Introduction
Myelodysplastic syndrome (MDS) is a group of
abnormal clonal disorders with ineffective hematopoiesis and
reduced peripheral blood cells (1). Myeloid cells in the bone marrow of
patients with MDS exhibit developmental abnormalities in one or
multiple lineages and high-risk patients may progress to acute
myeloid leukemia (AML) (2). Bone
marrow mesenchymal stem cells (BM-MSCs) are an important
constituent of the bone marrow hematopoietic microenvironment. They
may support the survival, self-renewal and differentiation of
hematopoietic stem cells through direct contact and cytokine
secretion (3,4). In MDS, abnormalities in the
hematopoietic stem cells and bone marrow hematopoietic
microenvironment are present (5,6).
There are significant gene expression profile differences between
the BM-MSCs from patients with MDS and those of healthy individuals
(7,8), which may selectively lead to
malignant clonal proliferation of hematopoietic stem cells in MDS.
Therefore, changes in BM-MSCs are associated with the pathogenesis
of MDS. BM-MSCs from patients with MDS and healthy individuals have
a similar cell morphology, proliferation capacity and immune
phenotype, and may be induced to transform into osteoblasts and
adipocytes in vitro. However, the ability of BM-MSCs to
support hematopoiesis is decreased in patients with MDS, which is
associated with the development and progression of the disease
(9).
Oxygen homeostasis is an essential prerequisite for
life activities in cells and hypoxia is one of the factors
affecting the bone marrow microenvironment. An essential regulatory
protein for sensing a hypoxic environment is hypoxia-inducible
factor-1 (HIF-1). HIF-1 is composed of the oxygen-sensitive HIF-1α,
as well as the constitutively expressed HIF-1β. HIF-1α is an
effector to a hypoxic environment that downregulates gene
expression in bone marrow cells, which enables the body to adapt to
a hypoxic environment (10).
HIF-1α has been considered to be an oncogenic protein, as hypoxic
regions are present in solid tumors and stabilize the HIF-1α
protein (11). However, it has
been indicated that hypoxia and HIF-1α facilitate the
differentiation of AML cells, suggesting that hypoxia has different
effects on leukemia (12). MDS
comprises heterogeneous hematopoietic disorders, which may be
identified based on their genetic, epigenetic, splicing and
metabolic aberrations in patients. Mutations in major
MDS-associated genes (Dnmt3a, Tet2, Asxl1, Runx1 and Mll1) activate
HIF-1α signaling (13). The
HIF-1α transcriptional signature was generally activated in bone
marrow stem/progenitor cells from patients with MDS (14). In vitro experiments
indicated that the HIF-1α signature was dysregulated in human
patients with MDS; the dysregulation of HIF-1α led to a clinically
relevant diversity of MDS phenotypes by functioning as a signaling
pathway for MDS-driving mutations. The genetic disruption of HIF-1α
resolves MDS phenotypes (15). In
addition, specifically inhibiting HIF-1α expression through RNA
interference may block hypoxia and HIF-1α-induced cell
differentiation (16). BM-MSCs
exist under hypoxic conditions and HIF-1α may be identified in the
cytoplasm of BM-MSCs, even under normoxic conditions (17). In BM-MSCs in a hypoxic state, IL-1
and TNF-α are activated via the PI3K and MAPK pathways to trigger
HIF-1α expression (17,18). In the present study, the
difference in the expression of HIF-1α in BM-MSCs between patients
with MDS and healthy subjects was examined to evaluate the
significance of the difference in HIF-1α expression. Furthermore,
the differential expression of HIF-1α was examined in bone marrow
biopsy specimens from patients with MDS of different risk groups
according to International Prognostic Scoring System (IPSS); those
patients may be categorized into different risk groups in order to
provide suitable treatment strategies, particularly in terms of
HIF-1α targeting therapy in different patients with MDS.
Patients and methods
Patients
The Ethics Committee of Jiading District Central
Hospital Affiliated to Shanghai University of Medicine & Health
Sciences (Shanghai, China) approved the present study. On
recruitment, the subjects were fully informed of the experimental
procedures, and the patients signed a consent document for their
excess samples to be used for scientific research. The rights and
privacy of the subjects were protected to the greatest extent. The
present study included 40 patients with MDS (mean age, 62 years;
age range, 40–83 years; 28 males and 12 females) and 20 patients
with hemocytopenia as the control group. The MDS patients were
diagnosed based on the 2008 World Health Organization's criteria.
The control group had suspected hematological disease whose
diagnosis was ruled out (mean age, 54 years; age range, 35–72
years; 12 males and 8 females). The 40 patients with MDS and 20
patients with hemocytopenia who were recruited at the Jiading
District Central Hospital Affiliated to Shanghai University of
Medicine and Health Sciences (Shanghai, China) between September
2017 and May 2019, were examined according to the IPSS (19). The patients with MDS were
classified into the lower-risk group and higher-risk group
(20), as the IPSS score divides
patients into a lower-risk subset (low and intermediate-1) and a
higher-risk subset (intermediate-2 and high). Among these MDS
cases, 8 cases had MDS with single lineage dysplasia (known as
MDS-SLD), 10 cases had MDS with multi-lineage dysplasia (known as
MDS-MLD), 4 cases had MDS with ring sideroblasts and MLD (known as
MDS-RS-MLD), 8 cases had MDS with excess blasts (EB)-1 (MDS-EB-1)
and 10 cases had MDS-EB-2. All MDS cases were treatment-naïve
(Table I).
 | Table I.Clinical manifestation of patients
with MDS (n=40). |
Table I.
Clinical manifestation of patients
with MDS (n=40).
| Item | Value |
|---|
| Sex,
female/male | 12/28 |
| Age, years | 66.9±10.85 |
| IPSS |
|
|
Low | 1 |
|
Intermediate-1 | 22 |
|
Intermediate-2 | 12 |
|
High | 5 |
| Diagnosis |
|
|
MDS-SLD | 8 |
|
MDS-MLD | 10 |
|
MDS-RS | 4 |
|
MDS-EB-1 | 8 |
|
MDS-EB-2 | 10 |
Isolation and culture of BM-MSCs
From each subject, 5 ml of bone marrow was aspirated
in a BD vacutainer containing heparin as an anticoagulant (cat. no.
367884; BD Biosciences). After centrifugation, the supernatant was
discarded and the cells were resuspended in PBS at 25°C.
Ficoll-Paque Plus (cat. no. 17-1440-02; Cytiva) with a density of
1.077 g/ml was used and the cells were centrifuged in a horizontal
centrifuge at 400 × g for 30 min at 25°C. Mononuclear cells were
collected and washed twice with PBS prior to seeding in the BM-MSC
growth culture medium (α-minimum essential medium + 10% FBS + 1%
penicillin/streptomycin; all from Gibco; Thermo Fisher Scientific,
Inc.) at a density of 1×106/cm2 in a culture
flask (Corning, Inc.). The culture flasks were placed in an
incubator a 37°C with 5% CO2 and saturated humidity for
28 days of culture. The BM-MSCs were adherent to the culture
flasks. The BM-MSCs were digested with 1 ml 0.25% trypsin for 3
min. Second- and third-generation cells were used in the
experiments.
BM-MSC flow-cytometry assay
BM-MSCs were digested with 0.25% trypsin for 3 min
at 25°C and resuspended in PBS at a density of 1×106/ml.
The cell suspension was added to test tubes at 500 µl/tube and
processed with a test kit, which contained mouse anti-human
CD45-PE, CD73-APC, CD90-FITC and CD105-PerCP-Cy5.5 antibodies (cat.
no. 562245; BD Biosciences), according to the manufacturer's
instructions. Mouse IgG isotype-PE, isotype-APC, isotype-FITC,
isotype-PerCP-Cy5.5 (cat. no. 562245; BD Biosciences) were also set
up. The tubes were incubated at 4°C for 30 min and washed four
times with PBS before they were loaded into the flow cytometer
(FACSCalibur; BD Biosciences). Flowjo7.6 software (BD Biosciences)
was used for data acquisition and analysis.
HIF-1α expression in BM-MSCs by
reverse transcription-quantitative PCR (RT-qPCR)
Fluorescence RT-qPCR was used to measure HIF-1α
expression in primary BM-MSCs. TRIpure (Aidlab Biotechnologies Co.,
Ltd, China) was used for RNA extraction. HiScript Reverse
Transcriptase (cat. no. R101-01/02; Vazyme Biotech Co., Ltd.) was
used for RT of RNA into cDNA according to the manufacturer's
instructions. AceQ qPCR SYBR-Green Master Mix (cat. no. Q111-02;
Vazyme Biotech Co., Ltd.) was used to analyze target gene
expression using the ABI 7500 system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) The PCR for each gene was conducted as
follows: 95°C for 30 sec, and then 95°C for 5 sec and annealing at
60°C for 30 sec for 45 cycles. The primer sequences are provided in
Table II. The 2−ΔΔCq
method (21) was used to
calculate the relative expression level of mRNA.
 | Table II.Primer sequences for reverse
transcription-quantitative PCR. |
Table II.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′-3′) |
|
|---|
|
| Forward | Reverse |
| HIF-1α |
TCCAAGAAGCCCTAACGTGT |
TGATCGTCTGGCTGCTGTAA |
| GAPDH |
TCAAGAAGGTGGTGAAGCAGG |
TCAAAGGTGGAGGAGTGGGT |
Western blot analysis of HIF-1α
expression in BM-MSCs
Western blot was used to determine the protein
expression of HIF-1α in BM-MSCs. Total protein was extracted from
third-generation BM-MSCs with RIPA lysis buffer. The protein from
the cell lysate was quantified using the BCA protein assay kit
(Thermo Fisher Scientific, Inc.). A total of 15 µg protein extract
was re-suspended in loading buffer prior to resolution by 10%
SDS-PAGE in a Tris-glycine buffer, followed by transfer onto a PVDF
membrane (EMD Millipore). The PVDF membrane was blocked by 5%
skimmed milk (cat. no. 232100; Difco; BD Biosciences) solution at
25°C for 1h. The membrane was incubated with antibodies to HIF-1α
(1:1,000 dilution; cat. no. 79233; Cell Signaling Technology, Inc.)
and β-actin (1:1,000 dilution; cat. no. G043; Applied Biological
Materials) at 4°C overnight. Then the membrane was washed thrice
with Tris-buffered saline containing Tween-20 (TBST) for 5 min each
time. Subsequently, the membrane was incubated with secondary
antibody IRDye 800 CW goat anti-mouse (1:10,000 dilution; cat. no.
926-32210; LI-COR Biosciences) or goat anti-rabbit (1:10,000
dilution; cat. no. 926-32211; LI-COR Biosciences) for 1 h at 25°C,
and then washed thrice with TBST. The membrane was then analyzed
using a two-color infrared fluorescence imaging system (LI-COR
Biosciences).
Cell apoptosis assay
The BM-MCS cells (1×106/ml) from each of
the two groups were re-suspended in culture medium and centrifuged
at 250 × g for 5 min at 25°C. PBS (3 ml) was used to re-suspend the
cells for 5 min prior to passing them through a 300-mesh nylon
sieve once. Alexa Fluor-488 -labeled Annexin V (5 µl; cat. no.
40305ES20; Shanghai Yeasen Biotechnology Co., Ltd.) was added to
each tube and cells were incubated in the dark at room temperature
for 20 min. Subsequently, 10 µl propidium iodide (PI) and 200 µl
working solution were added. The cells were loaded onto the flow
cytometer (FACSCalibur; BD Biosciences) within 30 min after they
were mixed with the solution and 10,000 cells were acquired for
analysis. According to the manufacturer's instructions, Annexin
V+ PI− cells are early apoptotic cells and
Annexin V+ PI+ cells are late apoptotic
cells. The sum of the proportions of these two types of cells is
the apoptosis rate. Annexin V− PI+ cells are
necrotic cells.
Cell cycle experiment
A 0.25% trypsin solution was used to digest the
BM-MSCs at 37°C for 3 min and the cells were then collected.
Subsequently, the cells were centrifuged at 250 × g for 5 min and
the culture medium was discarded. PBS was used to wash the cells
twice, 1 ml of pre-cooled 70% ethanol was added, and the cells were
fixed at 4°C for 30 min. The samples were then centrifuged at 250 ×
g for 5 min at 25°C. Ethanol was aspirated and PBS was added for
washing. The tubes were centrifuged at 250 × g for 5 min before the
supernatant was discarded. PBS (200 µl) and 2 µl (0.25 mg/ml) RNase
A (ST579; Beyotime Institute of Biotechnology) were added and the
mixture was incubated at 37°C for 30 min. PI (5 µl at 50 µg/ml) was
added and the tubes were incubated at room temperature in the dark
for 30 min. Cells were loaded onto the flow cytometer (FACSCalibur;
BD Biosciences) for measurement (FlowJo 7.6; BD Biosciences).
1×106 cells were tested in triplicate and means were
calculated.
Immunohistochemistry
Paraffin sections of bone marrow tissues
successively underwent three changes of xylenes and two changes of
absolute ethanol, 95, 90, 80 and 70% ethanol (5 min each) before
immersion in distilled water for 2 min at 25°C. The thickness of
paraffin sections was 4 µm. A 1% trypsin (Gibco; Thermo Fisher
Scientific, Inc.) retrieval solution was used for antigen retrieval
at 37°C for 30 min. A 3% hydrogen peroxide solution was added to
the tissue sections, followed by incubation at room temperature for
15 min. PBS was used to wash the sections thrice for 3 min each.
The glass slides were wiped dry, and the sections were blocked with
normal goat serum (1:20 dilution; cat. no. C0265; Beyotime
Institute of Biotechnology) at room temperature for 30 min. HIF-1α
antibody (1:100 dilution; cat. no. AF1009; Affinity Biosciences)
was added, followed by incubation at 4°C overnight. Absorbent
towels were used to dry the slides before HRP-labeled goat
anti-rabbit secondary antibody (1:5,000 dilution; cat. no.
074-1506; KPL) was added, after which slides were incubated at room
temperature for 20 min. Absorbent towels were used to dry the
slices before a freshly prepared DAB color development solution
(Shanghai Junrui Biotechnology Co., Ltd.) was added. Color
development was stopped by rinsing with distilled water. Harris
hematoxylin (cat. no. H9627; MilliporeSigma) was added for 3 min
for counterstaining. After washing, 1% of hydrochloric acid-alcohol
was used for differentiation before washing with PBS. The slices
were put into 75%, 85% and absolute ethanol for 6 min each and then
in xylene (Sinopharm Chemical Reagent Co., Ltd) for 5 min for
dehydration. The slices were then dried and sealed with neutral gum
(Sinopharm Chemical Reagent Co., Ltd). The sections were observed
and images were acquired under a microscope imaging system (DS-U3;
Nikon Corporation). The immunohistochemistry results were analyzed
using Image Pro Plus software 6.0 (Media Cybernetics, Inc.). The
mean optical density was obtained by dividing the integrated
optical density sum/area sum.
Statistical analysis
All of the experiments were repeated three times.
Statistical analysis was performed using GraphPad Prism 8
statistical software (GraphPad Software, Inc.). Values are
expressed as the mean ± standard deviation and an unpaired t-test
was used to compare independent samples between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
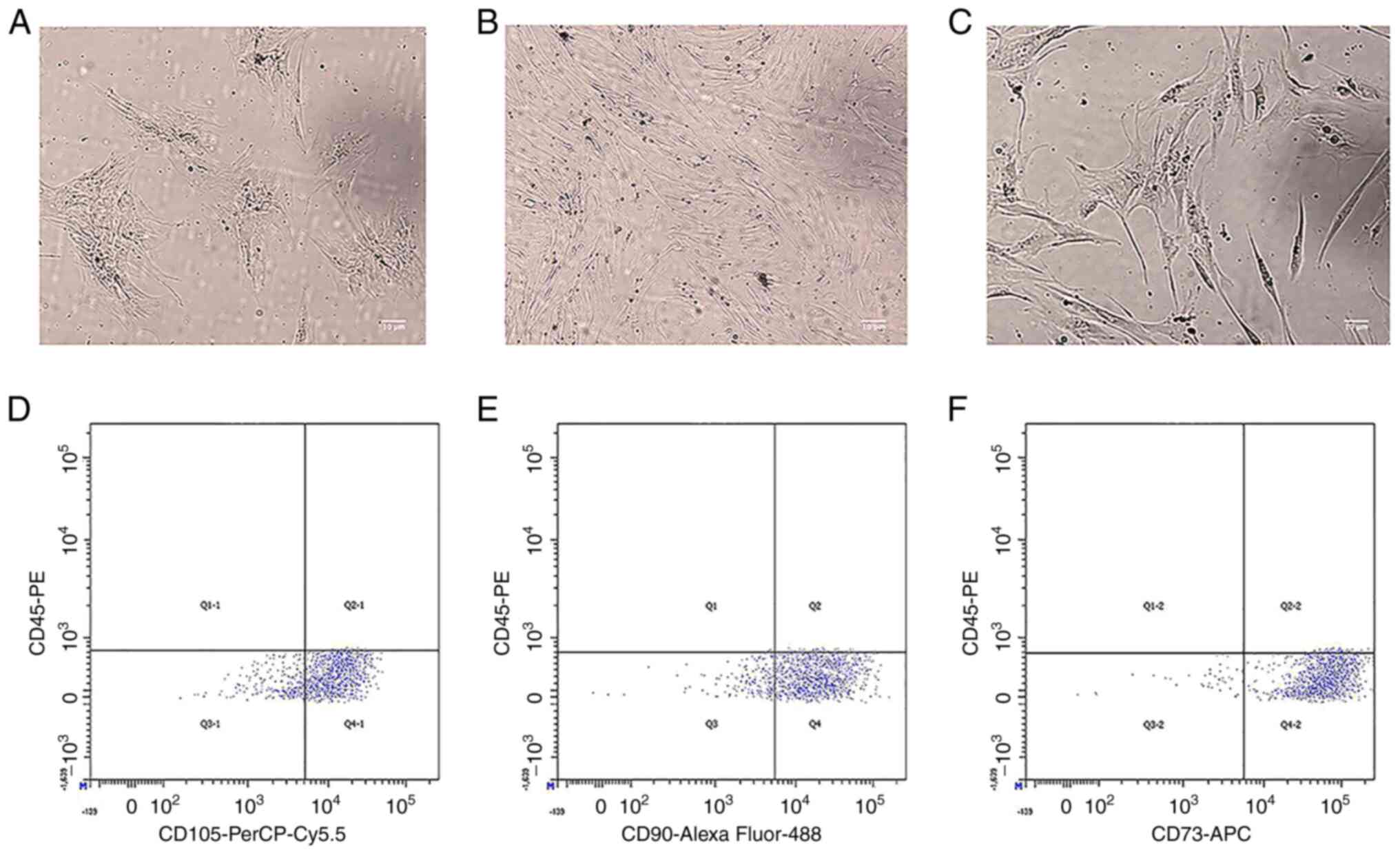
Morphological characteristics and flow
cytometry of BM-MSCs
Morphological examination revealed that the BM-MSCs
obtained from 40 patients with MDS and 20 control subjects appeared
as clusters of fibroblast-like cells. Fibroblast colony-forming
units were observed in 5 out of 40 MDS samples (Fig. 1A). No notable morphological
differences were observed between the MDS patient group and control
group (Fig. 1B and C). To verify
that the obtained cells were BM-MSCs, flow cytometry was used to
perform immunophenotypic analysis of primary cells cultured from
the bone marrow, and the cells were passaged to the third
generation prior to being used in this experiment. The flow
cytometry results evaluating the immunophenotype of BM-MSCs
isolated from the bone marrow of patients with MDS revealed
identical cell immune marker expression to that reported by
Flores-Figueroa et al (22) on BM-MSCs from normal adult bone
marrow. These findings included high expression of CD105, CD90 and
CD73 and no expression of CD45. The cells were positive for CD105
(79.6%), CD90 (83.2%) and CD73 (94.3%) (Fig. 1D-F) and negative for CD45.
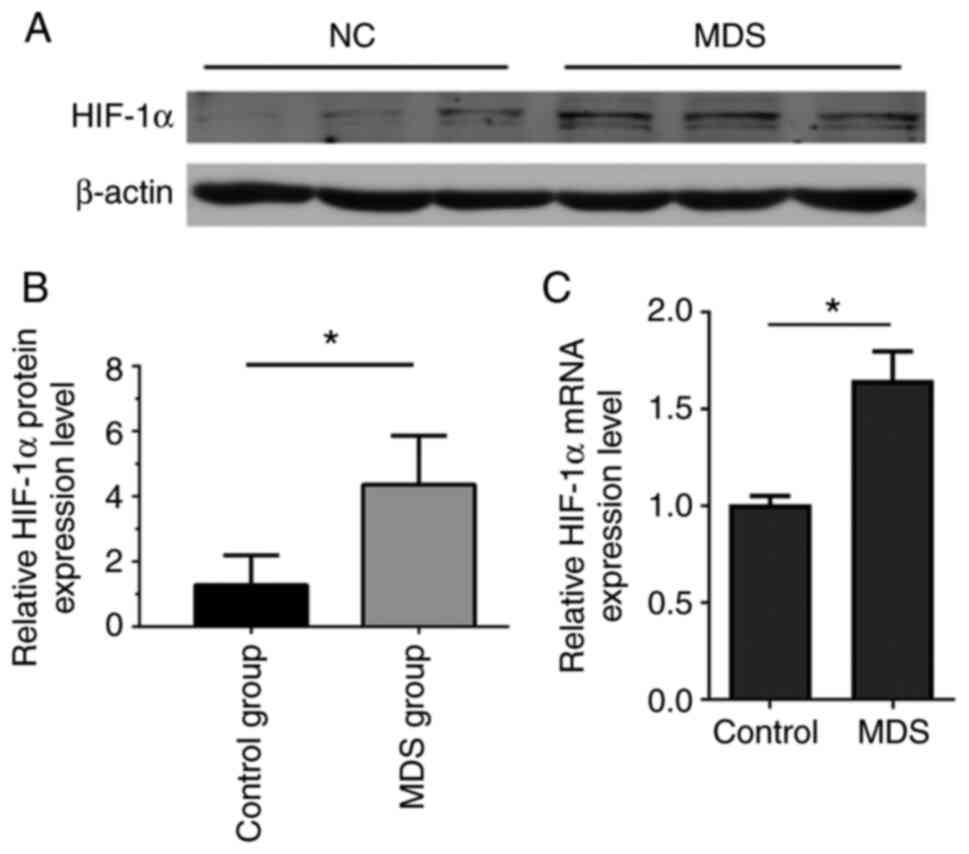
HIF-1α expression is upregulated in
BM-MSCs from the MDS group
To determine the differences in HIF-1α expression
between the MDS and control groups, western blot and RT-PCR were
used to measure HIF-1α protein and mRNA expression, respectively,
in BM-MSCs in the two groups (Fig.
2). The results indicated a statistically significant
difference in HIF-1α protein expression between the MDS group and
the control group (P<0.05; Fig. 2A
and B). BM-MSCs in the MDS group and control group were
passaged until the third generation before RT-qPCR was performed,
revealing a significant difference in HIF-1α mRNA expression
between the MDS group and the control group (P<0.05; Fig. 2C). These results indicated that
HIF-1α expression was altered in MDS BM-MSCs. Changes in the
hematopoietic microenvironment may lead to MDS. To determine
whether high expression of HIF-1α mRNA in BM-MSCs from patients
with MDS (and presumably the corresponding high HIF-1α protein
levels) affect apoptosis and the cell cycle, the subsequent
experiments were performed.
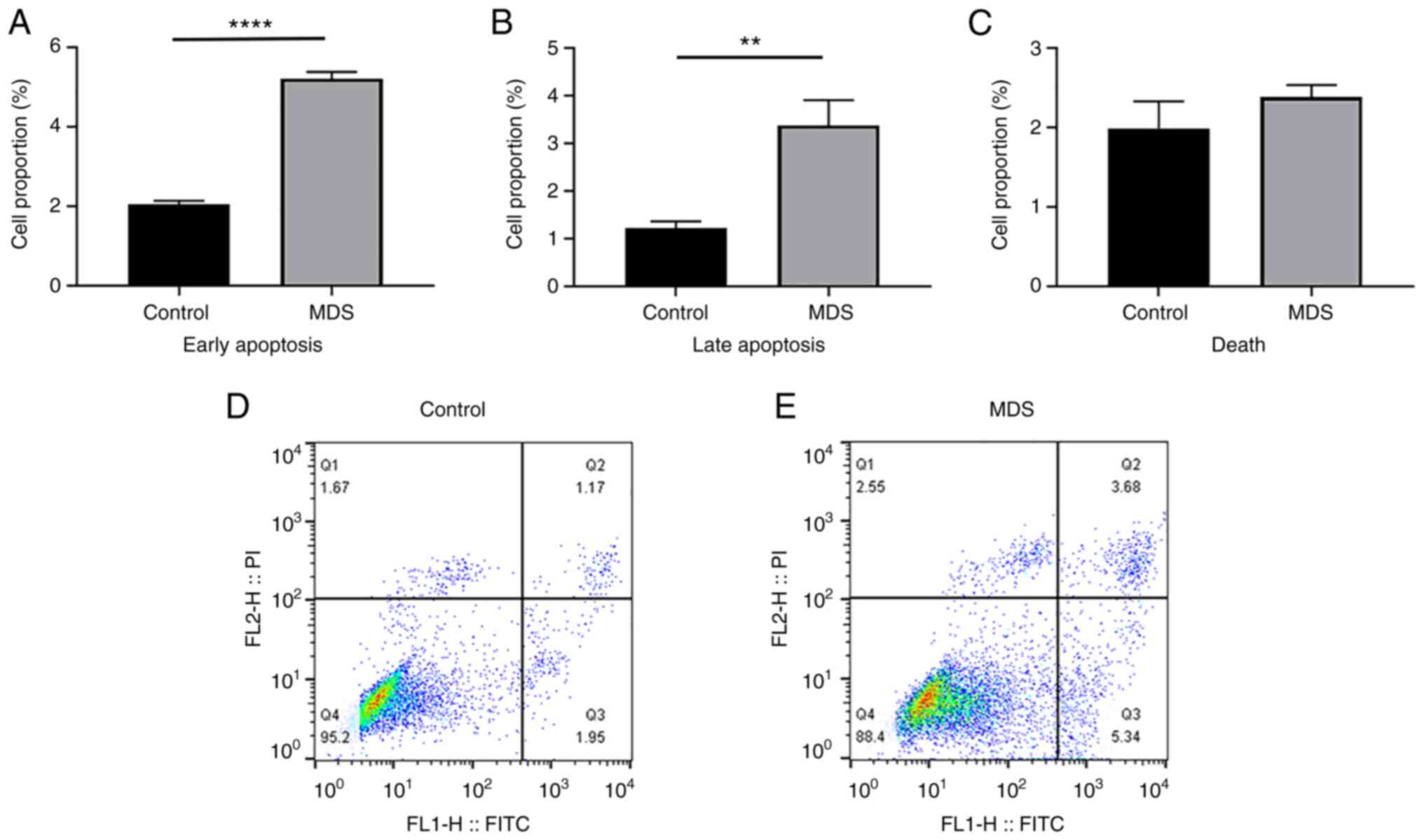
Apoptotic cell proportion of
BM-MSCs
BM-MSCs in the MDS and control groups were passaged
until the third generation prior to analysis by flow cytometry with
an apoptosis assay kit to measure the proportion of apoptotic
cells. Comparison of BM-MSCs from the control and MDS groups
indicated that the MDS group had a higher proportion of cells in
early apoptosis (2.04±0.08 vs. 5.22±1.34%; P<0.0001) and late
apoptosis (1.23±0.11 vs. 3.38±0.43%; P<0.01), but there was no
difference in the proportion of necrotic cells (1.99±0.28 vs.
2.38±0.12%; P=0.142; Fig. 3A-C).
This demonstrated that MDS BM-MSCs had a higher proportion of
apoptosis than normal BM-MSCs.
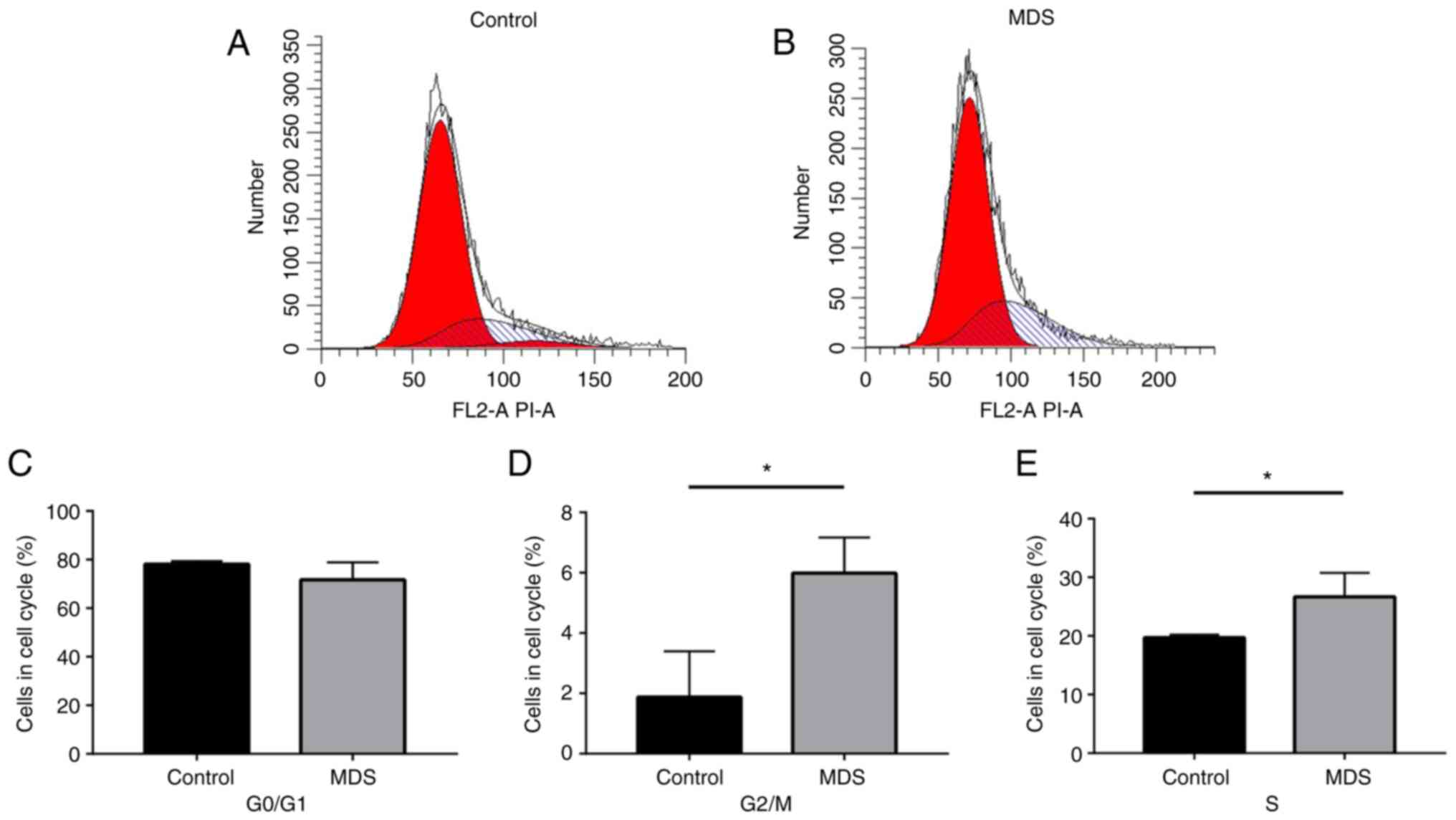
Cell cycle of BM-MSCs
To further identify changes in the cell cycle in
BM-MSCs in MDS, an assay kit and flow cytometry were used to
measure the cell cycle distribution of BM-MSCs from the MDS and
control groups (Fig. 4A and B).
The results suggested that the G0/G1 phase
populations were not significantly different between the MDS and
control groups (Fig. 4C).
However, the BM-MSCs of the MDS group had a significantly higher
proportion of cells in S phase and G2/M phase compared
with the control group (P<0.05; Fig. 4D and E).
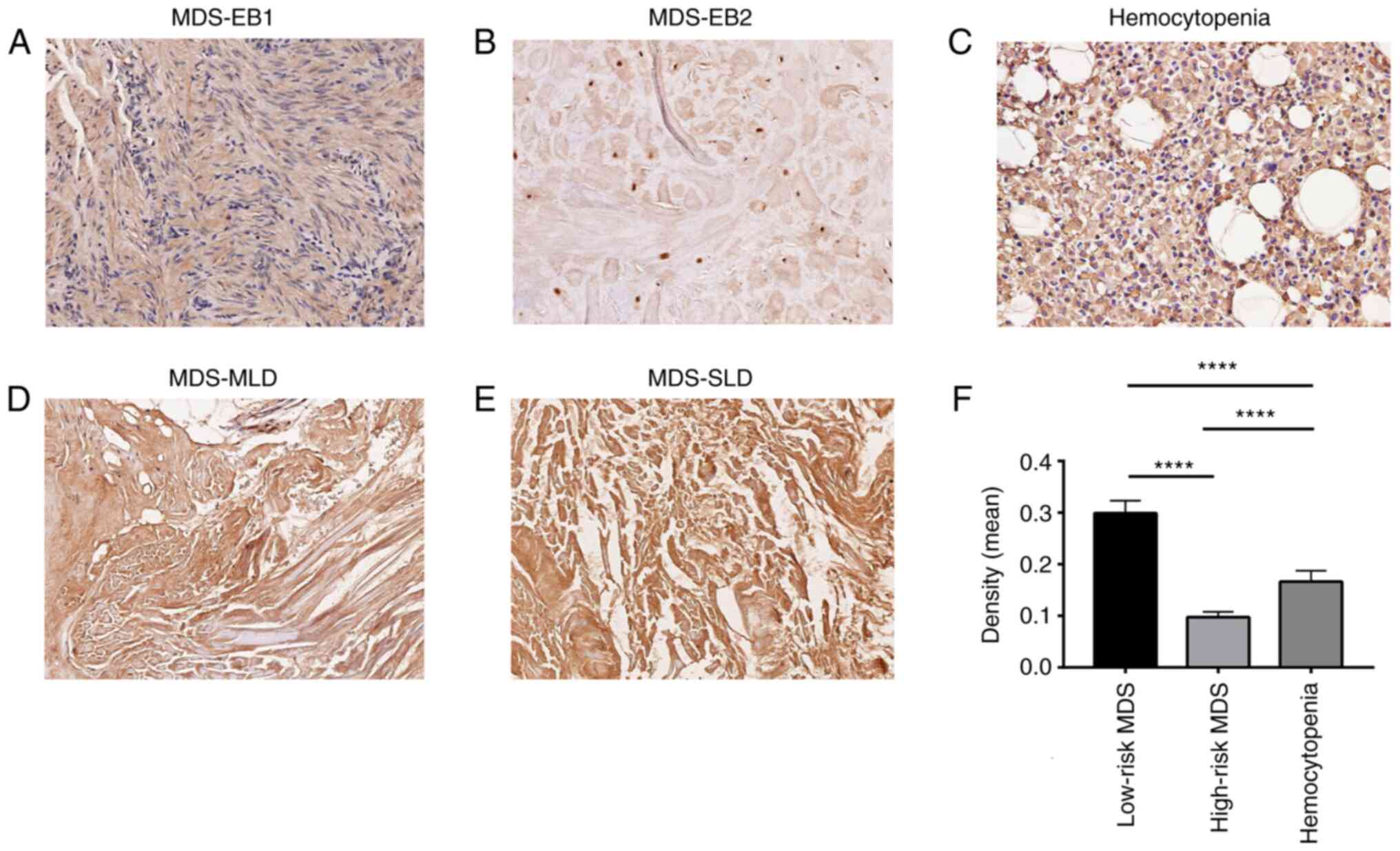
Immunohistochemical analysis
Immunohistochemistry was used to analyze HIF-1α
expression in bone marrow tissues from patients with MDS (Fig. 5). According to the prognosis
determined by IPSS, the patients with MDS were divided into two
groups: A total of 16 patients were assigned to the lower-risk MDS
group and 7 to the higher-risk MDS group. Furthermore, 17
hemocytopenia samples were used for analysis. The results indicated
that HIF-1α expression in bone marrow tissue sections from the MDS
lower-risk group (intermediate-1) (Fig. 5C and D) was higher than that in
the MDS higher-risk group (P<0.0001; Fig. 5A and B).
Discussion
MDS is a disease in which abnormalities are present
in hematopoietic stem cells and the bone marrow microenvironment
(23). BM-MSCs are an essential
constituent of the bone marrow microenvironment and possess
self-renewal and pluripotent potential (24). BM-MSCs are able to differentiate
into osteoblastic, adipogenic and chondroblastic cells (25), promote hematopoiesis and regulate
hematopoietic stem cells to ensure they maintain their
hematopoietic capabilities throughout their entire lifespan.
BM-MSCs also have immuno-regulatory functions, as they maintain the
stability of the bone marrow immune microenvironment and have been
indicated to reduce the damage caused by stress stimuli on
hematopoietic stem cells in in vitro experiments (26). BM-MSCs were able to protect
intramedullary blast cells from damage caused by NK cells. In
animal experiments, gene abnormalities in BM-MSCs have been
demonstrated to induce MDS (27).
Currently, there is a limited understanding of the genetics and
gene expression characteristics of BM-MSCs in MDS. However, the
importance of functional senescence in MDS BM-MSCs and its impact
on disease progression, prognosis evaluation and treatment effects
is widely recognized (28). This
may explain why current treatments that only target the multiple
mechanisms of clonal hematopoiesis in MDS resulted in poor efficacy
in certain patients (29).
Therefore, the effect of changes in the bone marrow
microenvironment, particularly concerning regulatory mechanisms of
BM-MSCs on MDS development, progression and treatment, is worth of
further investigation.
Under physiological conditions, there are multiple
pathways that regulate biological behavior in numerous
stem/progenitor cells to adapt to a hypoxic environment, which has
certain effects on gene expression in BM-MSCs (30,31). HIF-1α is an effector in a hypoxic
environment. A hypoxic environment may promote endothelial cell
proliferation, remodeling and the synthesis of pro-angiogenic
factors such as VEGF, bFGF, CXC12 through HIF-1, thereby inducing
angiogenesis (32). HIF-1α has
also been indicated to participate in enhanced innate immune
responses and it regulates the activation of macrophage migration
inhibitory factor (MIF) (33).
MIF was observed to be highly expressed in AML cells (34) and a number of solid tumor types,
including glioblastoma (35–37), neuroblastoma (38) and melanoma (39). However, overexpression of MIF is
associated with poor outcome in patients with MDS (40). HIF-1α is associated with AML
progression, and if treatment-naïve AML cells contain high levels
of HIF-1α, they have an increased tendency toward extramedullary
invasion and poor prognosis (41). It may be hypothesized that HIF-1α
participates in the oncogenesis and progression of myeloid leukemia
and the specific pathways and effects involved require further
study. Aberrant HIF-1α stabilization and abnormal mitochondrial and
autophagic death were observed in the bone marrow of patients with
MDS (41). The expression of
HIF-1α was associated with poor overall survival and disease
progression of MDS, as well as with the bone marrow blast
percentage and cytogenetics (42). Examining and evaluating the
effects of hypoxic factors and elucidating their mechanisms of
action may provide a potential therapeutic target for the diagnosis
and treatment of MDS (43).
In the present study, it was observed that there
were no notable morphological differences between BM-MSCs from
patients with MDS and those from healthy human bone marrow. BM-MSCs
from both groups exhibited long vortex-like fibers and were
arranged in a neat and orderly manner, which is similar to what has
been reported in the literature. Immuno-phenotype flow cytometry
confirmed that the cultured cells were BM-MSCs. Experiments
demonstrated that HIF-1α expression was significantly upregulated
in BM-MSCs from the MDS group compared with those from the control
group (P<0.05). HIF-1α staining of bone marrow biopsy tissues
suggested that HIF-1α expression in the low- and intermediate-risk
MDS groups was higher than that in the high-risk group
(P<0.0001). In addition, a higher proportion of apoptotic
BM-MSCs was present in the MDS group compared with that in the
control group, and cell cycle distribution differences were
simultaneously present. An increased proportion of BM-MSCs from the
MDS group in the S-phase and G2/M phase compared with
those from the control group was observed, while the
G0/G1-phase populations were similar.
Although the above results differ from those reported in the
literature (44), the present
results suggested that hematopoietic arrest is present in BM-MSCs
from patients with MDS. In the present study, the proportion of
apoptotic BM-MSCs was higher in the MDS group than in the control
group (45). Senescence of
BM-MSCs in the MDS bone marrow microenvironment may explain why
persistent peripheral blood cytopenia is present in lower patients
in the MDS group (45). It is
worth noting that there are significant differences in the
diagnosis and treatment strategies for low- and intermediate-risk
MDS compared with strategies for high-risk MDS. Supportive
treatment, immunotherapy and cytokine treatment are the mainstay
treatments for the former group, whose main clinical presentation
is a reduction in one to three lineages of peripheral blood cells
(46). In the present study,
HIF-1α expression was measured in the bone marrow biopsy tissues
from patients with MDS to reveal that the expression of HIF-1α in
the bone marrow biopsy tissues of patients with low- and
intermediate-risk MDS was significantly higher than that of
patients with high-risk MDS. This suggested that the
pathophysiology of low- and intermediate-risk MDS is different from
that of high-risk MDS, and accordingly, it was hypothesized that
HIF-1α may serve as a potential therapeutic target for MDS. Further
research on excessive apoptosis and senescence in BM-MSCs in
patients with low- and intermediate-risk MDS is required. In the
present study, 40 patients with MDS and 20 patients with
hemocytopenia were analyzed, but the number of cases may have been
insufficient; thus, the number of subjects may be increased in a
future study.
Certain studies observed increased apoptosis in
BM-MSCs in the early stage of MDS, resulting in ineffective
hematopoiesis and affecting the survival of hematopoietic cells,
which in turn resulted in pancytopenia (47). Reduced apoptosis and proliferation
of clonal cells occurring in the late stage and persistent clonal
proliferation may cause MDS to progress to AML. The
pathophysiological mechanisms underlying the early and late stages
of this disease have been elucidated. It has been noted that
increased apoptosis, which is associated with ineffective
progenitors and survival of hematopoietic cells, contributes to
cytopenia during the early stages (48). As a consequence of these defective
pathways, cytopenia and ineffective hemopoiesis takes place.
However, prolonged late-stage MDS may lead to AML, with a decrease
in apoptosis, and an increased degree of neoplastic cell survival
may be observed (49,50). The mechanisms promoting the
conversion of excessive apoptosis in the early stage to reduced
apoptosis and accelerated progression in the late stage remain
elusive and the effects of the bone marrow microenvironment during
progression cannot be ignored. The effects of hypoxia and HIF-1α
expression on clonal cells and the bone marrow microenvironment
require further investigation.
In the present study, HIF-1α overexpression occurred
in primary BM-MSCs from patients with MDS and these BM-MSCs
exhibited excessive apoptosis and senescence, which differs from
literature reports indicating that HIF-1α overexpression under
normoxic conditions may lead to higher survival and inhibit
apoptosis in MSCs (51). It was
previously reported that increased HIF-1 expression in a hypoxic
environment promoted the differentiation of MSCs into endothelial
cells as well as bone marrow angiogenesis, leading to increased
erythroid proliferation and an increased red blood cell count
(51), which differs from the
observations of the present study that indicated excessive
apoptosis and growth arrest of BM-MSCs from patients with MDS and
cannot explain cytopenia in MDS. Senescence of MDS-MSCs may explain
cytopenia in low- and moderate-risk patients. The mechanisms
underlying the clonal proliferation and the driving factors for
transformation in the high-risk group require further investigation
to ensure that screening for patients who are more suitable for
HIF-1α-targeted therapy will be possible in the future.
Acknowledgements
The authors would like to thank Dr Shuang Sha
(Shanghai Key Laboratory of Molecular Imaging, Shanghai University
of Medicine and Health Sciences) and Dr Yan Dong (Department of
Hematology, Tongji Hospital of Tongji University) for revising the
manuscript and providing advice regarding the data analysis.
Funding
The present study was supported by the Shanghai Jiading District
Health Bureau Research Project (grant no. 2016-KY-02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BQ participated in writing the manuscript and
performed the statistical analysis. XH participated in the
experimental design and data acquisition. LZ, FZ and QG performed
the experiments. BQ and XH confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Jiading District Central
Hospital Affiliated to Shanghai University of Medicine & Health
Sciences (Shanghai, China) approved the present study and all
subjects provided written informed consent for the use of their
samples in research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Platzbecker U, Kubasch AS,
Homer-Bouthiette C and Prebet T: Current challenges and unmet
medical needs in myelodysplastic syndromes. Leukemia. 35:2182–2198.
2021. View Article : Google Scholar
|
|
2
|
Adès L, Itzykson R and Fenaux P:
Myelodysplastic syndromes. Lancet. 383:2239–2252. 2014. View Article : Google Scholar
|
|
3
|
Raaijmakers MH: Myelodysplastic syndromes:
Revisiting the role of the bone marrow microenvironment in disease
pathogenesis. Int J Hematol. 95:17–25. 2012. View Article : Google Scholar
|
|
4
|
Ishibashi M, Tamura H and Ogata K: Disease
progression mechanism in myelodysplastic syndromes: Insight into
the role of the microenvironment. Leuk Res. 35:1449–1452. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Medyouf H, Mossner M, Jann JC, Nolte F,
Raffel S, Herrmann C, Lier A, Eisen C, Nowak V, Zens B, et al:
Myelodysplastic cells in patients reprogram mesenchymal stromal
cells to establish a transplantable stem cell niche disease unit.
Cell Stem Cell. 14:824–837. 2014. View Article : Google Scholar
|
|
6
|
Balderman SR, Li AJ, Hoffman CM, Frisch
BJ, Goodman AN, LaMere MW, Georger MA, Evans AG, Liesveld JL,
Becker MW and Calvi LM: Targeting of the bone marrow
microenvironment improves outcome in a murine model of
myelodysplastic syndrome. Blood. 127:616–625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raaijmakers MH, Mukherjee S, Guo S, Zhang
S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian
RP, Scadden EO, et al: Bone progenitor dysfunction induces
myelodysplasia and secondary leukaemia. Nature. 464:852–857. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roela RA, Carraro DM, Brentani HP, Kaiano
JH, Simão DF, Guarnieiro R, Lopes LF, Borojevic R and Brentani MM:
Gene stage-specific expression in the microenvironment of pediatric
myelodysplastic syndromes. Leuk Res. 31:579–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Corradi G, Baldazzi C, Očadlíková D,
Marconi G, Parisi S, Testoni N, Finelli C, Cavo M, Curti A and
Ciciarello M: Mesenchymal stromal cells from myelodysplastic and
acute myeloid leukemia patients display in vitro reduced
proliferative potential and similar capacity to suppotr leukemia
cell survival. Stem Cell Res Ther. 9:2712018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frolova O, Samudio I, Benito JM, Jacamo R,
Kornblau SM, Markovic A, Schober W, Lu H, Qiu YH, Buglio D, et al:
Regulation of HIF-1α signaling and chemoresistance in acute
lymphocytic leukemia under hypoxic conditions of the bone marrow
microenvironment. Cancer Biol Ther. 13:858–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Albadari N, Deng S and Li W: The
transcriptional factors HIF-1 and HIF-2 and their novel inhibitors
in cancer therapy. Expert Opin Drug Discov. 14:667–682. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Minami T, Matsumura N, Sugimoto K, Shimizu
N, De Velasco M, Nozawa M, Yoshimura K, Harashima N, Harada M and
Uemura H: Hypoxia-inducing factor (HIF)-1α-derived peptide capable
of inducing cancer-reactive cytotoxic T lymphocytes from
HLA-A24+ patients with renal cell carcinoma. Int
Immunopharmacol. 44:197–202. 2017. View Article : Google Scholar
|
|
13
|
Lotfinia M, Lak S, Mohammadi Ghahhari N,
Johari B, Maghsood F, Parsania S, Sadegh Tabrizi B and Kadivar M:
Hypoxia Pre-Conditioned embryonic mesenchymal stem cell secretome
reduces IL-10 production by peripheral blood mononuclear cells.
Iran Biomed J. 21:24–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashi Y, Zhang Y, Yokota A, Yan X, Liu
J, Choi K, Li B, Sashida G, Peng Y, Xu Z, et al: Pathobiological
pseudohypoxia as a putative mechanism underlying myelodysplastic
syndromes. Cancer Discov. 8:1438–1457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayashi Y: Role of HIF1A in the
development of myelodysplastic syndromes. Rinsho Ketsueki.
60:818–823. 2019.(In Japanese).
|
|
16
|
Ma CP, Liu H, Yi-Feng Chang I, Wang WC,
Chen YT, Wu SM, Chen HW, Kuo YP, Shih CT, Li CY and Tan BC: ADAR1
promotes robust hypoxia signaling via distinct regulation of
multiple HIF-1α-inhibiting factors. EMBO Rep. 20:e471072019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JH, Yoon YM and Lee SH: Hypoxic
preconditioning promotes the bioactivities of mesenchymal stem
cells via the HIF-1α-GRP78-Akt Axis. Int J Mol Sci. 18:13202017.
View Article : Google Scholar
|
|
18
|
Chen T, Zhu H, Wang Y, Zhao P, Chen J, Sun
J, Zhang X and Zhu G: Apoptosis of bone marrow mesenchymal
stromal/stem cells via the MAPK and endoplasmic reticulum stress
signaling pathways. Am J Transl Res. 10:2555–2566. 2018.PubMed/NCBI
|
|
19
|
Benton CB, Khan M, Sallman D, Nazha A,
Nogueras González GM, Piao J, Ning J, Aung F, Al Ali N, Jabbour E,
et al: Prognosis of patients with intermediate risk IPSS-R
myelodysplastic syndrome indicates variable outcomes and need for
models beyond IPSS-R. Am J Hematol. 93:1245–1253. 2018. View Article : Google Scholar
|
|
20
|
Garcia-Manero G: Myelodysplasic syndromes:
2011 update on diagnosis, risk-stratification, and management. Am J
Hematol. 86:490–498. 2011. View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flores-Figueroa E, Varma S, Montgomery K,
Greenberg PL and Gratzinger D: Distinctive contact between
CD34+ hematopoietic progenitors and CXCL12+
CD271+ mesenchymal stromal cells in benign and
myelodysplastic bone marrow. Lab Invest. 92:1330–1341. 2012.
View Article : Google Scholar
|
|
23
|
Purwaningrum M, Jamilah NS, Purbantoro SD,
Sawangmake C and Nantavisai S: Comparative characteristic study
from bone marrow-derived mesenchymal stem cells. J Vet Sci.
22:e742021. View Article : Google Scholar
|
|
24
|
Busser H, Najar M, Raicevic G, Pieters K,
Velez Pombo R, Philippart P, Meuleman N, Bron D and Lagneaux L:
Isolation and characterization of human mesenchymal stromal cells
subpopulations: Comparison of bone marrow and adipose tissue. Stem
Cells Dev. 24:2142–2157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cicione C, Muiños-López E, Hermida-Gómez
T, Fuentes-Boquete I, Díaz-Prado S and Blanco FJ: Effects of severe
hypoxia on bone marrow mesenchymal stem cells differentiation
potential. Stem Cells Int. 2013:2328962013. View Article : Google Scholar
|
|
27
|
Guang FR, Ling Z, Huang JS, Huang WX, Gong
BD, Fang XX, Zhang XY and Tang JP: Effect of mesenchymal stem cells
on Sjögren-like mice and the microRNA expression profiles of
splenic CD4+ T cells. Exp Ther Med. 13:2828–2838. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnson RC, Kurzer JH, Greenberg PL and
Gratzinger D: Mesenchymal stromal cell density is increased in
higher grade myelodysplastic syndromes and independently predicts
survival. Am J Clin Pathol. 142:795–802. 2014. View Article : Google Scholar
|
|
29
|
Cluzeau T, Sebert M, Rahmé R, Cuzzubbo S,
Lehmann-Che J, Madelaine I, Peterlin P, Bève B, Attalah H, Chermat
F, et al: Eprenetapopt plus azacitidine in TP53-mutated
myelodysplastic syndromes and acute myeloid leukemia: A phase II
Study by the Groupe Francophone des Myelodysplasies (GFM). J Clin
Oncol. 39:1575–1583. 2021. View Article : Google Scholar
|
|
30
|
Gars E, Yousry SM, Babu D, Kurzer JH,
George TI and Gratzinger D: A replicable CD271+
mesenchymal stromal cell density score: Bringing the dysfunctional
myelodysplastic syndrome niche to the diagnostic laboratory. Leuk
Lymphoma. 58:1730–1732. 2017. View Article : Google Scholar
|
|
31
|
Ohnishi S, Yasuda T, Kitamura S and Nagaya
N: Effect of hypoxia on gene expression of bone marrow-derived
mesenchymal stem cells and mononuclear cells. Stem Cells.
25:1166–1177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elshabrawy HA, Chen Z, Volin MV, Ravella
S, Virupannavar S and Shahrara S: The pathogenic role of
angiogenesis in rheumatoid arthritis. Angiogenesis. 18:433–448.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alonso D, Serrano E, Bermejo FJ and Corral
RS: HIF-1α-regulated MIF activation and Nox2-dependent ROS
generation promote Leishmania amazonensis killing by macrophages
under hypoxia. Cell Immunol. 335:15–21. 2019. View Article : Google Scholar
|
|
34
|
Abdul-Aziz AM, Shafat MS, Mehta TK, Di
Palma F, Lawes MJ, Rushworth SA and Bowles KM: MIF-Induced Stromal
PKCβ/IL8 is essential in human acute myeloid Leukemia. Cancer Res.
77:303–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mangano K, Mazzon E, Basile MS, Di Marco
R, Bramanti P, Mammana S, Petralia MC, Fagone P and Nicoletti F:
Pathogenic role for macrophage migration inhibitory factor in
glioblastoma and its targeting with specific inhibitors as novel
tailored therapeutic approach. Oncotarget. 9:17951–17970. 2018.
View Article : Google Scholar
|
|
36
|
Xu S, Guo X, Gao X, Xue H, Zhang J, Guo X,
Qiu W, Zhang P and Li G: Macrophage migration inhibitory factor
enhances autophagy by regulating ROCK1 activity and contributes to
the escape of dendritic cell surveillance in glioblastoma. Int J
Oncol. 49:2105–2115. 2016. View Article : Google Scholar
|
|
37
|
Presti M, Mazzon E, Basile MS, Petralia
MC, Bramanti A, Colletti G, Bramanti P, Nicoletti F and Fagone P:
Overexpression of macrophage migration inhibitory factor and
functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in
glioblastoma. Oncol Lett. 16:2881–2886. 2018.
|
|
38
|
Cavalli E, Ciurleo R, Petralia MC, Fagone
P, Bella R, Mangano K, Nicoletti F, Bramanti P and Basile MS:
Emerging role of the macrophage migration inhibitory factor family
of cytokines in neuroblastoma. Pathogenic effectors and novel
therapeutic targets? Molecules. 25:11942020.
|
|
39
|
Soumoy L, Kindt N, Ghanem G, Saussez S and
Journe F: Role of macrophage migration inhibitory factor (MIF) in
melanoma. Cancers (Basel). 11:5292019. View Article : Google Scholar
|
|
40
|
Falantes JF, Trujillo P, Piruat JI,
Calderón C, Márquez-Malaver FJ, Martín-Antonio B, Millán A, Gómez
M, González J, Martino ML, et al: Overexpression of GYS1, MIF, and
MYC is associated with adverse outcome and poor response to
azacitidine in myelodysplastic syndromes and acute myeloid
leukemia. Clin Lymphoma Myeloma Leuk. 15:236–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stergiou IE, Kambas K, Poulaki A,
Giannouli S, Katsila T, Dimitrakopoulou A, Vidali V, Mouchtouris V,
Kloukina I, Xingi E, et al: Exploiting the role of
hypoxia-inducible factor 1 and pseudohypoxia in the myelodysplastic
syndrome pathophysiology. Int J Mol Sci. 22:40992021. View Article : Google Scholar
|
|
42
|
Tong H, Hu C, Zhuang Z, Wang L and Jin J:
Hypoxia-inducible factor-1α expression indicates poor prognosis in
myelodysplastic syndromes. Leuk Lymphoma. 53:2412–2418. 2012.
View Article : Google Scholar
|
|
43
|
Liu Z, Tian M, Ding K, Liu H, Wang Y and
Fu R: High expression of PIM2 induces HSC proliferation in
myelodysplastic syndromes via the IDH1/HIF1-α signaling pathway.
Oncol Lett. 17:5395–5402. 2019.
|
|
44
|
Pleyer L, Valent P and Greil R:
Mesenchymal stem and progenitor cells in normal and dysplastic
hematopoiesis-masters of survival and clonality? Int J Mol Sci.
17:10092016. View Article : Google Scholar
|
|
45
|
Blau O, Baldus CD, Hofmann WK, Thiel G,
Nolte F, Burmeister T, Türkmen S, Benlasfer O, Schümann E, Sindram
A, et al: Mesenchymal stromal cells of myelodysplastic syndrome and
acute myeloid leukemia patients have distinct genetic abnormalities
compared with leukemic blasts. Blood. 118:5583–5592. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang H, Niu H, Zhang T, Xing L, Shao Z and
Fu R: Low- and intermediate-risk myelodysplastic syndrome with pure
red cell aplasia. Hematology. 26:444–446. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sato F, Miyaoka Y, Miyajima A and Tanaka
M: Oncostatin M maintains the hematopoietic microenvironment in the
bone marrow by modulating adipogenesis and osteogenesis. PLoS One.
9:e1162092014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tehranchi R, Fadeel B, Forsblom AM,
Christensson B, Samuelsson J, Zhivotovsky B and Hellstrom-Lindberg
E: Granulocyte colony-stimulating factor inhibits spontaneous
cytochrome C release and mitochondria-dependent apoptosis of
myelodysplastic syndrome hematopoietic progenitors. Blood.
101:1080–1086. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ferrer RA, Wobus M, List C, Wehner R,
Schönefeldt C, Brocard B, Mohr B, Rauner M, Schmitz M, Stiehler M,
et al: Mesenchymal stromal cells from patients with myelodyplastic
syndrome display distinct functional alterations that are modulated
by lenalidomide. Haematologica. 98:1677–1685. 2013. View Article : Google Scholar
|
|
50
|
Bakhtiari T, Ghaderi A, Safaee Nodehi SR,
Aghazadeh Z, Tofighi Zavareh F, Jafarnezhad-Ansariha F, Barati A
and Mirshafiey A: An in vitro assessment for evaluating the
efficiency of β-d-mannuronic acid (M2000) in myelodysplastic
syndrome. J Cell Physiol. 234:12971–12977. 2019. View Article : Google Scholar
|
|
51
|
da Silva-Coelho P, Kroeze LI, Yoshida K,
Koorenhof-Scheele TN, Knops R, van de Locht LT, de Graaf AO, Massop
M, Sandmann S, Dugas M, et al: Clonal evolution in myelodysplastic
syndromes. Nat Commun. 8:150992017. View Article : Google Scholar : PubMed/NCBI
|