Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, and non-small cell lung cancer (NSCLC) is the
most common type of lung cancer (1). Tyrosine kinase inhibitors, such as
gefitinib, are widely used as targeted therapeutics for the
treatment of NSCLC (2–4). Although most patients with NSCLC
initially respond to chemotherapy, their cancer gradually develops
resistance, leading to cancer progression, or recurrence and poorer
prognoses (5). Therefore, there is
an urgent need to identify an appropriate agent that could be
combined with gefitinib to effectively combat gefitinib resistance
(GR). However, most agents that have been combined with gefitinib,
such as pemetrexed (6), thalidomide
(7) or metformin (8), have variable degrees of toxicity and
several side effects.
Cucurbitacin B (CuB) is an oxidized tetracyclic
triterpenoid derived from plants of the Cucurbitaceae
family. This molecule exhibits antineoplastic activity in various
types of cancer (9,10), with low toxicity and fewer side
effects (9). In addition, CuB can
activate the protein expression of apoptosis-related factors
cleaved caspase-3 and cleaved caspase-9 (11), inhibit the metastatic ability of
NSCLC (12) and inhibit
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumors
(13). In addition, Liu et
al (5) found that CuB inhibited
GR NSCLC by inducing lysosomal degradation of epidermal growth
factor receptor. Therefore, gefitinib is a candidate for
combination therapy.
It is necessary to explore the mechanisms of action
of combined drug therapy for NSCLC. MicroRNAs (miRNAs/miRs) are a
type of small non-coding RNA. Dysregulation of specific miRNAs may
be involved in the development of resistance to a variety of cancer
treatments (i.e., modulating cancer cell sensitivity to such
therapies) (14,15). One such miRNA, miR-17-5p, is closely
associated with the occurrence, progression and prognosis of lung
cancer (16–19). miRNAs can modulate the expression of
target genes at both the transcriptional and post-transcriptional
levels (20). In the present study,
the TargetScan database was analyzed and it was found that among
the putative target genes, the signal transducer and activator of
transcription 3 (STAT3) gene is a potential target for
miR-17-5p.
STAT3 is a transcription factor known to promote
tumorigenesis (21). It is
frequently activated in pre-neoplastic and cancerous cells and is
frequently identified during research studies as being dysregulated
in lung cancer (22–24). Activation of STAT3 can promote
proliferation and metastasis of NSCLC cells (25). Studies have shown that miRNAs, such
as miR-34a and miR-519a, can inhibit the process of NSCLC by
regulating STAT3 expression (26,27).
In addition, some studies have shown that miR-17-5p can target
STAT3 to reduce the activity of rat cardiomyocytes (28), or promote the apoptosis of breast
cancer cells (29), and this
antagonistic regulatory relationship between miR-17-5p and STAT3
has been confirmed. However, the mechanism by which the
miR-17-5p/STAT3 axis regulates CuB to inhibit NSCLC progression
remains unclear.
In the present study, GR PC9 cells were cultured
in vitro to simulate GR in patients with lung cancer, and
the role of the miR-17-5p/STAT3 axis in regulating the effect of
CuB in GR NSCLC cells was explored from the perspective of
CuB-miRNA-mRNA interaction.
Materials and methods
Reagents, cells and biomolecules
CuB (cat. no. C8499) and gefitinib (cat. no.
SML1657) were purchased from Sigma-Aldrich (Merck KGaA). A human
lung adenocarcinoma-derived cell line (PC9) was purchased from
Procell Life Science & Technology Co., Ltd., and 293T cell
lines were purchased from the American Type Culture Collection.
Roswell Park Memorial Institute (RPMI)-1640 medium,
phosphate-buffered saline (PBS) and fetal bovine serum (FBS) were
purchased from Gibco (Thermo Fisher Scientific, Inc.). A
PrimeScript RT reagent kit and SYBR Premix ExTaq II kit were
purchased from Takara Biotechnology Co., Ltd. Lysis buffer and ECL
reagent were purchased from Thermo Fisher Scientific, Inc. A BCA
protein assay kit was purchased from Tiangen Biotech Co., Ltd.
Polyvinylidene fluoride (PVDF) membranes were purchased from
MilliporeSigma. Sodium dodecyl sulfate (SDS), bovine serum albumin
(BSA) blocking buffer (5%) and trypsin-EDTA (0.25%) solution and
trypan blue staining solution (0.4%) were purchased from Beijing
Solarbio Science & Technology Co., Ltd. Anti-STAT3 (cat. no.
ab68153), anti-phosphorylated (p)-STAT3 (cat. no. ab267373),
anti-caspase-3 (cat. no. ab32351), anti-cleaved caspase-3 (cat. no.
ab2302), anti-caspase-9 (cat. no. ab32539), anti-cleaved caspase-9
(cat. no. ab2324), anti-GAPDH antibodies (cat. no. ab181602) and
goat anti-rabbit (cat. no. ab205718) were purchased from Abcam.
Guangzhou RiboBio Co., Ltd., synthesized a miR-17-5p mimic
(5′-CAAAGUGCUUACAGUGCAGGUAG-3′), miR-17-5p scrambled mimic negative
control (NC; 5′-ACUAAUGAGCGAGUGAAUCCGUG-3′), miR-17-5p inhibitor
(5′-CTACCTGCACTGTAAGCACTTTG-3′) and miR-17-5p scrambled inhibitor
NC (5′-CAGUACUUUUGUGUAGUACAA-3′). Sangon Biotech Co., Ltd.,
synthesized a STAT3-encoding plasmid (ov-STAT3), a
corresponding NC plasmid (ov-NC) and PCR primer pairs targeting
STAT3, GAPDH, miR-17-5p and U6. TRIzol®,
Lipofectamine® 2000 transfection reagent and the
SYBR-Green I Real-Time PCR kit were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.). A Cell Counting Kit-8 (CCK-8) and
RIPA lysis buffer were purchased from Beyotime Institute of
Biotechnology. An Annexin V-FITC Apoptosis Detection Kit was
purchased from BD Biosciences. The wild-type (WT) and mutant type
(MUT) 3′-UTR of STAT3 were synthesized (Guangzhou RiboBio
Co., Ltd.) and inserted into psiCHECK™-2 vector plasmids and
pRL-SV40 reporter vector plasmids (all plasmids were purchased from
Promega Corporation). psiCHECK-2 plasmids carried two reporter
genes (Renilla and firefly luciferases), whereas pRL-SV40
plasmids carried only one (Renilla luciferase).
Culture conditions for PC9 or PC9/GR
cells, induction of GR and transfection with miRNAs
The PC9 or PC9/GR cell lines were cultured at 37°C
and 5% CO2 in RPMI-1640 medium supplemented with 10% FBS
(hereafter referred to as normal culture medium). To produce a GR
cell variant (PC9/GR), gefitinib concentration was progressively
increased, as previously described (30). Thereafter, resistance was maintained
by including 1 µg/ml gefitinib in culture media. Once cells reached
60–80% confluence, they were transfected with 50 nM ov-STAT3, 100
nM miR-17-5p mimic, mimic NC, inhibitor or inhibitor NC using
Lipofectamine 2000 liposomes at 37°C, according to the
manufacturer's instructions. Cells were incubated for 4 h, medium
was replaced with normal cell culture medium, and cells were
incubated for a further 48 h prior to analysis.
Confirmation of induced PC9/GR drug
resistance and evaluation of the impact of CuB
Following incubation of PC9 and PC9/GR cells with
varying concentrations of gefitinib (0, 0.39, 0.78, 1.56, 3.125,
6.25, 12.5, 25 and 50 µM) and CuB (2, 4, 6, 8, 10, 12 and 16 µg/ml)
for 48 h, the number of viable cells was determined via CCK-8
(according to the manufacturer's instructions). Concentrations of
gefitinib that produced 50% inhibitory concentration
(IC50) were used to calculate the drug resistance index
(RI): RI = (IC50 for P9/GR cells)/(IC50 for
PC9 cells). PC9/GR cells were cultured at IC25 of CuB or
gefitinib.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA was extracted from PC9 cells, PC9/GR cells
incubated in the presence or absence of varying concentrations of
CuB (2, 4, 6, 8, 10, 12 and 16 µg/ml) and PC9/GR cells treated with
a combination of CuB and gefitinib at IC25 using TRIzol
reagent, according to the manufacturer's instructions. Extracted
RNA was reverse transcribed to cDNA using a PrimeScript RT reagent
kit, according to the manufacturer's instructions. The RT-qPCR
procedure was performed using a SYBR Premix ExTaq II kit (according
to the manufacturer's instructions) in conjunction with a 7500
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Thermocycling conditions were as follows: 95°C for 5 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Primer
pair sequences were as follows: STAT3 forward,
5′-ATCCTGAAGCTGACCCAGG-3′ and reverse, 5′-CTGCAGGTCGTTGGTGTCA-3′;
GAPDH forward, 5′-GCTCATTTGCAGGGGGGAG-3′ and reverse,
5′-GTTGGTGGTGCAGGAGGCA-3′; miR-17-5p forward,
5′-ACACTCCAGCTGGGCAAAGTGCTTACAGTGC-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′; and U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Target RNA levels were normalized to those of housekeeping genes
GAPDH or U6. Relative mRNA expression levels were
calculated using the 2−ΔΔCq method (31).
Western blotting
PC9 or PC9/GR cells were lysed using ice-cold RIPA
lysis buffer. Lysate protein concentrations were determined using a
BCA protein assay kit. Equal amounts of denatured proteins (20 µg)
were resolved via 10% SDS-polyacrylamide gel electrophoresis and
separated proteins were subsequently transferred to a PVDF
membrane. The membrane was blocked with 5% BSA for 1 h at 20°C,
incubated with primary antibodies (anti-STAT3, 1:1,000;
anti-p-STAT3, 1:1,000; anti-caspase-3, 1:5,000; anti-caspase-3,
1:5000; anti-cleaved caspase-3, 1:500; anti- caspase-9, 1:2,000 and
anti-cleaved caspase-9, 1 µg/ml) overnight at 4°C, rinsed with TBS
containing 0.05% Tween-20 buffer (Beijing Solarbio Science &
Technology Co., Ltd.) twice for 10 min each time, and subsequently
incubated with the horseradish peroxidase-conjugated secondary
antibody (goat anti-rabbit; 1:10,000) for 2 h at 23±2°C. Protein
bands were visualized by the addition of ECL reagent in conjunction
with an imaging system (DNR Bio-Imaging Systems, Ltd.). Anti-GAPDH
antibody (1:10,000) was used as a loading control. ImageJ software
(version 1.49n; National Institutes of Health) was used for
densitometry.
Proliferation assay
A single-cell suspension was prepared via
trypsinization of PC9 or PC9/GR cells, and these cells were seeded
into six-well plates (Costar; Corning, Inc.) at a density of 500
cells per well in 2 ml culture medium. After cells were cultured
for 2 weeks, stained with trypan blue staining solution (23±2°C for
5 min) and counted. Survival rates were determined via CCK-8, using
96-well plates (cat. no. 3599; Costar; Corning, Inc.), according to
the manufacturer's instructions. Briefly, cells were seeded at a
density of 5×103 cells/well and cultured for 24–48 h, 10
µl CCK-8 solution was added per well, plates were incubated for 60
min at 23±2°C and absorbance at 450 nm was measured using an
enzyme-labeled instrument (Multiskan MK3, Thermo Fisher Scientific,
Inc.). The IC50 and IC25 values are obtained
by analyzing the survival rate of PC9 or PC9/GR cells by GraphPad
Prism 8 (GraphPad Software Inc.).
Apoptosis assay
Early + late apoptosis of PC9 or PC9/GR cells was
assessed using an Annexin V-FITC Apoptosis Detection Kit, according
to the manufacturer's instructions. Briefly, cells
(1×106 cells/ml) were harvested by trypsin digestion,
washed twice using ice-cold PBS and resuspended in 500 µl binding
buffer. Next, cells were incubated with 5 µl Annexin V-FITC and 5
µl propidium iodide (PI) in the dark for 15 min at 23±2°C, followed
by flow cytometry (BD FACSCalibur; BD Biosciences); FlowJo software
(version 10.6.2; FlowJo LLC) was used for analysis.
Binding site prediction
The TargetScan database 7.2 (http://www.targetscan.org/vert_72/) was used to
predict STAT3 binding sites for miR-17-5p.
Dual-luciferase reporter assay
293T cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS. Thereafter, 293T cells were transfected with 500 ng
each of a miR-17-5p mimic or inhibitor and their NCs, 1 µg each of
the vector plasmid containing WT or MUT STAT3, and 50 ng
pRL-SV40 reporter vector plasmid using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were
incubated for 48 h, and luciferase activity was measured using a
Dual-Luciferase Reporter Assay System (Promega Corporation)
according to the manufacturer's instructions. Briefly, absorbance
at 490 nm (determined by luciferase activity) was measured, and
target values were calculated with respect to the NC groups. The
ratio of firefly to Renilla activity was used to normalize
firefly luciferase values.
Statistical analysis
All experiments were performed in triplicate. All
data are expressed as the mean ± standard deviation. All
statistical analyses were performed using SPSS version 21.0
statistical analysis package (IBM Corp.). Comparison of multiple
groups was performed using one-way ANOVA followed by Dunnett's post
hoc test. Means of two groups were compared using an unpaired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Gefitinib-induced downregulation of
miRNA expression
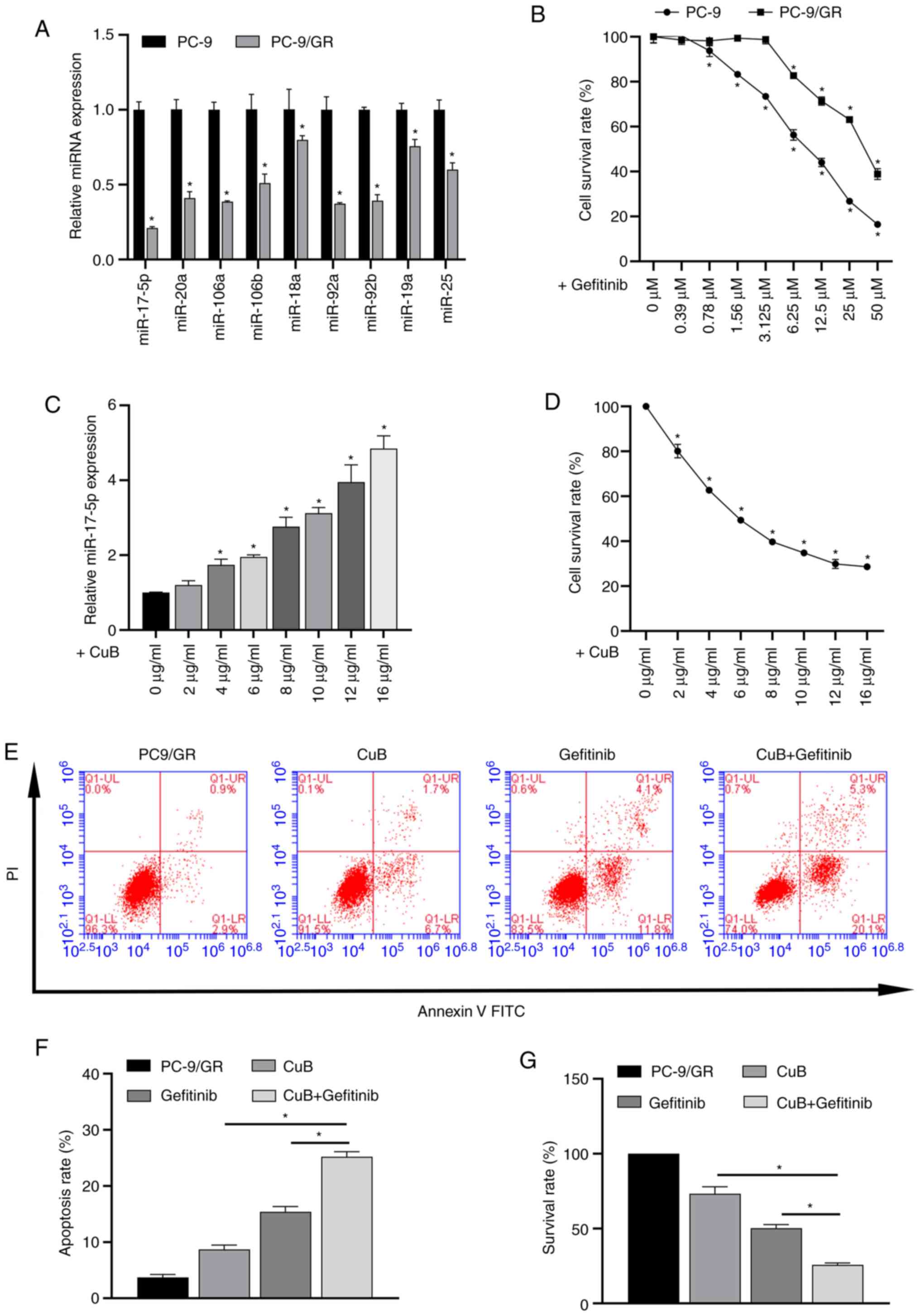
The differences in the expression levels of various
miRNAs in PC9 and PC9/GR cells were detected via RT-qPCR. The
results showed that miR-17-92 family levels were downregulated in
all PC9/GR cells compared with PC9 cells. Among them, miR-17-5p
downregulation was the most significant, so in this study,
miR-17-5p was selected for further study (Fig. 1A).
Confirmation of induced PC9/GR drug
resistance
Survival rates of PC9 and PC9/GR cells following
exposure to gefitinib were determined (Fig. 1B). Inhibitory concentrations of
gefitinib were as follows: IC50 for PC9 cells, 3.89
µg/ml; IC25 for PC9/GR cells, 10.4 µg/ml; and
IC50 for PC9/GR cells, 20.6 µg/ml. A calculated RI of
5.3 indicated successful induction of moderate GR in PC9/GR
cells.
Addition of CuB ameliorates the
gefitinib-induced downregulation of miR-17-5p expression
RT-qPCR results showed that the expression of
miR-17-5p in PC9/GR cells was gradually upregulated with a gradual
increase of CuB concentration (Fig.
1C).
Effect of CuB on gefitinib-induced
resistance in PC9/GR cells
The CCK-8 assay showed that the IC25 of
CuB on PC9/GR cells was 2.6 µg/ml. (Fig. 1D). Then, the effect of the combined
action of CuB (IC25) and gefitinib (IC50) on
PC9/GR cells was analyzed. The results showed that the combination
of CuB and gefitinib significantly increased apoptosis (Fig. 1E and F) and reduced survival rate
(Fig. 1G) in PC9/GR cells compared
with CuB (IC25) or gefitinib (IC50) treatment
alone.
Effect of CuB on miR-17-5p expression
and cell proliferation/apoptosis at gefitinib IC25 in
PC9/GR cells transfected with miR-17-5p mimic or inhibitor
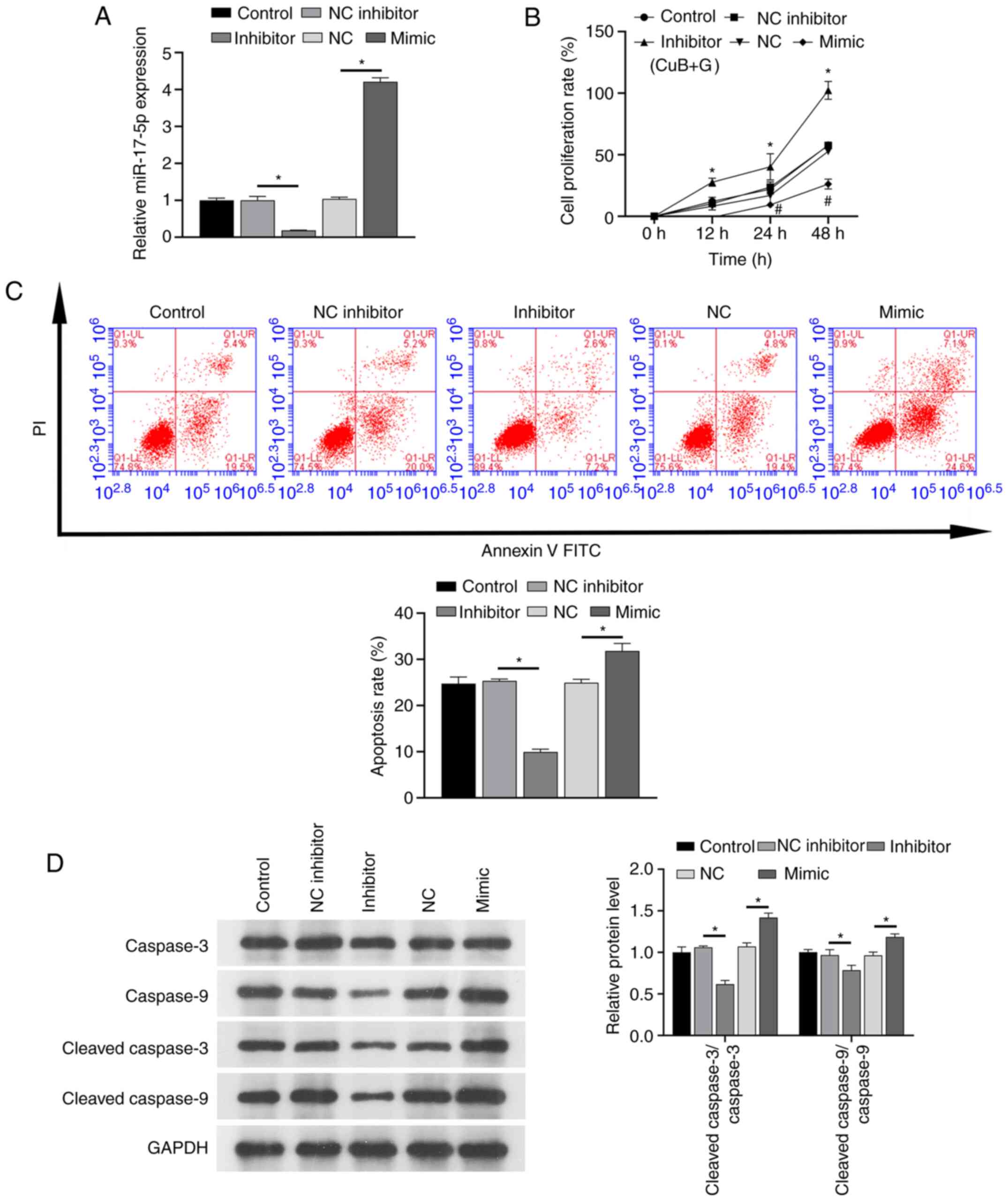
RT-qPCR investigating the effect of CuB on miR-17-5p
expression in PC9/GR cells transfected with miRNAs confirmed that
miR-17-5p expression levels were lower in the miR-17-5p inhibitor
group compared with in the inhibitor NC group, whereas levels were
higher in the miR-17-5p mimic group compared with the NC mimic
group (Fig. 2A). This result
demonstrated that the synthetic miR-17-5p mimics and inhibitors
were effective. Additionally, the results of CCK-8, flow cytometry
and western blotting showed that the miR-17-5p inhibitor promoted
the proliferation of PC-9/GR cells and inhibited apoptosis and
protein levels of cleaved caspase-3 and cleaved caspase-9 under the
combined action of CuB and gefitinib (Fig. 2B-D). Transfection with the miR-17-5p
mimic induced the opposite effects of those noted for the miR-17-5p
inhibitor.
STAT3 is directly targeted by
miR-17-5p
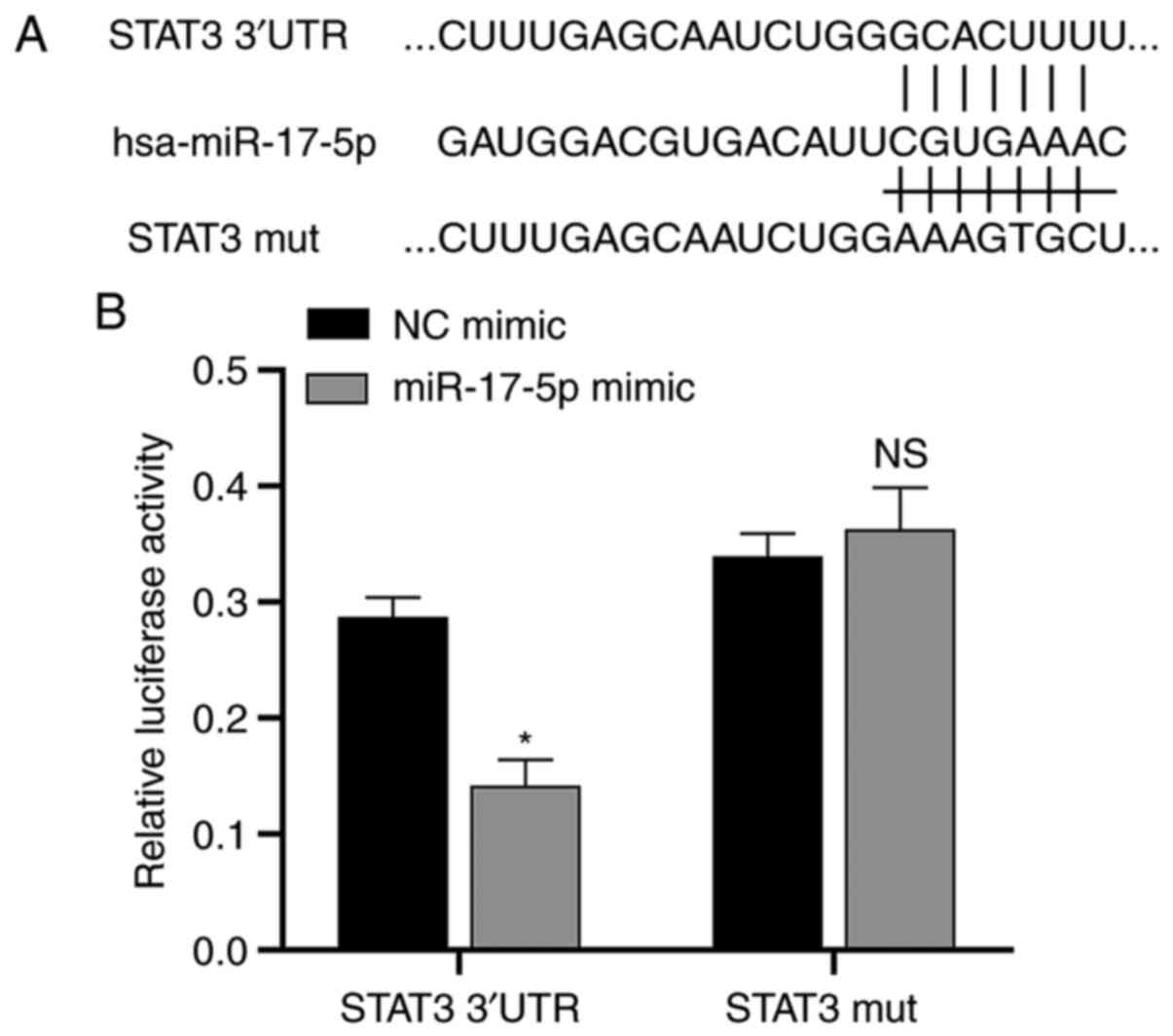
Since miRNAs usually inhibit protein synthesis by
binding to the 3′-UTR region of mRNA, the putative downstream
target genes of miR-17-5p were analyzed using the TargetScan
database. The results showed that the oncogene STAT3, which
has been demonstrated by a number of studies to play a key role in
the development of NSCLC (25,32),
is also one of the assumed target genes of miR-17-5p, so
STAT3 was selected in this study for further study into the
molecular mechanism of this miRNA. The TargetScan database analysis
predicted the putative miR-17-5p binding site of the STAT3
oncogene (Fig. 3A). The
dual-luciferase reporter assay showed that luciferase activity was
significantly downregulated after transfection of miR-17-5p mimics
in STAT3 3′-UTR group compared with NC mimic group.
Meanwhile, there was no significant difference in luciferase
activity after the transfection of mimics with the MUT STAT3
compared with the NC mimic group. This result demonstrated that
STAT3 was directly targeted by miR-17-5p (Fig. 3B).
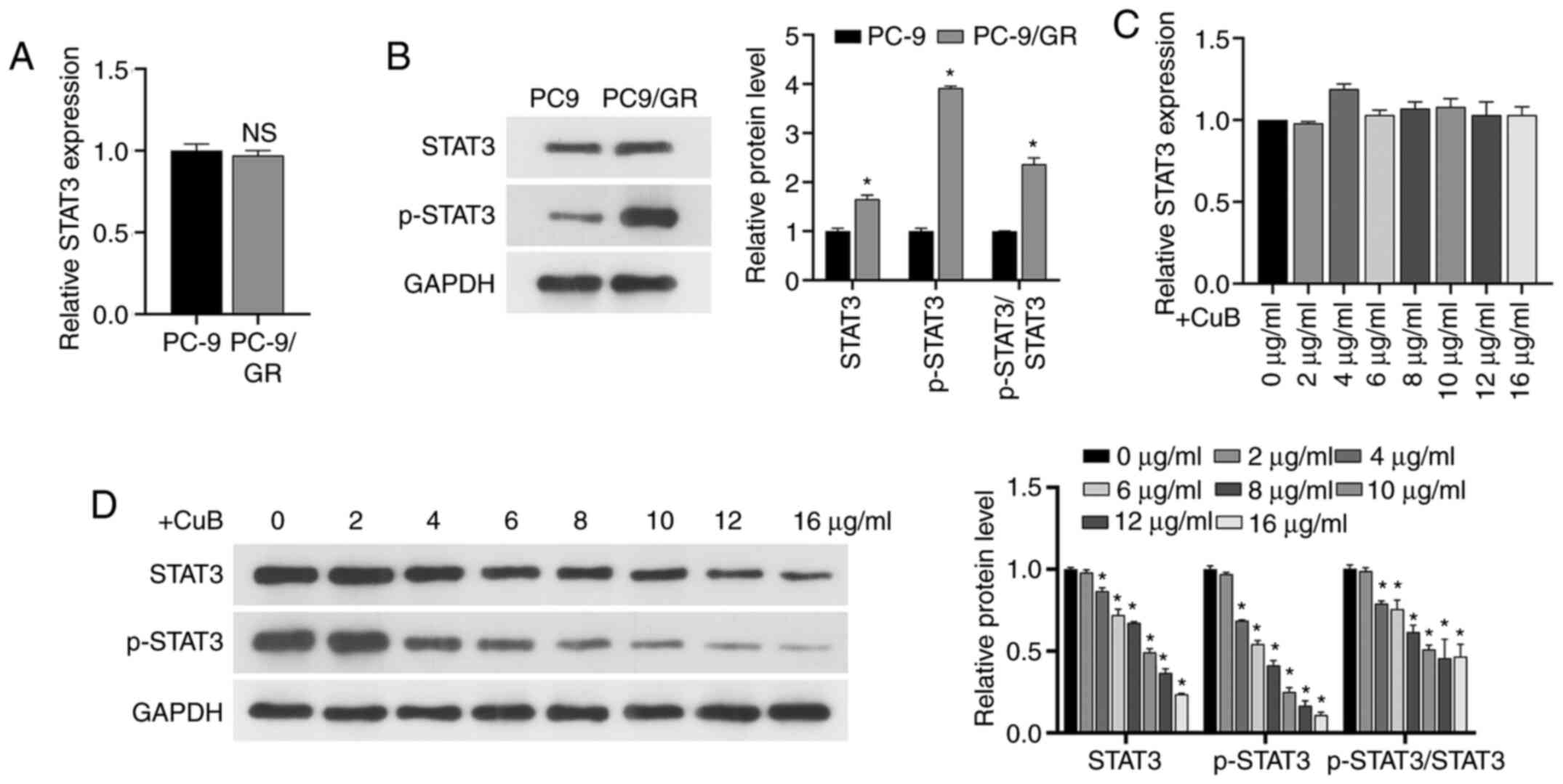
Effect of CuB on STAT3 transcription
and protein expression levels
The RT-qPCR assay demonstrated that GR did not
significantly alter STAT3 transcription in PC9/GR cells
(Fig. 4A), whereas western blotting
demonstrated that it significantly upregulated STAT3 expression
levels and promoted the phosphorylation of STAT3 (P<0.05;
Fig. 4B). Then, RT-qPCR and western
blotting confirmed that the STAT3 transcript level was not
significantly altered by CuB (P>0.05; Fig. 4C), but CuB decreased STAT3 protein
expression and inhibited STAT3 phosphorylation in a dose-dependent
manner (Fig. 4D).
miR-17-5p/STAT3 axis is modulated by
CuB and gefitinib combination treatment
In the presence of IC25 concentrations of
CuB and gefitinib, RT-qPCR and western blotting assays
investigating the impact of CuB on gefitinib sensitivity of PC9/GR
cells transduced with miRNAs demonstrated that while the
CuB-induced change in miR-17-5p expression did not significantly
alter STAT3 transcript level (Fig. 5A), STAT3 protein and phosphorylation
levels were lower in the miR-17-5p mimic group than in the mimic NC
group, and higher in the miR-17-5p inhibitor group than in the
inhibitor NC group (Fig. 5B).
Furthermore, transfection with ov-STAT3 increased the expression
levels of the STAT3 transcript (Fig. 5C), STAT3 and p-STAT3 protein
expression levels (Fig. 5D), and
negated the beneficial effects of CuB on gefitinib sensitivity in
PC9/GR cells, including restoring increased proliferation (Fig. 5E) and decreased apoptosis (Fig. 5F) and protein levels of cleaved
caspase-3 and cleaved caspase-9 (Fig.
5G) compared with the ov-NC group. These results confirmed that
overexpression of STAT3 could reverse the apoptotic effect of CuB
on PC9/GR cells and increase cell proliferation. Briefly, these
results indicated that CuB regulated the miR-17-5p/STAT3 axis to
increase gefitinib sensitivity in PC9/GR cells.
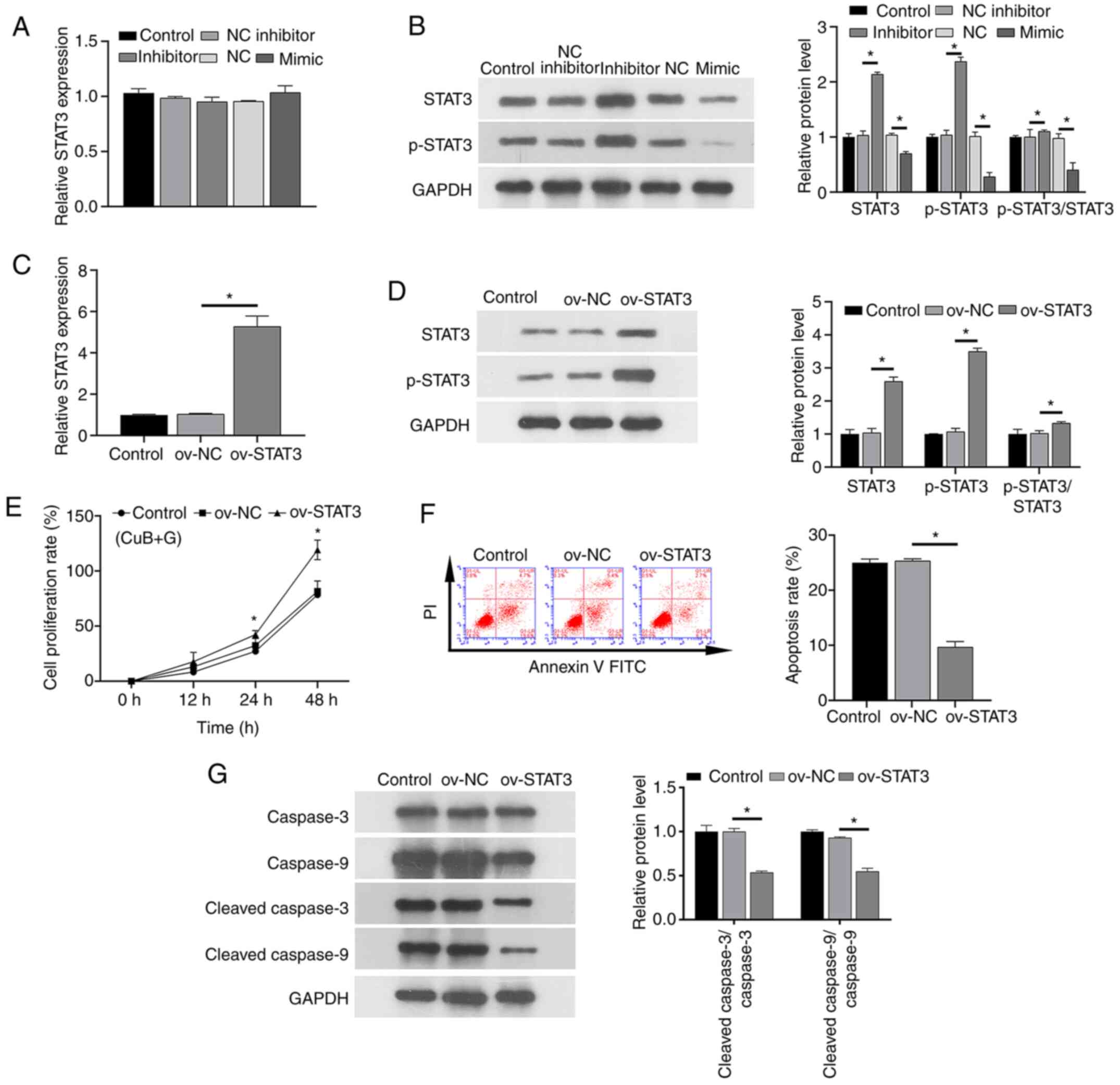 | Figure 5.Effect of CuB in the presence of
gefitinib at IC25 on PC9/GR cells transfected with
miR-17-5p mimic or inhibitor, or overexpressing STAT3. (A) Impact
of miR-17-5p mimic/inhibitor on STAT3 transcript levels. (B)
Impact of miR-17-5p mimic/inhibitor on STAT3 protein levels. (C)
Impact of STAT3 overexpression on STAT3 transcript levels. (D)
Impact of STAT3 overexpression on STAT3 protein expression. (E)
Impact of STAT3 overexpression on PC9/GR cell proliferation. (F)
Impact of STAT3 overexpression on PC9/GR cell apoptosis. (G) Impact
of STAT3 overexpression on caspase-3, cleaved caspase-3, caspase-9
and cleaved caspase-9 protein levels. All values are expressed as
mean ± standard deviation. *P<0.05. NS, not significant; CuB,
cucurbitacin B; GR, gefitinib-resistant; NC, negative control; miR,
microRNA; p-, phosphorylated; ov-, overexpression vector. |
Discussion
Multiple miRNAs, including the miR-17-92 family have
been implicated in various types of malignancies (33,34).
Abnormal expression of miR-17-92 family members is closely
associated with the occurrence and progression of lung cancer
(35,36). The present study investigated
whether one miR-17-92 family member, miR-17-5p, may be involved in
modulating NSCLC drug sensitivity. The findings demonstrated that,
compared with gefitinib-sensitive PC9 parent cells, PC9/GR cells
expressed lower levels of miR-17-5p, suggesting that miR-17-5p may
play a role in GR. This result is similar to the findings of a
study conducted by Gong et al (37) on miR-17-5p regulating GR in A549
cells. As miRNAs typically regulate cellular functions through
interaction with target genes (38), the present study identified
STAT3 as a putative miR-17-5p target, including a plausible
regulatory mechanism that may account for the modulation of GR.
Indeed, the present study demonstrated that knockdown of miR-17-5p
expression enhanced the expression of STAT3, thereby facilitating
GR. However, the addition of CuB could upregulate miR-17-5p
expression in GR PC9/GR cells, thereby ameliorating GR. Liu et
al (5) reported that CuB could
induce lysosomal stress-dependent death of GR NSCLC cells, which
was consistent with the results of the present study.
It has been shown that CuB exhibits potent
antineoplastic effects across a wide variety of cancer types,
including colon (9), bladder
(10), pancreatic (39), breast (40) and NSCLC (41,42).
It has been reported that miRNA can reverse the apoptotic effect of
CuB on cancer cells (39). The
present study demonstrated that inhibiting miR-17-5p negated the
beneficial effects of CuB on PC9/GR cell responses to gefitinib,
thereby upregulating proliferation, downregulating apoptosis and
protein levels of apoptosis-related factors cleaved caspase-3 and
cleaved caspase-9. This suggested that CuB-mediated miR-17-5p
expression modulation impacted the proliferation and apoptosis of
PC9/GR cells. As the underlying mechanisms remained unclear, it was
identified via a TargetScan database analysis that the putative
miR-17-5p target gene STAT3 may play a key role in such
mechanisms.
STAT3 is a known oncogene. Specifically, its
transcription promotes the occurrence and progression of NSCLC
(43–45). Although the present study employed a
dual-luciferase reporter assay to demonstrate that STAT3 is
directly targeted by miR-17-5p, the impact of the miR-17-5p/STAT3
axis on PC9/GR cell function requires further verification.
Findings of the present study also indicated that CuB
dose-dependently enhanced STAT3 protein expression and
phosphorylation, and during combined CuB and gefitinib (at
IC25) treatment miR-17-5p negatively regulated STAT3
protein expression. Furthermore, overexpression of STAT3 increased
proliferation and decreased apoptosis and protein levels of cleaved
caspase-3 and cleaved caspase-9 of PC9/GR cells. These results
indicated that miR-17-5p reversed the effect of CuB and gefitinib
combination therapy in PC9/GR cells by activating the STAT3
protein. Notably, neither GR nor CUB treatment, nor miR-17-5p
mimics or inhibitor transfection, affected the gene expression of
STAT3 in PC9 or PC9/GR cells, suggesting that these factors
could influence the post-transcriptional regulation of
STAT3, but not the transcriptional level of
STAT3.
This study has some shortcomings. First, there is no
further discussion on related pathways. Second, this study did not
analyze the combination of CuB and gefitinib in vivo to see
a true reflection of the effects of this RNA-drug interaction in a
complex biological system. These are also the goals of our future
research.
In conclusion, CuB ameliorated NSCLC resistance to
gefitinib by modulating the miR-17-5p/STAT3 axis in a manner that
decreased STAT3 protein levels and phosphorylation, inhibited
proliferation and promoted apoptosis. These initial preclinical
findings supported this axis as a potential novel target for
prevention or amelioration of GR. Further research is required to
confirm that the beneficial effects of CuB persist in vivo,
and such research should consider the associated biological
pathways in more detail.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Guangdong Province, China (grant no.
2018A030310169).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL designed the study and developed the methodology.
BY, LZ and HT performed the experiments and collected the data. BY
and WW analyzed and interpreted the data. BY drafted the original
manuscript. YL and BY confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CuB
|
cucurbitacin B
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
NSCLC
|
non-small cell lung cancer
|
|
IC
|
inhibitory concentration
|
|
NC
|
negative control
|
|
UTR
|
untranslated region
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
SDS
|
sodium dodecyl sulfate
|
|
PVDF
|
polyvinylidene fluoride
|
|
BSA
|
bovine serum albumin
|
References
|
1
|
Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y,
Zhao J, Kim DW, Soo RA, Kim SW, Pan H, et al INSIGHT Investigators,
: Tepotinib plus gefitinib in patients with EGFR-mutant
non-small-cell lung cancer with MET overexpression or MET
amplification and acquired resistance to previous EGFR inhibitor
(INSIGHT study): An open-label, phase 1b/2, multicentre, randomised
trial. Lancet Respir Med. 8:1132–1143. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park K, Tan EH, O'Byrne K, Zhang L, Boyer
M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, et al: Afatinib versus
gefitinib as first-line treatment of patients with EGFR
mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase
2B, open-label, randomised controlled trial. Lancet Oncol.
17:577–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al West Japan Oncology Group, : Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): An
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lopez Sambrooks C, Baro M, Quijano A,
Narayan A, Cui W, Greninger P, Egan R, Patel A, Benes CH, Saltzman
WM, et al: Oligosaccharyltransferase inhibition overcomes
therapeutic resistance to EGFR tyrosine kinase inhibitors. Cancer
Res. 78:5094–5106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu P, Xiang Y, Liu X, Zhang T, Yang R,
Chen S, Xu L, Yu Q, Zhao H, Zhang L, et al: Cucurbitacin B induces
the lysosomal degradation of EGFR and suppresses the CIP2A/PP2A/Akt
signaling axis in gefitinib-resistant non-small cell lung cancer.
Molecules. 24:242019.
|
|
6
|
Noronha V, Patil VM, Joshi A, Menon N,
Chougule A, Mahajan A, Janu A, Purandare N, Kumar R, More S, et al:
Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin
Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol. 38:124–136.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia X, Liu Y, Liao Y, Guo Z, Huang C,
Zhang F, Jiang L, Wang X, Liu J and Huang H: Synergistic effects of
gefitinib and thalidomide treatment on EGFR-TKI-sensitive and
-resistant NSCLC. Eur J Pharmacol. 856:1724092019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li L, Jiang L, Wang Y, Zhao Y, Zhang XJ,
Wu G, Zhou X, Sun J, Bai J, Ren B, et al: Combination of metformin
and gefitinib as first-line therapy for nondiabetic advanced NSCLC
patients with EGFR mutations: A randomized, double-blind phase II
trial. Clin Cancer Res. 25:6967–6975. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dandawate P, Subramaniam D, Panovich P,
Standing D, Krishnamachary B, Kaushik G, Thomas SM, Dhar A, Weir
SJ, Jensen RA, et al: Cucurbitacin B and I inhibits colon cancer
growth by targeting the Notch signaling pathway. Sci Rep.
10:12902020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurman Y, Kiliccioglu I, Dikmen AU,
Esendagli G, Bilen CY, Sozen S and Konac E: Cucurbitacin B and
cisplatin induce the cell death pathways in MB49 mouse bladder
cancer model. Exp Biol Med (Maywood). 245:805–814. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Wang R, Ma E, Deng Y, Wang X, Xiao J
and Jing Y: The induction of G2/M cell-cycle arrest and apoptosis
by cucurbitacin E is associated with increased phosphorylation of
eIF2alpha in leukemia cells. Anticancer Drugs. 21:389–400. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shukla S, Sinha S, Khan S, Kumar S, Singh
K, Mitra K, Maurya R and Meeran SM: Cucurbitacin B inhibits the
stemness and metastatic abilities of NSCLC via downregulation of
canonical Wnt/β-catenin signaling axis. Sci Rep. 6:218602016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shukla S, Khan S, Kumar S, Sinha S, Farhan
M, Bora HK, Maurya R, Meeran SM and Cucurbitacin B: Cucurbitacin B
Alters the expression of tumor-related genes by epigenetic
modifications in NSCLC and inhibits NNK-induced lung tumorigenesis.
Cancer Prev Res (Phila). 8:552–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Li M and Hu C: Exosomal transfer
of miR-214 mediates gefitinib resistance in non-small cell lung
cancer. Biochem Biophys Res Commun. 507:457–464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang W, Lin J, Wang P and Sun J:
miR-17-5p down-regulation contributes to erlotinib resistance in
non-small cell lung cancer cells. J Drug Target. 25:125–131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Si Q, Xiao S, Xie Q, Lin J, Wang
C, Chen L, Chen Q and Wang L: Prognostic significance of serum
miR-17-5p in lung cancer. Med Oncol. 30:3532013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chatterjee A, Chattopadhyay D and
Chakrabarti G: miR-17-5p downregulation contributes to paclitaxel
resistance of lung cancer cells through altering beclin1
expression. PLoS One. 9:e957162014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Wang W, Chang H, Han Z, Yu X and
Zhang T: Reciprocal regulation of miR-206 and IL-6/STAT3 pathway
mediates IL6-induced gefitinib resistance in EGFR-mutant lung
cancer cells. J Cell Mol Med. 23:7331–7341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen M, Xu Z, Xu W, Jiang K, Zhang F, Ding
Q, Xu Z and Chen Y: Inhibition of ATM reverses EMT and decreases
metastatic potential of cisplatin-resistant lung cancer cells
through JAK/STAT3/PD-L1 pathway. J Exp Clin Cancer Res. 38:1492019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tong M, Wang J, Jiang N, Pan H and Li D:
Correlation between p-STAT3 overexpression and prognosis in lung
cancer: A systematic review and meta-analysis. PLoS One.
12:e01822822017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong WJ, Ma LY, Hu L, Lv YN, Huang H, Xu
JQ, Huang DD, Liu RJ, Han Y, Zhang Y, et al: STAT3 rs4796793
contributes to lung cancer risk and clinical outcomes of
platinum-based chemotherapy. Int J Clin Oncol. 24:476–484. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Kwak KJ, Wu Z, Yang D, Li J, Chang
M, Song Y, Zeng H, Lee LJ, Hu J, et al: PLAUR confers resistance to
gefitinib through EGFR/P-AKT/survivin signaling pathway. Cell
Physiol Biochem. 47:1909–1924. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu X, Li D, Liu J, Ma Z, Huang H, Min L,
Dai L and Dong S: Downregulation of PTPRK promotes cell
proliferation and metastasis of NSCLC by enhancing STAT3
activation. Anal Cell Pathol (Amst). 2019:42650402019.PubMed/NCBI
|
|
26
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Chen L, Li JJ, Zhou Q, Huang A, Liu
WW, Wang K, Gao L, Qi ST and Lu YT: miR-519a enhances
chemosensitivity and promotes autophagy in glioblastoma by
targeting STAT3/Bcl2 signaling pathway. J Hematol Oncol. 11:702018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du W, Pan Z, Chen X, Wang L, Zhang Y, Li
S, Liang H, Xu C, Zhang Y, Wu Y, et al: By targeting Stat3
microRNA-17-5p promotes cardiomyocyte apoptosis in response to
ischemia followed by reperfusion. Cell Physiol Biochem. 34:955–965.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liao XH, Xiang Y, Yu CX, Li JP, Li H, Nie
Q, Hu P, Zhou J and Zhang TC: STAT3 is required for
miR-17-5p-mediated sensitization to chemotherapy-induced apoptosis
in breast cancer cells. Oncotarget. 8:15763–15774. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ogino A, Kitao H, Hirano S, Uchida A,
Ishiai M, Kozuki T, Takigawa N, Takata M, Kiura K and Tanimoto M:
Emergence of epidermal growth factor receptor T790M mutation during
chronic exposure to gefitinib in a non small cell lung cancer cell
line. Cancer Res. 67:7807–7814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peng W, He D, Shan B, Wang J, Shi W, Zhao
W, Peng Z, Luo Q, Duan M, Li B, et al: LINC81507 act as a competing
endogenous RNA of miR-199b-5p to facilitate NSCLC proliferation and
metastasis via regulating the CAV1/STAT3 pathway. Cell Death Dis.
10:5332019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu H, Wu Z, Zhou H, Cai W, Li X, Hu J,
Gao L, Feng T, Wang L, Peng X, et al: The SOX4/miR-17-92/RB1 axis
promotes prostate cancer progression. Neoplasia. 21:765–776. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu F, Zhang F, Li X, Liu Q, Liu W, Song
P, Qiu Z, Dong Y and Xiang H: Prognostic role of miR-17-92 family
in human cancers: Evaluation of multiple prognostic outcomes.
Oncotarget. 8:69125–69138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Li Y, Qi P and Ma Z: Biology of
miR-17-92 cluster and its progress in lung cancer. Int J Med Sci.
15:1443–1448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang C, Jia X, Zhou J, Sun Q and Ma Z: The
MiR-17-92 gene cluster is a blood-based marker for cancer detection
in non-small-cell lung cancer. Am J Med Sci. 360:248–260. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong J, He L, Ma J, Zhang J, Wang L and
Wang J: The relationship between miR-17-5p, miR-92a, and let-7b
expression with non-small cell lung cancer targeted drug
resistance. J BUON. 22:454–461. 2017.PubMed/NCBI
|
|
38
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou J, Liu M, Chen Y, Xu S, Guo Y and
Zhao L: Cucurbitacin B suppresses proliferation of pancreatic
cancer cells by ceRNA: Effect of miR-146b-5p and lncRNA-AFAP1-AS1.
J Cell Physiol. 234:4655–4667. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dittharot K, Dakeng S, Suebsakwong P,
Suksamrarn A, Patmasiriwat P, Promkan M and Cucurbitacin B:
Cucurbitacin B Induces Hypermethylation of Oncogenes in Breast
Cancer Cells. Planta Med. 85:370–378. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marostica LL, de Barros ALB, Oliveira J,
Salgado BS, Cassali GD, Leite EA, Cardoso VN, Lang KL, Caro MS,
Durán FJ, et al: Antitumor effectiveness of a combined therapy with
a new cucurbitacin B derivative and paclitaxel on a human lung
cancer xenograft model. Toxicol Appl Pharmacol. 329:272–281. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marostica LL, Silva IT, Kratz JM, Persich
L, Geller FC, Lang KL, Caro MS, Durán FJ, Schenkel EP and Simões
CM: Synergistic antiproliferative effects of a new cucurbitacin B
derivative and chemotherapy drugs on lung cancer cell line A549.
Chem Res Toxicol. 28:1949–1960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang X, Sai B, Wang F, Wang L, Wang Y,
Zheng L, Li G, Tang J and Xiang J: Hypoxic BMSC-derived exosomal
miRNAs promote metastasis of lung cancer cells via STAT3-induced
EMT. Mol Cancer. 18:402019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu W, Ru Z, Zhou Y, Xiao W, Sun R, Zhang
S, Gao Y, Li X, Zhang X and Yang H: Lung cancer-derived
extracellular vesicles induced myotube atrophy and adipocyte
lipolysis via the extracellular IL-6-mediated STAT3 pathway.
Biochim Biophys Acta Mol Cell Biol Lipids. 1864:1091–1102. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi J, Li J, Yang S, Hu X, Chen J, Feng J,
Shi T, He Y, Mei Z, He W, et al: LncRNA SNHG3 is activated by E2F1
and promotes proliferation and migration of non-small-cell lung
cancer cells through activating TGF-β pathway and IL-6/JAK2/STAT3
pathway. J Cell Physiol. 235:2891–2900. 2020. View Article : Google Scholar : PubMed/NCBI
|