Introduction
Liver fibrosis is the result of a healing response
to a variety of injury factors, such as chronic hepatitis,
cholestasis, alcohol and drugs. It has been reported that liver
fibrosis serves a significant role in the development of liver
cirrhosis and liver cancer (1,2).
When the liver is damaged, the injured hepatocytes, Kupffer cells
or inflammatory cells produce several endogenous profibrotic
cytokines or proinflammatory factors, which in turn activate
resting hepatic stellate cells (HSCs). Activated HSCs undergo
collagen overproduction, while collagen degradation is inhibited,
eventually leading to the accumulation of extracellular matrix
(ECM). ECM is considered to be the main pathophysiological process
leading to liver fibrosis (3).
Liver fibrosis has recently become the main focus in numerous
studies. Unfortunately, safe and effective anti-fibrotic drugs are
still lacking (4). Therefore,
further studies on the development of effective drugs for treating
liver fibrosis are urgently required.
TGF-β1 plays an important role in liver fibrosis
(5–7). Activated HSCs secrete TGF-β1 and
differentiate into myofibroblasts, which in turn promote the
synthesis and accumulation of ECM-related proteins leading to liver
fibrosis. Additionally, TGF-β1 can also activate HSCs, which
further increase the synthesis and secretion of TGF-β1, thereby
promoting the development of liver fibrosis (8). Furthermore, another study revealed
that TGF-β1 enhanced ECM by regulating the TGF-β/Smad signaling
pathway to further promote the development of fibrosis (6).
Aspirin is a common non-steroidal anti-inflammatory
drug, also known as acetylsalicylic acid; it is most commonly used
to decrease fever, relieve pain and attenuate inflammatory
responses (9). However, it is
also used to improve the cardiovascular system (10), central nervous system (11) and even certain types of cancer
(12,13). Emerging evidence has suggested
that aspirin exhibits notable antifibrotic effects on liver
diseases. For example, aspirin significantly reduced the liver
fibrosis index in US adults with chronic liver diseases (14). Additionally, aspirin was found to
attenuate the degree of hepatic fibrosis in a liver fibrosis rat
model (15). Nevertheless, the
mechanism underlying the effect of aspirin on regulating hepatic
fibrosis remains elusive. A recent study showed that aspirin could
alleviate liver fibrosis in rats by inhibiting the toll-like
receptor 4/NF-κB signaling pathway (16), although the effect of aspirin on
liver fibrosis by inhibiting the TGF-β1/Smad pathway has not been
previously investigated.
Therefore, in the present study, a rat model of
hepatic fibrosis was established by intraperitoneal injection of
thioacetamide (TAA) to evaluate the antifibrotic effect of aspirin.
In addition, the study further explored whether aspirin could
attenuate liver fibrosis through the TGF-β1/Smad signaling pathway
and the effects of aspirin on HSC collagen production, thus
uncovering the molecular mechanism underlying the protective effect
of aspirin against liver fibrosis. The results of the present study
may provide further knowledge for the use of aspirin in the
treatment of hepatic fibrosis.
Materials and methods
Drugs
TAA was obtained from MilliporeSigma, while aspirin
was purchased from Bayer AG.
Animals
A total of 30 healthy male Sprague-Dawley (SD) rats
(age, 6 weeks), weighing 200–220 g, were obtained from the
Experimental Animal Center of the Tongji Medical College, Huazhong
University of Science and Technology (Wuhan, China). The animals
were maintained under a 12 h light/dark cycle at room temperature
(20–25°C) and a humidity of 50–60%. All animals had free access to
food and water. The animal experiments were approved by the Ethics
Committee of Animal Experiments of the Tongji Medical College
(Wuhan, China; approval no. TJ-A20150803).
Cell culture
The rat HSC line, HSC-T6 (cat. no. CL-0116), was
purchased from the Shanghai Institute of Biochemistry and Cell
Biology, and was cultured in high-glucose DMEM supplemented with
10% FBS (both from Gibco; Thermo Fisher Scientific, Inc.), 100
µg/ml penicillin and 100 µg/ml streptomycin (Beyotime Institute of
Biotechnology) at 37°C in a 5% CO2 incubator. HSCs were
treated with different concentrations of aspirin (10, 20 and 40
mmol/l) at 37°C for 72 h, as previously described (16).
Biochemical analyses
Serum was isolated from rat blood samples at 1,200 ×
g for 5 min at 4°C and the serum levels of aspartate transaminase
(AST) and alanine transaminase (ALT) were measured using the
corresponding AST (cat. no. C010-2-1; Nanjing Jiancheng
Bioengineering Institute) and ALT (cat. no. C009-2-1; Nanjing
Jiancheng Bioengineering Institute) commercial kits, according to
the manufacturer's instructions. The levels of hepatic
hydroxyproline were measured using a hydroxyproline test kit (cat.
no. A030-2-1; Nanjing Jiancheng Bioengineering Institute),
according to the manufacturer's protocol.
TAA-induced liver fibrosis and aspirin
treatment
SD rats were randomly divided into four groups
(n=7/group). Rats in the TAA and TAA + aspirin groups received 200
mg/kg intraperitoneal TAA twice weekly for 8 weeks, while 1 ml
physiological saline and 1 ml aspirin (30 mg/kg), respectively,
were administered by gavage once every morning for 8 weeks
(15). In addition, rats in the
sham + aspirin group were treated with saline and aspirin as
aforementioned. However, rats in the sham group were treated with
intraperitoneal saline twice weekly for 8 weeks and then an
additional 1 ml physiological saline was administered by gavage
once every morning over a period of 8 weeks. At the end of the
eighth week, 72 h after the last TAA injection, all animals were
anaesthetized with 200 mg/kg intraperitoneal sodium pentobarbital
and sacrificed by cervical dislocation. All animal experiments in
the present study were performed in accordance with the Guide for
the Care and Use of Laboratory Animals. Blood was collected by
cardiac puncture and livers were subsequently removed. Liver and
blood samples were collected and stored at −80°C for further
analysis.
Histopathological and
immunohistochemical (IHC) analysis
Liver tissues were fixed in 4% paraformaldehyde
solution at 4°C for 24 h and embedded in paraffin.
Paraffin-embedded tissue samples were cut into 4-µm thick sections
followed by staining with H&E (hematoxylin 5 min, eosin 3 min)
and Masson's trichrome (Wuhan Servicebio Technology Co., Ltd.) for
15 min at room temperature according to the manufacturer's
protocol. Subsequently, the tissue sections were deparaffinized
with xylene and rehydrated in gradient ethanol at room temperature.
For antigen retrieval, the tissue was boiled in a microwave for 15
min and washed with PBS. The tissue sections were blocked with 10%
bovine serum albumin (Beijing Dingguo Changsheng Biotechnology Co.,
Ltd.) for 15 min at room temperature and were then incubated
overnight at 4°C with the following primary antibodies: Rabbit
anti-TGF-β1 (dilution 1:1,000; cat. no. 21898-1-AP; Wuhan Sanying
Biotechnology), rabbit anti-collagen I (dilution 1:500; cat. no.
ab34710; Abcam), rabbit anti-α-smooth muscle actin (α-SMA)
(dilution, 1:500; cat. no. ab32575; Abcam), rabbit
anti-phosphorylated (p)-Smad2 (dilution 1:500; cat. no. 3108; Cell
Signaling Technology, Inc.) and rabbit anti-p-Smad3 (dilution
1:500; cat. no. 1880-1; Epitomics; Abcam). Following rinsing in
PBS, the tissue sections were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:500; cat. no.
PV-9001; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
secondary antibody for 1 h at 37°C, followed by staining with 0.05%
diaminobenzidine for 90 sec and counterstaining with hematoxylin
for 5 min at room temperature. Finally, the tissue sections were
dehydrated again in gradient ethanol and mounted with neutral
rubber (17). Representative
images in three randomly selected fields per slide were captured
under the Nikon Digital ECLIPSE C1 confocal microscope (Nikon
Corporation). The positive staining to total area ratios were
calculated using the Image-Pro Plus 6.0 imaging software (Media
Cybernetics, Inc.). The average value from three ratios was used
for statistical analysis.
Western blot analysis
Total protein was extracted from liver tissue and
HSCs using RIPA buffer supplemented with protease inhibitors
(Beyotime Institute of Biotechnology). The protein concentration
was measured using BCA and 40 µg protein/lane was separated by
SDS-PAGE on a 8–10% gel and then transferred onto a PVDF membrane.
Following blocking with 5% skimmed milk in TBS at room temperature
for 1 h, the membranes were incubated overnight at 4°C with the
following primary antibodies: Rabbit anti-TGF-β1 (dilution 1:500;
cat. no. 21898-1-AP; Wuhan Sanying Biotechnology), rabbit
anti-collagen I (dilution, 1:500; cat. no. ab34710; Abcam), rabbit
anti-α-SMA (dilution 1:500; cat. no. ab32575; Abcam), rabbit
anti-p-Smad2 (dilution 1:1,000; cat. no. 3108; Cell Signaling
Technology, Inc.), rabbit anti-Smad2 (dilution 1:500; cat. no.
1641-1), rabbit anti-p-Smad3 (dilution, 1:1,000; cat. no. 1880-1)
and rabbit anti-Smad3 (dilution, 1:500; cat. no. 1735-1) (all from
Epitomics; Abcam) and rabbit anti-β-actin (dilution 1:1,000; cat.
no. sc-47778; Santa Cruz Biotechnology, Inc.). Following washing
with TBS containing 0.1% Tween-20, the membranes were incubated
with horseradish peroxidase-conjugated goat anti-rabbit IgG
secondary antibodies (dilution 1:5,000; cat. no. ZB-5301; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 1 h at room
temperature. The immunoreactive bands were visualised using an ECL
reagent (Thermo Fisher Scientific, Inc.) and detected using the
ChemiDoc™ Imaging System (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from liver tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). A SMA2000 spectrophotometer (Thermo Fisher Scientific, Inc.)
was used to measure RNA absorbance at 260 nm. Next, the
PrimeScript® RT reagent kit (Takara Biotechnology Co.,
Ltd.) was used for reverse transcription. The transcriptional
conditions were 37°C for 15 min and 98°C for 5 min, followed by
maintenance at 4°C. qPCR was performed using the SYBR Green PCR
Master Mix-PLUS kit (Toyobo Life Science) on the CFX96 Touch™
Real-Time PCR Detection System (Bio-Rad, Laboratories, Inc.). Each
20-µl reaction contained 10 µl SYBR Green Mix, 6 µl nuclease-free
H2O, 2 µl cDNA, 1 µl upstream primer and 1 µl downstream
primer. The thermocycling conditions for qPCR were as follows:
Initial denaturation at 95°C for 60 sec, followed by 40 cycles of
95°C for 15 sec, 60°C for 15 sec and extension at 72°C for 45 sec.
The primer sequences were synthesized by Sangon Biotech Co., Ltd.
The relative mRNA expression levels were calculated with the
2−ΔΔCq method (18).
GAPDH served as an internal reference gene. The primer sequences
used are listed in Table I.
 | Table I.Primer sequences for quantitative
PCR. |
Table I.
Primer sequences for quantitative
PCR.
| Gene | Sequence
(5′-3′) | Product size,
bp |
|---|
| COL1A1 |
| 251 |
|
Forward |
GAGAGAGCATGACCGATGGA |
|
|
Reverse |
CGTGCTGTAGGTGAATCGAC |
|
| α-SMA |
| 148 |
|
Forward |
TGTGCTGGACTCTGGAGATG |
|
|
Reverse |
GAAGGAATAGCCACGCTCAG |
|
| TGF-β1 |
| 72 |
|
Forward |
CGGACTACTACGCCAAAGAAGT |
|
|
Reverse |
TGGTTTTGTCATAGATTGCGTT |
|
| GAPDH |
| 124 |
|
Forward |
GACATCAAGAAGGTGGTG |
|
|
Reverse |
CAGCATCAAAGGTGGAAG |
|
Statistical analysis
All experimental data are expressed as the mean ±
SEM of three independent experiments. Comparisons among multiple
groups were performed with one-way ANOVA followed by Tukey's post
hoc test. All graphs were constructed by GraphPad Prism 5.0
software (GraphPad Software, Inc.). All statistical analyses were
performed using SPSS 22.0 software (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Aspirin attenuates TAA-induced liver
fibrosis in a rat model
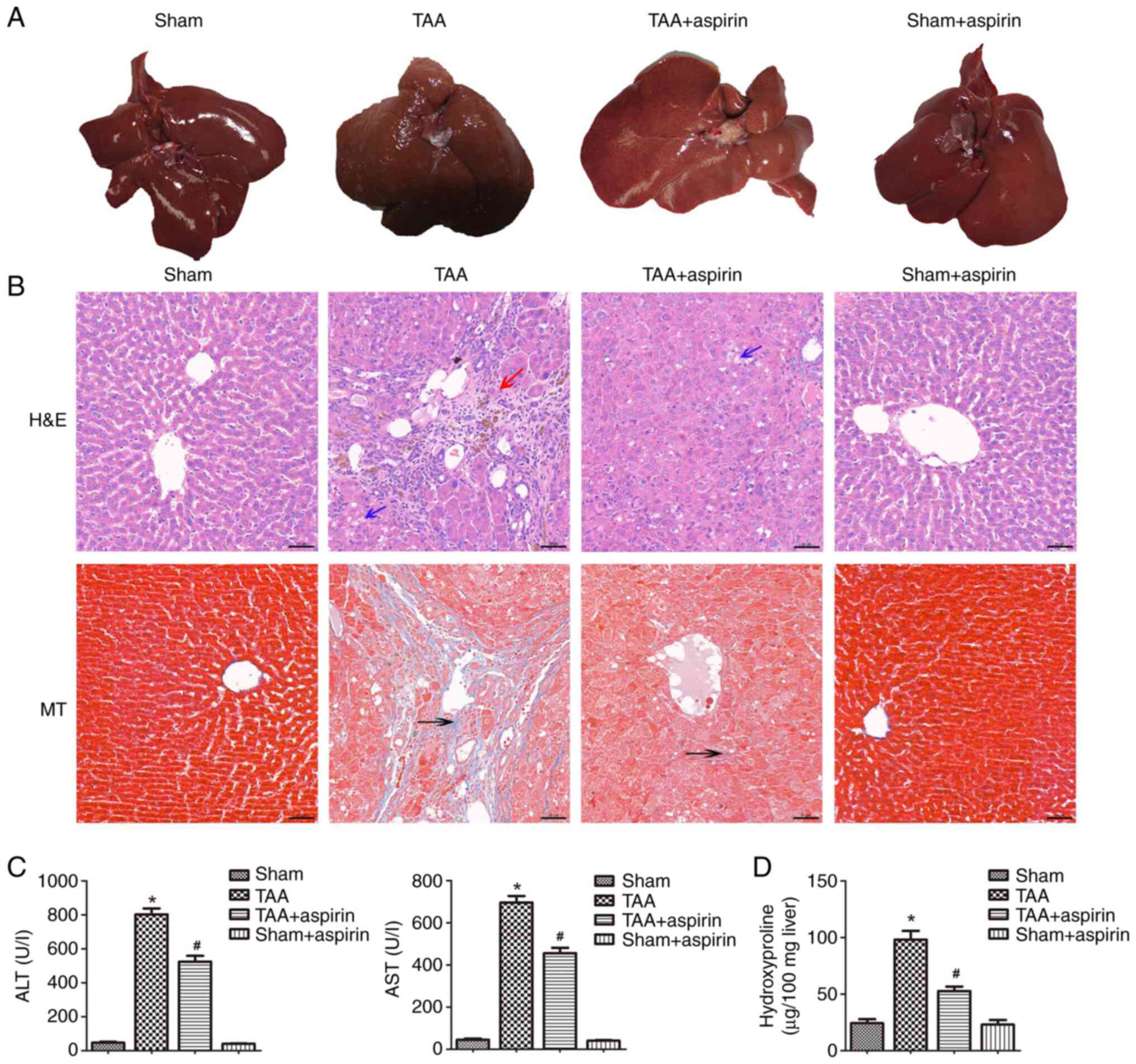
The livers in the sham and aspirin groups of the
in vivo rat model appeared bright red, soft, smooth and
sharp-edged at the eighth week after treatment (Fig. 1A). By contrast, the livers in the
TAA group were dark red, hard, rough with granular nodules and
blunt-edged (Fig. 1A). The livers
in the TAA + aspirin group were more yellow and slightly harder
compared with those in the sham and sham + aspirin group. However,
livers in the TAA + aspirin group were softer compared with those
in the TAA group, while no granular nodules were observed (Fig. 1A). Subsequently, H&E and
Masson's staining were performed to evaluate the degree of liver
fibrosis. Liver tissues derived from rats in the TAA group had
pathological changes, with hepatic steatosis and fat vacuoles,
while the liver lobule structure was abnormal or damaged as shown
by H&E staining. Masson's staining showed a marked increase in
collagen and the formation of pseudolobules (Fig. 1B). However, the aforementioned
damaged tissues and fibrotic areas were marked improved in the TAA
+ aspirin group (Fig. 1B). To
evaluate the effect of aspirin on liver injury, the levels of AST
and ALT in the serum were detected. As shown in Fig. 1C, the serum levels of AST and ALT
were significantly increased in the TAA group compared with that in
the sham group. However, the levels of both AST and ALT were
significantly decreased in the TAA + aspirin group compared with
that in the TAA group indicating that aspirin attenuated
TAA-induced liver injury. Furthermore, hydroxyproline, a marker of
total hepatic collagen content, was significantly increased in the
TAA group compared with that in the sham group, and then decreased
in the TAA + aspirin group compared with that in the TAA group
(Fig. 1D). Additionally, the
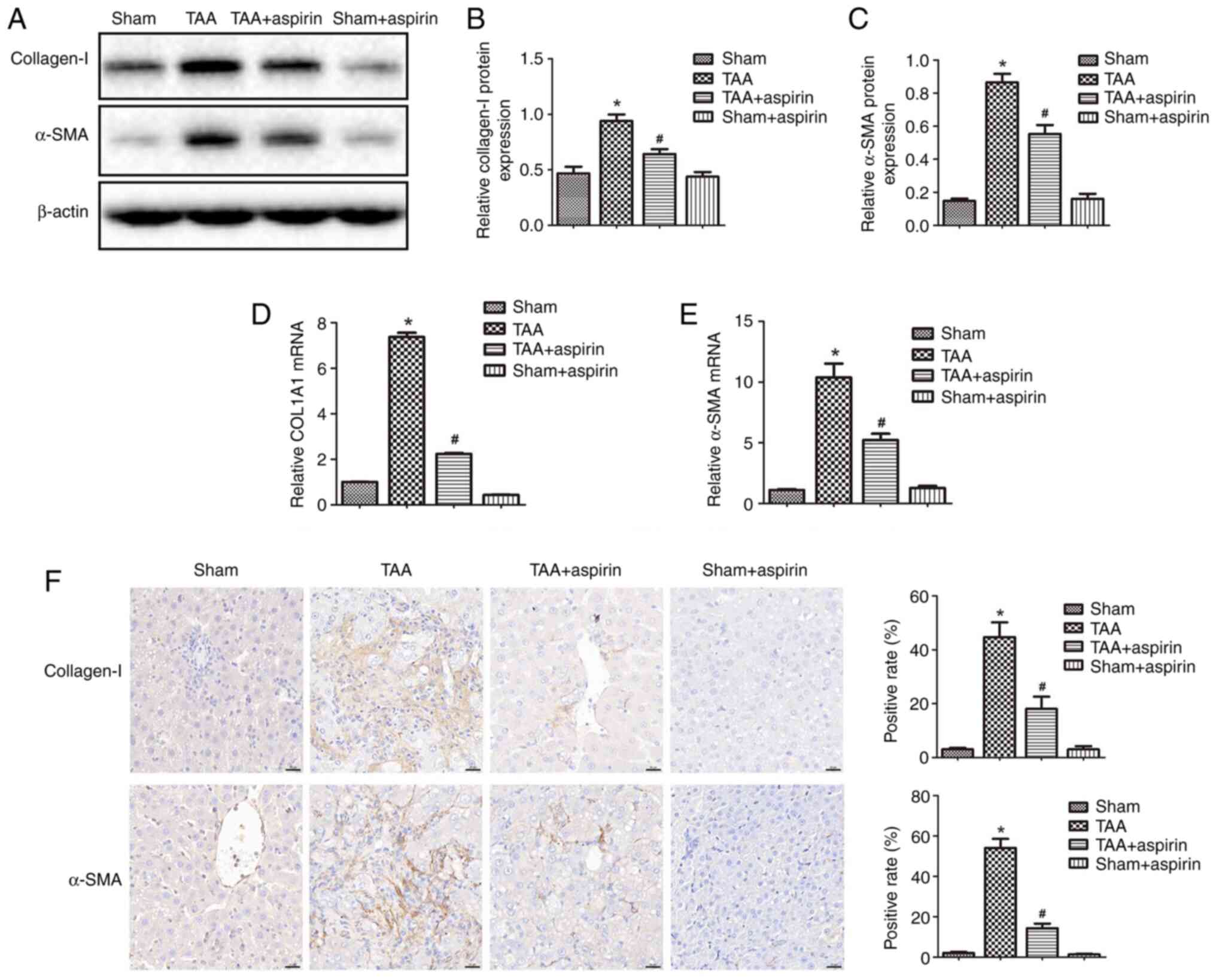
results demonstrated that the protein and mRNA expression levels of
α-SMA and collagen I were upregulated in the TAA group compared
with those in the sham group, and then downregulated again in the
TAA + aspirin group compared with those in the TAA group (Fig. 2A-E). Additionally, IHC staining
was performed to analyze the expression levels of α-SMA and
collagen I in the liver tissue. The results showed enhanced
staining of α-SMA and collagen I in the TAA group, which was then
decreased in the TAA + aspirin group (Fig. 2F). The aforementioned findings
suggested that aspirin could attenuate TAA-induced hepatic fibrosis
in rats.
Aspirin attenuates liver fibrosis by
regulating the TGF-β1/Smad signaling pathway
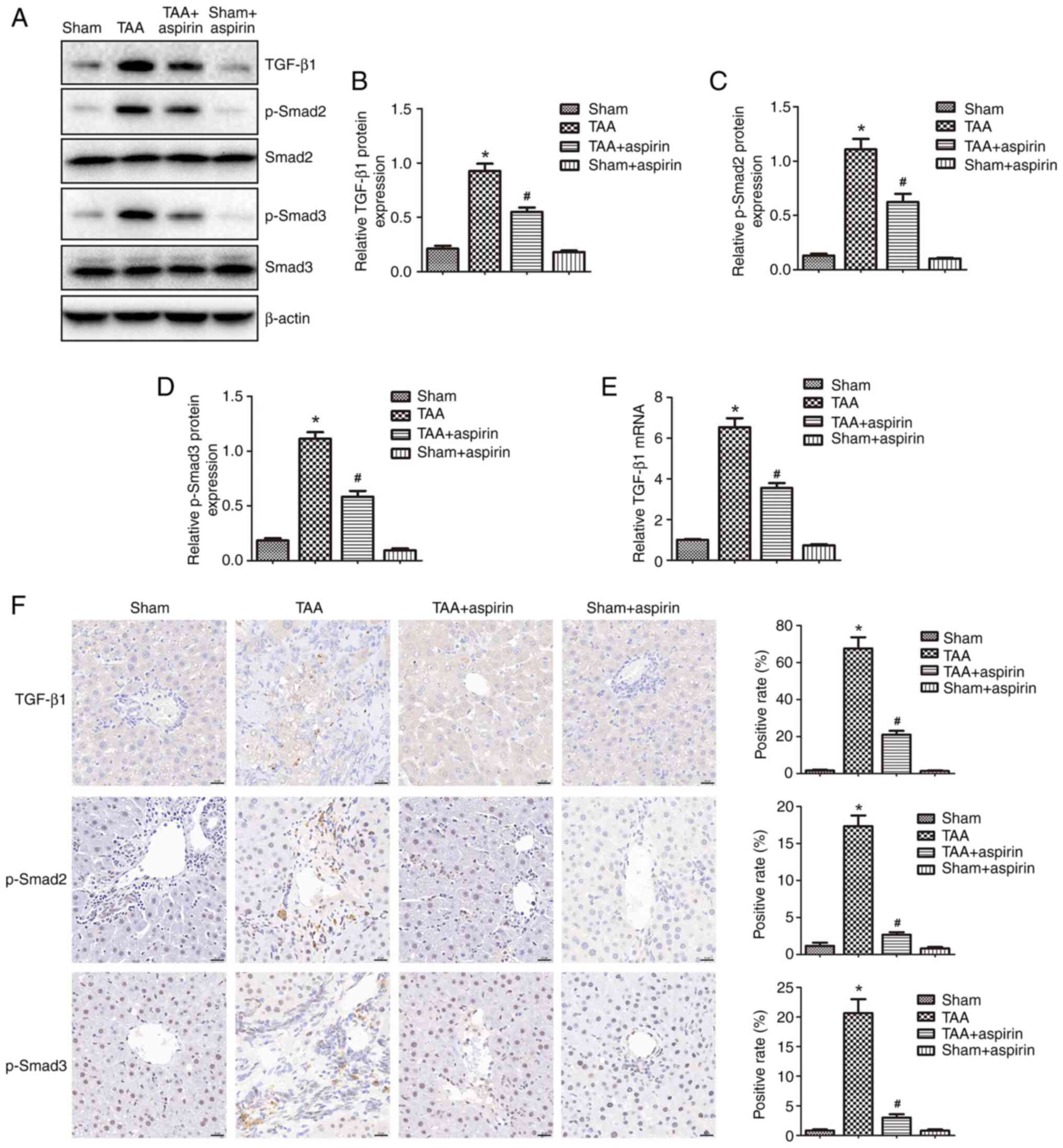
To explore the mechanisms underlying the effect of
aspirin on inhibiting liver fibrosis, the protein expression levels
of TGF-β1, p-Smad2, p-Smad3, Smad2 and Smad3 were determined by
western blotting and IHC staining (Fig. 3). The results showed that the
protein expression levels of TGF-β1, p-Smad2 and p-Smad3 were
significantly increased in the TAA group compared with those in the
sham group, while they were significantly reduced in the TAA +
aspirin group compared with those in the TAA group. No changes were
observed in the protein levels of total Smad2 and Smad3 (Fig. 3A-D and F). In addition, treatment
with aspirin significantly decreased the mRNA levels of TGF-β1
(Fig. 3E). These findings
indicated that aspirin could attenuate liver fibrosis by inhibiting
the TGF-β1/Smad signaling pathway.
In vitro effect of aspirin on
HSCs
To further investigate whether the antifibrotic
effect of aspirin was triggered by regulating the TGF-β1/Smad
signaling pathway in HSCs, HSCs were treated with different
concentrations of aspirin. As shown in Fig. 4, aspirin downregulated the protein
expression levels of α-SMA, collagen I, TGF-β1, p-Smad2 and p-Smad3
in a dose-dependent manner. These results were consistent with the
observations in the rat model of liver fibrosis.
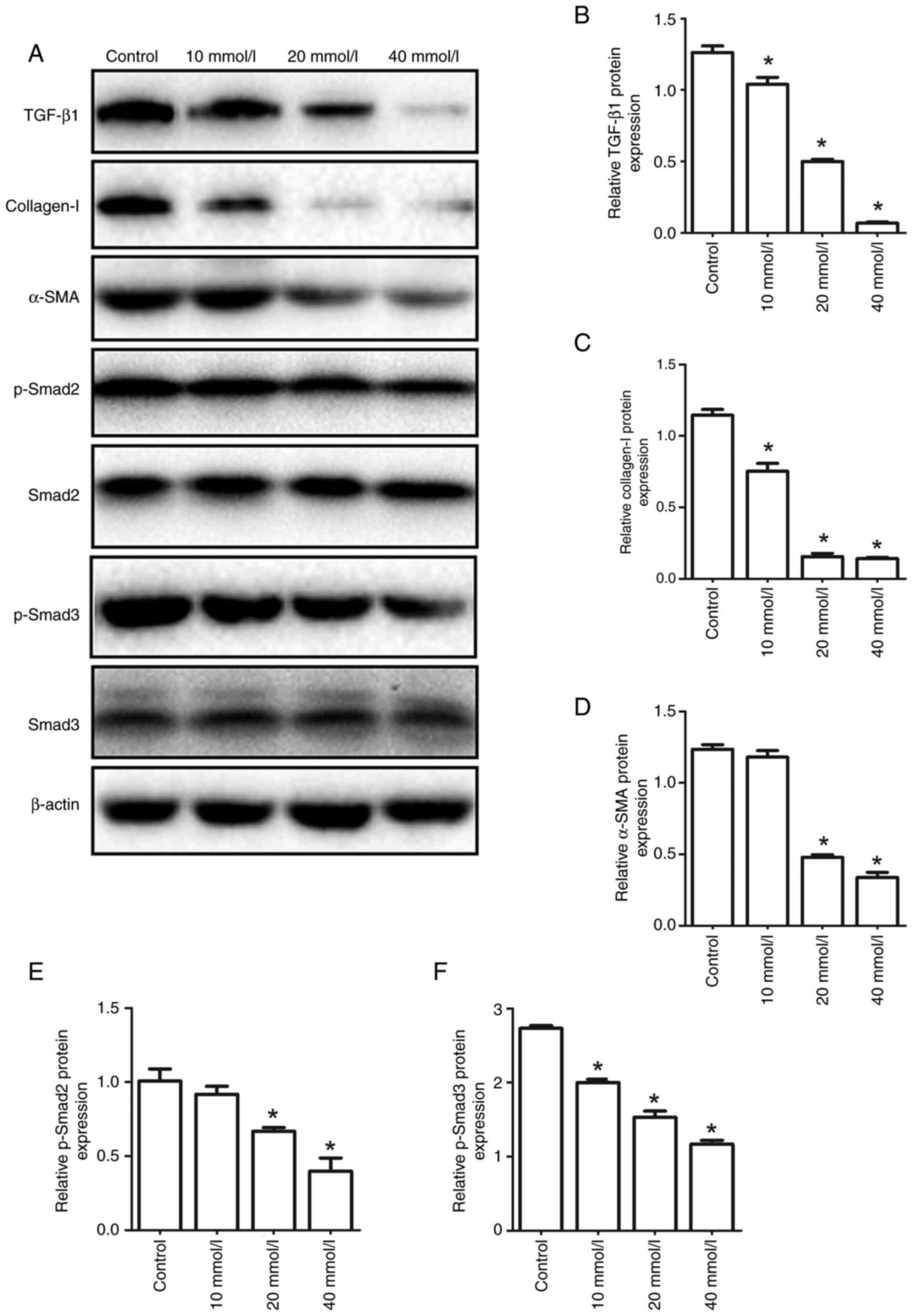 | Figure 4.Effect of different concentrations of
aspirin on the protein expression levels of α-SMA, collagen I,
TGF-β1, p-Smad2 and p-Smad3 in HSCs derived from rats. (A) The
protein expression levels of TGF-β1, collagen I, α-SMA, p-Smad2,
p-Smad3, Smad2 and Smad3 in HSCs were determined by western blot
analysis. (B-F) The relative protein expression of (B) TGF-β1, (C)
collagen I, (D) α-SMA, (E) p-Smad2 and (F) p-Smad3. *P<0.05 vs.
control. p-Smad2, phosphorylated Smad2; HSC, hepatic stellate cell;
p-, phosphorylated; α-SMA, α-smooth muscle actin. |
Discussion
Liver fibrosis, characterized by increased
deposition of ECM, is the repair and healing process during liver
injury caused by various factors (19). However, this process is also
associated with the transformation of chronic liver diseases into
cirrhosis and liver cancer (20).
Currently, there are no safe and effective drugs for the treatment
of liver cirrhosis. Most drugs can only reduce complications, but
they cannot treat the underlying cause of liver fibrosis.
Therefore, further studies on the development of novel antifibrotic
treatments are urgently needed to prevent and/or reverse liver
fibrosis. Although it has been reported that aspirin exhibits
antifibrotic effects, the mechanism underlying the effect on
hepatic fibrosis remains elusive. The present study revealed that
aspirin attenuated liver fibrosis by suppressing TGF-β1/Smad
signaling in rats.
Within the present study, a rat model of liver
fibrosis was established using TAA. A previous study demonstrated
that the liver morphology and function in TAA-induced liver
fibrosis rats is similar to that in humans (21). In the present study, treatment of
rats with TAA significantly increased the levels of AST and ALT in
the serum. A recent study revealed that the enhanced levels of AST
and ALT, two common markers of liver injury (22), were associated with disruption of
hepatocyte membrane integrity or hepatocyte injury (23). In the present study, the results
of H&E and Masson's staining revealed the onset of typical
fibrosis-related pathological changes in the livers of rats treated
with TAA for 8 weeks. Consistent with previous studies (15,24), the treatment of rats with aspirin
alleviated the pathological changes in the liver, thus indicating
that aspirin could exert an antifibrotic effect and improve liver
injury.
Emerging evidence has suggested that the activation
of HSCs plays an important role in liver fibrosis. Once the liver
is injured, quiescent HSCs are activated and differentiate into
myofibroblasts, which express high levels of α-SMA, collagen I and
collagen III, thus resulting in an increased deposition of ECM and
the development of liver fibrosis (25). Profibrogenic cytokines, such as
TGF-β1 and TNF-α, also play a critical role in the progress of
liver fibrosis (26). Liver
injury induces Kupffer cells to produce TGF-β1 (27). In addition, another study showed
that HSCs could be activated by TGF-β1 and differentiate into
myofibroblasts to further promote the development of fibrosis
(28). The present study
demonstrated that the expression levels of TGF-β1, p-Smad2,
p-Smad3, collagen I and α-SMA were significantly increased in the
TAA-induced liver fibrosis model, and then significantly reduced
following aspirin treatment. Furthermore, aspirin downregulated the
protein expression levels of TGF-β1, p-Smad2, p-Smad3, collagen I
and α-SMA in HSCs. The in vitro results were consistent with
those observed in vivo. These results suggested that aspirin
could attenuate liver fibrosis by inhibiting the TGF-β1/Smad
signaling pathway.
A recent study showed that aspirin downregulated
TGF-β1 in a carbon tetrachloride-induced chronic liver injury model
(29). TGF-β1 is a significant
mediator of liver fibrosis. TGF-β1 is secreted by Kupffer cells,
activated in HSCs and specific inflammatory cells (30). TGF-β1 activates resting HSCs,
which in turn secrete more TGF-β1 to maintain the activation status
of these cells, thus enhancing ECM deposition (31). TGF-β1 binds to type I and type II
TGF-β receptors (TβRs) on the surface of HSCs, while the
intracellular signal transduction of TGF-β1 is mediated by the Smad
protein family. When TGF-β1 is activated, it binds to TβRII on the
surface of HSCs to activate TβRI. In turn, activated TβRI primarily
induces the phosphorylation of Smad2 and Smad3 in quiescent and
activated HSCs, respectively (32). Subsequently, activated Smad2/3
bind to Smad4 and the complex translocates into the nucleus to
activate the transcription of downstream genes, inducing liver
fibrosis-related genes such as fibronectin, α-SMA and collagen I
(33,34). Previous studies suggested that the
increased expression of fibronectin, α-SMA and collagen I in liver
fibrosis could be associated with the TGF-β1/Smad signaling pathway
(35,36). The aforementioned molecules could
alleviate liver fibrosis in rats by inhibiting the TGF-β1 signaling
pathway (37). The present study
explored the effect of aspirin on the TGF-β1/Smad signaling
pathway. The results showed that treatment with TAA increased
TGF-β1 levels, which were restored following treatment with
aspirin. Accordingly, aspirin reduced the expression levels of the
downstream molecules p-Smad2 and p-Smad3. Therefore, these results
indicated that aspirin could effectively inhibit the TGF-β1/Smad
signaling pathway involved in liver fibrosis.
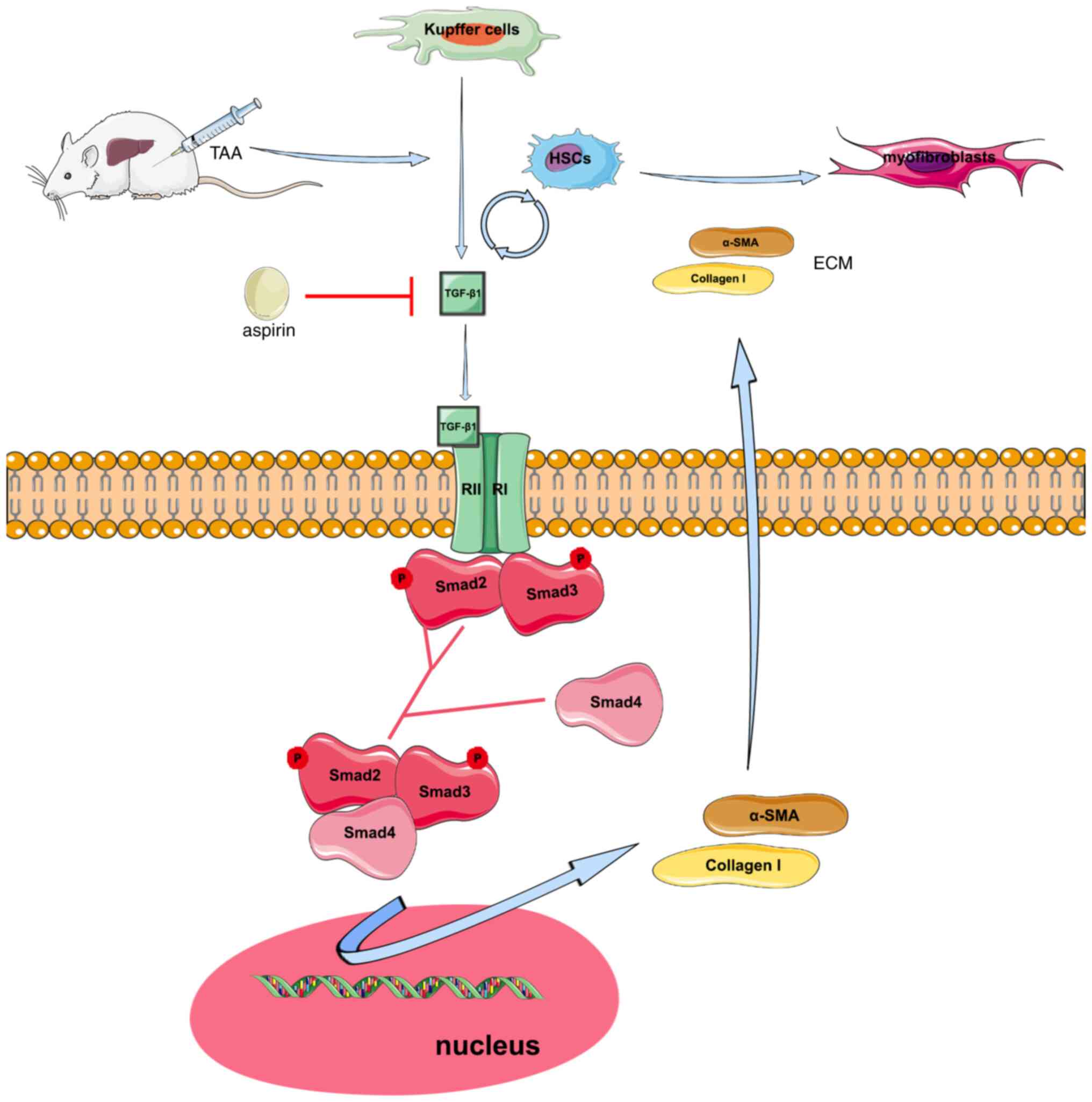
Aspirin alleviated TAA-induced liver fibrosis in
rats. Therefore, treatment with aspirin reduced the secretion of
TGF-β1 by activated HSCs and Kupffer cells, thus resulting in
p-Smad2, p-Smad3, α-SMA and collagen I downregulation by the
TGF-β1/Smad signaling pathway. Additionally, reduction of TGF-β1
reduced the activation of HSCs and myofibroblasts and attenuated
ECM production (Fig. 5).
The present study contains some limitations. First,
the results showed that aspirin alleviated TAA-induced liver
fibrosis in rats. However, there are also other liver fibrosis
models with different underlying mechanisms, such as the bile duct
ligation-induced liver fibrosis model. Therefore, the mechanism
underlying the antifibrotic effect of aspirin should be also
investigated in such models. Second, a previous study indicated
that aspirin activates AMP-activated protein kinase and upregulates
Smad 6 in FOP fibroblast cells (38). However, whether Smad 6, as a key
negative regulator of the TGF-β1/Smad signaling pathway, is
involved in the antifibrotic effects of aspirin should be further
investigated. Third, the present study revealed that aspirin
alleviated TAA-induced liver fibrosis. However, the underlying
mechanism of aspirin with regard to improving liver fibrosis was
only preliminarily investigated. Therefore, additional studies are
required to further investigate the involvement of the TGF-β1/Smad
signaling pathway in the antifibrotic effect of aspirin.
In summary, the present study suggested that aspirin
could exert a potential protective role against liver fibrosis. The
present study was the first to demonstrate that aspirin could
ameliorate TAA-induced liver fibrosis by inhibiting the TGF-β1/Smad
signaling pathways. These results indicated that aspirin could
provide novel insights into the development of new drugs for the
prevention and treatment of liver fibrosis. Furthermore, the safety
of aspirin in clinical practice and other mechanisms remain to be
studied.
Acknowledgements
Not applicable.
Funding
This work was supported by the Health and Family Planning
Research Foundation of Hubei Province of China (grant no.
WJ2016-Y-26).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS and BL designed and performed the experiments,
designed the figures and drafted the manuscript. JXie, XJ, BX and
XH performed the experiments. JXia designed the experiments,
analyzed the data, and critically revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript. YS and JXia confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of Animal Experiments of the Tongji Medical College
(approval no. TJ-A20150803).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Friedman SL: Liver fibrosis-from bench to
bedside. J Hepatol. 38 (Suppl 1):S38–S53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman SL: Seminars in medicine of the
Beth Israel Hospital, Boston. The cellular basis of hepatic
fibrosis. Mechanisms and treatment strategies. N Engl J Med.
328:1828–1835. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kisseleva T and Brenner DA: Role of
hepatic stellate cells in fibrogenesis and the reversal of
fibrosis. J Gastroenterol Hepatol. 22 (Suppl 1):S73–S78. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friedman SL: Evolving challenges in
hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 7:425–436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szabo G and Bala S: Alcoholic liver
disease and the gut-liver axis. World J Gastroenterol.
16:1321–1329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang D, Hao X, Xu L, Cui J, Xue L and
Tian Z: Intestinal flora imbalance promotes alcohol-induced liver
fibrosis by the TGFβ/smad signaling pathway in mice. Oncol Lett.
14:4511–4516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Kim B, Park YK, Koo SI and Lee JY:
Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression
by inhibiting Smad3 activation in hepatic stellate cells. Biochim
Biophys Acta. 1850:178–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Q, Duan ZP, Ha DK, Bengmark S,
Kurtovic J and Riordan SM: Synbiotic modulation of gut flora:
Effect on minimal hepatic encephalopathy in patients with
cirrhosis. Hepatology. 39:1441–1449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vonkeman HE and van de Laar MA:
Nonsteroidal anti-inflammatory drugs: Adverse effects and their
prevention. Semin Arthritis Rheum. 39:294–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berk M, Woods RL, Nelson MR, Shah RC, Reid
CM, Storey E, Fitzgerald S, Lockery JE, Wolfe R, Mohebbi M, et al:
Effect of aspirin vs. placebo on the prevention of depression in
older people: A randomized clinical trial. JAMA Psychiatry.
77:1012–1020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antman EM: Evaluating the cardiovascular
safety of nonsteroidal anti-inflammatory drugs. Circulation.
135:2062–2072. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henry WS, Laszewski T, Tsang T, Beca F,
Beck AH, McAllister SS and Toker A: Aspirin suppresses growth in
PI3K-mutant breast cancer by activating AMPK and inhibiting mTORC1
Signaling. Cancer Res. 77:790–801. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gray RT, Coleman HG, Hughes C, Murray LJ
and Cardwell CR: Low-dose aspirin use and survival in colorectal
cancer: Results from a population-based cohort study. BMC Cancer.
18:2282018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang ZG, Feldbrügge L, Tapper EB, Popov
Y, Ghaziani T, Afdhal N, Robson SC and Mukamal KJ: Aspirin use is
associated with lower indices of liver fibrosis among adults in the
United States. Aliment Pharmacol Ther. 43:734–743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li CJ, Yang ZH, Shi XL and Liu DL: Effects
of aspirin and enoxaparin in a rat model of liver fibrosis. World J
Gastroenterol. 23:6412–6419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Nong L, Jia Y, Tan A, Duan L, Lu Y
and Zhao J: Aspirin alleviates hepatic fibrosis by suppressing
hepatic stellate cells activation via the TLR4/NF-κB pathway. Aging
(Albany NY). 12:6058–6066. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Chen XP, Zhang WG, Zhang F, Xiang
S, Dong HH and Zhang L: Hepatic non-parenchymal cells and
extracellular matrix participate in oval cell-mediated liver
regeneration. World J Gastroenterol. 15:552–560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dranoff JA and Wells RG: Portal
fibroblasts: Underappreciated mediators of biliary fibrosis.
Hepatology. 51:1438–1444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sherman M and Klein A: AASLD single-topic
research conference on hepatocellular carcinoma: Conference
proceedings. Hepatology. 40:1465–1473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yanguas SC, Cogliati B, Willebrords J,
Maes M, Colle I, van den Bossche B, de Oliveira C, Andraus W, Alves
VAF, Leclercq I and Vinken M: Experimental models of liver
fibrosis. Arch Toxicol. 90:1025–1048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai N, Zou Y, Zhu L, Wang HF and Dai MG:
Antioxidant properties of proanthocyanidins attenuate carbon
tetrachloride (CCl4)-induced steatosis and liver injury in rats via
CYP2E1 regulation. J Med Food. 17:663–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li S, Zheng X, Zhang X, Yu H, Han B, Lv Y,
Liu Y, Wang X and Zhang Z: Exploring the liver fibrosis induced by
deltamethrin exposure in quails and elucidating the protective
mechanism of resveratrol. Ecotoxicol Environ Saf. 207:1115012021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simon TG, Henson J, Osganian S, Masia R,
Chan AT, Chung RT and Corey KE: Daily aspirin use associated with
reduced risk for fibrosis progression in patients with nonalcoholic
fatty liver disease. Clin Gastroenterol Hepatol.
17:2776–2784.e2774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiao W, Bai M, Yin H, Liu J, Sun J, Su X,
Zeng H and Wen J: Therapeutic effects of an inhibitor of
thioredoxin reductase on liver fibrosis by inhibiting the
transforming growth factor-β1/smads pathway. Front Mol Biosci.
8:6901702021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhangdi HJ, Su SB, Wang F, Liang ZY, Yan
YD, Qin SY and Jiang HX: Crosstalk network among multiple
inflammatory mediators in liver fibrosis. World J Gastroenterol.
25:4835–4849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai X, Li Z, Zhang Q, Qu Y, Xu M, Wan X
and Lu L: CXCL6-EGFR-induced Kupffer cells secrete TGF-β1 promoting
hepatic stellate cell activation via the SMAD2/BRD4/C-MYC/EZH2
pathway in liver fibrosis. J Cell Mol Med. 22:5050–5061. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wree A, Holtmann TM, Inzaugarat ME and
Feldstein AE: Novel drivers of the inflammatory response in liver
injury and fibrosis. Semin Liver Dis. 39:275–282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chávez E, Castro-Sánchez L, Shibayama M,
Tsutsumi V, Salazar EP, Moreno MG and Muriel P: Effects of acetyl
salycilic acid and ibuprofen in chronic liver damage induced by
CCl4. J Appl Toxicol. 32:51–59. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shek FW and Benyon RC: How can
transforming growth factor beta be targeted usefully to combat
liver fibrosis? Eur J Gastroenterol Hepatol. 16:123–126. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu N, Feng J, Lu X, Yao Z, Liu Q, Lv Y,
Han Y, Deng J and Zhou Y: Isorhamnetin inhibits liver fibrosis by
reducing autophagy and inhibiting extracellular matrix formation
via the TGF-β1/smad3 and TGF-β1/p38 MAPK pathways. Mediators
Inflamm. 2019:61750912019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Achyut BR and Yang L: Transforming growth
factor-β in the gastrointestinal and hepatic tumor
microenvironment. Gastroenterology. 141:1167–1178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahamed J and Laurence J: Role of
platelet-derived transforming growth factor-β1 and reactive oxygen
species in radiation-induced organ fibrosis. Antioxid Redox Signal.
27:977–988. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mao Y, Zhang S, Yu F, Li H, Guo C and Fan
X: Ghrelin attenuates liver fibrosis through regulation of TGF-β1
expression and autophagy. Int J Mol Sci. 16:21911–21930. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu X, Hu H and Yin JQ: Therapeutic
strategies against TGF-beta signaling pathway in hepatic fibrosis.
Liver Int. 26:8–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Zhao L, Jiao FZ, Zhang WB, Chen Q
and Gong ZJ: Histone deacetylase inhibitor suberoylanilide
hydroxamic acid alleviates liver fibrosis by suppressing the
transforming growth factor-β1 signal pathway. Hepatobiliary
Pancreat Dis Int. 17:423–429. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin H, Ying Y, Wang YY, Wang G, Jiang SS,
Huang D, Luo L, Chen YG, Gerstenfeld LC and Luo Z: AMPK
downregulates ALK2 via increasing the interaction between Smurf1
and Smad6, leading to inhibition of osteogenic differentiation.
Biochim Biophys Acta Mol Cell Res. 1864:2369–2377. 2017. View Article : Google Scholar : PubMed/NCBI
|