Introduction
Psoriasis is a chronic inflammatory skin disease
that affects 2–3% of the population in the United States, and its
prevalence has been increasing over the past few decades (1,2). A
lack of understanding exists regarding the mechanisms leading to
the increased immunological response in psoriasis, and a complete
cure is not available for this disease (3,4).
Patients are commonly prescribed steroids for treatment. However,
the use of steroids is not recommended by dermatological textbooks
and guidelines owing to the high number of side effects (5). Therefore, an effective psoriasis
treatment that is devoid of side effects is urgently required
(6).
Psoriasis is a T cell-mediated skin inflammatory
disorder that is characterized by the hyperproliferation and
abnormal differentiation of the epidermal keratinocytes (7). The condition results from the
continuous interactions between the infiltrated inflammatory cells
and the activated keratinocytes (8). The infiltration of T cells and
neutrophils leads to the production of various cytokines, such as
interleukin (IL)-17, IL-23 and interferon (IFN)-γ, which stimulate
the keratinocytes and exacerbate psoriasis (9). Moreover, these cytokines activate
the signal transducers and activators of transcription 1 (STAT1)
and nuclear factor-κB (NF-κB) in the psoriatic keratinocytes
(10). Therefore, pharmacological
interventions that effectively suppress the signaling molecules
that induce inflammatory mediators are potential targets for the
development of therapeutic compounds.
The fruit of Schisandra chinensis (Turcz).
Baill. (Schisandraceae; S. chinensis) is a commonly known
traditional Chinese herb that is widely used to treat asthma,
rheumatism, cough and arthritis (11,12). In addition, S. chinensis
has antiallergic, anti-inflammatory, antitumor and antiviral
effects (11,13,14). S. chinensis is rich in
bioactive components, including lignans, triterpenoids,
polysaccharides and sterols (15).
In particular, lignans, which function as
antioxidants and play a role in plant defenses, and exhibit
anti-inflammatory and antioxidant activities that can be utilized
to treat skin diseases (16).
Among the lignans, gomisin M2 (GM2) has shown antiallergic effects
by inhibiting mast cell activation and the human immunodeficiency
virus (17–19).
Although GM2 exerts diverse pharmacological effects,
its molecular mechanisms have not been demonstrated in studies on
skin inflammatory disorders, particularly psoriasis. Based on the
diverse pharmacological effects of GM2 extracted from S.
chinensis, the present study investigated the anti-inflammatory
effects of GM2 on an imiquimod (IMQ)-induced mouse model and
keratinocytes.
Materials and methods
Plant material, isolation of GM2 and
reagents
The fruits of S. chinensis were purchased
from the Yangnyeong Herbal Medicine Market (Daegu, Korea). The
fruits (20 kg) were subjected to 95% ethanol (10 l) extraction at
room temperature for 5 days. The ethanolic extract was evaporated
in vacuo to yield 5.7 kg residue, which was resuspended in
H2O and successively partitioned with dichloromethane,
ethyl acetate and n-butanol. The dichloromethane extract (525 g)
was then subjected to silica gel column chromatography with a
gradient of ether/acetone (20:1 to 1:2) to obtain five major
fractions (Fr. 1-Fr. 5). Fr. 1 was subjected to Sephadex LH-20
elution with methanol/H2O (1:1), yielding two fractions
(Fr. 1-1, Fr. 1-2). Medium-pressure liquid chromatography of Fr.
1-1 and Fr. 1-2 eluted with MeOH/H2O (10:1 to 1:1)
yielded GM2 (64 mg). GM2 was identified by 1H and
13C-nuclear magnetic resonance, and the spectral data
were compared with published data (20).
The purity of GM2 isolated from S. chinensis
was confirmed to be 94.8% pure by percentage of the peak area of
GM2 compared with the total peak area using high-performance liquid
chromatography with diode-array detection (Fig. S1A-C), and the analysis conditions
are described in Table I. All
reagents were purchased from Sigma-Aldrich (Merck KGaA) unless
otherwise stated, and recombinant human tumor necrosis factor
(TNF)-α and IFN-γ were procured from R&D Systems, Inc.
 | Table I.Conditions of HPLC-DAD. |
Table I.
Conditions of HPLC-DAD.
| Parameters | Conditions |
|---|
| Analytical
column | Phenomenex C18
(4.6×300 mm) |
| HPLC system | Gilson HPLC
system |
| Detector | Diode Array
detector (200~500 nm) |
| Mobile phase for
Solvent A | Water, % |
| 0
min | 60 |
| 30
min | 15 |
| 40
min | 15 |
| 60
min | 60 |
| Mobile phase for
Solvent B | Methanol, % |
| 0
min | 40 |
| 30
min | 85 |
| 40
min | 85 |
| 60
min | 40 |
| Flow rate,
ml/min | 1.0 |
| Column oven
temperature, °C | 30 |
| Injection volume,
µl | 20 |
| Run time, min | 60 |
Ethics statement and cell culture
C57BL/6J female mice (age, 8 weeks old; weight,
18–20 g) were purchased from the Dae-Han Experimental Animal Center
(Daejeon, Korea). Throughout the study period, four mice were
housed per cage in a laminar air flow room maintained at a
temperature of 23±2°C and a relative humidity of 55±5% with 12-h
light/dark cycles. The care and treatment of the mice were
performed in accordance with the guidelines established by the
Public Health Service Policy on the Humane Care and Use of
Laboratory Animals (21). The
study protocols were approved by the Institutional Animal Care and
Use Committee of Kyungpook National University (approval no.
KNU-2019-0001-5; Daegu, Korea).
Human keratinocyte cell line, HaCaT (American Type
Culture Collection), were maintained in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.), supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
antibiotics (100 U/ml penicillin G and 100 µg/ml streptomycin;
Gibco; Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2.
Cell viability
To quantify cell viability, human keratinocytes
(HaCaT cells) were seeded in 96-well plates at 1×104
cells/well. Cells were treated with CD or CA at 37°C for 24 h. A
total of 20 µl
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution (1 mg/ml) was added to each well. The plates were
incubated at 37°C for 4 h. The resulting formazan crystals were
dissolved using 100 µl DMSO per well, and the absorbance was
measured using a spectrophotometer (Molecular Devices, LLC) at a
wavelength of 570 nm.
IMQ-induced psoriasis-like skin
inflammation model and treatment with GM2
To induce psoriasis-like skin inflammation, the
hairs present in the back skin of each mouse were removed by
shaving. The mice (n=28) were randomly divided into seven groups of
four mice each: i) Control [phosphate buffered saline (PBS)]; ii)
10 mg/kg GM2; iii) IMQ + PBS; iv) IMQ + 0.1 mg/kg GM2; v) IMQ + 1
mg/kg GM2; vi) IMQ + 10 mg/kg GM2; and vii) IMQ + 1 mg/kg
dexamethasone (Dexa). IMQ cream (5%, Aldara™; Dong-A Pharmaceutical
Co., Ltd.), 62.5 mg, was applied on the back skin. GM2 and Dexa
were dissolved in PBS and orally administrated by gavage for 7
consecutive days. Dexa is used as a first-line treatment for
psoriasis and is administered by topical, oral, intravenous or
intramuscular injection (22–24). Therefore, Dexa was used as an
orally administered positive drug control under the same conditions
as GM2 in this study. Based on the use of Dexa (1 mg/kg) as a
positive drug control in various studies (25–27), 1 mg/kg Dexa was used in this
study.
Each day, the following parameters were measured
after 24 h: i) Skin thickness; ii) the Psoriasis Area Severity
Index (PASI); and iii) transepidermal water loss (TEWL). The
back-skin thickness was measured using a dial thickness gauge
(Mitutoyo Corporation), whereas TEWL was measured using a gpskin
Barrier Light. PASI was estimated using the erythema, scaling and
thickness parameters, and was scored independently on a 5-point
scale: i) 0, none; ii) 1, slight; iii) 2, moderate; iv) 3, marked;
and v) 4, very marked. Skin thickness, TEWL and the PASI score were
determined for 7 consecutive days.
To examine the characteristics associated with
psoriasis, photographs were taken of the back skin of mice at day
7. Next, the mice were put into a chamber and then 30% vol/min of
CO2 gas was injected into the chamber while controlling
the flowmeter at the end of the experiment (7 days). After
euthanasia, the back skin, spleen and whole blood from the
abdominal vena cava were collected. The back skin was used for
histological analysis and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR), and the whole blood was
centrifuged at 400 × g, for 15 min, at 4°C for the enzyme-linked
immunosorbent assay (ELISA).
ELISA
The serum levels of immunoglobulin (Ig) G2a (cat.
no. 552576; BD Biosciences), TNF-α (cat. no. 558534; BD
Biosciences) and myeloperoxidase (MPO; cat. no. DY3667; R&D
Systems, Inc.) were measured using a sandwich ELISA kit as per the
manufacturer's protocol. IL-6 (cat. no. DY206; R&D Systems,
Inc.) and CCL17 (cat. no. DY364; R&D Systems, Inc.) levels in
HaCaT cells were measured using ELISA kits. The cells
(2×105 cells/well in a 24-well plate) were pretreated
with or without GM2 (0.1, 1 or 10 µM) or Dexa (10 µM) for 1 h, and
then stimulated with TNF-α (10 ng/ml) and IFN-γ (10 ng/ml) at 37°C
for 15 h. These supernatants were collected and centrifuged at 400
× g at 4°C for 10 min. IL-6 and CCL17 levels were analyzed in
accordance with the manufacturer's instructions. Absorbance was
read at 450 nm using a spectrophotometer (VersaMax; Molecular
Devices, LLC). Data analysis was performed SoftMax Pro software
version 6 (Molecular devices, LLC).
RNA isolation and RT-qPCR
RNA samples were isolated from the HaCaT cells and
the mice back skin using the RNAiso Plus kit (Takara Bio, Inc.) as
per the manufacturer's protocol. The HaCaT cells (2×105
cells/well in a 24-well plate) were pretreated with GM2 (0.1, 1 and
10 µM) or Dexa (10 µM) at 37°C for 1 h and cotreated with TNF-α (10
ng/ml) and IFN-γ (10 ng/ml) at 37°C for 6 h. At the end of the
in vivo experimental period, the back skins of the mice were
collected and homogenized by a TissueLyser II (Qiagen GmbH).
For cDNA synthesis, the RevertAid RT kit (Thermo
Fisher Scientific, Inc.) was used as per the following conditions:
65°C for 5 min, 42°C for 60 min and 70°C for 5 min. RT-qPCR was
performed using the TB Green Premix Ex Taq (TIi RNaseH Plus; Takara
Bio, Inc.). The primer sequences are listed in Table II.
 | Table II.Sequences of primer pairs for reverse
transcription-quantitative PCR. |
Table II.
Sequences of primer pairs for reverse
transcription-quantitative PCR.
| A, In vitro
(Human) |
|---|
|
|---|
| Primer | Sequence
(5′→3′) | GenBank accession
number |
|---|
| CCL17 | F:
GTTCGGACCCCAACAACAAG | NM_002987.3 |
|
| R:
TGGCTCCAGTTCAGACAAGG |
|
| IL-1β | F:
GCTGATGGCCCTAAACAGATGAA | NM_000576.3 |
|
| R:
TGAAGCCCTTGCTGTAGTGGTG |
|
| IL-6 | F:
AAAGAGGCACTGGCAGAAAA | NM_001371096.1 |
|
| R:
ATCTGAGGTGCCCATGCTAC |
|
| IL-8 | F:
GGTGCAGTTTTGCCAAGGAG | NM_000584.4 |
|
| R:
TGCTTGAAGTTTCACTGGCATC |
|
| GAPDH | F:
CGACCACTTTGTCAAGCTCA | NM_002046.7 |
|
| R:
AGGGGAGATTCAGTGTGGTG |
|
|
| B, In
vivo (mouse) |
|
| Primer | Sequence
(5′→3′) | GenBank
accession number |
|
| CXCL1 | F:
TGTGGGAGGCTGTGTTTGTA | NM_008176.3 |
|
| R:
ACGAGACCAGGAGAAACAGG |
|
| TNF-α | F:
GGCAGGTCTACTTTGGAGTCATTGC | NM_001278601.1 |
|
| R:
ACATTCGAGGCTCCAGTGAATTCGG |
|
| IL-17A | F:
TTTAACTCCCTTGGCGCAAAA | NM_010552.3 |
|
| R:
CTTTCCCTCCGCATTGACAC |
|
| IFN-γ | F:
TCAAGTGGCATAGATGTGGAAGAA | NM_008337.4 |
|
| R:
TGGCTCTGCAGGATTTTCATG |
|
| IL-1β | F:
ATAACCTGCTGGTGTGTGAC | NM_008361.4 |
|
| R:
AGGTGCTGATGTACCAGTTG |
|
| IL-23 | F:
CATGGGGCTATCAGGGAGTA | NM_031252.2 |
|
| R:
AATAATGTGCCCCGTATCCA |
|
| GAPDH | F:
TGCTCCTCCCTGTTCCAGA | NM_008084.3 |
|
| R:
TACGGCCAAATCCGTTCACA |
|
The thermocycling conditions were as follows: 10 sec
at 95°C, 40 cycles of 5 sec at 95°C and 30 sec at 60°C, 15 sec at
95°C, 30 sec at 60°C and 15 sec at 95°C. Quantification analysis
was performed using the 2−∆∆Cq method (28). mRNA expression was normalized
against glyceraldehyde 3-phosphate dehydrogenase for both the HaCaT
cells and skin tissue, which were quantified by using the TP850
software (version 5.11; Takara Bio, Inc.).
Histological and immunohistochemical
(IHC) analysis
The mice back skins were fixed with 10% formaldehyde
at room temperature for 48 h and embedded in paraffin; 6-µm
sections were stained with toluidine blue (cat. no. 6586-04-5;
Sigma-Aldrich; Merck KGaA) at room temperature for 30 sec to
observe the mast cells, hematoxylin and eosin (H&E) at room
temperature for 40 min or IHC (for MPO or CD4) for histological
analysis. The epidermis and dermis of the tissues were observed
under the HBO 100 light microscope (Carl Zeiss AG). The epidermal
and dermal thickness were measured by a stage micrometer 10:100
lens (magnification, ×200; Carl Zeiss AG), which was equipped on
microscopic lens. The toluidine blue, H&E and IHC-stained
sections were observed under ×200 magnification. IHC analysis was
performed to detect the expression of MPO or CD4 in mice skin
tissue. In brief, sections were deparaffinized in xylene, followed
by treatment with graded ethanol solutions and rehydration in PBS.
Sections were treated with 3% hydrogen peroxide for 5 min, followed
by microwave antigen retrieval at 95°C for 15 min. Next, the slides
were blocked with blocking buffer [1X PBS/5% normal goat serum
(cat. no. ab7481; Abcam)/0.05% Tween-20] at room temperature for 1
h. The slides were then incubated with rabbit monoclonal antibodies
against MPO (1:100; cat. no. ab208670; Abcam) or CD4 (1:100; cat.
no. ab183685; Abcam) at 4°C overnight. Subsequently, the slides
were incubated with a secondary horseradish peroxidase-conjugated
anti-rabbit IgG (1:500; cat. no. 7074S; Cell Signaling Technology,
Inc.) at room temperature for 1 h. Positive reaction was observed
with 3,3′-diaminobenzidine (Vector Laboratories, Inc.; Maravai
LifeSciences), and the slides were counterstained with hematoxylin
at room temperature for 1 min. The IHC-stained sections were
observed under the HBO 100 light microscope (Carl Zeiss AG).
Western blotting
HaCaT cells (1×106 cells/well) were
seeded in a 6-well plate and pretreated with GM2 (10 µM) or Dexa
(10 µM) at 37°C for 1 h and then treated with TNF-α (10 ng/ml) and
IFN-γ (10 ng/ml) at 37°C for 15 min. After stimulation, cells were
washed twice with 1 ml cold PBS, centrifuged at 1,200 × g at 4°C
for 10 min, resuspended in 400 µl ice-cold hypotonic buffer (10 mM
HEPES/KOH, 2 mM MgCl2, 0.1 mM EDTA, 10 mM KCl, 1 mM DTT
and 0.5 mM PMSF; pH 7.9), left on ice for 10 min, vortexed and
centrifuged at 15,000 × g at 4°C for 5 min. Then, the supernatant
was used for the cytosolic proteins. After washing, the pelleted
nuclei were resuspended in 50 µl ice-cold lysis buffer (50 mM
HEPES/KOH, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 10% glycerol, 1 mM
DTT and 0.5 mM PMSF; pH 7.9), left on ice for 20 min, vortexed and
centrifuged at 15,000 × g for 5 min at 4°C; the supernatant was
collected. The supernatant was used as the nuclear proteins.
The cytosolic and nuclear proteins were quantified
using the Bradford protein assay (Bio-Rad Laboratories, Inc.).
Proteins (10 µg for STAT1 and phospho-STAT1Thy701, 30 µg
for IκBα and NF-κB p65) were loaded into each well and separated
via 10% SDS-PAGE. The separated proteins were then transferred to
0.45 µm nitrocellulose membranes (Pall Life Sciences). The
membranes were blocked with 3% bovine serum albumin (Sigma-Aldrich;
Merck KGaA) for 1 h at room temperature. Next, the membranes were
incubated with the primary antibodies overnight at 4°C, followed by
incubation with the secondary antibodies at room temperature for 1
h. Immunodetection was performed using G: Box Chemi XRQ (version
1.6.1.0; Syngene Europe) with a SuperSignal West Pico
chemiluminescent substrate (Thermo Fisher Scientific, Inc.).
β-actin and lamin B1 were used as the internal loading controls for
the cytosolic and nuclear proteins, respectively. Primary
antibodies against STAT1 (1:1,000; cat. no. 9172S),
phospho-STAT1Th701 (1:1,000; cat. no. 9167S), IκBα
(1:1,000; cat. no. 9242S) and NF-κB p65 (1:1,000; cat. no. 8242S)
and secondary antibodies [horseradish peroxidase-conjugated
anti-rabbit IgG (1:5,000; cat. no. 7074S) and anti-mouse IgG
(1:5,000; cat. no. 7076S)] were purchased from Cell Signaling
Technology, Inc. Primary antibodies against β-actin (1:1,000; cat.
no. SC47778) and lamin B1 (1:1,000; cat. no. SC374015) were
purchased from Santa Cruz Biotechnology, Inc.
Flow cytometry
After sacrifice, the spleen was weighed on a PAG214
analytical balance (Ohaus Corporation). Then, the mouse spleens
were carefully ground using 70-µm nylon cell strainers (Falcon;
Corning Life Sciences) to obtain regular single cells. Red blood
cells were removed by treating with the red blood cell lysis buffer
twice, and the single cells were counted. Subsequently, the cells
were stained using mouse CD4 PerCP-Cy™5.5-FITC-conjugated T helper
(Th)1 (IFN-γ), CD4 PerCP-Cy™5.5-APC-conjugated Th2 (IL-4) or CD4
PerCP-Cy™5.5-PE-conjugated Th17 (IL-17A) phenotyping kit (BD
Biosciences) as per the manufacturer's protocol. The fluorescence
intensity was detected using a FACSCalibur flow cytometer (BD
Biosciences), and the data were analyzed using BD CellQuest™ Pro
software (BD Biosciences) to determine the percentage of
CD4+ and gated IFN-γ+ plus IL-17A+
populations.
Statistical analyses
Statistical analyses were performed using Prism 8
software (GraphPad Software, Inc.). The treatment effects were
analyzed using one-way analysis of variance followed by Dunnett's
post hoc test. Additionally, PASI score data were analyzed using
Kruskal-Wallis test followed by Dunn's multiple comparison test.
Experiments were independently repeated in triplicate. P<0.05
was considered to indicate a statistically significant
difference.
Results
GM2 inhibits psoriasis-associated
characteristics in an IMQ-induced mouse model
The clinical characteristics of psoriasis include
scaling, erythema, dry skin, inflammatory keratosis and
histological changes in the epidermal and dermal thickness
(29). To assess the effects of
GM2 on psoriasis-like skin inflammation, an experimental psoriasis
mouse model was induced using IMQ cream.
The repeated application of IMQ exacerbated
characteristics of psoriasis, including: i) Epidermis, dermis and
skin thickness; ii) epidermal hyperplasia; iii) dry skin; and iv)
immune cell infiltration. However, the oral administration of GM2
not only alleviated these characteristics of psoriasis in a
dose-dependent manner, but also decreased the infiltration of
MPO-related cells (Figs. 1A and
S2A). Recently, GM2 has been
demonstrated to have anti-allergic effects (17). Therefore, to investigate whether
GM2 reduced the number of mast cells in IMQ-induced skin, toluidine
blue-stained tissues were microscopically examined. The
infiltration of mast cells into the skin was increased by IMQ,
however, it was decreased by GM2 (Fig. S2B). In addition, GM2 inhibited
the IMQ-induced infiltration of CD4+ cells into the skin
(Fig. S3). The IMQ-induced skin
had increased epidermal, dermal and skin thickness when compared
with that in the control group. However, the GM2-administered group
exhibited decreased epidermis, dermis and skin thickness in a
dose-dependent manner (Fig. 1B and
C). Moreover, GM2 decreased IMQ-induced TEWL (Fig. 1D).
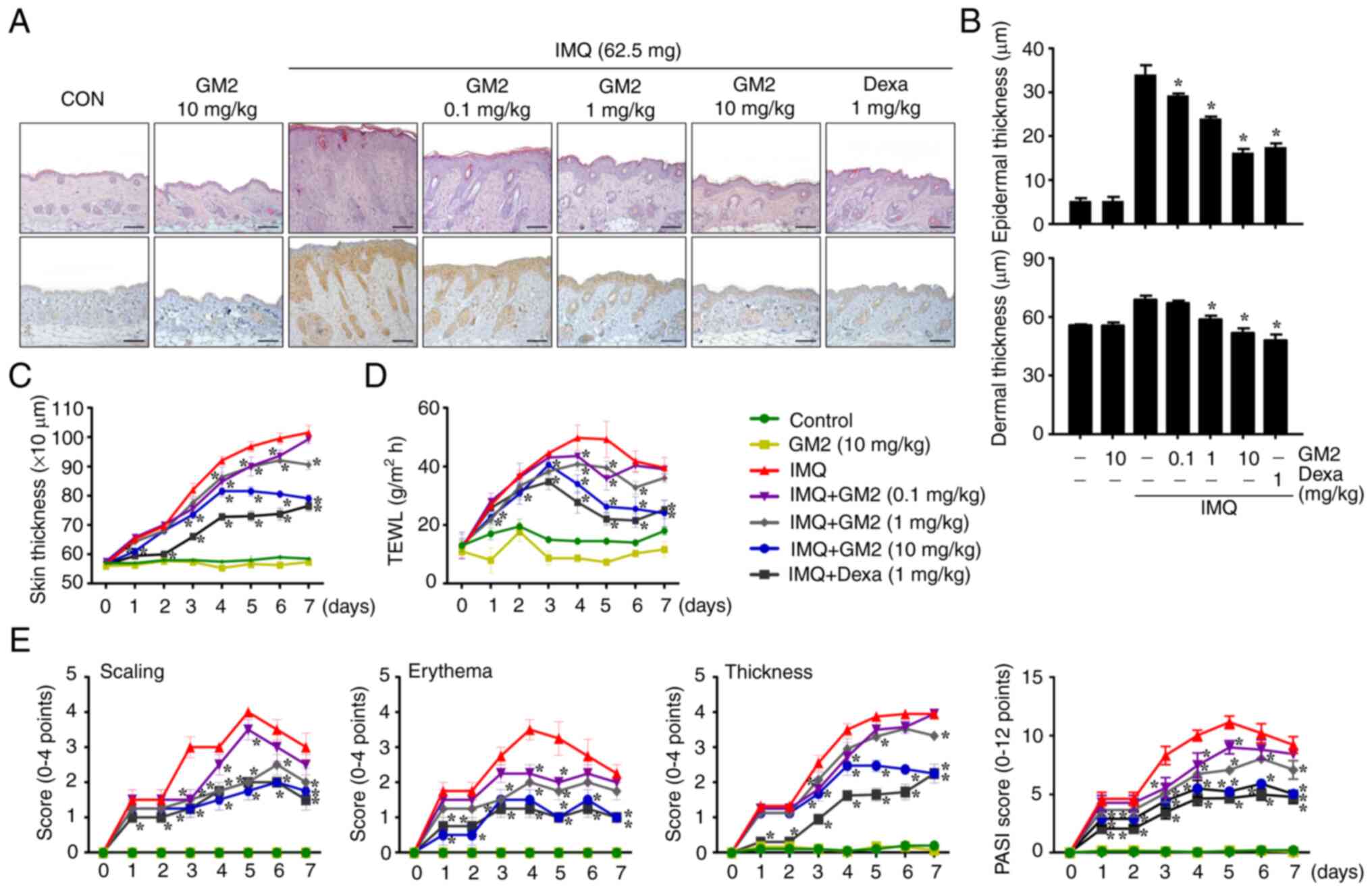 | Figure 1.GM2 attenuates symptoms of psoriasis
in an IMQ-induced mouse model. For histological observation, mouse
tissue was obtained at 7 days. (A) The mice skin tissues were
stained with hematoxylin and eosin or antibodies against
myeloperoxidase for histological observation and for the
determination of epidermal and dermal thickness and immune cell
infiltration. Original magnification, ×200; scale bar, 100 µm. (B)
Epidermal and dermal thickness were measured using a stage
micrometer under a brightfield microscope (magnification, ×200).
(C) Skin thickness. The skin thickness was measured 24 h after GM2
or Dexa application using a dial thickness gauge. (D) TEWL was
measured 24 h after GM2 or Dexa application using a gpskin Barrier
Light. (E) PASI score of the mice skin in different groups,
including scaling, erythema and thickness (scale of 0–4 and total
score were measured). The data are presented as the mean ± standard
error of the mean (n=4). *P<0.05 vs. IMQ only group. GM2,
gomisin M2; IMQ, imiquimod; Dexa, dexamethasone; TEWL,
transepidermal water loss; PASI, Psoriasis Area Severity Index. |
Psoriatic area and severity assessments primarily
relied on the PASI scoring to investigate scaling, erythema and
thickness (30). The continued
application of IMQ increased the PASI score; however, the oral
administration of GM2 alleviated the PASI score in a dose-dependent
manner (Fig. 1E).
GM2 decreases inflammatory-related
gene expression and serum levels
Several immune cells, including neutrophils and T
cells, infiltrate the site of the psoriatic lesion and exacerbate
the condition by releasing inflammatory cytokines and chemokines
(31). To evaluate the inhibitory
effects of GM2 on the IMQ-induced inflammatory cytokines and
chemokines, the expression levels of both were measured via
RT-qPCR. The application of IMQ increased the expression of several
cytokines and chemokines, including growth-regulated a protein
(CXCL1), IL-1β, IFN-γ, IL-17A, IL-23 and TNF-α. However, GM2
alleviated the gene expression levels of these cytokines and
chemokines (Fig. 2A).
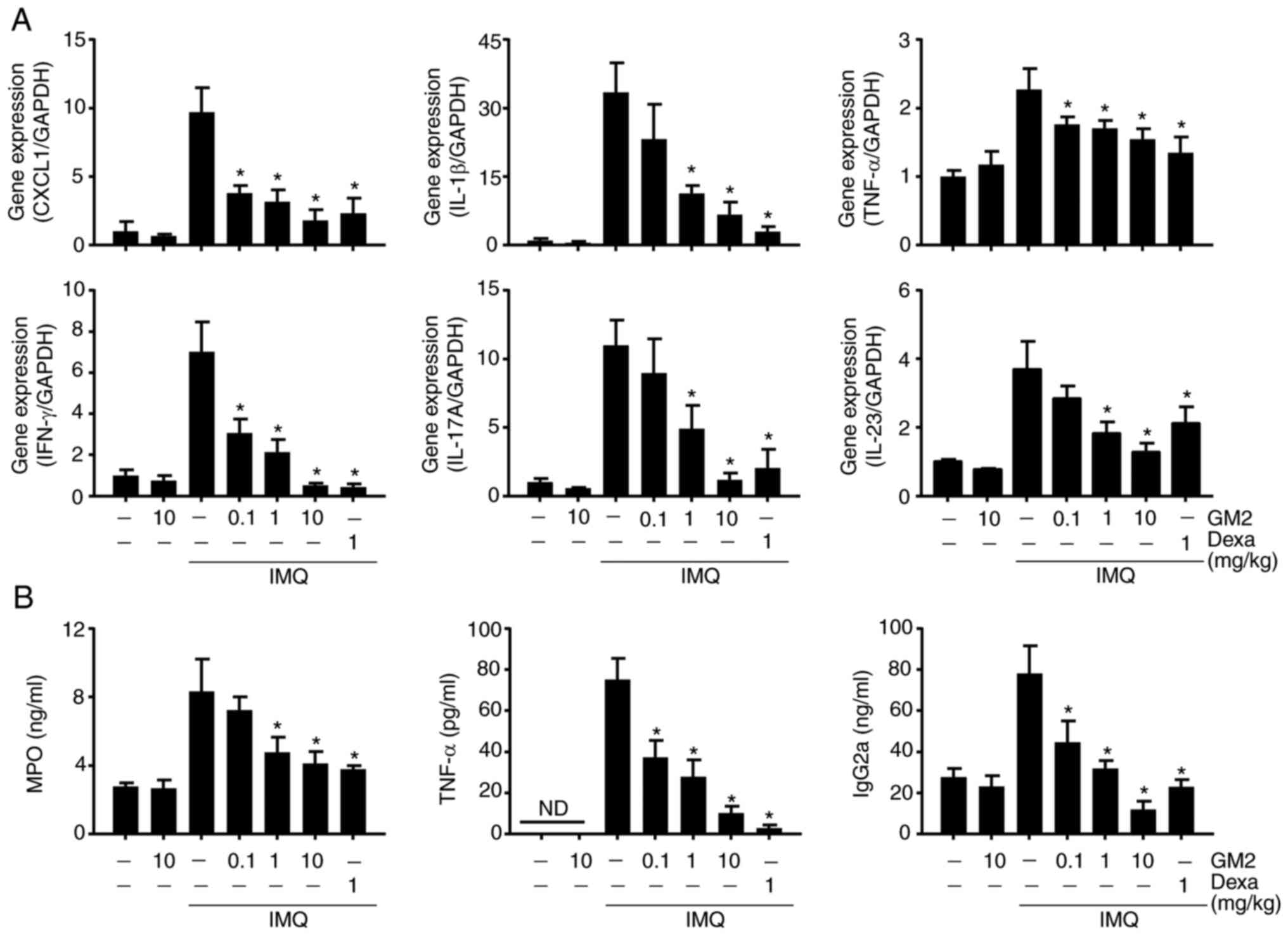 | Figure 2.GM2 inhibits psoriasis-associated
gene expression and serum levels. (A) mRNA expression was measured
via reverse transcription-quantitative PCR and normalized to GAPDH.
(B) MPO, TNF-α and IgG2a were measured using sandwich ELISA. The
data are presented as the mean ± standard error of the mean (n=4).
*P<0.05 vs. IMQ only group. GM2, gomisin M2; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; IgG2a, immunoglobulin
G2a; MPO, myeloperoxidase; IMQ, imiquimod; Dexa, dexamethasone;
CXCL1, growth-regulated a protein; TNF, tumor necrosis factor; IFN,
interferon; ND, not detected. |
To investigate the serum levels of the
inflammatory-related proteins, the serum levels of MPO, TNF-α and
IgG2a were measured using ELISA. The application of IMQ increased
the serum levels of these proteins. However, their levels were
reduced by GM2 in a dose-dependent manner (Fig. 2B).
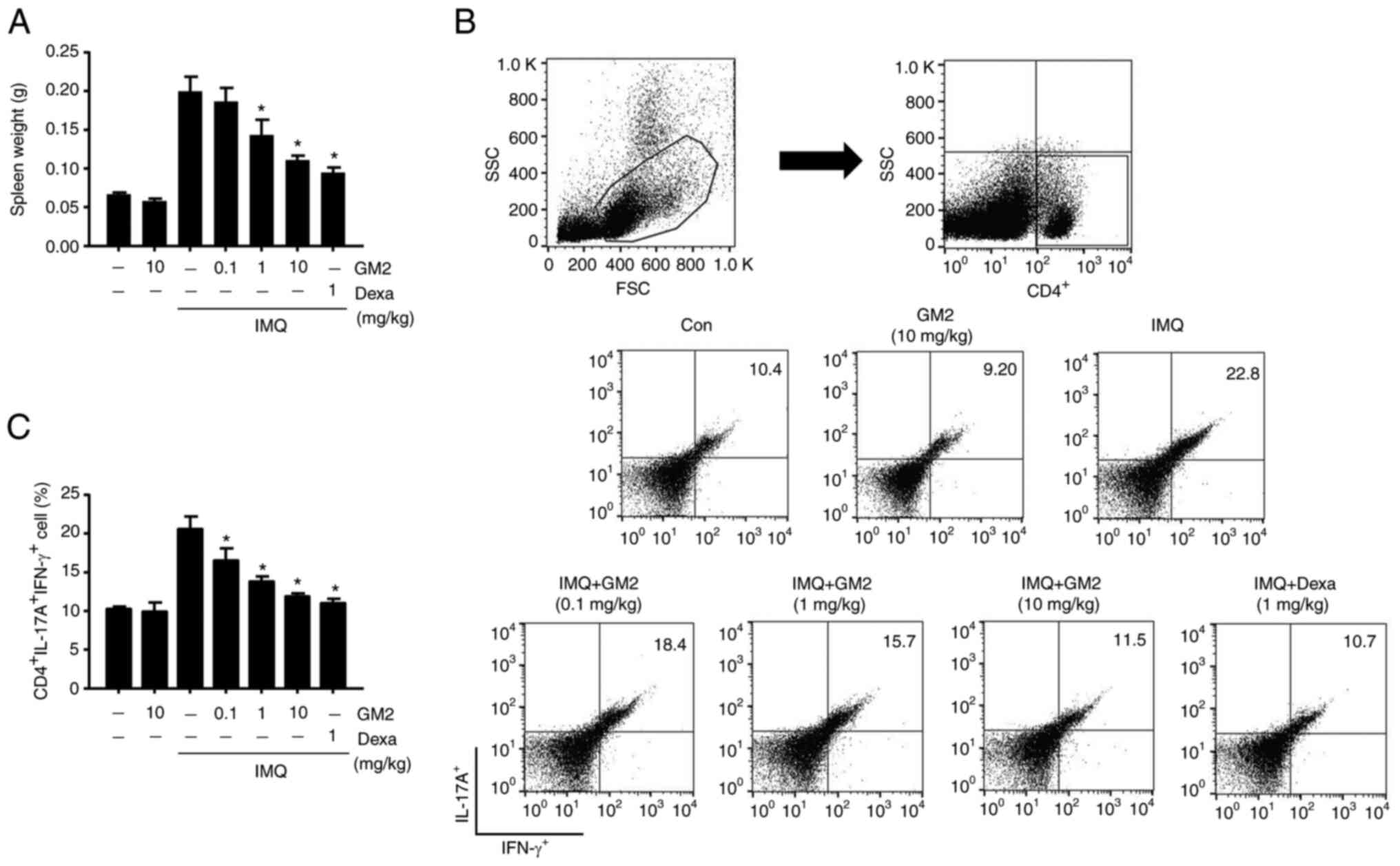
GM2 inhibits the alteration of Th1 and
Th17 cell populations in the spleen
The development of psoriasis is characterized by
alterations in the polarization of the T cell population, primarily
Th1 and Th17 cells (32). To
assess whether GM2 could influence systemic immune responses, the
weight of the spleen was measured and alterations in the
psoriasis-associated T cell populations in the spleen were
determined. IMQ-treated mice had increased spleen weights compared
with those in the control group. However, the oral administration
of GM2 significantly decreased the spleen weights (Fig. 3A). In addition, the population of
CD4+IFN-γ+IL-17A+ cells were
elevated in the IMQ-induced mice. However, the oral administration
of GM2 significantly decreased these cell numbers (Fig. 3B and C).
GM2 inhibits inflammatory gene
expression by inhibiting the STAT1 and NF-κB signaling pathways in
the keratinocytes
TNF-α and IFN-γ increase the promotion of
keratinocytes owing to the inflammatory environment of the skin and
stimulate the production and the release of diverse inflammatory
regulators (33). Therefore,
TNF-α- and IFN-γ-activated keratinocytes were used in the present
study.
The possible cytotoxicity of GM2 were initially
evaluated by performing an MTT assay. GM2 did not exhibit cytotoxic
effects on the keratinocytes at concentrations of up to 10 µM
(Fig. 4A). Therefore, the maximum
concentration of 10 µM was used for the in vitro
experiments. The effects of GM2 on the production and release of
inflammatory regulators in the TNF-α- and IFN-γ-stimulated
keratinocytes were then evaluated. However, pretreatment with GM2
significantly reduced the expression of cytokines and chemokines,
including C-C motif chemokine 17 (CCL17), IL-8, IL-6 and IL-1β, in
a dose-dependent manner compared with the TNF-α- and
IFN-γ-stimulated group (Fig. 4B).
In addition, GM2 significantly reduced the secretion of CCL17 and
IL-6 in a dose-dependent manner compared with the TNF-α- and
IFN-γ-stimulated group (Fig.
4C).
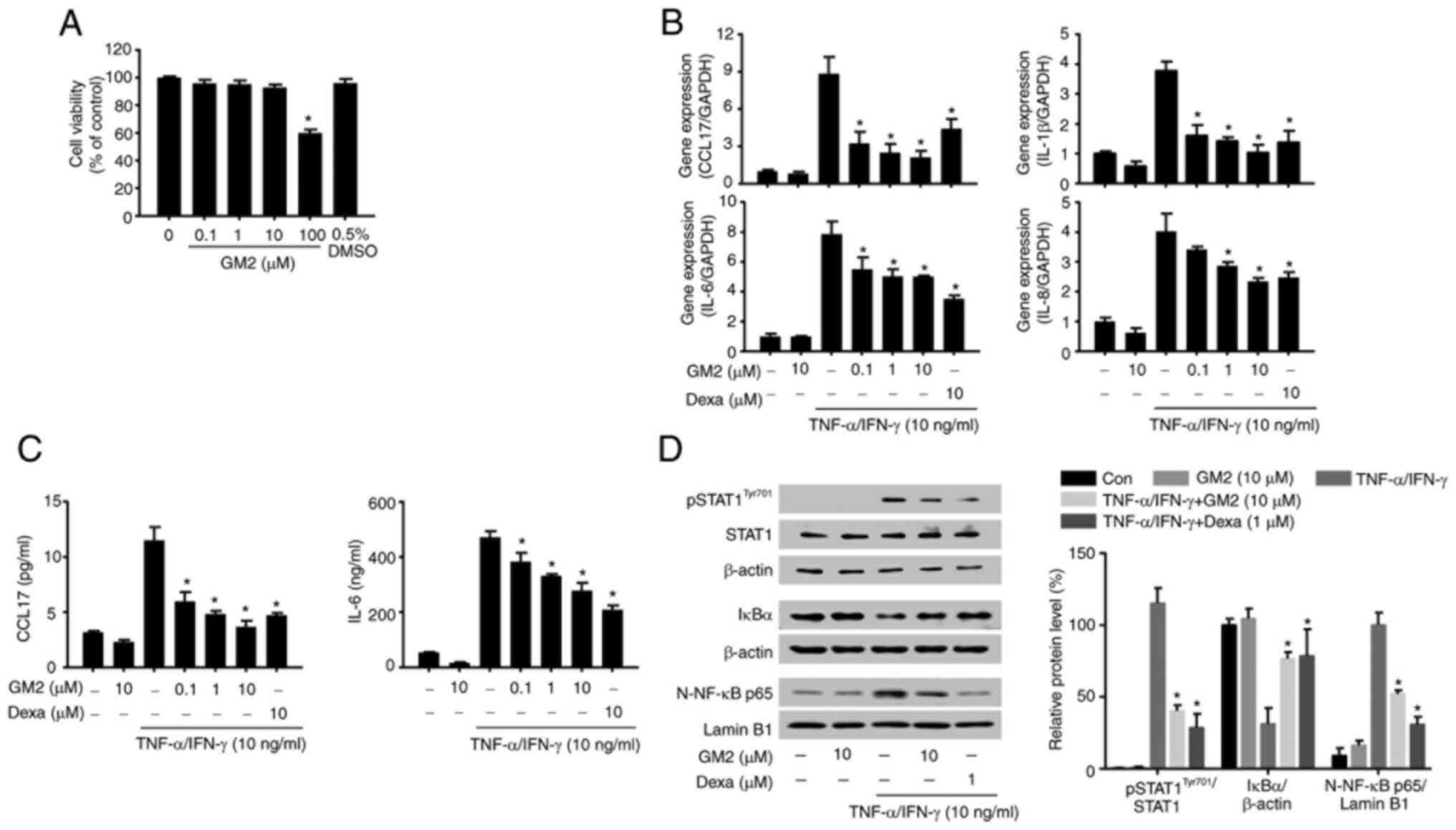 | Figure 4.GM2 inhibits inflammatory mediators
and the inflammatory signaling pathway in keratinocytes stimulated
by TNF-α and IFN-γ. Cells were pretreated with GM2 or Dexa before
stimulation with TNF-α (10 ng/ml) and IFN-γ (10 ng/ml). (A)
Viability of the HaCaT cells following GM2 treatment was determined
using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium
bromide assay. (B) mRNA expression levels of CCL17, IL-1β, IL-6 and
IL-8 in the keratinocytes were measured via reverse
transcription-quantitative PCR. (C) The cells were pretreated with
GM2 or Dexa for 1 h, followed by stimulation with TNF-α and IFN-γ
(10 ng/ml) for 15 h. The secretory protein levels of IL-6 and CCL17
were measured by ELISA. (D) The cells were pretreated with GM2 or
Dexa for 1 h and subsequently with TNF-α and IFN-γ for 15 min. The
phosphorylation of STAT1, degradation of IκBα and nuclear
translocation of NF-κB p65 were analyzed by western blotting.
β-actin and lamin B1 were used as loading controls, and the band
intensity was semi-quantified using ImageJ software. The data are
presented as the mean ± standard error of the mean (n=3).
*P<0.05 vs. TNF-α- and IFN-γ-stimulated group. GM2, gomisin M2;
Dexa, dexamethasone; TNF, tumor necrosis factor; IFN, interferon;
CCL17, C-C motif chemokine 17; p, phosphorylated; STAT1, signal
transducers and activators of transcription 1; NF-κB, nuclear
factor-κB. |
To investigate the inhibitory effects of GM2 on the
activated keratinocytes, western blotting was performed. The TNF-α-
and IFN-γ stimulation not only activated STAT1 and NF-κB, but also
induced IκBα degradation; however, GM2 significantly inhibited the
phosphorylation of STAT1, the translocation of NF-κB and the
degradation of IκBα compared with the TNF-α- and IFN-γ-stimulated
group (Fig. 4D).
Discussion
The annual incidence of psoriasis is increasing and
is aggravated by various factors, such as air pollution, smoking,
mechanical stress, dyslipidemia and hypertension (34,35). The topical application of
corticosteroids has been used as the main treatment for psoriasis.
However, the long-term application of corticosteroids is not
advisable owing to their diverse side effects, including atrophy,
hyperpigmentation, hypercoagulable state and dyslipidemia (22). Therefore, the attention of the
research community has turned toward natural products based on
traditional medicine (36). These
products are rich in bioactive compounds that encompass a wider
chemical space than the common synthetic small-molecule libraries
(37).
The fruits of S. chinensis have long been
used in traditional medicine in China, Japan and Korea to treat
diseases such as asthma and diabetes (38,39). Moreover, S. chinensis has
been certified as a safe nutritional ingredient by the European
Food Safety Agency, and its fruit has been reported to have diverse
pharmacological properties, including antioxidant,
anti-inflammatory and antiallergic effects (17,40). GM2 extracted from S.
chinensis has also been documented to exert various
pharmacological effects, including preventing liver damage and
reducing cancer cell proliferation (18,19,41). Based on these benefits, the
present study explored the inhibitory effects of GM2 on IMQ-induced
psoriasis-like skin inflammation and on TNF-α- and IFN-γ-stimulated
keratinocytes after the purity of the GM2 isolated from S.
chinensis was determined.
Psoriasis is characterized by prominent skin
inflammation involving neutrophils, which can potentiate T
cell-associated inflammation (42). T cells and mast cells can
infiltrate psoriatic lesions and accelerate the development of
psoriasis (43,44). In addition, neutrophils express a
number of different cytokines and chemokines, including CXCL1,
IL-17, TNF-α and IFN-γ, which can accelerate the progress of
psoriasis (45). Therefore,
controlling the infiltration of neutrophils in psoriatic lesions
and in the serum is important for the treatment of psoriasis. In
the current study, MPO was used as the marker for neutrophils as it
is a major protein found in neutrophilic granules (46). Thus, reducing the MPO levels in
psoriatic skin and in the serum may be a suitable approach to
reduce skin inflammation.
The present findings showed that GM2 decreased the
infiltration of MPO-associated cells in the psoriatic lesions as
well as the levels of MPO in the serum. In addition, the mRNA
expression levels of neutrophil-related cytokines and chemokines,
such as CXCL1, IL-1β, IL-17A, IL-23, TNF-α and IFN-γ, were
decreased in the lesions. These results suggested that GM2
alleviated psoriasis-like skin inflammation by inhibiting the
infiltration of neutrophils and neutrophil-related inflammatory
factors. In addition, IgG2a and TNF-α levels have been found to be
abnormally elevated in the serum of patients with psoriasis
(47,48). The current findings indicated that
GM2 reduced IgG2a and TNF-α in the serum of the IMQ-treated mice.
Therefore, GM2 may be able to alleviate psoriasis by controlling
the levels of inflammatory-related factors in the serum.
Additionally, this study showed that GM2 inhibited the infiltration
of mast cell and CD4+ cells. This suggested that GM2
alleviated characteristics of psoriasis by inhibiting skin
penetration of psoriasis-related cells. Furthermore, as skin
hydration is an index of skin barrier function in IMQ-induced
psoriasis-like skin (49), TEWL
was measured in the present study using the gpskin barrier light.
These results showed that TEWL was increased in IMQ-induced skin,
however, it was reduced by GM2. This result indicated that GM2 had
a beneficial effect on increasing moisture in the skin.
Several reports have provided evidence that Th1 and
Th17 cells are increased in psoriasis (32,50). Th1 cells are considered to be the
major factors in the development of psoriasis and are known to be
mainly involved in the early stages of the disease (46). Th17 cells also play an important
role in IL-17 production and psoriasis maintenance (46). Therefore, the inhibition of these
cells associated with inflammation is important for suppressing the
symptoms of psoriasis. GM2 significantly suppressed the number of
Th1 and Th17 cells. These findings implied that GM2 exerts its
anti-psoriasis effects by blocking Th1 and Th17 cell infiltration
during pathogenesis.
The current study evaluated the effects of GM2 on
activated keratinocytes, which are significant sources of cytokines
that can aggravate psoriasis development (29). TNF-α and IFN-γ induce the
production of various proinflammatory cytokines by activating
transcription factors, including STAT1 and NF-κB, in the
keratinocytes. The induced inflammatory cytokines can exacerbate
the skin lesions (51,52). In the present study, it was
established that GM2 decreased the expression levels of
inflammatory-related genes and the levels of secretory proteins in
the activated keratinocytes. In addition, GM2 inhibited the
phosphorylation of STAT1 and the translocation of NF-κB in TNF-α-
and IFN-γ-activated keratinocytes. Therefore, the anti-inflammatory
effects of GM2 on TNF-α- and IFN-γ-treated keratinocytes may be
exerted through a reduction in the production of cytokines and
chemokines by blocking the phosphorylation of STAT1 and the
translocation of NF-κB.
Collectively, these results indicated that a natural
compound containing GM2 could serve as a useful supplement for
treating psoriasis. However, further experiments are required to
explore the long term effects of GM2 in psoriasis-like skin
inflammation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Research
Foundation of Korea grants funded by the Korean government (grant
nos. 2019R1C1C1005172, 2019R1A2B5B01069444, 2019M3A9H1103690,
2017M3A9G8083382 and 2020M3A9D3038894).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NK performed the experiments and wrote the original
draft of the manuscript. SL conceived the research and analyzed the
data. JK performed the experiments. TKK designed the methodology.
DK and SHK performed the analysis and interpretation of data, and
revised the manuscript. DK and SHK confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Public Health Service Policy on the Humane Care and Use of
Laboratory Animals, and approved by the Institutional Animal Care
and Use Committee of Kyungpook National University (approval no.
KNU-2019-0001-5; Daegu, Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GM2
|
gomisin M2
|
|
IMQ
|
imiquimod
|
|
TNF
|
tumor necrosis factor
|
|
IFN
|
interferon
|
|
MPO
|
myeloperoxidase
|
|
STAT1
|
signal transducers and activators of
transcription 1
|
|
NF-κB
|
nuclear factor-κB
|
|
Dexa
|
dexamethasone
|
|
PASI
|
Psoriasis Area Severity Index
|
|
TEWL
|
transepidermal water loss
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemistry
|
|
PBS
|
phosphate buffered saline
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
References
|
1
|
Stockenhuber K, Hegazy AN, West NR, Ilott
NE, Stockenhuber A, Bullers SJ, Thrnton EE, Arnold IC, Tucci A,
Waldmann H, et al: Foxp3+ T reg cells control
psoriasiform inflammation by restraining an IFN-I-driven
CD8+ T cell response. J Exp Med. 215:1987–1998. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parisi R, Iskandar IYK, Kontopantelis E,
Augustin M, Griffiths CEM and Ascroft DM; Global Psoriasis Atlas, :
National, regional, and worldwide epidemiology of psoriasis:
Systematic analysis and modelling study. BMJ. 369:m15902020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Danielsen K, Olsen AO, Wilsgaard T and
Furberg AS: Is the prevalence of psoriasis increasing? A 30-year
follow-up of a population-based cohort. Br J Dermatol.
168:1303–1310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim WB, Jerome D and Yeung J: Diagnosis
and management of psoriasis. Can Fam Physician. 63:278–285.
2017.PubMed/NCBI
|
|
5
|
Mrowietz U and Domm S: Systemic steroids
in the treatment of psoriasis: What is fact, what is fiction? J Eur
Acad Dermatol Venereol. 27:1022–1025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghasemian M, Owlia S and Owlia MB: Review
of anti-inflammatory herbal medicines. Adv Pharmacol Sci.
2016:91309792016.PubMed/NCBI
|
|
7
|
Zhao J, Di T, Wang Y, Wang Y, Liu X, Liang
D and Li P: Paeoniflorin inhibits imiquimod-induced psoriasis in
mice by regulating Th17 cell response and cytokine secretion. Eur J
Pharmacol. 772:131–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gallais Sérézal I, Hoffer E, Ignatov B,
Martini E, Zitti B, Ehrström M and Eidsmo L: A skewed pool of
resident T cells triggers psoriasis-associated tissue responses in
never-lesional skin from patients with psoriasis. J Allergy Clin
Immunol. 143:1444–1454. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Huang L, Vergis AL, Ye H, Bajwa A,
Narayan V, Striter RM, Rosin DL and Okusa MD: IL-17 produced by
neutrophils regulates IFN-gamma-mediated neutrophil migration in
mouse kidney ischemia-reperfusion injury. J Clin Invest.
120:331–342. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hawkes JE, Yan BY, Chan TC and Krueger JG:
Discovery of the IL-23/IL-17 signaling pathway and the treatment of
psoriasis. J Immunol. 201:1605–1613. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee B, Bae EA, Trinh HT, Shin YW, Phuong
TT, Bae KH and Kim DH: Inhibitory effect of schizandrin on passive
cutaneous anaphylaxis reaction and scratching behaviors in mice.
Biol Pharm Bull. 30:1153–1156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim H, Ahn YT, Kim YS, Cho SI and An WG:
Antiasthmatic effects of schizandrae fructus extract in mice with
asthma. Pharmacogn Mag. 10 (Suppl 1):S80–S85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szopa A, Ekiert R and Ekiert H: Current
knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese
magnolia vine) as a medicinal plant species: A review on the
bioactive components, pharmacological properties, analytical and
biotechnological studies. Phytochem Rev. 16:195–218. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chae HS, Kang OH, Oh YC, Choi JG, Keum JH,
Kim SB, Kim YS, Mun SH, Shin DW, Han SH and Kwon DY: Gomisin N has
anti-allergic effect and inhibits inflammatory cytokine expression
in mouse bone marrow-derived mast cells. Immunopharmacol
Immunotoxicol. 33:709–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Opletal L, Sovová H and Bártlová M:
Dibenzo[a,c]cyclooctadiene lignans of the genus Schisandra:
Importance, isolation and determination. J Chromatogr B Analyt
Technol Biomed Life Sci. 812:357–371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Korkina L, Kostyuk V, De Luca C and
Pastore S: Plant phenylpropanoids as emerging anti-inflammatory
agents. Mini Rev Med Chem. 11:823–835. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dhakal H, Lee S, Kim EN, Choi JK, Kim MJ,
Kang J, Choi YA, Baek MC, Lee B, Lee HS, et al: Gomisin M2 inhibits
mast cell-mediated allergic inflammation via attenuation of
FcεRI-mediated Lyn and Fyn activation and intracellular calcium
levels. Front Pharmacol. 10:8692019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen M, Kilgore N, Lee KH and Chen DF:
Rubrisandrins A and B, lignans and related anti-HIV compounds from
Schisandra rubriflora. J Nat Prod. 69:1697–1701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou X, Deng J, Zhang Q, Wang D, Kennedy D,
Quinn RJ and Feng Y: Cytotoxic ethnic Yao medicine Baizuan, leaves
of Schisandra viridis A. C. Smith. J Ethnopharmacol.
194:146–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li F, Zhang T, Sun H, Gu H, Wang H, Su X,
Li C, Li B, Chen R and Kang J: A new nortriterpenoid, a
sesquiterpene and hepatoprotective lignans isolated from the fruit
of Schisandra chinensis. Molecules. 22:19312017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Public Health Service, . Public health
service policy on humane care and use of laboratory animals, US
Department of Health and Human Services. NIH publication No.
15-80137-25. 2015.
|
|
22
|
Uva L, Miguel D, Pinheiro C, Antunes J,
Cruz D, Ferreira J and Filipe P: Mechanisms of action of topical
corticosteroids in psoriasis. Int J Endocrinol. 2012:5610182012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Araki-Sasaki K, Katsuta O, Mano H, Nagano
T and Nakamura M: The effects of oral and topical corticosteroid in
rabbit corneas. BMC Ophthalmol. 16:1602016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Samtani MN and Jusko WJ: Comparison of
dexamethasone pharmacokinetics in female rats after intravenous and
intramuscular administration. Biopharm Drug Dispos. 26:85–91. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong NH, Lee S, Choi JK, Choi YA, Kim MJ,
Lee HS, Shin TY, Jang YH, Song KS and Kim SH: Polyozellin
alleviates atopic dermatitis-like inflammatory and pruritic
responses in activated keratinocytes and mast cells. Biomed
Pharmacother. 122:1097432020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Liu W, Gao S, Mao Y and Xin Y:
Application of imiquimod-induced murine psoriasis model in
evaluating interleukin-17A antagonist. BMC Immunol. 22:112021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lim JS, Kim JY, Lee S, Choi JK, Kim EN,
Choi YA, Jang YH, Jeong GS and Kim SH: Bakuchicin attenuates atopic
skin inflammation. Biomed Pharmacother. 129:1104662020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lowes MA, Suárez-Fariñas M and Krueger JG:
Immunology of psoriasis. Annu Rev Immunol. 32:227–255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feldman SR and Krueger GG: Psoriasis
assessment tools in clinical trials. Ann Rheum Dis. 64 (Suppl
2):ii65–ii73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiang CC, Cheng WJ, Korinek M, Lin CY and
Hwang TL: Neutrophils in psoriasis. Front Immunol. 10:23762019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Furiati SC, Catarino JS, Silva MV, Silva
RF, Estevam RB, Teodoro RB, Pereira SL, Ataide M, Rodrigues V Jr
and Rodrigues DBR: Th1, Th17, and Treg responses are differently
modulated by TNF-α inhibitors and methotrexate in psoriasis
patients. Sci Rep. 9:75262019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhattacharjee O, Ayyangar U, Kurbet AS,
Ashok D and Raghavan S: Unraveling the ECM-immune cell crosstalk in
skin diseases. Front Cell Dev Biol. 7:682019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schonmann Y, Ashcroft DM, Iskandar IYK,
Parisi R, Sde-Or S, Comaneshter D, Batat E, Shani M, Vinker S,
Griffiths CEM and Cohen AD: Incidence and prevalence of psoriasis
in Israel between 2011 and 2017. J Eur Acad Dermatol Venereol.
33:2075–2081. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kamiya K, Kishimoto M, Sugai J, Komine M
and Ohtsuki M: Risk factors for the development of psoriasis. Int J
Mol Sci. 20:43472019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng S, Lin Z, Wang Y, Wang Z, Li P and
Zheng Y: Psoriasis therapy by Chinese medicine and modern agents.
Chin Med. 13:162018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Atanasov AG, Zotchev SB, Dirsch VM;
International Natural Product Sciences Taskforce, ; Supuran CT:
Natural products in drug discovery: Advances and opportunities. Nat
Rev Drug Discov. 20:200–216. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim EJ, Jang M, Lee MJ, Choi JH, Lee SJ,
Kim SK, Jang DS and Cho IH: Schisandra chinensis stem
ameliorates 3-nitropropionic acid-induced striatal toxicity via
activation of the Nrf2 pathway and inhibition of the MAPKs and
NF-κB pathways. Front Pharmacol. 8:6732017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail.: An overview of Russian research and
uses in medicine. J Ethnopharmacol. 118:183–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Szopa A, Barnaś M and Ekiert H:
Phytochemical studies and biological activity of three Chinese
Schisandra species (Schisandra sphenanthera, Schisandra
henryi and Schisandra rubriflora): Current findings and
future applications. Phytochem Rev. 18:109–128. 2019. View Article : Google Scholar
|
|
41
|
Yang Y, Hao E, Pan X, Tan D, Du Z, Xie J,
Hou X, Deng J and Wei K: Gomisin M2 from Baizuan suppresses breast
cancer stem cell proliferation in a zebrafish xenograft model.
Aging (Albany NY). 11:8347–8361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tokuyama M and Mabuchi T: New treatment
addressing the pathogenesis of psoriasis. Int J Mol Sci.
21:74882020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Siiskonen H and Harvima I: Mast cells and
sensory nerves contribute to neurogenic inflammation and pruritus
in chronic skin inflammation. Front Cell Neurosci. 13:4222019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Diani M, Altomare G and Reali E: T helper
cell subsets in clinical manifestations of psoriasis. J Immunol
Res. 2016:76920242016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ogawa E, Sato Y, Minagawa A and Okuyama R:
Pathogenesis of psoriasis and development of treatment. J Dermatol.
45:264–272. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Odobasic D, Kitching AR and Holdsworth SR:
Neutrophil-mediated regulation of innate and adaptive immunity: The
role of myeloperoxidase. J Immunol Res. 2016:23498172016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dilek N, Dilek AR, Taşkın Y, Erkinüresin
T, Yalçın Ö and Saral Y: Contribution of myeloperoxidase and
inducible nitric oxide synthase to pathogenesis of psoriasis.
Postepy Dermatol Alergol. 33:435–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kyriakou A, Patsatsi A, Vyzantiadis TA and
Sotiriadis D: Serum levels of TNF-α, IL-12/23p40, and IL-17 in
plaque psoriasis and their correlation with disease severity. J
Immunol Res. 2014:4675412014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wallen-Russell C: Is there a relationship
between transepidermal water loss and microbial biodiversity on the
skin? Cosmetics. 6:182019. View Article : Google Scholar
|
|
50
|
Zhou F, Zhu Z, Gao J, Yang C, Wen L, Liu
L, Zuo X, Zheng X, Shi Y, Zhu C, et al: NFKB1 mediates Th1/Th17
activation in the pathogenesis of psoriasis. Cell Immunol.
331:16–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gröne A: Keratinocytes and cytokines. Vet
Immunol Immunopathol. 88:1–12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang L, Zhao Y, Liu Y, Akiyama K, Chen C,
Qu C, Jin Y and Shi S: IFN-γ and TNF-α synergistically induce
mesenchymal stem cell impairment and tumorigenesis via NFκB
signaling. Stem Cells. 31:1383–1395. 2013. View Article : Google Scholar : PubMed/NCBI
|