Introduction
Type 2 diabetes mellitus (T2DM) is a common chronic
metabolic disease (1). It is
estimated that there are ~382 million patients with T2DM worldwide.
By 2035, it is estimated that the number of patients will be 592
million (2). The liver is one of
the target organs affected by long-term hyperglycemia (3). Pronounced insulin resistance and
hyperglycemia have been observed in patients with T2DM accompanied
by nonalcoholic fatty liver disease (NAFLD) (4). Lipid accumulation caused by insulin
resistance can promote the development of NAFLD in patients with
T2DM (5–7). Furthermore, inflammation and oxidative
stress caused by liver lipid accumulation can accelerate liver cell
necrosis and fibrosis (8,9). Currently, the main treatment options
for T2DM or NAFLD include exercise, diet, and hypoglycemic and
hypolipidemic treatment; however, the therapeutic effects of these
strategies remain unsatisfactory (10). Increasing evidence has suggested
that the occurrence and development of T2DM-induced NAFLD is
associated with a complex pathogenesis and multiple factors
(10). There is currently no
effective drugs for the treatment of T2DM-induced NAFLD; therefore,
there is an urgent need to identify novel drugs to prevent NAFLD
caused by T2DM.
Through in-depth research, it has been revealed that
numerous active ingredients in traditional Chinese medicine have
the potential to relieve NAFLD. Increasing evidence has
demonstrated that carvacrol (2-methyl-5-isopropylphenol), a
monoterpenic phenol, may possess pharmacological properties,
including neuroprotective (11),
anti-inflammatory (12),
anti-oxidative (13),
anti-bacterial (14) and
anti-cancer (15) effects.
Carvacrol has been reported to relieve T2DM-related complications,
such as vascular inflammation (12), hypercontractility in the aorta
(16) and diabetic cardiomyopathy
(17). Nevertheless, to the best of
our knowledge, the effect of carvacrol on T2DM-induced liver injury
remains unknown. Because hyperglycemic db/db mice have been widely
utilized as T2DM models, the present study chose this method to
construct T2DM models (18,19). The present study hypothesized that
carvacrol may ameliorate liver injury induced by T2DM.
Materials and methods
Animals
A total of 30 male C57BL/KsJ db/db mice (age, 8
weeks; weight, 32–36 g) and 15 age-matched C57BL/6J control
non-diabetic db/m+ mice (weight, 16–18 g) were purchased from
Changzhou Cavans Experimental Animal Co., Ltd. (license number:
SCXK2001-0003). The mice were maintained under a 12-h light/dark
cycle, at 22±3°C and 70±10% humidity; they could freely move and
had free access to food and water. The animal experiment was
conducted according to the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (20). The present study was approved by the
Animal Care and Ethics Committee of The Second Affiliated Hospital
of Guizhou University of Traditional Chinese Medicine (approval no.
2018072; Guiyang, China).
Grouping and administration
All mice were routinely fed for 3 days. The 15 db/m+
mice were employed as the normal control group, and db/db mice were
randomly separated into two groups (n=15/group): Model group and
carvacrol group. Mice in the model and normal control groups were
administered saline daily by gavage. Mice in the carvacrol group
were administered an appropriate dose of carvacrol (10 mg/kg) daily
by gavage, as previously described (12). After daily treatment for 6 weeks,
all mice were fasted for 6 h, but were allowed to drink water.
Subsequently, venous blood was obtained from the tail vein and
fasting blood glucose was determined using an automatic blood
glucose meter (Johnson & Johnson). After taking blood, the mice
were euthanized through intraperitoneal injection of 200 mg/kg
pentobarbital sodium. Absence of a heartbeat and breathing was used
as the criterion for confirming euthanasia of rats. The blood
samples were left at room temperature for 2 h, and centrifuged at
3,500 × g for 15 min at 4°C. The supernatant was harvested for
serological analysis. In addition, liver tissues were removed and
weighed. Liver index was calculated in line with the ratio of liver
weight (g) and body weight (g). The tissue specimens were stored at
−80°C before further use.
Determination of serum glucose and
insulin
Mouse serum insulin concentration was examined using
a mouse insulin ELISA kit (cat. no. H203-1-2; Nanjing Jiancheng
Bioengineering Institute) according to manufacturer's protocol. The
absorbance value was examined at 450 nm using a microplate reader.
Homeostasis model assessment of insulin resistance (HOMA-IR) is one
of the most common methods used to evaluate insulin resistance
(21). HOMA-IR was determined
according to the following equation: HOMA-IR=fasting blood glucose
(mmol/l) × fasting insulin (mU/l)/22.5, where fasting insulin was
detected using the mouse insulin ELISA kit.
Oral glucose tolerance test
(OGTT)
After fasting for 6 h, the mice were given an oral
glucose solution (1.2 g/kg; body weight). Blood was drawn from the
tail vein and blood glucose was determined after glucose
administration for 0, 30, 60 and 120 min using a glucose monitor
(Ascensia ELITE; Bayer).
Insulin tolerance test (ITT)
ITT was conducted via intraperitoneally injecting 1
U/kg regular insulin (Novo Nordisk A/S) into mice that were fasted
for 6 h. Blood glucose levels were determined at 0, 15, 30, 60 and
120 min using a glucose monitor (Ascensia ELITE; Bayer).
Biochemical analyses
Serum alanine aminotransferase (ALT), aspartate
aminotransferase (AST), total cholesterol (TC), low-density
lipoprotein cholesterol (LDL-C), high-density lipoprotein
cholesterol (HDL-C), triglyceride (TG), glycosylated serum protein
(GSP), blood urea nitrogen (BUN), creatinine (Cre) and uric acid
(UA) levels were separately detected using ALT (cat. no. C009-2-1),
AST (cat. no. C010-1-1), TC (cat. no. A111-1-1), LDL-C (cat. no.
A113-1-1), HDL-C (cat. no. A112-1-1), TG (cat. no. A110-1-1), GSP
(cat. no. A037-2-1), BUN (cat. no. C013-2-1), Cre (cat. no.
C011-2-1) and UA ELISA kits (cat. no. C012-2-1) (all from Nanjing
Jiancheng Bioengineering Institute) according to manufacturer's
protocols.
Hematoxylin and eosin (H&E)
staining
Liver tissue specimens were fixed in 4%
paraformaldehyde solution at 4°C for 24 h, embedded in paraffin and
sectioned. Paraffin-embedded sections (5 µm) were treated with
xylene for 20 min twice, absolute ethanol for 5 min twice and 75%
ethanol for 5 min at 37°C. The sections were separately stained
with 0.5% hematoxylin and 0.5% eosin solution for 5 min at 37°C.
Neutral gum was used for sealing and images were captured under an
optical microscope (Olympus Corporation). The nucleus was stained
blue and the cytoplasm was stained red.
Masson's trichrome staining
Liver tissues were fixed overnight with 10% formalin
solution at 4°C and cut into 5-µm sections. Masson's trichrome
staining was conducted using the Masson's Trichrome Staining Kit
(cat. no. M1340; Beijing Solarbio Science & Technology Co.,
Ltd.). The tissues were stained with 0.5% Regaud hematoxylin for 10
min at 37°C. After washing for 15 min, 2% Masson lichun red acid
complex red fluid was added for 5–10 min. Subsequently, the tissues
were stained with 0.5% aniline blue, and then immersed in 1%
phosphotungstic acid-phosphomolybdic acid solution for 15 min. The
tissues were then washed with 1% glacial acetic acid solution for 5
min, and were further washed with 1% glacial acetic acid solution,
dehydrated, permeabilized and sealed in neutral resin. Images were
captured under a light microscope (Olympus Corporation). The nuclei
were stained black and the collagen fibers were stained blue.
Reticular fiber staining
Liver tissues were fixed overnight with 10% formalin
solution at 4°C and cut into 5-µm sections. Liver sections were
incubated with 0.5% potassium permanganate for 3 min, and 2% oxalic
acid and 2% ferric ammonium sulfate for 1 min at 37°C.
Subsequently, the sections were treated with ammonia silver
solution for 5 min and 10% formaldehyde solution for 30 sec. The
sections were then incubated with the gold chloride solution for 5
min, and sodium thiosulfate for 2 min. Finally, the sections were
stained with 0.5% eosin staining solution for 30 sec, and images
were captured under a light microscope (Olympus Corporation) and
were analyzed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.).
Periodic acid Schiff (PAS)
staining
Liver tissues were fixed, embedded and sectioned as
they were prior to H&E staining. Paraffin-embedded sections
were stained with 1% periodic acid for 15 min, 1% Chevron dye for
30 min and 0.5% hematoxylin for 3–5 min at 37°C. Images were
captured under a light microscope (Olympus Corporation). The
glycogen was stained purple-red and the cell nuclei were stained
light blue.
Immunohistochemistry
Liver tissues were fixed overnight with 10% formalin
solution at 4°C and cut into 5-µm sections. Liver tissue sections
were blocked with 5% skimmed milk powder for 1 h at 37°C.
Subsequently, the sections were incubated with the following
primary antibodies: Toll-like receptor 4 (TLR4; 1:100; cat. no.
ab13556; Abcam), NF-κB (1:100; cat. no. 10745–1-AP; Proteintech
Group, Inc.), NALP3 (1:100; cat. no. bs-6655R; BIOSS), AKT1 (1:150;
cat. no. 10176-2-AP; Proteintech Group, Inc.), phosphorylated
(p)-AKT1 (1:100; cat. no. 66444-1-Ig; Proteintech Group, Inc.),
insulin receptor (INSR; 1:80; cat. no. ab69508; Abcam), p-INSR
(1:80; cat. no. bs-16680R; BIOSS), mTOR (1:100; cat. no.
20657-1-AP; Proteintech Group, Inc.), p-mTOR (1:200; cat. no.
ab109268; Abcam), insulin receptor substrate 1 (IRS1; 1:100; cat.
no. ab245314; Abcam) and p-IRS1 (1:100; cat. no. bs-3200R; BIOSS)
at 4°C overnight. Subsequently, sections were incubated with
secondary antibodies (1:500; cat. nos. ab205719 and ab6721; Abcam)
for 1 h at 37°C, followed by staining with DAB for 5 min and
hematoxylin for 2 min at 37°C. Images were captured under a light
microscope (Olympus Corporation).
Immunofluorescence
Liver tissues were fixed overnight with 10% formalin
solution at 4°C and cut into 5 µm sections. Liver tissue sections
were blocked with 5% skimmed milk powder for 1 h at 37°C treated
with unconjugated primary antibodies, as follows: NALP3 (1:100;
cat. no. bs-6655R; BIOSS), mTOR (1:100; cat. no. 20657-1-AP;
Proteintech Group, Inc.), p-mTOR (1:200; cat. no. ab109268; Abcam),
TLR4 (1:100; cat. no. ab13556; Abcam), AKT1 (1:150; cat. no.
10176-2-AP; Proteintech Group, Inc.) and p-AKT1 (1:100; cat. no.
66444-1-Ig; Proteintech Group, Inc.) overnight at 4°C. After
washing with TBS-0.3% Tween-20 (TBST), sections were incubated with
secondary antibodies (1:500; cat. nos. ab150077 and ab150113;
Abcam) at room temperature for 2 h. Images were captured under an
immunofluorescence microscope.
Western blotting
Tissues were lysed using RIPA lysis buffer (Beyotime
Institute of Biotechnology) plus phosphatase and protease
inhibitors on ice. After centrifugation at 12,000 × g for 15 min at
4°C, the supernatant was harvested. Protein concentration was
examined using the BCA method (Pierce; Thermo Fisher Scientific,
Inc.). Subsequently, 120 µg protein was loaded per lane, samples
were separated by SDS-PAGE on 10% gels and were transferred onto
PVDF membranes (cat. no. IPVH00010; EMD Millipore). The membranes
were blocked with 5% skimmed milk powder for 2–3 h at room
temperature. The membranes were then incubated with the following
primary antibodies: TLR4 (1:1,000; cat. no. ab13556; Abcam), NF-κB
(1:1,000; cat. no. 10745-1-AP; Proteintech Group, Inc.), NALP3
(1:500; cat. no. bs-6655R; BIOSS), AKT1 (1:1,000; cat. no.
10176-2-AP; Proteintech Group, Inc.), p-AKT1 (1:500; cat. no.
66444-1-Ig; Proteintech Group, Inc.), INSR (1:400; cat. no.
ab69508; Abcam), p-INSR (1:400; cat. no. bs-16680R; BIOSS), mTOR
(1:2,000; cat. no. 20657-1-AP; Proteintech Group, Inc.), p-mTOR
(1:1,000; cat. no. ab109268; Abcam), IRS1 (1:500; cat. no.
ab245314; Abcam), p-IRS1 (1:500; cat. no. bs-3200R; BIOSS) and
β-actin (1:1,000; cat. no. bs-0061R; BIOSS) at 4°C overnight. After
washing three times with TBST, the membranes were incubated with
secondary antibodies (1:2,000; cat. nos. ab205719 and ab6721;
Abcam) at room temperature for 1 h. Blots were visualized utilizing
an ECL kit (cat. no. KGP1121; Nanjing KeyGen Biotech Co., Ltd.) and
a gel image detection analyzer (Invitrogen; Thermo Fisher
Scientific, Inc.). The results were semi-quantified using ImageJ
software (version 1.48; National Institutes of Health).
Statistical analysis
GraphPad Prism 8.0 (GraphPad Software, Inc.) was
utilized for statistical analysis. Data are presented as the mean ±
standard deviation and each assay was repeated at least three
times. One-way ANOVA was used for multiple comparisons, followed by
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Carvacrol decreases body weight, liver
weight and liver index of db/db mice
Compared with in the control group, the body weight
of db/db mice was significantly increased (Fig. 1A). Following treatment with 10 mg/kg
carvacrol, the body weight of db/db mice was gradually decreased.
Compared with that in the control group, db/db mice had a
significantly higher daily intake of water (Fig. 1B) and feed (Fig. 1C). Notably, carvacrol treatment
could ameliorate daily water intake for db/db mice; however, there
was no significant difference in feed intake between the model and
carvacrol groups. Furthermore, liver weight and liver index of
db/db mice were significantly increased compared with those in the
control group (Fig. 1D and E).
After 6 weeks of treatment with 10 mg/kg carvacrol, these two
indexes were significantly decreased in db/db mice (Fig. 1D and E). These results revealed that
T2DM could increase body and liver weight, which could be reversed
by carvacrol treatment.
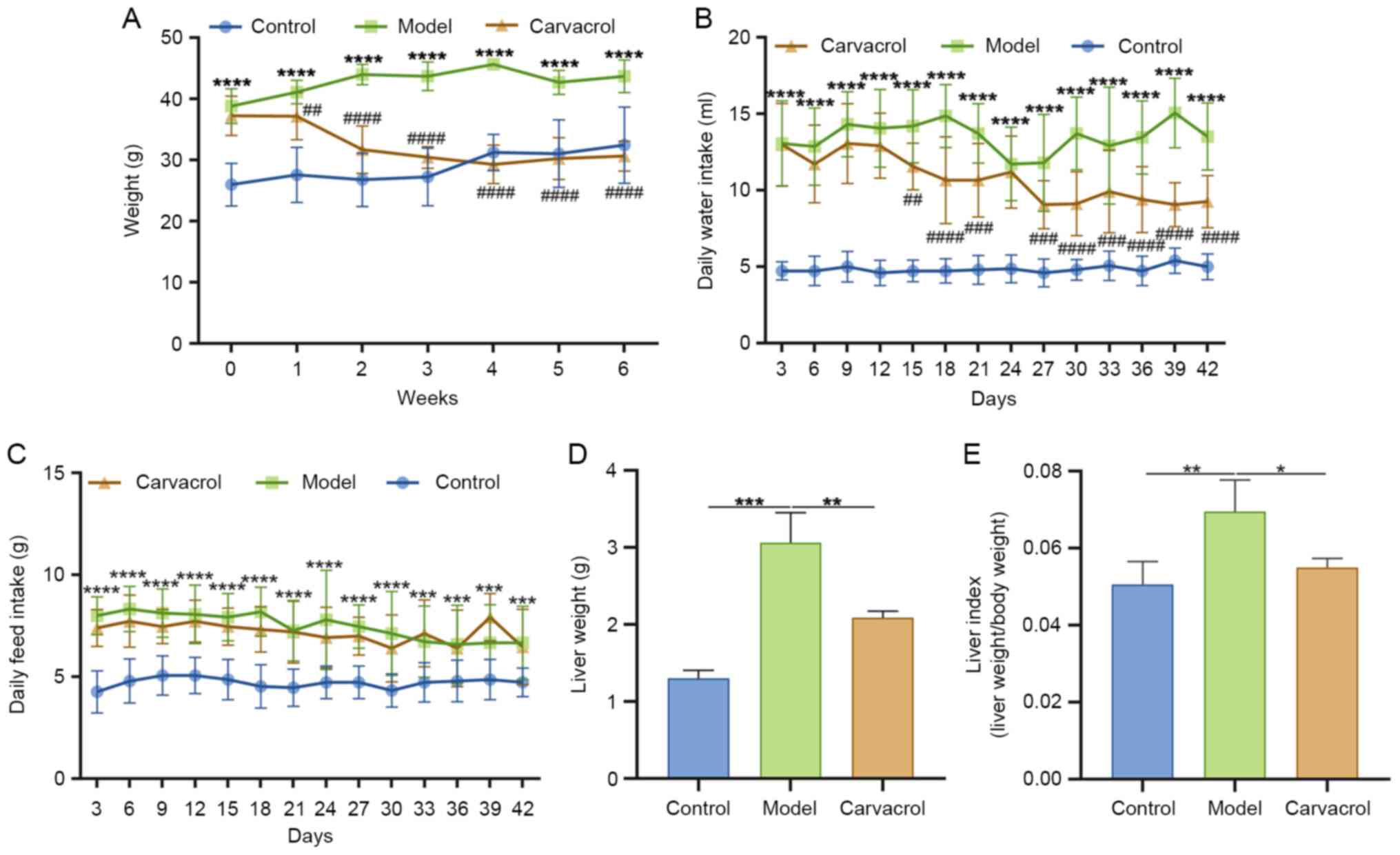 | Figure 1.Effect of carvacrol on body weight,
energy intake, liver weight and liver index of db/db mice. (A) Body
weight, (B) daily water intake, (C) daily feed intake, (D) liver
weight and (E) liver index. n=15/group. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 vs. control group or as indicated;
##P<0.01, ###P<0.001,
####P<0.0001 vs. model group. |
Carvacrol improves blood glucose and
insulin resistance of T2DM db/db mice
Fasting blood glucose and insulin levels can reflect
sensitivity to insulin at an overall level (22). Compared with in the control group,
the db/db mice exhibited higher fasting blood glucose levels
(Fig. 2A). Conversely, after 6
weeks of administration, carvacrol significantly reduced fasting
blood glucose levels (Fig. 2A).
Furthermore, GSP levels were detected in the three groups. As shown
in Fig. 2B, the db/db mice had
higher GSP levels compared with those in the control group.
Carvacrol administration significantly decreased the levels of GSP
in db/db mice. In addition, an OGTT was performed on the mice; as
expected, carvacrol markedly decreased the blood glucose levels of
db/db mice (Fig. 2C). Furthermore,
fasting blood insulin levels were significantly higher in the model
group compared with those in the control group (Fig. 2D), which were decreased by carvacrol
treatment. HOMA-IR levels were also significantly increased in the
model group compared with those in the control group (Fig. 2E). Conversely, HOMA-IR levels were
significantly suppressed in db/db mice following carvacrol
treatment. The mice also underwent an ITT. As shown Fig. 2F, compared with in the model group,
blood glucose levels were significantly decreased in
carvacrol-treated db/db mice at 0, 15, 30, 60 and 120 min after
insulin injection.
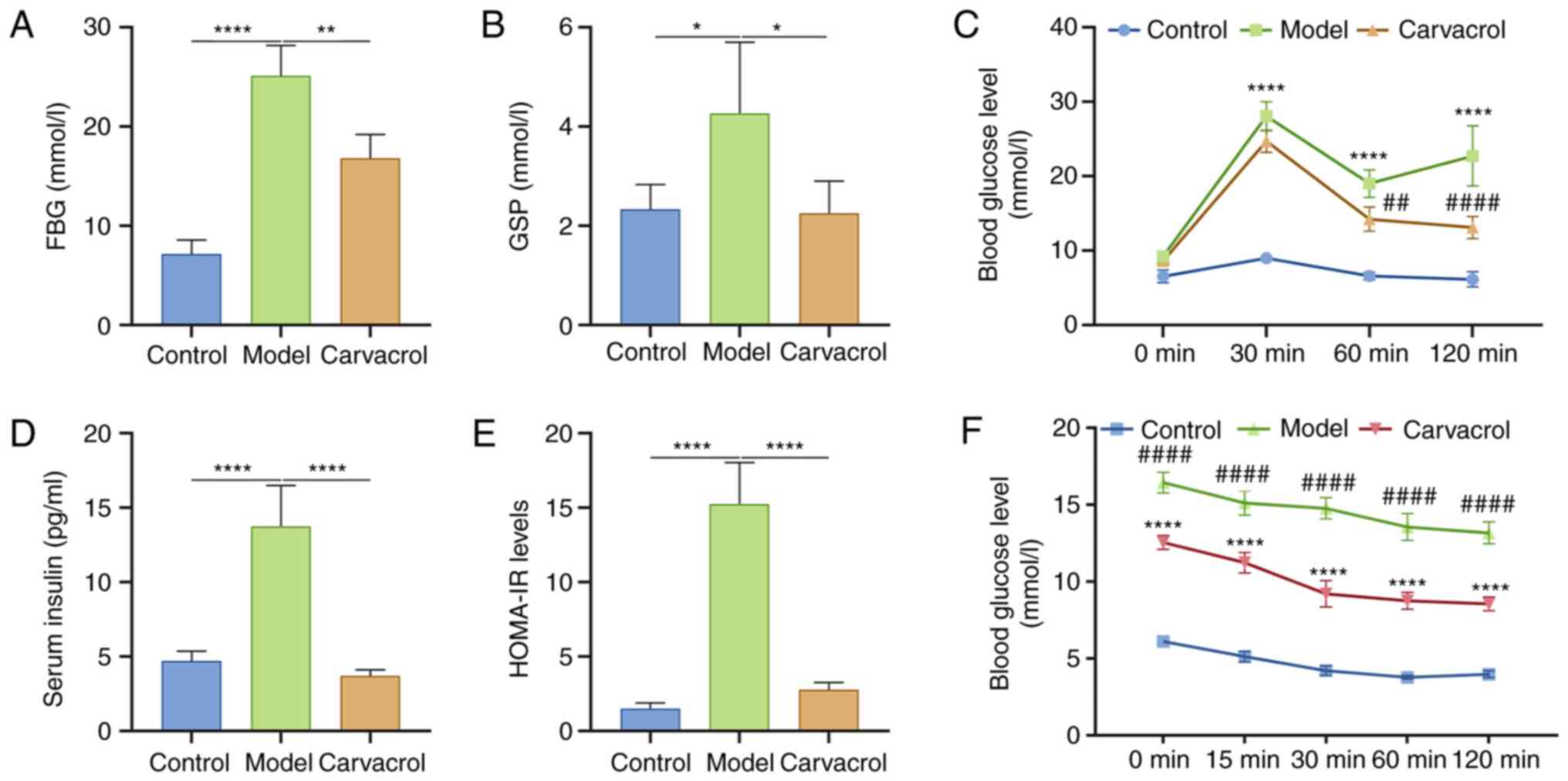 | Figure 2.Carvacrol improves FBG and insulin
resistance in db/db mice. (A) FBG, (B) GSP, (C) blood glucose
level, as determined by OGTT, (D) fasting insulin, (E) HOMA-IR and
(F) blood glucose level, as determined by ITT. n=15/group.
*P<0.05, **P<0.01, ****P<0.0001 vs. control group or as
indicated; ##P<0.01, ####P<0.0001 vs.
model group. FBG, fasting blood glucose; GSP, glycosylated serum
protein; HOMA-IR, homeostasis model assessment of insulin
resistance; ITT, insulin tolerance test; OGTT, oral glucose
tolerance test. |
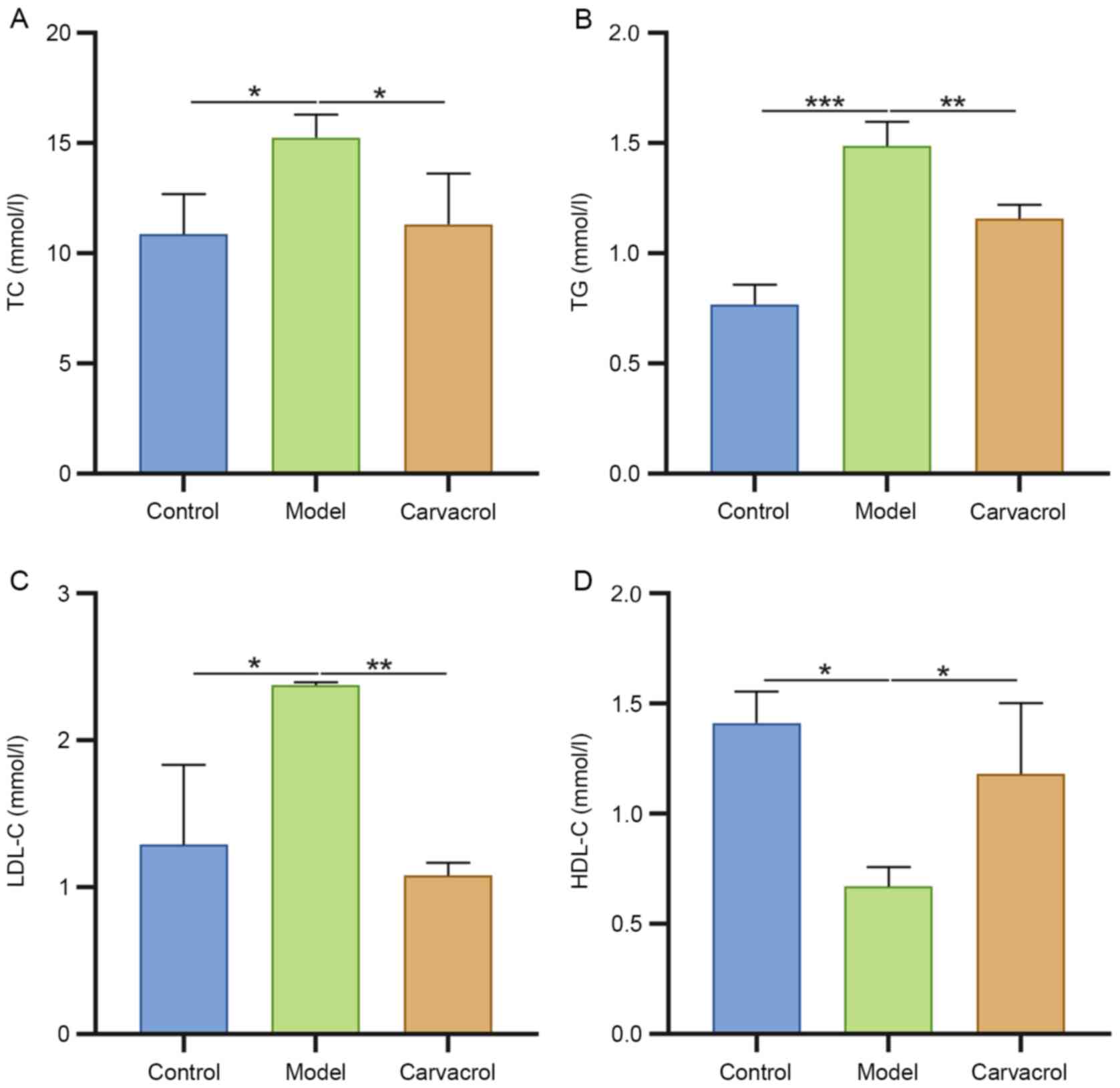
Carvacrol ameliorates dyslipidemia in
db/db mice
TC (Fig. 3A), TG
(Fig. 3B) and LDL-C levels
(Fig. 3C) were significantly
increased, and HDL-C levels (Fig.
3D) were significantly decreased in the serum of db/db mice
compared with those in the control group. Carvacrol administration
significantly reduced serum levels of TC, TG and LDL-C, and
increased serum levels of HDL-C in db/db mice, indicating that
carvacrol could ameliorate hyperlipidemia of db/db mice.
Carvacrol improves liver and kidney
damage in db/db mice
When liver cells are damaged, a large amount of AST
and ALT is released from the liver cells into the blood (23). Therefore, serum AST and ALT levels
can reflect the severity of liver damage. As shown in Fig. 4A and B, serum ALT and AST levels
were significantly higher in db/db mice compared with those in the
control group. Following administration with carvacrol, serum ALT
and AST levels were significantly reduced in db/db mice, suggesting
that carvacrol could improve T2DM-induced liver injury.
Furthermore, the renal function of the three groups was assessed.
The results revealed that serum BUN (Fig. 4C), Cre (Fig. 4D) and UA levels (Fig. 4E) were significantly higher in db/db
mice compared with those in the control group. Conversely,
carvacrol treatment significantly lowered these serum levels in
db/db mice. These findings demonstrated that carvacrol could
improve liver and kidney damage in db/db mice.
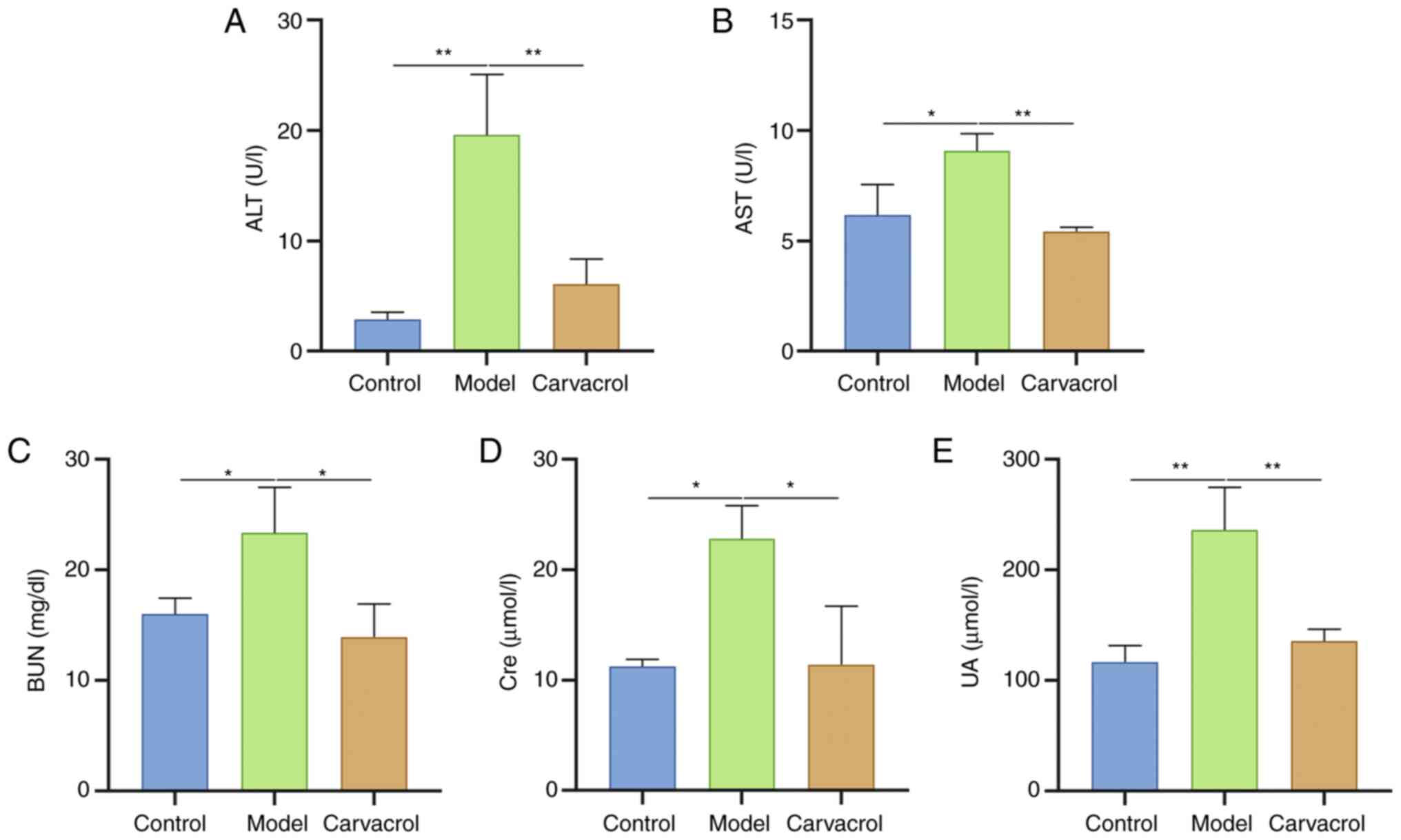 | Figure 4.Carvacrol improves liver and kidney
damage in db/db mice. (A) Serum ALT, (B) AST, (C) BUN, (D) Cre and
(E) UA levels were determined in the control, model and carvacrol
groups. n=15/group. *P<0.05, **P<0.01. ALT, alanine
aminotransferase; AST, aspartate aminotransferase; BUN, blood urea
nitrogen; Cre, creatinine; UA, uric acid. |
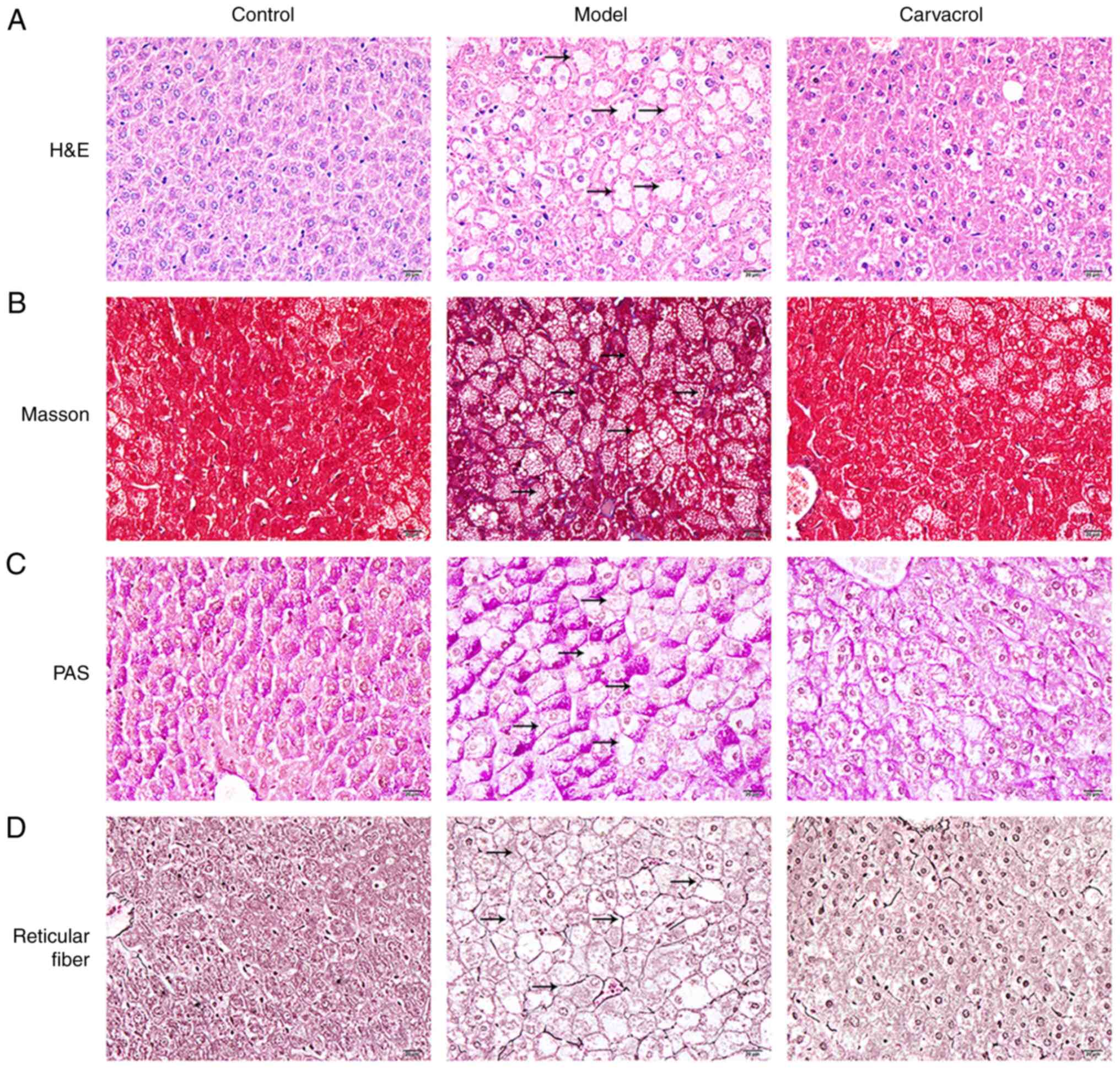
Carvacrol protects the liver of db/db
mice, as determined through histological examinations
Histological analysis of liver cell injury was
performed using H&E staining of the three groups (Fig. 5A). It was revealed that liver injury
of db/db mice was more serious compared with that in the control
group. Notably, vacuolar degeneration of hepatocytes was detected
in db/db mice. Conversely, carvacrol reduced the levels of liver
cell damage in db/db mice. Collagen fiber content in liver tissues
was detected utilizing Masson's trichrome staining (Fig. 5B). It was demonstrated that control
mice exhibited a normal liver lobule structure, whereas obvious
hyperplasia of collagen fibers in liver tissue was detected in
db/db mice. Following carvacrol treatment, the amount of collagen
fiber hyperplasia in the liver tissue of db/db mice was markedly
reduced. These findings indicated that diabetes may cause liver
fibrosis and carvacrol treatment may improve collagen fiber
hyperplasia in the liver tissues of db/db mice. In addition, PAS
was performed to detect glycogen in liver cells (Fig. 5C). Compared with in the control
group, there was obvious glycogen accumulation in the cytoplasm of
liver cells in db/db mice, whereas carvacrol treatment markedly
ameliorated glycogen accumulation in liver cells. In addition, as
shown in Fig. 5D, obvious reticular
fiber hyperplasia was detected in the liver tissues of db/db mice,
which was notably improved by carvacrol treatment.
Effects of carvacrol on insulin,
TLR4/NF-κB and AKT1/mTOR signaling pathways in db/db mice
To assess the potential mechanisms underlying the
effects of carvacrol on T2DM-induced liver injury, the expression
of insulin, TLR4/NF-κB and AKT1/mTOR signaling pathway components
were detected in the liver tissue specimens. Immunohistochemistry
results demonstrated that TLR4, NF-κB, NALP3 (Fig. 6A and B), AKT1, p-AKT1, INSR, p-INSR
(Fig. 6C and D), mTOR, p-mTOR, IRS1
and p-IRS1(Fig. 6E and F) were all
highly expressed in the liver tissues of db/db mice compared with
those in the control group. Following treatment with carvacrol, the
expression levels of these proteins were significantly suppressed
in the liver tissues of db/db mice. These data indicated that both
total and phosphorylated forms of AKT, mTOR, INSR and IRS1 were
activated in the liver tissues of db/db mice and were reduced after
carvacrol treatment, thus suggesting that carvacrol may inhibit the
expression and phosphorylation of these proteins in db/db mice.
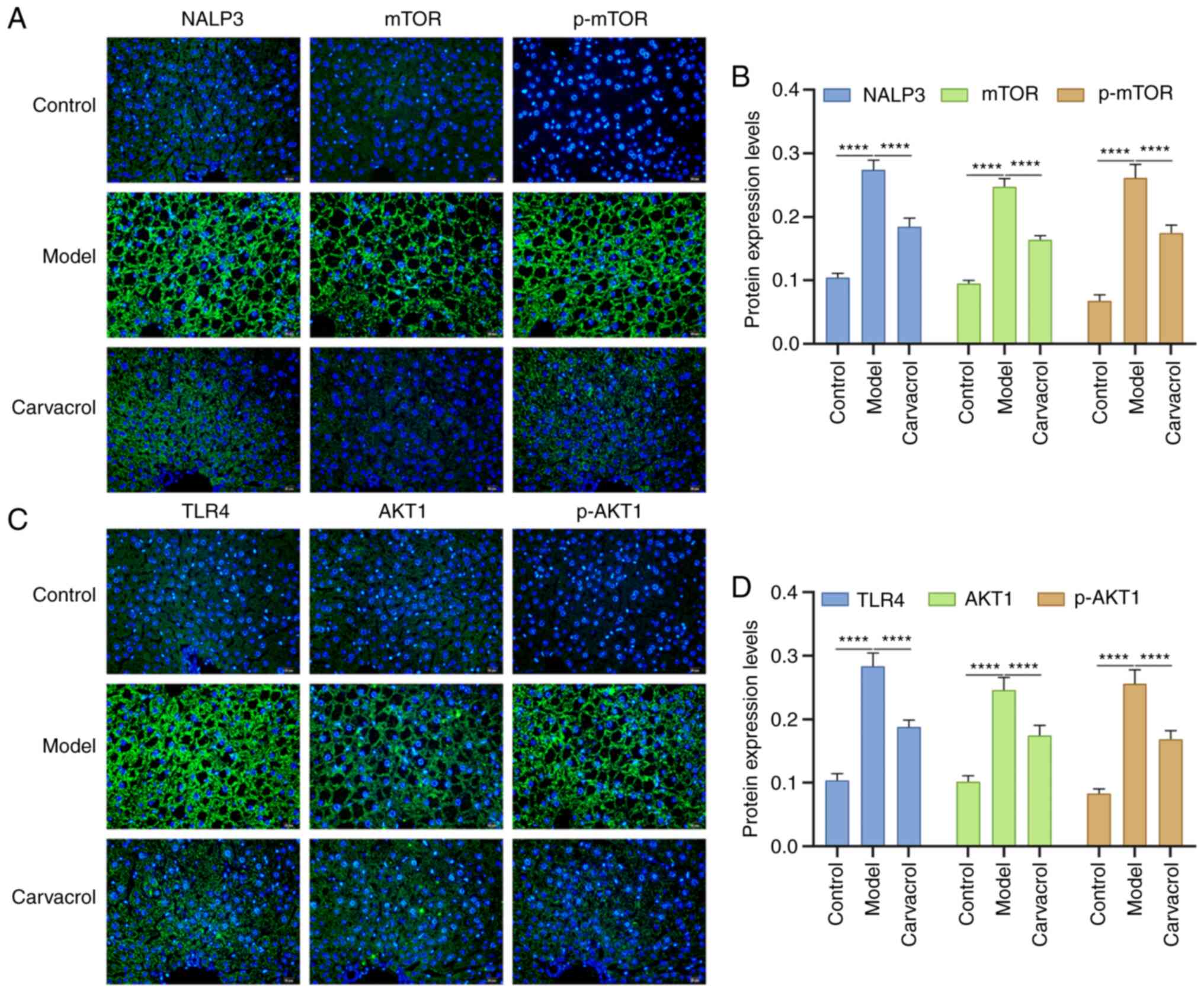
Consistently, immunofluorescence results confirmed that the
expression levels of NALP3, mTOR, p-mTOR (Fig. 7A and B), TLR4, AKT1 and p-AKT1
(Fig. 7C and D) were higher in the
liver tissues of db/db mice compared with those in the control
group. Conversely, carvacrol administration significantly decreased
the expression levels of these aforementioned proteins in db/db
mice. In addition, the expression levels of these proteins were
detected in the liver tissues of the three groups using western
blotting (Fig. 8A). As expected,
the expression levels and the phosphorylated/total protein ratios
of these proteins were significantly upregulated in db/db mice
compared with those in the control group (Fig. 8B-F). Nevertheless, carvacrol
treatment significantly decreased the expression levels and the
phosphorylated/total protein ratios of these proteins in db/db
mice. These findings indicated that carvacrol could ameliorate
T2DM-induced liver injury via insulin, TLR4/NF-κB and AKT1/mTOR
signaling pathways.
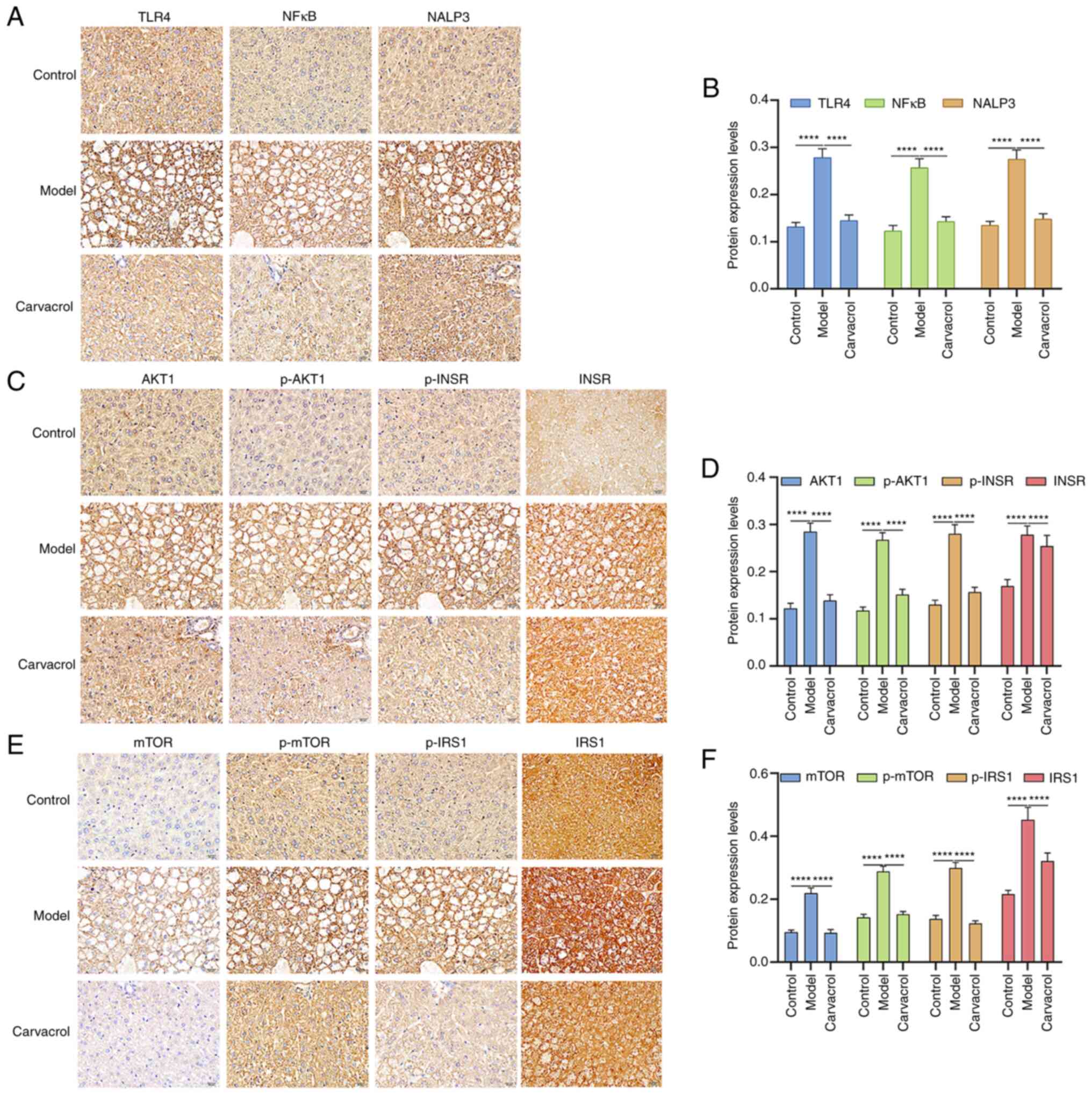 | Figure 6.Immunohistochemical analysis of the
expression levels of insulin, TLR4/NF-κB and AKT1/mTOR signaling
pathway components in the liver tissues of db/m+ and db/db mice. (A
and B) TLR4, NF-κB and NALP3; (C and D) AKT1, p-AKT1, INSR and
p-INSR; (E and F) mTOR, p-mTOR, IRS1 and p-IRS1. Scale bar, 20 µm;
magnification, ×200. ****P<0.0001. TLR4, Toll-like receptor 4;
INSR, insulin receptor; IRS1, insulin receptor substrate 1; p-,
phosphorylated. |
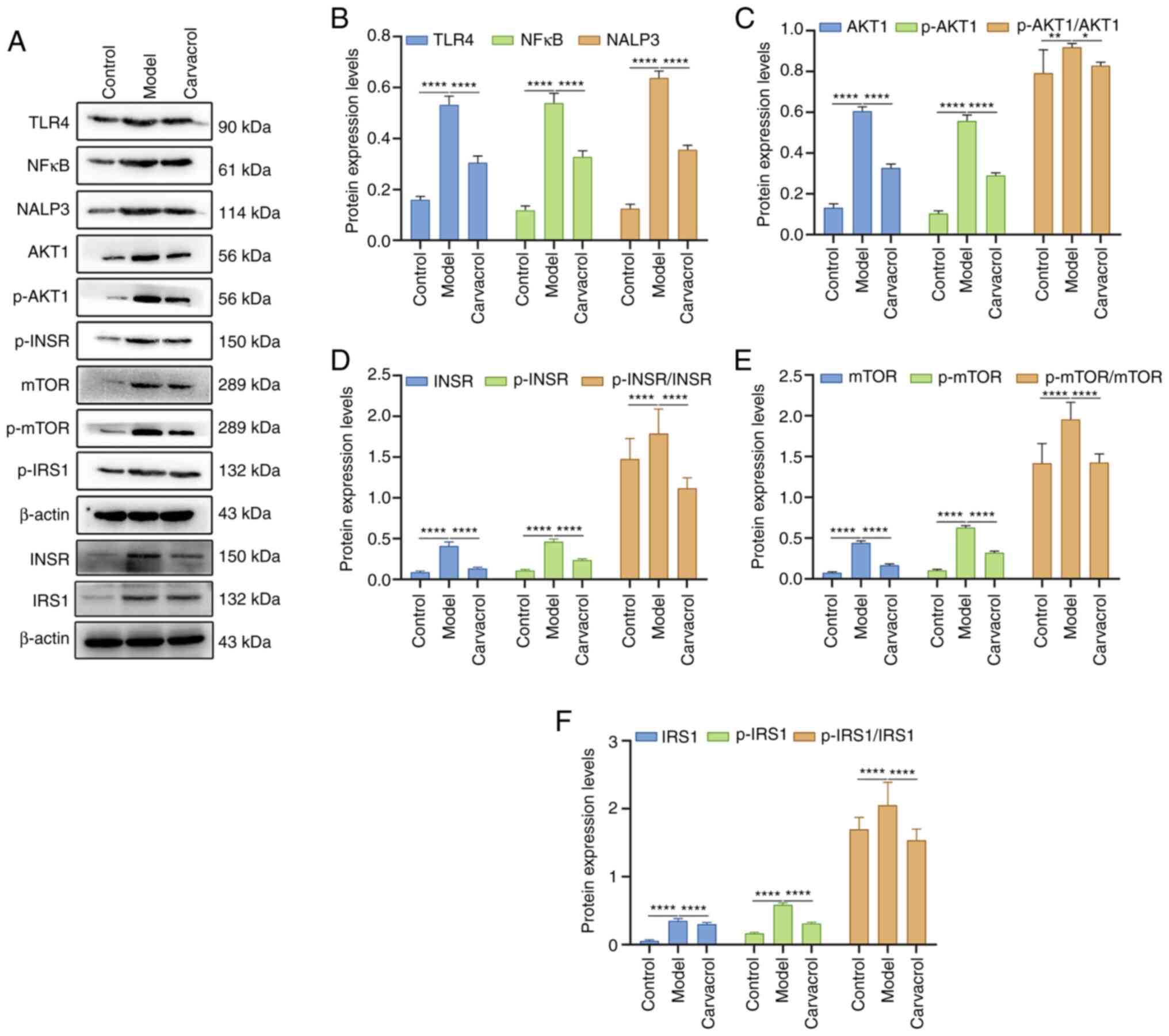 | Figure 8.Western blot analysis of the
expression levels of insulin, TLR4/NF-κB and AKT1/mTOR signaling
pathway components in the liver tissues of db/m+ and db/db mice.
(A) Representative western blot images. Total INSR and IRS1 (and
β-actin) were blotted for on a different membrane from the other
proteins. (B) TLR4, NF-κB and NALP3; (C) AKT1, p-AKT1 and
p-AKT1/AKT1; (D) INSR, p-INSR and p-INSR/INSR; (E) mTOR, p-mTOR and
p-mTOR/mTOR; (F) IRS1, p-IRS1 and p-IRS1/IRS1. β-actin was used as
a loading control. n=15/group. *P<0.05; **P<0.01,
****P<0.0001. TLR4, Toll-like receptor 4; INSR, insulin
receptor; IRS1, insulin receptor substrate 1; p-,
phosphorylated. |
Discussion
NAFLD has a very high prevalence worldwide and is
characterized by increased intrahepatic fat. Obesity and insulin
resistance, as the two main causes of T2DM, have been shown to be
significantly associated with the early stages of NAFLD, even
progressing to more severe steatohepatitis and cirrhosis (24). The present study investigated the
function of carvacrol in T2DM-related NAFLD treatment. The results
indicated that carvacrol effectively reduced liver damage, fibrosis
and liver dysfunction in db/db mice, which was associated with
insulin, TLR4/NF-κB and AKT1/mTOR signaling pathways.
In the present study, db/db mice were used as a
model of T2DM. It is well known that obesity and T2DM are both risk
factors of NAFLD (25). In the
present study, db/db mice exhibited high body weight and liver
weight, which was ameliorated following treatment with 10 mg/kg
carvacrol for 6 weeks. Blood glucose is one of the most important
indicators used to observe diabetes-induced liver injury as well as
the effect of drug treatment. The present study demonstrated that
carvacrol administration could significantly decrease blood glucose
levels in db/db mice. A previous study reported that carvacrol
treatment had no effect on blood glucose and body weight for db/m+
mice compared with the controls (17), indicating that carvacrol has no
toxic effect on healthy mice. Insulin resistance is a main
therapeutic target for T2DM (26).
In the present study, carvacrol significantly decreased serum
insulin and HOMA-IR levels of model mice. Important indicators of
blood lipid analysis include TC, TG, LDL-C and HDL-C (27). TG is the main energy storage unit of
the human body and is an important source of energy for day-to-day
activities. However, the continuous high concentration of TG in
serum may lead to excessive accumulation in liver cells and can
cause fatty liver disease (28).
Notably, carvacrol treatment significantly lowered serum TC, TG and
LDL-C levels, and increased HDL-C levels in db/db mice, suggesting
that carvacrol could ameliorate dyslipidemia.
High concentrations of AST and ALT exist in liver
cells; ~80% of AST is contained in the mitochondria and ALT is only
distributed in the cytoplasm (29).
If liver cells are seriously damaged, AST and ALT are released into
the blood; therefore, serum AST and ALT levels are important
indexes for liver injury. In the present study, db/db mice
exhibited high serum AST and ALT levels, suggesting liver cell
damage. Conversely, carvacrol significantly ameliorated liver
function in db/db mice, indicating that carvacrol may possess a
certain protective effect on the liver. In a previous study, obese
diabetic db/db mice manifested renal injury (30). The present findings indicated that
carvacrol could improve renal function of db/db mice. Furthermore,
the results of pathological analysis demonstrated that severe liver
steatosis and fibrosis was present in db/db mice; however,
following administration of carvacrol for 6 weeks, liver steatosis
and fibrosis were markedly ameliorated. In NAFLD, steatosis has
been reported to be present in >5% of liver cells in human
patients (31). It has been
reported that carvacrol could relieve the progress of carbon
tetrachloride-induced rat liver fibrosis; this was associated with
Hippo and TGF-β signaling pathways (32). Furthermore, carvacrol may exert a
positive effect on proliferation and regeneration of liver cells
following 24 and 48 h of partial hepatectomy (33). These findings suggested that
carvacrol could relieve the T2DM-induced liver damage.
Pharmacologically targeting signal molecules is an
attractive strategy against T2DM complications (24). The present study revealed that
carvacrol administration could ameliorate liver injury in db/db
mice, which was associated with insulin, TLR4/NF-κB and AKT1/mTOR
signaling pathways. Hyperglycemia, insulin deficiency and insulin
resistance are the main features of T2DM (34). As an insulin-sensitive organ, the
liver functions in maintaining a systemic glucose metabolism
balance (34). The present results
indicated that carvacrol could ameliorate liver insulin sensitivity
in a mouse model of T2DM by mediating insulin signaling pathway
components, including INSR, p-INSR, IRS1 and p-IRS1. A previous
study reported that the TLR4/NF-κB pathway may be involved in
diabetic liver injury (35). TLR4
is one of the key members of the TLR family, which can activate the
innate immune system (36). NF-κB,
as a dimeric transcription factor, can induce the expression of
inflammation-related cytokines, such as TNF-α and IL-6 (37). The present data suggested that
carvacrol could inactivate the TLR4/NF-κB pathway and suppress
NALP3 expression in the liver tissues of db/db mice, thereby
reducing the inflammatory response. The AKT1/mTOR pathway functions
in mediating insulin resistance (38,39).
The present study demonstrated that carvacrol could regulate
insulin resistance by targeting the AKT1/mTOR pathway.
In conclusion, the present study reported that
carvacrol administration could ameliorate liver injury,
particularly T2DM-induced dyslipidemia and insulin resistance.
Mechanistically, carvacrol could protect the liver in T2DM via
insulin, TLR4/NF-κB and AKT1/mTOR signaling pathways. Thus,
carvacrol may be considered a promising treatment strategy for
T2DM-associated liver injury and the therapeutic effect of
carvacrol on T2DM-induced liver injury is worthy of further
in-depth research.
Acknowledgements
Not applicable.
Funding
The present study was funded by grants from the
National Natural Science Foundation of China (grant no. 81760813)
and the Science and Technology Cooperation Plan of Guizhou
[Qiankehe LH (2016) grant no.7127].
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
WZ conceived and designed the study. LC, HZ and CD
conducted most of the experiments and data analysis, and wrote the
manuscript. QH, YC, QW and SL conducted a small number of
experiments and data analysis, and wrote and revised the
manuscript. WZ and LC confirm the authenticity of all the raw data.
All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Guizhou University
of Traditional Chinese Medicine (approval no. 2018072).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
T2DM
|
type 2 diabetes
|
|
NAFLD
|
nonalcoholic fatty liver disease
|
|
OGTT
|
oral glucose tolerance test
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
TC
|
total cholesterol
|
|
LDL
|
low-density lipoprotein
|
|
HDL
|
high-density lipoprotein
|
|
TG
|
triglyceride
|
|
GSP
|
glycosylated serum protein
|
|
BUN
|
blood urea nitrogen
|
|
Cre
|
creatinine
|
|
UA
|
uric acid
|
|
H&E
|
hematoxylin and eosin
|
|
PAS
|
periodic acid-Schiff
|
References
|
1
|
Liang W, Zhang D, Kang J, Meng X, Yang J,
Yang L, Xue N, Gao Q, Han S and Gou X: Protective effects of rutin
on liver injury in type 2 diabetic db/db mice. Biomed Pharmacother.
107:721–728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Younossi Z, Tacke F, Arrese M, Chander
Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George
J, Fan J and Vos MB: Global perspectives on nonalcoholic fatty
liver disease and nonalcoholic steatohepatitis. Hepatology.
69:2672–2682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang H, Yang T, Heng C, Zhou Y, Jiang Z,
Qian X, Du L, Mao S, Yin X and Lu Q: Quercetin improves
nonalcoholic fatty liver by ameliorating inflammation, oxidative
stress, and lipid metabolism in db/db mice. Phytother Res.
33:3140–3152. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang TC, Chiang H, Lai YH, Huang YL,
Huang HC, Liang YC, Liu HK and Huang C: Helminthostachys
zeylanica alleviates hepatic steatosis and insulin resistance
in diet-induced obese mice. BMC Complement Altern Med. 19:3682019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leung PS: The Modulatory action of Vitamin
D on the Renin-Angiotensin System and the determination of hepatic
insulin resistance. Molecules. 24:24792019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang CC, Cheng PN and Kao JH: Systematic
review: Chronic viral hepatitis and metabolic derangement. Aliment
Pharmacol Ther. 51:216–230. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li DJ, Liu J, Hua X, Fu H, Huang F, Fei
YB, Lu WJ, Shen FM and Wang P: Nicotinic acetylcholine receptor α7
subunit improves energy homeostasis and inhibits inflammation in
nonalcoholic fatty liver disease. Metabolism. 79:52–63. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu C, Liao JZ and Li PY: Traditional
Chinese herbal extracts inducing autophagy as a novel approach in
therapy of nonalcoholic fatty liver disease. World J Gastroenterol.
23:1964–1973. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scheen AJ: Beneficial effects of SGLT2
inhibitors on fatty liver in type 2 diabetes: A common comorbidity
associated with severe complications. Diabetes Metab. 45:213–223.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan X, Li X, Yang X, Yan J, Shi P, Ba L,
Cao Y and Wang P: The neuroprotective effects of carvacrol on
ischemia/reperfusion-induced hippocampal neuronal impairment by
ferroptosis mitigation. Life Sci. 235:1167952019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao W, Deng C, Han Q, Xu H and Chen Y:
Carvacrol may alleviate vascular inflammation in diabetic db/db
mice. Int J Mol Med. 46:977–988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Carvalho FO, Silva ÉR, Gomes IA,
Santana HS, do Nascimento Santos D, de Oliveira Souza GP, de Jesus
Silva D, Monteiro JC, de Albuquerque Júnior RLC, de Souza Araújo AA
and Nunes PS: Anti-inflammatory and antioxidant activity of
carvacrol in the respiratory system: A systematic review and
meta-analysis. Phytother Res. 34:2214–2229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cardoso AD, Santos EG, Lima AD, Temeyer
KB, Pérez de León AA, Costa LM Junior and Soares AM: Terpenes on
Rhipicephalus (Boophilus) microplus: Acaricidal activity and
acetylcholinesterase inhibition. Vet Parasitol. 280:1090902020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trindade GG, Thrivikraman G, Menezes PP,
França CM, Lima BS, Carvalho YM, Souza EP, Duarte MC, Shanmugam S,
Quintans-Júnior LJ, et al: Carvacrol/β-cyclodextrin inclusion
complex inhibits cell proliferation and migration of prostate
cancer cells. Food Chem Toxicol. 125:198–209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Wei J, Ma KT, Li CL, Mai YP, Qiu
XX, Wei H, Hou N and Luo JD: Carvacrol protects against
diabetes-induced hypercontractility in the aorta through activation
of the PI3K/Akt pathway. Biomed Pharmacother. 125:1098252020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou N, Mai Y, Qiu X, Yuan W, Li Y, Luo C,
Liu Y, Zhang G, Zhao G and Luo JD: Carvacrol attenuates diabetic
cardiomyopathy by modulating the PI3K/AKT/GLUT4 Pathway in diabetic
mice. Front Pharmacol. 10:9982019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kobayashi K, Forte TM, Taniguchi S, Ishida
BY, Oka K and Chan L: The db/db mouse, a model for diabetic
dyslipidemia: Molecular characterization and effects of Western
diet feeding. Metabolism. 49:22–31. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng BY, Wang Q, Luo YH, He JF, Tan T and
Zhu H: A novel and quick PCR-based method to genotype mice with a
leptin receptor mutation (db/db mice). Acta Pharmacol Sin.
39:117–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council Committee for
the Update of the Guide for the C, Use of Laboratory A. The
National Academies Collection, . Reports funded by National
Institutes of Health. Guide for the Care and Use of Laboratory
Animals. National Academies Press; Washington, DC: 2011, PubMed/NCBI
|
|
21
|
Emoto M, Nishizawa Y, Maekawa K, Hiura Y,
Kanda H, Kawagishi T, Shoji T, Okuno Y and Morii H: Homeostasis
model assessment as a clinical index of insulin resistance in type
2 diabetic patients treated with sulfonylureas. Diabetes Care.
22:818–822. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prasanna PL, Renu K and Valsala
Gopalakrishnan A: New molecular and biochemical insights of
doxorubicin-induced hepatotoxicity. Life Sci. 250:1175992020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sampath Kumar A, Maiya AG, Shastry BA,
Vaishali K, Ravishankar N, Hazari A, Gundmi S and Jadhav R:
Exercise and insulin resistance in type 2 diabetes mellitus: A
systematic review and meta-analysis. Ann Phys Rehabil Med.
62:98–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng Y, Liu T, Wang Z, Xu Y, Zhang Q and
Luo D: Low molecular weight fucoidan attenuates liver injury via
SIRT1/AMPK/PGC1α axis in db/db mice. Int J Biol Macromol.
112:929–936. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim KE, Jung Y, Min S, Nam M, Heo RW, Jeon
BT, Song DH, Yi CO, Jeong EA, Kim H, et al: Caloric restriction of
db/db mice reverts hepatic steatosis and body weight with divergent
hepatic metabolism. Sci Rep. 6:301112016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eckel RH, Grundy SM and Zimmet PZ: The
metabolic syndrome. Lancet. 365:1415–1428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng Q, Qi X, Fu Y, Chen Q, Cheng P, Yu X,
Sun X, Wu J, Li W, Zhang Q, et al: Flavonoids extracted from
mulberry (Morus alba L.) leaf improve skeletal muscle
mitochondrial function by activating AMPK in type 2 diabetes. J
Ethnopharmacol. 248:1123262020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berk PD and Verna EC: Nonalcoholic fatty
liver disease: Lipids and insulin resistance. Clin Liver Dis.
20:245–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sattar N, Fitchett D, Hantel S, George JT
and Zinman B: Empagliflozin is associated with improvements in
liver enzymes potentially consistent with reductions in liver fat:
Results from randomised trials including the EMPA-REG
OUTCOME® trial. Diabetologia. 61:2155–2163. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu HW, Kao HH and Wu CH: Exercise
training upregulates SIRT1 to attenuate inflammation and metabolic
dysfunction in kidney and liver of diabetic db/db mice. Nutr Metab
(Lond). 16:222019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cobbina E and Akhlaghi F: Non-alcoholic
fatty liver disease (NAFLD)-pathogenesis, classification, and
effect on drug metabolizing enzymes and transporters. Drug Metab
Rev. 49:197–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mohseni R, Karimi J, Tavilani H, Khodadadi
I and Hashemnia M: Carvacrol ameliorates the progression of liver
fibrosis through targeting of Hippo and TGF-β signaling pathways in
carbon tetrachloride (CCl4)-induced liver fibrosis in
rats. Immunopharmacol Immunotoxicol. 41:163–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ozen BD and Uyanoglu M: Effect of
carvacrol on IL-6/STAT3 pathway after partial hepatectomy in rat
liver. Bratisl Lek Listy. 119:593–601. 2018.PubMed/NCBI
|
|
34
|
Ding S, Xu S, Ma Y, Liu G, Jang H and Fang
J: Modulatory mechanisms of the NLRP3 inflammasomes in diabetes.
Biomolecules. 9:8502019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin H, Huang L, Ouyang T and Chen L:
Baicalein improves liver inflammation in diabetic db/db mice by
regulating HMGB1/TLR4/NF-κB signaling pathway. Int Immunopharmacol.
55:55–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu ZM, Zheng HY, Chen LH, Li YL, Wang Q,
Liao CF and Li XW: Low expression of miR-203 promoted diabetic
nephropathy via increasing TLR4. Eur Rev Med Pharmacol Sci.
22:5627–5634. 2018.PubMed/NCBI
|
|
37
|
Yi H, Peng R, Zhang LY, Sun Y, Peng HM,
Liu HD, Yu LJ, Li AL, Zhang YJ, Jiang WH and Zhang Z:
LincRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3
inflammasome-mediated inflammation in diabetic nephropathy. Cell
Death Dis. 8:e25832017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pomytkin I, Krasil'nikova I, Bakaeva Z,
Surin A and Pinelis V: Excitotoxic glutamate causes neuronal
insulin resistance by inhibiting insulin receptor/Akt/mTOR pathway.
Mol Brain. 12:1122019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Villalobos-Labra R, Silva L, Subiabre M,
Araos J, Salsoso R, Fuenzalida B, Sáez T, Toledo F, González M,
Quezada C, et al: Akt/mTOR role in human foetoplacental vascular
insulin resistance in diseases of pregnancy. J Diabetes Res.
2017:59478592017. View Article : Google Scholar : PubMed/NCBI
|