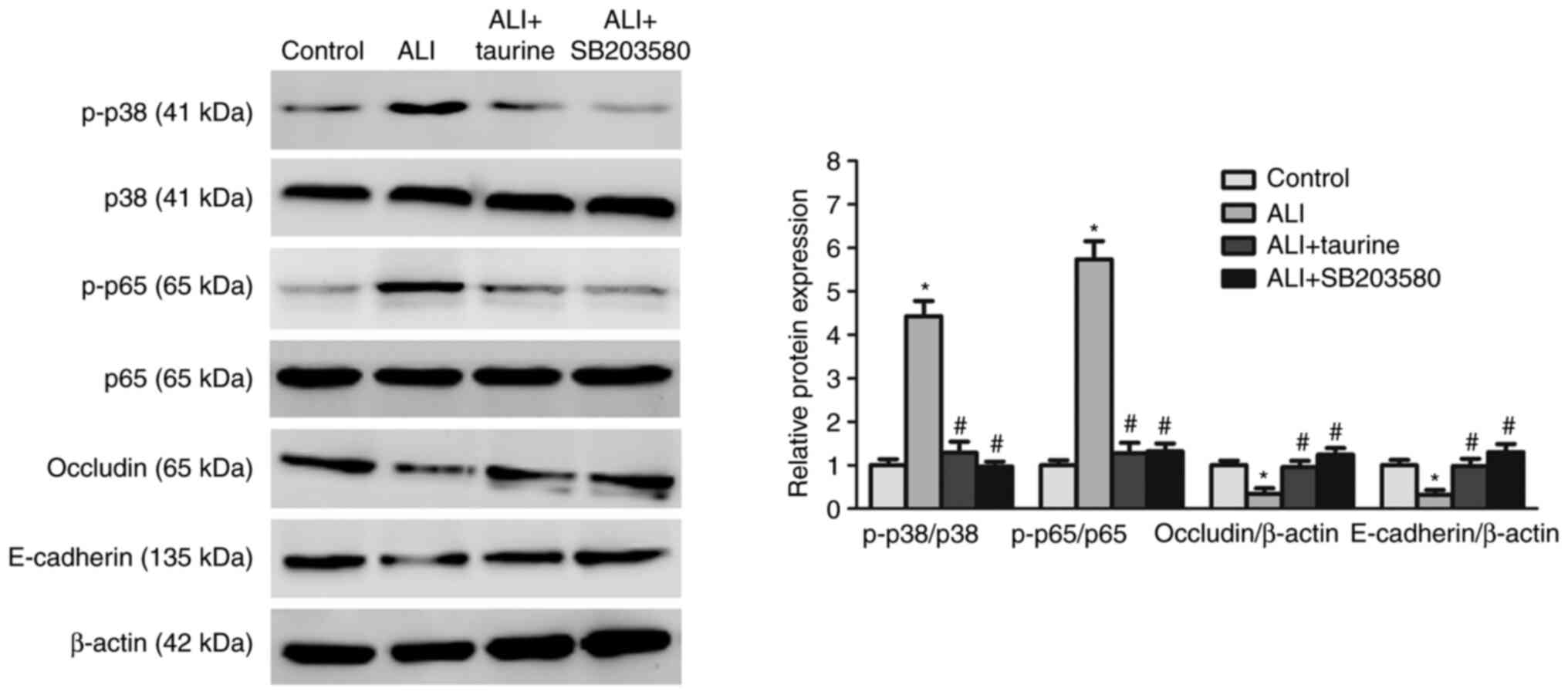
Taurine downregulates the expression
levels of p38/MAPK and NF-κB signaling pathway-related
proteins
The p38/MAPK and NF-κB signaling pathways are key
upstream pathways in the sepsis-induced inflammatory response
(30). To further investigate the
molecular mechanisms underlying the anti-inflammatory effects of
taurine, whether taurine inhibited sepsis-induced activation of the
p38/MAPK and NF-κB signaling pathways was investigated. Western
blotting was performed to detect the protein expression levels of
p-p38 and p-p65, which are key proteins of the p38/MAPK and NF-κB
signaling pathways (30),
respectively. Compared with those in the control group, the ratios
of p-p38/p38 and p-p65/p65 were significantly increased in the lung
tissues of septic rats, indicating that the p38/MAPK and NF-κB
signaling pathways were activated in the injured lung tissues
(Fig. 3). However, the protein
expression levels of the epithelial markers, E-cadherin and
occludin, were significantly decreased in the lung tissues of
CLP-induced septic ALI model rats compared with those in the
control group. By contrast, taurine significantly decreased the
protein expression levels of p-p38 and p-p65 and increased the
expression levels of E-cadherin and occludin in the ALI group.
Collectively, these results suggested that taurine inhibited
sepsis-induced lung injury and protected lung epithelium by
blocking the p38/MAPK and NF-κB signaling pathways, which were
activated by sepsis.
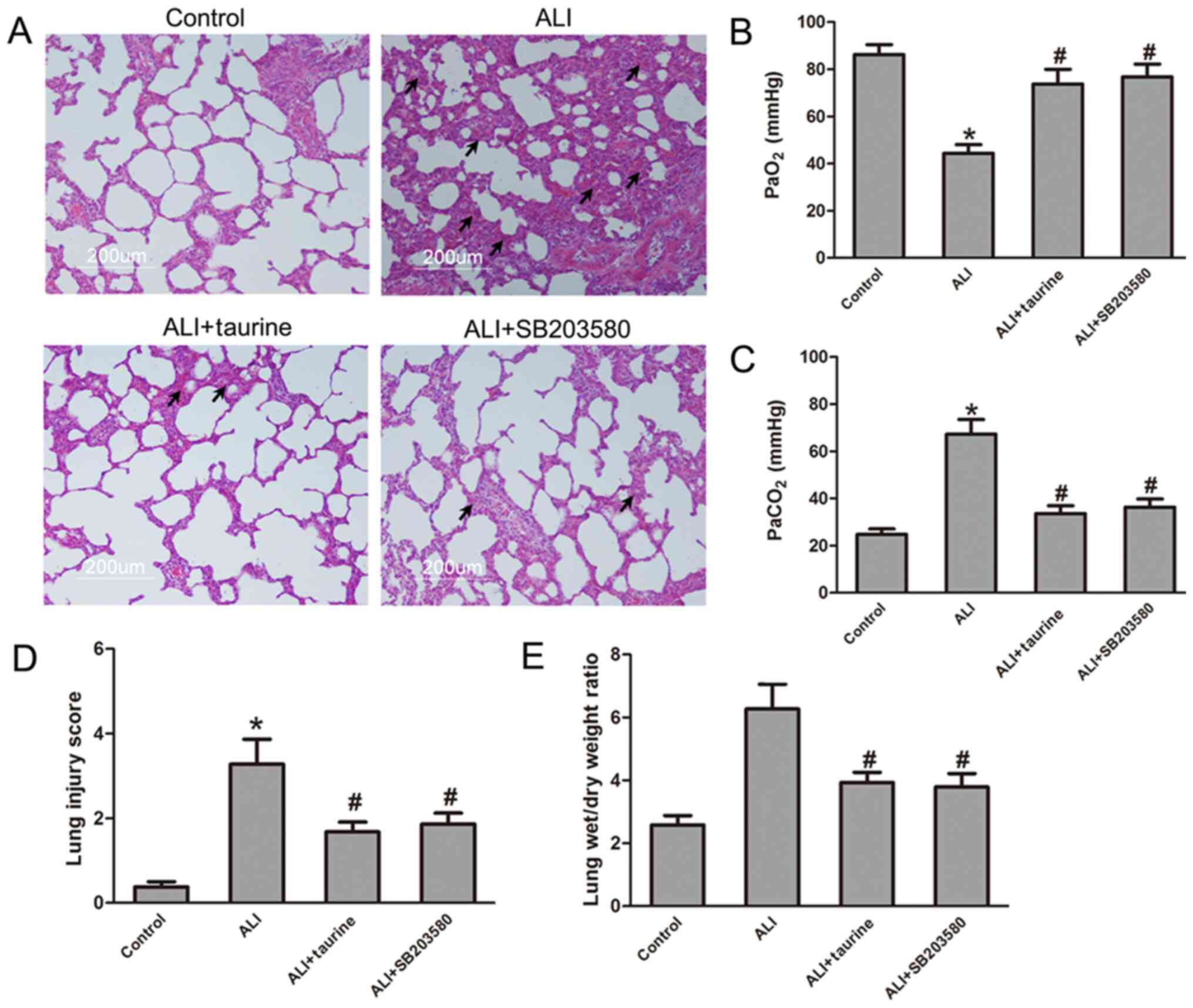
p38/MAPK inhibitor SB203580 attenuates
sepsis-induced lung injury
To identify the functions of the p38/MAPK signaling
pathway in sepsis-induced ALI, CLP-induced septic ALI model rats
were treated with or without the p38/MAPK inhibitor SB203580. The
H&E staining results demonstrated that SB203580 markedly
attenuated lung injury in CLP-induced septic ALI model rats treated
with SB203580 compared with the ALI group (Fig. 4A). SB203580 treatment significantly
increased PaO2 levels and decreased PaCO2
levels in the arterial blood of CLP-induced septic ALI model rats
compared with those in the ALI group (Fig. 4B and C), which suggested that
SB203580 suppressed sepsis-induced lung functions, a similar effect
to that observed in the taurine treatment group. Furthermore, the
lung injury score and lung wet/dry ratio were determined in each
group. As shown in Fig. 4D and E,
the results indicated that SB203580 significantly decreased the
lung injury score and lung wet/dry ratio in CLP-induced septic ALI
model rats compared with those in the ALI group. Notably, the
results of SB203580 treatment group were similar to those in the
taurine treatment group, which suggested that the p38/MAPK
signaling pathway may serve as an effective therapeutic target for
sepsis-induced ALI.
SB203580 suppresses oxidative stress
and the inflammatory response
To determine the effects of SB203580 on oxidative
stress and the inflammatory response, MDA and SOD expression levels
and IL-1β and TNF-α concentrations in the lung tissues of each
group were detected. The results demonstrated that SB203580
treatment significantly decreased MDA levels and the concentrations
of IL-1β and TNF-α, but restored SOD activities compared with those
in the ALI group (Fig. 5). The
results in the ALI + SB203580 group were similar to those in the
ALI + taurine group, which suggested that SB203580 may protect
against CLP-induced lung injury by inhibiting the p38/MAPK
signaling pathway. The results demonstrated that the p38/MAPK
signaling pathway may serve an essential role in the development of
sepsis-induced ALI.
SB203580 inhibits sepsis-activated
p38/MAPK signaling
The present study further indicated that treatment
with SB203580 significantly reduced the ratio of p-p38/p38 in
septic rats, suggesting that SB203580 inhibited activation of the
p38/MAPK signaling pathway mediated by CLP-induced sepsis (Fig. 6). In addition, the western blotting
results demonstrated that the protein expression levels of
E-cadherin and occludin were significantly increased in the ALI +
taurine group compared with those in the ALI group. The results of
the ALI + SB203580 group were also similar to those in the ALI +
taurine group, indicating that SB203580 protected lung epithelium
against sepsis-induced damage, which was similar to the protective
effects of taurine. Furthermore, it was observed that the ratio of
p-p65/p65 was also significantly decreased in the lung tissues of
CLP-induced septic ALI model rats treated with SB203580 compared
with those in the ALI group, which suggested that p38/MAPK may be
an upstream of the NF-κB signaling pathway. The results indicated
that the suppressed p38 MAPK signaling pathway was associated with
the expression level of NF-κB signaling-related proteins in
CLP-induced septic ALI model rats treated with SB203580, which was
similar to the taurine treatment group. Collectively, the results
indicated that taurine may serve a protective role in lung
epithelium damage in septic rats via modulating the p38 MAPK
signaling pathway.
Discussion
Sepsis, as one of the leading causes of death in
patients who are critically ill, induces a complex systemic
inflammatory response syndrome and subsequent multiple organ
failure (31,32). Multiple organ dysfunction syndromes,
particularly ALI/ARDS, are major causes of morbidity and mortality
in patients with sepsis (33).
ALI/ARDS, which is induced by sepsis, is characterized by the rapid
onset of respiratory insufficiency, acute inflammatory response,
alveolar epithelium damage, bleeding and lung interstitial edema
(6,34,35).
The pathogenesis of ALI/ARDS is not completely understood and there
are no specific effective therapeutic strategies available
(36,37). Therefore, it is important to
investigate the molecular mechanisms underlying sepsis-induced
ALI/ARDS to develop effective treatments.
Taurine, an endogenous free amino acid, is highly
expressed in numerous mammalian tissues. Taurine has been reported
to be an effective cell protector, which could protect against lung
damage by mitigating oxidative stress and reducing the expression
of inflammatory factors (20,21).
Previously, Li et al (21)
reported that taurine could reverse paraquat-induced disturbances
in glutathione (GSH) content and GSH peroxidase activity. Moreover,
taurine attenuated paraquat-induced A549 cell apoptosis via
modulation of oxidative stress. Although it has been reported that
taurine reverses paraquat-induced oxidative stress to protect
against cell apoptosis, the mechanisms underlying taurine-mediated
regulation of oxidative stress remain unclear. In contrast to the
CLP-induced septic ALI rat model established in the present study,
Men et al (26) demonstrated
that taurine treatment attenuated lung injury following limb
ischemia reperfusion (LIR). It has also been reported that taurine
prevents oxidative stress-induced cell apoptosis, decreases MDA
level, and increases SOD and catalase activities by inhibiting
endoplasmic reticulum stress. However, whether taurine attenuates
the expression levels of inflammatory factors to protect against
LIR-induced lung injury is not completely understood. Our previous
study indicated that taurine enhanced the protective effect of
dexmedetomidine on sepsis-induced lung injury by suppressing
activation of the NF-κB signaling pathway and decreased the
expression levels of IL-6 and IL-1β (20). Taurine serves an essential role in
regulating the T helper (Th)1/Th2 cytokine balance (38). In the present study, the protective
effects of taurine on sepsis-induced lung injury were investigated.
Taurine treatment preserved epithelium integrity by increasing the
expression levels of epithelium markers E-cadherin and occludin to
alleviate CLP-induced lung injury in rats. The proinflammatory
cytokines TNF-α and IL-1β are implicated in the pathogenesis of
sepsis-induced lung injury (39).
The present study demonstrated that taurine treatment decreased
sepsis-induced increases in TNF-α and IL-1β expression levels in
lung tissues, indicating that taurine treatment inhibited the
inflammatory response in CLP-induced septic ALI model rats.
Oxidative stress serves an essential role in the pathogenesis of
ALI (40). Therefore, oxidative
stress levels were assessed in the present study by detecting MDA
levels and SOD activities. MDA levels are an index of membrane
lipid peroxidation and can be used as a marker to indicate the
degree of tissue peroxidation damage. SOD serves an important role
in the balance of oxidation and antioxidation (25). The results of the present study
indicated that taurine effectively attenuated oxidative stress
damage in sepsis-induced lung injury, suggesting that taurine could
be used as a protective factor to attenuate lung injury by
decreasing sepsis-induced inflammatory responses and oxidative
stress. Moreover, taurine may protect epithelium integrity by
promoting the expression of epithelial markers. Therefore, the
present study further suggested that taurine may serve an essential
role in the lung repair process.
To clarify the mechanisms underlying
taurine-mediated protection of lung tissues after CLP induction,
p38/MAPK and NF-κB signaling pathways involved in sepsis-induced
inflammatory responses and oxidative stress were investigated.
Recent studies have demonstrated that the p38/MAPK signaling
pathway is one of the most important signaling pathways, serving a
critical role in the phosphorylation of substrates involved in the
sepsis-induced inflammatory response (12,39).
Certain studies have indicated that the p38/MAPK signaling pathway
also serves an essential role in regulating oxidative
stress-related signaling and participates in the pathogenesis of
ALI (12,41,42).
It is important to identify the changes in p38/MAPK activity
associated with sepsis-induced ALI; therefore, the protein
expression levels of p-p38 in the p38/MAPK signaling pathway were
detected in the lung tissues of CLP-induced septic ALI model rats.
The results demonstrated that taurine suppressed sepsis-induced
activation of the p38/MAPK signaling pathway. Furthermore, the
NF-κB signaling pathway, which was previously reported to be
involved in the regulation of inflammatory mediator generation and
immune responses (43,44), was also investigated in CLP-induced
septic ALI model rats. The results demonstrated that taurine
treatment decreased the ratio of p-p65/p65 to block NF-κB signaling
in CLP-induced septic ALI model rats. To investigate the role of
the p38/MAPK signaling pathway in the pathogenesis of
sepsis-induced lung injury, SB203580, an inhibitor of the p38/MAPK
signaling pathway, was used to treat the CLP-induced septic ALI
model rats. In the present study, the H&E staining and
respiratory function test results indicated that SB203580
attenuated lung injury, reduced inflammatory cell infiltration and
promoted lung restoration in CLP-induced septic ALI model rats.
Furthermore, MDA levels and SOD activity, which are important
indexes of oxidative stress, were significantly decreased after
SB203580 treatment in CLP-induced septic ALI model rats, which
indicated that inhibition of the p38/MAPK signaling pathway could
ameliorate oxidative stress. The concentrations of proinflammatory
cytokines, TNF-α and IL-1β, were significantly decreased in the
SB203580 treatment group compared with those in the ALI group,
suggesting that SB203580 administration reduced local inflammation
in CLP-induced septic ALI model rats. Moreover, sepsis-induced
p38/MAPK phosphorylation was attenuated by treatment with SB203580.
In addition, SB203580 administration also decreased the ratio of
p-p65/p65, a NF-κB signaling pathway-related protein, in
CLP-induced septic ALI model rats. Similarly, the results indicated
that inhibition of p38/MAPK signaling could restore the expression
levels of lung epithelium markers, suggesting that SB203580
protected against sepsis-induced lung epithelium damage. The
results observed following SB203580 treatment of septic rats were
similar to those observed in the taurine treatment group, which
suggested that taurine attenuated lung injury and promoted lung
repair by blocking the p38/MAPK signaling pathway. The present
study also revealed that inhibition of the p38/MAPK signaling
pathway could alter the activity of the NF-κB signaling pathway,
which suggested that the p38/MAPK signaling pathway may crosstalk
with the NF-κB signaling pathway for the generation of inflammatory
factors and the immune response in sepsis-induced ALI. However, the
present study only used the CLP-induced septic ALI rat model to
investigate the protective effects and molecular mechanisms
underlying taurine in sepsis-induced lung injury. Due to this
limitation, a lung epithelial cell line should be treated with LPS
to establish an in vitro experiment model to verify the
results of the present study and investigate the role of p38/MAPK
signaling or downstream signaling of p38/MAPK in regulating the
inflammatory response and oxidative stress during the pathogenesis
of LPS-induced lung epithelium damage.
In conclusion, to the best of our knowledge, the
present study was the first to investigate the protective effects
of taurine on sepsis-induced lung injury via suppressing the
p38/MAPK signaling pathway, inhibiting the inflammatory response
and oxidative stress levels, and preserving lung epithelium
integrity. The present study demonstrated that taurine displayed
potential therapeutic effects on sepsis-induced lung injury, and
the p38/MAPK signaling pathway may serve as a promising therapeutic
target for sepsis-induced lung injury.
Acknowledgements
The authors would like to thank Dr Zhaorui Sun
(Jinling Hospital, Nanjing, China) for their technical
assistance.
Funding
The present study was supported by the Science and
Technology Development Foundation of Nanjing Medical University
(grant no. NMUB2018288).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JCh and XX performed the majority of the
experiments. JCa and LJ performed the statistical analyses and
performed some of the experiments. BS and WZ designed the study and
analyzed the data. All authors contributed to preparing the
manuscript. All authors have read and approved the final
manuscript. BS and WZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All animal studies were conducted in accordance with
the Guide for the Care and Use of Experimental Animals established
by National Society for Medical Research and approved by the Ethics
Committee of Nanjing Medical University (approval no. SYXK
2019-0042).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALI
|
acute lung injury
|
|
CLP
|
cecal ligation and puncture
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
Kumar V: Pulmonary Innate Immune Response
Determines the Outcome of Inflammation During Pneumonia and
Sepsis-Associated Acute Lung Injury. Front Immunol. 11:17222020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lelubre C and Vincent JL: Mechanisms and
treatment of organ failure in sepsis. Nat Rev Nephrol. 14:417–427.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The Third International Consensus
Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fang WF, Huang CH, Chen YM, Hung KY, Chang
YC, Lin CY, Fang YT, Chang YT, Chen HC, Huang KT, et al:
Application of dynamic pulse pressure and vasopressor tools for
predicting outcomes in patients with sepsis in intensive care
units. J Crit Care. 52:156–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sterling SA, Puskarich MA, Glass AF,
Guirgis F and Jones AE: The Impact of the Sepsis-3 Septic Shock
Definition on Previously Defined Septic Shock Patients. Crit Care
Med. 45:1436–1442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li T and Zou C: The Role of
Deubiquitinating Enzymes in Acute Lung Injury and Acute Respiratory
Distress Syndrome. Int J Mol Sci. 21:48422020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K;
International Forum of Acute Care Trialists; Current Estimates and
Limitations, : Assessment of Global Incidence and Mortality of
Hospital-treated Sepsis. Am J Respir Crit Care Med. 193:259–272.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Liu S, Han S, Hu Y, Wu Z, Shi X,
Pang B, Ma Y and Jin J: The HDL from septic-ARDS patients with
composition changes exacerbates pulmonary endothelial dysfunction
and acute lung injury induced by cecal ligation and puncture (CLP)
in mice. Respir Res. 21:2932020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu X, Liang X, Li H and Sun R:
LPS-induced vein endothelial cell injury and acute lung injury have
Btk and Orai 1 to regulate SOC-mediated calcium influx. Int
Immunopharmacol. 90:1070392021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Dong L, Zhao D, Gao F and Yan J:
Classical dendritic cells regulate acute lung inflammation and
injury in mice with lipopolysaccharide induced acute respiratory
distress syndrome. Int J Mol Med. 44:617–629. 2019.PubMed/NCBI
|
|
11
|
Wang F, Wang M, Wang J, Chen M, Sun S, Yao
S and Xia H: Maresin1 ameliorates sepsis-associated lung injury by
inhibiting the activation of the JAK2/STAT3 and MAPK/ NF-κB
signaling pathways. Microb Pathog. 148:1044682020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu XH, Situ HL, Chen JP and Yu RH: Lipoxin
A4 alleviates lung injury in sepsis rats through p38/MAPK signaling
pathway. J Biol Reg Homeost Agents. 34:807–814. 2020.PubMed/NCBI
|
|
13
|
Zhang HF, Zhang HB, Wu XP, Guo YL, Cheng
WD and Qian F: Fisetin alleviates sepsis-induced multiple organ
dysfunction in mice via inhibiting p38 MAPK/MK2 signaling. Acta
Pharmacol Sin. 41:1348–1356. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao Y, Chen X, Liu Y, Chen X, Li C, Wang
L and Zhao W: Dexmedetomidine alleviates lung injury in sepsis mice
through regulating P38 MAPK signaling pathway. Panminerva Med. Mar
30–2020.(Epub ahead of print). doi:
10.23736/S0031-0808.20.03885-9.
|
|
15
|
Feng Y, Fang Z, Liu B and Zheng X: p38MAPK
plays a pivotal role in the development of acute respiratory
distress syndrome. Clinics (São Paulo). 74:e5092019. View Article : Google Scholar
|
|
16
|
Chen X, Hu J, Pan Y and Tang Z: Novel
noncoding RNAs biomarkers in acute respiratory distress syndrome.
Expert Rev Respir Med. 14:299–306. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jakaria M, Azam S, Haque ME, Jo SH, Uddin
MS, Kim IS and Choi DK: Taurine and its analogs in neurological
disorders: Focus on therapeutic potential and molecular mechanisms.
Redox Biol. 24:1012232019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schaffer S and Kim HW: Effects and
Mechanisms of Taurine as a Therapeutic Agent. Biomol Ther (Seoul).
26:225–241. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thirupathi A, Pinho RA, Baker JS, István B
and Gu Y: Taurine Reverses Oxidative Damages and Restores the
Muscle Function in Overuse of Exercised Muscle. Front Physiol.
11:5824492020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao W, Jia L, Yang HJ, Xue X, Xu WX, Cai
JQ, Guo RJ and Cao CC: Taurine enhances the protective effect of
Dexmedetomidine on sepsis-induced acute lung injury via balancing
the immunological system. Biomed Pharmacother. 103:1362–1368. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Wang J, Wei BK, Dong G and Wang X:
Protective Effect of Taurine on Paraquat-Induced Lung Epithelial
Cell Injury. Adv Exp Med Biol. 1155:739–746. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao Y, Li X, Gao J, Zhang Z, Feng Y, Nie
J, Zhu W, Zhang S and Cao J: Metabolomic Analysis of
Radiation-Induced Lung Injury in Rats: The Potential
Radioprotective Role of Taurine. Dose Response.
17:1559325819883479. 2019. View Article : Google Scholar
|
|
23
|
Sun Z, Yang Z, Wang M, Huang C, Ren Y,
Zhang W, Gao F, Cao L, Li L and Nie S: Paraquat induces pulmonary
fibrosis through Wnt/β-catenin signaling pathway and myofibroblast
differentiation. Toxicol Lett. 333:170–183. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Z, Ding X, Jin S, Pitt B, Zhang L,
Billiar T and Li Q: WISP1-αvβ3 integrin signaling positively
regulates TLR-triggered inflammation response in sepsis induced
lung injury. Sci Rep. 6:288412016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Q, Hua F, Deng C, Zhang J, Xu G and Hu
Y: Protective and therapeutic effects of Danhong injection on acute
pancreatitis associated lung injury. Mol Med Rep. 16:7603–7608.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Men X, Han S, Gao J, Cao G, Zhang L, Yu H,
Lu H and Pu J: Taurine protects against lung damage following limb
ischemia reperfusion in the rat by attenuating endoplasmic
reticulum stress-induced apoptosis. Acta Orthop. 81:263–267. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanino Y, Makita H, Miyamoto K, Betsuyaku
T, Ohtsuka Y, Nishihira J and Nishimura M: Role of macrophage
migration inhibitory factor in bleomycin-induced lung injury and
fibrosis in mice. Am J Physiol Lung Cell Mol Physiol.
283:L156–L162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Z, Gong X, Zhu H, Wang C, Xu X, Cui D,
Qian W and Han X: Inhibition of Wnt/β-catenin signaling promotes
engraftment of mesenchymal stem cells to repair lung injury. J Cell
Physiol. 229:213–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park I, Kim M, Choe K, Song E, Seo H,
Hwang Y, Ahn J, Lee SH, Lee JH, Jo YH, et al: Neutrophils disturb
pulmonary microcirculation in sepsis-induced acute lung injury. Eur
Respir J. 53:532019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cong Z, Li D, Tao Y, Lv X and Zhu X: α2A
-AR antagonism by BRL-44408 maleate attenuates acute lung injury in
rats with downregulation of ERK1/2, p38MAPK, and p65 pathway. J
Cell Physiol. 235:6905–6914. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rhodes A, Phillips G, Beale R, Cecconi M,
Chiche JD, De Backer D, Divatia J, Du B, Evans L, Ferrer R, et al:
The Surviving Sepsis Campaign bundles and outcome: results from the
International Multicentre Prevalence Study on Sepsis (the IMPreSS
study). Intens Care Med. 41:1620–1628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Machado FR, Cavalcanti AB, Bozza FA,
Ferreira EM, Angotti Carrara FS, Sousa JL, Caixeta N, Salomao R,
Angus DC, Pontes Azevedo LC, et al SPREAD Investigators; Latin
American Sepsis Institute Network, : The epidemiology of sepsis in
Brazilian intensive care units (the Sepsis PREvalence Assessment
Database, SPREAD): An observational study. Lancet Infect Dis.
17:1180–1189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan EKY and Fan J: Regulation of alveolar
macrophage death in acute lung inflammation. Respir Res. 19:502018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Magnani ND, Dada LA and Sznajder JI:
Ubiquitin-proteasome signaling in lung injury. Transl Res.
198:29–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matthay MA, Arabi YM, Siegel ER, Ware LB,
Bos LDJ, Sinha P, Beitler JR, Wick KD, Curley MAQ, Constantin JM,
et al: phenotypes and personalized medicine in the acute
respiratory distress syndrome. Intensive Care Med. 46:2136–2152.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Masterson C, Jerkic M, Curley GF and
Laffey JG: Mesenchymal stromal cell therapies: Potential and
pitfalls for ARDS. Minerva Anestesiol. 81:179–194. 2015.PubMed/NCBI
|
|
37
|
Wang YM, Qi X, Gong FC, Chen Y, Yang ZT,
Mao EQ and Chen EZ: Protective and predictive role of Mucin1 in
sepsis-induced ALI/ARDS. Int Immunopharmacol. 83:1064382020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu YF, Zhang QY, Wang LH, Liu XY and Zhang
SX: The protective effects of taurine on experimental autoimmune
myocarditis. Eur Rev Med Pharm. 21:1868–1875. 2017.PubMed/NCBI
|
|
39
|
Shao Z, Li Q, Wang S and Chen Z:
Protective effects of PNU 282987 on sepsis induced acute lung
injury in mice. Mol Med Rep. 19:3791–3798. 2019.PubMed/NCBI
|
|
40
|
Bhavsar TM, Cantor JO, Patel SN and
Lau-Cam CA: Attenuating effect of taurine on
lipopolysaccharide-induced acute lung injury in hamsters. Pharmacol
Res. 60:418–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cui S, Nian Q, Chen G, Wang X, Zhang J,
Qiu J and Zhang Z: Ghrelin ameliorates A549 cell apoptosis caused
by paraquat via p38-MAPK regulated mitochondrial apoptotic pathway.
Toxicology. 426:1522672019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang CY, Lin SQ, Liu FY, Ma JH, Jia FJ,
Han Z, Xie WD and Li X: The anti-inflammatory effect of
-kaur-15-en-17-al-18-oic acid on lipopolysaccharide-stimulated
RAW264.7 cells associated with NF-κB and P38/MAPK pathways. J Asian
Nat Prod Res. 23:570–583. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fang H, Zhang J, Ao M, He F, Chen W, Qian
Y, Zhang Y, Xu Y and Fang M: Synthesis and discovery of ω-3
polyunsaturated fatty acid- alkanolamine (PUFA-AA) derivatives as
anti-inflammatory agents targeting Nur77. Bioorg Chem.
105:1044562020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiao Q, Cui Y, Zhao Y, Liu L, Wang H and
Yang L: Orientin relieves lipopolysaccharide-induced acute lung
injury in mice: The involvement of its anti-inflammatory and
anti-oxidant properties. Int Immunopharmacol. 90:1071892021.
View Article : Google Scholar : PubMed/NCBI
|