Introduction
Ischemia causes an increase in intracellular and
mitochondrial calcium levels by impairing ATPase-dependent ion
transport, as well as reduces cellular ATP levels and intracellular
pH. The lack of ATP disrupts the mechanism regulating cell volume
and induces the lysis of organelles and plasma membranes (1). Reperfusion promotes the production of
reactive oxygen species (ROS), the sequestration of proinflammatory
immunocytes in ischemic tissues, endoplasmic reticulum stress and
the development of post-ischemic capillary no-reflow, which further
enhance tissue injury. These events finally lead to the opening of
mitochondrial permeability transition pores, which results in
ischemia/reperfusion (I/R)-induced cell lysis and death (1).
Ischemia can cause myocardial infarction, stroke and
peripheral vascular disease (1). At
present, alteplase, a tissue plasminogen activator, is the only
United States Food and Drug Administration (US FDA) approved
clot-busting medication used to recanalize the thrombosed or
occluded vasculature in ischemic stroke (2). Numerous factors can cause I/R, such as
acute mesenteric ischemia, small intestine transplantation,
intestinal obstruction, trauma or shock (3). The reperfusion following ischemia
further aggravates damage and results in more severe intestinal
injury compared with ischemia alone (4). The small intestine is highly sensitive
to I/R as mucosal intestinal injury often leads to bacterial
translocation and initiates the inflammatory response (5). Thus, regulation of inflammatory
responses and oxidative stress in the small intestine has
significant clinical implications for patients with I/R injury
(6). I/R is usually accompanied by
the extensive production of inflammatory cytokines, as well as with
the activation of both the innate and adaptive immune responses
(7), which further increases the
permeability of blood vessels, the tissue aggregation of
neutrophils (8), the production of
proinflammatory cytokines and hypotension (5).
Cyclooxygenase (COX)-derived prostanoids, such as
prostacyclin and prostaglandin E, have been revealed to exert
crucial roles in I/R-induced intestinal injuries (9,10).
Firstly, COX catalyzes arachidonic acid into prostaglandin
endoperoxide (PGH2), then PGH2is converted into prostaglandin
(11). COX-1 and COX-2, two COX
isoenzymes, have been identified and investigated (12). It has been revealed that COX-1 is
involved in the synthesis of prostanoids and exists in the majority
of cells under normal conditions. Conversely, COX-2 is often
undetectable under normal conditions, but its expression is
markedly increased under pathological conditions (13). For example, both COX-1 and COX-2 are
involved in I/R-induced myocardial (14) and gastric injuries (15). Moreover, COX-2-deficient mice
exhibit a significant reduction in damage after I/R insult
(16). However, the underlying
protective mechanisms remain unknown.
As a selective COX-2 inhibitor, parecoxib sodium has
exhibited potent capacities, such as relieving perioperative pain
and reducing I/R-induced hepatic (17), cerebral (18) or renal injuries (19). Thus, the present study aimed to
reveal the protective effect of parecoxib sodium in I/R-induced
intestinal injury, particularly on the inflammatory response and
oxidative stress. Moreover, the study aimed to further the
understanding of the role of COX-2 in intestinal I/R injury.
Materials and methods
Animals
A total of 60 adult male Sprague-Dawley rats (age,
8–10 weeks; weight, 250–280 g) were obtained from the Animal Centre
of Wenzhou Medical University (Wenzhou, China). Rats were
individually housed in standard cages in rooms with a constant
~21–25°C temperature and ~50-65% relative humidity and a 12
h-light/dark cycle. Rats were fed with standard chow and had free
access to water. All animals received humane care according to the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health. All animal care and experimental procedures
were approved by the Wenzhou Medical University Animal Policy and
Welfare Committee (approval no. 2013/APWC/0361). At the end of the
experimental period, animals were euthanized via CO2
asphyxiation with a flow rate of 30% chamber vol/min.
Drug treatment
In total, 60 rats were randomly divided into four
groups with 15 rats in each group: Control (sham operation) group,
intestinal I/R group, 10 mg/kg parecoxib sodium-pre-treated I/R
(I/R + Pare/10) group and 20 mg/kg parecoxib sodium-pre-treated I/R
(I/R + Pare/20) group. Parecoxib sodium (Pfizer, Inc.) was diluted
in isotonic saline. The prepared parecoxib sodium at 10 or 20 mg/kg
was intraperitoneally injected into rats once daily for 5
consecutive days prior to ischemia. The parecoxib dosage was
determined based on a previous study (19). Rats in sham and I/R groups were
injected intraperitoneally with the same volume of isotonic saline
at the same time. On the day of surgery, 1 h after the last
injection of parecoxib sodium or saline, all animals were
anesthetized with intraperitoneal injection of pentobarbital at 40
mg/kg. The superior mesenteric artery was isolated and then
completely occluded to induce ischemia. After 1 h of ischemia,
reperfusion was initiated by removing the occlusion and was
maintained for another 2 h. Rats were covered with warm blankets to
maintain their body temperature. At the end of reperfusion, blood
and small intestinal tissue samples were harvested and used for
subsequent assays. The time period for ischemia and reperfusion was
determined based on a previous study (20). The survival rate was calculated
daily for 7 consecutive days after reperfusion. Once rats reach the
criteria of humane endpoints [a reduction of ~4–6°C in body
temperature, a weight loss of >10%, decreased activity
(lethargy) and alertness, a rough coat and hunched posture, which
are direct signs of illness, pain or distress], they were
euthanized immediately. While rats that survived for 7 days
received euthanasia right after experiments via CO2
asphyxiation with a flow rate of 30% chamber vol/min (21).
Biochemical assays
The total antioxidant capacity (TAC) and
malondialdehyde (MDA) levels in the serum and small intestinal
samples were evaluated using a TAC Assay kit (cat. no. S0119;
Beyotime Institute of Biotechnology) and a MDA assay kit (cat. no.
S0131S; Beyotime Institute of Biotechnology), respectively. Tissues
stored at −80°C were homogenized in cold PBS at 2–8°C. Each
homogenized sample was centrifuged at 4,000 × g for 15 min at 4°C,
then the supernatant was collected for the detection of nitric
oxide (NO) levels using the nitrate reductase method.
The activity of superoxidase dismutase (SOD) in the
serum and small intestinal samples was assessed using the xanthine
oxidase method, with the Total Superoxide Dismutase Assay kit
supplied by Beyotime Institute of Biotechnology (cat. no. S0109).
SOD activity was measured using a microplate reader at a wavelength
of 560 nm. Myeloperoxidase (MPO) activity was measured using the
spectrophotometric method with 3,3-5,5 tetramethylbenzine as the
substrate, and the light absorbance was measured at 460 nm over a
period of 5 min (Elabscience Biotechnology, Inc.; cat. no.
E-BC-K074-S). The values of SOD and MPO activity were presented as
unit per µl/l of serum or unit per µg/g of tissue. All detection
procedures were performed in accordance with the manufacturer's
instructions.
ELISA
ELISA kits for interleukin (IL)-1β (cat. no. RLB00;
R&D Systems, Inc.), IL-8 (cat. no. RA20553; Bioswamp; Wuhan
Bienle Biotechnology Co., Ltd.), intercellular cell adhesion
molecule-1 (ICAM-1; cat. no. RIC100; R&D Systems, Inc.) and
IL-10 (cat. no. R1000; R&D Systems, Inc.) were commercially
purchased and used to determine the concentration of each cytokine
both in the plasma and small intestinal samples. In total, a 100-µl
aliquot of the supernatant from each well was collected for
measurement. The concentration of each cytokine in the supernatant
was standardized to the cell protein concentration in the
respective well. The absorbance was measured at 450 nm using a
Model 550 microplate reader (Bio-Rad Laboratories, Inc.). The
experiments were repeated four times using different batches of
rats.
Western blot analysis
Frozen small intestinal samples were homogenized in
RIPA buffer freshly supplemented with protease and phosphatase
inhibitor cocktails (Sigma-Aldrich; Merck KGaA) on ice. Lysates
were harvested after centrifugation at 12,000 × g for 30 min at
4°C. The protein concentration was determined with a Bradford
assay. Then, 30 µg protein for each sample was loaded and run via
10% SDS-PAGE, following which it was transferred onto a PVDF
membrane (Roche Diagnostics). After blocking with 5% fat-free milk
at room temperature for 1 h, membranes were incubated with specific
primary antibodies at their corresponding dilution (Table I) at 4°C overnight. The following
day, membranes were rinsed and incubated with HRP-conjugated
secondary antibodies at 1:1,000 dilution (bovine anti-rabbit
IgG-HRP, cat. no. sc-2370; bovine anti-mouse IgG-HRP, cat. no.
sc-2371; Santa Cruz Biotechnology, Inc.) at room temperature for 1
h. The protein bands were developed using ECL Advance Detection
kits (Thermo Fisher Scientific, Inc.) and detected with a Multi
Image Light Cabinet (Protein Simple). Densitometric analysis of
each band was performed using ImageJ software (version 1.46;
National Institutes of Health).
 | Table I.Summary of primary antibodies used in
the present study. |
Table I.
Summary of primary antibodies used in
the present study.
| Antibody | cat. no. | Host | Vendor | Dilution |
|---|
| Caspase-3 | sc-56053 | Mouse | Santa Cruz
Biotechnology, Inc. | 1:200 |
| Bcl-2 | ab194583 | Rabbit | Abcam | 1:500 |
| Bax | ab182733 | Rabbit | Abcam | 1:500 |
| β-actin | sc-47778 | Mouse | Santa Cruz
Biotechnology, Inc. | 1:200 |
Statistical analysis
Statistical analysis was conducted by using GraphPad
Prism 5.0 software (GraphPad Software, Inc.). All results were
presented as the mean ± standard deviation (SD) of at least three
independent experiments. All data were compared by one-way ANOVA
that was followed by Tukey's post hoc test. Survival rates were
analyzed by the Kaplan-Meier method using a log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Parecoxib sodium pre-administration
attenuates IR-induced oxidative stress both in the serum and small
intestinal tissues
In order to evaluate the protective effect of
parecoxib sodium against oxidative stress, several parameters in
the serum were detected, including the TAC, the concentrations of
MDA and NO, and the activities of SOD and MPO. As revealed in
Fig. 1A, I/R significantly
increased the serum concentration of MDA, which is a product of
lipid peroxidation, by ~2-fold, while the administration of
parecoxib sodium significantly inhibited I/R-induced MDA levels by
30–50%. The serum concentration of NO was increased by ~3.5-fold
post-I/R, while parecoxib sodium decreased NO levels by 50–70%
(Fig. 1B). Conversely, I/R
significantly decreased TAC in the serum by 60%, while parecoxib
sodium significantly increased TAC by 1.8-2-fold (Fig. 1C). Moreover, I/R significantly
suppressed the serum SOD activity by 75% and enhanced MPO activity
by 3.5-fold, while parecoxib sodium pre-treatment restored SOD
activity close to the control level and inhibited I/R-induced MPO
activation in a dose-dependent manner (Fig. 1D and E).
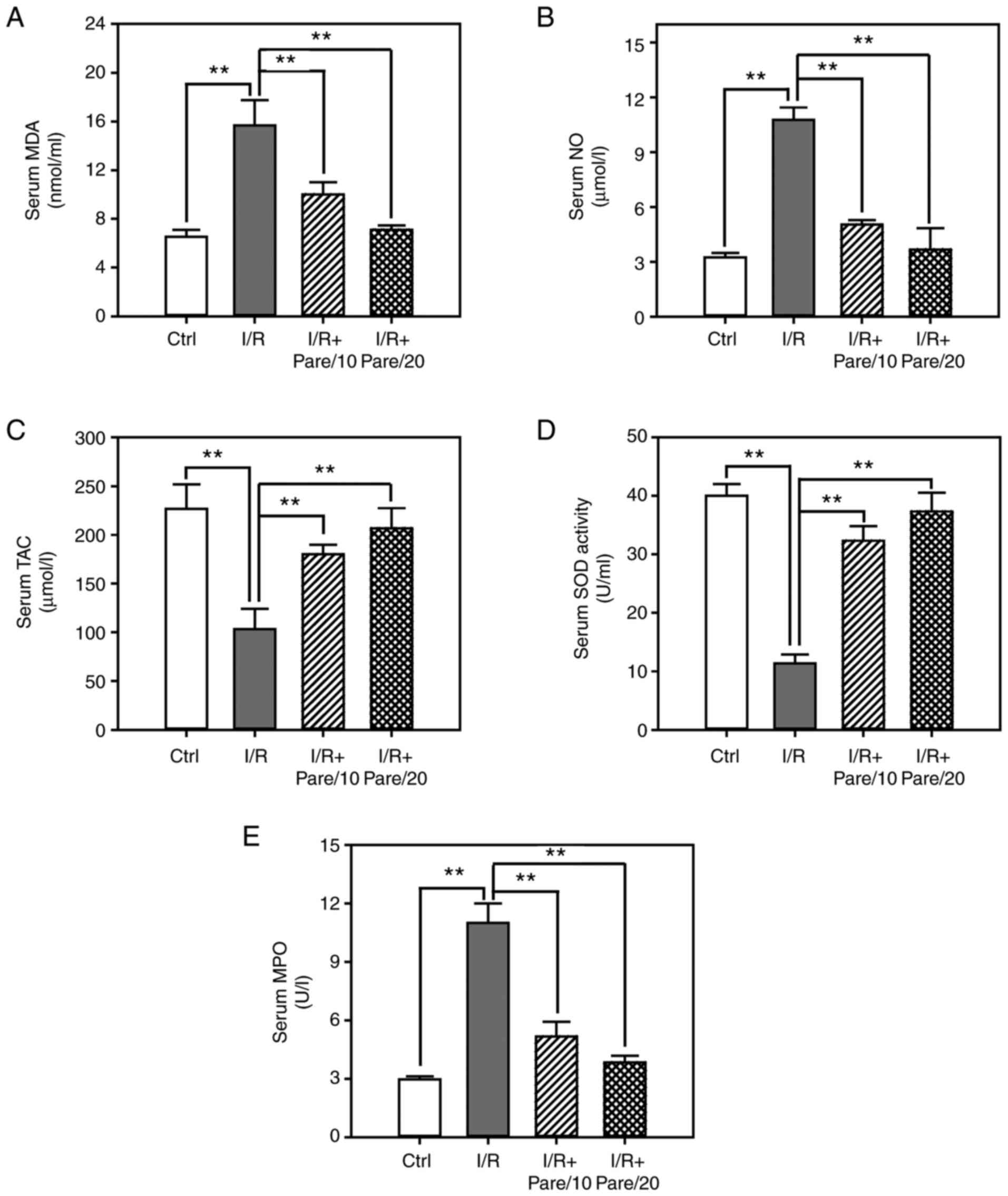 | Figure 1.Assessment of the oxidative stress
after I/R with/without parecoxib sodium pre-treatment in rat serum.
(A-C) The contents of malondialdehyde (A) nitric oxide (B) and
total antioxidant capacity (C) in rat serum in control, I/R, I/R +
Pare/10, and I/R + Pare/20 groups. (D and E) The activities of
superoxidase dismutase (D) and myeloperoxidase (E) in rat serum in
each group. Bars represent the means ± SD, n=3. **P<0.01 vs. the
I/R group. I/R, ischemia reperfusion; Pare, parecoxib; MDA,
malondialdehyde, NO, nitric oxide; TAC, total antioxidant capacity;
SOD, superoxidase dismutase; MPO, myeloperoxidase; Ctrl,
control. |
Next, the protective effect of parecoxib sodium
against oxidative stress in small intestinal tissues was examined.
As revealed in Fig. 2A and B,
parecoxib sodium re-treatment significantly suppressed I/R-induced
MDA and NO intestinal levels, and restored them close to the
control levels. Moreover, parecoxib sodium significantly enhanced
TAC intestinal levels and restored SOD activity (Fig. 2C and D). It was also revealed that
parecoxib sodium inhibited I/R-induced MPO activity to a level that
was close to that of the control (Fig.
2E). These results from intestinal tissues were consistent with
corresponding results from serum samples revealed in Fig. 1.
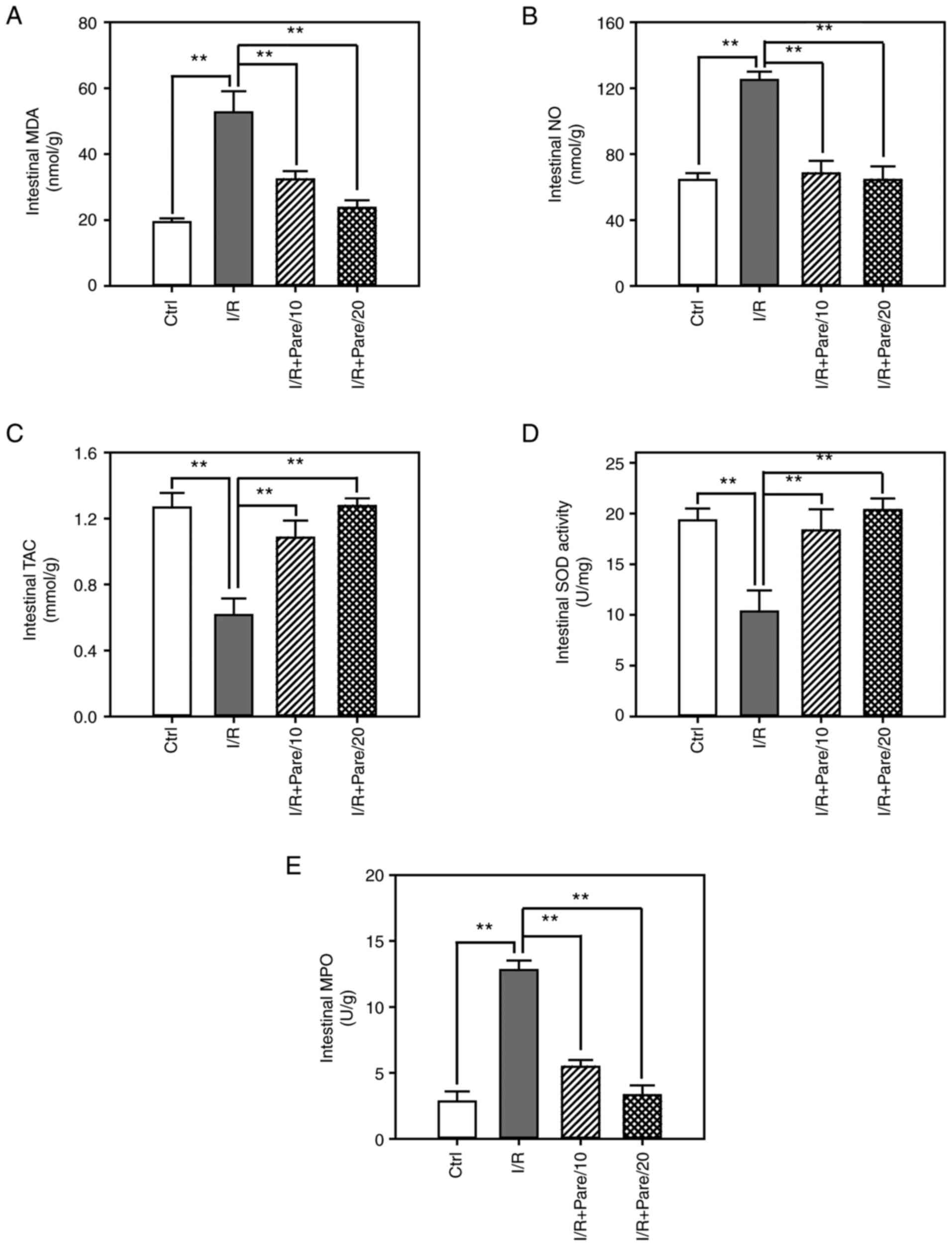 | Figure 2.Assessment of the oxidative stress
after I/R with/without parecoxib sodium pre-treatment in rat small
intestinal tissues. (A-C) The contents of malondialdehyde (A)
nitric oxide (B) and total antioxidant capacity (C) in the small
intestine of rats in control, I/R, I/R + Pare/10 and I/R + Pare/20
groups. (D and E) The activities of superoxidase dismutase (D) and
malondialdehyde (E) in rat small intestine in each group. Bars
represent the means ± SD, n=3. **P<0.01 vs. the I/R group. I/R,
ischemia reperfusion; Pare, parecoxib; MDA, malondialdehyde, NO,
nitric oxide; TAC, total antioxidant capacity; SOD, superoxidase
dismutase; MPO, myeloperoxidase; Ctrl, control. |
Parecoxib sodium pre-administration
decreases the inflammatory responses both in the serum and small
intestinal tissues
To evaluate the inhibitory effect of parecoxib
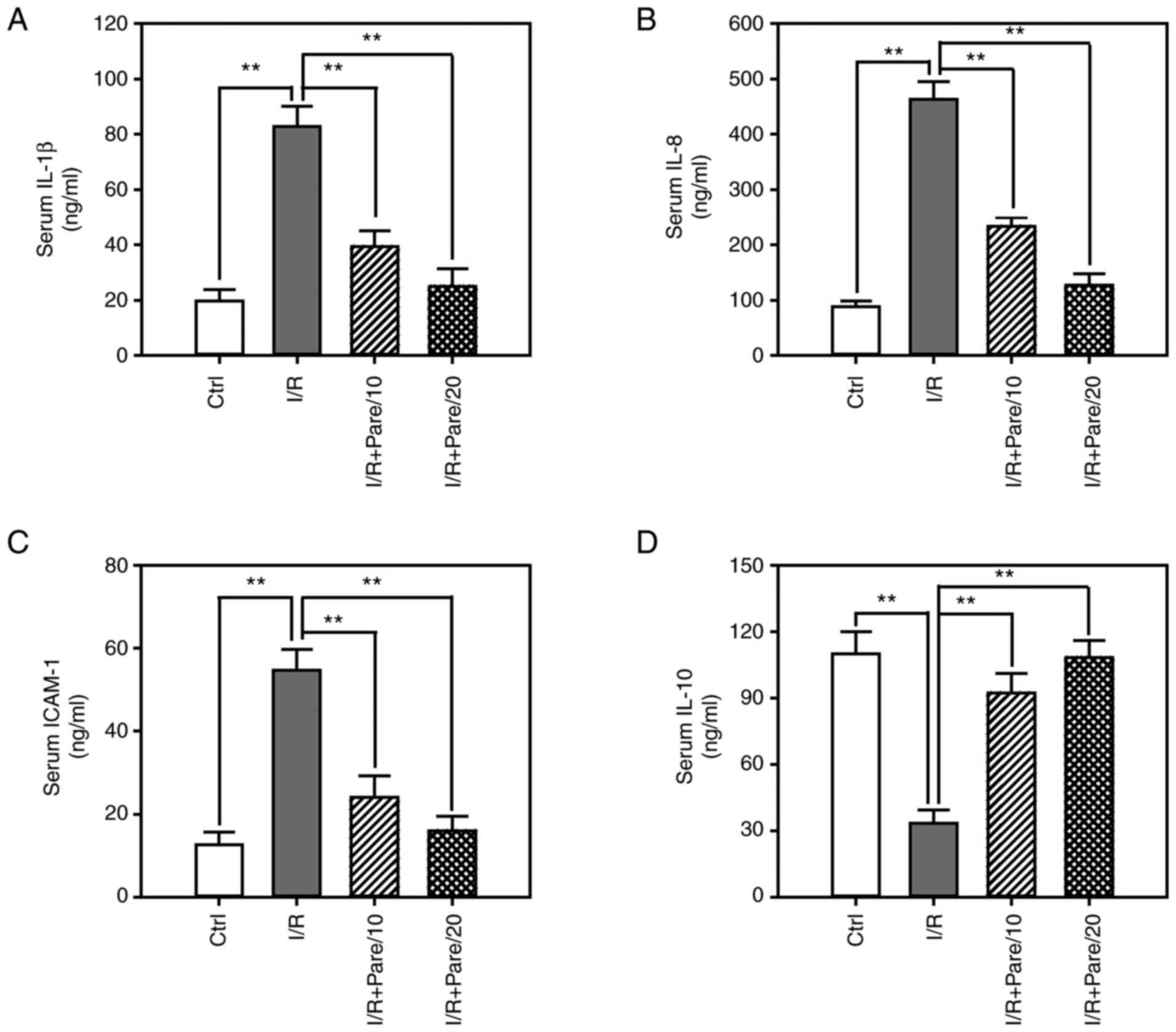
sodium on the inflammatory responses, the serum levels of
proinflammatory cytokines, including IL-1β (Fig. 3A), IL-8 (Fig. 3B) and ICAM-1 (Fig. 3C), as well as the serum level of the
anti-inflammatory cytokine IL-10 (Fig.
3D), were assessed. ELISA results demonstrated that the serum
levels of IL-1β, IL-8 and ICAM-1 were significantly increased by
4–5-fold after I/R injury, while pre-treatment with parecoxib
sodium could significantly suppress IL-1β, IL-8 and ICAM-1 levels
by 50–75% (Fig. 3A-C). Conversely,
I/R decreased IL-10 serum levels by 70%, while parecoxib sodium
exerted an inhibitory effect on I/R-induced IL-10 downregulation
(Fig. 3D).
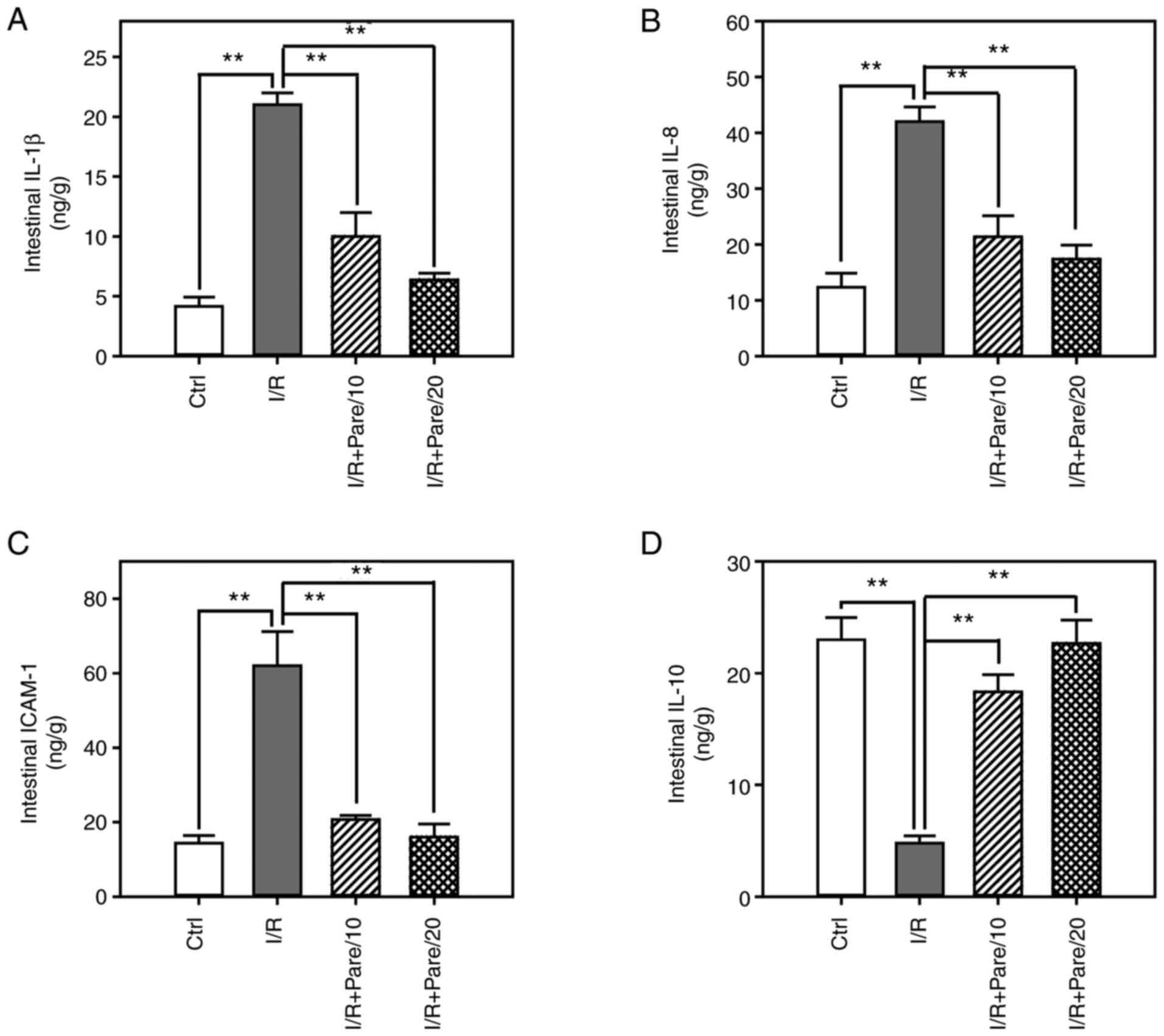
Next, the levels of IL-1β (Fig. 4A), IL-8 (Fig. 4B), ICAM-1 (Fig. 4C) and IL-10 (Fig. 4D) were assessed in small intestinal
tissues post-I/R or I/R + Pare treatment. The results demonstrated
that parecoxib sodium significantly decreased I/R-induced IL-1β,
IL-8 and ICAM-1 levels by 60–75% (Fig.
4A-C). In addition, parecoxib sodium significantly enhanced
IL-10 levels in the small intestine, which had been reduced by I/R
(Fig. 4D).
Parecoxib sodium pre-administration
reduces I/R-induced apoptosis
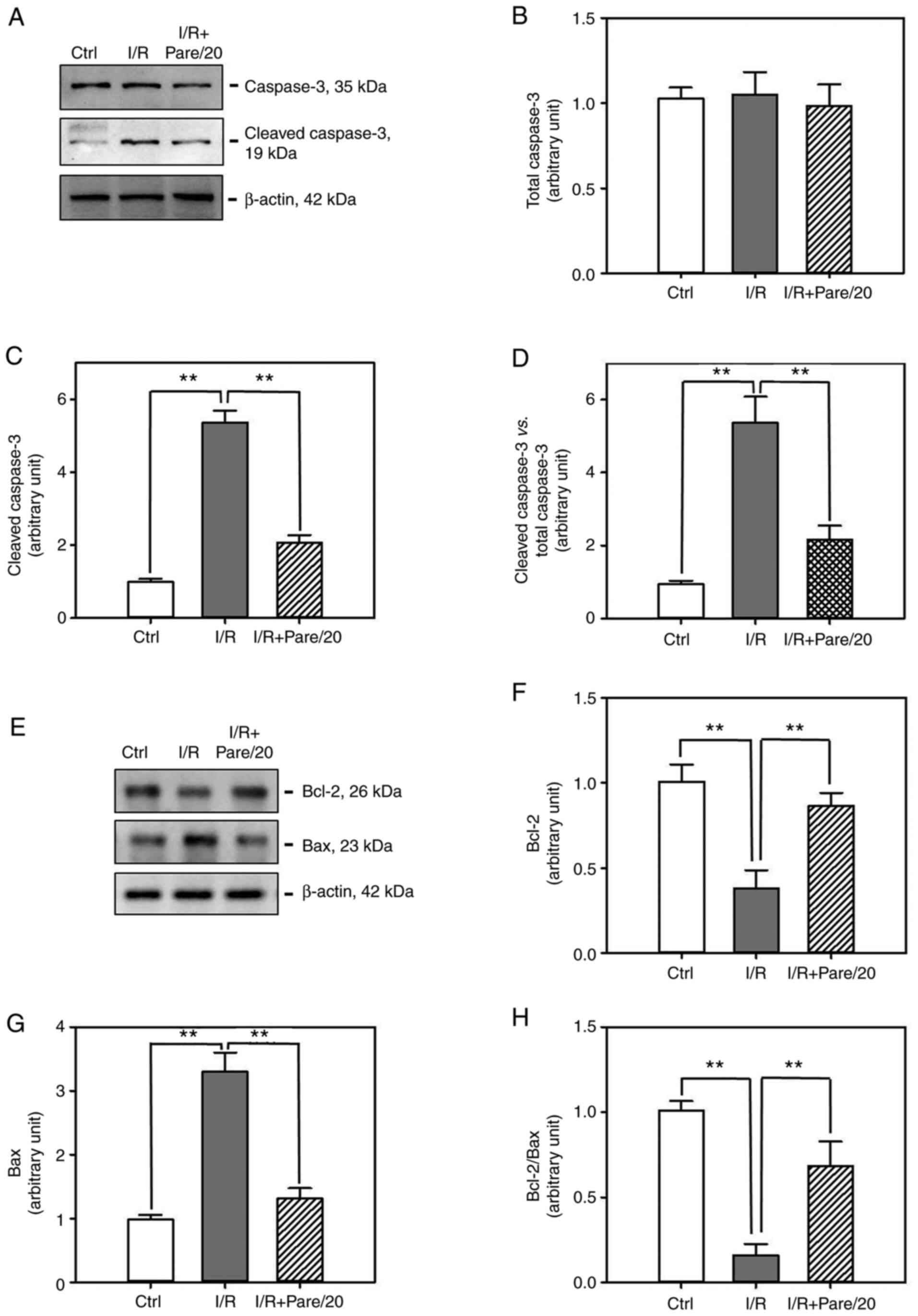
To evaluate the inhibitory effect of parecoxib
sodium on apoptosis, small intestinal tissues subjected to I/R or
I/R + Pare treatment were collected and the protein expression
levels of several apoptosis-related markers were examined,
including the total caspase-3 (Fig.
5A), cleaved caspase-3 (Fig.
5A), Bcl-2 (Fig. 5E) and Bax
(Fig. 5E). Western blot analysis
revealed that, although both I/R and I/R + Pare did not induce an
obvious change on total caspase-3 expression (Fig. 5B), I/R significantly induced the
production of the cleaved caspase-3 (Fig. 5C) and Bax (Fig. 5G) by 5-fold and 3-fold,
respectively. However, pre-treatment of parecoxib sodium
significantly inhibited I/R-induced cleaved caspase-3 activation
(Fig. 5C) and Bax expression
(Fig. 5G). Conversely, parecoxib
sodium could restore I/R-induced Bcl-2 downregulation (Fig. 5F). It is worth noting that the ratio
of cleaved caspase-3/total caspase-3, which indicates the
activation of caspase-3, was significantly decreased in the
parecoxib sodium-pre-treated group after I/R (Fig. 5D). Furthermore, the ratio of
Bcl-2/Bax was recovered in the parecoxib sodium-pre-treated group
after I/R (Fig. 5H). These results
indicated the enhanced apoptosis after I/R, and that parecoxib
sodium could efficiently suppress this effect.
Parecoxib sodium pre-treatment
improves the survival rate of rats after I/R
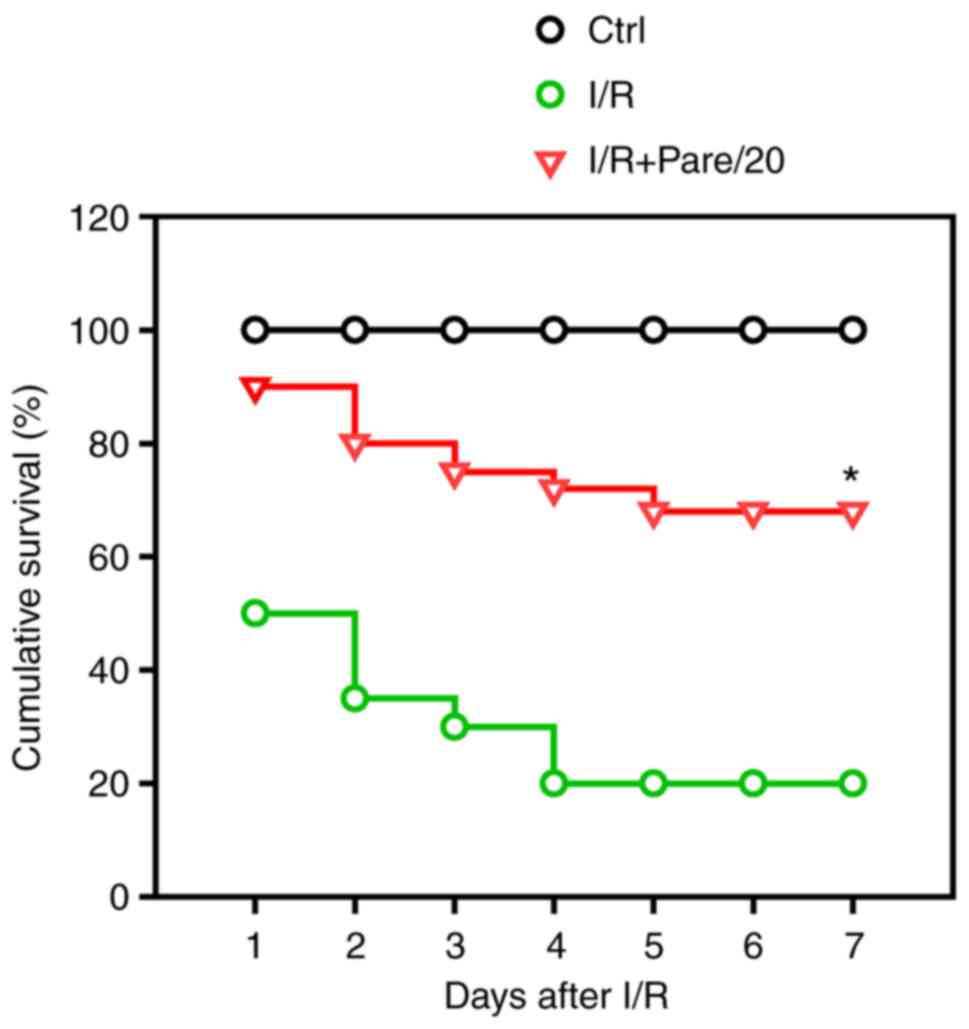
Finally, it was examined whether parecoxib sodium
exerted a protective role on the survival of rats after I/R
treatment. The results demonstrated that ~20% I/R model rats
survived 7 days after intestinal I/R, while parecoxib sodium
significantly improved the survival rate to >65% after I/R
(Fig. 6).
Discussion
The present study systematically examined the
antioxidant activity of parecoxib sodium and investigated its role
in I/R-induced small intestinal injuries. The results indicated
that parecoxib sodium was a potent free radical scavenger, as well
as a lipid peroxidation inhibitor. The pre-administration of
parecoxib sodium significantly ameliorated numerous antioxidative
biomarkers (such as MDA, NO, TAC, SOD and MPO), attenuated the
inflammatory responses (IL-1β, IL-6, ICAM-1 and IL-10), suppressed
intestinal apoptosis (caspase-3, Bcl-2 and Bax) and improved the
survival rate in rats after I/R injury. Thus, the administration of
parecoxib sodium prior to I/R appears to protect rats against
intestinal I/R injury.
I/R is a common pathophysiological process and is
accompanied with changes in the expression of COX in multiple
organs, such as the small intestine (10,22).
COX-1 and COX-2 have both been revealed to contribute to
I/R-induced intestinal injury. In the present study, the protective
role of parecoxib sodium, a widely used and selective COX-2
inhibitor during the perioperative period, in small intestinal I/R
injury was evaluated, and the results demonstrated the essential
role of COX in intestinal I/R injury.
Inflammatory cascades exert a critical role during
I/R-induced tissue damage, and COX-2 is an important inflammatory
mediator. Kamel et al (23)
reported that modafinil significantly downregulated I/R-induced
COX-2 upregulation and attenuated the elevated intestinal TNF-α and
IL-1β levels. Moreover, Duarte et al (24) observed that myeloid COX-2 deletion
led to a transient increase in IL-6 levels after hepatic I/R, while
administration of celecoxib, a selective COX-2 inhibitor,
significantly improved liver function in COX-2−/− mice.
Feng et al (25) also
revealed that pre-operative administration of rofecoxib, another
selective COX-2 inhibitor, decreased the serum levels of TNF-α and
IL-6 in patients, as well as inhibited both systemic and local
inflammation. IL-8 is one of the major inflammatory mediators in
intestinal I/R injury and it exerts deleterious effects on the
intestinal mucosa (26,27). A recent study revealed that when
COX-2 expression was suppressed by Ginkgo biloba, IL-8
levels were also reduced (28).
Accumulating evidence has shown that inflammatory
reactions exert a critical function in I/R-induced intestinal
damage, and inhibition of the inflammatory response efficiently
protects intestinal tissues against injury (29). Parecoxib sodium, as a COX-2
inhibitor, exerts a potent anti-inflammatory effect (30). In the present study, it was revealed
that parecoxib sodium pre-treatment decreased both the serum and
tissue contents of several pro-inflammatory cytokines, such as
IL-1β, IL-8 and ICAM-1, while it increased the level of the
anti-inflammatory cytokine IL-10. IL-1β and IL-8 are mainly
secreted by the activated M1-macrophages that possess various
biological activities, and they act as important inflammatory
mediators during I/R-induced intestinal injury (31). In I/R-induced tissue injury, both
the levels of IL-1β and IL-8 are increased at the early stage,
accompanied with the increased permeability of intestinal
epithelia, neutrophil adhesion and infiltration, and secretion of
other cytokines, which further deteriorate the injured tissue
(23,27). In the present study, the enhanced
ICAM-1 levels in the serum and small intestinal tissues after
intestinal I/R may cause increased neutrophil infiltration, while
inhibition of ICAM-1 by parecoxib sodium may ameliorate tissue
injury. This finding was consistent with a previous study that
reported leukocyte activation, neutrophil adhesion to endothelial
cells and ultimately infiltration into I/R-injured tissues
(32). Thus, these results
indicated that ICAM-1 may serve as a candidate for therapeutic
targeting. Collectively, the aforementioned findings demonstrated
that parecoxib sodium could potently suppress the inflammatory
response induced by I/R injury, which maybe characteristic of its
preventive role in the small intestine.
In addition, recent studies have confirmed the role
of parecoxib sodium in other diseases. For example, parecoxib
sodium was found to inhibit the inflammatory response by inhibiting
COX-2 expression and exhibited protective effects against sepsis in
mice (33). Administration of
parecoxib prior to hepatic I/R was revealed to attenuate hepatic
injury via the inhibition of the inflammatory response and
nitrosative stress (17).
Furthermore, modafinil, a US FDA-approved novel wake-promoting
agent, was revealed to exert a protective effect in intestinal
I/R-induced injury in rats (20).
MDA is the final product resulting from lipid
breakdown and oxidative stress, and it is considered as a good
indicator of free radical-induced lipid peroxidation (34). In the present study, the contents of
MDA were significantly increased both in the serum and small
intestinal tissues from rats after I/R induction, while parecoxib
sodium pre-administration significantly suppressed MDA levels in a
dose-dependent manner. This result indicated that parecoxib sodium
may suppress I/R-induced lipid peroxidation, enhance the
antioxidant defense system, and thus, alleviate the oxidative
injuries in small intestinal tissues.
NO acts as a double-edged sword in organisms. Under
normal physiological conditions, NO is a helpful messenger and
modulator. However, under oxidative stress, NO may be a toxic
metabolite and can be harmful for intestinal mucosa. For example, a
high level of exogenous NO was revealed to increase the degree of
intestinal mucosal injury (35).
Peroxynitrite, an oxidation product from the reaction of NO with
superoxide, can promote the apoptosis of intestinal epithelial
cells via the activation of caspases and the release of
apoptosis-activating factor-1 in mitochondria (36). In the present study, NO levels were
significantly enhanced, cleaved caspase-3 was markedly activated
and the ratio of Bcl-2/Bax was suppressed by I/R, which was
consistent with a previous study (37).
The present findings are important to clarifying the
antioxidant mechanism of parecoxib sodium in detail. There is an
intricate balance between the production of ROS and its consumption
in living organisms, and excessively released ROS will react with
multiple macromolecules, including DNA, lipids and proteins
(38). The body has developed an
antioxidant defense system against the harmful effects of ROS. SOD
is one of most critical enzymes in the cellular antioxidant system
that can remove excessive ROS in living organisms (39). Therefore, modulating the contents of
SOD in the serum or in the tissues may be able to protect against
oxidative stresses. The present results demonstrated that I/R
significantly inhibited SOD activity both in the serum and small
intestinal tissues, while parecoxib sodium significantly restored
the enzymatic activity of SOD in a dose-dependent manner, which may
be responsible for the increased resistance to oxidative stress.
Consistent with a recent study by Wu et al (40), parecoxib sodium reduced the levels
of ROS and lipid peroxidation in myocardial I/R injury rats,
thereby reducing oxidative stress. Similar findings were also
observed in the TAC both in the serum and tissues.
Along with the anti-inflammatory and anti-oxidative
features of parecoxib sodium, the present study also evaluated
whether parecoxib sodium was capable of improving I/R-induced
intestinal apoptosis. Caspase-3, as one of the major signaling
pathways involved in apoptosis, is a critical executing factor
during apoptosis (41). The current
results identified a significant activation of cleaved caspase-3
post-I/R, which was in agreement with a previous study reporting
that activation of caspase-3 was usually increased in intestinal
I/R (41). However, the
pre-treatment of parecoxib sodium effectively decreased cleaved
caspase-3 expression, and thus, inhibited intestinal apoptosis.
In conclusion, to the best of our knowledge, the
present study was the first to systematically examine the
antioxidant activity of parecoxib sodium using an in vivo
I/R rat model and to investigate the protective role against
I/R-induced intestinal injury. The results demonstrated that
parecoxib sodium decreased the inflammatory response, attenuated
oxidative stress, suppressed apoptosis, and thus, increased the
survival rate of rats. These results indicated a potential
protective effect of parecoxib sodium against oxidative tissue
damage, and that it could further improve the antioxidant defense
system. Therefore, parecoxib sodium pre-treatment may be an
effective strategy against I/R-induced intestinal injury in
patients with intestinal obstruction, acute mesenteric ischemia,
trauma, shock, neonatal necrotizing enterocolitis or small
intestine transplantation due to its anti-inflammatory,
anti-oxidative and anti-apoptotic properties. However, studies on
parecoxib sodium-involved signaling mechanism, the upstream and
downstream regulating molecules, cellular homeostasis and other
biological functions are urgently required. Moreover, whether the
molecular findings obtained from animal studies can be applied to
clinical practice needs further exploration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and ZZ conducted the experiments, analyzed the
data, and wrote and revised the manuscript. All authors read and
approved the final submission. ML and ZZ confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
All animal care and experimental procedures were
approved (approval no. 2013/APWC/0361) by the Wenzhou Medical
University Animal Policy and Welfare Committee (Wenzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Ischemia/reperfusion. Compr Physiol. 7:113–170. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mandalaneni K, Rayi A and Jillella DV:
Stroke reperfusion injury. StatPearls [Internet] Treasure Island
(FL): StatPearls Publishing; 2021, https://www.ncbi.nlm.nih.gov/books/NBK564350/
|
|
3
|
Guneli E, Cavdar Z, Islekel H, Sarioglu S,
Erbayraktar S, Kiray M, Sokmen S, Yilmaz O and Gokmen N:
Erythropoietin protects the intestine against ischemia/reperfusion
injury in rats. Mol Med. 13:509–517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tahir M, Arshid S, Fontes B, Castro MS,
Luz IS, Botelho KLR, Sidoli S, Schwämmle V, Roepstorff P and Fontes
W: Analysis of the effect of intestinal ischemia and reperfusion on
the rat neutrophils proteome. Front Mol Biosci. 5:892018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lenaerts K, Ceulemans LJ, Hundscheid IH,
Grootjans J, Dejong CH and Olde Damink SW: New insights in
intestinal ischemia-reperfusion injury: Implications for intestinal
transplantation. Curr Opin Organ Transplant. 18:298–303. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu DM, Sun BW, Sun ZW, Jin Q, Sun Y and
Chen X: Suppression of inflammatory cytokine production and
oxidative stress by CO-releasing molecules-liberated CO in the
small intestine of thermally-injured mice. Acta Pharmacol Sin.
29:838–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farmer DG, Ke B, Shen XD, Kaldas FM, Gao
F, Watson MJ, Busuttil RW and Kupiec-Weglinski JW: Interleukin-13
protects mouse intestine from ischemia and reperfusion injury
through regulation of innate and adaptive immunity.
Transplantation. 91:737–743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arndt H, Kubes P and Granger DN:
Involvement of neutrophils in ischemia-reperfusion injury in the
small intestine. Klin Wochenschr. 69:1056–1060. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ucar BI, Erikci A, Kosemehmetoglu K, Ozkul
C, Iskit AB, Ucar G and Zeren S: Effects of endothelin receptor
blockade and COX inhibition on intestinal I/R injury in a rat
model: Experimental research. Int J Surg. 83:89–97. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tóth Š, Jonecová Z, Čurgali K, Maretta M,
Šoltés J, Švaňa M, Kalpadikis T, Caprnda M, Adamek M, Rodrigo L and
Kruzliak P: Quercetin attenuates the ischemia reperfusion induced
COX-2 and MPO expression in the small intestine mucosa. Biomed
Pharmacother. 95:346–354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith WL: A seven-step plan for becoming a
moderately rich and famous biochemist. J Biol Chem. 294:1779–1793.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh R, Kumar R and Singh DP: Nitric
oxide-releasing nonsteroidal anti-inflammatory drugs:
Gastrointestinal-sparing potential drugs. J Med Food. 12:208–218.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeuchi K and Amagase K: Roles of
cyclooxygenase, prostaglandin E2 and EP receptors in mucosal
protection and ulcer healing in the gastrointestinal tract. Curr
Pharm Des. 24:2002–2011. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu L, Xu C, Huo X, Hao H, Wan Q, Chen H,
Zhang X, Breyer RM, Huang Y, Cao X, et al: The
cyclooxygenase-1/mPGES-1/endothelial prostaglandin EP4 receptor
pathway constrains myocardial ischemia-reperfusion injury. Nat
Commun. 10:18882019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Wang JC, Yang CM, Fan Q, Zheng J
and Liu H: Positive acceleration adaptive training attenuates
gastric ischemia-reperfusion injury through COX-2 and PGE2
expression. Exp Ther Med. 17:2901–2906. 2019.PubMed/NCBI
|
|
16
|
Hamada T, Tsuchihashi S, Avanesyan A,
Duarte S, Moore C, Busuttil RW and Coito AJ: Cyclooxygenase-2
deficiency enhances Th2 immune responses and impairs neutrophil
recruitment in hepatic ischemia/reperfusion injury. J Immunol.
180:1843–1853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang T, Ma Y, Xu KQ and Huang WQ:
Pretreatment of parecoxib attenuates hepatic ischemia/reperfusion
injury in rats. BMC Anesthesiol. 15:1652015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Dai Y, Zhou C and Zhu T: Parecoxib
exhibits anti-inflammatory and neuroprotective effects in a rat
model of transient global cerebral ischemia. J Toxicol Environ
Health A. 83:203–214. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel NS, Cuzzocrea S, Collino M,
Chaterjee PK, Mazzon E, Britti D, Yaqoob MM and Thiemermann C: The
role of cycloxygenase-2 in the rodent kidney following
ischaemia/reperfusion injury in vivo. Eur J Pharmacol. 562:148–154.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamel MY, Ahmed SM and Abdelzaher WY: The
potential protective effect of modafinil in intestinal ischemic
reperfusion-induced in rats. Int Immunopharmacol. 88:1069832020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boivin GP, Hickman DL, Creamer-Hente MA,
Pritchett-Corning KR and Bratcher NA: Review of CO2 as a euthanasia
agent for laboratory rats and mice. J Am Assoc Lab Anim Sci.
56:491–499. 2017.PubMed/NCBI
|
|
22
|
Tong F, Dong B, Chai R, Tong K, Wang Y,
Chen S, Zhou X and Liu D: Simvastatin nanoparticles attenuated
intestinal ischemia/reperfusion injury by downregulating BMP4/COX-2
pathway in rats. Int J Nanomedicine. 12:2477–2488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamel M, Ahmed SM and Abdelzaher W: The
potential protective effect of modafinil in intestinal ischemic
reperfusion-induced in rats. Int Immunopharmacol. 88:1069832020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duarte S, Kato H, Kuriyama N, Suko K,
Ishikawa TO, Busuttil RW, Herschman HR and Coito AJ: Hepatic
ischemia and reperfusion injury in the absence of myeloid
cell-derived COX-2 in mice. PLoS One. 9:e969132014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng Y, Ju H, Yang B and An H: Effects of
a selective cyclooxygenase-2 inhibitor on postoperative
inflammatory reaction and pain after total knee replacement. J
Pain. 9:45–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Öztürk T, Vural K, Tuğlu İ, Var A, Kurdal
T and Aydemir I: Acute and chronic pretreatment with atenolol
attenuates intestinal ischemia and reperfusion injury in
hypercholesterolemic rats. J Cardiothorac Vasc Anesth. 30:985–992.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Habes QLM, Linssen V, Nooijen S, Kiers D,
Gerretsen J, Pickkers P, Scheffer GJ and Kox M: Markers of
intestinal damage and their relation to cytokine levels in cardiac
surgery patients. Shock. 47:709–714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Ye T, Long T and Peng X: Ginkgetin
exerts anti-inflammatory effects on cerebral
ischemia/reperfusion-induced injury in a rat model via the
TLR4/NF-kB signaling pathway. Biosci Biotechnol Biochem.
83:675–683. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vollmar B and Menger MD: Intestinal
ischemia/reperfusion: Microcirculatory pathology and functional
consequences. Langenbecks Arch Surg. 396:13–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stichtenoth DO: The second generation of
COX-2 inhibitors: Clinical pharmacological point of view. Mini Rev
Med Chem. 4:617–624. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tyvold SS, Solligård E, Gunnes S, Lyng O,
Johannisson A, Grønbech JE and Aadahl P: Bronchial microdialysis of
cytokines in the epithelial lining fluid in experimental intestinal
ischemia and reperfusion before onset of manifest lung injury.
Shock. 34:517–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang GJ, Deng HY, Maier CM, Sun GH and
Yenari MA: Mild hypothermia reduces ICAM-1 expression, neutrophil
infiltration and microglia/monocyte accumulation following
experimental stroke. Neuroscience. 114:1081–1090. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun Y, Xu Q, Wu Z, Gong Y and Tang L:
Parecoxib inhibits inflammatory responses in a mouse model of
sepsis. FEBS Open Bio. Apr 3–2020.(Epub ahead of print). View Article : Google Scholar
|
|
34
|
Kimura M, Yokoyama A and Higuchi S:
Aldehyde dehydrogenase-2 as a therapeutic target. Expert Opin Ther
Targets. 23:955–966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wallace JL, Ianaro A and de Nucci G:
Gaseous mediators in gastrointestinal mucosal defense and injury.
Dig Dis Sci. 62:2223–2230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lau A, Arundine M, Sun HS, Jones M and
Tymianski M: Inhibition of caspase-mediated apoptosis by
peroxynitrite in traumatic brain injury. J Neurosci.
26:11540–11553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu W, Fan Z, Han Y, Lu S, Zhang D, Bai X,
Xu W, Li J and Wang H: Curcumin attenuates peroxynitrite-induced
neurotoxicity in spiral ganglion neurons. Neurotoxicology.
32:150–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Borisov VB, Siletsky SA, Nastasi MR and
Forte E: ROS defense systems and terminal oxidases in bacteria.
Antioxidants (Basel). 10:8392021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pérez S, Taléns-Visconti R, Rius-Pérez S,
Finamor I and Sastre J: Redox signaling in the gastrointestinal
tract. Free Radic Biol Med. 104:75–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu F, Wang W, Duan Y, Guo J, Li G and Ma
T: Effect of parecoxib sodium on myocardial ischemia-reperfusion
injury rats. Med Sci Monit. 27:e9282052021.PubMed/NCBI
|
|
41
|
Luo CC, Huang CS, Ming YC, Chu SM and Chao
HC: Calcitonin gene-related peptide downregulates expression of
inducible nitride oxide synthase and caspase-3 after intestinal
ischemia-reperfusion injury in rats. Pediatr Neonatol. 57:474–479.
2016. View Article : Google Scholar : PubMed/NCBI
|