Introduction
Chronic obstructive pulmonary disease (COPD) ranks
fourth among disease-related causes of death worldwide (1). COPD, regarded as a chronic systemic
inflammatory disease, is characterized by persistent limitation of
airflow due to airway and/or alveolar abnormalities (2). Cigarette smoking (CS) is the main
cause of COPD, although other factors have also been identified
(3). Furthermore, its pathogenesis
is based on genetic determinants, lung growth, as well as
environmental stimuli, such as oxidative stress, inflammation,
extracellular matrix proteolysis, and apoptotic and autophagic cell
death (4). In addition, bacterial
and/or viral infections have been reported to exacerbate COPD
(3). Airway inflammation is a key
driver of COPD progression, resulting in several phenotypes with
different patterns (5). Attempts
have been made to segregate patients with COPD into different
subtypes based on immune profiles, such as neutrophil- or
eosinophil-associated COPD (6),
with evidence indicating corticosteroids to be the most effective
in patients with COPD with eosinophilic inflammation (5,7). In
addition, novel biological therapies targeting specific
inflammatory biomarkers have been explored. For instance, anti-IL5
therapy in COPD reduces exacerbations in patients with a high blood
eosinophil count by ~20% (8).
Recently, potential biomarkers such as IL-5 and
TNF-α have been considered effective in targeted anti-inflammatory
therapy (9). Multiscale models of
CS-induced COPD have enabled identification of several positive
feedback loops, such as M1 and M2 transformation and balance, as
well as network elements, and these have served a determinant role
in CS-induced immune response and COPD progression (10,11).
An increased proportion of infiltrating macrophages, as well as a
reduction of CD4+ and CD8+T cells, are both
independently associated with smoking status or the level of
airflow limitation (10,11). CRP is associated with elevation of
acute exacerbated COPD (AECOPD), while CRP-guided prescription of
antibiotics for AECOPD results in less use of antibiotics with no
evidence of harm (12). Levels of
IL-6 are associated with the degree of airflow limitation (13), and both IL-6 and IL-8 are associated
with emphysema severity (14).
Similarly, IL-1β, IL-17A, TNF-α and IFN-γ have also been linked to
persistent airway inflammation and greater exacerbation (15–17).
In addition, pulmonary surfactant proteins might predict relapse of
patients with AECOPD (18), while
lack of club cell secretory protein (CC16) in the lungs results in
enhanced airway remodeling in COPD (19). Members of the extracellular MMP
family of proteins are associated with emphysema pathogenesis
(20). Consequently, MMP inhibitors
have been developed as novel therapeutics, with doxycycline
approved by the Food and Drug Administration (20). It is possible that more biomarkers
could promote the understanding of COPD pathogenesis and
development of targeted therapies. However, the complexity of these
biomarkers poses challenges in distinguishing between COPD
phenotypes and endotypes. It is, therefore, imperative to focus on
biomarkers that have a clear distinction of specific clinical
phenotypes or endotypes (21).
The present study analyzed the peripheral blood
expression of immune cells and serum levels of 20 inflammatory
factors with the aim to elucidate the complex network of immune
profiles in COPD and airway inflammatory patterns. The present
findings may provide insight to guide the future development of
novel therapies.
Materials and methods
Study patients and ethics
A total of 140 subjects with mean age of 65.13 were
enrolled in the study between September 2018 and June 2019. In
total, 87 patients with stable COPD (62 male, 25 female, age: Mean
± SEM 64.94±0.97) were recruited at the Outpatient Department of
West China Hospital, Sichuan University (Chengdu, China). Inclusion
criteria used for the recruitment included: i) Diagnosed with COPD
by respiratory physician; ii) performed pulmonary function test
[forced expiratory volume in 1 sec (FEV1)/forced vital capacity
(FVC) ≤70%] and chest CT scan; iii) no malignant tumor or
autoimmune disease; iv) no liver and kidney failure or special
infections such as HIV; v) no exacerbation in 3 months; vi) no
other chronic and acute severe respiratory diseases; and vii) no
chronic or acute systemic infections. A total of 24 patients with
AECOPD (17 male, 7 female, age: Mean ± SEM 67.00±2.13) admitted at
West China Hospital were enrolled using the following inclusion
criteria: i) Main diagnosis for admission was AECOPD; ii) pulmonary
function test (FEV1/FVC ≤70%) and chest CT scan were performed;
iii) no malignant tumor or autoimmune diseases; iv) no liver and
kidney failure or special infections such as HIV; and v) no other
severe chronic or acute respiratory disease. For those patients for
whom pulmonary function test and chest CT scan were not performed,
medical reports for the past year were required. COPD was defined
according to international guidelines (2), while severity of airflow obstruction
was graded using current Global Initiative for Chronic Obstructive
Lung Disease criteria (2). A total
of 29 healthy controls (18 non-smokers and 11 smokers, 18 male, 11
female, age: Mean ± SEM 64.15±1.34) were recruited at the Physical
Examination Center of West China Hospital (Chengdu, China) using
the following inclusion criteria: i) Pulmonary function test
(FEV1/FVC <70%) and chest CT scan were performed, indicating
normal pulmonary function; ii) no malignant tumor or autoimmune
disease; iii) no heart, liver and kidney failure or special
infections; iv) no other chronic or acute respiratory disease; and
v) no chronic or acute systemic infections. Spirometry parameters
were measured according to the recommendations of the European
Respiratory Society, and then expressed as a percentage of the
predicted (22). The modified
Medical Research Council dyspnea scale (mMRC) was adopted as the
classification criterion for symptom assessment (23), detailed description of baseline
characteristics was recorded by the respiratory physician.
According to smoking history, all the subjects were divided into
four groups: Smoking (73) and nonsmoking (24) COPD patients and smoking (8) and nonsmoking (17) controls. The present study was
approved by The Ethics Committee of West China Hospital, Sichuan
University (Chengdu, China) in accordance with the Declaration of
Helsinki (24). All patients
provided written informed consent before participating in the
study.
Flow cytometry
Fresh peripheral blood (2 ml) anticoagulated in EDTA
was collected from each patient and processed within 24 h.
Peripheral blood was divided into 2 tubes (1 ml). The corresponding
antibody (2 µl) was added to each tube (tube 1: CD45,PE-cy5.5;CD14,
FITC;CD64, PE;CD66, APC;CD16, PE-cy7; tube 2: CD45,PE-cy5.5;CD3,
PE; CD8, APC-cy7;CD4, FITC;CD56, PE-cy7;CD19, APC) and incubated
for 30 min in the dark at room temperature, then mixed with red
blood cell lysis solution (FCM Lysing solution for BC, Hangzhou
Multi Sciences (Lianke) Biotech Co., Ltd.) with the ratio of 1:3,
lysing for ~10 min until the solution was clear. Following
centrifugation (500 × g for 5 min at 4°C), the supernatant was
removed and washed by 500 µl-1 ml PBS for three times, centrifuged
at 400 × g for 5 min at 4°C, and then resuspended in 200 µl PBS.
The cells were stored on ice in the dark, or in a refrigerator at
4°C until use. Samples were analyzed using multicolor flow
cytometry (Navios EX flow cytometer; Beckman Coulter, Inc.) based
on CD45+ human leukocytes.
CD64+/CD14+ represented the
monocyte-macrophage system (MPS), CD3+ T lymphocytes
were divided into CD4+ and CD8+ subsets,
while CD3+CD4−CD8− was selected as
the marker for γδT cells. In addition,
CD3+CD56+ marked natural killer T lymphocytes
(NKTs), while CD3−CD56+ marked natural killer
(NK) cells. Antibodies ready to use were acquired from eBioscience
(Thermo Fisher Scientific, Inc.; CD14 Monoclonal Antibody, FITC,
human, cat. no. 11-0149-42; CD64 Monoclonal Antibody, PE, human,
cat. no. 12-0649-42; CD66 Monoclonal Antibody, APC, human, cat. no.
17-0668-42; CD16 Monoclonal Antibody, PE-cy7, human, cat. no.
25-0168-42; CD3 Monoclonal Antibody, PE, human, cat. no.
12-0038-42; CD8 Monoclonal Antibody, APC-cy7, human, cat. no.
A15448; CD4 Monoclonal Antibody, FITC, human, cat. no. 11-0049-42;
CD56 Monoclonal Antibody, PE-cy7, human, cat. no. 25-0567-42; CD19
Monoclonal Antibody, APC, human, cat. no. 17-0199-42; CD45
Monoclonal Antibody, PE-cy 5.5, human, cat. no. 35-0459-42), and
cells were stained according to the manufacturer's recommendations.
All the antigens were human source and stored at 4°C in the dark.
Monocyte-macrophage-granulocyte included antigens of CD45, CD14,
CD64, CD66 and CD16, while lymphocyte-NK-NK included CD45, CD3,
CD8, CD4, CD56 and CD19. FlowJo version 10.0.7 software (FlowJo
LLC) was used for analysis.
Analysis of inflammatory factors
A total of 5 ml peripheral EDTA-anticoagulated blood
was collected from each patient, 2 ml used for FCM, the rest (3 ml)
centrifuged at 1,600 × g for 15 min at 4°C. The resulting plasma
was stored at −79°C for subsequent ELISA tests and multi-plex panel
tests.
ELISA
The levels of five biomarkers were measured using
ELISAs. The plasma stored at −79°C was defrosted in a constant
temperature water bath to 37°C. CRP (Human C-Reactive Protein/CRP
Immunoassay kit; cat. no. DCRP00), CC16 (Human Uteroglobin
Quantikine ELISA kit; cat. no. DUGB00) and TGF-β (Human TGF-beta
Quantikine ELISA kit; cat. no. DB100B) levels were assessed using
Quantikine ELISA kits (all R&D Systems, Inc.), while
fibrinogen(Human Fibrinogen ELISA kit; ab208036) and neutrophil
elastase (Human PMN Elastase ELISA kit; ab119553) were analyzed
using Simple Step ELISA kits (Abcam) according to the to the
instructions of the respective manufacturer's instructions.
Multi-plex panel
Two multi-factor panels, including 15 biomarkers,
were tested using the Multiplexed kit (Magnetic Luminex®
Assay, Human Premixed Multi-Analyte kit; LXSAHM, R&D Systems,
Inc.) based on the Luminex 200 system with xPONENT 3.1 (Luminex
Corporation). One panel tested the levels of 13 analytes, including
IL-6, TNF-α, IL-1β, IFN-γ, IL-8, IL-33, IL-17A, IL-4, IL-5, IL-13,
TGF-α, Human growth-regulated oncogene α (GRO-α) and pulmonary
surfactant-associated protein D (SP-D), while the other was used to
assess myeloperoxidase (MPO) and MMP-9 (Supplementary
Materials).
Bioinformatics
Proteins (IL-6, IL-1β, TNF-α, IFN-γ, IL-8, IL-33,
IL-17A, TGF-α, GRO-α, CRP) and their functional interactions were
analyzed using the Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) database version 11.0 (25), and enriched Kyoto Encyclopedia of
Genes and Genomes pathways were determined using STRING [10 items
(human)-STRING interaction network (string-db.org)].
Statistical analysis
All samples were tested 3 times. Continuous
variables are presented as the mean ± standard error of the mean
and were analyzed using SPSS 22.0 (IBM Corp.). A normality test was
performed using a P-P diagram in SPSS 22.0. One-way ANOVA and
Tukey's post hoc test were used for comparisons of normally
distributed continuous variables among different groups. Kruskal
Wallis and Dunn's post hoc test were used for comparisons of
non-normally distributed continuous variables among different
groups. In addition, χ2 and Bonferroni's correction were
used for categorical variables. Pearson's correlation was used to
assess the relationship among continuous variables, while
Spearman's correlation test was used for categorical data. Figures
were generated using FlowJo version 10.0.7 software (FlowJo LLC)
and GraphPad Prism 8 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics of the
patients
A total of 87 patients with stable COPD and 24
patients with AECOPD, as well as 29 healthy controls were recruited
in the study. There were no significant differences in terms of
age, sex and BMI among the three groups (Table I). As expected, the proportion of
smokers was higher among patients with COPD compared with the
controls (COPD vs. AECOPD vs. controls; 75.9 vs. 62.5 vs. 37.9%,
respectively), and the lung function (FEV1/FVC, FEV1% and maximal
mid expiratory flow (MMEF)75/25*) of patients with COPD was worse
compared with controls (Table I).
AECOPD had more group D patients with severe airflow limitation
(stage III, 38.89 vs. 22.50%; stage IV, 50.00 vs. 28.75%) compared
with COPD (Table I). The score of
mMRC assessment was higher in patients with AECOPD compared with
patients with stable COPD (3.61 vs. 2.24). The most common drug
therapy for patients with COPD and AECOPD was long-acting
muscarinic antagonist (LAMA) + long-acting beta-2 agonist (LABA) +
inhaled corticosteroid (ICS; 63.29 and 45%, respectively), while
17.86% of patients with stable COPD did not receive drug therapy.
In conclusion, patients with COPD who smoked had worse lung
function compared with healthy controls, patients with AECOPD were
severe and needed more medication than stable patients with
COPD.
 | Table I.Clinical baseline characteristics of
patients with COPD, patients with AECOPD and healthy controls. |
Table I.
Clinical baseline characteristics of
patients with COPD, patients with AECOPD and healthy controls.
| Baseline
features | COPD (n=87) | AECOPD (n=24) | Healthy controls
(n=29) | P-value |
|---|
| Age, years (mean ±
SEM) | 64.94±0.97 | 67.00±2.13 | 64.15±1.34 | 0.519 |
| Male, n (%) | 62 (76.7) | 17 (75.0) | 18 (62.7) | 0.444 |
| BMI (mean ±
SEM) | 22.05±0.58 | 19.70±1.75 | 21.23±1.48 | 0.299 |
| Smoking history, n
(%) | 66 (75.9) | 15 (62.5) | 11 (37.9) | 0.001a |
| Smoking amount,
pack-year (mean ± SEM) | 31.59±3.26 | 22.38±4.23 | 9.71±3.57 | 0.001a |
| Exacerbation, n
(%) | 51 (58.62) | 24 (100) |
| 0.001a |
| Pulmonary
function |
|
|
|
|
| No. of
patients analyzed (n) | 78 | 16 | 22 |
|
|
FEV1/FVC (mean ± SEM) | 50.94±1.24 | 46.74±2.32 | 78.91±1.37 | 0.001a |
| FEV1%
(mean ± SEM) | 51.83±2.44 | 40.22±4.47 | 96.23±2.27 | 0.001a |
| RV/TLC
(mean ± SEM) | 142.14±8.61 | 145.06±14.72 | 110.58±4.34 | 0.281 |
|
MMEF75/25 (mean ± SEM) | 22.45±1.78 | 13.49±1.87 | 71.52±3.42 | 0.001a |
| GOLD
stageb |
|
|
|
|
| No. of
patients analyzed | 84 | 22 |
|
|
| A, n
(%) | 23 (27.38) | 0 (0.00) |
| 0.001a |
| B, n
(%) | 22 (26.19) | 3 (13.64) |
| 0.001a |
| C, n
(%) | 8 (9.52) | 1 (4.54) |
| 0.001a |
| D, n
(%) | 31 (36.9) | 18 (81.82) |
| 0.001a |
| Stage of Airflow
limitationb |
|
|
|
|
| No. of
patients analyzed | 80 | 18 |
|
|
| I
(mild), n (%) | 12 (15) | 1 (5.56) |
| 0.001a |
| II
(moderate), n (%) | 27 (33.75) | 1 (5.26) |
| 0.001a |
| III
(severe), n (%) | 18 (22.5) | 7 (38.89) |
| 0.001a |
| IV
(very severe), n (%) | 23 (28.75) | 9 (50) |
| 0.001a |
| Assessments |
|
|
|
|
| No. of
patients analyzed | 84 | 20 |
|
|
| mMRC
(mean ± SEM) | 2.24±0.11 | 3.61±0.14 |
| 0.001a |
| Cough
(mean ± SEM) | 1.88±0.14 | 2.09±0.45 |
| 0.528 |
| Sputum
(mean ± SEM) | 5.22±0.33 | 5.64±0.28 |
| 0.724 |
| Drug therapy |
|
|
|
|
| No. of
patients analyzed | 84 | 20 |
|
|
| 0
(none), n (%) | 15 (17.86) | 0 (0) |
|
|
| 1
(LAMA/LABA), n (%) | 8 (9.52) | 0 (0) |
|
|
| 2
(LABA+ICS), n (%) | 13 (15.48) | 3 (15) |
|
|
| 3
(LAMA+LABA+ICS), n (%) | 53 (63.29) | 9 (45) |
|
|
| 4
(LAMA+LABA), n (%) | 3 (3.57) | 8 (40) |
|
|
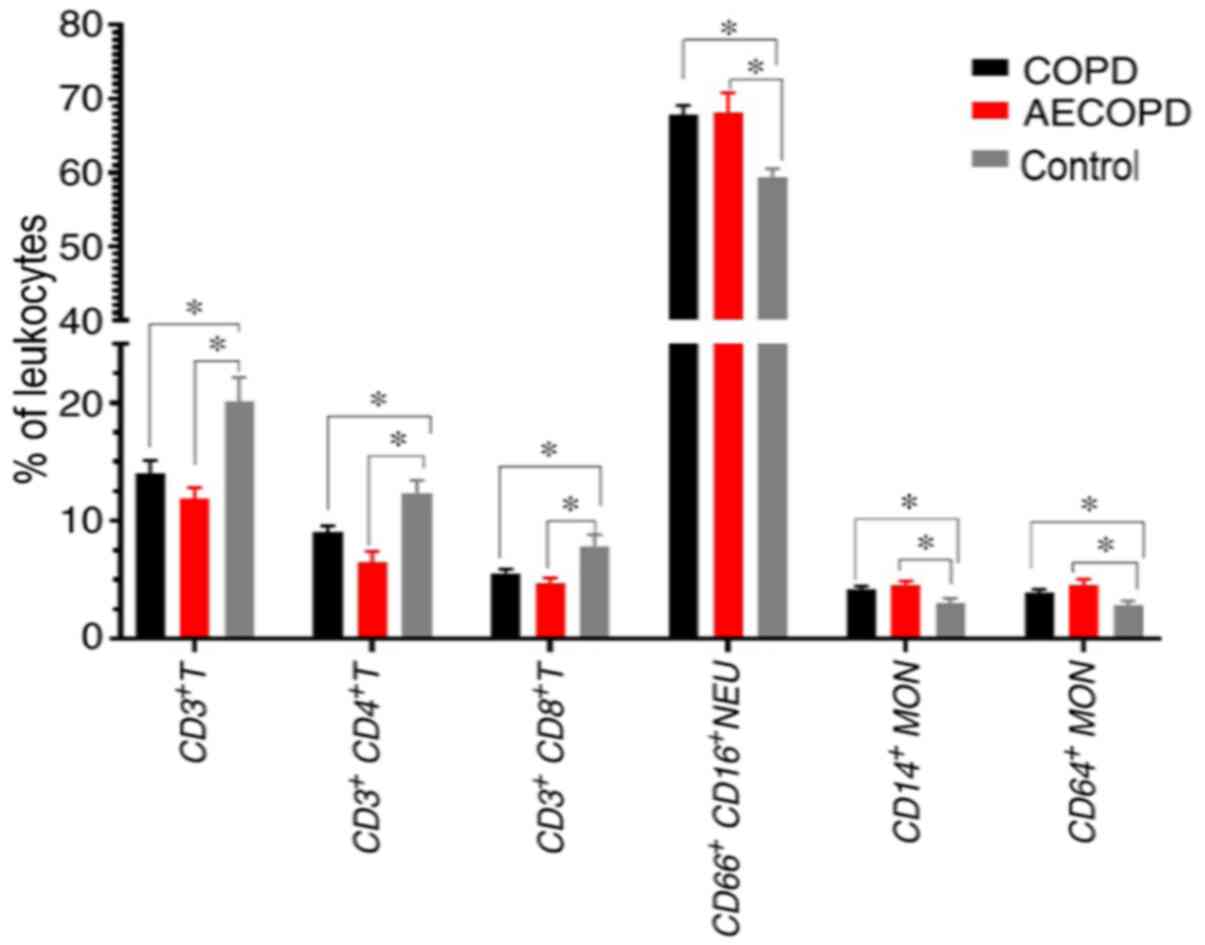
Immune cells expression profiles
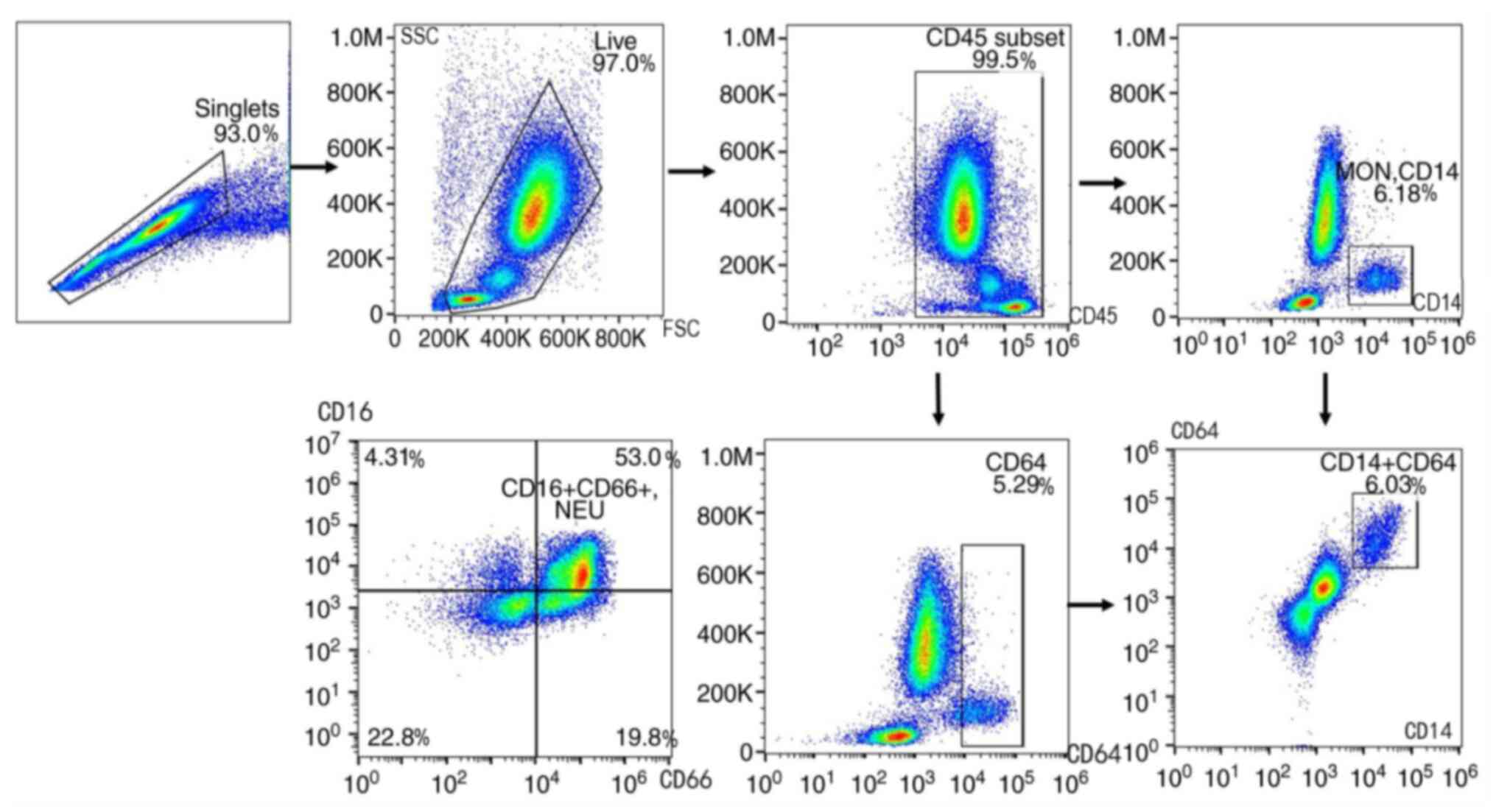
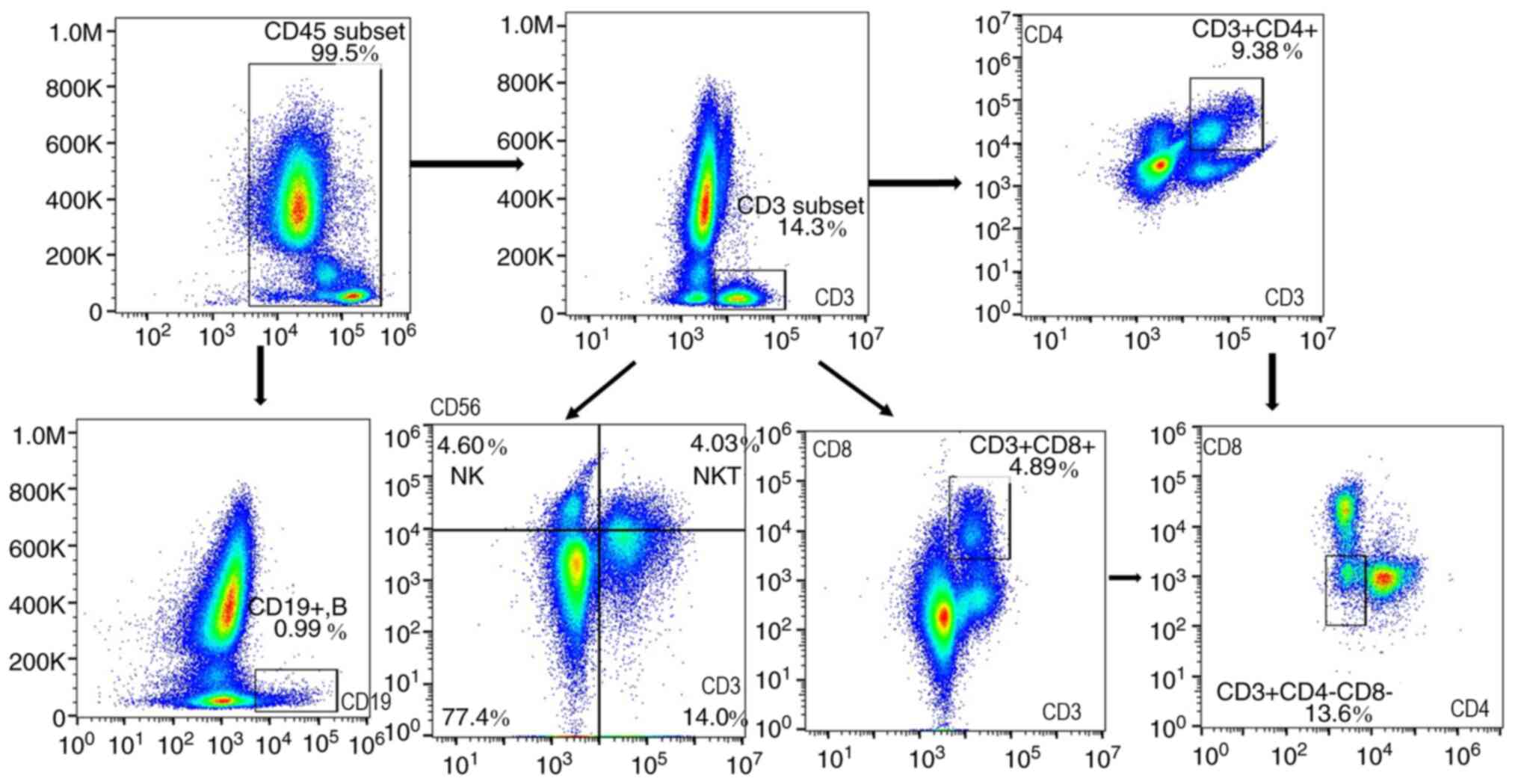
The flow cytometry results and workflow are shown in
Figs. 1 and 2. Following staining, living cells were
imaged and the CD45+ subset were gated as human
leukocyte There were significant differences in CD3+ T
lymphocytes (P<0.001), including the CD4+ T
(P<0.001) and CD8+ T (P=0.004) subsets,
CD14+ (P=0.027) and CD64+ (P=0.026) MON and
CD16+CD66+ neutrophil (P=0.011) proportions
across the study groups (Table II;
Fig. 3).
CD66+CD16+ neutrophils and MPS
(CD14+ and CD64+ subsets) were highly
expressed, while CD4+ and CD8+ T lymphocytes
were expressed at lower levels in patients with stable COPD and
patients with AECOPD compared with healthy controls (Table II). CD4+ T lymphocyte
counts were decreased in patients with stable COPD compared with
controls, and in AECOPD compared with stable COPD (AECOPD vs. COPD
vs. controls, 6.50 vs. 9.04 vs. 12.36%, respectively).
CD64+ and CD14+ MON tended to be increased in
AECOPD compared with stable COPD and controls (CD64+
MON: AECOPD vs. COPD vs. controls, 4.53 vs. 3.94 vs. 2.85%;
CD14+ MON: AECOPD vs. COPD vs. controls, 4.57 vs. 4.19
vs. 3.04%, respectively). CD14+CD64+ MON
levels were increased in AECOPD compared with controls (3.82 vs.
2.53%). Furthermore, CD19+ B lymphocytes,
CD3+CD56+ NKTs and
CD3−CD56+ NKs levels were not significantly
different among the three groups.
 | Table II.Peripheral blood immune profiles of
patients with COPD, patients with AECOPD and healthy controls. |
Table II.
Peripheral blood immune profiles of
patients with COPD, patients with AECOPD and healthy controls.
| Immune
profiles | COPD (mean ±
SEM) | AECOPD (mean ±
SEM) | Healthy control
(mean ± SEM) | P-value |
|---|
| Immune
cellsa, % |
|
|
|
|
|
CD3+ T cell | 14.01±1.08 | 11.90±0.9 |
20.11±2.04b,c | 0.001e |
|
CD3+CD4+
T cell |
9.04±0.53c |
6.50±0.89b |
12.36±1.06b,c | 0.001e |
|
CD3+CD8+
T cell | 5.55±0.36 | 4.70±0.47 |
7.85±0.97b,c | 0.004 |
|
CD4/CD8 |
1.97±0.13c |
1.47±0.21b | 1.89±0.17 | 0.160 |
|
CD3+CD4−CD8−γδT
cell | 1.13±0.17 | 1.46±0.45 | 1.40±0.23 | 0.586 |
|
CD3+CD56+
NKT | 2.88±0.24 | 3.01±0.32 | 2.93±0.48 | 0.976 |
|
CD3−CD56+
NK | 5.85±0.42 | 5.90±0.71 | 6.27±0.76 | 0.877 |
|
CD19+ B cell |
2.11±0.25c |
1.69±0.20b | 2.01±0.34 | 0.723 |
|
CD66+CD16+
NEU | 67.82±1.29 | 68.21±2.6 |
59.36±1.18b,c | 0.011 |
|
CD14+ MON | 4.19±0.26 | 4.57±0.33 |
3.04±0.41b,c | 0.027 |
|
CD64+ MON | 3.94±0.26 | 4.53±0.53 |
2.85±0.37b,c | 0.026 |
|
CD14+CD64+ | 3.19±0.23 | 3.82±0.38 |
2.53±0.37c | 0.070 |
| Inflammatory
factors, pg/ml (by multi-plex panel) |
|
|
|
|
|
IL-6 | 6.95±1.16 |
20.51±3.59b,d |
3.82±1.14b,c | 0.001e |
|
TNF-α | 11.23±1.79 |
21.71±3.31b,d |
8.56±2.09c | 0.023 |
|
IL-1β | 6.69±1.33 | 7.24±1.20 |
4.09±1.00c | 0.044 |
|
IFN-γ | 11.91±1.80 |
29.1±10.79b,d |
10.32±1.45c | 0.032 |
|
IL-8 | 17.58±3.71 |
53.39±14.61b,d |
12.87±3.11c | 0.008e |
|
IL-33 | 11.10±2.27 |
25.56±11.17d |
6.11±1.98c | 0.029 |
|
IL-17A | 4.57±0.60 |
9.56±0.89b,d |
4.15±1.02c | 0.047 |
|
IL-4 | 18.58±3.29 | 12.23±1.28 | 17.08±2.55 | 0.595 |
|
IL-5 | 12.18±1.04 | 12.71±1.13 | 11.5±0.6 | 0.843 |
|
IL-13 | 19.27±0.87 | 20.27±1.71 | 17.15±1.92 | 0.296 |
|
TGF-α | 2.15±0.57 | 2.20±0.73 |
1.46±0.25b,c | 0.006 |
|
GRO-α |
223.3±33.5c |
382.7±135.4b | 323.1±78.1 | 0.065 |
|
SP-D | 6671±926.7 | 7122±2273 | 7767±1948 | 0.800 |
|
MPO | 8856±843.8 | 7201±1084 | 8911±1156 | 0.405 |
|
MMP-9 | 10627±1901 | 6852.9±1367 | 7019.7±905 | 0.377 |
| Inflammatory
factors, pg/ml (by ELISA) |
|
|
|
|
|
CC16 |
18.77±1.31c |
34.03±12.43b | 19.43±3.76 | 0.342 |
|
Fibrinogen | 841.2±55.65 | 716.4±76.07 | 778.7±71.67 | 0.211 |
| NE | 499.3±80.34 | 742.5±267.1 | 634.8±178.6 | 0.432 |
|
CRP | 1882±412.4 |
4395±915.9b,d |
2020±958.6c | 0.010 |
|
TGF-β | 40.61±3.8 | 39.51±7.53 | 55.44±10.00 | 0.193 |
There were 73 smoking patients with COPD and 24
non-smoking patients with COPD, as well as 8 smoking controls and
17 non-smoking controls. The majority of smokers were male, and
their age was comparable among the four groups. There were also
significant differences in CD3+CD8+ T
lymphocyte (P<0.001), CD14+ (P=0.014) and
CD64+ (P=0.020) MON, and
CD16+CD66+ neutrophil (P=0.002) proportions
only between smokers and non-smokers (Table III). CD4+ T lymphocytes
levels were sharply decreased in patients with COPD compared with
non-smoking controls (smoking COPD vs. non-smoking COPD vs.
non-smoking controls: 8.16 vs. 9.42 vs. 12.75%). Levels of
CD8+ T lymphocytes were decreased in both patients with
COPD and smoking controls compared with non-smoking controls
(smoking COPD vs. non-smoking COPD vs. smoking controls vs.
non-smoking controls: 5.15 vs. 5.98 vs. 5.33 vs. 9.11%). While
CD66+CD16+ neutrophils (smoking COPD vs.
non-smoking COPD vs. smoking controls vs. non-smoking controls:
68.83 vs. 64.99 vs. 66.41% vs. 56.04%) MON (CD14+ and
CD64+ subsets) were more highly expressed in patients
with COPD and smoking controls compared with non-smoking controls
(Table III).
CD14+CD64+ MON levels were increased in
smokers compared with non-smokers (smoking COPD vs. non-smoking
COPD, 3.49 vs. 2.86%; smoking control vs. non-smoking control, 3.58
vs. 2.03%). Furthermore, the levels of CD19+ B
lymphocytes, CD3+CD56+ NKTs and
CD3−CD56+ NKs were not significantly
different among the four groups.
 | Table III.Peripheral blood immune cells of
smoking and non-smoking subjects. |
Table III.
Peripheral blood immune cells of
smoking and non-smoking subjects.
|
Characteristics | Smoking COPD
(n=73) | Non-smoking COPD
(n=24) | Smoking control
(n=8) | Non-smoking control
(n=17) | P-value |
|---|
| Age, years (mean ±
SEM) | 65.81±0.96 | 64.27±2.04 | 63.18±2.94 | 65.15±1.23 | 0.715 |
| Male, n (%) | 68 (93.15) | 8 (33.33) | 8 (100.00) | 7 (41.18) | 0.001a |
| Pack-years (mean ±
SEM) | 40.78±2.88 |
| 31.56±7.02 |
| 0.327 |
| CD3+ T
cell (mean ± SEM) | 12.88±0.65 | 15.43±1.67 | 15.82±2.57 |
22.25±2.66b–d | 0.001a |
|
CD3+CD4+ T cell
(mean ± SEM) | 8.16±0.48 | 9.42±1.24 | 11.58±1.95 |
12.75±1.29b,c | 0.003 |
|
CD3+CD8+ T cell
(mean ± SEM) | 5.15±0.31 | 5.98±0.74 | 5.33±1.01 |
9.11±1.30b–d | 0.001a |
| CD4/CD8 (mean ±
SEM) | 1.89±0.12 | 1.82±0.16 | 2.45±0.40 | 1.61±0.13 | 0.296 |
|
CD3+CD4−CD8−
(mean ± SEM) | 1.14±0.19 | 1.41±0.33 | 1.06±0.31 | 1.57±0.30 | 0.675 |
|
CD3+CD56+ NKT (mean
± SEM) | 2.91±0.24 | 2.87±0.39 | 2.41±0.49 | 3.19±0.68 | 0.854 |
|
CD3−CD56+ NK (mean ±
SEM) | 6.26±0.42 | 4.71±0.64 | 5.82±1.41 | 6.50±0.93 | 0.268 |
| CD19+ B
cell (mean ± SEM) |
1.74±0.13c |
2.41±0.34b | 1.58±0.63 | 2.23±0.41 | 0.125 |
|
CD66+CD16+ NEU (mean
± SEM) | 68.83±1.24 | 64.99±2.73 | 66.41±2.85 |
56.04±4.29b–d | 0.002 |
| CD14+
MON (mean ± SEM) | 4.42±0.27 | 3.85±0.25 | 4.05±0.69 |
2.56±0.47b–d | 0.014 |
| CD64+
MON (mean ± SEM) | 4.18±0.27 | 3.75±0.48 | 3.96±0.73 |
2.32±0.37b–d | 0.020 |
|
CD14+CD64+ (mean ±
SEM) | 3.49±0.24 |
2.86±0.32b | 3.58±0.66 |
2.03±0.40b,d | 0.029 |
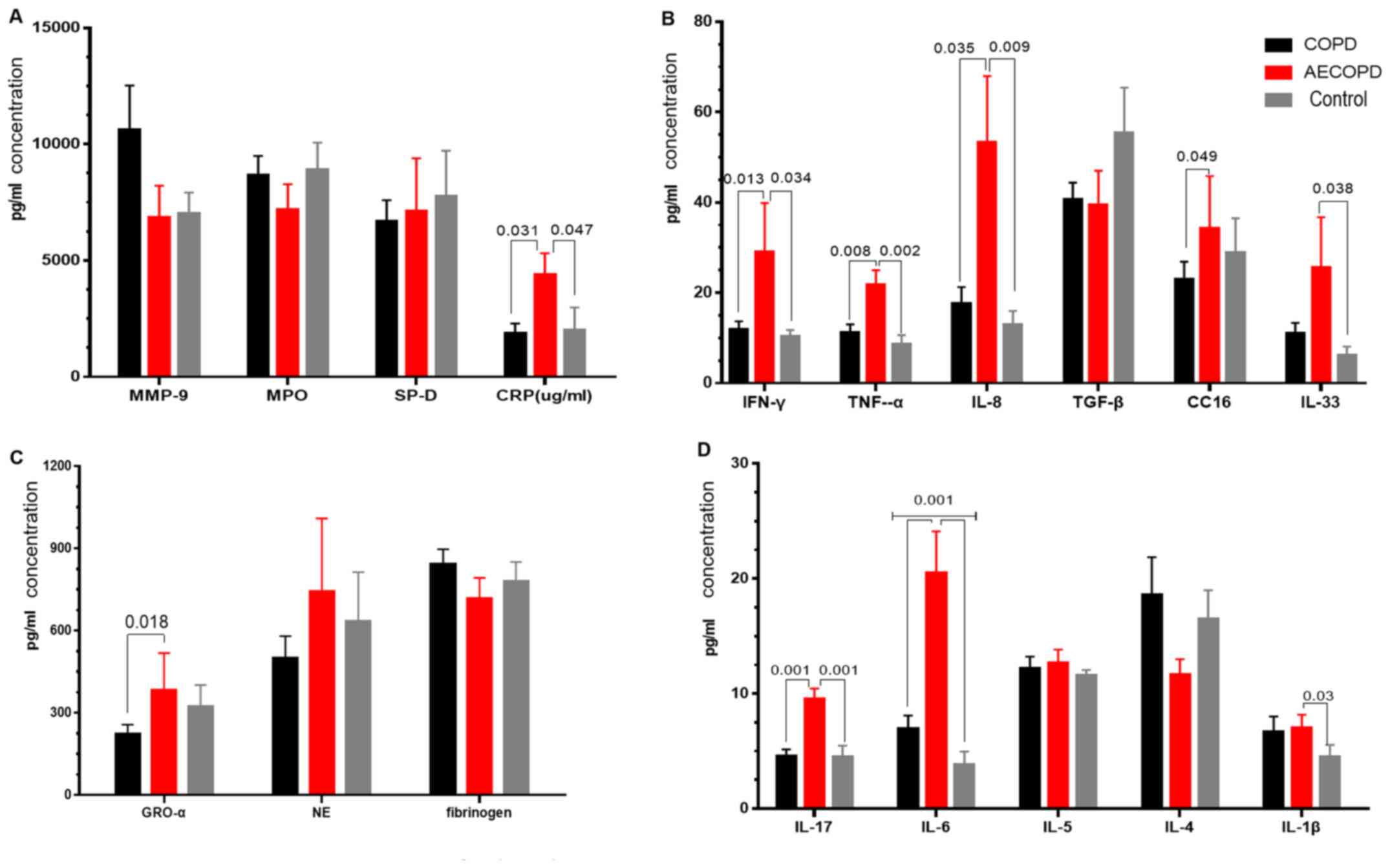
Inflammatory biomarkers
Higher IL-6 (COPD vs. AECOPD vs. controls, 6.95 vs.
20.51 vs. 3.82), IL-1β (COPD vs. AECOPD vs. controls, 6.69 vs. 7.24
vs. 4.09) and TGF-α (COPD vs. AECOPD vs. controls, 2.15 vs. 2.20
vs. 1.46) levels were observed in the COPD and AECOPD groups
compared with healthy controls. The levels of TNF-α (AECOPD vs.
COPD vs. controls, 21.71 vs. 11.23 vs. 8.56), IFN-γ (AECOPD vs.
COPD vs. controls, 29.10 vs. 11.91 vs. 10.32), IL-8 (AECOPD vs.
COPD vs. controls, 53.39 vs. 17.58 vs. 12.87), IL-17A (AECOPD vs.
COPD vs. controls, 9.56 vs. 4.57 vs. 4.15) and CRP (AECOPD vs. COPD
vs. controls, 4395 vs. 1882 vs. 2020) were markedly increased in
the AECOPD group compared with the COPD group and healthy controls.
IL-33 levels were higher in the AECOPD group compared with healthy
controls (AECOPD vs. controls, 25.56 vs. 6.11), while GRO-α was
higher in the AECOPD group compared with the COPD group (AECOPD vs.
COPD, 382.7 vs. 223.3). The other parameters exhibited no
significant differences among the groups (Table II; Fig.
4).
Bioinformatics analysis
Proteomic interaction analysis of key biomarkers was
performed by inputting 10 significant biomarkers (IL-6, IL-1β,
TNF-α, IFN-γ, IL-8, IL-33, IL-17A, TGF-α, GRO-α and CRP) into the
STRING database. The results indicated that there were three core
biomarkers of COPD: IL-6, TNF-α and IL-8. In addition, IFN-γ, CRP,
GRO-α, IL-33 and IL-17A were also essential in the complex network
(Fig. 5). The present study used
network expansion function through STRING and found some potential
genes closely associated with these proteins, including inhibitor
of NF-κB kinase regulatory subunit γ (IKBKG), inhibitor of kappa
light polypeptide gene enhancer in B-cells, kinase β (IKBKB),
receptor-interacting serine/threonine kinase 1 (RIPK1), TNF
receptor superfamily member 1A associated via death domain (TRADD)
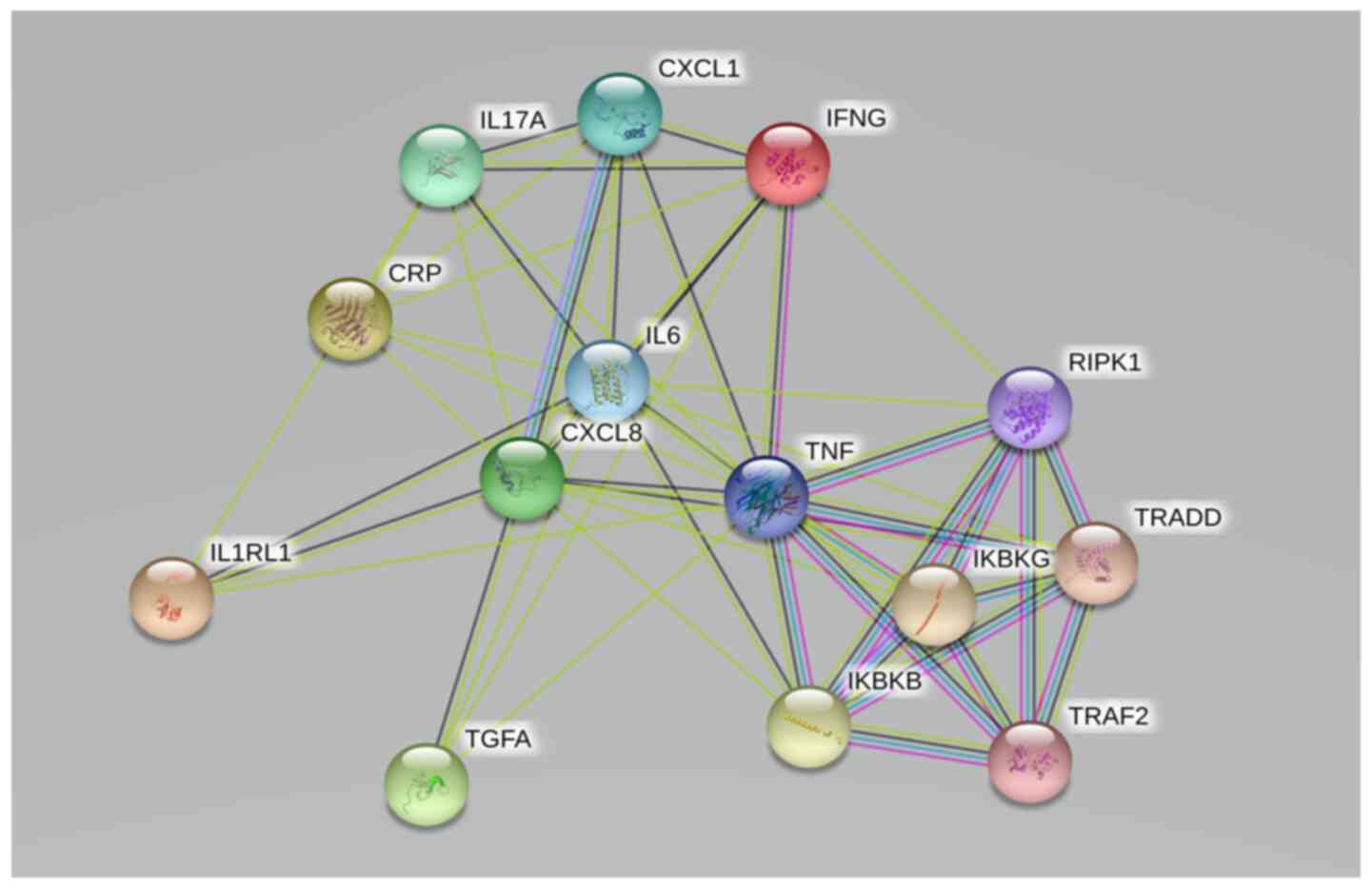
and TNF receptor-associated factor 2 (TRAF2; Fig. 6).
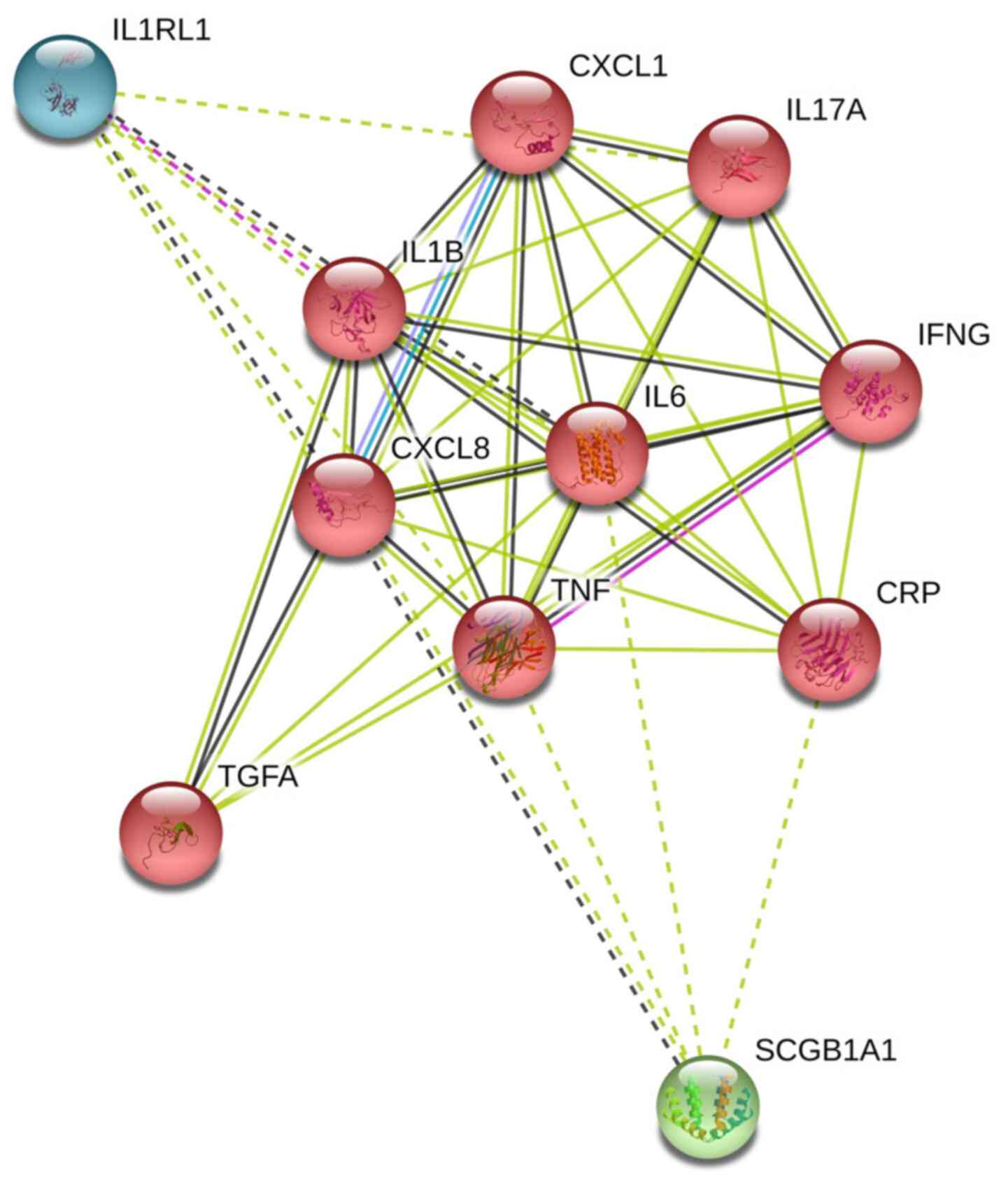 | Figure 5.Protein-protein interaction network
of significant inflammatory factors (IL-6, IL-1β, TNF-α, IFN-γ,
IL-8, IL-33, IL-17A, TGF-α, GRO-α and CRP). Network nodes represent
proteins (colored nodes: Query proteins and first shell of
interactors), while edges represent protein-protein associations
[from STRING website, 10 items (human)-STRING interaction network
(string-db.org)]. IL-6, TNF-α and IL-8 were
predominant in the network of significantly different inflammatory
factors (triangle). |
Correlation analysis
Pearson's correlation analysis revealed a mild
positive correlation between CD3+ T lymphocytes (mainly
CD4 subset) with FEV1/FVC (r=0.33), FEV1% (r=0.27) and maximal mid
expiratory flow (MMEF)75/25 (r=0.34) parameters; however,
Spearman's correlation analysis revealed a mild or weak negative
correlation with presence of smoking and exacerbation history.
Myeloid-derived immune cells, such as
CD16+CD66+ neutrophil (r=−0.26, r=−0.25) and
CD14+ (r=−0.22, r=−0.24)/CD64+ (r=−0.14,
r=−0.22) MON exhibited a weak negative correlation with pulmonary
function (FEV1/FVC, MMEF75/25). In addition, MMP-9 exhibited a mild
negative correlation with cough (r=−0.33) but was not correlated
with spirometry measurements. Furthermore, IL-8 (r=0.33) and IL-17A
(r=0.31) exhibited a mild positive correlation with cough, while
CRP was mildly negatively correlated with FEV1/FVC (r=−0.21), FEV1%
(r=−0.24) and MMEF75/25 (r=−0.26), smoking history (r=−0.62) but
mild positively correlated with exacerbations (r=0.26), mMRC
(r=0.26) and cough (r=0.28). Additionally, CC16 exhibited a
moderate negative correlation with TLC (total lung compacity)
(r=−0.44) and RV (residual volume)/TLC (r=−0.41; Tables IV and V; all P<0.05; correlation strength:
r=0.6–0.8 strong; r=0.4–0.6 moderate; r=0.2–0.4 mild and r=0.0–0.2
weak or uncorrelated).
 | Table IV.Pearson correlation coefficient
between immune profiles and clinical features. |
Table IV.
Pearson correlation coefficient
between immune profiles and clinical features.
| Correlation
(r) | FEV1/FVC | FEV1 | FVC | RV/TLC | TLC | MM75/25 |
|---|
| CD3+ T
cell | 0.33 | 0.27 | 0.14 | 0.13 | −0.10 | 0.34 |
| CD4+ T
cell | 0.35 | 0.28 | 0.24 | −0.01 | −0.15 | 0.38 |
| CD8+ T
cell | 0.24 | 0.13 | 0.05 | 0.19 | 0.02 | 0.23 |
| CD14+
MPS | −0.22 | −0.24 | −0.19 | −0.07 | −0.01 | −0.24 |
| CD64+
MPS | −0.14 | −0.12 | −0.10 | −0.12 | −0.47 | −0.22 |
|
CD16+CD66+ | −0.26 | −0.20 | −0.01 | −0.17 | 0.06 | −0.25 |
| IL-6 | −0.19 | −0.15 | 0.20 | 0.02 | 0.11 | −0.14 |
| CRP | −0.21 | −0.24 | −0.23 | −0.04 | 0.02 | −0.26 |
| CC16 | −0.03 | −0.02 | −0.07 | −0.41 | −0.44 | −0.05 |
| IL-8 | −0.09 | −0.08 | −0.18 | −0.17 | 0.21 | −0.07 |
| IL-17A | −0.24 | −0.31 | −0.28 | −0.44 | 0.41 | −0.26 |
| Fibrinogen | −0.05 | −0.04 | −0.01 | −0.13 | −0.13 | −0.11 |
 | Table V.Spearman's correlation coefficient
between immune profiles and clinical features. |
Table V.
Spearman's correlation coefficient
between immune profiles and clinical features.
| Correlation
coefficient | Smoking | Exacerbation | mMRC | Cough |
|---|
| CD3+ T
cell | −0.29a | −0.24a | −0.04 | 0.06 |
| CD4+ T
cell | −0.20a | −0.21 | −0.17 | 0.03 |
| CD8+ T
cell | −0.23a | −0.15 | 0.20 | −0.05 |
| CD14+
MPS | 0.27a | 0.16 | 0.13 | −0.04 |
| CD64+
MPS | 0.31a | 0.10 | 0.10 | −0.07 |
|
CD16+CD66+ | 0.24a | −0.09 | −0.06 | −0.07 |
| IL-6 | 0.15 | 0.22 | 0.16 | 0.24a |
| CRP | −0.62 | 0.26a | 0.26a | 0.28a |
| CC16 | −0.04 | 0.16 | 0.18 | −0.13 |
| IL-8 | 0.12 | 0.14 | 0.20 | 0.33a |
| IL-17A | −0.24 | 0.30a | 0.14 | 0.31a |
| Fibrinogen | 0.07 | −0.24 | −0.21 | −0.20 |
| MMP-9 | 0.07 | 0.02 | −0.06 | −0.33a |
Discussion
Inflammation serves a central role in the activation
and alteration of immune profiles in COPD (26). In the present study, significant
differences in the expression profiles of immune factors in
peripheral blood of patients with COPD were observed, based on
results from flow cytometry and analysis of multiple biomarker
panels. Specifically, there was a decrease in CD3+T
lymphocytes and an increase in CD14+/CD64+
MPS and CD16+CD66+ neutrophils in both the
COPD/AECOPD groups and smoking controls compared with non-smoking
controls. In addition, alterations of systemic inflammatory
markers, such as IL-6, TNF-α, IFN-γ, IL-17A and CRP, were reported,
suggesting that immune profiles were different among patients with
COPD, patients with AECOPD and healthy controls, CRP and IL-17A
were associated with smoking status.
A previous study reported an increase in the
proportion of macrophages (M1-M2) and a decrease in T-lymphocytes
(mainly CD4+T) in lung tissues of current smokers with
COPD compared with non-smokers and smokers without COPD (27). Furthermore, the proportion of
monocytes was markedly different between non-smokers and smokers in
the blood only (27). The present
study reported an increase in the proportion of
CD16+CD66+ neutrophils and
CD14+/CD64+ MPS, a decrease in the proportion
of CD3+T lymphocytes (both CD4+ and
CD8+ T Cell) in patients with COPD and smokers compared
with non-smoking controls in peripheral blood. This indicated the
predominant effects of smoking and cumulative immune composition
changes of COPD.
MPS is defined as a cell lineage, including
promonocytes and their precursors, in the bone marrow, monocytes in
circulation and macrophages in tissues (28). Once in the blood, monocytes undergo
transformation into tissue macrophages with phagocytic function,
and serve a central role in the immune regulation by generating
antigens to T lymphocytes and secreting proinflammatory factors
such as TNFα, IL-1β and IL-6 that are involved in host defense and
inflammation (28,29). Although the present study did not
assess macrophages in lung tissues and sputum, the increased ratio
of CD14+/CD64+ monocytes and
CD16+CD66+ neutrophils in blood revealed the
prominent systemic inflammation of smokers and patients with COPD,
and the decreased ratio of CD3+ T lymphocytes
(CD4+ and CD8+ subsets) in the blood revealed
the cumulative immune deficiency of smokers and patients with COPD.
Lower proportions of lymphocytes (CD3+ T cells)
indicated poorer lung function and a higher risk of exacerbation in
COPD. On the other hand, higher levels of myeloid cells
(CD16+CD66+ neutrophils and
CD14+/CD64+ MPS) indicated poorer lung
function in smokers with COPD compared with non-smoking controls.
However, the trigger of immune composition changes in non-smoking
patients with COPD remains unclear.
Blood biomarkers have a significant value in
diagnosis and prognosis of COPD (14). A study on COPD Gene and SPIROMCIS
measured 114 candidate plasma and serum biomarkers using the 13
panel Luminex assays (30). A total
of nine cytokines and chemokines were selected for further analysis
using a Meso Scale Discovery platform. These MSD multiplex panels
were used to measure Single nucleotide polymorphism in 2,123
subjects from COPD Gene and 1,117 subjects from SPIROMICS. The
results indicated a strong association between eotaxin and IL-6
with airflow obstruction (13). In
the present multiplex panel involving 20 biomarkers, it was
identified that IL-6, TNF-α and IL-8 (CXCL-8) served key roles in
the inflammation response network of COPD. Pearson's correlation
analysis revealed a close relationship between CRP and airway
airflow obstruction, while CC16 could be a novel marker of
emphysema. Furthermore, higher levels of IL-6, CRP, IL-8 and IL-17A
indicated more cough, sputum and dyspnea. Although no biomarker has
been demonstrated to be useful in the diagnosis of COPD to date, to
the best of our knowledge, blood-based biomarkers for predicting
progression of COPD remain relevant (31). The present findings may provide
novel insights into biomarkers that have potential for prediction
and evaluation of COPD pulmonary function, as well as its symptoms,
and are expected to be beneficial for development of novel
therapies for COPD phenotypes.
The present study analyzed the interaction among
biomarkers, identified the importance of IKBKG, RIPK1, TRADD and
TRAF2 and also investigated IL-17, TNF and NF-κB pathway, which are
associated with inflammatory cell chemotaxis and immune response
(25). The IL-17A, TNF and NF-κB
signaling pathways have been identified to be important in the
inflammatory response of COPD (32). Fisetin, which inhibits the TNF-α and
NF-κB signaling pathways, could be a good candidate drug for
improving lung function in patients with COPD (33). In addition, IL-17A-driven type-2
inflammation is another endotype of COPD, which could be an
indicator of steroid-unresponsive subgroup of COPD (34).
Previous studies have found T helper 17 (Th17)
cells, a subset of CD4+ T cells, to be positively
correlated with IL-17A levels, which are increased in patients with
COPD (35). The present study
reported elevated levels of IL-17A, although the proportion of
CD4+ T cells was decreased in patients with COPD
compared with healthy controls. A possible reason for this paradox
is that Th17 cells could be inhibiting the expansion of
CD4+ regulatory T (Treg) cells (36). An imbalance in Th17/Treg has been
reported to serve a pivotal role in COPD development and
progression (37). Studies have
also demonstrated a negative correlation between Th17 cell and Treg
cells in patients with COPD (34,38).
It is possible that the lower proportion of Treg cells could have
contributed to the decrease of CD4+ T cells in COPD in
the present study. Furthermore, a positive correlation between Treg
proportion and TGF-β level has been reported (38). However, the present study did not
observe a significant decrease of TGF-β in COPD. Further functional
experiments investigating the subset of immune cells are required
to investigate this.
The present study had several limitations. The wide
range of immune cells should be classified into more detailed
subgroups. The functional and dynamic evolution of various immune
components at different stages will need to be explored in future
studies. The biomarker panel used was selected from available
assays, based on possible mechanisms in COPD. This panel was
heavily weighted towards systemic inflammatory markers, not
lung-specific biomarkers. In addition, a larger population of
participants would be helpful in identifying subgroups to help
decrease the heterogeneity. Despite these limitations, the present
study identified COPD and smoking associated immune profiles, and
indicated those relevant to pulmonary functions and symptoms.
Potential pathways and genes of the inflammatory molecular network
were also identified.
Overall, the present study revealed changes in the
immune profiles in patients with COPD and smokers. A decrease in
CD3+ T cells and an increase in neutrophils and MPS were
also reported. In addition, levels of IL-6, TNF-α, IFN-γ, IL-8
IL-17A and CRP were higher in patients with AECOPD compared with
patients with COPD and healthy controls. Furthermore, IL-6, TNF-α
and IL-8 (CXCL8) were identified as core biomarkers in COPD
pathogenesis, while immune profiles were also relevant to pulmonary
functions and symptoms. Future studies will need to recruit more
patients with COPD in order to validate and expand the results.
Particular focus should be paid on investigating lung-specific
biomarkers to identify more COPD subgroups and help advance novel
therapies for COPD.
Acknowledgements
The authors thank Professor Dan Liu and Professor
Gang Wang of West China Hospital, Chengdu, China, for assistance
with the patients enrollment and experiments.
Funding
The study was supported by The National Key
Development Plan for Precision Medicine Research of China (grant
no. 2017YFC0910004).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SL and SZ conceived the present study, enrolled the
subjects, and recorded all the clinical data. ZW assisted in the
testing of inflammatory factors. FW performed the flow cytometry.
WL organized the study, supervised the study and was involved in
data aquisition, analysis and interpretation. SL and SZ confirm the
authenticity of all the raw data. All authors reviewed and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
West China Hospital, Sichuan University (Chengdu, China) and all
patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2017 Causes of Death Collaborators, .
Global, regional, and national age-sex-specific mortality for 282
causes of death in 195 countries and territories, 1980-2017: A
systematic analysis for the Global Burden of Disease Study 2017.
Lancet. 392:1736–1788. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh D, Agusti A, Anzueto A, Barnes PJ,
Bourbeau J, Celli BR, Criner GJ, Frith P, Halpin DMG, Han M, et al:
Global strategy for the diagnosis, management, and prevention of
chronic obstructive lung disease: The GOLD science committee report
2019. Eur Respir J. 53:19001642019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Decramer M, Janssens W and Miravitlles M:
Chronic obstructive pulmonary disease. Lancet. 379:1341–1351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tuder RM and Petrache I: Pathogenesis of
chronic obstructive pulmonary disease. J Clin Invest.
122:2749–2755. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brightling C and Greening N: Airway
inflammation in COPD-progress to precision medicine. Eur Respir J.
54:19006512019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gorska K, Paplinska-Goryca M, Nejman-Gryz
P, Goryca K and Krenke R: Eosinophilic and neutrophilic airway
inflammation in the phenotyping of mild-to-moderate asthma and
chronic obstructive pulmonary disease. COPD. 14:181–189. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Magnussen H: Therapy control of COPD by
eosinophilic granulocytes? Dtsch Med Wochenschr. 144:917–921.
2019.(In German). PubMed/NCBI
|
|
8
|
Pavord ID: Biologics and chronic
obstructive pulmonary disease. J Allergy Clin Immunol.
141:1983–1991. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yousuf A and Brightling CE: Biologic
drugs: A new target therapy in COPD? COPD. 15:99–107. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cruz T, Lopez-Giraldo A, Noell G,
Casas-Recasens S, Garcia T, Molins L, Juan M, Fernandez MA, Agustí
A and Faner R: Multi-level immune response network in mild-moderate
chronic obstructive pulmonary disease (COPD). Respir Res.
20:1522019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan Z, Yu H and Liao JL: Probing cellular
and molecular mechanisms of cigarette smoke-induced immune response
in the progression of chronic obstructive pulmonary disease using
multiscale network modeling. PLoS One. 11:e01631922016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Butler CC, Gillespie D, White P, Bates J,
Lowe R, Thomas-Jones E, Wootton M, Hood K, Phillips R, Melbye H, et
al: C-Reactive protein testing to guide antibiotic prescribing for
COPD exacerbations. N Engl J Med. 381:111–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bradford E, Jacobson S, Varasteh J,
Comellas AP, Woodruff P, O'Neal W, DeMeo DL, Li X, Kim V, Cho M, et
al: The value of blood cytokines and chemokines in assessing COPD.
Respir Res. 18:1802017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Regan EA, Hersh CP, Castaldi PJ, DeMeo DL,
Silverman EK, Crapo JD and Bowler RP: Omics and the search for
blood biomarkers in Chronic obstructive pulmonary disease: Insights
from COPDGene. Am J Respir Cell Mol Biol. 61:143–149. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou Y, Chen X, Liu J, Zhou DB, Kuang X,
Xiao J, Yu Q, Lu X, Li W, Xie B and Chen Q: Serum IL-1β and IL-17A
levels in patients with COPD: Associations with clinical
parameters. Int J Chron Obstruct Pulmon Dis. 12:1247–1254. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fermont JM, Masconi KL, Jensen MT, Ferrari
R, Di Lorenzo VAP, Marott JM, Schuetz P, Watz H, Waschki B,
Müllerova H, et al: Biomarkers and clinical outcomes in COPD: A
systematic review and meta-analysis. Thorax. 74:439–446. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai Y, Zhou Q, Fang Q, Song L and Chen K:
Inflammatory cytokines and T-Lymphocyte subsets in serum and sputum
in patients with bronchial asthma and chronic obstructive pulmonary
disease. Med Sci Monit. 25:2206–2210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papaioannou AI, Konstantelou E,
Papaporfyriou A, Bartziokas K, Spathis A, Bakakos P, Loukides S,
Koulouris N, Papiris S and Kostikas K: Serum surfactant protein
levels in patients admitted to the hospital with acute COPD
exacerbation. Lung. 196:201–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhai J, Insel M, Addison KJ, Stern DA,
Pederson W, Dy A, Rojas-Quintero J, Owen CA, Sherrill DL, Morgan W,
et al: Club cell secretory protein deficiency leads to altered lung
function. Am J Respir Crit Care Med. 199:302–312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rangasamy L, Geronimo BD, Ortin I, Coderch
C, Zapico JM, Ramos A and de Pascual-Teresa B: Molecular imaging
probes based on matrix metalloproteinase inhibitors (MMPIs).
Molecules. 24:29822019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stockley RA, Halpin DMG, Celli BR and
Singh D: Chronic obstructive pulmonary disease Biomarkers and their
interpretation. Am J Respir Crit Care Med. 199:1195–1204. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Graham BL, Steenbruggen I, Miller MR,
Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA,
McCarthy K, McCormack MC, et al: Standardization of spirometry 2019
update. An official American thoracic society and European
respiratory society technical statement. Am J Respir Crit Care Med.
200:e70–e88. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Munari AB, Gulart AA, Dos Santos K,
Venâncio RS, Karloh M and Mayer AF: Modified medical research
council dyspnea scale in GOLD classification better reflects
physical activities of daily living. Respir Care. 63:77–85. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Skierka AS and Michels KB: Ethical
principles and placebo-controlled trials-interpretation and
implementation of the Declaration of Helsinki's placebo paragraph
in medical research. BMC Med Ethics. 19:242018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caramori G, Casolari P, Barczyk A, Durham
AL, Di Stefano A and Adcock I: COPD immunopathology. Semin
Immunopathol. 38:497–515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cruz T, Lopez-Giraldo A, Noell G, Molins
L, Juan M, Fernandez MA, Canet MRF and Agusti A: Pulmonary and
systemic cellular immune response network in patients with
mild-moderate COPD. Eur Respiratory J Conf. 50:2017.
|
|
28
|
Hume DA, Irvine KM and Pridans C: The
mononuclear phagocyte system: The relationship between monocytes
and macrophages. Trends Immunol. 40:98–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang J, Zhang L, Yu C, Yang XF and Wang H:
Monocyte and macrophage differentiation: Circulation inflammatory
monocyte as biomarker for inflammatory diseases. Biomark Res.
2:12014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun W, Kechris K, Jacobson S, Drummond MB,
Hawkins GA, Yang J, Chen TH, Quibrera PM, Anderson W, Barr RG, et
al: Common genetic polymorphisms influence blood biomarker
measurements in COPD. PLoS Genet. 12:e10060112016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mannino DM: Biomarkers for chronic
obstructive pulmonary disease diagnosis and progression: Insights,
disappointments and promise. Curr Opin Pulm Med. 25:144–149. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garudadri S and Woodruff PG: Targeting
chronic obstructive pulmonary disease phenotypes, endotypes, and
biomarkers. Ann Am Thorac Soc. 15 (Suppl 4):S234–S238. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee S, Ro H, In HJ, Choi JH, Kim MO, Lee
J, Hong ST and Lee SU: Fisetin inhibits TNF-α/NF-κB-induced IL-8
expression by targeting PKCδ in human airway epithelial cells.
Cytokine. 108:247–254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Christenson SA, van den Berge M, Faiz A,
Inkamp K, Bhakta N, Bonser LR, Zlock LT, Barjaktarevic IZ, Barr RG,
Bleecker ER, et al: An airway epithelial IL-17A response signature
identifies a steroid-unresponsive COPD patient subgroup. J Clin
Invest. 129:169–181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roos AB, Sanden C, Mori M, Bjermer L,
Stampfli MR and Erjefalt JS: IL-17A is elevated in end-stage
chronic obstructive pulmonary disease and contributes to cigarette
smoke-induced lymphoid neogenesis. Am J Respir Crit Care Med.
191:1232–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng X, Zhang L, Chen J, Gu Y, Xu J and
Ouyang Y: Dendritic cells and Th17/Treg ratio play critical roles
in pathogenic process of chronic obstructive pulmonary disease.
Biomed Pharmacother. 108:1141–1151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ito JT, Cervilha DAB, Lourenco JD,
Goncalves NG, Volpini RA, Caldini EG, Landman G, Lin CJ, Velosa
APP, Teodoro WPR, et al: Th17/Treg imbalance in COPD progression: A
temporal analysis using a CS-induced model. PLoS One.
14:e02093512019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li XN, Pan X and Qiu D: Imbalances of Th17
and Treg cells and their respective cytokines in COPD patients by
disease stage. Int J Clin Exp Med. 7:5324–5329. 2014.PubMed/NCBI
|