Introduction
The skin is the first line of defense against
infection. Skin is a mechanical barrier between the body and the
surrounding environment (1). The
skin protects an organism from external hazards, toxins, and
pathogen infections by forming a barrier between the host and the
environment (2). However,
frequent, prolonged, and permanent contact with exogenous stimuli
will eventually activate immune responses. Hence, it is not
surprising that chronic immune-mediated skin diseases are among the
most common disorders of humans (3,4).
Keratinocytes are the major cell type of the
epidermis, the outermost layer of the skin that is involved in the
pathogenesis of inflammatory skin diseases. Keratinocytes are
involved in epidermal inflammatory responses upon exposure to
infectious agents and infiltration of immune cells (5). Inflammatory responses in
keratinocytes are initiated and maintained by pro-inflammatory
cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ
(IFN-γ) (6). Stimulation of
keratinocytes eventually leads to the production of cytokines,
chemokines, and adhesion molecules (7).
Interleukin (IL)-6 is a cytokine with a wide range
of effects on the immune system and the host. It is actively
researched as a promising target for clinical investigation
(8). IL-6 has been demonstrated
to activate Janus kinase 2 (JAK2) and signal transducer and
activator of transcription 3 (STAT3) in signaling cascades that
control inflammation (9). Nuclear
factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs) are
two major signaling pathways in the pathogenesis of inflammation.
NF-κB and MAPK activation mediates the response to critical
pro-inflammatory cytokines (such as TNF-α, IL-1β, IL-6, and IL-8)
and chemokines (10).
NF-κB is a transcription factor that plays an
important role in the inflammatory mechanism by regulating genes,
particularly inducible nitric oxide synthase (iNOS) and
cyclooxygenase-2 (COX-2), cytokines, and growth factors (11). The activation of NF-κB occurs
through association with an endogenous inhibitor protein of the IκB
(inhibitor of NF-κB) family (12). Once NF-κB is phosphorylated, it
translocate to the nucleus and binds to inflammation-related genes,
causing the production of pro-inflammatory mediators (13).
Mitogen-activated protein (MAP) kinases are a family
of serine/threonine kinase proteins regulated by phosphorylated
cascades of three kinases that serially phosphorylate one another:
p38 isoforms (p38s), c-Jun NH2-terminal kinases (JNKs), and
extracellular signal-regulated kinases (ERKs) (14). The activation of MAPKs through any
of the downstream signaling cascades (p38, JNK, or ERK) mediates
the regulation of the inflammatory mechanism (15). Thus, anti-inflammatory drug
modulators with phosphorylation activity on any of these cascades
can be an attractive strategy to inhibit chronic inflammation
(16).
The cosmetic and pharmaceutical industries have been
widely screening for products with favorable properties such as
anti-inflammatory, antiaging, and anti-melanogenic effects
(17). Adipose tissue-derived
stem cells (ADSCs) have shown potency in dermatological treatment
through topical application due to their improved ability to treat
inflammation (18). Recent
research has introduced improved and fascinating approaches to
modify ADSCs or component derivatives to be useful in treatment
options (19). Membrane-free stem
cell components (MFSCC) from ADSCs are considered as an alternative
source of therapeutics because they have better regenerative
effects than stem cells. In a previous study, our research group
reported the effects of MFSCC on lipopolysaccharide
(LPS)-stimulated Raw 264.7 cells through its preliminary
anti-inflammatory action (20).
Another study reported that MFSCC could regulate the NF-κB/MAPK
pathway in IL-1α-induced rat primary chondrocytes (21). In the present study, for the first
time, to the best of our knowledge, the inhibitory effect of MFSCC
on skin keratinocytes and fibroblast cells, was reported. The
anti-inflammatory mechanism of MFSCC through various inflammatory
pathways in human keratinocytes was also investigated using HaCaT
cells.
Materials and methods
Cell culture and reagents
HaCaT human keratinocytes cells and Detroit 551
human fibroblast cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) and minimum essential medium (MEM) containing 10%
fetal bovine serum (FBS; all Gibco; Thermo Fisher Scientific,
Inc.), supplemented with 100 U/ml penicillin and 100 µg/ml
streptomycin (both Thermo Fisher Scientific, Inc.). HaCaT human
keratinocytes cells were provided by Professor Hae Young Chung,
Department of Pharmacy, Longevity Science and Technology
Institutes, Research Institute for Drug Development, Pusan National
University, Korea (cat. no. 300493; CLS GmbH). Detroit 551 human
fibroblast cells were purchased from Korean Cell Line Bank (cat.
no. 10110; KCLB). The cells were incubated at 37°C and 5%
CO2. Recombinant human TNF-α and IFN-γ were obtained
from Enzynomics, Inc. and R&D systems, Inc. TNF-α and IFN-γ
were re-suspended in PBS (0 ng/ml). Antibodies IL-6 (product no.
12153S), JAK2 (product no. 3230S), STAT3 (product no. 4904S),
phosphorylated (p)-p65 (product no. 3033S), p65 (product no.
8242S), p-IκB-α (product no. 2859S), IκB-α (product no. 4812S),
p-JNK (product no. 4671S), JNK (product no. 9258S), p-p38 (product
no. 9216S), p38 (product no. 8690S), p-ERK1/2 (product no. 4370S),
ERK1/2 (product no. 4695S), collagen type I α1 chain (COL1A1)
(product no. 39952S), and matrix metalloproteinase (MMP)-1 (product
no. 54376S) were purchased from Cell Signaling Technology, Inc.
Antibodies p-JAK2 (product code ab195055), p-STAT3 (product code
ab30647), elastin (product code ab217356), and MMP-8 (product code
ab154507) were obtained from Abcam. Horseradish peroxidase
(HRP)-conjugated secondary antibodies to anti-rabbit (cat. no.
A120-101P) and anti-mouse (cat. no. A90-116P) were obtained from
Bethyl Laboratories, Inc.
Preparation of membrane-free stem cell
components (MFSCC)
MFSCC used in this study were prepared using
patented technology by T-Stem Co., Ltd. The adipose tissues used
for the preparation of MFSCC were obtained from Tiara Clinic
(Changwon, Korea) upon agreement from the respective donors. The
donors provided written informed consent and the Regional Ethics
Committee on Biomedical Research approved the clinical protocol.
The fat tissues were provided by females in their twenties with a
BMI of 25 to 29.9 (Overweight) considered appropriate based on
blood tests and physician diagnosis. The blood was tested for
viruses including hepatitis B virus (HVB), hepatitis C virus (HCV),
human immunodeficiency virus (HIV), human T-cell lymphocytic virus
(HTVL), parvovirus B19, cytomegalovirus (CMV), Epstein-Barr virus
(EBV), and Treponema pallidum. The cells were cultured at
37°C and 5% CO2, in a standard incubator using
serum-free cell culture medium. After the cell growth reached
70–80% confluence, the cells were sub-cultured until 6 to 8
passages. ADSCs were characterized using specific markers (positive
markers, CD105 and CD29; negative marker, CD34) by an
immunofluorescence assay (data not shown). A certain amount of stem
cells (1×106 cells/ml) was collected; the cell membranes
were removed by ultra-sonication, and the debris of the membranes
was eliminated by centrifugation at 800-1,500 × g (Room
temperature, 3 min.), following successive filtration. The final
product of MFSCC was obtained upon separating the stem cell
membranes, and performing nine non-toxic-based safety tests
conducted by accreditation authority certified under Good
Laboratory Practice (GLPs). The overall preparation of MFSCC in the
present study was based on the protocol by Venkatarame Gowda
Saralamma et al (20).
Cell viability assay
Cell viability was analyzed using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT).
HaCaT cells were seeded at a density of 5×104 cells/well
and Detroit 551 cells were seeded at a density of 3×104
cells/well in 48-well plates. After treatment with the indicated
concentrations of MFSCC (0, 1, 5, 10, 15, 20, and 25 µg/ml) with or
without TNF-α/INF-λ (10 ng/ml), cells were incubated for 24 h. MTT
solution was added to each well, and the cells were incubated for 2
h at 37°C. The formazan was dissolved in DMSO, and then the
absorbance was measured at 540 nm by microplate reader (BioTek
Instruments, Inc.).
Human inflammation antibody array
Antibody array was analyzed using RayBio Human
Inflammation Antibody Array C1 kit (cat. no. AAH-INF-1-2). HaCaT
cells were seeded at a density of 4×105 cells/well in a
100-mm plate, and treated with TNF-α/IFN-γ (10 ng/ml) with or
without MFSCC (10 µg/ml) for 24 h. The cell lysates were then
collected and processed according to manufacturer's instructions.
Arrays membranes were directly detected using a chemiluminescence
detection system (Bio-Rad Laboratories, Inc.) to obtain production
levels of the following cytokines/proteins: Eotaxin-1 (CCL11),
Eotaxin-2 (MPIF-2/CCL24), GCSF, GM-CSF, IFN-γ, IL-1α (IL-1 F1),
IL-1β (IL-1 F2), IL-2, IL-3, IL-4, IL-6, IL-7, IL-8 (CXCL8), IL-10,
IL-11, IL-12 p40, IL-12 p70, IL-13, I-309 (TCA-3/CCL1), and TIMP
metallopeptidase inhibitor 2 (TIMP2). Data were analyzed with the
Image Studio Lite software (version 5.2; LI-COR Biosciences). Data
are expressed as the relative signal intensity (RSI) between the
MFSCC co-treated test group and the only TNF-α/IFN-γ-treated
control group [RSI=(test group/control group)].
Western blot analysis
HaCaT cells were treated with the indicated
concentration of MFSCC with TNF-α/IFN-γ (10 ng/ml) for 24 h.
Detroit 551 cells were treated with the indicated concentration of
MFSCC for 24 h. Then the incubated cells were lysed using RIPA
buffer (iNtRON Biotechnology, Inc.) containing a protease inhibitor
cocktail and a phosphatase inhibitor (Thermo Fisher Scientific,
Inc.). The protein quantification of each cell lysate sample was
measured using BCA assay (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. Equal amounts of
protein (10 µg) were separated on 8–15% SDS-polyacrylamide gel
electrophoresis (SDS-PAGE), and then transferred to a
polyvinylidene fluoride (PVDF) membrane (Immunobilon-P, 0.45 mm;
EMD Millipore), using the semi-dry transfer system (Atto Corp). The
membranes were blocked with 5% bovine serum albumin (BSA) in
Tris-buffered saline containing 1% Tween-20 (TBS-T, pH 7.4) at room
temperature for 1 h, followed by incubation overnight at 4°C with a
1:1,000 dilution of the respective primary antibody. The membranes
were washed five times with TBS-T for 10 min each at room
temperature, and then incubated with a horseradish peroxidase
(HRP)-conjugated secondary antibody for 2 h at room temperature.
The membranes were then rewashed 5 times using TBS-T, detected by
chemiluminescence detection system and analyzed using Image Lab 4.1
program (both Bio-Rad Laboratories, Inc.). The densitometry
analysis using ImageJ software (version 1.50i) (National Institutes
of Health) of each of the protein bands was normalized by comparing
with the expression of β-actin.
Protein-protein interactions using the
Search Tool for the Retrieval of Interacting Genes/Proteins
(STRING) analysis
The protein-protein interaction network was
constructed using STRING (https://string-db.org/). The STRING database provides
the interactions of different proteins based on a confidence level
score. The interactive network with the most connected neighborhood
proteins of IL-6 were identified. The confidence score for the
interactive network was set up with a medium score of 0.4 to 0.9
respectively.
Statistical analysis
All experimental results are expressed as the mean ±
standard deviation (SD) of at least triplicate samples using
GraphPad Prism software (version 8.02; GraphPad Software, Inc., San
Diego,). Significant differences were calculated by one-way
factorial analysis of variance (ANOVA) followed by Bonferroni's
post hoc test. A value of P<0.05 was considered statistically
significant.
Results
Cytotoxicity of MFSCC to human
keratinocytes
To evaluate the cytotoxic effect of MFSCC, human
keratinocyte HaCaT cells were treated with MFSCC at indicated
concentrations (0, 1, 5, 10, 15, 20 and 25 µg/ml) for 24 h. Cell
viability assessment results indicated that MFSCC at concentrations
of up to 25 µg/ml did not cause 50% inhibition of HaCaT cells as
revealed in Fig. 1A. Co-treatment
with TNF-α/IFN-γ did not show significant cytotoxicity to HaCaT
cells either (Fig. 1B). Based on
these results, concentrations of 1, 5 and 10 µg/ml MFSCC were
selected for subsequent experiments.
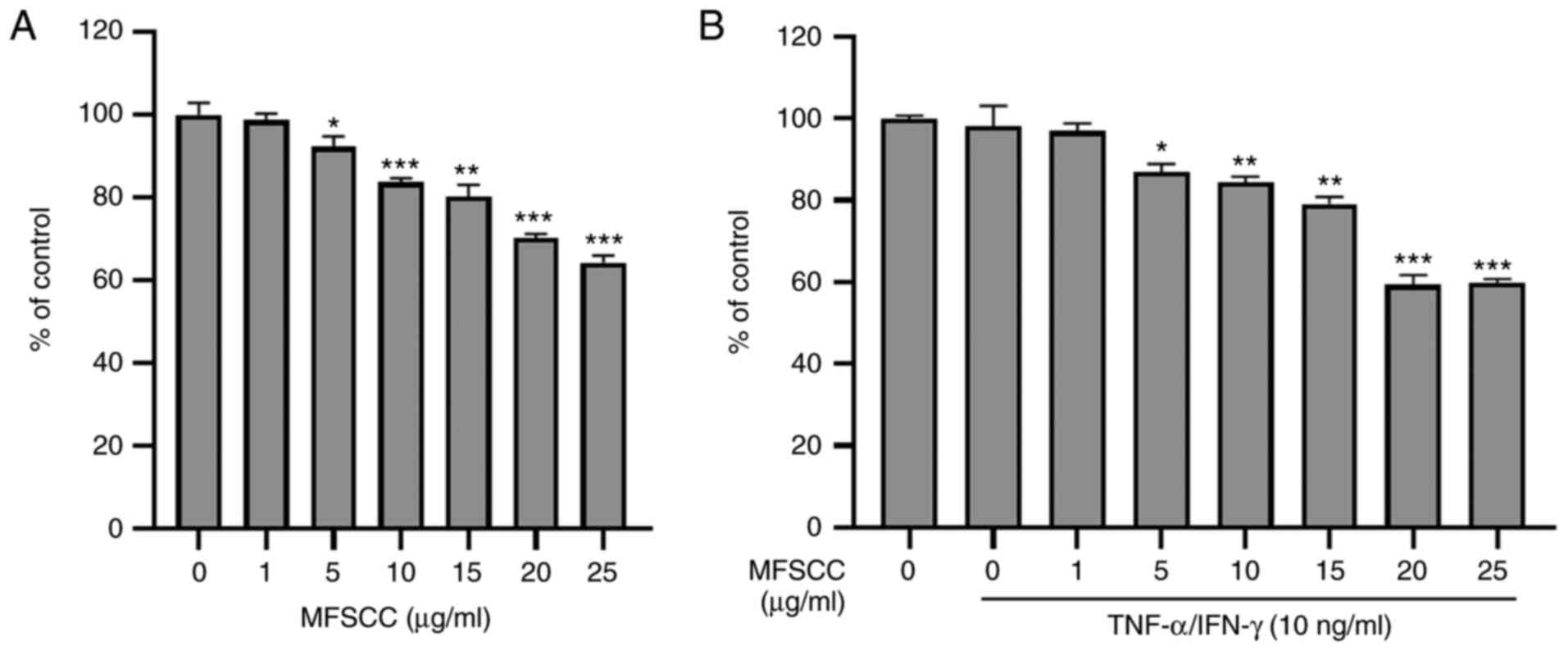 | Figure 1.Cytotoxic assessment of MFSCC in
HaCaT cells. (A) Effect of MFSCC on cell viability in HaCaT cells
treated with indicated concentrations (0, 1, 5, 10, 15, 20 and 25
µg/ml) of MFSCC for 24 h. (B) Effect of MFSCC (0, 1, 5, 10, 15, 20
and 25 µg/ml) on the cell viability of TNF-α/INF-γ-induced (10
ng/ml) HaCaT cells. Data are presented as the mean ± SD of three
independent experiments. *P<0.05, **P<0.01 and ***P<0.001
vs. the untreated group. MFSCC, membrane-free stem cell components;
TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ. |
Detection and identification of
proteins in TNF-α/IFN-γ-induced HaCaT cells associated with
inflammation using an antibody array
An antibody array was used for HaCaT cells treated
with only TNF-α/IFN-γ (10 ng/ml) considered the control, and those
co-treated with MFSCC (0, 1, 5, and 10 µg/ml) and TNF-α/IFN-γ (10
ng/ml) in order to identify proteins involved in the
anti-inflammatory action of MFSCC. Proteins were extracted from
cell lysates and subjected to Human Inflammation array using a kit
(RayBio® C-series Human Inflammation Array C1).
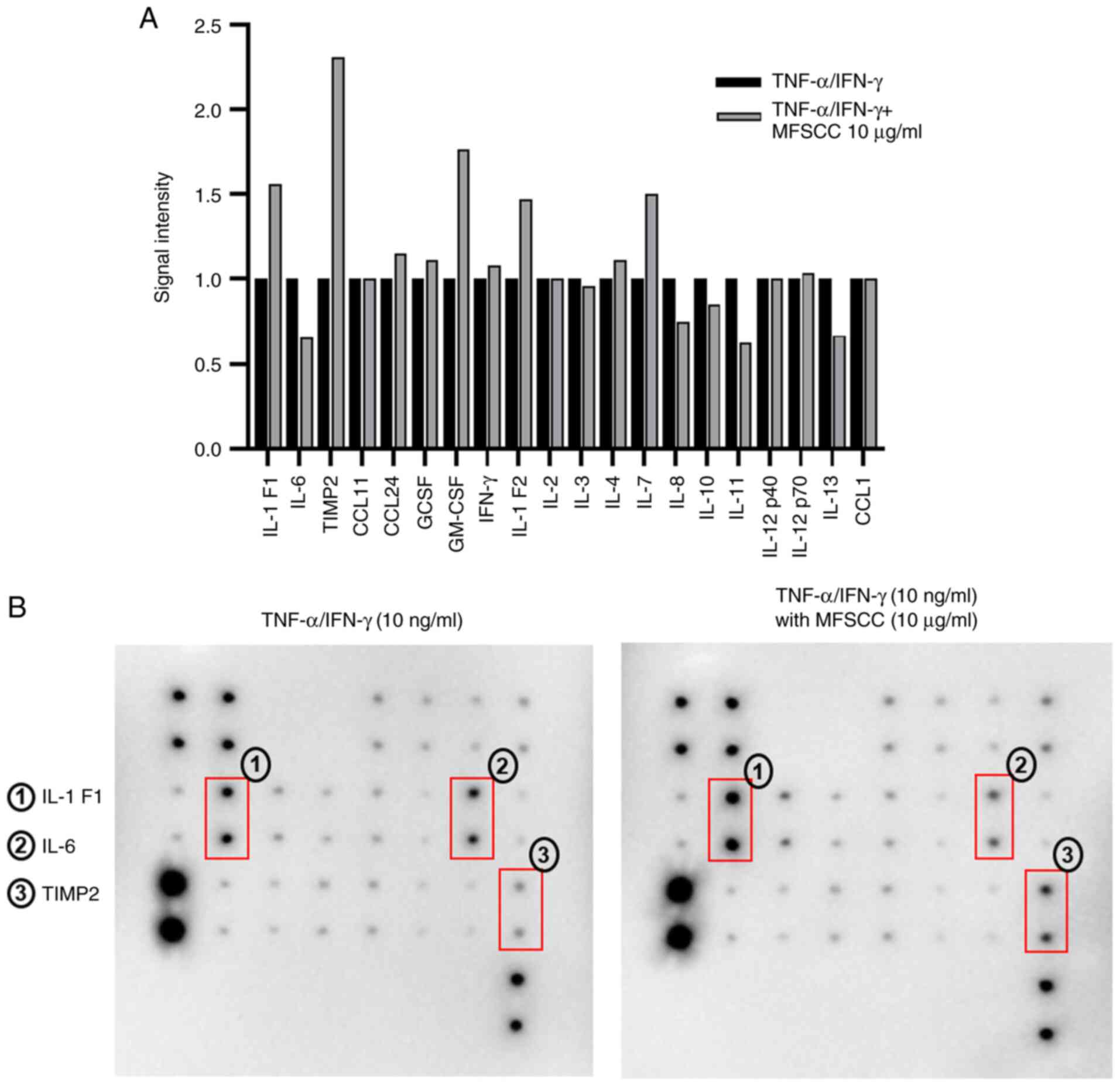
RayBio® C1 array contains 20 duplicate spots of
inflammatory-related proteins. A plot of all protein spots with
their expression is depicted graphically based on their signal
intensities (Fig. 2A). Among the
expressed protein spots, the protein expression levels with clear
signal intensities were selected to pivot and the significant
proteins related to anti-inflammatory roles were selected as shown
in Fig. 2B. These three protein
spots were found to be IL-1 F1 (IL-1α), IL-6, and TIMP2. IL-1 F1
(IL-1α) and IL-6 are involved in the activation of the acute phase
of inflammatory responses and cytokine-mediated pathways (22). TIMP2 is involved in regulation of
MMP proteins (23). Proteins
along with their biological functions are displayed in Table I. Among these three proteins, IL-6
was specifically focused on, which exhibited significant
downregulation in the MFSCC treatment group compared with that in
the TNF-α/IFN-γ-treated group. These results indicated the
anti-inflammatory action of MFSCC by inhibiting the inflammation
marker IL-6 upon its treatment. Thus, IL-6 was subjected to further
analysis.
 | Table I.List of proteins identified in varied
expression in HaCaT cells treated only with TNF-α/IFN-γ (10 ng/ml)
and co-treated with MFSCC (10 µg/ml) detected by antibody array
analysis. |
Table I.
List of proteins identified in varied
expression in HaCaT cells treated only with TNF-α/IFN-γ (10 ng/ml)
and co-treated with MFSCC (10 µg/ml) detected by antibody array
analysis.
| UniProt ID | Symbol | Protein name | Up/Down
regulation | Biological
function |
|---|
| P01583 | IL-1 F1
(IL-1α) | Interleukin-1α | ↑ | Inflammatory
response, cytokine-mediated signaling pathway |
| P05231 | IL-6 | Interleukin-6 | ↓ | Acute-phase
response, interleukin-6-mediated signaling pathway |
| P16035 | TIMP-2 | Metalloproteinase
inhibitor 2 | ↑ | Activation of
matrix metalloproteinases |
The STRING database search tool was used to analyze
protein-protein interactions and to retrieve genes/proteins
interacting with significant protein IL-6. An interactive
protein-protein network of IL-6 was constructed, with a score of
0.900 as the highest degree of confidence (Fig. S1). In the interactive network,
IL-6 was identified to be closely associated with nodes JAK2 and
STAT3. Both nodes, JAK2 and STAT3 were observed to have strong
associations with IL-6 through the JAK/STAT signaling pathway in
the network with a strength parameter of 1.96. Closely-associated
node partners and their functional parameters related to
inflammation are presented in Table
SI.
Western blot analysis of the
expression levels of IL-6, p-JAK2, and p-STAT3 proteins
To validate antibody array results, western blot
analysis was conducted using HaCaT cells treated with only
TNF-α/IFN-γ (10 ng/ml) or co-treated with MFSCC (0, 1, 5 and 10
µg/ml) and TNF-α/IFN-γ (10 ng/ml). Blots obtained from the western
blotting of protein IL-6 along with phosphorylated forms of JAK2
and STAT3 are shown in Fig. 3.
The expression levels of IL-6, p-JAK2, and p-STAT3 proteins were
decreased in the MFSCC-treated group compared with the group
treated only with TNF-α/IFN-γ. These western blot results were
consistent with the antibody array results which demonstrated IL-6
reduction and inhibition of inflammatory responses through JAK2 and
STAT3 phosphorylation in HaCaT cells.
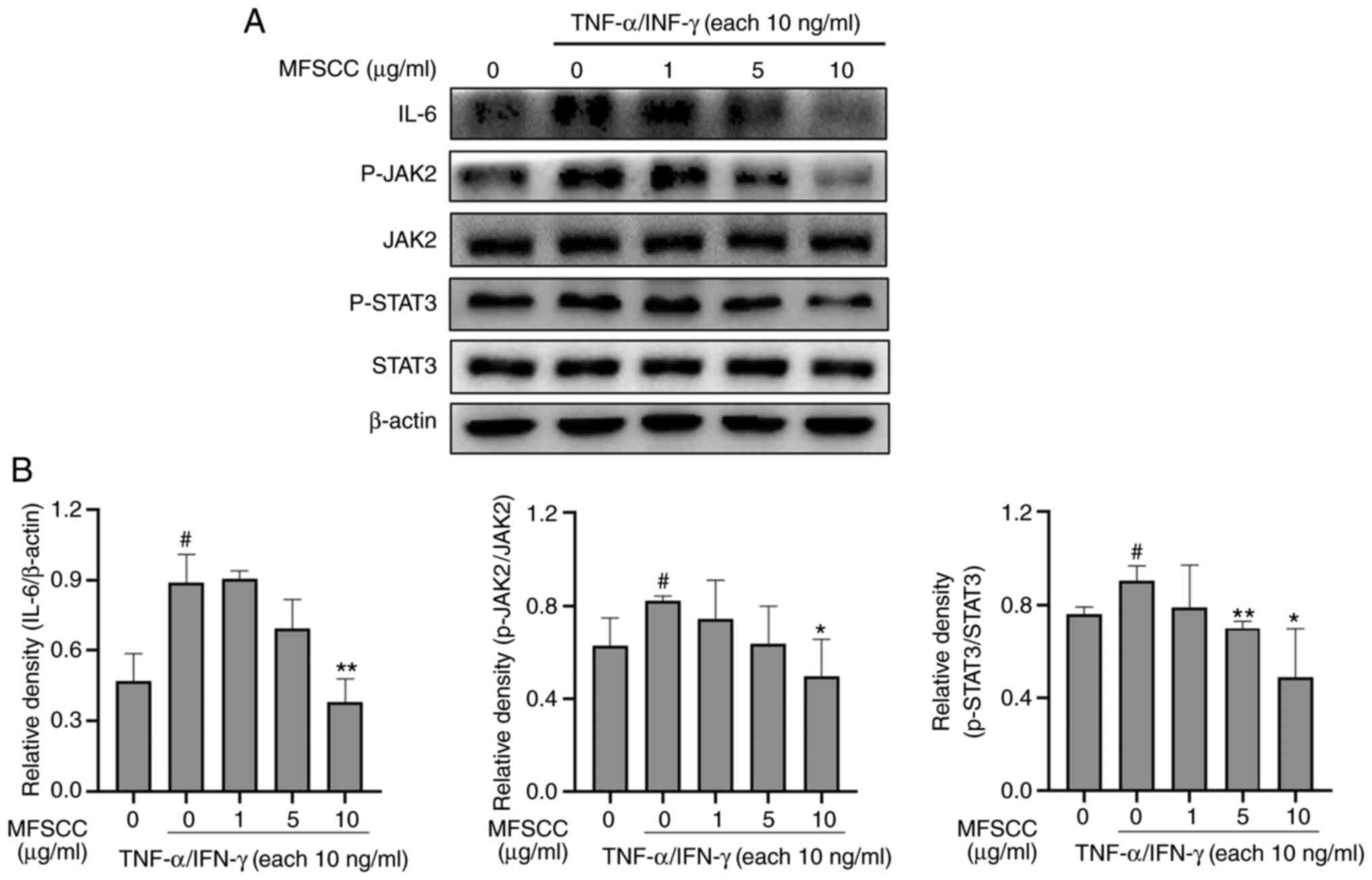 | Figure 3.Western blot analysis of the
expression of IL-6, p-JAK2, and p-STAT3 proteins in HaCaT cells.
(A) Protein levels of IL-6, JAK2, p-JAK2, STAT3, and p-STAT3 in the
TNF-α/IFN-γ-treated only group (10 ng/ml) and the co-treated MFSCC
(0, 1, 5 and 10 µg/ml) group. (B) The expression of the proteins
are shown graphically based on their relative density normalized
against β-actin which served as the acting and internal control.
#P<0.05 vs. the untreated group; *P<0.05 and
**P<0.01 vs. the TNF-α/IFN-γ-treated only group. IL,
interleukin; p-, phosphorylated; JAK2, Janus kinase 2; STAT3,
signal transducer and activator of transcription 3; TNF-α, tumor
necrosis factor-α; IFN-γ, interferon-γ; MFSCC, membrane-free stem
cell components. |
Effect of MFSCC on NF-κB signaling in
TNF-α/IFN-γ-stimulated HaCaT cells
Inflammatory responses through small regulator
molecules involve crucial mechanisms mediated by NF-κB signaling.
The activator of signal transcription factors through JAK/STAT
signaling also plays a pivotal role in the pathogenies of
inflammatory disorders (24). In
this aspect, the inhibitory potential of MFSCC on the NF-κB
signaling pathway was investigated by assessing protein markers
using western blotting. The results revealed that stimulation by
TNF-α/IFN-γ (10 ng/ml) increased the phosphorylation levels of
IκB-α and p65, which were decreased by co-treatment with MFSCC (0,
1, 5 and 10 µg/ml) (Fig. 4). The
reduction in the phosphorylation of NF-κB proteins by co-treatment
with MFSCC indicated that MFSCC could decrease inflammatory
responses by inhibiting the respective signaling pathway in HaCaT
cells.
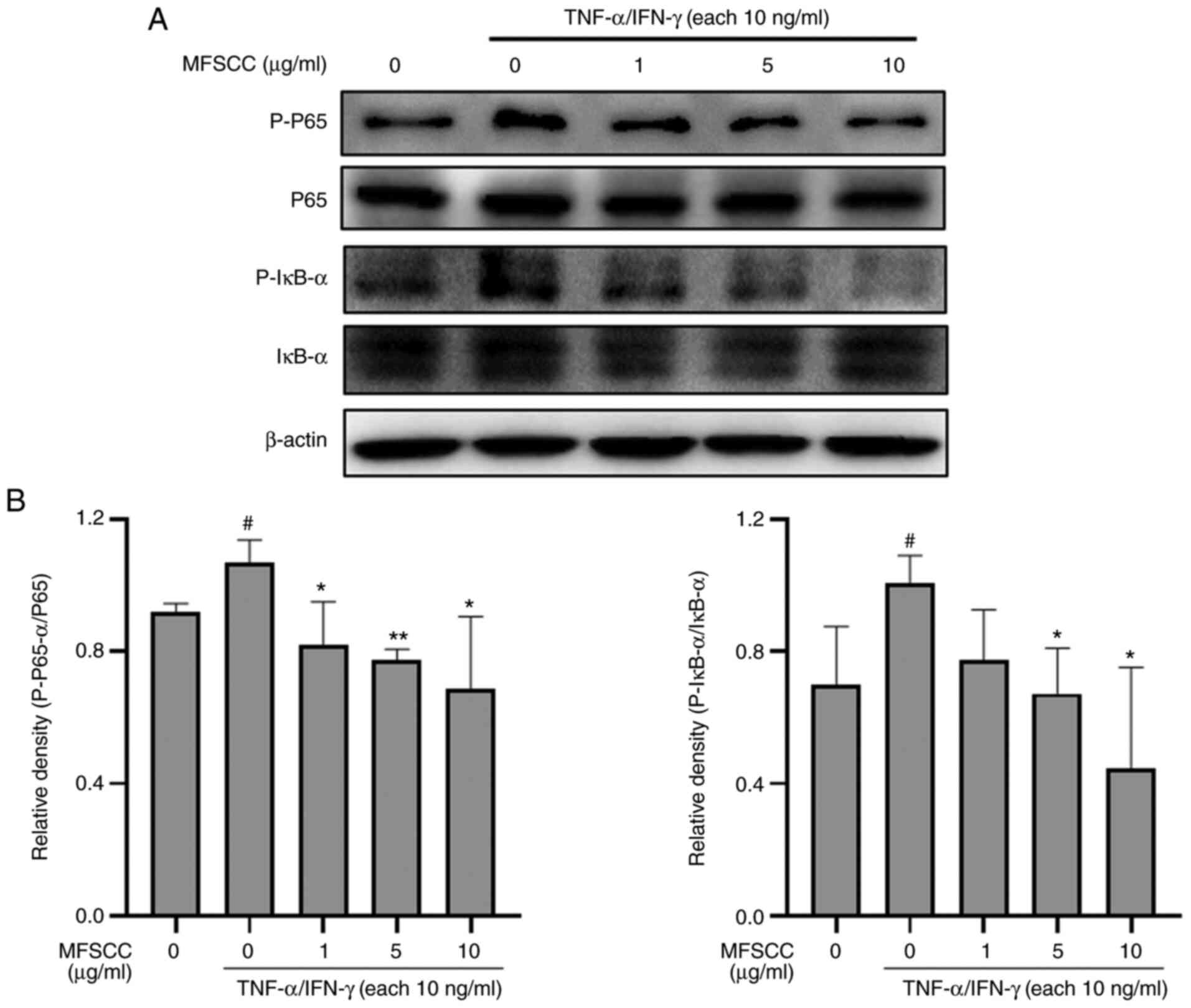 | Figure 4.Western blot analysis of the NF-κB
signaling pathway in HaCaT cells. (A) Protein levels of p65, p-p65,
IκB-α, and p-IκB-α in the TNF-α/IFN-γ-treated only group (10 ng/ml)
and the co-treated MFSCC (0, 1, 5, and 10 µg/ml) group. (B) The
expression of the proteins are shown graphically based on their
relative density normalized against the internal control.
#P<0.05 vs. the untreated group; *P<0.05 and
**P<0.01 vs. the TNF-α/IFN-γ-treated only group. NF-κB, nuclear
factor-κB; p-, phosphorylated; TNF-α, tumor necrosis factor-α;
IFN-γ, interferon-γ; MFSCC, membrane-free stem cell components. |
Effect of MFSCC on MAPK
phosphorylation in TNF-α/IFN-γ-stimulated HaCaT cells
Fundamental inflammatory responses mediated by any
stimuli are highly associated with MAPKs regulation in
anti-inflammatory mechanisms (25). To identify the association of the
MAPK pathway in HaCaT cells with MFSCC treatment, the
phosphorylation levels of JNK, p38, and ERK as downstream proteins
were examined. TNF-α/IFN-γ (10 ng/ml) treatment increased the
phosphorylation levels of MAPK proteins. However, co-treatment with
MFSCC (0, 1, 5 and 10 µg/ml) significantly reduced their
phosphorylation levels as shown in Fig. 5. These results indicated that
MFSCC could induce anti-inflammatory responses through regulation
of the MAPK pathway in HaCaT cells.
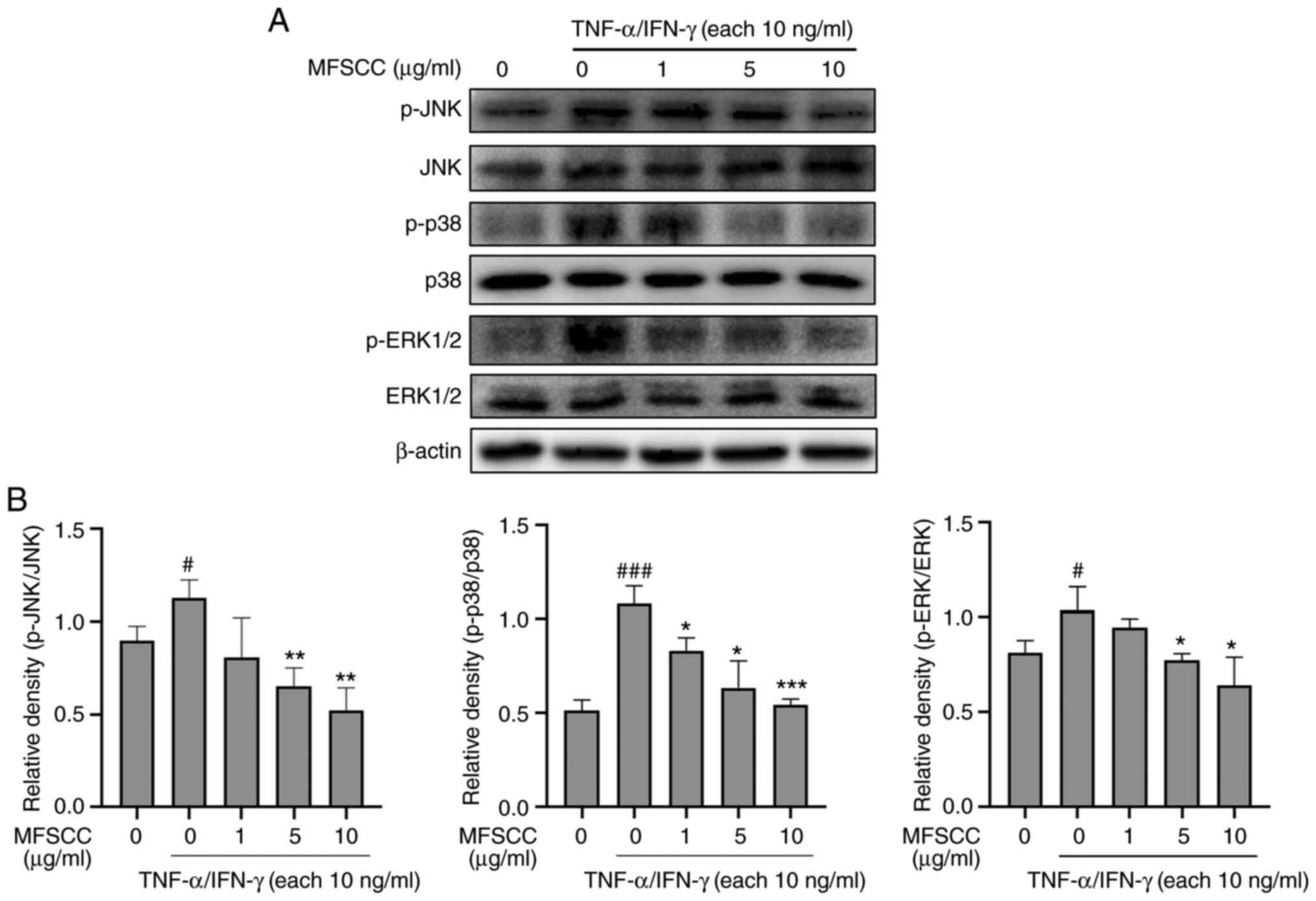 | Figure 5.Western blot analysis of the MAPK
signaling pathway in HaCaT cells. (A) Protein levels of JNK, p-JNK,
p38, p-p38, ERK1/2, and p-ERK1/2 in the TNF-α/IFN-γ-treated group
(10 ng/ml) and co-treated MFSCC (0, 1, 5 and 10 µg/ml) group. (B)
The expression of the proteins are shown graphically based on their
relative density normalized against the internal control.
#P<0.05 and ###P<0.001 vs. the
untreated group; *P<0.05, **P<0.01 and ***P<0.001 vs. the
TNF-α/IFN-γ-treated only group. MAPK, mitogen-activated protein
kinase; JNK, c-Jun NH2-terminal kinase; p-, phosphorylated; ERK,
extracellular signal-regulated kinase; TNF-α, tumor necrosis
factor-α; IFN-γ, interferon-γ; MFSCC, membrane-free stem cell
components. |
Expression of collagen and elastin in
MFSCC-treated Detroit 551 cells
The skin can be damaged by inflammatory reactions,
resulting in aging symptoms such as wrinkles (26). Thus, wrinkle-related proteins were
investigated using MFSCC-treated human fibroblast Detroit 551
cells. Cell viability assay results (Fig. 6A) demonstrated that MFSCC at
concentrations of 1, 5 and 10 µg/ml had no cytotoxicity. Therefore,
these concentrations were used in subsequent experiments. Wrinkle
formation is closely associated to the reduction of collagens and
the extracellular matrix (ECM) in dermal skin (27). According to previous studies, the
induction of collagen and ECM can lead to potent treatment for
anti-wrinkle conditions (24,28). Thus, the expression levels of
wrinkle-related proteins, including COL1A1, elastin, MMP-1, and
MMP-8 were determined. As revealed in Fig. 6B, MFSCC significantly
downregulated the protein levels of MMP-1 and MMP-8 but upregulated
the protein levels of COL1A1 and elastin. These findings indicated
that MFSCC could induce anti-wrinkle effects in Detroit 551 cells
by reducing collagen and ECM degradation.
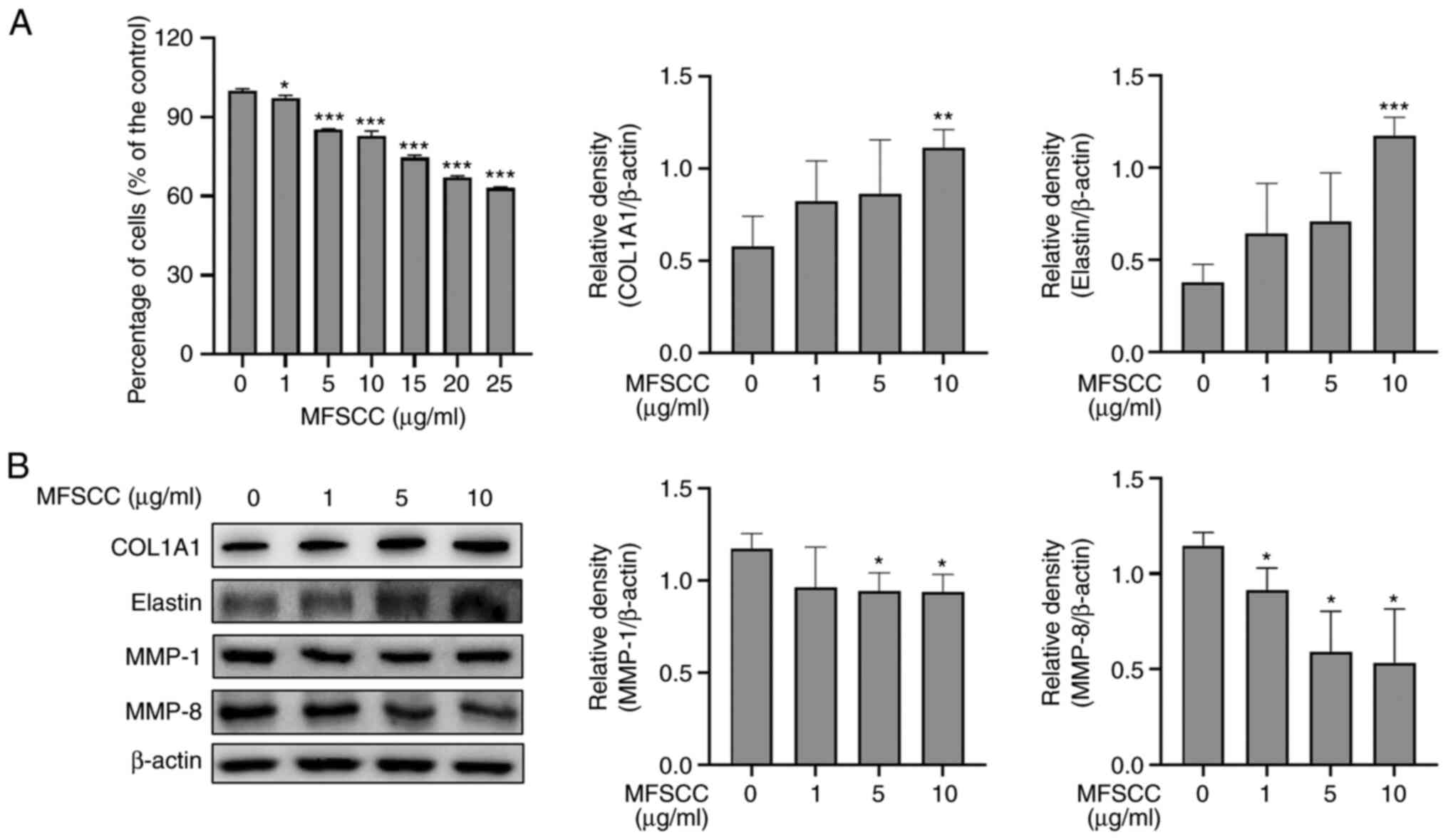 | Figure 6.Expression of collagen and elastin in
MFSCC-treated Detroit 551 cells. (A) Effect of MFSCC on the cell
viability of Detroit 551 cells treated with various concentrations
(0, 1, 5, 10, 15, 20 and 25 µg/ml). (B) Western blot analysis of
the expression levels of protein markers COL1A1, elastin, MMP-1,
and MMP-8 in MFSCC-treated Detroit 551 cells (0, 1, 5 and 10
µg/ml). The protein expression levels are shown graphically based
on their relative density normalized against β-actin as the acting
control. *P<0.05, **P<0.01 and ***P<0.001 vs. the
untreated group. MFSCC, membrane-free stem cell components; COL1A1,
collagen type I α1 chain; MMP, matrix metalloproteinase. |
Discussion
Skin forms the essential interface between the
intrinsic body and the extrinsic insults from the environment. It
can attenuate infections caused by microbes and radiation produced
by ultraviolet emission by activating innate immune responses
(1). The activation of the
inflammatory system can aid skin homeostasis and control
inflammatory disorders, allergic reactions, and skin cancer
(29).
Keratinocytes are highly active immunological cells
that execute responses to external stimuli in skin. They can
activate a variety of molecules involving different forms of
cytokines, pro-inflammatory chemokines, and other proteins
(30). Production levels of
chemokines and cytokines are elevated upon stimulation of
keratinocytes with inflammatory cytokines such as TNF-α and IFN-γ
(31). Previous research findings
on HaCaT cells have used TNF-α/IFN-γ activation to stimulate
various intracellular signaling pathways, including MAPKs, NF-κB,
and STAT-1/JAK-2 (32,33). Thus, keratinocyte-mediated
inflammatory molecules activated via immune responses are highly
associated with the pathology of skin inflammatory disorders
(34). In the present study, the
anti-inflammatory action of MFSCC was investigated through antibody
array-mediated analysis using TNF-α/IFN-γ-stimulated human
keratinocytes HaCaT cells.
Regarding the cytotoxic effect of MFSCC, cell
viability was moderately inhibited by MFSCC compared with the
control group of TNF-α/IFN-γ-stimulated HaCaT cells. Human
inflammatory antibody array analysis using TNF-α/IFN-γ-stimulated
HaCaT cells identified three differential proteins: IL-1 F1
(IL-1α), IL-6, and TIMP2. All these three identified proteins are
known to contribute to the inflammatory response pathway (35). IL-6 was found to be significantly
reduced in its expression upon treatment with MFSCC. IL-6 is one of
the well-characterized pro-inflammatory cytokines that exerts a
vital role in performing multiple functions of the immune system
(36). With the observed
downregulated expression in the array, it was hypothesized that
MFSCC could mediate the anti-inflammatory action through IL-6
(37). The host defense mechanism
involves the activation of several intracellular signaling
pathways, including JAK2/STAT3, mammalian MAPK, and NF-κB pathways
(38,39).
IL-6 is a prominent inflammatory mediator that can
activate JAK2 and STAT3 pathways with extracellular signals
regulated by the ERK pathway (40). The JAK2/STAT3 signaling pathway is
known to effectively contribute to inflammatory responses.
Considerable attention has been directed towards its inhibition for
the treatment of inflammation (41). The protein-protein interaction
network in the present study revealed that JAK2 and STAT3 are
closely associated interactors. Protein expression of IL-6, p-JAK2,
and p-STAT3 in HaCaT cells was determined to be downregulated by
MFSCC treatment. The present data indicated that MFSCC could
significantly inhibit the activation of the IL6-mediated JAK2/STAT3
signaling pathway.
Accumulating evidence has revealed that a variety of
signaling networks are involved in the preventive effect against
inflammation, such as NF-κB and MAPKs (42). Interestingly, in the present
study, MFSCC appeared to control the activation of NF-κB and MAPK
signaling pathways. The constitutive activation of NF-κB is driven
by major inflammatory factors such as TNF-α, IL-6, IL-1, and IL-8.
The most commonly triggered stimulant has been reported to be TNF-α
that can lead to complex activation (43). Furthermore, it has been suggested
that the interactive nature of STAT3 activation with NF-κB
signaling can lead to the induction of inflammation (44).
Inflammatory mediator production is primarily
managed by NF-κB signaling that is highly associated with MAPK
signaling (45). In terms of MAPK
activation, phosphorylation of ERK, p38, and JNK occurs in both the
nucleus and cytosolic layer to stimulate the expression of relevant
inflammatory factors (46). MAPKs
are also involved in the activation of JAK/STAT, a critical
signaling transduction pathway for the biological function of many
cytokines (47). Our experiments
also analyzed the impact of MAPKs on the expression of JNK, p38,
and ERK1/2. The results showed that their phosphorylated forms were
suppressed in MFSCC-treated HaCaT cells. Divergent active signals
from MAPK pathways are prominent mediators of inflammation. They
play a vital role in degenerative disorders (48). Conversely, there is evidence that
MAPK inhibitors are involved in treating systemic inflammation
caused by stimulation with TNF-α (49). Based on our findings, stimulation
with TNF-α/IFN-γ induced phosphorylation of p65, IκB-α, ERK, p38,
and JNK in HaCaT cells, although MFSCC treatment downregulated the
activation of MAPKs mediated by the NF-κB signaling pathway.
Inflammation of the skin is analogous with the
degeneration of elastic fibers and loss in elasticity, resulting in
wrinkle formation (50). Changes
associated with the microenvironment can impact the skin and lead
to aging and drying with loss of important ECM components (51). Inflammation in skin mediated by
cytokines such as IL-6 has been subsequently linked to the
formation of wrinkles, leading to poor integrity of skin structure
(50). The inflammatory mechanism
triggers responses of rapid collagen degradation by the family of
MMPs (52). Collagen degradation
in the phase of a wound response mechanism is followed by
fibroblast inducing new collagen synthesis to replace lost collagen
fibers in the ECM (53). The
present results also revealed that the expression levels of
pro-collagen and elastin were increased upon MFSCC treatment in
fibroblast cells. In addition, decreased expression levels of MMP-1
and MMP-8 were also observed in human fibroblast Detroit 551 cells
treated with MFSCC. This indicated that MFSCC could also trigger
anti-wrinkle effects by decreasing MMPs via IL-6 alteration. Thus,
the use MFSCC on skin inflammation has an added therapeutic
significance in that it also has a cosmetic function. In
consideration of overall outcomes, MFSCC provides a primary defense
against skin inflammation. It also tends to possess an anti-wrinkle
effect, indicating that it could be a potential treatment option
for inflammatory skin disorders in the future.
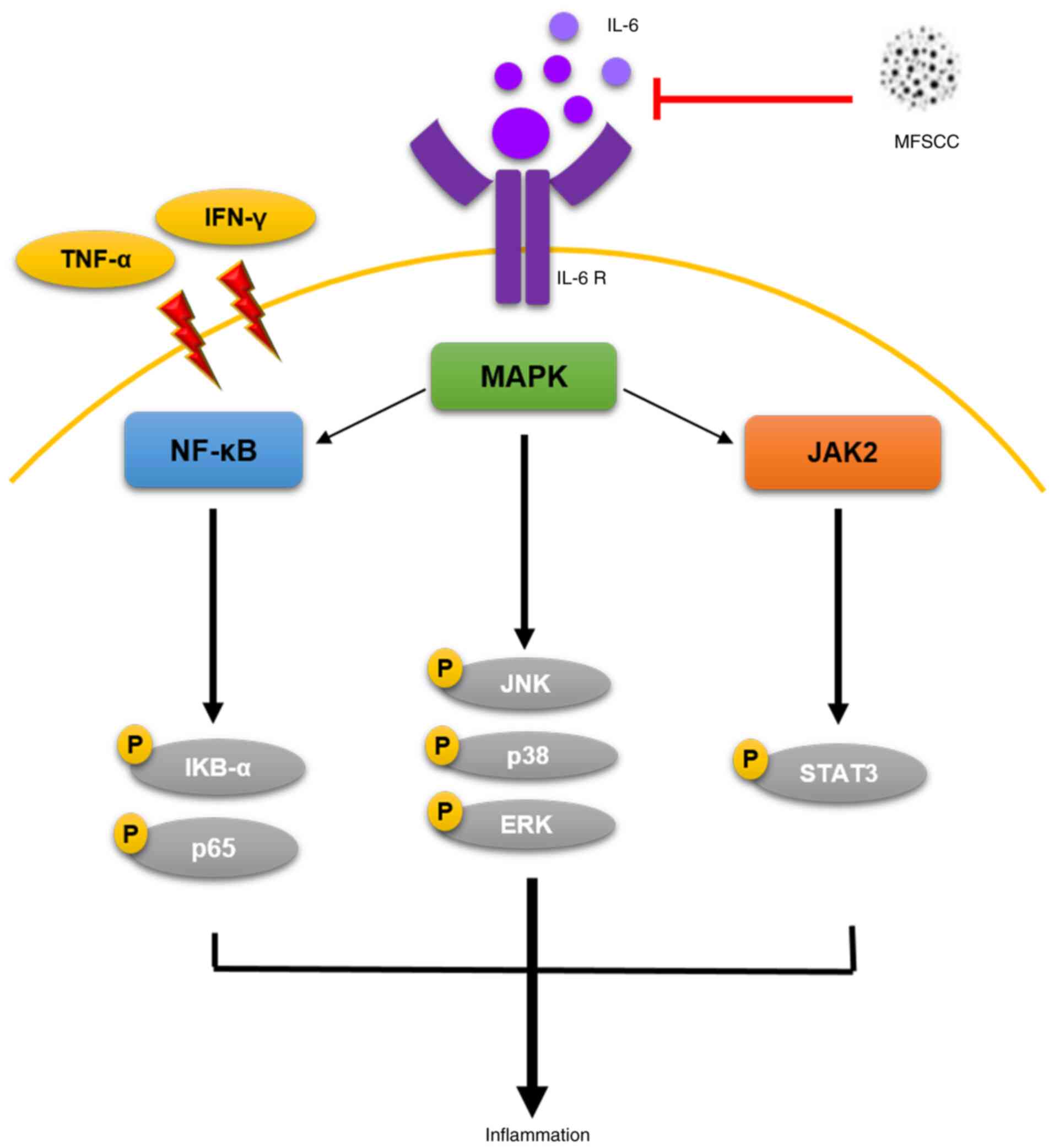
In conclusion, the results of the present study
suggest that MFSCC can inhibit primary inflammatory response
stimulated by TNF-α/IFN-γ through regulation of IL-6 and JAK2/STAT3
expression (Fig. 7). The present
study also revealed preliminary inhibitory effects on NF-κB and
MAPK signaling pathways in human keratinocyte HaCaT cells.
Furthermore, MFSCC treatment could improve collagen and elastin
expression, eventually leading to decreased expression of MMP
proteins in human fibroblast Detroit 551 cells. Collectively, these
findings form an initial study on the anti-inflammatory properties
of MFSCC and provide support for future studies about its
therapeutic effect on skin inflammation.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported and all the research activity
related to the study design, collection of data, analysis was
monitored by T-Stem Co., Ltd. and Tiara Clinic (Changwon, Korea).
Preparation and processing of membrane-free stem cell components
(MFSCC) were performed by T-Stem Co., Ltd., and Tiara Clinic. The
study was funded by the National Research Foundation of Korea by
the Ministry of Science and ICT (grant no.
2020R1A2B5B01001807).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GSK and YSK conceived the study and designed the
experiments. SEH performed the experiments and collected and
analyzed data. PV performed the experiments. SMK, PBB and HHK
analyzed data. JEP and JDH interpreted the results. GSK, YSK, SEH,
PV, SMK, PBB, HHK, JEP and JDH confirm the authenticity of all the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
All donors provided written informed consent and the
Regional Ethics Committee on Biomedical Research approved the
clinical protocol (Ministry of Health and Welfare, Sejong).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pasparakis M, Haase I and Nestle FO:
Mechanisms regulating skin immunity and inflammation. Nat Rev
Immunol. 14:289–301. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sabat R, Wolk K, Loyal L, Docke WD and
Ghoreschi K: T cell pathology in skin inflammation. Semin
Immunopathol. 41:359–377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parisi R, Webb RT, Kleyn CE, Carr MJ,
Kapur N, Griffiths CEM and Ashcroft DM: Psychiatric morbidity and
suicidal behaviour in psoriasis: A primary care cohort study. Br J
Dermatol. 180:108–115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weidinger S and Novak N: Atopic
dermatitis. Lancet. 387:1109–1122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wittmann M and Werfel T: Interaction of
keratinocytes with infiltrating lymphocytes in allergic eczematous
skin diseases. Curr Opin Allergy Clin Immunol. 6:329–334. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miodovnik M, Koren R, Ziv E and Ravid A:
The inflammatory response of keratinocytes and its modulation by
vitamin D: The role of MAPK signaling pathways. J Cell Physiol.
227:2175–2183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JH, Kim MS, Jeong GS and Yoon J:
Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines
production via blockade of NF-κB, STAT1 and p38-MAPK activation in
human epidermal keratinocytes. J Ethnopharmacol. 171:85–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hunter CA and Jones SA: IL-6 as a keystone
cytokine in health and disease. Nat Immunol. 16:448–457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ju SM, Song HY, Lee SJ, Seo WY, Sin DH,
Goh AR, Kang YH, Kang IJ, Won MH, Yi JS, et al: Suppression of
thymus- and activation-regulated chemokine (TARC/CCL17) production
by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose via blockade of
NF-kappaB and STAT1 activation in the HaCaT cells. Biochem Biophys
Res Commun. 387:115–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:170232017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mussbacher M, Salzmann M, Brostjan C,
Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, Basílio J,
Petzelbauer P, Assinger A and Schmid JA: Cell Type-specific roles
of NF-κB linking inflammation and thrombosis. Front Immunol.
10:852019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You P, Fu S, Yu K, Xia Y, Wu H, Yang Y, Ma
C, Liu D, Chen X, Wang J, et al: Scutellarin suppresses
neuroinflammation via the inhibition of the AKT/NF-κB and p38/JNK
pathway in LPS-induced BV-2 microglial cells. Naunyn Schmiedebergs
Arch Pharmacol. 391:743–751. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy - from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bianchi J and Cameron J: Assessment of
skin integrity in the elderly 1. Br J Community Nurs. 13 (Suppl
1):S26S28S30–S32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S, He Z and Xu J: Application of
adipose-derived stem cells in photoaging: Basic science and
literature review. Stem Cell Res Ther. 11:4912020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Venkatarame Gowda Saralamma V, Vetrivel P,
Kim SM, Ha SE, Lee HJ, Lee SJ, Kim YS, Pak JE, Lee HJ, Heo JD and
Kim GS: Proteome profiling of membrane-free stem cell components by
Nano-LS/MS analysis and its anti-inflammatory activity. Evid Based
Complement Alternat Med. 2019:46832722019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KH, Jo JH, Cho HJ, Park TS and Kim TM:
Therapeutic potential of stem cell-derived extracellular vesicles
in osteoarthritis: Preclinical study findings. Lab Anim Res.
36:102020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carlson NG, Wieggel WA, Chen J, Bacchi A,
Rogers SW and Gahring LC: Inflammatory cytokines IL-1 alpha, IL-1
beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin
through distinct pathways. J Immunol. 163:3963–3968.
1999.PubMed/NCBI
|
|
23
|
Lambert E, Dassé E, Haye B and Petitfrère
E: TIMPs as multifacial proteins. Crit Rev Oncol Hematol.
49:187–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SM, Ha SE, Vetrivel P, Kim HH, Bhosale
PB, Park JE, Heo JD, Kim YS and Kim GS: Cellular function of
Annexin A1 protein mimetic peptide Ac2-26 in human skin
keratinocytes HaCaT and fibroblast detroit 551 cells. Nutrients.
12:32612020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren Q, Guo F, Tao S, Huang R, Ma L and Fu
P: Flavonoid fisetin alleviates kidney inflammation and apoptosis
via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways
in septic AKI mice. Biomed Pharmacother. 122:1097722020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seif F, Khoshmirsafa M, Aazami H,
Mohsenzadegan M, Sedighi G and Bahar M: The role of JAK-STAT
signaling pathway and its regulators in the fate of T helper cells.
Cell Commun Signal. 15:232017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoo J, Park K, Yoo Y, Kim J, Yang H and
Shin Y: Effects of egg shell membrane hydrolysates on
anti-inflammatory, anti-wrinkle, anti-microbial activity and
moisture-protection. Korean J Food Sci Anim Resour. 34:26–32. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hwang E, Park SY, Jo H, Lee DG, Kim HT,
Kim YM, Yin CS and Yi TH: Efficacy and safety of enzyme-modified
panax ginseng for anti-wrinkle therapy in healthy skin: A
single-center, randomized, double-blind, placebo-controlled study.
Rejuvenation Res. 18:449–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Archer NK, Jo JH, Lee SK, Kim D, Smith B,
Ortines RV, Wang Y, Marchitto MC, Ravipati A, Cai SS, et al:
Injury, dysbiosis, and filaggrin deficiency drive skin inflammation
through keratinocyte IL-1 α release. J Allergy Clin Immunol.
143:1426–1443.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kennedy-Crispin M, Billick E, Mitsui H,
Gulati N, Fujita H, Gilleaudeau P, Sullivan-Whalen M, Johnson-Huang
LM, Suárez-Fariñas M and Krueger JG: Human keratinocytes' response
to injury upregulates CCL20 and other genes linking innate and
adaptive immunity. J Invest Dermatol. 132:105–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Albanesi C and Pastore S: Pathobiology of
chronic inflammatory skin diseases: Interplay between keratinocytes
and immune cells as a target for anti-inflammatory drugs. Curr Drug
Metab. 11:210–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang JH, Yoo JM, Cho WK and Ma JY:
Anti-inflammatory effects of Sanguisorbae Radix water extract on
the suppression of mast cell degranulation and STAT-1/Jak-2
activation in BMMCs and HaCaT keratinocytes. BMC Complement Altern
Med. 16:3472016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang JH, Yoo JM, Lee E, Lee B, Cho WK,
Park KI and Yeul Ma J: Anti-inflammatory effects of Perillae Herba
ethanolic extract against TNF-alpha/IFN-gamma-stimulated human
keratinocyte HaCaT cells. J Ethnopharmacol. 211:217–223. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Albanesi C: Keratinocytes in allergic skin
diseases. Curr Opin Allergy Clin Immunol. 10:452–456. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ravindran J, Agrawal M, Gupta N and Rao
PV: Alteration of blood brain barrier permeability by T-2 toxin:
Role of MMP-9 and inflammatory cytokines. Toxicology. 280:44–52.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmidt-Arras D and Rose-John S: IL-6
pathway in the liver: From physiopathology to therapy. J Hepatol.
64:1403–1415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6:a0162952014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cho SO, Lim JW and Kim H: Red ginseng
extract inhibits the expression of MCP-1 and iNOS in Helicobacter
pylori-infected gastric epithelial cells by suppressing the
activation of NADPH oxidase and Jak2/Stat3. J Ethnopharmacol.
150:761–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong C, Davis RJ and Flavell RA: MAP
kinases in the immune response. Annu Rev Immunol. 20:55–72. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taniguchi K and Karin M: IL-6 and related
cytokines as the critical lynchpins between inflammation and
cancer. Semin Immunol. 26:54–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weng L, Zhang H, Li X, Zhan H, Chen F, Han
L, Xu Y and Cao X: Ampelopsin attenuates lipopolysaccharide-induced
inflammatory response through the inhibition of the NF-κB and
JAK2/STAT3 signaling pathways in microglia. Int Immunopharmacol.
44:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chang X, Luo F, Jiang W, Zhu L, Gao J, He
H, Wei T, Gong S and Yan T: Protective activity of salidroside
against ethanol-induced gastric ulcer via the MAPK/NF-κB pathway in
vivo and in vitro. Int Immunopharmacol. 28:604–615. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fan Y, Mao R and Yang J: NF-κB and STAT3
signaling pathways collaboratively link inflammation to cancer.
Protein Cell. 4:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li L, Chen J, Lin L, Pan G, Zhang S, Chen
H, Zhang M, Xuan Y, Wang Y and You Z: Quzhou Fructus Aurantii
Extract suppresses inflammation via regulation of MAPK, NF-κB, and
AMPK signaling pathway. Sci Rep. 10:15932020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang TZ, Yang SH, Yao JF, Du J and Yan
TH: Sangxingtang inhibits the inflammation of LPS-induced acute
lung injury in mice by down-regulating the MAPK/NF-κB pathway. Chin
J Nat Med. 13:889–895. 2015.PubMed/NCBI
|
|
47
|
Castejon ML, Sanchez-Hidalgo M,
Aparicio-Soto M, Gonzalez-Benjumea A, Fernandez-Bolanos JG and
Alarcon-de-la-Lastra C: Olive secoiridoid oleuropein and its
semisynthetic acetyl-derivatives reduce LPS-induced inflammatory
response in murine peritoneal macrophages via JAK-STAT and MAPKs
signaling pathways. J Funct Foods. 58:95–104. 2019. View Article : Google Scholar
|
|
48
|
Plastira I, Bernhart E, Joshi L, Koyani
CN, Strohmaier H, Reicher H, Malle E and Sattler W: MAPK signaling
determines lysophosphatidic acid (LPA)-induced inflammation in
microglia. J Neuroinflammation. 17:1272020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yong HY, Koh MS and Moon A: The p38 MAPK
inhibitors for the treatment of inflammatory diseases and cancer.
Expert Opin Investig Drugs. 18:1893–1905. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Imokawa G: Mechanism of UVB-induced
wrinkling of the skin: Paracrine cytokine linkage between
keratinocytes and fibroblasts leading to the stimulation of
elastase. J Investig Dermatol Symp Proc. 14:36–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Oikarinen A: The aging of skin:
Chronoaging versus photoaging. Photodermatol Photoimmunol Photomed.
7:3–4. 1990.PubMed/NCBI
|
|
52
|
Nissinen LM and Kahari VM: Collagen
turnover in wound repair-a macrophage connection. J Invest
Dermatol. 135:2350–2352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cole MA, Quan T, Voorhees JJ and Fisher
GJ: Extracellular matrix regulation of fibroblast function:
redefining our perspective on skin aging. J Cell Commun Signal.
12:35–43. 2018. View Article : Google Scholar : PubMed/NCBI
|