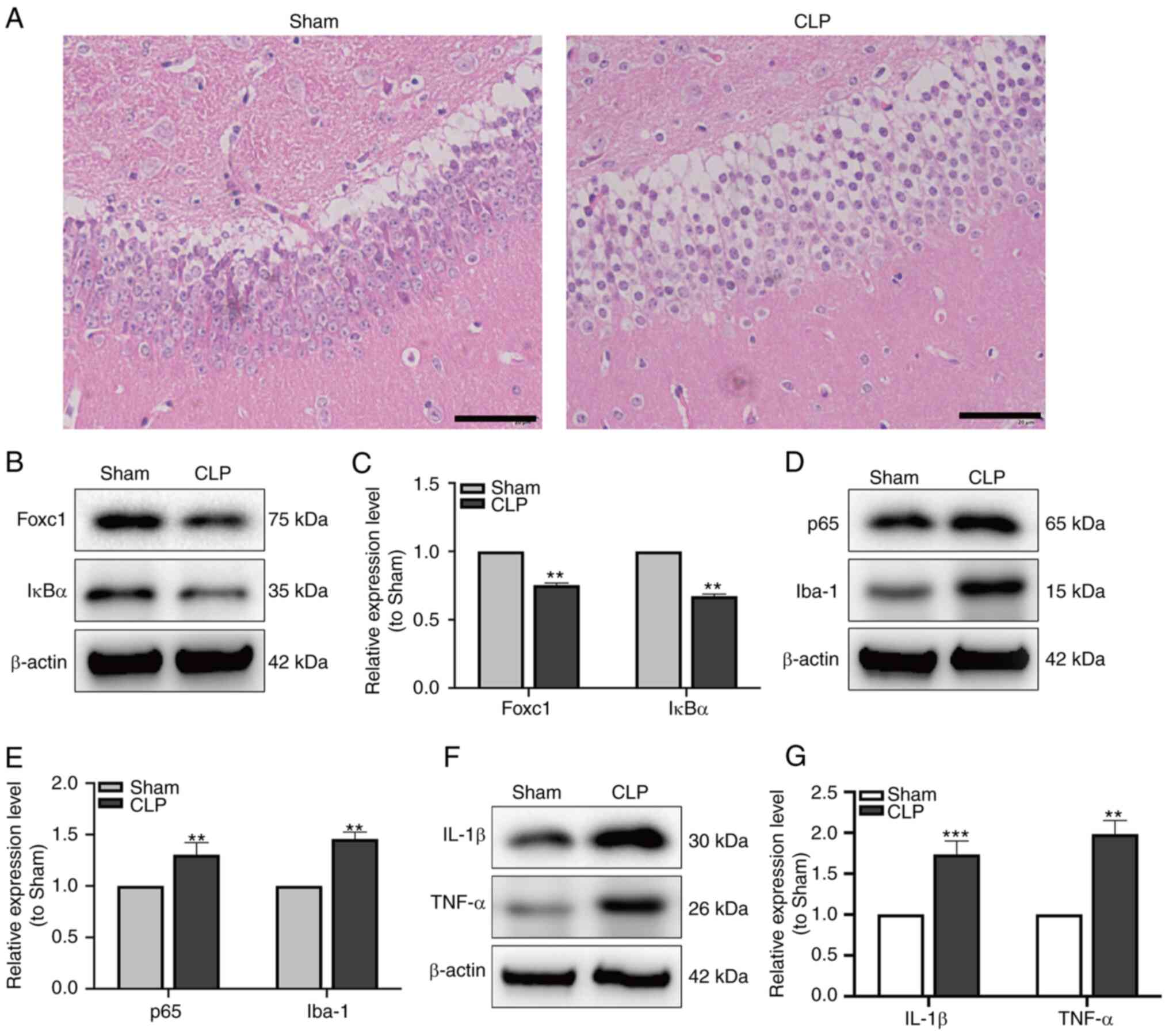
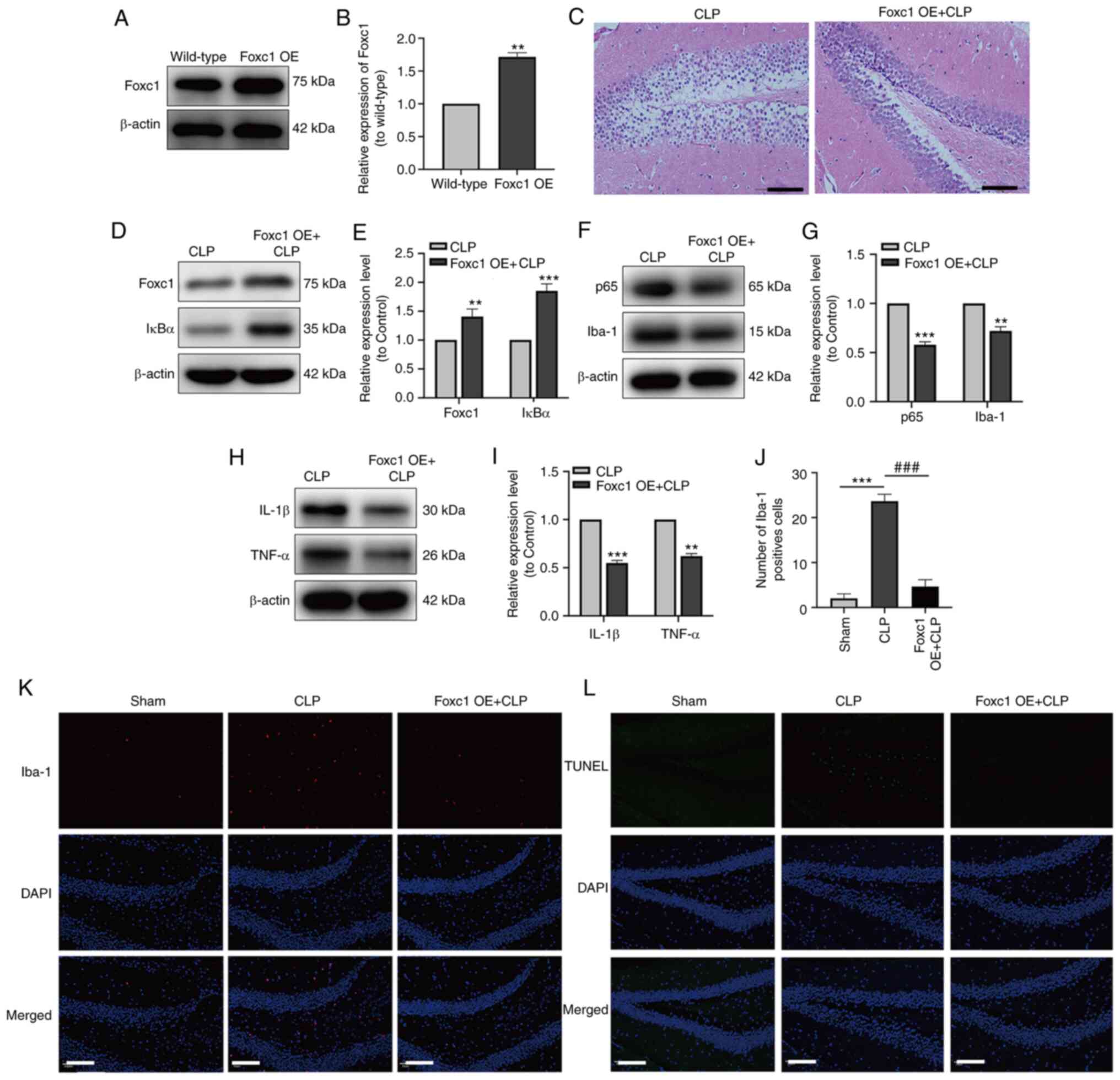
|
1
|
Gofton TE and Young GB: Sepsis-associated
encephalopathy. Nat Rev Neurol. 8:557–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Czempik PF, Pluta MP and Krzych ŁJ:
Sepsis-associated brain dysfunction: A review of current
literature. Int J Environ Res Public Health. 17:58522020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sonneville R, de Montmollin E, Poujade J,
Garrouste-Orgeas M, Souweine B, Darmon M, Mariotte E, Argaud L,
Barbier F, Goldgran-Toledano D, et al: Potentially modifiable
factors contributing to sepsis-associated encephalopathy. Intensive
Care Med. 43:1075–1084. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abe N, Nishihara T, Yorozuya T and Tanaka
J: Microglia and macrophages in the pathological central and
peripheral nervous systems. Cells. 9:21322020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michels M, Abatti M, Vieira A, Ávila P,
Goulart AI, Borges H, Córneo E, Dominguini D, Barichello T and
Dal-Pizzol F: Modulation of microglial phenotypes improves
sepsis-induced hippocampus-dependent cognitive impairments and
decreases brain inflammation in an animal model of sepsis. Clin Sci
(Lond). 134:765–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang LM, Wu Q, Kirk RA, Horn KP, Ebada
Salem AH, Hoffman JM, Yap JT, Sonnen JA, Towner RA, Bozza FA, et
al: Lipopolysaccharide endotoxemia induces amyloid-β and p-tau
formation in the rat brain. Am J Nucl Med Mol Imaging. 8:86–99.
2018.PubMed/NCBI
|
|
7
|
Gunther ML, Morandi A, Krauskopf E,
Pandharipande P, Girard TD, Jackson JC, Thompson J, Shintani AK,
Geevarghese S, Miller RR III, et al: The association between brain
volumes, delirium duration, and cognitive outcomes in intensive
care unit survivors: The VISIONS cohort magnetic resonance imaging
study*. Crit Care Med. 40:2022–2032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adam N, Kandelman S, Mantz J, Chrétien F
and Sharshar T: Sepsis-induced brain dysfunction. Expert Rev Anti
Infect Ther. 11:211–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maddux AB, Hiller TD, Overdier KH, Pyle LL
and Douglas IS: Innate immune function and organ failure recovery
in adults with sepsis. J Intensive Care Med. 34:486–494. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dal-Pizzol F, Tomasi CD and Ritter C:
Septic encephalopathy: Does inflammation drive the brain crazy?
Braz J Psychiatry. 36:251–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michels M, Vieira AS, Vuolo F, Zapelini
HG, Mendonça B, Mina F, Dominguini D, Steckert A, Schuck PF,
Quevedo J, et al: The role of microglia activation in the
development of sepsis-induced long-term cognitive impairment. Brain
Behav Immun. 43:54–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Margotti W, Giustina AD, de Souza Goldim
MP, Hubner M, Cidreira T, Denicol TL, Joaquim L, De Carli RJ,
Danielski LG, Metzker KL, et al: Aging influences in the
blood-brain barrier permeability and cerebral oxidative stress in
sepsis. Exp Gerontol. 140:1110632020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shulyatnikova T and Verkhratsky A:
Astroglia in sepsis associated encephalopathy. Neurochem Res.
45:83–99. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang SP, Yang RH, Shang J, Gao T, Wang R,
Peng XD, Miao X, Pan L, Yuan WJ, Lin L and Hu QK: FOXC1
up-regulates the expression of toll-like receptors in myocardial
ischaemia. J Cell Mol Med. 23:7566–7580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia W, Zhu J, Wang X, Tang Y, Zhou P, Wei
X, Chang B, Zheng X, Zhu W, Hou M and Li S: Overexpression of Foxc1
regenerates crushed rat facial nerves by promoting Schwann cells
migration via the Wnt/β-catenin signaling pathway. J Cell Physiol.
235:9609–9622. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Zhang Z, Li X, Chen J, Wang G, Tian
Z, Qian M, Chen Z, Guo H, Tang G, et al: Forkhead box C1 promotes
colorectal cancer metastasis through transactivating ITGA7 and
FGFR4 expression. Oncogene. 37:5477–5491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia S, Qu J, Jia H, He W, Li J, Zhao L,
Mao M and Zhao Y: Overexpression of Forkhead box C1 attenuates
oxidative stress, inflammation and apoptosis in chronic obstructive
pulmonary disease. Life Sci. 216:75–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao L, Zhang R, Su F, Dai L, Wang J, Cui
J, Huang W and Zhang S: FoxC1-induced vascular niche improves
survival and myocardial repair of mesenchymal stem cells in
infarcted hearts. Oxid Med Cell Longev. 2020:78653952020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao Q, Wang X, Shi Y, Zhang M, Yang J,
Dong M, Mi Y, Zhang Z, Liu K, Jiang L, et al: FOXC1 silencing
inhibits the epithelial-to-mesenchymal transition of glioma cells:
Involvement of β-catenin signaling. Mol Med Rep. 19:251–261.
2019.PubMed/NCBI
|
|
20
|
Nishimura DY, Swiderski RE, Alward WL,
Searby CC, Patil SR, Bennet SR, Kanis AB, Gastier JM, Stone EM and
Sheffield VC: The forkhead transcription factor gene FKHL7 is
responsible for glaucoma phenotypes which map to 6p25. Nat Genet.
19:140–147. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang L, Huang Z, Fan Y, He L, Ye M, Shi
K, Ji B, Huang J, Wang Y and Li Q: FOXC1 promotes proliferation and
epithelial-mesenchymal transition in cervical carcinoma through the
PI3K-AKT signal pathway. Am J Transl Res. 9:1297–1306.
2017.PubMed/NCBI
|
|
22
|
Ray PS, Wang J, Qu Y, Sim MS, Shamonki J,
Bagaria SP, Ye X, Liu B, Elashoff D, Hoon DS, et al: FOXC1 is a
potential prognostic biomarker with functional significance in
basal-like breast cancer. Cancer Res. 70:3870–3876. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Omatsu Y, Seike M, Sugiyama T, Kume T and
Nagasawa T: Foxc1 is a critical regulator of haematopoietic
stem/progenitor cell niche formation. Nature. 508:536–540. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nguyen PL, Bui BP, Lee H and Cho J: A
Novel 1,8-Naphthyridine-2-carboxamide derivative attenuates
inflammatory responses and cell migration in LPS-Treated BV2 Cells
via the suppression of ROS generation and TLR4/Myd88/NF-κB
signaling pathway. Int J Mol Sci. 22:25272021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Campesi I, Montella A and Franconi F:
Human monocytes respond to lipopolysaccharide (LPS) stimulation in
a sex-dependent manner. J Cell Physiol. Jul 12–2021.(Epub ahead of
print). doi: 10.1002/jcp.30503. PubMed/NCBI
|
|
28
|
National Research Council Institute for
Laboratory Animal R, . Guide for the Care and Use of Laboratory
Animals National Academies Press (US) Copyright 1996 by the
National Academy of Sciences. Washington, DC: 1996.
|
|
29
|
Yin J, Shen Y, Si Y, Zhang Y, Du J, Hu X,
Cai M, Bao H and Xing Y: Knockdown of long non-coding RNA SOX2OT
downregulates SOX2 to improve hippocampal neurogenesis and
cognitive function in a mouse model of sepsis-associated
encephalopathy. J Neuroinflammation. 17:3202020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao H, Han Z, Huang S, Bai R, Ge X, Chen F
and Lei P: Intermittent hypoxia caused cognitive dysfunction relate
to miRNAs dysregulation in hippocampus. Behav Brain Res. 335:80–87.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shan G, Tang T, Xia Y and Qian HJ: Long
non-coding RNA NEAT1 promotes bladder progression through
regulating miR-410 mediated HMGB1. Biomed Pharmacother.
121:1092482020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Yang T, Sun J, Zhang S and Liu S:
SENP1 modulates microglia-mediated neuroinflammation toward
intermittent hypoxia-induced cognitive decline through the
de-SUMOylation of NEMO. J Cell Mol Med. 25:6841–6854. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Ray PS, Sim MS, Zhou XZ, Lu KP,
Lee AV, Lin X, Bagaria SP, Giuliano AE and Cui X: FOXC1 regulates
the functions of human basal-like breast cancer cells by activating
NF-κB signaling. Oncogene. 31:4798–4802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang W, Chen Z, Zhang L, Tian D, Wang D,
Fan D, Wu K and Xia L: Interleukin-8 induces expression of FOXC1 to
promote transactivation of CXCR1 and CCL2 in hepatocellular
carcinoma cell lines and formation of metastases in mice.
Gastroenterology. 149:1053–1067.e14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Z and Trapp BD: Microglia and
neuroprotection. J Neurochem. 136 (Suppl 1):S10–S17. 2016.
View Article : Google Scholar
|
|
37
|
Kettenmann H, Hanisch UK, Noda M and
Verkhratsky A: Physiology of microglia. Physiol Rev. 91:461–553.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Colonna M and Butovsky O: Microglia
function in the central nervous system during health and
neurodegeneration. Annu Rev Immunol. 35:441–468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vaessen TJ, Overeem S and Sitskoorn MM:
Cognitive complaints in obstructive sleep apnea. Sleep Med Rev.
19:51–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kiernan EA, Smith SMC, Mitchell GS and
Watters JJ: Mechanisms of microglial activation in models of
inflammation and hypoxia: Implications for chronic intermittent
hypoxia. J Physiol. 594:1563–1577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Lima FFF, Mazzotti DR, Tufik S and
Bittencourt L: The role inflammatory response genes in obstructive
sleep apnea syndrome: A review. Sleep Breath. 20:331–338. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Calsolaro V and Edison P:
Neuroinflammation in Alzheimer's disease: Current evidence and
future directions. Alzheimers Dement. 12:719–732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Q, Wang Y, Feng J, Cao J and Chen B:
Intermittent hypoxia from obstructive sleep apnea may cause
neuronal impairment and dysfunction in central nervous system: The
potential roles played by microglia. Neuropsychiatr Dis Treat.
9:1077–1086. 2013.PubMed/NCBI
|
|
44
|
Zhai Q, Li F, Chen X, Jia J, Sun S, Zhou
D, Ma L, Jiang T, Bai F, Xiong L and Wang Q: Triggering receptor
expressed on myeloid cells 2, a novel regulator of immunocyte
phenotypes, confers neuroprotection by relieving neuroinflammation.
Anesthesiology. 127:98–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xia CY, Zhang S, Gao Y, Wang ZZ and Chen
NH: Selective modulation of microglia polarization to M2 phenotype
for stroke treatment. Int Immunopharmacol. 25:377–382. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hellström Erkenstam N, Smith PL, Fleiss B,
Nair S, Svedin P, Wang W, Boström M, Gressens P, Hagberg H, Brown
KL, et al: Temporal characterization of microglia/macrophage
phenotypes in a mouse model of neonatal hypoxic-ischemic brain
injury. Front Cell Neurosci. 10:2862016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dou Y, Wu HJ, Li HQ, Qin S, Wang YE, Li J,
Lou HF, Chen Z, Li XM, Luo QM and Duan S: Microglial migration
mediated by ATP-induced ATP release from lysosomes. Cell Res.
22:1022–1033. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mitchell S, Vargas J and Hoffmann A:
Signaling via the NF-κB system. Wiley Interdiscip Rev Syst Biol
Med. 8:227–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Viatour P, Merville MP, Bours V and
Chariot A: Phosphorylation of NF-kappaB and IkappaB proteins:
Implications in cancer and inflammation. Trends Biochem Sci.
30:43–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen S, Tang C, Ding H, Wang Z, Liu X,
Chai Y, Jiang W, Han Y and Zeng H: Maf1 ameliorates
sepsis-associated encephalopathy by suppressing the NF-κB/NLRP3
inflammasome signaling pathway. Front Immunol. 11:5940712020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Matthew SH and Sankar G: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Oliver KM, Garvey JF, Ng CT, Veale DJ,
Fearon U, Cummins EP and Taylor CT: Hypoxia activates
NF-kappaB-dependent gene expression through the canonical signaling
pathway. Antioxid Redox Signal. 11:2057–2064. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Regen F, Hellmann-Regen J, Costantini E
and Reale M: Neuroinflammation and Alzheimer's Disease:
Implications for microglial activation. Curr Alzheimer Res.
14:1140–1148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ho MS: Microglia in Parkinson's Disease.
Adv Exp Med Biol. 1175:335–353. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bjelobaba I, Savic D and Lavrnja I:
Multiple sclerosis and neuroinflammation: The overview of current
and prospective therapies. Curr Pharm Des. 23:693–730. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hong S, Beja-Glasser VF, Nfonoyim BM,
Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A,
Barres BA, et al: Complement and microglia mediate early synapse
loss in Alzheimer mouse models. Science. 352:712–716. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang H, Xiong W, Hang S, Wang Y, Zhang S
and Liu S: Depletion of SENP1-mediated PPARγ SUMOylation
exaggerates intermittent hypoxia-induced cognitive decline by
aggravating microglia-mediated neuroinflammation. Aging (Albany
NY). 13:15240–15254. 2021. View Article : Google Scholar : PubMed/NCBI
|