Introduction
Acute pancreatitis (AP) is a common disease, which
can be classified as mild, moderate and severe. Severe disease
develops in ~20% of patients with AP, which progresses rapidly and
can cause systemic inflammatory response syndrome and multiple
organ dysfunction, leading to increased mortality (1). The pathogenesis of AP is not yet
fully understood. Cell death caused by pyroptosis, necrosis,
apoptosis and necroptosis, is involved in the pathogenesis of AP.
Pyroptosis, apoptosis and necroptosis represent three pathways of
genetically encoded necrotic cell death (2). Apoptosis is a highly regulated form
of cell death, characterized by the activation of multiple caspases
(3), and necroptosis is a form of
regulated necrotic cell death mediated by receptor-interacting
protein kinase (RIP) 1 and RIP3 (4,5).
Unlike these, pyroptosis is a programmed cell death pathway, also
known as caspase-1-mediated cell death, that induces the release of
active IL-1β and IL-18 (2).
Increasing evidence indicates a close relationship between AP and
pyroptosis (6). However, the
underlying mechanism by which pyroptosis induces pancreatic injury
leading to AP has not been fully elucidated.
Pyroptosis is one of the main mechanisms of cell
death and causes an acute inflammatory response by activating
inflammasomes and releasing inflammatory cytokines (7). Previous studies have demonstrated
that pyroptosis is involved in the occurrence and development of
infectious diseases, cardiovascular diseases, cancer and numerous
other diseases (8–12). Emerging studies suggest that
toll-like receptors (TLRs) can be recognized as upstream signals by
nucleotide-binding and oligomerization domain-like receptors
(NLRs), which activate the assembly of the NLR pyrin domain
containing 3 (NLRP3) inflammasome and caspase-1 and subsequently
trigger pyroptosis (13–15). Tumor necrosis factor
receptor-associated factor (TRAF)6 is a member of the TRAF protein
family that serves a vital role in TLR signaling pathways (16,17). Furthermore, TRAF6 is required for
apoptosis-associated speck-like protein (ASC) oligomerization and
the assembly of the NLRP3 inflammasome; TRAF6 deficiency
specifically inhibits TLR/IL-1R-initiated NLRP3 inflammasome
activation, caspase-1 cleavage and pyroptosis (18). At present, the roles of TRAF6 in
AP and pyroptosis during AP are still unclear. Therefore, the
present study aimed to determine the role of TRAF6 in rat and cell
models of caerulein (CAE)-induced pancreatic injury. Pyroptosis was
also examined to identify its potential role in AP.
Materials and methods
Experimental rat model
In total, 24 Sprague-Dawley adult rats (male; age,
8–10 weeks; weight, 200–220 g) were purchased from the Experimental
Animal Center of Guangxi Medical University. All rats were housed
in normal barrier cages at a temperature of 25±2°C with 50–70%
humidity using a 12-h light/dark cycle and were allowed free access
to food and water. All rats were checked every 6 h. All animal
experiments were conducted in accordance with the Institutional
Animal Care and Use Committee of Guangxi Medical University
(Nanning, China; approval no. 201910023). The rats were randomly
divided into three groups: i) The control group; ii) the
CAE-induced AP for 24 h (AP24H) group; and iii) the CAE-induced AP
for 48 h (AP48H) group. The rats in the AP24H and AP48H groups were
induced by intraperitoneal injections of CAE (Sigma-Aldrich; Merck
KGaA). A total of seven injections at a dosage of 50 μg/kg CAE were
administered once per hour. Animals were sacrificed 24 or 48 h
after the last injection. The control group was similarly treated
with the same volume of saline.
Cell culture
The human pancreatic duct epithelial HPDE6C7 cell
line was purchased from Guangzhou Jennio Biotech Co., Ltd. HPDE6C7
cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco) at 37°C in a 5%
CO2 incubator. The cells were divided into four groups
according to different time points of stimulation with CAE: i
Control; ii) treatment for 12 h (12H); iii) treatment for 24 h
(24H); and iv) treatment for 48 h (48H). The optimal CAE
intervention time of 48 h was selected for subsequent experiments.
Subsequently, HPDE6C7 cells were once again divided into four
groups: i) The TRAF6 group; ii) the blank group; iii) the TRAF6 +
CAE group; and iv) the blank + CAE group. TRAF6 was overexpressed
by lentiviral infection in the TRAF6 group. The blank group was
used as the negative control by transfecting with blank virus
vector. Subsequently, the TRAF6 group and the blank group were
stimulated CAE for 48 h as the TRAF6 + CAE group and blank + CAE
group.
Lentivirus transfection
In order to facilitate the TRAF6 expression
analysis, the second generation lentiviral packaging system was
used. The study adopted the Ubi-MCS-3FLAG-CBh-gcGFP-IRES-puromycin
lentivirus expression vector (Shanghai Genechem Co., Ltd.), and
293T cells (ATCC) were transformed with 32 µg packaged plasmids
[maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS at 37°C in a 5% CO2 incubator]. The
ratio of packaging vector:envelope plasmid was 1:1. The supernatant
was collected 48 and 72 h after transfection, respectively. Next,
the supernatant was filtered into an ultracentrifuge tube using a
0.45-µm filter membrane. Centrifugation was performed at 72,000 × g
for 2 h at 4°C. The supernatants were then discarded and the
lentivirus deposition was resuspended with 500 µl fresh medium and
kept at −80°C. After 10 days, HPDE6C7 cells were infected with
TRAF6 lentivirus vector using 0.1% Polybrenne and Enhanced
Infection Solution (Shanghai Genechem Co., Ltd.), with a
multiplicity of infection of 5 at 37°C. At 72 h post-infection, the
medium was changed to fresh medium with 0.8 µg/ml puromycin. The
uninfected wild-type cells were set as the control group, with an
equal volume and concentration of puromycin. Fresh
puromycin-containing medium was used as replacement medium every 3
or 4 days until the control group cells died. The stable cell lines
were then cultured with 0.4 µg/ml puromycin. Reverse
transcription-quantitative PCR and western blotting analyses were
performed at 24 h post-transduction.
Blood and tissue preparation
All rats were anesthetized using 2% sodium
pentobarbital (45 mg/kg) intraperitoneally. The abdominal cavity
was opened and 2–3 ml blood was then collected from the abdominal
aorta. Subsequently, all rats were euthanized by rapid cervical
dislocation and checked closely to confirm respiratory arrest. The
pancreatic tissues around the pancreatic duct were removed. The
blood samples were centrifuged for 10 min at 3,500 × g at 4°C and
serum samples were collected. Serum and pancreatic tissues were
stored at −80°C for further experiments.
Histopathological analysis
Fresh pancreatic tissues were fixed in 4%
formaldehyde for 48 h at room temperature, followed by
paraffin-embedding, sectioning and cutting into 4 µm sections. The
sections were deparaffinized and stained with H&E (Beyotime
institute of Biotechnology) for 8 min at room temperature. Images
were captured using a light microscope (Olympus corporation).
According to Van Laethem criteria (19), pancreatic injury was scored in
terms of edema, inflammatory cell infiltration and acinar
necrosis.
Serum and supernatant assays
Amylase (AMY) activity was determined using an
automated clinical chemistry analyzer (Hitachi 7600; Hitachi,
Ltd.). Serum levels of interleukin-1β (IL-1β) (ELISA kit cat. no.
ml037361; Shanghai Enzyme-linked Biotechnology Co., Ltd.) and IL-18
(ELISA kit cat. no. ml002816; Shanghai Enzyme-linked Biotechnology
Co., Ltd.), and cell supernatants of IL-1β (ELISA kit cat. no.
CSB-E08053h; Cusabio Technology, LLC) were analyzed according to
the manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the pancreatic tissues
and HPDE6C7 cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). RNA concentration and purity were
determined using an ultra-micro spectrophotometer. Total RNA was
reverse-transcribed into cDNA using a PrimeScript™ RT reagent kit
with gDNA Eraser (cat. no. RR047A; Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. The Applied Biosystems
7500 qPCR system (Life Technologies Inc.) with SYBR-Green
fluorescence was subsequently used for the qPCR. Primer sequences
are listed in Table I. The
following thermocycling conditions were used to amplify the cDNA in
40 cycles: Pre-denaturation (95°C for 30 sec), annealing (95°C for
5 sec) and extension (60°C for 20 sec). mRNA expression levels were
calculated using the 2−ΔΔCq method (20) and normalized to the internal
reference genes GAPDH or β-actin.
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence (5′-3′) |
|---|
| Human- | F:
CGCGCATAGAACGACAAG |
| TRAF6 | R:
TTTCCAGGGGTGGGTCAAAC |
| Human- | F:
CGGCAAGACCAAGACGTGTGAG |
| NLRP3 | R:
CAGGCTCAGAATGCTCATCATCGG |
| Human- | F:
GAAGAAACACTCTGAGCAAGTC |
| caspase-1 | R:
GATGATGATCACCTTCGGTTTG |
| Human- | F:
AGATGTCCAGCCAGCTGCACC |
| caspase-3 | R:
TGACCCCACCGAACTCAAAGA |
| Human- | F:
CAAATTCCATGGCACCGTCA |
| GAPDH | R:
GACTCCACGACGTACTCAGC |
| Rat-TRAF6 | F:
TTTGGCGTCGGAGACACTTG |
|
| R:
TCGCTTGAAGACTGGCTGGA |
| Rat-NLRP3 | F:
CTGAAGCATCTGCTCTGCAACC |
|
| R:
AACCAATGCGAGATCCTGACAAC |
| Rat-caspase-1 | F:
ACTCGTACACGTCTTGCCCTCA |
|
| R:
CTGGGCAGGCAGCAAATTC |
| Rat-caspase-3 | F:
AGACAGACAGTGGAACTGACGATG |
|
| R:
GGCGCAAAGTGACTGGATGA |
| Rat-β-actin | F:
TTGCTGACAGGATGCAGAA |
|
| R:
ACCAATCCACACAGAGTACTT |
Western blotting
Total protein was extracted from pancreatic tissue
and HPDE6C7 cells using a RIPA buffer with 1% phenylmethylsulfonyl
fluoride. Total protein concentration was quantified using the
Bicinchoninic Acid Protein Assay kit (Wuhan Boster Biological
Technology Co., Ltd.). Protein samples (30 µg/well) were
transferred onto polyvinylidene fluoride membranes following 10%
and 15% SDS-PAGE. Membranes were blocked with 5% skimmed milk for
45 min at room temperature. The membranes were subsequently
incubated with primary antibodies, including those for TRAF6
(1:2,000; cat. no. ab33915; Abcam), NLRP3 (1:1,000; cat. no.
IMG-6668A; Novus Biologicals, Inc.), caspase-1 (1:1,000; cat. no.
ab179515; Abcam), caspase-3 (1:1,000; cat. no. ab14220; Cell
Signaling Technology, Inc.) and β-actin (1:5,000; cat. no. ab6276;
Abcam) overnight at 4°C. After washing with TBS-0.1% Tween-20
buffer, membranes were incubated with goat anti-rabbit IgG
(1:10,000; cat. no. ab4413; Cell Signaling Technology, Inc.) at
room temperature for 1 h. Blots were scanned using the
Odyssey® Fc Imager system (LI-COR Biosciences), and
protein expression was semi-quantified using imageJ software
(version 1.4.1; National Institutes of Health) with β-actin as the
loading control.
Hoechst/PI staining
HPDE6C7 cells were cultured with CAE for 48 h at
37°C and washed with PBS three times. Subsequently, 1 ml cell
staining buffer was added, followed by 5 µl Hoechst staining
solution and 5 µl propidium iodide (PI) staining solution (Beyotime
Institute of Biotechnology) at the same time and placed at 4°C for
30 min in the dark. After washing with PBS three times, the dye
mixture was discarded and the cells were observed under a
fluorescence microscope.
Statistical analysis
All data are presented as the mean ± standard of at
least three independent experiments. Data were analyzed using SPSS
24.0 software (IBM Corp.) and are expressed as the mean ± standard
deviation. One-way ANOVA was used for statistical comparisons
between more than two groups, followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Pancreatic histopathological
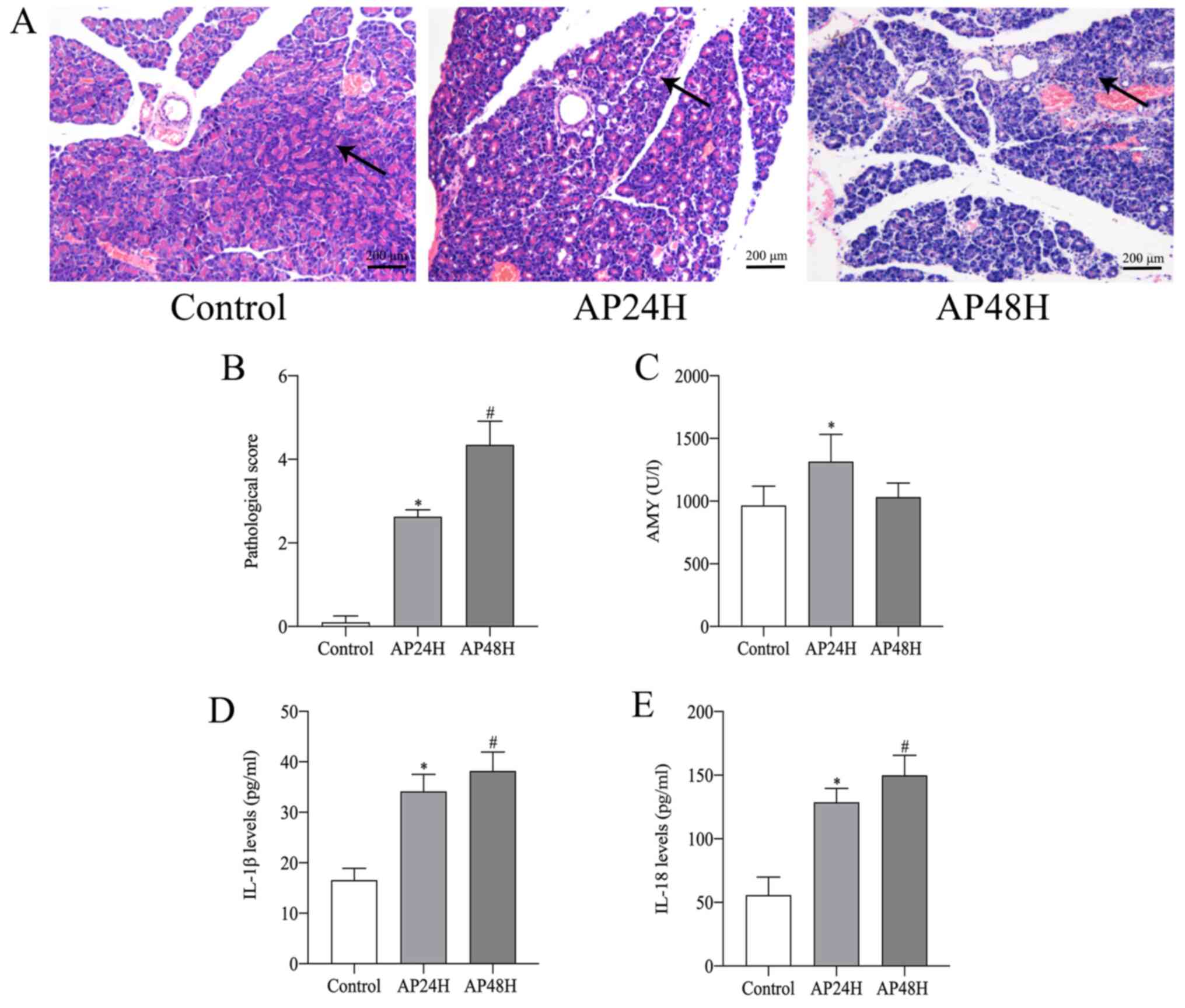
scores
Pancreatic injury was evaluated using a
histopathological scoring system. Histological changes confirmed
that AP was successfully established in the rats. Edema and
inflammatory cell infiltration were observed in the pancreatic
tissues of AP rats, and acinar necrosis was occasionally observed
in the AP48H group (Fig. 1A). The
pancreatic pathological scores of the AP24H group were
significantly higher compared with the control group. Compared with
the AP24H group, the AP48H group displayed significantly increased
pancreatic injury (Fig. 1B).
AMY, IL-1β and IL-18 serum levels
AMY activity and serum levels of IL-1β and IL-18
were detected. The serum activity of AMY was markedly higher in the
AP24H group compared with that in the control group (Fig. 1C). IL-1β and IL-18 serum levels in
the AP groups were significantly higher compared with those in the
control group. Furthermore, compared with the levels in the AP24H
group, the levels in the AP48H group increased to a greater extent
(Fig. 1D and E).
mRNA and protein expression levels of TRAF6, NLPR3,
caspase-1 and caspase-3 in pancreatic tissues. Following induction
with CAE for 24 and 48 h in rats, TRAF6 and caspase-1/3 pathways
were examined by RT-qPCR and western blotting. The mRNA and protein
expression levels of TRAF6, NLPR3, caspase-1 and caspase-3 were
significantly upregulated in the pancreatic tissues of the AP
groups compared with the control group. Moreover, the AP48H group
demonstrated significantly higher mRNA and protein expression
levels compared with the AP24H group (Fig. 2A-I).
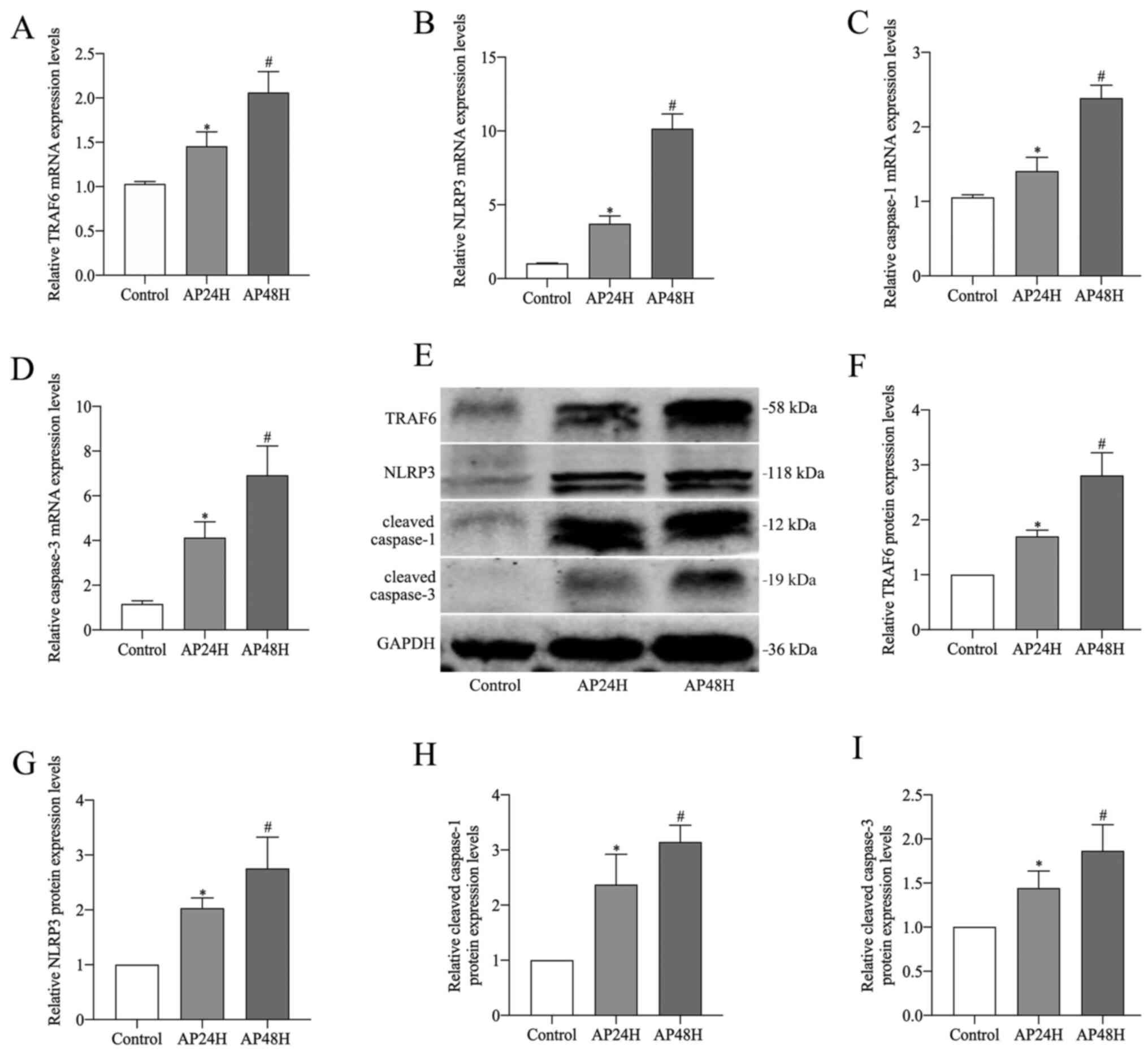 | Figure 2.TRAF6, NLPR3, caspase-1 and caspase-3
mRNA and protein expression levels in pancreatic tissue. Reverse
transcription-quantitative PCR analysis was performed to determine
the expression levels of (A) TRAF6, (B) NLRP3, (C) caspase-1 and
(D) caspase-3. (E) Western blotting was performed to determine the
protein expression levels of (F) TRAF6 (58 kDa), (G) NLRP3 (118
kDa), (H) cleaved caspase-1 (12 kDa) and (I) cleaved caspase-3 (19
kDa). *P<0.05 vs. control; #P<0.05 vs. control and
AP24H. TRAF6, tumor necrosis factor receptor-associated factor 6;
NLRP3, NLR pyrin domain containing 3; AP24H, caerulein-induced
acute pancreatitis for 24 h; AP48H, caerulein-induced acute
pancreatitis for 48 h. |
Construction of lentivirus
transfection vector for overexpression of TRAF6
In order to investigate the potential role of TRAF6
in vitro, TRAF6 was overexpressed in HPDE6C7 cells using
lentiviral infection, and the transfection data are described in
our previous study (21).
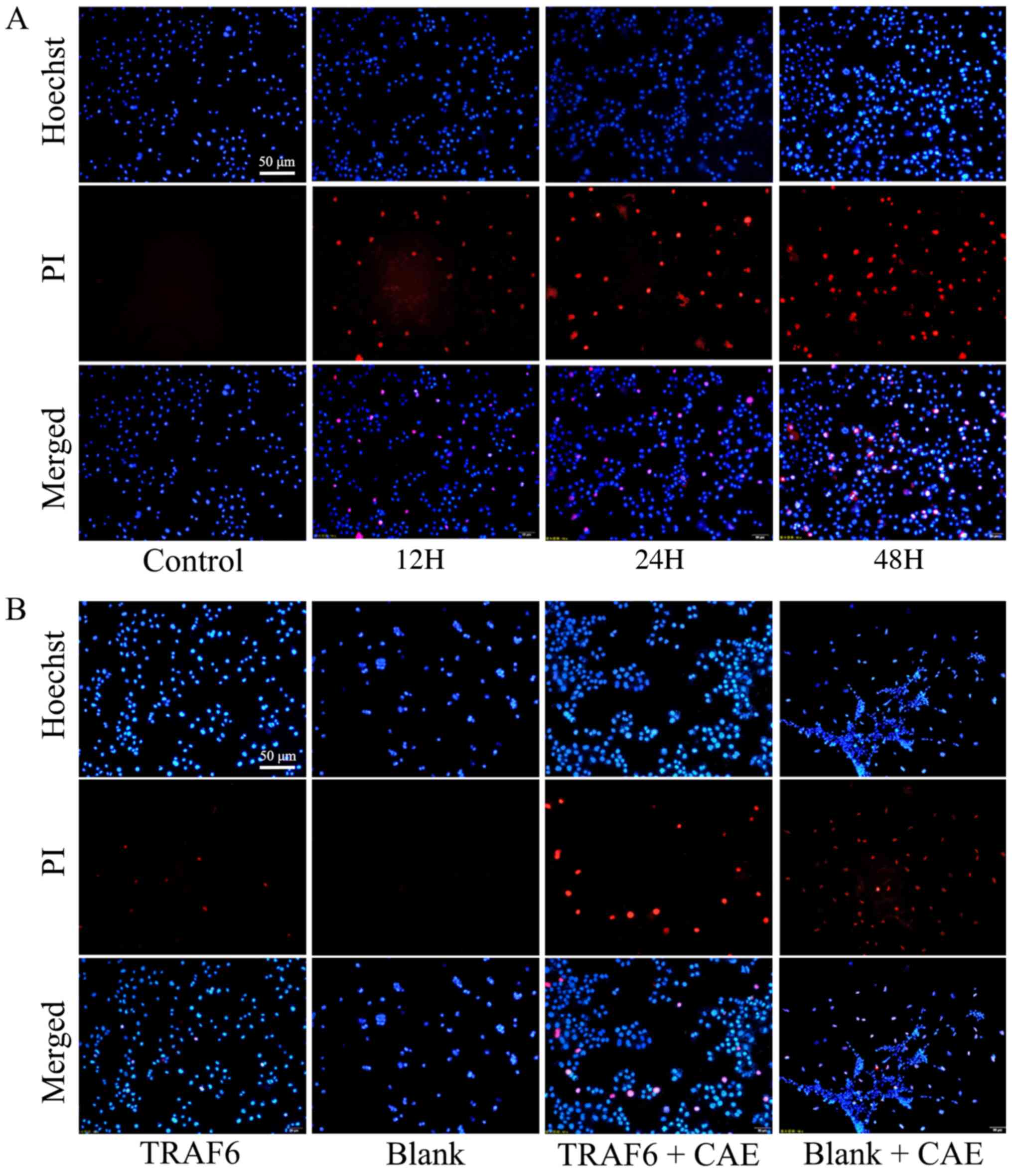
Pyroptosis morphology of HPDE6C7 cells
following CAE treatment and TRAF6 overexpression
HPDE6C7 cells were treated with CAE and TRAF6
overexpression vectors and visualized using Hoechst/PI staining.
Compared with the control, in the 12H group, PI could pass through
the cell membrane into the nucleus and displayed red fluorescence
following treatment with CAE. Red fluorescence was markedly
increased in the 24H and 48H groups compared with the control and
12H group (Fig. 3A). Following
TRAF6 overexpression, a small amount of red fluorescence was
observed in the TRAF6 group, compared with the blank group where
none was observed. After CAE treatment, the red fluorescence
increased in both groups, and that in the TRAF6 + CAE group was
significantly higher than that in the blank + CAE group (Fig. 3B).
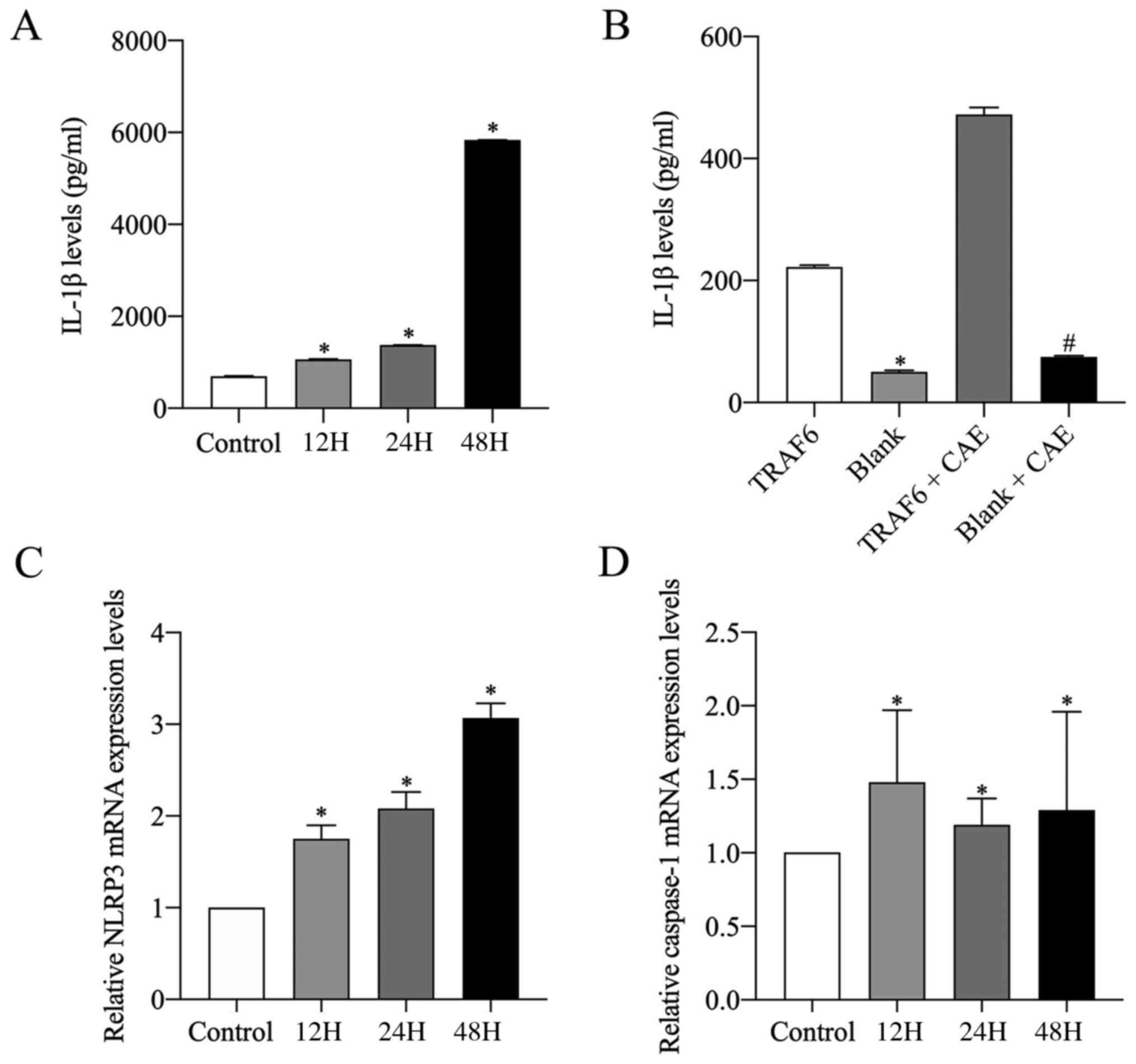
IL-1β levels in the supernatant of
HPDE6C7 cells
The level of IL-1β was also detected in the HPDE6C7
cell supernatant. IL-1β levels in CAE-stimulated groups were
significantly higher compared with the control group. Furthermore,
IL-1β levels increased with CAE stimulation time, with the highest
IL-1β level observed in the 48H group (Fig. 4A). Moreover, IL-1β levels in the
cell supernatants of the TRAF6 group and the TRAF6 + CAE group were
markedly higher compared with the blank group and the blank + CAE
group, respectively (Fig.
4B).
mRNA and protein expression levels of
NLPR3, caspase-1 and caspase-3 in HPDE6C7 cells
As the potential mechanisms of the caspase-1/3
signaling pathways were investigated in rats, this process was also
explored in vitro. NLPR3, caspase-1 and caspase-3 mRNA and
protein expression levels were analyzed via RT-qPCR and western
blotting in HPDE6C7 cells. mRNA expression levels of NLRP3 and
caspase-1 significantly increased with CAE stimulation compared
with the control. NLRP3 mRNA expression increased in a
time-dependent manner when exposed to CAE, with the highest
expression levels observed in the 48H group (Fig. 4C). Caspase-1 mRNA expression
levels were highest in the 12H group, with all CAE-stimulated
groups being significantly higher compared with the control
(Fig. 4D).
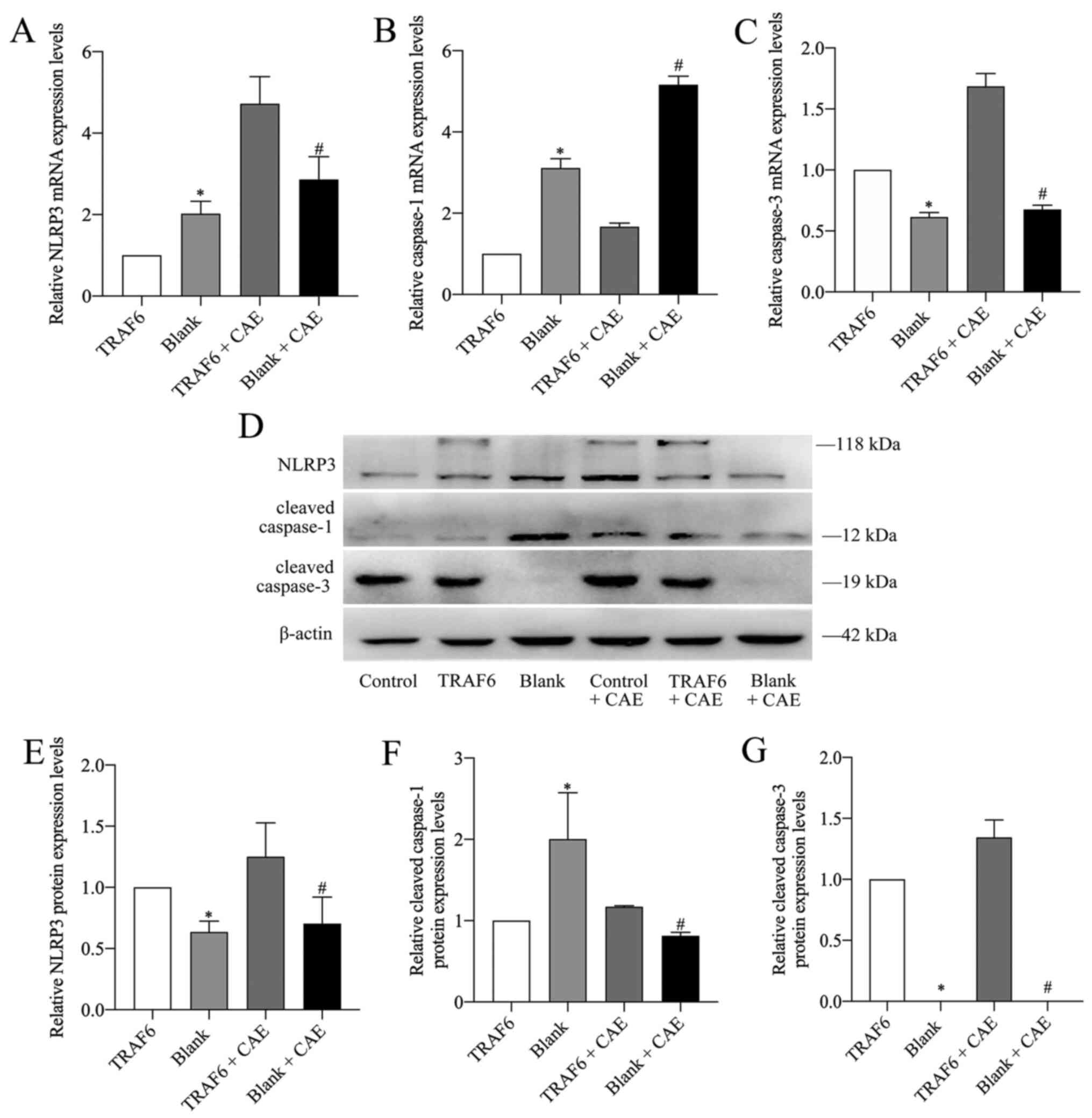
NLRP3 and caspase-1 mRNA and cleaved caspase-1
protein expression levels in the TRAF6 group were significantly
lower compared with the blank group (Fig. 5A, B and F), whereas the protein
expression levels of NLRP3 in the TRAF6 group were significantly
higher compared with the blank group (Fig. 5D and E). Caspase-3 mRNA and
cleaved caspase-3 protein expression levels in the TRAF6 group were
significantly higher compared with that in the blank group
(Fig. 5C, D and G). Following
TRAF6 overexpression, the mRNA expression levels of NLRP3 and
caspase-3 were significantly increased in the TRAF6 + CAE group
compared with the blank + CAE group (Fig. 5A and C). However, caspase-1 mRNA
expression was significantly decreased in the TRAF6 + CAE group
compared with the blank + CAE group (Fig. 5B). Protein expression levels of
NLRP3, cleaved caspase-1 and cleaved caspase-3 in the TRAF6 + CAE
group were significantly higher compared with the blank + CAE group
(Fig. 5D-G).
Discussion
AP is a common inflammatory disease that is often
characterized by abnormal activation of trypsin in the pancreas,
resulting in severe local inflammatory reactions, including edema
and bleeding and necrosis of pancreatic tissues (22). Further disease progression can be
accompanied by heart, lung, kidney and intestinal injury as well as
other complications, thereby increasing the risk of death (23–25).
Pyroptosis is morphologically characterized by both
necrosis and apoptosis. During pyroptosis, the swelling of cells
results in the formation of vesicular projections and numerous 1–2
nm pores on the cell membrane (26). With the rupture of the cell
membrane, cytoplasmic contents are released into the extracellular
space, which induces an inflammatory reaction, changes nuclear
concentration and causes DNA rupture, as well as other changes
(27). Pyroptosis activation
pathways are divided into classical caspase-1-dependent signaling
pathways and non-classical caspase-4/5/11-dependent signaling
pathways, although a previous study has shown that caspase-3 may
also be involved in pyroptosis (28).
NLRs are known to serve a significant role in
pyroptosis. The NLRP3 inflammasome consists of NLRP3 protein, ASC
and pro-caspase-1 (29).
Pathogen-associated molecular patterns, such as bacteria and
viruses, or damage-associated molecular patterns (DAMPs), are
recognized by intracellular and extracellular pattern recognition
receptors, which activate NLRP3 inflammasome assembly, after which
the NLRP3 inflammasome cleaves pro-caspase-1 into active caspase-1
(30). Furthermore, activated
caspase-1 converts pro-IL-1β and pro-IL-18 to mature IL-1β and
IL-18, respectively. Therefore, pyroptosis results in the release
of IL-1β and IL-18, which recruit inflammatory cells and amplify
the inflammatory response (8).
NLRP3-deficient mice display a greater reduction in the
inflammatory response of the AP model, suggesting that NLRP3 may
serve a pivotal role in AP (31).
A recent study suggested that NLRP3 induces the T helper 2
cell-mediated response via IL-18, thus facilitating the development
of the inflammatory response in mice with AP and inhibition of
NLRP3 may be useful to treat patients with severe AP (32). The present study demonstrated that
AP contributed to a significant increase in the mRNA and protein
expression levels of NLRP3, caspase-1 and caspase-3, and the serum
levels of IL-1β and IL-18. These results indicated that NLRP3,
caspase-1 and caspase-3 may serve a role in CAE-induced AP in
rats.
TLR is a crucial component of the innate immune
system, which is involved in the occurrence of AP through the
activation of the NLRP3 inflammasome (33). TLR9 is an important upstream DAMP
receptor that is involved in the pathogenesis of experimentally
induced pancreatitis, along with NLRP3, ASC and caspase-1, and more
importantly, that NLRP3, ASC and caspase-1 are required for
inflammation in AP (34), with
TRAF6 being the central confluence point and key adaptor protein of
most TLR signaling pathways. TRAF6 is a ubiquitin ligase that
mediates the occurrence of acute and chronic inflammatory diseases
(35). A previous study
demonstrated that TRAF6 is a convergence point of the TLR4
signaling pathway, which is critical in AP (36). Hence, in the present study TRAF6
expression levels were detected in the pancreatic tissues. The
results indicated that TRAF6 expression was significantly increased
in CAE-induced AP.
In the present study, the inflammatory response and
pancreatic injury worsened within 24 and 48 h following AP
induction in rats. Serum levels of AMY, IL-1β and IL-18 were
increased. Simultaneously, the mRNA and protein expression levels
of TRAF6, NLRP3, caspase-1 and caspase-3 were also significantly
elevated in pancreatic tissues. These results indicated that the
TRAF6, NLRP3 and caspase-1/3 signaling pathways, are also involved
in the pathogenesis of AP. It was therefore hypothesized that
pancreatic tissue injury may be associated with the activation of
pyroptosis, which leads to an inflammatory response.
To investigate the potential mechanisms by which
TRAF6 is involved in pyroptosis, HPDE6C7 cells were treated with
CAE to induce and observe pyroptosis. Hoechst/PI staining
demonstrated that following treatment with CAE, extensive red
fluorescence appeared in HPDE6C7 cells at 12, 24 and 48 h,
especially at 48 h. PI staining can also demonstrate the rounding,
swelling and necrosis of cells. Since the integrity of the cell
membrane is destroyed during necrosis, PI can freely pass through
the cell membrane and as a result cells exhibit red fluorescence,
which is considered to represent pyroptosis (37,38). Following TRAF6 overexpression, a
small amount of necrosis was found in the TRAF6 group, while
CAE-stimulated cells for 48 h exhibited higher red fluorescence in
the TRAF6 + CAE group and the Blank + CAE group.
The present study also confirmed that supernatant
IL-1β, NLRP3 and caspase-1 mRNA expression levels significantly
increased following CAE treatment of HPDE6C7 cells. However, TRAF6
overexpression significantly decreased NLRP3 mRNA expression in the
TRAF6 group compared with the blank group. The detection of the
mRNA expression levels of numerous genes cannot predict their
protein expression levels (39).
Since the effector molecule of a gene is the protein and not the
RNA, NLRP3 protein expression was analyzed via western blotting.
Western blotting demonstrated that NLRP3 protein expression levels
in the TRAF6 group were significantly higher compared with the
blank group. Furthermore, cleaved caspase-1 protein expression
levels were significantly decreased in the TRAF6 group compared
with the blank group. However, cleaved caspase-1 protein expression
levels were significantly higher in the TRAF6 + CAE group than in
the blank + CAE group following CAE treatment. This result
indicated that TRAF6 overexpression may lead to a decrease in
cleaved caspase-1 protein expression levels under viral
transduction and an increase in cleaved caspase-1 when treated with
CAE in the TRAF6 + CAE group. Significantly increased supernatant
IL-1β, caspase-3 mRNA and protein expression levels were also
observed in the TRAF6 and TRAF6 + CAE groups. The results of the
present study therefore indicated that CAE treatment and TRAF6
overexpression increased the aforementioned parameters and
therefore indicated the activation of pyroptosis.
In conclusion, TRAF6 and the caspase-1/3 signaling
pathways served a role in CAE-induced AP in rats. Pyroptosis in AP
was attributed to a combination of CAE and TRAF6 in the human
pancreatic ductal epithelial cells. In this study, it was
preliminarily found that TRAF6 may be related to the pyroptosis of
AP. These findings provided evidence to support further
investigations into TRAF6 in pyroptosis during AP.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81960126), the
Nanning Qingxiu District Science and Technology Project (grant no.
2019026) and the Guangxi Health Committee Self-Funded Research
Project (grant no. Z20190894).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW, YG and ZL contributed to the design, analysis
and interpretation of the study. BW, YG, HY and JZ contributed to
the data collection, data analysis and preparation of the
manuscript. ZS contributed materials and assisted in the data
analysis. BW, YG and ZS contributed to the writing and revision of
the manuscript. BW and YG confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Guangxi Medical University
(Nanning, China; approval no. 201910023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah AP, Mourad MM and Bramhall SR: Acute
pancreatitis: Current perspectives on diagnosis and management. J
Inflamm Res. 11:77–85. 2018. View Article : Google Scholar
|
|
2
|
Sendler M, Mayerle J and Lerch MM:
Necrosis, apoptosis, necroptosis, pyroptosis: It matters how acinar
cells die during pancreatitis. Cell Mol Gastroenterol Hepatol.
2:407–408. 2016. View Article : Google Scholar
|
|
3
|
Wang Q, Liu S and Han Z: miR-339-3p
regulated acute pancreatitis induced by caerulein through targeting
TNF receptor-associated factor 3 in AR42J cells. Open Life Sci.
15:912–922. 2020. View Article : Google Scholar
|
|
4
|
Wu K, Yao G, Shi X, Zhang H, Zhu Q, Liu X,
Lu G, Hu L, Gong W, Yang Q, et al: Asiaticoside ameliorates acinar
cell necrosis in acute pancreatitis via toll-like receptor 4
pathway. Mol Immunol. 130:122–132. 2021. View Article : Google Scholar
|
|
5
|
Song G, Ma Z, Liu D, Qian D, Zhou J, Meng
H, Zhou B and Song Z: Bone marrow-derived mesenchymal stem cells
attenuate severe acute pancreatitis via regulation of microRNA-9 to
inhibit necroptosis in rats. Life Sci. 223:9–21. 2019. View Article : Google Scholar
|
|
6
|
Lin T, Song J, Pan X, Wan Y, Wu Z, Lv S,
Mi L, Wang Y and Tian F: Downregulating gasdermin D reduces severe
acute pancreatitis associated with pyroptosis. Med Sci Monit.
27:e9279682021.
|
|
7
|
Fischer FA, Chen KW and Bezbradica JS:
Posttranslational and therapeutic control of gasdermin-mediated
pyroptosis and inflammation. Front Immunol. 12:6611622021.
View Article : Google Scholar
|
|
8
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017. View Article : Google Scholar
|
|
9
|
Jia C, Chen H, Zhang J, Zhou K, Zhuge Y,
Niu C, Qiu J, Rong X, Shi Z, Xiao J, et al: Role of pyroptosis in
cardiovascular diseases. Int Immunopharmacol. 67:311–318. 2019.
View Article : Google Scholar
|
|
10
|
Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R,
Qiu WQ, Pan R, Law BY, Wong VK, Yu CL, et al: Polyphyllin VI
induces caspase 1 mediated pyroptosis via the induction of ROS/NF
κB/NLRP3/GSDMD signal axis in non small cell lung cancer. Cancers
(Basel). 12:1932020. View Article : Google Scholar
|
|
11
|
Wu C, Chen H, Zhuang R, Zhang H, Wang Y,
Hu X, Xu Y, Li J, Li Y, Wang X, et al: Betulinic acid inhibits
pyroptosis in spinal cord injury by augmenting autophagy via the
AMPK-mTOR-TFEB signaling pathway. Int J Biol Sci. 17:1138–1152.
2021. View Article : Google Scholar
|
|
12
|
Cheng L and Zhang W: DJ-1 affects
oxidative stress and pyroptosis in hippocampal neurons of
Alzheimer's disease mouse model by regulating the Nrf2 pathway. Exp
Ther Med. 21:5572021. View Article : Google Scholar
|
|
13
|
Lin KM, Hu W, Troutman TD, Jennings M,
Brewer T, Li X, Nanda S, Cohen P, Thomas JA and Pasare C: IRAK-1
bypasses priming and directly links TLRs to rapid NLRP3
inflammasome activation. Proc Natl Acad Sci USA. 111:775–780.
2014.Erratum in: Proc Natl Acad Sci USA 111: 3195, 2014. View Article : Google Scholar
|
|
14
|
Bortoluci KR and Medzhitov R: Control of
infection by pyroptosis and autophagy: Role of TLR and NLR. Cell
Mol Life Sci. 67:1643–1651. 2010. View Article : Google Scholar
|
|
15
|
Ringel-Scaia VM, McDaniel DK and Allen IC:
The goldilocks conundrum: NLR inflammasome modulation of
gastrointestinal inflammation during inflammatory bowel disease.
Crit Rev Immunol. 36:283–314. 2016. View Article : Google Scholar
|
|
16
|
Walsh MC, Lee J and Choi Y: Tumor necrosis
factor receptor-associated factor 6 (TRAF6) regulation of
development, function, and homeostasis of the immune system.
Immunol Rev. 266:72–92. 2015. View Article : Google Scholar
|
|
17
|
Yoon K, Jung EJ, Lee SR, Kim J, Choi Y and
Lee SY: TRAF6 deficiency promotes TNF-induced cell death through
inactivation of GSK3beta. Cell Death Differ. 15:730–738. 2008.
View Article : Google Scholar
|
|
18
|
Xing Y, Yao X, Li H, Xue G, Guo Q, Yang G,
An L, Zhang Y and Meng G: Cutting edge: TRAF6 mediates TLR/IL 1R
signaling induced nontranscriptional priming of the NLRP3
inflammasome. J Immunol. 199:1561–1566. 2017. View Article : Google Scholar
|
|
19
|
Van Laethem JL, Marchant A, Delvaux A,
Goldman M, Robberecht P, Velu T and Devière J: Interleukin 10
prevents necrosis in murine experimental acute pancreatitis.
Gastroenterology. 108:1917–1922. 1995. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
21
|
Wei BW, Gong YH, Su Z, Yang HY, Qin MB and
Liang ZH: Involvement of miR 125b in human pancreatic ductal
epithelial barrier affected by TRAF6. Basic Clin Med. 41:1272–1276.
2021.(In Chinese).
|
|
22
|
Yu JH and Kim H: Oxidative stress and
inflammatory signaling in cerulein pancreatitis. World J
Gastroenterol. 20:17324–17329. 2014. View Article : Google Scholar
|
|
23
|
Garg PK and Singh VP: Organ failure due to
systemic injury in acute pancreatitis. Gastroenterology.
156:2008–2023. 2019. View Article : Google Scholar
|
|
24
|
Wang B, Li XH, Song Z, Li ML, Wu XW, Guo
MX, Zhang XH and Zou XP: Isoacteoside attenuates acute kidney
injury induced by severe acute pancreatitis. Mol Med Rep. 23:1–10.
2021. View Article : Google Scholar
|
|
25
|
Hu J, Zhang YM, Miao YF, Zhu L, Yi XL,
Chen H, Yang XJ, Wan MH and Tang WF: Effects of Yue-Bi-Tang on
water metabolism in severe acute pancreatitis rats with acute
lung-kidney injury. World J Gastroenterol. 26:6810–6821. 2020.
View Article : Google Scholar
|
|
26
|
Fink SL and Cookson BT:
Caspase-1-dependent pore formation during pyroptosis leads to
osmotic lysis of infected host macrophages. Cell Microbiol.
8:1812–1825. 2006. View Article : Google Scholar
|
|
27
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar
|
|
28
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar
|
|
29
|
Sutterwala FS, Ogura Y, Zamboni DS, Roy CR
and Flavell RA: NALP3: A key player in caspase-1 activation. J
Endotoxin Res. 12:251–256. 2006. View Article : Google Scholar
|
|
30
|
Martins JD, Liberal J, Silva A, Ferreira
I, Neves BM and Cruz MT: Autophagy and inflammasome interplay. DNA
Cell Biol. 34:274–281. 2015. View Article : Google Scholar
|
|
31
|
Fu Q, Zhai Z, Wang Y, Xu L, Jia P, Xia P,
Liu C, Zhang X, Qin T and Zhang H: NLRP3 deficiency alleviates
severe acute pancreatitis and pancreatitis associated lung injury
in a mouse model. BioMed Res Int. 2018:12949512018. View Article : Google Scholar
|
|
32
|
Sendler M, van den Brandt C, Glaubitz J,
Wilden A, Golchert J, Weiss FU, Homuth G, De Freitas Chama LL,
Mishra N, Mahajan UM, et al: NLRP3 inflammasome regulates
development of systemic inflammatory response and compensatory anti
inflammatory response syndromes in mice with acute pancreatitis.
Gastroenterology. 158:253–269.e14. 2020. View Article : Google Scholar
|
|
33
|
Liu Y and Li Y, Chen KL, Zhou B, Lv ZY,
Zhou ZG and Li Y: Knockdown of myeloid differentiation factor 88
attenuates lipopolysaccharide Induced inflammatory response in
pancreatic ductal cells. Pancreas. 45:755–760. 2016. View Article : Google Scholar
|
|
34
|
Hoque R, Sohail M, Malik A, Sarwar S, Luo
Y, Shah A, Barrat F, Flavell R, Gorelick F, Husain S, et al: TLR9
and the NLRP3 inflammasome link acinar cell death with inflammation
in acute pancreatitis. Gastroenterology. 141:358–369. 2011.
View Article : Google Scholar
|
|
35
|
Abdullah M, Berthiaume JM and Willis MS:
Tumor necrosis factor receptor-associated factor 6 as a nuclear
factor kappa B-modulating therapeutic target in cardiovascular
diseases: At the heart of it all. Transl Res. 195:48–61. 2018.
View Article : Google Scholar
|
|
36
|
Zhou XY, Zhou ZG, Ding JL, Wang L, Wang R,
Zhou B, Gu J, Sun XF and Li Y: TRAF6 as the key adaptor of TLR4
signaling pathway is involved in acute pancreatitis. Pancreas.
39:359–366. 2010. View Article : Google Scholar
|
|
37
|
Brennan MA and Cookson BT: Salmonella
induces macrophage death by caspase-1-dependent necrosis. Mol
Microbiol. 38:31–40. 2000. View Article : Google Scholar
|
|
38
|
Yang Y, Liu PY, Bao W, Chen SJ, Wu FS and
Zhu PY: Hydrogen inhibits endometrial cancer growth via a
ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer.
20:282020. View Article : Google Scholar
|
|
39
|
Chen G, Gharib TG, Huang CC, Taylor JM,
Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB,
Hanash SM, et al: Discordant protein and mRNA expression in lung
adenocarcinomas. Mol Cell Proteomics. 1:304–313. 2002. View Article : Google Scholar
|