Introduction
Diabetic foot ulcers (DFUs) are the most common
manifestation of diabetic foot disease. Peripheral neuropathy,
lower extremity arterial diseases and foot deformities are the main
reasons for the increased risk of DFU (1). Studies have revealed that diabetic
patients are adversely affected by their high-glucose environment
and oxidative stress response (2), leading to impaired cell phagocytosis
and antibacterial function, higher bacterial loads surrounding
wounds, the release of large amounts of procoagulant substances
into the blood, disorders of local circulation and deteriorated
ischemic and hypoxic symptoms (3), which severely interfere with wound
healing. Therefore, controlling infection and promoting wound
healing are the most important steps in the treatment of DFU
(4).
Negative-pressure wound therapy (NPWT) is a method
that has been widely used for the treatment of various wounds,
including DFU (5). NPWT
facilitates the drainage of wound exudate, provides wounds with a
wet and closed healing environment to isolate external bacteria and
reduce wound infection, increases blood flow to wound margins and
removes healing inhibitors in wound exudate, such as matrix
metalloproteinases and inflammatory factors, to reduce tissue
edema, resulting in the promoted growth of granulation tissue and
accelerated wound healing (6,7).
However, the specific mechanism remains to be elucidated.
Proteomics is a new research field that analyzes the
dynamic changes in the protein composition, expression levels and
modification status in cells from a holistic perspective.
Proteomics is widely used to understand the interactions and
connections between proteins and unravel the protein functions and
physiological activities of cells (8). Therefore, proteomic research can
provide a theoretical basis for the clarification of pathogenesis
and clues regarding solutions for curing diseases, such as diabetes
mellitus (9).
The present study used liquid chromatography tandem
mass spectrometry (LC-MS/MS) combined with label-free
quantification (label-free) to screen and identify differentially
expressed proteins (DEPs) in the granulation tissue of DFU wounds
prior to and following NPWT treatment for one week. A
bioinformatics analysis was performed to discover the key proteins
present in DFU granulation tissue and the signaling pathways
involved in wound healing. It is suggested that the results could
help further reveal the mechanism of NPWT, identify specific
biomarkers related to DFU and provide guidance for the early
diagnosis, monitoring of treatment efficacy and prognostic
evaluation of DFUs.
Materials and methods
Study subjects
In total, three patients with DFU hospitalized in
the Department of Endocrinology at The First Affiliated Hospital of
Anhui Medical University (Hefei, China) between March 2019 and
April 2019 were selected; the patients included two male patients
and one female patient with type 2 diabetes, with an average age of
56.0 years old. The duration of foot ulcers was >6 weeks. The
ulcer area was 6–12 cm2, the Wagner grade was 3
(10), the ankle-brachial ratio
(ABI) was 0.97–1.13, the percutaneous oxygen partial pressure
(TcPO2) was 69.8~76.5 mmHg and the duration of diabetes was 13–18
years. All subjects had no heart, liver or kidney dysfunction and
no noncancerous ulcer wounds. The subjects had no history of foot
ulcers and were not treated with NPWT. No glucocorticoids,
immunosuppressants or exogenous cytokines, such as epidermal growth
factor, erythropoietin, recombinant human granulocyte macrophage
colony-stimulating factor, or recombinant human granulocyte
colony-stimulating factor, were used in the past six months. The
present study was approved by the medical ethics committee of the
First Affiliated Hospital of Anhui Medical University (approval no.
CDEC000004982) and signed informed consent was obtained from the
subjects.
DFU treatment and collection of wound
specimens
All subjects received routine systemic treatment
after admission, including anti-infection, antihypertensive and
hypoglycemic treatment and correction of hypoproteinemia.
Concurrent debridement was performed to remove black necrotizing
soft tissue and bone tissue. A previous study (11) reported applying NPWT using a
VAC® negative-pressure-assisted healing system (Kinetic
Concepts, Inc.) with a negative pressure of 125 mmHg (1 mmHg=0.133
kPa). NPWT was not started until the wound infection could be
effectively controlled after routine systemic treatment. The course
of NPWT was one week. Granulation tissue was collected prior to and
following NPWT for one week and frozen at −80°C for
examination.
Protein extraction and quantitative
quality control
A mammalian tissue Total Protein Extraction kit
(cat. no. AP0601-50; Beijing Bang Fei Biotechnology Co., Ltd.) was
used to extract granulation tissue protein. A standard curve was
prepared from the standard product (0, 1, 2, 4 and 6 g), a 1 µl
sample was mixed with 19 µl water and the total product in 20 µl
solution was added to the detection labeling plate. Each sample was
tested in duplicate. Then, 200 µl Bradford working fluid was added
to each pore and the mixture was lightly pipetted up and down. The
sample was incubated for 10 min at room temperature. When the
purple gradient appeared, the wavelength was set to 595 nm. Total
protein was detected according to the standard curve and the
corresponding concentration was calculated.
SDS-PAGE method
Each 35 µg protein sample was added to 5X sample
buffer at a 5:1 (v/v) ratio, heated in a boiling water bath for 5
min and centrifuged at 14,000 × g at room temperature for 10 min.
The supernatant was collected and 10% SDS-PAGE was carried out at a
constant current of 14 mA for 90 min. Then, 0.25% Coomassie
brilliant blue staining at room temperature for 1 h.
Protein trypsin enzymolysis
procedure
Following protein quantification, 60 µl protein
solution was placed in a centrifuge tube, mixed with 5 µl 1 M DTT
solution, incubated for 1 h at 37°C and then added to 20 µl 1 M IAA
solution. After mixing, the sample was added to an ultrafiltration
tube at room temperature and kept in the dark for 1 h. Following
centrifugation at room temperature at 14,000 × g for 15 min, the
filtrate in the collection tube was discarded. Then, 100 µl UA (8 M
urea, 100 mM Tris-HCl, pH 8.0) was added and the ultrafiltration
tube was centrifuged at room temperature and 14,000 × g for 15 min,
the filtrate was discarded. Finally, 50 mM
NH4HCO3 was added, and the ultrafiltration
tube was centrifugated at room temperature at 14,000 × g for 15
min, the filtrate was discarded again while the supernatant
(concentrated solution) was collected and placed in a new
collection tube. Trypsin was added to the new collection tube at a
protein:enzyme ratio of 50:1 and hydrolysis was performed for 12–16
h at 37°C.
Mass spectrometry analysis
Each fraction was resuspended in buffer A (0.1%
formic acid, FA). The separations were performed using an Ultimate
3000 UPLC system (Thermo Fisher Scientific, Inc.) coupled online to
a Q-Extractive HF mass spectrometer (Thermo Fisher Scientific,
Inc.; the limit of detecting polypeptide signals >100 fg) for 78
min. The peptides were subjected to a C18 trap column (3 µm, 0.1×20
mm; Thermo Fisher Scientific, Inc.) at a flow rate of 0.6 µl/min.
The peptides were desalted online and loaded onto a C18 column (1.9
µm, 150 µm ×120 mm) using a gradient from 6–95% buffer B [0.08% FA
and 80% acetonitrile (ACN)] for 90 min. The mass spectrometer was
operated in the positive mode using a data-dependent acquisition
method. A full MS scan (300–1,400 m/z) was acquired in the Orbitrap
with the resolution set to a value of 120,000. The database
download date was January 2, 2019. These data included 20,412
reviewed and 157,167 unreviewed protein sequences. The proteins
were characterized using Proteome Discoverer 2.2 (Thermo Fisher
Scientific, Inc.). The parameters were as follows: Peptide mass
tolerance of ±10 ppm, fragment mass tolerance of 0.6 Da, number of
allowed maximum missed tryptic cleavage sites of two,
carbamidomethyl as fixed modification and acetyl and oxidation on
methionine as the variable modification. The search results were
then filtered using an False Discovery Rate (FDR) cutoff of 1% for
the peptide false identification rate. The collected data were
analyzed using Protein Pilot Software v. 5 (AB Sciex LLC). The
UniProt database (https://www.uniprot.org/uniprot/?query=homo%20sapiens&fil=organism%3A%22Homo+sapiens+%28Human%29+%5B9606%5D%22&sort=score)
was used to identify each peptide segment. According to the signal
intensity, the protein quality was determined in two repeated
samples. Proteins with a fold change in expression ≥1.2 or <0.7
at P<0.05 were considered differential proteins. Finally, an
enrichment analysis was performed to analyze the DEPs. In addition,
Gene Ontology (GO) analysis (12,13) includes the following three
ontologies: biological processes, cell localization and molecular
function (http://metascape.org). A GO analysis was
conducted using the website to understand the functions of the
different proteins. A Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analysis (14)
based on the KOBAS online analysis database (http://kobas.cbi.pku.edu.cn/) was conducted to analyze
the pathways of the differential proteins. P<0.05 indicated a
statistically significant difference. Through collation of the GO
analysis and KEGG analysis results, it was possible to determine
the possible important proteins by comparison with the
literature.
Western blot analysis and the
detection of activity of MMP2 and MMP9
A total of eight patients included five males and
three females with DFUs, with an average age of 54.3 years old,
were consecutively enrolled from the Department of Endocrinology at
the First Affiliated Hospital of Anhui Medical University.
Granulation tissue samples were collected before and after NPWT for
one week between June 2019 and July 2019. The protocol was approved
by the Ethics Committee of The First Affiliated Hospital of Anhui
Medical University (approval no. LLSC20191038) and all patients
provided signed informed consent to participate. Cathepsin S
(CTSS), peroxiredoxin-2 (PRDX2), protein S isoform 1 (PROS1) and
inter α-trypsin inhibitor heavy chain H4 (ITIH4) proteins were
selected for further validation by western blot analysis. In brief,
frozen granulation tissue of DFU wounds was homogenized on ice and
RIPA lysate containing phenylmethylsulfonyl fluoride was added for
30 min (1 ml RIPA lysate per 100 mg tissue). The mixture was
centrifuged at 12,000 × g and 4°C for 10 min and the supernatants
removed. Loading buffer was added and the sample was boiled for 10
min. After cooling to room temperature, the proteins (30 µg) were
separated by 10% SDS-PAGE, transferred onto PVDF membranes and
blocked with 5% skimmed milk at room temperature for 2 h. CTSS,
PRDX2, PROS1, ITIH4 and fibronectin primary antibodies diluted to
1:200, 1:500, 1:500, 1:500 and 1:1,000 respectively, were added and
the samples were incubated overnight at 4°C. Horseradish peroxidase
(HRP)-labeled secondary antibodies (OriGene Technologies, Inc.)
diluted to 1:5,000, 1:4,000, 1:3,000 and 1:5,000 were added for a 2
h incubation at room temperature for ECL autography, which was
performed the following day. The bands obtained from western
blotting were scanned into images and the gray values of the target
bands were analyzed with ImageJ 1.36b (National Institutes of
Health). β-actin was used as a housekeeping protein and was
determined following the same procedures mentioned above using a
primary antibody at 1:1,000 and a secondary antibody at 1:2,000.
The ratios of the CTSS, PRDX2, PROS1 and ITIH4 proteins to β-actin
in the granulation tissue were used as the relative expression
level of each target gene. The following antibodies were used in
this process: CTSS (cat. no. sc-271619; Santa Cruz Biotechnology,
Inc.), goat anti-mouse IgG (cat. no. ZB-2305; OriGene Technologies,
Inc.), goat anti-rabbit IgG (cat. no. ZB-2301; OriGene
Technologies, Inc.), rabbit anti-goat IgG (cat. no. ZB-2306;
OriGene Technologies, Inc.), β-Actin (cat. no. TA09; OriGene
Technologies, Inc.), PRDX2 (cat. no. DF6691; Affinity Biosciences),
PROS1 (cat. no. DF6487; Affinity Biosciences), ITIH4 (cat. no.
AF8157; R&D Systems, Inc.) and fibronectin (cat. no. AF5335;
Affinity Biosciences).
Gelatin zymography
To investigate the effect of NPWT on the activities
of MMP2 and MMP9 in granulation tissue, gelatin zymography was used
to detect the changes in MMP2 and MMP9 activity in granulation
tissue of the eight patients with diabetic foot ulcers prior to and
following NPWT for one week. The procedure for gelatin zymography
is follows: The granulation tissue of eight patients with DFUs
prior to and following NPWT for one week was lysed on ice and
homogenate. The mixture was centrifuged at room temperature at
12,000 × g for 10 min and then, the supernatants were aspirated and
the total protein was extracted. The protein content was detected
by a BCA protein quantitative kit (Shanghai Biyuntian Biotechnology
Co., Ltd.). Subsequently, according to the instructions of the
matrix metalloproteinase (MMP2, MMP2) gelatin zymogram kit
(Shanghai Xin Fan Biological Technology Co., Ltd.), the activities
of MMP2 and MMP9 were determined. Briefly, 60 µg protein samples
were directly loaded and separated by 10% SDS-PAGE. Following
electrophoresis, the gel was washed in eluent three times, for 10
min each time and then incubated at 37°C for 6 h. After incubation,
0.05% Coomassie brilliant blue was stained for 2 h at room
temperature and then decolorizing for 2 h and the gray value was
analyzed using ImageJ 1.36b (National Institutes of Health).
ELISA
A total of 17 patients with DFUs (including the
above eight patients with DFU with granulation tissue) were
consecutively enrolled from the Department of Endocrinology at The
First Affiliated Hospital of Anhui Medical University. The samples
were collected between June 2019 and October 2019. The baseline
information was: Age range 52–75 years-old; sex, 14 males and three
females; glycosylated hemoglobin A1c (HbA1c) 7.8–12.4%; course of
diabetes, 8–18 years; course of DFU, 6–14 weeks; ulcer area, 2–15
cm2; Wagner grade, 2–3; and ABI, 0.9–1.3. Similarly, the
ethics committee of the Department of Endocrinology at The First
Affiliated Hospital of Anhui Medical University approved the
experiment (approval no. LLSC20191038) and signed informed consent
from the subjects was obtained. Blood samples (5 ml) were collected
from the anterior cubital vein of the patients prior to and
following NPWT for one week. The levels of CTSS, PRDX2, PROS1 and
ITIH4 in the peripheral blood of the subjects were determined using
the following ELISA kits (Cusabio Biotech Co., Ltd.) according to
the protocols provided by the manufacturer: CTSS (cat. no.
CSB-E13722h), PRDX2 (cat. no. CSB-EL018654HU), PROS1 (cat. no.
CSB-E09903h) and ITIH4 (cat. no. CSB-E17022h).
Statistical methods
SPSS 17 (SPSS, Inc.) software was used for
statistical analysis. A Shapiro normality test was performed on the
data from the participants prior to and following NPWT for one
week. In the process of screening DEPs, if the data followed a
normal distribution, they were expressed as the mean ± standard
deviation and a paired-samples t-test was used for the statistical
analysis. If the data did not follow a normal distribution, a
Wilcoxon signed rank test was used for the statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General information on the three
subjects subjected to label-free quantitative mass
spectrometry
The three subjects (labeled Patient 1, Patient 2 and
Patient 3) had type 2 diabetes with a long disease course, poor
blood glucose control, a long DFU time, moderate wound infection
and normal blood perfusion. Overall, the wounds of the three
patients had similar infection statuses and blood supplies at the
time of enrollment (Table I). In
addition, after routine systemic treatment, the wound infection of
the three patients prior to NPWT was similar. Subsequently, the
three patients were treated with NPWT for 1 week. The laboratory
results demonstrated that the white blood cell levels, granulocyte
ratio and C-reactive protein levels were decreased, indicating that
the wound infection of the three patients was improved to a certain
extent following NPWT (Table
II).
 | Table I.Clinical characteristics of three
patients with diabetic foot ulcers for label-free quantitative mass
spectrometry. |
Table I.
Clinical characteristics of three
patients with diabetic foot ulcers for label-free quantitative mass
spectrometry.
| Clinical
feature | Patient 1 | Patient 2 | Patient 3 |
|---|
| Sex | Male | Female | Male |
| Age, years | 47 | 56 | 65 |
| Duration of ulcer,
days | 30 | 60 | 30 |
| Ulcer area,
cm2 | 6.0 | 10.0 | 12.0 |
| Wagner grading | Level 3 | Level 3 | Level 3 |
| Infection
gradea | Moderate | Moderate | Moderate |
| Duration of
diabetes mellitus, years | 13 | 13 | 18 |
| Type of
diabetes | Type 2 | Type 2 | Type 2 |
| ABIb |
|
|
|
|
Right | 1.13 | 0.98 | 1.08 |
|
Left | 1.05 | 1.02 | 0.97 |
| TcPO2, mmHg |
|
|
|
|
Right | 71.7 | 73.9 | 74.3 |
|
Left | 69.8 | 72.4 | 76.5 |
| HbAlc, % | 9.2 | 8.8 | 9.3 |
| WBC,
1×109/l | 10.9 | 11.1 | 10.2 |
| Granulocyte ratio,
% | 82.3 | 88.7 | 80.5 |
| CRP, mg/dl | 13.7 | 15.2 | 12.6 |
| ESR, mm/h | 25 | 30 | 22 |
| Microbial culture
of wound tissue | No bacterial
growth | No bacterial
growth | No bacterial
growth |
 | Table II.Laboratory tests for three patients
who received label-free quantitative mass spectrometry prior to and
following negative-pressure wound therapy for 1 week. |
Table II.
Laboratory tests for three patients
who received label-free quantitative mass spectrometry prior to and
following negative-pressure wound therapy for 1 week.
|
| Patient 1 | Patient 2 | Patient 3 |
|
|---|
|
|
|
|
|
|
|---|
| Laboratory
results | Before | After | Before | After | Before | After | Normal reference
value |
|---|
| WBC,
1×109/l | 6.5 | 5.6 | 7.2 | 6.0 | 6.0 | 5.8 | 3.5–9.5 |
| Granulocyte ratio,
% | 74.3 | 70.1 | 78.2 | 72.6 | 72.5 | 69.8 | 50.0–70.0 |
| CRP, mg/l | 13.7 | 10.6 | 15.2 | 11.1 | 12.6 | 9.9 | 0.0–10.0 |
| ESR, mm/h | 15.0 | 13.0 | 18.0 | 16.0 | 13.0 | 13.0 | 0.0–20.0 |
Identification of total proteins in
granulation tissues prior to and following NPWT for 1 week
The quantification of the protein samples, which
were extracted from granulation tissues, verified that there was a
sufficient amount of protein (Table
SI) and the SDS-PAGE images demonstrated clear protein bands
and no degradation in the protein samples (Fig. S1). These protein samples were
graded A and suitable for further MS experiments. The MS results
prior to and following NPWT for 1 week were analyzed. In six
samples from three patients, the total number of characterized
peptides was 16,978 and the total number of identified proteins was
1,762 (unique peptides ≥2, protein FDR ≤0.01; Table SII). These proteins were selected
for further screening for DEPs. Notably, because the content of a
few proteins in the sample was lower than the detection limit of
mass spectrometry, quantitative information could not be obtained,
resulting in missing values. Therefore, the average interpolation
method was used to replace the missing values with the average of
the effective quantitative values of the three groups of proteins
before and after NPWT.
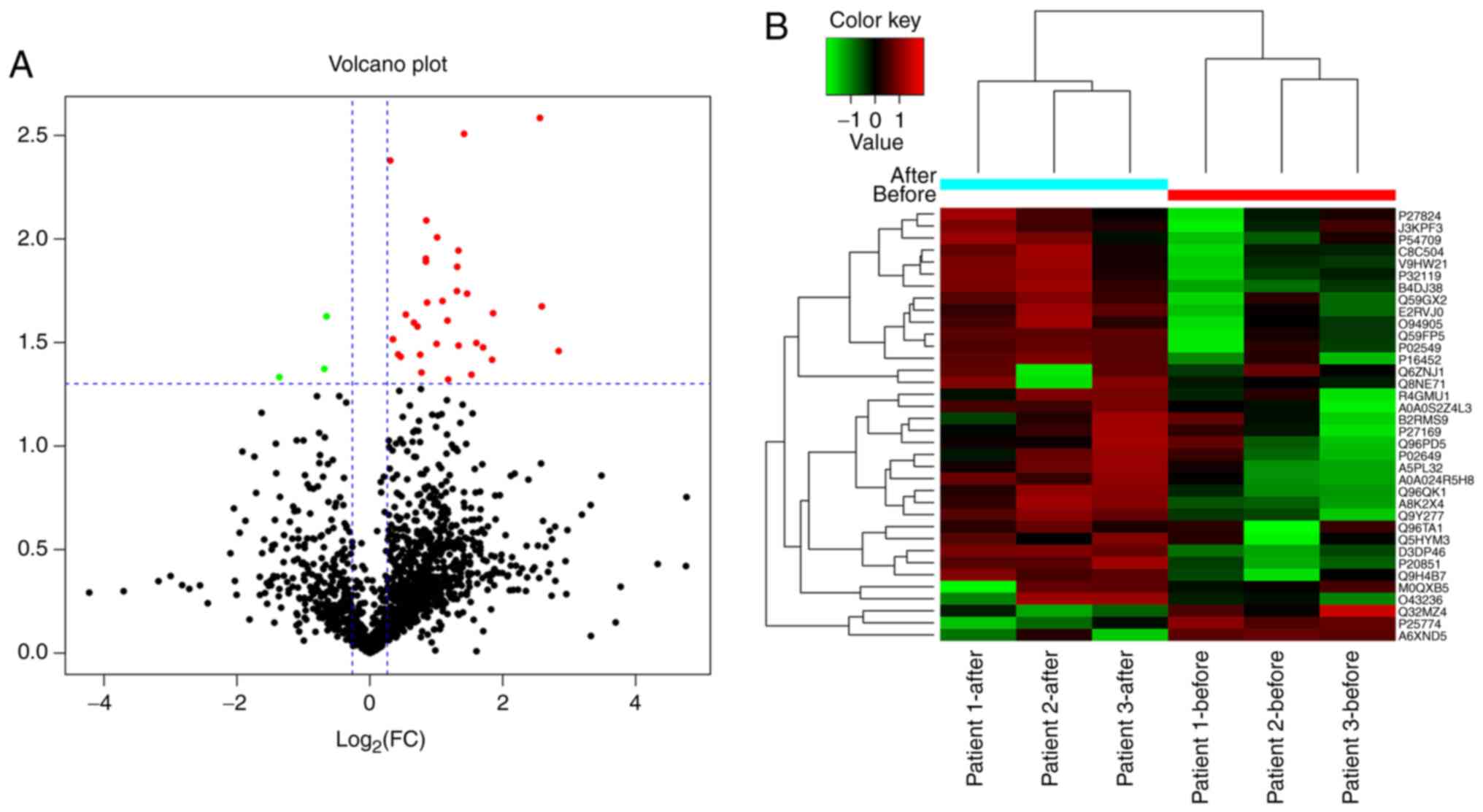
Screening for DEPs
Following the quantitative analysis, it was found
that 36 DEPs in the granulation tissue of three patients with DFU
were statistically significant following NPWT for one week compared
with their corresponding granulation specimens prior to NPWT
(P<0.05); of these proteins, 33 proteins were upregulated and
three were downregulated (Table
SIII; Fig. 1A). Only DEPs
with a change of 1.2-fold or greater were selected. The cluster
grams clearly demonstrated distinct protein expression levels prior
to and following NPWT for one week in each subject (Fig. 1B).
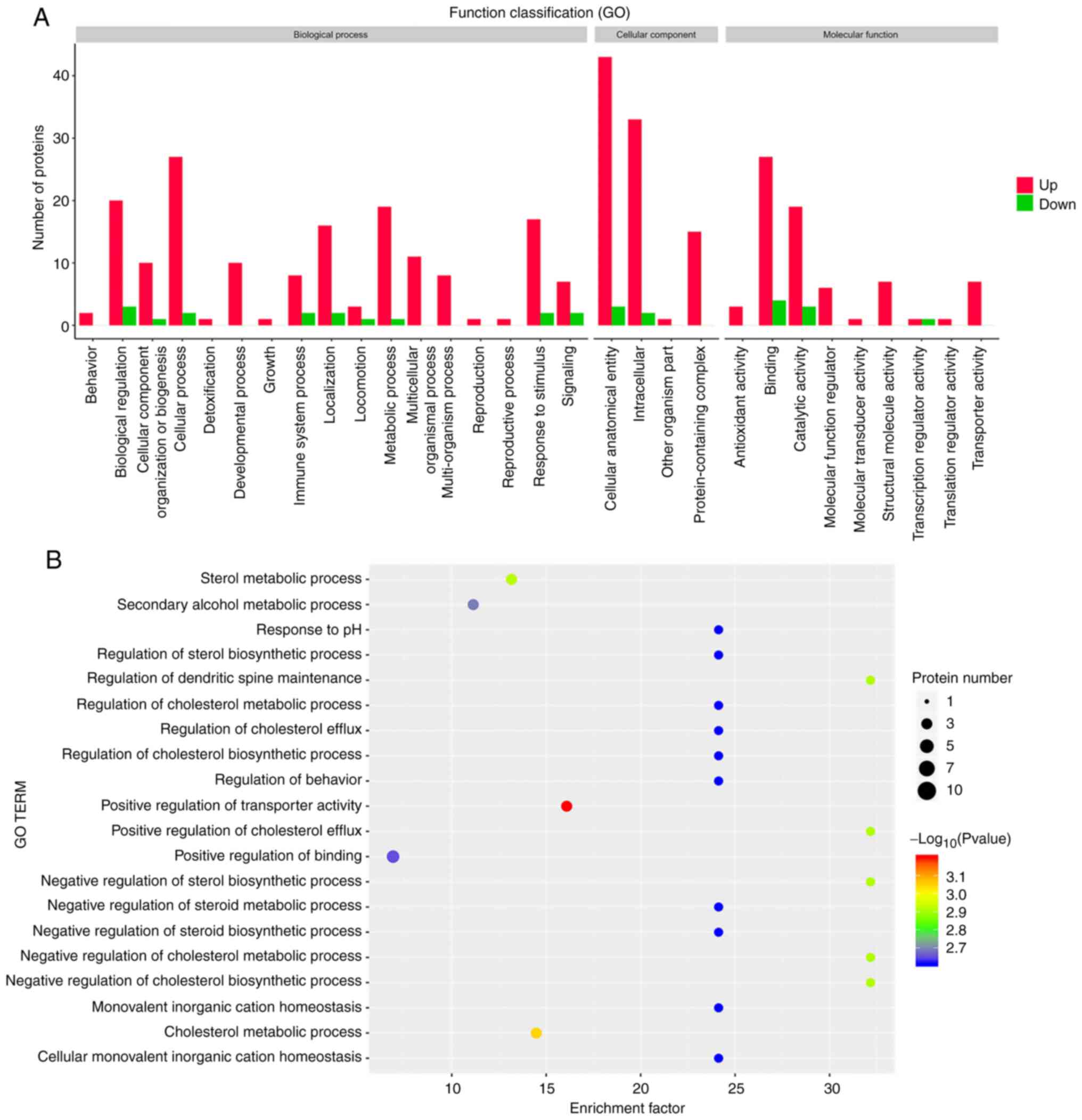
GO functional annotations and
enrichment analysis
The identified DEPs were annotated based on a GO
analysis. As shown in Fig. 2A, in
the biological process category, NPWT mainly influenced proteins in
the cellular process. Proteins involved in immune system processes
were also altered by NPWT. Notably, one protein annotated with the
term ‘detoxification’ (PRDX2) was significantly increased after
NPWT. In the cellular component category, most proteins altered
following NPWT were associated with the GO terms ‘cellular
anatomical entity’ and ‘intracellular’. In addition, in the
molecular function category, a number of DEPs following NPWT were
associated with the terms ‘binding’ and ‘catalytic activity’.
Notably, the proteins annotated with the term ‘antioxidant
activity’ were also upregulated after NPWT.
A GO functional enrichment analysis was further
conducted to identify the significantly enriched GO terms for which
the DEPs were annotated. 20 GO terms with the lowest P-values are
depicted in Fig. 2B. Important GO
terms are shown in Table III.
The most enriched DEPs were annotated with the GO term ‘cell
periphery’ (15 of 36 DEPs). Notably, the GO terms ‘structural
constituent of cytoskeleton’ [e.g., spectrin α chain erythrocytic 1
(SPTA1), leucine-rich repeat flightless-interacting protein 1
(LRRFIP1), erythrocyte membrane protein band 42 (EPB42) and tubulin
β-1 chain (TUBB1)], ‘negative regulation of response to stimulus’
[e.g., C4b-binding protein β chain (C4BPB), apolipoprotein E
(APOE), N-acetylmuramoyl-L-alanine amidase (PGLYRP2) and PROS1],
‘regulation of inflammatory response’ [e.g., vacuolar protein
sorting-associated protein 35 (VPS35) and PGLYRP2] and ‘lipid
binding’ [e.g., ERLIN2, paraoxonase/arylesterase 1 (PON1), APOL1
protein (APOL1) and APOE] were also significantly enriched
(Table III).
 | Table III.GO functional terms and enrichment
for differentially expressed proteins. |
Table III.
GO functional terms and enrichment
for differentially expressed proteins.
| GO ID | Description | UniProt ID | Symbol | P-value |
|---|
| GO:0071944 | Cell periphery | O94905, P16452,
O43236, Q6ZNJ1, J3KPF3, V9HW21, Q59FP5, P27824, P54709, Q96QK1,
Q32MZ4, Q96TA1, P02549, P02649, P20851 | ERLIN2, EPB42,
SEPTIN4, NBEAL2, SLC3A2, HEL-76, N/A, CANX, ATP1B3, VPS35, LRRFIP1,
FAM129B, SPTA1, APOE, C4BPB | 0.02 |
| GO:0048585 | Negative regulation
of response to stimulus | P20851, P02649,
Q96PD5, Q96TA1, Q96QK1, A0A0S2Z4L3 | C4BPB, APOE,
PGLYRP2, NBEAL2, VPS35, PROS1 | 0.05 |
| GO:0008289 | Lipid binding | O94905, P27169,
Q59FP5, A5PL32, P02649 | ERLIN2, PON1, N/A,
APOL1, APOE | 0.03 |
| GO:0005200 | Structural
constituent of cytoskeleton | Q59FP5, P02549,
P16452, Q9H4B7 | N/A, SPTA1, EPB42,
TUBB1 | 0.01 |
| GO:0050727 | Regulation of
inflammatory response | P02649, Q96QK1,
Q96PD5 | APOE, VPS35,
PGLYRP2 | 0.04 |
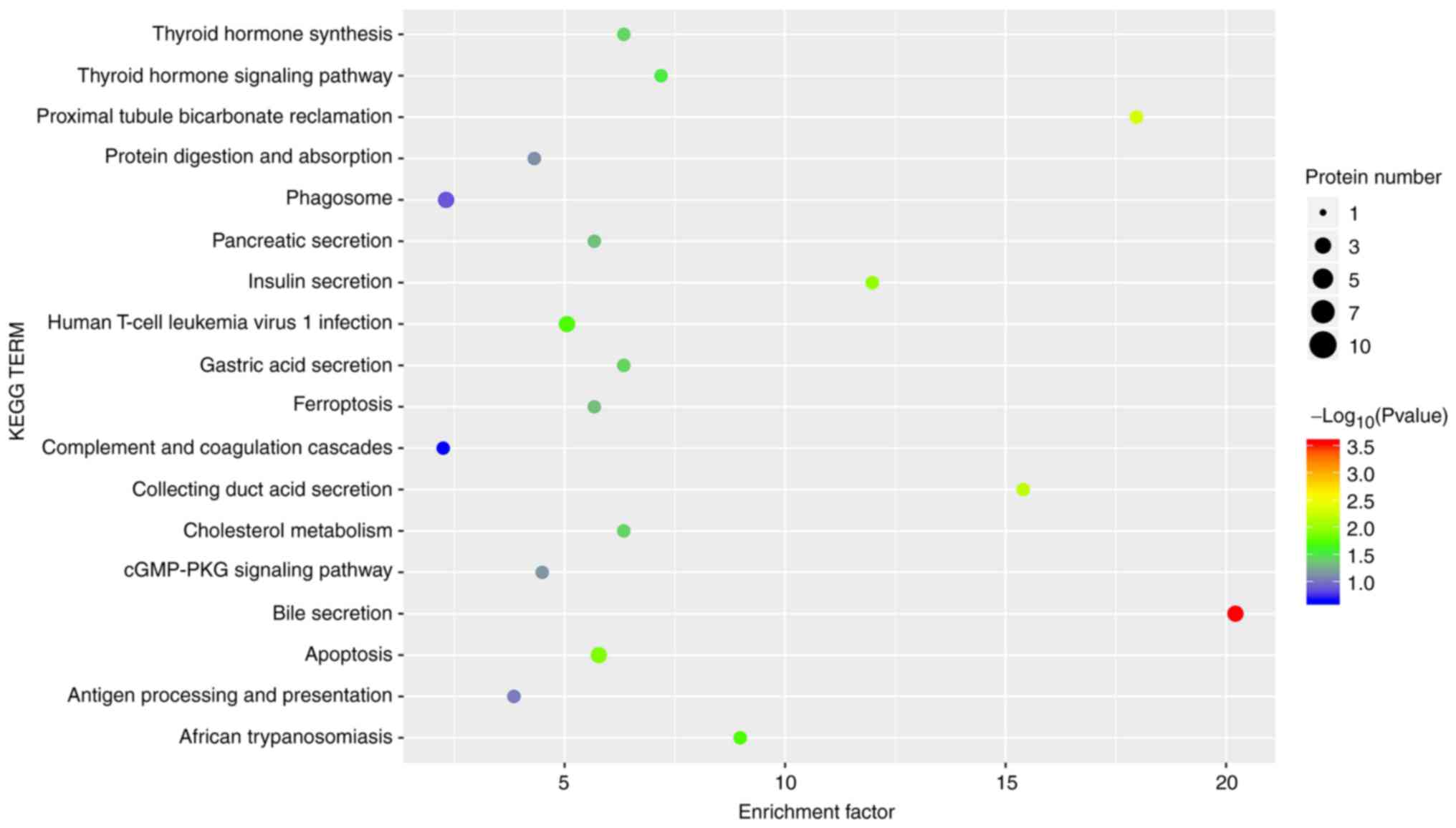
Enrichment analysis of KEGG signaling
pathways
To analyze the functions of these DEPs, the Kobas
online analysis tool was used to search for KEGG signaling
pathways. The results of the KEGG enrichment analysis demonstrated
that NPWT influenced pathways, such as ‘complement and coagulation
cascades’ (e.g., PROS1 and C4BPB), ‘apoptosis’ [e.g., Septin-4
(SEPT4), SPTA1 and CTSS] and ‘cholesterol metabolism’ [e.g.,
voltage-dependent anion-selective channel protein 3 (VDAC3) and
APOE] (Fig. 3; Table IV), indicating functional changes
induced by NPWT in granulation tissues. surprisingly, some
signaling pathways that are less related to wound healing, such as
‘thyroid hormone synthesis’, ‘thyroid hormone signaling pathway’,
‘human T-cell leukemia virus 1 infection’ and ‘African
trypanosomiasis’, were also found.
 | Table IV.Enrichment analysis of KEGG signaling
pathway and the differentially expressed proteins included. |
Table IV.
Enrichment analysis of KEGG signaling
pathway and the differentially expressed proteins included.
| KEGG ID | Description | UniProt ID | Symbol | P-value |
|---|
| ko04976 | Bile secretion | Q59GX, V9HW21,
P54709 | N/A, HEL-76,
ATP1B3 | 0.0003 |
| ko04964 | Proximal tubule
bicarbonate reclamation | V9HW21, P54709 | HEL-76, ATP1B3 | 0.0047 |
| ko04966 | Collecting duct
acid secretion | E2RVJ0, V9HW21 | SLC4A1, HEL-76 | 0.0065 |
| ko04911 | Insulin
secretion | Q59GX2, P54709 | N/A, ATP1B3 | 0.0109 |
| ko04210 | Apoptosis | O43236, P02549,
P25774 | SEPT4, SPTA1,
CTSS | 0.0133 |
| ko05166 | Human T-cell
leukemia virus 1 infection | Q59GX2, Q9Y277,
P27824 | N/A, VDAC3,
CANX | 0.0192 |
| ko05143 | African
trypanosomiasis | A5PL32, C8C504 | APOL1, HBB | 0.0193 |
| ko04919 | Thyroid hormone
signaling pathway | Q59GX2, P54709 | N/A, ATP1B3 | 0.0297 |
| ko04918 | Thyroid hormone
synthesis | P54709, P27824 | ATP1B3, CANX | 0.0377 |
| ko04971 | Gastric acid
secretion | V9HW21, P54709 | HEL-76, ATP1B3 | 0.0377 |
| ko04979 | Cholesterol
metabolism | Q9Y277, P02649 | VDAC3, APOE | 0.0377 |
| ko04216 | Ferroptosis | Q9Y277, J3KPF3 | VDAC3, SLC3A2 | 0.0463 |
| ko04972 | Pancreatic
secretion | V9HW21, P54709 | V9HW2, ATP1B3 | 0.0463 |
| ko04022 | cGMP-PKG signaling
pathway | Q9Y277, P54709 | VDAC3, ATP1B3 | 0.0707 |
| ko04974 | Protein digestion
and absorption | P54709, J3KPF3 | ATP1B3, SLC3A2 | 0.0760 |
| ko04612 | Antigen processing
and presentation | P27824, P25774 | CANX, CTSS | 0.0927 |
| ko04145 | Phagosome | Q9H4B7, P27824,
P25774 | TUBB1, CANX,
CTSS | 0.1353 |
| ko04610 | Complement and
coagulation cascades | P20851,
A0A0S2Z4L3 | C4BPB, PROS1 | 0.2225 |
Functional validation of DEPs
To verify the results obtained from the mass
spectrometry analysis, western blot assays was conducted to further
confirm the results from mass spectrometry. As a substrate of CTSS,
protein levels of fibronectin in the granulation tissues of eight
patients with DFUs prior to and following NPWT for 1 week were
detected. As expected, fibronectin protein was found to be
upregulated after NPWT in the granulation tissues, which was
consistent with the decreased levels of CTSS after NPWT (Fig. S2A-C). CTSS, PRDX2, PROS1 and
ITIH4 in the granulation tissues of eight patients with DFUs prior
to and following NPWT for 1 week (Figs. 4A-D and S3A-D) were then detected. As shown in
Fig. 4A1-3, compared with
pre-NPWT, the expression level of the CTSS protein in the
granulation tissue of DFU wounds was significantly decreased
following NPWT for 1 week, whereas the protein expression levels of
PROS1, ITIH4 and PRDX2 in the granulation tissues were elevated
following NPWT (Fig. 4B-D),
confirming the accuracy and validity of the mass spectrometry data.
In addition, the activities of MMP2 and MMP9 in granulation tissue
were significantly decreased following NPWT compared with those
prior to NPWT (Fig. S4A and B).
Furthermore, as CTSS, ITIH4, PROS1 and PRDX2 are secretory
proteins, blood samples were also collected from 17 patients with
DFU prior to and following NPWT and ELISA performed to measure the
serum CTSS, ITIH4, PROS1 and PRDX2 levels (Table SIV,Table SV,Table SVI,Table SVII,Table SVIII,Table SIX). Notably, following NPWT for
1 week, the serum CTSS levels were significantly decreased
(Fig. 5A1 and 2; Tables SVIII and SIX) and the serum ITIH4, PROS1 and
PRDX2 levels were clearly enhanced (Fig. 5B1 and 2, C1 and 2 and D1 and 2, respectively; Table SVIII), which was consistent with
the results of the western blot analysis. These results are
consistent with the mass spectrometry analysis and further increase
the reliability of the results obtained from the mass spectrometry
analysis.
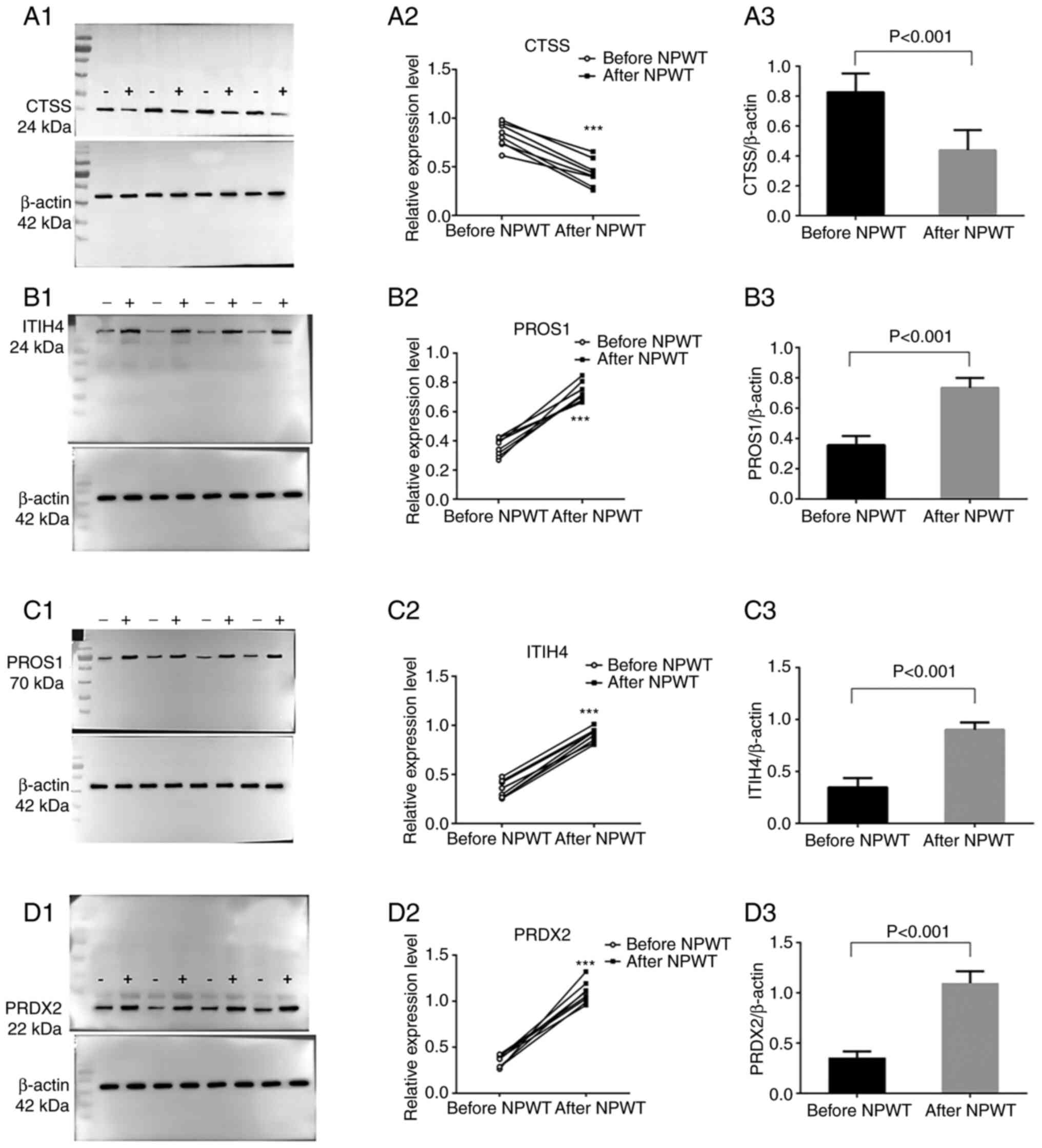 | Figure 4.Validation of the protein expression
levels of CTSS, PROS1, ITIH4 and PRDX2. Wound granulation tissues
from eight patients with diabetic foot ulcers before and after NPWT
were collected and subjected to western blotting. Primary
antibodies against (A) CTSS, (B) PROS1, (C) ITIH4 and (D) PRDX2
were used to detect differences in protein levels. (A1-D1) Protein
bands from four representative patients prior to NPWT (designated
as minus symbol ‘−’) and following NPWT (designated as plus symbol
‘+’) are presented in the left panel. Protein samples from the same
patients were loaded next to each other. (A2-D2) Then, eight pairs
of protein samples from eight patients were analyzed by ImageJ and
are depicted as paired dots in the right panel. (A3-D3) The gray
level of the target protein was compared with the internal
reference (β-actin) gray level to obtain the relative expression
value. Paired t-tests were performed and significant changes are
indicated by ***P<0.001 vs. before NPWT. CTSS, cathepsin; PROS1,
protein S isoform 1; ITIH4, inter α-trypsin inhibitor heavy chain
H4; PRDX2, peroxiredoxin-2; NPWT, negative-pressure wound
therapy. |
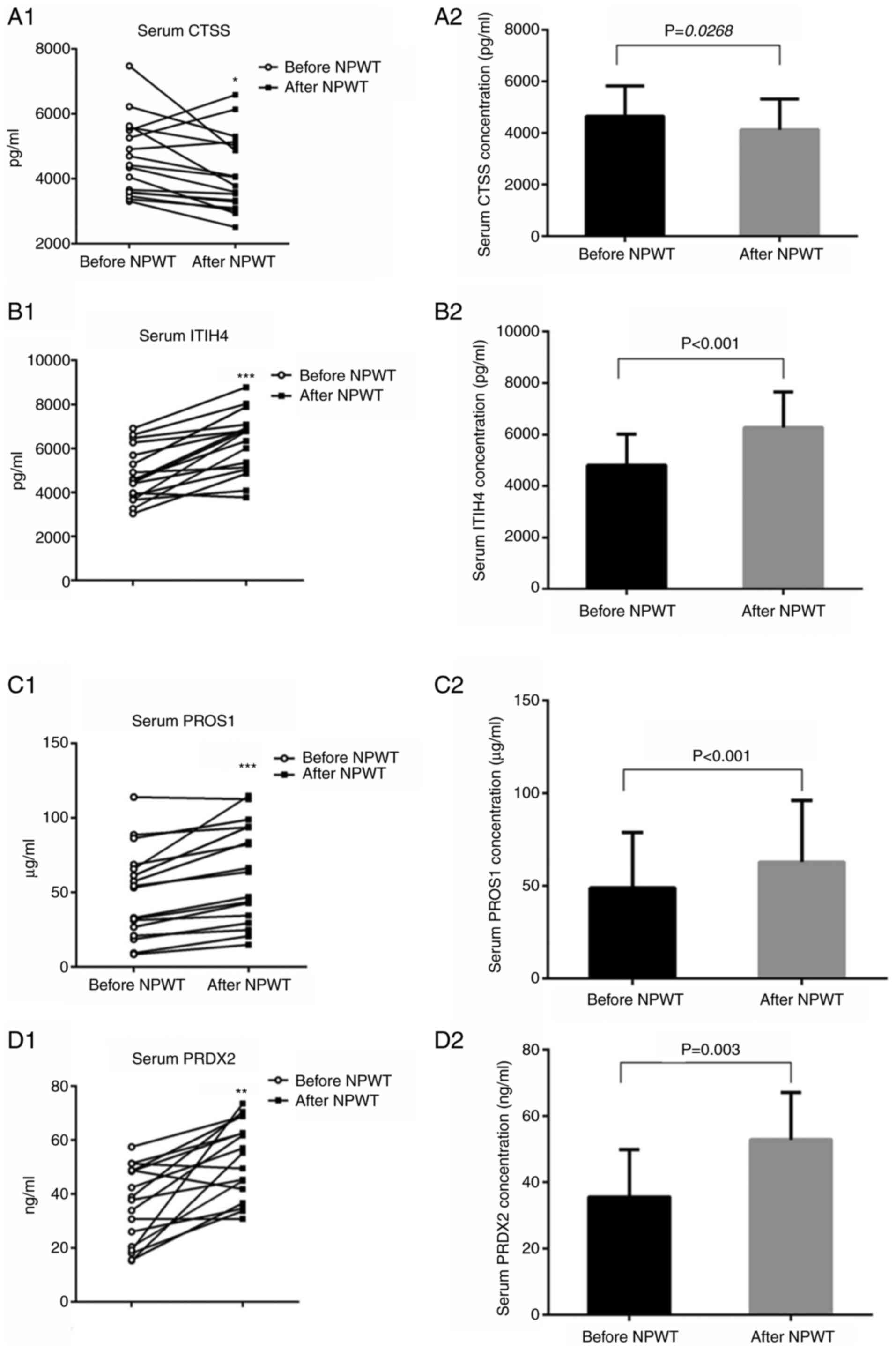 | Figure 5.ELISA analysis of serum CTSS, ITIH4,
PROS1 and PRDX2 levels in patients before and after NPWT. Blood
samples from 17 patients were collected and serum CTSS, ITIH4,
PROS1 and PRDX2 levels were measured with ELISA. Serum (A1) CTSS,
(B1) ITIH4, (C1) PROS1 and (D1) PRDX2 prior to and following NPWT
are depicted as paired dots. The changes in serum (A2) CTSS, (B2)
ITIH4, (C2) PROS1 and (D2) PRDX2 levels prior to and following NPWT
are shown by histograms. Paired t-tests were performed and
significant changes are indicated by *P<0.05, **P<0.01,
***P<0.001 vs. before NPWT. CTSS, cathepsin; PROS1, protein S
isoform 1; ITIH4, inter α-trypsin inhibitor heavy chain H4; PRDX2,
peroxiredoxin-2; NPWT, negative-pressure wound therapy. |
Discussion
As a noninvasive technology, NPWT has been widely
used for the clinical treatment of chronic wounds. A previous study
showed that NPWT can effectively promote DFU healing and reduce
amputation risk (15); however,
the mechanism by which NPWT accelerates DFU healing remains to be
elucidated. Therefore, searching for the therapeutic targets of
NPWT is of great significance for further elucidating the
pathogenesis of DFUs and developing novel therapeutic strategies.
In the present study, label-free quantitative mass spectrometry was
used to analyze the differences in protein expression in
granulation tissue from three patients with DFUs prior to and
following NPWT for one week. A total of 36 statistically
significant differentially expressed proteins were identified.
Among these proteins, 33 proteins were upregulated and 3 proteins
were downregulated. The analysis indicated that NPWT altered
multiple proteins in the granulation tissue of feet; these proteins
were associated with antioxidation and detoxification [e.g.,
hemoglobin subunit β (HBB) and PRDX2], the cytoskeleton (e.g.,
SPTA1 and EPB42), regulation of the inflammatory response (e.g.,
VPS35 and PGLYRP2), complement and coagulation cascades (e.g.,
PROS1, C4BPB and CTSS), lipid metabolism (e.g., ERLIN2, PON1, APOL1
and APOE) and extracellular matrix (e.g., ITIH4 and CTSS). To the
best of the authors' knowledge, the present study for the first
time systematically characterized the changes in protein expression
profiling in the granulation tissue of DFU wounds following NPWT
using label-free quantitative mass spectrometry.
Notably, among the 36 DEPs, CTSS was one of the few
downregulated DEPs and was related to the apoptosis pathway in the
KEGG signaling pathway. In addition, PRDX2 was related to
antioxidant activity in the GO terms. In addition, in the GO
enrichment analysis, PROS1 was associated with intravascular
thrombosis, while ITIH4 was associated with the stability of the
extracellular matrix. Therefore, CTSS, PRDX2, PROS1 and ITIH4 can
reflect the pathophysiological mechanism of wound healing from
different perspectives. Based on the above findings, combined with
a large number of literature reviews (16–19). It was suggested that the CTSS,
PRDX2, PROS1 and ITIH4 proteins are closely related to wound
healing. Finally, these four representative proteins were selected
for the functional validation of the DEPs.
The GO enrichment analysis performed in the present
study was consistent with a number of previous studies showing that
the wound healing process of diabetic ulcers is associated with
altered extracellular matrix deposition (20), cytoskeletal deregulation (21), dyslipidemia (22) and prolonged inflammation response
(23). The present study
unexpectedly found some signaling pathways that seemed weakly
relevant to the curative effect of wounds in the enrichment
analysis of KEGG signaling pathways, such as thyroid hormone
synthesis, thyroid hormone signaling pathway, human T-cell leukemia
virus 1 infection and African trypanosomiasis. In fact, it is
generally accepted that thyroid hormone signaling is important in
skin pathophysiology. Epidermal proliferation, hair cycling, wound
healing and stem cell function can be adversely affected by the
absence of thyroxine nuclear receptor (24,25). Liu et al (26) report that thyroxine can increase
the expression of bFGF mRNA via the αvβ3/protein kinase D/histone
deacetylase 5 signaling pathway and serves an important role in
angiogenesis, which is closely associated with wound healing. Human
T-cell lymphotropic virus type 1 (HTLV-1) can cause dysfunction of
T lymphocytes and HTLV-1-related infectious dermatitis, which is
characterized by chronic exudative eczematous eruption and
persistent infection with Staphylococcus aureus and
Staphylococcus aureus β hemolytic streptococcus (27,28). These skin lesions and pathogens
are related to the occurrence and healing of chronic wounds.
Additionally, a previous study revealed that skin is a potential
reservoir for African trypanosomes, which can cause chronic and
persistent pathological damage to the skin (29). Microarray data demonstrates that
chemokines ccl8, CCL19, CCL21, CCL27 and CXCL12 are highly
expressed in the skin of mice infected with African trypanosomes
(30). These chemokines may be
involved in the pathophysiological mechanism of wound healing.
Although the bile secretion signaling pathway has not been reported
in the literature related to wound repair, a previous report
demonstrated that bile salts can enhance the migration ability of
cells and physiological concentrations of bile salts can increase
the migration of intestinal epithelial cells by activating the
NF-kB signaling pathway, which serves a role in maintaining the
integrity of the intestinal mucosa (31). The proximal tubule bicarbonate
reclamation signaling pathway may affect the growth and
proliferation of vascular endothelial cells (32). Insulin stimulates a change in the
macrophage phenotype from proinflammatory (M1) to anti-inflammatory
(M2) and downregulates the inflammatory response by upregulating
the expression of PPAR-γ and inducing P38-mediated
dephosphorylation of PPAR-γ (Ser112), which, as a result, reduces
inflammation and improves chronic wound healing (33). Meanwhile, nitric oxide regulates
Rho GTPase through cGMP-PKG signaling and enhances keratinocyte
migration, thereby promoting wound healing (34). Cholesterol homeostasis is critical
to the functional integrity of cells (35). The cell surface delivery of
extracellular matrix (ECM) and integrins is the basis of cell
migration in wound healing (36).
This process is not only driven by several soluble NSF attachment
protein (SNAP) receptor (snare) proteins, which are key players in
the transport of vesicles but also tightly regulated by cholesterol
(36). Ferroptosis, a new type of
nonapoptotic cell death that is characterized by lipid
peroxidation, is primarily dependent on iron and reactive oxygen
species (ROS). The iron-promoting effect is mainly triggered by the
activation of antioxidants in the cell, leading to the accumulation
of lipid ROS and destruction of the membrane structure (37). For ferroptosis, the dynamic
balance between oxidative stress and antioxidants determines the
lipid peroxidation level in cells. This new type of cell death is
thus closely related to the lipid peroxidation of cells (37). In the metabolic pathway of protein
digestion and absorption, the protein SLC3A2 was enriched, which is
a heterodimeric amino acid transporter that regulates integrin
signaling in vitro (38).
The proliferation and migration of keratinocytes in the process of
wound re-epithelialization depend on SLC3A2. A study has shown that
the lack of SLC3A2 in the mouse epidermis leads to skin homeostasis
and improper epidermal wound healing (38).
In summary, the aforementioned GO terms and KEGG
pathways were enriched in DEPs and related to the healing process
of chronic ulcers, which are worthwhile to be further studied.
Previous studies provide evidence showing that DFUs
and other chronic wounds do not heal in an orderly and
physiological process (39,40). It is currently speculated that the
pathophysiological mechanism of DFU wound healing is complex and
involves a number of factors. Tissue hypoxia is one of these
factors (41). Hypoxia can
increase the level of ROS by amplifying acute inflammatory
reactions. The balance between ROS formation and antioxidant enzyme
activities can maintain redox homeostasis. However, this balance
can easily be disrupted, resulting in oxidative stress and damage
to cells, thereby prolonging the healing process (42). A previous study demonstrated that
hemoglobin performs an antioxidant peroxidase function and can
reduce the oxidative stress induced by hydrogen peroxide (43). Another study involving cervical
cancer patients demonstrated that hemoglobin may be an integral
part of the endogenous antioxidant defense system (44). The overexpression of hemoglobin
can reduce oxidative stress and protect cells from oxidative damage
(45). It is known that
intracellular superoxide can be dismutated into hydrogen peroxide,
which is further detoxified by peroxidases 1, 2 and 6. Among them,
PRDX2 has been shown to scavenge hydrogen peroxide and protect
cells from oxidative stress (46,47). In the present study, although
GO/KEGG enrichment analysis did not enrich the hypoxia-ROS model,
it was found that the expression of HBB and PRDX2 was significantly
upregulated after NPWT. Previous studies (48,49) have shown that PRDX2 upregulation
can reduce hypoxia-ROS damage to the skin. Therefore, it is
suggested that NPWT can help improve the antioxidant capacity of
granulation tissue and promote wound healing. A previous report
concerning the beneficial effects of some antioxidant treatments on
wound healing also support our findings (50).
In addition, the protein profiling analysis of the
present study revealed the following DEPs associated with
thrombosis: LRRFIP1 and PROS1. LRRFIP1 serves a key role in
regulating the platelet cytoskeleton and then affects the
activation process and function of platelets. A previous study
showed that LRRFIP1 triggers platelet aggregation by enhancing the
expression of α IIb β 3 (51).
Silencing the LRRFIP1 gene can lead to a significant reduction in
thrombosis (52). PROS1 is a
vitamin K-dependent glycoprotein and a key regulator of the
coagulation/fibrinolysis cascade. In general, free PROS (fPS) is a
cofactor of activated protein C (APC). The combination of fPS and
APC can lead to the inactivation of factors Va and VIIIa and
inhibit the production of thrombin (53,54). The circulating level of PROS1 has
been shown to decrease in diseases, such as venous thrombosis
(55). Despite its role in
anticoagulation as a cofactor of APC, a previous report
demonstrated that PROS is a pleiotropic anticoagulant with protein
C-independent activities that contributes to vascular development
(56). Notably, silencing PROS1
has also been shown to inhibit cell proliferation and induce
apoptosis in glioblastoma multiforme cells (18). A previous study also indicated
that PROS1 has a protective effect against glomerular injury in the
progression of diabetic complications, such as diabetic nephropathy
(57). The present study found
that NPWT upregulated the expression levels of PROS1 in granulation
tissue while downregulating the expression levels of LRRFIP1 in
granulation tissue. These proteins may participate in the
coagulation process, platelet production and cell proliferation in
the granulation tissue of chronic wounds. More importantly, these
results may indicate that NPWT serves a potential preventive role
in thrombosis. Thus, investigating whether NPWT can improve local
circulation by reducing the risk of intravascular microthrombosis
may be warranted in the future.
The currently recognized physiological processes
that affect wound healing mainly include growth factor production,
angiogenesis, phagocytic cell activity, collagen deposition,
epidermal barrier function, granulation tissue mass, keratinocyte
and fibroblast migration and proliferation, epidermis activation
and wound remodeling mediated by MMPs and the ECM (58,59). Compared with acute wounds, a
prominent feature of chronic DFUs is abnormally increased levels of
MMPs in the wound, which can promote tissue degeneration and
ultimately inhibit the normal wound repair process (60). In addition, diabetes can lead to
cell dysfunction, including T cell immune defects, leukocyte
chemotaxis defects, a reduced ability to perform phagocytosis to
destroy bacterial pathogens and fibroblast cell and epidermal cell
dysfunction (61). These factors
all hinder the wound healing process in DFU patients. The present
study explored changes in the activity of MMP2 and MMP9 in the
granulation tissue of eight patients with diabetic foot ulcers
prior to and following NPWT for one week by gelatin zymography and
observed that the activities of MMP2 and MMP9 in granulation tissue
were significantly decreased following NPWT compared with those
prior to NPWT, suggesting that NPWT can affect wound remodeling by
changing MMPs. Notably, it was also found that several DEPs were
associated with the regulation of MMPs, ECM and the inflammatory
response, such as CTSS, ITIH4, VPS35 and PGLYRP2. These results
also provide a new understanding of the mechanism of NPWT.
CTSS, an important member of the cysteine protease
family, is mainly expressed in lymph node antigen-presenting cells,
spleen cells and other immune cells. It participates in the
degradation of antiangiogenic peptides and adhesion proteins and
promotes neovascularization and tumor cell invasion and metastasis
(62). Previous studies found
that CTSS can interact with MMPs and serine proteases and
participates in the degradation of the ECM (63,64). An important physiological feature
of the healing process after tissue injury is increased ECM
synthesis and aggregation surrounding the wound. Fibroblasts are
the main cells that synthesize and secrete ECM. During acute skin
trauma, collagen, proteoglycan and glycosaminoglycan replace the
temporary ECM and participate in the formation of granulation
tissue. A large amount of ECM aggregation stimulates the migration
of epidermal cells and promotes wound re-epidermalization (59). As CTSS can promote the degradation
of the ECM, the downregulation of CTSS increases the expression of
the ECM and promotes wound healing. The present study found that
the protein expression of CTSS was significantly decreased after
NPWT, suggesting that NPWT may help wound healing by downregulating
CTSS in granulation tissue. To further verify this hypothesis, the
protein expression of fibronectin, which is a downstream protein of
CTSS was also measured. Previous studies have found that CTSS can
degrade fibronectin (65), which
is closely related to wound healing (66). The results of the present study
demonstrated that the expression of fibronectin was upregulated
following NPWT (Fig. S2) and the
change trend was opposite to that of CTSS. To the best of the
authors' knowledge, the present study is the first to report that
CTSS expression in DFU wound granulation tissue significantly
changes after NPWT. The specific mechanism by which CTSS is
involved in wound healing requires further research.
ITIH4, which belongs to the inter-α-inhibitor (ITIH)
family of proteins, was first reported to be elevated following
NPWT in the present study. The ITIH family consists of bikunin and
six different heavy chain proteins (67). Members of the ITIH family of
proteins help stabilize the extracellular matrix by binding
hyaluronic acid (68). In
contrast to other ITIHs, ITIH4 lacks a consensus
bikunin-recognizing sequence in the C-terminal region but still
contains a hyaluronic acid binding site in the von Willebrand-type
(vWA) domain and several sites are found in collagen (69). Therefore, it is still possible for
ITIH4 to bind hyaluronic acid or other components of the
extracellular matrix. Meanwhile, ITIH4 has also been shown to be
cleaved by MMP-13 and antagonize the effect of MMPs (70). Since MMPs are abnormally increased
in DFUs, our data indicate that the increased ITIH4 level in
granulation tissue of DFUs also contributes to the promoting effect
of NPWT on DFUs.
Notably, in the present study, during the
verification of the function of the DEPs, it was also found that
the levels of PRDX2, PROS1 and ITIH4 in the peripheral blood of DFU
patients were significantly increased following NPWT, while the
levels of CTSS were significantly decreased. On the one hand, these
results further supported the changes in PRDX2, PROS1, CTSS and
ITIH4 expression in wound granulation tissue after NPWT; on the
other hand, these data suggested that PRDX2, PROS1, CTSS and ITIH4
in serum can be used as biomarkers of NPWT in patients with DFUs.
Notably, after conducting a literature review, it was found that
some reports determining the PRDX2, PROS1, CTSS and ITIH4 levels in
peripheral blood by ELISA (71–74) further support these results.
There are some limitations in the present study.
First, due to limited funding, the mass spectrometry analysis was
only based on three samples per group, without expanding the sample
size. Therefore, the sample size of proteomics may not be strong
enough to select more DEPs at the given significance level.
Similarly, due to a lack of adequate financial support, only the
PRDX2, PROS1, CTSS and ITIH4 proteins were selected for the
functional validation of the DEPs; other highlighted target
proteins, such as LRRFIP1 and HBB, were not chosen for validation;
and the sample size in the validation process was small. Hence, it
is necessary to expand the sample size and select more target
proteins to verify the changes in tissue expression and serum
concentration. Second, the specimens were collected from
granulation tissues, which may not reflect changes in the
expression profiles of other tissues, such as the epidermis, during
wound healing of foot ulcers. It could be worthwhile to collect
more types of tissues during the wound healing process to validate
our results in future studies. Third, during the screening for
DEPs, only a quantitative, but not a qualitative, analysis of the
proteins was performed; thus, some bioactive proteins may have been
missed. Further analyses and improvements are needed in the future.
Fourth, screened was performed according to the P-value sequence of
KEGG enrichment analysis and showed all 18 enrichment pathways that
met the conditions. However, some of the metabolic pathways do not
seem to be closely related to wound treatment. The scientific value
of these findings needs to be confirmed by more studies.
In summary, the present study analyzed the changes
in protein expression profiles in granulation tissues of diabetic
feet following NPWT using the label-free mass spectrometry method
and identified 36 DEPs. Among these DEPs, 33 proteins were
upregulated and 3 proteins were downregulated. Further analysis
demonstrated that these DEPs were related to cell antioxidant
oxidation and detoxification, the cytoskeleton, regulation of the
inflammatory response, complement and coagulation cascades and
lipid metabolism. In addition, it was also reported for the first
time, to the best of the authors' knowledge that the levels of
PRDX2, PROS1 and ITIH4 in peripheral blood from DFU patients were
significantly increased after NPWT, while the levels of CTSS were
significantly decreased, suggesting that PRDX2, PROS1, CTSS and
ITIH4 in serum can be used as biomarkers of NPWT in patients with
DFUs. The present study lays a solid foundation for clarifying the
mechanism of NPWT and provides a new idea for further studies
concerning biomarkers of NPWT efficacy.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Key
Research and Development Program of Anhui Province (grant no.
202004a07020016) and the Natural Science Foundation of Anhui
Province (grant no. 2108085MH269).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The datasets generated and/or analyzed during the current
study are available in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the
iProX partner repository with the dataset identifier PXD020929.
Authors' contributions
ZJ and MC conceived and designed the experiments.
ZJ, LLi and XZ performed the experiments. ZJ, BS and SZ
participated in the data processing and provided technical
assistance during the experimental process. LLu and YT completed
the data analysis and interpretation, and drafted the manuscript.
MC and BS revised the manuscript and determined the final published
version. All authors have read and approved the final version of
the manuscript. ZJ and MC confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All procedures performed involving human
participants were in accordance with the ethical standards of the
committee of the First Affiliated Hospital of Anhui Medical
University (Hefei, China) and the 1964 Helsinki declaration and its
later amendments or comparable ethical standards. The present study
was approved by the medical ethics committee of the First
Affiliated Hospital of Anhui Medical University (approval nos.
CDEC000004982 and LLSC20191038) and signed informed consent was
obtained from all subjects
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang P, Lu J, Jing Y, Tang S, Zhu D and
Bi Y: Global epidemiology of diabetic foot ulceration: A systematic
review and meta-analysis. Ann Med. 49:106–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhong A, Chang M, Yu T, Gau R, Riley DJ,
Chen Y and Chen PL: Aberrant DNA damage response and DNA repair
pathway in high glucose conditions. J Can Res Updates. 7:64–74.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uccioli L, Izzo V, Meloni M, Vainieri E,
Ruotolo V and Giurato L: Non-healing foot ulcers in diabetic
patients: General and local interfering conditions and management
options with advanced wound dressings. J Wound Care. 24
(Suppl):S35–S42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Noor S, Khan RU and Ahmad J: Understanding
diabetic foot infection and its management. Diabetes Metab Syndr.
11:149–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isaac AL and Armstrong DG: Negative
pressure wound therapy and other new therapies for diabetic foot
ulceration: The current state of play. Med Clin North Am.
97:899–909. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borys S, Hohendorff J, Frankfurter C,
Kiec-Wilk B and Malecki MT: Negative pressure wound therapy use in
diabetic foot syndrome-from mechanisms of action to clinical
practice. Eur J Clin Invest. 49:e130672019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schintler MV: Negative pressure therapy:
Theory and practice. Diabetes Metab Res Rev. 28 (Suppl 1):S72–S77.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Savage N: Proteomics: High-protein
research. Nature. 527:S6–S7. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang N, Zhu F, Chen L and Chen K:
Proteomics, metabolomics and metagenomics for type 2 diabetes and
its complications. Life Sci. 212:194–202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oyibo SO, Jude EB, Tarawneh I, Nguyen HC,
Harkless LB and Boulton AJ: A comparison of two diabetic foot ulcer
classification systems: The wagner and the university of Texas
wound classification systems. Diabetes Care. 24:84–88. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mu S, Hua Q, Jia Y, Chen MW, Tang Y, Deng
D, He Y, Zuo C, Dai F and Hu H: Effect of negative-pressure wound
therapy on the circulating number of peripheral endothelial
progenitor cells in diabetic patients with mild to moderate degrees
of ischaemic foot ulcer. Vascular. 27:381–389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gene Ontology Consortium, . The gene
ontology resource: Enriching a GOld mine. Nucleic Acids Res.
49:D325–D334. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Z, Dumville JC, Hinchliffe RJ, Cullum
N, Game F, Stubbs N, Sweeting M and Peinemann F: Negative pressure
wound therapy for treating foot wounds in people with diabetes
mellitus. Cochrane Database Syst Rev. 10:D103182018.PubMed/NCBI
|
|
16
|
Memmert S, Nokhbehsaim M, Damanaki A,
Nogueira AV, Papadopoulou AK, Piperi C, Basdra EK, Rath-Deschner B,
Götz W, Cirelli JA, et al: Role of cathepsin S in periodontal wound
healing-an in vitro study on human PDL cells. BMC Oral Health.
18:602018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han YH, Jin MH, Jin YH, Yu NN, Liu J,
Zhang YQ, Cui YD, Wang AG, Lee DS, Kim SU, et al: Deletion of
peroxiredoxin II inhibits the growth of mouse primary mesenchymal
stem cells through induction of the G0/G1 cell-cycle arrest and
activation of AKT/GSK3β/β-catenin signaling. In Vivo. 34:133–141.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Che MM, Abdul MN, Ibrahim K, Mohd MN, Wan
NW, Harun R and Jamal R: Silencing of PROS1 induces apoptosis and
inhibits migration and invasion of glioblastoma multiforme cells.
Int J Oncol. 49:2359–2366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hennies HC: All is balanced:
Inter-α-trypsin inhibitors as unseen extracellular matrix proteins
in epidermal morphology and differentiation. Exp Dermatol.
24:661–662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maione AG, Smith A, Kashpur O, Yanez V,
Knight E, Mooney DJ, Veves A, Tomic-Canic M and Garlick JA: Altered
ECM deposition by diabetic foot ulcer-derived fibroblasts
implicates fibronectin in chronic wound repair. Wound Repair Regen.
24:630–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jozic I, Abujamra BA, Elliott MH,
Wikramanayake TC, Marjanovic J, Stone RC, Head CR, Pastar I,
Kirsner RS, Andreopoulos FM, et al: Glucocorticoid-mediated
induction of caveolin-1 disrupts cytoskeletal organization,
inhibits cell migration and re-epithelialization of non-healing
wounds. Commun Biol. 4:7572021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yazdanpanah L, Shahbazian H, Nazari I,
Hesam S, Ahmadi F, Cheraghian B, Arti HR and Mohammadianinejad SE:
Risk factors associated with diabetic foot ulcer-free survival in
patients with diabetes. Diabetes Metab Syndr. 12:1039–1043. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gouin JP and Kiecolt-Glaser JK: The impact
of psychological stress on wound healing: Methods and mechanisms.
Immunol Allergy Clin North Am. 31:81–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruiz-Llorente L, Contreras-Jurado C,
Martinez-Fernandez M, Paramio JM and Aranda A: Thyroid hormone
receptors regulate the expression of microRNAs with key roles in
skin homeostasis. Thyroid. 28:921–932. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Contreras-Jurado C, Lorz C, Garcia-Serrano
L, Paramio JM and Aranda A: Thyroid hormone signaling controls hair
follicle stem cell function. Mol Biol Cell. 26:1263–1272. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Zheng N, Shi YN, Yuan J and Li L:
Thyroid hormone induced angiogenesis through the integrin
αvβ3/protein kinase D/histone deacetylase 5 signaling pathway. J
Mol Endocrinol. 52:245–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McGill NK, Vyas J, Shimauchi T, Tokura Y
and Piguet V: HTLV-1-associated infective dermatitis: Updates on
the pathogenesis. Exp Dermatol. 21:815–821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimauchi T and Piguet V: DC-T cell
virological synapses and the skin: Novel perspectives in
dermatology. Exp Dermatol. 24:1–4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Capewell P, Cren-Travaille C, Marchesi F,
Johnston P, Clucas C, Benson RA, Gorman TA, Calvo-Alvarez E,
Crouzols A, Jouvion G, et al: The skin is a significant but
overlooked anatomical reservoir for vector-borne African
trypanosomes. Elife. 5:e177162016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alfituri OA, Ajibola O, Brewer JM, Garside
P, Benson RA, Peel T, Morrison LJ and Mabbott NA: Effects of
host-derived chemokines on the motility and viability of
Trypanosoma brucei. Parasite Immunol. 41:e126092019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Strauch ED, Bass BL, Rao JN, Vann JA and
Wang JY: NF-kappaB regulates intestinal epithelial cell and bile
salt-induced migration after injury. Ann Surg. 237:494–501. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen K, Xu L, Chen D, Tang W and Huang Y:
Human cytomegalovirus-encoded miR-UL112 contributes to
HCMV-mediated vascular diseases by inducing vascular endothelial
cell dysfunction. Virus Genes. 54:172–181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu T, Gao M, Yang P, Liu D, Wang D, Song
F, Zhang X and Liu Y: Insulin promotes macrophage phenotype
transition through PI3K/Akt and PPAR-γ signaling during diabetic
wound healing. J Cell Physiol. 234:4217–4231. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhan R, Yang S, He W, Wang F, Tan J, Zhou
J, Yang S, Yao Z, Wu J and Luo G: Nitric oxide enhances
keratinocyte cell migration by regulating Rho GTPase via cGMP-PKG
signalling. PLoS One. 10:e1215512015. View Article : Google Scholar
|
|
35
|
Ikonen E and Jansen M: Cellular sterol
trafficking and metabolism: Spotlight on structure. Curr Opin Cell
Biol. 20:371–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Enrich C, Rentero C, Hierro A and Grewal
T: Role of cholesterol in SNARE-mediated trafficking on
intracellular membranes. J Cell Sci. 128:1071–1081. 2015.PubMed/NCBI
|
|
37
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boulter E, Estrach S, Errante A, Pons C,
Cailleteau L, Tissot F, Meneguzzi G and Féral CC: CD98hc (SLC3A2)
regulation of skin homeostasis wanes with age. J Exp Med.
210:173–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baltzis D, Eleftheriadou I and Veves A:
Pathogenesis and treatment of impaired wound healing in diabetes
mellitus: New insights. Adv Ther. 31:817–836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rekha PD, Rao SS, Sahana TG and Prabhu A:
Diabetic wound management. Br J Community Nurs. 23 (Suppl
9):S16–S22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Catrina SB and Zheng X: Disturbed hypoxic
responses as a pathogenic mechanism of diabetic foot ulcers.
Diabetes Metab Res Rev. 32 (Suppl 1):S179–S185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kimmel HM, Grant A and Ditata J: The
presence of oxygen in wound healing. Wounds. 28:264–270.
2016.PubMed/NCBI
|
|
43
|
Widmer CC, Pereira CP, Gehrig P, Vallelian
F, Schoedon G, Buehler PW and Schaer DJ: Hemoglobin can attenuate
hydrogen peroxide-induced oxidative stress by acting as an
antioxidative peroxidase. Antioxid Redox Signal. 12:185–198. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moreno-Acosta P, Carrillo S, Gamboa O,
Romero-Rojas A, Acosta J, Molano M, Balart-Serra J, Cotes M,
Rancoule C and Magné N: Novel predictive biomarkers for cervical
cancer prognosis. Mol Clin Oncol. 5:792–796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li X, Wu Z, Wang Y, Mei Q, Fu X and Han W:
Characterization of adult α- and β-globin elevated by hydrogen
peroxide in cervical cancer cells that play a cytoprotective role
against oxidative insults. PLoS One. 8:e543422013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu W, Fu Z, Wang H, Feng J, Wei J and Guo
J: Peroxiredoxin 2 is upregulated in colorectal cancer and
contributes to colorectal cancer cells' survival by protecting
cells from oxidative stress. Mol Cell Biochem. 387:261–270. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Low FM, Hampton MB, Peskin AV and
Winterbourn CC: Peroxiredoxin 2 functions as a noncatalytic
scavenger of low-level hydrogen peroxide in the erythrocyte. Blood.
109:2611–2617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ryu J, Park SG, Park BC, Choe M, Lee KS
and Cho JW: Proteomic analysis of psoriatic skin tissue for
identification of differentially expressed proteins: Up-regulation
of GSTP1, SFN and PRDX2 in psoriatic skin. Int J Mol Med.
28:785–792. 2011.PubMed/NCBI
|
|
49
|
Ranieri D, Avitabile D, Shiota M, Yokomizo
A, Naito S, Bizzarri M and Torrisi MR: Nuclear redox imbalance
affects circadian oscillation in HaCaT keratinocytes. Int J Biochem
Cell Biol. 65:113–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dunnill C, Patton T, Brennan J, Barrett J,
Dryden M, Cooke J, Leaper D and Georgopoulos NT: Reactive oxygen
species (ROS) and wound healing: The functional role of ROS and
emerging ROS-modulating technologies for augmentation of the
healing process. Int Wound J. 14:89–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yin X, Liu P, Liu YY, Liu MY, Fan WL, Liu
BY and Zhao JH: LRRFIP1 expression triggers platelet agglutination
by enhancing alphaIIbbeta3 expression. Exp Ther Med. 18:269–277.
2019.PubMed/NCBI
|
|
52
|
Goodall AH, Burns P, Salles I, Macaulay
IC, Jones CI, Ardissino D, de Bono B, Bray SL, Deckmyn H, Dudbridge
F, et al: Transcription profiling in human platelets reveals
LRRFIP1 as a novel protein regulating platelet function. Blood.
116:4646–4656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sanchez-Pernaute O, Esparza-Gordillo J,
Largo R, Calvo E, Alvarez-Soria MA, Marcos ME, Herrero-Beaumont G
and de Córdoba SR: Expression of the peptide C4b-binding protein
beta in the arthritic joint. Ann Rheum Dis. 65:1279–1285. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Koedam JA, Meijers JC, Sixma JJ and Bouma
BN: Inactivation of human factor VIII by activated protein C.
Cofactor activity of protein S and protective effect of von
Willebrand factor. J Clin Invest. 82:1236–1243. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Broekmans AW, Bertina RM, Reinalda-Poot J,
Engesser L, Muller HP, Leeuw JA, Michiels JJ, Brommer EJ and Briët
E: Hereditary protein S deficiency and venous thrombo-embolism. A
study in three Dutch families. Thromb Haemost. 53:273–277. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Burstyn-Cohen T, Heeb MJ and Lemke G: Lack
of protein S in mice causes embryonic lethal coagulopathy and
vascular dysgenesis. J Clin Invest. 119:2942–2953. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhong F, Chen H, Xie Y, Azeloglu EU, Wei
C, Zhang W, Li Z, Chuang PY, Jim B, Li H, et al: Protein S protects
against podocyte injury in diabetic nephropathy. J Am Soc Nephrol.
29:1397–1410. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Valacchi G, Zanardi I, Sticozzi C, Bocci V
and Travagli V: Emerging topics in cutaneous wound repair. Ann N Y
Acad Sci. 1259:136–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Reinke JM and Sorg H: Wound repair and
regeneration. Eur Surg Res. 49:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lopez-Lopez N, Gonzalez-Curiel I,
Trevino-Santa CM, Rivas-Santiago B, Trujillo-Paez V, Enciso-Moreno
JA and Serrano CJ: Expression and vitamin D-mediated regulation of
matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs) in healthy skin and in diabetic foot
ulcers. Arch Dermatol Res. 306:809–821. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gary SR and Woo KY: The biology of chronic
foot ulcers in persons with diabetes. Diabetes Metab Res Rev. 24
(Suppl 1):S25–S30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wilkinson RD, Young A, Burden RE, Williams
R and Scott CJ: A bioavailable cathepsin S nitrile inhibitor
abrogates tumor development. Mol Cancer. 15:292016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yao X, Cheng F, Yu W, Rao T, Li W, Zhao S,
Zhou X and Ning J: Cathepsin S regulates renal fibrosis in mouse
models of mild and severe hydronephrosis. Mol Med Rep. 20:141–150.
2019.PubMed/NCBI
|
|
64
|
Klinngam W, Fu R, Janga SR, Edman MC and
Hamm-Alvarez SF: Cathepsin S alters the expression of
pro-inflammatory cytokines and MMP-9, partially through
protease-activated receptor-2, in human corneal epithelial cells.
Int J Mol Sci. 19:35302018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Taleb S, Cancello R, Clement K and Lacasa
D: Cathepsin S promotes human preadipocyte differentiation:
Possible involvement of fibronectin degradation. Endocrinology.
147:4950–4959. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lenselink EA: Role of fibronectin in
normal wound healing. Int Wound J. 12:313–316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhuo L and Kimata K: Structure and
function of inter-alpha-trypsin inhibitor heavy chains. Connect
Tissue Res. 49:311–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhuo L, Hascall VC and Kimata K:
Inter-alpha-trypsin inhibitor, a covalent
protein-glycosaminoglycan-protein complex. J Biol Chem.
279:38079–38082. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Soury E, Olivier E, Daveau M, Hiron M,
Claeyssens S, Risler JL and Salier JP: The H4P heavy chain of
inter-alpha-inhibitor family largely differs in the structure and
synthesis of its prolin-rich region from rat to human. Biochem
Biophys Res Commun. 243:522–530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pihl R, Jensen RK, Poulsen EC, Jensen L,
Hansen AG, Thøgersen IB, Dobó J, Gál P, Andersen GR, Enghild JJ, et
al: ITIH4 acts as a protease inhibitor by a novel inhibitory
mechanism. Sci Adv. 7:eaba73812021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Taleb S, Cancello R, Poitou C, Rouault C,
Sellam P, Lev P, Bouillot JL, Coussieu C, Basdevant A, Guerre-Millo
M, et al: Weight loss reduces adipose tissue cathepsin S and its
circulating levels in morbidly obese women. J Clin Endocrinol
Metab. 91:1042–1047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
El Eter E, Al Masri A, Habib S, Al Zamil
H, Al Hersi A, Al Hussein F and Al Omran M: Novel links among
peroxiredoxins, endothelial dysfunction, and severity of
atherosclerosis in type 2 diabetic patients with peripheral
atherosclerotic disease. Cell Stress Chaperones. 19:173–181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wen W, Sun H, Yang Y, Jia Y, Fang F, Qin
Y, Zhang M and Wei Y: Usefulness of cathepsin S to predict risk for
obstructive sleep apnea among patients with type 2 diabetes. Dis
Markers. 2020:88191342020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ma Y, Li R, Wang J, Jiang W, Yuan X, Cui J
and Wang C: ITIH4, as an inflammation biomarker, mainly increases
in bacterial bloodstream infection. Cytokine. 138:1553772021.
View Article : Google Scholar : PubMed/NCBI
|