Introduction
Osteoarthritis (OA) is a chronic, age-related
osteoarthropathy characterized by loss of cartilage, remodeling of
subchondral bone, formation of articular marginal osteophytes and
the presence of synovitis (1,2). OA in
joints is a major cause of deformity, pain and disability, which
results in considerable socioeconomic costs worldwide due to its
high incidence (3,4). Degeneration of the cartilage structure
is associated with changes in the extracellular matrix (ECM),
including matrix metalloproteinase-3 (MMP3), collagen and
glycosaminoglycan (GAG), which are all regarded as biological
markers of OA (5). The severity of
OA is correlated with MMP3 accumulation and GAG reduction. OA can
be induced by various physical and biochemical triggers (6,7), with
pathological changes being irreversible at the middle and late
stages of the disease. Therefore, it is important to develop
therapeutic strategies to treat the early stage of the disease
(8).
Autophagy is a metabolic process in which
cytoplasmic organelles and cytosolic components are degraded to
protect cells from stress, and it is a mechanism that is present in
numerous diseases (9–12). The expression levels of UNC-51-like
kinase 1 (ULK1), Beclin1 and microtubule-associated protein 1 light
chain 3 (LC3), which function as the initiator, regulator and
executor of autophagy, respectively, are commonly used as a
biomarker of the autophagic process (13). Autophagy has been considered to
serve as a protective mechanism in maintaining normal cartilage
function, and compromised autophagy has been demonstrated to lead
to OA and numerous other age-related diseases (14–18).
Therefore, targeting autophagy may serve as a promising therapy for
OA.
Mitogen-activated protein kinases (MAPKs) are a
family of Ser/Thr protein kinases thar are widely found in
eukaryotes and include extracellular signal-regulated kinase (ERK),
p38 and c-Jun NH2-terminal kinase (JNK). MAPKs regulate multiple
cellular processes, such as proliferation, differentiation,
survival and apoptosis (19,20).
Previous studies have demonstrated that JNK and p38 contribute to
apoptosis, whereas ERK mediates proliferation in diseases such as
OA, cancer, diabetes and Alzheimer's disease (19,21–23).
However, the role of MAPKs in autophagy varies among diseases
(24–31). Previous studies have also reported
that p38 acts synergistically with autophagy, whereas other studies
have demonstrated that MAPKs attenuate autophagy under certain
conditions (32–34). The effect of MAPKs on autophagy in
the pathogenesis of OA remains unclear.
In our previous study, compromised autophagy was
demonstrated to be closely related to the progression of OA, and
JNK and p38 MAPKs increased MMP3 expression and inhibited autophagy
(18). Selective inhibition of the
MAPK/MMP3 signaling pathway and restoration of impaired autophagy
may serve as a novel therapeutic strategy for OA and other
degenerative disorders.
In the present study, the effects of MAPK inhibitors
on autophagy in a rabbit model of early-stage OA, using
intra-articular injections of U0126 (an ERK inhibitor), SP600125 (a
JNK inhibitor) and SB203580 (a p38 inhibitor), were explored.
Materials and methods
Ethics
All animal experiments were approved by the
Institutional Animal Care and Use Committee of The Second Xiangya
Hospital of Central South University (Changsha, China; approval no.
2018005). All experimental procedures were performed in accordance
with the Animal Research: Reporting of in vivo Experiments
guidelines (35) and the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (36).
Animals
Healthy male New Zealand rabbits (age, 4 months;
weight, 2 kg; purchased from Hunan Pacific Biological Technology
Co., Ltd.) were used in the present study. The animals (n=40) were
randomly assigned into the following five groups: i) the control
group; ii) the OA group; iii) the OA + U0126 group; iv) the OA +
SP600125 group; and v) the OA + SB203580 group. In the control
group, the animals received an articular injection of 0.9% NaCl
(0.1 ml) once a day for 3 consecutive days. In the OA groups, an
articular injection of 0.1 ml 4% papain enzyme was administered
once a day for 3 consecutive days to establish the respective OA
model. For the OA + U0126, OA + SP600125 and OA + SB203580 groups,
at 7 days after the last injection of papain, animals received an
intra-articular injection of 0.1 ml (100 µmol/l) of U0126 (an ERK
inhibitor; Abcam; cat. no. ab120241), SP600125 (a JNK inhibitor;
Abcam; cat. no. ab120065) or SB203580 (a p38 inhibitor; Abcam; cat.
no. ab120162), respectively, once a day for 3 days. All animals
were fed in cages at the Animal Center of Second Xiangya Hospital
with a 12-h light/dark cycle, with free access to water and
standard chow, and were monitored every day. The environmental
temperature was maintained at 20–25°C and the relative humidity was
50±5%. Animals were euthanized at 15 days after the last
intra-articular injection of papain by 20 ml/kg of air embolism
under anesthesia (30 mg/kg of 3% pentobarbital sodium via the ear
vein). Cartilage of the medial condyles of the femur was then
removed for general observation, histological examination and
western blotting. Changes in rabbit weight were calculated before
and after the experiment.
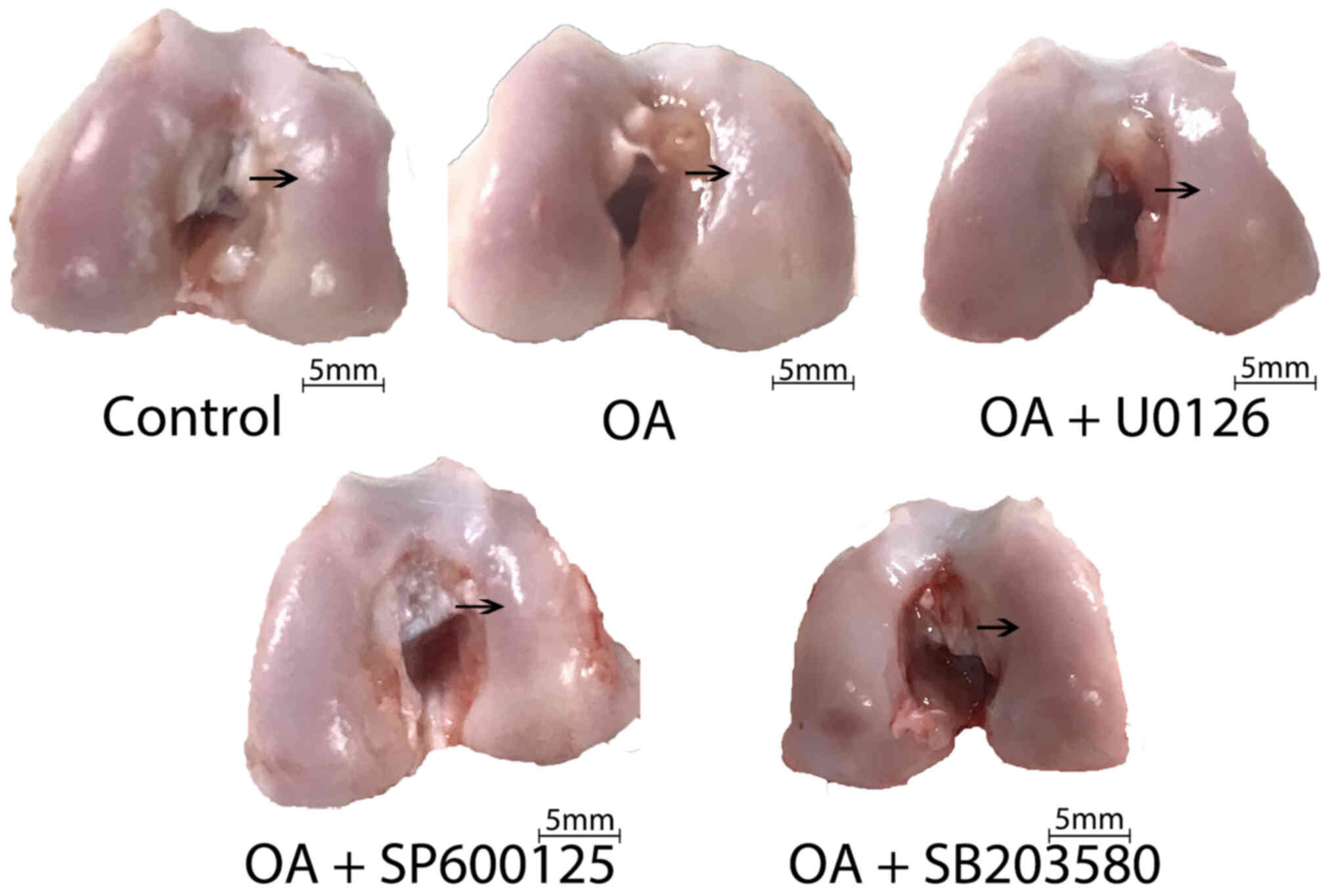
General observation of OA
severity
The distal epiphyses of the femurs were collected
following animal euthanasia and washed in normal saline at room
temperature. For the macroscopic observations, photographs of the
rabbit knees were recorded using a digital camera from the same
distance for each sample, and descriptive methods were used for
examining the thickening of cartilage, cartilage rough surface and
exudation of synovial fluid that were observed by the naked eye. OA
cartilage was characterized by joint swelling, cartilage hyperemia,
thickening of cartilage, a rough cartilage surface and the abundant
exudation of synovial fluid.
Histology
The medial condyles of the femurs were fixed in 4%
paraformaldehyde solution for 24 h and then decalcified in 11% EDTA
disodium buffer. The buffer was replaced every 3 days until the
cartilage was completely decalcified after 15 days. The condyles
were then dehydrated and embedded in paraffin. Frontal serial
sections (thickness, 6 µm) were cut and mounted on polysine
adhesion glass slides. Toluidine blue staining, H&E staining
and safranin-o/fast green staining were performed to evaluate
morphological changes in chondrocytes. Sections were observed using
a light microscope. All the procedures carried out at room
temperature.
To evaluate the pathological changes of early-stage
OA, cartilage was subjected to histological examination of lesion
depth, lesion extent over cartilage surface, matrix staining,
chondrocyte clustering, surface fibrillation and abrasion
quantification of GAG. For semi-quantitative analysis of GAG,
Image-Pro Plus version 6 (Media Cybernetics, Inc.) software was
used to measure the average optical density of the toluidine blue
staining (at wavelength of 255 nm). Five randomly selected fields
of view were analyzed from each slice and the average optical
density of each group was compared. The severity of OA was
evaluated based on the OA Research Society International (OARSI)
scoring system as previously described (35,36).
Western blotting
Total protein was extracted from the medial condyles
in lysis buffer (Cell Signaling Technology, Inc.) and used to
detect the protein expression levels of phosphorylated (p)-ERK,
ERK, p-p38, p38, p-JNK, JNK, MMP3, Beclin1, ULK1 and LC3B
(including LC3-II and LC3-I). Total protein concentration was
quantified using the BCA Kit (Thermo Fisher Scientific, Inc.).
Equal amounts of protein (20 µg) were separated via 10% SDS-PAGE
and transferred onto PVDF membranes via electroblotting. The
membranes were blocked for 1 h at room temperature in 5% BSA
solution (cat. no. 9048-46-8; GENVIEW) in 1 mol/l TBS-Tween 20
[TBST; pH 7.5; 0.1% (v/v) Tween-20]. The membranes were
subsequently incubated overnight at 4°C with the following primary
antibodies: Anti-β-actin (1:5,000; cat. no. ab8227; Abcam),
anti-ERK (1:500; cat. no. bs-0022R; BIOSS), anti-p38 (1:500; cat.
no. bs-0637R; BIOSS), anti-JNK (1:500; cat. no. bs-2900R; BIOSS),
anti-p-ERK (1:500; cat. no. bs-3016R; BIOSS), anti-p-p38 (1:500;
bs-0636R; BIOSS), anti-p-JNK (1:1,000; cat. no. ab124956; Abcam),
anti-ULK1 (1:1,000; cat. no. bs-3602R; BIOSS), anti-LC3B (1:500;
cat. no. NB100-2220; Novus Biologicals, LLC), anti-Beclin1
(1:1,000; cat. no. LS-C117076; LifeSpan Biosciences, Inc.) and
anti-MMP3 (1:500; cat. no. 17873-1-AP; ProteinTech Group, Inc.). To
visualize the bands following the primary incubation, the membranes
were incubated with horseradish peroxidase-conjugated secondary
antibodies (1:6,000; cat. no. SA00001-2; ProteinTech Group, Inc.)
for 1 h at room temperature. Membranes were washed three times, for
10 min each time, in 1 mol/l TBST after incubation with the primary
and secondary antibodies. Protein bands were visualized using
chemiluminescence (Thermo Fisher Scientific, Inc.). Grayscale
densitometry analysis was performed using Image Pro-Plus version 6
software with β-actin as the loading control.
Statistical analysis
GraphPad Prism 7.0 (GraphPad Software, Inc.) was
used to analyze the data. Analysis of three or more independent
groups was performed by one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference. Experiments were repeated ≥3 times and all
animals were included in the analysis to avoid biased parameter
estimates.
Results
General observation of OA
severity
Table I displays the
average body weight of the animals in each group before the study
and at the time of sacrifice. Within each group, the change in body
weight between the two time points was <0.5 kg, which was not
statistically significant. Compared with the healthy cartilage in
the control group, the cartilage in the OA group exhibited joint
swelling, cartilage hyperemia, thickening of cartilage, rough
cartilage surfaces and abundant exudation of synovial fluid. The
severity of OA was markedly reduced in animals receiving an
intra-articular injection of U0126, SP600125 or SB203580 compared
with that in the OA group (Fig.
1).
 | Table I.Changes in rabbit weight before and
after the experiment. |
Table I.
Changes in rabbit weight before and
after the experiment.
| Group (n=8) | Before study,
kg | After sacrifice,
kg |
|---|
| Control | 1.93±0.05 | 2.36±0.08 |
| OA | 1.99±0.05 | 2.38±0.07 |
| OA + U0126 | 2.00±0.06 | 2.44±0.07 |
| OA + SP600125 | 2.01±0.06 | 2.39±0.08 |
| OA + SB203580 | 2.00±0.06 | 2.39±0.03 |
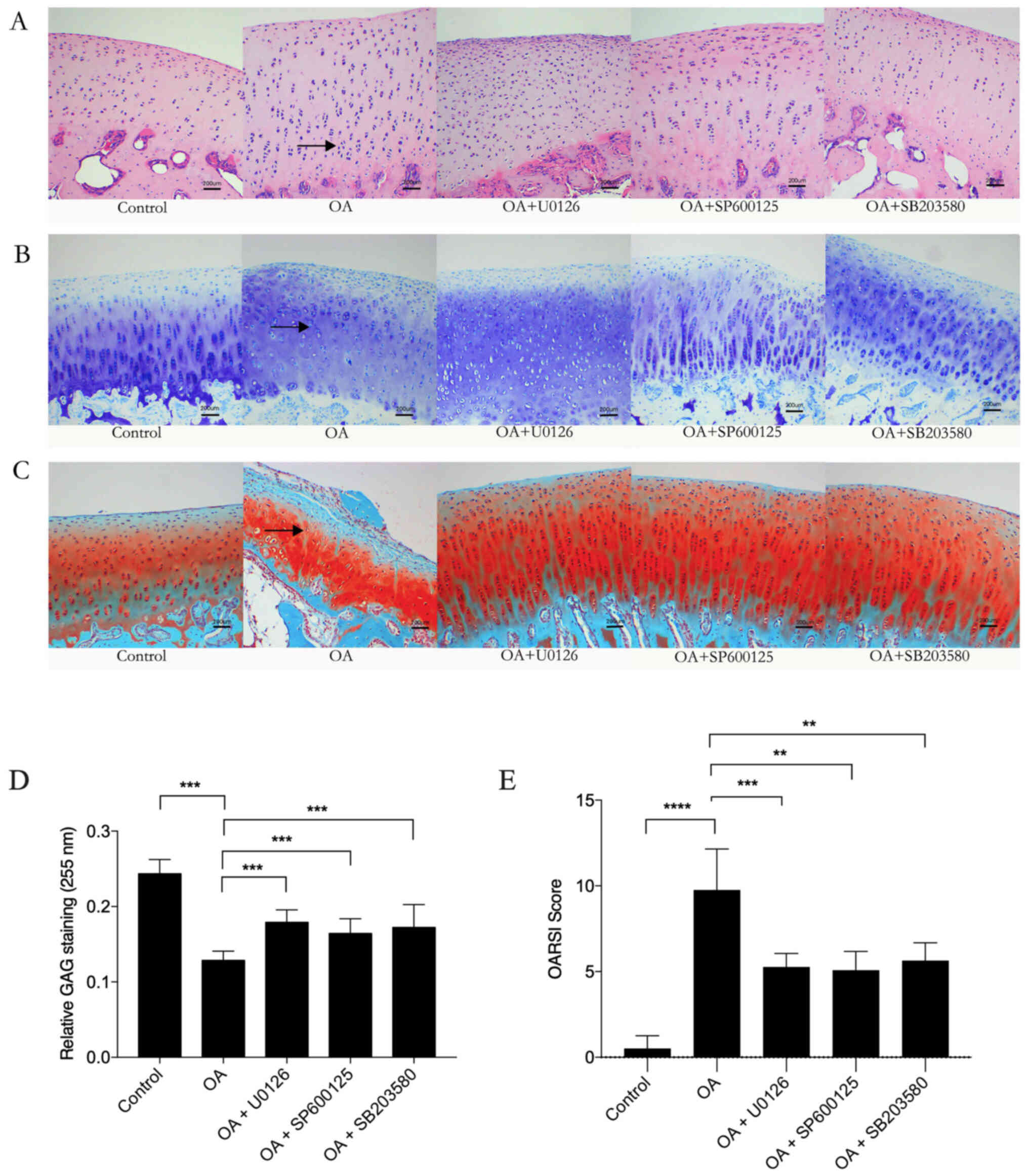
U0126, SP600125 and SB203530
ameliorate OA severity
Histological OA damage was evaluated by H&E,
toluidine blue and safranin-o/fast green staining. Cartilage from
the OA group exhibited pathological changes characterized by
thickening of the cartilage surface, cell disorientation, cell
clustering and uneven matrix distribution compared with the control
group. However, these pathological changes were markedly attenuated
in OA model animals treated with the MAPK inhibitors U0126,
SP600125 and SB203530 (Fig. 2A-C).
Semi-quantitative analysis of GAG was performed based on the
optical density of the toluidine blue stain and was analyzed using
Image-Pro Plus. The GAG staining in the OA group was significantly
lower compared with that in the control group. However, the
OA-induced decreases in GAG staining were significantly inhibited
by U0126, SP600125 and SB203530 treatment (Fig. 2D). The OARSI scoring system was used
to assess the severity and extent of OA based on H&E staining
and safranin-O/fast green staining. The results demonstrated that
U0126, SP600125 and SB203530 treatment significantly ameliorated
the severity of OA compared with that in the OA group (Fig. 2E).
Inhibition of the ERK signaling
pathway by U0126 restores autophagy in OA
Western blotting was used to analyze protein
expression levels in cartilaginous tissues (Fig. 3). p-ERK protein expression levels
were significantly increased in OA animals compared with those in
the control group. Moreover, the OA-induced increase in p-ERK
protein expression levels was significantly reduced by the ERK
inhibitor U0126. However, no statistically significant differences
were observed in the total ERK protein expression levels among the
control, OA and OA + U0126 groups. MMP3 protein expression levels
were significantly increased in the OA group compared with those in
the control group. Administration of U0126 significantly suppressed
MMP3 protein expression levels compared with those in the OA group,
confirming that U0126 had a protective effect against OA
pathogenesis. The cartilaginous tissue of OA model rabbits had
significantly reduced LC3II/I protein expression levels compared
with those in the control group, which suggested that autophagy was
compromised in OA. However, U0126 rescued the impaired autophagy
process in OA, as demonstrated by the significantly increased
LC3II/I protein expression levels in the OA + U0126 group compared
with those in the OA group. U0126 treatment also significantly
increased the expression of ULK1 and Beclin1 in the OA group
compared with that in the control group. Overall, these results
suggested that selectively blocking the ERK/MMP3 signaling pathway
may protect against compromised autophagy induced by OA.
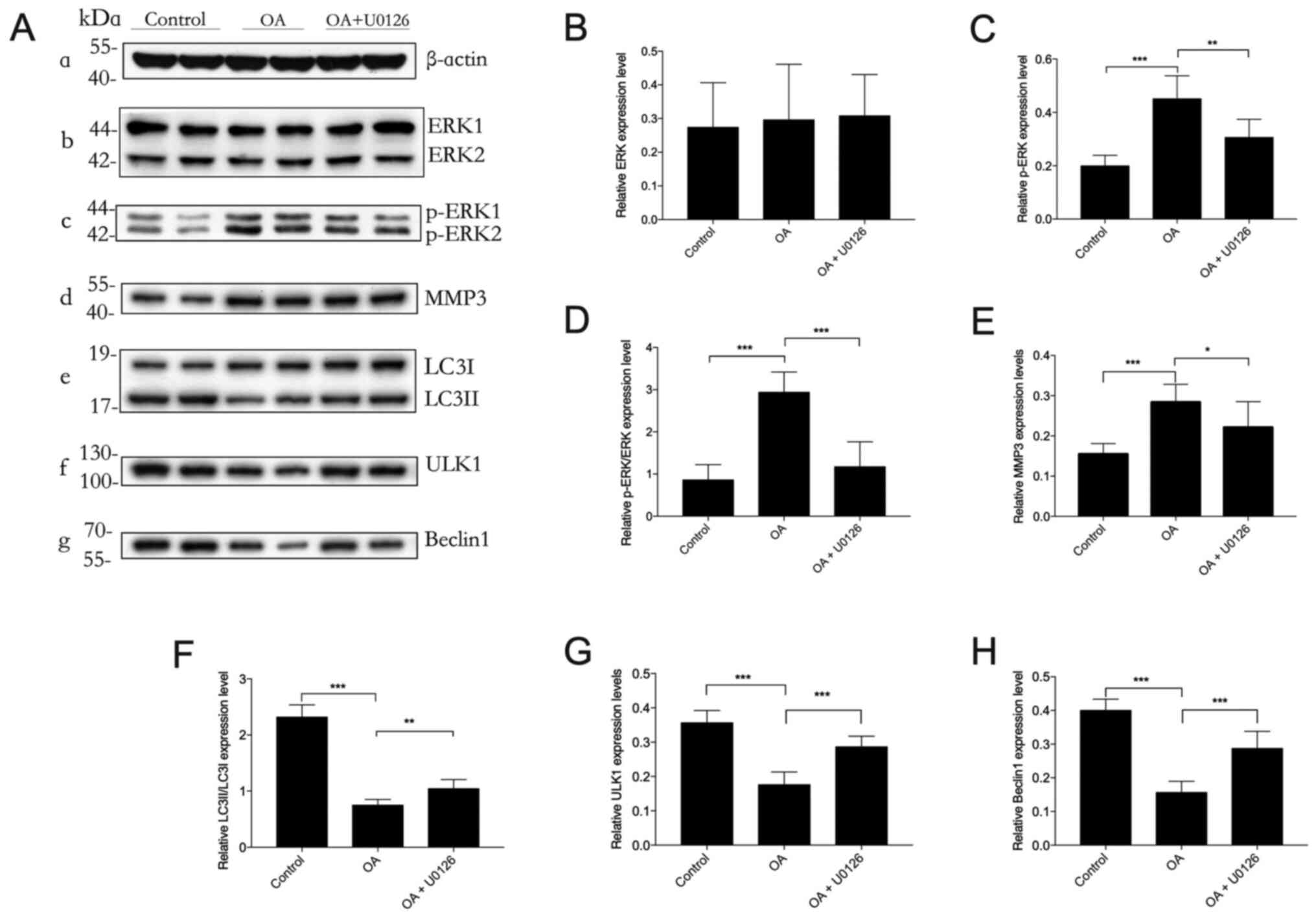 | Figure 3.Effect of U0126 on autophagy in OA.
Western blotting was performed to determine the protein expression
levels (A-a) β-actin (loading control), (A-b) ERK, (A-c) p-ERK,
(A-d) MMP3, (A-e) LC3II/LC3I, (A-f) ULK1 and (A-g) Beclin1 in
cartilage lysates in each experimental group of rabbits. (B-H)
Semi-quantification of band density. p-ERK protein expression
levels were normalized to β-actin. n=3. *P<0.05, **P<0.01,
***P<0.001. OA, osteoarthritis; p, phosphorylated; ULK1,
UNC-51-like kinase 1; MMP3, matrix metalloproteinase-3; LC3,
microtubule-associated protein 1 light chain 3; ERK, extracellular
signal-regulated kinase. |
Inhibition of the JNK signaling
pathway by SP600125 restores autophagy in OA
Western blotting demonstrated that the protein
expression levels of MMP3 and p-JNK were significantly upregulated
in the OA group compared with those in the control group. These
upregulated protein expression levels were significantly reduced by
SP600125 treatment (Fig. 4). The
significant downregulation of the protein expression levels of
autophagy markers LC3II/I, ULK1 and Beclin1 in the OA group,
compared with those in the control group, were significantly
attenuated by SP600125 treatment. These results suggested that the
protective effect of the JNK inhibitor SP600125 against OA may be
mediated via restoration of impaired autophagy in OA.
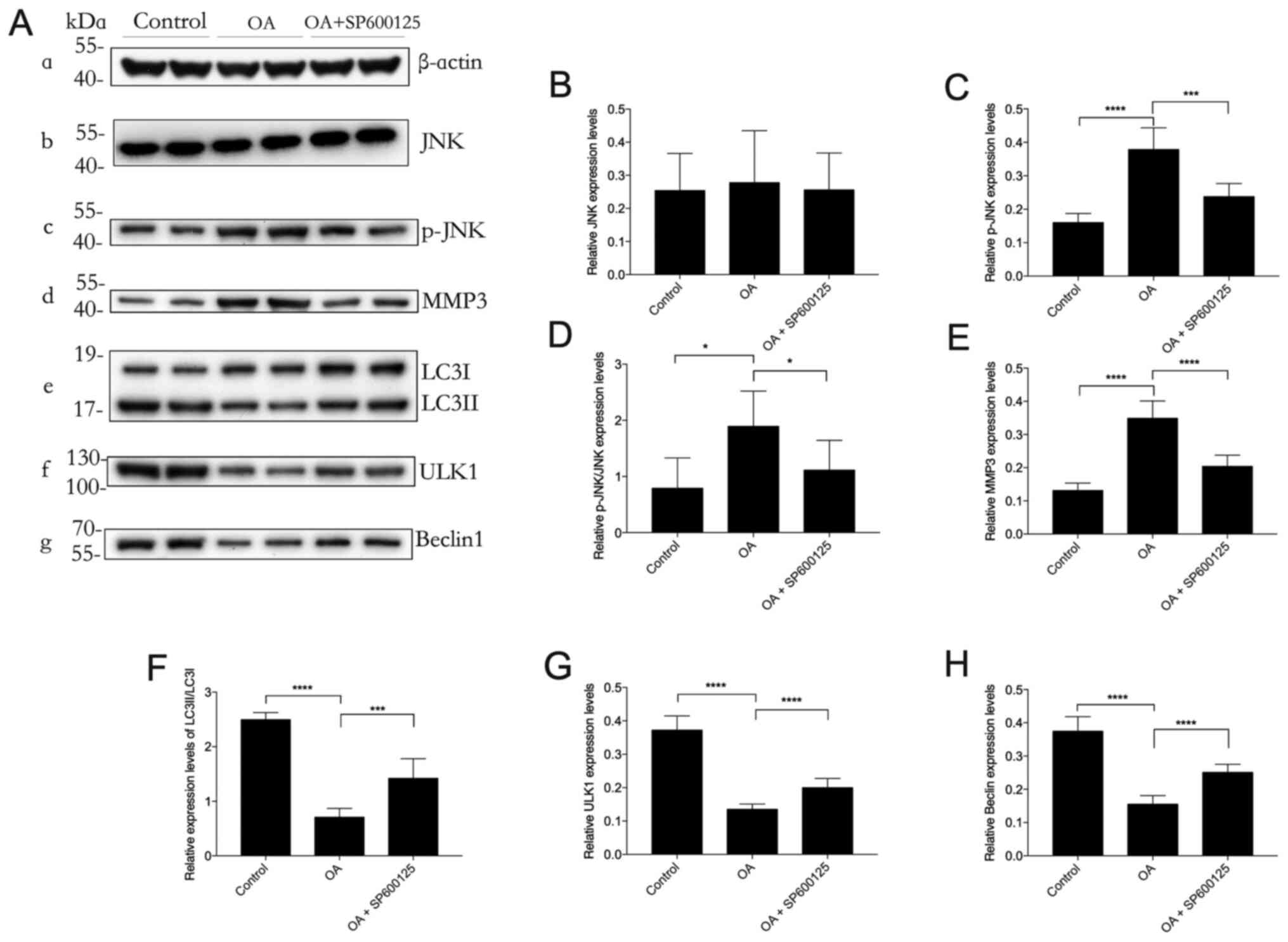 | Figure 4.Effect of SP600125 on autophagy in
OA. Western blotting was performed to determine the protein
expression levels (A-a) β-actin (loading control), (A-b) JNK, (A-c)
p-JNK, (A-d) MMP3, (A-e) LC3II/LC3I, (A-f) ULK1 and (A-g) Beclin1
in cartilage lysates in each experimental group of rabbits. (B-H)
Semi-quantification of band density. p-JNK protein expression
levels were normalized to β-actin. n=3. *P<0.05,
***P<0.001 and ****P<0.0001. OA, osteoarthritis;
p, phosphorylated; ULK1, UNC-51-like kinase 1; JNK, Jun
NH2-terminal kinase; MMP3, matrix metalloproteinase-3; LC3,
microtubule-associated protein 1 light chain 3. |
Inhibition of the p38 signaling
pathway by SB203580 restores autophagy in OA
Western blotting demonstrated that the protein
expression levels of MMP3 and p-JNK were significantly increased in
the OA group compared with those in the control group. This effect
was significantly decreased by SB203580 treatment (Fig. 5). The protein expression levels of
LC3II/I, ULK1 and Beclin1 were significantly downregulated in the
OA group compared with those in the control group, which was
significantly reduced by SB203580 treatment. These results
demonstrated that the selective p38 inhibitor SB203580 potentially
protected against OA by positively regulating autophagy in
cartilaginous tissues with OA.
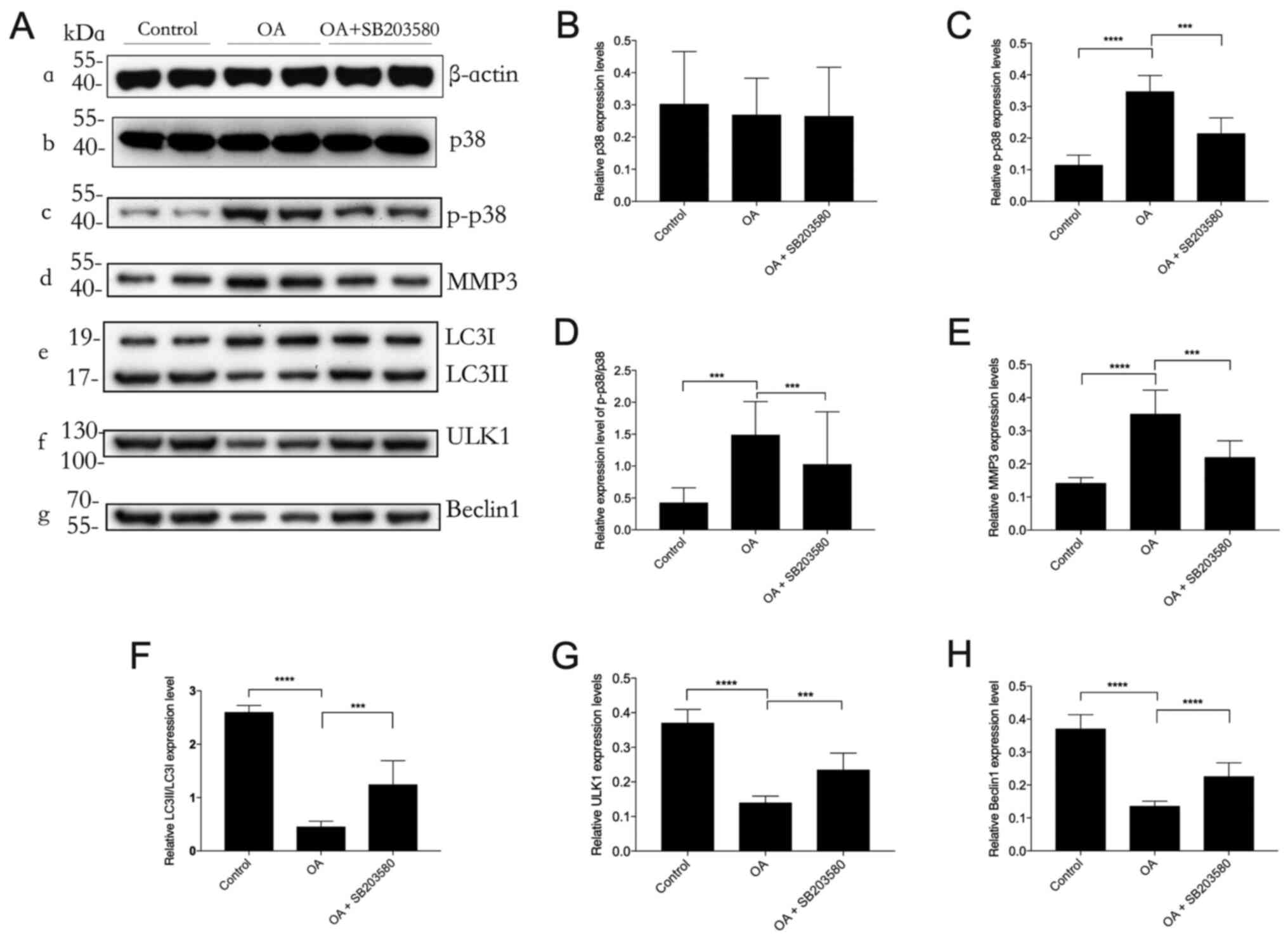 | Figure 5.Effect of SB203580 on autophagy in
OA. Western blotting was performed to determine the protein
expression levels (A-a) β-actin (loading control), (A-b) p38, (A-c)
p-p38, (A-d) MMP3, (A-e) LC3II/LC3I, (A-f) ULK1 and (A-g) Beclin1
in cartilage lysates in each experimental group of rabbits. (B-H)
Semi-quantification of band density. p-p38 protein expression
levels were normalized to β-actin. n=3. ***P<0.001 and
****P<0.0001. OA, osteoarthritis; p, phosphorylated; ULK1,
UNC-51-like kinase 1; MMP3, matrix metalloproteinase-3; LC3,
microtubule-associated protein 1 light chain 3. |
Discussion
OA is highly prevalent and has become a leading
cause of disability (37). The
healthcare resources and costs associated with managing OA can be
substantial. Early intervention with the purpose of preventing
disease onset and minimizing its progression is of vital importance
(4,6). In the present study, a rabbit model of
early-stage OA was established via intra-articular injection of
papain, and papain is a canonical reagent for inducing OA model
(38,39). The current results indicated that
early-stage OA could be characterized by joint swelling, cartilage
hyperemia, cartilage thickening, rough cartilage surfaces and
abundant exudation of synovial fluid. Although cartilage lesions
could be easily identified in the early stages, we found there were
no obvious symptoms or behavioral changes observed in the rabbits.
The successful establishment of the OA model was further confirmed
by subsequent histopathological assessment and the presence of
chondrocyte proliferation, cell disorientation, loss of ECM,
cartilage fibrillation and fissure formation. The OARSI scoring
system was first proposed by Pritzker et al (40) in 2006 and is a useful methodology
for assessing histopathological changes in OA cartilage. In
contrast to the Mankin grading system (41), which primarily reflects the late
stages of OA, the OARSI system, which is based on six grades
reflecting the depth of the lesion and four stages reflecting the
extent of OA over the joint surface, can identify differences in
early or mild OA. An OARSI score <12 is considered to indicate
early-stage OA. In 2016, a new OARSI grading system was established
that reflects the differences in cartilage stiffness between OARSI
grade 0 (intact surface and no signs of degeneration) and grade 1
(intact surface and early signs of arthritis) (42). Based on this new scoring system and
our previous findings (18), the
OARSI score in the rabbit OA model on the 15th day after papain
injection was determined to be ~12. Therefore, in the present
study, rabbits were euthanized and cartilage was obtained at this
time point for all experiments. The results demonstrated that
early-stage OA was successfully established in rabbits. Moreover,
the administration of MAPK pathway inhibitors (U0126, SP600125, or
SB203580) significantly ameliorated OA-induced injury, as
demonstrated by significantly diminished OARSI scores and
significantly increased GAG levels.
Autophagy is an evolutionarily conserved catabolic
process required for cellular homeostasis that determines cell
viability in response to stress. Autophagic systems in mammals are
broadly categorized into three types: Macroautophagy,
microautophagy and chaperone-mediated autophagy (43). Macroautophagy is the most common and
best characterized form of autophagy in mammalian cells (9–12). The
process of autophagy initiates with the formation of the
phagophore. Under stressful conditions, mTOR is inactivated, which
consequently facilitates the formation of the ULK complex, namely
ULK1-FAK family-interacting protein of 200 kDa-autophagy-related
protein (Atg) 13. The ULK complex subsequently recruits other
autophagy-related proteins, and Beclin1 actively forms a complex
with vacuolar protein sorting (VPS) 34 and the associated protein
VPS15 to induce the formation of a phagophore. The elongation of
the phagophore is facilitated by two ubiquitin conjugation systems:
i) The Atg12-Atg5-Atg16 complex; and ii) the
phosphatidylethanolamine (PE)-conjugated LC3II system. LC3I is
formed by the cleavage of pro-LC3 at the C-terminus by Atg4 and is
subsequently conjugated to PE with the help of Atg7 and Atg3 to
form LC3II (9–12). LC3II binds to the autophagosome
membrane and enables its fusion with the lysosome to form
autophagolysosomes that subsequently degrade the accumulated
cytosolic contents. In this way, autophagy serves an important role
in cellular homeostasis and has been considered a protective
mechanism in maintaining the function of cartilage (14–18).
The present study demonstrated that the inhibition of ERK, JNK and
p38 resulted in a significant increase in the protein expression
levels of ULK1, Beclin1 and LC3II/I in OA model rabbits, which
suggested that the inhibition of MAPKs exerted a protective effect
against OA by restoring impaired autophagy in OA.
Numerous signaling pathways and kinases regulate
autophagy, including Atg9, BCL-2, the forkhead box O family of
transcription factors, AMP-activated protein kinase, Akt, protein
kinase C and the MAPKs (ERK, p38 and JNK) (9,30). The
MAPK cascades are intracellular transduction pathways that respond
to various extracellular stimuli and control multiple fundamental
cellular processes, including growth, proliferation,
differentiation, motility, stress responses and cell death
(20). MAPKs not only serve an
important role in regulating cell death by apoptosis but have also
been implicated in autophagy (30).
Hence, the MAPK signaling pathway has become an area of high
interest in the field of autophagy research in recent years.
However, the effect of MAPKs on autophagy varies among different
diseases (24–31). Our previous study of early-stage OA
demonstrated that with the development of OA, the key markers of
the JNK and p38 MAPK pathways were elevated and the biomarkers of
autophagy were decreased, suggesting that JNK and p38 may
negatively regulate the autophagic process but positively regulate
MMP3 in OA (18). These findings
are shown in the current study as well. ERK was also included in
the present study in order to fully elucidate MAPK pathways in
regulating autophagy in early-stage OA. It was found that p-ERK1/2
was also increased in OA, while the ERK inhibitor, similar to JNK
and p38 inhibitors, enhanced the autophagic process in OA.
To the best of our knowledge, the present study was
the first to investigate the effect of MAPK signaling on autophagy
in early-stage OA via intra-articular injection of specific MAPK
inhibitors in rabbits. The results indicated that selective
inhibition of the ERK, JNK, or p38 MAPK signaling pathways
significantly increased the protein expression levels of autophagy
biomarkers (Beclin1, ULK1 and LC3II/I) in OA model rabbits.
Overall, these results suggested that the inhibition of MAPKs may
restore impaired autophagy in OA. Therefore, MAPK inhibitors
exerted a protective effect against OA by restoring impaired
autophagy, suggesting a potential therapeutic strategy for OA, as
well as other degenerative disorders.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding authors on
reasonable request.
Authors' contributions
ZJY and CNL designed the study, CNL and JS conducted
the experiments and JS and WJC analyzed the data. ZJY and WJC
interpreted the results of the experiments. CNL generated the
figures and drafted the manuscript and ZJY and JS edited and
revised the manuscript. All authors reviewed and approved the final
version of the manuscript. ZJY and JS confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of The Second Xiangya
Hospital of Central South University (Changsha, China; approval no.
2018005). All experimental procedures were performed in accordance
with the Animal Research: Reporting of in vivo Experiments
guidelines and the National Institutes of Health Guide for the Care
and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MAPK
|
mitogen-activated protein kinase
|
|
OA
|
osteoarthritis
|
|
MMP3
|
matrix metalloproteinase-3
|
|
GAG
|
glycosaminoglycan
|
|
ULK1
|
UNC-51-like kinase 1
|
|
LC3
|
microtubule-associated protein 1 light
chain 3
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JNK
|
Jun NH2-terminal kinase
|
|
OARSI
|
Osteoarthritis Research Society
International
|
References
|
1
|
Mathiessen A and Conaghan PG: Synovitis in
osteoarthritis: Current understanding with therapeutic
implications. Arthritis Res Ther. 19:182017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vila S: Inflammation in osteoarthritis. P
R Health Sci J. 36:123–129. 2017.PubMed/NCBI
|
|
3
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vina ER and Kwoh CK: Epidemiology of
osteoarthritis: Literature update. Curr Opin Rheumatol. 30:160–167.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saberi Hosnijeh F, Bierma-Zeinstra SM and
Bay-Jensen AC: Osteoarthritis year in review 2018: Biomarkers
(biochemical markers). Osteoarthritis Cartilage. 27:412–423. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hunt MA, Charlton JM and Esculier JF:
Osteoarthritis year in review 2019: Mechanics. Osteoarthritis
Cartilage. 28:267–274. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanwar JR, Samarasinghe RM, Kumar K, Arya
R, Sharma S, Zhou SF, Sasidharan S and Kanwar RK: Cissus
quadrangularis inhibits IL-1beta induced inflammatory responses on
chondrocytes and alleviates bone deterioration in osteotomized rats
via p38 MAPK signaling. Drug Des Devel Ther. 9:2927–2940. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bay-Jensen AC, Hoegh-Madsen S, Dam E,
Henriksen K, Sondergaard BC, Pastoureau P, Qvist P and Karsdal MA:
Which elements are involved in reversible and irreversible
cartilage degradation in osteoarthritis? Rheumatol Int. 30:435–442.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saha S, Panigrahi DP, Patil S and Bhutia
SK: Autophagy in health and disease: A comprehensive review. Biomed
Pharmacother. 104:485–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lapaquette P, Guzzo J, Bretillon L and
Bringer MA: Cellular and molecular connections between autophagy
and inflammation. Mediators Inflamm. 2015:3984832015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deretic V, Saitoh T and Akira S: Autophagy
in infection, inflammation and immunity. Nat Rev Immunol.
13:722–737. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levine B and Kroemer G: Biological
functions of autophagy genes: A disease perspective. Cell.
176:11–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeon H and Im GI: Autophagy in
osteoarthritis. Connect Tissue Res. 58:497–508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo P, Gao F, Niu D, Sun X, Song Q, Guo C,
Liang Y and Sun W: The role of autophagy in chondrocyte metabolism
and osteoarthritis: A comprehensive research review. Biomed Res
Int. 2019:51716022019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carames B, Taniguchi N, Otsuki S, Blanco
FJ and Lotz M: Autophagy is a protective mechanism in normal
cartilage, and its aging-related loss is linked with cell death and
osteoarthritis. Arthritis Rheum. 62:791–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Filfan M, Sandu RE, Zavaleanu AD, GresiTa
A, Glavan DG, Olaru DG and Popa-Wagner A: Autophagy in aging and
disease. Rom J Morphol Embryol. 58:27–31. 2017.PubMed/NCBI
|
|
18
|
Shi J, Zhang C, Yi Z and Lan C: Explore
the variation of MMP3, JNK, p38 MAPKs, and autophagy at the early
stage of osteoarthritis. IUBMB Life. 68:293–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI
|
|
20
|
Plotnikov A, Zehorai E, Procaccia S and
Seger R: The MAPK cascades: Signaling components, nuclear roles and
mechanisms of nuclear translocation. Biochim Biophys Acta.
1813:1619–1633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu K, Ma C, Xu L, Ran J, Jiang L, He Y,
Adel Abdo Moqbel S, Wang Z and Wu L: Polygalacic acid inhibits MMPs
expression and osteoarthritis via Wnt/beta-catenin and MAPK signal
pathways suppression. Int Immunopharmacol. 63:246–252. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mei S, Gu H, Ward A, Yang X, Guo H, He K,
Liu Z and Cao W: p38 mitogen-activated protein kinase (MAPK)
promotes cholesterol ester accumulation in macrophages through
inhibition of macroautophagy. J Biol Chem. 287:11761–11768. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen T, Miao Y, Ding C, Fan W, Liu S, Lv
Y, Gao X, De Boevre M, Yan L, Okoth S, et al: Activation of the
p38/MAPK pathway regulates autophagy in response to the
CYPOR-dependent oxidative stress induced by zearalenone in porcine
intestinal epithelial cells. Food Chem Toxicol. 131:1105272019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He Y, She H, Zhang T, Xu H, Cheng L, Yepes
M, Zhao Y and Mao Z: p38 MAPK inhibits autophagy and promotes
microglial inflammatory responses by phosphorylating ULK1. J Cell
Biol. 217:315–328. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo T, Zhang H, Yu Q, Liu G, Long M, Zhang
K, Liu W, Song R, Bian J, Gu J, et al: ERK1/2 MAPK promotes
autophagy to suppress ER stress-mediated apoptosis induced by
cadmium in rat proximal tubular cells. Toxicol In Vitro. 52:60–69.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barutcu SA, Girnius N, Vernia S and Davis
RJ: Role of the MAPK/cJun NH2-terminal kinase signaling pathway in
starvation-induced autophagy. Autophagy. 14:1586–1595. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan H, Gao Y and Zhang Y: Inhibition of
JNK suppresses autophagy and attenuates insulin resistance in a rat
model of nonalcoholic fatty liver disease. Mol Med Rep. 15:180–186.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He Q, Mei D, Sha S, Fan S, Wang L and Dong
M: ERK-dependent mTOR pathway is involved in berberine-induced
autophagy in hepatic steatosis. J Mol Endocrinol. 59:X12017.Erratum
for: J Mol Endocrinol 57: 251–260, 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sridharan S, Jain K and Basu A: Regulation
of autophagy by kinases. Cancers (Basel). 3:2630–2654. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Webber JL and Tooze SA: Coordinated
regulation of autophagy by p38alpha MAPK through mAtg9 and p38IP.
EMBO J. 29:27–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsuzawa T, Fujiwara E and Washi Y:
Autophagy activation by interferon-γ via the p38 mitogen-activated
protein kinase signalling pathway is involved in macrophage
bactericidal activity. Immunology. 141:61–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Wang N, Zhang S and Liang Q:
Autophagy protects bone marrow mesenchymal stem cells from
palmitate-induced apoptosis through the ROS-JNK/p38 MAPK signaling
pathways. Mol Med Rep. 18:1485–1494. 2018.PubMed/NCBI
|
|
34
|
Zhu J, Yu W, Liu B, Wang Y, Shao J, Wang
J, Xia K, Liang C, Fang W, Zhou C and Tao H: Escin induces
Caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK
signalling pathway in human osteosarcoma cells in vitro and in
vivo. Cell Death Dis. 8:e31132017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
NC3Rs Reporting Guidelines Working Group,
. Animal research: Reporting in vivo experiments: The ARRIVE
guidelines. J Physiol. 588:2519–2521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals, 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
37
|
Sharma L: Osteoarthritis of the Knee. N
Engl J Med. 384:51–59. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu XB, Kang RR, Tang TT, Li YJ, Wu JY,
Wang JM, Liu XY and Xiang DX: Topical delivery of
3,5,4′-trimethoxy-trans-stilbene-loaded Microemulsion-based
hydrogel for the treatment of osteoarthritis in a rabbit model.
Drug Deliv Transl Res. 9:357–365. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Higashiguchi T and Go K: Effect of
neurotropin on experimental osteoarthritis. Nihon Yakurigaku
Zasshi. 96:153–1561. 1990.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van der Sluijs JA, Geesink RG, van der
Linden AJ, Bulstra SK, Kuyer R and Drukker J: The reliability of
the Mankin score for osteoarthritis. J Orthop Res. 10:58–61. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Waldstein W, Perino G, Gilbert SL, Maher
SA, Windhager R and Boettner F: OARSI osteoarthritis cartilage
histopathology assessment system: A biomechanical evaluation in the
human knee. J Orthop Res. 34:135–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dash S, Aydin Y and Moroz K:
Chaperone-Mediated autophagy in the liver: Good or Bad? Cells.
8:13082019. View Article : Google Scholar : PubMed/NCBI
|