Introduction
Liver cancer is a leading cancer that occurs most
commonly and is considered as the fourth most prevalent human tumor
globally (1). Owing to increasing
number of new liver cancer cases, it has become major public health
challenge (2). In addition, weak
prognosis of major hepatic malignancies results in the increase in
the rate of mortality in primary liver cancer (1). Hepatocellular carcinoma (HCC) is the
most common form of liver cancer, accounting for up to 90% of all
primary hepatic malignancies (3).
Despite advances in medical and surgical therapies, HCC remains one
of the most common causes of cancer-related deaths worldwide since
therapies do not always produce optimal patient outcomes (4). Therefore, more effective treatment
methods of HCC are required, particularly certain specific targeted
agents.
Traditional Chinese medicine (TCM) is an important
therapy for liver cancer in China. Owing to its unique overall
concept, treatment based on syndrome differentiation and abundant
natural medicine resources, it has become a characteristic method
throughout the entire process of liver cancer prevention and
treatment (5,6). Ophiopogonin-B (OP-B) is a bioactive
component from the root of Ophiopogon japonicus, which is
widely used in TCM to treat pulmonary disease (7,8).
In the last decade, an increasing number of studies reported the
anticancer effect of OP-B on multiple malignant tumors, including
lung, colon, gastric and ovarian cancer (7–10).
It was previously demonstrated that OP-B induced HCC cell apoptosis
and decreased invasion through inhibition of the JAK2/STAT3
signaling pathway, indicating the potential value of OP-B in
treating HCC (11). In the
present study, further in vivo and in vitro studies
were performed to confirm whether OP-B exhibits an inhibitory
effect on the malignant processes of HCC.
Protein tyrosine phosphatase 1B [PTP1B; encoded by
protein tyrosine phosphatase non-receptor type 1 (PTPN1)] is an
important member in PTP superfamily of proteins. PTPs catalyze
phosphate monoesters hydrolysis on tyrosine residues; they are also
known to act as signaling molecules to regulate a number of
cellular processes, including cell growth, differentiation, mitotic
cycle and oncogenic transformation (12). To date, PTP1B has been reported to
be also involved in the development of obesity, diabetes, cancers
and cardiovascular diseases (13). Recently, Xu et al (14) showed that PTPN1 was upregulated in
HCC and its silencing suppressed proliferation and induced
apoptosis of HCC cells. A similar result was reported by Yang et
al (15), which suggested
that downregulation of PTP1B inhibited HCC development. These
results indicated an oncogenic role of PTP1B in HCC. Notably, it is
predicted by SwissTargetPrediction online database (16) that PTPN1 (PTP1B) is one of the
targets of OP-B (data not shown). Therefore, the present study
aimed to investigate whether OP-B exhibited an inhibitory effect on
the malignant process of HCC via targeting PTP1B.
Materials and methods
Cell culture and treatment
The human normal hepatocyte cell line HHL-5 and the
HCC cell line MHCC97-H were obtained from the American Type Culture
Collection. Both cell lines were cultured in DMEM (Sigma-Aldrich;
Merck KGaA) supplemented with 10% fetal bovine serum (FBS; Wisent
Biotechnology) and 1% penicillin-streptomycin (Sigma-Aldrich; Merck
KGaA) in an incubator with 5% CO2 at 37°C. For OP-B
treatment, cells were cultured in DMEM containing different
concentrations (5, 10, 20 and 40 µM) of OP-B (purity >97%;
Shanghai Yuanye Bio-Technology Co., Ltd.) for 24 h as previously
described (8,11,17,18). Cells cultured in normal DMEM
without OP-B were used as a control.
Cell transfection
A PTP1B overexpression vector (Ov-PTP1B) was
constructed by cloning human PTP1B cDNA into the pcDNA3.1 vector
(Thermo Fisher Scientific, Inc.). Empty pcDNA3.1 vector was used as
negative control (Ov-NC). MHCC97-H cells were grown in six-well
plates and subsequently transfected with 2 µg of either vector
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 24 h according to the manufacturer's
protocol. At 48 h post-transfection, monoclonal cells were then
selected and examined for PTP1B overexpression.
Xenograft tumor model
A total of 15 male BALB/c nude mice (age, 4–5 weeks;
15–20 g; Shanghai Laboratory Animal Center) were maintained under
specific pathogen-free conditions at room temperature under a
controlled 12/12 h light/dark cycle, and received food and water
ad libitum. The animal study protocol was approved by the
Ethics Committee of Suzhou Hospital of Integrated Traditional
Chinese and Western Medicine (Suzhou, China; approval no.
20180901). To establish the HCC xenograft model, MHCC97-H cells
(2×106 cells/200 µl) were subcutaneously injected into
the right flank of the nude mice. After six days, mice were treated
by oral gavage with normal saline (control group; n=5), 15 mg/kg
OP-B (OP-B 15 mg/kg group; n=5) or 75 mg/kg OP-B (OP-B 75 mg/kg
group; n=5) once a day for 21 days, as previously described
(8,17,18). Mouse body weight and tumor volume
were recorded every 7 days; at 21 days post-treatment, the mice
were sacrificed by cervical dislocation under anesthesia with 1%
sodium pentobarbital (50 mg/kg intraperitoneally). Tumor volumes
for each mouse were calculated as follows: Tumor volume=1/2 ×
length × width2.
Immunohistochemistry (IHC)
Xenografted tumor tissues were fixed with 4%
paraformaldehyde at 4°C for 12 h and embedded in paraffin. After
being deparaffinized with xylene and rehydrated with a descending
ethanol series, slides (3 µm thickness) were stained with the IHC
Assay Kit (cat. no. KGOS300; Nanjing KeyGen Biotech Co., Ltd.)
according to the manufacturer's protocol and then incubated at 4°C
overnight with the following primary antibodies purchased from
Abcam: Anti-Ki67 (1:1,000; cat. no. ab15580), anti-CD31 (1:50; cat.
no. ab28364) and anti-VEGFA (1:200; cat. no. ab52917). Secondary
antibody goat anti-rabbit IgG (1:1,000; cat. no. ab6721; Abcam) was
applied at room temperature for 2 h. Images of the tissue sections
were then captured with a fluorescence microscope (DMi8; Leica
Microsystems GmbH; ×200 magnification).
Cell viability
Cell Counting Kit-8 (CCK8; Beyotime Institute of
Biotechnology) was used to measure cell viability. Briefly, cells
were seeded into 96-well plates at a density of 1,000 cells/well at
37°C for 24 h. After treatment with different concentrations of
OP-B (0, 5, 10, 20 and 40 µM) at 37°C for 24 h, cells were
incubated with 10 µl of CCK-8 solution at 37°C for 2 h. The optical
density was calculated at 450 nm using a microplate reader
(Model550; Bio-Rad Laboratories, Inc.).
Colony formation
For the colony formation assay, 1×103
MHCC97-H cells were cultivated in six-well plates in a 37°C
incubator with 5% CO2. After 14 days, cell colonies were
fixed with 95% alcohol at room temperature for 30 min and stained
with 0.1% crystal violet at room temperature for 30 min
(Sigma-Aldrich; Merck KGaA). Images were captured with a light
microscope (Nikon Corporation; ×200 magnification) and colonies
were counted using ImageJ software (version 1.8.0; National
Institutes of Health).
TUNEL staining
According to the manufacturer's protocol, MHCC97-H
cell apoptosis was analyzed using TUNEL Assay kit (Abcam). Briefly,
cells were fixed in 4% paraformaldehyde solution for 30 min at room
temperature, treated with 0.2% Triton X-100 for 5 min at room
temperature, washed twice in PBS at room temperature, and labeled
with fluorescein-12-dUTP using terminal deoxynucleotidyl
transferase for 2 h at room temperature. Subsequently, cell nuclei
were stained with 5 µg/ml DAPI at room temperature for 3 min. All
images were obtained using a fluorescence microscope (DMi8; Leica
Microsystems GmbH; ×200 magnification).
Wound healing assay
Briefly, MHCC97-H cells were seeded in a six-well
plate at a density of 5×105 cells/well in DMEM. When the
cells were ~80% confluent, scratches were made in the middle of
slides using a sterile 10-µl pipette tip. After incubation for
another 48 h with or without 20 µM OP-B in serum-free DMEM at 37°C,
images were captured to estimate closure of the gap using a light
microscope (Nikon Corporation; ×200 magnification). Migration
distance was evaluated using ImageJ software (version 1.8.0;
National Institutes of Health) and calculated as follows: Migration
distance=(width of gap at 0 h-width of gap at 48 h)/width of gap at
0 h. Data are shown as relative migration distance by normalization
to the control group.
Transwell invasion assay
The invasive ability of MHCC97-H cells was evaluated
by the Biocoat invasion assay kit (Corning, Inc.) strictly
following the manufacturer's protocol. Firstly, 5×105
cells per well were plated in the upper chamber followed by
treatment with 20 µM OP-B or not. After 12 h of treatment, the
cells in the upper chamber were incubated with serum-free DMEM
medium, whereas media supplemented with 10% FBS was placed at the
lower chamber. After 48 h, the invasive cells at the lower chamber
were stained with 0.1% crystal violet at room temperature and
observed using an optical light microscope (Leica Microsystems
GmbH; ×200 magnification).
Tube formation assay
Matrigel (BD Biosciences) was thawed at 4°C,
pipetted into 96-well plates and allowed to polymerize at 37°C for
1 h. Cells (1×104 per well) were suspended in DMEM
containing 0 or 20 µM OP-B and seeded onto the Matrigel. After
incubation for 24 h at 37°C, tube formation was analyzed by
counting nodes and measuring total tube numbers using ImageJ
software (version 1.8.0; National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol, and then reverse transcribed into cDNA
using a High Capacity cDNA Reverse Transcription Kit (Applied
Biosystems) according to the manufacturer's protocol. SYBR-Green
Supermix (Takara Biotechnology Co., Ltd.) was used for qPCR in a 10
µl reaction volume on the Roche Light Cycler R480 System (Roche
Diagnostics). The following thermocycling conditions were used for
the qPCR: Initial denaturation at 95°C for 30 sec; followed by 40
cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 20
sec and extension at 70°C for 10 sec. Target gene expression levels
were analyzed using the 2−ΔΔCq (19) method and normalized to GAPDH. The
primers used for RT-qPCR were as follows: PTP1B forward,
5′-CCAGCCAAAGGGGAGCCGTC-3′ and reverse, 5′-CTATGTGTTGCTGTTGAACA-3′;
and GAPDH forward, 5′-AATGGGCAGCCGTTAGGAAA-3′ and reverse,
5′-GCGCCCAATACGACCAAATC-3′.
Western blot assay
Total protein was extracted from cells or tumor
tissues by RIPA lysis buffer (Beyotime Institute of Biotechnology).
Total protein was quantified using a BCA Kit (Beyotime Institute of
Biotechnology), mixed with 5X sample buffer and boiled at 95°C for
5 min. Equal amounts (40 µg) of protein lysate per sample were
separated on 10% gels using SDS-PAGE and transferred onto the PVDF
membranes. After blocking with 5% skimmed milk for 2 h at room
temperature, membranes were incubated with primary antibodies at
4°C overnight and secondary antibodies (goat anti-rabbit IgG HRP;
1:10,000; cat. no. ab6721; Abcam) at room temperature for 2 h. An
enhanced chemiluminescence (ECL) detection kit (Amersham; Cytiva)
was used to visualize the protein bands. Band intensity was
semi-quantified by ImageJ software (v1.8.0; National Institutes of
Health). Primary antibodies (all from Abcam) included: anti-PTP1B
(1:1,000; cat. no. ab252928), anti-Bcl-2 (1:1,000; cat. no.
ab32124), anti-Bax (1:5,000; cat. no. ab32503), anti-cleaved
caspase 3 (1:500; cat. no. ab32042), anti-caspase-3 (1:1,000; cat.
no. ab184787), anti-cleaved poly ADP-ribose polymerase (PARP;
1:5,000; cat. no. ab32064), anti-PARP (1:5,000; cat. no. ab191217),
anti-E-cadherin (1:10,000; cat. no. ab40772), anti-N-cadherin
(1:10,000; cat. no. ab76011), anti-Vimentin (1:1,000; cat. no.
ab45939), anti-VEGFA (1:10,000; cat. no. ab52917),
anti-phosphorylated (p)-phosphatidylinositol 3 kinase (PI3K;
1:1,000; cat. no. ab191606), anti-total (t)-PI3K (1:1,000; cat. no.
ab278545), anti-p-AKT (1:1,000; cat. no. ab38449), anti-t-AKT
(1:1,000; cat. no. ab8805), anti-p-adenosine
5′-monophosphate-activated protein kinase (AMPK; 1:1,000; cat. no.
ab109402), anti-t-AMPK (1:1,000; cat. no. ab214425) and anti-GAPDH
(1:10,000; cat. no. ab181602).
Statistical analysis
Data are expressed as the mean ± standard deviation.
One-way ANOVA followed by Tukey's post hoc test was used for
analysis between multiple groups. Calculations were performed using
GraphPad Prism software (version 5.0; GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
OP-B inhibits tumor growth and PTP1B
expression in HCC xenografted mice
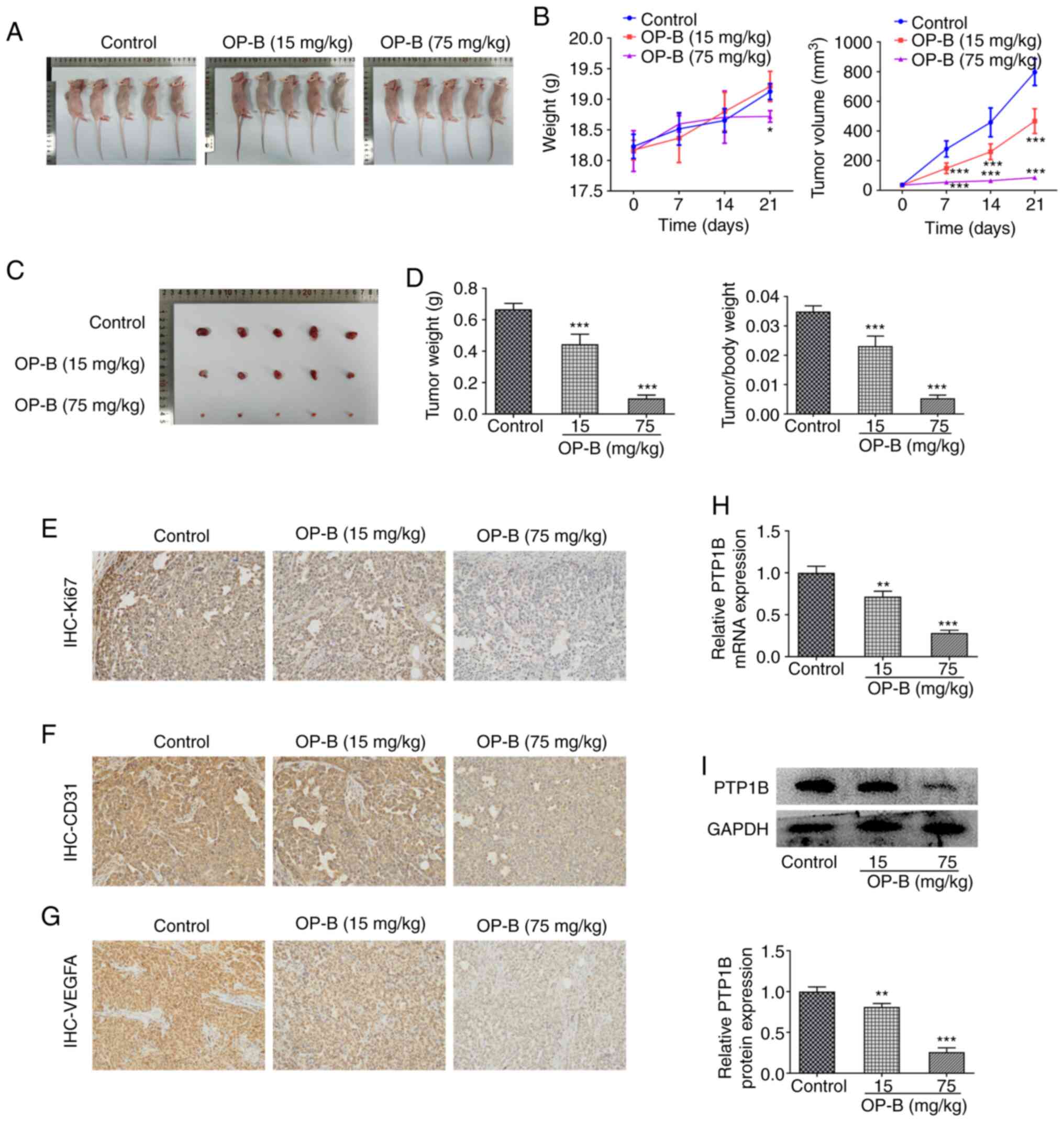
The anticancer effects of OP-B on HCC were first
investigated in vivo. HCC xenografts were injected into mice
(Fig. 1A) that were subsequently
treated orally with 0, 15 or 75 mg/kg OP-B. Tumor volume and mouse
body weight were examined for 21 days (Fig. 1B). The tumors were excised
(Fig. 1C) and the final tumor
weight and the tumor/body weight ratio were measured. (Fig. 1D), which were significantly
reduced in mice treated with both 15 and 75 mg/kg OP-B compared
with the control mice. Additionally, it was observed from IHC
results that the expression level of Ki67 (Fig. 1E), CD31 (Fig. 1F) and VEGFA (Fig. 1G) in HCC tumor tissues of the
xenografted mice was markedly decreased upon OP-B (15 and 75 mg/kg)
administration compared with the control mice. In addition, the
mRNA and protein expression levels of PTP1B in tumor samples of
xenograft mice model was significantly inhibited by OP-B (Fig. 1H and I, respectively). These
results showed that OP-B exerted anticancer effect on HCC in
vivo.
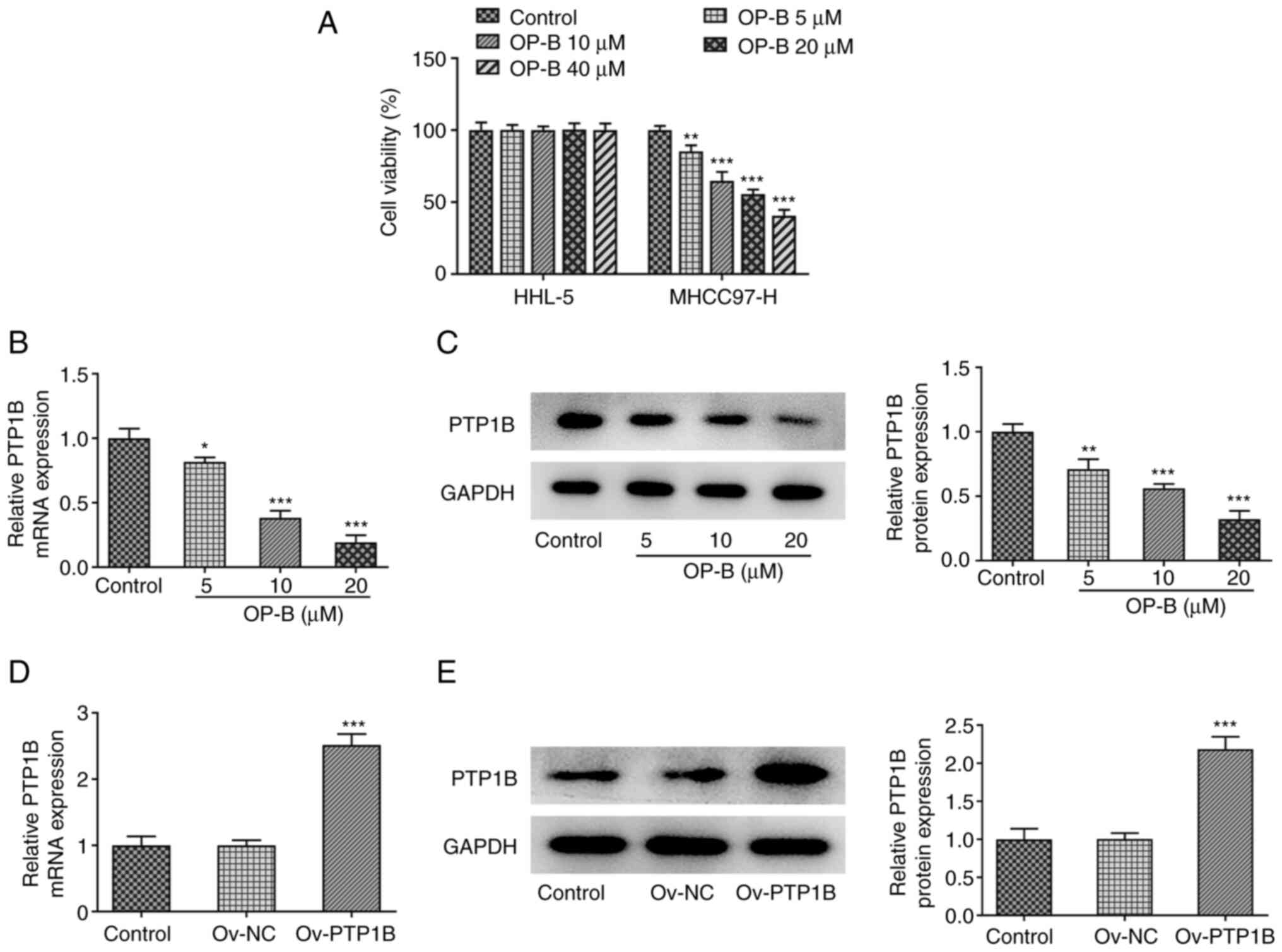
OP-B reduces cell viability and PTP1B
expression in HCC cells
To validate the effect of OP-B on HCC and PTP1B
expression in vitro, HHL-5 human normal hepatocyte and
MHCC97-H HCC cells were exposed to different concentrations of OP-B
(5, 10, 20 and 40 µM) for 24 h, then cell viability was measured.
As revealed in Fig. 2A, OP-B
treatment did not cause significant effect on HHL-5 cell viability,
but significantly reduced MHCC97-H cell viability in a
concentration-dependent manner. In addition, both the mRNA and the
protein expression levels of PTP1B were significantly downregulated
by OP-B (Fig. 2B and C,
respectively).
Subsequently, PTP1B was overexpressed in MHCC97-H
cells by transfection of Ov-PTP1B vector and then the
overexpression efficiency was confirmed by RT-qPCR and western
blotting (Fig. 2D and E,
respectively).
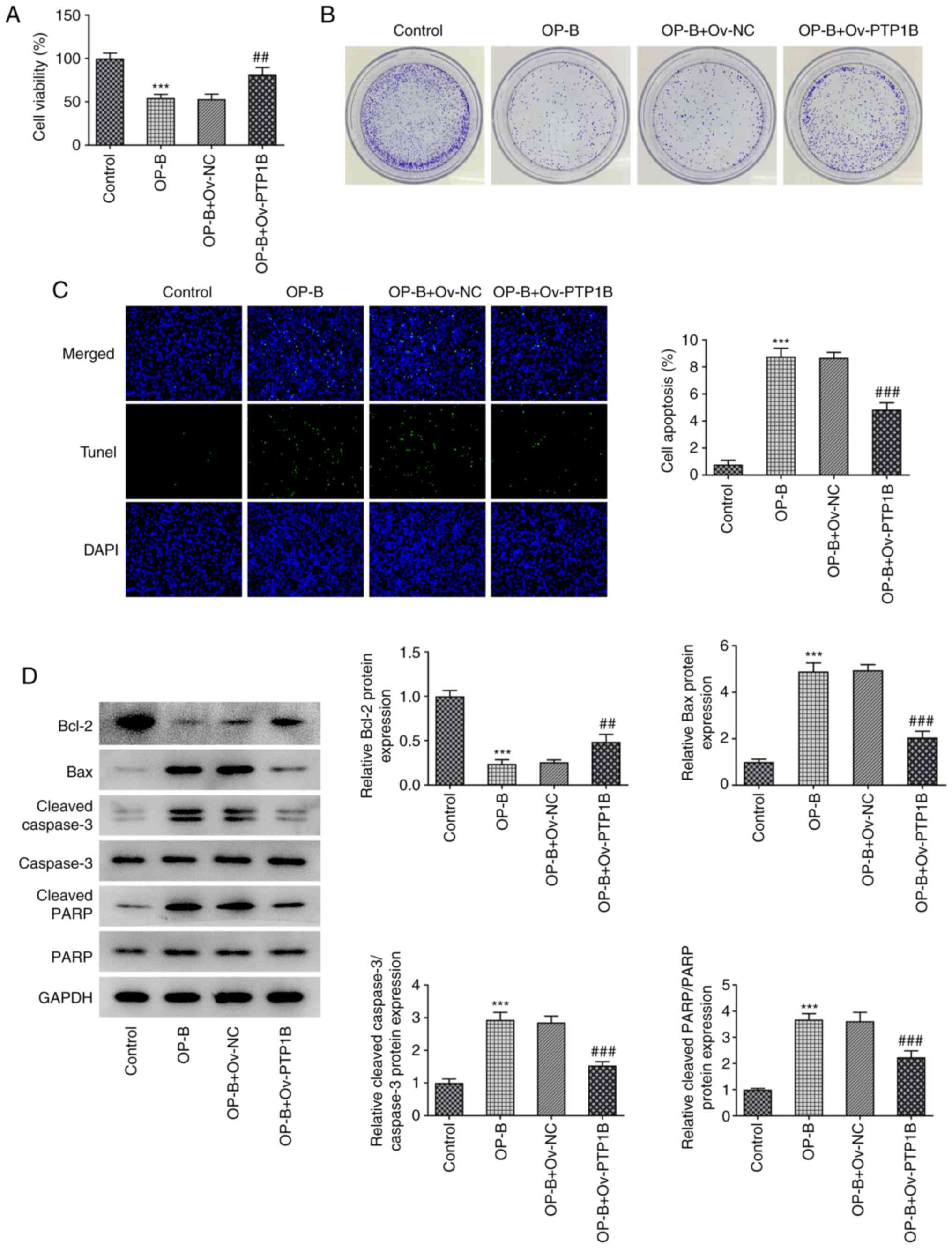
Decreased PTP1B expression may be
responsible for the anticancer effect of OP-B on HCC
To observe the effect of OP-B on the malignant
processes of HCC, MHCC97-H cells overexpressing PTP1B were treated
with 20 µM OP-B, then cell proliferation, apoptosis, migration,
invasion and angiogenesis were evaluated. As revealed in Fig. 3A, OP-B significantly reduced cell
viability compared with the untreated control group, whereas OP-B +
Ov-PTP1B treatment significantly enhanced the cell viability
compared with the OP-B + Ov-NC group. These results indicated that
the inhibitory effect of OP-B on HCC cell viability was reversed by
PTP1B overexpression. Furthermore, OP-B markedly suppressed the
colony formation compared with control MHCC97-H cells, whereas
PTP1B overexpression markedly reduced this effect (Fig. 3B). TUNEL staining revealed that
apoptosis was significantly increased upon OP-B treatment compared
with the control group, and PTP1B overexpression reduced the level
of apoptosis compared with OP-B + Ov-NC (Fig. 3C). Consistently, OP-B treatment
resulted in decreased protein expression of Bcl-2, but increased
expression levels of Bax, cleaved caspase 3 and cleaved PARP,
suggesting the induction of cell apoptosis (Fig. 3D). However, PTP1B overexpression
caused a reverse effect on the expression of these proteins in
OP-B-treated cells (Fig. 3D).
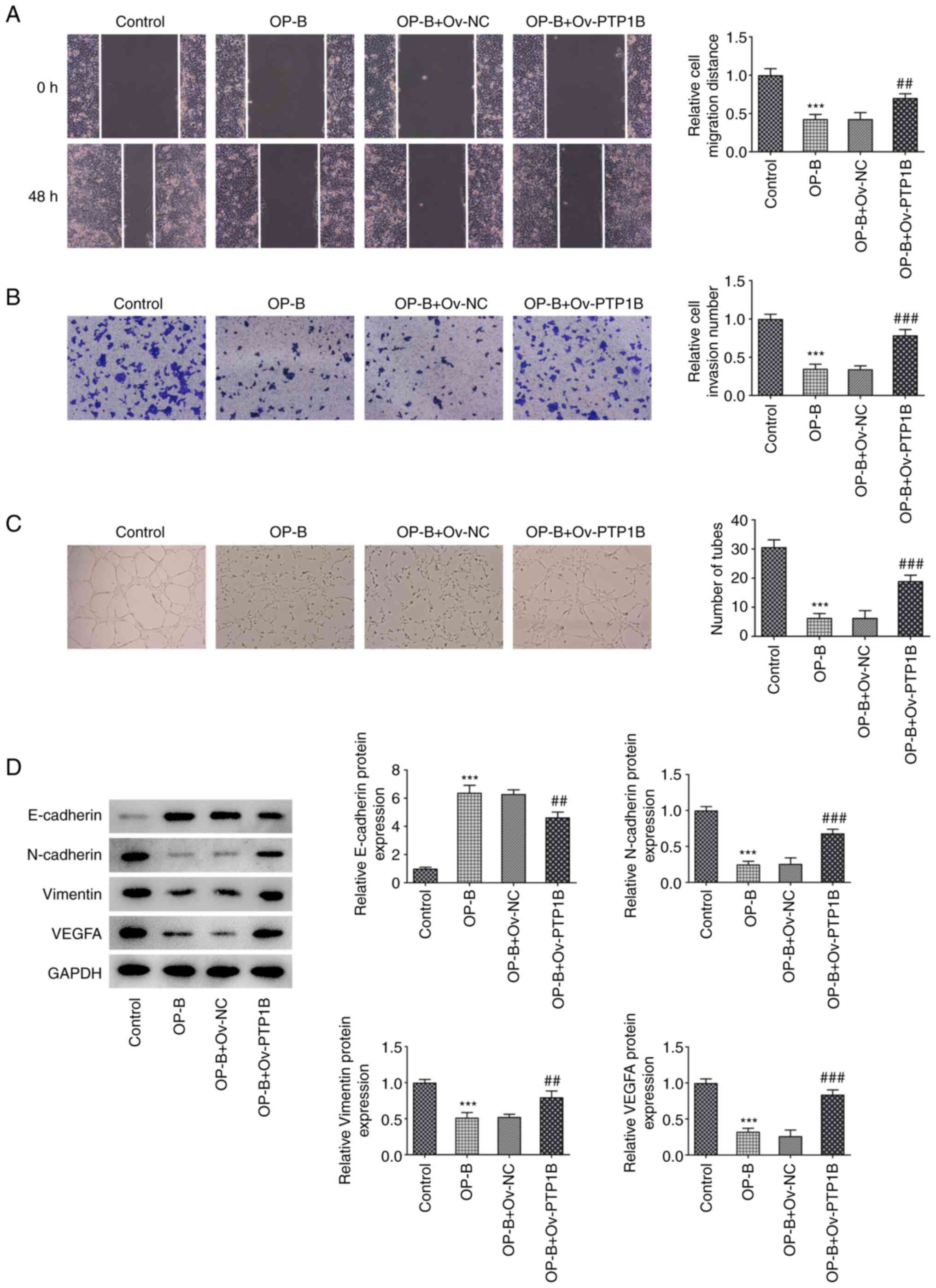
Results from wound healing, invasion and tube
formation assays demonstrated that OP-B significantly inhibited
MHCC97-H cell migration, invasion and angiogenesis compared with
untreated control cells (Fig.
4A-C, respectively). By contrast, OP-B + Ov-PTP1B treatment
significantly increased these processes compared with OP-B + Ov-NC
group (Fig. 4A-C). In addition,
OP-B treatment significantly increased E-cadherin expression but
decreased N-cadherin, Vimentin and VEGFA expression (Fig. 4D), whereas PTP1B overexpression
reversed the effect on the expression levels of these proteins
caused by OP-B.
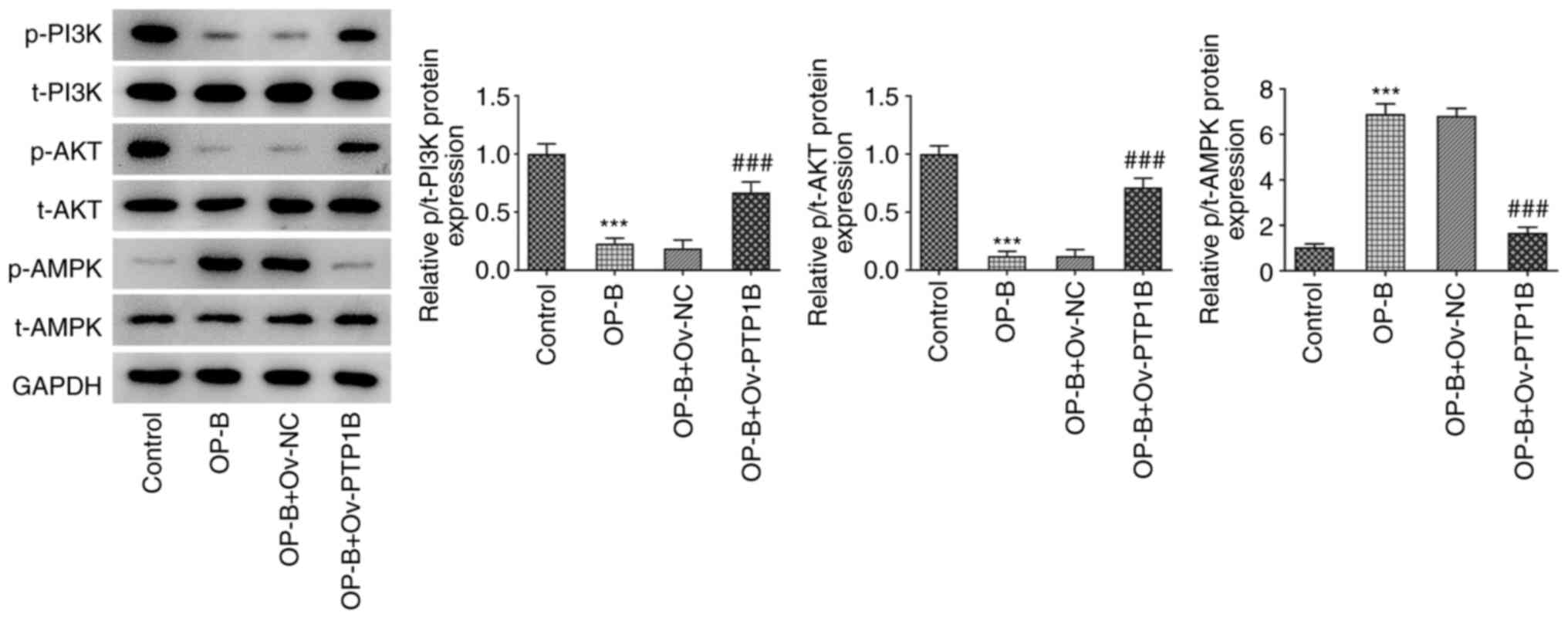
OP-B inactivates the PI3K/AKT
signaling and activates AMPK signaling by reducing PTP1B
expression
To uncover the underlying related signaling
mechanism of OP-B, the expression of the PI3K/AKT and AMPK
signaling pathways was detected. As demonstrated in Fig. 5, MHCC97-H cells showed decreased
p/t-PI3K and p/t-AKT expression levels as well as increased
p/t-AMPK expression in response to OP-B treatment. Conversely, when
compared with OP-B + Ov-NC, OP-B + Ov-PTP1B treatment significantly
upregulated p/t-PI3K and p/t-AKT expression levels and
downregulated p/t-AMPK expression. These data indicated that OP-B
may inhibit PI3K/AKT activation and activate the AMPK signaling,
which is supported by the data showing that these effects were
blocked by PTP1B overexpression.
Discussion
OP-B is a natural active compound extracted from the
TCM Ophiopogon japonicus root, which was found to exert
inhibitory effect on non-small cell lung cancer cell lines
(20). In the present study, OP-B
was demonstrated to inhibit HCC development both in vitro
and in vivo. Further experiments indicated that OP-B may
exert its anticancer effect on HCC by targeting PTP1B and
regulating the PI3K/AKT and AMPK signaling pathways.
Natural compounds from TCM have been revealed to
exert remarkable effects in the treatment of HCC (21–24). For example, PHY906 has been
reported to reduce the adverse effects of capecitabine in advanced
HCC patients in a phase I/II clinical study (25). These studies reflected the
satisfactory curative effect and huge potential of TCM in the
treatment of HCC. In the present study, 15 and 75 mg/kg OP-B was
selected for treating HCC-xenografted mice by oral gavage according
to previous studies (8,17,18). OP-B at these two doses had no
toxic effect on mice; it did not affect the mental state, life
activity and weight gain of rats with the exception of 75 mg/kg
OP-B, which significantly reduced body weight at 21 days
post-treatment and this may due to the significant reduction in
tumor weight caused by 75 mg/kg OP-B treatment. Additionally, it
was found that in HCC xenograft tumors, OP-B effectively inhibited
the growth of tumor and the expression of Ki67, CD31 and VEGFA. In
in vitro experiments, OP-B did not affect the cell viability
of normal hepatocyte HHL-5, but significantly impaired that of the
HCC cell line MHCC97-H. Meanwhile, it was observed that 40 µM OP-B
reduced the cell viability of MHCC97-H to less than 50%, indicating
the excessive toxicity caused by 40 µM OP-B. Thus 5, 10 and 20 µM
OP-B was utilized for subsequent functional analysis and results
showed that treatment with OP-B significantly suppressed
proliferation, migration, invasion and angiogenesis, but induced
apoptosis of HCC cells. In addition, the expression of E-cadherin
was enhanced and that of N-cadherin, vimentin and VEGFA was
decreased upon OP-B treatment. Ki67 is a well-known cell
proliferation marker; CD31 is a well-known marker of endothelial
cells and a key factor for adhesion and accumulation of platelets
(26); VEGFA is the key regulator
of angiogenesis during the growth of solid tumors (26). Loss of E-cadherin expression
results in loss of contact inhibition and is associated with
increased cell motility and advanced stages of cancer (27); N-cadherin and Vimentin are
recognized as a markers for epithelial-mesenchymal transition (EMT)
(28). Consistently, the in
vivo and in vitro results of the present study
demonstrated the anticancer effect of OP-B on HCC.
To uncover the mechanism through which OP-B exert
its anticancer effect, its potential downstream targets were
predicted using SwissTargetPrediction online database and PTP1B was
identified as one of the targets of OP-B (data not shown). Notably,
the participation of PTP1B in liver cancer initiation and
progression has been previously reported (15,29,30). Results from the present study
showed that OP-B reduced the expression of PTP1B both in HCC tumor
tissues and in cells in a concentration-depend manner. It was
therefore speculated that OP-B may suppress the progression of HCC
by targeting PTP1B. Further functional analysis revealed that
overexpression of PTP1B significantly reversed the effect of OP-B
treatment on malignant processes of HCC cells, indicating that
PTP1B downregulation may be responsible for the anticancer effect
of OP-B on HCC.
Finally, the mechanisms underlying the actions of
OP-B/PTP1B in HCC were uncovered. Jin et al (31) reported that PTP1B promoted the
progression of glioma by activating the MAPK/ERK and PI3K/AKT
pathways. Xu et al (32)
demonstrated that inhibition of PTP1B blocked pancreatic cancer
progression by targeting the PKM2/AMPK/mTOC1 pathway. OP-B was also
found to inhibit the PI3K/Akt signaling pathway in non-small cell
lung cancer cells (20). The
PI3K/AKT pathway had been extensively studied and found to be
commonly activated in human cancer. Inhibition of this pathway
leads to regression of human tumors (33). AMPK signaling is generally
considered a key effector that mediates the tumor-suppressive
function of liver kinase B1 (34). The inhibition of PI3K/AKT pathway
or the activation of AMPK pathway has been found to inhibit
proliferation, migration, invasion and EMT of cancer cells by
regulating the expression of related markers such as Ki67, CD31,
VEGFA, E-cadherin, N-cadherin and Vimentin (35–37). In the present study, OP-B
inhibited the phosphorylation of PI3K/AKT and enhanced the
phosphorylation of AMPK, whereas PTP1B overexpression reversed the
effect of OP-B on the PI3K/AKT and AMPK signaling pathways.
Although the present results were opposite with certain previous
studies (38,39), which showed that PTP1B inhibited
the phosphorylation of PI3K/AKT signaling, the effect of PTP1B on
PI3K/AKT and AMPK phosphorylation in cancer cells was demonstrated
for the first time, to the best of our knowledge. Further studies
are required to investigate whether it is due to the particularity
of cancer cells compared with normal healthy cells that led to this
opposite finding. Combined with previous findings (11), it was demonstrated that OP-B could
indeed suppress the malignant processes of HCC through regulating
multiple pathways. However, the involvement of the PI3K/AKT and
AMPK signaling pathways needs further validation using an agonist
or antagonist of these two pathways. Whether OP-B could inhibit HCC
through regulating other pathways involved in HCC progression, such
as TGF-β, mTOR and Wnt/β-catenin signaling pathways (40–42) needs to be elucidated in future
studies.
Collectively, the present study demonstrated that
OP-B suppressed the progression of HCC both in vivo and
in vitro through targeting PTP1B. Additionally, the PI3K/AKT
and AMPK signaling pathways may be involved in this effect. These
results may provide insight into the underlying mechanism involved
in the antitumor effects of OP-B on HCC and may support the
application of OP-B in a clinical setting.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Research on Medical
and Health Application of Suzhou Science and Technology Program
(grant no. sys2018003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS and YZ conceived and designed the study. FY, QG
and HT performed the experiments. FY and QG analyzed and
interpreted the data. JS and YZ drafted the manuscript and revised
it for critically important intellectual content. JS and YZ confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal study protocol was approved by the Ethics
Committee of Suzhou Hospital of Integrated Traditional Chinese and
Western Medicine (Suzhou, China; approval no. 20180901).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grandhi MS, Kim AK, Ronnekleiv-Kelly SM,
Kamel IR, Ghasebeh MA and Pawlik TM: Hepatocellular carcinoma: From
diagnosis to treatment. Surg Oncol. 25:74–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartke J, Johnson M and Ghabril M: The
diagnosis and treatment of hepatocellular carcinoma. Semin Diagn
Pathol. 34:153–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sim HW and Knox J: Hepatocellular
carcinoma in the era of immunotherapy. Curr Probl Cancer. 42:40–48.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao X, Bu Y and Jia Q: Traditional
Chinese medicine as supportive care for the management of liver
cancer: Past, present, and future. Genes Dis. 7:370–379. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li HM: Microcirculation of liver cancer,
microenvironment of liver regeneration, and the strategy of Chinese
medicine. Chin J Integr Med. 22:163–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang S, Li H, Li L, Gao Q, Gu L, Hu C,
Chen M and Zhang X: Ophiopogonin B inhibits migration and invasion
in non-small cell lung cancer cells through enhancing the
interaction between Axin and β-catenin. J Cancer. 12:6274–6284.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao GY, Ma J, Lu P, Jiang X and Chang C:
Ophiopogonin B induces the autophagy and apoptosis of colon cancer
cells by activating JNK/c-Jun signaling pathway. Biomed
Pharmacother. 108:1208–1215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Zhang Q, Jiang Y, Li F and Xin H:
Effects of ophiopogonin B on the proliferation and apoptosis of
SGC-7901 human gastric cancer cells. Mol Med Rep. 13:4981–4986.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan S, Xu Y, Yi T and Wang H: The
anti-tumor effect of OP-B on ovarian cancer in vitro and in vivo,
and its mechanism: An investigation using network
pharmacology-based analysis. J Ethnopharmacol. 283:1147062021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi J, Zhu L, Tang HL, Zhou YQ, Xue BY and
Chen C: Ophiopogonin Binduceshepatocellular carcinoma MHCC97-H cell
apoptosis and decreases invasion through inhibition of the
JAK2/STAT3 signaling pathway. Int J Clin Exp Med. 11:1825–1834.
2018.PubMed/NCBI
|
|
12
|
Kumar A, Rana D, Rana R and Bhatia R:
Protein tyrosine phosphatase (PTP1B): A promising drug target
against life-threatening ailments. Curr Mol Pharmacol. 13:17–30.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma B, Xie L, Yang F, Wang W, Zhou Q,
Xiang M, Zhou S, Lv W, Jia Y, Pokhrel L, et al: Recent advance on
PTP1B inhibitors and their biomedical applications. Eur J Med Chem.
199:1123762020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu X, Tao Y, Niu Y, Wang Z, Zhang C, Yu Y
and Ma L: miR-125a-5p inhibits tumorigenesis in hepatocellular
carcinoma. Aging. 11:7639–7662. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Q, Zhang L, Zhong Y, Lai L and Li X:
miR-206 inhibits cell proliferation, invasion, and migration by
down-regulating PTP1B in hepatocellular carcinoma. Biosci Rep.
39:BSR201818232019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daina A, Michielin O and Zoete V:
SwissTargetPrediction: Updated data and new features for efficient
prediction of protein targets of small molecules. Nucleic Acids
Res. 47:W357–W364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen M, Guo Y, Zhao R, Wang X, Jiang M, Fu
H and Zhang X: Ophiopogonin B induces apoptosis, mitotic
catastrophe and autophagy in A549 cells. Int J Oncol. 49:316–324.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen M, Hu C, Guo Y, Jiang R, Jiang H,
Zhou Y, Fu H, Wu M and Zhang X: Ophiopogonin B suppresses the
metastasis and angiogenesis of A549 cells in vitro and in
vivo by inhibiting the EphA2/Akt signaling pathway. Oncol Rep.
40:1339–1347. 2018.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen M, Du Y, Qui M, Wang M, Chen K, Huang
Z, Jiang M, Xiong F, Chen J, Zhou J, et al: Ophiopogonin B-induced
autophagy in non-small cell lung cancer cells via inhibition of the
PI3K/Akt signaling pathway. Oncol Rep. 29:430–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Li M, Wang X, Dang Z, Yu L, Wang X,
Jiang Y and Yang Z: Effects of adjuvant traditional Chinese
medicine therapy on long-term survival in patients with
hepatocellular carcinoma. Phytomedicine. 62:1529302019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu C, Yang S, Wang K, Bao X, Liu Y, Zhou
S, Liu H, Qiu Y, Wang T and Yu H: Alkaloids from traditional
Chinese medicine against hepatocellular carcinoma. Biomed
Pharmacother. 120:1095432019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang M, Ye Q, Mao D and Li H: Research
progress in liver-regenerating microenvironment and DNA methylation
in hepatocellular carcinoma: The role of traditional Chinese
medicine. Med Sci Monit. 26:e9203102020.PubMed/NCBI
|
|
24
|
Gong Y: Identifying the targets for
treatment of liver fibrosis and hepatocellular carcinoma from both
western medicine and Chinese medicine. Chin J Integr Med.
18:245–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yen Y, So S, Rose M, Saif MW, Chu E, Liu
SH, Foo A, Jiang Z, Su T and Cheng YC: Phase I/II study of
PHY906/capecitabine in advanced hepatocellular carcinoma.
Anticancer Res. 29:4083–4092. 2009.PubMed/NCBI
|
|
26
|
Menon SS, Guruvayoorappan C, Sakthivel KM
and Rasmi RR: Ki-67 protein as a tumour proliferation marker. Clin
Chim Acta. 491:39–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mendonsa AM, Na TY and Gumbiner BM:
E-cadherin in contact inhibition and cancer. Oncogene.
37:4769–4780. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bartolomé RA, Martín-Regalado Á, Jaén M,
Zannikou M, Zhang P, de Los Ríos V, Balyasnikova IV and Casal JI:
Protein tyrosine phosphatase-1B inhibition disrupts
IL13Rα2-promoted invasion and metastasis in cancer cells. Cancers
(Basel). 12:5002020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tai WT, Chen YL, Chu PY, Chen LJ, Hung MH,
Shiau CW, Huang JW, Tsai MH and Chen KF: Protein tyrosine
phosphatase 1B dephosphorylates PITX1 and regulates p120RasGAP in
hepatocellular carcinoma. Hepatology. 63:1528–1543. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin T, Li D, Yang T, Liu F, Kong J and
Zhou Y: PTPN1 promotes the progression of glioma by activating the
MAPK/ERK and PI3K/AKT pathways and is associated with poor patient
survival. Oncol Rep. 42:717–725. 2019.PubMed/NCBI
|
|
32
|
Xu Q, Wu N, Li X, Guo C, Li C, Jiang B,
Wang H and Shi D: Inhibition of PTP1B blocks pancreatic cancer
progression by targeting the PKM2/AMPK/mTOC1 pathway. Cell Death
Dis. 10:8742019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alzahrani AS: PI3K/Akt/mTOR inhibitors in
cancer: At the bench and bedside. Semin Cancer Biol. 59:125–132.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan J, Dong X, Yap J and Hu J: The MAPK
and AMPK signalings: Interplay and implication in targeted cancer
therapy. J Hematol Oncol. 13:1132020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang B, Wu J, Guo P, Wang Y, Fang Z, Tian
J, Yu Y, Teng W, Luo Y and Li Y: Down-regulation of SREBP via
PI3K/AKT/mTOR pathway inhibits the proliferation and invasion of
non-small-cell lung cancer cells. Onco Targets Ther. 13:8951–8961.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu J, Zhao X, Sun Q, Jiang Y, Zhang W, Luo
J and Li Y: Synergic effect of PD-1 blockade and endostar on the
PI3K/AKT/mTOR-mediated autophagy and angiogenesis in Lewis lung
carcinoma mouse model. Biomed Pharmacother. 125:1097462020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q, Kong J, Dong S, Xu W and Sun W:
Metformin exhibits the anti-proliferation and anti-invasion effects
in hepatocellular carcinoma cells after insufficient radiofrequency
ablation. Cancer Cell Int. 17:482017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li H, Dusseault J and Larose L: Nck1
depletion induces activation of the PI3K/Akt pathway by attenuating
PTP1B protein expression. Cell Commun Signal. 12:712014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun T, Wang Q, Yu Z, Zhang Y, Guo Y, Chen
K, Shen X and Jiang H: Hyrtiosal, a PTP1B inhibitor from the marine
sponge Hyrtios erectus, shows extensive cellular effects on
PI3K/AKT activation, glucose transport, and TGFbeta/Smad2
signaling. Chembiochem. 8:187–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen J, Gingold JA and Su X:
Immunomodulatory TGF-β signaling in hepatocellular carcinoma.
Trends Mol Med. 25:1010–1023. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferrín G, Guerrero M, Amado V,
Rodríguez-Perálvarez M and De la Mata M: Activation of mTOR
signaling pathway in hepatocellular carcinoma. Int J Mol Sci.
21:12662020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vilchez V, Turcios L, Marti F and Gedaly
R: Targeting Wnt/β-catenin pathway in hepatocellular carcinoma
treatment. World J Gastroenterol. 22:823–832. 2016. View Article : Google Scholar : PubMed/NCBI
|