Introduction
Necrotizing enterocolitis (NEC) is a severe neonatal
disease with a high mortality rate (1). A previous study reported that low
SIRT1 expression in the intestinal tissues of patients with NEC,
which may be due to the suppression of the SIRT1 signaling pathway,
thereby promoting the occurrence of NEC (2). In addition, the serum SIRT1 level in
children with NEC was low and continued to decrease with disease
progression (2). According to
previous results, serum SIRT1 is highly sensitive and specific to
the diagnosis of NEC, which is of certain reference value for the
early diagnosis of NEC (3). In a
rat model, the results indicated that the expression level of SIRT1
was markedly decreased in the intestine of rats with NEC induced by
LPS, but this effect was reversed with SIRT1 activator treatment to
protect the intestine by inhibiting the inflammatory response
(3,4). Therefore, SIRT1 may be a novel
diagnostic biomarker of NEC and serves an important role in the
development of NEC. However, the mechanism has not been fully
elucidated.
In recent years, microRNA (miRNAs) has been revealed
to serve an important regulatory role in the occurrence and
development of NEC (5). The miRNAs
are a class of small RNA molecules that regulate the translational
level of genes. They can inhibit the translation of protein-coding
gene expression to repress protein translation by binding to the
target mRNAs (6–8). To date, it has been revealed that
certain miRNAs are associated with the occurrence and development
of NEC, including miR-429, miR-431 and miR-1290 (9,10). A
previous study demonstrated that miRNAs are associated with cancer
development, progression and treatment (11). The miR-34a is highly expressed and
serves an important role in a variety of tumor types and diseases
(12–16). A previous study reported that the
miR-34a and CSF1R inhibit each other and are lost in tumor cells,
which may be associated with the treatment and prognosis of
colorectal cancer (12). The
miR-34a is an important small RNA molecule and is recognized as a
master regulator of tumor suppression (13). Notably, the miR-34a expression is
governed by p53, but it may be regulated by multiple
p53-independent mechanisms (14–16).
Another study demonstrated that propofol may induce apoptosis and
inhibit the migration of PANC-1 cells in pancreatic cancer by
promoting the upregulation of miR-34a-dependent LOC285194 and
E-cadherin, respectively (17). In
addition, miR-34a may target the WNT1 protein, and promote the
proliferation and invasion of E-P cadherin through the WNT1
switch/catenin pathway in cervical squamous cell carcinoma cells
(18).
Additionally, miR-34a regulation in inflammation is
exemplified by direct repression of them iR-34a gene, which may
provide a conserved STAT3-binding site in the first intron in
colorectal cancer cells (19,20).
Notably, the miR-34a is a crucial regulator of the suppression of
tumor progression by inhibiting the IL-6/STAT3/miR-34a pathway
(19). A previous study reported
that melatonin may be effective in the hepatotoxicity induced by
BAP through the miR-34a/Sirt1/autophagy molecular pathway (21). Additionally, CO decreases cell
senescence and liver senescence by regulating miR-34a and Sirt1
expression (22). Another previous
study indicated that miR-34a-5p may target SIRT1 and serves an
important role in decreasing the damage of nucleus pulpoda cells
induced by compression load (23).
Serum miR-34a in patients with chronic hepatitis C (CHC) may
promote liver fibrosis by mediating the Sirt1/P53 pathway, which
may be a key biomarker for the prognosis and diagnosis of patients
with CHC (16). These studies
revealed an important function of miR-34a providing a potential
therapeutic strategy for inflammation and also demonstrated that
the miR-34a/SIRT1 signaling axis is important in tumors and
diseases. However, the function of miR-34a and its mechanism in NEC
remain unclear. Therefore, in the present study, the function of
the miR-34a/SIRT1 signaling axis was evaluated in the NEC patient
serum and NEC rat models.
Materials and methods
Blood samples
Blood samples were collected from control subjects
and patients (Table I) with
necrotizing enterocolitis (NEC) from January 2019 to January 2020.
The present study was approved by the Fujian Provincial Hospital
Ethics Committee (K2019-01-030). It was performed in accordance
with the International Ethical Guidelines for Human Biomedical
Research (2012). The information regarding the patients with NEC
was provided by the guardians of the patients. Written informed
consent was obtained from participants involved in the study. Study
subjects (n=30 per group) comprised normal subjects and patients
with NEC. Patient clinical information was collected, and the
correlations were analyzed. The sample (1 ml of fasting peripheral
venous blood) was collected from the radial vein of the newborn.
The biochemical analyses were performed immediately after the
sample collection, and the samples were stored at −80°C for further
analyses. Plasma albumin, glucose, C-reactive protein (CRP) and
procalcitonin (PCT) were measured using an auto analyzer (Hitachi
912 Autoanalyser; Hitachi, Ltd.).
 | Table I.NEC clinical indices. |
Table I.
NEC clinical indices.
| Characteristic | NEC group
(n=30) | Control group
(n=30) | Statistics | P-value |
|---|
| Gestation,
weeks |
|
|
χ2=0.067 | 0.7952 |
|
<34 | 19 | 11 |
|
|
|
≥34 | 11 | 19 |
|
|
| Birth weight,
g |
|
|
χ2=4.267 | 0.0389a |
|
<1,500 | 16 |
7.0 |
|
|
|
≥1,500 | 14 | 23.0 |
|
|
| Sex |
|
|
χ2=5.711 | 0.0169a |
|
Male | 16 | 17 |
|
|
|
Female | 14 | 13 |
|
|
| Albumin, g/l | 24.30±4.14 | 30.27±4.40 | t=5.320 |
<0.001c |
| Glucose,
mmol/l |
2.68±2.80 |
3.85±1.52 | z=−3.260 | 0.0011b |
| CRP, mg/l |
32.50±17.81 |
4.89±2.20 | z=−6.653 |
<0.001c |
| PCT, ng/ml |
7.77±1.93 |
0.30±0.12 | t=−20.811 |
<0.001c |
Construction and evaluation of the NEC
rat model
Newborn SPF Sprague-Dawley (SD) rats that did not
eat colostrum within 2 h of birth were used for NEC model
construction. Newborn rats were placed in a self-made incubator
(temperature 37°C, humidity 45–55%), and artificially fed with milk
substitutes, which were prepared according to previous studies
(24,25). SD rats were purchased from Shanghai
Slake Laboratory Animal Co., Ltd. (license no. SCXK 2017-0005). The
40SD rats (body weight, 5.6–8.5 g) were divided into five groups
(n=8 per group), including control group, NEC model group,
NEC+miR34a inhibitor group (inhibitor, ACAACCAGCUAAGACACUGCCA;
provided by BD Biosciences, NEC+SIRT1 activator group, and
NEC+miR34a+SIRT1 inhibitor group (Guangzhou Baisaike Biotechnology
Co., Ltd.). The rat model was used according to the Animal Care and
Use Committee of Provincial Clinical College of Fujian Medical
University. The intestinal tissue and blood were sampled on the
sixth day. The paraformaldehyde-fixed intestinal tissue was stained
with hematoxylin for 8 min and 0.5% eosin for 2 min at 24°C.
Intestinal function was assessed by a double-blinded scoring method
using the Shiou scoring standard (26). Morphologically, indicators of the
integrity and tissue structure of intestinal mucosa villi were
recorded the five fields each pathological tissue section and
analyzed by a light microscope (Olympus Corporation, magnification,
×40).
Analysis of inflammatory and oxidative
stress cytokines
The serum concentrations of tumor necrosis factor
(TNF) α, interleukin (IL) 1β, IL-6, IL-8, IL-10, monocyte
chemoattractant protein (MCP) 1 and vascular cell adhesion molecule
(VCAM) was determined using ELISA method and according to the
manufacturer's protocols. The TNF-α (cat. no. ab181421), IL-1β
(cat. no. ab214025), IL-6 (cat. no. ab46027), IL-8 (cat. no.
ab214030), were purchased from Abcam, the rat IL-8 (cat. no.
SEKR-0071), IL-10 (cat. no. SEKR-0006) and MCP-1 (cat. no.
SEKR-0024) kits were purchased from Suolaibao Technology Co., Ltd.
and the VCAM1 kits (cat. no. CSB-E07275r) were purchased from Wuhan
Huamei Biological Engineering Co., Ltd.
The serum concentrations of superoxide dismutase
(SOD) and malondialdehyde (MDA) were determined using commercial
kits (Beijing Solarbio Science & Technology Co., Ltd.)
according to the manufacturer's protocols.
Total RNA isolation and qPCR
analysis
Total RNA was isolated from the serum of patients
with NEC and normal controls using RNAiso Reagent (Takara Bio,
Inc.) according to the manufacturer's protocols. Then, 2 µg of RNA
was used as input material to synthesize cDNA. For qPCR, 2.0 µl
cDNA was mixed with 12.5 µl qPCR SYBR® Green Master Mix
(A6001; Promega Corporation), 2.0 µl primers and 8.5 µl
nuclease-free water to a final volume of 25.0 µl. The miR-34a
analysis was conducted in a final volume of 20.0 µl. The primers
were designed and synthesized by Sangon Biotech Co., Ltd. and the
primer sequences were as follows: SIRT1 forward,
AGATCCTCAAGCCATGTTCG and reverse, CAGAGCTGCTCATGAATGCTG; GAPDH
forward, GTCATCCCTGAGCTGAACGG and reverse, CCACCTGGTGCTCAGTGTAG;
miR-34a forward, CGCGTGGCAGTGTCTTAGCT and reverse,
AGTGCAGGGTCCGAGGTATT; miR-34a RT,
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAACC; U6 forward,
CTCGCTTCGGCAGCACATATACT and reverse, ACGCTTCACGAATTTGCGTGTC; and U6
RT, AAAATATGGAACGCTTCACGAATTTG. The qPCR conditions were 95°C for 5
min, followed by 40 cycles at 95°C for 10 sec, 60°C for 30 sec,
72°C for 30 sec and 95°C for 15 sec. According to a previous study,
the mRNA expression was assayed using the ABI 7500 PCR system (ABI)
and calculated using the2−ΔΔCq method of
ΔΔCt=(Ct.Target-Ct.
GAPDH)X-(Ct.Target-Ct.GAPDH)Control
(27).
Western blot analysis
Total protein was isolated from cells using RIPA
buffer (cat. no. R0278-50ML, Sigma-Aldrich; Merck KGaA) containing
the PMSF. The protein sample concentration was detected using a BCA
Protein Assay Kit PC0020-500 (Beijing Solarbio Science &
Technology Co., Ltd.). SDS-PAGE (12%) was performed to separate
proteins (15 µg) according to a previous study (28). Following separation, the blots were
blocked and transferred onto nitrocellulose membranes. The
membranes were blocked with 5% bovine serum albumin (BSA) for 1 h
at room temperature. Following washing with TBS, the membranes were
incubated with the antibodies (Abcam) against SIRT1 (1:1,000
dilution; cat. no. ab189494), TNF-a (1:1,500 dilution; cat. no.
ab205587), IL-1β (1:1,500 dilution; cat. no. ab205924), IL-6
(1:1,500 dilution; cat. no. ab9770), IL-8 (1:1,000 dilution; cat.
no. ab170381) and IL-10 (1:1,000 dilution; cat. no. ab9969)
overnight at 4°C. The anti-mouse HRP-conjugated secondary antibody
(1:1,000; cat. no. ab8227) was used and incubated for 2 h at room
temperature. Next, the signal was detected by chemiluminescence
with Thermo ECL (34080; Thermo Fisher Scientific, Inc.).
Anti-β-actin was used as a loading control. All antibodies were
purchased from Abcam. The results were collected by the Versa Doc
imaging system (Shanghai Peiqing Science & Technology Co.,
Ltd.) and analyzed using ImageJ software (V1.8.0.112, National
Institutes of Health).
Statistical analysis
The results were analyzed by SPSS 22.0 statistical
software (IBM Corp.) using Student's t-test or one-way analysis of
variance by the LSD model. P<0.05 was considered to indicate a
statistically significant difference. All data are expressed as the
mean ± standard deviation. The clinical index was analyzed using
SPSS 22.0 statistical software using the models of Chi-square test,
Z nonparametric test and T-test. Correlation analysis was performed
between miR-34a and SIRT1, SOD, IL-10, TNF-a, IL-1β, IL-6, IL-8,
MCP-1, MDA and VCAM-1 by SPSS 22.0 statistical software using
Pearson's correlation model.
Results
Correlation between miR-34a and the
NEC clinical index
The results of the NEC clinical index, including
birth weight and the concentrations of albumin and glucose, were
significantly decreased compared with the control group, but the
CRP and PCT concentrations were significantly increased (Table I). The miR-34a and SIRT1 expression
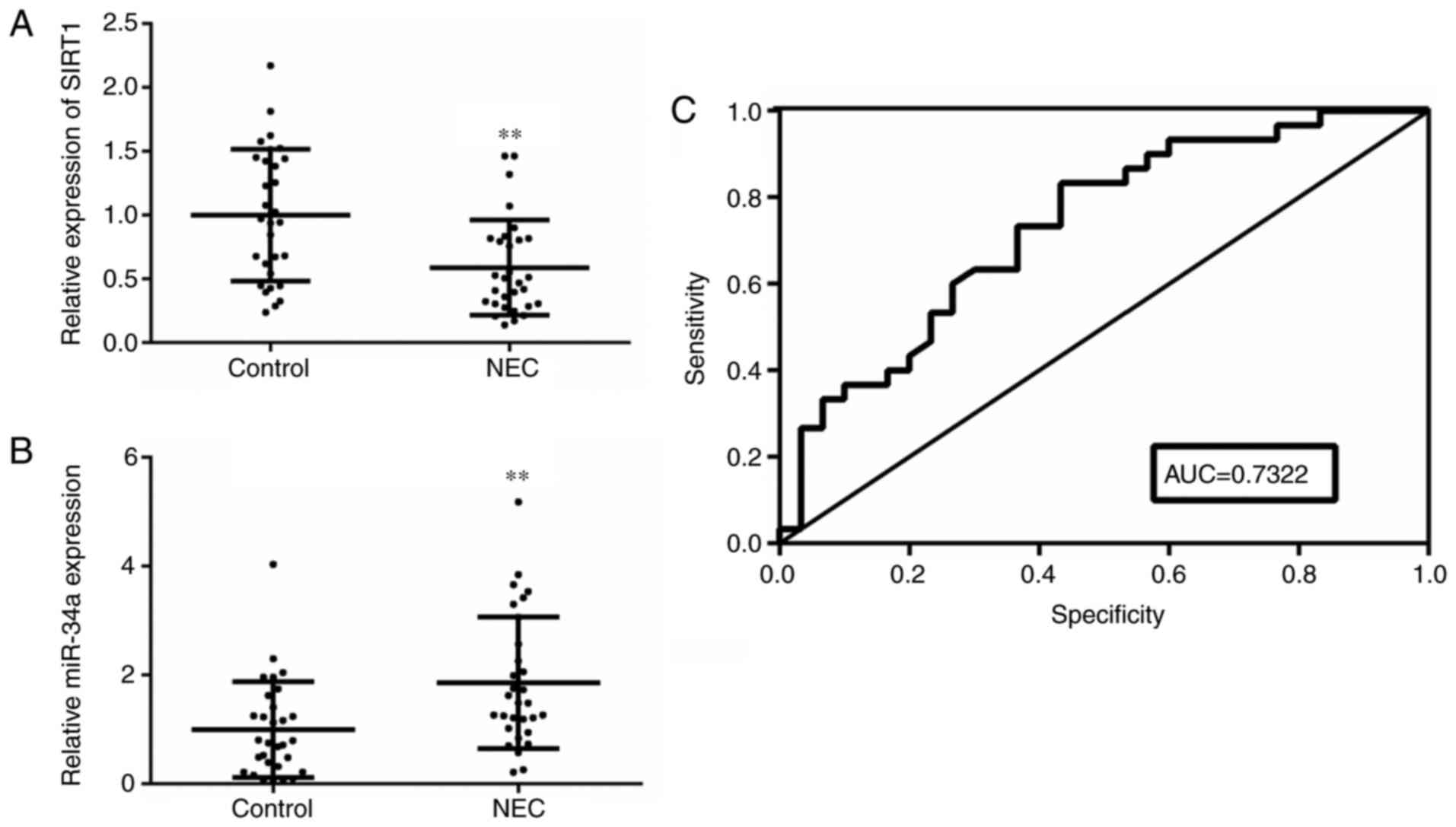
levels in the blood of patients with NEC are shown in Fig. 1. The SIRT1 gene expression level was
markedly decreased in the NEC group, but the miR-34a gene
expression level was notably increased in the NEC group (P<0.01;
Fig. 1A and B). According to
Table II, miR-34a was negatively
correlated with albumin (P=0.0285) and glucose (P=0.0458), and
positively correlated with CRP (P=0.0326) and PCT (P=0.0121).
Notably, miR-34a showed a significant positive correlation with NEC
severity (P=0.0034). ROC analysis results demonstrated that the AUC
result was 0.7322, which indicated that miR-34a has a high accuracy
in the diagnosis of patients with NEC (P<0.01; Fig. 1C).
 | Table II.Correlation analysis between the
miR-34a and clinical indicators. |
Table II.
Correlation analysis between the
miR-34a and clinical indicators.
| Characteristic | has-miR-34a | r | P-value |
|---|
| Sex |
| −0.09755 | 0.6081 |
|
Male | 16 |
|
|
|
Female | 13 |
|
|
| Gestational,
weeks | 33+1 | −0.1118 | 0.5564 |
| Birth weight,
g |
1,494.7±540.33 | −0.0982 | 0.6057 |
| Severity,
bellstage |
| 0.5169 | 0.0034b |
| Ia
phase | 19 |
|
|
| Ib
phase | 11 |
|
|
| Albumin, g/l | 24.30±4.14 | −0.3999 | 0.0285a |
| Glucose,
mmol/l |
2.68±2.80 | −0.3674 | 0.0458a |
| CRP, mg/l |
32.50±17.81 | 0.3911 | 0.0326a |
| PCT (ng/ml) |
7.77±1.93 | 0.4523 | 0.0121a |
Correlation between miR-34a and the
inflammatory cytokine levels and oxidative stress in patients with
NEC
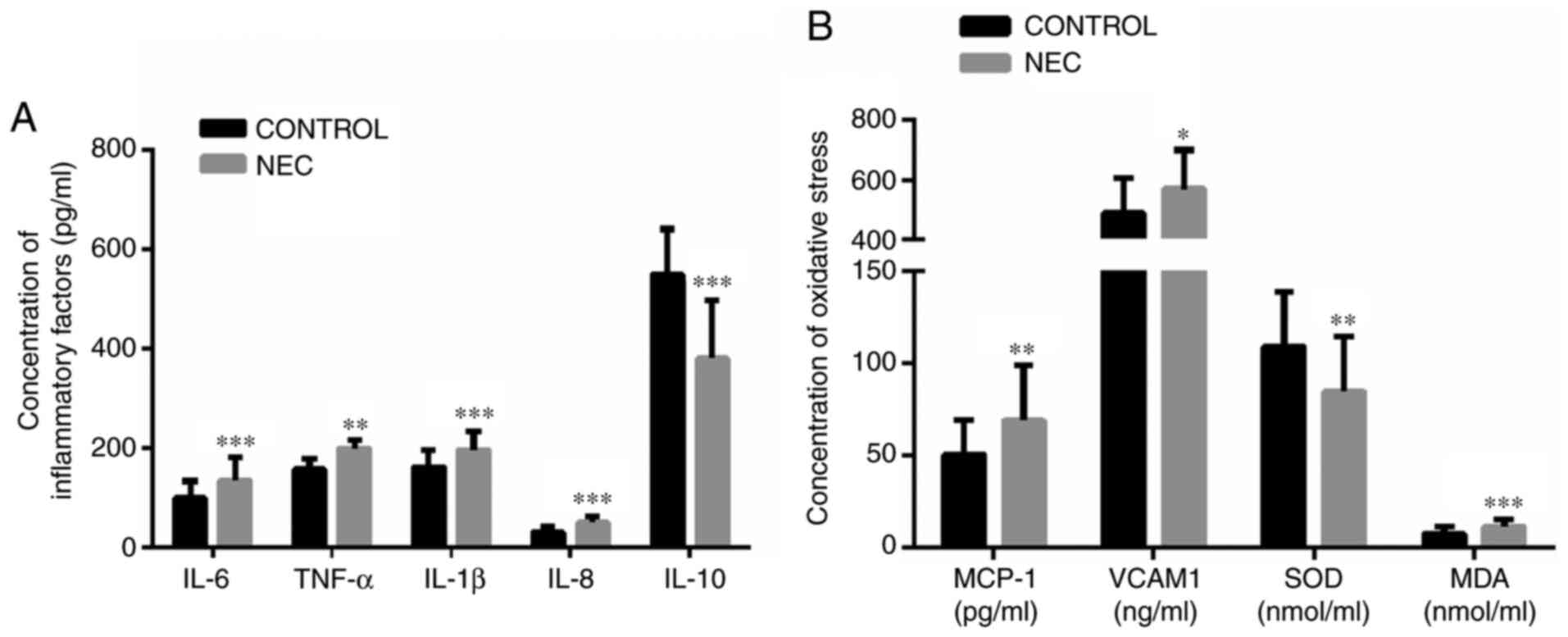
The concentrations of IL-6, TNF-α, IL-1β and IL-8
were significantly increased in the blood of patients with NEC,
compared with the blood of control patients, but the IL-10 was
significantly decreased in the blood of patients with NEC (Fig. 2A). The MCP-1, VCAM-1 and MDA
concentration were significantly increased in the blood of patients
with NEC, compared with the control group, but the SOD
concentration was significantly decreased in the blood of patients
with NEC (Fig. 2B). Based on the
blood of the patients with NEC, the correlation analysis results
demonstrated that the miR-34a was significantly negatively
correlated with SIRT1 gene expression and the concentrations of
IL-10 and SOD but was significantly positively correlated with CRP
(P=0.0326) and PCT (P=0.0121). Notably, the miR-34a and IL-6,
TNF-α, IL-1β, IL-8, MCP-1, VCAM1 and MDA demonstrated significant
positive correlations (Table
III).
 | Table III.Correlation analysis among miR-34a
and SIRT1, inflammatory factors and oxidative stress factors. |
Table III.
Correlation analysis among miR-34a
and SIRT1, inflammatory factors and oxidative stress factors.
| hsa-miR-34a | r | P-value |
|---|
| SIRT1 | −0.4114 | 0.0239a |
| TNF-a | 0.3907 | 0.0328a |
| IL-1β | 0.4828 | 0.4606b |
| IL-6 | 0.4606 | 0.0104a |
| IL-8 | 0.5740 |
<0.001c |
| IL-10 | −0.3781 | 0.0394a |
| MCP-1 | 0.5661 | 0.0011b |
| MDA | 0.4029 | 0.0273a |
| SOD | −0.3936 | 0.0314a |
| VCAM-1 | 0.4553 | 0.0115a |
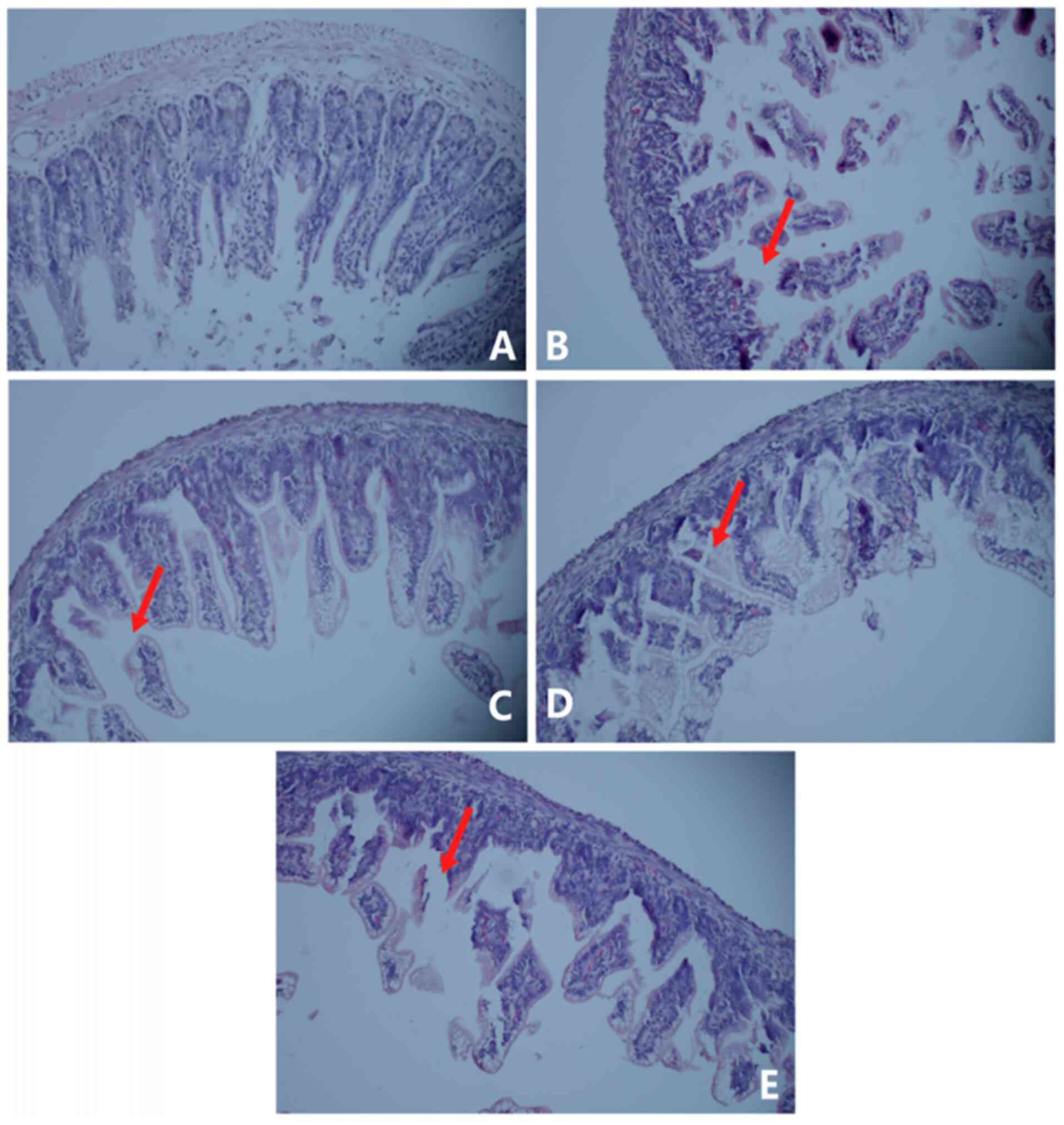
Pathological analysis of the rat NEC
model
As shown in Fig. 3,
compared with the control group (Fig.
3A), the intestinal tissue histological examination
demonstrated that the intestinal villi were seriously damaged and
shed in the NEC model group (Fig.
3B) and the double-blind score was 4. Based on the NEC group,
miR-34a inhibitor and SIRT1 activator treatment decreased the
double-blind score to 2 and demonstrated that the intestinal villus
damage was decreased (Fig. 3C and
D). However, the intestinal villus damage was significantly
aggravated when the SIRT1 activators were replaced with SIRT1
inhibitors, and the double-blind score increased to 3 (Fig. 3E). These results indicated that
miR-34a inhibitors and SIRT1 activators effectively improved NEC
intestinal mucosal necrosis, and SIRT1 inhibitors reversed the
efficacy of miR-34a inhibitors in an NEC rat model.
Inflammatory cytokine levels and
oxidative stress in the NEC rat model
To evaluate the effect of miR-34a and SIRT1 on
inflammatory cytokine levels and oxidative stress in the NEC rat
model, the miR-34a inhibitors, the SIRT1 activators and SIRT1
inhibitors were used. The results demonstrated that the
concentrations of IL-1β, TNF-α, IL-6 and IL-8 were significantly
increased in the NEC group, compared with the control group, but
they were significantly reversed in the groups treated with the
miR-34a inhibitors and the SIRT1 activators (Fig. 4A and B). The IL-10 concentration was
significantly decreased in the NEC group, compared with the control
group, but it was significantly reversed in the groups treated with
the miR-34a inhibitors and SIRT1 activators and the group treated
with miR-34a inhibitors and the SIRT1 inhibitors (Fig. 4B). The MDA, MCP-1 and VCAM-1
concentrations were significantly increased in the NEC group,
compared with the control group, but they were significantly
reversed in the groups treated with the miR-34a inhibitors and
SIRT1 activators (Fig. 4C and D).
However, the SOD concentration was different from the MDA, MCP-1
and VCAM-1 results (Fig. 4A). In
particular, miR-34a inhibitors and the SIRT1 treatment decreased
the SOD concentration and increased MDA, MCP-1 and VCAM-1
concentrations, compared with the miR-34a inhibitor group. These
data indicated that the miR-34a inhibitors and SIRT1 activators may
decrease oxidative stress levels in the NEC rat model and that the
SIRT1 inhibitors may reverse the effect of miR-34a inhibitors on
the NEC-induced oxidative stress response.
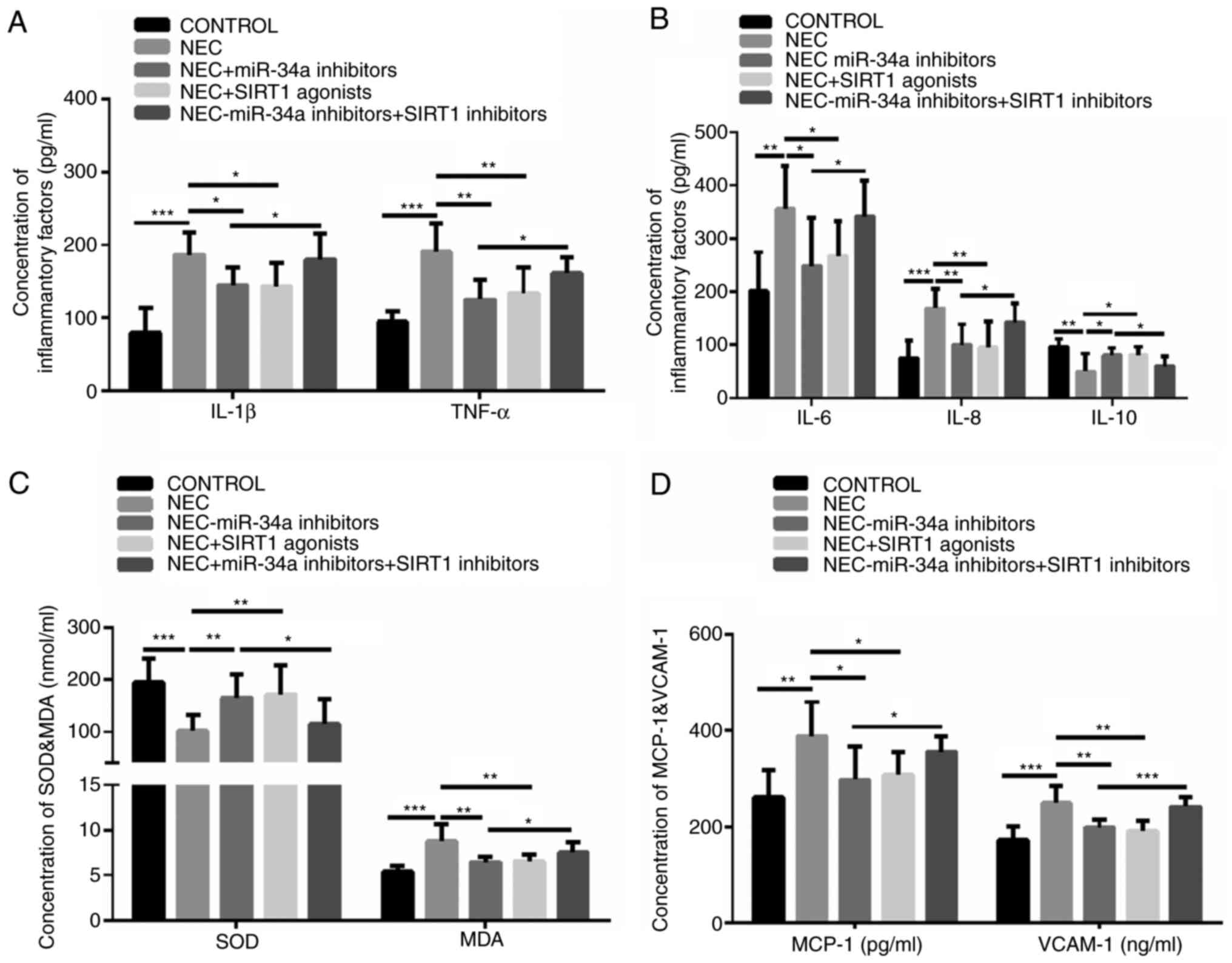 | Figure 4.The inflammatory cytokine levels and
oxidative stress markers in the NEC rat model. (A) IL-1β and TNF-α
concentrations. (B) IL-6, IL-8 and IL-10 concentrations. (C) SOD
and MDA concentrations. (D) MCP-1 and VCAM-1 concentrations. All
the data are presented as the mean ± standard deviation. Compared
with the control group, *P<0.05, **P<0.01 and ***P<0.001.
NEC, necrotizing enterocolitis; IL, interleukin; TNF, tumor
necrosis factor; SOD, superoxide dismutase; MDA, malondialdehyde;
MCP-1, monocyte chemoattractant protein-1; VCAM-1, vascular cell
adhesion molecule 1. |
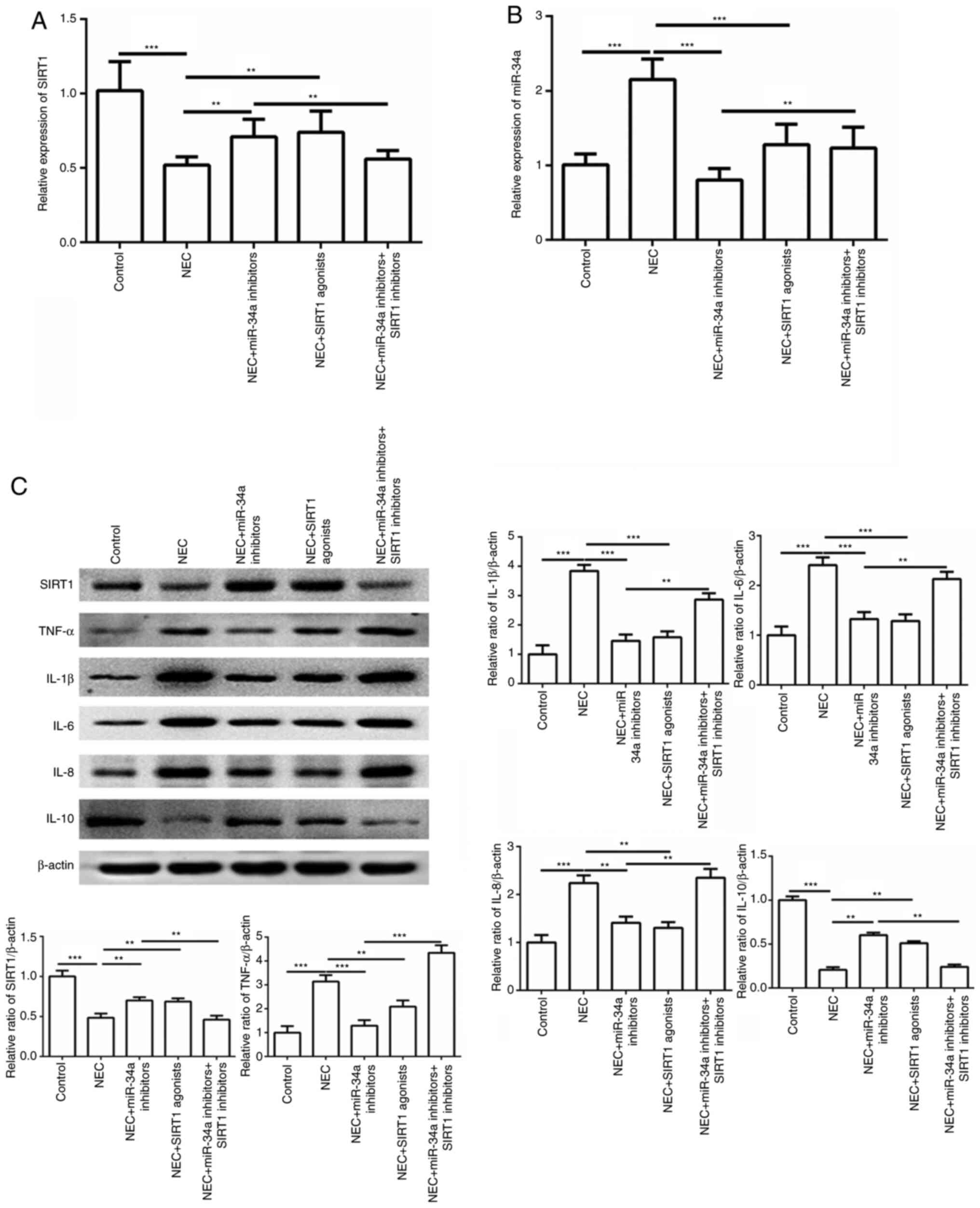
Effect of miR-34a and SIRT1 on the
gene and protein expression levels of inflammatory cytokines and
oxidative stress markers in a rat NEC model
As shown in Fig. 5,
the SIRT1 gene expression level was markedly decreased in the NEC
group, but was significantly increased in the group treated with
miR-34a inhibitors and the SIRT1 activators (P<0.001 or
P<0.0001; Fig. 5A). The miR-34a
gene expression level was the opposite of the SIRT1 expression
(P<0.001 or P<0.0001; Fig.
5B). In particular, the expression of the SIRT1 gene was
markedly decreased in the group treated with inhibitors of miR-34a
and the SIRT1 group, compared with the miR-34a inhibitor group.
However, the miR-34a expression levels were significantly increased
when the SIRT1 inhibitor supplementation was used. The protein
expression results demonstrated that SIRT1, TNF-α, IL-1β, IL-6,
IL-8 and IL-10 were consistent with the serum results. These data
indicated that miR-34a and SIRT1 maybe an important index in
regulating NEC inflammation (Fig.
5C).
Discussion
NEC is a severe neonatal gastrointestinal disease
with a high mortality rate in preterm newborns (1). At present, the pathogenesis of NEC is
considered to result from inappropriate proinflammation,
prematurity and bacterial colonization (29). A previous study indicated that the
function of microRNA is an important regulatory factor in the
occurrence and development of NEC (5). Su et al (30) indicated that miR-874 may
downregulate the aquaporin-3 expression in intestinal barrier
dysfunction (30). In the present
study, the miR-34a expression was markedly increased in the NEC
group, compared with the control group. It was also demonstrated
that inhibition of miR-34a expression may decrease intestinal
damage in the NEC rat model according to the pathological results.
Previous results have indicated that miR-7-5p, miR-181a-5p,
miR-194-5p and miR-362-3p are associated with intestinal diseases
(31–33).
NEC is also a severe inflammatory disorder in
preterm infants based on severe gut barrier damage (34). In the blood of patients with NEC,
the miR-34a was negatively correlated with albumin and glucose and
positively correlated with CRP and PCT. Notably, miR-34a showed a
significant positive correlation with NEC severity. Based on the
ROC analysis results, miR-34a was highly accurate in the diagnosis
of patients with NEC. Additionally, the concentrations of IL-6,
TNF-α, IL-1β, IL-8, MCP-1, VCAM-1 and MDA were significantly
increased in the blood of patients with NEC, but IL-10 was
significantly decreased. This result is consistent with those of a
previous study, which reported that miR-34a may direct the binding
of the transcription factor NF-кB to regulate inflammation
(35). Notably, the
anti-inflammatory factor IL-10 was markedly decreased in the NEC
group. Previous studies have demonstrated that
miR-34a-overexpression inhibited cell proliferation in certain
experimental models (36,37). In other words, inhibition of
miRNA-34a lead to increased cell proliferation, which was
accompanied by inflammatory suppression.
SIRT1 is an important apoptosis gene and a target
gene of miR-34a. As in previous study, the histone deacetylase
SIRT1 was repressed by the miR-34a, thereby transactivating its
target genes (38). Recent studies
suggested that SIRT1 is a potential treatment target for human
degenerative intervertebral disc disease and myocardial infarction
by inhibiting apoptosis (39,40). A
previous study also indicated that activation of SIRT1 may suppress
cellular senescence and promote cell proliferation (41). In the present study, SIRT1
expression was significantly downregulated in cells treated with
miR-34a and the SIRT1 inhibitor, which was markedly reversed by
inhibiting miR-34a. Furthermore, in the blood of patients with NEC,
a significant downregulation of SIRT1 expression and significant
upregulation of miR-34a expression was also observed. Notably, the
negative correlation between miR-34a and SIRT1 mRNA expression was
verified, which indicated that miR-34a upregulation may abrogate
the repressive effect of the target gene SIRT1. These findings were
also proven in the NEC rat model. Therefore, it was proposed that
the miR-34a/SIRT1 axis serves a potential therapeutic role in
NEC.
In conclusion, the results suggested that miR-34a
may affect the occurrence of NEC by regulating the inflammatory
factor balance and SIRT1 expression. Therefore, targeting the
miR-34a/SIRT1 axis offers novel therapeutic opportunities for
treating NEC.
Acknowledgements
Not applicable.
Funding
The study was supported by the Health and Young
Backbone Talents Training Project of Fujian Province (grant no.
2019-ZQN-2).
Availability of data and materials
The datasets used/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ designed the research, analyzed and interpreted
the patient data regarding the NEC disease. HZ, YLin and YLiu
preformed the experimental analyses, and analyzed and interpreted
the data in NEC rat model. YLiu performed the ELISA to detect the
cytokine concentration. HZ was a major contributor in writing the
manuscript. All authors read and approved the final manuscript. All
authors confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Fujian
Provincial Hospital Ethics Committee (K2019-01-030). It was
performed in accordance with the International Ethical Guidelines
for Human Biomedical Research (2012). The information of patients
with NEC was provided by the guardians of the patient. Written
informed consent was obtained from volunteers involved in the
study.
Patient consent for publication
All patients provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neu J and Walker WA: Necrotizing
enterocolitis. N Engl J Med. 364:255–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bein A, Eventov-Friedman S, Arbell D and
Schwartz B: Intestinal tight junctions are severely altered in NEC
preterm neonates. Pediatr Neonatol. 59:464–473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin Y, Wu X, Peng B, Zou H, Li S, Wang J
and Cao J: Curcumin improves necrotising microscopic colitis and
cell pyroptosis by activating SIRT1/NRF2 and inhibiting the TLR4
signalling pathway in newborn rats. Innate Immun. 26:609–617. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mikuš P, Pecher D, Rauová D, Horváth C,
Szobi A and Adameova A: Determination of novel highly effective
necrostatin Nec-1s in rat plasma by high performance liquid
chromatography hyphenated with quadrupole-time-of-flight mass
spectrometry. Molecules. 23:19462018. View Article : Google Scholar
|
|
5
|
Xu Y, Liu Y, Xie H, Zhou Y, Yan X, Chen W,
Wang X, Yu Z, Wang F, Chen X, et al: Profile analysis reveals
endogenous RNAs regulate necrotizing enterocolitis progression.
Biomed Pharmacother. 125:1099752020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen JF, Mandel EM, Thomson JM, Wu Q,
Callis TE, Hammond SM, Conlon FL and Wang DZ: The role of
microRNA-1 and microRNA-133 in skeletal muscle proliferation and
differentiation. Nat Genet. 38:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee RC, Feinbaum RL and Ambros V: The C.
Elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu H and Wang YB: Systematic large-scale
meta-analysis identifies miRNA-429/200a/b and miRNA-141/200c
clusters as biomarkers for necrotizing enterocolitis in newborn.
Biosci Rep. 39:BSR201915032019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ng PC, Chan KYY, Yuen TP, Sit T, Lam HS,
Leung KT, Wong RPO, Chan LCN, Pang YLI, Cheung HM, et al: Plasma
miR-1290 is a novel and specific biomarker for early diagnosis of
necrotizing enterocolitis-biomarker discovery with prospective
cohort evaluation. J Pediatr. 205:83–90.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vandenboom Ii TG, Li Y, Philip PA and
Sarkar FH: MicroRNA and cancer: Tiny molecules with major
implications. Curr Genomics. 9:97–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi XL, Kaller M, Rokavec M, Kirchner T,
Horst D and Hermeking H: Characterization of a
p53/miR-34a/CSF1R/STAT3 feedback loop in colorectal cancer. Cell
Mol Gastroenterol Hepatol. 10:391–418. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Slabáková E, Culig Z, Remšík J and Souček
K: Alternative mechanisms of miR-34a regulation in cancer. Cell
Death Dis. 8:e31002017. View Article : Google Scholar
|
|
14
|
Mathé E, Nguyen GH, Funamizu N, He P,
Moake M, Croce CM and Hussain SP: Inflammation regulates microRNA
expression in cooperation with p53 and nitric oxide. Int J Cancer.
131:760–765. 2012. View Article : Google Scholar
|
|
15
|
Rokavec M, Li H, Jiang L and Hermeking H:
The p53/miR-34 axis in development and disease. J Mol Cell Biol.
6:214–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li XJ, Zhang WY, Xu K and Lu J: miR-34a
promotes liver fibrosis in patients with chronic hepatitis via
mediating Sirt1/p53 signaling pathway. Pathol Res Pract.
216:1528762020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Jiao H, Jiang Z and Chen R:
Propofol inhibits migration and induces apoptosis of pancreatic
cancer PANC-1 cells through miR-34a-mediated E-cadherin and
LOC285194 signals. Bioengineered. 11:510–521. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B, Guo X, Li N, Chen Q, Shen J, Huang
X, Huang G and Wang F: WNT1, a target of miR-34a, promotes cervical
squamous cell carcinoma proliferation and invasion by induction of
an E-P cadherin switch via the WNT/β-catenin pathway. Cell Oncol
(Dordr). 43:489–503. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Concepcion CP, Han YC, Mu P, Bonetti C,
Yao E, D'Andrea A, Vidigal JA, Maughan WP, Ogrodowski P and Ventura
A: Intact p53-dependent responses in miR-34-deficient mice. PLoS
Genet. 8:e10027972012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barangi S, Mehri S, Moosavi Z, Hayesd AW,
Reiter RJ, Cardinali DP and Karimi G: Melatonin inhibits
Benzo(a)pyrene-induced apoptosis through activation of the
Mir-34a/Sirt1/autophagy pathway in mouse liver. Ecotoxicol Environ
Saf. 196:1105562020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park J, Kim J, Chen Y, Song HC, Chen Y,
Zheng M, Surh YJ, Kim UH, Park JW, Joe Y and Chung HT: CO
ameliorates cellular senescence and aging by modulating the
miR-34a/Sirt1 pathway. Free Radic Res. 54:848–858. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiang Q, Kang L, Wang J, Liao Z, Song Y,
Zhao K, Wang K, Yang C and Zhang Y: CircRNA-CIDN mitigated
compression loading-induced damage in human nucleus pulposus cells
via miR-34a-5p/SIRT1 axis. EBioMedicine. 53:1026792020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Auestad N, Korsak RA, Bergstrom JD and
Edmond J: Milk-substitutes comparable to rat's milk; their
preparation, composition and impact on development and metabolism
in the artificially reared rat. Br J Nutr. 61:495–518. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ozdemir R, Yurttutan S, Sari FN, Oncel MY,
Erdeve O, Unverdi HG, Uysal B and Dilmen U: All-trans-retinoic acid
attenuates intestinal injury in a neonatal rat model of necrotizing
enterocolitis. Neonatology. 104:22–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Afrazi A, Sodhi CP, Good M, Jia H, Siggers
R, Yazji I, Ma C, Neal MD, Prindle T, Grant ZS, et al:
Intracellular heat shock protein-70 negatively regulates TLR4
signaling in the newborn intestinal epithelium. J Immunol.
188:4543–4557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie Z, Zhang J, Ma S, Huang X and Huang Y:
Effect of Chinese herbal medicine treatment on plasma lipid profile
and hepatic lipid metabolism in Hetian broiler. Poult Sci.
96:1918–1924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie Z, Wang Y, Huang J, Qian N, Shen G and
Chen L: Anti-inflammatory activity of polysaccharides from
phellinus linteus by regulating the NF-κB translocation in
LPS-stimulated RAW264.7 macrophages. Int J Biol Macromol.
129:61–67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Emami CN, Petrosyan M, Giuliani S,
Williams M, Hunter C, Prasadarao NV and Ford HR: Role of the host
defense system and intestinal microbial flora in the pathogenesis
of necrotizing enterocolitis. Surg Infect (Larchmt). 10:407–417.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su ZR, Zhi XF, Zhang Q, Yang L, Xu H and
Xu ZK: LncRNA H19 functions as a competing endogenous RNA to
regulate AQP3 expression by sponging miR-874 in the intestinal
barrier. FEBS Lett. 590:1354–1364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heverhagen AE, Legrand N, Wagner V,
Fendrich V, Bartsch DK and Slater EP: Overexpression of MicroRNA
miR-7-5p is a potential biomarker in neuroendocrine neoplasms of
the small intestine. Neuroendocrinology. 106:312–317. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vaira V, Roncoroni L, Barisani D, Gaudioso
G, Bosari S, Bulfamante G, Doneda L, Conte D, Tomba C, Bardella MT,
et al: microRNA profiles in coeliac patients distinguish different
clinical phenotypes and are modulated by gliadin peptides in
primary duodenal fibroblasts. Clin Sci (Lond). 126:417–423. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bansal A, Hong XM, Lee IH, Krishnadath KK,
Mathur SC, Gunewardena S, Rastogi A, Sharma P and Christenson LK:
MicroRNA expression can be a promising strategy for the detection
of Barrett's esophagus: A pilot study. Clin Transl Gastroenterol.
5:e652014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martin CR and Walker WA: Intestinal immune
defences and the inflammatory response in necrotising
enterocolitis. Semin Fetal Neonatal Med. 11:369–377. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Wang K, Chen X, Meng H, Song M, Wang
Y, Xu X and Bai Y: Transcriptional activation of microRNA-34a by
NF-kappa B in human esophageal cancer cells. BMC Mol Biol.
13:42012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tazawa H, Tsuchiya N, Izumiya M and
Nakagama H: Tumor-suppressive miR-34a induces senescence-like
growth arrest through modulation of the E2F pathway in human colon
cancer cells. Proc Natl Acad Sci USA. 104:15472–15477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bommer GT, Gerin I, Feng Y, Kaczorowski
AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ji ML, Jiang H, Zhang XJ, Shi PL, Li C, Wu
H, Wu XT, Wang YT, Wang C and Lu J: Preclinical development of a
microRNA-based therapy for intervertebral disc degeneration. Nat
Commun. 9:50512018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gupta SK, Foinquinos A, Thum S, Remke J,
Zimmer K, Bauters C, de Groote P, Boon RA, de Windt LJ, Preissl S,
et al: Preclinical development of a MicroRNA-based therapy for
elderly patients with myocardial infarction. J Am Coll Cardiol.
68:1557–1571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia X, Guo J, Lu F and Jiang J: SIRT1
plays a protective role in intervertebral disc degeneration in a
puncture-induced rodent model. Spine (Phila Pa 1976). 40:E515–E524.
2015. View Article : Google Scholar : PubMed/NCBI
|